Introduction
Alcoholic fatty liver disease (AFLD) is a disease of
the liver caused by long-term heavy drinking. Over 90% of long-term
alcoholics suffer from AFLD (1).
Without proper treatment, AFLD continues to deteriorate and
develops into inflammation, fibrosis and cirrhosis, eventually
leading to hepatocellular carcinoma. Fatty liver is usually
accompanied by insulin resistance (2), obesity (3) and dyslipidemia (4). AFLD is a worldwide medical problem
associated with high morbidity and mortality rates. However, to
date, no approved treatments for patients with AFLD are available,
and the only strategy for the management of patients with alcoholic
liver is to avoid alcohol intake (5). Therefore, the development of novel
drugs for the treatment of AFLD is urgently required.
Cytoglobin (Cygb) was first discovered in 2001
(6) and named in 2002 (7). The majority of research indicates that
Cygb is a positive regulator of tissue repair and regeneration in
multiple organ systems (8). It has
been reported that Cygb functions as a peroxidase (6), active oxygen scavenger (9) and nitric oxide plus dioxygenase
(10). In animal experiments,
recombinant human (rh)Cygb protected hepatic stellate cells from
oxidative stress damage and inhibited their differentiation into
fibroblasts (11). This has been
confirmed by previous experiments in our laboratory, demonstrating
that rhCygb improved alcohol-induced liver damage in model rats and
significantly reversed serum index elevation (12). Furthermore, it significantly reduced
the proliferation of Kupffer cells (KCs) and tumor necrosis factor
(TNF)-α expression (12). However,
the molecular mechanisms underlying the effects of rhCygb in the
treatment of AFLD have remained to be fully elucidated.
In order to explore the underlying molecular
mechanisms, a rat model of AFLD was established through 30 weeks of
free oral administration of alcohol and the rats were subsequently
treated with rhCygb for 10 weeks. The liver and serum samples were
extracted to perform 2-dimensional electrophoresis (2-DE) and
matrix-assisted laser desorption ionization time of flight
(MALDI-TOF) mass spectrometry (MS). Bioinformatics analysis was
also performed to predict the possible mechanisms of rhCygb based
on the differential expression of the proteins. The present study
may assist in elucidating the molecular mechanisms of rhCygb and
lay a foundation for the development of treatments for AFLD.
Materials and methods
Animals
Male Wistar rats (n=40; body weight, 125–155 g; age,
4 weeks) were purchased from the Experimental Animal Center of
Southern Medical University. Rats were housed at a constant
temperature of 24–25°C and 70% humidity under a controlled 12-h
light-dark cycle; they had free access to water and standard rat
chow. The experimental protocol followed the guidelines approved by
the Chinese Association of Laboratory Animal Care and was approved
by the Southern Medical University Animal Care and Use Committee
(IACUC).
Induction of AFLD and treatments
Liquor (56% vol; Beijing Red Star Co., Ltd.), was
mixed with distilled water to reach a concentration of 40% (v/v).
The rats in the alcohol modeling (AM) group (n=30) were
administered a liquid diet containing ethanol (liquor) instead of
water for 30 weeks. The rats in the negative control (NC) group
(n=10) were administered an isocaloric liquid diet containing
glucose to replace ethanol. During the 30 weeks, rats had free
access to normal food. After 30 weeks, one rat was randomly
selected from the NC group and the AM group and the liver was
obtained for pathological sectioning to confirm the formation of
AFL. After modeling, the rats in the AM group were randomly divided
into the AFLD group (n=15; saline 0.5 ml/kg) and the rhCygb group
(n=15; rhCygb, 3 mg/kg). Each group of rats was treated by
subcutaneous injection every day for 10 weeks. The animals in the
AFLD group and rhCygb group were given alcohol continuously during
this period.
Chemical analysis
Blood samples were collected for serum separation as
described previously (13). Samples
were stored at −20°C for further analysis. Aspartate
aminotransferase (AST), alanine aminotransferase (ALT), total
cholesterol (T-CHO), triglyceride (TG), high-density lipoprotein
cholesterol (HDL-C) and low-density lipoprotein-cholesterol (LDL-C)
levels were measured spectrophotometrically using an Olympus AU
5200 (Olympus Corp.).
Pathological evaluation
All rats were anesthetized and sacrificed. The liver
tissues were obtained and fixed in 10% neutral formalin, embedded,
cut into 4-µm sections and stained with H&E for assessment of
steatosis, inflammation and necrosis. Histopathological
examinations of the liver sections were performed using an Olympus
BX41 image system (Olympus Corp.) as described previously (14,15).
2-DE
Liver samples and serum samples were obtained from
the NC group, AFLD group and rhCygb group. A ProteinMiner Protein
Enrichment kit (Bio-Rad Laboratories, Inc.) was used to remove
high-abundance proteins from serum samples and these were further
purified using the 2-D Clean-up kit (Bio-Rad Laboratories, Inc.)
for isoelectric focusing (IEF). Serum samples were loaded onto a
17-cm non-linear immobilized pH gradient (IPG) strip (pH 3–10) and
subjected to one-dimensional IEF. The IEF program used was 250 V
for 1 h, 500 V for 1 h, 1,000 V for 1 h, 10,000 V for 3 h and 500 V
for 1 h.
The liver tissues were thoroughly washed with PBS to
remove the effects of serum proteins and the liver samples were
frozen in liquid nitrogen and homogenized with a mortar and pestle.
Subsequently, the protein samples were treated with protein lysate,
nuclease and ultrasound, and finally centrifuged at 40,000 g and
4°C for 1 h to collect the supernatant. After purification with the
2-D Clean-up kit, liver samples loaded onto a 17-cm linear IPG
strip (pH 5–8) for IEF. The IEF program used was the same as that
specified above.
After IEF, all of the samples were loaded on a 12.5%
homogeneous SDS gel. The gel was run in parallel at 10 mA for 60
min, and then at 28 mA until the bromophenol blue dye reached the
bottom. Each experiment was performed in triplicate. The 2-DE gels
were stained using the Vorum silver staining method.
Image analysis
A PowerLook 2100XL-USBTM (UMAX) was used for image
acquisition, with an optical resolution of 300 DPI and a pixel
depth of 8 bits. PDQuest version 8.0.1 (Bio-Rad Laboratories, Inc.)
was used for image analysis of 2-DE results. Protein false spots
were manually removed and protein classes that were not recognized
by the software were added. According to the position, size, shape
and other parameters of the spots, the image analysis software
automatically matched the same protein spots in different maps and
the unmatched protein spots were regarded as the difference
points.
In-gel digestion
Gels were eluted with 500 µl 25 mM ammonium
bicarbonate containing 50% acetonitrile and the supernatant was
discarded; this procedure was repeated 3 times, lasting for 60 min
each time. Deposits were eluted once with 500 µl H2O,
the supernatant was discarded and the pellet was dehydrated using
500 µl acetonitrile. To break the disulfide bonds of proteins, 10
mM dithiothreitol (DTT) was added for 1 h and 55 mM iodoacetamide
(IAM) was added for alkylation of cysteine in a dark room for 45
min; trypsin solution (10 ng/µl in 25 mM ammonium bicarbonate
solution) was used to cover it. After 30 min on ice, excess enzyme
solution was removed, and 25 µl 25 mM ammonium bicarbonate was
added to digest deposits overnight at 37°C. Formic acid (FA; 5%)
was used to terminate the reactions. The samples were eluted with
500 µl 25 mM ammonium bicarbonate containing 50% acetonitrile and
the supernatant was discarded; this procedure was repeated 3 times,
lasting for 60 min each time. The samples were further eluted once
with 500 µl of H2O, the supernatant was discarded and
the pellet was dehydrated using 500 µl acetonitrile; 10 mM DTT was
applied for 1 h to break the disulfide bonds and subsequently,
alkylation of cysteine was performed in a dark room using 55 mM IAM
for 45 min. The solution was covered with trypsin solution, left to
incubate on ice for 30 min, excess enzyme solution was removed and
25 µl 25 mM ammonium bicarbonate was added to digest overnight at
37°C. 5% FA was added to terminate the reaction.
