Introduction
Intracranial aneurysm is one of the common disorders
in clinical neuroscience with an incidence of approximately 3.2%
worldwide (1). The incidence of
intracranial aneurysm in the female population is at ≤12.9%,
significantly higher compared with the male population (2). Notably, intracranial aneurysm is a
non-ignorable factor for subarachnoid hemorrhage following cerebral
thrombosis and hypertensive cerebral hemorrhage in cerebrovascular
accidents (3,4). Indeed, >85% of spontaneous
subarachnoid hemorrhage is caused by rupture of aneurysms (3,4).
Therefore, it is of great clinical significance to explore the
pathogenesis of intracranial aneurysms and find effective
therapeutic targets.
There are many types of Toll-like receptor (TLRs)
subfamilies. TLR1, TLR2, TLR4, TLR5, TLR6 and TLR10 are found on
the cell surface and can be activated by a variety of extracellular
ligands, while TLR3, TLR7, TLR8 and TLR9 are intracellularly
expressed and generally recognized by nucleic acid structure
(5). TLRs serve important roles in
the inflammatory response of central nervous system (6). TLR2 and TLR4 expression increases in
patients with ischemic stroke and TLR4 knockdown attenuates brain
edema, inflammation and damage after intracerebral hemorrhage (ICH)
(7). In addition, TLR2 promotes
inflammatory damage after ICH (8).
Hemoglobin can induce the formation of TLR2/TLR4 heterodimer after
ICH, which can amplify the harmful effects of TLR2 and TLR4 in the
brain (9).
The major myeloid differentiation response gene 88
(MyD88) is an important adaptor that is downstream of TLR2 and TLR4
(10). Activated MyD88 can promote
the phosphorylation and nuclear translocation of NF-κB, thus
upregulating the expression of pro-inflammatory factors and matrix
metalloproteinases and aggravating tissue damage (11–13).
Therefore, TLR2/4 and its downstream signaling pathway serve an
important regulatory role in nerve tissue injury. It has also been
reported that TLR4 is upregulated during cerebral aneurysm
formation in endothelial cells (14,15).
How TLR2/4 and their downstream signaling pathway is involved in
the pathogenesis of intracranial aneurysm remains to be
elucidated.
The present study focused on the association between
the TLR2/4-MyD88-NF-κB signaling pathway and intracranial aneurysm.
The expression of the TLR2/4-MyD88-NF-κB signaling pathway was
detected in the serum from intracranial aneurysm patients. Through
an in vitro model, the involvement of the TLR2/4-MyD88-NF-κB
signaling pathway in the pathogenesis of intracranial aneurysm was
investigated.
Materials and methods
Patients
Venous blood (5 ml; upper extremity) was collected
from patients with intracranial aneurysm (n=222) and age- and
sex-matched normal controls (n=200). The inclusion criteria were as
follows: Intracranial aneurysm diagnosed by digital subtraction
angiography following initial screening of magnetic resonance
angiogram or computerized tomography angiography; ruptured
aneurysm; Hunt and Hess grade 1–3 (16); and patients receiving endovascular
embolization. The exclusion criteria included: Delayed neurological
deficits; Glasgow Coma Scale score (17) decreased by ≥2 points; with or
without cerebral infarction unrelated to aneurysm treatment or
other causes; neurogenic pulmonary edema, hydrocephalus and
epilepsy. In total, 222 patients (50% male; age: 45–65 years) were
enrolled and 200 normal controls (50% male; age: 44–65 years) were
recruited from the Health Examination Center of the First
Affiliated Hospital of Nanchang University between January 1, 2018
and May 1, 2019. Written informed consents were obtained from the
participants. All experimental procedures were approved by the
Ethics Committee of Nanchang University (approval no.
NCU-20201213).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA from blood cells (3×105
cells/ml) was extracted using a TRIzol® assay kit
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The purity of RNA was determined using a
NanoDrop spectrophotometer (NanoDrop Technologies; Thermo Fisher
Scientific, Inc.) based on the optical density (OD): OD280/260.
Thereafter, RNA was transcribed into cDNA according to the
instructions of the reverse transcription kit (cat. no. 639522;
Takara Biotechnology Co., Ltd.). qPCR was used to detect the
expression level of the targeted genes using the synthesized cDNA
as templates. The primers (5′→3′) are listed in Table I. The PCR was processed in a 20 µl
reaction system including 1 µl cDNA, 2 µl primers, 10 µl 2X
ULtraSYBR Mixture (CoWin Biosciences) and 7 µl dH2O with
thermocycling as follows: Denaturing 10 sec at 95°C, annealing 30
sec at 58°C and extension 30 sec at 72°C for 40 cycles. Relevant
expression of MyD88, TLR2 and TLR4 was normalized to GAPDH using
the 2−ΔΔCq method (18).
Experiments were repeated 6 times.
 | Table I.Primer sequences of MyD88, TLR2 and
TLR4. |
Table I.
Primer sequences of MyD88, TLR2 and
TLR4.
| Genes | Sequence
(5′→3′) | Primer length
(bp) | Product length
(bp) | Annealing
temperature (°C) |
|---|
| MyD88 F |
CAGCGACATCCAGTTTGTGC | 20 | 153 | 59.7 |
| MyD88 R |
GGCCTTCTAGCCAACCTCTT | 20 |
|
|
| TLR2 F |
ATGCTGCCATTCTCATTCTTC | 21 | 100 | 57.8 |
| TLR2 R |
TCCAGGTAGGTCTTGGTGTTC | 21 |
|
|
| TLR4 F |
GACCTGTCCCTGAACCCTAT | 20 | 136 | 57.6 |
| TLR4 R |
CTAAACCAGCCAGACCTTGA | 20 |
|
|
| GAPDH F |
CAATGACCCCTTCATTGACC | 20 | 106 | 57.2 |
| GAPDH R |
GAGAAGCTTCCCGTTCTCAG | 20 |
|
|
ELISA
TLR2 (cat. no. ab131556; Abcam), TLR4 (cat. no.
MBS2702401; MyBioSource, Inc.), MyD88 (cat. no. ab171341; Abcam)
and NF-κB (cat. no. ab133112; Abcam) levels in serum were detected
by ELISA method following the instructions of the assay kits.
Experimental groups
Human brain vascular smooth muscle cells (BVSMCs)
were purchased from Shanghai Maisha Biotechnology Co., Ltd, and
cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(FBS) and 100 U/ml penicillin-streptomycin in 5% CO2 at
37°C. The experiments were divided into four groups: A control
group, an angiotensin II (Ang II) group, an Ang II + small
interfering (si)RNA control group and an Ang II + TLR2-siRNA group.
The plasmids were transfected when cell confluence reached 80%. The
transfection solution included 125 µl optiMEM, 5 µl
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) and 2.5 µg pLV Puro vector (cat. no. VL3103;
Beijing Inovogen Tech. Co., Ltd.) or 1 µg siRNA, and the mixture
was incubated at room temperature for 5 min. The mixture was added
into the corresponding well (final concentration of plasmid: 1
µg/ml). The culture medium (medium containing 20% FBS) was
refreshed 6 h following transfection. Following 24 h of
transfection, BVSMCs were treated with Ang II to induce cell
remodeling as previously described (19). The sequences of three TLR2 siRNAs
and negative control (NC) were synthesized by Universal Biological
Systems (Anhui) Co., Ltd. and were as follows: TLR2 siRNA-1,
5′-GCUGACAUCCAAUGGAAUUAAUUCCAUUGGAUGUCAGC-3′; TLR2 siRNA-2,
5′-GGGACUUCAUUCCUGGCAAUUGCCAGGAAUGAAGUCCC-3′; TLR2 siRNA-3,
5′-GCAAGCUGCGGAAGAUAAUAUUAUCUUCCGCAGCUUGC-3′; and NC,
5′-UUCUCCGAACGUGUCACGUTTACGUGACACGUUCGGAGAATT-3′.
