Introduction
Acute lung injury (ALI) is a serious illness
resulting from pulmonary inflammation caused by various conditions,
including sepsis, acid aspiration and trauma (1–3).
Infection by gram-positive bacteria is one of the main causes of
pulmonary inflammation (4).
Although extensive research on the mechanism and treatment of
gram-positive-bacteria-induced ALI has been carried out, the
specific mechanism remains to be elucidated and effective drugs and
treatments remain unavailable, resulting in high ALI-associated
morbidity and mortality worldwide (5–8).
Lipoteichoic acid (LTA) is expressed on the surface of
gram-positive bacteria (9). Mice
treated with LTA develop gram-positive-bacteria-induced pneumonia,
a common and serious type of pneumonia that affects humans of all
ages (7,10).
Shikonin is a major active ingredient isolated from
the roots of Lithospermum erythrorhizon (11). The antitumor and anti-bacterial
properties of shikonin have been studied in vitro and in
vivo (12,13). Previous studies have indicated that
the anti-inflammatory effect of shikonin results from the reduction
of oxidative stress (14), the
inhibition of Th2 cytokine production and the release of histamine
from mast cells (15). Recently,
the protective role of shikonin against lipopolysaccharide
(LPS)-induced ALI was demonstrated in a mouse model (16). However, the effect of shikonin on
LTA-induced ALI and the underlying mechanisms have not been
studied.
Neutrophils are the first line of immune-defense
cells against pathogen infection (17). However, the defensive response by
overactivated neutrophils can also have a damaging effect on the
lung tissue (18) due to the
release of excessive amounts of inflammatory factors such as TNF-α,
IL-1β and IL-6 causing serious lung injury (17–19).
Previous studies have shown that apoptosis is caused by bacterial
infection (20–22). The continuous release of
inflammatory factors in ALI is in part ascribed to the inhibition
or delay of neutrophil apoptosis in the lung tissue (23,24).
This suggests that promoting neutrophil apoptosis could reduce lung
inflammation and damage. Apoptosis is an orderly cell death process
controlled by multiple genes (25).
Caspase-3 is a key regulatory protein involved in apoptosis. Poly
(ADP-ribose) polymerase (PARP) DNA-repairing enzyme can be cleaved
into different fragments by caspase-3, resulting in abnormal DNA
repair and apoptosis (26). Caspase
3-mediated apoptosis can be inhibited by myeloid cell leukemia-1
(Mcl-1) and Bcl-2 (27). Therefore,
identification and action on targets related to neutrophil
apoptosis is an alternative strategy for preventing and treating
ALI.
In the present study, the protective effect of
shikonin in LTA-induced ALI was identified. Pretreatment with
shikonin in LTA-induced ALI mouse model markedly reduced lung
inflammation and decreased pro-inflammatory cytokine expression.
The findings of the present study confirmed that shikonin promoted
neutrophil apoptosis mediated by increased caspase-3 and decreasing
Mcl-1 expression, consequently reducing inflammation in
gram-positive-bacteria-induced ALI.
Materials and methods
Animals
Male C57BL/6 mice (6–8 weeks old, 22±3 g, specific
pathogen-free, n=20) were purchased from SLAC Laboratory Animal
Corporation and acclimated to laboratory conditions (25°C, 50–60%
humidity) for two weeks under a 12-h light/dark cycle and with free
access to food and water before the experiments. The present study
adhered to the Principles of Laboratory Animal Care (NIH
publication No. 85-23, revised 1996) (28). All experimental protocols described
in the present study were approved by the Animal Care and Use
Committee of Bengbu Medical College (approval no. LABMT-019).
Compound and reagents
Shikonin was prepared at 20 mM in DMSO
(Sigma-Aldrich; Merck KGaA) and stored at −20°C. The primers used
in the present study were synthesized by Shanghai HuaGen Biotech
Co., Ltd. Cleaved caspase-3 (Asp175; cat. no. 9664), cleaved PARP
(cat. no. 5625), Mcl-1 (cat. no. 94296), Bcl-2 (cat. no. 15071),
p53 (cat. no. 2527) and β-actin (cat. no. 4970) primary antibodies
were purchased from Cell Signaling Technology, Inc. The peroxidase
AffiniPure goat anti-rabbit IgG (H+L) (cat. 111-035-003) and
peroxidase AffiniPure goat anti-mouse IgG (H+L) (cat. 115-035-003)
secondary antibodies were purchased from Jackson ImmunoResearch
Laboratories, Inc. and diluted in EZ-Buffers (cat. no.
C520011-0100; Sangon Biotech Co., Ltd. Other chemical reagents were
obtained from Sigma-Aldrich (Merck KGaA).
LTA-induced ALI mouse model
Shikonin was dissolved in a vehicle (castor
oil:ethanol:PBS=1:1:8). Mice were randomly divided into four groups
and anesthetized by intraperitoneal injection of 50 mg/kg
pentobarbital sodium before intratracheal injection. Each group
contained 5 mice. The LTA group mice were intratracheally
challenged with 5 mg/kg LTA (Sigma-Aldrich; Merck KGaA). The LTA +
shikonin group mice were intraperitoneally injected with shikonin
at 10 mg/kg for 30 min, then administered an intratracheal
injection of 5 mg/kg LTA. The vehicle group mice were
intraperitoneally injected with the same volume of castor
oil:ethanol:PBS (1:1:8) as the LTA + shikonin group mice and, 30
min later, intratracheally challenged with the same volume of PBS
as used for the LTA + shikonin group mice. The vehicle + shikonin
group mice were intraperitoneally injected with 10 mg/kg shikonin
for 30 min, then administrated an intratracheal injection of the
same volume of PBS as the LTA + shikonin group mice. Animal health
and behavior were monitored all the time during the experiment. The
four group mice appeared healthy without obvious abnormal behavior
during the experiment. After 6 h, all the mice were sacrificed with
CO2 inhalation (30% cage volume/min, 5–6 min) until the
mice ceased breathing and had faded eyes. The samples were then
collected. The experiments were conducted in March 2019.
Acquisition and analysis of
bronchoalveolar lavage fluid (BALF)
The BALF of mice were collected according to a
previous study (29). Briefly, the
lungs were lavaged three times with 50 µM EDTA. The BALF was
centrifuged at 300 × g at 4°C for 5 min. The cell-free supernatants
were harvested and analyzed for the total protein content using a
BCA protein assay kit (Beyotime Institute of Biotechnology).
Neutrophils were incubated with Gr-1(Ly6G)-FITC antibody (1:200;
cat. no. 11-5931-82; eBioscience; Thermo Fisher Scientific, Inc.)
and analyzed by flow cytometry (LSRFortessa X-20; BD Biosciences)
to determine the percentage of neutrophils in BALF.
Histopathology
Lung tissues (left lobe) were fixed with 4%
paraformaldehyde overnight in 4°C. Following dehydration in 80%
alcohol for 1 h, 90% alcohol for 2 h and 100% alcohol for 2 h at
room temperature, then washing in 100% xylene for 1 h in room
temperature, the lung tissues were embedded in paraffin and cut
into 5-µm sections with a microtome (cat. no. RM2235; Leica
Microsystems GmbH). Prior to staining, the sections were heated in
a drying oven to 90°C for 30 min and then washed with 100% xylene
for 15 min following immersion in a descending series (100, 95, 95,
80 and 70%) of alcohol for 3 min at room temperature, at each
concentration. Then, the sections were stained with 100%
hematoxylin for 5 min and 100% eosin for 1 min, both at room
temperature (Beyotime Institute of Biotechnology) and images were
captured by a light microscope (RX51; Olympus Corporation).
