Introduction
Abdominal aortic aneurysm (AAA) is a disease
characterized by enlargement of the abdominal aorta and commonly
occurring in individuals >50 years old, typically in males
(1). AAA is usually asymptomatic
but can be lethal upon rupture (1,2), which
is a considerable threat to the health of the elderly. It has been
reported that high-salt diet is positively correlated with the risk
of AAA both in patients and in animal models (3,4).
However, the underlying mechanism of high salt inducing AAA remains
to be elucidated.
Endothelial dysfunction of abdominal aorta is highly
associated with AAA (5).
Particularly, apoptosis of endothelial cells of blood vessels
increases the permeability of vessel walls, enhancing the migration
and binding of inflammatory cells to the vascular smooth muscle
layer, which is believed to be a major cause of aneurysms and
rupture (6,7). Thus, investigation on the apoptosis of
endothelial cells should contribute to the understanding of
pathogenesis of AAA. Human umbilical vein endothelial cells
(HUVECs) are a commonly used cell model in studies related to
endothelial cells (8). Blocking
NF-κB signaling protects HUVEC cells from high glucose-induced
apoptosis (9), indicating the
potential involvement of NF-κB pathway in endothelial
apoptosis.
Nuclear factor of activated T cells 5 (NFAT5) is a
transcriptional factor mainly induced by hypertonic stress
(10). Although body fluid usually
remains isotonic due to the balance of intra- and extracellular
solutes, excess intake of salts causes hypertonicity of blood,
leading to various clinical manifestations which may elevate the
expression NFAT5 in the cells of vessel walls (11). NFAT5 is closely associated with the
NF-κB pathway and enhances NF-κB activity by forming NF-κB-NFAT5
complexes, which promotes the binding of NF-κB to κB elements of
NF-κB-responsive genes, including various proinflammatory genes
such as vascular cell adhesion molecule-1 (VCAM-1), intercellular
adhesion molecule-1 (ICAM-1) and inducible nitric oxide synthase
(iNOS) (12). Nevertheless, the
role of NFAT5 in endothelial apoptosis remains to be
elucidated.
The present study identified that hypertonic culture
medium elevated expression of NFAT5 in HUVECs and induced
celldeath. Furthermore, overexpression of NFAT5 by plasmid
transfection also led to the apoptosis of HUVECs. In addition,
knockdown of NFAT5 using specific small interfering (si) RNA
relieved cell death induced by hypertonic medium. Finally, it was
identified that NFAT5 enhanced the activity of NF-κB signaling
pathway and inhibited the expression of Bcl-2, an anti-apoptotic
protein, in HUVECs. Altogether, the present study demonstrated a
novel mechanism underlying hypertonicity-induced apoptosis of
HUVECs, mediated by NFAT5 and the NF-κB signaling pathway. These
findings extend the current knowledge about the pathogenesis of
AAA, especially the role of high-salt diet in the progression of
AAA.
Materials and methods
Patient samples
Human AAA (n=9) and adjacent healthy aorta
abdominalis (n=9) were collected by aneurysmectomy and prosthetic
vascular graft repair at Hangzhou First Affiliated Hospital between
January 2015 and December 2019. Six of the patients were male and 3
were female (age range, 57–71 years). Each sample was homogenized
for RNA extraction. The study was approved by the Ethics Committee
of Hangzhou First Affiliated Hospital and written informed consent
was obtained from each patient.
Cell culture
HUVECs were a kind gift from Dr Ye Qiu (College of
Biology, Hunan University, China). They were maintained in complete
culture medium consisting of Dulbecco's modified Eagle's medium
(DMEM, Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
fetal bovine serum (FBS, Sigma-Aldrich; Merck KGaA), 100,000 U/l
penicillin (Sigma-Aldrich; Merck KGaA) and 100 mg/l streptomycin
(Sigma-Aldrich; Merck KGaA). The cell culture was incubated in 37°C
with 5% of CO2.
The hypertonic medium was made by dissolving NaCl
(Sigma-Aldrich; Merck KGaA) in the complete culture medium at a
final concentration of 100 mM, which was equal to 30 g salt
ingestion by a human of 60 kg. This dose was demonstrated to
elevate NFAT5 expression in a previous study (13). The hypertonic medium was then
filtered using Millex-GP Syringe Filter Unit (0.22 µm; EMD
Millipore) to avoid contamination. Normal complete culture medium
was subjected to the same process of filtration to serve as control
medium for hypertonic treatment.
RNA extraction and reverse
transcription-quantitative (RT-q) PCR
Total cellular RNA was extracted from HUVECs (90%
confluence) using PureLink RNA Mini kit (Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. cDNA was then
synthesized by reverse transcription using the SuperScript III
First-Strand Synthesis System (Invitrogen; Thermo Fisher
Scientific, Inc.) and detected by RT-qPCR using the SYBR-Green qPCR
kit (Bimake.com) according to the manufacturer's
instructions. Briefly, the synthesized cDNA was mixed with
SYBR-Green qPCR master mix and the primers to a total reaction
volume of 20 µl. Then the two-step qPCR program was run as follows:
Activation at 95°C for 30 sec, denaturation at 95°C for 15 sec,
followed by annealing and extension at 60°C for 60 sec, with a
total of 40 cycles. The Cq values were collected and converted to
gene expression levels by calculating 2−ΔΔCq (14). GAPDH mRNA levels were used as the
endogenous control to normalize the data. All RT-qPCR experiments
were performed in triplicate with no template as a negative
control. The primers used were: Human NFAT5-forward,
5′-GAAGTGGACATTGAAGGCACT−3′ and reverse,
5′-CTGGCTTCGACATCAGCATT-3′; human VCAM-1-forward,
5′-CAGTAAGGCAGGCTGTAAAAGA-3′ and reverse,
5′-TGGAGCTGGTAGACCCTCG-3′; human ICAM-1-forward,
5′-GTATGAACTGAGCAATGTGCAAG-3′ and reverse,
5′-GTTCCACCCGTTCTGGAGTC-3′; human GAPDH-forward,
5′-AATCCCATCACCATCTTCCA-3′ and reverse, 5′-TGGACTCCACGACGTACTCA-3′;
human iNOS-forward, 5′-TCATCCGCTATGCTGGCTAC-3′ and reverse,
5′-CCCGAAACCACTCGTATTTGG−3′.
