Introduction
Thyroid cancer (TC) is the most frequent endocrine
malignancy in the world (1,2). Over the last decade, the incidence
rate of TC incidence has been increasing by ~2% per year, with a
slow but steady increase in the mortality rate (~0.7% per year)
(3). There are several risk factors
that are closely associated with TC occurrence and development,
including genetic factors, age, sex, environmental exposure and
epigenetic alterations. Even though there have been improvements in
the diagnosis and surgical treatment of TC, the disadvantages
associated with treatment strategies should not be ignored
(4,5). Furthermore, TC demonstrates an
increasing morbidity year on year, along with high rate of
recurrence and a younger age at diagnosis, indicating a requirement
for additional research on TC (3).
Therefore, it is necessary to elucidate the underlying mechanisms
associated with the progression and pathogenesis of TC and in turn
help its treatment.
Sld5, Psf1 (GINS1), Psf2 (GINS2) and Psf3 (GINS3)
constitute the DNA replication complex (6–8). Among
them, GINS2 is an important subunit of GINS DNA replication complex
and is located on human chromosome at 16q24 (9). The relative molecular mass of GINS2 is
21 kDa and the mRNA length is 1,196 bp (8,10,11).
GINS2 mediates the interaction between MCM complexes and Cdc45
during the initiation of DNA replication in eukaryotic cells
(9). Previous reports have
identified that GINS2 is associated with a number of malignant
tumors (12,13). It mainly affects the initiation and
progression of malignancy. For example, the expression of GINS2 is
remarkably upregulated in acute promyelocytic leukemia (14,15).
In addition, upregulation of GINS2 is found in lung cancer tissues
and is in turn associated with cancer metastasis (16). GINS2 can enhance the ability of
growth and proliferation in cervical cancer (6). As a consequence, GINS2 could act as a
potential prognosis indicators and drug target in some types of
malignant tumors. The connection between GINS2 and the development
of TC requires further elucidation.
MAPK belongs to serine/threonine protein kinases
(17,18). The MAPK signaling cascade acts as a
critical pathway for tumor cell proliferation, differentiation,
apoptosis and drug-resistance (19,20).
MAPK is activated by a variety of stimulants including cytokines,
growth factors, neurotransmitters and hormones (21,22).
In addition, MAPK activation can phosphorylate nuclear
transcription factors and protein kinases, regulate the
transcription of related genes and participate in various
physiological processes (22).
Previous studies have shown that the MAPK signaling pathway is
activated in a various types of cancer, including gastric (23), lung (24), ovarian (25) and liver cancers (26). The MAPK signaling pathway consists
of four sub-pathways: ERK, JNK, BMK and p38 (27). Studies have shown that activation of
the ERK signaling pathway facilitates cell growth, differentiation,
migration and survival (28,29).
Activation of JNK and p38 involves changes of cell differentiation,
apoptosis and cell survival (30).
However, the function and regulation of BMK1/ERK5 have been
explored by fewer studies although this pathway has been reported
in the regulation of cell growth, differentiation and survival
(31).
The present study aimed to investigate the
relationship between GINS2 and TC and explore the effects of MAPK
signaling pathway in the process of GINS2 that affects TC cells,
offering a new potential diagnosis target for TC.
Materials and methods
Cell lines and authentication
TC cell lines (K1 and SW579) were obtained from The
Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences, Shanghai, China. K1 cells were maintained in 5%
CO2 at 37°C with RPMI-1640 medium (HyClone; Cytiva)
supplemented with 10% fetal bovine serum (10% FBS; Gibco; Thermo
Fisher Scientific, Inc.) and penicillin/streptomycin (HyClone;
Cytiva). SW579 cells were maintained in 0% CO2 at 37°C
with L-15 medium (HyClone; Cytiva) supplemented with 10% FBS and
penicillin/streptomycin. In order to ensure the accuracy of
results, STR profiling was performed for K1 cells. DNA was
extracted with genomic extraction kit (Axygen; Corning, Inc.) and
amplified with 21-STR. Data were analyzed using an ABI 3730XL
genetic analyzer (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The EXPASY database (www.expasy.org/) confirmed that the cell name was K1
and the cell number corresponded to CVCL_2537. No multiple allelic
groups were found in the cell line.
Gene interference
Small interfering (si)RNA was used to knock down
specific sequences. The oligonucleotides against GINS2 sequences
were designed and synthesized by Shanghai GenePharma Co., Ltd. The
following sequences were included: GINS2 siRNA (sense:
GCUCAACCACAUGUACAAATT and antisense: UUUGUACAUGUGGUUGAGCTT); and
negative control (NC) sequence (sense: UUCUCCGAACGU GUCACGUTT and
antisense: ACGUGACACGUUCGG AGAATT). These are not homologous to any
human DNA sequences. K1 and SW579 cells were seeded into culture
plate and incubated at 37°C overnight, followed by transfection
with siRNA-GINS2, blank and respective controls (80 nM) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols. After
24–48 h transfection, the subsequent experiments were
performed.
