Introduction
Thyroid hormones (THs), synthesized and released by
the thyroid, are vital in physiological systems, including growth,
development and basal metabolism (1). Thyroid dysfunction is one of the
leading endocrine disorders in the world (2). Endocrine disturbance, including
disruption of the TH system, has been studied in western countries
since 1996 (3). Iodine deficiency
and autoimmune diseases are the pivotal causes of thyroid
dysfunction and numerous studies indicate that genetic and
environmental factors interfere with endocrine signaling by
affecting TH levels, thereby causing thyroid disease (4–7). The
use of chemicals has increased and pollutants have consequently
become a major health problem worldwide, especially those that
alter the function of the thyroid gland and secretion of THs
(8).
Phthalates are widely used as plasticizers and
softeners in various commercial products such as pharmaceutical
devices, food packaging, make-up and cosmetics (9–13).
Di-(2-ethylhexyl)phthalate (DEHP), one of the most common
phthalates, is added to plastics to make them flexible, but it is
an environmental endocrine disruptor (14–16).
DEHP binds non-covalently to plastic matrices that allow it to
leach from end-products, hence it readily pollutes air, food and
water (17–20). DEHP exposure is mainly by inhalation
of polluted air, ingestion of contaminated water or food and
contacted with the skin (21). DEHP
is a toxic chemical and following absorption, toxic effects occur
when it is metabolized to dangerous metabolites such as
monoethylhexyl phthalate (22–27).
Thus, human exposure to DEHP is a worldwide concern.
The hypothalamus-pituitary-thyroid (HPT) axis
regulates the thyroid endocrine system and all the evidence
indicates that the thyroid is vulnerable to DEHP disrupting the
endocrine system through its effects on TH biosynthesis, transport,
secretion and metabolism (28). The
hypothalamus secretes thyrotropin-releasing hormone (TRH), which
stimulates the pituitary to secrete TSH and THs regulate TSH
secretion through a negative feedback loop in mammals (29). The biosynthesis and secretion of THs
relies on iodine and iodine absorption by thyroid follicular cells
(30). TSH regulates these kinase
pathways, which mediate the expression of genes associated with
thyroid gland development, including the actions of paired box
protein 8 (PAX-8), sodium iodide symporter (NIS), thyroid
transcription factor 1 (TTF-1), iodide transporter pendrin (PDS)
and thyroid peroxidase (TPO) (31,32).
In animal experiments, including DEHP as a contaminant in rat feed,
decreased the plasma concentration of tetraiodothyronine (T4) and
its metabolites and induced histological changes in the thyroid
gland (33,34). Cell culture experiments revealed
that changes in TH activity and iodide uptake by thyroid follicular
cells following exposure to DEHP may be associated with its
antagonistic activity (35,36). Recent epidemiological studies have
indicated that there is a correlation between DEHP exposure and
THs, in which levels of free thyroxine (FT4) and triiodothyronine
(T3) in serum were negatively correlated with the concentration of
DEHP (37–39). TH activation and inactivation is
mediated by the deiodinase family of enzymes. When T3 and T4 enter
the cell through the thyroid hormone transporter, the concentration
of thyroid hormone is controlled by three iodothyroxine deiodinases
[deiodinases 1 (D1), deiodinases 2 (D2) and deiodinases 3 (D3)]. D1
and D2 convert thyroxine T4 into active T3, while D3 prevents T4
from activating and terminates T3 operation (40). DEHP disrupts the stability of THs
and the HPT axis by altering the expression of genes, hormone
levels and enzyme activities. These alterations can be reliably
used to evaluate the effects of chemicals used on the thyroid
endocrine system.
Despite encouraging progress, our understanding of
DEHP toxicity remains poor and the underlying mechanisms remain to
be elucidated. The present study reported the risk of thyroid
diseases from exposure to DEHP and explored the potential of
mechanisms by which DEHP affects the HPT axis and the
thyroid-stimulating hormone (TSH)/TSH receptor (TSHr) pathways.
Materials and methods
Chemicals and reagents
DEHP (C24H38O4, CAS: 117-81-7, purity 99.0%) was
purchased from the National Institute of Standards and Technology.
Alanine aminotransferase (ALT), aspartate aminotransferase (AST),
total protein (TP), albumin (ALB), blood glucose (GLU), urea
nitrogen (BUN), creatinine (CREA), total cholesterol (TC),
triglycerides (TG) reagent kits were obtained from Maccura
Biotechnology Co., Ltd. Total antioxidant capacity (T-AOC),
superoxide dismutase (SOD), malondialdehyde (MDA), catalase (CAT)
and glutathione (GSH) contents were measured by using assay kits
(Jiancheng Haihao Biotechnology Co., Ltd.). Serum transthyretin
protein (TTR; cat. no. GD-S0712-A), TRH (cat. no. GD-S2054-A) and
thyroid-stimulating antibody (TSAb; cat. no. GD-S1768-A) levels
were measured using ELISA kits (Shanghai Guduo Biological
Technology Co. Ltd.). Serum T4, T3, free triiodothyronine (FT3),
FT4 and TSH levels were measured using radioimmunoassay kits
(Beijing North Institute of Biotechnology Co., Ltd.). Anti-NIS
polyclonal (cat. no. ab83816), anti-TSHr polyclonal (cat. no.
ab202960), anti-thyroglobulin (Tg) monoclonal (cat. no. ab156008),
TPO (cat. no. ab203057), anti-GAPDH (cat. no. ab8245) and anti-α
tubulin (cat. no. ab7291) antibodies were obtained from Abcam.
