Osteoarthritis (OA) is the most common form of
arthritis. OA is mainly characterized by the loss of structure in
the articular cartilage, remodeling of the subchondral bone and
osteophyte formation (1,2). According to a review in 2017, OA has
been reported to affect 240 million individuals worldwide (3). The etiology of OA is multifarious,
including age, sex, genetic, mechanical stress on the joint, and
loss of functional integrity of cellular organelles (4,5).
Treatment options of OA have expanded and their availability has
greatly been improved. However, these treatments are not always
satisfactory, since a complete cure for OA is not yet possible
(6,7). Therefore, there is a large demand for
alternative therapeutics for OA. A better understanding of the
underlying mechanisms of OA pathogenesis may facilitate the
discover of more crucial targets, and may reduce the effect the
devastating symptoms of OA (8,9).
Currently, the role of proteins associated with
lipid metabolism have been identified in health and disease. Among
these proteins, peroxisome proliferator-activated receptor (PPAR)
has been reported to be involved in reducing inflammatory responses
in human OA cartilage (10–12). PPAR is a ligand-activated
transcription factor and a member of the nuclear receptor
superfamily. It is originally identified to play a key role in
lipid homeostasis. There are three isotypes of PPAR: α, γ and β/δ
(13,14). PPARα is present in a wide range of
cells including endothelial cells, hepatocytes, myocardiocytes and
chondrocytes, and exerts anti-inflammatory effects on various
tissues (15,16). PPARγ has potent anti-inflammatory
properties and regulates energy storage (17,18).
PPARδ is the most widely expressed in whole body tissues, and
regulates energy expenditure in cells (19,20).
The present review discusses the association between
PPAR and OA, as well as evaluating the protective effects of PPAR
on the prevention of OA.
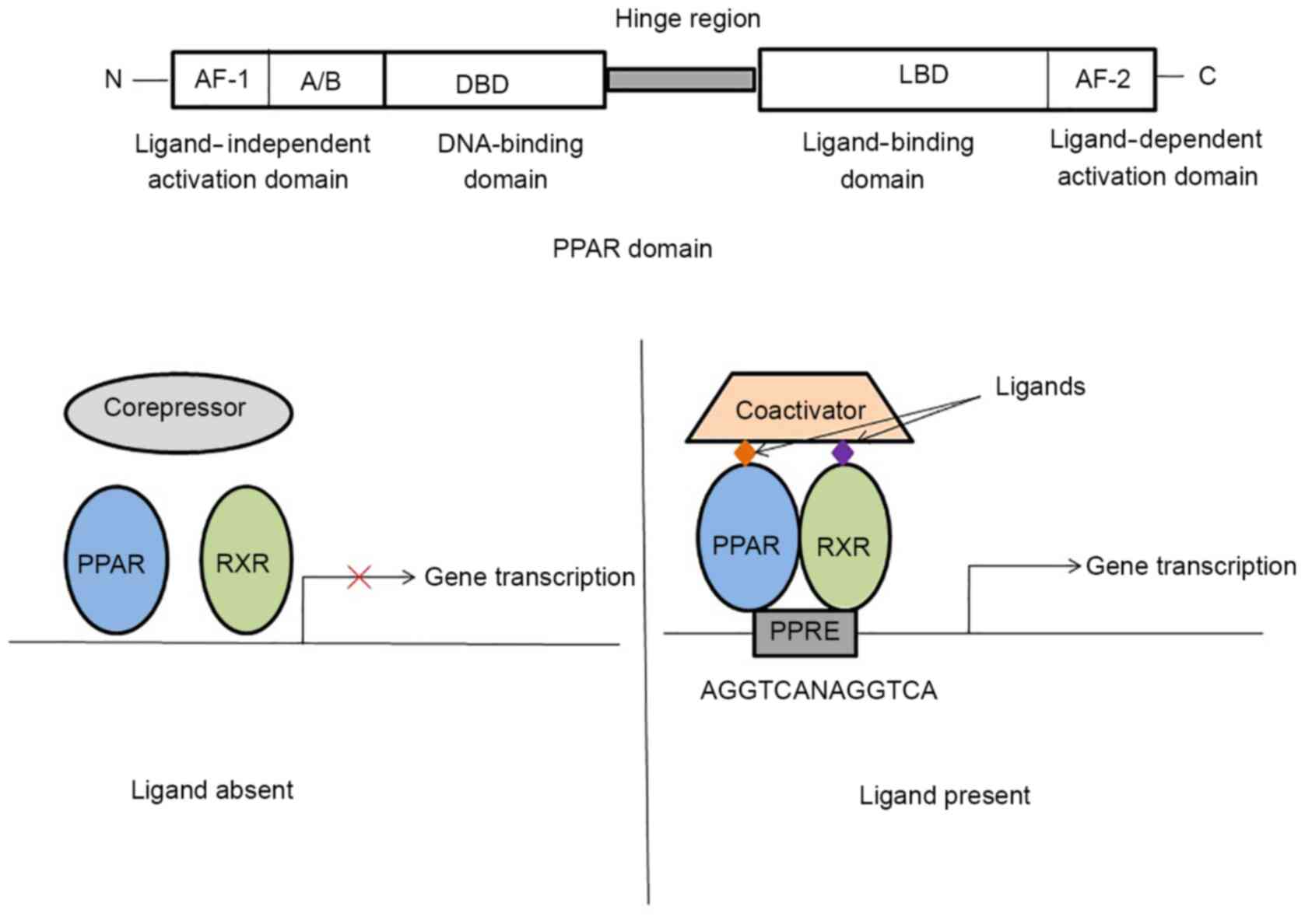
PPARs heterodimerize with the retinoid X receptor
(RXR) and bind to specific regions on the DNA termed peroxisome
proliferators response elements (PPREs). The DNA consensus sequence
of PPRE is 5′-AGGTCANAGGTCA-3′, which occurs in the promoter region
of target genes. The function of PPAR/RXR heterodimers is modified
by a number of coregulator complexes, which leads to
transactivation and transrepression of various genes, for example,
cytokine genes or glucocorticoid response element-driven genes
(24–26). When activated by a ligand, the
PPAR/RXR heterodimer is associated with coactivator protein
complexes (such as cAMP response element-binding protein, PPARs
coactivators, cAMP response element-binding protein binding
protein, and steroid receptor coactivator-1), and the rate of is
transcription of target genes is increased (27,28).