MALDI-TOF-MS data analysis
Peptide mixtures of each gel spot were dissolved in
0.1% TFA, desalted and concentrated. The samples were then mixed
with an equivalent volume of matrix (α-cyano-4-hydroxycinnamic acid
in 30% acetonitrile/0.1% trifluoracetic acid), spotted on a target
disk and allowed to air-dry. Samples were analyzed using a Bruker
ultrafleXtreme MALDI-TOF MS (Bruker Daltonics). The protein
database search was performed using the MASCOT search engine
(matrixscience.com; Rat_Uniprot-20190313). Mass
tolerance was allowed within 150 parts/million. Proteins matching
>5 peptides and with a MASCOT score >60 were considered
significant (P<0.05).
Bioinformatics analysis
The UNIPROT database (uniprot.org/20190404) was searched for confirming the
basic information and function of the protein. To cluster and
analyze the function of proteins, metascape (metascape.org/20190515), an online tool, was used for
gene ontology (GO) and pathway analysis. In order to clearly
illustrate the connection of the clustering network, Cytoscape
(version 3.1.2; National Institute of General Medical Sciences of
the National Institutes of Health; cytoscape.org)
was used to map the analysis results. Briefly, all differentially
expressed proteins with enriched GO terms were identified, and
accumulative hypergeometric P-values and enrichment factors were
calculated and used for filtering. Remaining significant terms were
then hierarchically clustered into a tree based on
Kappa-statistical similarities among their gene memberships.
Subsequently, 0.3 kappa score was applied as the threshold to cast
the tree into term clusters. Each term is represented by a circle
node, where its size is proportional to the number of input genes
fall into that term, and its color represents its cluster identity
(nodes of the same color belong to the same cluster). Terms with a
similarity score >0.3 are linked by an edge (the thickness of
the edge represents the similarity score). The network was
visualized using Cytoscape (version 3.1.2) with ‘force-directed’
layout and with edge bundled for clarity. One term from each
cluster is selected to have its term description shown as
label.
Statistical analysis
Each assay was repeated three times. Values are
expressed as the mean ± standard deviation. The Student's t-test
was used to compare differences between two groups. One-way
analysis of variance (ANOVA) was used to compare the differences
between the vol % values (fold changes of spot volume) of each
identified protein, followed by the least-significant difference
(LSD) post-hoc multiple-comparisons test. GraphPad Prism version 5
(GraphPad Software, Inc.) was used for statistical analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
AFLD treatment with rhCygb
An AFLD rat model was constructed as described
previously (12). Compared with
those in the NC group, the serum levels of ALT and AST and the
AST/ALT ratio were increased in the AM group (Fig. 1A-C). The rat liver of the AM group
exhibited notable severe steatosis on H&E staining (Fig. 1D), indicating that the rats had
developed AFLD. After treatment with rhCygb for 10 weeks, compared
with that in the AFLD group, the AST/ALT ratio in the rhCygb group
was decreased and there was no statistically significant difference
between the NC and rhCygb groups (Fig.
