Introduction
Kidney stone (KS) disease (KSD) is one of the most
common urological diseases. In Western countries, 5–10% of the
population experience ≥1 urinary calculus episode during their
lifetime (1,2). KSD is the result of metabolic
disorders that occur for multiple reasons, but its exact
pathogenesis remains elusive. Genetic variations, dietary habits
and geographic distributions all contribute to the formation of KSs
(3), with diet being an important
factor. Different global regions and dietary cultures result in
complex and diverse stone compositions, including calcium oxalate,
calcium phosphate, calcium carbonate, cysteine and uric acid stones
(2,3). The incidence of KSD may be decreased
by changing to a low-protein and low-salt diet, and by increasing
the intake of fruit, whole grain cereal fiber and magnesium
(4–6).
Eating habits and types of food can directly affect
intestinal micro-organisms. Numerous studies (2,3,5,7–15)
have reported that intestinal microbes can participate in the
synthesis and metabolic breakdown of molecules involved in
physiological and pathophysiological processes, including
short-chain fatty acids, amino acids, vitamins, neurotransmitters
and hormones, amongst others (7).
Changes in metabolites or the induction of the immune response due
to the disruption caused by intestinal dysbacteriosis may
contribute to the regulation of various diseases. For example,
short-chain fatty acids secreted by intestinal bacteria affect the
metabolism of foreign substances, branched-chain amino acid
transport, oxidative stress and the mucin degradation mechanism
that regulates obesity and diabetes (7–13).
Overconsumption of choline or carnitine generates trimethylamine
(TMA) during intestinal metabolism, which subsequently produces TMA
N-oxide (TMAO). The presence of TMAO promotes platelet activation
and increases the incidence of atherosclerosis (14–16).
Thus, blocking the intestinal TMA metabolic enzyme may
significantly inhibit atherosclerotic plaque formation (15). These findings suggest that
intestinal microbial disorders may be the cause of disease onset
and are not only associated with disease symptoms.
Stern et al (2) used the 16s RNA method to determine the
presence of intestinal microbes in 23 cases of KSs, and the levels
of Bacteroides and Prevotella in the experimental
group were found to be significantly increased. In addition,
Suryavanshi et al (17)
reported that the level of Oxalobacter in the feces was
lower in the KSD group compared with that in the healthy control
group (HLT), and this lower abundance was observed among patients
with KSD with oxalate metabolism disorder and calcium oxalate
components. These aforementioned results suggest that calcium
oxalate stones in Western populations may be caused by i) a close
correlation between intestinal dysbacteriosis and the incidence of
KSD, and ii) low levels of Oxalobacter in the intestinal
tract. Since diets differ between Chinese and Western populations,
KSs exhibit marked diversity and no significant distribution
characteristics, and therefore, it remains unknown whether
different intestinal bacterial disorders may lead to the formation
of different types of KSs. Thus, the aim of the present study was
to analyze the diversity of intestinal flora in KSD and HLT
subjects in northeastern China, and further analyze the variability
of intestinal flora and gene abundance among patients with calcium
oxalate KS (COKS), uric acid KS (UAKS) and carbonated apatite KS
(CCKS), in order to investigate the association between the
different types of KSs and intestinal dysbacteriosis. The findings
of the present study may enable the design of KS preventive
treatments by control of the intestinal flora via diet, precise
medical treatment of KS and relatively cost-effective simple
intestinal flora sequencing analysis paired with diet
intervention.
Materials and methods
Ethics statement
The protocol of the present study was approved by
the Ethics Committee of the Second Affiliated Hospital of Harbin
Medical University (approval no. 2015-yan-221) and complied with
the World Medical Association Declaration of Helsinki regarding
ethical conduct of research involving human subjects. The study was
performed in accordance with the approved local guidelines and
regulations, and written informed consent was obtained from all the
participants.
Patients
The study involved 91 individuals with KSD (age,
26–80 years; 48 male patients and 43 female patients), including 42
with COKS, 12 with UAKS and 18 with CCKS, and 75 HLT (age, 21–85
years; 44 male patients and 31 female patients). Stone samples were
only collected from the patients who were treated with percutaneous
nephrolithotomy. It was not possible to collect stone samples from
the remainder of the patients who were treated with holmium laser
lithotripsy. The participants provided blood samples (5 ml), urine
samples (10 ml) and stool samples (using sterile cryopreserved
tubes) after 6 h of fasting. The fresh samples were stored in a
freezer at −80°C until analysis. All samples were collected from
individuals who had not undergone gastrointestinal surgery and
before the use of antibiotics. Patients with KSD included both
those with newly developed and those with recurrent KSs. All stone
composition types were registered. Classification of stones was
based on the components with the largest proportions (18). HLT participants had not used
medications over the past year, and those who had undergone
gastrointestinal surgery or received antibiotics within the past 2
weeks were excluded. The samples were collected by two surgical
methods, percutaneous nephrolithotomy and holmium laser
lithotripsy. Stone specimens of the patients were successfully
collected and stone analysis was conducted via percutaneous
nephrolithotomy. However, the stone was divided into 1–2 mm size
after holmium laser lithotripsy and failed to be successfully
collected, which is the reason for incomplete samples.
Gut microbiome analysis
Fecal samples (100 µl) were collected in Specimen
Transport Medium (Qiagen, Inc.) and microbiome DNA was extracted
using two methods, with a bead-beating based PowerSoil DNA
isolation kit (MO BIO Laboratories, Inc.) and a chemical-lysis
based QIAamp DNA mini kit (Qiagen, Inc.), following the
manufacturer's protocol. Upon finishing, the purified DNA was
rinsed in 100 µl elution buffer (pH 8.0) and its concentration was
measured using Qubit 2.0 (Thermo Fisher Scientific, Inc.). The
microbiome DNA was PCR-amplified using barcoded primers spanning
the V4 variable region of the 16s rRNA gene, as previously
described (19). Barcoded PCR
products from all samples were pooled at approximately equal molar
DNA concentrations and sequenced on an Illumina MiSeq (Illumina
Inc.) by the Albert Einstein Epigenomics Shared Facility using
paired-end reads.
