Introduction
Cervical cancer is the fourth most common type of
malignant cancer among women worldwide, resulting in ~570,000 new
cases in 2018. Among the 311,000 global cervical cancer-related
deaths in 2018, ~90% occurred in low and middle income countries
(1,2). Therefore, identifying high quality and
inexpensive agents to prevent and treat cervical cancer is
important. Previous research has reported that the occurrence of
cervical carcinoma was associated with numerous factors, including
persistent human papillomavirus (HPV) infection, chromosomal
telomerase activation, genetic mutations and immune system
dysfunction (3–8). However, the pathogenesis of cervical
carcinoma is not completely understood. Therefore, the pathogenesis
of cervical carcinoma should be further investigated to improve the
clinical diagnosis and efficacy of treatments.
Chemotherapy has been shown to be effective for
treating cervical carcinoma, particularly suitable for the
treatment of patients with terminal disease or metastatic tumors.
However, chemotherapy is often accompanied by various adverse
reactions, including toxicity and drug resistance (9,10),
which severely impacts the quality of life of surviving patients
with cancer. Therefore, it remains necessary to discover anticancer
drugs with high efficiency and low toxicity, which can improve
patient immunity, enhance the efficacy of chemotherapy and prolong
and improve the quality of life of patients.
Panax notoginsenoside, a traditional Chinese
medicine, was found to serve as an antioxidant to scavenge free
radicals, prevent and treat cardiovascular, central neural and
cerebrovascular diseases, promote angiogenesis and immunity,
decrease the levels of blood fat and blood pressure and exert tumor
suppressive effects (11–17). Panax notoginsenoside has been
used in combination with chemotherapy due to its ability to boost
the body's innate immunity to improve the curative effect against
aplastic anemia and solid tumor (15,17).
Notoginsenoside R1 (NGR1) is one of the major active constituents
of Panax notoginsenoside. A previous study reported that
NGR1 significantly suppressed the proliferation of colorectal
cancer cells and arrested cells in the S phase (18). Moreover, NGR1 also effectively
reduced the invasion and metastasis of lung cancer (19). However, few studies have
investigated the role of NGR1 in cervical cancer, and the mechanism
of action of NGR1 in cervical carcinoma remains to be elucidated.
In a preliminary study, NGR1 was discovered to exhibit moderate
toxicity, which inhibited cervical carcinoma cell viability.
Therefore, the present study further investigated NGR1 and its
cytotoxic mechanisms.
Materials and methods
Reagents
pEGFP-C1-plant homeodomain finger protein 6 (PHF6)
was gifted by Dr DJ Picketts (Regenerative Medicine Program, Ottawa
Hospital Research Institute). NGR1 (cat. no. 110745) was obtained
from Nanjing SenBeiJia Biological Technology Co., Ltd.; the purity
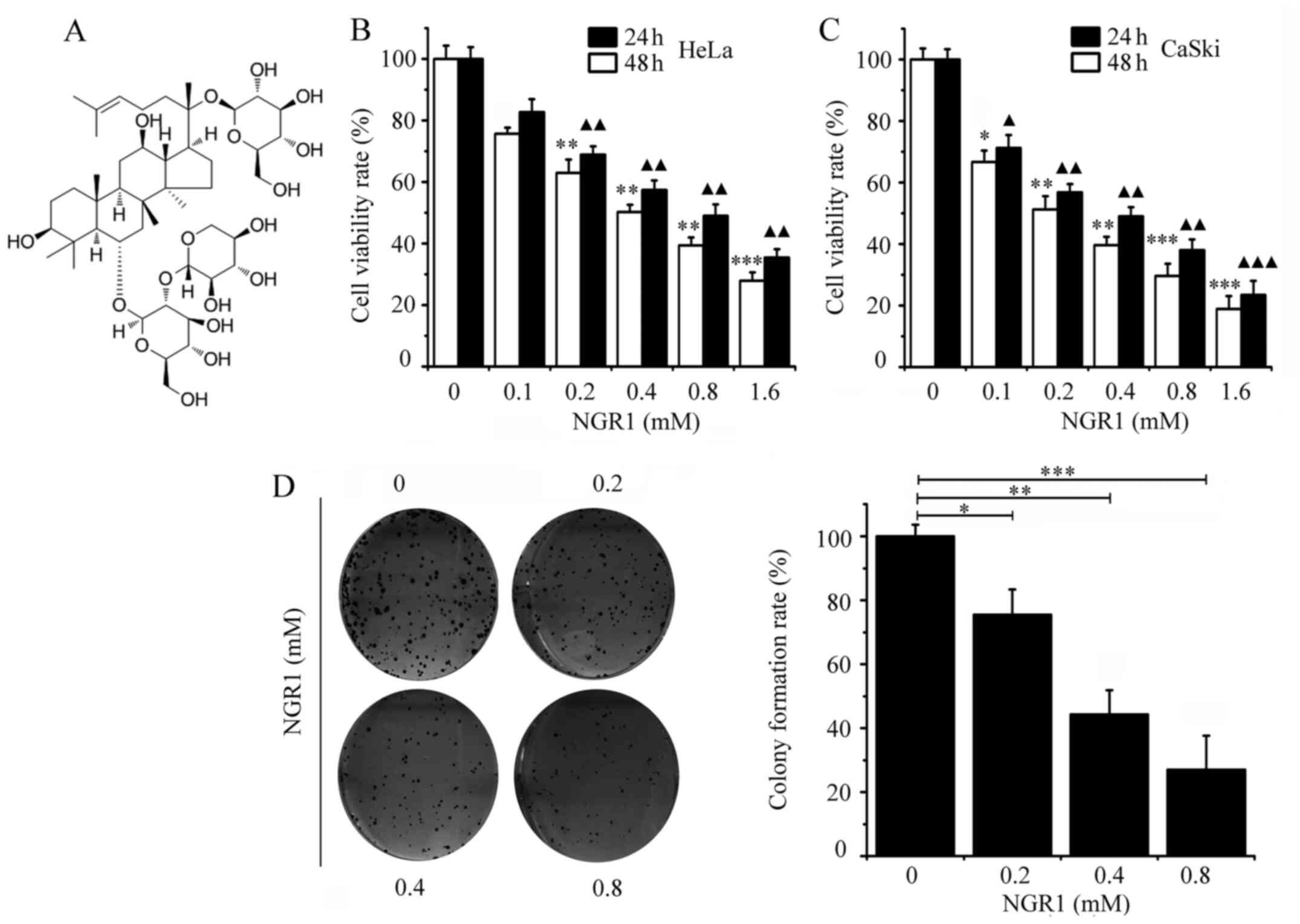
of NGR1 was ≥98% and the chemical structure is shown in Fig. 1A. NGR1 was dissolved in DMSO to a
final concentration of 10 mM, and was stored at −20°C. DMEM was
purchased from Hyclone; Cytiva, FBS was obtained from Gibco; Thermo
Fisher Scientific, Inc., and Cell Counting Kit-8 (CCK-8), DAPI and
BCA kits were purchased from Meilunbio. The Annexin V-FITC
Apoptosis Detection kit was purchased from Beyotime Institute of
Biotechnology. The following primary antibodies were purchased from
Abcam: Anti-γH2A.X variant histone [H2AX; phosphorylated (p) on
serine 139] (cat. no. ab81299), anti-H2AX (cat. no. ab229914),
anti-Bcl-2 (cat. no. ab182858), anti-ATR serine/threonine (ATR)
(cat. no. ab184137), anti-p-ATR (S428) (cat. no. ab178407),
anti-PHF6 (cat. no. ab173304), anti-cyclin A2 (cat. no. ab181591),
anti-cyclin D1 (cat. no. ab16663), anti-CDK2 (cat. no. ab32147),
anti-p53 (cat. no. ab179477), anti-PARP1 (cat. no. ab191217),
anti-nucleolin (cat. no. ab22758) and anti-GAPDH (cat. no.
ab181602). Anti-Lamin B1 (cat. no. 13435) and anti-cleaved
caspase-3 (cat. no. 9661) were purchased from Cell Signaling
Technology. DMSO, SDS and Triton™ X-100 were purchased from
Sigma-Aldrich; Merck KGaA. Goat anti-rabbit IgG H&L
(DyLight® 488) secondary antibody (cat. no. ab96899) was
obtained from Abcam. HRP-conjugated anti-rabbit IgG secondary
antibody (cat. no. sc-2357) and PE-conjugated anti-rabbit IgG
secondary antibody (cat. no. sc-3753) were obtained from Santa Cruz
Biotechnology, Inc. Small interfering RNA (siRNA/si) targeting PHF6
(siPHF6) and negative control siRNA were purchased from Guangzhou
RiboBio Co., Ltd. NE-PER Nuclear and Cytoplasmic Extraction
reagents (cat. no. 78835), Opti-MEM (cat. no. 11058021) and
Lipofectamine® 2000 transfection reagent (cat. no.
