Introduction
As a major cause of death and disability worldwide,
ischemic stroke is a serious clinical condition with poor prognosis
(1). At present, tissue plasminogen
activator is the only accepted treatment used in the clinic, but
long-term use leads to reperfusion injury; therefore, the
identification of novel therapeutic strategies for reperfusion
injury, including neuroprotection, neurogenesis and angiogenesis,
is important (2). By improving the
current understanding of the epigenetic mechanism underlying
ischemic stroke, novel strategies for the early diagnosis and
treatment of ischemic stroke may be identified.
Long non-coding (lnc)RNAs, which were initially
considered as noise from the translational process, are non-protein
coding transcripts that are >200 nucleotides in length (3). Previous studies have revealed that
lncRNAs are critical transcriptional and post-transcriptional
regulators that participate in the regulation of protein expression
in different types of diseases, such as cancer, osteoporosis and
cardiovascular diseases (4–6). lncRNAs are important and effective
regulators of disease progression and various biological
activities, such as angiogenesis, macrophage M2 polarization and
inflammatory responses (3).
Increasing evidence also demonstrated that lncRNAs serve important
regulatory roles in cell differentiation and tissue regeneration
(7,8). lncRNAs have been reported to serve
important roles in the cerebrovascular system (9). Several specific lncRNAs, including
lncRNA H19 imprinted maternally expressed transcript (H19), were
confirmed to be upregulated in cerebral ischemic model animals and
oxygen-glucose deprived (OGD) cells (10–12),
promoting cell apoptosis, angiogenesis, inflammation and cell
death.
As one of the best characterized lncRNA, lncRNA H19
is a maternally imprinted gene that is primarily expressed during
embryonic development (13).
However, under certain pathological conditions, including tissue
regeneration, carcinogenesis and hypoxia, H19 expression is
reactivated (14–18). Hypoxia induces cerebral ischemia and
reperfusion injury, followed by stimulating lncRNA H19 expression
via activating hypoxia induced factor 1α (19). In a previous study, lncRNA H19
expression levels were significantly upregulated in patients who
had suffered from a stroke compared with healthy controls,
displaying a high diagnostic value (9). In addition, lncRNA H19 knockdown
promoted microglial M1 to M2 polarization by downregulating histone
deacetylase 1 in OGD-treated BV2 microglial cells (9). Moreover, an association among the SNP
in H19, rs217727 and the higher risk of ischemic stroke was
identified (20).
lncRNA H19 acts on various microRNAs (miRNAs/miRs),
including let-7, miR-22, miR-141, miR-183, miR-200a and miR-29b,
resulting in the restoration of the target genes of the miRNAs
(21,22). A previous study demonstrated that
lncRNA H19 directly targeted miR-29b to activate TGF-β1 signaling,
further accelerating tenogenic differentiation and promoting tendon
healing (23). Furthermore, lncRNA
H19 mediates the protective effect of hypoxic post-conditioning
against hypoxia-reoxygenation injury by inhibiting miR-29b-3p
expression in aged cardiomyocytes (24). Therefore, it was hypothesized that
there might be an association between lncRNA H19 and miR-29b in
regulating ischemic stroke. As an NADC-dependent protein
deacetylase, silent mating-type information regulation 2 homolog 1
(SIRT1) serves important roles in metabolic regulation and
adaptation (25). SIRT1 regulates
inflammation, oxidative stress, autophagy and cell apoptosis via
deacetylation of various transcription factors, including
peroxisome proliferator-activated receptor-g co-activator-1α
(PGC-1α) (26,27).
The present study investigated whether there were
alterations in lncRNA H19 expression levels in the middle cerebral
artery occlusion (MCAO) mouse model and OGD-treated HT22 cells.
Subsequently, the effects of lncRNA H19 knockdown on OGD-induced
expression levels of inflammatory cytokines, miR-29b, SIRT1 and
PGC-1α levels were also assessed.
Materials and methods
Animals
All experimental animal procedures were approved by
the Animal Ethics Committee of the Tianjin Medical University.
C57BL/6 mice (male; age, 10–12 weeks; weight, 21–23 g; n=8 in each
group; Charles River Laboratories, Inc.) were housed at 22±2°C and
50±15% relative humidity and a 12-h light/dark cycle with adequate
food and water.
MCAO model
Animals were randomly divided into the following two
groups (n=10 per group): i) MCAO; and ii) sham-operated. To
establish the MCAO mouse model, mice were anesthetized by the
intraperitoneal injection of 45 mg/kg sodium pentobarbital (2%). An
uncoated 6-0 monofilament nylon suture (diameter, 0.20 mm) was
inserted to occlude the MCA for 1 h. Subsequently, the suture was
removed for 24 h of reperfusion. Mice in the sham-operated group
underwent the same procedure, but the suture was not inserted. Mice
were euthanized 24 h following ischemia by intraperitoneal
injection of 150 mg/kg sodium pentobarbital (2%). Death was
verified by dilated pupils and cessation of the heartbeat. The
infarct ipsilateral hemisphere brain was isolated and used for
subsequent experiments.
Transfection and establishment of the
OGD model
The HT22 mouse hippocampal neuronal cell line was
purchased from Procell Life Science & Technology Co., Ltd.
Cells were incubated in a humidified incubator in a normal culture
medium containing DMEM solution (Gibco; Thermo Fisher Scientific,
Inc.) mixed with 10% fetal serum (Gibco; Thermo Fisher Scientific,
Inc.) and 7.5% horse serum (Gibco; Thermo Fisher Scientific, Inc.)
at 37°C and 5% CO2. HT22 cells were seeded at a density
of 4×105 cells/well were transfected with 100 mmol/l H19
small interfering (si)RNA or scrambled siRNA negative control
(si-NC) using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) for 24 h at 37°C with 5% CO2.
The siRNA sequences were as follows: i) H19 siRNA,
5′-CCCUCAAGAUGAAAGAAAUTTAUUUCUUUCAUCUUGAGGGTT-3′; ii) si-NC,
5′-UUCUCCGAACGUGUCACGUTT-3′. Subsequently, HT22 cells were exposed
to OGD to mimic ischemic-like conditions. Briefly, cells in the OGD
group were cultured in a hypoxic incubator with 95% N2
and 5% CO2 for 3, 6 or 9 h. For reperfusion, cells were
transferred to normal culture medium for 24 h and kept at 37°C in
an incubator with 5% CO2.