Western blot analysis
Total proteins were isolated from cell lysates using
RIPA buffer containing protease inhibitors and phosphatase
inhibitors (Beyotime Institute of Biotechnology). The protein
concentrations were determined using a BCA protein assay kit
(Beyotime Institute of Biotechnology). Protein (~40 µg) from each
sample was separated to 10% SDS-PAGE and then transferred onto a
PVDF membrane (EMD Millipore). The membranes were washed with pH
7.5, 50 mM Tris-HCL buffer saline containing 0.05% Tween-20 (TBST)
and blocked with 5% skimmed milk powder for 1 h at room
temperature. The PVDF membrane was incubated with primary
antibodies at 4°C overnight with gentle agitation. The following
primary antibodies were used in the present study: GINS2 (1:1,000;
cat. no. ab197123; Abcam), ERK (1:1,000; cat. no. 4695; Cell
Signaling Technology, Inc.), phosphorylated (p-)ERK (1:2,000; cat.
no. 4370; Cell Signaling Technology, Inc.), JNK (1:1,000; cat. no.
9252; Cell Signaling Technology, Inc.), p-JNK (1:2,000, cat. no.
9255, Cell Signaling Technology, Inc.), p38 (1:1,000; cat. no.
8690; Cell Signaling Technology, Inc.), p-p38 (1:1,000; cat. no.
4511; Cell Signaling Technology, Inc.), and GAPDH (1:1,000; cat.
no. 5174; Cell Signaling Technology, Inc.). The samples were washed
with TBST (containing 0.1% Tween-20), then incubated with secondary
antibodies conjugated with horseradish peroxidase (1:3,000; cat.
nos. 7074 and 7076; Cell Signaling Technology, Inc.) at room
temperature for 1.5 h. The blots were observed using an enhanced
chemiluminescence detection kit (Beyotime Institute of
Biotechnology). The levels of the proteins of interest were
normalized to GAPDH and analyzed using FluorChem FC3 software
(version 3.4.0, ProteinSimple).
MTT assay
Cell proliferation was measured using MTT assay
according to the manufacturer's protocols (Beyotime Institute of
Biotechnology). The TC cell lines were cultivated in 96-well plates
at a density of ~5×103/well, followed by treatment with
siGINS2 at 12, 24, 48 and 72 h. CCK-8 reagent (10 µl) was added to
each well and incubation for 4 h at 37°C. The optical density (OD)
value of each well was measured at 450 nm by using a microplate
reader (Promega Corporation). The cell viability results from three
independent experiments were normalized to the medium control group
and expressed as the mean ± standard deviation.
Apoptosis assay
Cell apoptosis rate was detected by a fluorescein
isothiocyanate (FITC)-Annexin V/propidium iodide (PI) apoptosis kit
(Beyotime Institute of Biotechnology). TC cells were seeded into a
24-well plate at a density of ~2×105 cells/well for
apoptosis assay. Following treatment with NC sequence or GINS2
siRNA, the cells were washed twice with PBS, followed by addition
of Annexin V-FITC (5 µl) into the well according to the
manufacturer's protocols and maintained for 15 min at room
temperature for the reaction to take place. Propidium iodide (10
µl) was added into the tube and left for 15 min at room
temperature. Cell apoptosis was determined by using an inverted
fluorescence microscope (Olympus Corporation) within 1 h. Data were
analyzed using ImageJ 1.46 (National Institutes of Health).
Transwell assay
A Transwell 24-well Boyden chamber (8 µm pore size,
Corning, Inc.) with or without Matrigel was used to determine the
capacity of migration and invasion. For migration assay, the cells
after treatment were digested with 0.25% trypsin (Thermo Fisher
Scientific, Inc.) and then suspended in serum-free medium. The
cells were counted and seeded at a density of 1×105
cells/well in the upper chamber. A total of 700 µl of complete
medium was placed in the lower chamber as a source of
chemo-attractant. After 24 h, the non-migratory cells in the upper
layer of the chamber were removed, while the cells which had
migrated through the membrane were fixed with 4% paraformaldehyde
for 10 min and stained with 0.5% crystal violet for 30 min at room
temperature. A total of five random high power fields were observed
and images captured under a light microscope (magnification, ×100,
Olympus Corporation), then the migration through the membrane
calculated as the average number of cells/field. For invasion
assay, the steps were similar to that of the cell migration, with
the only difference being that the Transwell membranes were
precoated with Matrigel for 30 min at 37°C (diluted at 1:2; BD
Biosciences).
Wound healing assay
A wound healing assay was used to assess the cell
migration ability in TC cell lines. Following the transfection of
K1 and SW579 cells, the cells were trypsinized and seeded into a
6-well plate and cultured until they reached 80% confluence in a
complete medium. Each well was then scratched by a 200 µl sterile
pipette tip and washed with PBS several times to remove cell
debris. In the next 48 h, the cells were incubated with serum-free
medium and the cells that migrated to the wound surface were
considered as producing an in vitro healing process. Images
of the wound healing were captured under a light microscope
(magnification, ×100; Olympus Corporation) and the rate of closure
was assessed. The relative migratory ability was evaluated using
ImageJ 1.46 (National Institutes of Health) based on the width at 0
h time point (the wound width of (0–48 h)/0 h wound width ×
100%.
Statistical analysis
All the data are shown as means ± standard deviation
and analyzed by one-way analysis of variance followed by Tukey's
test. Statistical analyses were performed using SPSS v16.0 software
(SPSS, Inc.). The experiments were repeated ≥3 times. P<0.05 was
considered to indicate a statistically significant difference.