The short Illumina reads were processed for an
operational taxonomic unit (OTU) classification according to
previously described methods (20–23).
Compositions of microbiome communities were summarized by a
proportion at the genus level.
Statistical analysis
The OTU results of all the samples in one group are
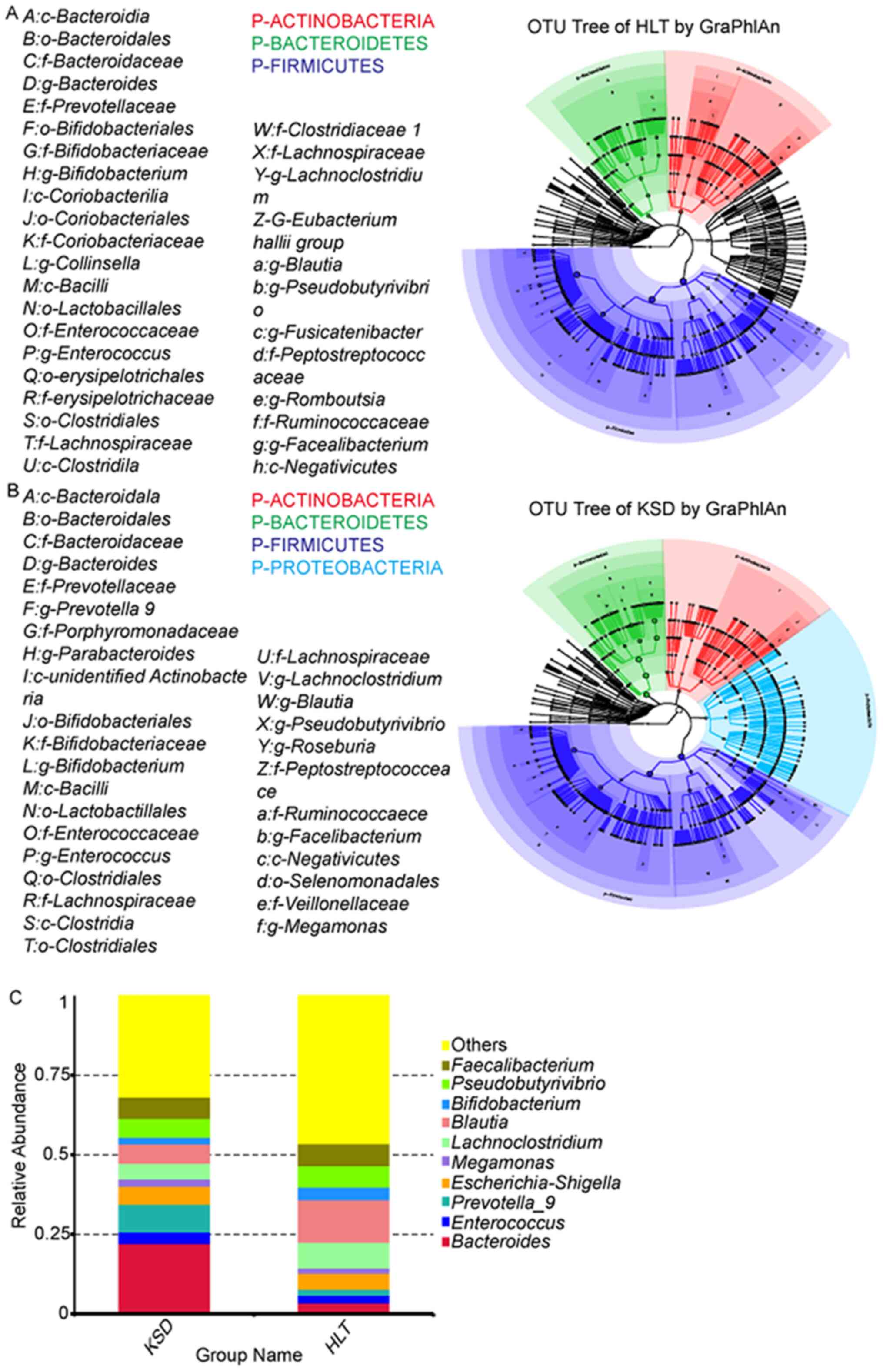
presented in Fig. 1 and were based
on individual OTUs. The relative levels of the species were plotted
with GraphPad software (version 6; GraphPad Software, Inc.).
Heatmaps were generated from the specimen and samples. Unpaired
Student's t-tests were used to identify significant differences
between groups. P<0.05 was considered to indicate a
statistically significant difference. χ2 tests were used
to compare the main intestinal flora types between the KSD and HLT
groups.
To determine the major intestinal flora types in the
experiment and control groups, age, sex, presence of diabetes
mellitus (DM) and body mass index (BMI) were added to the
independent logistic regression model of KSD associated with
Bacteroides, Pseudomonas and Blautia. Unpaired
Student's t-tests were also used to compare the mean abundance
levels of Bacteroides, Pseudomonas and Blautia with
DM, hypertension and BMI. A non-parametric test was used to compare
the COKS, UAKS and CCKS groups. An unpaired two-tailed Student
t-test was used to compare the difference between two groups.
One-way ANOVA followed by Tukey's post hoc test was used for
multiple comparisons. The statistical methods used in Table III were ANOVAs (one-way) and post
hoc Tukey's tests. All examinations were bilateral (P<0.05).
Data were statistically analyzed using SPSS v.20 (IBM Corp.).
 | Table III.Clinical characteristics of the COKS,
UAKS and CCKS stone groups. |
Table III.