MTT assay
HT22 cell viability was assessed by performing an
MTT assay. Briefly, HT22 cells were seeded (5×104
cells/ml) into 96-well plates. Following OGD and H19 siRNA
transfection, 20 µl MTT (5 mg/ml) was added to each well and
incubated for 4 h at 37°C. Subsequently, 150 µl DMSO was used to
dissolve the purple formazan. The absorbance was measured at a
wavelength of 490 nm using a microplate reader. Cell viability in
the control group was set at 100% and cell viability in OGD and H19
siRNA transfection groups were normalized to the control group.
Flow cytometry analysis
Flow cytometry was performed to detect the rate of
apoptosis. Cell apoptosis was assessed using the Annexin
V-FITC/propidium iodide double staining kit (Beijing Solarbio
Science & Technology, Co., Ltd.) according to the
manufacturer's protocol.
ELISA
The concentrations of inflammatory cytokines,
including interleukin (IL)-6, IL-1β, TNF-α, IL-10 and TGF-β1, were
measured using ELISA kits (R&D Systems, Inc.) according to the
manufacturer's protocols.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from mouse brain tissue and
HT22 cells using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Total RNA was reverse transcribed into cDNA using the PrimeScript
RT Master Mix kit (Takara Biotechnology Co., Ltd.). Subsequently,
qPCR was performed using the SYBR Premix Ex Taq II kit (Takara
Biotechnology Co., Ltd.). The following primers were used for qPCR:
H19 forward, 5′-GTCAAACAGGGCAAGATGGG-3′ and reverse,
5′-ATTACGGTGGGTGGGATGTT-3′; miR-29b forward,
5′-CAGACCTGTAGCACCATTTGAA-3′ and reverse,
5′-TATCCTTGTTCACGACTCCTTCAC-3′; SIRT1 forward,
5′-TCCTTGGAGACTGCGATGTT-3′ and reverse,
5′-ATATGAAGAGGTGTTGGTGGC-3′; PGC-1a forward,
5′-CAATACCTCATGGGACAGCG-3′ and reverse, 5′-GCCTCCAGGGAAAGCAAA-3′;
U6 forward, 5′-CGCTTCGGCAGCACATATAC-3′ and reverse,
5′-AAATATGGAACGCTTCACGA-3′ and GAPDH forward,
5′-AGGTCGGTGTGAACGGATTTG-3′ and reverse
5′-TGTAGACCATGTAGTTGAGGTCA-3′. All samples were run in triplicate.
miRNA and mRNA expression levels were quantified using the
2−ΔΔCq method and normalized to the internal reference
genes U6 and GAPDH, respectively.
Western blotting
Total protein was extracted from HT22 cells. Protein
concentrations were determined using a Protein Assay kit (Bio-Rad
Laboratories, Inc.). Proteins were separated via SDS-PAGE and
transferred onto nitrocellulose membranes, which were blocked with
5% skimmed milk in TBS-Tween-20 (TBST) at room temperature for 1 h.
Subsequently, the membranes were incubated overnight at 4°C with
primary antibodies targeted against: SIRT1 (1:1,000; GeneTex,
Inc.), PGC-1α (1:500; GeneTex, Inc.) and GAPDH (1:5,000; OriGene
Technologies, Inc.). Following washing three times for 10 min each
time, the membranes were incubated with a goat anti-rabbit
secondary antibody (1:2,000; GeneTex, Inc.) at room temperature for
1 h. The membranes were washed three times with TBST for 10 min
each time. Protein expression was semi-quantified via densitometry
(Bio-Rad Laboratories, Inc.) with GAPDH as the loading control.
Statistical analysis
Data are presented as the mean ± SD. Comparisons
among multiple groups were analyzed using one-way ANOVA analysis
followed by the Bonferroni post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
lncRNA H19 expression levels are
upregulated, whereas miR-29b, SIRT1 and PGC-1α expression levels
are downregulated in the MCAO mouse model
To investigate whether lncRNA H19 mRNA expression
level were altered in ischemic brain tissue isolated from the MCAO
mouse model, RT-qPCR was performed. The results indicated that
lncRNA H19 expression levels were significantly elevated in
ischemic brain tissue isolated from the MCAO mouse model compared
with the sham group (P<0.05; Fig.
1A). Furthermore, miR-29b, SIRT1 and PGC-1α expression levels
were significantly decreased in the MCAO mouse model compared with
the sham group (P<0.05; Fig.
1B).
