Introduction
Ischemic stroke is a common disease that affects
human health, with high morbidity, mortality and disability rates
worldwide (1,2). After cerebral ischemia, the blood
supply is restored for a certain period of time. However, brain
function cannot be restored, and cerebral ischemia causes serious
neurological dysfunction. Furthermore, secondary injury plays a key
role in the process of cerebral ischemia-reperfusion nerve function
damage, and the inflammatory reaction after stroke is an important
aspect of secondary injury (3).
Apoptosis is an active process of cell death that
occurs under physiological or pathological conditions (4). During the first few minutes to several
days after cerebral ischemia, increased cell apoptosis occurs in
ischemic areas, especially in ischemic penumbra areas, which also
constitutes an important part of cerebral ischemic damage (5).
The inflammatory reaction and apoptosis are key
components in cerebral ischemia-reperfusion injury (5–7). The
severity of the inflammatory reaction and apoptosis has an
important impact on the prognosis of stroke (5,8).
Furthermore, inflammation is expected to become a target for
treatment of ischemic stroke. Thus, understanding the temporal and
spatial changes of the inflammatory response and apoptosis in
cerebral ischemia-reperfusion injury is important to help treatment
of cerebral ischemia-reperfusion injury (9). Magnetic resonance scanning is a
non-invasive, free-from-radiation technique that can be used to
display cerebral ischemic foci from multiple angles (10). A T1-weighted image (T1WI) is a
magnetic resonance image weighted by longitudinal relaxation time
T1; T1WI can highlight the difference of tissue T1 relaxation
(longitudinal relaxation). Moreover, a T2-weighted image (T2WI) is
a magnetic resonance image weighted by transverse relaxation
(spin-spin relaxation) time T2; T2WI can highlight the difference
of tissue T2 relaxation (transverse relaxation). Furthermore,
ischemic brain tissue shows low signals on T1WI, and a high signal
on T2WI.
The ultrasmall superparamagnetic iron oxide particle
(USPIO) is a new blood pool contrast agent, and an effective MRI
molecular imaging method for dynamic observation of the cell
infiltration process in vivo (11,12).
USPIO MRI can detect macrophages and inflammatory reactions in
vivo, and has been used in the research of various diseases,
such as stroke, prostate cancer and experimental autoimmune
encephalomyelitis (13–18). USPIO particles can be phagocytized
by macrophages, and thus can be used for imaging inflammatory
reactions after cerebral ischemia (19). After cerebral ischemia, microglia,
which exist in brain tissue and are the macrophages of the brain,
are activated in large numbers, and these activated microglia can
engulf specific paramagnetic USPIOs (20). The superparamagnetism of iron oxide
nanoparticles means these particles exhibit high transverse
relaxivity, and are thus used to produce signal loss in T2-weighted
images (21). However, some
particles exhibit significant longitudinal relaxivity and can
produce hyperintensity in T1-weighted images under certain
conditions (22).
Therefore, the present study used ferumoxytol to
enhance MRIs to observe the inflammatory response in the acute
stage of cerebral ischemia-reperfusion injury. The present study
also investigated the dynamic changes of the inflammatory response
and apoptosis after cerebral ischemia-reperfusion injury.
Materials and methods
Experimental animals
All experiments and procedures were approved by the
Animal Ethics Committee of Shanghai University of Traditional
Chinese Medicine (registration no. SZY201712006). In total, 54
wild-type, specific-pathogen free male C57BL/6n mice (age, 8–10
weeks; weight, 20–25 g) were purchased from Vital River Laboratory
Animal Technology Co., Ltd. [license nos. SCXK (Beijing) 2016-0006
and SCXK (Zhejiang) 2018-0001]. Mice were reared in the Animal
Experimental Centre of Shanghai University of Traditional Chinese
Medicine, Animals were housed in a temperature (22±2°C), humidity
(55±10%) and 12 h light/dark cycle-controlled environment and given
ad libitum access to food and water. The environmental
facility license no. was SYXK (Shanghai): 2014-0008.
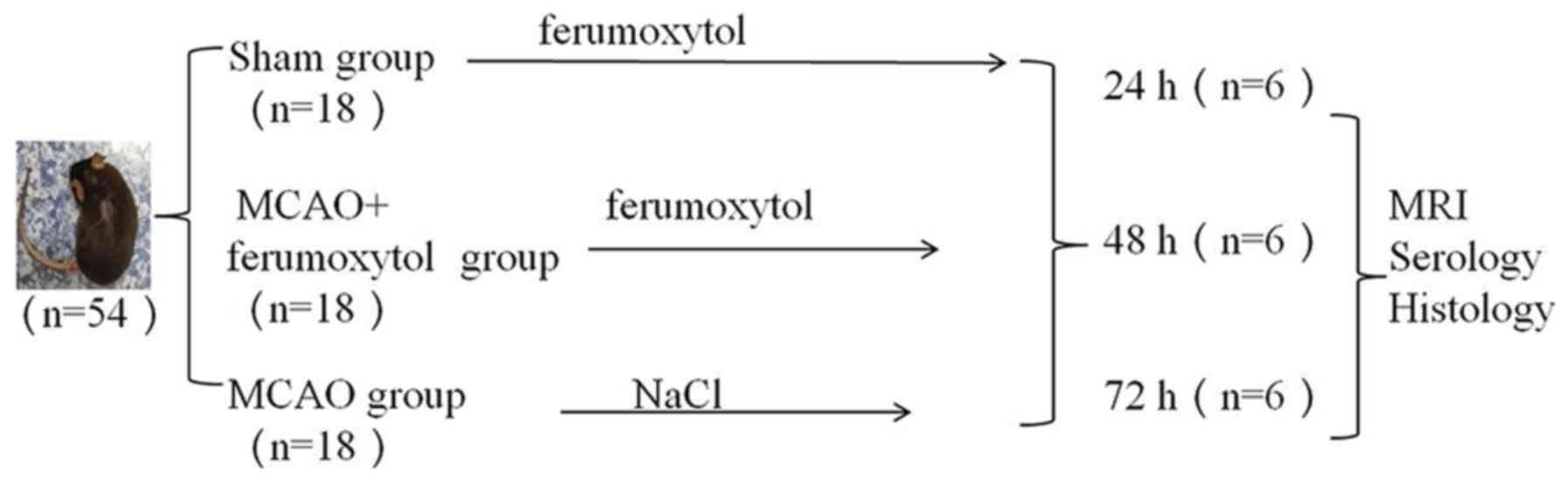
Experimental design
The 54 mice used in the study were divided into the
sham-operation group (sham group), middle cerebral artery occlusion
(MCAO) and MCAO + ferumoxytol groups (n=18 mice in each group)
according to their body weight. Each group was further divided into
24, 48 and 72 h subgroups, with 6 mice in each group. In the sham
group, the common carotid artery, external carotid artery and
internal carotid artery were separated; the specific separation
steps were as the same as with the MCAO group, without the
insertion of an occluding suture, and the specific separation steps
are described in detail in the following paragraph. In the MCAO and
MCAO + ferumoxytol groups, the right middle cerebral artery was
blocked using a modified Longa suture method (23) to establish a cerebral
ischemia-reperfusion model. Subsequently, mice in the sham and MCAO
+ ferumoxytol groups were injected with ferumoxytol (trade name,
Feraheme; 1 mg/ml; purchased from Canada by Shanghai So-Fe
Biomedicine Technology Co., Ltd.; injection dose, 18 mg Fe/kg) via
the tail vein after successful modelling. Mice in the MCAO group
were injected through the tail vein with 0.9% NaCl solution of
equal volume after model establishment. The vital signs monitored
after the operation included the body temperature and activity of
the mice. The average time between suturing the animals and
stabilizing the vital signs was 30–120 min; therefore, the
injection was started ~2 h after operation. During this time period
(2 h after the surgery) the body temperature of the mouse was
stable, and when this returned to normal, and the mice started
eating and drinking; the injection dose was 0.02 ml USPIO
solution/g mice. The mice were first scanned using MRI at the
specified time, and then 0.5–1 ml blood was collected by
retroorbital exsanguination for the detection of serum inflammatory
factors. The mice were sacrificed immediately after retroorbital
exsanguination by cervical dislocation (n=6/time point) at
three-time points, and the brain tissues were taken for
histological examination. During the experiment, the number of dead
mice and the cause of death was recorded, and the mice in which
MCAO was not established, which was observed in the T2-weighted
images of the MRI, were removed. The model was subsequently
re-established to supplement the sample size of the corresponding
group. Fig. 1 shows the
experimental design in further detail.
Improved method for establishing the
cerebral ischemia model
All mice drank freely and were fasted for 12 h prior
to the operation to prevent postoperative intestinal obstruction.
