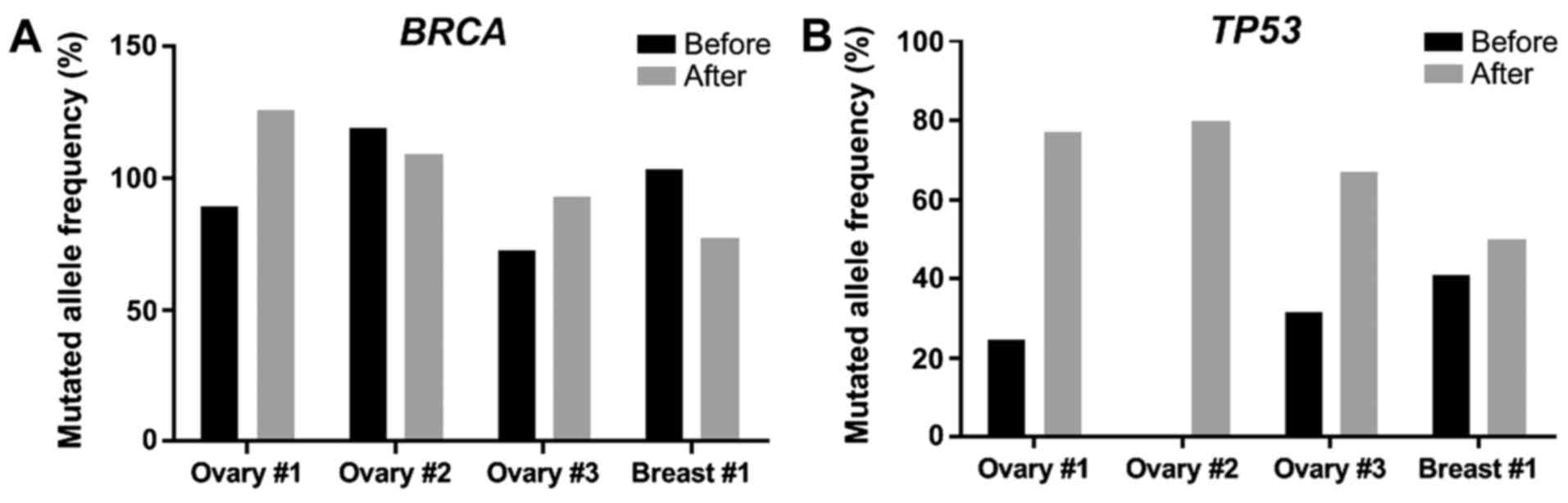
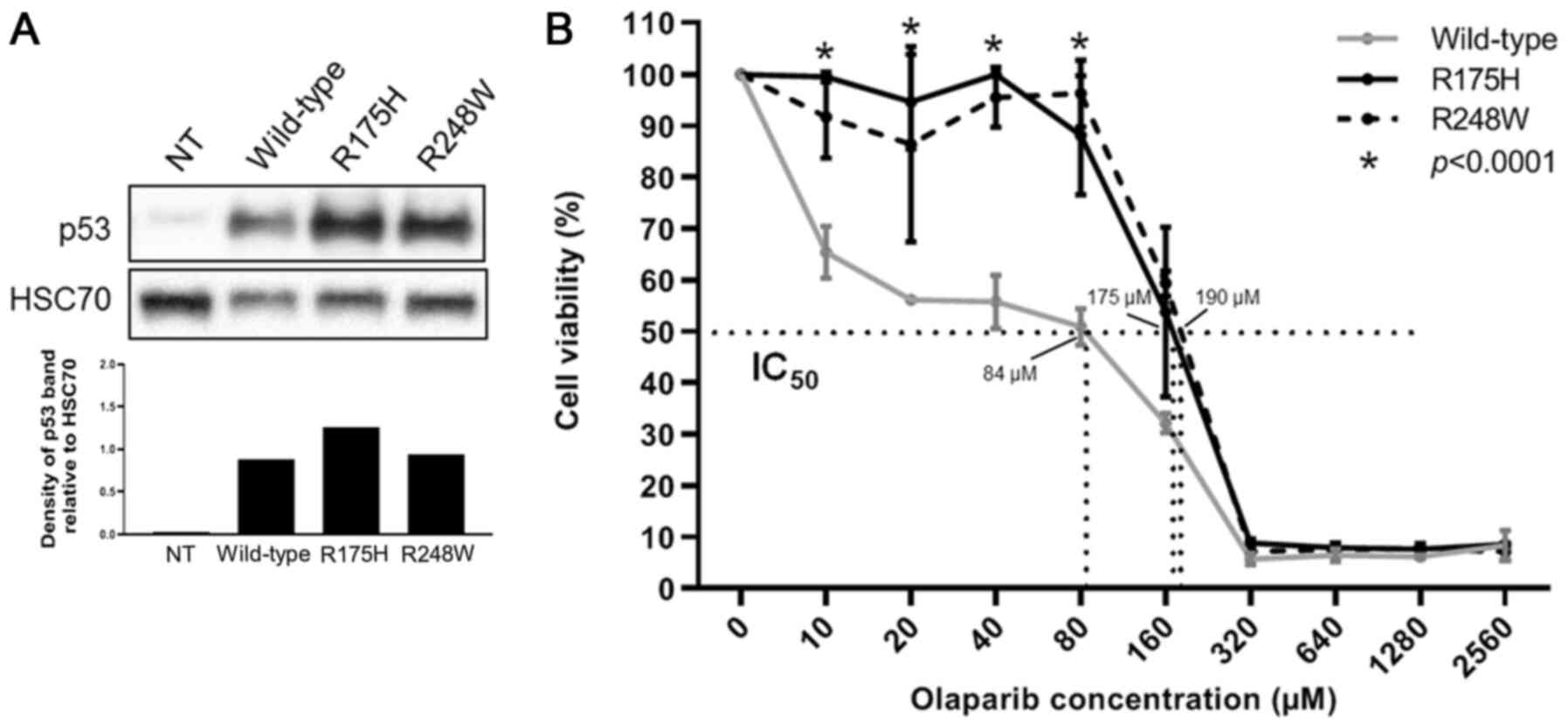
|
1
|
Paul A and Paul S: The breast cancer
susceptibility genes (BRCA) in breast and ovarian cancers. Front
Biosci. 19:605–618. 2014. View
Article : Google Scholar
|
|
2
|
Manchana T, Phoolcharoen N and Tantbirojn
P: BRCA mutation in high grade epithelial ovarian cancers. Gynecol
Oncol Rep. 29:102–105. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bishop AJ and Schiestl RH: Role of
homologous recombination in carcinogenesis. Exp Mol Pathol.
74:94–105. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Helleday T: Homologous recombination in
cancer development, treatment and development of drug resistance.
Carcinogenesis. 31:955–960. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gelmon KA, Tischkowitz M, Mackay H,
Swenerton K, Robidoux A, Tonkin K, Hirte H, Huntsman D, Clemons M,
Gilks B, et al: Olaparib in patients with recurrent high-grade
serous or poorly differentiated ovarian carcinoma or
triple-negative breast cancer: A phase 2, multicentre, open-label,
non-randomised study. Lancet Oncol. 12:852–861. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ledermann J, Harter P, Gourley C,
Friedlander M, Vergote I, Rustin G, Scott C, Meier W,
Shapira-Frommer R, Safra T, et al: Olaparib maintenance therapy in
platinum-sensitive relapsed ovarian cancer. N Engl J Med.