MTS cell viability assay
HUVEC morphology was observed and images captured at
room temperature under a TMS-F phase-contrast microscope (Nikon
Corporation) connected to Coolpix 8400 a camera (Nikon
Corporation). HUVEC viability was further quantified using an MTS
assay kit following the manufacturer's instructions (Promega
Corporation). Briefly, HUVECs were incubated with MTS solution (2
mg/ml) for 2 h at 37°C in an atmosphere containing 5%
CO2. Subsequently, the formazan crystals were dissolved
in DMSO and diluted to 5X in phospate-buffered saline (PBS).
Absorbance of formazan was measured at 492 nm using an ELISA plate
reader (Tecan Group Ltd.). The results were normalized to the
corresponding controls which were set as a viability of 100%.
Western blot analysis
HUVECs were washed with cold PBS before the addition
of an appropriate volume (50 µl) of RIPA lysis buffer (Santa Cruz
Biotechnology, Inc.). After incubation for 20 min on ice, the cell
lysates were centrifuged at 13,000 × g for 15 min at 4°C, and
protein-containing supernatant was collected. Protein concentration
was determined using the Bradford Assay. The isolated proteins (50
µg) were separated by 8–12% SDS-PAGE and transferred onto
polyvinylidene difluoride (PVDF) membranes (Sigma-Aldrich; Merck
KGaA). Membranes were blocked with 5% skimmed milk in TBS buffer
containing 5% Tween-20 (Sigma-Aldrich; Merck KGaA) at 4°C for 2h
and incubated with one of the following primary antibodies in 4°C
overnight: Monoclonal mouse anti-NFAT5 (cat. no. sc-398171; Santa
Cruz Biotechnology, Inc.), polyclonal rabbit anti-Bcl-2 (cat. no.
A5010; Bimake.com), polyclonal rabbit anti-caspase-3-p12
(cat. no. A5357; Bimake.com; 1:500 dilution),
HRP-conjugated monoclonal mouse anti-β-actin (cat. no. A5092;
Bimake.com; 1:3,000 dilution). After several washes
with TBST, each blot was further incubated with secondary antibody
(goat anti-mouse, cat. no. sc-2005 or donkey anti-rabbit, cat. no.
sc-2313; 1:5,000 dilution) conjugated to horseradish peroxidase
(Santa Cruz Biotechnology, Inc.) in room temperature for 2 h.
Detection was carried out by enhanced chemiluminescence (Amersham;
Cytiva) as per the manufacturer's instructions. β-actin was used as
a loading control. Signal intensities were quantified using the
ImageJ 2 (National Institutes of Health) program and normalized to
the control samples. All the western blots were conducted in three
biological repeats.
Constructs, siRNAs and
transfection
pEGFP-NFAT5 containing the coding region for
myc-tagged NFAT5 (EGFP removed during construction; cat. no. 13627;
Addgene, Inc.) was a kind gift from Dr Anjana Rao (Department of
Pathology, Harvard Medical School) (15). pEGFP-N1, the empty vector, was a
kind gift from Dr Ye Qiu (College of Biology, Hunan University).
pSI-Check2-hRluc-NF-κB-firefly, the NF-κB luciferase reporter
construct, was a gift from Dr Qing Deng (Department of Biological
Sciences, Purdue University) (cat. no. 106979; Addgene, Inc.)
(16).
The plasmids were transfected using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Briefly, 1×106 HUVEC cells were grown at 37°C overnight
to 80–90% confluence in 6-well plates, washed with PBS and overlaid
by the transfection medium (DMEM with 10% FBS and the transfection
complex containing plasmids and Lipofectamine® 2000) for
6 h. The transfection medium was then replaced with the complete
culture medium for further incubation for 42 h before the following
experiments.
The siRNAs targeting human NFAT5 (cat. no. sc-43968;
10 µM) and the scrambled control siRNAs (cat. no. sc-37007; 10 µM)
were purchased from Santa Cruz Biotechnology, Inc. All the siRNAs
were transfected into cells using Lipofectamine® 2000
according to the manufacturer's instructions. Briefly, HUVEC cells
were incubated in 6-well plates at 37°C overnight to 40%
confluence, then washed with PBS and re-cultured in Opti-MEM with
transfection complex containing Lipofectamine 2000® and
siRNAs. After 6 h of incubation, the transfection medium was
replaced with DMEM containing 10% FBS and the incubation was
continued for 48 h.
Dual-luciferase assay
pSI-Check2-hRluc-NF-κB-firefly, a luciferase
reporter construct for NF-κB activity in which the expression of
firefly luciferase (FLuc) is controled by the responsive promoter
of NF-κB was used in this assay, and Renilla luciferase
(RLuc), which is constitutively expressed served as internal
control in this assay. HUVECs were transfected with the luciferase
reporter constructs together with pEGFP-NFAT5 or the control vector
pEGFP using Lipofectamine® 2000. At 48 h
post-transfection, HUVECs were lysed by passive lysis buffer
(Promega Corporation). The cell lysates were in a dual-luciferase
assay to determine the relative luciferase activity
(Renilla/firefly) using the Dual-Luciferase Reporter Assay
System (Promega Corporation) following the manufacturer's
instructions. The luminescence was measured using an ELISA plate
reader (Tecan Group, Ltd.).
Statistical analysis
All experiments were repeated three times.
Quantitative results are presented in bar graphs as the mean ± SD.
Data were analyzed by Microsoft Excel 2010 (Microsoft Corporation)
using unpaired Student's t-test unless otherwise stated. P<0.05
was considered to indicate a statistically significant
difference.
Results
Hypertonic culture medium induces
apoptosis of HUVECs
To explore changes in NFAT5 expression in AAA, AAA
samples and adjacent healthy aorta abdominalis samples were
collected from nine patients, and the mRNA levels of NFAT5 detected
in the samples by RT-qPCR. As shown in Fig. 1A, compared with the adjacent healthy
tissue, NFAT5 was upregulated to ~3 times in the AAA samples,
indicating an obvious increase of NFAT5 in AAA.