RNA isolation and reverse
transcription-quantitative (RT-q) PCR
Frozen lungs (one of the right upper lobe) were
homogenized and the total RNA was isolated using TRIzol®
Reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. cDNA was prepared using ReverTra Ace qPCR
RT kit (Toyobo Life Science) according to the manufacturer's
protocol and amplified by qPCR with a TOROGreen® Qpcr
Master Mix (TOROIVD TECHNOLOGY COMPANY LIMITED) and primer sets for
TNF-α (forward, 5′-TTCTCATTCCTGCTTGTGG-3′ and reverse,
5′-ACTTGGTGGTTTGCTACG-3′); IL-1β (forward,
5′-CCAGCTTCAAATCTCACAGCAG-3′ and reverse,
5′-CTTCTTTGGGTATTGCTTGGGATC-3′); IL-6 (forward,
5′-CTTCTTGGGACTGATG-3′ and reverse, 5′-CTGGCTTTGTCTTTCT-3′); and
GAPDH (forward, 5′-TGCGACTTCAACAGCAACTC-3′ and reverse,
5′-CTTGCTCAGTGTCCTTGCTG-3)′. Thermocycling of RT-qPCR was: 95°C for
2 min, 95°C for 10 sec, 58°C for 30 sec and 72°C for 20 sec,
repeated for 40 cycles. The 2−ΔΔCq method (30) was used to analyze the expression of
mRNAs normalized to the GAPDH internal reference gene. The
experiments were independently repeated in triplicate.
Myeloperoxidase (MPO) activity
assay
The MPO activity was determined according to a
previous study (31). Briefly, lung
tissues were collected and subjected to three freeze-thaw cycles.
Supernatants were collected at 4°C. Protein concentration in
supernatants was determined using a BCA protein assay kit (Beyotime
Institute of Biotechnology). After adding the substrate and
catalyst to the supernatants, changes in absorbance at 655 nm were
measured using a microplate reader (FlexStation 3; Molecular
Devices, LLC). MPO activity was defined as the absorbance change
per min per gram of protein.
Isolation of neutrophils and cell
culture
Mouse bone-marrow neutrophils were obtained by
flushing the bone marrow from mouse tibias and femurs. Neutrophils
were purified (>95% purity) using a one-step Nycoprep/Percoll
gradient (TBD, Tianjin Haoyang Biological Products Technology Co.,
Ltd.). According to the manufacturer's protocol, cells were
suspended and cultured in RPMI-1640 (HyClone; Cytiva) with 10%
fetal bovine serum (FBS; Biological Industries) and treated with
the indicated concentrations of shikonin according to the
experimental requirements. DMSO (1%) was added to the culture
medium as the solvent control.
Annexin-V/PI-binding apoptosis
assay
The neutrophil is the terminal differential cell
with short survival time (32). The
primary cultured neutrophils in vitro can be preserved for
only 1–2 days due to activation from culture environment (32). Given the short survival time of
primary-cultured neutrophil, 0.3, 1 and 3 µM shikonin was used to
treat neutrophils for 24 h according to previous studies (33–37).
The neutrophils were collected, centrifuged at 300 × g for 5 min at
4°C and washed with cold PBS. Then, neutrophils were resuspended
with 400 µl PBS and incubated with 2 µl Annexin V-FITC and 4 µl
propidium iodide for 30 min at room temperature according to the
manufacturer's instructions of the Annexin V-FITC apoptosis assay
kit (Beyotime Institute of Biotechnology). Samples were analyzed by
flow cytometry (LSRFortessa X-20; BD Biosciences). Data were
analyzed using FlowJo 7.6 software (FlowJo LLC).
Western blot analysis
Following treatment with shikonin for 24 h,
neutrophils were collected and lysed in RIPA buffer (Xi'an Weiao
Biotechnology Co., Ltd.) with 1 mM PMSF and quantified using a BCA
assay (Beyotime Institute of Biotechnology). Following heating at
99°C for 10 min, each sample (15 µg) was loaded on 10% SDS/PAGE
gels and then transferred to nitrocellulose filter membranes. Blots
were blocked with 5% skim milk at room temperature for 1 h and then
incubated with primary antibodies (1:1,000) overnight at 4°C.
Subsequently, blots were probed with the corresponding secondary
antibodies (1:10,000) at room temperature for 1 h in dark. The
protein signals were detected using an ECL kit (Shanghai Share-Bio
Biotechnology Co., Ltd.). Semi-quantification of western blots was
performed using ImageJ software (1.4.3.67; National Institute of
Mental Health).
Statistical analysis
Data are presented as mean ± standard error of the
mean obtained from ≥3 independent experiments. One-way analysis of
variance (ANOVA) and Bonferroni's Multiple Comparison Test was
performed using GraphPad Prism 6 (GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Shikonin attenuates LTA-induced ALI
and inflammatory response
An LTA-induced ALI mouse model was established to
determine the effect of shikonin on gram-positive
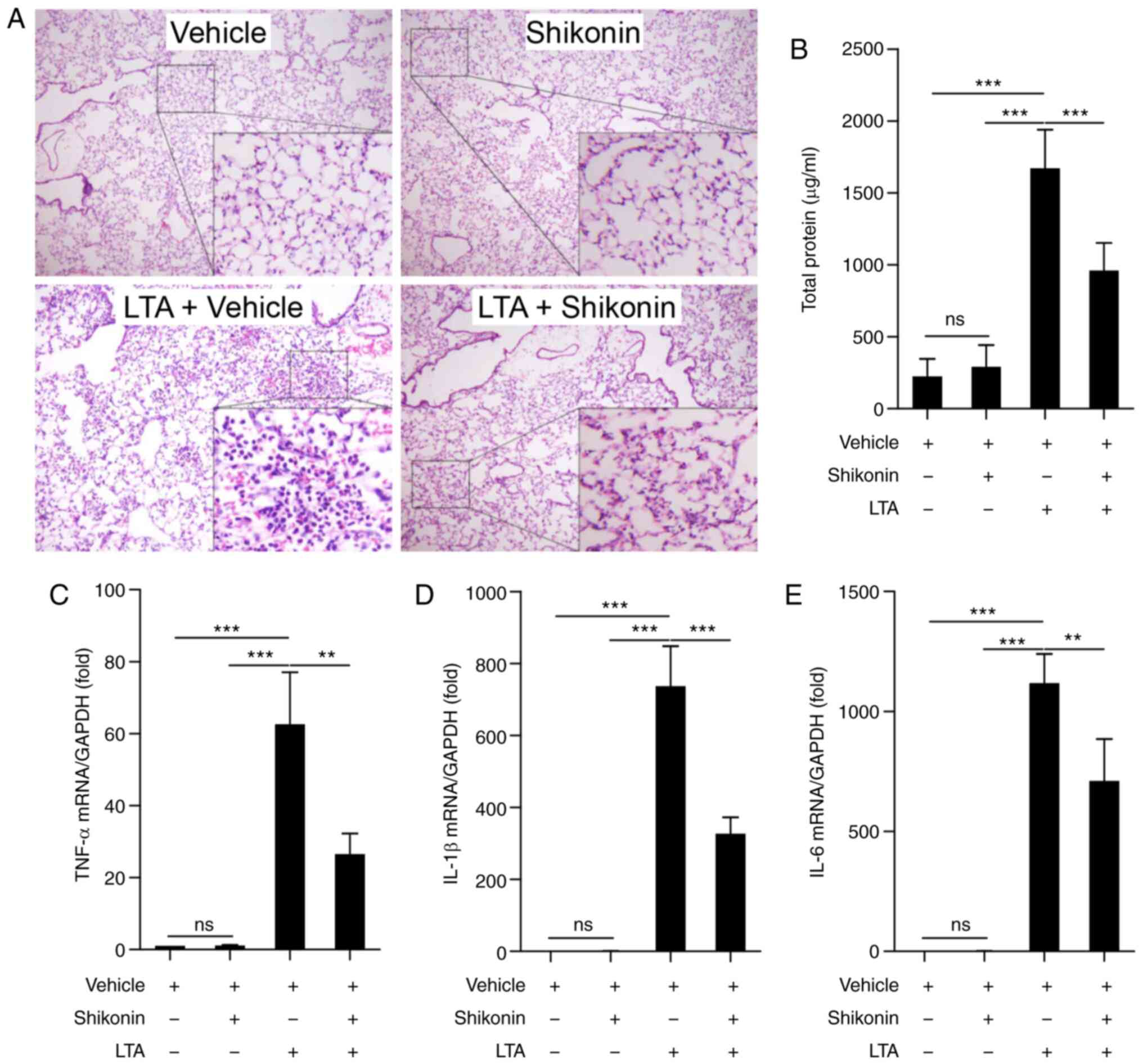
bacterial-infection-induced ALI. As shown in Fig. 1A, the administration of LTA for 6 h
induced inflammatory cell infiltration, inter-alveolar septal
thickening and alveolar collapse. However, the infiltration of
inflammatory cells was significantly reduced following pretreatment
with shikonin (Fig. 1A). The total
protein concentration in the BALF of the LTA-challenged group was
~10-fold higher compared with the vehicle group (Fig. 1B). Pretreatment with shikonin
significantly reduced the total protein concentration in BAL cells
(Fig. 1B). The expression of
pro-inflammatory cytokines in lung tissues was measured following
LTA treatment for 6 h; compared with that in the mice that received
vehicle treatment, the expression of TNF-α, IL-1β and IL-6 was
significantly elevated (Fig. 1C-E).
