Introduction
Pneumonia is a lower respiratory illness, which is
characterized by the following symptoms: Cough, fever, chest pain
and in severe cases, heart failure (1). Pneumonia is a common infectious
disease, with high morbidity rates (~0.02%) worldwide, particularly
in children (2,3). Inflammation is a key defense mechanism
to injury that prevents the entry of hazardous substances into the
body; however, inflammation also displays the potential to cause
injury (4). The respiratory tract
is easily infected on account of poor immune function in pediatric
cases (5). Inflammation from
endotoxins is a leading cause of pneumonia (6). As a potent endotoxin for inflammation,
lipopolysaccharide (LPS) is the primary bioactive component of the
cell wall of gram-negative bacterium (7). Therefore, developing novel therapeutic
strategies to inhibit the progression of pneumonia is
important.
MicroRNAs (miRNAs/miRs) are a subgroup of non-coding
RNAs ≤200 nucleotides in length, which control gene expression at
the transcriptional and post-transcriptional levels, thus affecting
cellular processes (8). miRNAs,
such as miR-3941, have been reported to regulate inflammatory
responses and diseases (9). In
addition, certain miRNAs have been identified in the pathogenesis
of pneumonia. For example, Abd-El-Fattah et al (10) demonstrated that miR-155, miR-21 and
miR-197 are upregulated in patients with pneumonia, but their
targets and respective functions have not been identified. In 2017,
Huang et al (11) identified
miRNA biomarkers for pneumonia via RNA-sequencing and
bioinformatics analysis, which demonstrated that let7f is
downregulated in the peripheral blood of patients with severe
pneumonia. Therefore, the present study aimed to investigate the
role of let7f in pneumonia.
Materials and methods
Serum samples
As pneumonia is also frequent in children (2), the present study aimed to identify a
potential therapeutic target for pneumonia in children. Peripheral
venous blood (3 ml) was collected from 29 healthy children (22 male
patients and 7 female patients; age range, 1–8 years; mean age, 4.7
years) and 29 patients with pneumonia (22 male patients and 7
female patients; age range, 1–7 years; mean age, 3.8 years) at
Guizhou Provincial People's Hospital (Guiyang, China) between March
2016 and February 2018. The exclusion criteria were as follows: i)
Patients with immunodeficiency, tuberculosis infection or asthma;
and ii) patients with respiratory tract infection or inflammatory
disease. Patients aged 1–8 years who were diagnosed with pneumonia
were included in the present study. Blood samples were centrifuged
at 1,000 × g for 10 min at 4°C and the supernatant was collected
for subsequent experimentation. The present study was approved by
the Ethical Committee of Guizhou Provincial People's Hospital
(approval no. 2016011605). The parents or legal guardians of all
participants provided written informed consent.
Cell culture
The human lung adenocarcinoma A549 cell line and
normal human fibroblast WI-38 cell line were purchased from
American Type Culture Collection. Cells were maintained in DMEM
supplemented with 10% FBS and 1% penicillin/streptomycin (all
purchased from Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C
with 5% CO2.
Cell transfection
The full length of MAPK6 was reconstructed into a
pcDNA3.1 empty vector (Invitrogen; Thermo Fisher Scientific, Inc.).
let7f-5p mimic (5′-UUGAUAUGUUAGAUGAUGGAGU-3′) and negative control
(NC) mimic (5′-UCACAACCUCCUAGAAAGAGUAGA-3′) were synthesized by
Shanghai GenePharma Co., Ltd. A549 and WI-38 cells
(1×104 cells/well) were transfected with 2 µg pcDNA3.1,
2 µg pcDNA3.1-MAPK6, 100 nM NC mimic or 100 nM let7f-5p mimic using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. After
transfection for 48 h at 37°C, cells were used for subsequent
experiments.
LPS treatment
Briefly, cells were seeded (2×105
cells/well) into 6-well plates and cultured at 37°C for 24 h. To
establish the in vitro pneumonia model, WI-38 cells were
incubated with 10 µg/ml LPS (Beijing Solarbio Science &
Technology Co., Ltd.) for 12 h at 37°C as previously described
(12,13), whereas A549 cells were incubated
with 10 µg/ml LPS for 12 h at 37°C as previously described
(14).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from peripheral venous
blood, and A549 and WI-38 cells using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was reverse
transcribed into cDNA using the PrimeScript™ RT reagent kit (cat.
no. RR047A; Takara Biotechnology Co., Ltd.), according to the
manufacturer's protocol. Subsequently, qPCR was performed using the
SYBR Premix Ex Taq (cat. no. RR003A; Takara Biotechnology
Co., Ltd.). The following thermocycling conditions were used for
qPCR: Initial denaturation at 94°C for 5 min; followed by 40 cycles
of degeneration at 94°C for 30 sec, annealing at 60°C for 30 sec
and extension at 72°C for 1 min. The following primers were used
for qPCR: let7f-5p forward, 5′-TTGATATGTTAGATGATGGAGT-3′ and
reverse, 5′-ACTCCATCATCTAACATATCAA-3′; IL-6 forward,
5′-AGCCACTCACCTCTTCAGAACGAA-3′ and reverse,
5′-TACTCATCTGCACAGCTCTGGCTT-3′; TNF-α forward,
5′-GCCAATGGCATGGATCTCAAAG-3′ and reverse,
5′-CAGAGCAATGACTCCAAAGT-3′; U6 forward, 5′-GTGCTCGCTTCGGCAGCACAT-3′
and reverse, 5′-AATATGGAACGCTTCACGAAT-3′; MAPK6 forward,
5′-GTACACATGTGTTATCTACCTCA-3 and reverse,
5′-TACAATAAACGCTGGCTAA-3′; and GAPDH forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse, 5′-TGGTGAAGACGCCAGTGGA-3′.
let7f-5p and IL-6/TNF-α/MAPK6 mRNA expression levels were
quantified using the 2−ΔΔCq method (15) and normalized to the internal
reference genes U6 and GAPDH, respectively.
Western blotting
Total protein was extracted from peripheral venous
blood, and A549 and WI-38 cells using RIPA lysis buffer
(Sigma-Aldrich; Merck KGaA) supplemented with protease inhibitors.
Total protein was quantified using the BCA method (Beyotime
Institute of Biotechnology). Equal amounts of protein (15 µg/lane)
were separated via 8% SDS-PAGE and transferred onto PVDF membranes.
Following blocking with 5% non-fat milk at room temperature for 2
h, the membranes were incubated overnight at 4°C with primary
antibodies (all purchased from Cell Signaling Technology, Inc.)
targeted against: MAPK6 (cat. no. 4067; 1:1,000), STAT3 (cat. no.
12640; 1:1,000), phosphorylated-STAT3 (cat. no. 9145; 1:1,000) and
GAPDH (cat. no. 5174; 1:1,000). Following washing three times with
TBST, the membranes were incubated with HRP-conjugated goat
anti-rabbit secondary antibodies (cat. no. 7074; 1:3,000; Cell
Signaling Technology, Inc.) at room temperature for 1 h. Protein
bands were visualized using an ECL system (Beyotime Institute of
Biotechnology). Protein expression was semi-quantified using ImageJ
software (version 1.50; National Institutes of Health).