Clinical characteristics of the COKS,
UAKS and CCKS stone groups.
| Variable | COKS | UAKS | CCKS | P-value |
|---|
| Age, years | 56.4±10.6 | 46.0±4.8 | 55.7±13.6 | 0.043 |
| Sex |
|
|
| 0.034 |
|
Female | 9 | 6 | 15 |
|
|
Male | 33 | 6 | 3 |
|
| BMI | 24.7±2.8 | 28.8±4.7 | 24.1±2.8 | 0.223 |
| Urine pH | 5.7±0.6 | 6.1±1.0 | 6.9±0.6 | 0.434 |
| Biochemical
index |
|
|
|
|
| Ca | 2.33±0.11 | 2.25±0.11 | 2.20±0.12 | 0.328 |
| P | 1.10±0.16 | 1.04±0.15 | 1.17±0.12 | 0.651 |
| Mg | 0.99±0.07 | 1.36±0.64 | 1.02±0.07 | 0.448 |
| TC | 7.76±5.36 | 5.05±1.32 | 4.68±1.83 | 0.292 |
| TG | 3.33±2.04 | 3.52±1.34 | 2.30±2.42 | 0.151 |
|
HDL | 1.44±0.37 | 1.02±0.24 | 1.29±0.53 | 0.471 |
|
LDL | 3.02±1.17 | 2.31±0.24 | 2.65±1.08 | 0.252 |
|
CRP | 7.25±9.21 | 4.77±3.97 | 8.41±7.81 | 0.439 |
Results
KSD and intestinal dysbacteriosis
Fecal samples were collected from 91 patients with
KSD and 75 HLT subjects (patient information is presented in
Table I). A total of 178 genera
were identified by 16s rRNA sequencing, and the top 10 bacteria
with the highest levels were statistically analyzed. At the phylum
level, the abundance of Firmicutes and Bacteroides
was highest in the KSD group. Moreover, the HLT group exhibited
higher abundance levels of Actinobacteria (Fig. 1A and B). At the genus level, the
abundance of Bacteroides and Prevotella-9 was highest
in the KSD group, accounting for 31% of all bacteria present. The
abundance of Bacteroides in the KSD group was higher
compared with that in the HLT group (22.2 vs. 3.6%; P<0.001).
The Prevotella-9 abundance was 4.65-fold higher in the KSD
group compared with that in the HLT group (8.8 vs. 1.9%;
P<0.001). The two bacteria with the highest levels in the HLT
group were Blautia and Lachnoclostridium, accounting
for 21% of total bacteria (Fig. 1C;