However, the expression of TNF-α (Fig.
1C), IL-1β (Fig. 1D) and IL-6
(Fig. 1E) was markedly decreased
following pretreatment with shikonin. These results indicate that
shikonin protects against LTA-induced ALI and the corresponding
inflammatory response.
Shikonin inhibits LTA-induced
infiltration of pulmonary neutrophils
Infiltration of pulmonary neutrophils is one of the
most important symptoms of pneumonia that directly leads to
lung-tissue damage (38). As shown
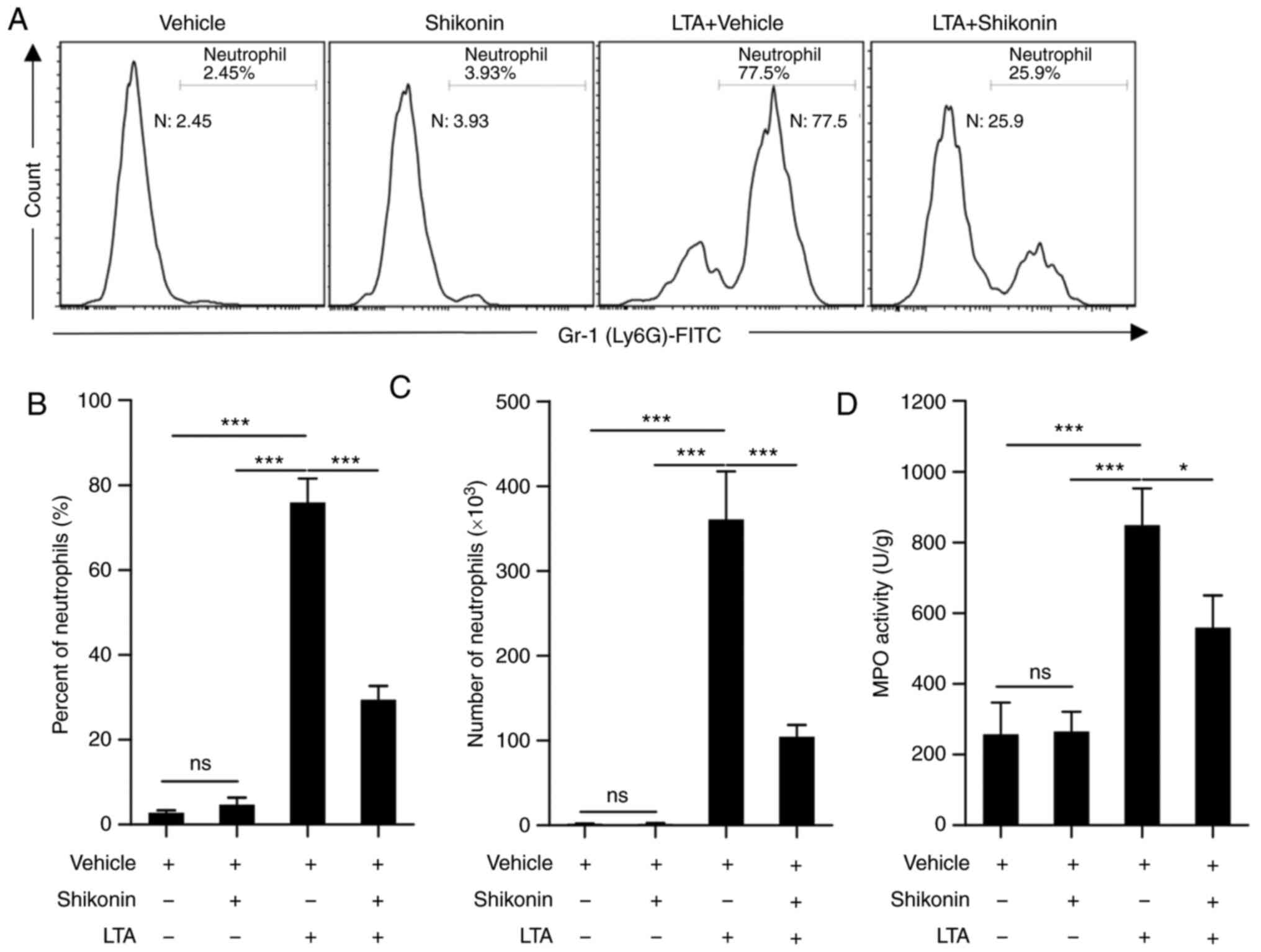
in Fig. 2, neutrophil infiltration
increased ~20 times compared with the vehicle group following a 6-h
LTA challenge. However, the neutrophil percentage (Fig. 2A and B) and amounts (Fig. 2C) were significantly reduced
following pre-injection of shikonin compared with those in mice
receiving LTA and vehicle. The activity of MPO-specific marker
representing neutrophil infiltration is associated with the
severity of ALI (39). MPO activity
in lung tissue was tested. LTA-challenged mice had higher MPO
activities compared with the mice that received the vehicle
(Fig. 2D). However, the activity
was significantly reduced by pretreatment with shikonin for 6 h
(Fig. 2D). These results indicated
that shikonin inhibits the accumulation of pulmonary
neutrophils.
Shikonin accentuates neutrophil
apoptosis
Neutrophils serve an important function in
infection-induced inflammation (20). Moderately activated neutrophils
protect against pathogen infection (40,41)
but inflammatory factors released by overactivated neutrophils
induce tissue injury (24). To
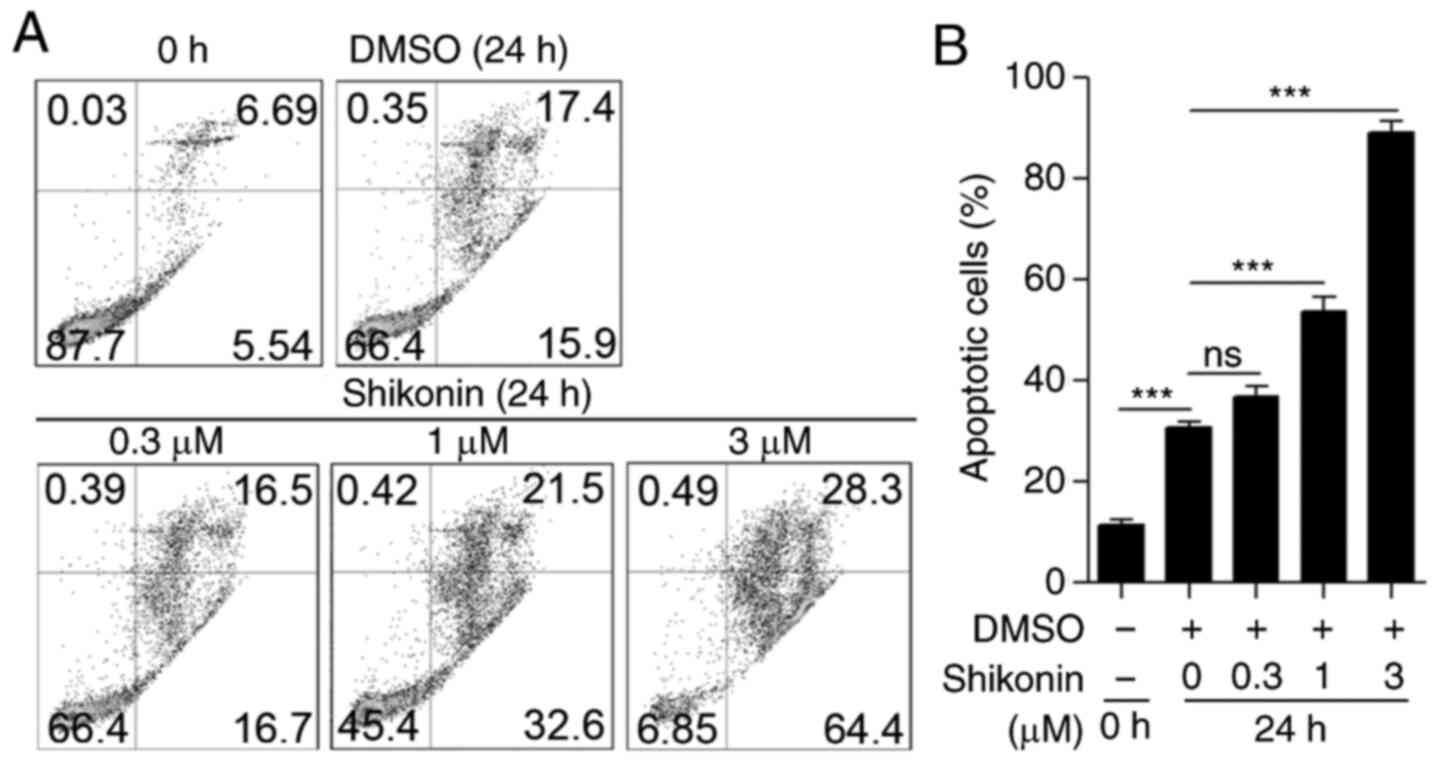
determine the effect of shikonin, neutrophils were isolated from
bone marrow and treated with 0.3, 1 or 3 µM shikonin. After 24 h of
incubation, neutrophils were stained with Annexin V/PI and analyzed
by flow cytometry. As shown in Fig.
