Introduction
Co-culturing cells with vascular endothelial cells
(VECs) has the dual advantage of improving vascularization rates,
and increasing the promotion of osteoblast and bone marrow
mesenchymal stem cell differentiation (1). Osteoblasts secrete vascular
endothelial growth factor (VEGF) to promote cell proliferation and
differentiation of endothelial cells (ECs), while ECs can affect
osteogenic differentiation by secreting bone morphogenetic proteins
(BMPs) (2,3). This facilitates the interaction
between osteoblasts and ECs to promote the formation of new bone
and blood vessels, with VEGF being a key mediator for angiogenesis
(4,5). Co-culture studies showed that a human
bone marrow EC line increased the cell proliferation of human bone
marrow-derived fibroblasts using gelatine-coated and
hydroxyapatite-coated substrates (6). Adipose-derived stem cells (ADSCs)
express VEGF and hepatocyte growth factor so a co-culture of VECs
and ADSCs has the potential to differentiate into fat, bone,
cartilage, and skeletal and smooth muscle cells; therefore, these
cells could be useful sources for bone engineering (7). Increased melanocyte proliferation and
migration, as well as reduced differentiation, was observed when
they were co-cultured with ADSCs compared with melanocyte
mono-cultures (8).
Various different scaffolds have been proposed for
tissue engineering. Scaffolds are typically composed of natural or
human-made polymers, bioceramics and hybrid materials (9). Partially deproteinized biologic bone
(PDPBB) is a relatively novel scaffold used in bone tissue
engineering that is prepared using fibronectin combined with
partially deproteinized bone (PDPB) (10). PDPB is a natural bioderived bone
scaffold material obtained by natural bone physicochemical
treatment, which maintains the natural reticulated pore structure
of the original bone (11). It is
comprised of 22.4% protein, has a calcium-phosphorus ratio of 1:74
and contains hydroxyapatite for improved histocompatibility during
osteogenesis (12). Preparation of
the scaffold material removes the antigenicity of the material, and
also removes the matrix necessary for cell and scaffold material
adhesion (13). It has been found
that when PDPB is co-cultured with cells in vitro, cell
activity decreases gradually, and PDPB ages and sheds from the
scaffold over a period of time (14). Therefore, in order to improve the
efficacy of PDPB, fibronectin, which exists in normal bone matrix
and is secreted by osteoblasts, has been used to prepare PDPBB
(15). It is hypothesized that
PDPBB is biocompatible because fibronectin improves the
histocompatibility of PDPB. It has been demonstrated that PDPBB
seeded in a co-culture with bone marrow stromal cells and
endothelial progenitor cells accelerates bone healing by promoting
vascularized biological bone regeneration (16).
To the best of our knowledge, there are no studies
that examine the advantages of using PDPBB scaffolds co-cultured
with VECs and ADSCs. Therefore, the current preliminary study aimed
to assess the adhesion and cell viability of the co-culture of VECs
and ADSCs in vitro on PDPBB scaffolds, and determine the
optimal time period for maximum cell viability that could be used
as a point of reference for in vivo experiments.
Materials and methods
Materials
A total of two female 18-week-old Sprague-Dawley
(SD) rats (weight, 200±10 g) at full-term pregnancy were provided
by Animal Section of Kunming Medical University (animal production
license no. SCXK; approval no. 2005-0008). The experimental
protocols of this study were approved by Kunming Medical University
and the study was carried out in accordance with the
recommendations presented in the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health (17). The rats were housed at 25°C and
45–60% humidity in standard housing, with a 12-h light/dark cycle
and access to food and water ad libitum.
Instruments and reagents
Biosafety clean benches and a constant-temperature
CO2 incubator were purchased from Thermo Fisher
Scientific, Inc.. The low temperature automatic balance centrifuge
was from Beijing Medical Centrifuge Factory. An inverted microscope
was purchased from Olympus Corporation. Electro-thermal constant
water tanks were supplied by Shanghai Medical Instruments Factory.
Ultrasonic tissue pulverizer (Sonics & Materials, Inc.). An
automated reader for enzyme plate was purchased from Bio-Rad
Laboratories, Inc. A scanning electron microscope (S-3000N;
Hitachi, Ltd.), automatic biochemical analysis instrument (Olympus
2700) and a fluorescence microscope (Applied Imaging; Molecular
Devices LLC) were used. The following reagents were used: Low sugar
DMEM (Gibco; Thermo Fisher Scientific, Inc.); trypsin
(Sigma-Aldrich; Merck KGaA); EDTA (Sigma-Aldrich; Merck KGaA);
newborn calf serum (NBCS; Gibco; Thermo Fisher Scientific, Inc.);
fetal calf serum (Abcam Bioleaf); MTT (Amresco, LLC); SDS
(Sigma-Aldrich; Merck KGaA); isobutanol (Tianjin Fucheng Chemical
Reagent Factory); VEGF (PeproTech, Inc.); insulin-like growth
factor-1 (IGF-1; PeproTech, Inc.); basic fibroblast growth factor
(bFGF; PeproTech, Inc.); and epidermal growth factor (EGF;
PeproTech, Inc.). The following antibodies were purchased: Anti-von
Willebrand factor (vWF) antibody (cat. no. SC-365712; Santa Cruz
Biotechnology, Inc.); mouse anti-CD90 antibody (cat. no. SC-53116;
Santa Cruz Biotechnology, Inc.); cy3-labeled goat anti-mouse IgG
(cat. no. BA1031; Wuhan Boster Biological Technology, Ltd.).
Preparation and scanning electron
microscopy (SEM) of PDPBB
Following the method described previously (14), fresh pork vertebrae from Diannan
small-ear pigs, obtained from the Experimental Animal Center
Department of Laboratory Animal Science, Kunming Medical
University, were used to make bone strips with a cross-sectional
area of 0.5 cm2. After washing repeatedly with distilled
water, PDPB was prepared according to the method described in
Table I. PDPB was dipped in
distilled water. The pH value of the 30% H2O2
was adjusted to 7.0–7.2, and PDPB was air-dried in a drying box;
then, the PDPB was cut to obtain bone pieces of 0.5×0.4×0.3 cm and
bone fragments of 0.5×0.5×0.1 cm, which were rinsed in saline using
an ultrasonic cleaning tank.
 | Table I.Preparation of partially
deproteinized biologic bone. |
Table I.
Preparation of partially
deproteinized biologic bone.
| Reagents | Time | Temperature
(°C) |
|---|
| 30%
H2O2 | 72 h (switch every
24 h) | 38 |
| Cleaning with
distilled water | 30 min | 25 |
| Ethyl alcohol | 24 h | 25 |
| Cleaning with
distilled water | 30 min | 25 |
| Acetone | 24 h | 25 |
| Cleaning with
distilled water | 30 min | 25 |
| Air-dried in drying
box | 8 h | 25 |
Fibronectin was dissolved in PBS to get a final
stock solution of 150 µg/ml. PDPBB was prepared by soaking PDPB in
the fibronectin solution for 12 h; it was then air-dried in a
drying box and radioactively sterilized with cobalt-60. Then, two
pieces of PDPBB were fixed with 3.5% glutaraldehyde for 6 h at 25°C
and rinsed three times with distilled water (15 min each time),
following which conductive staining with 4% tannic acid and 3.5%
glutaraldehyde was performed for 48 h at 25°C. After this, they
were rinsed three times with distilled water (30 min each time),
fixed with 1% citric acid for 4 h at 25°C, rinsed four times with
distilled water (20 min each time), rehydrated using 30–100%
ethanol and subsequently tert-butanol, and finally pieces were
freeze-dried. After platinum-palladium alloy was sprayed onto the
samples, the morphology of fibronectin attached to the stent
material, as well as the morphology and behaviour of the cells
grown in the PDPBB, were observed using a scanning electron
microscope (SEM S-3000N; Hitach, Ltd.). All samples were analyzed
at 15 kV (18).