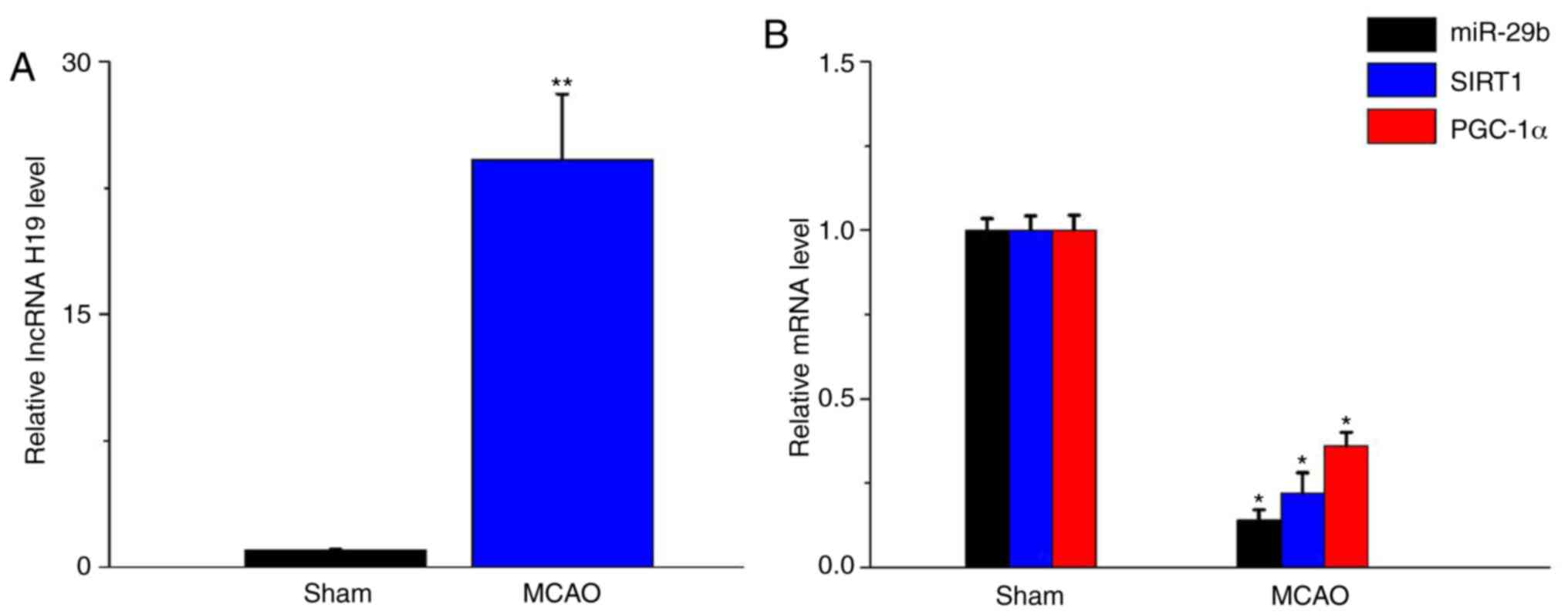 | Figure 1.lncRNA H19, miR-29b, SIRT1 and PGC-1α
expression levels in the MCAO mouse model. (A) lncRNA H19, (B)
miR-29b, SIRT1 and PGC-1α expression levels were determined by
performing reverse transcription-quantitative PCR. Data are
presented as the mean ± SD. *P<0.05 and **P<0.01 vs. sham.
lncRNA, long non-coding RNA; H19, H19 imprinted maternally
expressed transcript; miR, microRNA; SIRT1, silent mating-type
information regulation 2 homolog 1; PGC-1α, peroxisome
proliferator-activated receptor-g co-activator-1α; MCAO, middle
cerebral artery occlusion. |
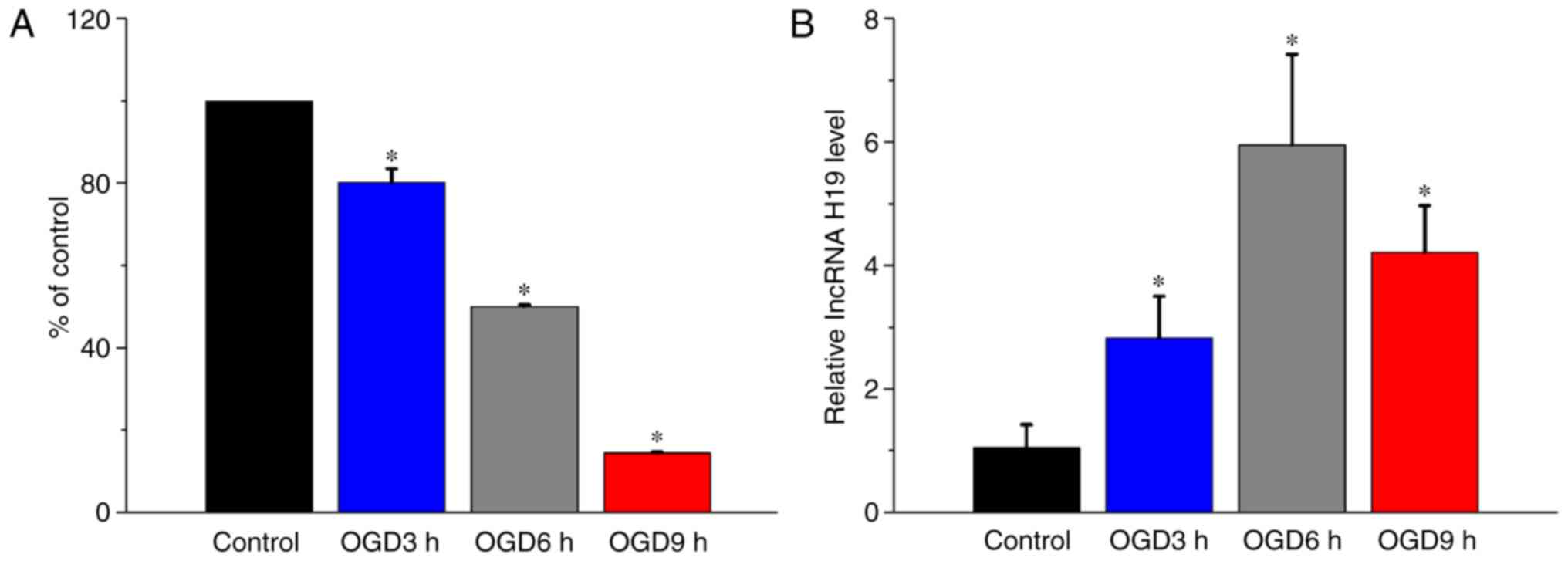
OGD treatment induces cytotoxicity and
increases lncRNA H19 expression levels in HT22 cells
To investigate the effect of OGD treatment on HT22
cells, cell viability and lncRNA H19 expression levels were
measured following OGD treatment at different times. Following
treatment for 3, 6 or 9 h, OGD significantly decreased HT22 cell
viability in a time-dependent manner compared with the control
group (P<0.05; Fig. 2A). The
RT-qPCR results indicated that lncRNA H19 expression levels were
significantly elevated in OGD-treated cells compared with control
cells (P<0.05; Fig. 2B). The
aforementioned results indicated that elevated lncRNA H19 mRNA
expression levels may aggravate cerebral ischemia injury.