Cell viability assay
A549 and WI-38 cell viability were assessed using
the Cell Counting Kit-8 (CCK-8) detection kit (cat. no. CSP04;
Dojindo Molecular Technologies, Inc.) according to the
manufacturer's protocol. Briefly, cells were seeded
(5×103 cells/well) into 96-well plates and cultured for
24 h at 37°C. Subsequently, 20 µl CCK-8 solution was added to each
well for 1 h at 37°C. The absorbance was measured at a wavelength
of 450 nm using a microplate reader.
ELISA
A549 and WI-39 cell culture media were collected.
The levels of inflammatory cytokines, including IL-6 (cat. no.
D6050) and TNF-α (cat. no. MTA00B), were detected using ELISA kits
(R&D Systems, Inc.) according to the manufacturer's
protocol.
Dual-luciferase reporter assay
TargetScan software (version 7.1; www.targetscan.org/vert_71) was used to predict
the complementary binding sites between let7f-5p and the
3′-untranslated region (UTR) of MAPK6. To validate the interaction
between let7f-5p and MAPK6, A549 and WI-38 cells were seeded
(1×104 cells/well) into 24-well plates and
co-transfected with wild-type (WT) MAPK6 (0.2 mg) or mutant MAPK6
(0.2 mg) pmiRGLO dual-luciferase vectors (Promega Corporation) and
let7f-5p mimic (20 nM) or NC mimic (20 nM) using Lipofectamine
2000. Following incubation for 24 h at 37°C, luciferase activities
were detected using a Dual-Luciferase Reporter assay system
(Promega Corporation) according to the manufacturer's protocol.
Firefly luciferase activity was normalized to Renilla
luciferase activity.
Statistical analysis
Statistical analyses were performed using GraphPad
software (version 6.0; GraphPad Software, Inc.). All experiments
were performed in triplicate. Data are presented as the mean ± SD.
The unpaired Student's t-test was performed to compare the
difference of let7f-5p and MAPK6 between healthy volunteers and
patients with pneumonia. The unpaired Student's t-test was
performed to compare differences between two groups for in
vitro experiments. One-way ANOVA followed by Tukey's post hoc
test was used to compare differences among multiple groups.
Pearson's correlation analysis was performed to determine the
correlation between let7f-5p and MAPK6 in the peripheral venous
blood of patients with pneumonia. P<0.05 was considered to
indicate a statically significant difference.
Results
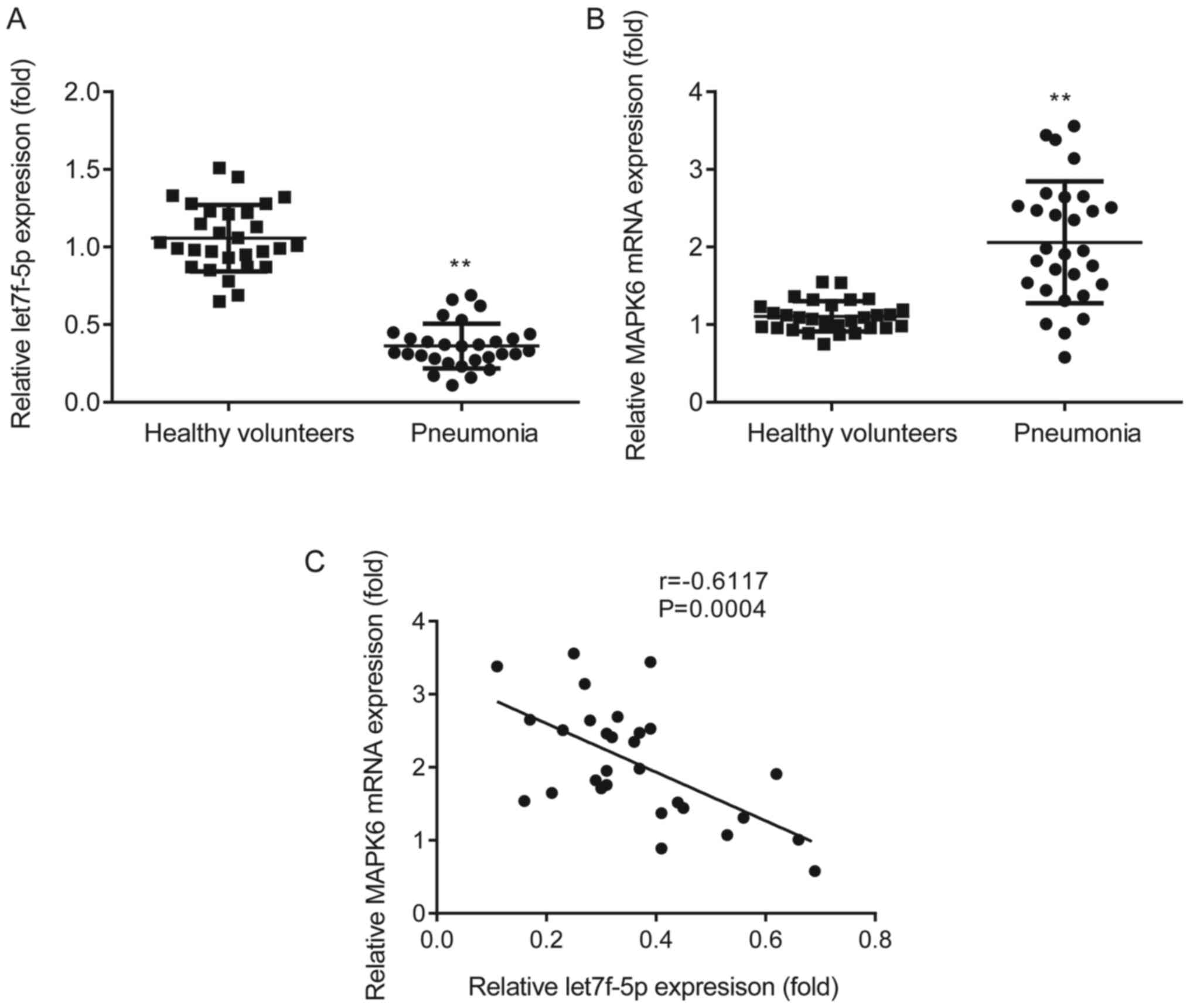
let7f-5p expression is negatively
correlated with MAPK6 expression in patients with pneumonia
The expression profiles of let7f-5p and MAPK6 in
patients with pneumonia and healthy volunteers were assessed via
RT-qPCR. let7f-5p expression was significantly decreased (Fig. 1A) and MAPK6 expression was
significantly increased (Fig. 1B)
in the peripheral venous blood of patients with pneumonia compared
with healthy volunteers. Pearson's correlation analysis
demonstrated that let7f-5p expression was negatively correlated
with MAPK6 expression in patients with pneumonia (Fig. 1C).