11668500) were purchased from Thermo Fisher Scientific, Inc.
Cell culture
HeLa and CaSki cells were purchased from the
American Type Culture Collection. Cells were cultured in DMEM
supplemented with 10% FBS and 1% penicillin (100 U/ml) and 1%
streptomycin (100 µg/ml). Cells were maintained in a suitable
environment at 37°C, with an atmosphere containing 5% (v/v)
CO2.
CCK-8 assay
Cell viability of HeLa and CaSki cells was
determined using a CCK-8 assay. Briefly, 5×104 HeLa and
CaSki cells/well were cultured in 96-well plates at 37°C for 12 h.
Subsequently, cells were treated with different concentrations of
NGR1 (0, 0.1, 0.2, 0.4, 0.8, 1.6 mM) at 37°C for 24 and 48 h.
Alternatively, cells were treated for 24 h with: i) siPHF6; ii)
siPHF6 + 0.4 mM NGR1; iii) PHF6; iv) PHF6 + 0.4 mM NGR1; or v) 0 mM
NGR1 (control group). Following the incubation, cells were washed
twice with PBS and 100 µl DMEM supplemented with 10% CCK-8 reagent
was added to each well and incubated at 37°C for 2 h. The
absorbance was measured at a wavelength of 460 nm on an Epoch™ 2
microplate reader (BioTek Instruments, Inc.). The cell viability
rate was calculated according to the following formula:
[(As-Ab)/(Ac-Ab)] ×100%, where As represents the absorbance of the
NGR1 treatment groups, Ac represents the absorbance of the control
group and Ab represents the absorbance of material background. The
half maximal inhibitory concentration (IC50) of NGR1 in
the HeLa and CaSki cell lines was calculated using Origin Pro 8
software (OriginLab).
Soft agar cell colony formation
assay
The cell colony forming assay was performed as
previously described (20).
Briefly, basal agarose was prepared with 1X DMEM, 0.6%
low-melting-point agarose (Amresco, LLC), 10% FBS, 1% penicillin
and 1% streptomycin. The basal agarose contained 0.2, 0.4 or 0.8 mM
NGR1. The basal agarose compounds were poured into a 6-well plate.
The top agarose layer was prepared with 1X DMEM, 0.3%
low-melting-point agarose, 10% FBS, 1% penicillin and 1%
streptomycin. Subsequently, cells (1×103 cells/well)
were added to make the top agarose. The top agarose also contained
0.2, 0.4 or 0.8 mM NGR1. The top agarose compounds were poured onto
the basal agarose. Cells were incubated at 37°C for 12 days.
Subsequently, cell colonies were fixed with 4% paraformaldehyde for
20 min at room temperature, and stained with 0.5% crystal violet
for 15 min at room temperature. Data were analyzed using ImageJ
software (version 1.8.0; National Institutes of Health).
DAPI staining
HeLa cells (1×105 cells/well) were seeded
into a 6-well culture plate and treated with 0, 0.2, 0.4 or 0.8 mM
NGR1 for 24 h at 37°C. Cells were fixed with 4% formaldehyde for 20
min at room temperature. Nuclei were stained with 4 µg/ml DAPI for
20 min at room temperature and washed with PBS. Cell nuclear
morphology was visualized using a BX43 fluorescence microscope
(Olympus Corporation).
Flow cytometric analysis of
apoptosis
Cells (1×105 cells/well) were cultured in
6-well plates until reaching 90% confluence, and were then treated
with 0, 0.2, 0.4 or 0.8 mM NGR1 for 12 h at 37°C. Apoptosis was
subsequently evaluated using the Annexin V-FITC Apoptosis Detection
kit according to the manufacturer's protocol. Briefly,
1×106 cells were collected, centrifuged at 1,000 × g for
5 min at room temperature, washed with PBS and resuspended in 195
µl Annexin V-FITC binding buffer. Subsequently, cells were stained
with 5 µl Annexin V-FITC and 10 µl PI solution at room temperature
in the dark for 15 min. Cells were filtered with a 300-mesh nylon
film and maintained on ice until analysis using an Accuri C6 flow
cytometer (BD Biosciences). Early and late apoptosis was analyzed
using FlowJo software (version 7.6; FlowJo, LLC). All experiments
were performed independently and repeated three times.
Cell cycle analysis
Following the treatment of cells with NGR1, the cell
cycle distribution was analyzed via flow cytometry. Briefly, cells
were treated with 0, 0.2, 0.4 or 0.8 mM NGR1 for 24 h at 37°C.
Subsequently, 1×106 cells were collected, centrifuged at
1,000 × g for 5 min at 4°C, washed with cold PBS and fixed with
precooled 70% ethanol at 4°C for overnight. After washing, cells
were resuspended in 0.535 ml dye buffer, which contained 10 µl
RNase (50X) and 25 µl PI (25X), and incubated in the dark at room
temperature for 30 min. Cells were filtered with a 300-mesh nylon
film, and analyzed using an Accuri C6 flow cytometer (BD
Biosciences) and ModFit LT software (version 3.2; Verity Software
House).
Cell transfection
HeLa cells (1×105 cells/well) were seeded
into 6-well plates and cultured to 80% confluence.
Lipofectamine® 2000 (5 µl) was used to transfect the
cells with specific small interfering RNA (siRNA/si) targeting PHF6
(siPHF6; 20 nM), pEGFP-C1 empty plasmid (1 µg), pEGFP-C1 plasmid
overexpressing PHF6 (pEGFP-C1-PHF6; 1 µg) or the non-specific
control siRNA [negative control (NC); 20 nM]. siRNAs or plasmids
were mixed with Lipofectamine® 2000 in Opti-MEM culture
medium at room temperature for 20 min. Subsequently, the mixtures
were added to each well for 6 h at 37°C. After 6 h, the mixtures
were removed and replaced with complete medium (1X DMEM
supplemented with 10% FBS and 1X antibiotics) to culture for 24 h
at 37°C. siPHF6s and siRNA NC were obtained from Guangzhou RiboBio
Co., Ltd. and the sequences were as follows: siPHF6-1,
5′-GGACAGTTACTAATATCTG-3′; siPHF6-2, 5′-GCACGAAGCTGATGTGTTC-3′;
siPHF6-3, 5′-CCACTGTGCATTGCATGAT-3-′; and NC,
5′-UUCUCCGAACGUGUCACGU-3′.
Immunofluorescence staining
Immunofluorescence staining was performed as
previously described (20).
