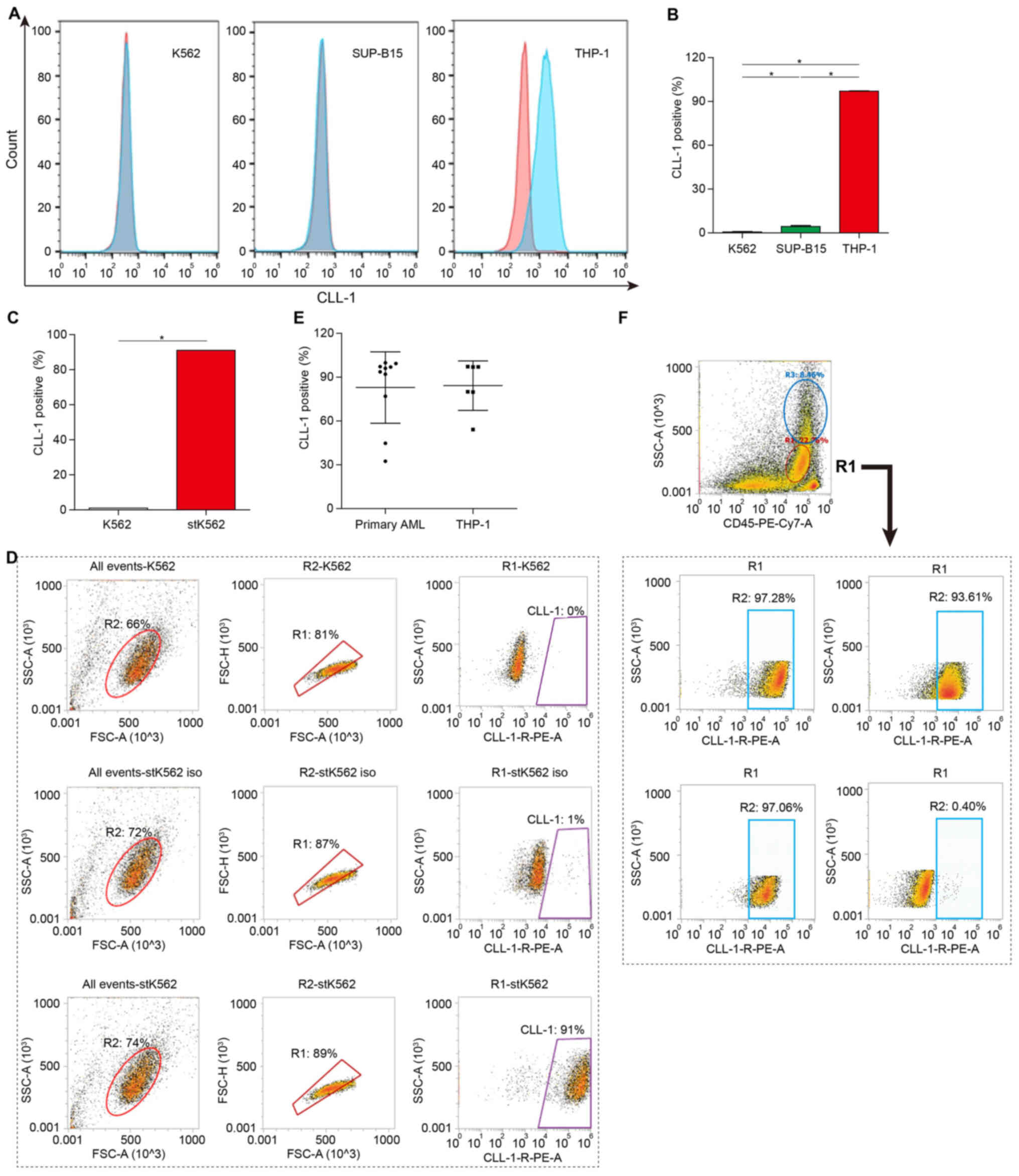
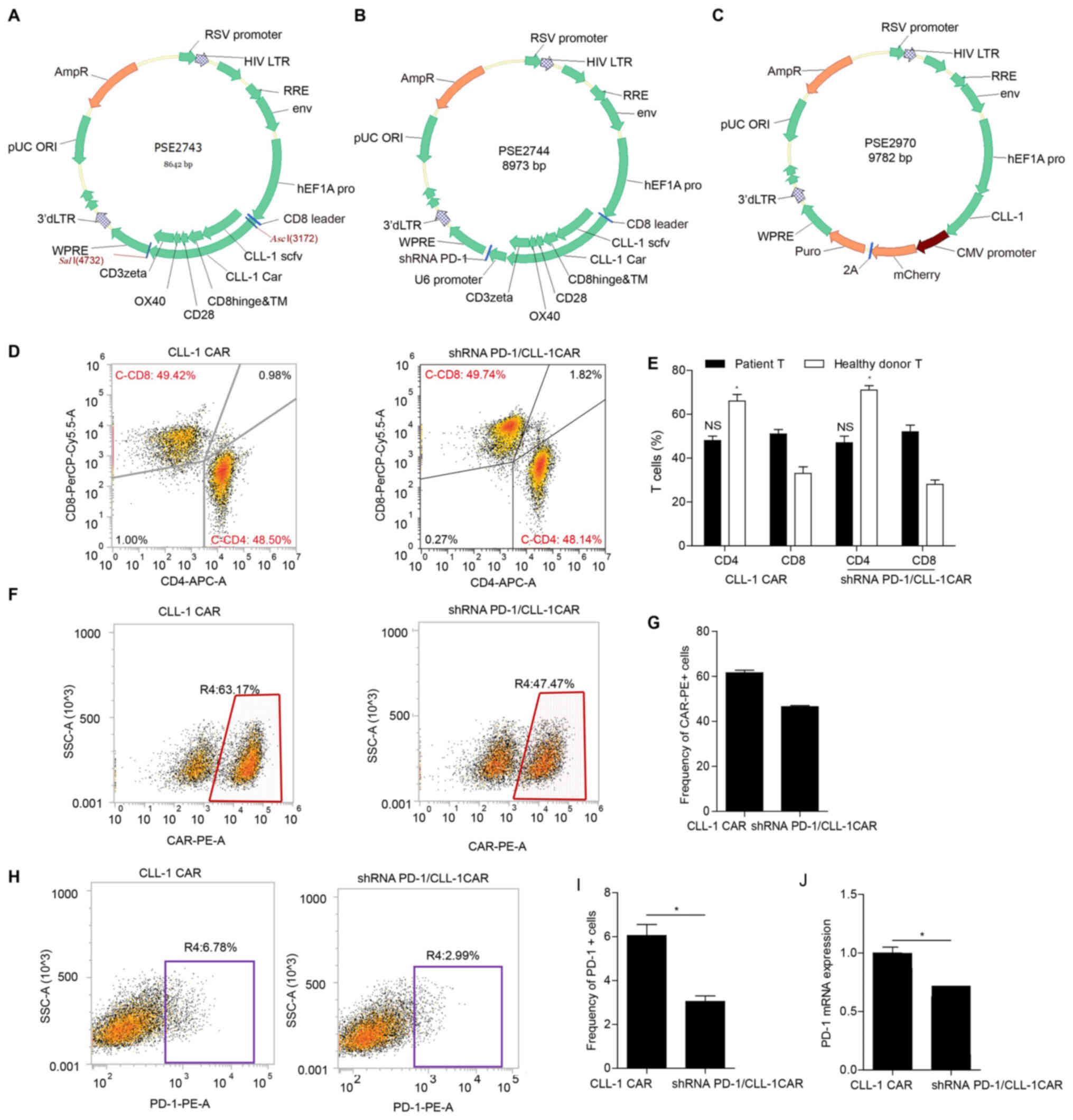
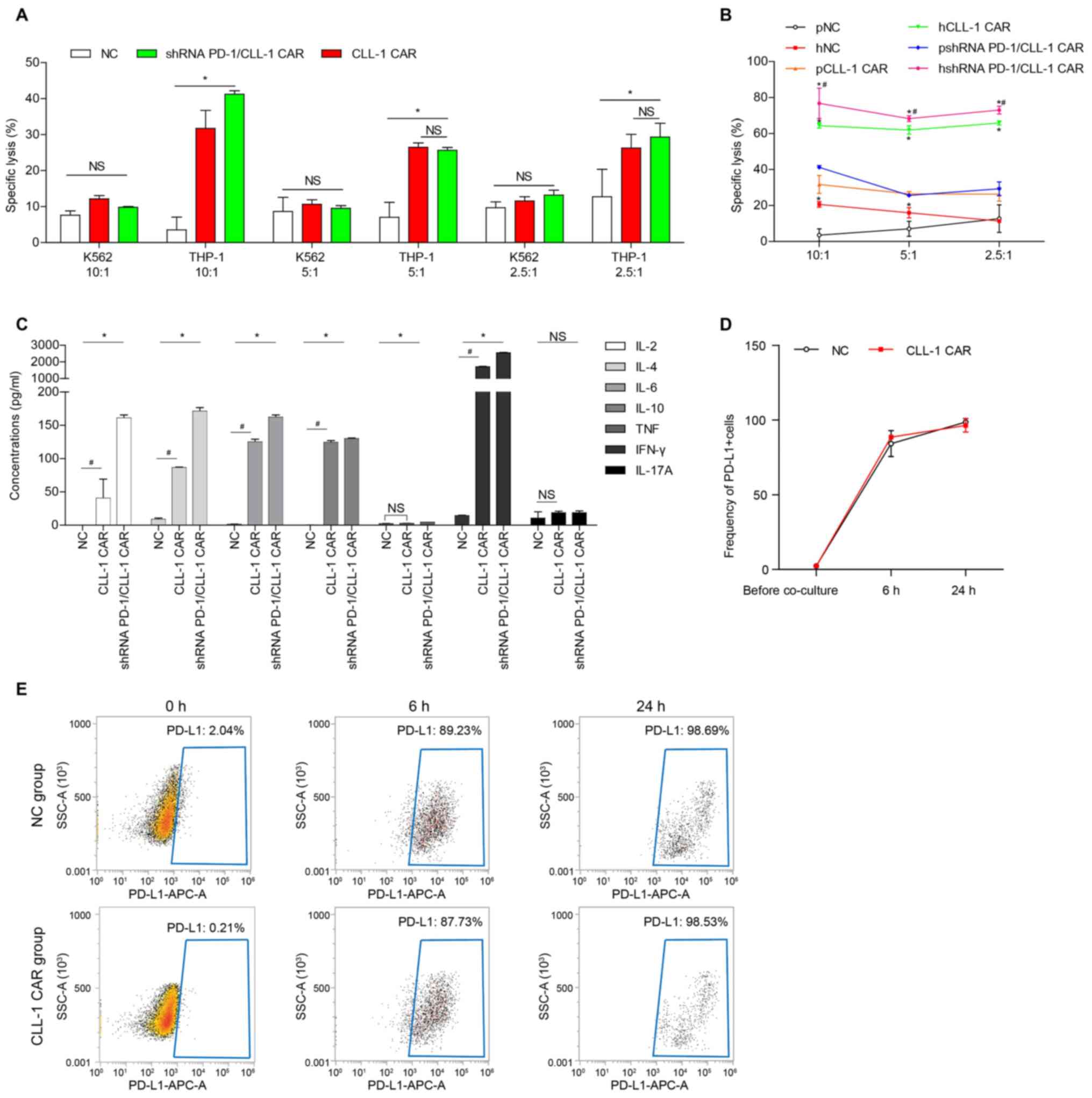
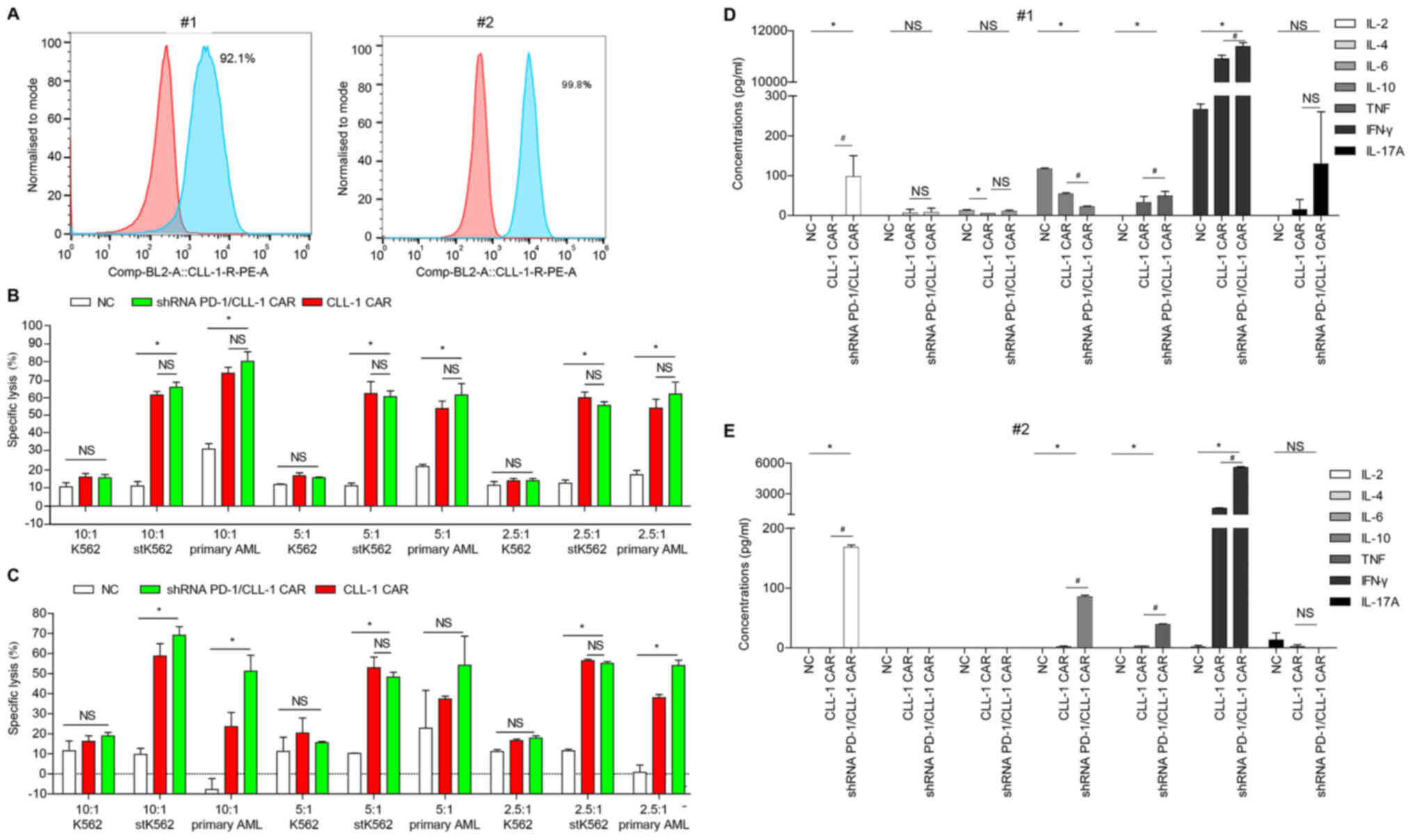
|
1
|
Juliusson G, Lazarevic V, Hörstedt AS,
Hagberg O and Höglund M; Swedish Acute Leukemia Registry Group, :
Acute myeloid leukemia in the real world: Why population-based
registries are needed. Blood. 119:3890–3899. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shallis RM, Wang R, Davidoff A, Ma X and