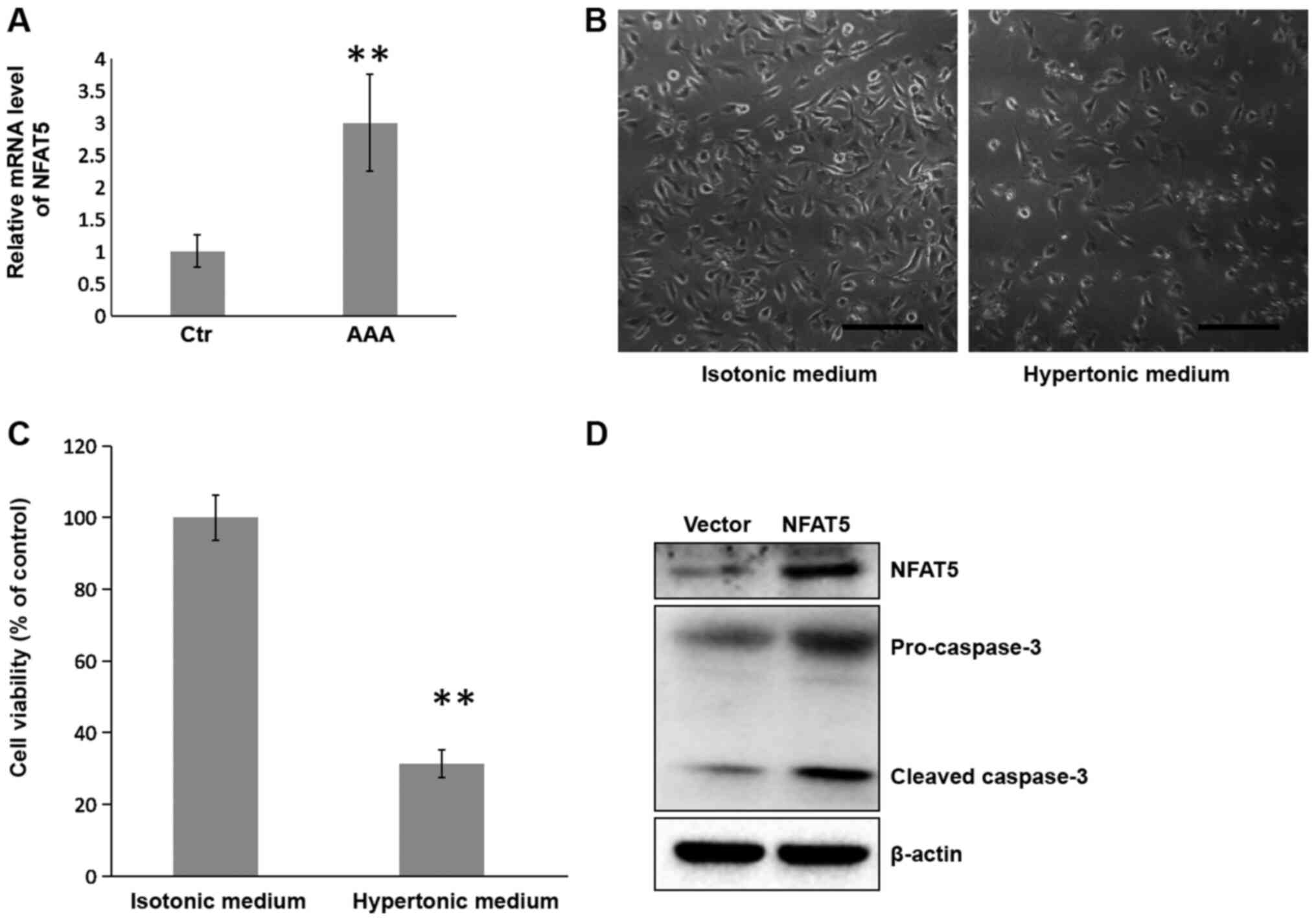 | Figure 1.Hypertonic culture medium causes cell
death of HUVECs and induces caspase-3 cleavage. (A) AAA tissue
samples and adjacent healthy aorta abdominalis Ctr were collected
from patients and subjected to RT-qPCR to detect the mRNA levels of
NFAT5. Data were normalized to GAPDH mRNA levels. All RT-qPCR
experiments were performed in triplicate and the results were
presented as the fold change relative to Ctr. **P<0.01, paired
t-test. HUVECs were cultured in isotonic or hypertonic medium.
After 8 h of incubation, the cells were subjected to (B) phase
contrast microscopy (scale bar, 200 µm), (C) MTS cell viability
assay and (D) western blotting detection of NFAT5 and cleaved
caspase-3. β-actin was used as a loading control. Cell viability is
presented as a percentage of the Ctr. n=9 in each group.
**P<0.01, Student's t-test. HUVEC, human umbilical vein
endothelial cell; AAA, abdominal aortic aneurysm; Ctr, control;
RT-qPCR, reverse transcription-quantitative PCR; NFAT5, nuclear
factor of activated T cells 5. |
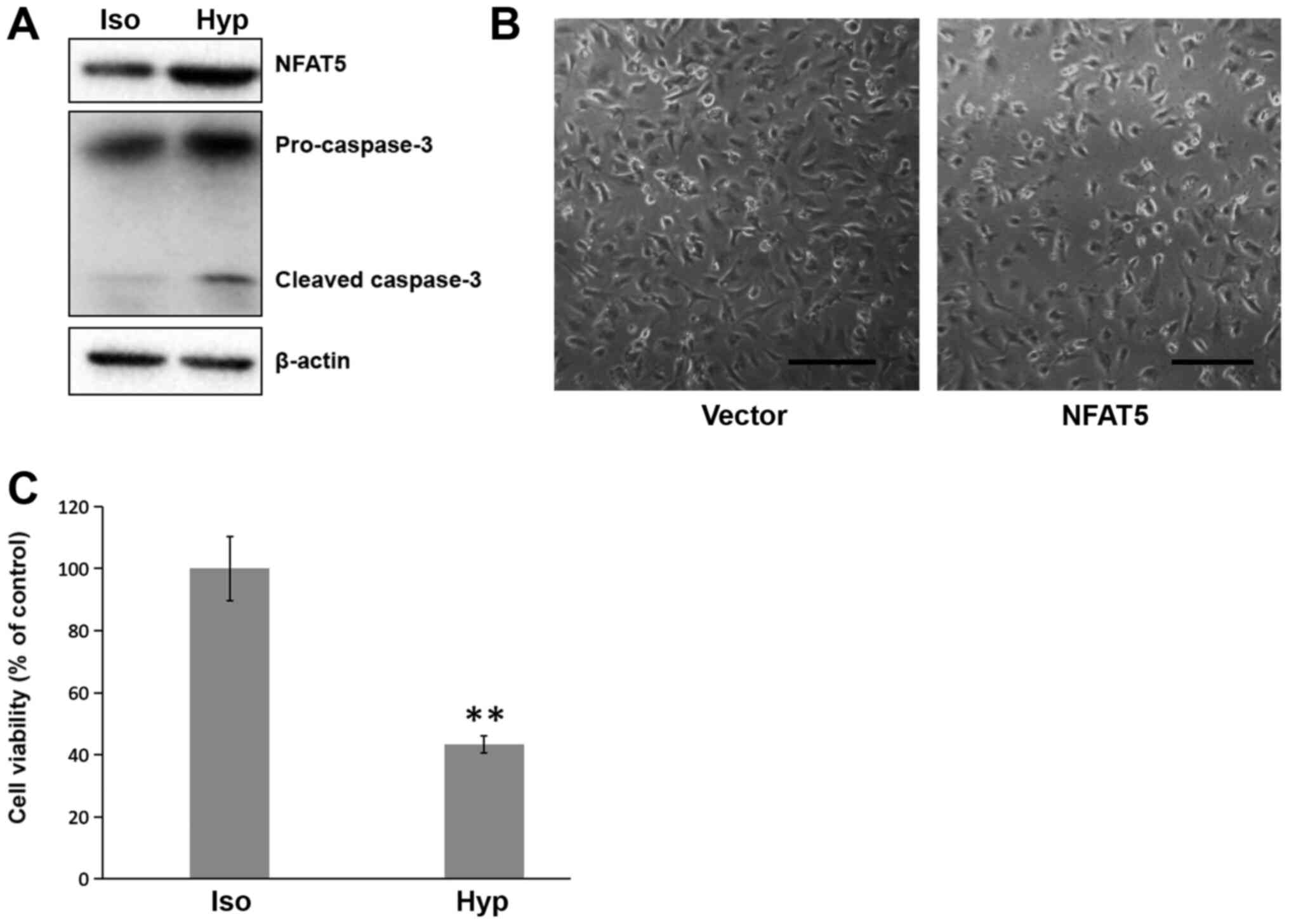
As NFAT5 is induced by hypertonicity (10) and the dysfunction of the aorta
endothelial cells is critical for AAA generation (5), the effect of hypertonicity on
endothelial cells was studied. It has previously been suggested
that cell culture medium with additional 100 mM of NaCl can elevate
the expression of NFAT5 but will not induce apoptosis in HeLa cells
within 8 h of treatment (13). In
order to elucidate the effect of hypertonic environment on HUVECs,
the HUVECs were cultured in the medium with additional 100 mM of
NaCl for 8 h. HUVECs in hypertonic medium underwent significant
cell death (P=0.00034) compared with those in isotonic medium
(Fig. 1B). An MTS assay