Briefly, cells were fixed with 4% paraformaldehyde for 20 min at
room temperature and slides were subsequently incubated with 0.1%
Triton X-100 for 5 min at room temperature. Cells were then
incubated at 4°C overnight with anti-PHF6 (1:100), anti-nucleolin
(1:100) and anti-γH2AX (1:100) primary antibodies. Following the
incubation, the cells were washed and further incubated with a
fluorescein-labeled secondary antibody (1:100) for 1 h at room
temperature. The cell nucleus was subsequently stained with DAPI (4
µg/ml) for 30 min at room temperature. Stained cells were observed
under a confocal laser scanning microscope (Olympus
Corporation).
Western blotting
Western blotting was performed as previously
described (20). Briefly,
cytoplasmic and nuclear protein extraction (H2AX, γH2AX, Lamin B1
and PHF6) were performed using the NE-PER™ Nuclear and Cytoplasmic
Extraction kit (Thermo Fisher Scientific, Inc.) and other total
proteins were extracted from cells using RIPA lysis buffer (Thermo
Fisher Scientific, Inc.). Total protein concentrations were
quantified using a BCA kit (Beyotime Institute of Biotechnology).
Equal amounts of cell lysate (40 µg/lane) were separated by 10%
SDS-PAGE, transferred onto a PVDF membrane and blocked with 5% skim
milk solution at room temperature for 1 h. Then, after washing with
PBS, the PVDF membranes were incubated with the following primary
antibodies (all 1:1,000 except anti-GAPDH) overnight at 4°C:
Anti-cleaved caspase-3, anti-poly (ADP-ribose) polymerase 1
(PARP1), anti-Bcl-2, anti-cyclin D1, anti-CDK2, anti-cyclin A2,
anti-ATR, anti-p-ATR, anti-p53, anti-H2AX, anti-γH2AX, anti-Lamin
B1, anti-PHF6 and anti-GAPDH (1:5,000). Following the primary
antibody incubation at 4°C for overnight, the membranes were washed
with PBS and incubated with an IgG HRP-conjugated secondary
antibody (1:5,000) at room temperature for 1 h. Protein bands were
visualized using an ECL detection kit (Thermo Fisher Scientific,
Inc.). Densitometric analysis was performed using ImageJ software
(version 1.8.0; National Institutes of Health).
Cytoplasmic and nuclear protein
extraction
HeLa cells were cultured in a 10-cm culture dish
(~5×106 cells/well). Cytoplasmic and nuclear protein
extraction were performed using the NE-PER™ Nuclear and Cytoplasmic
Extraction Kit (Thermo Fisher Scientific, Inc.). Briefly, the cells
were collected and washed with pre-cooled PBS and resuspended in
200 µl CER I supplemented with PMSF, protease and phosphatase
inhibitors (Halt™ Protease and Phosphatase Inhibitor Cocktail;
Thermo Fisher Scientific, Inc.). Cells were incubated on ice for 10
min, then 11 µl ice-cold CER II was added to the cell suspension
and centrifuged at 16,000 × g for 5 min at 4°C. The supernatant
containing the cytoplasmic extract was immediately transferred to a
new pre-chilled tube and stored at −80°C. The precipitate
containing the nuclear component was suspended in 100 µl ice-cold
NER with protease inhibitors and the suspension was oscillated in
the tube for 15 sec every 10 min for a total of 40 min.
Subsequently, the suspension was centrifuged at 16,000 × g for 10
min at 4°C The obtained supernatant contained the nuclear
component, which was immediately transferred to a new pre-chilled
tube and stored at −80°C.
Statistical analysis
All experimental protocols were repeated at least
three times. Statistical analyses were performed using Origin Pro 8
software (OriginLab). Experimental data are presented as the mean ±
SD. Statistical differences between groups were determined using a
one-way ANOVA followed by a Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
NGR1 inhibits the proliferation of
cervical carcinoma cells
It was first determined whether NGR1 could inhibit
the proliferation of the cervical carcinoma cell lines, HeLa and
CaSki were treated with NGR1 (0, 0.1 0.2, 0.4, 0.8, 1.6 mM) for 24
and 48 h. The chemical structure of NGR1 is presented in Fig. 1A. Cell viability was evaluated by
performing the CCK-8 assay. As shown in Fig. 1B, NGR1 exhibited moderate
cytotoxicity towards inhibiting HeLa cell viability, with an
IC50 of 0.8 mM at 24 h and an IC50 of 0.41 mM
at 48 h However, the CaSki cells were more sensitive to NGR1, with
an IC50 of 0.4 mM at 24 h and an IC50 of 0.19
mM at 48 h (Fig. 1C). The results
suggested that NGR1 inhibited HeLa and CaSki cell viability in a
time- and dose-dependent manner. The exact IC50 of NGR1
in cervical carcinoma cell lines is presented in Table I.
 | Table I.IC50 of Notoginsenoside R1
in cervical carcinoma cells. |
Table I.
IC50 of Notoginsenoside R1
in cervical carcinoma cells.
| Cell line | IC50 at
24 h (mM) | IC50 at
48 h (mM) |
|---|
| HeLa | 0.809±0.037 | 0.400±0.023 |
| CaSki | 0.413±0.032 | 0.194±0.041 |
Soft agar cell colony formation experiments are
often used to evaluate the proliferation of malignant cancer cells
(20). Subsequently, the effect of
NGR1 on CaSki cell colony formation was investigated. The present
results revealed that NGR1 also significantly inhibited the colony
forming ability of CaSki cells in a dose-dependent manner compared
with the control cells. The number of CaSki cell colonies was
significantly decreased by 0.2 mM NGR1 compared with the control
group (Fig. 1D).
NGR1 induces the apoptosis of cervical
carcinoma cells
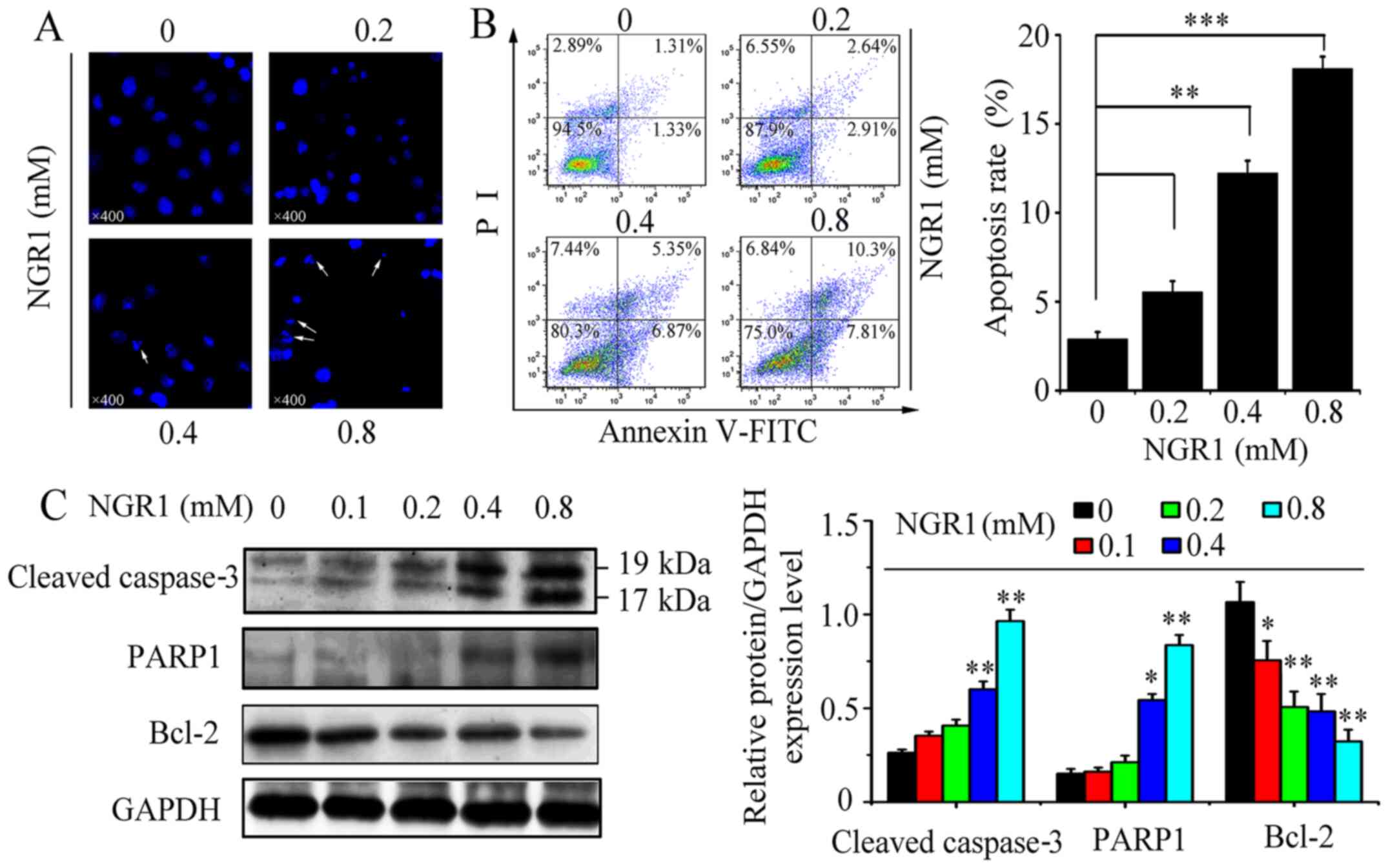
To examine whether NGR1 altered cervical cancer cell
apoptosis, HeLa cells were treated with NGR1 (0, 0.2, 0.4 and 0.8
mM) for 24 h. Cell morphological apoptosis were evaluated by DAPI
staining and cell apoptotic rates were evaluated via flow
cytometry. As shown in Fig. 2A, the
cell nuclei exhibited irreversible condensation, and some typical
apoptotic bodies were observed in the nuclei following 0.8 mM NGR1.