Zeidan AM: Epidemiology of acute myeloid leukemia: Recent progress
and enduring challenges. Blood Rev. 36:70–87. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
National Cancer Institute: Surveillance,
Epidemiology, and End Results (SEER) Program Cancer Stat Facts:
Leykemia-Acute myeloid leukemia (AML). https://seer.cancer.gov/statfacts/html/amyl.htmlApril
18–2019
|
|
5
|
Chen C, Wang P and Wang C: Prognostic
nomogram for adult patients with acute myeloid leukemia: A SEER
database analysis. Medicine (Baltimore). 98:e158042019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen W, Zheng R, Zhang S, Zeng H, Xia C,
Zuo T, Yang Z, Zou X and He J: Cancer incidence and mortality in
China, 2013. Cancer Lett. 401:63–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rodriguez-Abreu D, Bordoni A and Zucca E:
Epidemiology of hematological malignancies. Ann Oncol. 18 (Suppl
1):i3–i8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sant M, Allemani C, Tereanu C, De Angelis
R, Capocaccia R, Visser O, Marcos-Gragera R, Maynadié M, Simonetti
A, Lutz JM, et al HAEMACARE Working Group, : Incidence of
hematologic malignancies in Europe by morphologic subtype: Results
of the HAEMACARE project. Blood. 116:3724–3734. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Xiao Q, Wang Z and Feng WL: CAR-T
therapy for leukemia: Progress and challenges. Transl Res.