Results
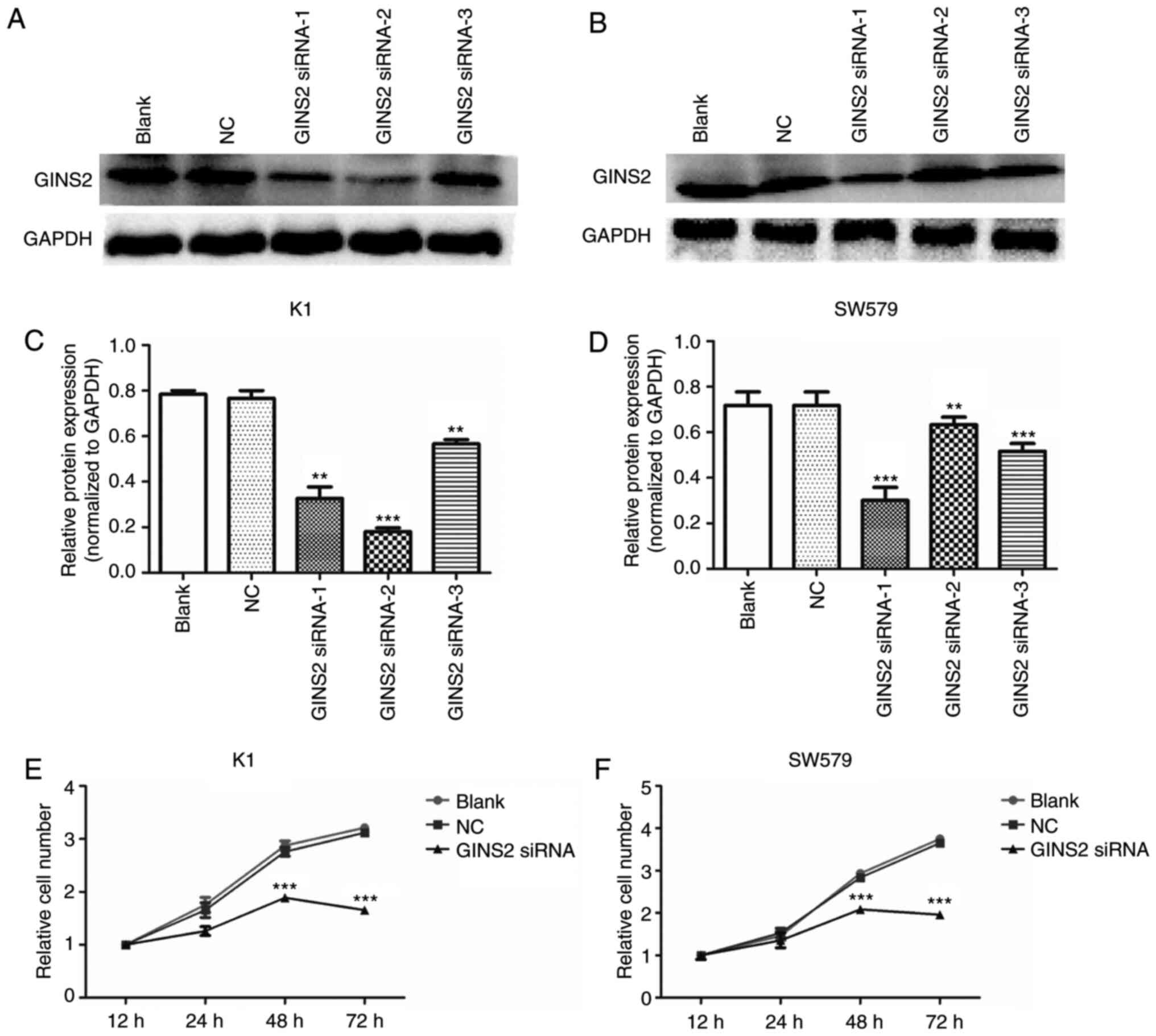
Suppression of GINS2 markedly inhibits
the cell proliferation of TC cells
K1 and SW579 cells were treated with GINS2 siRNA to
inhibit the expression of GINS2. Fig.
1A and C (K1 cells) and Fig. 1B and
D (SW579 cells) show the western blotting images of GINS2
interference by different siRNAs. The most effective sequence
(GINS2 siRNA-1) was selected to conduct further experiments. To
examine whether GINS2 has an effect on TC cell proliferation, MTT
assay was performed. TC cells were transfected with GINS2 siRNA and
incubated for 12, 24, 48 and 72 h. The results demonstrated that
the absorbance in K1 (Fig. 1E) and
SW579 (Fig. 1F) cells was increased
from 12 to 72 h in the control group, while the OD 450 nm value was
increased from 12 to 48 h and decreased from 48 to 72 h in the
GINS2 siRNA group. From 24 to 72 h, the number of TC cells in the
GINS2 siRNA group was clearly lower compared with the control
(P<0.001). These results demonstrated that cell viability was
significantly inhibited following silencing of the GINS2 gene by
siRNA in K1 and SW579 cells.