Table II). The Blautia
abundance was 2.12-fold higher in the HLT group compared with the
KSD group (13.4 vs. 6.0%; P<0.001). The abundance of
Lachnoclostridium was 1.58-fold higher in the HLT group
compared with the KSD group (8.0 vs. 5.0%; P=0.013 Fig. 1C; Table
II). High abundance of Prevotella-9 (OR=3.78;
P<0.001) and Blontia (OR=−3.04, P=0.002), and low
abundance of Bacteroides (OR=6.86; P<0.001) were
independent influencing factors in the KSD group according to
multivariate analysis (Table
II).
 | Table I.Key characteristics of the KSD and
HLT groups. |
Table I.
Key characteristics of the KSD and
HLT groups.
| Variable | KSD | HLT | P-value |
|---|
| Age, years | 56.3±11.4 | 57.0±13.3 | 0.695 |
| Sex |
|
| 0.445 |
|
Female | 43 | 31 |
|
|
Male | 48 | 44 |
|
| BMI | 24.7±3.4 | 25.1±4.1 | 0.419 |
| Comorbidities |
|
|
|
| DM | 11±12 | 10±13 | 0.810 |
|
HTN | 23±25 | 23±30 | 0.210 |
 | Table II.Top ten differences of intestinal
flora (abundance, %) between KSD and HLT groups at the genus
level. |
Table II.
Top ten differences of intestinal
flora (abundance, %) between KSD and HLT groups at the genus
level.
| Genus | Case | Control | P-value |
|---|
|
Bacteroides | 0.222±0.183 | 0.036±0.057 |
<0.001 |
|
Enterococcus | 0.036±0.104 | 0.024±0.099 | 0.474 |
|
Prevotella_9 | 0.088±0.152 | 0.019±0.069 |
<0.001 |
|
EscherichiaShigella | 0.057±0.097 | 0.049±0.104 | 0.623 |
|
Megamonas | 0.023±0.054 | 0.018±0.074 | 0.633 |
|
Lachnoclostridium | 0.050±0.043 | 0.080±0.094 | 0.013 |
| Blautia | 0.060±0.064 | 0.134±0.072 |
<0.001 |
|
Bifidobacterium | 0.022±0.050 | 0.041±0.072 | 0.043 |
|
Pseudobutyrivibrio | 0.060±0.071 | 0.066±0.063 | 0.530 |
|
Faecalibacterium | 0.067±0.065 | 0.070±0.063 | 0.721 |
Different types of intestinal
dysbacteriosis are associated with different types of KSs
