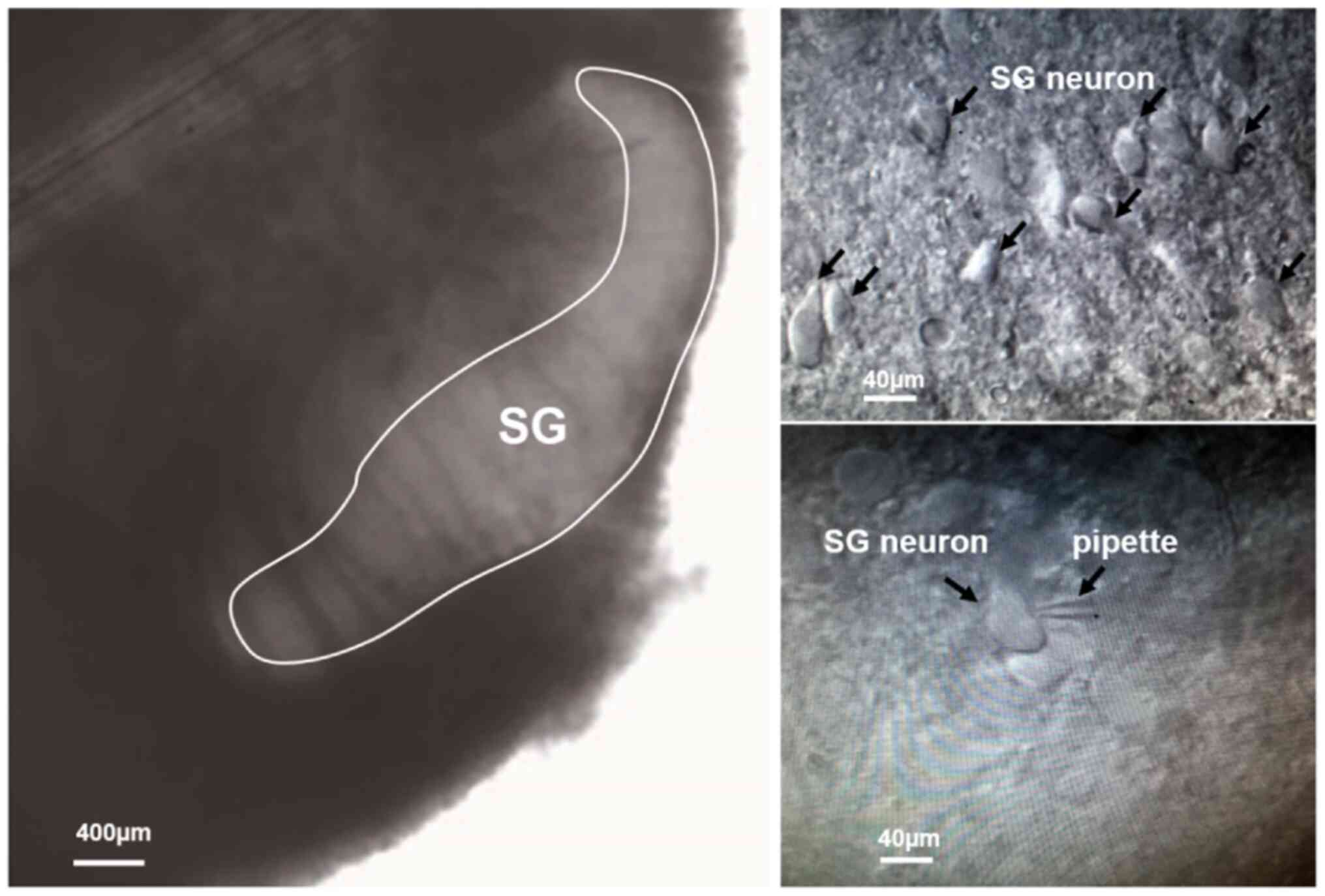
Introduction
Electroacupuncture (EA) is used to treat neuropathic
pain (NP) induced by peripheral nerve injury (PNI) (1,2). To
the best of our knowledge, however, the mechanism has not yet been
identified. Previous studies have noted that neuronal activities
are associated with neurotransmitters and neuromodulators, such as
opioids, interleukins, chemokines, serotonin and adenosine, and
that the appearance and persistence of NP depends on hyperactive
microglia (3–8). Suppression of microglia activation
attenuates pain induced by nerve injury (9,10).
Previous studies have demonstrated that the P2X4 receptor (P2X4R)
triggers allodynia following PNI, and that relief of NP occurs both
in mice injected intrathecally with a P2X4R antisense
oligonucleotide and in mice lacking P2X4R (8,11). ATP
is a transmitter that conveys sensory information between
hyperactive microglia and nociceptive neurons. Hyperactive
microglia induce or promote increased expression levels of P2X4R
(8). In response to extracellular
ATP, P2X4R can mediate a number of effects, such as the production
and diffusion of bioactive factors, including cytokines and
neurotrophic factors that can induce depolarization of dorsal horn
sensory neurons (12). The
physiological process of EA analgesia involves numerous
transmitters and modulators, including acetylcholine opioid
peptides, substance P, glutamate, γ-amino-butyric acid and other
associated peptides (13). Previous
studies have indicated that EA analgesia is associated with
decreased purine and purinergic receptors, especially P2X4R
(3,14). In light of this association, EA
treatment may have a role in the relief of NP, which may be
mediated in part by P2X4R in microglia in the spinal dorsal horn
(SDH) (15,16). The present study demonstrated that
the pain behavior and expression levels of P2X4R in microglia in
the SDH were altered in spinal nerve ligation (SNL) rats.
Whole-cell patch clamp techniques were used to investigate the
variation in the frequency of spontaneous excitatory postsynaptic
currents (sEPSC) in SNL rat spinal substantia gelatinosa (SG)
neurons. Studies have demonstrated that EA treatment at ‘Zusanli’
(ST-36, at the posterolateral aspect of the knee joint, ~5 mm below
the humeral head) and ‘Kunlun’ (BL-60, ~10 mm above the prominence
of the lateral malleolus of the hind limb) points can relieve
neuropathic pain, but its specific mechanism has not yet been
elucidated (1,2). The aim of the present study was to
investigate the potential mechanism by which EA treatment at
‘Zusanli’ and ‘Kunlun’ points relieves NP via the action on
P2X4R.
Materials and methods
Experimental animals
The Institutional Animal Care and Use Committee of
Wenzhou Medical University approved all experiments. A total of 72
male Sprague-Dawley rats (weight, 180–200 g; age, 6–8 weeks),
bought from Wenzhou Medical University (Wenzhou, China), were kept
on a standard laboratory diet at room temperature (20–22°C) and
12-h alternative light-dark cycle conditions in a pathogen-free
room. The behavioral experiment was performed between 2:00 p.m. and
4:00 p.m. All experimental rats were randomly distributed into four
groups (n=6): Control, sham, SNL and ipsilateral EA groups. All
surgical procedures were performed using a microscope (Leica S8
APO; Leica Microsystems, Ltd; magnification, ×4). The experimental
rats were anesthetized with 5% chloral hydrate [350 mg/kg,
intraperitoneal (i.p.)]. SNL surgery was performed as previously
described (17–19). Briefly, an incision was made in the
midline lumbar region of animals placed in a prone position. In
order to expose the right L4-L5 spinal nerves completely, the right
L5 vertebral transverse was cut. Following right L5 spinal nerve
separation, it was ligated with 5-0 silk, and the incision was
closed. In the sham group, the right L5 spinal nerve was exposed
but not ligated. Pain thresholds were measured at days 0, 3, 5, 7,
10, 12 and 14 post-SNL.