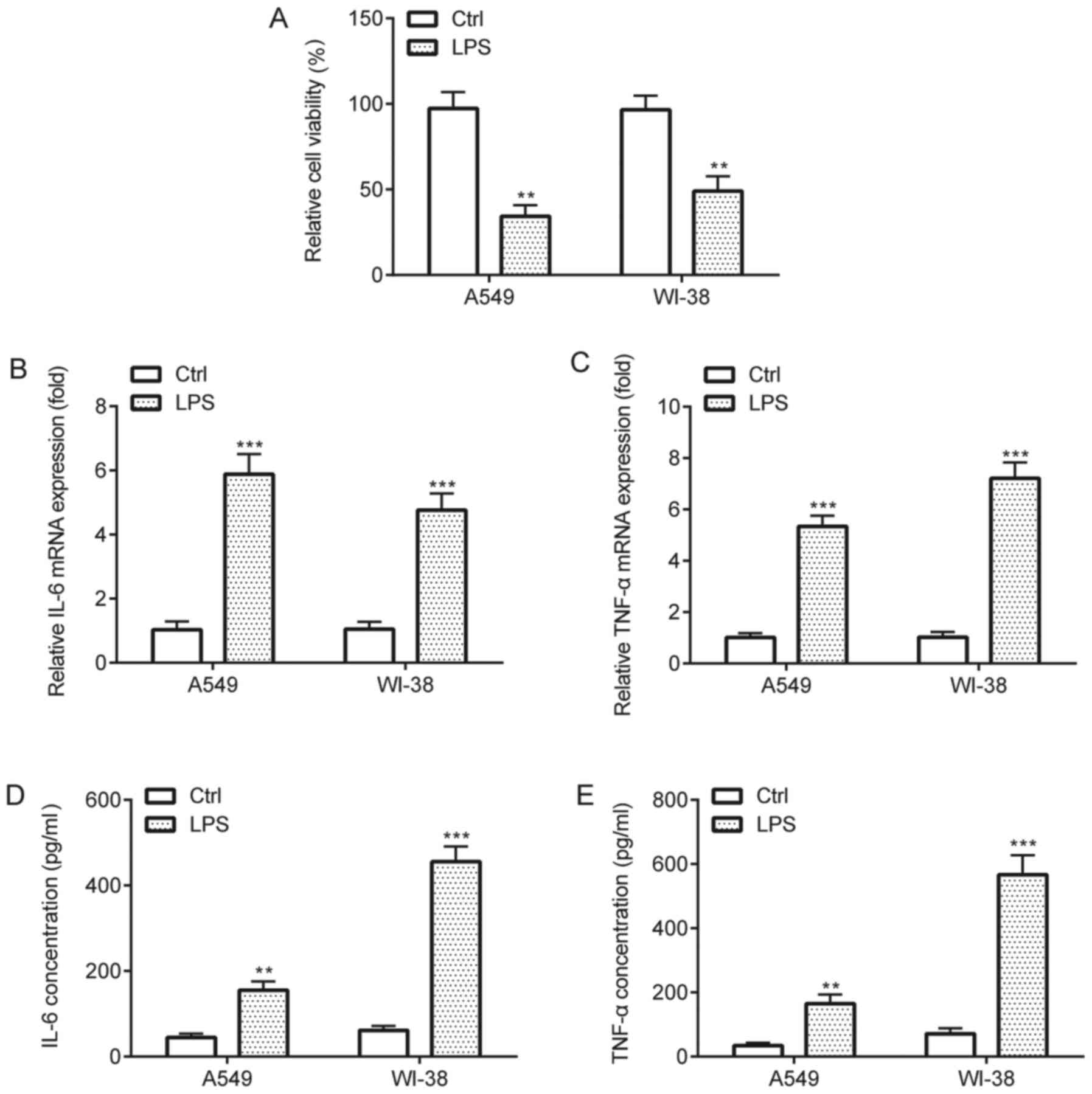
LPS-induced inflammatory injury in
A549 and WI-38 cells
The effects of LPS on A549 and WI-38 cells were
assessed by performing CCK-8 and ELISA assays. The CCK-8 assay
results indicated that LPS treatment significantly decreased cell
viability compared with the control group (Fig. 2A). In addition, the RT-qPCR and
ELISA results indicated that LPS treatment significantly increased
the expression and release of proinflammatory cytokines TNF-α and
IL-6 in A549 and WI-38 cells compared with the control group
(Fig. 2B-E). Collectively, the
results suggested that LPS induced inflammatory injury in A549 and
WI-38 cells.
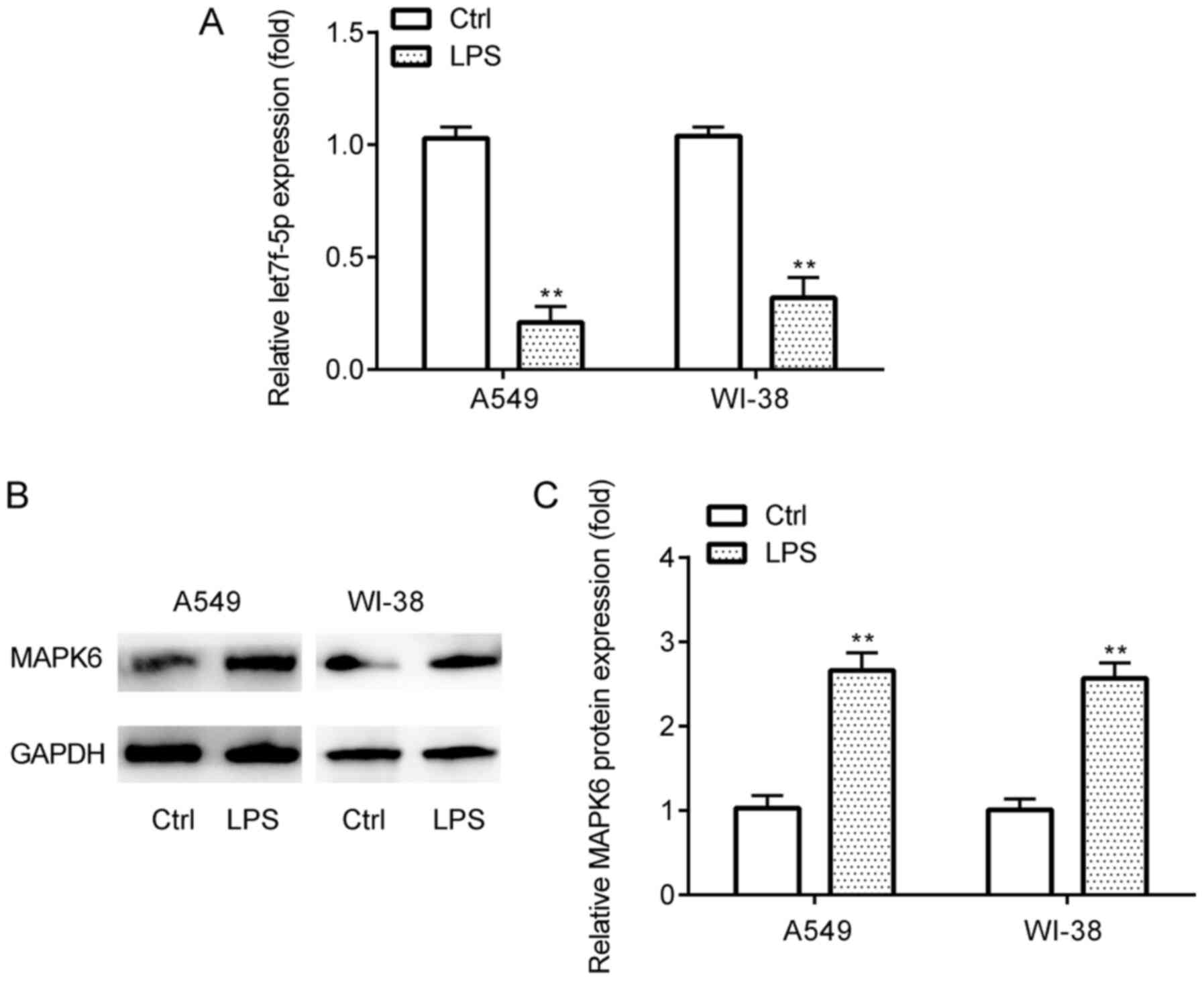
let7f-5p and MAPK6 expression in
LPS-induced inflammatory injury
The expression profiles of let7f-5p and MAPK6 in the
in vitro pneumonia model in A549 and WI-38 cells were
assessed via RT-qPCR and western blotting. The results demonstrated
that let7f-5p expression levels were significantly lower (Fig. 3A), whereas MAPK6 protein expression
levels were significantly higher (Fig.