3, the proportion of apoptotic cells increased significantly
with increased shikonin concentration (Fig. 3A and B). The effect of shikonin on
neutrophil apoptosis was examined further by determining the
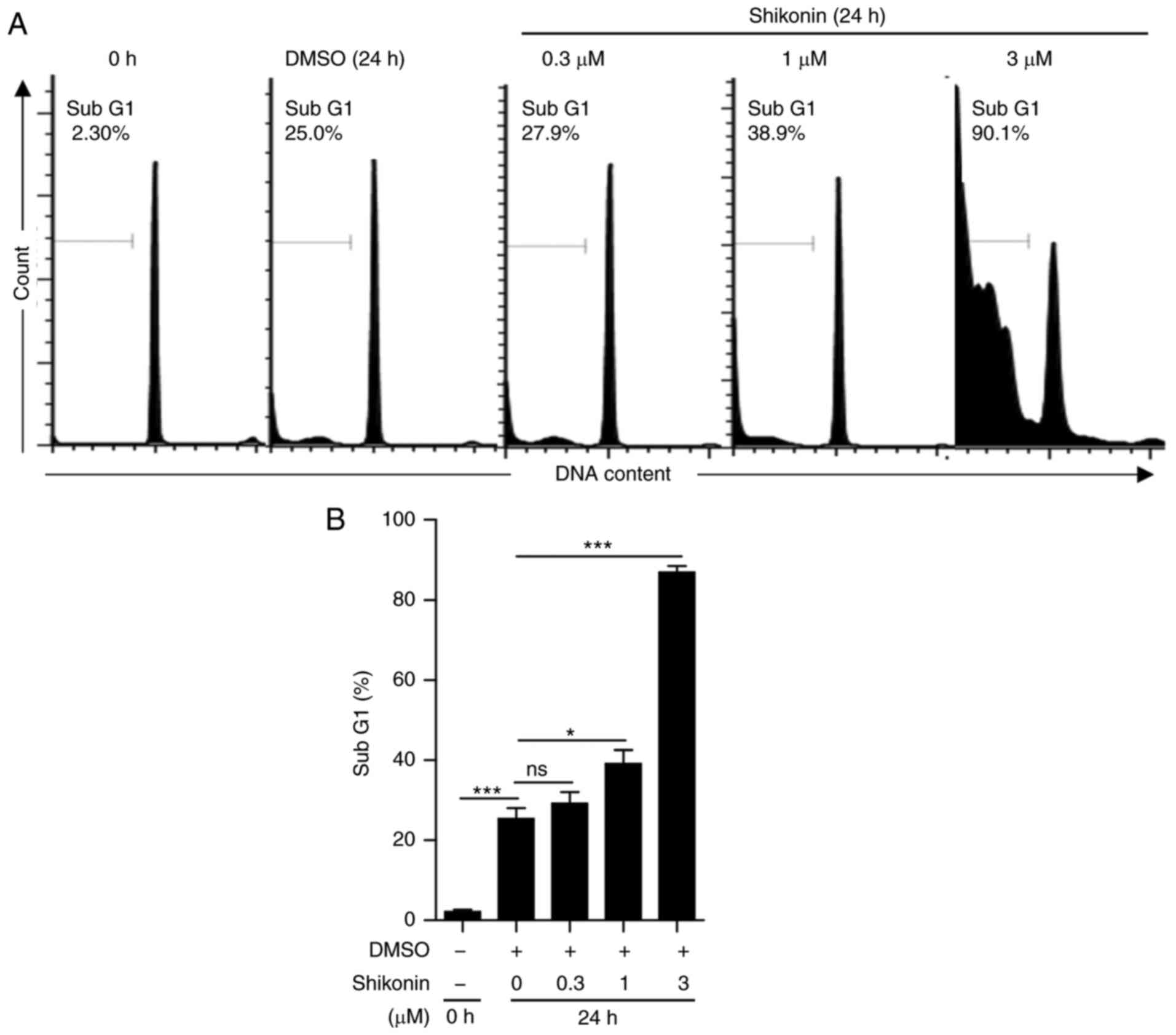
fractional DNA content (sub-G1) in the late stage of apoptosis
(Fig. 4A). The results showed that
shikonin dose-dependently enhanced DNA fragmentation (Fig. 4B). The proportion of sub-G1
neutrophils was markedly increased by 10 µM Shikonin from
25.33±2.72% (untreated) to 86.87±1.64% (Fig. 4B). These results suggested that
shikonin directly induces neutrophil apoptosis.