Behavioral tests
The EA treatment time was fixed at 9:00-10:00 a.m.
MWT and TWL tests time were fixed at 2:00-4:00 p.m. The Electronic
von Frey anesthesiometer (IITC Life Science Inc.) was used to
measure MWT to judge mechanical hyperalgesia. The experimental rats
were allowed to acclimatize in the wire mesh-bottom cages (20×14×16
cm) for 30 min. The test probe was positioned at the base of third
and fourth toes, and the pressure of the Electronic von Frey
anesthesiometer was set to 0.1–70.0 g. Both lifting and licking the
paw were considered to be positive responses. The maximum pressure
was also recorded. Each hind paw was tested alternately six times
at 5 min intervals. The average value was used for statistical
analysis. Following the MWT test, rats were placed in a square,
transparent, bottomless acrylic box (16.0×12.5×14.0 cm) and allowed
to acclimatize for 15 min before being subjected to a TWL test. The
TWL was used to test thermal hyperalgesia using Plantar Test
apparatus (Ugo-Basile S.R.L.) (13). The infrared source was set at 60°C
under a glass plate and directed towards the plantar surface of the
hind paw. Withdrawal of the paw led to the infrared source breaking
off, at which point latency was measured. The hind paw of the
experimental rats was tested five times at 15-min intervals and the
TWL was expressed as the mean value.
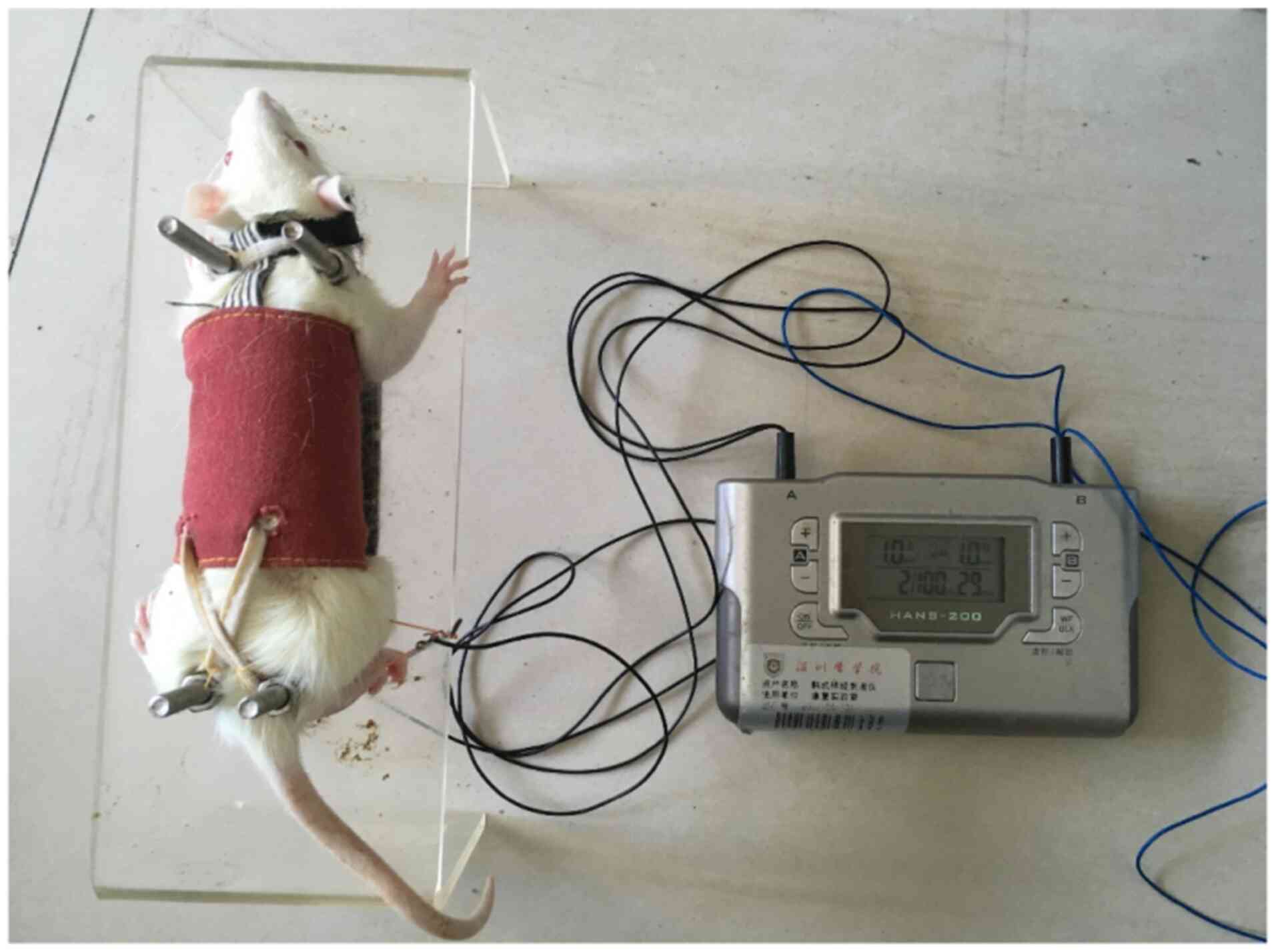
EA treatment
EA treatment was started on day 7 post-SNL in the EA
groups. The rats were maintained in fixation equipment (patent no.
201110021482.5; State Intellectual Property Office) (13). Acupuncture needles were
percutaneously inserted 2–3 mm at the Zusanli and Kunlun points. EA
stimulus (2/100 Hz; 1.5 mA) was delivered using an electrical
stimulation device (HANS-200E; Nanjing Jisheng Medical Technology,
Ltd.) for 30 min daily. The intensity was set at 1 mA, and the
total stimulation period was 30 min for 7 days, which ensured the
best curative effect of EA (Fig.
1).
Reverse transcription-quantitative
PCR
Real-time amplification using SYBR-Green Supermix
(Toyobo Life Science) and a Light Cycler 480 system (Roche
Diagnostics GmbH) was performed using 4 ng cDNA extracted from
L4-L5 segments with TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. RNA
preparation and cDNA synthesis were performed as previously
described (20). The PCR conditions
consisted of an initial melting cycle at 95°C for 15 min, followed
by 40 cycles of amplification at 95°C for 15 sec (denaturation),
60°C for 30 sec (annealing) and 72°C for 30 sec (extension).
Primers were procured from Invitrogen (Thermo Fisher Scientific,
Inc.): P2X4R forward, 5′-GGGTGAAGTTTTATTCCAGC-3′; P2X4R reverse,
5′-GGGTGAAGTTTTCTGCAGCC-3′; GAPDH forward,
5′-CTTCACCACCATGGAGAAGGC-3′; and GAPDH reverse,
5′-GGCATGGACTGTGGTCATGAG-3′. The quantification values were
obtained from the quantification cycle (Cq) number at which the
increase in the signal was associated with exponential growth of
the PCR products. All samples were run in triplicate and repeated
three times. RPS16 quantification was used as an internal control
for normalization. Fold differences in mRNA levels over vehicle
control were calculated using the 2−ΔΔCq method
(21).
Western blotting
Western blotting was performed as previously
described (22) with minor
modifications. The rats were deeply anesthetized with i.p.
injection of 30 mg/kg pentobarbital sodium. The Rat Anesthesia
Guidelines of University Minnesota (https://www.researchservices.umn.edu/services-name/research-animal-resources/research-support/guidelines/anesthesia-rats)
were used to determine when the rat had entered deep anesthesia.