The preparation method of cerebral ischemia-reperfusion in MCAO and
MCAO + ferumoxytol groups were as described below. Mice were
anaesthetized by intraperitoneal injection (1% sodium
pentobarbital, 35–40 mg/kg (Sinopharm Chemical Reagent Co., Ltd.),
and tweezers were used to gently pinch the toes of the mouse. If
the mouse did not have any reaction after pinching, it was
considered to have entered a deep state of anesthesia. The skin was
sterilized using an alcohol cotton ball after the mice were fixed
in the supine position. The skin in the middle of neck was cut
1.0–1.5 cm using ophthalmology scissors. The subcutaneous tissues
and glands were separated with ophthalmologic tweezers until the
carotid sheath was exposed, to separate the right common carotid
artery and the external carotid artery. Then, the proximal end of
the common carotid artery and the external carotid artery were
ligated. The occluding suture (diameter, 0.14±0.02 mm; Beijing
Cinotech Co., Ltd.) was placed into the 27G syringe needle (outer
diameter, 0.4 mm; needle length, 13 mm), and the needle was
inserted into the common carotid artery; the occluding suture was
pushed from the tail of the needle, and the common carotid artery
and its internal occluding suture were clamped with tweezers. The
needle was subsequently withdrawn, and the occluding suture
continued to be inserted into the internal carotid artery; the
insertion process was stopped when resistance was encountered. At
this point, the head end of the occluding suture was ~1 cm from the
bifurcation of the common carotid artery, and the beginning of the
middle cerebral artery was blocked. The occluding suture and the
common carotid artery were fixed together with a thin surgical
suture, and the incision was sterilized and sewn up. After 30 min,
the nylon thread was withdrawn back to the bifurcation of the
common carotid artery to cause reperfusion. The room temperature
was kept at ~25°C during and after the operation. The mice were
irradiated with an incandescent lamp to keep the rectal temperature
at 37.0±0.5°C after the operation. The mice were housed in a single
cage and the neurological function score of each mouse was recorded
using the Longa 5 grade method (24): i) 0, no obvious symptoms of
neurologically defective function; ii) 1, on lifting the tail
vertically, the opposite front paws could not be extended; iii) 2,
the mouse turned to the hemiplegic side when walking; iv) 3, when
walking, the body fell to the hemiplegic side; v) 4, the mouse
could not walk spontaneously, and there was loss of consciousness;
and vi) 5, death. Mice with a score of 1–3 were included in the
experiment, and mice with a score of 0, 4 or 5, or those that died
before the observation time point, were excluded. The health status
of mice was observed every 4 h; if mice were unable to eat,
nutritional support was given by intraperitoneal injection of
0.2–0.5 ml physiological saline, according to their physical
condition. If mice died before the MRI scan or before the serum
samples were collected, mice of the same weight were randomly added
to the group to ensure the same sample number of experimental
animals in each subgroup. Neurological function of mice was also
evaluated prior to MRI.
MRI acquisition and processing
All scans were performed on a Siemens Skyra3.0T
machine with an 8-channel transverse coil for small animals (cat.
no. 5000049101/001006; Shanghai Chenguang Medical Technology Co.,
Ltd.). The mice were anaesthetized by intraperitoneal injection (1%
pentobarbital sodium, 35–40 mg/kg; Sinopharm Chemical Reagent Co.,
Ltd.). The mice were fixed at the center of the coil in a prone
position, and fast spin echo sequence scanning (25,26)
was performed. The sequence and specific parameters of MRI are
shown in Table I. After MRI, the
changes of MRI signals in the mouse brain tissues were observed,
and a clinician with 5 years' clinical experience independently
measured the signal values of the right negative enhancement region
and the left corresponding region on T2WI images. The signal values
of the left and right sides in the same region as the MCAO group
were measured in the sham operation group. Calculations were
performed as follows: The signal ratio of the sham operation group
in T2WI=right signal value/left signal value. The signal ratio of
the MCAO + ferumoxytol group in T2WI=right negative enhancement
region signal value/left corresponding region signal value.
 | Table I.Sequences and parameters of magnetic
resonance scanning. |
Table I.
Sequences and parameters of magnetic
resonance scanning.
|
Sequence/parameter | TR (msec) | TE (msec) | Flip angle (°) | THK (mm) | Gap (mm) | FOV (mm) | Bandwidth
(Hz/Px) | NEX | Matrix | Slice number | Acquisition time
(min, sec) |
|---|
| T1WI TSE-TRA | 500 | 13 | 131 | 1.0 | 0.1 | 60×36 | 244 | 3 | 256×256 | 8 | 2,47 |
| T2WI TSE-TRA | 3,800 | 80 | 150 | 1.0 | 0.1 | 60×36 | 260 | 2 | 256×256 | 6 | 2,59 |
| T1WI TSE-COR | 761 | 13 | 131 | 1.0 | 0.1 | 60×48 | 244 | 4 | 256×256 | 10 | 2,51 |
| T2WI TSE-COR | 3,800 | 80 | 150 | 1.0 | 0.1 | 60×48 | 260 | 4 | 256×256 | 10 | 2,51 |
| DWI-COR | 3,010 | 50/70 | 180 | 0.9 | 0.09 | 75×58 | 591 | 1 | 94×94 | 10 | 11,37 |
Serological tests
After the MRI scan, the mice were still anesthetized
and the skin was disinfected around the orbit. Then, blood samples
were collected by retroorbital exsanguination, and samples were
centrifuged at 5 × g for 15–20 min after standing for 30 min. The
upper serum was collected, and the serum levels of interleukin-1β
(IL-1β) and tumor necrosis factor-α (TNF-α) were determined using
their respective ELISA kits (cat. nos. 88-7013 and 88-7324; Thermo
Fisher Scientific, Inc.).
Histological examination
After blood collection, the mice were euthanized by
cervical dislocation. The brain tissue was removed, and the tissue
samples were fixed at room temperature with 4% paraformaldehyde for
24 h, prior to being embedded in paraffin. Brain tissues with
negative enhancement corresponding to MRI coronal fracture images
were sectioned (5-µm thick) for hematoxylin and eosin (HE)
staining. Briefly, the tissue slides were placed in an oven at 60°C
for 1 h, deparaffinized with xylene 3 times for 20 min each at room
temperature, rehydrated in a descending series of ethanol and
washed in tap water. The sections were subsequently stained with
hematoxylin (cat. no. H9627; Sigma-Aldrich; Merck KGaA) for 10 min
at room temperature and washed in running tap water for 5 min.
Then, the tissue slides were counterstained with eosin (cat. no.
E4009; Sigma-Aldrich; Merck KGaA) for 3 min at room temperature.
The slides were dehydrated in 95 and 100% alcohol twice for 2 min
each or until the excess eosin was removed. Slides were washed with
xylene twice for 2 min each and mounted in Permount (Sinopharm
Chemical Reagent Co., Ltd.).
Immunohistochemistry
IBA1 is a microglial cell/macrophage specific
protein antibody (27–29). Brain tissue sections were prepared
as described above for HE staining. The sections were
deparaffinized with xylene three times for 20 min each at room
temperature and rehydrated using a descending alcohol series, prior
to undergoing heat-mediated antigen retrieval with boiled sodium
citrate buffer (pH 6; cat. no. G1202; Wuhan Servicebio Technology
Co., Ltd.) in a microwave oven for 20 min. The sections were washed
in PBS (pH 7.4; cat. no. G0002; Wuhan Servicebio Technology Co.,
Ltd.) 3 times for 5 min each time. The sections were blocked with
5% rabbit serum (cat. no. G1209; Wuhan Servicebio Technology Co.,
Ltd.) for 30 min at room temperature before being incubated with
the primary IBA1 antibody (1:2,000; cat. no. ab178846; Abcam)
overnight at 4°C to identify the microglial cells. Following the
primary antibody incubation, a goat anti-rabbit
horseradish-peroxidase secondary antibody (cat. no. K5007; Dako;
Agilent Technologies, Inc.), without a dilution, was incubated with
the sections for 30 min at room temperature. The slides were
subsequently stained with 3,3′-diaminobenzidine and the
phagocytized ferumoxytol was analyzed following the described
method below.
Identification of phagocytized
ferumoxytol
The phagocytized ferumoxytol was subsequently
identified. The chemical formula of ferumoxytol is Fe3O4, which is
an inactive material, in which the Fe is trivalent iron (30). Potassium ferrocyanide solution can
separate trivalent iron ions from proteins using dilute
hydrochloric acid, and the trivalent iron is able to react with
potassium ferrocyanide; this reaction produces an insoluble blue
compound (31). In this experiment,
after washing the sections that were stained with IBA1 antibody
with running water, the Prussian blue iron stain kit (cat. no.
GP1068; Wuhan Servicebio Technology Co., Ltd.) was used to identify
phagocytized ferumoxytol in cells, according to the manufacturer's
protocol. Briefly, a mixture of 2% potassium ferrocyanide and 2%
hydrochloric acid was used to stain the trivalent iron ions for 1 h
at room temperature, prior to washing with tap water. The sections
were subsequently dehydrated with an ascending alcohol series,
deparaffinized with xylene three times for 5 min each at room
temperature and mounted in Permount (Sinopharm Chemical Reagent
Co., Ltd.).
TUNEL staining
TUNEL was used to detect apoptotic cells using an
apoptosis kit (in situ cell death detection kit; cat. no.
11684817910; Roche Applied Science). Firstly, paraffin-embedded
sections of brain tissues were prepared according to the method in
HE staining. The sections were deparaffinized with xylene twice for
5 min each at room temperature and rehydrated using a descending
alcohol series, prior to being rinsed with PBS twice. Subsequently,
50 µl TUNEL reaction mixture (50 µl TdT + 450 µl dUTP) was
incubated with the sections for 1 h at 37°C in the dark. After
being washed with PBS 3 times, 100 µl 3′3-Diaminobenzidine was
added to the tissue and a light microscope (NIKON Eclipse 50i;
Nikon Corporation) was used to observe the color change; the
reaction was stopped when a yellow color appeared. The cell nuclei
were counterstained with Harris hematoxylin (cat. no. H9627;
Sigma-Aldrich; Merck KGaA) for 30 sec at room temperature. The
sections were dehydrated with an ascending series of alcohol,
deparaffinized with xylene three times for 5 min each at room
temperature and mounted as previously described.
Counting method
After all the samples were stained, all the sections
(HE, IBA1, Prussian blue and TUNEL staining) were visualized using
a digital pathology slide scanner (KFBIO KF-PRO-120; KFBIO Konfoong
Bioinformation Tech Co., Ltd.) and analyzed using the digital slice
reading software K-viewer version 1.1 (KFBIO Konfoong
Bioinformation Tech Co., Ltd.). The HE staining focused on
observing the presence of necrosis, edema and inflammatory cell
infiltration in the brain tissue using ×10, ×200 and ×400
magnification. IBA1 immunohistochemical staining and Prussian blue
staining were mainly observed in the hippocampus at a magnification
of ×400, whereas TUNEL staining was mainly observed in the cerebral
cortex and brain tissue with a magnification of ×400. Each animal
had four non-repeated fields of view around the lesion. The high
magnification images were taken from the low magnification of their
panoramic images using K-viewer version 1.1 software. The
immunohistochemical staining images were semi-quantitatively
analyzed using ImageJ software version 1.8.0 (National Institutes
of Health). and the specimens were scored according to the
intensity of the dye color and the number of positive cells for
IBA1 (32): The intensity of the
dye color was graded as 0 (no color), 1 (light yellow), 2 (light
brown), or 3 (brown), and the number of positive cells was graded
as 0 (<5%), 1 (5–25%), 2 (25–50%), 3 (51–75%), or 4 (>75%).