Following treatment for 48 h, cell remodeling of
BVSMCs was detected based on cell proliferation, migration and
apoptosis. The expressions of TLR2, TLR4, MyD88, NF-κB and p-p65
were detected by RT-qPCR and western blotting.
Cell Counting Kit-8 (CCK-8) assay
Medium (10 µl) with CCK-8 (Gibco; Thermo Fisher
Scientific, Inc.) was added into each well following drug treatment
or transfection. After an additional 4-h incubation in a
CO2 incubator at 37°C, the absorbance at 570 nm was
recorded by a microplate reader (Thermo Fisher Scientific, Inc.).
Cell proliferation was calculated based on OD values.
Flow cytometry
The cells in each group were collected after
digestion by trypsin (Gibco; Thermo Fisher Scientific, Inc.).
Thereafter, the cells were incubated with Annexin V-FITC and
propidium iodide using an Annexin V-FITC apoptosis detection kit
(cat. no. C1062; Beyotime Institute of Biotechnology) for 30 min in
the dark at room temperature. Apoptosis was detected using an
Accuri C6 flow cytometer (BD Biosciences) within 1 h. Data were
analyzed using FlowJo software (version 7.6; FlowJo, LLC). The
apoptotic rate was calculated based on the percentages of early
apoptotic cells (EA) and late apoptotic cells (LA). The equation
was as follows: Apoptotic rate (%) = [(Number of EA + Number of
LA)/Total number of EA and LA] ×100.
Cell migration
Cells were seeded into a 6-well plate
(1×106 cells/well) and following 24 h of incubation at
37°C, a uniform line was made across the center of the well using a
200-µl pipette tip. Following 24 or 48 h incubation in DMEM (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS in a
CO2 incubator at 37°C, the images were captured under a
light microscope (magnification, ×200; Olympus Corporation). The
migration speed was calculated based on the following formula: Cell
mobility (µm/h) = [Width(1)-width(2)]/72 h.
Width(1) represented the width measured immediately (0
h) after drawing the line. Width(2) represented the
width measured 24 or 48 h after drawing the line.
Western blotting
Protein was extracted by a protein isolation kit
(cat. no. 28-9425-44; ReadyPrep) which was purchased from Cytiva.
The concentration of the protein was quantified with a
bicinchoninic acid protein assay kit. Thereafter, 25 µg protein was
separated via 12% sodium dodecyl sulfate polyacrylamide gel
electrophoresis. The proteins were then transferred onto PVDF
membranes and blocked in 5% milk (2 h at room temperature). The
following primary antibodies were incubated with the membranes
overnight at 4°C: Rabbit polyclonal anti-TLR2 (1:500; cat. no.
ab213676, Abcam); rabbit polyclonal anti-TLR4 (1:1,000; cat. no.
bs-20594R, BIOSS); rabbit polyclonal anti-MyD88 (1:1,000; cat. no.
bs-1047R, BIOSS); rabbit polyclonal anti-NF-κB p65 (1:1,000; cat.
no. bs-0465R, BIOSS); rabbit polyclonal anti-p-p65 (1:1,000;
AF2006, affinity). The secondary antibody (1:2,000; ZB-2305;
OriGene Technologies, Inc.) was co-incubated for 2 h at room
temperature. Protein bands were visualized using ECL solution (cat.
no. SW2010-1; Beijing Solarbio Science & Technology Co., Ltd.).
Densitometric analysis was performed using Quantity One software
(version 4.6; Bio-Rad Laboratories, Inc.).
Statistical analyses
Data in the present study were presented as the mean
± standard deviation and analyzed using SPSS version 17.0 (SPSS,
Inc.). All data were in normal distribution and unpaired t-test or
one-way analysis of variance followed by the Bonferroni's test was
employed in the data analysis. P<0.05 was considered to indicate
a statistically significant difference.
Results
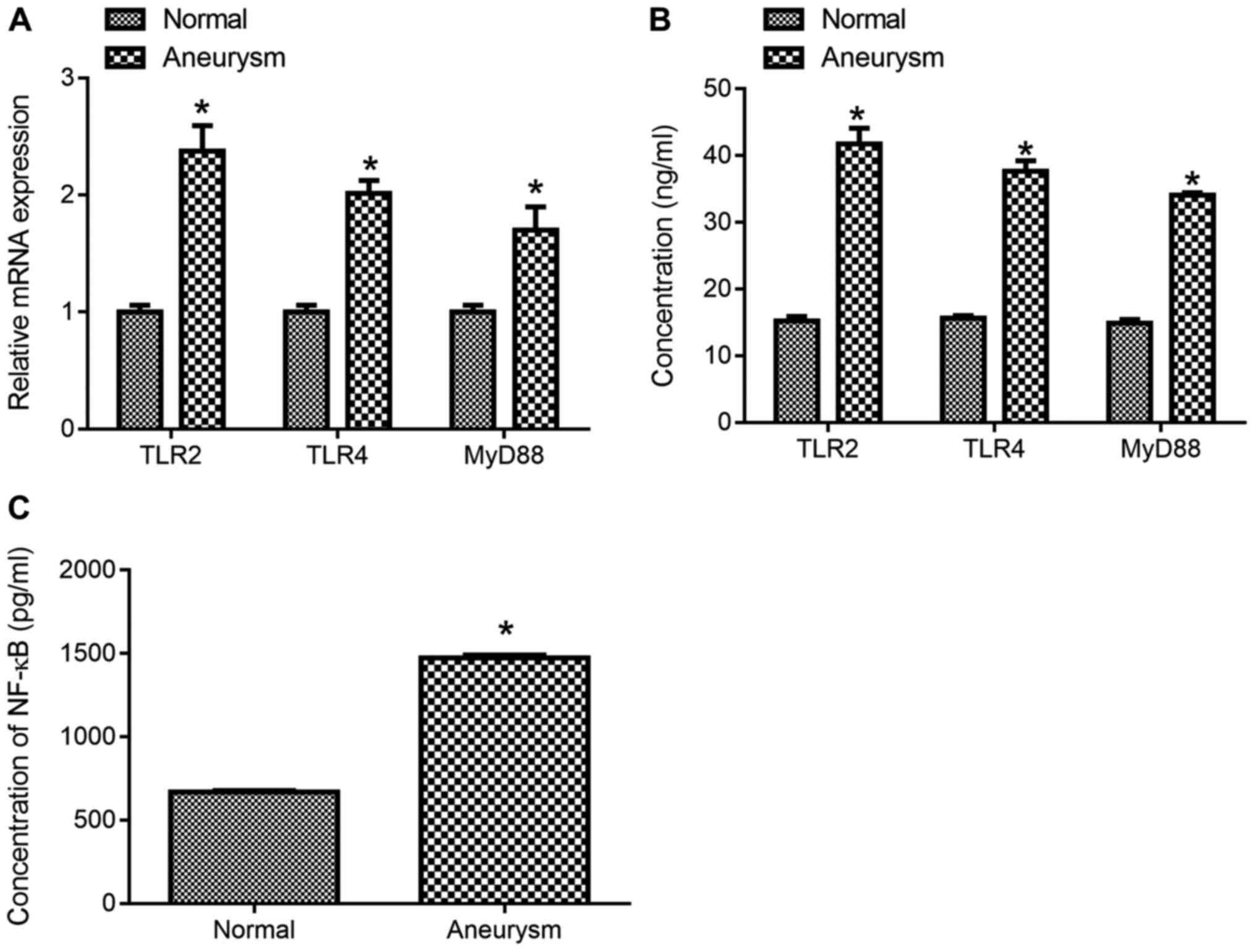
Expression of TLR2, TLR4, MyD88 and
NF-κB in the serum of patients with intracranial aneurysms
The expression of TLR2, TLR4 and MyD88 mRNA in
patients with intracranial aneurysm was significantly higher
compared with normal controls (Fig.