Corneal reflexes were observed to disappear in the rat eye. Rats
were observed to have no response after lightly clamping the fourth
toe with tweezers, thereby confirming that rats had entered a state
of deep anesthesia. The rats were sacrificed by decapitation. The
proteins extracted from L4-L5 segments were quantified using a
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology). The proteins (42 kDa actin and 62 kDa P2X4R) were
subjected to 8% SDS-PAGE and transferred to a PVDF membrane. The
membranes were blocked with 5% non-fat milk for 2 h at 4°C and
incubated overnight at 4°C with anti-rat P2X4R polyclonal antibody
(1:1,000; Alomone Labs) or GAPDH antibody (1:4,000; Sigma-Aldrich,
Merck). Membranes were subsequently incubated with horseradish
peroxidase (HRP)-conjugated secondary antibody (goat anti-rabbit
IgG; 1:3,000; cat. no. 7074; Cell Signaling Technology, Inc.) for 2
h at 4°C. These membranes were washed 3 times with TBST (0.1%
Tween-20) (5 min/time) after incubating with HRP-conjugated
antibody. The bands were detected using the ECL method (BeyoECL
Plus; cat. no. P0018S; Beyotime Institute of Biotechnology) and
exposed to radiography films.
Immunofluorescence staining
Immunofluorescence staining was performed as
previously described with minor modifications (23). On day 14 post-SNL, the rats were
anesthetized with 10% chloral hydrate (350 mg chloral hydrate/kg).
The rats exhibited no signs of peritonitis following administration
of chloral hydrate. The rats were perfused through the ascending
aorta with physiological saline. Subsequently, rats were fixed with
4% paraformaldehyde for 4 h in 0.1 M phosphate buffer at pH
7.2-7.4, 4°C. Following fixation, the heartbeat disappeared and the
body was stiff. The lumbosacral section was dehydrated, cleared and
embedded in paraffin for transverse paraffin sections. Transverse
spinal cord sections (5 µm) were excised and mounted on
poly-L-lysine-coated slides. Sections were deparaffinized and
rehydrated in descending alcohol series. Then, sections were
immersed in antigen repair buffer (sodium citrate; pH 6.0) and
heated in a microwave oven at 100°C for 20 min and allowed to cool
naturally. The slides were blocked with 3%
H2O2 for 10 min at room temperature and 10%
normal goat serum (Gibco; Thermo Fisher Scientific, Inc.) with 0.3%
Triton X-100 in PBS for 1 h at 4°C. The sections were incubated
with rabbit anti-P2X4 (1:200; cat. no. 13534-1-AP; ProteinTech
Group Inc.) and mouse anti-ionized calcium-binding adapter molecule
1 (Iba-1; 1:400; cat. no. ab15690; Abcam) antibodies for 16 h at
4°C. The secondary antibodies were tetraethyl rhodamine
isothiocyanate (1:1,000; cat. no. AP31444TC-N; OriGene
Technologies, Inc.) conjugated to rabbit anti-P2X4 IgG and
fluorescein conjugated to goat anti-mouse IgG (1:5,000; cat. no.
BL003A; Biosharp Life Sciences), and the incubation was performed
for 1 h at 37°C. Slides were washed three times (5 min/time) with
PBS and incubated with DAPI staining solution (1:1,000; cat. no.
C1005; Beyotime Institute of Biotechnology) for 10 min at 25°C,
then washed a further three times with PBS (5 min/time). Images
were captured using a BX41 fluorescence microscope (Olympus
Corporation; magnification, ×10 and ×40). Image-Pro Plus software
(version 5.1; Media Cybernetics, Inc.) was used to determine the
staining intensity.
Section preparation
The spinal cord sections from rats were prepared as
previously described (13,24). Briefly, rats were anesthetized as
aforementioned, then transcardially perfused with ~70 ml of
ice-cold, oxygenated (95% O2, 5% CO2) cutting
solution containing: 105.0 N-methyl-D-glucamine, 105.0 HCl, 2.5.0
KCl, 1.2 NaH2PO4, 26.0 NaHCO3,
25.0 glucose, 10.0 MgSO4, 0.5 CaCl2, 5.0
L-ascorbic acid, 3.0 sodium pyruvate and 2.0 mM thiourea (pH 7.4,
295–305 mOsm). The lumbosacral section was removed in the cutting
solution. All ventral and dorsal roots were cut and the
pia-arachnoid membrane was removed. Transverse spinal sections (300
µm) were cut using a vibratome (VT1200S; Leica Microsystems, Ltd.)
and placed in an incubator filled with normal oxygenated (95%
O2, 5% CO2) Krebs solution for at least 30
min at 32°C. The normal Krebs solution contained: 117.0 NaCl, 3.6
KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2
NaH2PO4, 25.0 NaHCO3, 11.0
D-glucose, 0.4 ascorbic acid and 2.0 mM pyruvate.
Patch-clamp recordings
The patch-clamp recording procedures were performed
as previously described (24,25).
Specifically, the section was placed in a recording chamber beneath
a BX51W1 upright light microscope (Olympus Corporation;
magnification, ×20). The procedure of tight-seal, whole-cell
patch-clamp and the recordings were performed at room temperature
(22–24°C) with artificial cerebrospinal fluid perfusion. SG neurons
were identified using an infrared and differential interference
contrast camera (cat. no. BX51WI; Olympus Corporation;
magnification, ×20 and ×100). The recording pipettes were made from
borosilicate glass capillaries (optical density, 1.5 mm; inner
diameter, 1.12 mm; Sutter Instrument Company) with a micropipette
puller (P-97; Sutter Instrument Company) and had a resistance of
4–6 MΩ when filled with a solution containing: 130.0 K-gluconate,
5.0 KCl, 4.0 Mg-ATP, 10.0 phosphocreatine, 0.3 Li-GTP and 10.0 mM
HEPES (pH 7.4 adjusted with KOH, 300 mOsm). The frequency of
spontaneous excitatory postsynaptic currents (sEPSC) was recorded
using an EPC-10 amplifier with a lowpass filter at 5 kHz using
Patchmaster software (version UI325; HEKA Elektronik GmbH; Harvard
Bioscience, Inc.).