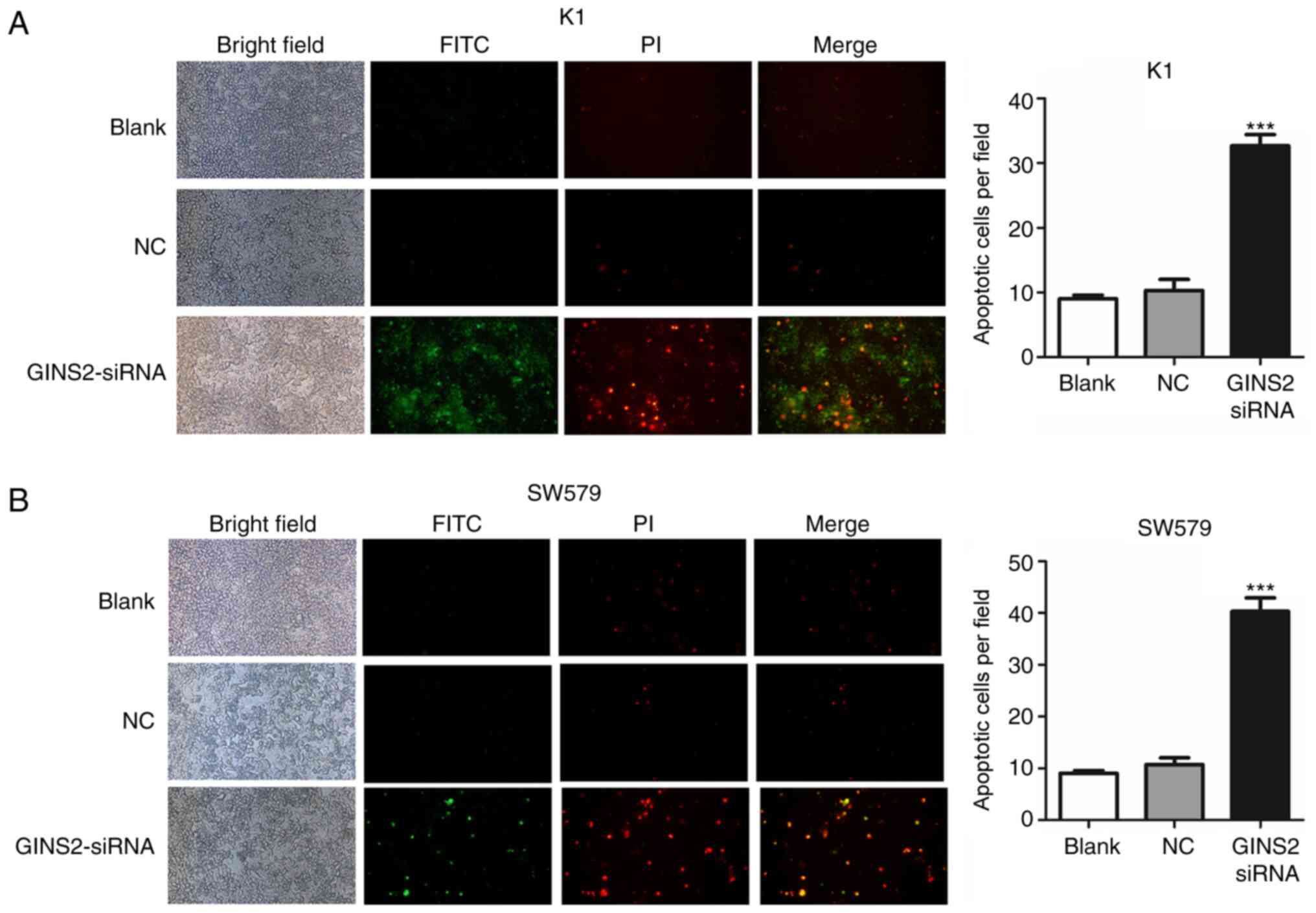
Suppression of GINS2 induces cell
apoptosis in TC cells
Whether GINS2 interference could affect cell
apoptosis in K1 and SW579 cells was studied. The cells were
subjected to Annexin V-FITC/PI staining. The results revealed that
the GINS2 suppression induced apoptotic cell death of TC cells
(Fig. 2A and B), suggesting that
inhibition of GINS2 initiated cell death.
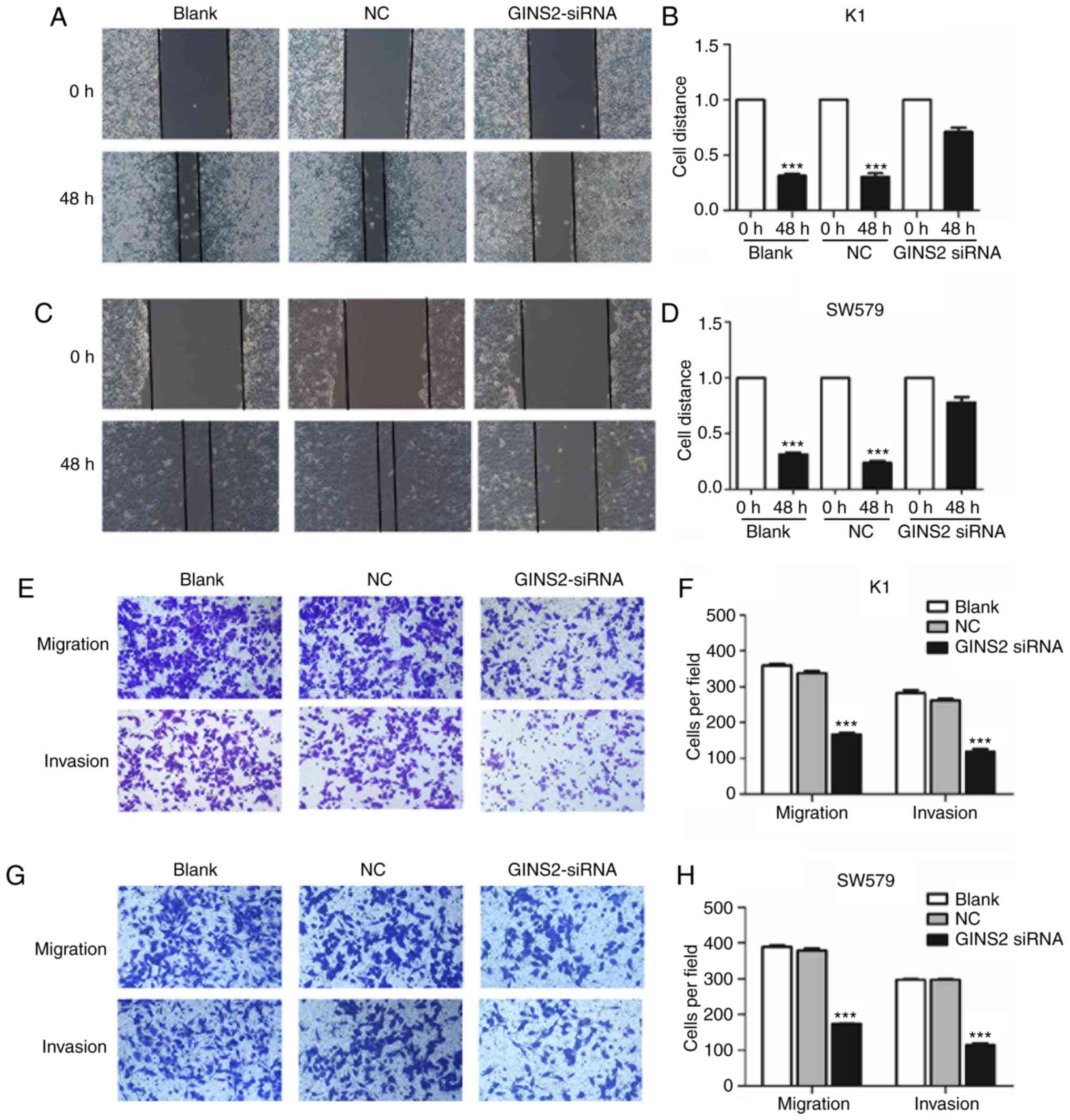
Suppression of GINS2 decreases
migration and invasion of TC cells
The ability of migration and invasion is a key
indicator of tumor metastasis. The present study used wound healing
and Transwell assays to determine the capacity of tumor metastasis.
The results of wound healing assay demonstrated that silencing of
GINS2 significantly inhibited the migratory capacity in K1 and
SW579 cells (P<0.01; Fig. 3A-D).
In addition, Transwell assay with or without Matrigel was used to
investigate the effect of GINS2 on the migratory and invasive
abilities of K1 and SW579 cells. As demonstrated in Fig. 3E-H, interference of GINS2
significantly suppressed the migratory and invasive abilities of K1
and SW579 cells (P<0.001). These results suggested that GINS2
acted as a promoter gene of migration and invasion in TC cells.
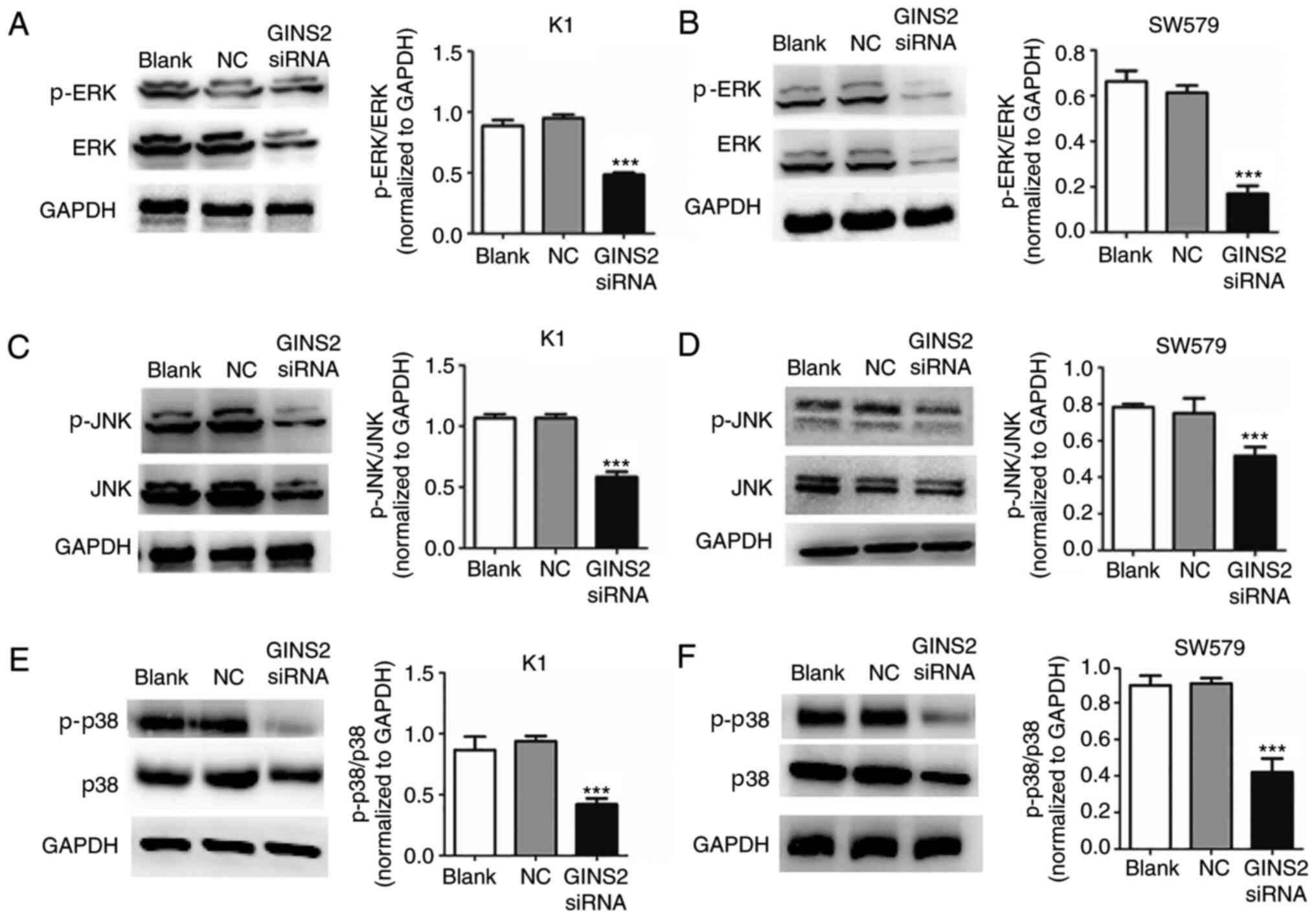
Suppression of GINS2 affects TC
proliferation through the MAPK signaling pathway
The forgoing results demonstrated that
downregulation of GINS2 caused changes in the physiological
function of the cells, including cell proliferation, apoptosis,
migration and invasion. To determine the mechanism between GINS2
and TC, signaling molecules of the MAPK pathway associated with
proliferation and migration (ERK, JNK and p38) were detected by
western blotting. This demonstrated that the phosphorylation levels
of ERK, JNK and p38 were significantly decreased with GINS2
interference in K1 and SW579 cell lines when compared to control
cells (***P<0.001; Fig. 4A-F).
These results indicated that downregulation of GINS2 inhibited