However, in the control group (0 mM), the morphology of the cell
nuclei was oval and regular, with homogenous color distribution. As
shown in Fig. 2B, the cell
apoptotic rate increased in a dose-dependent manner; compared with
the control group (2.64%), treatment with 0.2, 0.4 and 0.8 mM NGR1
increased the apoptotic rate to 5.55, 12.22 and 18.11%,
respectively (Fig. 2B).
Cleaved caspase-3 is the activated form of
caspase-3, which is an important marker of apoptosis (21). Therefore, the expression levels of
the active form of caspase-3 following NGR1 treatment in HeLa cells
for 24 h were investigated. The active form of caspase-3 was
significantly upregulated by 0.4 and 0.8 mM NGR1, compared with the
control group (Fig. 2C). PARP1 has
been discovered to serve a crucial role in DNA repair and apoptosis
(22,23). Therfore, the effect of NGR1 on the
expression of PARP1 was also assessed. The expression levels of
PARP1 were also significantly upregulated by 0.4 and 0.8 mM NGR1,
compared with the control group. Conversely, the expression levels
of Bcl-2, which is an oncogene that inhibits apoptosis (24), were significantly downregulated by
0.4 and 0.8 mM NGR1 compared with the control group (Fig. 2C).
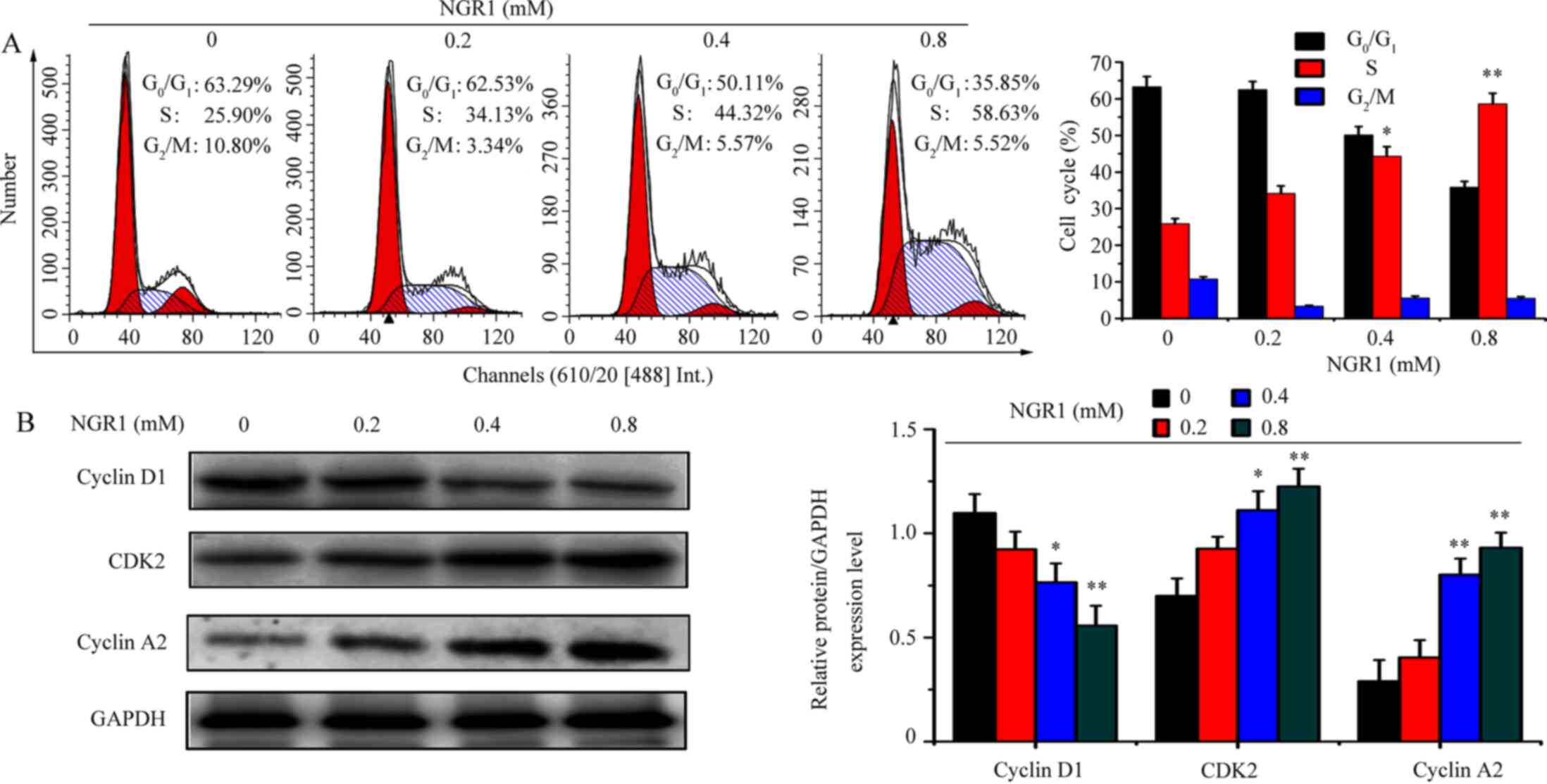
NGR1 arrests cervical carcinoma cells
in the S phase of the cell cycle and activates cyclin A2 and
CDK2
The aforementioned results demonstrated that NGR1
inhibited HeLa and CaSki cell viability. Therefore, whether NGR1
was able to inhibit cell viability by regulating the cell cycle was
investigated. HeLa cells were treated with 0, 0.2, 0.4 and 0.8 mM
NGR1 for 24 h. Compared with the control group (0 mM; S phase,
25.90%), treatment with 0.2, 0.4 and 0.8 mM NGR1 increased the S
phase proportion to 34.13, 44.32, 58.63%, respectively (Fig. 3A). Furthermore, the expression
levels of cell cycle-related proteins were analyzed using western
blotting. The expression levels of cyclin A2 and CDK2 were
significantly upregulated by 0.4 and 0.8 mM NGR1, compared with the
control group (Fig. 3B). However,
the expression levels of cyclin D1, a protein involved in the
G0/G1 phase of the cell cycle, were
significantly decreased by 0.4 and 0.8 mM NGR1 compared with the
control group (Fig. 3B).