3B and C) in LPS-treated A549 and WI-38 cells compared with the
control group.
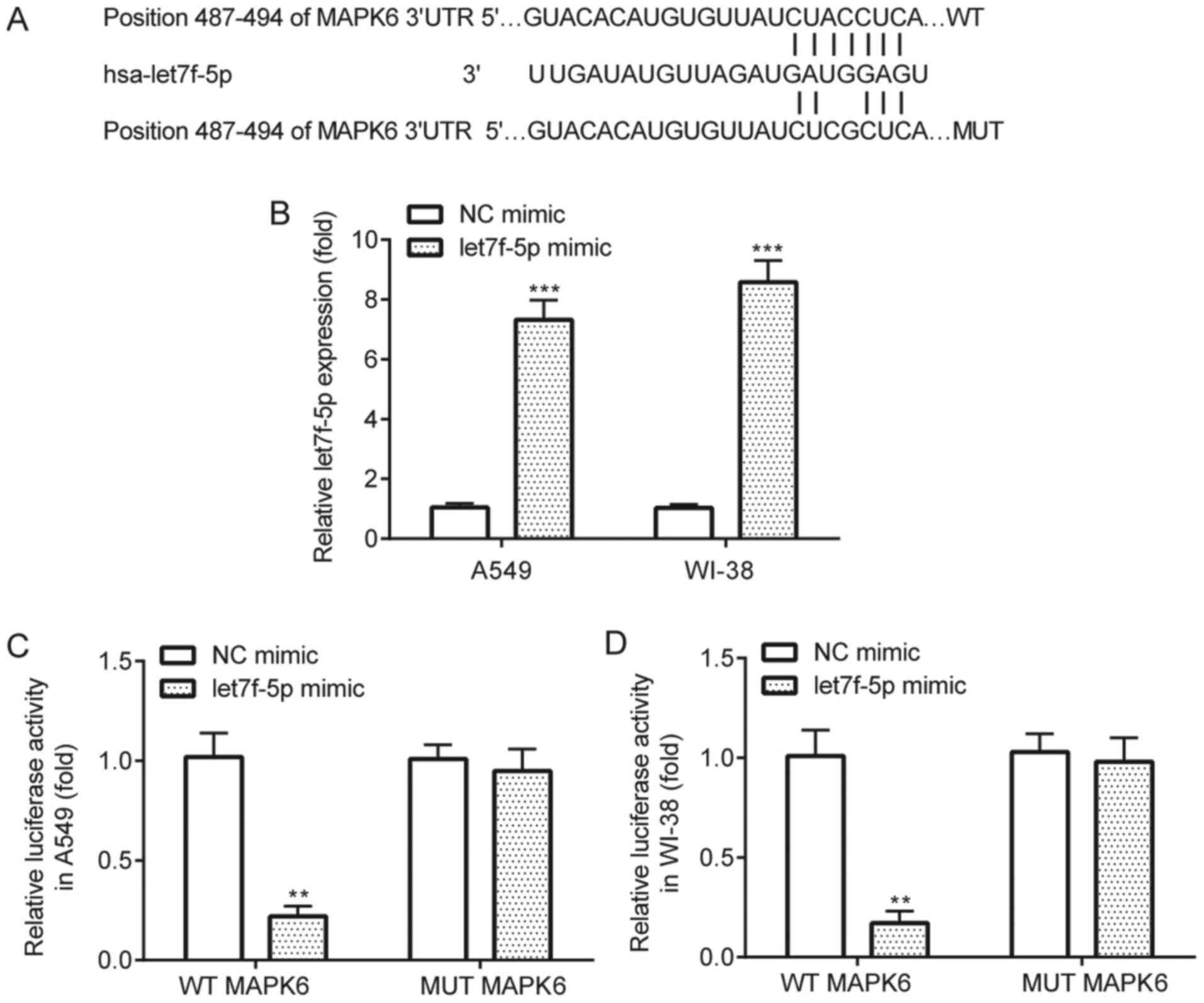
let7f-5p targets MAPK6
The interaction between let7f-5p and MAPK6 was
verified by performing the dual-luciferase reporter assay.
TargetScan software identified complementary binding sites between
let7f-5p and the 3′UTR of MAPK6 (Fig.
4A). The results demonstrated that let7f-5p mimic significantly
increased let7f-5p expression in A549 and WI-38 cells compared with
NC mimic (Fig. 4B). The
dual-luciferase reporter assay results indicated that compared with
NC mimic, let7f-5p mimic significantly decreased the luciferase
activity of WT MAPK6 in A549 and WI-38 cells, but did not
significantly alter the luciferase activity of MUT MAPK6 in A549 or
WI-38 cells (Fig. 4C and D).