Shikonin induces an apoptotic
signaling pathway
To further investigate the mechanism by which
shikonin promotes neutrophil apoptosis, the activation of caspase-3
(cleaved caspase-3) and PARP (cleaved PARP), which serve important
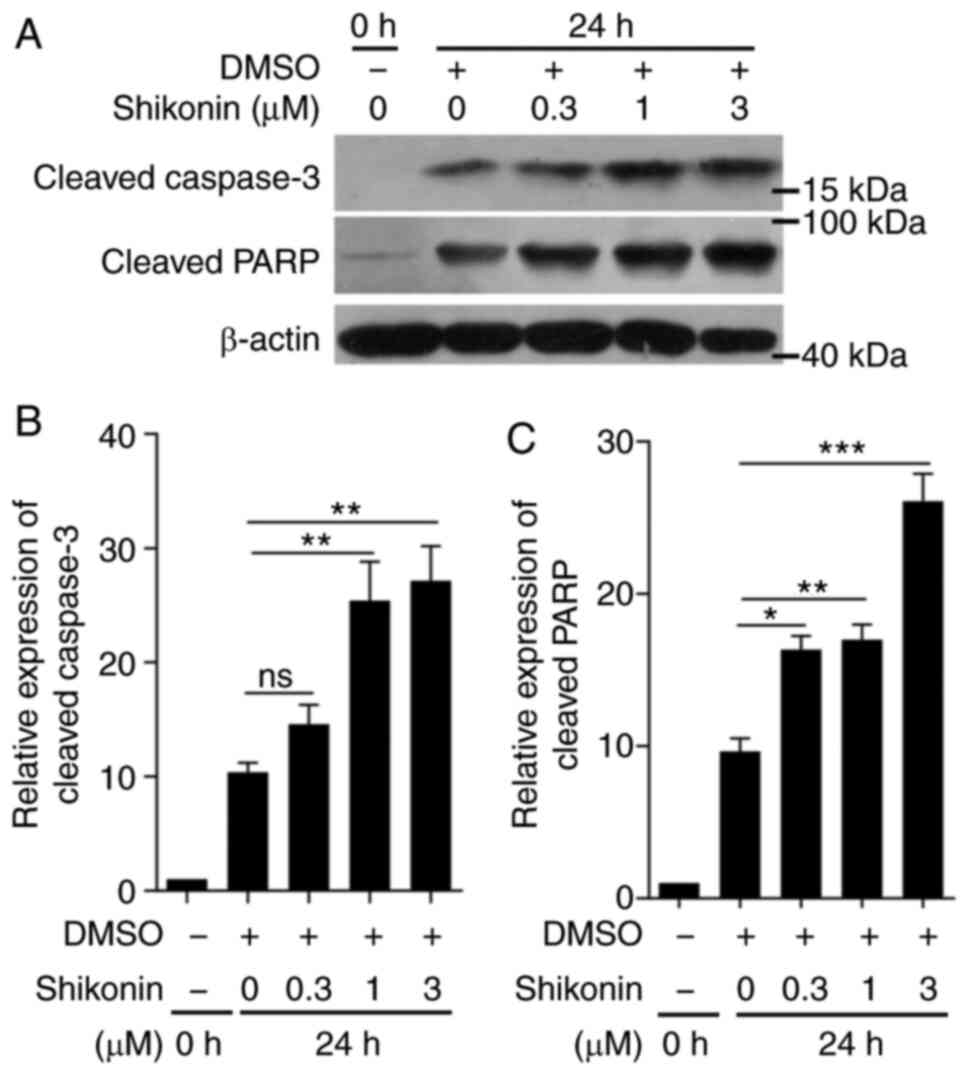
roles in apoptosis, were measured. As shown in Fig. 5, the expression of cleaved caspase-3
and cleaved PARP increased in a dose-dependent manner following
treatment with different concentrations of shikonin for 24 h. PARP
is a DNA-repair-related enzyme and the major substrate of
caspase-3. It can be cleaved into different fragments by cleaved
caspase-3 resulting in abnormal DNA repair and apoptosis (26). The content of cleaved PARP was
determined and, as expected, the expression of cleaved PARP was
elevated in a dose-dependent manner following shikonin treatment
(Fig. 5C). As the full-long
caspase-3 and PARP were decreased following cleavage, the
expression of cleaved caspase-3 and cleaved PARP was normalized by
using β-actin (42,43). These data suggested that shikonin
induced apoptosis by activating the caspase-3 signaling
pathway.
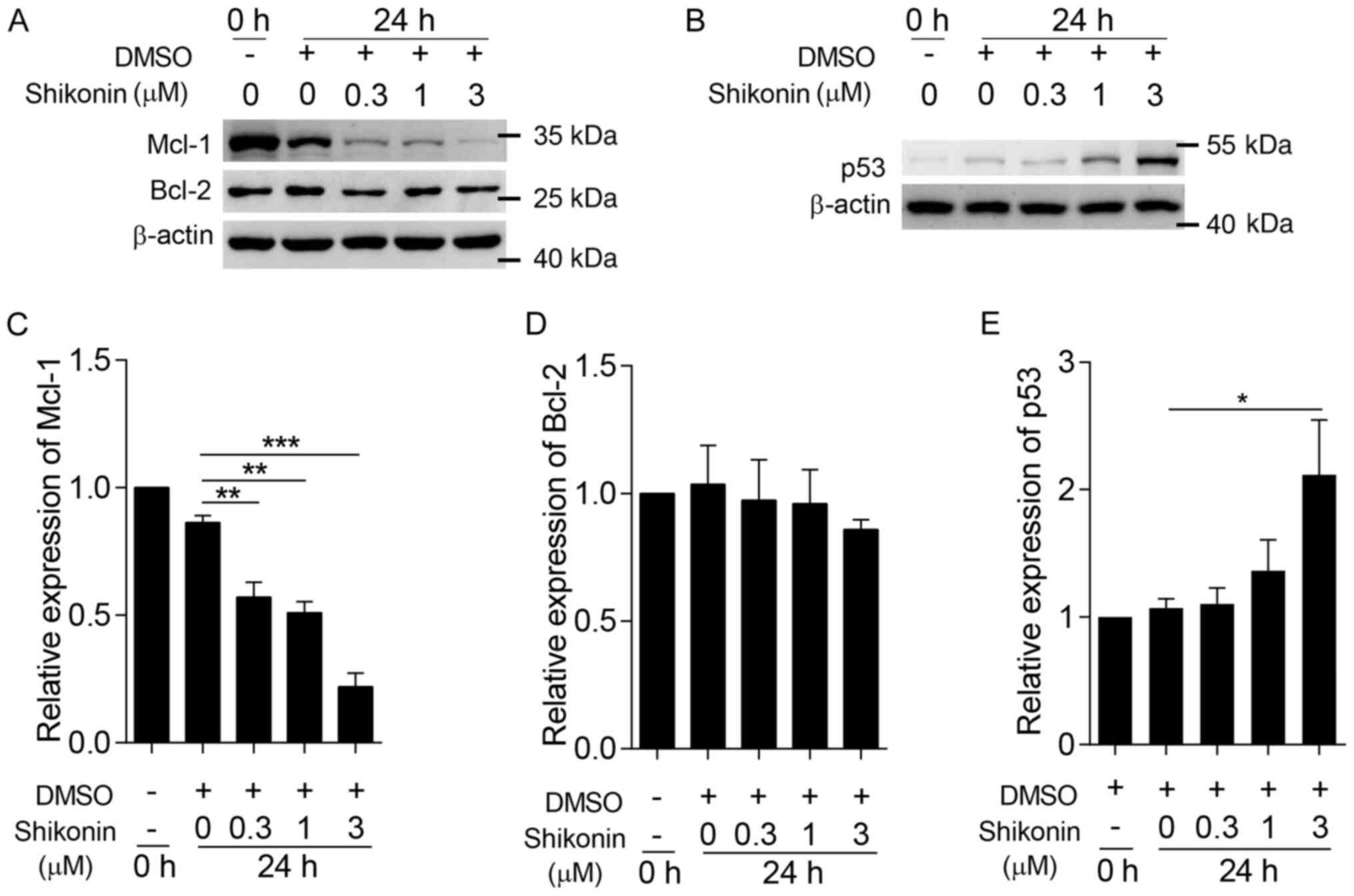
Shikonin inhibits the expression of
Mcl-1 and increases the expression of p53
Studies have shown that the apoptosis caused by
caspase-3 can be inhibited by the Bcl-2 family proteins (27), with Mcl-1 and Bcl-2 being important
proteins of the BCL-2 group (44).
To confirm the pro-apoptosis mechanism of shikonin, the effect of
shikonin on the expression of the antiapoptotic proteins Mcl-1 and
Bcl-2 was examined. Following treatment with different
concentrations of shikonin for 24 h, the expression of Mcl-1 was
significantly reduced in a dose-dependent manner (Fig. 6A and B); the expression of Mcl-1 was
four times lower compared with untreated neutrophils following
treatment with 3 µM shikonin for 24 h (Fig. 6B). However, shikonin did not affect
the expression of Bcl-2 (Fig. 6A and
C). The expression of p53 in neutrophils treated with 0.3, 1 or
3 µM shikonin was also analyzed. The expression of p53 was
increased significantly following treatment with 3 µM shikonin for
24 h compared with the DMSO control group (Fig. 6A and D). These results indicated
that shikonin inhibited the expression of Mcl-1 and increased the
expression of p53 in neutrophils.
Discussion
Sepsis-induced ALI and Acute respiratory distress
syndrome are associated with serious inflammation. TNF-α, IL-6 and
IL-1β serve important roles in the initiation and development of
pneumonia (45,46). TNF-α is the earliest
pro-inflammatory cytokine produced mainly by monocytes and can
induce the inflammatory cascade in endothelial and epithelial cells
thereby accelerating the production of other cytokines, including
IL-6 and IL-1β (45,47). The present study identified that the
production of TNF-α, IL-6 and IL-1β markedly decreased with
shikonin treatment in mice with LTA-induced ALI. A recent study
suggested that shikonin serves an anti-inflammatory role in
LPS-induced mice by inhibiting the NK-κB signaling pathway in
vitro and in vivo (48).