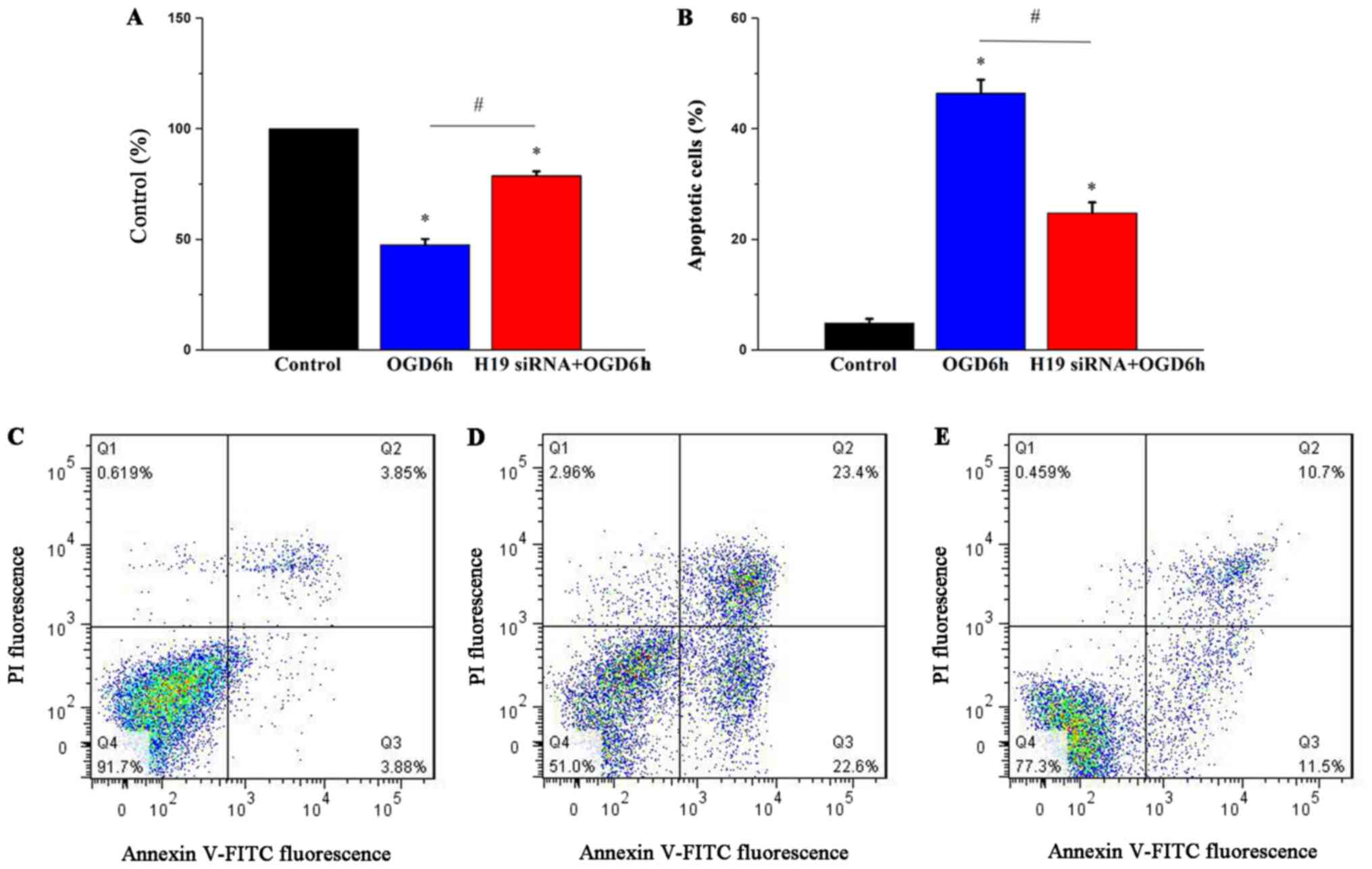
H19 knockdown relieves OGD-induced
HT22 cell cytotoxicity
To investigate the role of lncRNA H19 in OGD-induced
cytotoxicity, H19 siRNA was used to knock down lncRNA H19
expression. H19 siRNA significantly decreased lncRNA H19 expression
levels compared with the si-NC group (P<0.05; Fig. S1). The MTT assay results
demonstrated that OGD treatment for 6 h significantly reduced cell
viability compared with the control group (P<0.05), which was
significantly ameliorated by H19 knockdown (P<0.05; Fig. 3A). Flow cytometry was performed to
evaluate the effects of lncRNA H19 on cell apoptosis. The results
indicated that OGD treatment for 6 h significantly increased cell
apoptosis compared with the control group (P<0.05), which was
also significantly ameliorated by H19 knockdown (P<0.05;
Fig. 3B). The results suggested
that OGD may induce cell injury by increasing lncRNA H19 expression
levels.
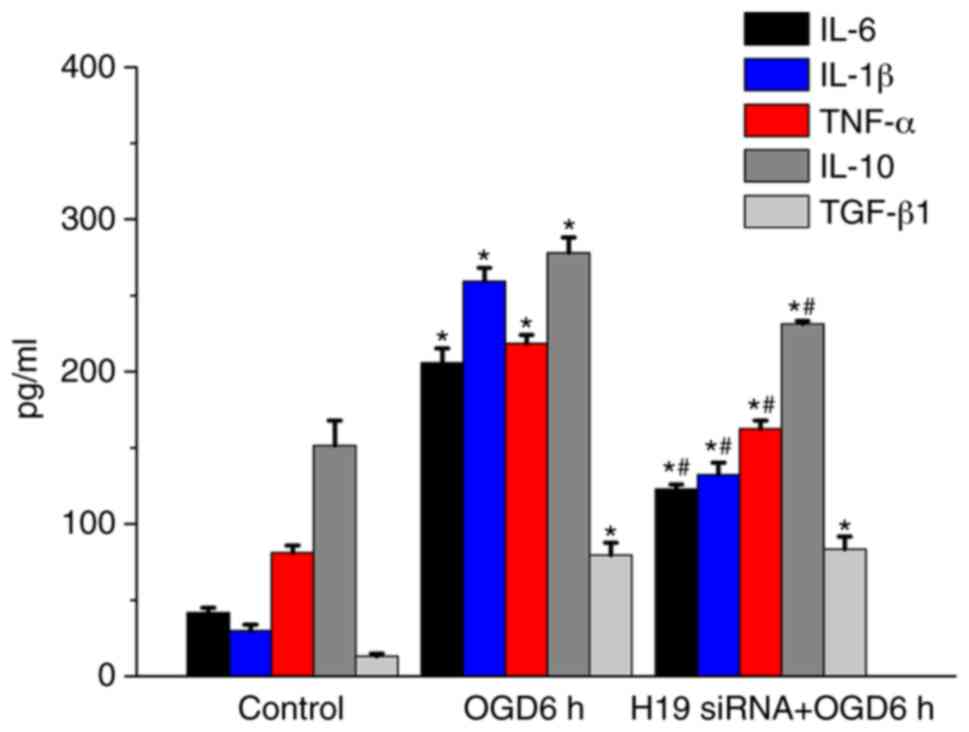
H19 knockdown decreases inflammatory
cytokine concentrations in HT22 cells
Increasing evidence has demonstrated that
inflammatory cytokines participate in the pathogenesis of cerebral
ischemia (28,29). In the present study, the
concentrations of several inflammatory cytokines were detected to
investigate whether H19 knockdown altered the inflammatory response
in HT22 cells (Fig. 4). IL-6,
IL-1β, TNF-α, IL-10 and TGF-β1 concentrations were significantly
increased following OGD treatment for 6 h compared with the control
group (P<0.05). However, H19 knockdown significantly inhibited
OGD-induced increases in IL-6, IL-1β, TNF-α and IL-10
concentrations following treatment for 6 h (P<0.05). IL-10 is an
anti-inflammatory cytokine (30).