1E). In addition, blood lipid levels in each group of rats were
analyzed. Compared with those in the NC group, the rats in the AFLD
group exhibited abnormal downregulation of HDL-C (Fig. 1F) and abnormal upregulation of TG,
T-CHO and LDL-C in the serum (Fig.
1G-I). rhCygb treatment reversed these dyslipidemias and
restored the levels of the laboratory indexes to levels similar to
those in the NC group (Fig. 1F-I).
Fig. 1D displays the complete
hepatic lobule structure; there was no fatty degeneration in the NC
group, hepatocyte boundaries were clear, the nucleus was located at
the center of the cell and hepatocytes were present radially around
the central vein. However, in the AM group, the volume of
hepatocytes increased, there were lipid vacuoles of different sizes
in the cytoplasm, the nucleus of the hepatocytes was squeezed to
the side of the cells by vacuoles, the hepatic cord was disorderly
arranged and the portal area was accompanied by inflammatory cell
infiltration, which was characterized by severe steatosis. The
livers of rats treated with rhCygb for 10 weeks displayed a
significant reduction in lipid vacuoles and hepatic cords were
neatly arranged compared with the AFLD group (Fig. 1J). The results suggested that rhCygb
was able to reverse AFLD and dyslipidemias in rats.
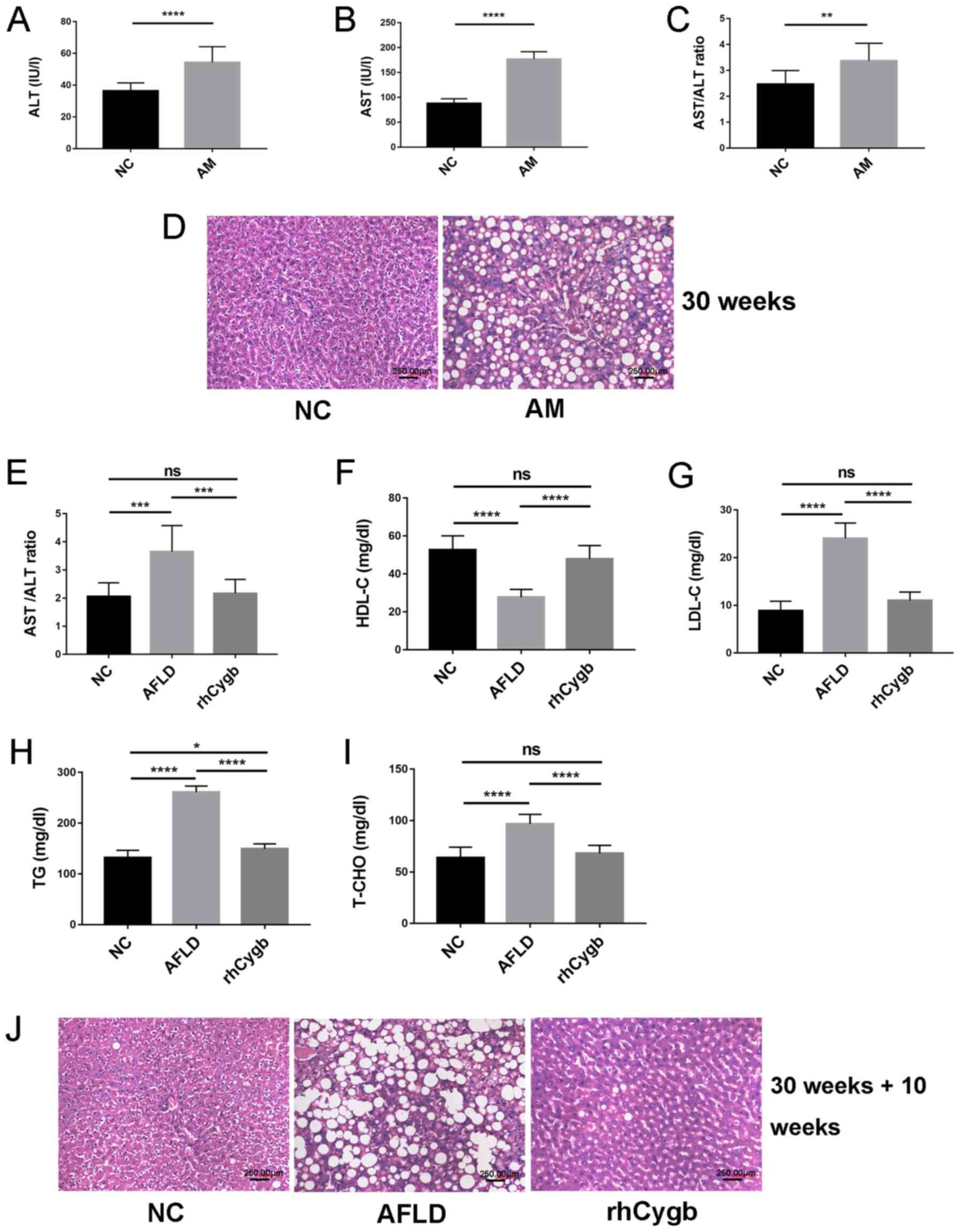 | Figure 1.Effect of rhCygb on histology and
serum biomarkers in rats with AFLD. ALT levels in the NC and AM
groups (A) prior to and (B) after treatment. (C) Comparison of the
AST/ALT ratio in the NC group and AM group prior to treatment. (D)
Representative images for the NC group and AM group after 30 weeks
of modeling. (E) Comparison of the AST/ALT ratio in the NC group,
AFLD group and rhCygb group after treatment. (F) HDL-C, (G) LDL-C,
(H) TG and (I) T-CHO values of the NC, AFLD and rhCygb groups after
treatment. (D and J) Representative images of H&E staining to
observe the morphology of livers from the different groups
(magnification, ×200). (J) Representative images for the NC group,
AFLD group and rhCygb group after 30 weeks of modeling followed by
10 weeks of treatment (scale bar, 250 µM). Values are expressed as
the mean ± standard deviation (n=3). *P<0.05, **P<0.01;
***P<0.001; ****P<0.0001. ns, no significance; AST, aspartate
aminotransferase; ALT, alanine aminotransferase; TG, triglycerides;
T-CHO, total cholesterol; HDL-C, high-density lipoprotein
cholesterol; LDL-C, low-density lipoprotein cholesterol; NC,
negative control; rhCygb, recombinant human cytoglobin; AFLD,
alcoholic fatty liver disease; AM, AFLD model. |
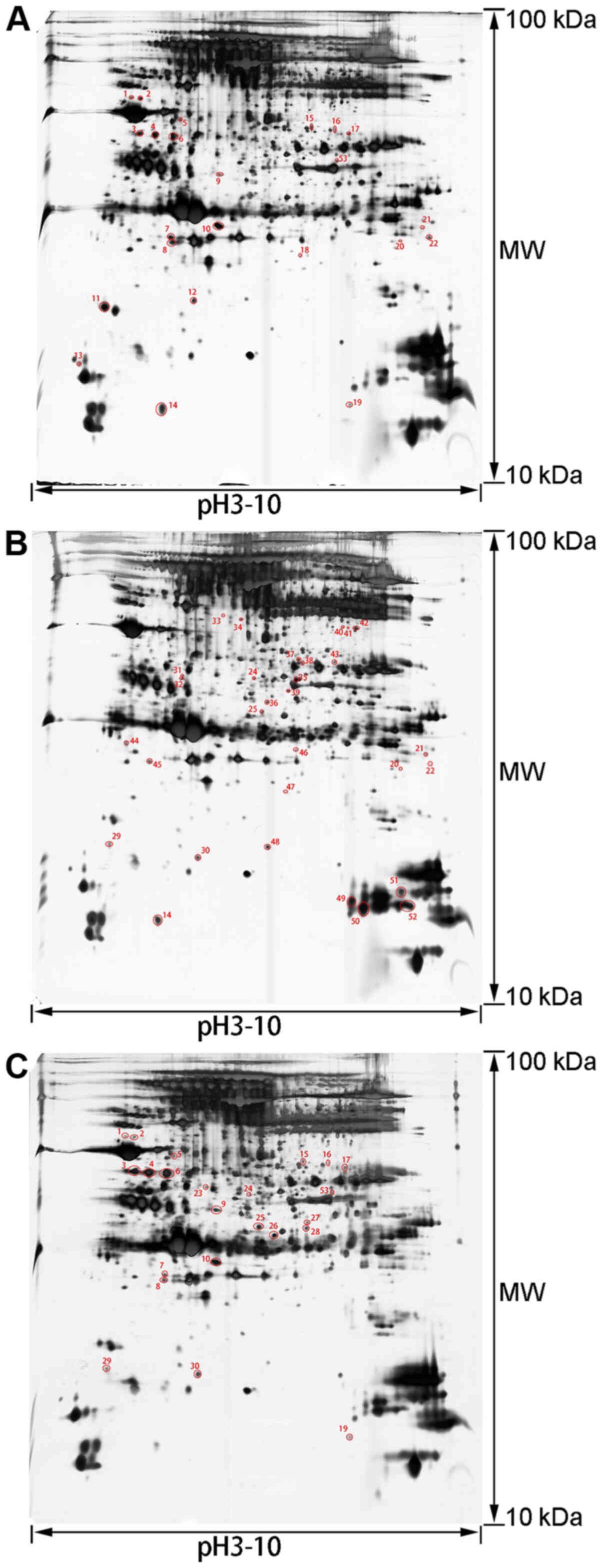
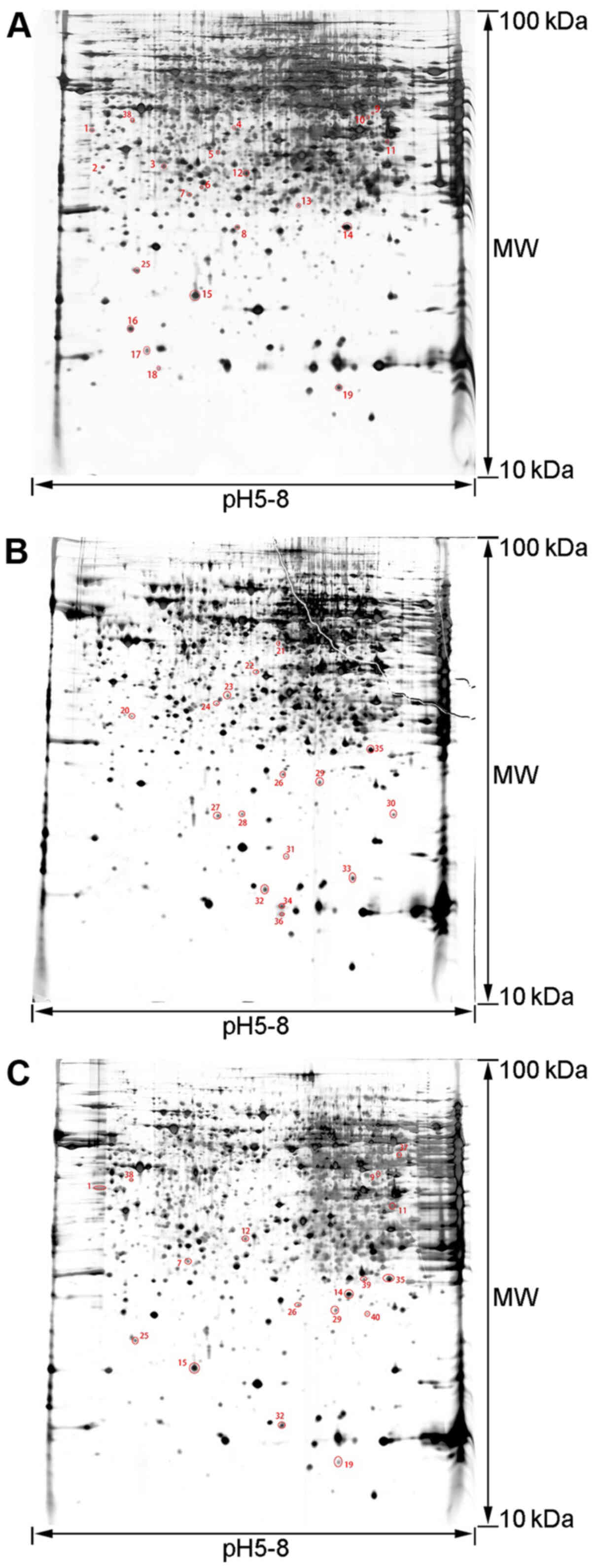
2-DE pattern
To analyze the mechanism of the therapeutic effects
of rhCygb, protein expression in the serum and liver of animals in
the different groups was assessed using 2-DE-based proteomics. The
proteins in the three groups had similar patterns, the protein
spots were evenly distributed, the proteins from the serum samples
were concentrated at the isoelectric point pH 3.5–9.5 and the
proteins from the liver samples were concentrated at the
isoelectric point pH 5–8. Based on three repeats, 53 differentially
expressed protein spots were obtained in serum samples (Fig. 2) and 40 differentially expressed
protein spots in liver samples (Fig.