Isolation of cord blood mononuclear
cells and induced differentiation culture of VECs
The two full-term pregnancy SD rats were
administered with anesthesia intraperitoneally using 3%
pentobarbital sodium (30 mg/kg) and a 1.5±0.5 cm skin incision was
made in the middle of the abdomen to open up the abdominal cavity;
the incision was cleaned with 75% alcohol for 10 min to ensure
sterile conditions. Umbilical cord blood mononuclear cells were
isolated according to the method described previously, under
aseptic conditions (19,20). Following skin incision, the
peritoneal cavity was opened, and 3 ml of rat umbilical cord blood
was collected in heparin-coated tubes, and mixed with PBS at a
ratio of 1:1 and with 0.5% methylcellulose at a ratio of 4:1. This
was left at room temperature for 30 min for the sedimentation of
red blood cells to take place, following which the supernatant was
collected, layered on to the rat lymphocyte separation solution (MP
Biomedicals, LLC) with a density of 1.803 g/ml and then centrifuged
at 241.5 × g for 20 min at 25°C. PBS was added to the interface
layer to resuspend cells for washing to remove lymphocyte
separation medium and platelets. Then, 5 ml low sugar DMEM (L-DMEM;
Gibco; Thermo Fisher Scientific, Inc.) containing VECs inducing
solution [10% fetal bovine serum (Abcam Bioleaf), 1% penicillin, 1%
streptomycin, 20 µg/l VEGF, 2 µg/l IGF-1, 2 µg/l bFGF and 20 µg/l
EGF] was added. Afterwards, cells were seeded into a culture flask
with a bottom area of 25 cm2 and incubated at 37°C, with
5% CO2 in saturated humidity. When the primary cells
were fused and sufficiently confluent to cover >90% of the
culture flask, they were digested with 0.25% trypsin and 0.01%
EDTA, passaged and maintained for 6 weeks (20,21).
Lastly, the morphology of the cells was observed under an inverted
microscope (magnification, ×40).
Isolation and culture of ADSCs
The two SD rats were administered anesthesia
intraperitoneally with 3% pentobarbital sodium (30 mg/kg) and
sacrificed by cervical dislocation. ADSCs were isolated from
adipose tissue according to the method described previously
(22–24), and were placed in DMEM containing
10% NBCS. The cells were cultured to the third generation at 37°C
with 5% CO2 in saturated humidity to observe