let7f-5p attenuates LPS-induced
inflammatory injury by targeting MAPK6
The effects of let7f-5p and MAPK6 in the LPS-induced
in vitro pneumonia model were investigated in A549 and WI-38
cells. The RT-qPCR and western blotting results demonstrated that
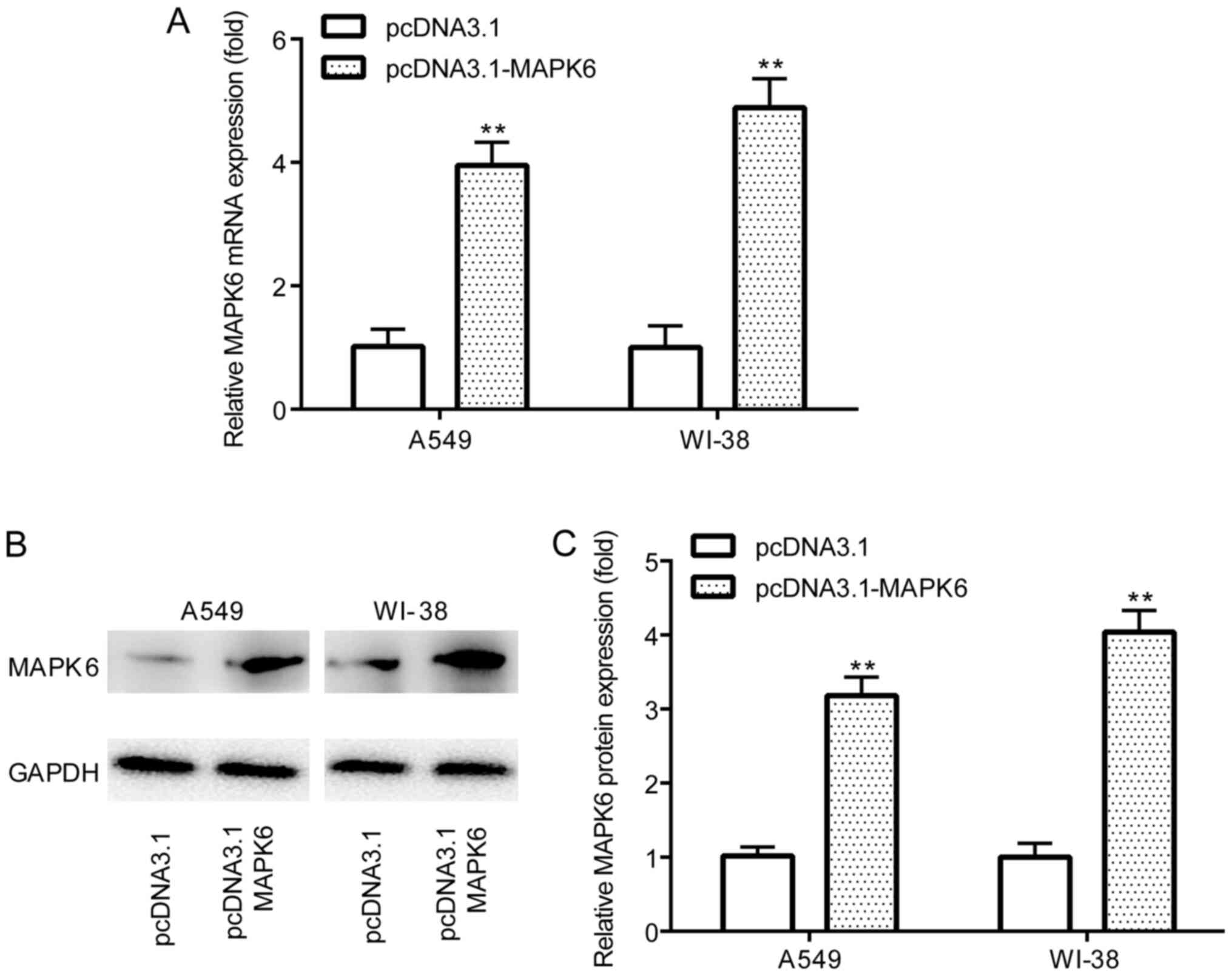
pcDNA3.1-MAPK6 significantly increased MAPK6 mRNA and protein
expression levels (Fig. 5A-C)
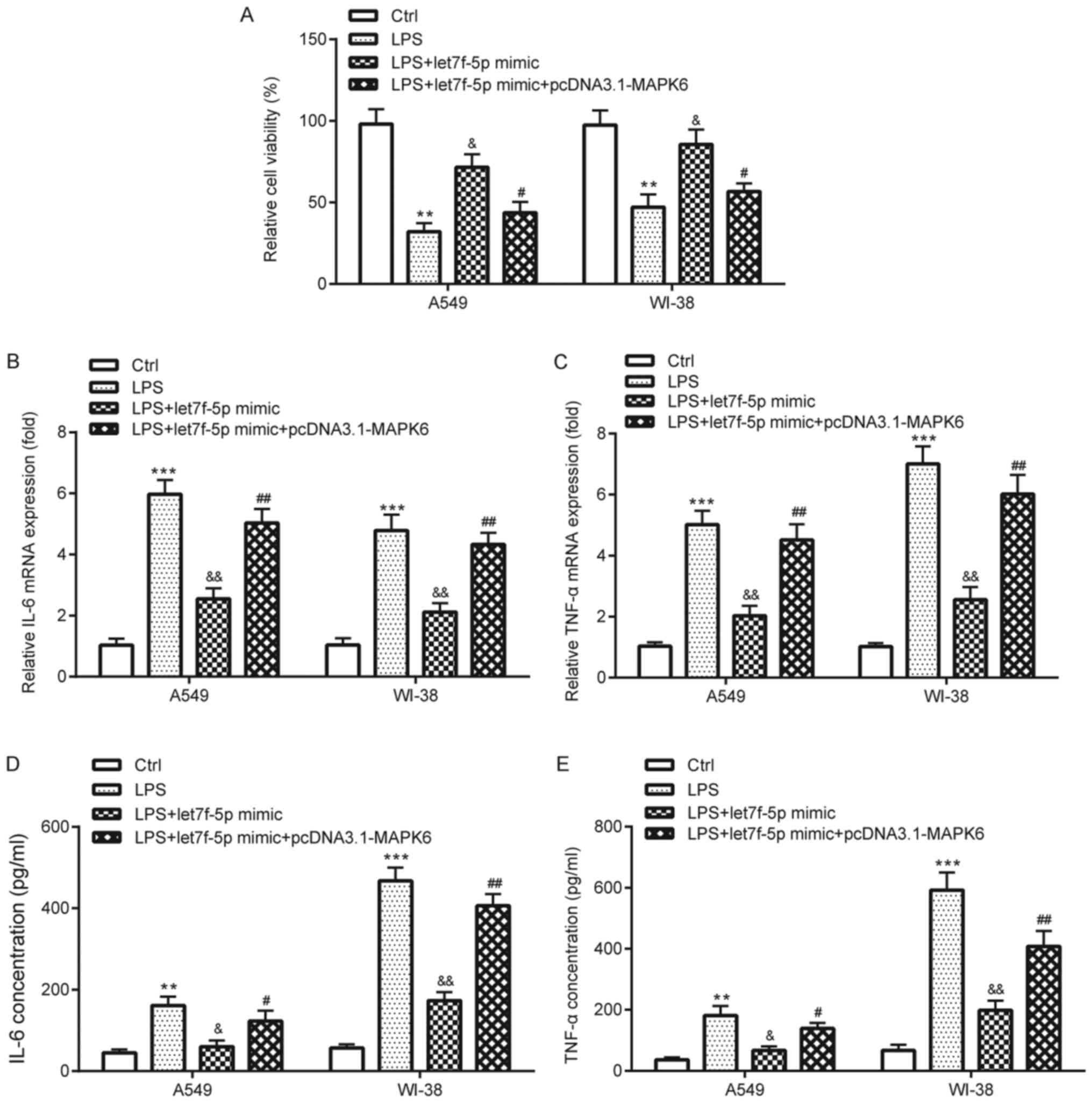
compared with pcDNA3.1 in A549 and WI-38 cells. The CCK-8 assay
results suggested that let7f-5p mimic significantly ameliorated
LPS-induced reductions in cell viability in A549 and WI-38 cells,
but let7f-5p mimic-mediated effects were significantly reversed by
MAPK6 overexpression (Fig. 6A). The
RT-qPCR and ELISA results demonstrated that let7f-5p mimic
significantly ameliorated LPS-induced TNF-α and IL-6 expression and
release in A549 and WI-38 cells, which was also significantly
reversed by transfection with pcDNA3.1-MAPK6 (Fig. 6B-E).