1A; P<0.05). The expression levels of TLR2, TLR4, MyD88
(Fig. 1B) and NF-κB p65 (Fig. 1C) in patients with intracranial
aneurysm were significantly higher compared with normal control
(Fig. 1B and C; P<0.05).
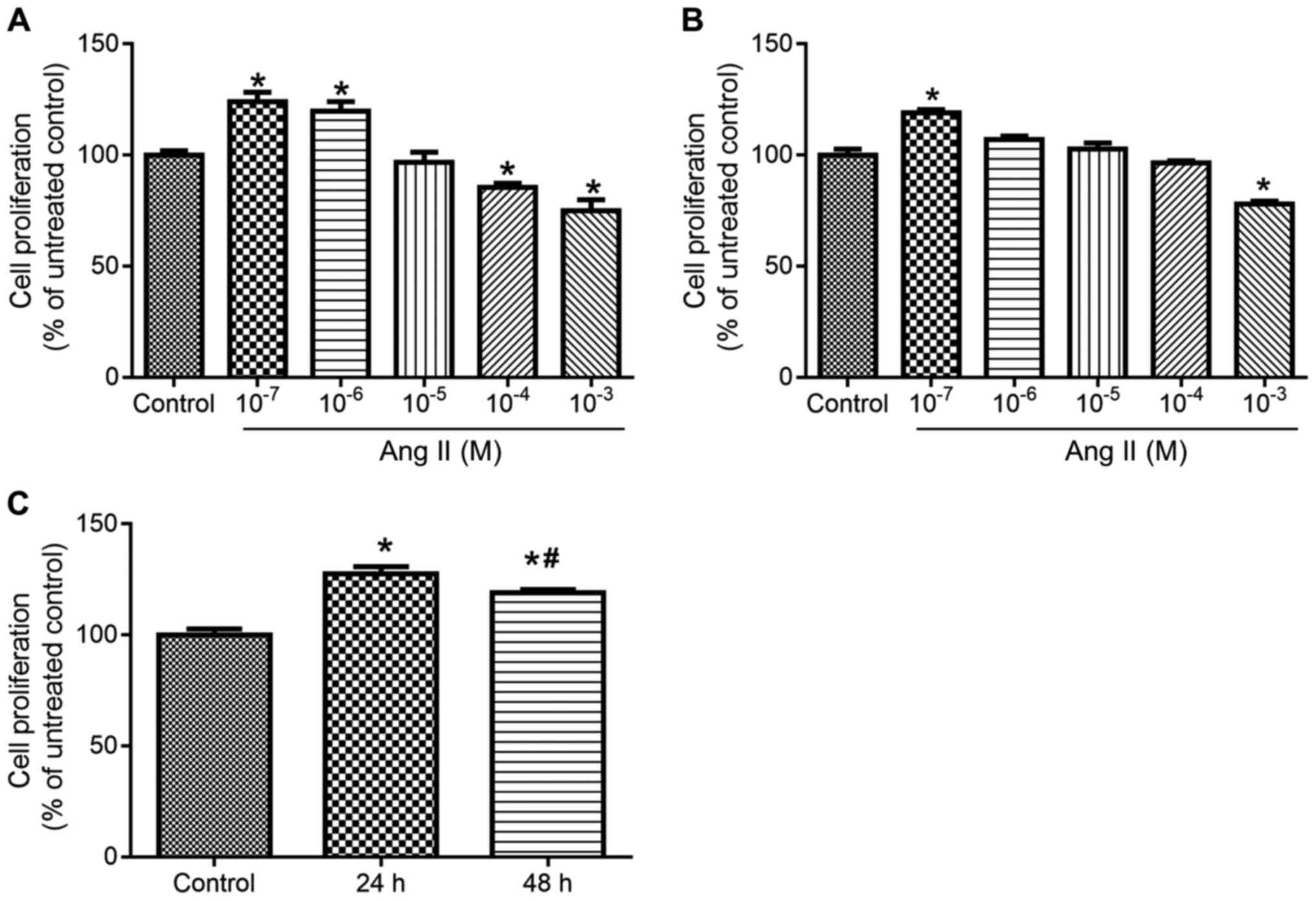
TLR2-siRNA attenuates Ang II-induced
cell proliferation of BVSMCs
As shown in Fig. 2A and
B, 10−7 M Ang II facilitated cell proliferation
after administration for 24 and 48 h compared with control group.
Thus, this concentration of Ang II was selected to produce the
remodeling model of BVSMCs. As shown in Fig. 2C, the cell proliferation increased
significantly 24 h after Ang II administration. Therefore,
10−7 M Ang II was selected to treat the cells for 24
h.
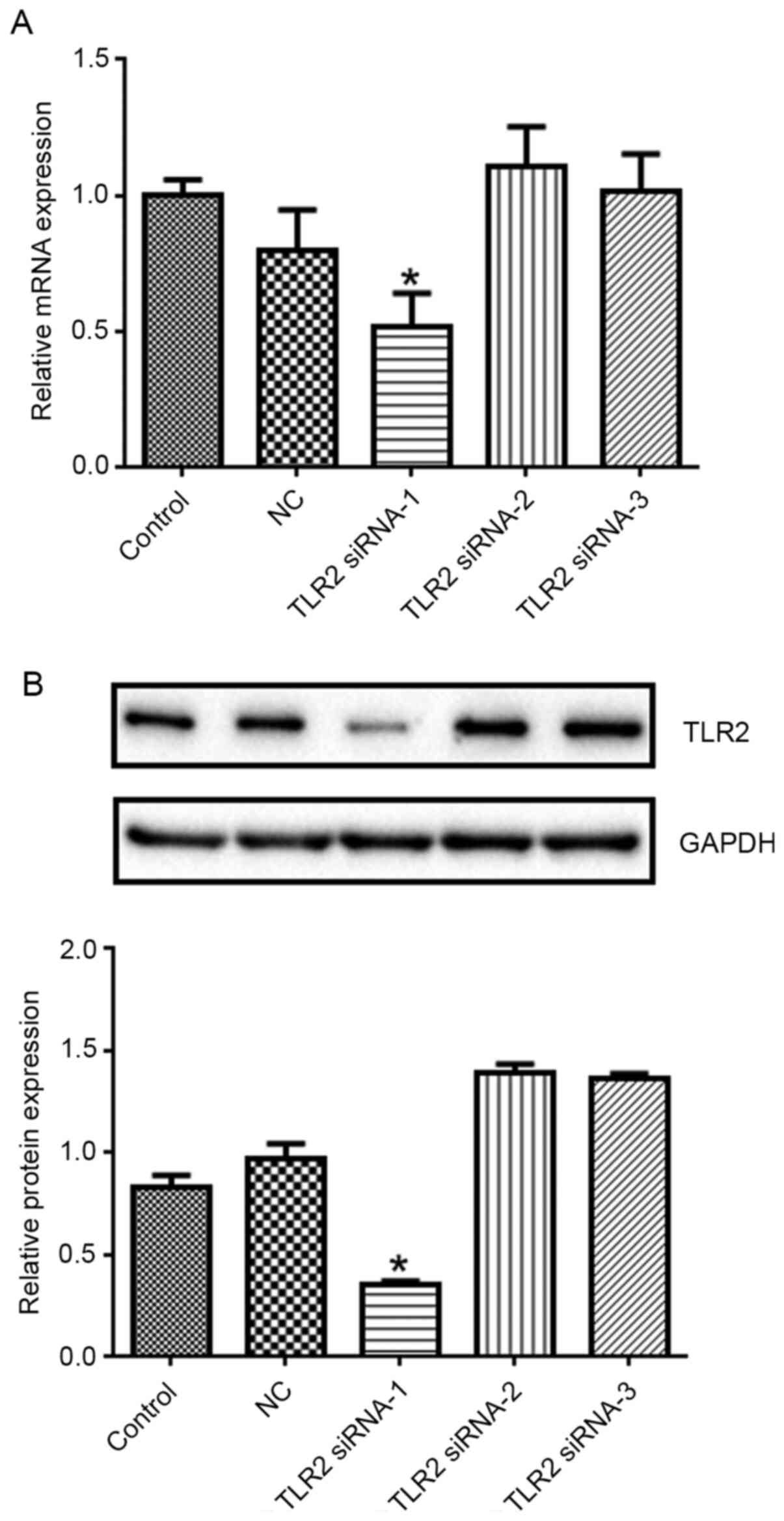
TLR2 expression in the TLR2 siRNA-1 group decreased
significantly (Fig. 3; P<0.05
vs. control). Therefore, TLR2 siRNA-1 was selected in the
subsequent experiments.