adipogenesis.
Identification of umbilical VECs and
ADSCs
The umbilical VECs and ADSCs (1:1, 1:3 and 3:1) were
seeded into two 6-well cover slips; each population was seeded into
6 wells. Four wells of cord blood-derived VECs were identified
using anti-vWF antibody immunofluorescence, 4 wells of ADSCs were
identified using anti-CD90 antibody immunofluorescence and 2 wells
of each population were used as negative controls (only secondary
antibodies were added). Briefly, the adherent cells of each group
were washed twice with PBS, fixed with 4% paraformaldehyde for 30
min at 25°C, dried for 10 min and incubated with 3% newborn serum
albumin (Gibco; Thermo Fisher Scientific, Inc.) for 20 min at 25°C.
Cells were then incubated for 60 min at 25°C with anti-CD90
(1:100), anti-vWF (1:500) or without a primary antibody for the
negative control. Following the primary antibody incubation, the
cells were washed three times with PBS (5 min each time), then
incubated with an anti-mouse cy3 fluorescein-labeled secondary
antibody (1:500) at 37°C for 30 min, washed three times with PBS
(for 5 min each time) and counterstained with DAPI for 1 min at
25°C. Following this, they were mounted by 50% buffered glycerol
and observed under a fluorescence microscope (magnification,
×630).
Determination of the transplantation
time of the co-culture system combined with PDPBB in vitro
A total of 144 PDPBB bone pieces (0.5×0.5×0.1 cm)
were randomly divided into the following four groups (with 36
pieces per group): group A, PDPBB and ADSCs; group B, PDPBB and
VECs; group C, PDPBB, and the co-culture of VECs and ADSCs in the
ratio of 1:1; and group D, PDPBB without cells as a control group.
The PDPBB pieces were added to each group with a 20-µl solution at
a concentration of 5×106 cells/ml (25).
The cells were incubated for 4 h at 37°C and 5%
CO2 with saturated humidity, and then transferred to
96-well plates, to each of which 24 pieces of PDPBB were added. The
cells were cultured at 37°C and 80 µl DMEM, which was changed on
alternate days.
On the 2nd, 4, 6, 8, 10 and 12th day, 10 µl MTT
(0.5%) was added to one 96-well plate and 100 µl MTT formazan
solution (10 g of SDS containing sodium lauryl sulfate, 5 ml of
isobutanol, 120 µl of 0.01 µmol/l hydrochloric acid per 100 ml of