STAT3 is involved in
let7f-5p/MAPK6-mediated regulation of LPS-induced inflammatory
injury
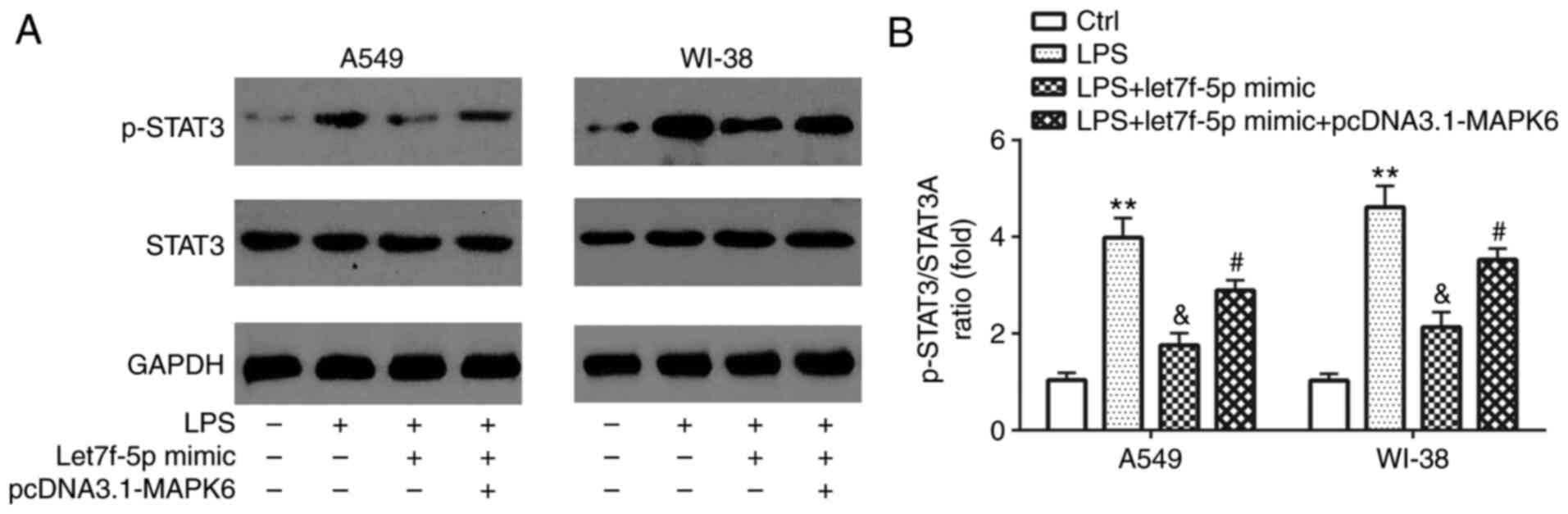
Compared with the control group, LPS significantly
increased STAT3 activation in A549 and WI-38 cells, which was
reversed by transfection with let7f-5p mimic (Fig. 7A and B). However, let7f-5p
mimic-mediated effects on STAT3 activation were significantly
reversed by transfection with pcDNA3.1-MAPK6.
Discussion
Pneumonia is a common infectious disease, with high
mortality and morbidity rates worldwide (2,3);
therefore, developing novel therapeutic strategies for the
management of pneumonia is important. Targeted therapy is
extensively applied in the treatment of several diseases (16). For example, the let7 family
participates in multiple carcinogenic signaling pathways (17). let-7 serves as a tumor suppressor,
whereby let-7 downregulation promotes lung cancer cell
proliferation (18), whereas let-7b
and let-7c are crucial for lung restoration in influenza pneumonia
model mice (18). let7f inhibition
facilitates neuroprotection in ischemic stroke (19), and let7f expression is elevated
following ischemia-reperfusion (20), but decreased in patients with
papillary thyroid cancer (21). By
performing RNA-sequencing and bioinformatics analysis, a previous
study demonstrated that let7f expression is decreased in the
peripheral blood of patients with severe pneumonia compared with
healthy volunteers (11). More
recently, it has been reported that let7f expression is decreased
in the blood of extracellular vesicles of alcohol-drinkers without
liver injury compared with non-drinkers (22). To the best of our knowledge, the
present study was the first to investigate the role of let7f-5p in
pneumonia.
MAPK6 serves as a promoter of several diseases:
Small nucleolar RNA host gene 6 facilitates breast cancer cell
proliferation and metastasis by regulating the miR-26a-5p/MAPK6
axis (23); miR-144-3p inhibits the
progression of cervical cancer by targeting MAPK6 (24); nuclear paraspeckle assembly
transcript 1 enhances myocardial ischemia-reperfusion injury via
the miR-495-3p/MAPK6 axis (25);
and miR-26a-5p negatively regulates neuropathic pain by targeting
MAPK6 (26). As for the function of
MAPK6 in inflammation, hierarchical clustering in tongue tissue
with hyperplasia suggests that cytokine-mediated inflammation may
be associated with MAPK6 (27).
However, the exact role of MAPK6 in pneumonia is not completely
understood.
The present study aimed to investigate the role of
let7f-5p and MAPK6 by recruiting healthy volunteers and patients
with pneumonia, and using in vitro pneumonia model. The
results demonstrated that let7f-5p expression was significantly
decreased, whereas MAPK6 expression was significantly increased in
the peripheral venous blood of patients with pneumonia and in
LPS-induced WI-38 and A549 cells compared with healthy volunteers
and control cells, respectively. The results also demonstrated that
let7f-5p targeted MAPK6 in WI-38 and A549 cells, and let7f-5p
expression was negatively correlated with MAPK6 expression in the
peripheral venous blood of patients with pneumonia.
Pneumonia is associated with inflammation (6). TNF-α, a predominant cytokine that is
produced by monocytes and macrophages, is an important inflammatory
mediator (28,29). TNF-α initiates the inflammatory
response by inducing local infiltration, neutrophil chemotaxis,
phagocytosis and killing of pathogens (30). IL-6, an important cytokine that is
produced by monocytes, macrophages and lymphocytes, is a pivotal
mediator during the acute phase of inflammatory response (28,30).
The results of the present study demonstrated that, compared with
the control group, LPS treatment significantly increased the
expression levels of TNF-α and IL-6 in A549 and WI-38 cells, which
were reversed by transfection with let7f-5p mimic. However,
let7f-5p mimic-mediated effects were reversed by MAPK6
overexpression.