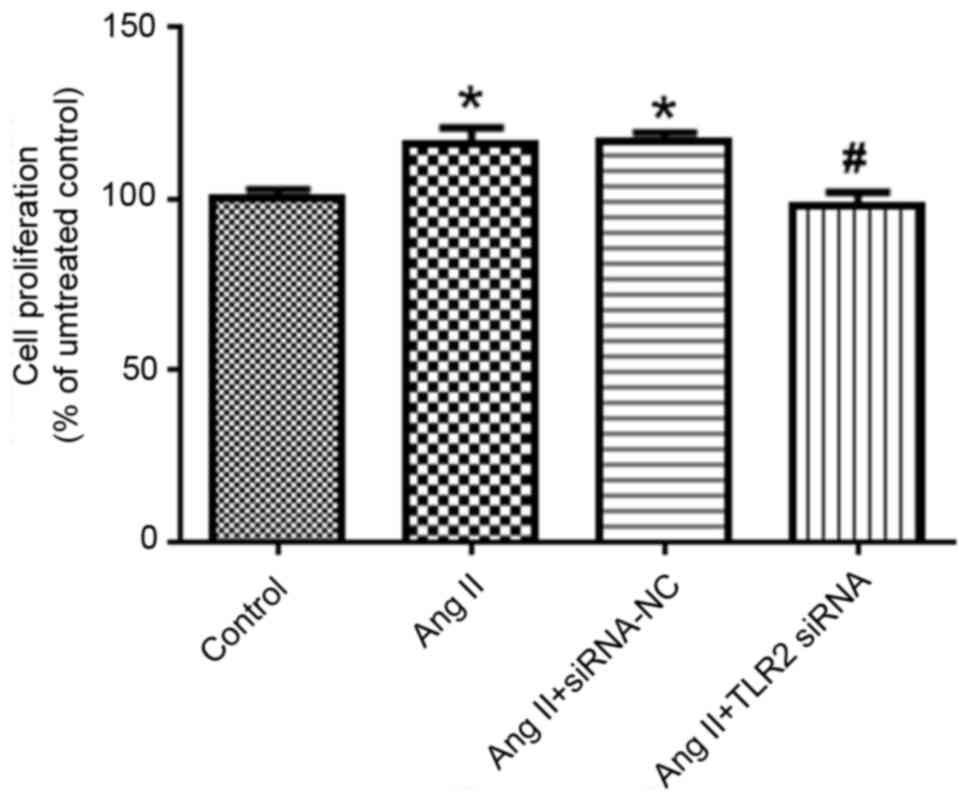
Compared with the control group, the proliferation
of Ang II group increased significantly (P<0.05; Fig. 4). By contrast, the cell
proliferation of Ang II + TLR2 siRNA group decreased significantly
(P<0.05 vs. Ang II + siRNA NC group).
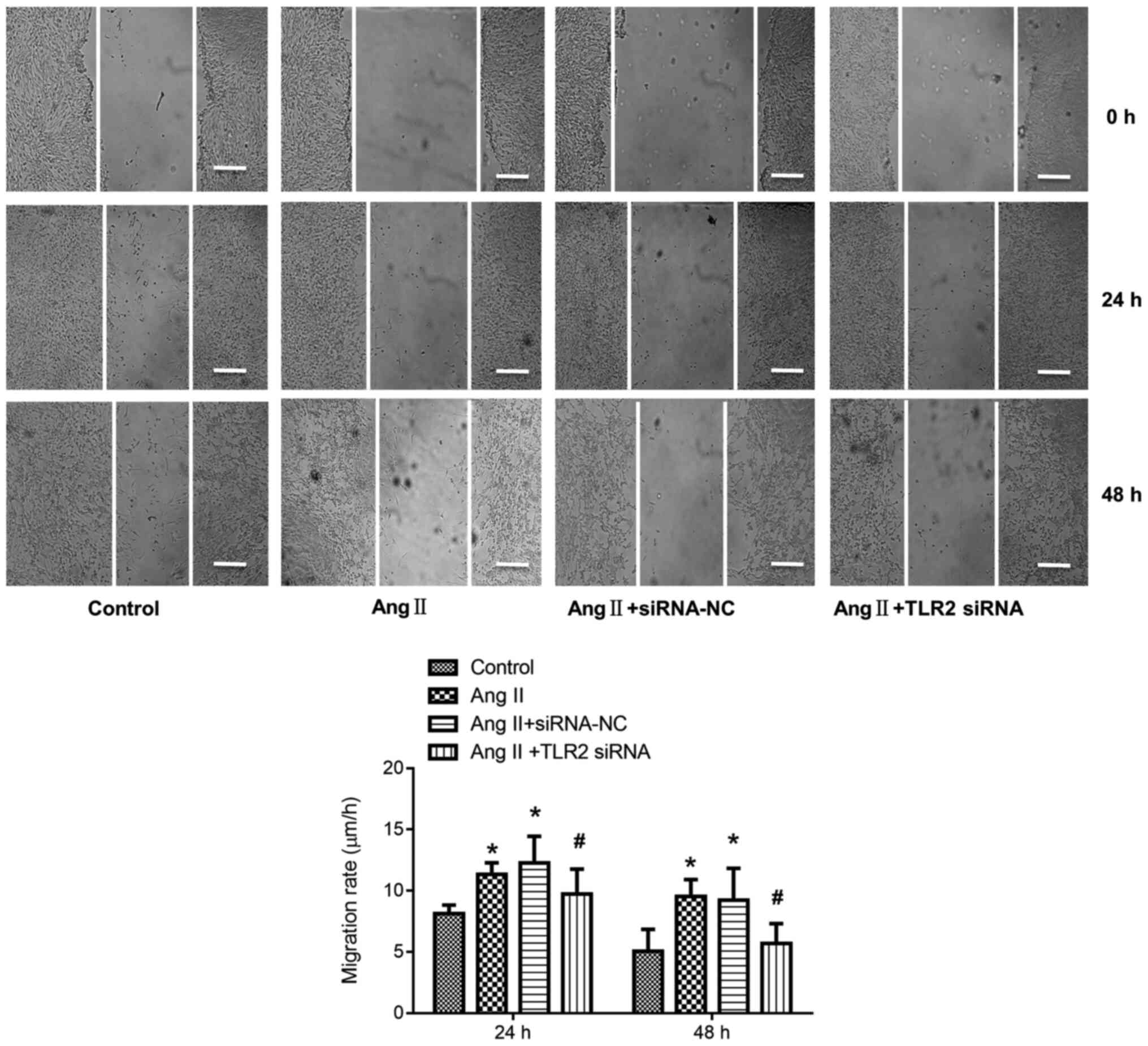
TLR2-siRNA attenuates Ang II-induced
cell migration
Compared with control group, the cell migration rate
of Ang II group increased significantly (P<0.05). By contrast,
the cell migration rate of Ang II + TLR2 siRNA group decreased
significantly compared with Ang II + siRNA NC group (P<0.05;
Fig. 5).