366:1382–1392. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaufman B, Shapira-Frommer R, Schmutzler
RK, Audeh MW, Friedlander M, Balmaña J, Mitchell G, Fried G,
Stemmer SM, Hubert A, et al: Olaparib monotherapy in patients with
advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol.
33:244–250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Farmer H, McCabe N, Lord CJ, Tutt AN,
Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I,
Knights C, et al: Targeting the DNA repair defect in BRCA mutant
cells as a therapeutic strategy. Nature. 434:917–921. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ashworth A: A synthetic lethal therapeutic
approach: Poly(ADP) ribose polymerase inhibitors for the treatment
of cancers deficient in DNA double-strand break repair. J Clin
Oncol. 26:3785–3790. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choi YE, Meghani K, Brault ME, Leclerc L,
He YJ, Day TA, Elias KM, Drapkin R, Weinstock DM, Dao F, et al:
Platinum and PARP inhibitor resistance due to overexpression of
microRNA-622 in BRCA1-mutant ovarian cancer. Cell Rep. 14:429–439.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bunting SF, Callén E, Wong N, Chen HT,
Polato F, Gunn A, Bothmer A, Feldhahn N, Fernandez-Capetillo O, Cao
L, et al: 53BP1 inhibits homologous recombination in
Brca1-deficient cells by blocking resection of DNA breaks. Cell.