NGR1 upregulates the expression levels
of the DNA damage regulatory proteins, γH2AX and ATR and
downregulates PHF6 expression levels
Following treatment with NGR1, the expression levels
of DNA damage regulatory proteins in HeLa cells were measured. HeLa
cells were treated with NGR1 (0, 0.1, 0.2, 0.4 and 0.8 mM) for 24
h. As shown in Fig. 4A and B, NGR1
induced H2AX and ATR phosphorylation and significantly upregulated
the expression levels of p53 in a dose-dependent manner, In
addition, NGR1 downregulated the expression levels of PHF6 in a
dose-dependent manner. Compared with the control group, NGR1
significantly increased the expression levels of H2AX and ATR
phosphorylation and upregulated the levels of p53 at 0.2 mM, but
significantly downregulated PHF6 expression levels at 0.4 mM. To
assess alterations in DNA damage regulatory proteins after NGR1
treatment, HeLa cells were treated with 0.4 mM NGR1 for 0, 3, 6,
12, 24 or 48 h. As shown in Fig. 4C and
D, NGR1 increased H2AX and ATR phosphorylation, upregulated the
expression levels of p53 and downregulated the expression levels of
PHF6 in a time-dependent manner. However, compared with the control
group, H2AX phosphorylation was significantly increased by NGR1
until 24 h, returning to normal levels at 48 h, which might be
associated with NGR1 inducing excessive HeLa cell death.
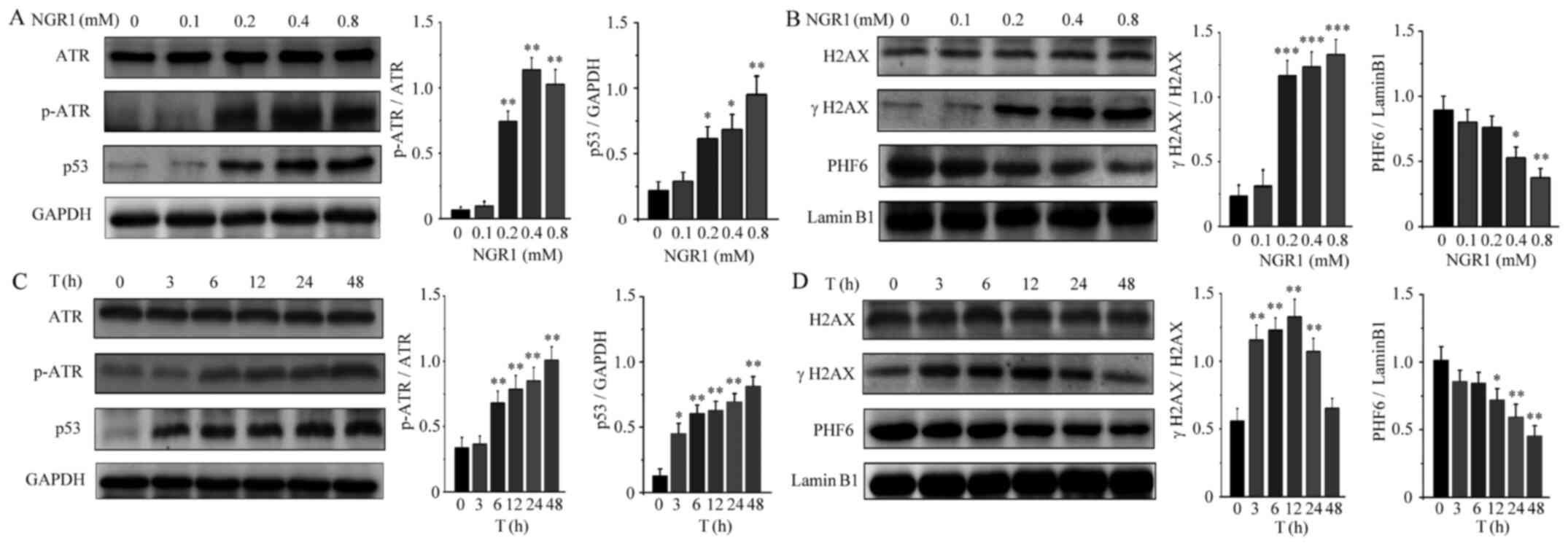 | Figure 4.Effects of NGR1 on the expression
levels of DNA damage-related proteins. HeLa cells were treated with
a series of concentrations of NGR1 (0, 0.1, 0.2, 0.4 or 0.8 mM) for
12 h and the expression levels of (A) ATR, p-ATR and p53 and (B)
γH2AX, H2AX and PHF6 were analyzed using western blotting. HeLa
cells were treated for different durations (0, 3, 6, 12, 24 or 48
h) with 0.4 mM NGR1, and western blotting was used to analyze the
expression levels of (C) ATR, p-ATR and p53 and (D) γH2AX, H2AX and
PHF6. Data are expressed as the mean ± SD of three independent
experiments. *P<0.05, **P<0.01, ***P<0.001 vs. 0 mM. NGR1,
Notoginsenoside R1; ATR, ATR serine/threonine kinase; p-,
phosphorylated; H2AX, H2A.X variant histone; PHF6, plant
homeodomain finger protein 6. |
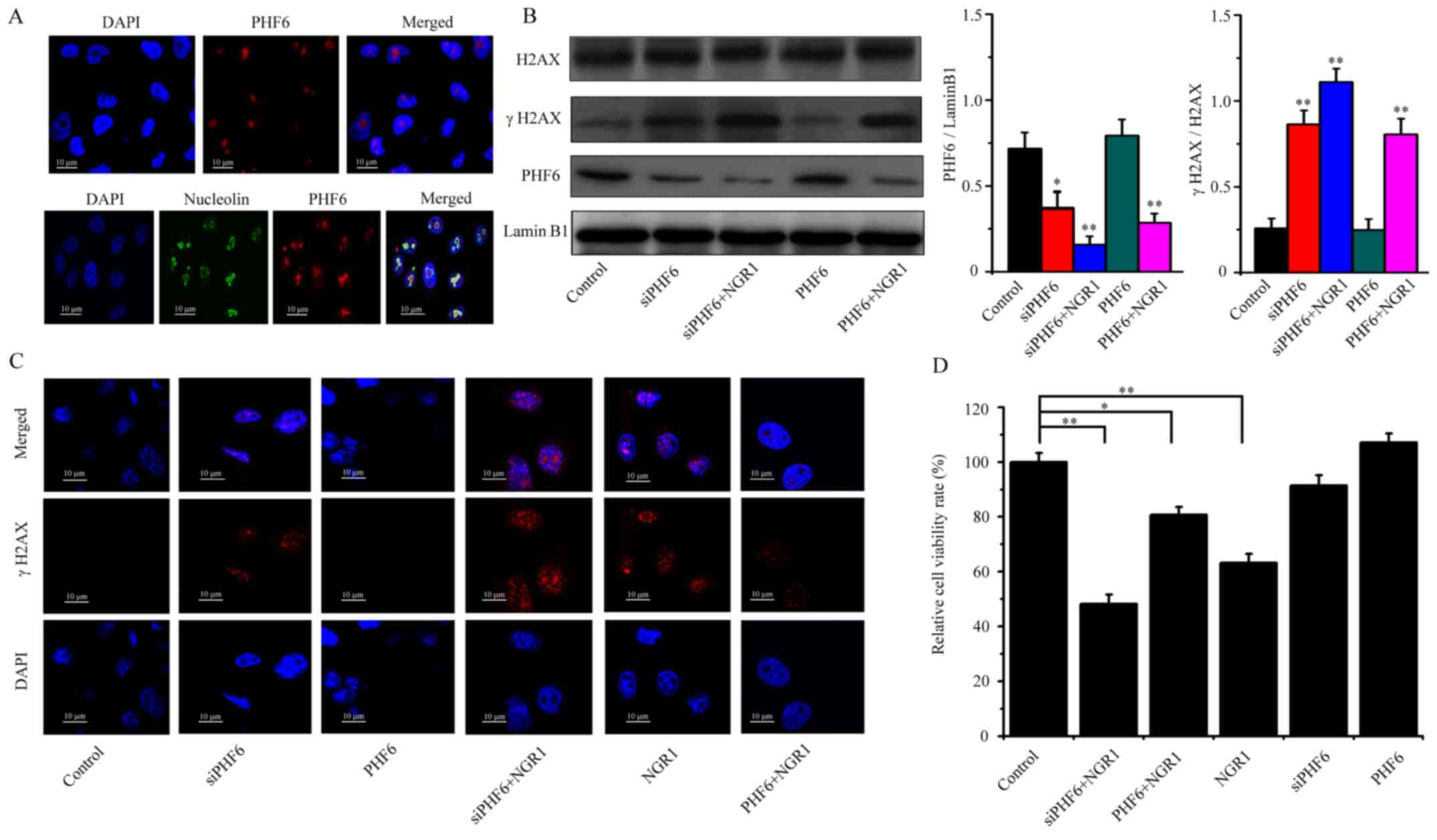
NGR1 induces DNA damage to inhibit
cell viability via the downregulation of PHF6 expression in the
nucleolus
PHF6 is a nucleolus protein involved in numerous
important biological processes, such as transcriptional regulation
and chromatin remodeling (25–27).