3).
Protein identification and
bioinformatics analysis
The gel spots of differentially expressed proteins
were excised and identified by MALDI-TOF-MS. By using the MASCOT
search engine to query the NCBInr protein database, 26 proteins in
the serum samples were determined to be differentially expressed
with confidence (Table I) and 20
proteins in the liver samples with confidence (Table II). A comparative analysis of the
expression levels of each proteins in the tables between different
groups was conducted. Comparing the AFLD group with the NC group
indicated that there were abnormal expression level changes of
these proteins in the AFLD group. Comparing the rhCygb group with
the AFLD group suggested whether rhCygb could reverse the abnormal
changes of these proteins.
 | Table I.Differentially expressed proteins in
the serum samples. |
Table I.
Differentially expressed proteins in
the serum samples.
|
|
|
|
|
|
|
| Trend of
expression |
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Code | Protein name | Swiss-prot
accession | Encoding gene | MW | PI | Protein score | AFLD (vs. NC) | rhCygb (vs.
AFLD) | Protein
function |
|---|
| 33 |
Phenylalanine-4-hydroxylase | P04176 | PAH | 52302.51 | 6 | 59.4 | ↑ | ↓ | Catalyzes the
hydroxylation of L-phenylalanine to L-tyrosine. |
| 7 | Glutathione
peroxidase 3 | P23764 | GPX3 | 25636.91 | 8.28 | 107 | ↓ | ↑ | Protects cells and
enzymes from oxidative damage, by catalyzing the reduction of
hydrogen peroxide, lipid peroxides and organic hydroperoxide, by
glutathione. |
| 2 | Serum albumin | P02770 | ALB | 70681.83 | 6.45 | 67.9 | ↓ | ↑ | Its main function
is the regulation of the colloidal osmotic pressure of blood. Binds
to the bacterial siderophore enterobactin and inhibits
enterobactin-mediated iron uptake of E. coli from ferric
transferrin and may thereby limit the utilization of iron and
growth of enteric bacteria such as E. coli (by
similarity). |
| 17 | Psmd1 protein | Q5PPJ7 | PSMD1 | 94231.96 | 6.35 | 101 | ↓ | ↑ | Component of the
26S proteasome, a multiprotein complex involved in the
ATP-dependent degradation of ubiquitinated proteins. |
| 30 |
Inter-alpha-inhibitor H4 heavy chain | O35802 | ITIH4 | 103884.72 | 6.48 | 125 | ↑ | – | Possible
serine-type endopeptidase inhibitor activity and may participate in
the hyaluronan metabolic process. |
| 25 | Ig kappa chain C
region, B allele | P01835 | IGKC | 11764.60 | 4.76 | 92.5 | ↑ | ↑ | In the recognition
phase of humoral immunity, the membrane-bound immunoglobulins serve
as receptors, which, upon binding of a specific antigen, trigger
the clonal expansion and differentiation of B lymphocytes into
immunoglobulin-secreting plasma cells. |
| 14 | Apolipoprotein
A-II | P04638 | APOA2 | 11488.89 | 6.66 | 138 | – | ↓ | May stabilize HDL
structure by its association with lipids and affect the HDL
metabolism. |
| 20 | Proteasome subunit
beta type-5 | P28075 | PSMB5 | 28738.28 | 7.04 | 87.7 | – | ↓ | Within the 20S core
complex, PSMB5 displays a chymotrypsin-like activity. |
| 49 | Zero
beta-globin | Q63011 |
| 15953.20 | 7.35 | 286 | ↑ | ↓ | Involved in oxygen
transport from the lung to the various peripheral tissues. |
| 10 | Complement C4 | P08649 | C4 | 193638.91 | 7.34 | 68.7 | ↓ | ↑ | It induces the
contraction of smooth muscle, increases vascular permeability and
causes histamine release from mast cells and basophilic
leukocytes. |
| 31 | Actin, cytoplasmic
1 | P60711 | ACTB | 42051.86 | 5.15 | 78 | ↑ | ↓ | Forms the
cytoskeleton and participates in cell movement and has a role in
regulating gene transcription and movement and repair of damaged
DNA when localized in the nucleus. |
| 35 | Sulfotransferase
1A1 | P17988 | SULT1A1 | 34169.13 | 6.85 | 131 | ↑ | ↓ | Sulfotransferase
that utilizes 3′-phospho-5′-adenylyl sulfate as a sulfonate donor
to catalyze the sulfate conjugation of catecholamines, phenolic
drugs and neurotransmitters. Also has estrogen sulfotransferase
activity. Responsible for the sulfonation and activation of
minoxidil. |
| 11 | Apolipoprotein
N | Q5M890 | APON | 28499.71 | 5 | 155 | ↓ | ↑ | Similar to
apolipoprotein F-like, related to lipid metabolism. |
| 48 | Galectin-5 | P47967 | LGALS5 | 16414.11 | 6.66 | 236 | ↑ | ↓ | May function in
erythrocyte differentiation. |
| 22 | Complement C8 gamma
chain | D3ZPI8 | C8G | 18573.51 | 8.49 | 120 | – | ↓ | C8 is a constituent
of the membrane attack complex. |
| 46 | Carboxypeptidase
B2 | Q9EQV9 | CPB2 | 49194.70 | 8.56 | 105 | ↑ | ↓ | Cleaves C-terminal
arginine or lysine residues from biologically active peptides such
as kinins or anaphylatoxins in the circulation, thereby regulating
their activities. Downregulates fibrinolysis by removing C-terminal
lysine residues from fibrin that has already been partially
degraded by plasmin. |
| 29 | Apolipoprotein
E | P02650 | APOE | 35788.35 | 4.93 | 120 | ↑ | – | Has a
heparin-binding activity and binds heparan-sulfate proteoglycans on
the surface of cells, a property that supports the capture and the
receptor-mediated uptake of apolipoprotein E-containing
lipoproteins by cells. |
| 3,4,6 | Haptoglobin | P06866 | HP | 39051.73 | 6.51 | 113 | ↓ | ↑ | Combines with the
free plasma hemoglobin from the blood to prevent kidney damage. It
has antioxidant activity and antibacterial activity. |
| 50 | Hemoglobin subunit
alpha-1/2 | P01946 | HBA1 | 15489.83 | 8.14 | 463 | ↑ | ↓ | Involved in oxygen
transport from the lung to the various peripheral tissues. |
| 39 | Cyclic
nucleotide-gated channel | Q9QWN7 | CNGA3 | 70754.48 | 7.82 | 65.7 | ↑ | ↓ | Participation in
cation transcellular membrane transport. |
| 51 | Serum amyloid A
protein | Q5M878 | SAA4 | 15077.44 | 9.39 | 126 | ↑ | ↓ | Major acute-phase
reactant. Apolipoprotein of the HDL complex. |
| 15 | Transforming growth
factor beta-1 proprotein | P17246 | TGFB1 | 44984.90 | 8.63 | 65.5 | ↓ | ↑ | Precursor of the
latency-associated peptide and transforming growth factor beta-1
chains. |
| 8 | Glutathione
peroxidase 3 | P23764 | GPX3 | 25636.91 | 8.28 | 69 | ↓ | ↑ | Protects cells and
enzymes from oxidative damage, by catalyzing the reduction of
hydrogen peroxide, lipid peroxides and organic hydroperoxide, by
glutathione. |
| 52 | Hemoglobin subunit
alpha-1/2 | P01946 | HBA1 | 15489.83 | 8.14 | 314 | ↑ | ↓ | Involved in oxygen
transport from the lung to the various peripheral tissues. |
 | Table II.Differentially expressed proteins in
the liver samples. |
Table II.