over-activated MAPK signaling pathways in TC cells.
Discussion
The present study presented a comprehensive
investigation on the role of GINS2 in regulating TC tumorigenesis.
Functional analyses demonstrated that silencing of GINS2 inhibited
cell proliferation, migration and invasion and induced cell
apoptosis in TC cell lines. Furthermore, GINS2 interference
affected the biological function of TC cells by regulating the MAPK
signaling pathway. These results implied that GINS2 might be a
potential therapeutic target for treating TC.
Overproliferation, migration and invasion of tumor
cells are important problems that urgently require clarification
and resolution in the clinical and experimental research of
malignant tumors and this is also the case in thyroid cancer
(32,33). Changes in the physiological
functions of tumor cells is a complex process that involves
multiple genes and pathways. GINS is a ring-like protein complex
that involves PSF1, PSF2 (GINS2), PSF3 and SLD5 and is initially
extracted from budding yeast (34).
GINS2 acts as an important subunit of GINS complexes and mediates
the initiation of DNA replication in eukaryotic cells (35,36).
Previous studies have revealed that GINS2 is associated with the
malignancy of some cancer types and is highly expressed in several
malignant tumors (6,12), including TC (9). Furthermore, overexpression of GINS2
demonstrates an association with prognostic survival period and
distant metastasis (12).
Previous experimental and clinical studies have
shown that GINS2 is closely associated with tumorigenesis (6,12) and
the present study hypothesized that GINS2 might be involved in
tumor malignancy. Cell proliferation, migration and invasion are
regarded as the most important biological characteristics of
malignant cell behavior (13).
Tumor cells have the characteristics of limitless replicative
potential, tissue invasion and metastasis, resisting cell death
(37). In particular, limitless
proliferation of tumor cells promotes the progression of
metastasis, which is one of the major causes of poor prognosis