IL-10 concentrations were significantly increased by OGD treatment
for 6 h compared with the control group (P<0.05), which
suggested a potential self-protection or compensation mechanism.
The results indicated that lncRNA H19 may prompt the inflammatory
response in ischemia stroke.
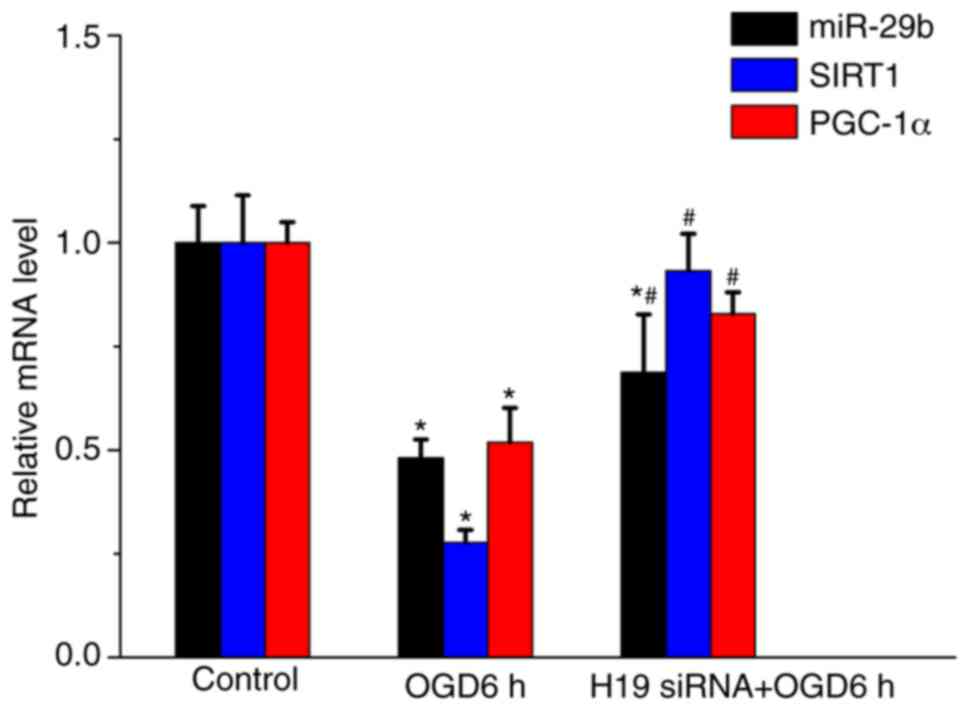
H19 knockdown alters miR-29b, SIRT1
and PGC-1α expression levels in OGD-treated HT22 cells
The effects of lncRNA H19 on miR-29b, SIRT1 and
PGC-1α expression levels in OGD-treated HT22 cells were
investigated by performing RT-qPCR and western blotting. Following
OGD treatment for 6 h, miR-29b, SIRT1 and PGC-1α expression levels
were significantly decreased compared with the control group
(P<0.05); however, OGD-mediated effects on expression were
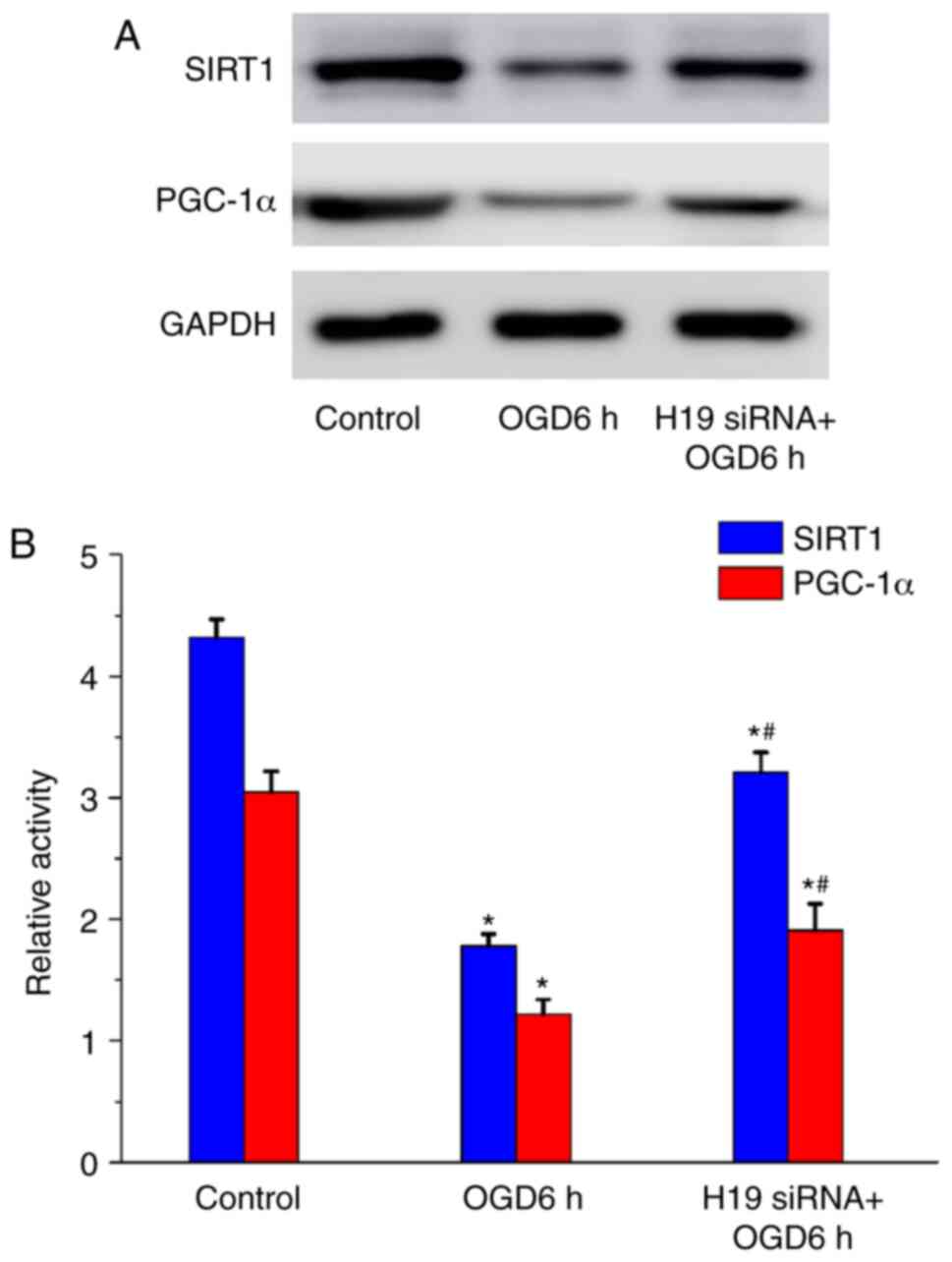
significantly inhibited by H19 knockdown (P<0.05; Fig. 5). Consistent with the RT-qPCR
results, the western blotting results demonstrated that OGD
treatment for 6 h significantly decreased SIRT1 and PGC-1α protein
expression levels compared with the control group (P<0.05;
Fig. 6). However, H19 knockdown
significantly increased SIRT1 and PGC-1α expression levels compared
with the OGD 6 h group (P<0.05).
Discussion
In the present study, lncRNA H19 expression levels
in the MCAO mouse model and OGD-treated HT22 cells were
investigated. The results demonstrated that lncRNA H19 mRNA
expression levels in MCAO model mice and OGD-treated cells were
significantly elevated compared with sham mice and control cells,
respectively. Moreover, in OGD-treated cells, H19 knockdown
significantly increased cell viability and significantly decreased
cell apoptosis. H19 knockdown also significantly increased miR-29b,
SIRT1 and PGC-1α expression levels, and significantly decreased
inflammatory cytokine concentrations in OGD-treated cells.
Collectively, the results of the present study indicated a
potential role of lncRNA H19 in regulating the OGD-induced immune
response, which may participate in subsequent pathological
processes. However, the results of the present study should be
verified using patient samples in future studies.
Ischemic stroke remains one of the leading causes of
morbidity and mortality worldwide (31), with multiple causes leading to
neuronal injury. Emerging evidence indicates that inflammation
serves an important role in the pathogenesis of brain ischemia