demonstrated a viability of ~31% for cells cultured in hypertonic
medium (Fig. 1C). These results
indicate that hypertonicity induces HUVEC death.
To further investigate the underlying mechanism of
HUVEC death, the cleavage of caspase-3, an executor of apoptosis,
was detected by western blotting. The results demonstrated that the
levels of the 17-kDa cleavage band of caspase-3 increased in the
cells cultured in hypertonic medium (Fig. 1D), indicating that cell death was
due to apoptosis. Notably, an increase of NFAT5 protein in cells
subjected to hypertonic culture was also observed (Fig. 1C).
Overexpression of NFAT5 aggravates
apoptosis of HUVECs
To explore the role of NFAT5 in the
hypertonicity-induced apoptosis of HUVECs, NFAT5 was overexpressed
in HUVECs by transfection of pEGFP-NFAT5. The cell viability was
evaluated by MTS assay and the protein extracted from the cells was
then subjected to western blotting of caspase-3 cleavage. As shown
in Fig. 2A, 48 h post transfection,
compared with the control cells transfected with pEGFP-N1 (empty
vector), the cells transfected with pEGFP-NFAT5 demonstrated an
increase of NFAT5 protein and caspase-3 cleavage (Fig. 2A), as well as more severe cell death
compared with those in isotonic medium (Fig. 2B). MTS assay further supported this
finding by showing a 50% reduction of cell viability upon NFAT5
overexpression (Fig. 2C). These
results implied a pro-apoptotic role for NFAT5 in HUVECs.
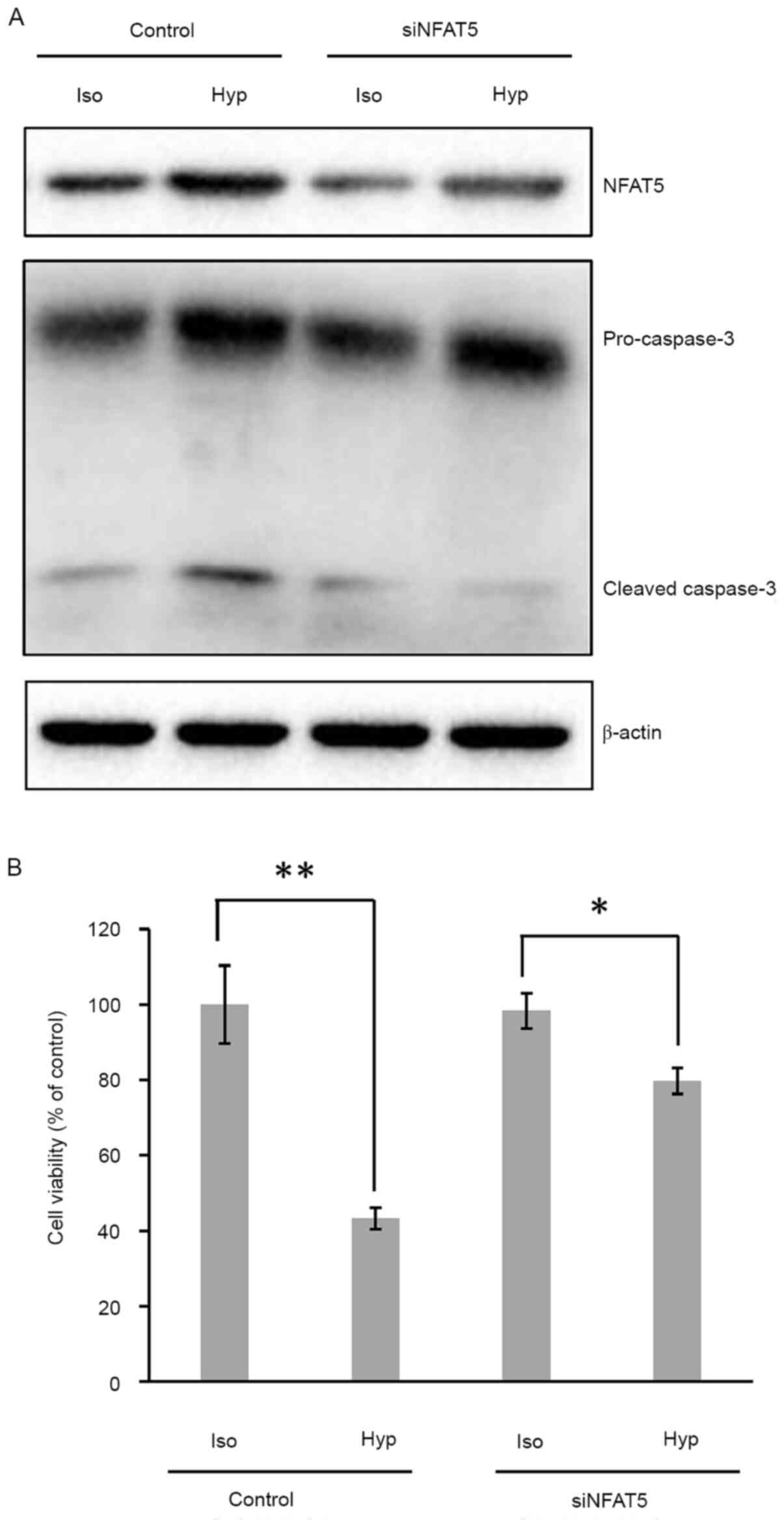
Knockdown of NFAT5 relieves
hypertonicity-induced apoptosis of HUVECs
To verify the role of NFAT5 in hypertonicity-induced
apoptosis of HUVECs, the cells were transfected with NFAT5 siRNA,
then subjected to the treatment of hypertonic medium as
aforementioned. As shown in Fig.