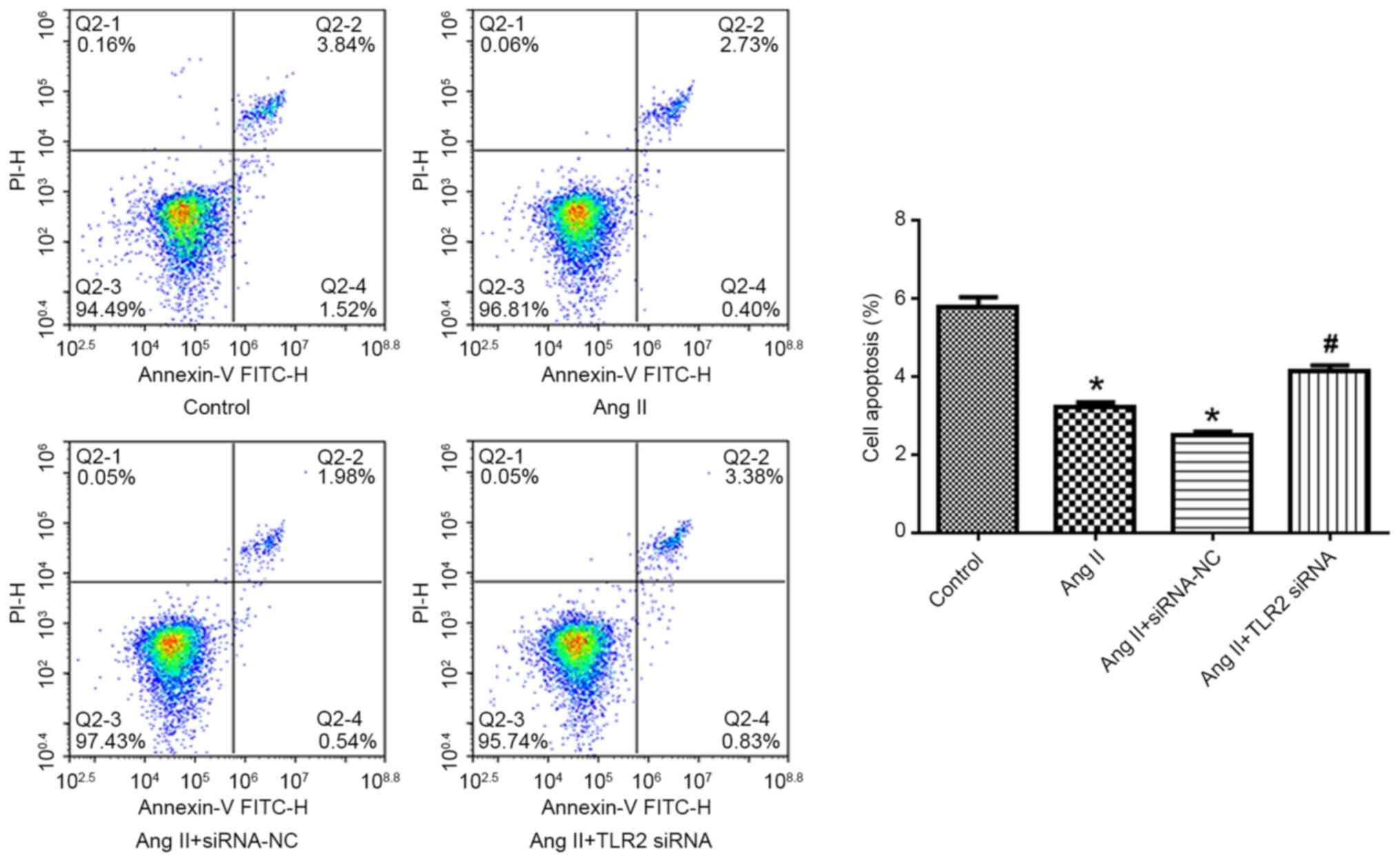
TLR2-siRNA attenuates Ang II-induced
decrease of apoptosis of BVSMCs
Compared with the control group, the apoptosis rate
of Ang II group decreased significantly (P<0.05). By contrast,
the apoptosis rate of Ang II + TLR2 siRNA group increased
significantly (Fig. 6; P<0.05
vs. Ang II + siRNA NC group).
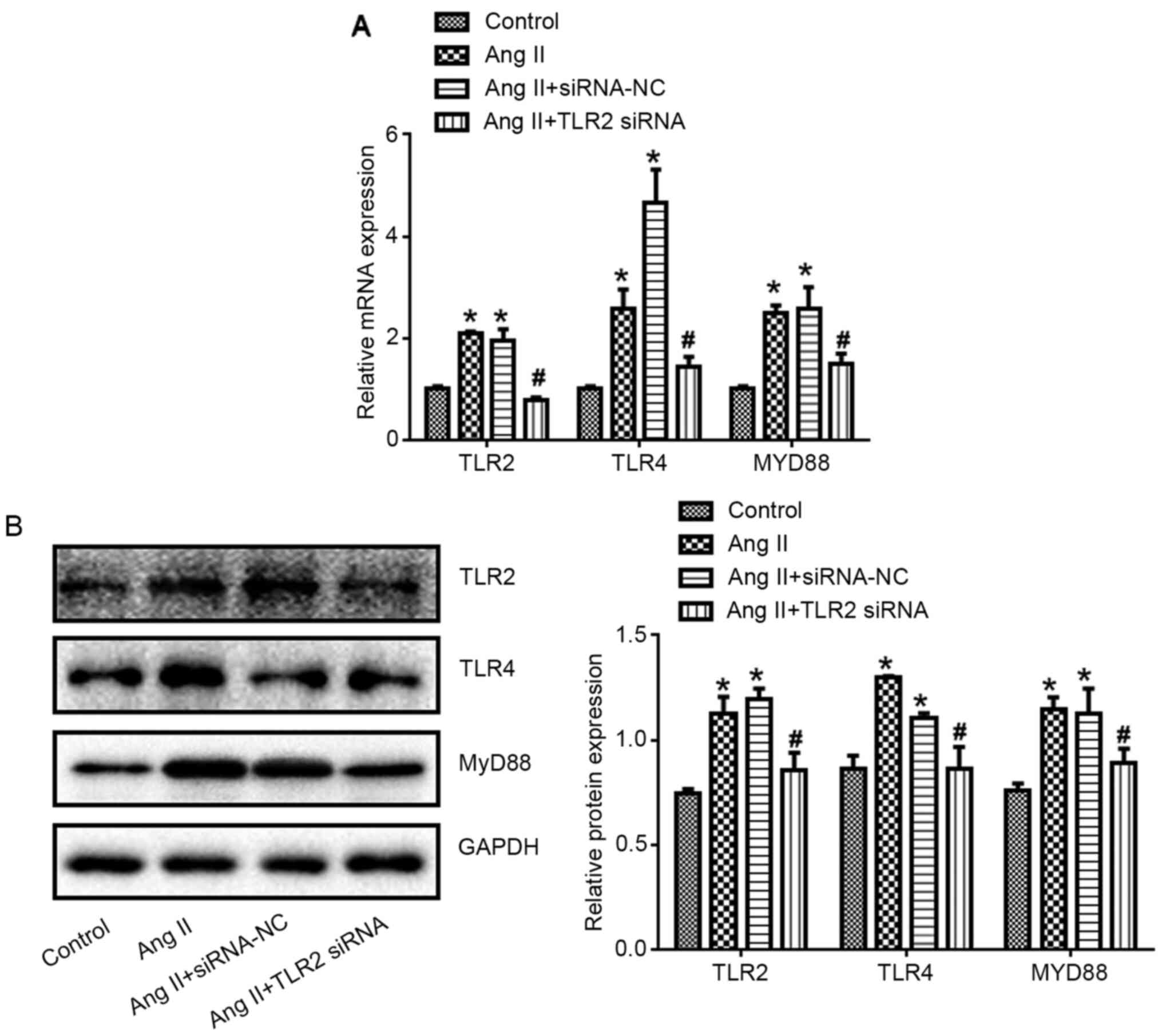
TLR2-siRNA reduced Ang II-induced
expression of TLR2, TLR4 and MyD88
Compared with control group, TLR2, TLR4 and MyD88
expression at mRNA and protein levels in Ang II group increased
significantly (P<0.05). Compared with Ang II + siRNA NC group,
TLR2, TLR4 and MyD88 expression in Ang II + TLR2 siRNA group
decreased significantly (P<0.05; Fig. 7).