182:135–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tasian SK, Kenderian SS, Shen F, Ruella M,
Shestova O, Kozlowski M, Li Y, Schrank-Hacker A, Morrissette JJD,
Carroll M, et al: Optimized depletion of chimeric antigen receptor
T-cells in murine xenograft models of human acute myeloid leukemia.
Blood. 129:2395–2407. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clarke CA and Glaser SL: Acute myeloid
leukemia. N Engl J Med. 342:358–359. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rowe JM and Tallman MS: How I treat acute
myeloid leukemia. Blood. 116:3147–3156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stahl M, Kim TK and Zeidan AM: Update on
acute myeloid leukemia stem cells: New discoveries and therapeutic
opportunities. World J Stem Cells. 8:316–331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lichtenegger FS, Krupka C, Haubner S,
Köhnke T and Subklewe M: Recent developments in immunotherapy of
acute myeloid leukemia. J Hematol Oncol. 10:1422017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taussig DC, Pearce DJ, Simpson C,
Rohatiner AZ, Lister TA, Kelly G, Luongo JL, Danet-Desnoyers GA and
Bonnet D: Hematopoietic stem cells express multiple myeloid
markers: Implications for the origin and targeted therapy of acute
myeloid leukemia. Blood. 106:4086–4092. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tettamanti S, Marin V, Pizzitola I,
Magnani CF, Giordano Attianese GM, Cribioli E, Maltese F,
Galimberti S, Lopez AF, Biondi A, et al: Targeting of acute myeloid
leukaemia by cytokine-induced killer cells redirected with a novel
CD123-specific chimeric antigen receptor. Br J Haematol.
161:389–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghaffari S, Smadja-Joffe F, Oostendorp R,
Lévesque JP, Dougherty G, Eaves A and Eaves C: CD44 isoforms in
normal and leukemic hematopoiesis. Exp Hematol. 27:978–993. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kikushige Y, Shima T, Takayanagi S, Urata
S, Miyamoto T, Iwasaki H, Takenaka K, Teshima T, Tanaka T, Inagaki
Y, et al: TIM-3 is a promising target to selectively kill acute
myeloid leukemia stem cells. Cell Stem Cell. 7:708–717. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hosen N, Park CY, Tatsumi N, Oji Y,
Sugiyama H, Gramatzki M, Krensky AM and Weissman IL: CD96 is a
leukemic stem cell-specific marker in human acute myeloid leukemia.
Proc Natl Acad Sci USA. 104:11008–11013. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bonardi F, Fusetti F, Deelen P, van
Gosliga D, Vellenga E and Schuringa JJ: A proteomics and
transcriptomics approach to identify leukemic stem cell (LSC)
markers. Mol Cell Proteomics. 12:626–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saito Y, Kitamura H, Hijikata A,
Tomizawa-Murasawa M, Tanaka S, Takagi S, Uchida N, Suzuki N, Sone
A, Najima Y, et al: Identification of therapeutic targets for
quiescent, chemotherapy-resistant human leukemia stem cells. Sci
Transl Med. 2:17ra92010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moshaver B, van Rhenen A, Kelder A, van
der Pol M, Terwijn M, Bachas C, Westra AH, Ossenkoppele GJ,
Zweegman S and Schuurhuis GJ: Identification of a small
subpopulation of candidate leukemia-initiating cells in the side
population of patients with acute myeloid leukemia. Stem Cells.