The two grades were added together and the specimens were assigned
to one of four levels: 0–1 (−), 2 (+), 3–4 (++) or >5 (+++); a
score of ≥3 was defined as IBA1-positive and when blue particles
were observed in the cells, the cells were considered as Prussian
blue staining positive. The number of cells was counted as the
number of IBA1 + Prussian blue-positive and IBA1-positive cells,
respectively; the ratio of these was taken as the positive cell
percentage. The average value of the four graphs was taken as the
positive cell percentage in the mouse. TUNEL staining was used to
calculate the ratio of apoptotic cells/total cells in four
non-repeating fields.
Statistical analysis
All statistical analyses were performed using SPSS
25.0 software (IBM Corp.). The neurological score of mice was
described as the mode number. The MRI signal ratio, serum
inflammatory factor level and activated microglia number were
presented as the mean ± SD. Serological comparisons were conducted
using the one-way ANOVA; the least significant difference method
was used for pairwise analysis. The Pearson correlation test was
used to examine the correlation between T2WI minimum signal ratio
and positive microglia ratio in the MCAO + ferumoxytol group. The
graphs were created using GraphPad Prism version 7.04 (GraphPad
Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
During the modeling process, two mice died due to
excessive bleeding during the modeling process, and two mice were
excluded on the basis that no MCAO was observed in the T2-weighted
images. The model was re-established in two additional mice to
supplement the sample size of the corresponding group.
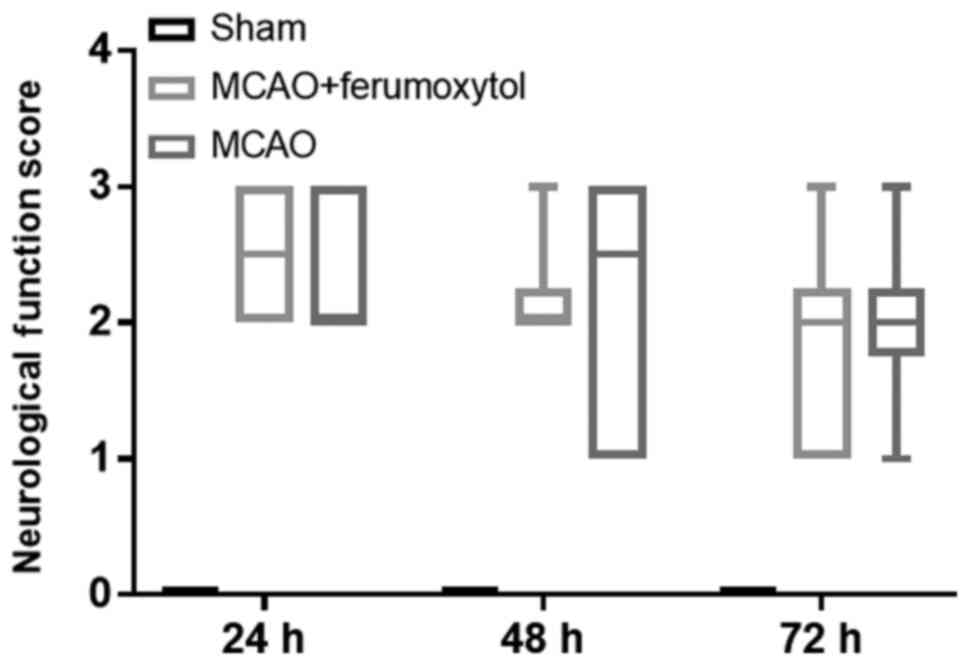
Neurological function score
The mice in the sham group did not show neurological
function deficits at the different time points of reperfusion (all
scores, 0 points). The mice in the MCAO and MCAO + Ferumoxytol
groups had different degrees of neurological function defect.
Moreover, the neurological function score was not normally
distributed (Fig. 2); therefore,
data were presented as the mode. The neurological function scores
in the MCAO group were all 2 points at 24, 48 and 72 h, and the
neurological function scores in the MCAO + Ferumoxytol group were
3, 2 and 2 points at 24, 48 and 72 h, respectively (Fig. 2).
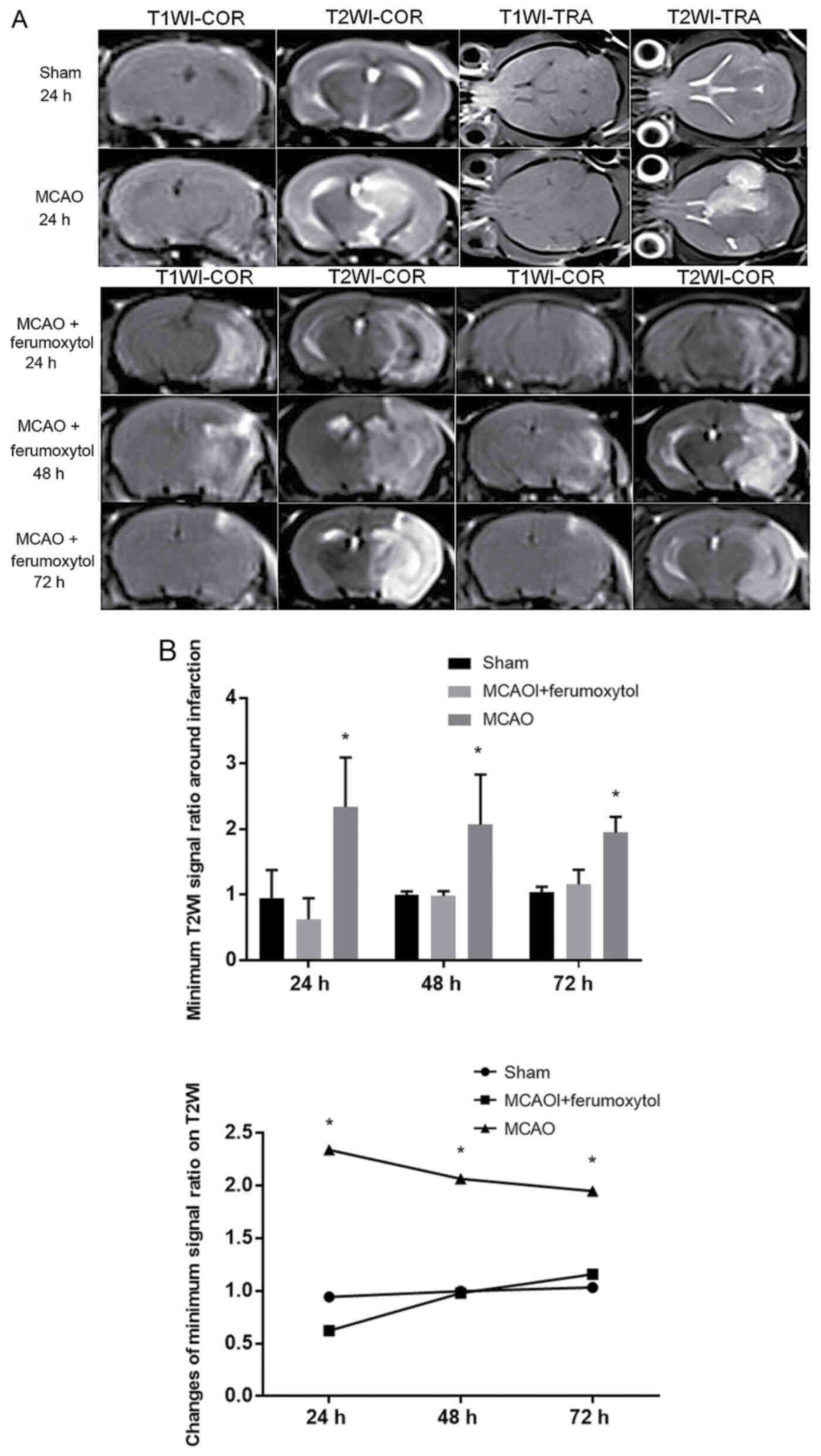
MRI signal changes in mice of each
group
In the sham group, there were no significant signal
changes in T1WI and T2WI at 24–72-h reperfusion. In the MCAO +
ferumoxytol group, there were hyperintense areas in the right brain
tissue on T2WI ~24 h after reperfusion, with speckles and stripes
of negative enhancement around this area. Furthermore, negative
enhancement areas were observed on the infarct edge 48 h after
reperfusion, and remained until 72 h after infarction. Moreover,
these negative enhancement areas showed high signal on T1WI. In
MCAO group, there were no obvious signal changes on T1WI, and large
areas of hyperintense region were identified in the right brain
tissue on T2WI, with no obvious negative enhancement surrounding
the area (Fig. 3A). The signal
ratio around the infarction in the MCAO + ferumoxytol group was the
lowest at 24 h after reperfusion, and gradually increased over
time. The signal ratio in the MCAO group was significantly higher
compared with the sham and MCAO + ferumoxytol groups (P<0.05),
and signal ratio in the MCAO group showed a slow downward trend at
48 and 72 h after reperfusion. However, there was no significant
change in the signal ratio of the right side and the left side of
T2WI at each time point in the sham group, and the signal ratio was
close to 1 (Fig. 3B).