The STAT3 signaling pathway is pivotal for the
inflammatory response (31). During
acute inflammatory injury, the STAT3 signaling pathway is
implicated in lung injury (32) and
LPS-induced inflammatory injury, as well as in the circular RNA
(circ)_0038467/miR-338-3p axis (33). The results of the present study
demonstrated that LPS significantly increased STAT3 activation
compared with the control group, which was reversed by transfection
with let7f-5p mimic. Moreover, let7f-5p mimic-mediated effects on
STAT3 activation were reversed by MAPK6 overexpression.
As for the association between the
let7f-5p/MAPK6/STAT3 axis and inflammation, several reports in
other inflammation-related diseases have been published. In 2019,
Tan et al (34) reported
that let7f-5p attenuated inflammation in systemic lupus
erythematosus by targeting NLR family pyrin domain containing 3. In
2020, Yao et al (35)
demonstrated that MAPK6 was involved in circ_0000285-induced
inflammation in diabetic nephropathy. The STAT3 signaling pathway
is pivotal for the inflammatory response (31). In 2019, Li et al (36) reported that let7f-5p reduced Th17
differentiation in multiple sclerosis by targeting STAT3. In 2018,
Kim et al (37) demonstrated
that orientin repressed breast cancer cell invasion via the
MAPK6/STAT3 signaling pathway (37). However, the relationship between
let7f-5p and MAPK6, as well as the interactions between let7f-5p
and MAPK6, let7f-5p and STAT3 or MAPK6 and STAT3 in pneumonia have
not been previously reported. Therefore, to the best of our
knowledge, the present study indicated for the first time that the
let7f-5p/MAPK6/STAT3 axis may serve an inhibitory role in
inflammation in pneumonia.
The present study had two key limitations. The
results of the present study demonstrated that let7f-5p expression
was significantly decreased in patients with pneumonia and in
LPS-induced A549 and WI-38 cells compared with healthy volunteers
and control cells, respectively. Therefore, the present study aimed
to investigate the effect of let7f-5p overexpression on pneumonia
and to assess whether MAPK6 overexpression could rescue the effects
of let7f-5p overexpression on pneumonia. The results indicated that
let7f-5p inhibited pneumonia-associated inflammation in
vitro by targeting MAPK6. However, the effects of MAPK6
knockdown or knockout on inflammation could be similar to the
effects mediated by let7f-5p overexpression, but this was not
investigated in the present study, thus further investigation is
required. Secondly, although the WI-38 cell line is widely used for
the study of pneumonia in vitro (13,38,39), a
future study using a normal non-cancerous human lung cell line
should be performed to verify the results of the present study.
Collectively, the results of the present study
suggested that let7f-5p inhibited pneumonia-associated inflammation
in vitro by targeting MAPK6 and inactivating the STAT3
signaling pathway. Therefore, let7f-5p may serve as a potential
target for anti-inflammatory therapeutic strategies for
pneumonia.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Respiratory
Disease Clinical Research Center of Guizhou Province (grant no.
2016-2907) and the Science and Technology Project of Guizhou
Province (grant nos. 2017-1100 and 2019-1195).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
LX, QS and ZO performed the experiments and analyzed
the data. XZ and CZ designed the present study and supervised the
experiments. CZ drafted the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of Guizhou Provincial People's Hospital (approval no.
2016011605). The parents or legal guardians of all participants
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cillóniz C, Torres A, Niederman M, van der
Eerden M, Chalmers J, Welte T and Blasi F: Community-acquired
pneumonia related to intracellular pathogens. Intensive Care Med.
42:1374–1386. 2016. View Article : Google Scholar
|
|
2
|
Korppi M: Diagnosis and treatment of
community-acquired pneumonia in children. Acta Paediatr.
101:702–704. 2012. View Article : Google Scholar
|
|
3
|
Simonetti AF, Viasus D, Garcia-Vidal C and
Carratalà J: Management of community-acquired pneumonia in older
adults. Ther Adv Infect Dis. 2:3–16. 2014.
|
|
4
|
Kim GD: Myricetin inhibits angiogenesis by
inducing apoptosis and suppressing PI3K/Akt/mTOR signaling in
endothelial cells. J Cancer Prev. 22:219–227. 2017. View Article : Google Scholar
|
|
5
|
Yu B, Shen Y, Qiao J and Cui Q: Geniposide
attenuates Staphylococcus aureus-induced pneumonia in mice by
inhibiting NF-κB activation. Microb Pathog. 112:117–121. 2017.
View Article : Google Scholar
|
|
6
|
Rojas M, Woods CR, Mora AL, Xu J and
Brigham KL: Endotoxin-induced lung injury in mice: Structural,
functional, and biochemical responses. Am J Physiol Lung Cell Mol
Physiol. 288:L333–L341. 2005. View Article : Google Scholar
|
|
7
|
Didier D and Jean-Damien R: Acute lung
injury and bacterial infection. Clin Chest Med. 26:105–112. 2005.
View Article : Google Scholar
|
|
8
|
Wong KY, Huang X and Chim CS: DNA
methylation of microRNA genes in multiple myeloma. Carcinogenesis.
33:1629–1638. 2012. View Article : Google Scholar
|
|
9
|
Fei S, Cao L and Pan L: microRNA3941
targets IGF2 to control LPS-induced acute pneumonia in A549 cells.
Mol Med Rep. 17:4019–4026. 2018.
|
|
10
|
Abd-El-Fattah AA, Sadik NA, Shaker OG and
Aboulftouh ML: Differential microRNAs expression in serum of
patients with lung cancer, pulmonary tuberculosis, and pneumonia.
Cell Biochem Biophys. 67:875–884. 2013. View Article : Google Scholar
|
|
11
|
Huang S, Feng C, Zhai YZ, Zhou X, Li B,
Wang LL, Chen W, Lv FQ and Li TS: Identification of miRNA
biomarkers of pneumonia using RNA-sequencing and bioinformatics
analysis. Exp Ther Med. 13:1235–1244. 2017. View Article : Google Scholar
|
|
12
|
Bai D, Han A and Cong S: The effect of
down-regulation of CCL5 on lipopolysaccharide-induced WI-38
fibroblast injury: A potential role for infantile pneumonia. Iran J
Basic Med Sci. 21:449–454. 2018.
|
|
13
|
Zhou Z, Zhua Y, Gao G and Zhang Y: Long
noncoding RNA SNHG16 targets miR-146a-5p/CCL5 to regulate LPS
induced WI-38 cell apoptosis and inflammation in acute pneumonia.
Life Sci. 228:189–197. 2019. View Article : Google Scholar
|
|
14
|
Wang Q, Li D, Han Y, Ding X, Xu T and Tang
B: MicroRNA-146 protects A549 and H1975 cells from LPS-induced
apoptosis and inflammation injury. J Biosci. 42:637–645. 2017.
View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Pérez-Herrero E and Fernández-Medarde A:
Advanced targeted therapies in cancer: Drug nanocarriers, the
future of chemotherapy. Eur J Pharm Biopharm. 93:52–79. 2015.