As with LPS stimulation, LTA activates the MAPK and NK-κB signaling
pathways by binding to the Toll-like receptor (TLR)4 and TLR2,
respectively (49–53). Therefore, the present study
hypothesized that shikonin could inhibit LTA-induced cytokine
generation in vitro. Shikonin is used in traditional Chinese
herbal medicine for its various medical properties, including
bactericidal activity, promotion of wound healing and anti-cancer
effects (54–56). Previous findings have shown that
shikonin alleviates LPS-induced ALI (49). LPS is isolated from gram-negative
bacteria while LTA is from gram-positive bacteria (57,58).
Together with previous research, the findings of the present study
demonstrated that shikonin is a protective agent against
bacteria-induced pneumonia and ALI.
Pathological features of ALI include injured
capillary endothelial and pulmonary epithelial cells, increased
pulmonary capillary permeability and impaired alveolar gas-exchange
(59). Although the pathogenesis of
gram-positive-bacteria-induced ALI is not entirely clear, excessive
inflammatory responses are considered to be the critical factor in
inducing lung injury (60). In
addition to cytokine storm, the infiltration of inflammatory cells
into lung tissue is widely accepted as a typical characteristic of
ALI that leads directly to lung-tissue damage (61). Neutrophils are the dominant
leucocytes and provide vital protection against body infection
(20). Activated neutrophils
release various injurious molecules, including proteolytic enzymes,
pro-inflammatory cytokines, oxidants and NO, that can cause damage
to the surrounding tissues (17).
Primary cultured neutrophils in vitro are easily activated
by the cultured environment including the Matrigel plate (32). Sustained accumulation of neutrophils
contributes to the development of ALI (32). Previous research has confirmed that
promoting neutrophil apoptosis is a promising way to treat ALI
(62). For example, Rahman et
al (63) reported that
inhibiting erBb (a family of receptor tyrosine kinases) reduces
pulmonary inflammation by increasing neutrophil apoptosis in a
murine ALI model. Harris et al (64) identified that IL-4 accelerates human
neutrophil apoptosis through modulated interleukin 4 receptor
α-dependent type 2 cytokine signaling that contributes to the ALI
resolution. In addition, several compounds, including emodin
(65) andrographolide (66) and matrine (67) alleviate ALI by promoting neutrophil
apoptosis. The present study indicated that shikonin inhibited
LTA-induced ALI by promoting neutrophil apoptosis. Therefore,
shikonin is a promising compound for the treatment of
neutrophil-related inflammation.
The protective role of shikonin has been researched
in previous studies. Lu et al (68) identified that shikonin inhibits
LPS-induced expression of TNF-α in rat primary cultured
macrophages. Prasad et al (34) identified that pro-inflammatory
mediators including NO, PGE2, TNF-α, inducible nitric oxide
synthase and cyclooxygenase-2 were significantly downregulated by
shikonin pretreatment in bv-2 microglia. A previous study
demonstrates a growth-enhancing effect of shikonin on human dermal
fibroblasts (69). Together, those
studies suggest that shikonin acts on multiple cell targets. The
present study identified that shikonin inhibited inflammation in an
LTA-induced ALI mice model by inducing neutrophil apoptosis.
Endotoxin-induced apoptosis is partly dependent on the intrinsic
mitochondrial pathway (70). The
activation of caspase-3 and PARP is the key point of the
mitochondrial pathway (26). The
effect of shikonin on the mitochondrial pathway was further
analyzed. As expected, the levels of cleaved caspase-3 and
cleaved-PARP significantly increased with the treatment of
shikonin. Previous findings showed that shikonin induces apoptosis
of colon cancer (71) and chronic
myeloid leukemia cells (72) by
reducing the Bcl-2 level. In the present study, however, the
expression of Bcl-2 was unchanged but the content of Mcl-1 was
significantly increased following treatment with shikonin. This is
because the level of Bcl-2 was lower in neutrophils than in cancer
cells (27). Thus, its effect is
not as important as that of Mcl-1 (73). The decrease in Mcl-1 contributed to
the body's anti-inflammatory response; this result is consistent
with that of a previous study (74). Furthermore, a recent study suggested
that inhibition of neutrophil apoptosis is closely related to
upregulated Mcl-1 and results in increased pulmonary disease
(75). Consistent with previous
studies, the data from the present study indicated that shikonin
enhanced neutrophil apoptosis by increasing cleaved caspase 3 and
decreasing Mcl-1 expression. Therefore, Mcl-1 is a more effective
target than Bcl-2 in neutrophil apoptosis.
Neutrophils are typically the first leukocytes to be
recruited to an inflammatory site and are capable of eliminating
pathogens as well as accelerating inflammation (32). Although the present study identified
that shikonin inhibited the recruitment of neutrophils to the lung
tissues, the mechanisms remain to be elucidated. Furthermore, the
results of the present study demonstrated that Mcl-1 was a
potential target of shikonin for inducing neutrophil apoptosis.
However, it is still unknown how shikonin induces neutrophil
apoptosis. The special signal pathways and banding sites of
shikonin should to be determined in future studies.
Collectively, the present study demonstrated that
pretreatment with shikonin in an LTA-induced murine ALI model
alleviated pathological changes in lung tissue, reduced
infiltration of inflammatory cells and decreased expression of
pro-inflammatory cytokines. Shikonin promoted neutrophil apoptosis
by triggering mitochondrial-mediated apoptosis signaling pathways,
specifically increasing the level of cleaved caspase-3 and
decreasing the expression of Mcl-1. The present study suggested
that shikonin is a therapeutic candidate for treating ALI.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key Research
and Development Program of Anhui Province (grant no.
1804h08020287), the National Key Clinical Specialty Construction
Project of Pulmonary Critical Care Medicine (grant no. 2012-649)
and the National Natural Science Foundation of China (grant nos.
81973329, 81773741 and 81573438).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YoZ and FQ designed the study. YoZ and HZ performed
the experiments and drafted the manuscript. MW and SG participated
in the animal experiments. LH and TH participated in data analysis.
YaZ and YuZ were involved in the interpretation of the data from
the experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The experimental protocols of the present study were
approved by the Animal Care and Use Committee of Bengbu Medical
College (Anhui, China) and conducted in accordance with the
guidelines of this committee. The present study adhered to the
Principles of Laboratory Animal Care (NIH publication No. 85-23,
revised 1996).
Patient consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing
interests.
References
|
1
|
Rubenfeld GD, Caldwell E, Peabody E,
Weaver J, Martin DP, Neff M, Stern EJ and Hudson LD: Incidence and
outcomes of acute lung injury. New Engl J Med. 353:1685–1693. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ware LB and Matthay MA: Medical
progress-the acute respiratory distress syndrome. New Engl J Med.
342:1334–1349. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goligher EC, Brochard LJ, Reid WD, Fan E,
Saarela O, Slutsky AS, Kavanagh BP, Rubenfeld GD and Ferguson ND:
Diaphragmatic myotrauma: A mediator of prolonged ventilation and
poor patient outcomes in acute respiratory failure. Lancet Respir
Med. 7:90–98. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schreiber MP, Chan CM and Shorr AF:
Bacteremia in Staphylococcus aureus pneumonia: Outcomes and
epidemiology. J Crit Care. 26:395–401. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Knapp S, Wieland CW, van't Veer C,
Takeuchi O, Akira S, Florquin S and van der Poll T: Toll-like
receptor 2 plays a role in the early inflammatory response to
murine pneumococcal pneumonia but does not contribute to
antibacterial defense. J Immunol. 172:3132–3138. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ghimire L, Paudel S, Jin LL, Baral P, Cai
SS and Jeyaseelan S: NLRP6 negatively regulates pulmonary host
defense in Gram-positive bacterial infection through modulating
neutrophil recruitment and function. Plos Pathog. 14:e10073082018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen ZX, Zhang D, Li M and Wang BL:
Costunolide ameliorates lipoteichoic acid-induced acute lung injury
via attenuating MAPK signaling pathway. Int Immunopharmacol.
61:283–289. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Beltramo F and Khemani RG: Definition and
global epidemiology of pediatric acute respiratory distress
syndrome. Ann Transl Med. 7:5022019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cohen J: The immunopathogenesis of sepsis.