In the absence of ligands, the PPAR/RXR heterodimer is associated
with corepressor complexes (such as nuclear receptor co-repressor,
and silencing mediator of retinoid acid and thyroid hormone
receptor) and represses gene transcription by chromatin remodeling
(27,29). It was reported that activated
PPAR/RXR heterodimer may also repress target gene transcription
through DNA-independent protein-protein interactions with other
transcription factors or coactivators (30,31).
For the activation of PPARs, a number of natural or
synthetic PPAR ligands, named agonists, have been identified. The
mostwell-studied natural PPAR ligands include polyunsaturated fatty
acids, eicosanoids, endocannabinoids and endogenous specialized
pro-resolving mediators. The synthetic PPAR ligands include
fibrates and thiazolidinediones (32,33).
PPAR antagonists could also be used as an interesting PPAR
modulator. Antagonists are compounds that bind to the LBD but
interfere with H12 folding, which inhibits the binding of
co-activators or subsequent transcriptional activation. Several
antagonists have been identified including MK886, GW6471, BADGE,
GW9662, PD068235, SR-202, LG100641, indomethacin, GSK0660, SR13904
and NSC636948 (34).
PPARs play a critical role in regulating diverse
biological processes such as development, differentiation,
inflammation and wound healing. They also may act as lipid sensors
and regulators of energy (lipid and carbohydrate) metabolism
(28,35). However, PPARs may cause the
metabolic energy imbalance in disease conditions such as
inflammation, diabetes, obesity, dyslipidemia, neurodegenerative
disorder and cancer (20,36,37).
PPARs play a major regulatory role in lipid
metabolism and energy homeostasis by modulating target genes
encoding lipid metabolism enzymes or lipid transporters, triggering
a conformational change (38,39).
Activated PPARs are known to have the protective and detrimental
effect against various types of diseases, including diabetes,
dyslipidemia, inflammation, pain, obesity, cancer and
neurodegenerative disorders (40,41).
PPARs play an important role in the immune response by inhibiting
the expression of pro-inflammatory genes by peripheral immune cells
through trans-repressive mechanisms. Several factors have been
involved in regulating inflammatory signaling pathways mediated by
different PPARs. During the inflammatory reaction, PPARs promote
the inactivation of NF-κB. Activation of all PPARs by different
pro-inflammatory factors causes the inhibition of NF-κB activation,
which leads to the inhibition of inflammatory reactions. Activated
PPARs bind with and thus inactivate p65 NF-κB through the
proteolytic degradation of p65 NF-κB, leading to the reduction of
the pro-inflammatory response. PPARα and PPARγ can inhibit the
acetylation of p65 NF-κB by binding with p300 and inhibits
activation of this pro-inflammatory factor. PPARα and PPARγ can
also inhibit NF-κB activation by increasing the expression of IκBα
and the activity of SIRT1. Activated PPARβ/δ inactivates NF-κB p65
by disrupting the assembly of TAK1, TAB1 and HSP27 into a complex.
PPARγ increases the activity of the E3 ubiquitin ligase, which
leads to proteolytic degradation of NF-κB (42–44).
PPARs also cause the inhibition of inflammatory reactions by
inactivating STATs. Activated PPARα disrupts the activity of STAT1
and PPAR-γ blocks the pro-inflammatory action of IFN-γ, as well as
increase the expression of the suppressor of cytokine signaling 3,
by inhibiting the JAK-STAT pathway (45).
PPARs also inhibit the proliferation of several
types of human cancer cell lines (46,47).
PPARs control the expression of genes involved in differentiation,
and negatively regulates the cell cycle. PPARs have also shown
efficacy in neurodegenerative disorders by inhibiting the
activation of microglial cells (20,48).