Statistical analysis
Statistical significance was determined using SPSS
Statistics software (version 16.0; SPSS, Inc.). Data are presented
as the mean ± standard error of the mean of three experimental
repeats. Behavioral results with multiple comparisons were
statistically analyzed by a mixed analysis of variance (ANOVA) for
repeated measures, followed by Sidak's test. The other data were
carried out using one-way analysis of variance, followed by Tukey's
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
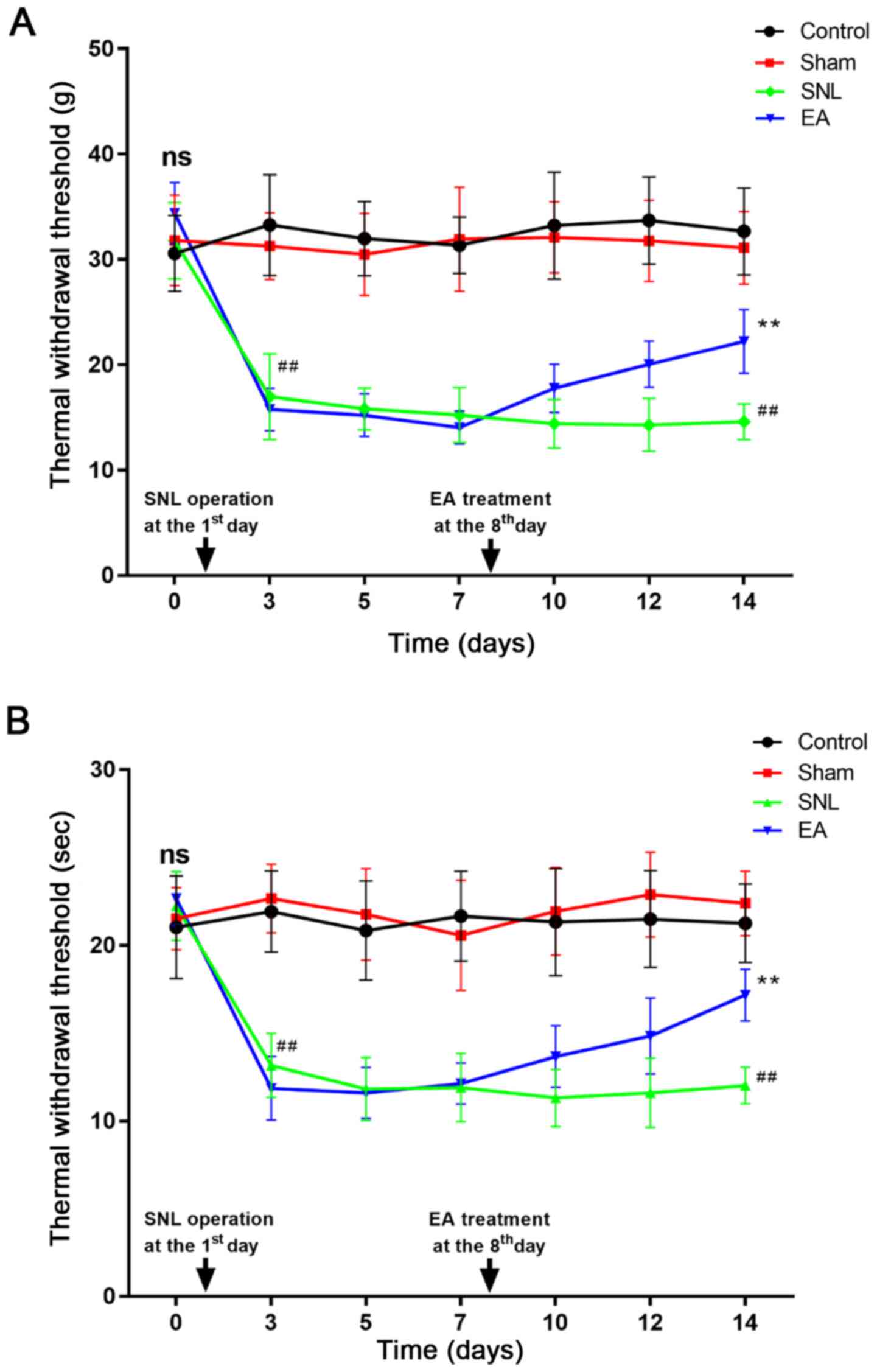
EA reverses SNL-induced mechanical
allodynia and thermal hyperalgesia
Baseline measures of MWT and TWL did not differ
between groups (Fig. 2). MWT and
TWL were recorded on day 3 post-SNL to avoid measuring the effects
of postoperative pain, as previously described (13). Mechanical allodynia and thermal
allodynia developed at day 3 post-SNL and were sustained until day
14. In the EA groups, all rats were tested 30 min post-EA
treatment. As presented in Fig. 2A and
B, the values of MWT and TWL in SNL rats notably decreased from
day 3 to day 14 post-SNL compared with the control and sham groups
(P<0.01). In the EA groups, the values of MWT and TWL notably
increased from day 7 to day 14 compared with those of the SNL
groups (P<0.01). The results indicate that EA relieved pain
behavior in SNL rats.
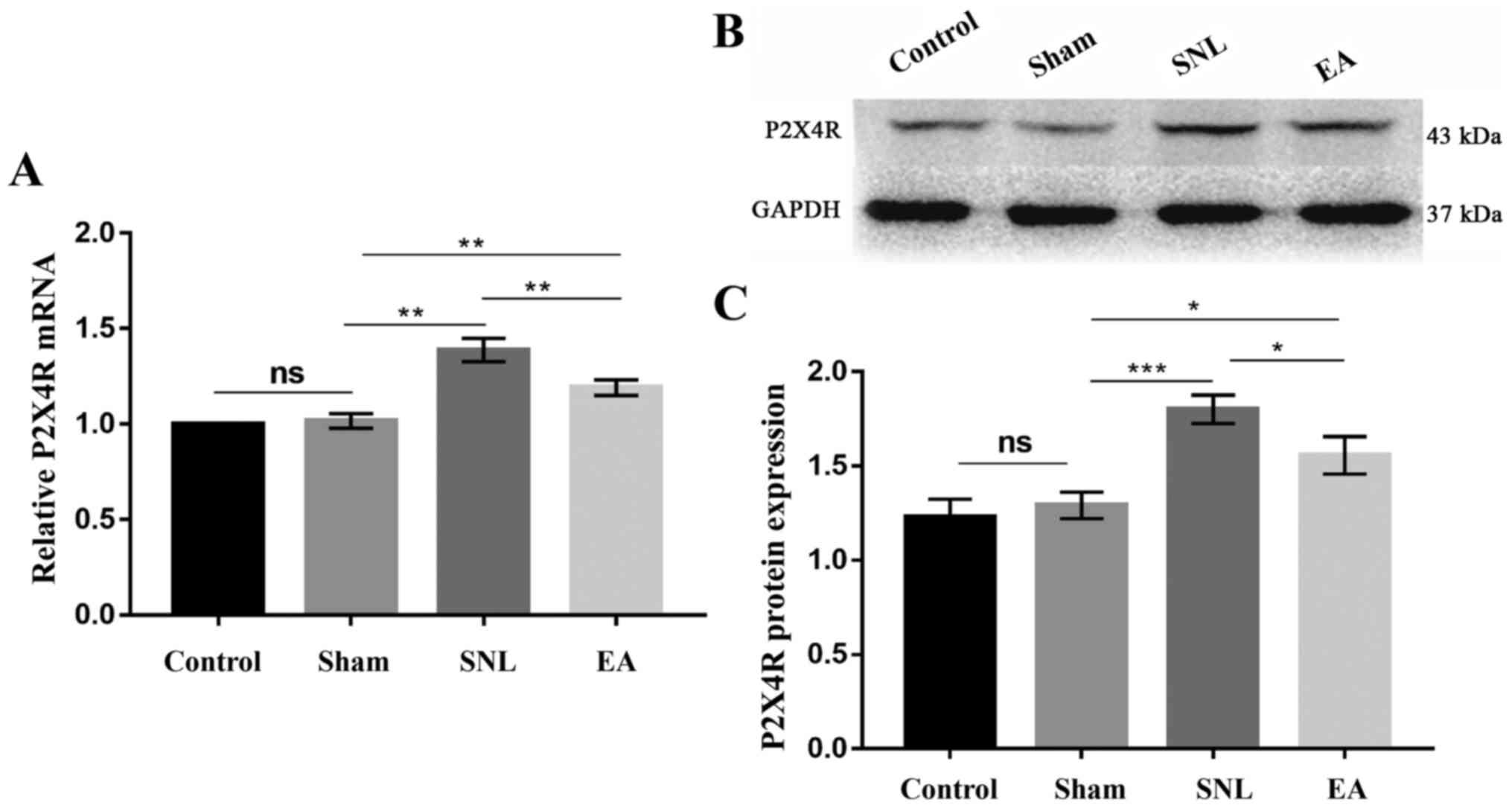
EA decreases expression levels of
P2X4R mRNA and protein in the spinal cord of SNL rats
In order to detect the effect of EA and to
demonstrate the role of P2X4R in maintaining NP, the mRNA and
protein levels of P2X4R in the spinal cord were investigated. As
presented in Fig. 3A, P2X4R mRNA
expression levels in the SNL group were higher than those in the
control and sham groups (P<0.01). However, the relative
expression levels of P2X4R mRNA in the EA group decreased in
comparison with those in the SNL group (P<0.01) following 7 days
of EA treatment. The results of P2X4R protein expression level
analysis are presented in Fig. 3B and
C. The relative expression levels of P2X4R protein in the
control, sham, SNL and EA groups were 1.229±0.043, 1.291±0.031,
1.800±0.034 and 1.557±0.044, respectively. The SNL group exhibited
upregulated P2X4R protein levels compared with those in the control
and sham groups (P<0.001). The P2X4R protein levels in the EA
group were significantly lower than those in the SNL group
(P<0.05). The results indicate that EA inhibited the
upregulation of P2X4R protein expression levels in SNL rats.