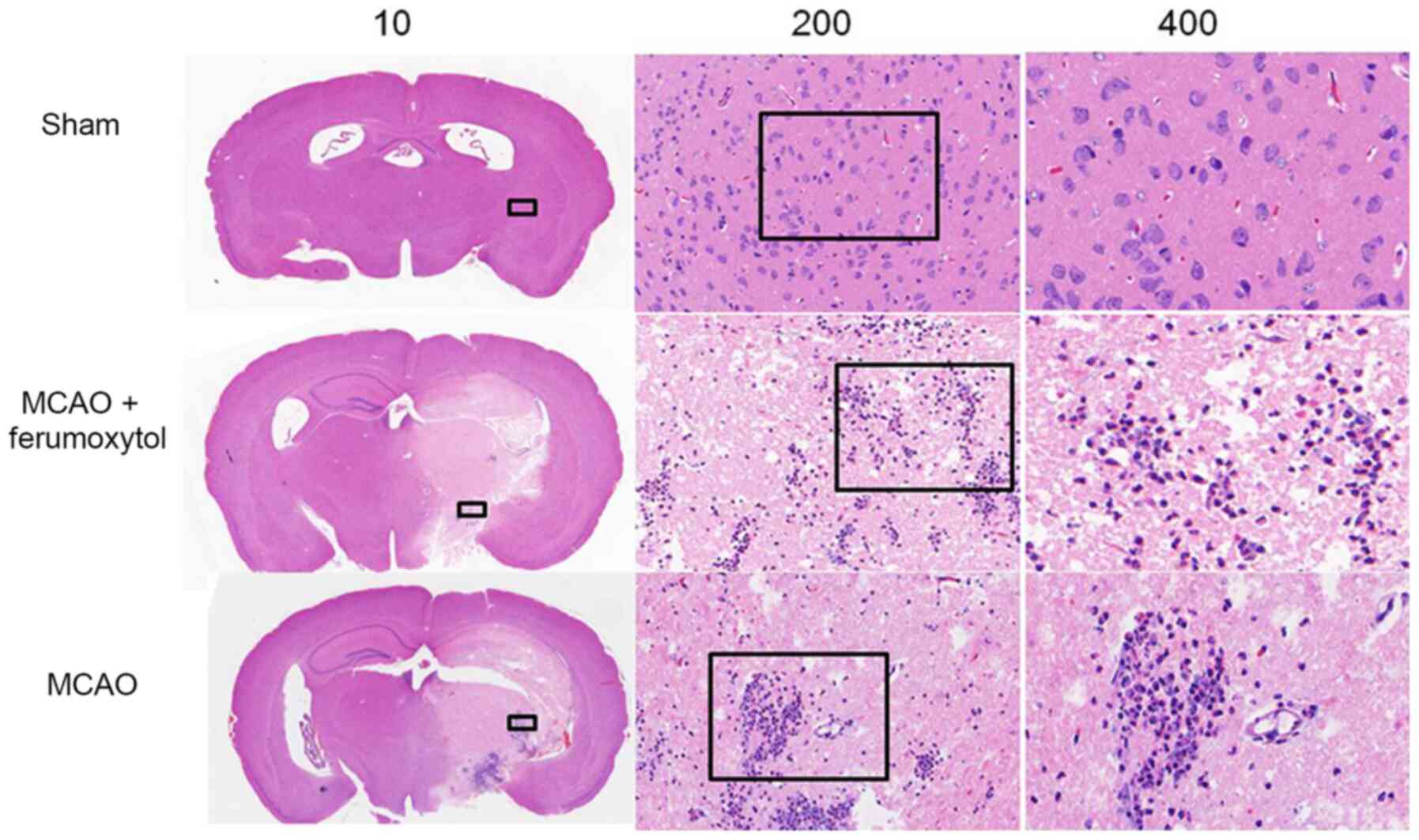
Histological examination
Mice in the sham group had a defined structure, neat
cell arrangement, no obvious necrosis, compact intercellular space
and very few inflammatory cells. However, in the MCAO and MCAO +
ferumoxytol groups, the brain tissue was highly damaged with a
large number of necrotic cells, disordered cell arrangement,
interstitial edema and large numbers of inflammatory cells
(Fig. 4). The IBA1
immunohistochemistry + Prussian blue staining results suggested
that in the sham group there were a large number of stationary
branched microglia with smaller cell bodies and radially tapering
branches from the cell bodies, and also a small number of large,
round or irregular activated microglia. It was demonstrated that in
the MCAO and MCAO + ferumoxytol groups there were a large number of
stationary branched microglia in the left cerebral parenchyma with
smaller cell bodies and radially tapering branches from the cell
bodies, and also a small number of large, round or irregular
activated microglia. Moreover, a large number of activated
microglia can be seen around the right cerebral parenchymal infarct
in the MCAO group (Fig. 5A). In the
MCAO + ferumoxytol group, there were a large number of activated
microglia at the infarct edge after reperfusion at 24 h, and blue
iron particles were found in the microglia. In addition, the number
of activated microglia at the infarct edge decreased after
reperfusion for 48 and 72 h, a small number of activated positive
cells were found in the infarct center, and a small number of blue
iron particles were found in the intercellular substance (Fig. 5B and C).
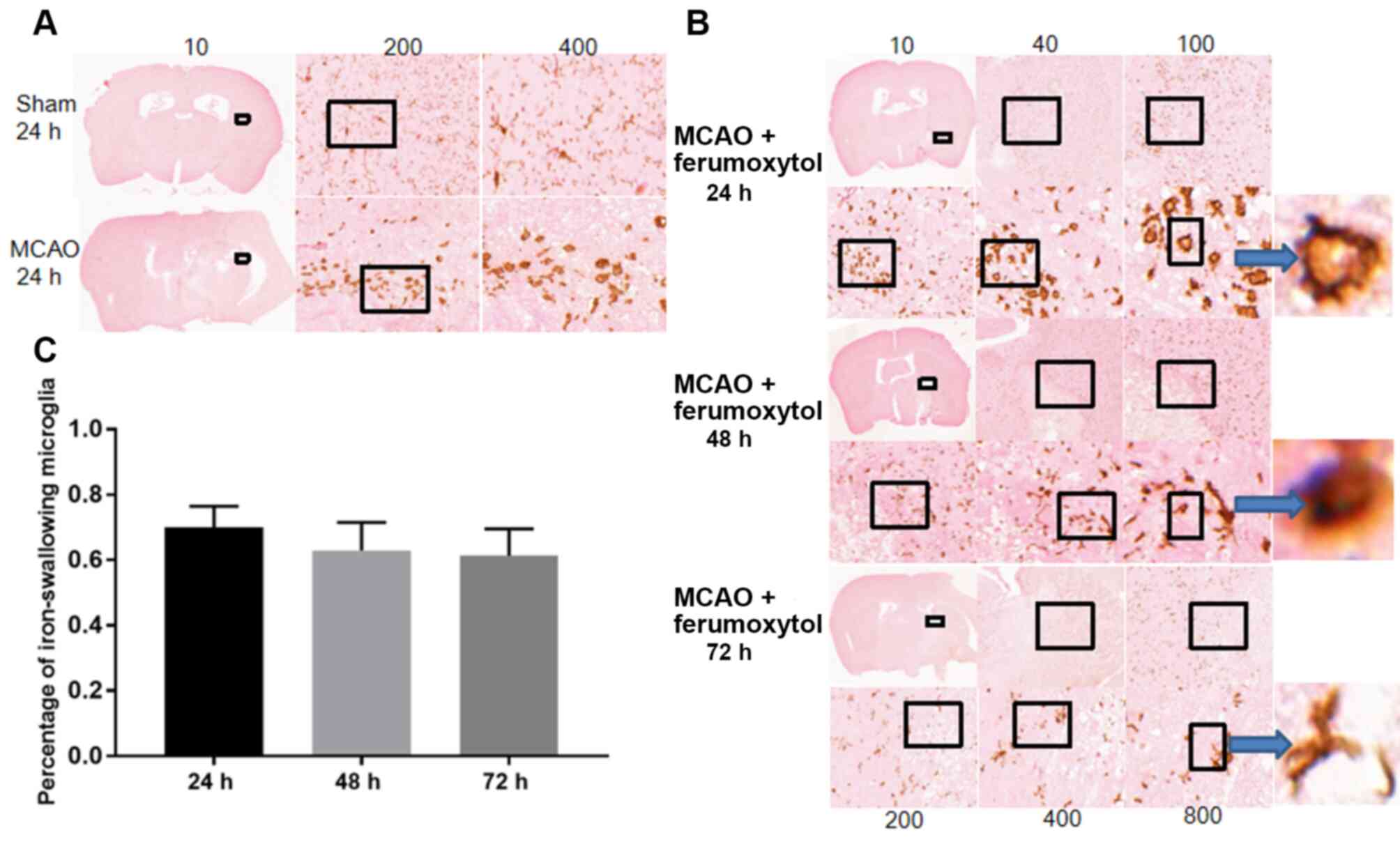 | Figure 5.IBA1 + Prussian blue staining.
Staining identified a small number of large, round or irregular
activated microglia in the sham group, and a large number of
branched inactivated microglia in MCAO group. (A) Results of IBA1 +
Prussian blue staining in the MCAO + ferumoxytol group.
Magnification, ×10, ×200 or ×400. (B) Staining images and (C)
quantification of the percentages of iron-engulfing microglia.