3A, NFAT5 protein was decreased in NFAT5-siRNA-transfected
cells, compared with scramble-siRNA-transfected control cells; in
addition, the caspase-3 cleavage was reduced in hypertonic medium
when NFAT5 was knocked down. MTS assay also demonstrated higher
cell viability of ~80% for NFAT5-knocked-down cells, compared with
~43% for control cells (Fig. 3B).
These results substantiated the involvement of NFAT5 in
hypertonicity-induced apoptosis of HUVECs.
NFAT5 activates NF-κB signaling
pathway in HUVECs
As aforementioned, the NF-κB signaling pathway is
closely associated with the apoptosis of HUVECs, and NFAT5 enhances
NF-κB activity. Thus, it was hypothesized that NFAT5 induced
apoptosis of HUVECs through the NF-κB pathway. To verify this, the
HUVECs were co-transfected with pEGFP-NFAT5 and
pSI-Check2-hRluc-NF-κB-firefly. At 48 h post transfection, the
cells were used in a Dual-luciferase assay. The results
demonstrated that the activity of NF-κB increased 8-fold upon NFAT5
overexpression compared with cells transfected with the empty
vector (Fig. 4A). Hypertonic medium
treatment demonstrated a similar activation of NF-κB (5-fold).
However, this activation was notably reduced when NFAT5 was knocked
down using specific siRNA (Fig.
4B), indicating that hypertonicity activated the NF-κB pathway
through NFAT5.
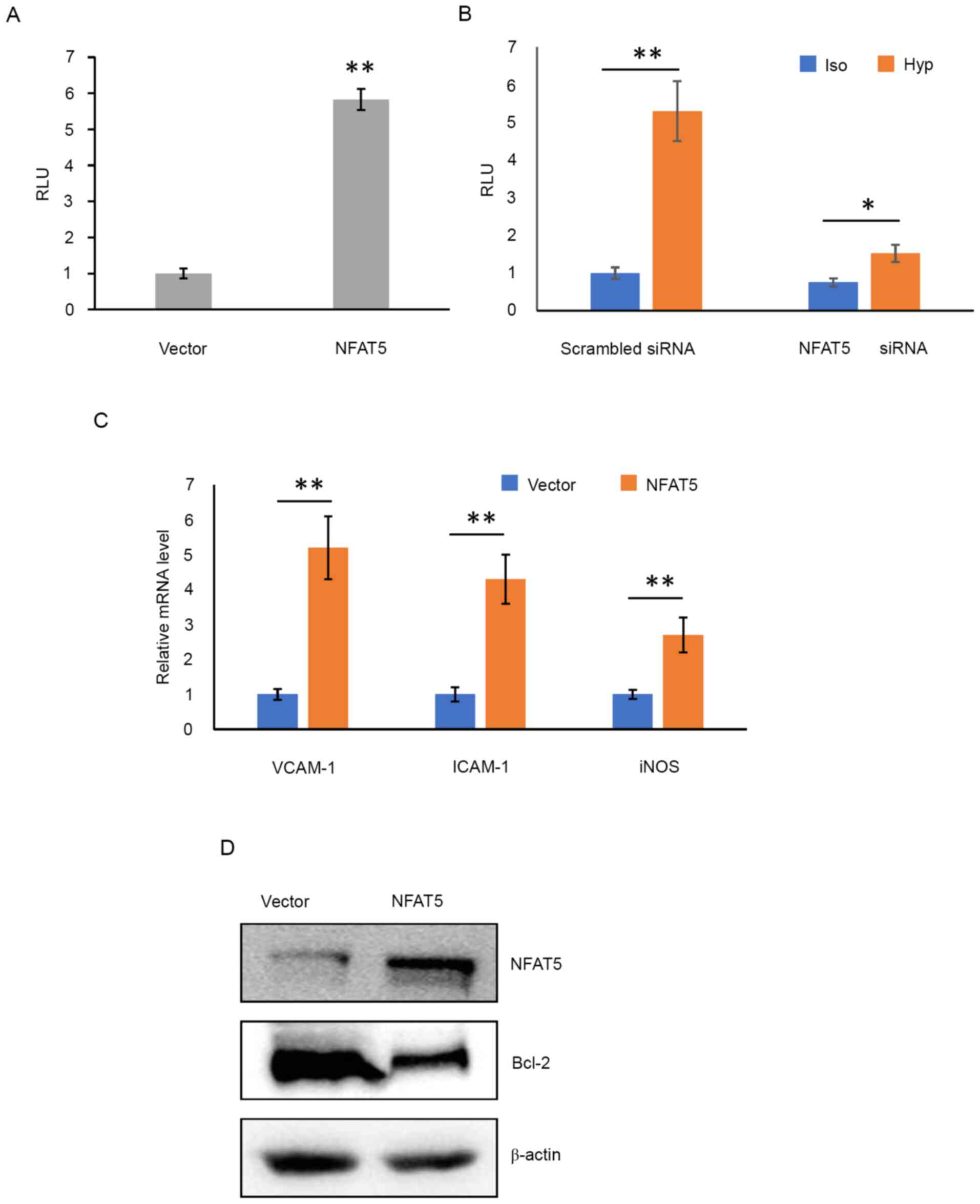 | Figure 4.Overexpression of NFAT5 or
hyptertonic condition enhances the activity of NF-κB in HUVECs. (A)
HUVECs were cotransfected with pSI-Check2-hRluc-NF-κB-firefly and
pEGFP-NFAT5 (NFAT5) or pEGFP-N1 empty vector (Vector). At 48 h post
transfection, the cells were lysed and subjected to dual-luciferase
assay. (B) HUVECs were co-transfected with
pSI-Check2-hRluc-NFκB-firefly and scrambled siRNA or NFAT5 siRNA.