Nature. 420:885–891. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ginsburg I: Role of lipoteichoic acid in
infection and inflammation. Lancet Infect Dis. 2:171–179. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Papageorgiou VP, Assimopoulou AN,
Couladouros EA, Hepworth D and Nicolaou KC: The chemistry and
biology of alkannin, shikonin, and related naphthazarin natural
products. Angew Chem Int Edit. 38:270–301. 1999. View Article : Google Scholar
|
|
12
|
Yang Y, Gao WY, Tao SY, Wang YX, Nin JZ,
Zhao PW, Rao CC and Lei Y: ER-mediated anti-tumor effects of
shikonin on breast cancer. Eur J Pharmacol. 863:1726672019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vegara S, Funes L, Marti N, Saura D, Micol
V and Valero M: Bactericidal activities against pathogenic bacteria
by selected constituents of plant extracts in carrot broth. Food
Chem. 128:872–877. 2011. View Article : Google Scholar
|
|
14
|
Guo H, Sun J, Li D, Hu Y, Yu X, Hua H,
Jing X, Chen F, Jia ZJ and Xu J: Shikonin attenuates
acetaminophen-induced acute liver injury via inhibition of
oxidative stress and inflammation. Biomed Pharmacother.
112:1087042019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fan XH, Cheng L and Yan AH: Ameliorative
effect of acetylshikonin on ovalbumin (OVA)-induced allergic
rhinitis in mice through the inhibition of Th2 cytokine production
and mast cell histamine release. Apmis. 127:688–695. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Xu T, Pan Z, Ge X, Sun C, Lu C,
Chen H, Xiao ZX, Zhang B, Dai YR and Liang G: Shikonin inhibits
myeloid differentiation protein 2 to prevent LPS-induced acute lung
injury. Brit J Pharmacol. 175:840–854. 2018. View Article : Google Scholar
|
|
17
|
Potey PM, Rossi AG, Lucas CD and Dorward
DA: Neutrophils in the initiation and resolution of acute pulmonary
inflammation: Understanding biological function and therapeutic
potential. J Pathol. 247:672–685. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abraham E: Neutrophils and acute lung
injury. Crit Care Med. 31 (4 Suppl):S195–S199. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong L, Xu J, Lu X, Yang Y and Qiu H:
Inflammation by regulating neutrophil infiltration and balance of
th1/th2 response in acute lung injury. Int Care Med. 38:S16.
2012.
|
|
20
|
Marshall JC: Neutrophils in the
pathogenesis of sepsis. Crit Care Med. 33 (12 Suppl):S502–S505.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dockrell DH, Marriott HM, Prince LR,
Ridger VC, Ince PG, Hellewell PG and Whyte MK: Alveolar macrophage
apoptosis contributes to pneumococcal clearance in a resolving
model of pulmonary infection. J Immunol. 171:5380–5388. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leiber A, Schwarz J, Kostlin N, Spring B,
Fehrenbach B, Katava N, Poets CF and Gille C: Neonatal myeloid
derived suppressor cells show reduced apoptosis and
immunosuppressive activity upon infection with Escherichia
coli. Eur J Immunol. 47:1009–1021. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi W, Xu C, Hussain M, Wu F, Lu M, Wu X,
Tang L, Wu X and Wu J: Inhibition of myosin light-chain kinase
enhances the clearance of lipopolysaccharide-induced lung
inflammation possibly by accelerating neutrophil apoptosis. Shock.
48:377–386. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang K, Lai C, Li T, Wang C, Wang W, Ni B,
Bai CQ, Zhang SG, Han L, Gu H, et al: Basic fibroblast growth
factor protects against influenza A virus-induced acute lung injury
by recruiting neutrophils. J Mol Cell Biol. 10:573–585. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Porter AG and Janicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cory S and Adams JM: The BCL2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bayne K: Revised guide for the care and
use of laboratory animals available. American Physiological
Society. Physiologist. 39:208–211. 1996.
|
|
29
|
Wu YX, He HQ, Nie YJ, Ding YH, Sun L and
Qian F: Protostemonine effectively attenuates
lipopolysaccharide-induced acute lung injury in mice. Acta
Pharmacol Sin. 39:85–96. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu Y, He H, Ding Y, Liu S, Zhang D, Wang
J, Jiang H, Zhang D, Sun L, Ye RD and Qian F: MK2 mediates
macrophage activation and acute lung injury by regulating let-7e
miRNA. Am J Physiol Lung Cell Mol Physiol. 315:L371–L381. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kolaczkowska E and Kubes P: Neutrophil
recruitment and function in health and inflammation. Nat Rev
Immunol. 13:159–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang J, Wang Z and Chen DL: Shikonin
ameliorates isoproterenol (ISO)-induced myocardial damage through
suppressing fibrosis, inflammation, apoptosis and ER stress. Biomed
Pharmacother. 93:1343–1357. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Prasad RG, Choi YH and Kim GY: Shikonin
Isolated from Lithospermum erythrorhizon downregulates
proinflammatory mediators in lipopolysaccharide-stimulated BV2
microglial cells by suppressing crosstalk between reactive oxygen
species and NF-κB. Biomol Ther (Seoul). 23:110–118. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Taguchi A, Koga K, Kawana K, Makabe T, Sue
F, Miyashita M, Yoshida M, Urata Y, Izumi G, Tkamura M, et al:
Resveratrol enhances apoptosis in endometriotic stromal cells. Am J
Reprod Immunol. 75:486–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim SS, Jang HJ and Oh MY: Quercetin
enhances the function and reduces apoptosis of mouse islets.
Transplant Proc. 51:1451–1457. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sophonnithiprasert T, Nilwarangkoon S,
Nakamura Y and Watanapokasin R: Goniothalamin enhances
TRAIL-induced apoptosis in colorectal cancer cells through DR5
upregulation and cFLIP downregulation. Int J Oncol. 47:2188–2196.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Matthay MA, Ware LB and Zimmerman GA: The
acute respiratory distress syndrome. J Clin Invest. 122:2731–2740.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lv H, Liu Q, Wen Z, Feng H, Deng X and Ci
X: Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute
lung injury via induction of AMPK/GSK3 β-Nrf2 signal axis. Redox
Bio. 12:311–324. 2017. View Article : Google Scholar
|
|
40
|
Tan Z, Jiang R, Wang X, Wang Y, Lu L, Liu
Q, Zheng SG, Sun B and Ryffel B: RORγt+IL-17+ neutrophils play a
critical role in hepatic ischemia-reperfusion injury. J Mol Cell
Biol. 5:143–146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brinkmann V, Reichard U, Goosmann C,
Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y and Zychlinsky A:
Neutrophil extracellular traps kill bacteria. Science.
303:1532–1535. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Salaun C, Leroy C, Rousseau A, Boitez V,
Beck L and Friedlander G: Identification of a novel
transport-independent function of PiT1/SLC20A1 in the regulation of
TNF-induced apoptosis. J Biol Chem. 285:34408–34418. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang H, Rivera Z, Jube S, Nasu M, Bertino
P, Goparaju C, Franzoso G, Lotze MT, Krausz T, Pass HI, et al:
Programmed necrosis induced by asbestos in human mesothelial cells
causes high-mobility group box 1 protein release and resultant
inflammation. Proc Natl Acad Sci USA. 107:12611–12616. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Certo M, Del Gaizo Moore V, Nishino M, Wei
G, Korsmeyer S, Armstrong SA and Letai A: Mitochondria primed by
death signals determine cellular addiction to antiapoptotic BCL-2
family members. Cancer Cell. 9:351–365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li X, Ye C, Mulati M, Sun L and Qian F:
Ellipticine blocks synergistic effects of IL-17A and TNF-α in
epithelial cells and alleviates severe acute
pancreatitis-associated acute lung injury. Biochem Pharmacol.