Brown represents IBA1 positive expression and blue represents
Prussian blue iron positive staining. Magnification, ×10, ×40,
×100, ×200, ×400 or ×800. IBA1, ionized calcium binding adapter
molecule 1; MCAO, middle cerebral artery occlusion. |
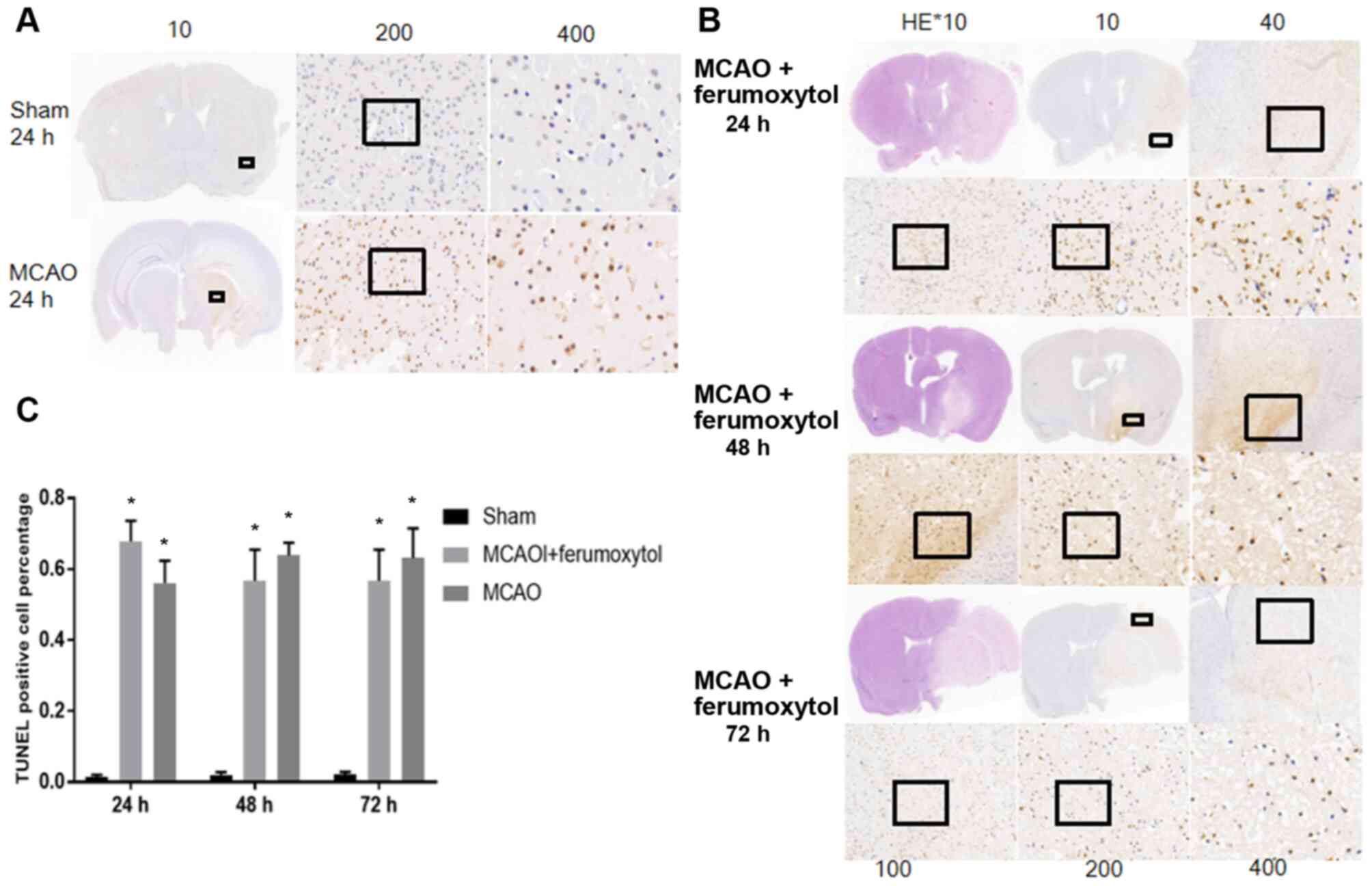
TUNEL staining results indicated that there were no
significant levels of positive apoptotic cells in the brain tissue
of the sham group mice at various time points (Fig. 6A). The number of TUNEL cells in MCAO
group and MCAO + ferumoxytol group was significantly higher
compared with the sham group at the same time point (P<0.05).
The aim of the present study was to investigate the level of the
inflammatory reaction and apoptosis in the MCAO + ferumoxytol
group, to see if ferumoxytol has influence on the inflammatory
response. Therefore, only TUNEL images at 24 h reperfusion in the
sham operation group and the MCAO group were shown (Fig. 6A). The present results suggested
that a large number of positive apoptotic cells were identified in
the infarct area and its surroundings in MCAO + ferumoxytol group
~24 h after reperfusion, and the number of the apoptotic cells in
the infarct area decreased over time (Fig. 6B and C).
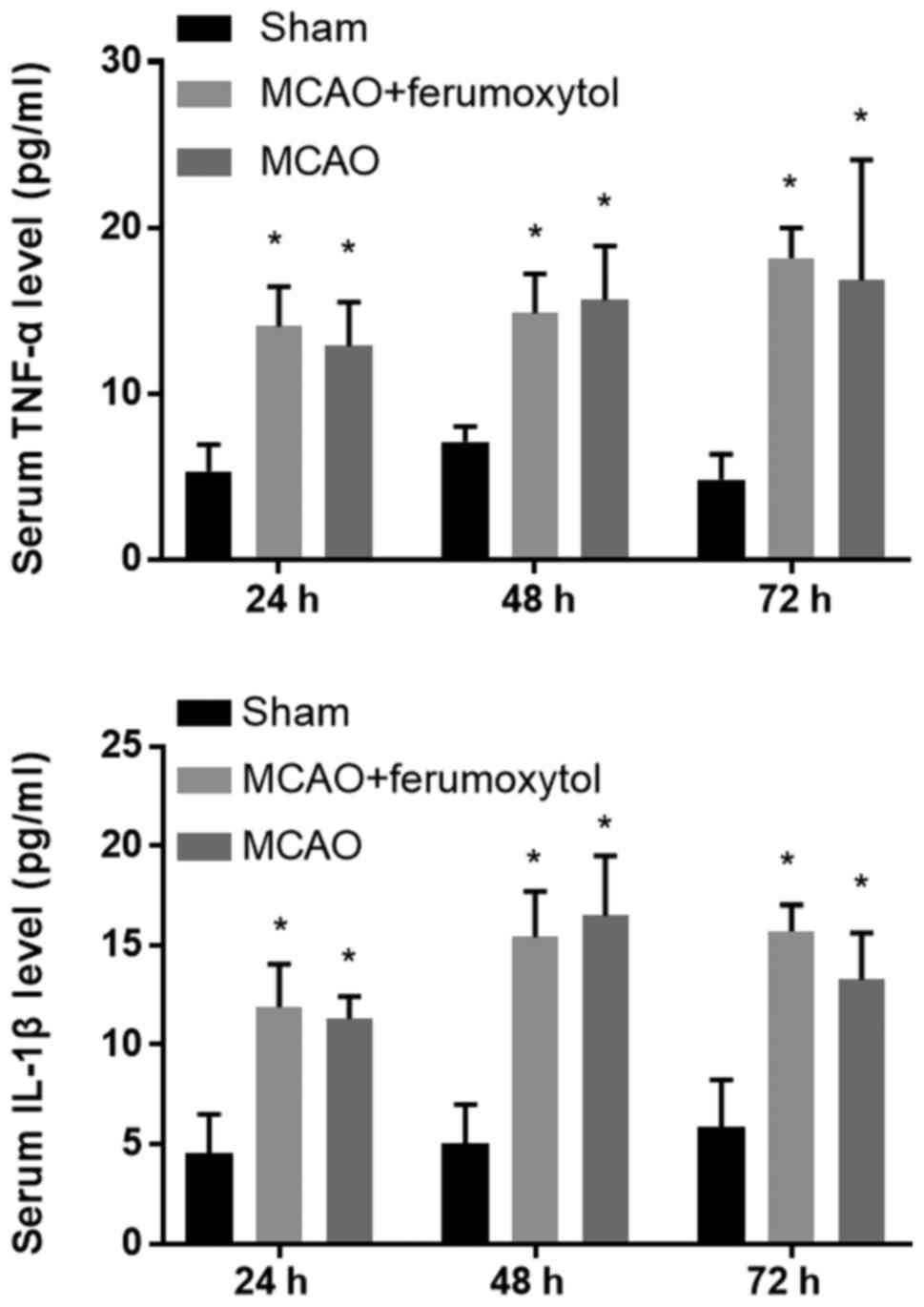
Detection of the serum inflammatory
factors TNF-α and IL-1β
The inflammatory response is an important cause of
secondary injury after stroke (33). Moreover, the release of various
proinflammatory factors after cerebral ischemia aggravates ischemic
injury (34). It was demonstrated
that the serum levels of the inflammatory factors TNF-α and IL-1β
in the MCAO and MCAO + ferumoxytol groups were significantly higher
compared with the sham group at 24, 48 and 72 h after reperfusion
(P<0.05; Table II). To
investigate the effect of 18 mg Fe/kg ferumoxytol injection on the
serum inflammatory factors in mice, the levels of the serum
inflammatory factors TNF-α and IL-1β were detected in the MCAO and
MCAO + ferumoxytol groups (Fig. 7).
It was found that the serum levels of TNF-α and IL-1β were not
significantly different between these two groups (Fig. 7).
 | Table II.Levels of serum inflammatory factors
TNF-α and IL-1β in mice. |
Table II.
Levels of serum inflammatory factors
TNF-α and IL-1β in mice.
|
| TNF-α | IL-1β |
|---|
|
|
|
|
|---|
| Group | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h |
|---|
| Sham group | 5.305±1.610 | 7.085±0.925 | 4.822±1.509 | 4.563±1.957 | 5.008±1.980 | 5.838±2.406 |
| MCAO +
ferumoxytol |
14.035±2.410a |
14.838±2.373a |
18.153±1.844a |
11.882±2.171a |
15.403±2.303a |
15.690±1.345a |
| MCAO |
13.453±2.481a |
15.630±3.250a |
16.812±7.272a |
11.300±1.122a |
16.483±3.005a |
13.255±2.364a |
Correlation between the MRI signal
ratio and positive cell ratio
The present study tested the correlation between
T2WI signal ratio and IBA1 + Prussian blue positive cell ratio, at
each time point in MCAO + ferumoxytol group. It was found that the
correlation coefficients R at 24, 48 and 72 h after reperfusion
were 0.271, 0.386 and 0.410, respectively, with no significant
correlation (Table III).
 | Table III.Correlation between the T2WI signal
ratio and the IBA1 + Prussian blue positive cell ratio in the
middle cerebral artery occlusion + ferumoxytol group. |
Table III.