At 40 h post transfection, the culture medium was replaced with
hypertonic medium or isotonic medium as a control. After another 4
h of incubation, the cells were lysed and subjected to
dual-luciferase assay. The luminescence of firefly luciferase was
normalized by the luminescence of Renilla luciferase to
generate RLU. (C) HUVECs were transfected with pEGFP-NFAT5 (NFAT5)
or pEGFP-N1 empty vector. At 48 h post transfection, the cells were
lysed and subjected to RT-qPCR detection of VCAM-1, ICAM-1 and
iNOS. GAPDH mRNA levels were used as the endogenous control to
normalize the data. All RT-qPCR experiments were performed in
triplicate and the results were presented as the fold change of
each vector group. n=9 in each group. *P<0.05, **P<0.01,
Student's t-test. (D) HUVECs were transfected with pEGFP-NFAT5
(NFAT5) or pEGFP-N1 empty vector. At 48 h post transfection, the
cells were subjected to western blotting detection of NFAT5 and
Bcl-2. β-actin was used as a loading control. NFAT5, nuclear factor
of activated T cells 5; HUVEC, human umbilical vein endothelial
cell; si, small interfering; RLU, relative luminescence unit;
RT-qPCR, reverse transcription-quantitative PCR; VCAM-1, vascular
cell adhesion molecule 1; ICAM-1, intercellular adhesion molecule
1; iNOS, inducible nitric oxide synthase; Vector, empty vector. |
NF-κB activation leads to the expression of various
pro-inflammatory genes, contributing to the progression of AAA
(17). To further clarify the
activation of NF-κB signaling cascade induced by NFAT5, the mRNA
levels of VCAM-1, ICAM-1 and iNOS, the three pro-inflammatory genes
downstream of NF-κB, were detected in HUVECs upon NFAT5
overexpression. As shown in Fig.
4C, when NFAT5 was overexpressed, mRNA levels of all the three
genes were significantly elevated (P=0.00058 for VCAM-1, P=0.0012
for ICAM-1 and P=0.0088 for iNOS), indicating an increased
expression of these genes induced by NFAT5.
Overexpression of NFAT5 decreases the
expression of Bcl-2
Bcl-2 is a typical anti-apoptotic protein. It is
reported that NF-κB pathway enhances apoptosis of HUVECs by
downregulating the expression of Bcl-2 (9). Thus, the present study verified the
effect of NFAT5 on Bcl-2 expression in HUVECs. NFAT5 was
overexpressed by transfection as aforementioned. At 48 h post
transfection, the cells were subjected to western blotting
detection of Bcl-2 protein. These results demonstrated that Bcl-2
was dramatically decreased in NFAT5-overexpressed cells compared
with control cells (Fig. 4D),
indicating that NFAT5 inhibited the expression of Bcl-2.
Discussion
AAA is the most common form of aortic aneurysm and
is characterized by enlargement of the abdominal aorta with a
diameter >3 cm or >50% larger than normal (2 cm) (1). The susceptible population of AAA is
elderly males and 2–8% of males over the age of 65 are affected by
AAA; however, AAA is also identified in women with a rate of
one-quarter of that in men (1). AAA
usually causes no symptoms but is accompanied by the risk of
rupture. Upon AAA rupture, the mortality can be as high as 85–90%,
which resulted in 168,200 mortalities around the world in 2013, and
the mortality rate continues to increase (18,19).
Currently, AAA is a great threat to public health, especially to
seniors and surgical treatment is the only option for patients with
large AAA (20).
Although the exact pathogenic causes of AAA are
still unclear, the risk factors for the progress of AAA usually
include tobacco smoking, alcohol, hypertension, genetic background
and atherosclerosis (21,22). High salt intake is also reported to
be closely associated with increased prevalence of AAA (3). As high salt intake is an important
cause of atherosclerosis and hypertension (23,24),
it is reasonable to propose that high salt intake contributes to
the progression of AAA. However, the pathophysiological link
between high salt intake and AAA has rarely been investigated.
Endothelial dysfunction is reported to be critical
for the development of AAA (5).
Briefly, endothelial dysfunction impairs the bioavailability of NO,
which increases oxidative stress and inflammatory infiltration; the
inflammatory cells secretes various proteolytic enzymes and
interleukins that degrades the aortic wall, further leading to
vascular smooth cells transformation and apoptosis, a typical
pathogenic change of AAA. Endothelial dysfunction can be caused by
a variety of factors (5). The
present study used HUVECs as a model and identified that the
extracellular medium with excess NaCl causes the apoptosis of
endothelial cells, which may, at least partly, explain how high
salt intake raises the risk of AAA.
High sodium in the extracellular fluid leads to a
hypertonic environment and brings stress to the cells which will
activate NFAT5, the master transcriptional factor regulating
hypertonic response (10). NFAT5
transcriptionally regulates the expression of various downstream
genes by directly binding to tonicity-responsive enhancer element
in the promoters to relieve hypertonic stress (25). For instance, some genes downstream
of NFAT5 are responsible for the biosynthesis of organic osmolytes
which balance cellular osmotic pressure, including aldose reductase
(26), taurine transporter
(27), betaine/GABA transporter
(28) and sodium/myo-inositol
transporter (29,30). In addition, NFAT5 also induces the
expression of molecular chaperones, such as Hsp70-2 (31) and Osp94 (32), in order to prevent the accumulation
of misfolding proteins in the stress condition. Therefore, NFAT5 is
usually considered as a pro-survival protein that protects the
cells in hypertonic stress. However, the present study identified
that high level of NFAT5 induced the apoptosis of endothelial
cells, indicating diverse functions of NFAT5 on different
cells.