triple solution) was added after 4 h; after 12 h, the absorbance
value of each well was measured by an enzyme labeling detector at a
wavelength of 570 nm. The optimal time for maximum cell viability
was recorded.
Observation of the co-cultured cells
and PDPBB under SEM
When the cell viability on the scaffold was the
highest, 2 pieces of PDPBB in each group were selected, fixed with
3.5% glutaraldehyde at 25°C for 6 h and rinsed 3 times with
distilled water (15 min each time). Then, conductive staining with
4% tannic acid and 3.5% glutaraldehyde was performed for 48 h at
25°C, PDPBB was rinsed 3 times with distilled water (30 min each
time), fixed with 1% citric acid for 4 h at 25°C, rinsed 4 times
with distilled water (20 min each time), rehydrated using 30–100%
ethanol and subsequently tert-butanol, freeze-dried and sprayed
with platinum-palladium alloy. Finally, the adhesion of the cells
to the PDPBB was observed by SEM.
Statistical analysis
Data were expressed as the mean ± SD, and the data
was analyzed using SPSS 17.0 statistical software package (SPSS,
Inc.). Two-way mixed ANOVA was performed to determine statistically
significant differences between the cell viability of treatment
groups, with post hoc pairwise comparisons performed using
Bonferroni's test, with α=0.05. P<0.001 was considered to be
statistically significant.
Results
SEM showing the structure of
PDPBBs
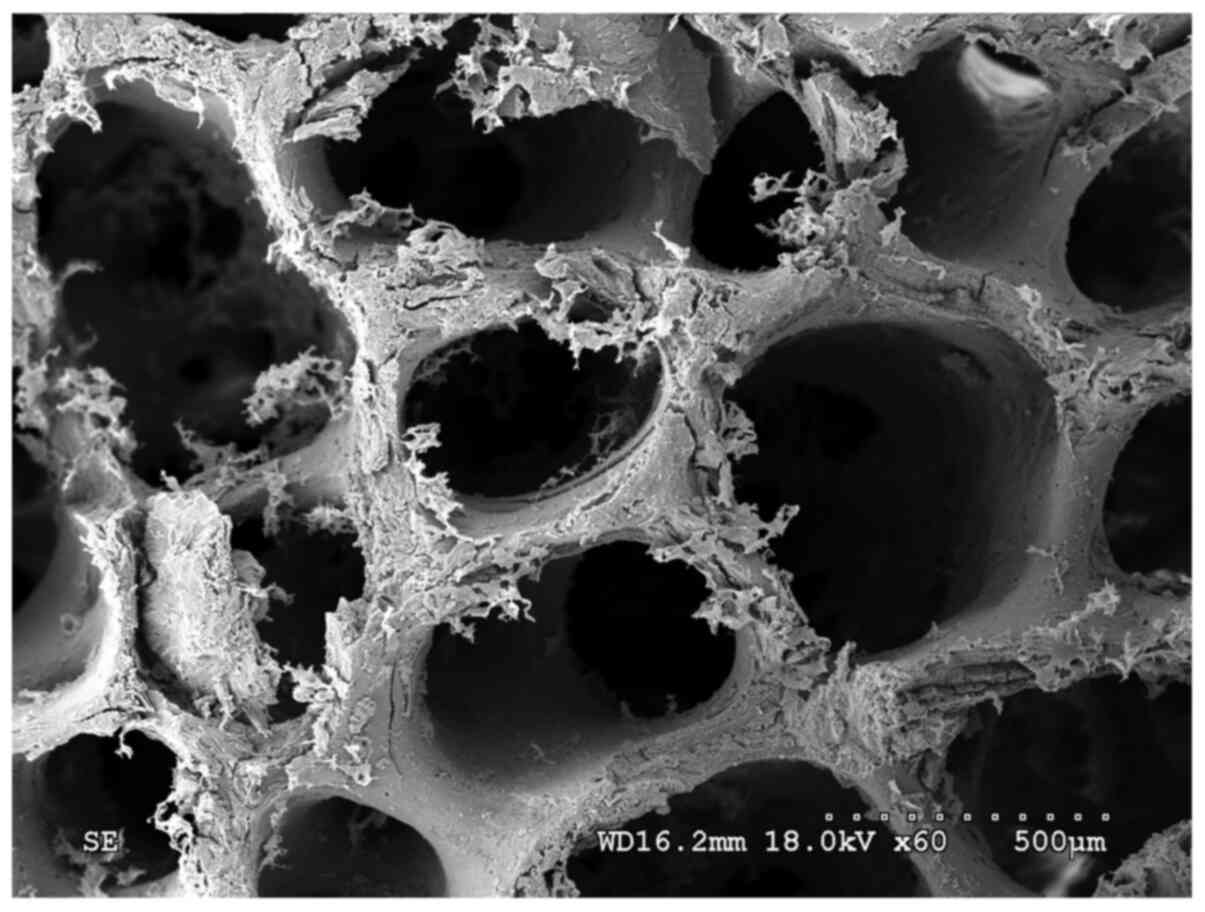
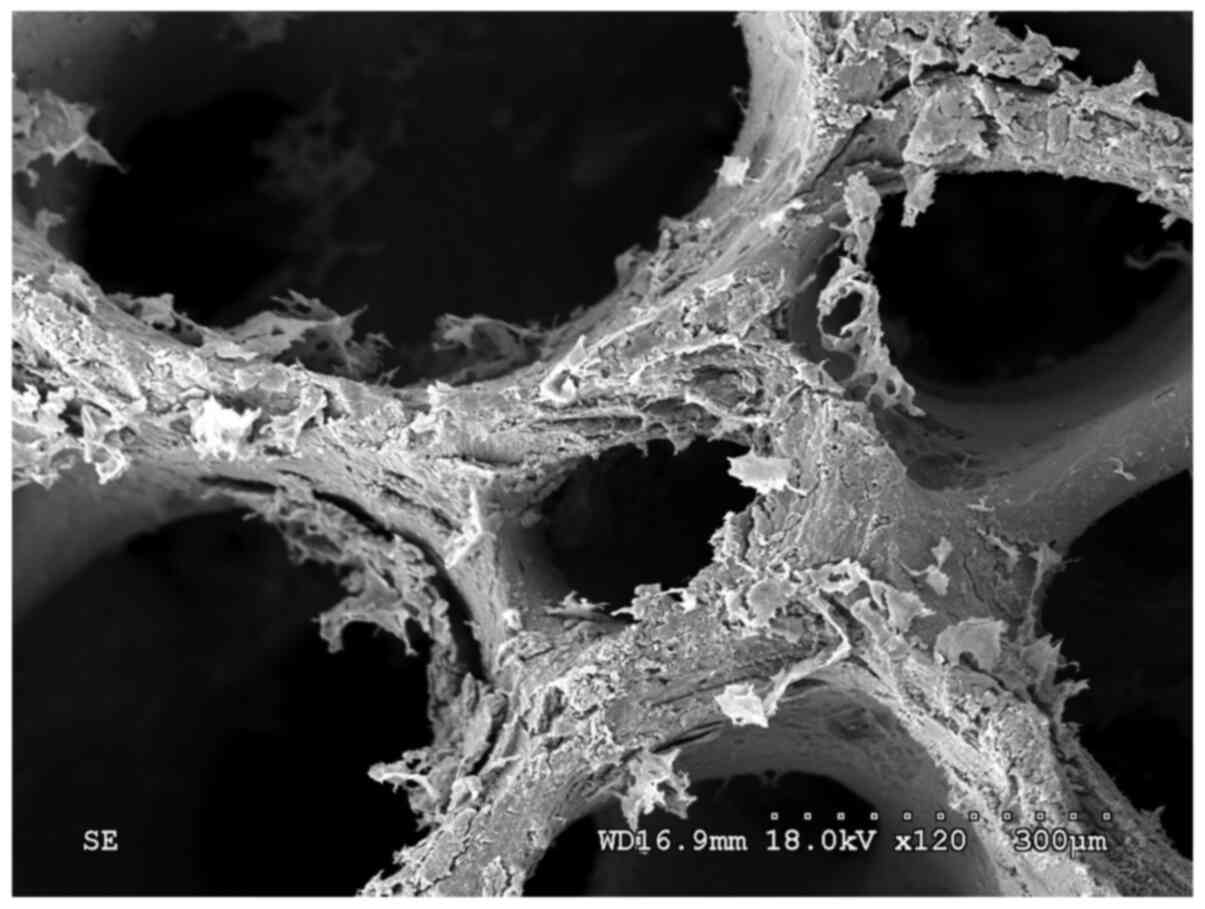
The fibronectin-modified PDPBB was observed by SEM.
The lacunas among the materials were interconnected internally
until the blood vessels and cells on normal bone tissues
disappeared. A large amount of flaky protein crystals were loosely
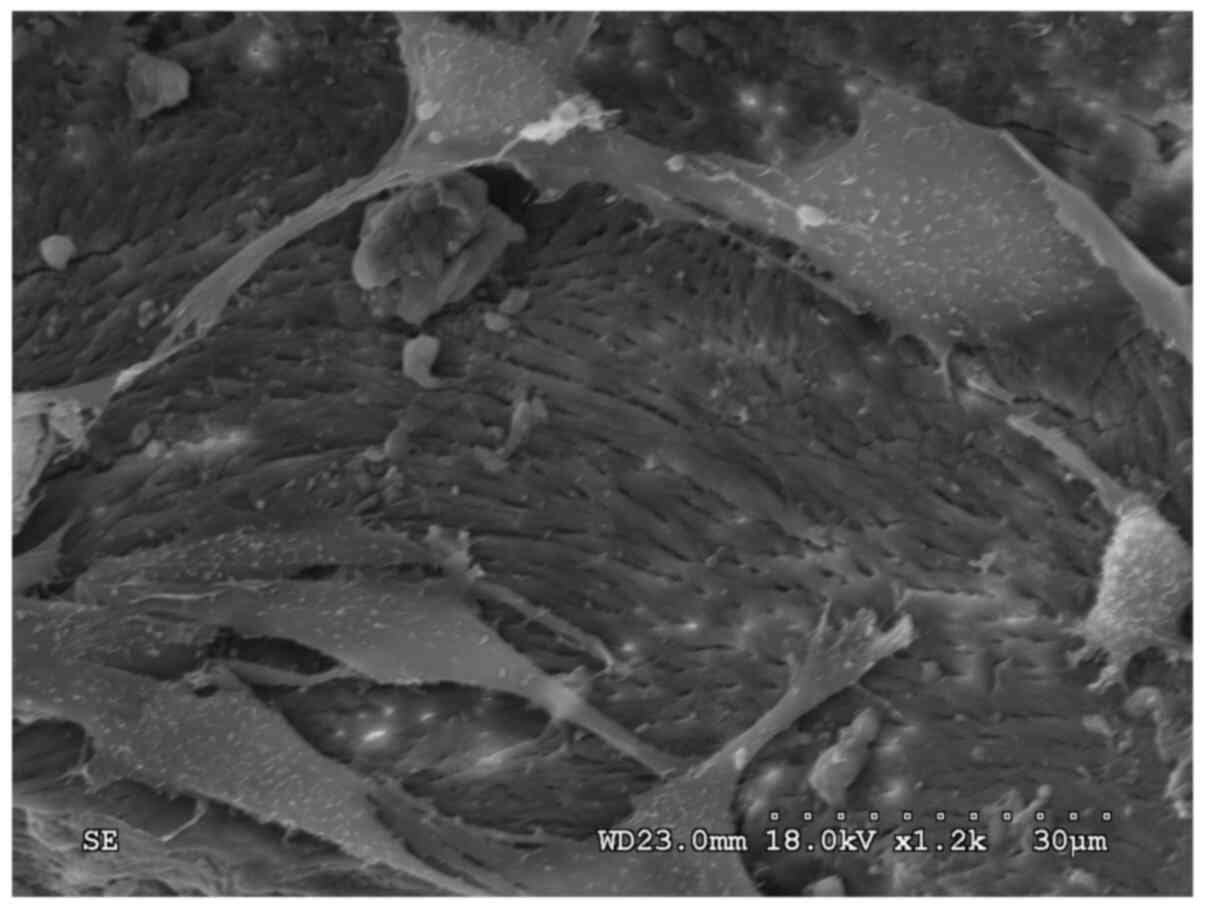
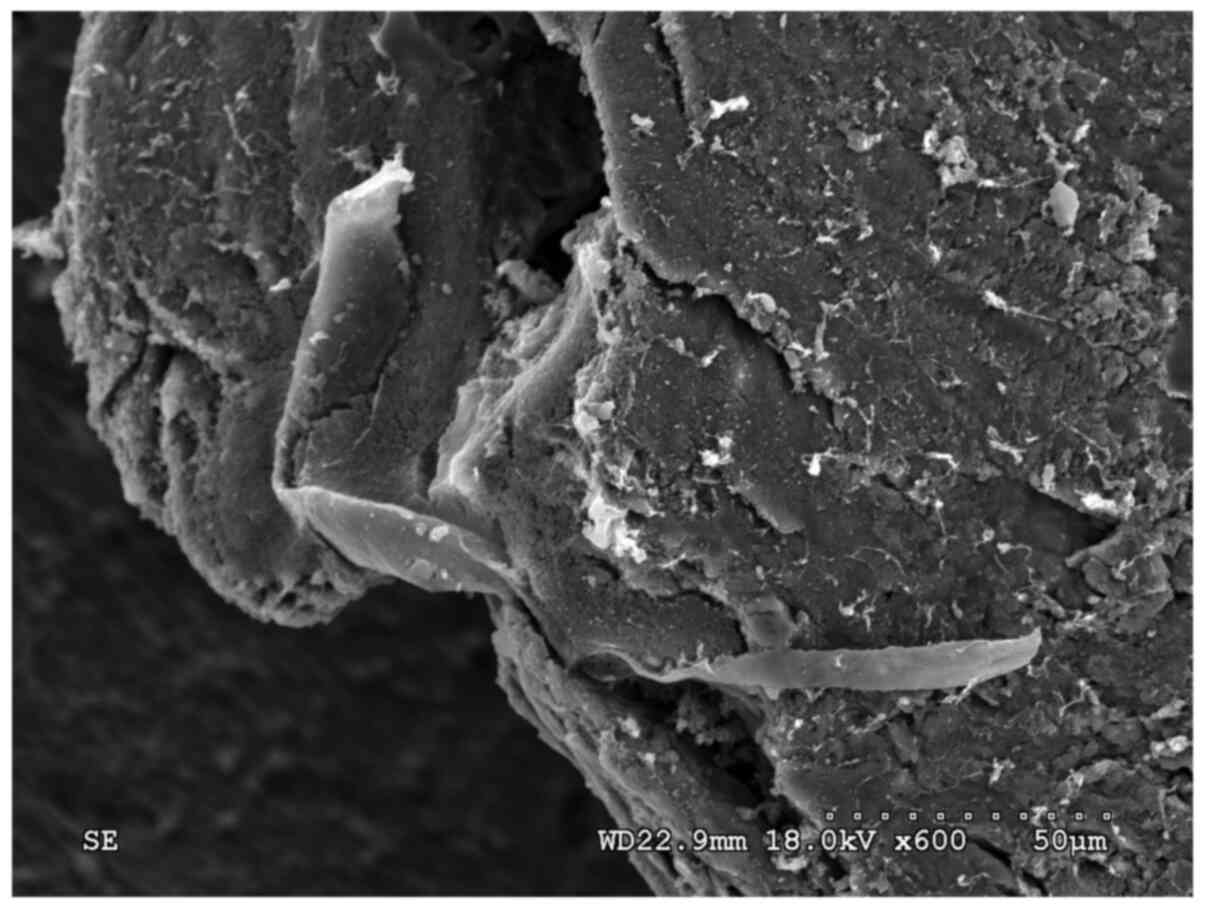
attached to the surface of the scaffold (Fig. 1). The protein was distributed
non-uniformly and was not attached stably to the stent material
(Fig. 2).
Structure of umbilical cord blood
mononuclear cells
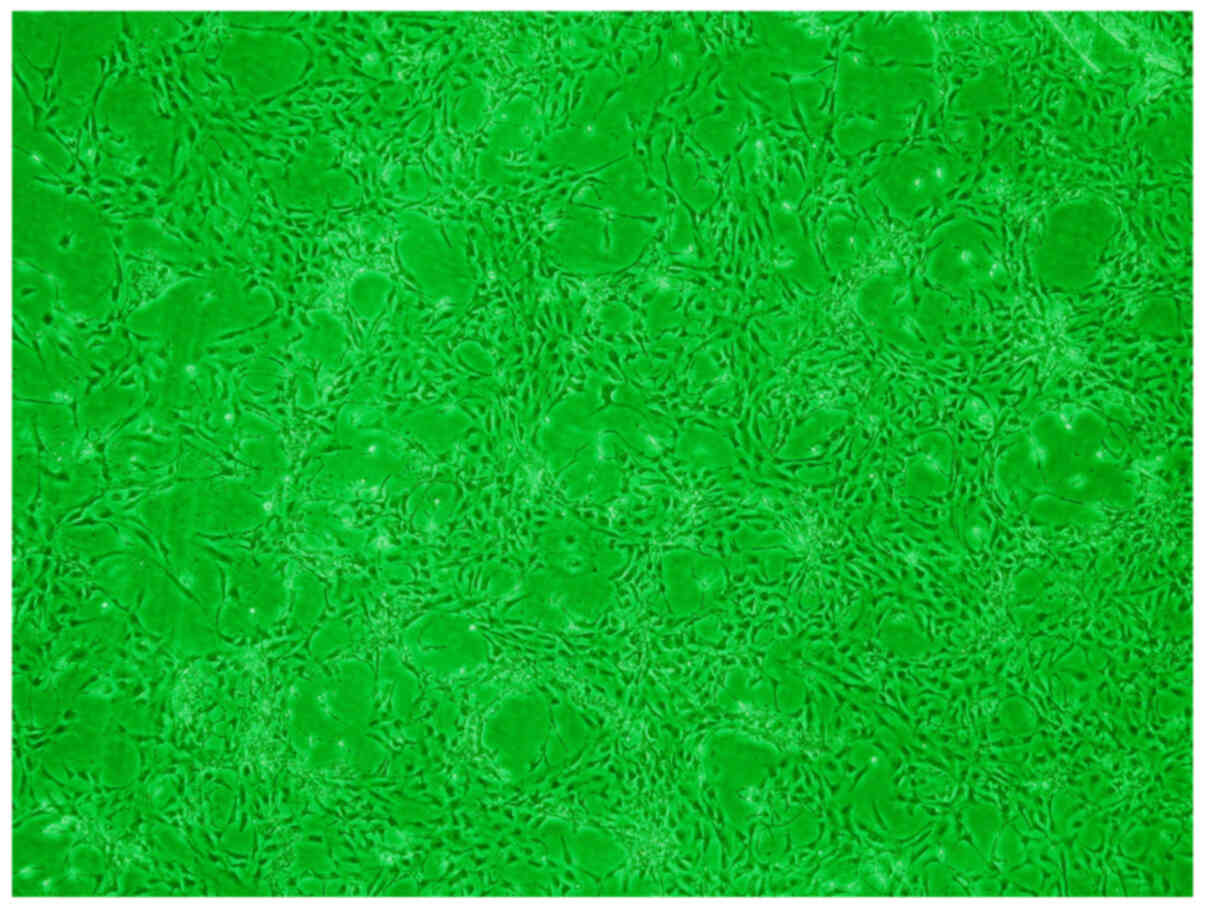
Umbilical cord blood mononuclear cells were induced
to grow in a vortex structure for 6 weeks. It was observed that the
majority of cells were shortened from long spindles and had a
polygonal-like morphology (Fig. 3).