Correlation between the T2WI signal
ratio and the IBA1 + Prussian blue positive cell ratio in the
middle cerebral artery occlusion + ferumoxytol group.
| Parameter | 24 h after
reperfusion | 48 h after
reperfusion | 72 h after
reperfusion |
|---|
| T2WI signal
ratio | 0.9432±0.4321 | 0.9968±0.0507 | 0.10326±0.8729 |
| IBA1 + Prussian
blue positive cell ratio | 0.6999±0.6043 | 0.6295±0.8616 | 0.6136±0.8152 |
| Correlation
coefficient, r | 0.271 | 0.386 | 0.410 |
Discussion
Cerebrovascular disease is a common, frequently
occurring clinical disease with high mortality and disability
rates. Using animal models to study cerebrovascular disease has
become an important research method for investigating cerebral
ischemia. The Longa modified thread embolism method is the most
commonly used technique in cerebral ischemia research (35). This method has a constant ischemic
site and can be used for reperfusion, simulating different states
of permanent and transient focal cerebral ischemia in humans.
Furthermore, this method allows for accurate control of ischemia
and reperfusion time.
The present study aimed to improve some of the
methodology of cerebral ischemia modeling in mice. During the
modeling process, the present study used a suitable syringe needle
to draw the occluding suture into the common carotid artery, which
replaced the traditional ophthalmic scissors, which reduced
intraoperative bleeding and greatly improved the success rate. The
MCAO group and the MCAO + Ferumoxytol group had different degrees
of neurological deficits after the model was established, which
showed that the use of the improved thread plug method to create
the model was beneficial. However, the present results suggested
that the neural function scoring data did not conform to normal
distribution, and the SD was large. The reason for this may be due
to the shortcomings of Longa 5 grade scoring method, which is
subjective. Furthermore, in our present study, the evaluation is
not sufficiently comprehensive, and the score is not detailed to
the required degree, which leads to poor sensitivity in terms of
evaluating neurological deficits in mice.
In previous studies that investigated changes in
cerebral ischemia, the animals were typically euthanized after
establishment of the cerebral ischemia model, and then sections
were obtained to observe the pathological changes; this procedure
requires a large number of experimental animals (24,36).
Furthermore, non-invasive and dynamic monitoring of cerebral
ischemia, and inflammatory injury in vivo, allows for
continuous and dynamic observation in the same organism (37). MRI is a high-resolution technique
for soft tissue, and further advantages are that there is no
radiation damage and the method can be used repeatedly. Moreover,
MRI is a multi-parameter, multi-sequence and multi-slice imaging
technique, making it an important method for in vivo
research (38).
USPIO is a new type of MRI negative contrast agent
with several advantages, including being effective at the
nano-scale, blood pooling, specific targeting, biocompatibility,
longer blood half-life and no long-term toxicity (12,39).
USPIO is able to change the longitudinal and transverse relaxation
time, and can also change the MRI signal (40). Therefore, the pathological changes
involved in the inflammatory response can be detected in
vivo using this imaging technology, and it has gained
increasing attention for use as a mononuclear phagocyte
system-specific MRI contrast agent. Currently, USPIO has been used
for cell tracing-related research, including autoimmune
encephalomyelitis, atherosclerosis and other diseases (18,41–44).
For inflammatory diseases of the central nervous system, USPIO can
be used as both a marker of cell infiltration and a marker of
blood-brain barrier destruction, which is a new method for
inflammatory imaging of the central nervous system (45,46).
Previous studies have used USPIO to investigate whether the
inflammatory reaction has a correlation with cerebral infarction
volume (47).
The component of USPIO selected in the present study
was ferumoxytol, which is an USPIO approved by the Food and Drug
Administration to treat iron deficiency anemia caused by chronic
kidney disease in adults; the chemical formula of ferumoxytol is
Fe3O4 (30,48).
Ferumoxytol, as an ultrasmall, ultra-paramagnetic material, has
become more commonly used for investigating central nervous system
diseases, including post-stroke perfusion imaging or cell
integration (49,50). Previous studies have used USPIO to
investigate the in vivo mechanisms of inflammatory response,
but have not observed the dynamic changes of the inflammatory
response and apoptosis in depth (51–53).
The inflammatory response and apoptosis are the most important
pathological changes after stroke. Thus, in the present study it
was important to assess the dynamic changes of the inflammatory
response and apoptosis. Previous studies have found that USPIO can
play an anti-inflammatory and pro-inflammatory role, or have no
effect on inflammation (54–57).
However, in vitro studies revealed that the influence of
USPIO on the inflammatory reaction is associated with particle
size, different surface modifications, injection concentration and
time (54–56,58).
While Doyle et al (59)
found that ferumoxytol did not affect the cerebral infarction
volume and inflammatory response of mice, results that are
inconsistent with the present study. There are a couple of major
differences between the present study and that by Doyle et
al (59): i) the injection
concentration and injection time differed (i.e., in the previous
study, 7 mg/kg ferumoxytol were injected 48 h after MCAO); and ii)
the present study used C57BL/6n mice, whereas the previous study
used BALB/CJ mice. Therefore, at present, there are no reliable
data to show the effect of 18 mg Fe/kg ferumoxytol on the
inflammatory response following cerebral ischemia in C57BL/6n mice.
If the volume of ferumoxytol used in the present study were to have
intensified or alleviated the inflammatory reaction, it would not
have been appropriate to have used this concentration to study the
inflammatory reaction in itself. The present study used the MCAO +
normal saline group as a control to compare the differences between
the serum inflammatory factors TNF-α and IL-1β between experimental
groups; this represents the most novel aspect of the present
study.
The present results suggested that there were no
significant differences in the serum levels of the inflammatory
factors TNF-α and IL-1β comparing between the MCAO and MCAO +
ferumoxytol groups at 24, 48 and 72 h after cerebral
ischemia-reperfusion. Therefore, the present results indicated that
18 mg Fe/kg ferumoxytol, which was used to assess the inflammatory
reaction, does not affect the release of TNF-α and IL-1β.
Consequently, in the present study it was feasible to inject 18 mg
Fe/kg ferumoxytol to investigate in vivo the MRI of the
inflammatory response after cerebral ischemia. A previous clinical
study showed that USPIO-enhanced MRI may facilitate a more specific
treatment of inflammation in patients with stroke (60). Microglia are macrophages in the
brain that can react rapidly to changes in the brain
microenvironment (20). Microglia
are important imaging targets for studying the inflammatory
response after stroke (61). The
region where USPIO aggregation causes signal changes on magnetic
resonance images represents the activated microglia aggregation
region (62). While ferumoxytol has
a long half-life in blood, its half-life is only 45 min in mice,
and previous studies have shown that almost no ferumoxytol is
present in cerebral vessels 24 h after injection (63). Moreover, another previous study
demonstrated that the signal changes in MRI at the early stage are
not caused by phagocytosis of iron particles by blood-derived
macrophages (64). In addition, the
inflammatory response after cerebral ischemia is a dynamic process
(65). Following cerebral
ischemia-reperfusion, the metabolic and vasoactive substances
increase vascular permeability and vascular endothelial cells
release adhesion factors (66).
Furthermore, white blood cell aggregation-adhesion-exudation is
initiated, with neutrophils arriving first, monocytes predominating
24 h later, and blood-derived macrophages infiltrating in large
quantities 3 days after cerebral ischemia (66). Inflammatory cells in brain tissue
within 24–72 h following cerebral ischemia-reperfusion are mainly
inherent microglial cells in the brain (67). Therefore, MRI signal changes within
24–72 h after cerebral ischemia are mainly caused by activated
phagocytosis of iron particles by microglia (64). However, the dynamic changes of
microglial cells at 24–72 h after cerebral ischemia-reperfusion
have yet to be fully elucidated. Therefore, the present study
observed the activation of endogenous microglial cells during the
24–72 h period after cerebral ischemia-reperfusion, and compared
changes in the MRI signals with brain histopathology. The present
study used the lowest signal ratio of T2WI to reflect the degree of
activation in microglial cells that engulfed ferumoxytol; the lower
the ratio, the higher the degree of activation of the
microglia.
Apoptosis is a relatively ordered, delayed
energy-dependent cell death process. Following cerebral ischemia,
due to the collateral circulation blood supply in the area
surrounding the necrotic region, the degree of ischemia is lower
compared with the central area, there is provision of a partial
energy supply, and cell damage is relatively slight (68). Reperfusion injury can produce
calcium ion imbalance, endoplasmic reticulum and mitochondrial
dysfunction, and enhanced peroxidation, which leads to DNA damage
(69). Moreover, a large degree of
reperfusion injury can initiate cell apoptosis (5,70).
The present results suggested that the negative
enhancement areas of T2WI in the cerebral ischemic area are
primarily concentrated around the infarction in the MCAO +
ferumoxytol group. Immunohistochemical staining results showed a
large number of activated microglia in these negative enhancement
areas, with positive iron particles visible in the microglia.
Furthermore, TUNEL staining results identified a large number of
apoptotic cells in these areas, indicating that apoptosis and the
activation of microglia occur consistently 24–72 h after cerebral
ischemia-reperfusion. Moreover, the MRI T2WI-enhanced region signal
ratio results suggested that the signal was lowest 24 h after
reperfusion, and the degree of microglial cell activation and
apoptosis were highest at this time point. Thus, the present
results suggested that ferumoxytol-enhanced MRI may be used as an
effective strategy to monitor the inflammatory response in the
acute stage of cerebral ischemia-reperfusion injury. It was
demonstrated that there is a peak in the inflammatory response at
24 h after cerebral ischemia-reperfusion, which was accompanied by
a peak of apoptosis. Thus, the activation of the inflammatory
response after cerebral ischemia-reperfusion injury is consistent
with apoptosis.