View Article : Google Scholar
|
|
17
|
Wang X, Cao L, Wang Y, Wang X, Liu N and
You Y: Regulation of let-7 and its target oncogenes (Review). Oncol
Lett. 3:955–960. 2012. View Article : Google Scholar
|
|
18
|
Tan KS, Choi H, Jiang X, Yin L, Seet JE,
Patzel V, Engelward BP and Chow VT: Micro-RNAs in regenerating
lungs: An integrative systems biology analysis of murine influenza
pneumonia. BMC Genomics. 15:5872014. View Article : Google Scholar
|
|
19
|
Selvamani A, Sathyan P, Miranda RC and
Sohrabji F: An antagomir to microRNA Let7f promotes neuroprotection
in an ischemic stroke model. PLoS One. 7:e326622012. View Article : Google Scholar
|
|
20
|
Wang L, Niu X, Hu J, Xing H, Sun M, Wang
J, Jian Q and Yang H: After myocardial ischemia-reperfusion,
miR-29a, and Let7 could affect apoptosis through regulating IGF-1.
Biomed Res Int. 2015:2454122015. View Article : Google Scholar
|
|
21
|
Damanakis AI, Eckhardt S, Wunderlich A,
Roth S, Wissniowski TT, Bartsch DK and Di Fazio P: MicroRNAs let7
expression in thyroid cancer: Correlation with their deputed
targets HMGA2 and SLC5A5. J Cancer Res Clin Oncol. 142:1213–1220.
2016. View Article : Google Scholar
|
|
22
|
Eguchi A, Franz N, Kobayashi Y, Iwasa M,
Wagner N, Hildebrand F, Takei Y, Marzi I and Relja B: Circulating
extracellular vesicles and their miR ‘Barcode’ differentiate
alcohol drinkers with liver injury and those without liver injury
in severe trauma patients. Front Med (Lausanne). 6:302019.
View Article : Google Scholar
|
|
23
|
Lv P, Qiu X, Gu Y, Yang X, Xu X and Yang
Y: Long non-coding RNA SNHG6 enhances cell proliferation, migration
and invasion by regulating miR-26a-5p/MAPK6 in breast cancer.
Biomed Pharmacother. 110:294–301. 2019. View Article : Google Scholar
|
|
24
|
Wu J, Zhao Y, Li F and Qiao B: miR-144-3p:
A novel tumor suppressor targeting MAPK6 in cervical cancer. J
Physiol Biochem. 75:143–152. 2019. View Article : Google Scholar
|
|
25
|
Luo M, Sun Q, Zhao H, Tao J and Yan D:
Long noncoding RNA NEAT1 sponges miR-495-3p to enhance myocardial
ischemia-reperfusion injury via MAPK6 activation. J Cell Physiol.
235:105–113. 2020. View Article : Google Scholar
|
|
26
|
Zhang Y, Su Z, Liu HL, Li L, Wei M, Ge DJ
and Zhang ZJ: Effects of miR-26a-5p on neuropathic pain development
by targeting MAPK6 in in CCI rat models. Biomed Pharmacother.
107:644–649. 2018. View Article : Google Scholar
|
|
27
|
Liu YC, Ho HC, Lee MR, Lai KC, Yeh CM, Lin
YM, Ho TY, Hsiang CY and Chung JG: Early induction of
cytokines/cytokine receptors and Cox2, and activation of NF-κB in
4-nitroquinoline 1-oxide-induced murine oral cancer model. Toxicol
Appl Pharmacol. 262:107–116. 2012. View Article : Google Scholar
|
|
28
|
Khan J, Noboru N, Young A and Thomas D:
Pro and antiinflammatory cytokine levels (TNF-α, IL-1β, IL-6 and
IL-10) in rat model of neuroma. Pathophysiology. 24:155–159. 2017.
View Article : Google Scholar
|
|
29
|
Berg AS, Inchley CS, Fjaerli HO, Leegaard
TM, Lindbaek M and Nakstad B: Clinical features and inflammatory
markers in pediatric pneumonia: A prospective study. Eur J Pediatr.
176:629–638. 2017. View Article : Google Scholar
|
|
30
|
Dai JP, Wang QW, Su Y, Gu LM, Zhao Y, chen
XX, Chen C, Li WZ, Wang GF and Li KS: Emodin inhibition of
influenza A virus replication and influenza viral pneumonia via the
Nrf2, TLR4, p38/JNK and NF-kappaB pathways. Molecules. 22:17542017.
View Article : Google Scholar
|
|
31
|
Yu Q, Zeng K, Ma X, Song F, Jiang Y, Tu P
and Wang X: Resokaempferol-mediated anti-inflammatory effects on
activated macrophages via the inhibition of JAK2/STAT3, NF-κB and
JNK/p38 MAPK signaling pathways. Int Immunopharmacol. 38:104–114.
2016. View Article : Google Scholar
|
|
32
|
Gao H and Ward PA: STAT3 and suppressor of
cytokine signaling 3: Potential targets in lung inflammatory
responses. Expert Opin Ther Targets. 11:869–880. 2007. View Article : Google Scholar
|
|
33
|
Liu G, Wan Q, Li J, Hu X, Gu X and Xu S:
Circ_0038467 regulates lipopolysaccharide-induced inflammatory
injury in human bronchial epithelial cells through sponging
miR-338-3p. Thorac Cancer. 11:1297–1308. 2020. View Article : Google Scholar
|
|
34
|
Tan W, Gu Z, Leng J, Zou X, Chen H, Min F,
Zhou W, Zhang L and Li G: Let-7f-5p ameliorates inflammation by
targeting NLRP3 in bone marrow-derived mesenchymal stem cells in
patients with systemic lupus erythematosus. Biomed Pharmacother.
118:1093132019. View Article : Google Scholar
|
|
35
|
Yao T, Zha D, Hu C and Wu X: Circ_0000285
promotes podocyte injury through sponging miR-654-3p and activating
MAPK6 in diabetic nephropathy. Gene. 747:1446612020. View Article : Google Scholar
|
|
36
|
Li ZH, Wang YF, He DD, Zhang XM, Zhou YL,
Yue H, Huang S, Fu Z, Zhang LY, Mao ZQ, et al: Let-7f-5p suppresses
Th17 differentiation via targeting STAT3 in multiple sclerosis.
Aging (Albany NY). 11:4463–4477. 2019. View Article : Google Scholar
|
|
37
|
Kim SJ, Pham TH, Bak Y, Ryu HW, Oh SR and
Yoon DY: Orientin inhibits invasion by suppressing MMP-9 and IL-8
expression via the PKCα/ERK/AP-1/STAT3-mediated signaling pathways
in TPA-treated MCF-7 breast cancer cells. Phytomedicine. 50:35–42.
2018. View Article : Google Scholar
|
|
38
|
Zhang Y, Zhu Y, Gao G and Zhou Z:
Knockdown XIST alleviates LPS-induced WI-38 cell apoptosis and
inflammation injury via targeting miR-370-3p/TLR4 in acute
pneumonia. Cell Biochem Funct. 37:348–358. 2019. View Article : Google Scholar
|
|
39
|
Zhang L, Dong L, Tang Y, Li M and Zhang M:
miR-146b protects against the inflammation injury in pediatric
pneumonia through MyD88/NF-κB signaling pathway. Infect Dis (Lond).
52:23–32. 2020. View Article : Google Scholar
|