Fu et al (49) reported that
PPARα has a protective role in obesity by initiating the
transcription of proteins involved in lipid metabolism and
repressing inducible nitric oxide synthase to repress feeding
stimulation. Michalik et al (50) reported that PPARα plays a role in
wound healing by controlling inflammation at the wound site. Lee
et al (51) demonstrated
that activation of PPARα regulated hepatic autophagy by nutrient
status. Lee et al (52)
showed that activation of PPARα synergizes with the glucocorticoid
receptor (GR) to promote self-renewal of early committed erythroid
progenitors.
Mitochondrial dysfunction plays an important role in
the initiation and progression of cartilage degeneration in OA by
impairing chondrocyte growth, increasing chondrocyte oxidative
stress and enhancing inflammatory responses (53,54).
PPARs have been implicated in regulating articular cartilage
homeostasis through the modulation of various signaling pathway.
The reduction of PPARα may promote inflammatory and destructive
responses in OA cartilage. Loss of PPARα increases the expression
of MMP1 and MMP13, as well as enhances the production of
triglycerides and cholesterol levels in plasma, and thereby induces
cartilage degradation in OA. PPARδ can act as a promoter of
cartilage degeneration in O (55).
Ratneswaran et al (56)
reported that PPARδ activation by GW501516 (a selective PPARδ
agonist) resulted in enhanced expression of several proteases in
chondrocytes, increased aggrecan degradation and glycosaminoglycan
release; whereas cartilage-specific PPARδ-knockout mice showed
strong protection from cartilage degeneration in a mouse model of
posttraumatic OA.
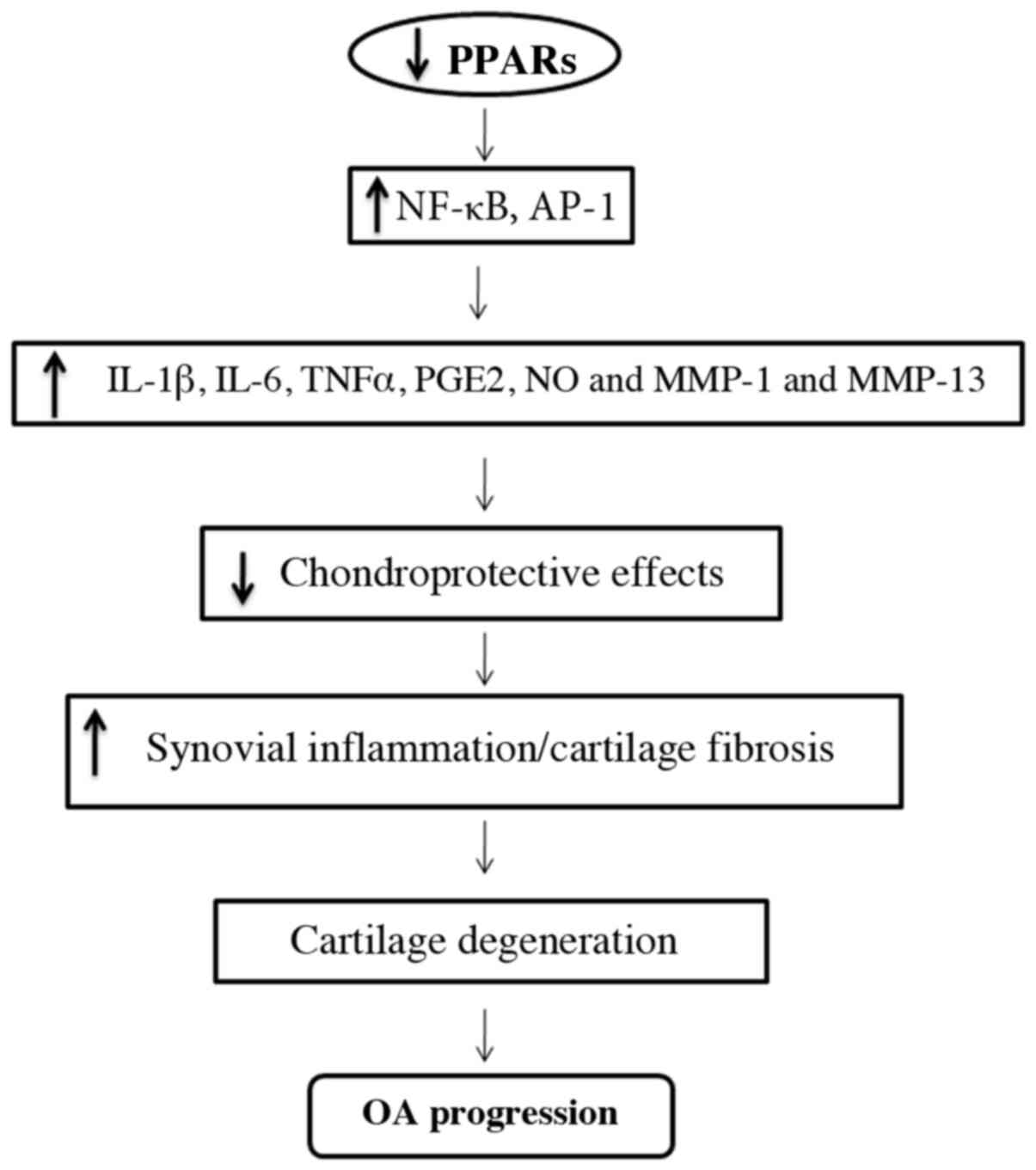
The deficiency of PPARγ in the articular cartilage
may be responsible for the acceleration of severe OA by increasing
catabolic activity and the suppression of chondroprotection
(Fig. 2) (57,58).