(29,32). In particular, several specific
lncRNAs have been reported to participate cerebral ischemia-induced
cell apoptosis, inflammation, cell death and angiogenesis (10). An example is lncRNA H19, which is
abundantly expressed in embryonic development and growth control,
but downregulated following birth (14). However, the expression of lncRNA H19
can be reactivated under certain specific conditions, such as
tumorigenesis and oxidative stress (17,18,33).
Wang et al (9) reported that
lncRNA H19 expression levels were elevated in patients who had
suffered from a stroke compared with healthy controls. In the
present study, lncRNA H19 expression was significantly upregulated
in MCAO model mice and OGD-treated HT22 cells compared with sham
mice and control cells, respectively. In addition, H19 knockdown
protected cells against OGD-induced cell apoptosis. However, the
possible regulatory mechanism underlying lncRNA H19 is not
completely understood.
A previous study demonstrated that lncRNA H19
regulated autophagy via the dual specificity phosphatase 5/ERK1/2
axis in ischemic stroke (20). Wang
et al (34) reported that
H19 knockdown promotes the transcriptional activity of p53,
resulting in increased Notch1 expression levels in ischemic stroke.
lncRNA H19 serves as a competing endogenous RNA by sponging miRNA
in several types of carcinoma, such as cholangiocarcinoma, oral
squamous cell carcinoma, osteosarcoma (18,35,36).
miRNAs, including the miR-29 family, serve a vital role during
cerebral ischemia (37,38). In the present study, miR-29b
expression levels were significantly decreased in the MCAO mouse
model and OGD-treated HT22 cells compared with the sham group and
control cells, respectively. In addition, H19 knockdown attenuated
OGD-mediated alterations in miR-29b expression levels, which
suggested that lncRNA H19 may affect ischemic stroke by regulating
miR-29b.
As a key member of the sirtuin family of
NAD+-dependent enzymes, SIRT1 serves a pivotal role in
cerebral ischemia. SIRT1-overexpression mice display less
hippocampal damage following severe ischemic damage (39). Several compounds exert a
neuroprotective effect against cerebral ischemia by activating or
upregulating SIRT1 (40,41). SIRT1 could directly phosphorylate
and deacetylate PGC-1α to form a transcription complex, which may
control the expression of specific metabolic genes (42,43).