Differentially expressed proteins in
the liver samples.
|
|
|
|
|
|
|
| Trend of
expression |
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| ID | Protein name | Swiss-prot
accession | Gene name | MW | PI | Protein score | AFLD (vs. NC) | rhCygb (vs.
AFLD) | Protein
function |
|---|
| 1 | Haptoglobin | P06866 | HP | 39051.73 | 6.51 | 92.1 | ↓ | ↑ | As described in
Table I. |
| 7 | Proteasome subunit
beta type-10 | Q4KM35 | PSMB10 | 29305.06 | 6.62 | 69.6 | ↓ | ↑ | This subunit is
involved in antigen processing to generate class I binding
peptides. |
| 8 | Glutathione
peroxidase | Q6PDW8 | GPX1 | 16649.44 | 7.35 | 109 | ↓ | – | Glutathione
peroxidase activity participates in the response to oxidative
stress. |
| 11 |
4-Hydroxy-2-oxoglutarate aldolase 1 | D4A2K1 | HOGA1 | 34784.99 | 8.35 | 78.8 | ↓ | ↑ | Glyoxylate
metabolism and glycine degradation. |
| 12 | Deaminated
glutathione amidase | Q5PQK6 | NIT1 | 36811.42 | 7.04 | 303 | ↓ | ↑ | Catalyzes the
hydrolysis of the amide bond in
N-(4-oxoglutarate)-L-cysteinylglycine (deaminated glutathione), a
metabolite repair reaction to dispose of the harmful deaminated
glutathione. |
| 14 | Glutathione
peroxidase 1 | P04041 | GPX1 | 22463.37 | 8.05 | 231 | ↓ | ↑ | Protects the
hemoglobin in erythrocytes from oxidative breakdown. |
| 15 | Major urinary
protein | P02761 | MUP5 | 21008.61 | 6.13 | 339 | ↓ | ↑ | Major urinary
proteins bind and release pheromones. They may also protect
pheromones from oxidation. |
| 16 | Retinol-binding
protein 1 | P02696 | RBP1 | 15994.87 | 4.87 | 261 | ↓ | – | Accepts retinol
from the transport protein STRA6 and thereby contributes to retinol
uptake, storage and retinoid homeostasis. |
| 20 | Glutamate-cysteine
ligase regulatory subunit | P48508 | GCLM | 30870.7 | 5.2 | 83.1 | ↑ | ↓ | It is responsible
for the synthesis of glutathione from L-cysteine and L-glutamic
acid in the glutathione biosynthesis pathway. |
| 25 | ATP synthase
subunit alpha, mitochondrial | F1LP05 | ATP5FLA | 59830.66 | 9.64 | 173 | ↓ | ↑ | Produces ATP from
ADP in the presence of a proton gradient across the membrane. |
| 26 | ATP synthase
subunit alpha, mitochondrial | F1LP05 | ATP5A1 | 59830.66 | 9.64 | 354 | ↑ | – | Produces ATP from
ADP in the presence of a proton gradient across the membrane. |
| 27 | Acyl-coenzyme A
synthetase ACSM2, mitochondrial | F1M1W1 | ACSM1 | 40093.31 | 8.8 | 275 | ↑ | ↓ | It has a catalytic
role in the conjugation process of benzoate with glycine. |
| 29 | Glutathione
S-transferase | Q9JLX3 | GST | 26028.71 | 9.33 | 117 | ↑ | – | Catalytic action
occurs when a thiol group on glutathione undergoes a substitution
reaction. |
| 31 | Galectin-5 | P47967 | LGALS5 | 16414.11 | 6.66 | 257 | ↑ | ↓ | May function in
erythrocyte differentiation. |
| 32 |
D-beta-hydroxybutyrate dehydrogenase,
mitochondrial | P29147 | BDH1 | 38576.47 | 9.09 | 91.7 | ↑ | – | 3-hydroxybutyrate
dehydrogenase activity. |
| 33 | Histidine triad
nucleotide binding protein 2 (predicted), isoform CRA_a | D4AB01 | HINT2 | 17426.28 | 9.93 | 215 | ↑ | ↓ | Participates in the
lipid catabolic process and in the negative regulation of
peptidyl-lysine acetylation. |
| 35 | Hemoglobin subunit
alpha-1/2 | P01946 | HBA1 | 15489.83 | 8.14 | 120 | ↑ | – | Involved in oxygen
transport from the lung to the various peripheral tissues. |
| 37 | Bucs1 protein | B5DFA3 | ACSM1 | 65999.74 | 7.71 | 63.7 | – | ↑ | May have a
catalytic role in the conjugation process of benzoate with
glycine. |
| 38 | Plectin | Q6ZYE7 | PLEC1 | 187344.9 | 6.03 | 75.6 | ↓ | ↑ | Cytoskeletal
protein binding. |
| 39 | Coiled-coil
domain-containing 13 | M0R4S7 | CCDC13 | 69912.7 | 9.38 | 63.4 | – | ↑ | It is speculated to
be involved in the maintenance of non-motor cilia assembly and
genomic stability. |
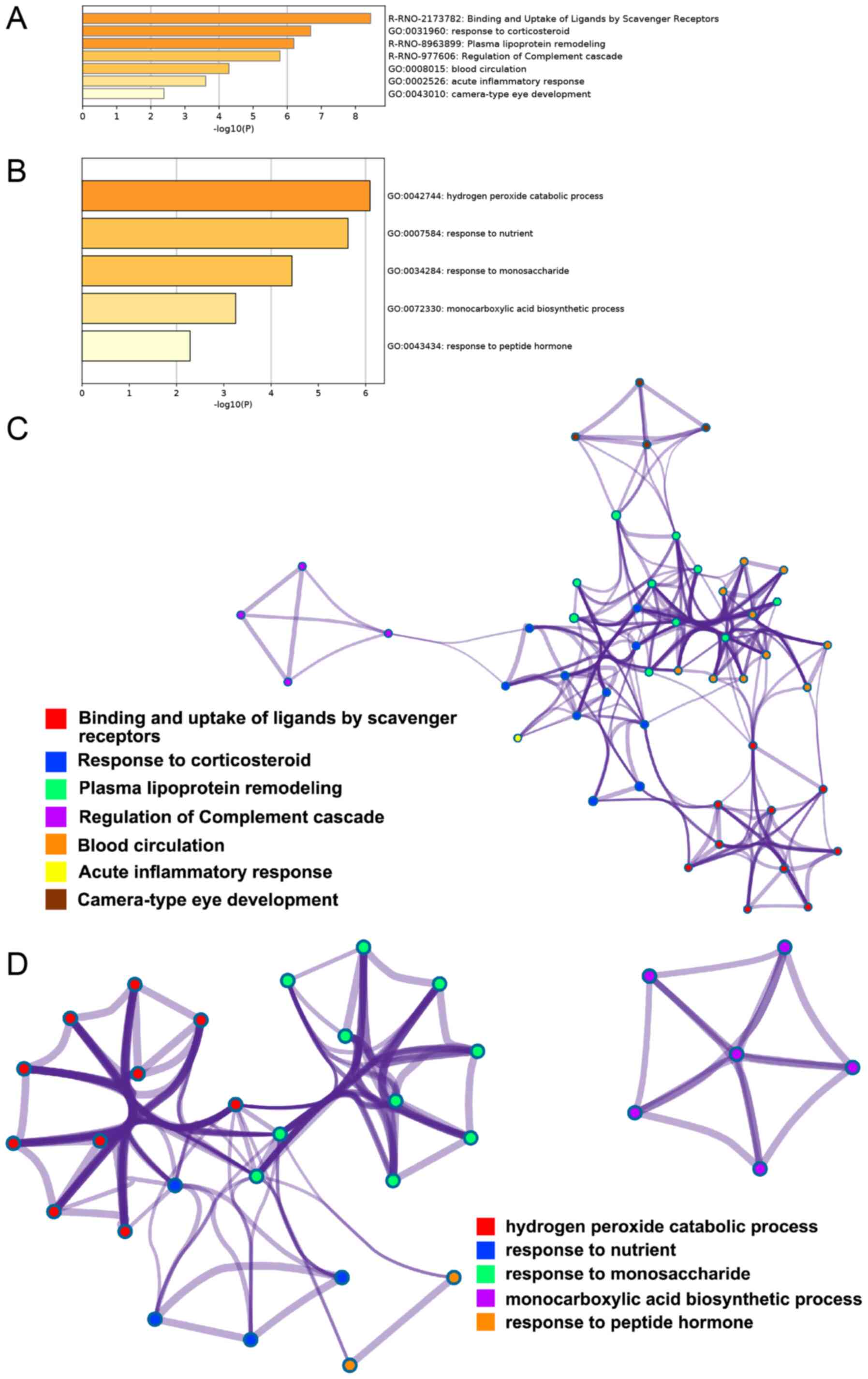
GO enrichment and Reactome Gene Sets analysis of the
26 differentially expressed proteins in the serum samples was
performed and 7 classifications were obtained: Binding and uptake
of ligands by scavenger receptors, response to corticosteroid,
plasma lipoprotein remodeling, regulation of the complement
cascade, blood circulation, acute inflammatory response and
camera-type eye development (Fig.