26:3059–3067. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu J, Wang L, Zhao F, Tseng S, Narayanan
C, Shura L, Willingham S, Howard M, Prohaska S, Volkmer J, et al:
Pre-clinical development of a humanized anti-CD47 antibody with
anti-cancer therapeutic potential. PLoS One. 10:e01373452015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Amadori S, Suciu S, Selleslag D, Aversa F,
Gaidano G, Musso M, Annino L, Venditti A, Voso MT, Mazzone C, et
al: Gemtuzumab ozogamicin versus best supportive care in older
patients with newly diagnosed acute myeloid leukemia unsuitable for
intensive chemotherapy: Results of the randomized phase III
EORTC-GIMEMA AML-19 trial. J Clin Oncol. 34:972–979. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park JH, Rivière I, Gonen M, Wang X,
Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, et
al: Long-term follow-up of CD19 CAR therapy in acute lymphoblastic
leukemia. N Engl J Med. 378:449–459. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cao J, Wang G, Cheng H, Wei C, Qi K, Sang
W, Zhenyu L, Shi M, Li H, Qiao J, et al: Potent anti-leukemia
activities of humanized CD19-targeted chimeric antigen receptor T
(CAR-T) cells in patients with relapsed/refractory acute
lymphoblastic leukemia. Am J Hematol. 93:851–858. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sotillo E, Barrett DM, Black KL, Bagashev
A, Oldridge D, Wu G, Sussman R, Lanauze C, Ruella M, Gazzara MR, et
al: Convergence of acquired mutations and alternative splicing of
CD19 enables resistance to CART-19 immunotherapy. Cancer Discov.
5:1282–1295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fischer J, Paret C, El Malki K, Alt F,
Wingerter A, Neu MA, Kron B, Russo A, Lehmann N, Roth L, et al:
CD19 isoforms enabling resistance to CART-19 immunotherapy are
expressed in B-ALL patients at initial diagnosis. J Immunother.
40:187–195. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fan M, Li M, Gao L, Geng S, Wang J, Wang
Y, Yan Z and Yu L: Chimeric antigen receptors for adoptive T cell
therapy in acute myeloid leukemia. J Hematol Oncol. 10:1512017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Beavis PA, Sek K and Darcy PK: A novel
target antigen for the treatment of acute myeloid leukemia by CAR-T
cells. Mol Ther. 25:1997–1998. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Prommersberger S, Jetani H, Danhof S,
Monjezi R, Nerreter T, Beckmann J, Einsele H and Hudecek M: Novel
targets and technologies for CAR-T cells in multiple myeloma and
acute myeloid leukemia. Curr Res Transl Med. 66:37–38. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu H, Zhou Q, Deshmukh V, Phull H, Ma J,
Tardif V, Naik RR, Bouvard C, Zhang Y, Choi S, et al: Targeting
human C-type lectin-like molecule-1 (CLL1) with a bispecific
antibody for immunotherapy of acute myeloid leukemia. Angew Chem
Int Ed Engl. 53:9841–9845. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bakker AB, van den Oudenrijn S, Bakker AQ,
Feller N, van Meijer M, Bia JA, Jongeneelen MA, Visser TJ, Bijl N,
Geuijen CA, et al: C-type lectin-like molecule-1: A novel myeloid
cell surface marker associated with acute myeloid leukemia. Cancer
Res. 64:8443–8450. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang J, Chen S, Xiao W, Li W, Wang L, Yang
S, Wang W, Xu L, Liao S, Liu W, et al: CAR-T cells targeting CLL-1
as an approach to treat acute myeloid leukemia. J Hematol Oncol.