Elevated PGC-1α expression levels could protect against neuronal
death during cerebral ischemia (44). Consistent with a previous study
(45), SIRT1 and PGC-1α mRNA and
protein expression levels were significantly reduced in the MCAO
mouse model and OGD-treated HT22 cells compared with the sham group
and control cells, respectively. H19 knockdown inhibited
OGD-induced cell apoptosis and suppressed OGD-mediated
downregulation of SIRT1 and PGC-1α, which suggested a
neuroprotective effect against ischemic injury. The present study
also indicated that elevated SIRT1 and PGC-1α mRNA and protein
expression levels may protect against neuronal death during
cerebral ischemia. However, a limitation of the present study was
that the expression levels of apoptosis- and inflammation-related
genes were not measured using an animal model; therefore, further
investigation is required.
In conclusion, the present study demonstrated that
H19 knockdown ameliorated OGD-induced cell apoptosis and increases
in inflammatory cytokine concentrations, which suggested an
immunomodulatory effect of lncRNA H19 in ischemic stroke.
Furthermore, to the best of our knowledge, the present study
demonstrated for the first time that H19 knockdown also attenuated
OGD-mediated downregulation of miR-29b, SIRT1 and PGC-1α expression
levels, which indicated that lncRNA H19 may participate in
neuroprotection by regulating miR-29b, SIRT1 and PGC-1α expression
levels.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81601041, 81601411
and 81603404), the Medical Foundation of Jieping Wu (grant no.
320.6750.19089-56) and the Youth Incubation Fund of General
Hospital of Tianjin Medical University (grant no.
zyyfy2019007).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JX supervised and designed the study. CW, FM and PX
performed the experiments. JX revised the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental animal procedures were approved by
The Animal Ethics Committee of Tianjin Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Writing Group Members, ; Mozaffarian D,
Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de
Ferranti S, Despres JP, Fullerton HJ, et al: Heart disease and
stroke statistics-2016 update: A report from the American heart
association. Circulation. 133:e38–e360. 2016.PubMed/NCBI
|
|
2
|
Mo Y, Sun YY and Liu KY: Autophagy and
inflammation in ischemic stroke. Neural Regen Res. 15:1388–1396.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yao RW, Wang Y and Chen LL: Cellular
functions of long noncoding RNAs. Nat Cell Biol. 21:542–551. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi X, Sun M, Liu H, Yao Y and Song Y:
Long non-coding RNAs: A new frontier in the study of human
diseases. Cancer Lett. 339:159–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang Y, Yujiao W, Fang W, Linhui Y, Ziqi
G, Zhichen W, Zirui W and Shengwang W: The roles of miRNA, lncRNA
and circRNA in the development of osteoporosis. Biol Res.
53:402020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang Y: The novel regulatory role of
lncRNA-miRNA-mRNA axis in cardiovascular diseases. J Cell Mol Med.
22:5768–5775. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu L and Xu PC: Downregulated LncRNA-ANCR
promotes osteoblast differentiation by targeting EZH2 and
regulating Runx2 expression. Biochem Biophys Res Commun.
432:612–617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Xu Z, Jiang J, Xu C, Kang J, Xiao
L, Wu M, Xiong J, Guo X and Liu H: Endogenous miRNA sponge
lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem
cell self-renewal. Dev Cell. 25:69–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Zhao H, Fan Z, Li G, Ma Q, Tao Z,
Wang R, Feng J and Luo Y: Long noncoding RNA H19 promotes
neuroinflammation in ischemic stroke by driving histone deacetylase
1-dependent M1 microglial polarization. Stroke. 48:2211–2221. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bao MH, Szeto V, Yang BB, Zhu SZ, Sun HS
and Feng ZP: Long non-coding RNAs in ischemic stroke. Cell Death
Dis. 9:2812018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang X, Tang X, Liu K, Hamblin MH and Yin
KJ: Long noncoding RNA malat1 regulates cerebrovascular pathologies
in ischemic stroke. J Neurosci. 37:1797–1806. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan H, Yuan J, Gao L, Rao J and Hu J: Long
noncoding RNA MEG3 activation of p53 mediates ischemic neuronal
death in stroke. Neuroscience. 337:191–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bartolomei MS, Zemel S and Tilghman SM:
Parental imprinting of the mouse H19 gene. Nature. 351:153–155.
1991. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gabory A, Jammes H and Dandolo L: The H19
locus: Role of an imprinted non-coding RNA in growth and
development. Bioessays. 32:473–480. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dey BK, Pfeifer K and Dutta A: The H19
long noncoding RNA gives rise to microRNAs miR-675-3p and
miR-675-5p to promote skeletal muscle differentiation and
regeneration. Genes Dev. 28:491–501. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Voellenkle C, Garcia-Manteiga JM, Pedrotti
S, Perfetti A, De Toma I, Da Silva D, Maimone B, Greco S, Fasanaro
P, Creo P, et al: Implication of long noncoding RNAs in the
endothelial cell response to hypoxia revealed by RNA-sequencing.
Sci Rep. 6:241412016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matouk IJ, Raveh E, Abu-lail R, Mezan S,
Gilon M, Gershtain E, Birman T, Gallula J, Schneider T, Barkali M,
et al: Oncofetal H19 RNA promotes tumor metastasis. Biochim Biophys
Acta. 1843:1414–1426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang WT, Ye H, Wei PP, Han BW, He B, Chen
ZH and Chen YQ: LncRNAs H19 and HULC, activated by oxidative
stress, promote cell migration and invasion in cholangiocarcinoma
through a ceRNA manner. J Hematol Oncol. 9:1172016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matouk IJ, Mezan S, Mizrahi A, Ohana P,
Abu-Lail R, Fellig Y, Degroot N, Galun E and Hochberg A: The
oncofetal H19 RNA connection: Hypoxia, p53 and cancer. Biochim
Biophys Acta. 1803:443–451. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Cao B, Han D, Sun M and Feng J:
Long non-coding RNA H19 induces cerebral ischemia reperfusion
injury via activation of autophagy. Aging Dis. 8:71–84. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ding D, Li C, Zhao T, Li D, Yang L and
Zhang B: LncRNA H19/miR-29b-3p/PGRN axis promoted
epithelial-mesenchymal transition of colorectal cancer cells by
acting on wnt signaling. Mol Cells. 41:423–435. 2018.PubMed/NCBI
|
|
22
|
Wang X, Zou M, Li J, Wang B, Zhang Q, Liu
F and Lu G: LncRNA H19 targets miR-22 to modulate
H2O2-induced deregulation in nucleus pulposus
cell senescence, proliferation, and ECM synthesis through Wnt
signaling. J Cell Biochem. 119:4990–5002. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu YF, Liu Y, Fu WM, Xu J, Wang B, Sun YX,
Wu TY, Xu LL, Chan KM, Zhang JF and Li G: Long noncoding RNA H19
accelerates tenogenic differentiation and promotes tendon healing
through targeting miR-29b-3p and activating TGF-β1 signaling. FASEB
J. 31:954–964. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang X, Cheng L, Xu L, Zhang Y, Yang Y,
Fu Q, Mi W and Li H: The LncRNA, H19 mediates the protective effect
of hypoxia postconditioning against hypoxia-reoxygenation injury to
senescent cardiomyocytes by targeting microRNA-29b-3p. Shock.