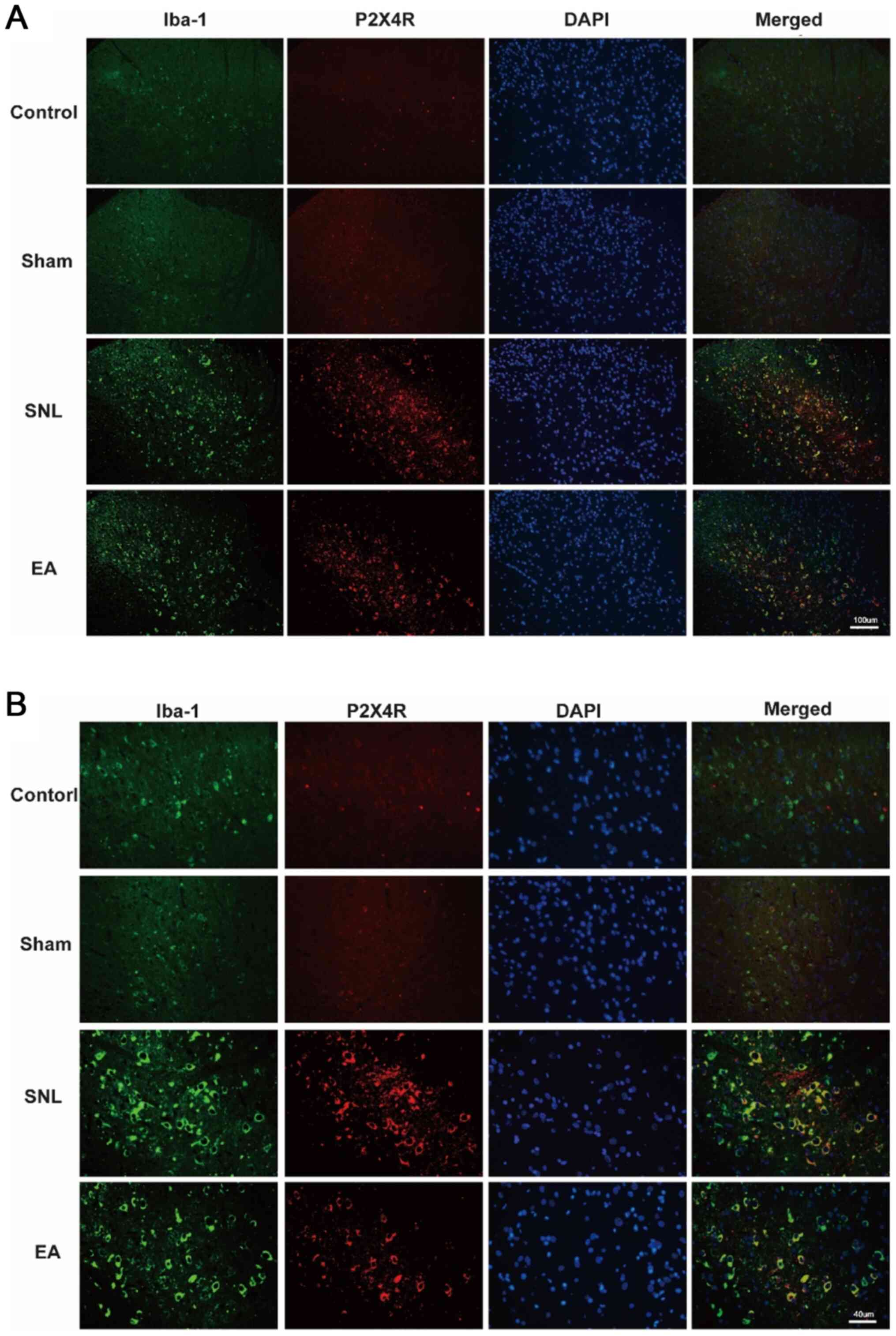
EA decreases immunofluorescence
staining of P2X4R and Iba-1
Upregulation of ionized calcium-binding adapter
molecule 1 (Iba-1) is a marker of microglia activation (26). The results of double
immunofluorescence staining are presented in Fig. 4A (magnification, ×20) B
(magnification, ×40). The co-expression of P2X4R and Iba-1 was
notable in the SNL group in contrast with the control group. The
number of P2X4R+ microglia in the EA group was
significantly lower following EA treatment. These results indicate
that EA inhibited microglia activation and suppressed the
expression levels of the P2X4R receptor.