(24). In the present study, GINS2
silencing resulted in reduced cell proliferation, decreased
migration rate and impaired invasive ability in TC cells.
Furthermore, cell apoptosis was increased in K1 and SW579 cells
following GINS2 silencing by in situ fluorescence detection.
Previous studies have demonstrated that in situ fluorescence
assay can verify the change of apoptosis rate (38,39).
Alternatively, caspase-3 activity and other techniques can also be
applied for cell apoptosis assay, which will be performed in
further studies.
The results of the present study confirmed that
GINS2 might serve an important role in TC progression and the
related molecular mechanisms in this process require further
investigation. Cells encounter a variety of signals in their
environment and respond to each stimulus appropriately by
modulating gene and protein expression expression levels (17,26).
Numerous external signals are transduced by a highly conserved
eukaryotic signaling mechanism, including the well-known MAPK
cascade (20). MAPK, which is a
type of serine/threonine protein kinase that can be activated by
different extracellular stimuli, including cytokines,
neurotransmitters, hormones, cell stress and cell adhesion, is an
important transporter of signals from cell surface to nucleus
(17). MAPK regulates cell
proliferation, differentiation, development and apoptosis through
signaling transmission (23,40).
In our previous study, it was found that GINS2 interference
inhibited cell viability, induced cell cycle arrest and promoted
cell apoptosis in pancreatic cancer cell lines via the MAPK/ERK
pathway (41). In the present
study, the relationship between GINS2 and MAPK pathway was
investigated in TC cells. The results demonstrated that GINS2
silencing resulted in significantly decreased expression levels of
phosphorylated ERK, JNK and p38 in K1 and SW579 cells. These
results indicated that silencing of GINS2 could inhibit the
activation of MAPK pathway and then affect proliferation,
apoptosis, migration and invasion of cells.