Wang et al (59) reported
that PPAR-γ coactivator (PGC)-1α is the master regulator of
mitochondrial biogenesis that critically mediates anti-catabolic
activity in chondrocytes. Mitochondrial biogenesis has been
impaired in human OA chondrocytes that promote chondrocyte
pro-catabolic responses. PPARγ was also reported as a master
adipogenic regulator that may influence the deposition of fat in
both skeletal muscle and connective tissues. Deposition of fat is a
strong risk factor for OA in the knee. The major adipose tissue in
knee joint is infrapatellar fat pad (IPFP) that can produce
inflammatory cytokines and adipokines. Consequently, PPARγmay
associate with the pathological changes of IPFP in OA by triggering
adipogenesis, via the activation of different signaling pathways
(60–63). It is also reported that loss of
PPARγ can enhance the synthesis of various catabolic and
inflammatory factors, including inflammatory cytokines such as
interleukin (IL)-1b, IL-6, tumor necrosis factor-α, prostaglandin
E2, nitric oxide (NO) and matrix metalloproteinases (MMPs) involved
in the pathogenesis of OA (64,65).
Moreover, loss of PPARγ reduces chondroprotective effects,
anti-inflammatory and antifibrogenic effects, resulting in
increased synovial inflammation (accumulation of macrophages) and
increased synovial and cartilage fibrosis; this could be a
contributing factor resulting in cartilage destruction and the
progression of OA (57,66).
As we have already discussed that OA is a
progressive degenerative joint disorder and the most common form of
arthritis, it has become a socioeconomic and clinical concern.
Traditional OA treatments are still unsatisfactory to stimulate the
regeneration of cartilage. PPARs play a critical role in regulating
cartilage health, and the lack of PPARs leads to the degeneration
of cartilage in OA (12,67,68).
Several studies have found that PPARs may be a therapeutic target
to counteract the degradative mechanisms associated with OA
(Table I) (12,15,55,56,58,65,69–73).
These studies have showed that PPAR agonists can reduce the
development of cartilage lesions by inhibiting the synthesis of
various catabolic and inflammatory factors involved in the
pathogenesis of OA (74,75).
Several other studies focused on naturally occurring
plant products that may activate PPARs and provide a preventive
strategy for the treatment of OA. Qu et al (71) investigated the role of mangiferin
(MFN) in human OA chondrocytes. Cells were treated with various
concentrations of MFN and found that MFN inhibited IL-1β-induced
inflammatory response in human OA chondrocytes by activating PPARγ
(71). Wang et al (72) suggested that Antarctic krill oil
(AKO) improves articular cartilage degeneration via activating
chondrocyte autophagy and inhibiting apoptosis in mice with OA. It
was also shown that AKO upregulates PPARγ and reduces mTOR
signaling, and thereby maintains cartilage homeostasis in OA model
mouse (72). Wang et al
(73) reported that the
downregulation of galectin-3 (Gal-3) protects from
lipopolysaccharide-induced chondrocytes injury in OA via the
regulation of TLR4 and PPARγ-mediated NF-κB signaling pathway. This
indicated that the activation of PPARγ effectively increases
anti-inflammatory and antiapoptotic effect in human OA
chondrocytes, through the depletion of Gal-3 (73). Jingbo et al (74) investigated the protective effect of
betulinic acid (BA; a triterpenoid isolated from birch bark)
against OA progression. It was suggested that BA inhibited
IL-1β-induced inflammation in OA chondrocytes by activating PPARγ
(74). Kang et al (75) reported that hyperglycemia-induced
cartilage degeneration induces OA. It was suggested that oleanolic
acid (OLA) prevents high-glucose-induced cartilage degeneration via
PPARγ-associated mitochondrial stabilization. It was also reported
that OLA treatment inhibited apoptosis and decreased SOD2 protein
degradation via PPARγ (75).
OA is the most prevalent chronic human health
disorder that is characterized by cartilage degeneration. It is a
leading cause of disability, which reduces mobility and increases
dependency (76,77). Due to the lack of PPARs playing a
critical role in the pathogenesis of OA, the activation of PPARs
using PPAR agonists may be interesting therapeutic targets for the
prevention of OA progression (78,79).
Investigation of novel physiological roles of PPARs and the
identification of specific PPAR agonists, which reduce the risk of
OA by limiting cartilage degeneration, may provide exciting
therapeutic strategies in the future. Moreover, the precise
molecular mechanisms through which PPARs exert their actions
require clarification. For example, the detailed signal
transduction mechanism from ligand binding (PPAR-agonists) to gene
transcription should be clarified. In addition, clinical
investigations on PPAR activation in patients with OA should be
performed for the establishment of this therapeutic approach.