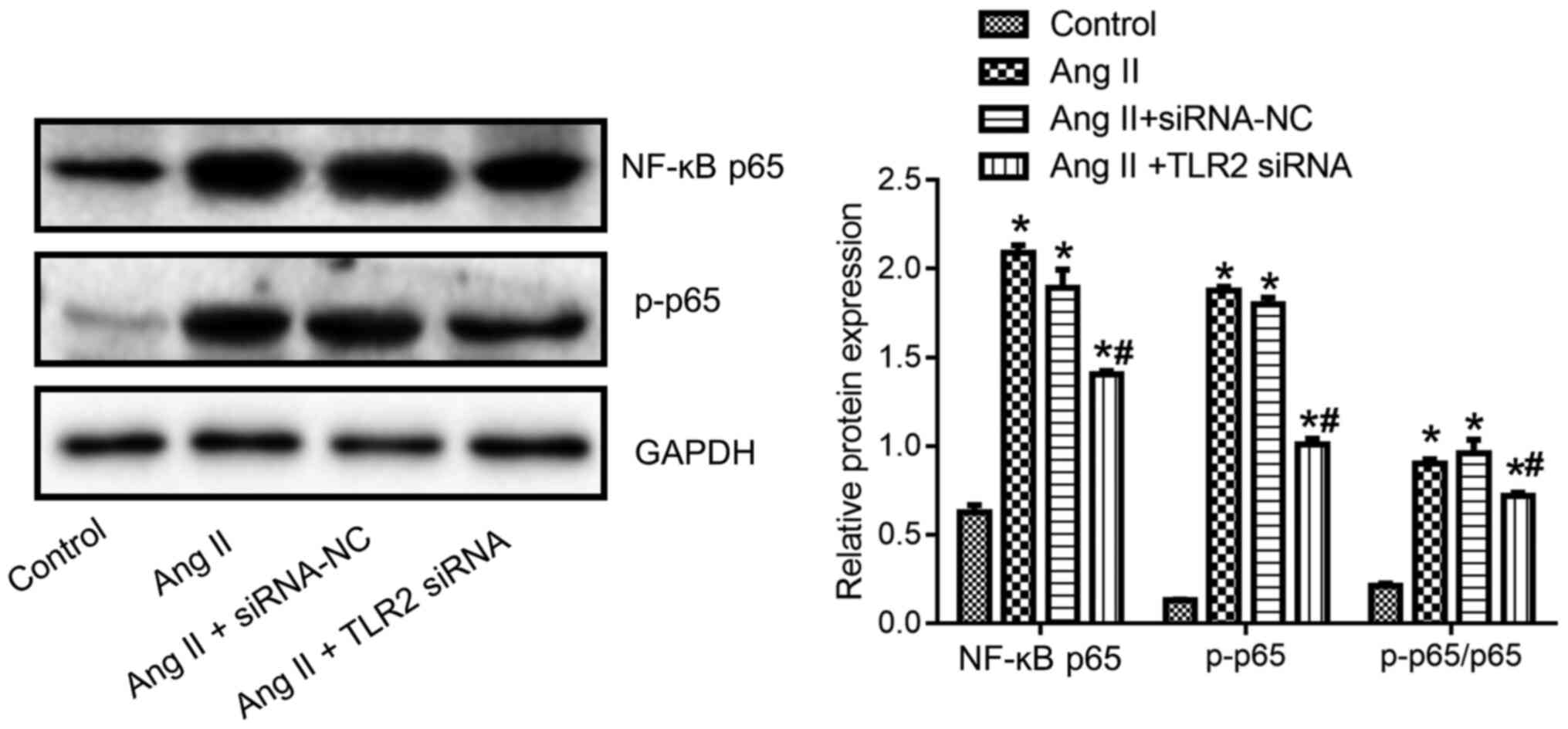
TLR2-siRNA attenuates Ang II-induced
expression of NF-κB p65 and p-p65 of BVSMCs
Compared with control group, the expression of NF-κB
p65, p-p65 and p-p65/p65 in the Ang II group was significantly
higher (P<0.05; Fig. 8).
Compared with Ang II + siRNA NC group, the expression of NF-κB p65,
p-p65 and p-p65/p65 in Ang II + TLR2 siRNA group was significantly
lower (P<0.05; Fig. 8).
Discussion
The present study reported that the expression of
TLR2/4-MyD88-NF-κB was promoted in patients with intracranial
aneurysm. In vitro experiments revealed that Ang II-induced
remodeling of BVSMCs was ameliorated by TLR2 silencing. Clinical
evidence and results from basic experiments revealed that
TLR2/4-MyD88-NF-κB signaling pathway was potentially involved in
the development of intracranial aneurysm.
The pathogenesis of intracranial aneurysm remains to
be fully elucidated, although genetic, environmental, estrogen
level, hypertension, smoking and alcohol are closely related to the
occurrence and development of intracranial aneurysm (20). Ang II can cause vascular endothelial
cell remodeling and induce vascular tissue dysfunction (21,22).
Ang II serves an important role in myocardial remodeling by
promoting the growth of cardiac fibroblasts and expression of
extracellular matrix protein (23).
It has been shown that Ang II stimulates the secretion of
aldosterone in the adrenal cortex to regulate vasomotion, promotes
the inflammatory response and oxidative stress of blood vessels and
leads to the occurrence of vascular remodeling by acting on the
type 1 receptor of angiotensin (24). Ang II stimulation can also promote
the proliferation and migration of vascular smooth muscle (25). Thus, Ang II-stimulated BVSMCs were
used to investigate the mechanisms underlying the proliferation and
migration of vascular smooth muscle (25).