However, there are several limitations to the
present study. Firstly, the T2WI signal ratio was used to evaluate
the level of iron consumed by cells, but SWI is the most sensitive
and non-invasive method for iron quantification (71). However, the mouse brain tissue
voxels used in the present study are small, and thus we failed to
obtain satisfactory SWI images on 3T machines. In addition, the
weak linear correlation is due to the fact that cells that
internalize USPIO are primarily activated macrophages. However,
there are other cell types involved, and parts of USPIO that are
not engulfed reach the site directly via the damaged blood-brain
barrier. In addition, previous studies have shown that USPIO in the
cell stroma prove to be difficult to stain using Prussian blue
(72): A technique that was used in
the present study. Moreover, the T2WI scanning plane may not be
identical to the IBA1 immunohistochemical iron counterstain
section, and due to the heterogeneity of microglial cell
distribution, the proportion of positive cells obtained in this
plane cannot fully represent the overall situation of the lowest
signal area. In addition, while no visible hemorrhage foci are
found upon MRI scanning, HE detection showed that some mice had
gastric hemorrhage. Therefore, these factors may explain why the
lowest signal ratio in the subsequent MR T2WI and the positive cell
ratio were not correlated.
Collectively, the present results suggested that
ferumoxytol-enhanced MRI may reflect the activation of microglial
cells in brain tissue 24–72 h after cerebral ischemia-reperfusion
in vivo. Thus, the present results may provide a useful
imaging method to observe the inflammatory response after cerebral
ischemia. Moreover, the histological examination results indicated
that the dynamic changes of microglial cell activation and
apoptosis are highly consistent; therefore, further studies are
required to investigate the pathological changes, diagnosis and
treatment of cerebral ischemia.
Acknowledgements
The authors would like to thank Miss Liu Mengxiao,
the Siemens Shanghai engineer of MR Scientific Marketing, Siemens
Healthcare, for adjusting the magnetic resonance scanning
parameters in this study, and Shanghai Runnerbio Technology Co.,
Ltd. for helping with the histological examination.
Funding
This research was supported by The General Program
of China National Natural Science Foundation (grant no.
81573782).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SZ conceived and designed the experiments. LZ
performed the experiments and was a major contributor in writing
the manuscript. YK participated in establishing the model and
analyzed the data. SY and FL analyzed the data and critically
revised the manuscript. ZG and ML helped with the magnetic
resonance sequence in the mice. SZ supervised all research and
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Animal Care and Use Committee of Shanghai University of Traditional
Chinese Medicine (registration no. SZY201712006).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MCAO
|
middle cerebral artery occlusion
|
|
USPIO
|
ultrasmall superparamagnetic particles
of iron oxide
|
|
TNF-α
|
tumor necrosis factor-α
|
|
IL-1β
|
interleukin-1β
|
|
IBA1
|
ionized calcium binding adapter
molecule 1
|
|
T1WI
|
T1-weighted image
|
|
T2WI
|
T2-weighted image
|
References
|
1
|
Guzik A and Bushnell C: Stroke
epidemiology and risk factor management. Continuum (Minneapolis,
Minn.). 23:15–39. 2017.PubMed/NCBI
|
|
2
|
Wang Y, Cui L, Ji X, Dong Q, Zeng J, Wang
Y, Zhou Y, Zhao X, Wang C, Liu L, et al: The China national stroke
registry for patients with acute cerebrovascular events: Design,
rationale, and baseline patient characteristics. Int J Stroke.
6:355–361. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Duris K and Jurajda M: Evolutionary
concept of inflammatory response and stroke. J Neurosci Res.
98:98–104. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Radak D, Katsiki N, Resanovic I, Jovanovic
A, Sudar-Milovanovic E, Zafirovic S, Mousad SA and Isenovic ER:
Apoptosis and acute brain ischemia in ischemic stroke. Curr Vasc
Pharmacol. 15:115–122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim E and Cho S: Microglia and
monocyte-derived macrophages in stroke. Neurotherapeutics.
13:702–718. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin R, Yang G and Li G: Inflammatory
mechanisms in ischemic stroke: Role of inflammatory cells. J Leukoc
Biol. 87:779–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Duris K, Splichal Z and Jurajda M: The
role of inflammatory response in stroke associated programmed cell
death. Curr Neuropharmacol. 16:1365–1374. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chauveau F, Cho TH, Berthezene Y,
Nighoghossian N and Wiart M: Imaging inflammation in stroke using
magnetic resonance imaging. Int J Clin Pharmacol Ther. 48:718–728.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Antonucci MU and Yazdani M: A helpful tool
in diagnosing stroke mimics: Arterial spin labeled perfusion
magnetic resonanceimaging. J Emerg Med. Mar 18–2020.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rausch M, Sauter A, Fröhlich J, Neubacher
U, Radü EW and Rudin M: Dynamic patterns of USPIO enhancement canbe
observed in macrophages after ischemic braindamage. Magn Reson Med.
46:1018–1022. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weissleder R, Stark DD, Engelstad BL,
Bacon BR, Compton CC, White DL, Jacobs P and Lewis J:
Superparamagnetic iron oxide: Pharmacokinetics and toxicity. AJR Am
J Roentgenol. 152:167–173. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hedgire S, Krebill C, Wojtkiewicz GR,
Oliveira I, Ghoshhajra BB, Hoffmann U and Harisinghani MG:
Ultrasmall superparamagnetic iron oxide nanoparticle uptake as
noninvasive marker of aortic wall inflammation on MRI: Proof of
concept study. Br J Radiol. 91:201804612018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Duan X, Wang Y, Zhang F, Lu L, Cao M, Lin
B, Zhang X, Mao J, Shuai X and Shen J: Superparamagnetic Iron
Oxide-loaded cationic polymersomes for cellular MR imaging of
therapeutic stem cells in stroke. J Biomed Nanotechnol.
12:2112–2124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Debats OA, Fortuin AS, Meijer HJ, Hambrock
T, Litjens GJ, Barentsz JO and Huisman HJ: Intranodal signal
suppression in pelvic MR lymphography of prostate cancer patients:
A quantitative comparison of ferumoxtran-10 and ferumoxytol. PeerJ.
4:e24712016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Farr TD, Lai CH, Grünstein D, Orts-Gil G,
Wang CC, Boehm-Sturm P, Seeberger PH and Harms C: Imaging early
endothelial inflammation following stroke by core shell silica
superparamagnetic glyconanoparticles that target selectin. Nano
Lett. 14:2130–2134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Beckmann N, Cannet C, Babin AL, Blé FX,
Zurbruegg S, Kneuer R and Dousset V: In vivo visualization of
macrophage infiltration and activity in inflammation using magnetic
resonance imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol.
1:272–298. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brochet B, Deloire MS, Touil T, Anne O,
Caillé JM, Dousset V and Petry KG: Early macrophage MRI of
inflammatory lesions predicts lesion severity and disease
development in relapsing EAE. Neuroimage. 32:266–274. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marinescu M, Chauveau F, Durand A, Riou A,
Cho TH, Dencausse A, Ballet S, Nighoghossian N, Berthezène Y and
Wiart M: Monitoring therapeutic effects in experimental stroke by
serial USPIO-enhanced MRI. Eur Radiol. 23:37–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luther EM, Petters C, Bulcke F, Kaltz A,
Thiel K, Bickmeyer U and Dringen R: Endocytotic uptake of iron
oxide nanoparticles by cultured brain microglial cells. Acta
Biomater. 9:8454–8465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gkagkanasiou M, Ploussi A, Gazouli M and
Efstathopoulos EP: USPIO-Enhanced MRI Neuroimaging: A Review. J
Neuroimaging. 26:161–168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Strobel K, Hoerr V, Schmid F, Wachsmuth L,
Löffler B and Faber C: Early detection of lung inflammation:
Exploiting T1-effects of iron oxide particles using UTE MRI. Magn
Reson Med. 68:1924–1931. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Belayev L, Alonso OF, Busto R, Zhao W and
Ginsberg MD: Middle cerebral artery occlusion in the rat by
intraluminal suture. Neurological and pathological evaluation of an
improved model. Stroke. 27:1616–1623. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu W, Wang X, Zheng Y, Shang G, Huang J,
Tao J and Chen L: Electroacupuncture inhibits inflammatory injury
by targeting the miR-9-mediated NF-κB signaling pathway following
ischemic stroke. Mol Med Rep. 13:1618–1626. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sage JE, Samii VF, Abramson CJ, Green EM,
Smith M and Dingus C: Comparison of conventional spin-echo and fast
spin-echo magnetic resonance imaging in the canine brain. Vet
Radiol Ultrasound. 47:249–253. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Attenberger UI, Runge VM, Stemmer A,
Williams KD, Naul LG, Michaely HJ, Schoenberg SO, Reiser MF and
Wintersperger BJ: Diffusion weighted imaging: A comprehensive
evaluation of a fast spin echo DWI sequence with BLADE (PROPELLER)
k-space sampling at 3T, using a 32-channel head coil in acute brain
ischemia. Invest Radiol. 44:656–661. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ohsawa K, Imai Y, Sasaki Y and Kohsaka S:
Microglia/macrophage-specific Protein Iba1 Binds to Fimbrin and
Enhances Its Actin-bundling activity. J Neurochem. 88:844–856.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Annovazzi L, Mellai M, Bovio E, Mazzetti
S, Pollo B and Schiffer D: Microglia immunophenotyping in gliomas.
Oncol Lett. 15:998–1006. 2018.PubMed/NCBI
|
|
29
|
Konishi H, Kobayashi M, Kunisawa T, Imai
K, Sayo A, Malissen B, Crocker PR, Sato K and Kiyama H: Siglec-H is
a microglia-specific marker that discriminates microglia from
CNS-associated macrophages and CNS-infiltrating monocytes. Glia.