4A). Table III presents the
gene symbols corresponding to the proteins contained in the GO
enrichment analysis. GO enrichment analysis and Reactome Gene Sets
of the 20 differentially expressed proteins from the liver samples
indicated 5 classifications: Hydrogen peroxide catabolic process,
response to nutrient, response to monosaccharide, monocarboxylic
acid biosynthetic process, and response to peptide hormone
(Fig. 4B); Table IV shows the gene symbols
corresponding to the proteins contained in the GO enrichment
analysis.
 | Table III.Gene symbols included in each GO term
and reactome gene sets of the serum samples. |
Table III.
Gene symbols included in each GO term
and reactome gene sets of the serum samples.
| GO term | Term name | List of genes |
|---|
| R-RNO-2173782 | Binding and uptake
of ligands by scavenger receptors | ALB, HP, HBA1,
APOE |
| GO:0031960 | Response to
corticosteroid | HP, APOA2, TGFB1,
GPX3, SULT1A1, CNGA3 |
| R-RNO-8963899 | Plasma lipoprotein
remodeling | ALB, APOA2,
APOE |
| R-RNO-977606 | Regulation of
complement cascade | C4A, CPB2, C8G |
| GO:0008015 | Blood
circulation | ALB, HBA1, APOE,
TGFB1, SULT1A1 |
| GO:0002526 | Acute inflammatory
response | HP, APOA2,
SAA4 |
| GO:0043010 | Camera-type eye
development | TGFB1, ACTB,
CNGA3 |
 | Table IV.Gene symbols included in each GO term
of the liver samples. |
Table IV.
Gene symbols included in each GO term
of the liver samples.
| GO term | Term name | List of genes |
|---|
| GO:0042744 | Hydrogen peroxide
catabolic process | GPX1, HP, HBA1,
GCLM, BDH1 |
| GO:0007584 | Response to
nutrient | GPX1, HP, RBP1,
GCLM, BDH1 |
| GO:0034284 | Response to
monosaccharide | GPX1, HP, GCLM,
LOC259246 |
| GO:0072330 | Monocarboxylic acid
biosynthetic process | RBP1, HOGA1,
ACSM1 |
| GO:0043434 | Response to peptide
hormone | HP, BDH1,
LOC259246 |
Metascape was used to perform statistically
terminological enrichment of the 12 upregulated proteins and 14
downregulated proteins in the serum proteins and the 14 upregulated
proteins and 6 downregulated proteins in the liver samples of rats
treated with rhCygb. A subset of representative terms was selected
from the enriched terms and converted to a network layout. For the
serum samples, the terms contained in the network included binding
and uptake of ligands by scavenger receptors, response to
corticosteroid, plasma lipoprotein remodeling, regulation of
complement cascade, blood circulation, acute inflammatory response
and camera-type eye development (Fig.
4C). For the liver samples, the terms contained in the network
included hydrogen peroxide catabolic process, response to nutrient,
response to monosaccharide, monocarboxylic acid biosynthetic
process and response to peptide hormone (Fig. 4D).
In the visualization network of the serum samples
(Fig. 4C), rhCygb may have
exhibited its effects primarily via two parts: i) The purple
cluster represented by regulation of complement cascade, which was
notably distinct from the other six clusters; and ii) the remaining
six clusters (not including the purple cluster) consisted of red
cluster represented by binding and uptake of ligands by scavenger
receptor, a blue cluster represented by response to corticosteroid,
green cluster represented by plasma lipoprotein remodeling, orange
cluster represented by blood circulation, yellow cluster
represented by acute inflammatory response and a brown cluster
represented by camera-type eye development. In the six clusters on
the right, three groups of blue, green and orange were more closely
associated with each other and even intertwined (the thickness of
the edge represents the similarity score), indicating that rhCygb
may be capable of affecting metabolites in serum. In the
visualization network of liver samples (Fig. 4D), red term clusters represented by
hydrogen peroxide catabolic process, blue term clusters represented
by response to nutrient, green term clusters represented by
response to monosaccharide and orange term clusters represented by
response to peptide hormone were associated with each other (these
three clusters linked with each other and had wide edge), where red
term clusters were most closely associated with green term clusters
(the purple link is the darkest). Blue term clusters and orange
term clusters were less closely associated with other term clusters
(purple lines are lighter). In addition, purple term clusters
represented by monocarboxylic acid biosynthetic process were not
associated with other term clusters.
Discussion
The results of a previous study by our group
indicated that rhCygb reduces alcohol-induced liver injury in rats
and significantly reverses the AFLD-associated changes in the
levels of serum biomarkers; in vitro, rhCygb significantly
reduced the proliferation of KCs and TNF-α expression in
lipopolysaccharide (LPS)-induced KCs (12). However, the underlying mechanisms of
the effects of rhCygb on AFLD have remained to be determined. In
the present study, a rat model of AFLD was established and treated
with rhCygb. Rat livers and serum were collected from the different
treatment groups and the differentially expressed proteins were
identified in rat livers and serum using 2-DE and MALDI-TOF-MS.
A total of 53 differentially expressed proteins were
observed in the serum samples and the identities of 26 proteins
were successfully uncovered using mass spectrometry and the NCBI
databases. According to GO categories and Reactome Gene Sets,
differentially expressed proteins were primarily involved in
binding and uptake of ligands by scavenger receptors, response to
corticosteroid, plasma lipoprotein remodeling, regulation of
complement cascade, blood circulation, acute inflammatory response
and camera-type eye development. The clusters obtained were
primarily associated with the regulation of lipoproteins in the
blood and immune responses, suggesting that Cygb may serve a
therapeutic role by regulating the body's immune response and lipid
metabolism.
In addition, 40 differentially expressed proteins
were observed in the liver samples and the identities of 20
proteins were successfully determined using mass spectrometry and
the NCBI databases. According to the GO categories, differentially
expressed proteins were primarily involved in the hydrogen peroxide
catabolic process, response to nutrient, response to
monosaccharide, monocarboxylic acid biosynthetic process and
response to peptide hormone. These five major GO categories belong
to cellular metabolic processes, indicating that Cygb may serve its
therapeutic role primarily by affecting cell metabolism in the
liver. The partially identified proteins are discussed further
below.