However, there are still some limitations in the
present study. Rescue assays were not performed, wherein MAPK was
overexpressed at the same time as GINS2 downregulation, in order to
investigate if MAPK overexpression can overcome the effect of GINS2
knockdown. Also, GINS2 may also inhibit or activate other signaling
pathways, which will be further investigated in the future.
The present study preliminarily clarified that GINS2
silencing suppressed cell proliferation, migration and invasion and
induced cell apoptosis in TC by regulating MAPK signaling pathways.
More studies should be conducted to illuminate the function of
GINS2 and its related mechanisms in the future.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from
National Natural Science Foundation of China (grant nos. 81873178
and 81904044), Shanghai Municipal Commission of Health and Family
Planning (grant no. 201740084), Science and Technology Development
Fund of Shanghai Pudong New Area (grant no. PKJ2019-Y15), Key
Specialty Construction Project of Pudong Health and Family Planning
Commission of Shanghai (grant no. PWZzk2017-21), Shanghai Pudong
Commission of Health and Family Planning (grant no. PW2017B-11) and
Talents Training Program of Seventh People's Hospital of Shanghai
University of TCM (grant nos. XX2017-06 and XX2019-01).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WX and BZ conceived the experiments. SH, MZ, XM and
GW conducted the experiments. YY, YS and JZ analyzed the data. MZ
and YS wrote and revised the paper. WX and BZ confirm the
authenticity of the data used in the present manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xiao Y, Zhou Q, Xu Y, Yuan SL and Liu QA:
Positive thyroid antibodies and risk of thyroid cancer: A
systematic review and meta-analysis. Mol Clin Oncol. 11:234–242.
2019.PubMed/NCBI
|
|
2
|
Xing M: Molecular pathogenesis and
mechanisms of thyroid cancer. Nat Rev Cancer. 13:184–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bonjoc KJ, Young H, Warner S, Gernon T,
Maghami E and Chaudhry A: Thyroid cancer diagnosis in the era of
precision imaging. J Thorac Dis. 12:5128–5139. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reale C, Russo F, Credendino SC, Cuomo D,
De Vita G, Mallardo M, Pennino F, Porreca I, Triassi M, De Felice
M, et al: A toxicogenomic approach reveals a novel gene regulatory
network active in in vitro and in vivo models of thyroid
carcinogenesis. Int J Environ Res Public Health. 16:162019.
View Article : Google Scholar
|
|
5
|
Samimi H, Haghpanah V, Irani S, Arefian E,
Sohi AN, Fallah P and Soleimani M: Transcript-level regulation of
MALAT1-mediated cell cycle and apoptosis genes using dual
MEK/Aurora kinase inhibitor ‘BI-847325’ on anaplastic thyroid
carcinoma. Daru. 27:1–7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ouyang F, Liu J, Xia M, Lin C, Wu X, Ye L,
Song L, Li J, Wang J, Guo P, et al: GINS2 is a novel prognostic
biomarker and promotes tumor progression in early-stage cervical
cancer. Oncol Rep. 37:2652–2662. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan T, Liang W, Jiang E, Ye A, Wu Q and Xi
M: GINS2 regulates cell proliferation and apoptosis in human
epithelial ovarian cancer. Oncol Lett. 16:2591–2598.
2018.PubMed/NCBI
|
|
8
|
Peng L, Song Z, Chen D, Linghu R, Wang Y,
Zhang X, Kou X, Yang J and Jiao S: GINS2 regulates matrix
metallopeptidase 9 expression and cancer stem cell property in
human triple negative breast cancer. Biomed Pharmacother.
84:1568–1574. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ye Y, Song YN, He SF, Zhuang JH, Wang GY
and Xia W: GINS2 promotes cell proliferation and inhibits cell
apoptosis in thyroid cancer by regulating CITED2 and LOXL2. Cancer
Gene Ther. 26:103–113. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu C, Wang R and Zhang Y: GINS complex
subunit 2 (GINS2) plays a protective role in alcohol-induced brain
injury. Artif Cells Nanomed Biotechnol. 47:1–9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng M, Zhou Y, Yang X, Tang J, Wei D,
Zhang Y, Jiang JL, Chen ZN and Zhu P: High GINS2 transcript level
predicts poor prognosis and correlates with high histological grade
and endocrine therapy resistance through mammary cancer stem cells
in breast cancer patients. Breast Cancer Res Treat. 148:423–436.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rantala JK, Edgren H, Lehtinen L, Wolf M,
Kleivi K, Vollan HK, Aaltola AR, Laasola P, Kilpinen S, Saviranta
P, et al: Integrative functional genomics analysis of sustained
polyploidy phenotypes in breast cancer cells identifies an
oncogenic profile for GINS2. Neoplasia. 12:877–888. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen YL, Li HZ, Hu YW, Zheng L and Wang Q:
Loss of GINS2 inhibits cell proliferation and tumorigenesis in
human gliomas. CNS Neurosci Ther. 25:273–287. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao Y, Wang S, Liu B and Zhong L: Roles of
GINS2 in K562 human chronic myelogenous leukemia and NB4 acute
promyelocytic leukemia cells. Int J Mol Med. 31:1402–1410. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Zhong L, Liu BZ, Gao YJ, Gao YM
and Hu XX: Effect of GINS2 on proliferation and apoptosis in
leukemic cell line. Int J Med Sci. 10:1795–1804. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
MacNeill SA: Structure and function of the
GINS complex, a key component of the eukaryotic replisome. Biochem
J. 425:489–500. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim EK and Choi EJ: Compromised MAPK
signaling in human diseases: An update. Arch Toxicol. 89:867–882.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun J and Nan G: The mitogen-activated
protein kinase (MAPK) signaling pathway as a discovery target in
stroke. J Mol Neurosci. 59:90–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Burotto M, Chiou VL, Lee JM and Kohn EC:
The MAPK pathway across different malignancies: A new perspective.