OA is a slowly progressive disease that is becoming
a worldwide epidemic. Early identification and administration of
effective treatment, to inhibit the destructive or inflammatory
responses in cartilage, may be the best strategies against OA. A
better understanding of the pathogenic mechanisms may provide the
knowledge to identify new targets to develop therapeutic drugs for
OA. PPARs are affected in OA and targeting PPARs might be an
innovative approach for the treatment of OA. The use of selective
targets of PPARs may minimize the side effects and might be a
promising therapeutic avenue for the treatment of OA. More studies
are necessary to identify selective agonists for efficiently
targeting PPARs in the prevention and treatment of OA.
Not applicable.
The present study was supported by the National
Natural Science Foundation of China (grant no. 81902303), the
Guangdong Basic and Applied Basic Research Foundation (grant no.
2020A151501048), the Shenzhen Science and Technology Project (grant
nos. JCYJ20190806164216661, GJHZ20180416164801042 and
JCYJ20180305124912336) and the Clinical Research Project of Shezhen
Second People's Hospital (grant no. 20203357028).
Not applicable.
ZD and GH reviewed the design of the review, and
drafted and proofread the article. WJ created the figures and
revised the article. WX, WL and WZ participated in literature
collection, analysis and summary. ZD supervised the project. All
authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Cucchiarini M, de Girolamo L, Filardo G,
Oliveira JM, Orth P, Pape D and Reboul P: Basic science of
osteoarthritis. J Exp Orthop. 3:222016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hügle T and Geurts J: What drives
osteoarthritis?-synovial versus subchondral bone pathology.
Rheumatology (Oxford). 56:1461–1471. 2017.PubMed/NCBI
|
|
3
|
Nelson AE: Osteoarthritis year in review
2017: Clinical. Osteoarthritis Cartilage. 26:319–325. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xia B, Di Chen, Zhang J, Hu S, Jin H and
Tong P: Osteoarthritis pathogenesis: A review of molecular
mechanisms. Calcif Tissue Int. 95:495–505. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen D, Shen J, Zhao W, Wang T, Han L,
Hamilton JL and Im HJ: Osteoarthritis: Toward a comprehensive
understanding of pathological mechanism. Bone Res. 5:160442017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vargas Negrín F, Medina Abellán MD,
Hermosa Hernán JC and de Felipe Medina R: Treatment of patients
with osteoarthritis. Aten Primaria. 46 (Suppl 1):39–61. 2014.(In
Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun MM, Beier F and Pest MA: Recent
developments in emerging therapeutic targets of osteoarthritis.
Curr Opin Rheumatol. 29:96–102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chevalier X, Eymard F and Richette P:
Biologic agents in osteoarthritis: Hopes and disappointments. Nat
Rev Rheumatol. 9:400–410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mobasheri A and Batt M: An update on the
pathophysiology of osteoarthritis. Ann Phys Rehabil Med.
59:333–339. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu J, Liu W, Bemis A, Wang E, Qiu Y,
Morris EA, Flannery CR and Yang Z: Comparative proteomic
characterization of articular cartilage tissue from normal donors
and patients with osteoarthritis. Arthritis Rheum. 56:3675–3684.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boileau C, Martel-Pelletier J, Fahmi H,
Mineau F, Boily M and Pelletier JP: The peroxisome
proliferator-activated receptor gamma agonist pioglitazone reduces
the development of cartilage lesions in an experimental dog model
of osteoarthritis: In vivo protective effects mediated through the
inhibition of key signaling and catabolic pathways. Arthritis
Rheum. 56:2288–2298. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Monemdjou R, Vasheghani F, Fahmi H, Perez
G, Blati M, Taniguchi N, Lotz M, St-Arnaud R, Pelletier JP,
Martel-Pelletier J, et al: Association of cartilage-specific
deletion of peroxisome proliferator-activated receptor γ with
abnormal endochondral ossification and impaired cartilage growth
and development in a murine model. Arthritis Rheum. 64:1551–1561.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guan Y: Peroxisome proliferator-activated
receptor family and its relationship to renal complications of the
metabolic syndrome. J Am Soc Nephrol. 15:2801–2815. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dubois V, Eeckhoute J, Lefebvre P and
Staels B: Distinct but complementary contributions of PPAR isotypes
to energy homeostasis. J Clin Invest. 127:1202–1214. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
François M, Richette P, Tsagris L, Fitting
C, Lemay C, Benallaoua M, Tahiri K and Corvol MT: Activation of the
peroxisome proliferator-activated receptor alpha pathway
potentiates interleukin-1 receptor antagonist production in
cytokine-treated chondrocytes. Arthritis Rheum. 54:1233–1245. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kono K, Kamijo Y, Hora K, Takahashi K,
Higuchi M, Kiyosawa K, Shigematsu H, Gonzalez FJ and Aoyama T:
PPAR{alpha} attenuates the proinflammatory response in activated
mesangial cells. Am J Physiol Renal Physiol. 296:F328–F336. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sha W, Thompson K, South J, Baron M and
Leask A: Loss of PPARγ expression by fibroblasts enhances dermal
wound closure. Fibrogenesis Tissue Repair. 5:52012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Majdalawieh A and Ro HS: PPARgamma1 and
LXRalpha face a new regulator of macrophage cholesterol homeostasis
and inflammatory responsiveness, AEBP1. Nucl Recept Signal.