141:243–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baker SJ, Markowitz S, Fearon ER, Willson
JK and Vogelstein B: Suppression of human colorectal carcinoma cell
growth by wild-type p53. Science. 249:912–915. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang M, Zhuang G, Sun X, Shen Y, Wang W,
Li Q and Di W: TP53 mutation-mediated genomic instability induces
the evolution of chemoresistance and recurrence in epithelial
ovarian cancer. Diagn Pathol. 12:162017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alsadoun N, MacGrogan G, Truntzer C,
Lacroix-Triki M, Bedgedjian I, Koeb MH, El Alam E, Medioni D,
Parent M, Wuithier P, et al: Solid papillary carcinoma with reverse
polarity of the breast harbors specific morphologic,
immunohistochemical and molecular profile in comparison with other
benign or malignant papillary lesions of the breast: A comparative
study of 9 additional cases. Mod Pathol. 31:1367–1380. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dong Z, Dong H, Zhong X, Peng Z, Zhu X,
Sun Y, Chen Y, Liu C, Yin X, Zhu G, et al: Development of a
compehensive NGS workflow for the analysis of tumor BRCA1 and BRCA2
mutations and large rearrangements. J Genet Genome Res. Sept
28–2015.(Epub ahead of print). doi: 10.23937/2378-3648/1410019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Végran F, Boidot R, Solary E and
Lizard-Nacol S: A short caspase-3 isoform inhibits
chemotherapy-induced apoptosis by blocking apoptosome assembly.
PLoS One. 6:e290582011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Végran F, Mary R, Gibeaud A, Mirjolet C,
Collin B, Oudot A, Charon-Barra C, Arnould L, Lizard-Nacol S and
Boidot R: Survivin-3B potentiates immune escape in cancer but also
inhibits the toxicity of cancer chemotherapy. Cancer Res.
73:5391–5401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Andreassen PR, Ho GP and D'Andrea AD: DNA
damage responses and their many interactions with the replication
fork. Carcinogenesis. 27:883–892. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bryant HE, Schultz N, Thomas HD, Parker
KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ and Helleday T:
Specific killing of BRCA2-deficient tumours with inhibitors of
poly(ADP-ribose) polymerase. Nature. 434:913–917. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fong PC, Boss DS, Yap TA, Tutt A, Wu P,
Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O'Connor MJ, et
al: Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA
mutation carriers. N Engl J Med. 361:123–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cancer Genome Atlas Research Network, .
Integrated genomic analyses of ovarian carcinoma. Nature.
474:609–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Norquist B, Wurz KA, Pennil CC, Garcia R,
Gross J, Sakai W, Karlan BY, Taniguchi T and Swisher EM: Secondary
somatic mutations restoring BRCA1/2 predict chemotherapy resistance
in hereditary ovarian carcinomas. J Clin Oncol. 29:3008–3015. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Levine AJ: Reviewing the future of the P53
field. Cell Death Differ. 25:1–2. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hakem R, de la Pompa JL, Elia A, Potter J
and Mak TW: Partial rescue of Brca1 (5–6) early embryonic lethality
by p53 or p21 null mutation. Nat Genet. 16:298–302. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jonkers J, Meuwissen R, van der Gulden H,
Peterse H, van der Valk M and Berns A: Synergistic tumor suppressor
activity of BRCA2 and p53 in a conditional mouse model for breast
cancer. Nat Genet. 29:418–425. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Greenblatt MS, Chappuis PO, Bond JP, Hamel
N and Foulkes WD: TP53 mutations in breast cancer associated with
BRCA1 or BRCA2 germ-line mutations: Distinctive spectrum and
structural distribution. Cancer Res. 61:4092–4097. 2001.PubMed/NCBI
|
|
27
|
Bernardini MQ, Baba T, Lee PS, Barnett JC,
Sfakianos GP, Secord AA, Murphy SK, Iversen E, Marks JR and
Berchuck A: Expression signatures of TP53 mutations in serous
ovarian cancers. BMC Cancer. 10:2372010. View Article : Google Scholar : PubMed/NCBI
|