Cancer. 120:3446–3456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gyurkó MD, Steták A, Sőti C and Csermely
P: Multitarget network strategies to influence memory and
forgetting: The Ras/MAPK pathway as a novel option. Mini Rev Med
Chem. 15:696–704. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cuadrado A and Nebreda AR: Mechanisms and
functions of p38 MAPK signalling. Biochem J. 429:403–417. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma Y, Zhao Q, Shao Y, Cao MZ, Zhao M and
Wang D: Melatonin inhibits the inflammation and apoptosis in rats
with diabetic retinopathy via MAPK pathway. Eur Rev Med Pharmacol
Sci. 23 (Suppl):1–8. 2019.PubMed/NCBI
|
|
23
|
Shang H, Cao Z, Zhao J, Guan J, Liu J,
Peng J, Chen Y, Joseph Sferra T, Sankararaman S and Lin J: Babao
Dan induces gastric cancer cell apoptosis via regulating MAPK and
NF-κB signaling pathways. J Int Med Res. 47:5106–5119. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang G, Zeng Z, Sun W, Li S, You C, Tang
F, Peng S, Ma S, Luo Y, Xu J, et al: Small nucleolar RNA 71A
promotes lung cancer cell proliferation, migration and invasion via
MAPK/ERK pathway. J Cancer. 10:2261–2275. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yasui H, Kajiyama H, Tamauchi S, Suzuki S,
Peng Y, Yoshikawa N, Sugiyama M, Nakamura K and Kikkawa F: CCL2
secreted from cancer-associated mesothelial cells promotes
peritoneal metastasis of ovarian cancer cells through the P38-MAPK
pathway. Clin Exp Metastasis. 37:145–158. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jin C, Chen Z, Shi W and Lian Q:
Tropomodulin 3 promotes liver cancer progression by activating the
MAPK/ERK signaling pathway. Oncol Rep. 41:3060–3068.
2019.PubMed/NCBI
|
|
27
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lavoie H, Gagnon J and Therrien M: ERK
signalling: A master regulator of cell behaviour, life and fate.
Nat Rev Mol Cell Biol. 21:607–632. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Semba T, Sammons R, Wang X, Xie X, Dalby
KN and Ueno NT: JNK signaling in stem cell self-renewal and
differentiation. Int J Mol Sci. 21:212020. View Article : Google Scholar
|
|
31
|
Yu Z, Ye S, Hu G, Lv M, Tu Z, Zhou K and
Li Q: The RAF-MEK-ERK pathway: Targeting ERK to overcome obstacles
to effective cancer therapy. Future Med Chem. 7:269–289. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou C, Yang C and Chong D: E-cadherin
expression is associated with susceptibility and
clinicopathological characteristics of thyroid cancer: A
PRISMA-compliant meta-analysis. Medicine (Baltimore).
98:e161872019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kawai T, Iwata K, Shinotsuka Y, Kubo S,
Masuoka H, Yabuta T, Hirokawa M, Nakamura H, Miyauchi A and Komai
K: CD44v8-10 and CD44s are age-dependently expressed in primary
cultured papillary thyroid carcinoma cells and are associated with
cell proliferation. Kobe J Med Sci. 65:E1–E9. 2019.PubMed/NCBI
|
|
34
|
Hashimoto Y, Puddu F and Costanzo V:
RAD51- and MRE11-dependent reassembly of uncoupled CMG helicase
complex at collapsed replication forks. Nat Struct Mol Biol.
19:17–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moiseeva T, Hood B, Schamus S, O'Connor
MJ, Conrads TP and Bakkenist CJ: ATR kinase inhibition induces
unscheduled origin firing through a Cdc7-dependent association
between GINS and And-1. Nat Commun. 8:13922017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chmielewski JP, Henderson L, Smith CM and
Christensen TW: Drosophila Psf2 has a role in chromosome
condensation. Chromosoma. 121:585–596. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pennati M, Cimino-Reale G, Gatti L and
Cassinelli G: Strategies to strike survival networks in cancer.
Crit Rev Oncog. 21:269–308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Duan Z, Chen Q, Du L, Tong J, Xu S, Zeng
R, Ma Y, Chen X and Li M: Phagocytosis of Candida albicans
inhibits autophagic flux in macrophages. Oxid Med Cell Longev.
2018:49386492018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen H, Lin W, Lin P, Zheng M, Lai Y, Chen
M, Zhang Y, Chen J, Lin X, Lin L, et al: IL-10 produces a dual
effect on OGD-induced neuronal apoptosis of cultured cortical
neurons via the NF-κB pathway. Aging (Albany NY). 11:10796–10813.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang M, He S, Ma X, Ye Y, Wang G, Zhuang
J, Song Y and Xia W: GINS2 affects cell viability, cell apoptosis,
and cell cycle progression of pancreatic cancer cells via MAPK/ERK
pathway. J Cancer. 11:4662–4670. 2020. View Article : Google Scholar : PubMed/NCBI
|