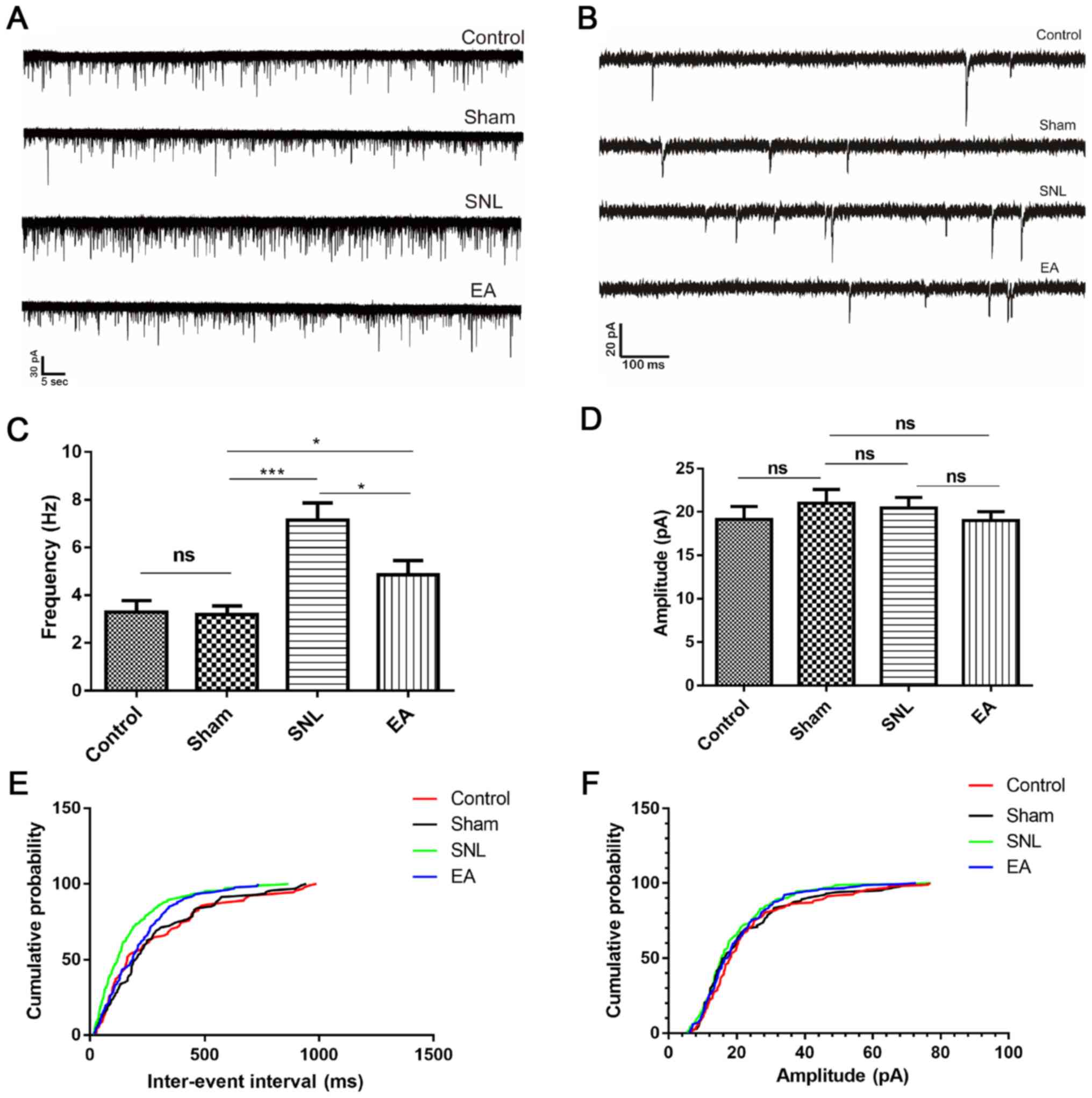
EA decreases the frequency of sEPSCs
in spinal cord SG neurons in the SNL group
Spinal cord SG neurons are predominantly excitatory
neurons (24) and form a
nociceptive circuit, which receives input from afferent C-fibers
and sends output to lamina I projection neurons (27). It was hypothesized that EA may
modulate neurotransmitter release and synaptic transmission by
increasing expression levels of P2X4R in the spinal cord. The
present study recorded sEPSCs in SG neurons in spinal cord sections
from rats (Figs. 5 and 6A and B). The SNL group exhibited an
enhanced frequency of sEPSCs in SG neurons (P<0.001; Fig. 6C and E) compared with the control
and sham groups. In the EA group the frequency of sEPSCs was
significantly decreased (P<0.05; Fig. 6C and E) but the amplitude of sEPSCs
was not significantly altered (P>0.05; Fig. 6D and F) compared with the SNL
group.
Glutamate AMPA/kainate receptors mediate sEPSCs, and
excitatory synaptic transmission causes frequency changes in sEPSCs
(28–30). Therefore, EA may inhibit excitatory
synaptic transmission by decreasing glutamate release from
presynaptic terminals, which may result in EA-induced suppression
of P2X4R expression levels in microglia.
Discussion
The results of the present study demonstrated that
EA treatment alleviated nerve injury-induced tactile allodynia and
thermal hyperalgesia by inhibiting activation of spinal
microglia-mediated P2X4R and by regulating the excitability of
neurons in the SG region of SNL rats. The findings of the present
study demonstrated the underlying mechanisms of the therapeutic
effect of EA on NP in regards to purinergic receptor family
modulation.
Acupuncture is used worldwide as a treatment for a
number of conditions, particularly for pain (4,31).
Zusanli and Kunlun points, first described in
‘HuangDiNeiJing·LingShu·BenShu’ (an ancient Chinese book, recorded
in 200 BC), are commonly used acupoints to treat a number of
symptoms (including pain relief) both in clinical practice and in
research. It has been reported that EA stimulates the Zusanli point
to relieve NP via inhibition of COX2 expression levels, activation
of opioid receptors M1 mAChR, β2 nAChR and endothelin-B receptors,
and secretion of neuroactive mediators (32–34).
Studies have also demonstrated that acupuncture at the Kunlun point
can alleviate NP by inhibiting the p38 MAPK pathway and the
expression levels of prostaglandin E2 and G protein-coupled kinase
2 (35,36). In the present study, increased
sensitivity to thermal and mechanical stimulation was observed in
SNL rats. SNL rats exhibit abnormal hyperalgesia and mechanical
irritation, which is similar to human NP symptoms and behavior
induced by injury and dysfunction of the peripheral nervous system
(37). In the present study, the
MWT and TWL in the EA group were significantly increased compared
with the SNL group, indicating that EA may relieve mechanical pain
and thermal pain in SNL rats. These findings indicated that EA
treatment at the Zusanli and Kunlun points may be beneficial in the
treatment of NP.
Spinal microglia have been demonstrated to be
immediately activated following nerve injury and are necessary for
the initiation and maintenance of pain hypersensitivity (38). The ATP receptors P2X4R and P2X7R
have been demonstrated to be predominately expressed in the
microglia of the spinal cord (11,39,40).
Furthermore, a previous study has demonstrated that DRG P2X3R is
involved in the analgesic effect of EA in rat models of chronic
constriction injury (13).
Following binding of ATP, microglial ionotropic P2X4R leads to
increased microglia activation, which exaggerates pain states
(41). Inhibition of spinal
P2X4R+ microglia significantly alleviates tactile
allodynia induced by nerve injury but not that induced by thermal
hyperalgesia (11,42). Moreover, P2X4R knockout has been
demonstrated to increase sensitivity to thermal hyperalgesia in an
inflammatory mouse model (43). In
the present study, the expression levels of P2X4R protein and mRNA
were notably decreased compared with those in SNL rats following EA
treatment. Therefore, the effects of EA on NP may be associated
with the expression levels of P2X4R. Moreover, the results of the
present study indicate that P2X4R was co-expressed with Iba-1 in
the SDH. These results are consistent with the results of previous
studies (11,44). The present study also demonstrated
that co-expression of P2X4R and Iba-1 in the SDH of SNL rats was
increased compared with that in control rats. Upregulated Iba-1 was
associated with the activation of microglia. The results of the
present study indicated that microglia were activated following
PNI. Following EA treatment, the co-expression of P2X4R with Iba-1
in the SDH was decreased compared with that in the SNL group. EA
may attenuate the transmission of nociceptive information by
inhibiting the expression levels of P2X4R in SDH microglia, thus
relieving pain behaviors in SNL rats.
sEPSCs were the most important indicator reflecting
the excitatory transmission of neurons recorded. In general, the
frequency of sEPSC changes was associated with presynaptic
mechanisms. Increased presynaptic transmitter release led to
increased sEPSC frequency. The amplitude of sEPSC was associated
with pre- and post-synaptic mechanisms. In the present study,
whole-cell patch clamp results demonstrated no significant
difference in the amplitude of sEPSCs in spinal SG neurons in the
four groups, but the frequency of sEPSCs was significantly
different. Compared with the control group, the SNL group exhibited
higher sEPSC frequency in SG nociceptive neurons. Additionally, the
present study demonstrated that EA significantly decreased the
frequency of sEPSCs but did not affect the amplitude of sEPSCs.