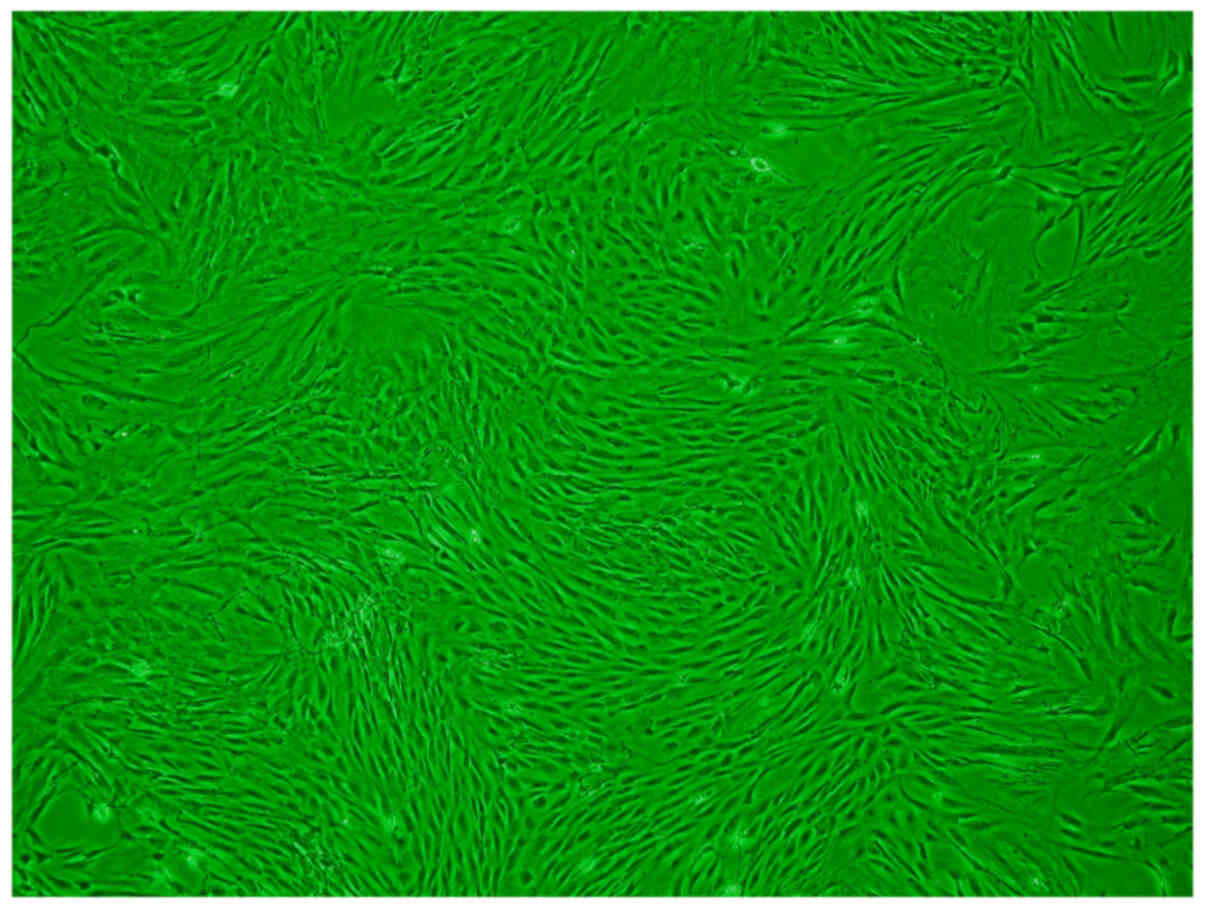
The primary ADSCs showed a slender fusiform shape (Fig. 4). The third-generation ADSCs were
observed to be spindle-shaped, with a fusiform morphology, and the
cells were arranged in a spiral shape without cell overlap
(Fig. 5).
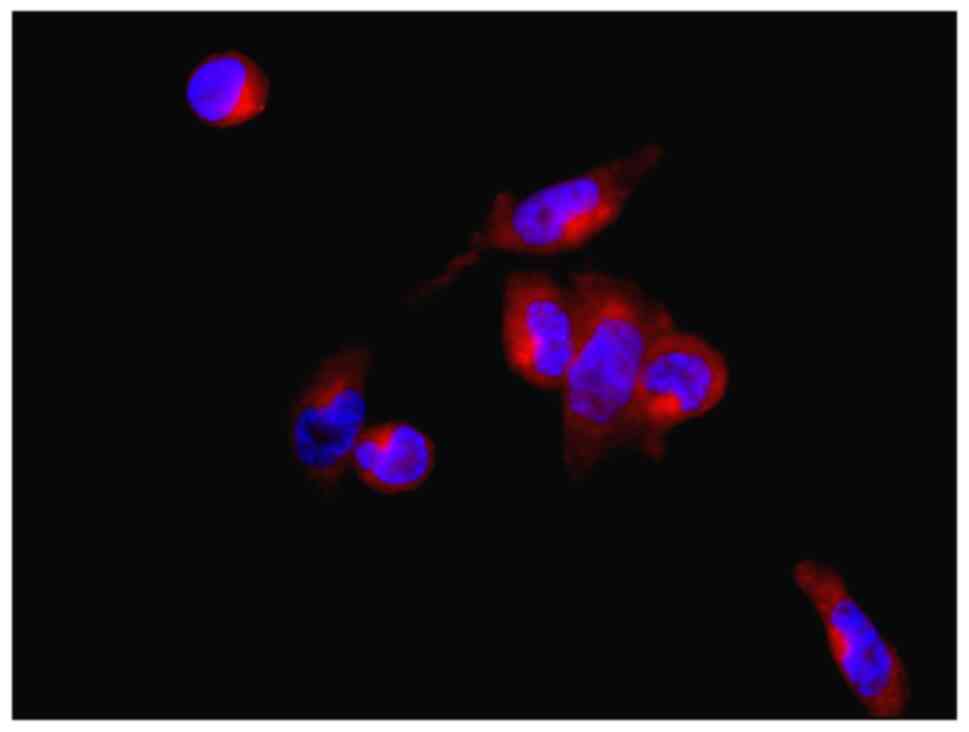
Immunofluorescent staining
Immunofluorescent staining of VECs showed that the
cells were polygon-shaped and positive for vWF, which is a
glycoprotein and a useful marker for ECs (26) (Fig.
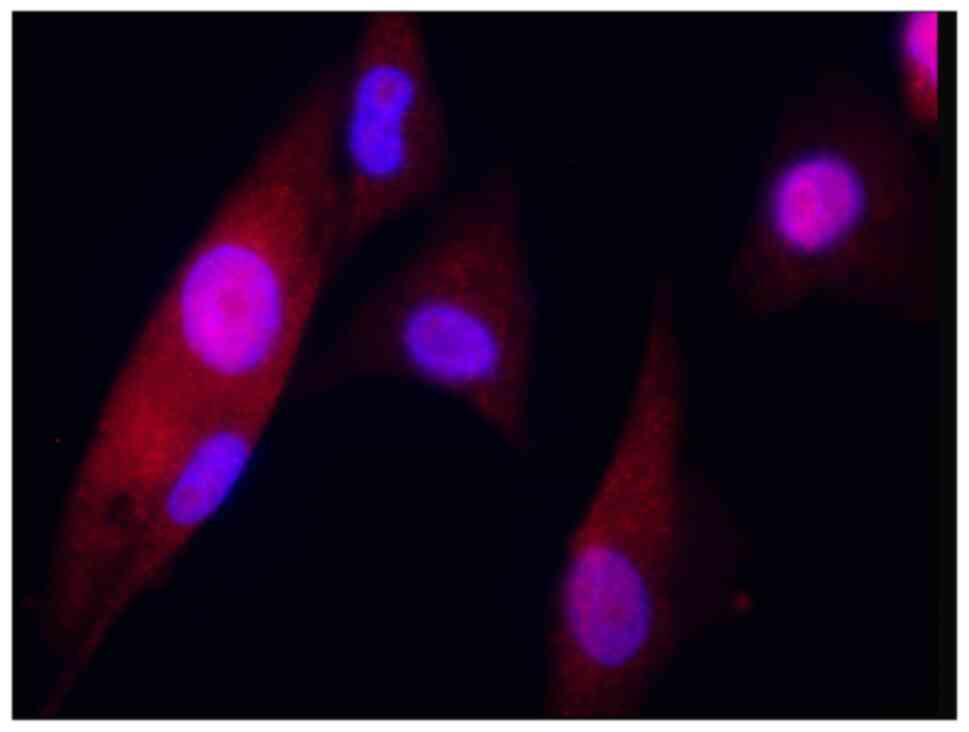
6). ADSCs showed a long, spindle-shape morphology and were
positive for CD90, which is an established stem cell marker in the
hematopoietic system (27)
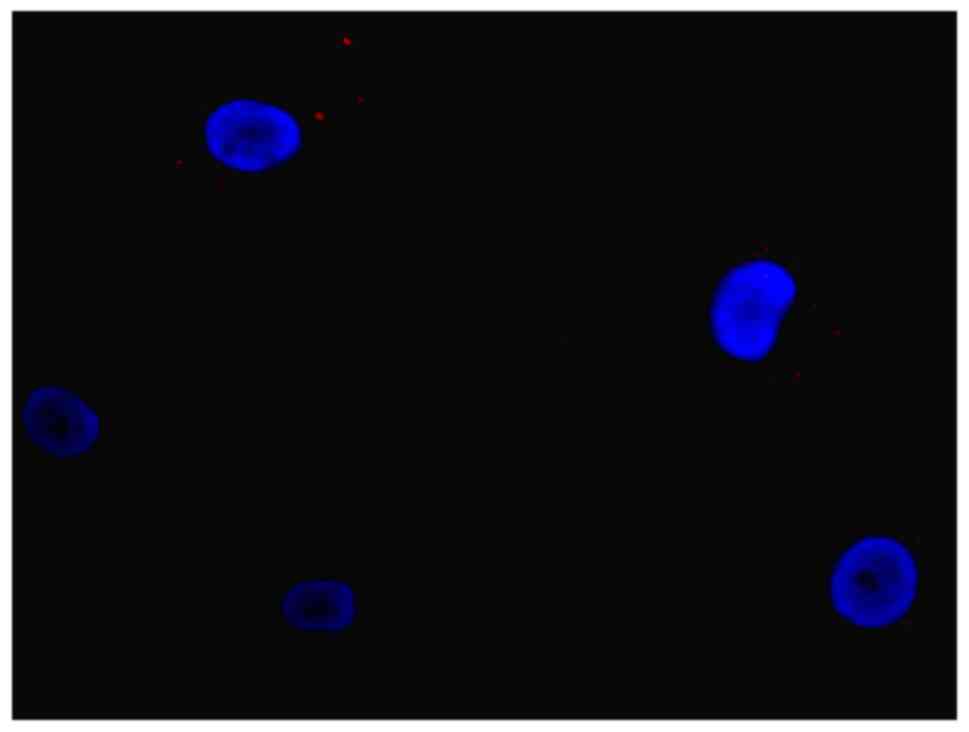
(Fig. 7). In the control group,
cells were not positive for vWF or CD90 (Fig. 8).