The NF-κB pathway is reported to inhibit the
proliferation and aggravate the apoptosis of HUVECs (9). The NF-κB pathway is multifunctional
and can be either pro-survival or pro-apoptotic for cells in
different circumstances (33,34).
For HUVECs in stress, the NF-κB pathway is likely to be
pro-apoptotic in that it suppresses the expression of Bcl-2, an
anti-apoptotic protein, and thus induces apoptosis (9). Notably, NFAT5 positively regulates the
NF-κB pathway by forming NF-κB-NFAT5 complexes, which promote the
binding of NF-κB to κB elements of NF-κB-responsive genes (12). The results of the present study
demonstrated that overexpression of NFAT5 enhanced the activity of
NF-κB and reduced the levels of Bcl-2, which may explain the
pro-apoptotic role of NFAT5 in HUVECs. In addition, it is also
reported that NFAT5 negatively regulates Bcl-2 and promotes cell
apoptosis in hepatocellular carcinoma (35), which is consistent with the results
of the present study and further supports the mechanism that
NFAT5-Bcl2 mediates hypertonicity-induced apoptosis of HUVECs.
Nevertheless, the present study was based on the
HUVEC cell model and was not verified in animal models, due to the
lack of NFAT5 knockout mice. Conventional NFAT5 knockout mice are
reported to be prenatally lethal due to impaired renal and heart
development (36,37). Additionally, these mice demonstrate
impaired immune responses (38,39),
which hinders their application in the study of inflammatory
diseases, such as AAA. A tamoxifen-inducible NAFT5 knockout mouse
model was established by Kuper et al (40), but tamoxifen itself might affect the
development of AAA (41).
Establishing an adequate NFAT5-deficient animal model for AAA
research, perhaps by using a specific NFAT5 inhibitor, is a major
direction of our future studies.
In summary, the present study suggested a novel
mechanism underlying the apoptosis of endothelial cells in a
hypertonic condition. It identified that hypertonicity induced the
expression of NFAT5 in HUVECs, subsequently activating the NF-κB
pathway and inhibiting Bcl-2 expression, resulting in the apoptosis
of the cells. These findings uncovered a distinctive role of NFAT5
in promoting apoptosis of endothelial cells and provided a possible
explanation for the risk of high salt intake on AAA. In the
prevention and treatment of AAA, more attention should be on
hypertonic conditions in the blood, which may also serve as a
therapeutic target for drug development.
Acknowledgements
The authors would like to thank Dr Qiu Ye (College
of Biology of Hunan University) for providing the cell lines and
plasmids, as mentioned in the manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XX and XF designed the experiments and wrote the
manuscript. CH and DX performed the in vitro work. YL and MH
performed the abdominal aortic bypass surgery and collected patient
samples. JL performed the manuscript check and the data analysis.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Affiliated Hangzhou First People's Hospital, Zhejiang University
School of Medicine. Informed consent was signed by all patients who
participated in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kent KC: Clinical practice. Abdominal
aortic aneurysms. N Eng J Med. 371:2101–2108. 2014. View Article : Google Scholar
|
|
2
|
Spangler R, Van Pham T, Khoujah D and
Martinez JP: Abdominal emergencies in the geriatric patient. Int J
Emerg Med. 7:432014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Golledge J, Hankey GJ, Yeap BB, Almeida
OP, Flicker L and Norman PE: Reported high salt intake is
associated with increased prevalence of abdominal aortic aneurysm
and larger aortic diameter in older men. PLoS One. 9:e1025782014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu S, Gong MC and Guo Z: A new mouse
model for introduction of aortic aneurysm by implantation of
deoxycorticosterone acetate pellets or aldosterone infusion in the
presence of high salt. Methods Mol Biol. 1614:155–163. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siasos G, Mourouzis K, Oikonomou E,
Tsalamandris S, Tsigkou V, Vlasis K, Vavuranakis M, Zografos T,
Dimitropoulos S, Papaioannou TG, et al: The role of endothelial
dysfunction in aortic aneurysms. Curr Pharm Des. 21:4016–4034.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krischek B, Kasuya H, Tajima A, Akagawa H,
Sasaki T, Yoneyama T, Ujiie H, Kubo O, Bonin M, Takakura K, et al:
Network-based gene expression analysis of intracranial aneurysm
tissue reveals role of antigen presenting cells. Neuroscience.
154:1398–1407. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishibashi R, Aoki T, Nishimura M,
Hashimoto N and Miyamoto S: Contribution of mast cells to cerebral
aneurysm formation. Curr Neurovasc Res. 7:113–124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao Y, Gong Y, Liu L, Zhou Y, Fang X,
Zhang C, Li Y and Li J: The use of human umbilical vein endothelial
cells (HUVECs) as an in vitro model to assess the toxicity of
nanoparticles to endothelium: A review. J Appl Toxicol.
37:1359–1369. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen G, Chen Y, Chen H, Li L, Yao J, Jiang
Q, Lin X, Wen J and Lin L: The effect of NF-κB pathway on
proliferation and apoptosis of human umbilical vein endothelial
cells induced by intermittent high glucose. Mol Cell Biochem.
347:127–133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lopez-Rodríguez C, Aramburu J, Rakeman AS
and Rao A: NFAT5, a constitutively nuclear NFAT protein that does
not cooperate with Fos and Jun. Proc Natl Acad Sci USA.
96:7214–7219. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rondon-Berrios H, Argyropoulos C, Ing TS,
Raj DS, Malhotra D, Agaba EI, Rohrscheib M, Khitan ZJ, Murata GH,
Shapiro JI and Tzamaloukas AH: Hypertonicity: Clinical entities,
manifestations and treatment. World J Nephrol. 6:1–13. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roth I, Leroy V, Kwon HM, Martin PY,
Féraille E and Hasler U: Osmoprotective transcription factor
NFAT5/TonEBP modulates nuclear factor-kappaB activity. Mol Biol
Cell. 21:3459–3474. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qiu Y, Ye X, Zhang HM, Hanson P, Zhao G,
Tong L, Xie R and Yang D: Cleavage of osmosensitive transcriptional
factor NFAT5 by Coxsackieviral protease 2A promotes viral
replication. PLoS Pathog. 13:e10067442017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jauliac S, Lopez-Rodriguez C, Shaw LM,
Brown LF, Rao A and Toker A: The role of NFAT transcription factors
in integrin-mediated carcinoma invasion. Nat Cell Biol. 4:540–544.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou W, Pal AS, Hsu AY, Gurol T, Zhu X,
Wirbisky-Hershberger SE, Freeman JL, Kasinski AL and Deng Q:
MicroRNA-223 suppresses the canonical NF-κB pathway in basal
keratinocytes to dampen neutrophilic inflammation. Cell Rep.