Haptoglobin acts as an antioxidant and antibacterial
agent and is able to remove free plasma hemoglobin to prevent
kidney damage (16). In the present
study, the expression of haptoglobin in the ALFD group was impaired
in the 2-DE analysis of both liver and serum. It is hypothesized
that this may have been due to the stimulation of alcohol that
resulted in a decrease in the expression of haptoglobin, which in
turn reduced its protective effects on the liver. The liver
continues to accumulate lesions under other stimuli, eventually
developing into AFLD. In the rhCygb group, the expression levels of
haptoglobin returned to normal levels and this may underlie the
effects of Cygb. In addition to the results of the present study,
previous studies reported that Cygb affects the expression of
haptoglobin in liver fibrosis (11)
and in an atherosclerosis model (17).
The primary function of glutathione peroxidase 3
(Gpx3) is to protect cells and enzymes from oxidative damage by
catalyzing the reduction of hydrogen peroxide, lipid peroxides and
organic hydroperoxides by glutathione (18,19).
The expression of Gpx3 was decreased in rats with continuous
administration of highly concentrated alcohol, suggesting reduced
body antioxidant capacity and damage. Treatment with rhCygb
recovered the expression levels of Gpx3 and thus, the body's
antioxidant capacity. 2-DE analysis indicated differentially
expressed protein levels of glutathione peroxidase 1 (Gpx1),
glutamate-cysteine ligase regulatory subunit (Gclm), glutathione
S-transferase (GST) and Gpx3, all of which are involved in the
metabolism of glutathione. The primary role of Gpx1 is to protect
hemoglobin in red blood cells from oxidative damage (20). In the present study, in the liver
samples, Gpx1 was downregulated in the AFLD group, whereas in the
rhCygb group, the levels were similar to those in the NC group;
Gclm expression was increased in the AFLD group and the levels in
the rhCygb group were similar to those in the NC group; GST
exhibited an increase in expression levels in both the AFLD group
and the rhCygb group.
The primary function of serum albumin (Alb) is to
regulate the colloid osmotic pressure of blood and it may also
inhibit the growth of enteric bacteria (21). In addition, the ability of Alb to
inhibit the growth of enteric bacteria may indirectly reduce the
activation of LPS-induced KC caused by heavy drinking, after which
intestinal permeability is altered and enteric bacteria enter the
blood (12). In the AFLD group, the
expression of Alb decreased, whereas in the rhCygb group, the
expression of Alb increased, suggesting that Cygb may reduce the
imbalance of blood colloid osmotic pressure caused by long-term
drinking by increasing the expression of Alb.
Psmd1 is a component of the 26S proteasome. The 26S
proteasome maintains protein homeostasis by removing misfolded or
damaged proteins that may disrupt cell function (22–25).
The protein expression levels of Psmd1 were decreased in the AFLD
group and in the serum of the rhCygb group, they were restored to
levels similar to those in the NC group. A decrease in the levels
of Psmd1 may result in aberrant accumulation of erroneously
misfolded proteins, which may impair cell function, thus resulting
in a series of pathological changes (22–25).
Proteasome subunit β type-5 (Psmb5) is a component
of the 20S core proteasome complex and is involved in the
proteolytic degradation of the majority of intracellular proteins
(26). Psmb5 may be associated with
two 19S regulatory particles to form a 26S proteasome. The String
database indicates that Psmb5 interacts with Psmd1 (27). In the present study, it was
suggested that rhCygb treatment reduced the expression levels of
Psmb5.
Serum amyloid A protein (Saa4) is an apolipoprotein
of the major acute phase reactants and HDL complexes (28,29).
Saa4 is primarily expressed in the liver (30), recruits immune cells to the site of
inflammation and transports cholesterol to the liver for secretion
into the bile (31). In the present
study, the expression of Saa4 in the AFLD group was significantly
increased compared with that in the NC group and Saa4 expression in
the rhCygb group was similar to the levels observed in the NC
group. The change in the expression levels of Saa4 in serum
indirectly reflects the therapeutic effect of Cygb.
Complement C4 (C4) is a major histocompatibility
complex class-III protein, which is essential for the propagation
of the classical complement pathway (32,33).
Derived from proteolytic degradation of complement C4, C4a
anaphylatoxin is a mediator of local inflammatory processes. C4a
induces the contraction of smooth muscle, increases vascular
permeability and causes histamine release from mast cells and
basophilic leukocytes (34). In the
present study, the expression of complement C4 was decreased in the
AFLD group and the expression of rhCygb was similar to that in the
NC group.
Cygb regulates the concentration of lipoproteins in
serum by regulating the expression of apolipoprotein A-II (Apoa2),
apolipoprotein N (Apon) and apolipoprotein E (Apoe), where Apoa2
and Apoe are the major apolipoproteins and are involved in the
regulation of lipoprotein metabolism (35). The String database indicates that
Apon2 is co-expressed with Apon. The ability of Cygb to regulate
lipoprotein in serum was reported in a previous study by our group
(12).
To better understand the association between
differentially expressed proteins following Cygb treatment, the GO
terms were identified and cast into term clusters. For the
differentially expressed proteins in the liver, by hierarchically
clustering significant terms into the tree, the five term clusters
were divided into two parts, of which the purple term clusters
represented by the monocarboxylic acid biosynthetic process are
separate parts, indicating that the purple term clusters are a
separate metabolic process affected by Cygb. The remaining 4 term
clusters are linked to each other to form a network of
relationships, indicating that the differential proteins of these
four term clusters act together on a biological process. Therefore,
the effect of Cygb on intracellular metabolism is primarily divided
into two parts-one is monocarboxylic acid biosynthetic process
(purple term clusters) and the other is primarily related to the
reaction of intracellular metabolites, of which red term clusters
(hydrogen peroxide catabolic process) are the decomposition of
hydrogen peroxide, which is a detoxification mechanism of Cygb.
From the differentially expressed proteins in serum,
by hierarchically clustering important terms into a tree, seven
term clusters were divided into three parts, where the purple term
cluster represented by the adjustment of the complement cascade was
the most independent, indicating that the purple term cluster is an
independent biological process of Cygb. The remaining six term
clusters were joined to each other to form a network of
relationships, indicating that the differentially expressed
proteins contained in the six terminology clusters act together on
biological processes. The blue term cluster representing the
response to corticosteroids, the green term cluster representing
plasma lipoprotein remodeling and the orange term cluster
representing the blood circulation were closely intertwined with
each other. The differentially expressed proteins contained in the
three terminologies may together form a pathway that is affected by
Cygb. Therefore, the influence of Cygb on the biological process of
the organism is primarily divided into three parts: i) Regulation
of the complement cascade (purple term cluster), ii) the steady
state of the blood system (six term clusters other than the purple
term cluster) and iii) maintenance of dynamic balance of blood
components (blue, green and orange term clusters).
In conclusion, the present study continuing on from
the previous study by our group (12), indicated that Cygb exhibited
beneficial effects in a rat model of AFLD. To further explore the
mechanisms of action of Cygb, 2-DE, MS identification and
bioinformatics analysis of differentially expressed proteins was
performed. A total of 20 differentially expressed proteins were
identified in the liver and all of these were linked to
intracellular metabolism, indicating that Cygb primarily exerts its
effects in AFLD by affecting cellular metabolic pathways (such as
response to nutrient and monocarboxylic acid biosynthetic process).
In particular, Cygb affected the decomposition of hydrogen peroxide
to exert a detoxifying effect. In addition, 26 differentially
expressed proteins were identified in the serum, which were
primarily involved in the body's immune response and regulation of
blood components, suggesting that Cygb may affect biological
processes including regulation of immune function and maintenance
of the blood component balance (e.g. of scavengers) body-to-ligand
binding and uptake, response to corticosteroids and plasma
lipoprotein remodeling, to exert its therapeutic effects in
AFLD.
Acknowledgements
Not applicable.
Funding
This work was supported by a grant from Guangdong
Province Science and Technology Plan Project (grant no.