Among the COKS, UAKS and CCKS groups, female
morbidity was highest in the CCKS group (n=5; P=0.034; Table III). The classification of stones
was based on the components with the largest proportion (18). The majority of the enrolled patients
had a urinary tract infection when they were hospitalized, but
urea-positive bacteria were not found in clinical urine bacterial
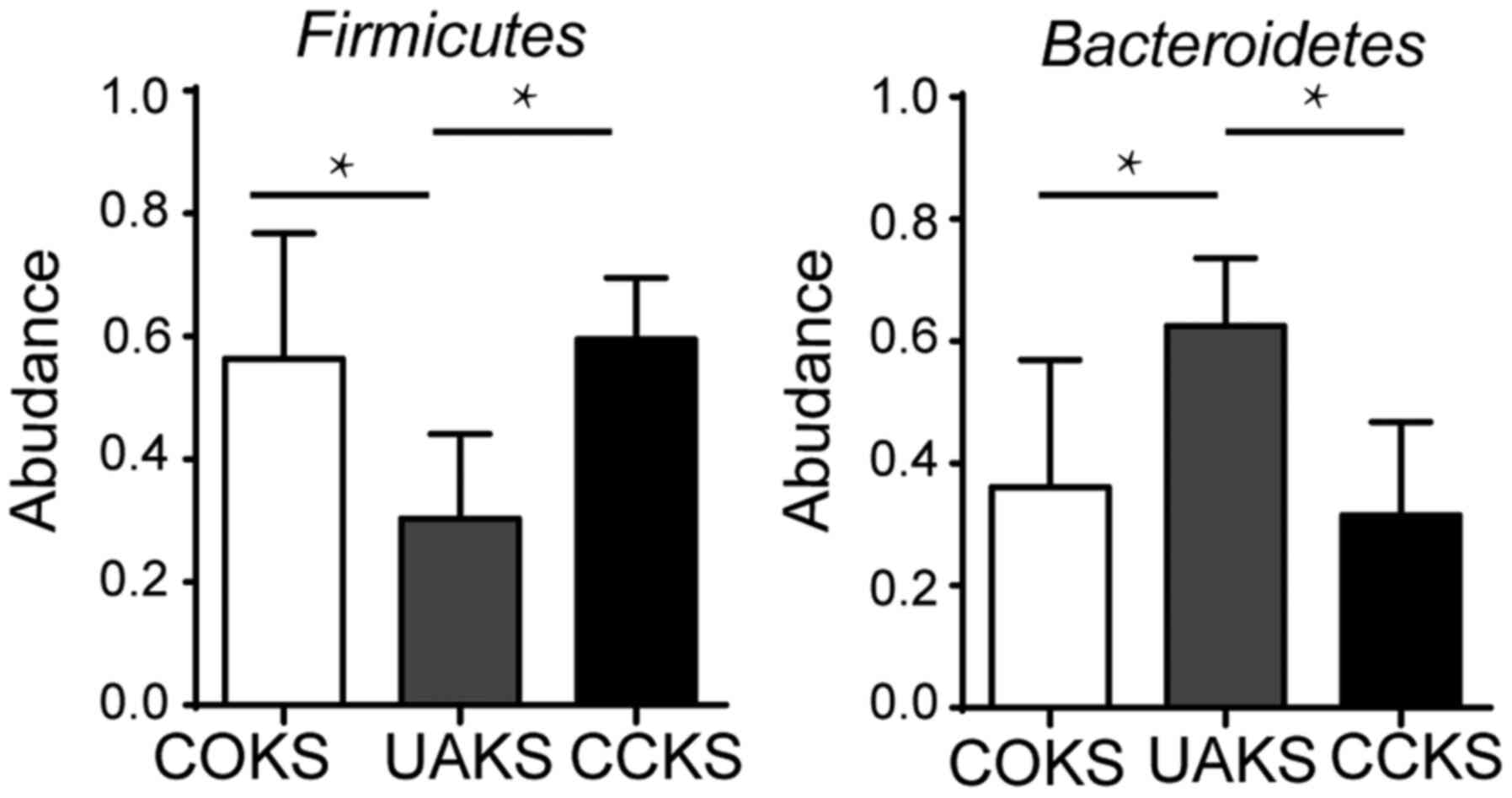
culture. The abundance of Firmicutes (56.4 vs. 30.4%;
P=0.029) and Bacteroides (36.2 vs. 62.6%; P=0.009) differed
significantly between the COKS and UAKS groups. In the CCKS and
UAKS groups, there were significant differences in the abundance
levels of Firmicutes (59.5 vs. 30.4%; P=0.014) and
Bacteroidetes (31.5 vs. 62.6%; P=0.006). There was no
significant difference in the abundance of Firmicutes (56.4
vs. 59.5%; P>0.05) and Bacteroides (36.2 vs. 31.5%;
P>0.05) between the COKS and CCKS groups (Fig. 2).
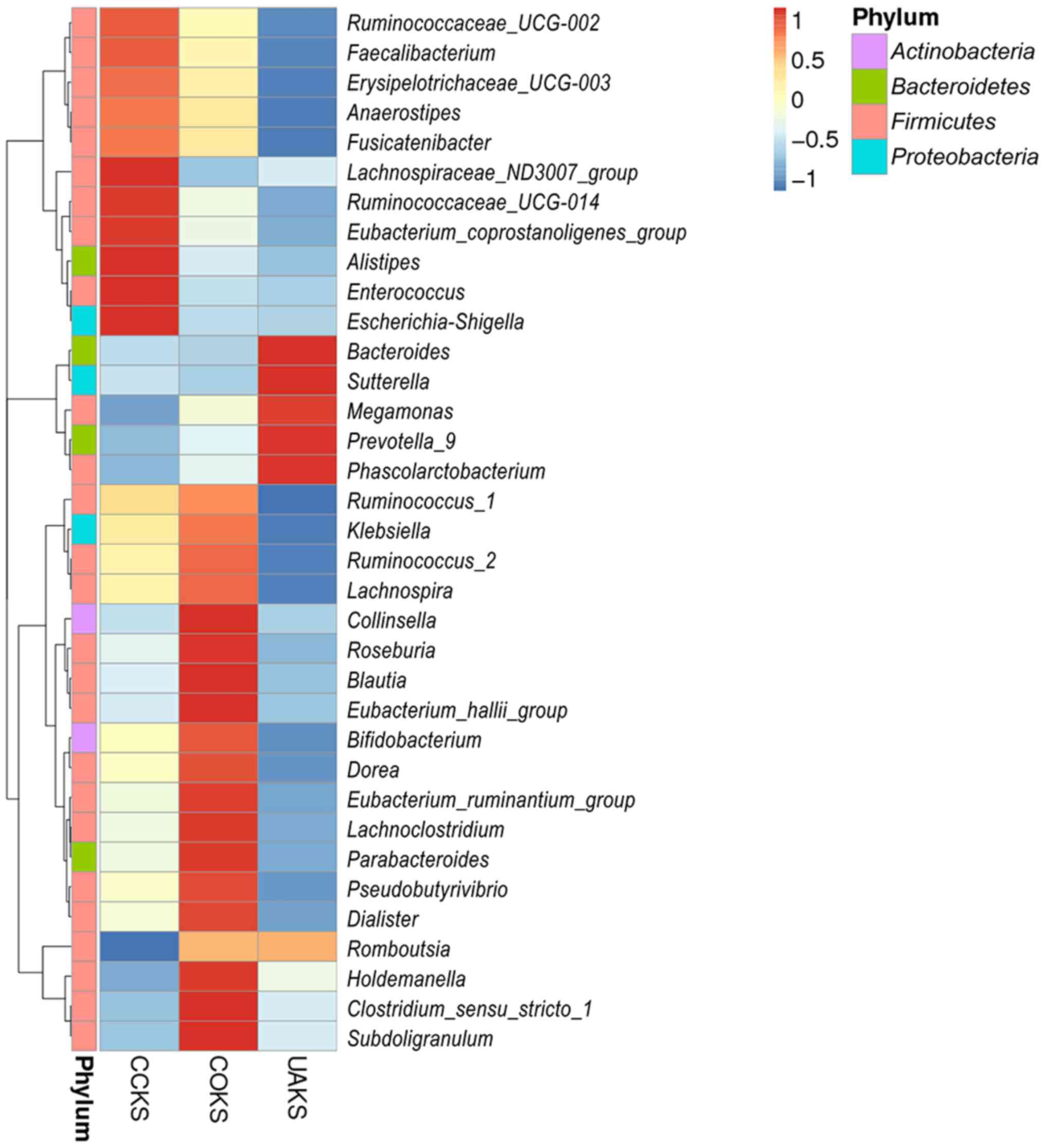
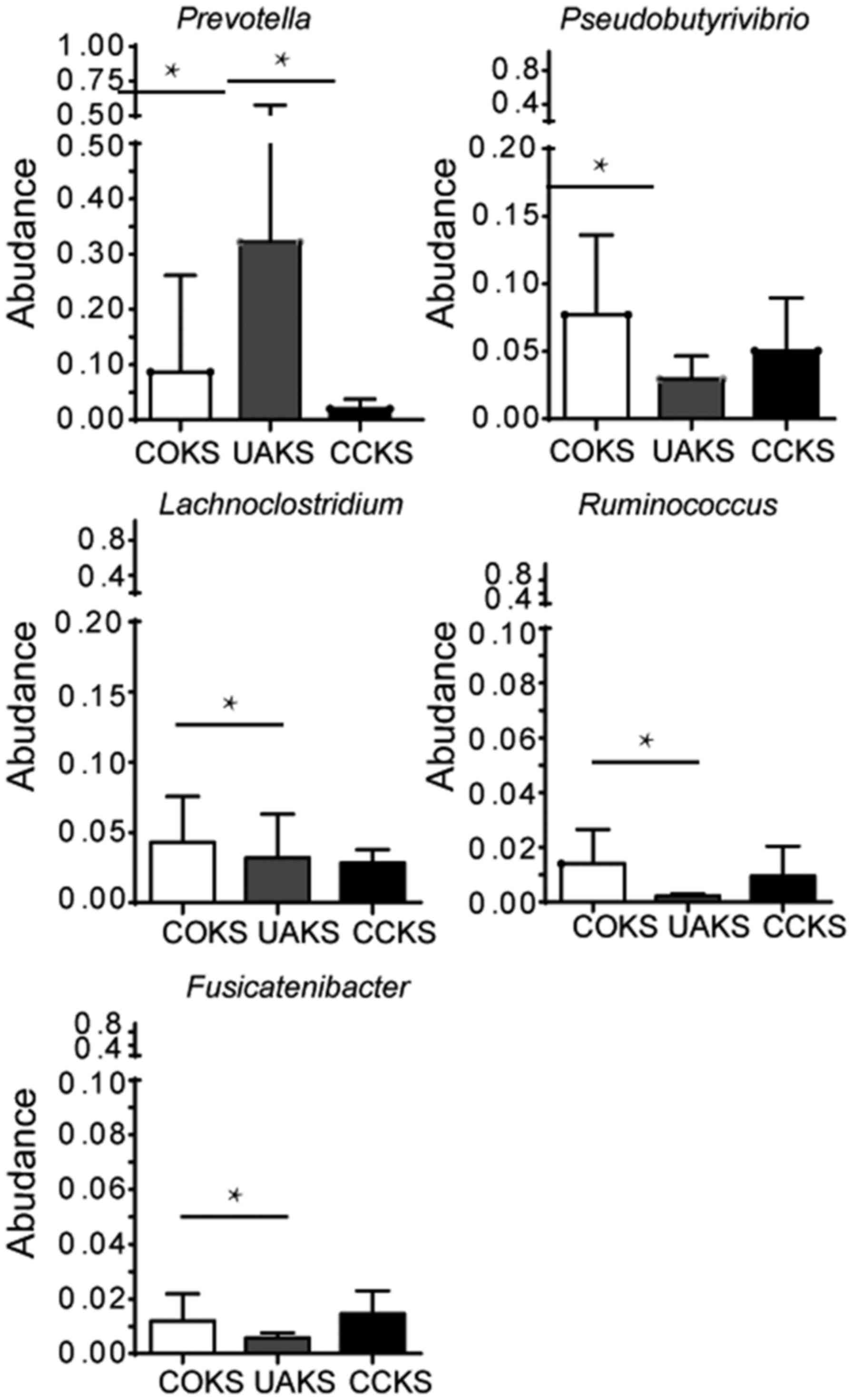
Among COKS, UAKS and CCKS groups, Prevotella
had the highest abundance in the UAKS group,
Pseudobutyrivibrio and Lachnoclostridium had the
highest abundance in the COKS group, and Ruminococcus-2 had
the lowest abundance in the UAKS group (Fig. 3). Moreover, no significant
differences in the abundance of bacteria were identified between
the COKS and CCKS groups containing calcium (all P>0.05;
Fig. 4). The Prevotella
abundance levels differed between the non-calcium-containing UAKS
and the calcium-containing COKS and CCKS groups (32.2 vs. 8.6 and
2.0%, respectively; all P<0.005; Fig. 4). In addition, there were
differences in abundance levels between the COKS and the UAKS
groups for Pseudobutyrivibrio, Lachnoclostridium,
Ruminococcus and Fusicatenibacter (7.7 vs. 2.9%, 4.5 vs.