52:249–256. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boutant M and Canto C: SIRT1 metabolic
actions: Integrating recent advances from mouse models. Mol Metab.
3:5–18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kitada M, Ogura Y, Monno I and Koya D:
Sirtuins and type 2 diabetes: Role in inflammation, oxidative
stress, and mitochondrial function. Front Endocrinol (Lausanne).
10:1872019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fang WJ, Wang CJ, He Y, Zhou YL, Peng XD
and Liu SK: Resveratrol alleviates diabetic cardiomyopathy in rats
by improving mitochondrial function through PGC-1α deacetylation.
Acta Pharmacol Sin. 39:59–73. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu H, Wu X, Luo J, Wang X, Guo H, Feng D,
Zhao L, Bai H, Song M, Liu X, et al: Pterostilbene attenuates
astrocytic inflammation and neuronal oxidative injury after
ischemia-reperfusion by inhibiting NF-ΚB phosphorylation. Front
Immunol. 10:24082019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang Q, Huang Q, Hu Z and Tang X:
Potential neuroprotective treatment of stroke: Targeting
excitotoxicity, oxidative stress, and inflammation. Front Neurosci.
13:10362019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fang D and Zhu J: Molecular switches for
regulating the differentiation of inflammatory and IL-10-producing
anti-inflammatory T-helper cells. Cell Mol Life Sci. 77:289–303.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grysiewicz RA, Thomas K and Pandey DK:
Epidemiology of ischemic and hemorrhagic stroke: Incidence,
prevalence, mortality, and risk factors. Neurol Clin. 26871–895.
(vii)2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Anrather J and Iadecola C: Inflammation
and stroke: An overview. Neurotherapeutics. 13:661–670. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matouk IJ, DeGroot N, Mezan S, Ayesh S,
Abu-lail R, Hochberg A and Galun E: The H19 non-coding RNA is
essential for human tumor growth. PLoS One. 2:e8452007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang J, Cao B, Zhao H, Gao Y, Luo Y, Chen
Y and Feng J: Long noncoding RNA H19 prevents neurogenesis in
ischemic stroke through p53/Notch1 pathway. Brain Res Bull.
150:111–117. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hong Y, He H, Sui W, Zhang J, Zhang S and
Yang D: (Corrigendum) Long noncoding RNA H19 promotes cell
proliferation and invasion by acting as a ceRNA of miR138 and
releasing EZH2 in oral squamous cell carcinoma. Int J Oncol.
53:9152018.PubMed/NCBI
|
|
36
|
Li M, Chen H, Zhao Y, Gao S and Cheng C:
H19 Functions as a ceRNA in promoting metastasis through decreasing
miR-200s activity in osteosarcoma. DNA Cell Biol. 35:235–240. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li L and Stary CM: Targeting glial
mitochondrial function for protection from cerebral ischemia:
Relevance, mechanisms, and the role of microRNAs. Oxid Med Cell
Longev. 2016:60323062016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ouyang YB, Xu L, Yue S, Liu S and Giffard
RG: Neuroprotection by astrocytes in brain ischemia: Importance of
microRNAs. Neurosci Lett. 565:53–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hattori Y, Okamoto Y, Nagatsuka K,
Takahashi R, Kalaria RN, Kinoshita M and Ihara M: SIRT1 attenuates
severe ischemic damage by preserving cerebral blood flow.
Neuroreport. 26:113–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fusco R, Scuto M, Cordaro M, D'Amico R,
Gugliandolo E, Siracusa R, Peritore AF, Crupi R, Impellizzeri D,
Cuzzocrea S and Di Paola R: N-Palmitoylethanolamide-oxazoline
protects against middle cerebral artery occlusion injury in
diabetic rats by regulating the SIRT1 pathway. Int J Mol Sci.
20:48452019. View Article : Google Scholar
|
|
41
|
Duan J, Cui J, Zheng H, Xi M, Guo C, Weng
Y, Yin Y, Wei G, Cao J, Wang Y, et al: Aralia taibaiensis protects
against I/R-induced brain cell injury through the Akt/SIRT1/FOXO3a
pathway. Oxid Med Cell Longev. 2019:76097652019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Canto C and Auwerx J: Caloric restriction,
SIRT1 and longevity. Trends Endocrinol Metab. 20:325–331. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rodgers JT, Lerin C, Gerhart-Hines Z and
Puigserver P: Metabolic adaptations through the PGC-1 alpha and
SIRT1 pathways. FEBS Lett. 582:46–53. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen SD, Yang DI, Lin TK, Shaw FZ, Liou CW
and Chuang YC: Roles of oxidative stress, apoptosis, PGC-1α and
mitochondrial biogenesis in cerebral ischemia. Int J Mol Sci.
12:7199–7215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yan X, Yu A, Zheng H, Wang S, He Y and
Wang L: Calycosin-7-O-β-D-glucoside attenuates OGD/R-induced damage
by preventing oxidative stress and neuronal apoptosis via the
SIRT1/FOXO1/PGC-1α pathway in HT22 cells. Neural Plast.
2019:87980692019. View Article : Google Scholar : PubMed/NCBI
|