TLR2 and TLR4 are associated with the pathogenesis
of vascular inflammation, atherosclerosis and diabetes (26–28).
These receptors share a common downstream signaling pathway and
serve a synergistic role in the activation of pre-inflammatory
response (29,30). TLR2 and TLR4 are highly expressed in
macrophages and endothelial cells in human atherosclerotic plaques,
both of which promote the proliferation of vascular smooth muscle
cells in animal atherosclerotic models (31,32).
By knocking down the expression of TLR2, it was found that the
effects of Ang II stimulation on human BVSMCs were blocked. These
data suggested that TLR2 was involved in Ang II-induced cell
remodeling.
As an inflammatory transcription factor, NF-κB is
involved in many cell activities, including cell proliferation,
stress response, immune response, apoptosis and inflammation
(33,34). The activity of NF-κB in aneurysms is
markedly increased (35,36), suggesting that NF-κB signaling
pathway serves an important role in the occurrence and development
of aneurysms. TLRs recognize the ligands and send signals to the
intracellular domain through leucine rich repetitive sequences in
the extracellular domain, and induce the corresponding signal
conversion cascade reaction (37).
Its signal transduction mechanism may include MyD88-dependent and
independent mechanisms, which eventually lead to the activation of
NF-κB (37). Using clinical
samples, in the present study it was found that the expression
levels of TLR2, TLR4, MyD88 and NF-κB p65 protein in patients with
intracranial aneurysm were higher compared with normal control.
These results also indicated that the TLR2/4/NF-κB signaling
pathway served important roles in intracranial aneurysm.
The in vitro data of the present study
demonstrated that the expression of TLR2, TLR4, MyD88, NF-κB p65
and p-p65 in BVSMCs stimulated by Ang II increased, but were
downregulated by TLR2 silencing. Ang II can activate TLR4 and
increase its expression (38).
MyD88 can independently regulate cell activation and movement, and
the TLR4/MyD88 signaling pathway is involved in Ang II-induced
apoptosis (39). Ang II
pretreatment results in upregulation of NF-κB and p-p65 expression
(40). NF-κB is considered to be a
typical pro-inflammatory molecule, regulating the transcription of
a number of inflammatory cytokines (41). The results of the present study
indicated that the TLR2/4-MyD88-NF-κB signaling pathway can be
activated by Ang II treatment.
There remained some limitations to the present
study. First, more clinical data ought to be collected to verify
that the TLR2/4-MyD88-NF-κB signaling pathway is specific for the
occurrence or development of intracranial aneurysm, but not
subarachnoid hemorrhage. Subarachnoid hemorrhage can be caused by a
ruptured aneurysm, arteriovenous malformation, or head injury
(3,4). The present study did not distinguish
subarachnoid hemorrhage patients from intracranial aneurysm
patients. Future research should investigate the influence of
subarachnoid hemorrhage caused by other factors on this signaling
pathway. Second, Ang II treatment could affect the biological
activities of BVSMCs, including the proliferation, apoptosis and
migration. The TLR2/4-MyD88-NF-κB signaling pathway is an important
pathway for inflammatory response (40). Whether inflammatory reaction takes
part in the process deserves future study. Finally, 10% FBS was
used in the cell migration assay. FBS may have also promoted the
migration of the cells, which may cause bias or reduce the ability
to observe the full effect of the knockdown of TLR2.
In conclusion, TLR2, TLR4, MyD88 and NF-κB p65
protein expression increased in patients with aneurysms, and the
occurrence of aneurysms was related to the TLR2/4-MyD88-NF-κB
signaling pathway. Interference of TLR2/4-MyD88-NF-κB signaling
pathway will affect aneurysms.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ, YW, JF and ML performed the experiments and
analyzed the data. XZ and ZJ designed the study, wrote the
manuscript and confirmed the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consents were obtained from the
participants. All experimental procedures were approved by the
Ethics Committee of Nanchang University (approval no.
NCU-20201213).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Jabbarli R, Dinger TF, Darkwah Oppong M,
Pierscianek D, Dammann P, Wrede KH, Kaier K, Köhrmann M, Forsting
M, Kleinschnitz C and Sure U: Risk factors for and clinical
consequences of multiple intracranial aneurysms: A systematic
review and meta-analysis. Stroke. 49:848–855. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lather HD, Gornik HL, Olin JW, Gu X, Heidt
ST, Kim ESH, Kadian-Dodov D, Sharma A, Gray B, Jaff MR, et al:
Prevalence of intracranial aneurysm in women with fibromuscular
dysplasia: A report from the US registry for fibromuscular
dysplasia. JAMA Neurol. 74:1081–1087. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brown RD Jr and Broderick JP: Unruptured
intracranial aneurysms: Epidemiology, natural history, management
options, and familial screening. Lancet Neurol. 13:393–404. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khey KMW, Huard A and Mahmoud SH:
Inflammatory pathways following subarachnoid hemorrhage. Cell Mol
Neurobiol. 40:675–693. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takeda K and Akira S: Toll-like receptors.
Curr Protoc Immunol. 109:14 12 11–14 12 10. 2015. View Article : Google Scholar
|
|
6
|
Kielian T: Toll-like receptors in central
nervous system glial inflammation and homeostasis. J Neurosci Res.
83:711–730. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fang H, Chen J, Lin S, Wang P, Wang Y,
Xiong X and Yang Q: CD36-mediated hematoma absorption following
intracerebral hemorrhage: Negative regulation by TLR4 signaling. J
Immunol. 192:5984–5992. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goering J, Pope MR and Fleming SD: TLR2
regulates complement-mediated inflammation induced by blood loss
during hemorrhage. Shock. 45:33–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang YC, Zhou Y, Fang H, Lin S, Wang PF,
Xiong RP, Chen J, Xiong XY, Lv FL, Liang QL and Yang QW: Toll-like
receptor 2/4 heterodimer mediates inflammatory injury in
intracerebral hemorrhage. Ann Neurol. 75:876–889. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ramstead AG, Robison A, Blackwell A,
Jerome M, Freedman B, Lubick KJ, Hedges JF and Jutila MA: Roles of
toll-like receptor 2 (TLR2), TLR4, and MyD88 during pulmonary
Coxiella burnetii infection. Infect Immun. 84:940–949. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su Q, Lv X, Sun Y, Ye Z, Kong B and Qin Z:
Role of TLR4/MyD88/NF-κB signaling pathway in coronary
microembolization-induced myocardial injury prevented and treated
with nicorandil. Biomed Pharmacother. 106:776–784. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo J, Liang W, Li J and Long J: Knockdown
of FSTL1 inhibits oxLDL-induced inflammation responses through the
TLR4/MyD88/NF-κB and MAPK pathway. Biochem Biophys Res Commun.
478:1528–1533. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen F, Zhu X, Sun Z and Ma Y: Astilbin
inhibits high glucose-induced inflammation and extracellular matrix
accumulation by suppressing the TLR4/MyD88/NF-κB pathway in rat
glomerular mesangial cells. Front Pharmacol. 9:11872018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aoki T, Nishimura M, Ishibashi R, Kataoka
H, Takagi Y and Hashimoto N: Toll-like receptor 4 expression during
cerebral aneurysm formation. Laboratory investigation. J Neurosurg.