22:1810–1823. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parodi FE, Mao D, Ennis TL, Bartoli MA and
Thompson RW: Suppression of experimental abdominal aortic aneurysms
in mice by treatment with pyrrolidine dithiocarbamate, an
antioxidant inhibitor of nuclear factor-kappaB. J Vasc Surg.
41:479–489. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
GBD 2015 Mortality and Causes of Death
Collaborators: Global, regional, and national life expectancy,
all-cause mortality, and cause-specific mortality for 249 causes of
death, 1980–2015: A systematic analysis for the Global Burden of
Disease Study 2015. Lancet. 388:1459–1544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
GBD 2013 Mortality and Causes of Death
Collaborators Global, regional, and national age-sex specific
all-cause and cause-specific mortality for 240 causes of death,
1990–2013: A systematic analysis for the Global Burden of Disease
Study 2013. Lancet. 385:117–171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sakalihasan N, Limet R and Defawe OD:
Abdominal aortic aneurysm. Lancet. 365:1577–1589. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Keisler B and Carter C: Abdominal aortic
aneurysm. Am Fam Physician. 91:538–543. 2015.PubMed/NCBI
|
|
22
|
Greenhalgh RM and Powell JT: Endovascular
repair of abdominal aortic aneurysm. N Engl J Med. 358:494–501.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao X, Yang X, Zhang X, Li Y, Zhao X, Ren
L, Wang L, Gu C, Zhu Z and Han Y: Dietary salt intake and coronary
atherosclerosis in patients with prehypertension. J Clin Hypertens
(Greenwich). 16:575–580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ketonen J, Merasto S, Paakkari I and
Mervaala EM: High sodium intake increases vascular superoxide
formation and promotes atherosclerosis in apolipoprotein
E-deficient mice. Blood Press. 14:373–382. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Halterman JA, Kwon HM and Wamhoff BR:
Tonicity-independent regulation of the osmosensitive transcription
factor TonEBP (NFAT5). Am J Physiol Cell Physiol. 302:C1–C8. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ko BC, Turck CW, Lee KW, Yang Y and Chung
SS: Purification, identification, and characterization of an
osmotic response element binding protein. Biochem Biophys Res
Commun. 270:52–61. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ito T, Fujio Y, Hirata M, Takatani T,
Matsuda T, Muraoka S, Takahashi K and Azuma J: Expression of
taurine transporter is regulated through the TonE
(tonicity-responsive element)/TonEBP (TonE-binding protein) pathway
and contributes to cytoprotection in HepG2 cells. Biochem J.
382:177–182. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miyakawa H, Woo SK, Dahl SC, Handler JS
and Kwon HM: Tonicity-responsive enhancer binding protein, a
rel-like protein that stimulates transcription in response to
hypertonicity. Proc Natl Acad Sci USA. 96:2538–2542. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bosman DK, Deutz NE, Maas MA, van Eijk HM,
Smit JJ, de Haan JG and Chamuleau RA: Amino acid release from
cerebral cortex in experimental acute liver failure, studied by in
vivo cerebral cortex microdialysis. J Neurochem. 59:591–599. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rim JS, Atta MG, Dahl SC, Berry GT,
Handler JS and Kwon HM: Transcription of the sodium/myo-inositol
cotransporter gene is regulated by multiple tonicity-responsive
enhancers spread over 50 kilobase pairs in the 5′-flanking region.
J Biol Chem. 273:20615–20621. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Woo SK, Lee SD, Na KY, Park WK and Kwon
HM: TonEBP/NFAT5 stimulates transcription of HSP70 in response to
hypertonicity. Mol Cell Biol. 22:5753–5760. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kojima R, Randall JD, Ito E, Manshio H,
Suzuki Y and Gullans SR: Regulation of expression of the stress
response gene, Osp94: Identification of the tonicity response
element and intracellular signalling pathways. Biochem J.
380:783–794. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sheikh MS and Huang Y: Death receptor
activation complexes: It takes two to activate TNF receptor 1. Cell
Cycle. 2:550–552. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yeung F, Hoberg JE, Ramsey CS, Keller MD,
Jones DR, Frye RA and Mayo MW: Modulation of NF-kappaB-dependent
transcription and cell survival by the SIRT1 deacetylase. EMBO J.
23:2369–2380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qin X, Wang Y, Li J, Xiao Y and Liu Z:
NFAT5 inhibits invasion and promotes apoptosis in hepatocellular
carcinoma associated with osmolality. Neoplasma. 64:502–510. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mak MC, Lam KM, Chan PK, Lau YB, Tang WH,
Yeung PK, Ko BC, Chung SM and Chung SK: Embryonic lethality in mice
lacking the nuclear factor of activated T cells 5 protein due to
impaired cardiac development and function. PLoS One. 6:e191862011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
López-Rodríguez C, Aramburu J, Jin L,
Rakeman AS, Michino M and Rao A: Bridging the NFAT and NF-kappaB
families: NFAT5 dimerization regulates cytokine gene transcription
in response to osmotic stress. Immunity. 15:47–58. 2001.PubMed/NCBI
|
|
38
|
Go WY, Liu X, Roti MA, Liu F and Ho SN:
NFAT5/TonEBP mutant mice define osmotic stress as a critical
feature of the lymphoid microenvironment. Proc Natl Acad Sci USA.
101:10673–10678. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Trama J, Go WY and Ho SN: The
osmoprotective function of the NFAT5 transcription factor in T cell
development and activation. J Immunol. 169:5477–5488. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kuper C, Beck FX and Neuhofer W:
Generation of a conditional knockout allele for the NFAT5 gene in
mice. Front Physiol. 5:5072014.PubMed/NCBI
|
|
41
|
Grigoryants V, Hannawa KK, Pearce CG,
Sinha I, Roelofs KJ, Ailawadi G, Deatrick KB, Woodrum DT, Cho BS,
Henke PK, et al: Tamoxifen up-regulates catalase production,
inhibits vessel wall neutrophil infiltration, and attenuates
development of experimental abdominal aortic aneurysms. J Vasc
Surg. 41:108–114. 2005. View Article : Google Scholar : PubMed/NCBI
|