1.9%, 1.4 vs. 0.2% and 1.2 vs. 0.6%, respectively; all P<0.05;
Fig. 4). There were differences
between the three species of Anaerotruncus, Tyzzerell-4 and
Turicibacter, but the abundance level was very low (data not
shown).
The differences in gastrointestinal flora were
analyzed and compared between men and women (Table IV). There were no statistically
significant differences in gastrointestinal flora between the KSD
and the HLT groups under the phylum level classification (all
P>0.05; data not shown). Under the genus classification,
significant differences were observed in the KSD groups between men
and women. Statistically significant differences were identified
among Collinsella, Peptostreptococcus, Sutterella, Barnesiella,
Peptococcus, Senegalimassilia, Butyricimonas, Bilophila,
Ruminiclostridium-9, Coprobacter, Mogibacterium and
Cupriavidus (all P<0.05; Table IV). The abundance levels of
Collinsella (0.7 vs. 0.2%; P=0.031), Sutterella (0.3
vs. 0.1%; P=0.028) and others (data not shown) were very low. In
the HLT group, there were significant differences among
unidentified-Lachnospiraceae, Paraprevotella, Haemophilus,
Sphingomonas, Ruminiclostridium-1, Streptomyces, Johnsonella
and Acholeplasma (all P<0.05; Table IV), but the abundance levels were
very low (data not shown).
 | Table IV.Sex differences in KSD groups (male,
n=48; female, n=43) and HLT groups (male, n=44; female, n=31) under
genus classification. |
Table IV.
Sex differences in KSD groups (male,
n=48; female, n=43) and HLT groups (male, n=44; female, n=31) under
genus classification.
| A, KSD groups |
|---|
|
|---|
| Taxonomy | Male | Female | P-value |
|---|
|
Collinsella | 427.631±946.67 |
115.771±226.548 | 0.031 |
|
Peptostreptococcus | 2.501±5.772 | 0.491±0.883 | 0.021 |
|
Sutterella | 84.771±132.766 |
207.531±334.135 | 0.028 |
|
Barnesiella | 24.150±59.532 | 88.811±169.650 | 0.022 |
|
Peptococcus | 1.791±3.175 | 0.671±1.796 | 0.040 |
|
Senegalimassilia | 4.171±9.924 | 0.951±2.663 | 0.035 |
|
Butyricimonas | 9.001±14.388 | 27.441±55.455 | 0.040 |
|
Bilophila | 11.351±12.790 | 28.491±46.608 | 0.024 |
|
Ruminiclostridium-9 | 15.311±19.333 | 33.811±46.321 | 0.018 |
|
Coprobacter |
2.981±8.721 | 12.001±26.265 | 0.037 |
|
Mogibacterium | 0.331±0.753 | 0.051±0.213 | 0.014 |
|
Cupriavidus | 0.081±0.279 | 0.001±0.000 | 0.044 |
|
| B, HLT
groups |
|
|
Taxonomy | Male | Female | P-value |
|
|
unidentified-Lachnospiraceae | 19.141±29.890 | 49.190±79.644 | 0.049 |
|
Paraprevotella | 23.720±44.767 | 6.441±6.730 | 0.016 |
|
Haemophilus | 28.741±72.885 | 4.130±5.780 | 0.033 |
|
Sphingomonas | 0.261±0.658 | 0.031±0.177 | 0.038 |
|
Ruminiclostridium-1 | 0.140±0.351 | 0.001±0.000 | 0.013 |
|
Streptomyces | 0.231±0.611 | 0.001±0.000 | 0.017 |
|
Johnsonella | 0.161±0.433 | 0.001±0.000 | 0.018 |
|
Acholeplasma | 0.121±0.324 | 0.001±0.000 | 0.024 |
Discussion
The composition of KSs is complex and diverse, and
pathogenic mechanisms are affected by multiple factors, with diet
being the most important factor affecting the formation of KSs
(4–6,24,25).
The differences in ethnicity and dietary structure markedly affect
the composition and function of intestinal ecosystems (26–29).
The present study demonstrated that patients with KSs had
intestinal dysbacteriosis, and Oxalobacter levels were
upregulated in patients with COKS. However, the prevalence of
specific bacteria was not obvious among other patients with KSD.
The present study analyzed the differences in gastrointestinal
bacteria among patients with KSD, and performed further comparative
analyses of bacterial abundance and the specific composition of
KSs.
The mechanism of KS formation is complex. A variety
of stone compositions contribute to metabolic regulation under
control of the susceptibility gene (30). Similar to other researchers
(4–6,25), the
present results suggested that diet was one of the most important
factors affecting the formation of kidney stones. Endogenous and
exogenous oxalic acid metabolic disorders lead to formation of
COKS, with Oxalobacter being one of the most important
contributors (17). Few studies
have reported other types of bacteria causing gastrointestinal
disorders to be involved in KS formation.
It was hypothesized that the abnormal changes in
metabolic products and bacterial intestinal colonization may
trigger mucosal immune inflammation mechanisms, which may cause
further changes in the body's renal function when intestinal
dysbacteriosis is present. When the levels of the components of
kidney stones increases, renal tubular epithelial injury,
inflammatory responses and calculus susceptibility gene regulation
occur, eventually leading to KS formation (31,32).
The high incidence and recurrence of KSD may be
closely associated with ethnicity and diet; thus, it is important
to investigate changes in intestinal dysbacteriosis (33). In the present study, the abundance
of Bacteroides and Prevotella in the KSD group was
significantly higher compared with that in the HLT group (6.1- and
4.6-fold, respectively). The trend of the obtained results was
consistent with that reported by Stern et al (2) (6.1- vs. 3.4-fold and 4.6- vs.
2.8-fold, respectively), and variations may be due to differences
in ethnicity and dietary cultures of the patients recruited. In
addition, Lachnoclostridium, Blautia and
Bifidobacterium were 1.5-, 2.1- and 1.9-fold higher,
respectively, in patients compared with the HLT group.