8:e0042010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tanaka T, Yamamoto J, Iwasaki S, Asaba H,
Hamura H, Ikeda Y, Watanabe M, Magoori K, Ioka RX, Tachibana K, et
al: Activation of peroxisome proliferator-activated receptor delta
induces fatty acid beta-oxidation in skeletal muscle and attenuates
metabolic syndrome. Proc Natl Acad Sci USA. 100:15924–15929. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tyagi S, Gupta P, Saini AS, Kaushal C and
Sharma S: The peroxisome proliferator-activated receptor: A family
of nuclear receptors role in various diseases. J Adv Pharm Technol
Res. 2:236–240. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Issemann I and Green S: Activation of a
member of the steroid hormone receptor superfamily by peroxisome
proliferators. Nature. 347:645–650. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Michalik L, Auwerx J, Berger JP,
Chatterjee VK, Glass CK, Gonzalez FJ, Grimaldi PA, Kadowaki T,
Lazar MA, O'Rahilly S, et al: International Union of Pharmacology.
LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev.
58:726–741. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zoete V, Grosdidier A and Michielin O:
Peroxisome proliferator-activated receptor structures: Ligand
specificity, molecular switch and interactions with regulators.
Biochim Biophys Acta. 1771:915–925. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guan Y and Breyer MD: Peroxisome
proliferator-activated receptors (PPARs): Novel therapeutic targets
in renal disease. Kidney Int. 60:14–30. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Michalik L and Wahli W: Involvement of
PPAR nuclear receptors in tissue injury and wound repair. J Clin
Invest. 116:598–606. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bougarne N, Paumelle R, Caron S, Hennuyer
N, Mansouri R, Gervois P, Staels B, Haegeman G and De Bosscher K:
PPARalpha blocks glucocorticoid receptor alpha-mediated
transactivation but cooperates with the activated glucocorticoid
receptor alpha for transrepression on NF-kappaB. Proc Natl Acad Sci
USA. 106:7397–7402. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qi C, Zhu Y and Reddy JK: Peroxisome
proliferator-activated receptors, coactivators, and downstream
targets. Cell Biochem Biophys. 32:187–204. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu S and Reddy JK: Transcription
coactivators for peroxisome proliferator-activated receptors.
Biochim Biophys Acta. 1771:936–951. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Balakumar P, Rose M, Ganti SS, Krishan P
and Singh M: PPAR dual agonists: Are they opening Pandora's Box?
Pharmacol Res. 56:91–98. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Berger J and Moller DE: The mechanisms of
action of PPARs. Annu Rev Med. 53:409–435. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oliveira AC, Bertollo CM, Rocha LT,
Nascimento EB Jr, Costa KA and Coelho MM: Antinociceptive and
antiedematogenic activities of fenofibrate, an agonist of PPAR
alpha, and pioglitazone, an agonist of PPAR gamma. Eur J Pharmacol.
561:194–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kytikova OY, Perelman JM, Novgorodtseva
TP, Denisenko YK, Kolosov VP, Antonyuk MV and Gvozdenko TA:
Peroxisome Proliferator-Activated Receptors as a Therapeutic Target
in Asthma. PPAR Res. 2020:89069682020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peraza MA, Burdick AD, Marin HE, Gonzalez
FJ and Peters JM: The toxicology of ligands for peroxisome
proliferator-activated receptors (PPAR). Toxicol Sci. 90:269–295.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ammazzalorso A, De Filippis B, Giampietro
L and Amoroso R: Blocking the peroxisome proliferator-activated
receptor (PPAR): An overview. ChemMedChem. 8:1609–1616.
2013.PubMed/NCBI
|
|
35
|
Ferré P: The biology of peroxisome
proliferator-activated receptors: Relationship with lipid
metabolism and insulin sensitivity. Diabetes. 53 (Suppl 1):S43–S50.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Racke MK and Drew PD: PPARs in
Neuroinflammation. PPAR Res. 2008:6383562008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Terauchi Y and Kadowaki T: PPAR and
diabetes. Nihon Rinsho. 63:623–629. 2005.(In Japanese). PubMed/NCBI
|
|
38
|
Fajas L, Debril MB and Auwerx J:
Peroxisome proliferator-activated receptor-gamma: From adipogenesis
to carcinogenesis. J Mol Endocrinol. 27:1–9. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gross B, Pawlak M, Lefebvre P and Staels
B: PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat Rev
Endocrinol. 13:36–49. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jones AB: Peroxisome
proliferator-activated receptor (PPAR) modulators: Diabetes and
beyond. Med Res Rev. 21:540–552. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gurnell M, Savage DB, Chatterjee VK and
O'Rahilly S: The metabolic syndrome: Peroxisome
proliferator-activated receptor gamma and its therapeutic
modulation. J Clin Endocrinol Metab. 88:2412–2421. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Korbecki J, Bobiński R and Dutka M:
Self-regulation of the inflammatory response by peroxisome
proliferator-activated receptors. Inflamm Res. 68:443–458. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hou Y, Moreau F and Chadee K: PPARγ is an
E3 ligase that induces the degradation of NFκB/p65. Nat Commun.