11:72018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tashiro H, Sauer T, Shum T, Parikh K,
Mamonkin M, Omer B, Rouce RH, Lulla P, Rooney CM, Gottschalk S, et
al: Treatment of acute myeloid leukemia with T-cells expressing
chimeric antigen receptors directed to C-type lectin-like
molecule-1. Mol Ther. 25:2202–2213. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Heczey A, Louis CU, Savoldo B, Dakhova O,
Durett A, Grilley B, Liu H, Wu MF, Mei Z, Gee A, et al: CAR-T cells
administered in combination with lymphodepletion and PD-1
inhibition to patients with neuroblastoma. Mol Ther. 25:2214–2224.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ankri C, Shamalov K, Horovitz-Fried M,
Mauer S and Cohen CJ: Human T-cells engineered to express a
programmed death 1/28 costimulatory retargeting molecule display
enhanced antitumor activity. J Immunol. 191:4121–4129. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arber DA, Orazi A, Hasserjian R, Thiele J,
Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M and Vardiman JW:
The 2016 revision to the World Health Organization classification
of myeloid neoplasms and acute leukemia. Blood. 127:2391–2405.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jin HT, Ahmed R and Okazaki T: Role of
PD-1 in regulating T cell immunity. Curr Top Microbiol Immunol.
350:17–37. 2011.PubMed/NCBI
|
|
41
|
van Rhenen A, van Dongen GA, Kelder A,
Rombouts EJ, Feller N, Moshaver B, Stigter-van Walsum M, Zweegman
S, Ossenkoppele GJ and Jan Schuurhuis G: The novel AML stem cell
associated antigen CLL-1 aids in discrimination between normal and
leukemic stem cells. Blood. 110:2659–2666. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Larsen HO, Roug AS, Just T, Brown GD and
Hokland P: Expression of the hMICL in acute myeloid leukemia-a
highly reliable disease marker at diagnosis and during follow-up.
Cytometry B Clin Cytom. 82:3–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Darwish NH, Sudha T, Godugu K, Elbaz O,
Abdelghaffar HA, Hassan EE and Mousa SA: Acute myeloid leukemia
stem cell markers in prognosis and targeted therapy: Potential
impact of BMI-1, TIM-3 and CLL-1. Oncotarget. 7:57811–57820. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang YY, Chen WL, Weng XQ, Sheng Y, Wu J,
Hao J, Liu ZY, Zhu YM, Chen B, Xiong SM, et al: Low CLL-1
expression is a novel adverse predictor in 123 patients with de
novo CD34+ acute myeloid leukemia. Stem Cells Dev.
26:1460–1467. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Laborda E, Mazagova M, Shao S, Wang X,
Quirino H, Woods AK, Hampton EN, Rodgers DT, Kim CH, Schultz PG, et
al: Development of a chimeric antigen receptor targeting C-type
lectin-like molecule-1 for human acute myeloid leukemia. Int J Mol
Sci. 18:182017. View Article : Google Scholar
|
|
46
|
Dunn GP, Old LJ and Schreiber RD: The
immunobiology of cancer immunosurveillance and immunoediting.
Immunity. 21:137–148. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Park Y, Lim J, Kim S, Song I, Kwon K, Koo
S and Kim J: The prognostic impact of lymphocyte subsets in newly
diagnosed acute myeloid leukemia. Blood Res. 53:198–204. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Alcasid M, Ma L, Gotlib JR, Arber DA and
Ohgami RS: The clinicopathologic significance of lymphocyte subsets
in acute myeloid leukemia. Int J Lab Hematol. 39:129–136. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gray KD, Vedvyas Y, Kalloo O, Shevlin E
and Min IM: Abstract 2738: PD-L1/PD-1 checkpoint inhibition in
anaplastic thyroid cancer and enhancement of ICAM-1-targeted
chimeric antigen receptor (CAR)-T cell tumor lysis. Cancer Res.
78:2738. 2018.
|
|
50
|
Williams P, Basu S, Garcia-Manero G,
Hourigan CS, Oetjen KA, Cortes JE, Ravandi F, Jabbour EJ, Al-Hamal
Z, Konopleva M, et al: The distribution of T cell subsets and the
expression of immune checkpoint receptors and ligands in patients
with newly diagnosed and relapsed acute myeloid leukemia. Cancer.
125:1470–1481. 2019. View Article : Google Scholar : PubMed/NCBI
|