65:1927–1943. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Castaneda RT, Khurana A, Khan R and
Daldrup-Link HE: Labeling stem cells with ferumoxytol, an
FDA-approved iron oxide nanoparticle. J Vis Exp.
e34822011.PubMed/NCBI
|
|
31
|
Cui L, Hu J, Li CC, Wang CM and Zhang CY:
An electrochemical biosensor based on the enhanced quasi-reversible
redox signal of prussian blue generated by self-sacrificial label
of iron metal-organic framework. Biosens Bioelectron. 122:168–174.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ma Y, Ma L, Guo Q and Zhang S: Expression
of bone morphogenetic protein-2 and its receptors in epithelial
ovarian cancer and their influence on the prognosis of ovarian
cancer patients. J Exp Clin Cancer Res. 29:852010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Anrather J and Iadecola C: Inflammation
and stroke: An overview. Neurotherapeutics. 13:661–670. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Petrovic-Djergovic D, Goonewardena SN and
Pinsky DJ: Inflammatory disequilibrium in stroke. Circ Res.
119:142–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Benedek A, Móricz K, Jurányi Z, Gigler G,
Lévay G, Hársing LG Jr, Mátyus P, Szénási G and Albert M: Use of
TTC staining for the evaluation of tissue injury in the early
phases of reperfusion after focal cerebral ischemia in rats. Brain
Res. 1116:159–165. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Moraga A, Gómez-Vallejo V, Cuartero MI,
Szczupak B, San Sebastián E, Markuerkiaga I, Pradillo JM, Higuchi
M, Llop J, Moro MÁ, et al: Imaging the role of toll-like receptor 4
on cell proliferation and inflammation after cerebral ischemia by
positron emission tomography. J Cereb Blood Flow Metab. 36:702–708.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Weinstein JS, Varallyay CG, Dosa E,
Gahramanov S, Hamilton B, Rooney WD, Muldoon LL and Neuwelt EA:
Superparamagnetic iron oxide nanoparticles: Diagnostic magnetic
resonance imaging and potential therapeutic applications in
neurooncology and central nervous system inflammatory pathologies,
a review. J Cereb Blood Flow Metab. 30:15–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shah A and Dobrovolskaia MA: Immunological
effects of iron oxide nanoparticles and iron-based complex drug
formulations: Therapeutic benefits, toxicity, mechanistic insights,
and translational considerations. Nanomedicine. 14:977–990. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Weissleder R, Elizondo G, Wittenberg J,
Rabito CA, Bengele HH and Josephson L: Ultrasmall superparamagnetic
iron oxide: Characterization of a new class of contrast agents for
MR imaging. Radiology. 175:489–493. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Clemente-Casares X and Santamaria P:
Nanomedicine in autoimmunity. Immunol Lett. 158:167–174. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Toyota T, Ohguri N, Maruyama K, Fujinami
M, Saga T and Aoki I: Giant vesicles containing superparamagnetic
iron oxide as biodegradable cell-tracking MRI probes. Anal Chem.
84:3952–3957. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Himmelreich U and Dresselaers T: Cell
labeling and tracking for experimental models using magnetic
resonance imaging. Methods. 48:112–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
von Zur Muhlen C, von Elverfeldt D,
Bassler N, Neudorfer I, Steitz B, Petri-Fink A, Hofmann H, Bode C
and Peter K: Superparamagnetic iron oxide binding and uptake as
imaged by magnetic resonance is mediated by the integrin receptor
Mac-1 (CD11b/CD18): Implications on imaging of atherosclerotic
plaques. Atherosclerosis. 193:102–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stoll G and Bendszus M: New approaches to
neuroimaging of central nervous system inflammation. Curr Opin
Neurol. 23:282–286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang YM, Feng X, Yin le K, Li CC, Jia J
and Du ZG: Comparison of USPIO-enhanced MRI and Gd-DTPA enhancement
during the subacute stage of focal cerebral ischemia in rats. Acta
Radiol. 55:864–873. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nighoghossian N, Wiart M, Cakmak S,
Berthezène Y, Derex L, Cho TH, Nemoz C, Chapuis F, Tisserand GL,
Pialat JB, et al: Inflammatory response after ischemic stroke: A
USPIO-enhanced MRI study in patients. Stroke. 38:303–307. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Balakrishnan VS, Rao M, Kausz AT, Brenner
L, Pereira BJ, Frigo TB and Lewis JM: Physicochemical properties of
ferumoxytol, a new intravenous iron preparation. Eur J Clin Invest.
39:489–496. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Toth GB, Varallyay CG, Horvath A, Bashir
MR, Choyke PL, Daldrup-Link HE, Dosa E, Finn JP, Gahramanov S,
Harisinghani M, et al: Current and potential imaging applications
of ferumoxytol for magnetic resonance imaging. Kidney Int.
92:47–66. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bashir MR, Bhatti L, Marin D and Nelson
RC: Emerging applications for ferumoxytol as a contrast agent in
MRI. J Magn Reson Imaging. 41:884–898. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wiart M, Davoust N, Pialat JB, Desestret
V, Moucharrafie S, Cho TH, Mutin M, Langlois JB, Beuf O, Honnorat
J, et al: MRI monitoring of neuroinflammation in mouse focal
ischemia. Stroke. 38:131–137. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sekerdag E, Solaroglu I and Gursoy-Ozdemir
Y: Cell death mechanisms in stroke and novel molecular and cellular
treatment options. Curr Neuropharmacol. 16:1396–1415. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kuschinsky W and Gillardon F: Apoptosis
and cerebral ischemia. Cerebrovasc Dis. 10:165–169. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Müller K, Skepper JN, Posfai M, Trivedi R,
Howarth S, Corot C, Lancelot E, Thompson PW, Brown AP and Gillard
JH: Effect of ultrasmall superparamagnetic iron oxide nanoparticles
(Ferumoxtran-10) on human monocyte-macrophages in vitro.
Biomaterials. 28:1629–1642. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hsiao JK, Chu HH, Wang YH, Lai CW, Chou
PT, Hsieh ST, Wang JL and Liu HM: Macrophage physiological function
after superparamagnetic iron oxide labeling. NMR Biomed.
21:820–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Siglienti I, Bendszus M, Kleinschnitz C
and Stoll G: Cytokine profile of iron-laden macrophages:
Implications for cellular magnetic resonance imaging. J
Neuroimmunol. 173:166–173. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Oude Engberink RD, van der Pol SM, Döpp
EA, de Vries HE and Blezer EL: Comparison of SPIO and USPIO for in
vitro labeling of human monocytes: MR detection and cell function.
Radiology. 243:467–474. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Eamegdool SS, Weible MW II, Pham BT,
Hawkett BS, Grieve SM and Chan-Ling T: Ultrasmall superparamagnetic
iron oxide nanoparticle prelabelling of human neural precursor
cells. Biomaterials. 35:5549–5564. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Doyle KP, Quach LN, Arceuil HE and
Buckwalter MS: Ferumoxytol administration does not alter infarct
volume or the inflammatory response to stroke in mice. Neurosci
Lett. 584:236–240. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Saleh A, Schroeter M, Ringelstein A,
Hartung HP, Siebler M, Mödder U and Jander S: Iron oxide
particle-enhanced MRI suggests variability of brain inflammation at
early stages after ischemic stroke. Stroke. 38:2733–2737. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang Y, Wang B, Zhu MT, Li M, Wang HJ,
Wang M, Ouyang H, Chai ZF, Feng WY and Zhao YL: Microglial
activation, recruitment and phagocytosis as linked phenomena in
ferric oxide nanoparticle exposure. Toxicol Lett. 205:26–37. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Pohland M, Glumm R, Wiekhorst F, Kiwit J
and Glumm J: Biocompatibility of very small superparamagnetic iron
oxide nanoparticles in murine organotypic hippocampal slice
cultures and the role of microglia. Int J Nanomedicine.
12:1577–1591. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Christen T, Ni W, Qiu D, Schmiedeskamp H,
Bammer R, Moseley M and Zaharchuk G: High-resolution cerebral blood
volume imaging in humans using the blood pool contrast agent
ferumoxytol. Magn Reson Med. 70:705–710. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Desestret V, Brisset JC, Moucharrafie S,
Devillard E, Nataf S, Honnorat J, Nighoghossian N, Berthezène Y and
Wiart M: Early-stage investigations of ultrasmall superparamagnetic
iron oxide-induced signal change after permanent middle cerebral
artery occlusion in mice. Stroke. 40:1834–1841. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
van der Zijden JP, van der Toorn A, van
der Marel K and Dijkhuizen RM: Longitudinal in vivo MRI of
alterations in perilesional tissue after transient ischemic stroke
in rats. Exp Neuro. 212:207–212. 2008. View Article : Google Scholar
|
|
66
|
Mishra SK, Kumar BS, Khushu S, Singh AK
and Gangenahalli G: Early monitoring and quantitative evaluation of
macrophage infiltration after experimental traumatic brain injury:
A magnetic resonance imaging and flow cytometric analysis. Mol Cell
Neurosci. 78:25–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Perego C, Fumagalli S and De Simoni MG:
Temporal pattern of expression and colocalization of
microglia/macrophage phenotype markers following brain ischemic
injury in mice. J Neuroinflammation. 8:1742011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ortiz de Mendivil A, Alcalá-Galiano A,
Ochoa M, Salvador E and Millán JM: Brainstem stroke: Anatomy,
clinical and radiological findings. Semin Ultrasound CT MR.
34:131–141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Nakamura K and Shichita T: Cellular and
molecular mechanisms of sterile inflammation in ischaemic stroke. J
Biochem. 165:459–464. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hou K, Xu D, Li F, Chen S and Li Y: The
progress of neuronal autophagy in cerebral ischemia stroke:
Mechanisms, roles and research methods. J Neurol Sci. 400:72–82.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Haacke EM, Ayaz M, Khan A, Manova ES,
Krishnamurthy B, Gollapalli L, Ciulla C, Kim I, Petersen F and
Kirsch W: Establishing a baseline phase behavior in magnetic
resonance imaging to determine normal vs. abnormal iron content in
the brain. J Magn Reson Imaging. 26:256–264. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Schroeter M, Saleh A, Wiedermann D, Hoehn
M and Jander S: Histochemical detection of ultrasmall
superparamagnetic iron oxide (USPIO) contrast medium uptake in
experimental brain ischemia. Magn Reson Med. 52:403–406. 2004.
View Article : Google Scholar : PubMed/NCBI
|