3:13002012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Scirpo R, Fiorotto R, Villani A, Amenduni
M, Spirli C and Strazzabosco M: Stimulation of nuclear receptor
peroxisome proliferator-activated receptor-γ limits NF-κB-dependent
inflammation in mouse cystic fibrosis biliary epithelium.
Hepatology. 62:1551–1562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang S, Awad KS, Elinoff JM, Dougherty EJ,
Ferreyra GA, Wang JY, Cai R, Sun J, Ptasinska A and Danner RL: G
protein-coupled receptor 40 (GPR40) and peroxisome
proliferator-activated receptor γ (PPARγ): An Integrated
Two-Receptor Signaling Pathway. J Biol Chem. 290:19544–19557. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Badr MZ: PPAR research: Successful
launching and promising future. PPAR Res. 2009:5435842009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Colin C, Salamone S, Grillier-Vuissoz I,
Boisbrun M, Kuntz S, Lecomte J, Chapleur Y and Flament S: New
troglitazone derivatives devoid of PPARγ agonist activity display
an increased antiproliferative effect in both hormone-dependent and
hormone-independent breast cancer cell lines. Breast Cancer Res
Treat. 124:101–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Grommes C, Landreth GE and Heneka MT:
Antineoplastic effects of peroxisome proliferator-activated
receptor gamma agonists. Lancet Oncol. 5:419–429. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fu J, Gaetani S, Oveisi F, Lo Verme J,
Serrano A, Rodríguez De Fonseca F, Rosengarth A, Luecke H, Di
Giacomo B, Tarzia G, et al: Oleylethanolamide regulates feeding and
body weight through activation of the nuclear receptor PPAR-alpha.
Nature. 425:90–93. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Michalik L, Desvergne B, Tan NS,
Basu-Modak S, Escher P, Rieusset J, Peters JM, Kaya G, Gonzalez FJ,
Zakany J, et al: Impaired skin wound healing in peroxisome
proliferator-activated receptor (PPAR)alpha and PPARbeta mutant
mice. J Cell Biol. 154:799–814. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee HY, Gao X, Barrasa MI, Li H, Elmes RR,
Peters LL and Lodish HF: PPAR-α and glucocorticoid receptor
synergize to promote erythroid progenitor self-renewal. Nature.
522:474–477. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lee JM, Wagner M, Xiao R, Kim KH, Feng D,
Lazar MA and Moore DD: Nutrient-sensing nuclear receptors
coordinate autophagy. Nature. 516:112–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Blanco FJ, Rego I and Ruiz-Romero C: The
role of mitochondria in osteoarthritis. Nat Rev Rheumatol.
7:161–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gavriilidis C, Miwa S, von Zglinicki T,
Taylor RW and Young DA: Mitochondrial dysfunction in osteoarthritis
is associated with down-regulation of superoxide dismutase 2.
Arthritis Rheum. 65:378–387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Clockaerts S, Bastiaansen-Jenniskens YM,
Feijt C, Verhaar JA, Somville J, De Clerck LS and Van Osch GJ:
Peroxisome proliferator activated receptor alpha activation
decreases inflammatory and destructive responses in osteoarthritic
cartilage. Osteoarthritis Cartilage. 19:895–902. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ratneswaran A, LeBlanc EA, Walser E, Welch
I, Mort JS, Borradaile N and Beier F: Peroxisome
proliferator-activated receptor δ promotes the progression of
posttraumatic osteoarthritis in a mouse model. Arthritis Rheumatol.
67:454–464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Vasheghani F, Monemdjou R, Fahmi H, Zhang
Y, Perez G, Blati M, St-Arnaud R, Pelletier JP, Beier F,
Martel-Pelletier J, et al: Adult cartilage-specific peroxisome
proliferator-activated receptor gamma knockout mice exhibit the
spontaneous osteoarthritis phenotype. Am J Pathol. 182:1099–1106.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Vasheghani F, Zhang Y, Li YH, Blati M,
Fahmi H, Lussier B, Roughley P, Lagares D, Endisha H, Saffar B, et
al: PPARγ deficiency results in severe, accelerated osteoarthritis
associated with aberrant mTOR signalling in the articular
cartilage. Ann Rheum Dis. 74:569–578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang Y, Zhao X, Lotz M, Terkeltaub R and
Liu-Bryan R: Mitochondrial biogenesis is impaired in osteoarthritis
chondrocytes but reversible via peroxisome proliferator-activated
receptor γ coactivator 1α. Arthritis Rheumatol. 67:2141–2153. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cordani N, Pisa V, Pozzi L, Sciorati C and
Clementi E: Nitric oxide controls fat deposition in dystrophic
skeletal muscle by regulating fibro-adipogenic precursor
differentiation. Stem Cells. 32:874–885. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Reggio A, Spada F, Rosina M, Massacci G,
Zuccotti A, Fuoco C, Gargioli C, Castagnoli L and Cesareni G: The
immunosuppressant drug azathioprine restrains adipogenesis of
muscle Fibro/Adipogenic Progenitors from dystrophic mice by
affecting AKT signaling. Sci Rep. 9:43602019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Cerquone Perpetuini A, Giuliani G, Reggio
A, Cerretani M, Santoriello M, Stefanelli R, Palma A, Vumbaca S,
Harper S, Castagnoli L, et al: Janus effect of glucocorticoids on
differentiation of muscle fibro/adipogenic progenitors. Sci Rep.