To demonstrate the localization of PHF6 in the nucleus, an
immunofluorescence assay was conducted. The results of the
immunofluorescence assay demonstrated that PHF6 was localized in
the cell nucleus. Furthermore, the results revealed that PHF6 was
specifically localized in the nucleoli (Fig. 5A). Prior to the cell transfection
assay, the interference efficiencies of the siPHF6s transfection
were presented in Fig. S1.
Meanwhile, the transfection efficiencies of pEGFP-C1-PHF6 and
siPHF6 were presented in Fig. S2.
As shown in Fig. 5B, PHF6
overexpression did not alter the expression levels of γH2AX
compared with the control group. but NGR1 treatment (PHF6 + 0.4 mM
NGR1) significantly increased γH2AX expression levels compared with
the control group (Fig. 5B).
Therefore, the results suggested that PHF6 overexpression did not
alter DNA damage. However, following transfection with siPHF6, the
expression level of the γH2AX protein was significantly upregulated
compared with the control group. The addition of NGR1 (siPHF6 + 0.4
mM NGR1 group) to si-PHF6-transfected HeLa cells markedly increased
γH2AX protein expression levels compared with the siPHF6 group.
Thus, the results suggested that PHF6 knockdown damaged DNA, but
also cooperated with NGR1 to induce DNA damage (Fig. 5B). The aforementioned results were
further supported by the immunocytochemistry results, which
indicated that the number of γH2AX foci was not markedly difference
between the PHF6 and control groups. However, the number of γH2AX
foci was markedly increased in the PHF6 + NGR1, NGR1 and siPHF6 +
NGR1 groups compared with the control group, especially in siPHF6 +
NGR1 group (Fig. 5C). The results
indicated that NGR1 might induce DNA damage via downregulating PHF6
expression. Furthermore, the association between PHF6, NGR1 and
cell viability was also investigated. PHF6 overexpression did not
alter HeLa cell viability compared with the control group. However,
PHF6 knockdown slightly inhibited HeLa cell viability compared with
the control group, although this was not significant. In all the
experimental groups, the siPHF6 + NGR1 group displayed the most
obvious significant inhibitory effect on cell viability compared
with the control group. The results revealed that siPHF6 promoted
NGR1-induced inhibition of cell viability (Fig. 5D).
Discussion
NGR1 has been extracted from the Panax
notoginsenoside complex and is used as a valuable Chinese
herbal medicine due to its reported multiple beneficial effects on
human health, such as inhibiting inflammatory responses,
antimyocardial ischemia and hypoxia, antiatherosclerosis,
antiplatelet aggregation effects (28–32).
Several previous studies have reported that NGR1 exerted
antihepatoma effects and inhibited human colorectal cancer
metastasis (33,34); however, few have reported the role
of NGR1 in cervical carcinoma. The present study demonstrated that
NGR1 had moderate antitumor activity and inhibited the viability of
cervical carcinoma cells in a time- and dose-dependent manner. In
addition, NGR1, as a immunologic adjuvant to enhance immunity
(35,36), may serve as a beneficial candidate
for chemotherapy to treat cervical carcinoma.
Apoptosis, a widespread phenomenon that occurs in
the developmental stages of prokaryotic and eukaryotic cells, is
controlled by specific genes, such as caspase-3, caspase-9, Bcl-2
and Bax (21,24,37–39).
It also plays a pivotal role in biological evolution, embryonic
development and dynamic homeostatic balance maintenance (40,41).
It was previously observed that NGR1 induced apoptosis in SW480
human colorectal cancer cells (17). In the present study, the results
revealed that NGR1 induced apoptosis in cervical carcinoma cells.
This conclusion was supported by the observed presence of apoptotic
bodies that appeared in the nucleus, an increased rate of
apoptosis, the upregulation of cleaved caspase-3 and PARP1 protein
expression levels, and the downregulation of Bcl-2 expression
levels following NGR1 treatment.
Panax notoginseng extract, which includes
notoginsenoside R1, ginsenosides Rg1, Re, Rb1, Rc and Rd, and
isomeric ginsenosides Rb2 and Rb3, caused cell cycle arrest at S
phase (16). The results of the
current study also revealed that NGR1 arrested cells in the S
phase, while simultaneously upregulating the expression levels of
cyclin A2 and CDK2, and downregulating the expression levels of
cyclin D1. DNA damage in cells is typically due to the biological
environment or endogenous metabolic cell products (42). An intricate DNA repair system has
since evolved to protect genomic stability, whereby ATR/ATM, p53
and PARP1 of the DDR signaling network are activated to participate
in the repair of damaged DNA (43,44).
γH2AX, a marker of double-strand breaks (DSBs), is crucial in the
cellular stress response to DNA damage and acts as a focal point
for the recruitment of other protein assemblages to repair the DSBs
(45–47). The present study also revealed that
NGR1 induced H2AX phosphorylation in a dose- and time-dependent
manner within the early experimental period (<24 h); however,
with longer exposure, the expression of γH2AX decreased until it
gradually returned to the normal levels (from 24 to 48 h). These
findings indicated that NGR1 may induce the DNA damage in the early
stage, but the DNA double strands were completely degraded as the
duration of NGR1 treatment increased. In addition, NGR1 upregulated
p53 expression levels and the phosphorylation of ATR in a dose- and
time-dependent manner, but downregulated the expression levels of
PHF6 in a dose- and time-dependent manner. These results indicated
that the downregulation of PHF6 expression may be negatively
associated with DNA damage.
Mutations in PHF6 were first discovered in
Borjeson-Forssman-Lehmann syndrome, which is a rare X-linked
intellectual disability syndrome (48). Previous studies have revealed that
PHF6 may be crucial for nucleolar transcriptional regulation and/or
chromatin remodeling (26,49) The knockdown of PHF6 was discovered
to trigger a series of biochemical signaling pathways to
participate in the repair of damaged ribosomal DNA in the nucleus,
thus delaying the progression of the cell cycle and inhibiting the
proliferation of cells (25,27).