177:1139922020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ding YH, Song YD, Wu YX, He HQ, Yu TH, Hu
YD, Zhang DP, Jiang HC, Yu KK, Li XZ, et al: Isoalantolactone
suppresses LPS-induced inflammation by inhibiting TRAF6
ubiquitination and alleviates acute lung injury. Acta Pharmacol
Sin. 40:64–74. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhou P, Xie W, Luo Y, Lu S, Dai Z, Wang R,
Sun G and Sun X: Protective effects of total saponins of Aralia
elata (Miq.) on endothelial cell injury induced by TNF-α via
modulation of the PI3K/Akt and NF-κB signalling pathways. Int J Mol
Sci. 20:362018. View Article : Google Scholar
|
|
48
|
Yang C, Liu P, Wang S, Zhao G, Zhang T,
Guo S, Jiang K, Wu H and Deng G: Shikonin exerts anti-inflammatory
effects in LPS-induced mastitis by inhibiting NF-κB signaling
pathway. Biochem Biophys Res Commun. 505:1–6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lin MX, Yi YX, Fang PP, Huang SS, Pan CW,
Jin LX, Zhang T and Zhou GY: Shikonin protects against
D-Galactosamine and lipopolysaccharide-induced acute hepatic injury
by inhibiting TLR4 signaling pathway. Oncotarget. 8:91542–91550.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mayerhofer R, Frohlich EE, Reichmann F,
Farzi A, Kogelnik N, Frohlich E, Sattler W and Holzer P: Diverse
action of lipoteichoic acid and lipopolysaccharide on
neuroinflammation, blood-brain barrier disruption, and anxiety in
mice. Brain Behav Immun. 60:174–187. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bulgari O, Dong X, Roca AL, Caroli AM and
Loor JJ: Innate immune responses induced by lipopolysaccharide and
lipoteichoic acid in primary goat mammary epithelial cells. J Anim
Sci Biotechno. 8:292017. View Article : Google Scholar
|
|
52
|
Zhang D, Li X, Hu Y, Jiang H, Wu Y, Ding
Y, Yu K, He H, Xu J, Sun L and Qian F: Tabersonine attenuates
lipopolysaccharide-induced acute lung injury via suppressing TRAF6
ubiquitination. Biochem Pharmacol. 154:183–192. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xu C, Chen G, Yang W, Xu Y, Xu Y, Huang X,
Liu J, Feng Y, Xu Y and Liu B: Hyaluronan ameliorates LPS-induced
acute lung injury in mice via Toll-like receptor (TLR) 4-dependent
signaling pathways. Int Immunopharmacol. 28:1050–1058. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhao Q, Assimopoulou AN, Klauck SM,
Damianakos H, Chinou I, Kretschmer N, Rios JL, Papageorgiou VP,
Bauer R and Efferth T: Inhibition of c-MYC with involvement of
ERK/JNK/MAPK and AKT pathways as a novel mechanism for shikonin and
its derivatives in killing leukemia cells. Oncotarget.
6:38934–38951. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Han X, Kang KA, Piao MJ, Zhen AX, Hyun YJ,
Kim HM, Ryu YS and Hyun JW: Shikonin exerts cytotoxic effects in
human colon cancers by inducing apoptotic cell death via the
endoplasmic reticulum and mitochondria-mediated pathways. Biomol
Ther (Seoul). 27:41–47. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Thakur R, Trivedi R, Rastogi N, Singh M
and Mishra DP: Inhibition of STAT3, FAK and Src mediated signaling
reduces cancer stem cell load, tumorigenic potential and metastasis
in breast cancer. Sci Rep. 5:101942015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Oh KS, Patel H, Gottschalk RA, Lee WS,
Baek S, Fraser IDC, Hager GL and Sung MH: Anti-inflammatory
chromatinscape suggests alternative mechanisms of glucocorticoid
receptor action. immunity. 47:298–309 e5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kim JH, Lee J, Park J and Gho YS:
Gram-negative and Gram-positive bacterial extracellular vesicles.
Semin Cell Dev Biol. 40:97–104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wheeler AP and Bernard GR: Acute lung
injury and the acute respiratory distress syndrome: A clinical
review. Lancet. 369:1553–1564. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Goodman RB, Pugin J, Lee JS and Matthay
MA: Cytokine-mediated inflammation in acute lung injury. Cytokine
Growth Factor Rev. 14:523–535. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Luce JM: Acute lung injury and the acute
respiratory distress syndrome. Critical Care Medicine. 26:369–376.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kurdowska AK and Florence JM: Promoting
neutrophil apoptosis to treat acute lung injury. Am J Respir Crit
Care Med. 200:399–400. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Rahman A, Henry KM, Herman KD, Thompson
AA, Isles HM, Tulotta C, Sammut D, Rougeot JJ, Khoshaein N, Reese
AE, et al: Inhibition of ErbB kinase signalling promotes resolution
of neutrophilic inflammation. Elife. 8:e509902019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Harris AJ, Mirchandani AS, Lynch RW,
Murphy F, Delaney L, Small D, Coelho P, Watts ER, Sadiku P,
Griffith D, et al: IL4Rα signaling abrogates hypoxic neutrophil
survival and limits acute lung injury responses in vivo. Am
J Resp Crit Care. 200:235–246. 2019. View Article : Google Scholar
|
|
65
|
Cui H, Li S, Xu C, Zhang J, Sun Z and Chen
H: Emodin alleviates severe acute pancreatitis-associated acute
lung injury by decreasing pre-B-cell colony-enhancing factor
expression and promoting polymorphonuclear neutrophil apoptosis.
Mol Med Rep. 16:5121–5128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Li X, Yuan K, Zhu Q, Lu Q, Jiang H, Zhu M,
Huang G and Xu A: Andrographolide ameliorates rheumatoid arthritis
by regulating the apoptosis-NETosis balance of neutrophils. Int J
Mol Sci. 20:50352019. View Article : Google Scholar
|
|
67
|
Yu X, Seow HJ, Wang H, Anthony D,
Bozinovski S, Lin L, Ye JM and Vlahos R: Matrine reduces cigarette
smoke-induced airway neutrophilic inflammation by enhancing
neutrophil apoptosis. Clin Sci (Lond). 133:551–564. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lu L, Qin A, Huang H, Zhou P, Zhang C, Liu
N, Li S, Wen G, Zhang C, Dong W, et al: Shikonin extracted from
medicinal Chinese herbs exerts anti-inflammatory effect via
proteasome inhibition. Eur J Pharmacol. 658:242–247. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yan Y, Furumura M, Gouya T, Iwanaga A,
Teye K, Numata S, Karashima T, Li XG and Hashimoto T: Shikonin
promotes skin cell proliferation and inhibits nuclear factor-κB
translocation via proteasome inhibition in vitro. Chin Med J
(Engl). 128:2228–2233. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mantzarlis K, Tsolaki V and Zakynthinos E:
Role of oxidative stress and mitochondrial dysfunction in sepsis
and potential therapies. Oxid Med Cell Longev. 2017:59852092017.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Andujar I, Marti-Rodrigo A, Giner RM, Rios
JL and Recio MC: Shikonin prevents early phase inflammation
associated with azoxymethane/dextran sulfate sodium-induced colon
cancer and induces apoptosis in human colon cancer cells. Planta
Med. 84:674–683. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chen Y, Wang T, Du J, Li Y, Wang X, Zhou
Y, Yu X, Fan W, Zhu QJ, Tong X and Wang Y: The critical role of
PTEN/PI3K/AKT signaling pathway in shikonin-induced apoptosis and
proliferation inhibition of chronic myeloid leukemia. Cell Physiol
Biochem. 47:981–993. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Mollet L, Robinet P, Dubois M, Aurouet A,
Normand T, Charpentier S, Sureau A, Grandclement C, Garnache-Ottou
F, Deconinck E, et al: Opposing Mcl-1, the GALIG proapoptotic gene
is upregulated as neutrophils die and underexpressed in acute
myeloid leukemia cells. Mole Immunol. 56:123–128. 2013. View Article : Google Scholar
|
|
74
|
Lucas CD, Dorward DA, Tait MA, Fox S,
Marwick JA, Allen KC, Robb CT, Hirani N, Haslett C, Duffin R and
Rossi AG: Downregulation of Mcl-1 has anti-inflammatory
pro-resolution effects and enhances bacterial clearance from the
lung. Mucosal Immunol. 7:857–868. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhao H, Ma Y and Zhang L:
Low-molecular-mass hyaluronan induces pulmonary inflammation by
up-regulation of Mcl-1 to inhibit neutrophil apoptosis via
PI3K/Akt1 pathway. Immunol. 155:387–395. 2018. View Article : Google Scholar
|