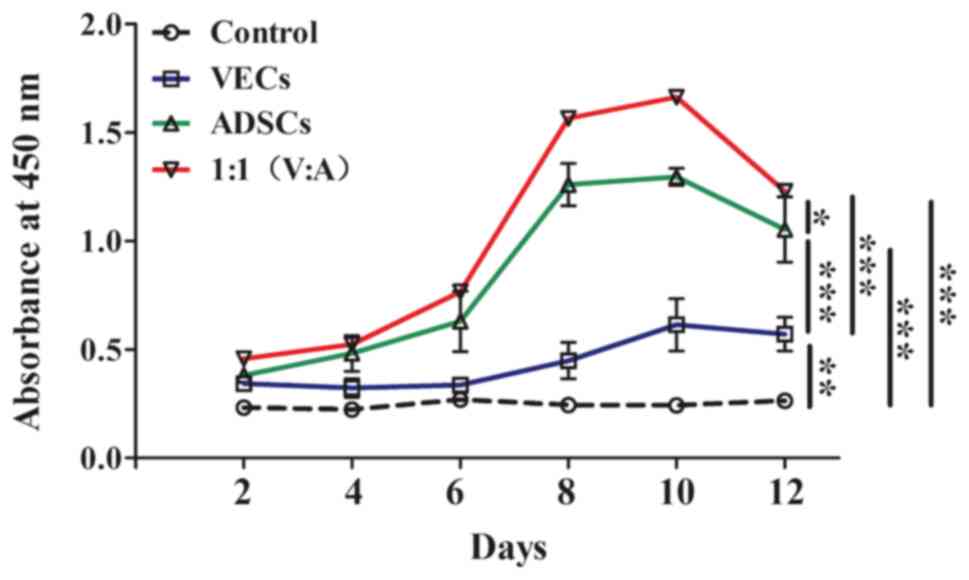
Cell viability
Cell viability was measured in all groups, as shown
in Fig. 9. In all groups, except
the control group, cell viability in each group gradually increased
and peaked on the 10th day. The 1:1 (VECs:ADSCs) co-cultured cells
exhibited the highest cell viability, followed by the ADSCs group.
After the 10th day, the absorbance gradually decreased. The
viability of the control group remained unchanged throughout. It
was revealed that there was a significant effect of treatment on
cell viability (F(3,20)=1282.6; P<0.001), as well as
a significant interaction between treatment and time. Pairwise
comparisons revealed that all groups were significantly different
from each other (P<0.001), with the highest cell viability in
the 1:1 co-culture group and the lowest in the control group
(Table II).
 | Table II.Results of data analysis of each cell
culture group on partially deproteinized biologic bone. |
Table II.
Results of data analysis of each cell
culture group on partially deproteinized biologic bone.
|
| Absorbance at 450
nm, mean ± SD |
|---|
|
|
|
|---|
| Group | 2nd day | 4th day | 6th day | 8th day | 10th day | 12th day |
|---|
| VECs | 0.34±0.02 | 0.32±0.04 | 0.34±0.02 | 0.45±0.05 | 0.61±0.12 | 0.57±0.07 |
| ADSCs | 0.38±0.01 | 0.48±0.08 | 0.63±0.14 | 1.26±0.10 | 1.36±0.04 | 1.05±0.05 |
| 1:1 | 0.46±0.01 | 0.52±0.13 | 0.77±0.03 | 1.57±0.02 | 1.66±0.01 | 1.23±0.10 |
| Control | 0.25±0.01 | 0.22±0.13 | 0.27±0.03 | 0.24±0.02 | 0.24±0.01 | 0.26±0.10 |
SEM images showing cells adhered to
the surface of PDPBBs
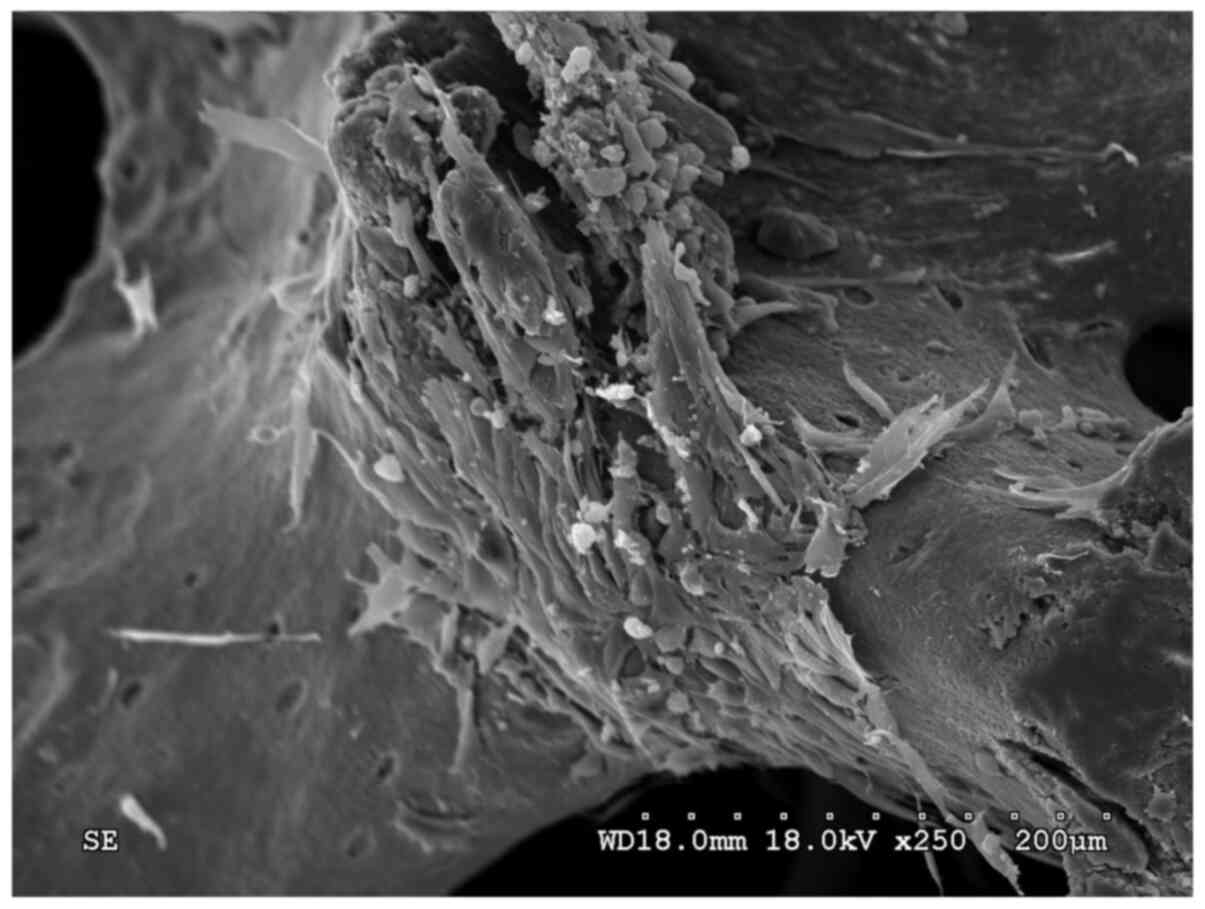
On the 10th day, ADSCs that adhered to the surface
of the PDPBB were spindle-shaped and protruded into the micropores
on the surface of the PDPBB (Fig.