10:53632020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Reggio A, Rosina M, Palma A, Cerquone
Perpetuini A, Petrilli LL, Gargioli C, Fuoco C, Micarelli E,
Giuliani G, Cerretani M, et al: Adipogenesis of skeletal muscle
fibro/adipogenic progenitors is affected by the
WNT5a/GSK3/β-catenin axis. Cell Death Differ. 27:2921–2941. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Boileau C, Martel-Pelletier J, Fahmi H,
Mineau F, Boily M and Pelletier JP: The peroxisome
proliferator-activated receptor gamma agonist pioglitazone reduces
the development of cartilage lesions in an experimental dog model
of osteoarthritis: In vivo protective effects mediated through the
inhibition of key signaling and catabolic pathways. Arthritis
Rheum. 56:2288–2298. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Fahmi H, Martel-Pelletier J, Pelletier JP
and Kapoor M: Peroxisome proliferator-activated receptor gamma in
osteoarthritis. Mod Rheumatol. 21:1–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Giaginis C, Giagini A and Theocharis S:
Peroxisome proliferator-activated receptor-gamma (PPAR-gamma)
ligands as potential therapeutic agents to treat arthritis.
Pharmacol Res. 60:160–169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hellio Le Graverand-Gastineau MP: OA
clinical trials: current targets and trials for OA. Choosing
molecular targets: what have we learned and where we are headed?
Osteoarthritis Cartilage. 17:1393–1401. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Malemud CJ: Biologic basis of
osteoarthritis: State of the evidence. Curr Opin Rheumatol.
27:289–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kobayashi T, Notoya K, Naito T, Unno S,
Nakamura A, Martel-Pelletier J and Pelletier JP: Pioglitazone, a
peroxisome proliferator-activated receptor gamma agonist, reduces
the progression of experimental osteoarthritis in guinea pigs.
Arthritis Rheum. 52:479–487. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Afif H, Benderdour M, Mfuna-Endam L,
Martel-Pelletier J, Pelletier JP, Duval N and Fahmi H: Peroxisome
proliferator-activated receptor gamma1 expression is diminished in
human osteoarthritic cartilage and is downregulated by
interleukin-1beta in articular chondrocytes. Arthritis Res Ther.
9:R312007. View
Article : Google Scholar : PubMed/NCBI
|
|
71
|
Qu Y, Zhou L and Wang C: Mangiferin
inhibits IL-1β-induced inflammatory response by activating PPAR-γ
in human osteoarthritis chondrocytes. Inflammation. 40:52–57. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wang K, Han L, Zhu Y, Liu Y, Wang J and
Xue C: Antarctic Krill Oil improves articular cartilage
degeneration via activating chondrocyte autophagy and inhibiting
apoptosis in osteoarthritis mice. J Funct Foods. 46:413–422. 2018.
View Article : Google Scholar
|
|
73
|
Wang JS, Xiao WW, Zhong YS, Li XD, Du SX,
Xie P, Zheng GZ and Han JM: Galectin-3 deficiency protects
lipopolysaccharide-induced chondrocytes injury via regulation of
TLR4 and PPAR-γ-mediated NF-κB signaling pathway. J Cell Biochem.
120:10195–10204. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Jingbo W, Aimin C, Qi W, Xin L and
Huaining L: Betulinic acid inhibits IL-1β-induced inflammation by
activating PPAR-γ in human osteoarthritis chondrocytes. Int
Immunopharmacol. 29:687–692. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kang X, Yang Z, Sheng J, Liu JB, Xie QY,
Zheng W and Chen K: Oleanolic acid prevents cartilage degeneration
in diabetic mice via PPARγ associated mitochondrial stabilization.
Biochem Biophys Res Commun. 490:834–840. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Karsdal MA, Michaelis M, Ladel C, Siebuhr
AS, Bihlet AR, Andersen JR, Guehring H, Christiansen C, Bay-Jensen
AC and Kraus VB: Disease-modifying treatments for osteoarthritis
(DMOADs) of the knee and hip: Lessons learned from failures and
opportunities for the future. Osteoarthritis Cartilage.
24:2013–2021. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Vitale ND, Vandenbulcke F, Chisari E,
Iacono F, Lovato L, Di Matteo B and Kon E: Innovative regenerative
medicine in the management of knee OA: The role of Autologous
Protein Solution. J Clin Orthop Trauma. 10:49–52. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Stienstra R, Mandard S, Patsouris D, Maass
C, Kersten S and Müller M: Peroxisome proliferator-activated
receptor alpha protects against obesity-induced hepatic
inflammation. Endocrinology. 148:2753–2763. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhou JL, Liu SQ, Qiu B, Hu QJ, Ming JH and
Peng H: The protective effect of sodium hyaluronate on the
cartilage of rabbit osteoarthritis by inhibiting peroxisome
proliferator-activated receptor-gamma messenger RNA expression.
Yonsei Med J. 50:832–837. 2009. View Article : Google Scholar : PubMed/NCBI
|