The differences between the CCKS, UAKS and COKS
groups were further analyzed. No lower abundance levels of
Oxalobacter were found in the KSD group when compared with
the HLT group, which was consistent with the findings of
Suryavanshi et al (17).
However, in the present study, statistically significant
differences in the levels of Oxalobacter between these two
groups were not observed, but there were significant differences in
Prevotella-9 abundance between the calcium-containing COKS
and CCKS groups and the non-calcium-containing UAKS group.
Prevotella-9 is positively correlated with serum uric acid
levels, while UAKS are a product of metabolic disorders of uric
acid (34). Thus, it was suggested
that abnormally high abundance of Prevotella-9 may lead to
the formation of uric acid stones. However, it should be noted that
Stern et al (2) did not
identify any significant differences in bacteria when comparing
uric acid and non-UAKS, which may be attributed to the daily
high-fiber diet of the study population. Additionally, in the
present study, differences were observed in the abundance levels of
Pseudobutyrivibrio, Lachnoclostridium and Ruminococcus
2 between the COKS and UAKS groups, all of which belong to the
Helicobacter pylori family. One of the major metabolites is
short-chain fatty acids, which participates in the metabolism
(35) and inflammatory responses of
the body (36,37). It was hypothesized that the bacteria
present in intestinal dysbacteriosis may interfere with the body's
normal inflammatory response and material metabolism via uric acid
short-chain fatty acid metabolic disorders, which ultimately lead
to calcium and UAKS formation in the kidney.
In general, the composition of intestinal microbiota
changes according to age, diet and drug use, and differs across
ethnicities and geographic regions (2,3,5,8,17).
Loss of Oxalobacter formigeneses is hypothesized to be a
factor that produces COKS, as Oxalobacter formigeneses
metabolizes oxalate in the intestine; however, several studies,
including the present, question the validity of this hypothesis
(2,38). The mechanism underlying the
formation of calculi has yet to be fully elucidated. Further
extensive basic research and clinical data are required for
confirmation. Although individuals residing in the same area tend
to consume similar foods, individual dietary variations also affect
the statistical results (4,6). The present study randomly recruited
participants in both the KSD and HLT groups. The stones collected
were mostly of mixed composition. The main stone composition was
based on infrared spectrum analysis. The participants did not
strictly control their daily diets in the present study. Since some
patients opted for holmium laser lithotripsy and some did not agree
to undergo stone composition analysis, and thus, there was an
insufficient number of patients in the COKS, UAKS and CCKS groups
to establish statistical significance.
In conclusion, the present study indicated a key
association between specific KS components and intestinal flora,
providing a theoretical basis for novel treatment strategies for
KSs. Moreover, the results of the present study indicated that
differences and interactions between bacteria might predict
specific types of urolithiasis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of Heilongjiang Province (grant no.
H201407).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XL was responsible for designing the experiments,
and analyzing and interpreting the data. EZ was responsible for
compiling articles, performing the experiments and analyzing the
data. WZ, BG, BY and WW collected clinical specimens and
interpreted the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The protocol of the present study was approved by
the Ethics Committee of the Second Affiliated Hospital of Harbin
Medical University (approval no. 2015-yan-221) and complied with
the World Medical Association Declaration of Helsinki regarding
ethical conduct of research involving human subjects. The study was
performed in accordance with the approved local guidelines and
regulations, and written informed consent was obtained from all the
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Scales CD Jr, Smith AC, Hanley JM and
Saigal CS; Urologic Diseases in America Project, : Prevalence of
kidney stones in the United States. Eur Urol. 62:160–165. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stern JM, Moazami S, Qiu Y, Kurland I,
Chen Z, Agalliu I, Burk R and Davies KP: Evidence for a distinct
gut microbiome in kidney stone formers compared to non-stone
formers. Urolithiasis. 44:399–407. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Daudon M, Dore JC, Jungers P and Lacour B:
Changes in stone composition according to age and gender of
patients: A multivariate epidemiological approach. Urol Res.
32:241–247. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taylor EN, Stampfer MJ and Curhan GC:
Dietary factors and the risk of incident kidney stones in men: New
insights after 14 years of follow-up. J Am Soc Nephrol.
15:3225–3232. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tracy CR, Best S, Bagrodia A, Poindexter
JR, Adams-Huet B, Sakhaee K, Maalouf N, Pak CY and Pearle MS:
Animal protein and the risk of kidney stones: A comparative
metabolic study of animal protein sources. J Urol. 192:137–141.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Borghi L, Schianchi T, Meschi T, Guerra A,
Allegri F, Maggiore U and Novarini A: Comparison of two diets for
the prevention of recurrent stones in idiopathic hypercalciuria. N
Engl J Med. 346:77–84. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brunkwall L and Orho-Melander M: The gut
microbiome as a target for prevention and treatment of
hyperglycaemia in type 2 diabetes: From current human evidence to
future possibilities. Diabetologia. 60:943–951. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karlsson FH, Tremaroli V, Nookaew I,
Bergstrom G, Behre CJ, Fagerberg B, Nielsen J and Backhed F: Gut
metagenome in European women with normal, impaired and diabetic
glucose control. Nature. 498:99–103. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F,
Liang S, Zhang W, Guan Y, Shen D, et al: A metagenome-wide
association study of gut microbiota in type 2 diabetes. Nature.