In the present study, PHF6 was also discovered to be localized in
the nucleoli. Hence, the relationship between PHF6 and γH2AX
expression levels was subsequently investigated. The results
demonstrated that PHF6 knockdown upregulated γH2AX, but PHF6
overexpression downregulated γH2AX, suggesting that PHF6 expression
levels were negatively associated with γH2AX expression levels. In
addition, compared with the control group, the expression levels of
γH2AX were significantly increased in the PHF6 + NGR1 and siPHF6 +
NGR1 groups; in particular, γH2AX was more abundant in the siPHF6 +
NGR1 group. These findings indicated that the downregulation of
PHF6 may enhance NGR1-mediated induction of DNA damage. The same
findings were obtained from the immunofluorescence assays, as the
number of γH2AX foci was highest in the siPHF6 + NGR1 group. In
addition, PHF6 knockdown enhanced NGR1-mediated inhibition of
cervical carcinoma cell viability. Altogether, the current data
indicated that NGR1 may induce DNA damage to inhibit cell viability
by downregulating PHF6.
In conclusion, the findings of the present study
revealed that NGR1 was able to effectively inhibit the viability of
cervical carcinoma cells in a dose- and time-dependent manner,
resulting in apoptosis, the arrest of cells in the S phase, the
upregulation of cyclin A2 and CDK2 expression levels, and
downregulation of cyclin D1 expression levels. In addition, the
data also indicated that NGR1 induced DNA damage by downregulating
the nucleolus protein PHF6, which enhanced NGR1-induced DNA damage
and inhibited cervical carcinoma cell viability. The DNA repair
function can resist the anticancer effects of chemotherapy drugs,
leading to drug resistances50,51). Therefore, these findings may
provide a novel target and therapeutic strategy for cancer
therapies. NGR1 may have the potential to be applied in clinical
settings for the treatment of cervical carcinoma in the future.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by grants from the
Traditional Chinese Medicine Bureau of Guangdong Province (grant
no. 20181223), The Key Laboratory for Innovative Research on
Medical Laboratory Technology of Longhua District, Shenzhen (grant
no. 20150925A0410015) and The Science and Technology Innovation
Project of Longhua District, Shenzhen (grant no. 2017115).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PM conceived and designed the study. TC performed
the molecular experiments and wrote the manuscript. WW, YX, LZ and
FP performed some of the experiments and analyzed the data. LG and
XJ provided some reagents and performed flow cytometry experiments.
TC and PM interpreted the data and critically revised the
manuscript and confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Ervik M, Lam F, Colombet M, Mery
L, Pineros M, Znaor A, Soerjomataram I and Bray F: Global Cancer
Observatory: Cancer Today. International Agency for Research on
Cancer; Lyon: 2018
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Silver MI and Kobrin S: Exacerbating
disparities? Cervical cancer screening and HPV vaccination. Prev
Med. 130:1059022020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cervical cancer analysis reveals new
mutations. Cancer Discov. 7:3442017.
|
|
5
|
Kailash U, Soundararajan CC, Lakshmy R,
Arora R, Vivekanandhan S and Das BC: Telomerase activity as an
adjunct to high-risk human papillomavirus types 16 and 18 and
cytology screening in cervical cancer. Br J Cancer. 95:1250–1257.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharma A, Rajappa M, Saxena A and Sharma
M: Telomerase activity as a tumor marker in Indian women with
cervical intraepithelial neoplasia and cervical cancer. Mol Diagn
Ther. 11:193–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li C, Ma C, Zhang W and Wang J: The immune
function differences and high-risk human papillomavirus infection
in the progress of cervical cancer. Eur J Gynaecol Oncol.
35:557–561. 2014.PubMed/NCBI
|
|
8
|
Lindström AK and Hellberg D:
Immunohistochemical LRIG3 expression in cervical intraepithelial
neoplasia and invasive squamous cell cervical cancer: Association
with expression of tumor markers, hormones, high-risk
HPV-infection, smoking and patient outcome. Eur J Histochem.
58:22272014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Falletta P, Sanchez-del-Campo L, Chauhan
J, Effern M, Kenyon A, Kershaw CJ, Siddaway R, Lisle R, Freter R,
Daniels MJ, et al: Translation reprogramming is an evolutionarily
conserved driver of phenotypic plasticity and therapeutic
resistance in melanoma. Genes Deve. 31:18–33. 2017. View Article : Google Scholar
|
|
10
|
Wang F, Li L, Liu B, Chen Z and Li C:
Hyaluronic acid decorated pluronic P85 solid lipid nanoparticles as
a potential carrier to overcome multidrug resistance in cervical
and breast cancer. Biomed Pharmacother. 86:595–604. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang BR, Cheung KK, Zhou X, Xie RF, Cheng
PP, Wu S, Zhou ZY, Tang JY, Hoi PM, Wang YH and Lee SM:
Amelioration of acute myocardial infarction by saponins from flower
buds of Panax notoginseng via pro-angiogenesis and
anti-apoptosis. J Ethnopharmacol. 181:50–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang P, Zhang L, Yao J, Shi Y, Li P and
Ding K: An arabinogalactan from flowers of Panax notoginseng
inhibits angiogenesis by BMP2/Smad/Id1 signaling. Carbohydr Polym.
121:328–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Su P, Wang L, Du SJ, Xin WF and Zhang WS:
Advance in studies of Panax notoginseng saponins on
pharmacological mechanism of nervous system disease. Zhongguo Zhong
Yao Za Zhi. 39:4516–4521. 2014.(In Chinese). PubMed/NCBI
|
|
14
|
Xia W, Sun C, Zhao Y and Wu L:
Hypolipidemic and antioxidant activities of sanchi (Radix
notoginseng) in rats fed with a high fat diet. Phytomedicine.
18:516–520. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao Y, Sun X, Yu X, Gao R and Yin L:
Saponins from Panax notoginseng leaves improve the symptoms
of aplastic anemia and aberrant immunity in mice. Biomed
Pharmacother. 102:959–965. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He NW, Zhao Y, Guo L, Shang J and Yang XB:
Antioxidant, antiproliferative, and Pro-apoptotic activities of a
Saponin extract derived from the roots of Panax notoginseng
(Burk.) F.H. Chen. J Med Food. 15:350–359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan Z, Zhu ZL, Wang HQ, Li W, Mi YX and
Liu CX: Pharmacokinetics of panaxatrol disuccinate sodium, a novel
anti-cancer drug from Panax notoginseng, in healthy
volunteers and patients with advanced solid tumors. Acta Pharmacol
Sin. 31:1515–1522. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wan CZ, Xie JT, Fishbein A, Aung HH, He H,
Mehendale SR, He TC, Du W and Yuan CS: Antiproliferative effects of
different plant parts of Panax notoginseng on SW480 human
colorectal cancer cells. Phytother Res. 23:6–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cong S, Xiang L, Yuan X, Bai D and Zhang
X: Notoginsenoside R1 up-regulates microRNA-132 to protect human
lung fibroblast MRC-5 cells from lipopolysaccharide-caused injury.
Int Immunopharmacol. 68:137–144. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ming P, Cai T, Li J, Ning Y, Xie S, Tao T
and Tang F: A novel arylbenzofuran induces cervical cancer cell
apoptosis and G1/S arrest through ERK-mediated Cdk2/cyclin-A
signaling pathway. Oncotarget. 7:41843–41856. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen DL, Engle JT, Griffin EA, Miller JP,
Chu W, Zhou D and Mach RH: Imaging Caspase-3 activation as a marker
of apoptosis-targeted treatment response in cancer. Mol Imaging
Biol. 17:384–393. 2014. View Article : Google Scholar
|
|
22
|
Wang Y, Luo W and Wang Y: PARP-1 and its
associated nucleases in DNA damage response. DNA Repair (Amst).