These results indicated that EA may attenuate the transmission
efficiency between synapses in the SG region of the SDH during NP
by decreasing the excitability of neurons that transmit pain
signals from the peripheral nerves to the spinal cord. In addition,
previous studies have also demonstrated that EA has
anti-inflammatory effects (45–48)
and that microglia activation is associated with inflammation
(49). The SNL rat model can induce
both NP and inflammatory pain (50–52).
The present study indicates that the mechanism underlying EA
treatment of NP involves the transmission efficiency between
synapses.
In light of the downregulated P2X4R mRNA and protein
expression levels in the EA group, the present study demonstrated
that the analgesic effects of EA analgesia may be mediated in part
by P2X4R. In future, pharmacological, chemogenic and optogenetic
methods may be used to further characterize the analgesic effects
of EA mediated by P2X4R.
In conclusion, the present study demonstrated that
mechanical allodynia and thermal hyperalgesia may be attenuated by
the analgesic effects of EA. EA may exert analgesic effects by
inhibiting P2X4R-mediated activation of spinal microglia and
decreasing the excitability of neurons in the SG region of SNL
rats. However, further research is required in order to verify
these effects and to identify the underlying molecular mechanism of
EA in animals with PNI.
Acknowledgements
The authors would like to thank Dr Yu Su and Dr
Lixiu Lv in the Scientific Research Center of The Second Affiliated
Hospital and Yuying Children's Hospital of Wenzhou Medical
University, Wenzhou, China.
Funding
The current study was supported by National Natural
Science Foundation of China (grant nos. 81574074 and 81873376) and
the Basic Research Program of Wenzhou City (grant no.
Y20190192).
Availability of data and materials
The datasets generated during and/or analyzed during
the current study are available from the corresponding author on
reasonable request.
Authors' contributions
YZ, CJ, XJ, JC, XC and XY performed the laboratory
experiments, collected and analyzed the data and interpreted the
results. KZ wrote the manuscript. JW, MJ and GY analyzed the data.
MJ and GY revised the manuscript. KZ, WT and SJ designed the
experiments, supervised the study and revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All applicable international, national, and/or
institutional guidelines for the care and use of animals were
followed. All experiments were approved by the Institutional Animal
Care and Use Committee of Wenzhou Medical University (approval no.
WMU 174890).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang JY, Chen R, Chen SP, Gao YH, Zhang
JL, Feng XM, Yan Y, Liu JL, Gaischek I, Litscher D, et al:
Electroacupuncture reduces the effects of acute noxious stimulation
on the electrical activity of pain-related neurons in the
hippocampus of control and neuropathic pain rats. Neural Plast.
2016:65210262016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Y, Zhao Y, Ma X, Li J, Hou J and Lv
X: Beneficial Effects of Electroacupuncture on Neuropathic Pain
Evoked by Spinal Cord Injury and Involvement of PI3K mTOR
Mechanisms. Biol Res Nurs. 21:5–13. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen XM, Xu J, Song JG, Zheng BJ and Wang
XR: Electroacupuncture inhibits excessive interferon-γ evoked
up-regulation of P2X4 receptor in spinal microglia in a CCI rat
model for neuropathic pain. Br J Anaesth. 114:150–157. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao ZQ: Neural mechanism underlying
acupuncture analgesia. Prog Neurobiol. 85:355–375. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park JH, Kim SK, Kim HN, Sun B, Koo S,
Choi SM, Bae H and Min BI: Spinal cholinergic mechanism of the
relieving effects of electroacupuncture on cold and warm allodynia
in a rat model of neuropathic pain. J Physiol Sci. 59:291–298.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim SK, Park JH, Bae SJ, Kim JH, Hwang BG,
Min BI, Park DS and Na HS: Effects of electroacupuncture on cold
allodynia in a rat model of neuropathic pain: Mediation by spinal
adrenergic and serotonergic receptors. Exp Neurol. 195:430–436.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Padi SS and Kulkarni SK: Minocycline
prevents the development of neuropathic pain, but not acute pain:
Possible anti-inflammatory and antioxidant mechanisms. Eur J
Pharmacol. 601:79–87. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsuda M, Tozaki-Saitoh H and Inoue K:
Purinergic system, microglia and neuropathic pain. Curr Opin
Pharmacol. 12:74–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beggs S, Trang T and Salter MW:
P2X4R+ microglia drive neuropathic pain. Nat Neurosci.
15:1068–1073. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun S, Cao H, Han M, Li TT, Zhao ZQ and
Zhang YQ: Evidence for suppression of electroacupuncture on spinal
glial activation and behavioral hypersensitivity in a rat model of
monoarthritis. Brain Res Bull. 75:83–93. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsuda M, Shigemoto-Mogami Y, Koizumi S,
Mizokoshi A, Kohsaka S, Salter MW and Inoue K: P2X4 receptors
induced in spinal microglia gate tactile allodynia after nerve
injury. Nature. 424:778–783. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsuda M and Inoue K: Neuron microglia
interaction by purinergic signaling in neuropathic pain following
neurodegeneration. Neuropharmacology. 2015.
|
|
13
|
Tu WZ, Cheng RD, Cheng B, Lu J, Cao F, Lin
HY, Jiang YX, Wang JZ, Chen H and Jiang SH: Analgesic effect of
electroacupuncture on chronic neuropathic pain mediated by P2X3
receptors in rat dorsal root ganglion neurons. Neurochem Int.
60:379–386. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu J, Chen XM, Zheng BJ and Wang XR:
Electroacupuncture relieves nerve injury-induced pain
hypersensitivity via the inhibition of spinal P2X7
receptor-positive microglia. Anesth Analg. 122:882–892. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grace PM, Rolan PE and Hutchinson MR:
Peripheral immune contributions to the maintenance of central glial
activation underlying neuropathic pain. Brain Behav Immun.
25:1322–1332. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsuda M, Masuda T, Kitano J, Shimoyama H,
Tozaki-Saitoh H and Inoue K: IFN-γ receptor signaling mediates
spinal microglia activation driving neuropathic pain. Proc Natl
Acad Sci USA. 106:8032–8037. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Phelps CE, Navratilova E, Dickenson AH,
Porreca F and Bannister K: Kappa opioid signaling in the right
central amygdala causes hind paw specific loss of diffuse noxious
inhibitory controls in experimental neuropathic pain. Pain.
160:1614–1621. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Navratilova E, Ji G, Phelps C, Qu C, Hein
M, Yakhnitsa V, Neugebauer V and Porreca F: Kappa opioid signaling
in the central nucleus of the amygdala promotes disinhibition and
aversiveness of chronic neuropathic pain. Pain. 160:824–832. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sosanya NM, Kumar R, Clifford JL, Chavez
R, Dimitrov G, Srinivasan S, Gautam A, Trevino AV, Williams M,
Hammamieh R, et al: Identifying Plasma Derived Extracellular
Vesicle (EV) Contained Biomarkers in the Development of Chronic
Neuropathic Pain. J Pain. Jun 19–2019.(Epub ahead of print).
PubMed/NCBI
|
|
20
|
Gofman L, Fernandes NC and Potula R:
Relative Role of Akt, ERK and CREB in Alcohol-Induced Microglia
P2X4R Receptor Expression. Alcohol Alcohol. 51:647–654. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou K, Wu J, Chen J, Zhou Y, Chen X, Wu
Q, Xu Y, Tu W, Lou X, Yang G, et al: Schaftoside ameliorates oxygen
glucose deprivation-induced inflammation associated with the
TLR4/Myd88/Drp1-related mitochondrial fission in BV2 microglia
cells. J Pharmacol Sci. 139:15–22. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou K, Chen J, Wu J, Wu Q, Jia C, Xu YXZ,
Chen L, Tu W, Yang G, Kong J, et al: Atractylenolide III
ameliorates cerebral ischemic injury and neuroinflammation
associated with inhibiting JAK2/STAT3/Drp1-dependent mitochondrial
fission in microglia. Phytomedicine. 59:1529222019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rivera-Arconada I, Roza C and Lopez-Garcia
JA: Characterization of hyperpolarization-activated currents in
deep dorsal horn neurons of neonate mouse spinal cord in vitro.