490:55–60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dumas ME, Barton RH, Toye A, Cloarec O,
Blancher C, Rothwell A, Fearnside J, Tatoud R, Blanc V, Lindon JC,
et al: Metabolic profiling reveals a contribution of gut microbiota
to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad
Sci USA. 103:12511–12516. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pedersen HK, Gudmundsdottir V, Nielsen HB,
Hyotylainen T, Nielsen T, Jensen BA, Forslund K, Hildebrand F,
Prifti E, Falony G, et al: Human gut microbes impact host serum
metabolome and insulin sensitivity. Nature. 535:376–381. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Le Chatelier E, Nielsen T, Qin J, Prifti
E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy
S, et al: Richness of human gut microbiome correlates with
metabolic markers. Nature. 500:541–546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ussar S, Griffin NW, Bezy O, Fujisaka S,
Vienberg S, Softic S, Deng L, Bry L, Gordon JI and Kahn CR:
Interactions between gut microbiota, host genetics and diet
modulate the predisposition to obesity and metabolic syndrome. Cell
Metab. 22:516–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang WH, Wang Z, Shrestha K, Borowski AG,
Wu Y, Troughton RW, Klein AL and Hazen SL: Intestinal
microbiota-dependent phosphatidylcholine metabolites, diastolic
dysfunction, and adverse clinical outcomes in chronic systolic
heart failure. J Card Fail. 21:91–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Z, Klipfell E, Bennett BJ, Koeth R,
Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, et al:
Gut flora metabolism of phosphatidylcholine promotes cardiovascular
disease. Nature. 472:57–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu W, Gregory JC, Org E, Buffa JA, Gupta
N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, et al: Gut microbial
metabolite TMAO enhances platelet hyperreactivity and thrombosis
risk. Cell. 165:111–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suryavanshi MV, Bhute SS, Jadhav SD,
Bhatia MS, Gune RP and Shouche YS: Hyperoxaluria leads to dysbiosis
and drives selective enrichment of oxalate metabolizing bacterial
species in recurrent kidney stone endures. Sci Rep. 6:347122016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mandel NS, Mandel IC and Kolbach-Mandel
AM: Accurate stone analysis: The impact on disease diagnosis and
treatment. Urolithiasis. 45:3–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y and Qian PY: Conservative fragments
in bacterial 16S rRNA genes and primer design for 16S ribosomal DNA
amplicons in metagenomic studies. PLoS One. 4:e74012009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smith BC, McAndrew T, Chen Z, Harari A,
Barris DM, Viswanathan S, Rodriguez AC, Castle P, Herrero R,
Schiffman M and Burk RD: The cervical microbiome over 7 years and a
comparison of methodologies for its characterization. PLoS One.
7:e404252012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Caporaso JG, Kuczynski J, Stombaugh J,
Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich
JK, Gordon JI, et al: QIIME allows analysis of high-throughput
community sequencing data. Nat Methods. 7:335–336. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Edgar RC: Search and clustering orders of
magnitude faster than BLAST. Bioinformatics. 1:2460–2461. 2010.
View Article : Google Scholar
|
|
23
|
Matsen FA, Kodner RB and Armbrust EV:
Pplacer: Linear time maximum-likelihood and Bayesian phylogenetic
placement of sequences onto a fixed reference tree. BMC Bioinform.
11:5382010. View Article : Google Scholar
|
|
24
|
Org E, Blum Y, Kasela S, Mehrabian M,
Kuusisto J, Kangas AJ, Soininen P, Wang Z, Ala-Korpela M, Hazen SL,
et al: Relationships between gut microbiota, plasma metabolites,
and metabolic syndrome traits in the METSIM cohort. Genome Biol.
18:702017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pak CY: Kidney stones. Lancet.
351:1797–1801. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Parks BW, Nam E, Org E, Kostem E, Norheim
F, Hui ST, Pan C, Civelek M, Rau CD, Bennett BJ, et al: Genetic
control of obesity and gut microbiota composition in response to
high-fat, high-sucrose diet in mice. Cell Metab. 17:141–152. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu GD, Chen J, Hoffmann C, Bittinger K,
Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R,
et al: Linking long-term dietary patterns with gut microbial
enterotypes. Science. 334:105–108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Daniel H, Gholami AM, Berry D,
Desmarchelier C, Hahne H, Loh G, Mondot S, Lepage P, Rothballer M,
Walker A, et al: High-fat diet alters gut microbiota physiology in
mice. ISME J. 8:295–308. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
David LA, Maurice CF, Carmody RN,
Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y,
Fischbach MA, et al: Diet rapidly and reproducibly alters the human
gut microbiome. Nature. 505:559–563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Palsson R, Indridason OS, Edvardsson VO
and Oddsson A: Genetics of common complex kidney stone disease:
Insights from genome-wide association studies. Urolithiasis.
47:11–21. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ratajczak W, Rył A, Mizerski A,
Walczakiewicz K, Sipak O and Laszczyńska M: Immunomodulatory
potential of gut microbiome-derived short-chain fatty acids
(SCFAs). Acta Biochim Pol. 66:1–12. 2019.PubMed/NCBI
|
|
32
|
Okada A, Yasui T, Fujii Y, Niimi K,
Hamamoto S, Hirose M, Kojima Y, Itoh Y, Tozawa K, Hayashi Y and
Kohri K: Renal macrophage migration and crystal phagocytosis via
inflammatory-related gene expression during kidney stone formation
and elimination in mice: Detection by association analysis of
stone-related gene expression and microstructural observation. J
Bone Miner Res. 12:2701–2711. 2010. View Article : Google Scholar
|
|
33
|
Fakhoury MQ, Gordon B, Shorter B, Renson
A, Borofsky MS, Cohn MR, Cabezon E, Wysock JS and Bjurlin MA:
Perceptions of dietary factors promoting and preventing
nephrolithiasis: A cross-sectional survey. World J Urol.
37:1723–1731. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lim MY, Rho M, Song YM, Lee K, Sung J and
Ko G: Stability of gut enterotypes in Korean monozygotic twins and
their association with biomarkers and diet. Sci Rep. 4:73482014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Teixeira TF, Grzeskowiak L, Franceschini
SC, Bressan J, Ferreira CL and Peluzio MC: Higher level of faecal
SCFA in women correlates with metabolic syndrome risk factors. Br J
Nutr. 109:914–919. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Winter SE and Baumler AJ: Why related
bacterial species bloom simultaneously in the gut: Principles
underlying the ‘like will to like’ concept. Cell Microbiol.
16:179–184. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Winter SE and Baumler AJ: Dysbiosis in the
inflamed intestine: Chance favors the prepared microbe. Gut
Microbes. 5:71–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee JA and Stern JM: Understanding the
link between gut microbiome and urinary stone disease. Curr Urol
Rep. 20:192019. View Article : Google Scholar : PubMed/NCBI
|