81:1026512019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Caron MC, Sharma AK, O'Sullivan J, Myler
LR, Ferreira MT, Rodrigue A, Coulombe Y, Ethier C, Gagné JP,
Langelier MF, et al: Poly(ADP-ribose) polymerase-1 antagonizes DNA
resection at double-strand breaks. Nat Commun. 10:29542019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Siddiqui WA, Ahad A and Ahsan H: The
mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update.
Arch Toxicol. 89:289–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Todd MA, Huh MS and Picketts DJ: The
sub-nucleolar localization of PHF6 defines its role in rDNA
transcription and early processing events. Eur J Human Genet.
10:1453–1459. 2016. View Article : Google Scholar
|
|
26
|
Todd MA and Picketts DJ: PHF6 interacts
with the nucleosome remodeling and deacetylation (NuRD) complex. J
Proteome Res. 11:4326–4337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang J, Leung JWC, Gong Z, Feng L, Shi X
and Chen J: PHF6 regulates cell cycle progression by suppressing
ribosomal RNA synthesis. J Biol Chem. 288:3174–3183. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao J, Cui L, Sun J, Xie Z, Zhang L, Ding
Z and Quan X: Notoginsenoside R1 alleviates oxidized low-density
lipoprotein-induced apoptosis, inflammatory response, and oxidative
stress in HUVECS through modulation of XIST/miR-221-3p/TRAF6 axis.
Cell Signall. 76:1097812020. View Article : Google Scholar
|
|
29
|
Zhong L, Zhou XL, Liu YS, Wang YM, Ma F,
Guo BL, Yan ZQ and Zhang QY: Estrogen receptor α mediates the
effects of notoginsenoside R1 on endotoxin-induced inflammatory and
apoptotic responses in H9c2 cardiomyocytes. Mol Med Rep.
12:119–126. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He K, Yan L, Pan CS, Liu YY, Cui YC, Hu
BH, Chang X, Li Q, Sun K, Mao XW, et al: ROCK-dependent ATP5D
modulation contributes to the protection of notoginsenoside NR1
against ischemia-reperfusion-induced myocardial injury. Am J
Physiol Heart Circ Physiol. 307:H1764–H1776. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu Y, Sun G, Luo Y, Wang M, Chen R, Zhang
J, Ai Q, Xing N and Sun X: Cardioprotective effects of
Notoginsenoside R1 against ischemia/reperfusion injuries by
regulating oxidative stress- and endoplasmic reticulum
stress-related signaling pathways. Sci Rep. 6:217302016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peng Y, Li SN, Pei X and Hao K: The
multivariate regression statistics strategy to investigate
content-effect correlation of multiple components in Traditional
Chinese Medicine based on a partial least squares method.
Molecules. 23:5452018. View Article : Google Scholar
|
|
33
|
Li Y, Li Z, Jia Y, Ding B and Yu J: In
vitro Anti-hepatoma activities of Notoginsenoside R1 through
downregulation of tumor promoter miR-21. Dig Dis Sci. 65:1364–1375.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee CY, Hsieh SL, Hsieh S, Tsai CC, Hsieh
LC, Kuo YH and Wu CC: Inhibition of human colorectal cancer
metastasis by notoginsenoside R1, an important compound from
Panax notoginseng. Oncol Rep. 37:399–407. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Limsuwanchote S, Wungsintaweekul J,
Yusakul G, Han JY, Sasaki-Tabata K, Tanaka H, Shoyama Y and
Morimoto S: Preparation of a monoclonal antibody against
Notoginsenoside R1, a distinctive saponin from Panax
notoginseng, and its application to indirect competitive ELISA.
Planta Medica. 80:337–342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun HX, Chen Y and Ye Y: Ginsenoside Re
and notoginsenoside R1: Immunologic adjuvants with low haemolytic
effect. Chem Biodivers. 3:718–726. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dwyer DJ, Camacho DM, Kohanski MA, Callura
JM and Collins JJ: Antibiotic-induced bacterial cell death exhibits
physiological and biochemical hallmarks of apoptosis. Mol Cell.
46:561–572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li Y, Zhou M, Hu Q, Bai XC, Huang W,
Scheres SH and Shi Y: Mechanistic insights into caspase-9
activation by the structure of the apoptosome holoenzyme. Proc Natl
Acad Sci USA. 114:1542–1547. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Funk K, Czauderna C, Klesse R, Becker D,
Hajduk J, Oelgeklaus A, Reichenbach F, Fimm-Todt F, Lauterwasser J,
Galle PR, et al: BAX redistribution induces apoptosis resistance
and selective stress sensitivity in human HCC. Cancers (Basel).
12:14372020. View Article : Google Scholar
|
|
40
|
Kulkarni S, Micci MA, Leser J, Shin C,
Tang SC, Fu YY, Liu L, Li Q, Saha M, Li C, et al: Adult enteric
nervous system in health is maintained by a dynamic balance between
neuronal apoptosis and neurogenesis. Proc Natl Acad Sci USA.
114:E3709–E3718. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marchi S, Patergnani S, Missiroli S,
Morciano G, Rimessi A, Wieckowski MR, Giorgi C and Pinton P:
Mitochondrial and endoplasmic reticulum calcium homeostasis and
cell death. Cell Calcium. 69:62–72. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stadler J and Richly H: Regulation of DNA
repair mechanisms: How the chromatin environment regulates the DNA
damage response. Int J Mol Sci. 18:17152017. View Article : Google Scholar
|
|
43
|
Matsuoka S, Ballif BA, Smogorzewska A,
McDonald ER III, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini
N, Lerenthal Y, et al: ATM and ATR substrate analysis reveals
extensive protein networks responsive to DNA damage. Science.
316:1160–1166. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zheng L, Dai H, Zhou M, Li X, Liu C, Guo
Z, Wu X, Wu J, Wang C, Zhong J, et al: Polyploid cells rewire DNA
damage response networks to overcome replication stress-induced
barriers for tumour progression. Nat Commun. 3:8152012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lowndes NF and Toh GW: DNA repair: The
importance of phosphorylating histone H2AX. Curr Biol. 15:R99–R102.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jakob B, Splinter J, Conrad S, Voss KO,
Zink D, Durante M, Löbrich M and Taucher-Scholz G: DNA
double-strand breaks in heterochromatin elicit fast repair protein
recruitment, histone H2AX phosphorylation and relocation to
euchromatin. Nucleic Acids Res. 39:6489–6499. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Müller B, Ellinwood NM, Lorenz B and
Stieger K: Detection of DNA double strand breaks by γH2AX does not
result in 53bp1 recruitment in mouse retinal tissues. Front
Neurosci. 12:2862018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang C, Mejia LA, Huang J, Valnegri P,
Bennett EJ, Anckar J, Jahani-Asl A, Gallardo G, Ikeuchi Y, Yamada
T, et al: The X-linked intellectual disability protein PHF6
associates with the PAF1 complex and regulates neuronal migration
in the mammalian brain. Neuron. 78:986–993. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Vallée D, Chevrier E, Graham GE, Lazzaro
MA, Lavigne PA, Hunter AG and Picketts DJ: A novel PHF6 mutation
results in enhanced exon skipping and mild
Borjeson-Forssman-Lehmann syndrome. J Med Genet. 41:778–783. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rocha CRR, Silva MM, Quinet A, Cabral-Neto
JB and Menck CFM: DNA repair pathways and cisplatin resistance: An
intimate relationship. Clinics (Sao Paulo). 73 (Suppl 1):e478s2018.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Salehan MR and Morse HR: DNA damage repair
and tolerance: A role in chemotherapeutic drug resistance. Br J
Biomed Sci. 70:31–40. 2016. View Article : Google Scholar
|