2013A022100027) and Guangzhou Science and Technology Project (grant
no. 201804010046).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WQD and ZL conceived and designed the experiments.
ZRZ, JW and BHC performed the experiments and analyzed the data.
ZGY and WW analyzed the data and contributed
reagents/materials/analysis tools. YMX and ZYW performed the
experiments. PW and YMX analyzed the data. WQD, ZL and ZRZ drafted
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experimental procedures were performed in
accordance with the institutional guidelines approved by the
Chinese Association of Laboratory Animal Care. All experimental
protocols were approved by the IACUC at Southern Medical University
(Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Basra S and Anand BS: Definition,
epidemiology and magnitude of alcoholic hepatitis. World J Hepatol.
3:108–113. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gaggini M, Morelli M, Buzzigoli E,
DeFronzo RA, Bugianesi E and Gastaldelli A: Non-alcoholic fatty
liver disease (NAFLD) and its connection with insulin resistance,
dyslipidemia, atherosclerosis and coronary heart disease.
Nutrients. 5:1544–1560. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jung UJ and Choi MS: Obesity and its
metabolic complications: The role of adipokines and the
relationship between obesity, inflammation, insulin resistance,
dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci.
15:6184–6223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fon Tacer K and Rozman D: Nonalcoholic
fatty liver disease: Focus on lipoprotein and lipid deregulation. J
Lipids. 2011:7839762011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suk KT, Kim MY and Baik SK: Alcoholic
liver disease: Treatment. World J Gastroenterol. 20:12934–12944.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawada N, Kristensen DB, Asahina K,
Nakatani K, Minamiyama Y, Seki S and Yoshizato K: Characterization
of a stellate cell activation-associated protein (STAP) with
peroxidase activity found in rat hepatic stellate cells. J Biol
Chem. 276:25318–25323. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Burmester T, Ebner B, Weich B and Hankeln
T: Cytoglobin: A novel globin type ubiquitously expressed in
vertebrate tissues. Mol Biol Evol. 19:416–421. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoshizato K, Thuy le TT, Shiota G and
Kawada N: Discovery of cytoglobin and its roles in physiology and
pathology of hepatic stellate cells. Proc Jpn Acad Ser B Phys Biol
Sci. 92:77–97. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fordel E, Thijs L, Moens L and Dewilde S:
Neuroglobin and cytoglobin expression in mice. Evidence for a
correlation with reactive oxygen species scavenging. FEBS J.
274:1312–1317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gardner AM, Cook MR and Gardner PR:
Nitric-oxide dioxygenase function of human cytoglobin with cellular
reductants and in rat hepatocytes. J Biol Chem. 285:23850–23857.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Z, Wei W, Chen B, Cai G, Li X, Wang P,
Tang J and Dong W: The effect of rhCygb on CCl4-induced hepatic
fibrogenesis in rat. Sci Rep. 6:235082016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wen J, Wu Y, Wei W, Li Z, Wang P, Zhu S
and Dong W: Protective effects of recombinant human cytoglobin
against chronic alcohol-induced liver disease in vivo and in vitro.
Sci Rep. 7:416472017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Liang X, Yang J, Wang H, Tan D,
Chen S, Cheng J, Chen Y, Sun J, Rong F, et al: Improved performance
of quantitative collagen parameters versus standard histology in
longitudinal assessment of nonadvanced liver fibrosis for chronic
hepatitis B. J Viral Hepat. 25:598–607. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou J, Li J, Yu Y, Liu Y, Li H, Liu Y,
Wang J, Zhang L, Lu X, Chen Z and Zuo D: Mannan-binding lectin
deficiency exacerbates sterile liver injury in mice through
enhancing hepatic neutrophil recruitment. J Leukoc Biol.
105:177–186. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He WQ, Chen XJ, Wen YQ, Li YZ, He H and
Chen Q: Detection of hepatitis B virus-like nucleotide sequences in
liver samples from murine rodents and asian house shrews. Vector
Borne Zoonotic Dis. 19:781–783. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fagoonee S, Gburek J, Hirsch E, Marro S,
Moestrup SK, Laurberg JM, Christensen EI, Silengo L, Altruda F and
Tolosano E: Plasma protein haptoglobin modulates renal iron
loading. Am J Pathol. 166:973–983. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ou L, Li X, Chen B, Ge Z, Zhang J, Zhang
Y, Cai G, Li Z, Wang P and Dong W: Recombinant human cytoglobin
prevents atherosclerosis by regulating lipid metabolism and
oxidative stress. J Cardiovasc Pharmacol Ther. 23:162–173. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qi X, Ng KT, Lian QZ, Liu XB, Li CX, Geng
W, Ling CC, Ma YY, Yeung WH, Tu WW, et al: Clinical significance
and therapeutic value of glutathione peroxidase 3 (GPx3) in
hepatocellular carcinoma. Oncotarget. 5:11103–11120. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arthur JR: The glutathione peroxidases.
Cell Mol Life Sci. 57:1825–1835. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lubos E, Loscalzo J and Handy DE:
Glutathione peroxidase-1 in health and disease: From molecular
mechanisms to therapeutic opportunities. Antioxid Redox Signal.
15:1957–1997. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dich J, Hansen SE and Thieden HI: Effect
of albumin concentration and colloid osmotic pressure on albumin
synthesis in the perfused rat liver. Acta Physiol Scand.
89:352–358. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rock KL, Gramm C, Rothstein L, Clark K,
Stein R, Dick L, Hwang D and Goldberg AL: Inhibitors of the
proteasome block the degradation of most cell proteins and the
generation of peptides presented on MHC class I molecules. Cell.
78:761–771. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen B, Retzlaff M, Roos T and Frydman J:
Cellular strategies of protein quality control. Cold Spring Harb
Perspect Biol. 3:a0043742011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Voges D, Zwickl P and Baumeister W: The
26S proteasome: A molecular machine designed for controlled
proteolysis. Annu Rev Biochem. 68:1015–1068. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rodriguez KA, Edrey YH, Osmulski P,
Gaczynska M and Buffenstein R: Altered composition of liver
proteasome assemblies contributes to enhanced proteasome activity
in the exceptionally long-lived naked mole-rat. PLoS One.
7:e358902012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fujito NT and Nonaka M: Highly divergent
dimorphic alleles of the proteasome subunit beta type-8 (PSMB8)
gene of the bichir Polypterus senegalus: Implication for evolution
of the PSMB8 gene of jawed vertebrates. Immunogenetics. 64:447–453.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
da Fonseca PC, He J and Morris EP:
Molecular model of the human 26S proteasome. Mol Cell. 46:54–66.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang L and Colón W: The interaction
between apolipoprotein serum amyloid A and high-density
lipoprotein. Biochem Biophys Res Commun. 317:157–161. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de Beer MC, Yuan T, Kindy MS, Asztalos BF,
Roheim PS and de Beer FC: Characterization of constitutive human
serum amyloid A protein (SAA4) as an apolipoprotein. J Lipid Res.
36:526–534. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Uhlar CM and Whitehead AS: Serum amyloid
A, the major vertebrate acute-phase reactant. Eur J Biochem.
265:501–523. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eklund KK, Niemi K and Kovanen PT: Immune
functions of serum amyloid A. Crit Rev Immunol. 32:335–348. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mayilyan KR: Complement genetics,
deficiencies, and disease associations. Protein Cell. 3:487–496.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Birmingham DJ and Hebert LA: The
complement system in lupus nephritis. Semin Nephrol. 35:444–454.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hugli TE, Marceau F and Lundberg C:
Effects of complement fragments on pulmonary and vascular smooth
muscle. Am Rev Respir Dis. 135:S9–S13. 1987.PubMed/NCBI
|
|
35
|
Mahley RW, Innerarity TL, Rall SC Jr and
Weisgraber KH: Plasma lipoproteins: Apolipoprotein structure and
function. J Lipid Res. 25:1277–1294. 1984. View Article : Google Scholar : PubMed/NCBI
|