10), whereas only a small number of VECs adhered, which were
polygonal in shape (Fig. 11). In
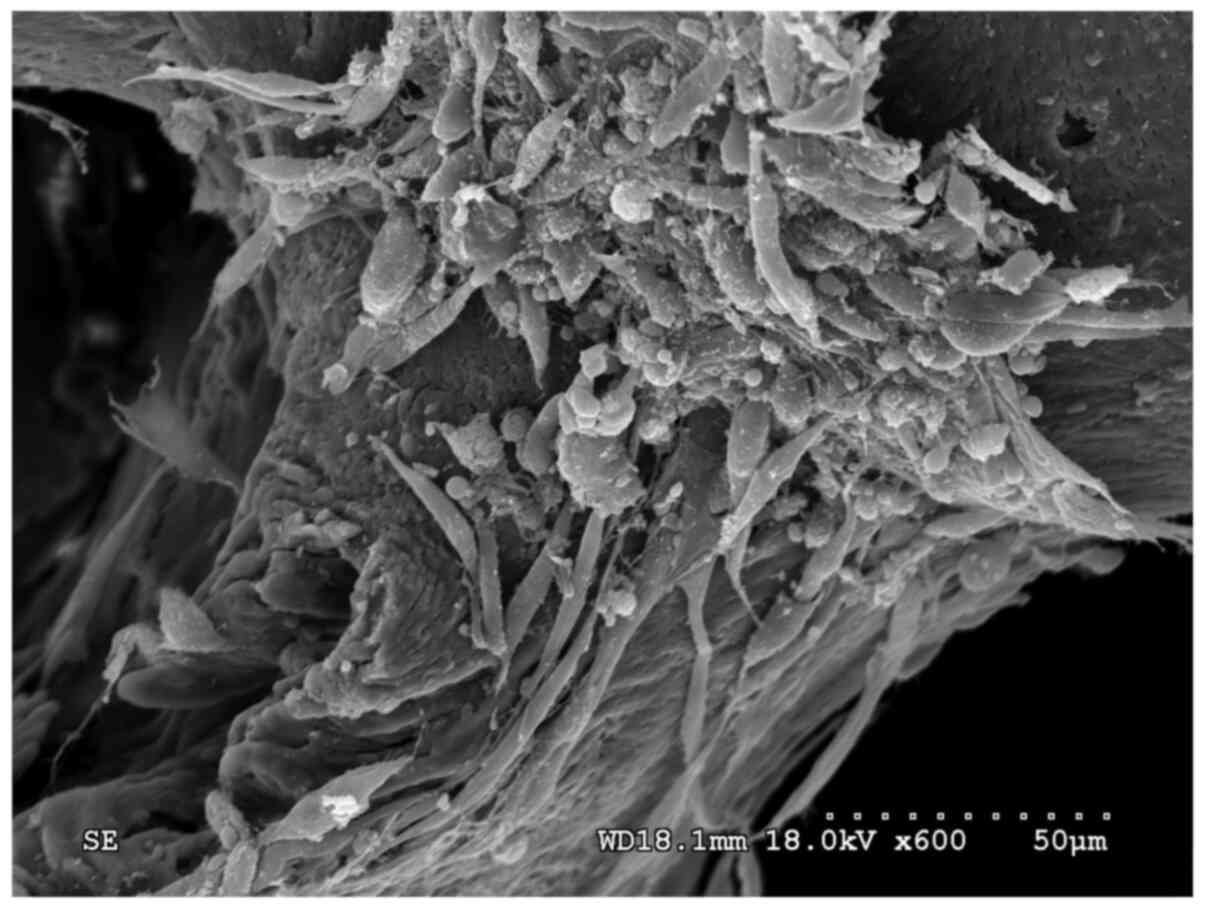
the co-culture cell group, a large number of cells were observed to
adhere to the PDPBB in a nest-like distribution, and the cells had
accumulated to form a cell cluster. The cell morphology was
diverse, with a mixed population of both polygonal and spindle
cells (Fig. 12). In Fig. 13, a large number of granular
materials of different sizes can be observed; furthermore, it was
shown that the cells grew along the trabecular bone to form a cell
layer (Fig. 14).
Discussion
The present preliminary study sought to evaluate the
adhesion and proliferation of the co-culture system of VECs and
ADSCs in vitro on PDPBB scaffolds, and to determine the
optimal time period for maximum cell proliferation that could be
used for transplanting cells during in vivo experiments. It
was observed that the adhesion and cell viability of the
co-cultured cells was higher, in terms of the total number of cells
adhered to the PDPBB, as well as the morphologically formed cell
layers as compared with those of the single cell types.
Furthermore, it was found that the cell viability was the highest
on the 10th day.
Previous studies have demonstrated that co-culturing
VECs and ADSCs influences the differentiation of osteogenic cells
and is a useful source for bone engineering (7,10,28).
Therefore, in the present study, the same system was adopted to
verify the cell viability and adhesion efficiency of VECs and
ADSCs. VECs secrete factors such as BMP and VEGF (29,30);
BMP-7 stimulates angiogenesis by increasing VEGF expression in ECs
via direct and indirect mechanisms (31). ADSCs, when used in a co-culture, can
increase bone regeneration, and show high levels of CD90 and CD105
expression. Additionally, ADSCs show high expression levels of
stemness genes, including SOX2, octamer-binding transcription
factor 4, NANOG and Kruppel-like factor 4, and they are able to
differentiate into osteogenic, chondrogenic and adipogenic cells
(32). The present study is
consistent with a previous study that found that PDPBB seeded with
a co-cultured system provided an ideal environment for cell growth
and osteogenesis, along with cytocompatibility (16). Therefore, it is necessary to use a
co-culture system instead of single cells for tissue engineering
experiments. Furthermore, it was found that a 1:1 ratio of the
cells yielded favorable results. Although the present study did not
explore molecular pathways, an MTT assay revealed that the cell
viability of the co-culture on the PDBPP scaffold was significantly
higher than either of the individual cell types.
Accurate timing of the transplantation steps
(implantation of scaffolds and transplantation of cells) is
important to reach sufficient vascularization and a proper level of
tissue ingrowth prior to transplantation. When cells are
transplanted too early, premature cells with poor adherence grow,
whereas when cells are transplanted too late, cell apoptosis
increases (33). The adhesion and
function of ECs on smooth muscle cells in a co-culture, with the
addition of fibronectin, could be consistently maintained for up to
10 days, although there was no change in the growth rate of ECs
(34). In the present study, it was
observed that cell viability gradually increased over time and was
the highest on the 10th day, so this is hypothesized to be the
optimum length of time for the transplantation of tissue-engineered
bone grafts to an in vivo system, though further experiments
are needed.
Fibronectin mediates several cellular interactions
with the extracellular matrix and plays important roles in cell
adhesion, migration, growth and differentiation (35). Fibronectin, together with
transforming growth factor-b1, may affect bone formation, in part
by regulating the survival of osteoblasts (36). Additionally, fibronectin coating on
discs of hydroxyapatite or a-type alumina as scaffolds led to
enhanced adhesion and the spreading of MC3T3-E1 osteoblastic cells
(37). In the present study, it was
shown that the modification of PDPB scaffold material using
fibronectin promoted adhesion and viability of cells on the
scaffold material, and also induced osteogenic differentiation in
the co-culture, as evidenced by the SEM images.
The present study has certain limitations. It is
preliminary, and requires further in-depth molecular and cellular
experiments to validate the results. Nevertheless, this study
provides an initial understanding of the benefit of co-culturing
VECs and ADSCs in bone grafting experiments. Additionally, future
studies are required to address molecular pathways involved and the
behavior, safety and efficacy of the PDBPP scaffold in an in
vivo system.
In conclusion, this study demonstrated that VECs and
ADSCs as a co-culture with a PDBPP scaffold leads to increased
adhesion and cell viability, which is the highest on the 10th day,
thus indicating that until this time point, the cells can be
maintained in a healthy condition.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GY, FW, YL, JH and DL analyzed and interpreted the
experimental data, contributed equally in writing the manuscript
and revised it critically for important intellectual content. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocols of this study were
approved by Kunming Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gurel PG, Torun KG and Hasirci V:
Influence of co-culture on osteogenesis and angiogenesis of bone
marrow mesenchymal stem cells and aortic endothelial cells.