113:851–858. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okada T and Suzuki H: Toll-like receptor 4
as a possible therapeutic target for delayed brain injuries after
aneurysmal subarachnoid hemorrhage. Neural Regen Res. 12:193–196.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bracard S, Lebedinsky A, Anxionnat R, Neto
JM, Audibert G, Long Y and Picard L: Endovascular treatment of Hunt
and Hess grade IV and V aneuryms. AJNR Am J Neuroradiol.
23:953–957. 2002.PubMed/NCBI
|
|
17
|
Catapano JS, Zeoli T, Frisoli FA,
Burkhardt JK and Lawton MT: Long-term independence in older
patients with aneurysmal subarachnoid hemorrhage in the Barrow
Ruptured Aneurysm Trial (BRAT). World Neurosurg.
S1878-S8750(20)32524-9, Dec 1, 2020 (Epub ahead of print). doi:
10.1016/j.wneu.2020.11.139.
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yaghini FA, Song CY, Lavrentyev EN,
Ghafoor HU, Fang XR, Estes AM, Campbell WB and Malik KU:
Angiotensin II-induced vascular smooth muscle cell migration and
growth are mediated by cytochrome P450 1B1-dependent superoxide
generation. Hypertension. 55:1461–1467. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sathyan S, Koshy LV, Srinivas L, Easwer
HV, Premkumar S, Nair S, Bhattacharya RN, Alapatt JP and Banerjee
M: Pathogenesis of intracranial aneurysm is mediated by
proinflammatory cytokine TNFA and IFNG and through stochastic
regulation of IL10 and TGFB1 by comorbid factors. J
Neuroinflammation. 12:1352015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma A, Wang D, An Y, Fang W and Zhu H:
Comparative transcriptomic analysis of mice liver treated with
different AMPK activators in a mice model of atherosclerosis.
Oncotarget. 8:16594–16604. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu C, Chen B, Jin X, Liu X, Wang F, Guo R,
Chen Z, Zheng H, Wang L and Zhang Y: Puerarin protects endothelial
progenitor cells from damage of angiotensin II via activation of
ERK1/2Nrf2 signaling pathway. Mol Med Rep. 17:3877–3883.
2018.PubMed/NCBI
|
|
23
|
Zhang ZZ, Cheng YW, Jin HY, Chang Q, Shang
QH, Xu YL, Chen LX, Xu R, Song B and Zhong JC: The sirtuin 6
prevents angiotensin II-mediated myocardial fibrosis and injury by
targeting AMPK-ACE2 signaling. Oncotarget. 8:72302–72314. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Allen AM, Zhuo J and Mendelsohn FA:
Localization and function of angiotensin AT1 receptors. Am J
Hypertens. 13:S31–S38. 2000. View Article : Google Scholar
|
|
25
|
Yu S, Chen Y, Chen S, Ye N, Li Y and Sun
Y: Klotho inhibits proliferation and migration of angiotensin
II-induced vascular smooth muscle cells (VSMCs) by modulating NF-κB
p65, akt, and extracellular signal regulated kinase (ERK) signaling
activities. Med Sci Monit. 24:4851–4860. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Allam R, Scherbaum CR, Darisipudi MN,
Mulay SR, Hägele H, Lichtnekert J, Hagemann JH, Rupanagudi KV, Ryu
M, Schwarzenberger C, et al: Histones from dying renal cells
aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrol.
23:1375–1388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bielinski SJ, Hall JL, Pankow JS,
Boerwinkle E, Matijevic-Aleksic N, He M, Chambless L and Folsom AR:
Genetic variants in TLR2 and TLR4 are associated with markers of
monocyte activation: The atherosclerosis risk in communities MRI
study. Hum Genet. 129:655–662. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Y, Yin H, Zhao M and Lu Q: TLR2 and
TLR4 in autoimmune diseases: A comprehensive review. Clin Rev
Allergy Immunol. 47:136–147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peng C, Wang H, Zhang WJ, Jie SH, Tong QX,
Lu MJ and Yang DL: Inhibitory effect of miR-125b on hepatitis C
virus core protein-induced TLR2/MyD88 signaling in THP-1 cells.
World J Gastroenterol. 22:4354–4361. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee IT, Lin CC, Hsu CK, Wu MY, Cho RL and
Yang CM: Resveratrol inhibits staphylococcus aureus-induced
TLR2/MyD88/NF-κB-dependent VCAM-1 expression in human lung
epithelial cells. Clin Sci (Lond). 127:375–390. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He M, Ichinose T, Yoshida Y, Arashidani K,
Yoshida S, Takano H, Sun G and Shibamoto T: Urban PM2.5 exacerbates
allergic inflammation in the murine lung via a
TLR2/TLR4/MyD88-signaling pathway. Sci Rep. 7:110272017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kollgaard T, Enevold C, Bendtzen K, Hansen
PR, Givskov M, Holmstrup P and Nielsen CH: Cholesterol crystals
enhance TLR2- and TLR4-mediated pro-inflammatory cytokine responses
of monocytes to the proatherogenic oral bacterium Porphyromonas
gingivalis. PLoS One. 12:e01727732017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sen R and Baltimore D: Multiple nuclear
factors interact with the immunoglobulin enhancer sequences. Cell
1986. 46: 705–716. J Immunol. 177:7485–7496. 2006.PubMed/NCBI
|
|
34
|
Song Z, Shen F, Zhang Z, Wu S and Zhu G:
Calpain inhibition ameliorates depression-like behaviors by
reducing inflammation and promoting synaptic protein expression in
the hippocampus. Neuropharmacology. 174:1081752020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wei H, Mao Q, Liu L, Xu Y, Chen J, Jiang
R, Yin L, Fan Y, Chopp M, Dong J and Zhang J: Changes and function
of circulating endothelial progenitor cells in patients with
cerebral aneurysm. J Neurosci Res. 89:1822–1828. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aoki T, Frosen J, Fukuda M, Bando K, Shioi
G, Tsuji K, Ollikainen E, Nozaki K, Laakkonen J and Narumiya S:
Prostaglandin E2-EP2-NF-κB signaling in macrophages as a potential
therapeutic target for intracranial aneurysms. Sci Signal.
10:eaah60372017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li HB, Li X, Huo CJ, Su Q, Guo J, Yuan ZY,
Zhu GQ, Shi XL, Liu JJ and Kang YM: TLR4/MyD88/NF-κB signaling and
PPAR-ү within the paraventricular nucleus are involved in the
effects of telmisartan in hypertension. Toxicol Appl Pharmacol.
305:93–102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ji YY, Wang ZD, Liu JT and Liu N:
Inhibitory effect of fenofibrate on angiotensin II-induced
toll-like receptor 4 expression, myeloperoxidase activity and
expression in RAW264.7 cells. Yao Xue Xue Bao. 44:462–467. 2009.(In
Chinese). PubMed/NCBI
|
|
39
|
Gao W, Wang H, Zhang L, Cao Y, Bao JZ, Liu
ZX, Wang LS, Yang Q and Lu X: Retinol-binding protein 4 induces
cardiomyocyte hypertrophy by activating TLR4/MyD88 pathway.
Endocrinology. 157:2282–2293. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang J, Jiang H, Chen SS, Chen J, Xu SK,
Li WQ and Wang JC: CBP knockdown inhibits angiotensin II-induced
vascular smooth muscle cells proliferation through downregulating
NF-κB transcriptional activity. Mol Cell Biochem. 340:55–62. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang H, Ding J, Fan Q and Liu S: TRPC6
up-regulation in Ang II-induced podocyte apoptosis might result
from ERK activation and NF-kappaB translocation. Exp Biol Med
(Maywood). 234:1029–1036. 2009. View Article : Google Scholar : PubMed/NCBI
|