Neuropharmacology. 70:148–155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yasaka T, Tiong SY, Hughes DI, Riddell JS
and Todd AJ: Populations of inhibitory and excitatory interneurons
in lamina II of the adult rat spinal dorsal horn revealed by a
combined electrophysiological and anatomical approach. Pain.
151:475–488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sankar SB, Pybus AF, Liew A, Sanders B,
Shah KJ, Wood LB and Buckley EM: Low cerebral blood flow is a
non-invasive biomarker of neuroinflammation after repetitive mild
traumatic brain injury. Neurobiol Dis. 124:544–554. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Todd AJ: Neuronal circuitry for pain
processing in the dorsal horn. Nat Rev Neurosci. 11:823–836. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kohno T, Wang H, Amaya F, Brenner GJ,
Cheng JK, Ji RR and Woolf CJ: Bradykinin enhances AMPA and NMDA
receptor activity in spinal cord dorsal horn neurons by activating
multiple kinases to produce pain hypersensitivity. J Neurosci.
28:4533–4540. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang K, Kumamoto E, Furue H and Yoshimura
M: Capsaicin facilitates excitatory but not inhibitory synaptic
transmission in substantia gelatinosa of the rat spinal cord.
Neurosci Lett. 255:135–138. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kawasaki Y, Zhang L, Cheng JK and Ji RR:
Cytokine mechanisms of central sensitization: Distinct and
overlapping role of interleukin-1β, interleukin-6, and tumor
necrosis factor-α in regulating synaptic and neuronal activity in
the superficial spinal cord. J Neurosci. 28:5189–5194. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Berman BM, Langevin HM, Witt CM and Dubner
R: Acupuncture for chronic low back pain. N Engl J Med.
363:454–461. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lau WK, Lau YM, Zhang HQ, Wong SC and Bian
ZX: Electroacupuncture versus celecoxib for neuropathic pain in rat
SNL model. Neuroscience. 170:655–661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen SP, Kan Y, Zhang JL, Wang JY, Gao YH,
Qiao LN, Feng XM, Yan YX and Liu JL: Involvement of hippocampal
acetylcholinergic receptors in electroacupuncture analgesia in
neuropathic pain rats. Behav Brain Funct. 12:132016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vieira JS, Toreti JA, de Carvalho RC, de
Araújo JE, Silva ML and Silva JRT: Analgesic Effects Elicited by
Neuroactive Mediators Injected into the ST 36 Acupuncture Point on
Inflammatory and Neuropathic Pain in Mice. J Acupunct Meridian
Stud. 11:280–289. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liang Y, Du JY, Qiu YJ, Fang JF, Liu J and
Fang JQ: Electroacupuncture attenuates spinal nerve
ligation-induced microglial activation mediated by p38
mitogen-activated protein kinase. Chin J Integr Med. 22:704–713.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang H, Yu X, Ren X and Tu Y:
Electroacupuncture alters pain related behaviors and expression of
spinal prostaglandin E2 in a rat model of neuropathic pain. J
Tradit Chin Med. 36:85–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gao Y, Xu C, Liang S, Zhang A, Mu S, Wang
Y and Wan F: Effect of tetramethylpyrazine on primary afferent
transmission mediated by P2X3 receptor in neuropathic pain states.
Brain Res Bull. 77:27–32. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Inoue K and Tsuda M: Microglia and
neuropathic pain. Glia. 57:1469–1479. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
He WJ, Cui J, Du L, Zhao YD, Burnstock G,
Zhou HD and Ruan HZ: Spinal P2X(7) receptor mediates microglia
activation-induced neuropathic pain in the sciatic nerve injury rat
model. Behav Brain Res. 226:163–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kobayashi K, Takahashi E, Miyagawa Y,
Yamanaka H and Noguchi K: Induction of the P2X7 receptor in spinal
microglia in a neuropathic pain model. Neurosci Lett. 504:57–61.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Masuda T, Iwamoto S, Yoshinaga R,
Tozaki-Saitoh H, Nishiyama A, Mak TW, Tamura T, Tsuda M and Inoue
K: Transcription factor IRF5 drives P2X4R+-reactive
microglia gating neuropathic pain. Nat Commun. 5:37712014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Biber K, Tsuda M, Tozaki-Saitoh H,
Tsukamoto K, Toyomitsu E, Masuda T, Boddeke H and Inoue K: Neuronal
CCL21 up-regulates microglia P2X4 expression and initiates
neuropathic pain development. EMBO J. 30:1864–1873. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ulmann L, Hirbec H and Rassendren F: P2X4
receptors mediate PGE2 release by tissue-resident macrophages and
initiate inflammatory pain. EMBO J. 29:2290–2300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Williams WA, Linley JE, Jones CA, Shibata
Y, Snijder A, Button J, Hatcher JP, Huang L, Taddese B, Thornton P,
et al: Jones Antibodies binding the head domain of P2X4 inhibit
channel function and reverse neuropathic pain. Pain. 160:1989–2003.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gao F, Xiang HC, Li HP, Jia M, Pan XL, Pan
HL and Li M: Electroacupuncture inhibits NLRP3 inflammasome
activation through CB2 receptors in inflammatory pain. Brain Behav
Immun. 67:91–100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhan J, Qin W, Zhang Y, Jiang J, Ma H, Li
Q and Luo Y: Upregulation of neuronal zinc finger protein A20
expression is required for electroacupuncture to attenuate the
cerebral inflammatory injury mediated by the nuclear factor-kB
signaling pathway in cerebral ischemia/reperfusion rats. J
Neuroinflammation. 13:2582016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang R, Lao L, Ren K and Berman BM:
Mechanisms of acupuncture-electroacupuncture on persistent pain.
Anesthesiology. 120:482–503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang Y, Zhang RX, Zhang M, Shen XY, Li A,
Xin J, Ren K, Berman BM, Tan M and Lao L: Electroacupuncture
inhibition of hyperalgesia in an inflammatory pain rat model:
Involvement of distinct spinal serotonin and norepinephrine
receptor subtypes. Br J Anaesth. 109:245–252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Harrison C: Inflammatory disorders:
Steroids modulate microglia-mediated inflammation. Nat Rev Drug
Discov. 10:492–493. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhuang ZY, Gerner P, Woolf CJ and Ji RR:
ERK is sequentially activated in neurons, microglia, and astrocytes
by spinal nerve ligation and contributes to mechanical allodynia in
this neuropathic pain model. Pain. 114:149–159. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu X, Liu H, Xu S, Tang Z, Xia W, Cheng
Z, Li W and Jin Y: Spinal translocator protein alleviates chronic
neuropathic pain behavior and modulates spinal astrocyte-neuronal
function in rats with L5 spinal nerve ligation model. Pain.
157:103–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Burke NN, Kerr DM, Moriarty O, Finn DP and
Roche M: Minocycline modulates neuropathic pain behaviour and
cortical M1-M2 microglial gene expression in a rat model of
depression. Brain Behav Immun. 42:147–156. 2014. View Article : Google Scholar : PubMed/NCBI
|