Microvasc Res. 108:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weyand B and Von SHP: Altered VEGF-A and
receptor mRNA expression profiles, and identification of VEGF144 in
foetal rat calvaria cells, in coculture with microvascular
endothelial cells. Cell Biol Int. 37:713–724. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu X, Neoh KG, Zhang J, Kang ET and Wang
W: Immobilization strategy for optimizing VEGF's concurrent
bioactivity towards endothelial cells and osteoblasts on implant
surfaces. Biomaterials. 33:8082–8093. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Geuze RE, Theyse LF, Kempen DH, Hazewinkel
HA, Kraak HY, Oner FC, Dhert WJ and Alblas J: A differential effect
of bone morphogenetic protein-2 and vascular endothelial growth
factor release timing on osteogenesis at ectopic and orthotopic
sites in a large-animal model. Tissue Eng Part A. 18:2052–2062.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiao C, Zhou H, Liu G, Zhang P, Fu Y, Gu
P, Hou H, Tang T and Fan X: Bone marrow stromal cells with a
combined expression of BMP-2 and VEGF-165 enhanced bone
regeneration. Biomed Mater. 6:0150132011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choong CS, Hutmacher DW and Triffitt JT:
Co-culture of bone marrow fibroblasts and endothelial cells on
modified polycaprolactone substrates for enhanced potentials in
bone tissue engineering. Tissue Eng. 12:2521–2531. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao X, Liu L, Wang FK, Zhao DP, Dai XM
and Han XS: Coculture of vascular endothelial cells and
adipose-derived stem cells as a source for bone engineering. Ann
Plast Surg. 69:91–98. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim JY, Park CD, Lee JH, Lee CH, Do BR and
Lee AY: Co-culture of melanocytes with adipose-derived stem cells
as a potential substitute for co-culture with keratinocytes. Acta
Derm Venereol. 92:16–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eltom A, Zhong G and Ameen M: Scaffold
techniques and designs in tissue engineering functions and
purposes: A review. Adv Mater Sci Eng. 2019:1–13. 2019. View Article : Google Scholar
|
|
10
|
Wang F, Zhang H, Li Y, Liu L, He C, Cai G
and Song E: Heterotopic osteogenesis study of tissue engineered
bone by co-culture of vascular endothelial cells and
adipose-derived stem cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za
Zhi. 33:1310–1319. 2019.(In Chinese). PubMed/NCBI
|
|
11
|
Liu J, Zhou P, Long Y, Huang C and Chen D:
Repair of bone defects in rat radii with a composite of allogeneic
adipose-derived stem cells and heterogeneous deproteinized bone.
Stem Cell Res Ther. 9:792018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang Y, Wu C, Zhang X, Chang J and Dai K:
Regulation of immune response by bioactive ions released from
silicate bioceramics for bone regeneration. Acta Biomater.
66:81–92. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng J, Chen L, Peng K, Chen X, Wu J, He Z
and Xiang Z: Bone marrow mesenchymal stem cells and endothelial
progenitor cells co-culture enhances large segment bone defect
repair. J Biomed Nanotechnol. 15:742–755. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han X, Liu L, Wang F, Zhao X, Zhao D, Dai
X and Li Y: Reconstruction of tissue-engineered bone with bone
marrow mesenchymal stem cells and partially deproteinized bone in
vitro. Cell Biol Int. 36:1049–1053. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang FK, Yang G, Liu L, Li YL, Han R, Zhao
X and He C: Fibronectin improved the cytocompatibility of partially
deproteinized bone. J Biomater Tissue Eng. 9:534–536. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu L, Zhao X, He B, Jiang J, Xie XJ and
Liu L: The possible roles of biological bone constructed with
peripheral blood derived EPCs and BMSCs in osteogenesis and
angiogenesis. Biomed Res Int. 2016:81689432016.PubMed/NCBI
|
|
17
|
National Research Council: Guide for the
Care and Use of Laboratory Animals. 8th edition. Washington, DC:
The National Academies Press; 2011, simplehttps://doi.org/10.17226/12910
|
|
18
|
Thrivikraman G, Athirasala A, Twohig C,
Boda SK and Bertassoni LE: Biomaterials for craniofacial bone
regeneration. Dent Clin North Am. 61:835–856. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang C, Li Y, Yang M, Zou Y, Liu H, Liang
Z, Yin Y, Niu G, Yan Z and Zhang B: Efficient differentiation of
bone marrow mesenchymal stem cells into endothelial cells in vitro.
Eur J Vasc Endovasc Surg. 55:257–265. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ai WJ, Li J, Lin SM, Li W, Liu CZ and Lv
WM: R-Smad signaling-mediated VEGF expression coordinately
regulates endothelial cell differentiation of rat mesenchymal stem
cells. Stem Cells Dev. 24:1320–1331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Berendsen AD and Olsen BR: How vascular
endothelial growth factor-A (VEGF) regulates differentiation of
mesenchymal stem cells. J Histochem Cytochem. 62:103–108. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Priya N, Sarcar S, Majumdar AS and
SundarRaj S: Explant culture: A simple, reproducible, efficient and
economic technique for isolation of mesenchymal stromal cells from
human adipose tissue and lipoaspirate. J Tissue Eng Regen Med.
8:706–716. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Raposio E and Bertozzi N: Isolation of
ready-to-use adipose-derived stem Cell (ASC) pellet for clinical
applications and a comparative overview of alternate methods for
ASC isolation. Curr Protoc Stem Cell Biol. 41:1F.17.1–1F.17.12.
2017. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bajek A, Gurtowska N, Olkowska J,
Kazmierski L, Maj M and Drewa T: Adipose-derived stem cells as a
tool in cell-based therapies. Arch Immunol Ther Exp (Warsz).
64:443–454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang F, Liu L, Li Y, Dai X, Li Y and Li Z:
Optimal condition of in vitro constructing tissue-engineered bone
with bone marrow stromal cells and partially deproteinised bone.
Zhongguo Zu Zhi Gong Cheng Yan Jiu. 33:6401–6405. 2008.(In
Chinese).
|
|
26
|
Randi AM, Smith KE and Castaman G: von
Willebrand factor regulation of blood vessel formation. Blood.
132:132–140. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kawamoto K, Konno M, Nagano H, Nishikawa
S, Tomimaru Y, Akita H, Hama N, Wada H, Kobayashi S, Eguchi H, et
al: CD90-(Thy-1-) high selection enhances reprogramming capacity of
murine adipose-derived mesenchymal stem cells. Dis Marker.
35:573–579. 2013. View Article : Google Scholar
|
|
28
|
Wang F, Liu L, Zhao D, He X, Dai X and Li
Y and Li Y: Influence of co-culturing vascular endothelial cells
and adipose-derived stromal cells on osteogenic differentiation in
vitro. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 24:399–405.
2010.(In Chinese). PubMed/NCBI
|
|
29
|
Aksel H and Huang GT: Combined effects of
vascular endothelial growth factor and bone morphogenetic protein 2
on odonto/osteogenic differentiation of human dental pulp stem
cells in vitro. J Endod. 43:930–935. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma XW, Cui DP and Zhao DW: Vascular
endothelial growth factor/bone morphogenetic protein-2 bone marrow
combined modification of the mesenchymal stem cells to repair the
avascular necrosis of the femoral head. Int J Clin Exp Med.
8:15528–15534. 2015.PubMed/NCBI
|
|
31
|
Akiyama I, Yoshino O, Osuga Y, Shi J,
Harada M, Koga K, Hirota Y, Hirata T, Fujii T, Saito S and Kozuma
S: Bone morphogenetic protein 7 increased vascular endothelial
growth factor (VEGF)-a expression in human granulosa cells and VEGF
receptor expression in endothelial cells. Reprod Sci. 21:477–482.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu PH, Chung HY, Wang JH, Shih JC, Kuo MY,
Chang PC, Huang YD, Wang PC and Chang CC: Amniotic membrane and
adipose-derived stem cell co-culture system enhances bone
regeneration in a rat periodontal defect model. J Formos Med Assoc.
115:186–194. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gálisová A, Fábryová E, Sticová E,
Kosinová L, Jirátová M, Herynek V, Berková Z, Kříž J, Hájek M and
Jirák D: The optimal timing for pancreatic islet transplantation
into subcutaneous scaffolds assessed by multimodal imaging.
Contrast Media Mol Imaging. 2017:54184952017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lavender MD, Pang Z, Wallace CS, Niklason
LE and Truskey GA: A system for the direct co-culture of
endothelium on smooth muscle cells. Biomaterials. 26:4642–4653.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pankov R and Yamada KM: Fibronectin at a
glance. J Cell Sci. 115:3861–3863. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Globus RK, Doty SB, Lull JC, Holmuhamedov
E, Humphries MJ and Damsky CH: Fibronectin is a survival factor for
differentiated osteoblasts. J Cell Sci. 111:1385–1393.
1998.PubMed/NCBI
|
|
37
|
Kawashita M, Hasegawa M, Kudo TA, Kanetaka
H, Miyazaki T and Hashimoto M: Effect of fibronectin adsorption on
osteoblastic cellular responses to hydroxyapatite and alumina.
Mater Sci Eng C Mater Biol Appl. 69:1268–1272. 2016. View Article : Google Scholar : PubMed/NCBI
|