TTF-1 (D2E8 rabbit mAb cat. no. 12373) and PAX-8 (D2S2I rabbit mAb
cat. no. 59019) were purchased from Cell Signaling Technology, Inc.
TSH-β antibody (cat. no. MAB4507) was purchased from R&D
Systems Inc. Alkaline phosphatase (AP)-conjugated goat anti-rabbit
IgG (cat. no. ZB-2301), AP-conjugated rabbit anti-goat IgG (cat.
no. ZB-2306) and AP-conjugated rabbit anti-mouse IgG (cat. no.
ZB-2305) secondary antibody were purchased from ZSGB-BIO.
Animals and experimental design
A total of 40 healthy 2-week-old male Wistar rats
(70±10 g) were purchased from Vital River Laboratory Animal
Technology Co. Ltd. All animals were housed under a constant
temperature (23±1°C) and humidity (50–60%) and a 12-h light/dark
cycle. All rats were provided with distilled water and standard
AIN-93M diet ad libitum. Rats were randomly assigned to
experimental groups (n=10): A control group, a 150 mg/kg/day DEHP
group [~ five times the no-observed-adverse-effect level (NOAEL)],
300 mg/kg/day DEHP group (~10 times the NOAEL) and a 600 mg/kg/day
DEHP group (~20 times the NOAEL). The NOAEL was obtained from a
104-week study on the chronic toxicity of DEHP in rats using a
previously described method (41).
Based on the investigation of DEPH properties in different
experiments, a concentration of 600 mg/kg/day, which is 1/40 of the
half lethal dose of DEHP for rats, was used in subsequent
experiments (42). DEHP was
administered to rats via gavage and control animals received peanut
oil without phthalate. DEHP was intragastrically administered to
rats daily for 90 consecutive days. All protocols were performed in
accordance with the Regulations of the Ethical Committee for
Research on Laboratory Animals, as assessed and approved by the
Ministry of Health of China and the Institute of Zoology Animal and
Medical Ethics Committee of Harbin Medical University. Subsequent
experiments were conducted under the strict principles of Good
Laboratory Practice to ensure good quality in vivo
toxicology studies.
Sample collection and measurement of
biochemical indices
The body weight of the rats was measured weekly. On
day 89, the rats were transferred to the metabolic cage, and urine
samples were collected for 24 h. Rats were anesthetized by
intraperitoneal injection of 2% pentobarbital sodium (35 mg/kg).
Euthanasia was performed by abdominal aortic bleeding. Blood
samples (~5 ml/per rat) were collected from the abdominal aorta.
Thyroid, pituitary, hypothalamus, liver, kidney and testis tissue
samples were obtained and frozen in liquid nitrogen, and then were
transferred to a −80°C refrigerator for storage until analysis.
Serum biochemical indicators were detected using an automatic
biochemical analyzer (Hitachi High-Tech Corporation) according to
the manufacturer's instructions. Measurements of ALT, AST, TC and
TG were performed according to the manufacturer's protocol.
Determination of oxidative stress
indices
The content of oxidation products and the activity
of antioxidant enzymes are indicators of the level of oxidation and
therefore the degree of oxidative damage (43). Antioxidant enzyme activities of SOD
and CAT and antioxidant levels of MDA, GSH and T-AOC in serum were
measured according to the manufacturer's protocols.
Effects of DEHP exposure on THs
Serum T3, T4, FT3, FT4 and TSH levels were obtained
using radioimmunoassay kits (Beijing North Institute Of
Biotechnology Co., Ltd.) according to the instructions supplied
with the Automatic Gamma Counter (Wallac Wizard-2 2470 Automatic
Gamma Counter; PerkinElmer, Inc.).
Serum TTR, TRH and TSAb were measured according to
the ELISA kit instructions. A BioTek 3 MFD instrument (BioTek
Instruments, Inc.) was used to repeatedly measure standards
solution and samples.
The urine iodine (UI) determination method was
performed according to the principle of Ascerium-Cerium Catalytic
Spectrophotometric Determination of Urine Iodine (WS/T 107–2006)
and the analysis standard of iodine in urine (GBW09109h; National
Institute of Standard Substance, Beijing, China) was based on the
reference analysis provided by the National Iodine Deficiency
Reference Laboratory (44,45). A TU-1901 double beam ultraviolet
visible-visible spectrophotometer (Beijing Puxi General Instrument
Co., Ltd.) was used to determine the iodine content in standard and
diluted samples.
Histology and histopathology
The thyroid, liver, kidney and testes samples from
the four groups were fixed in 10% paraformaldehyde for 12 h,
dehydrated and trimmed and embedded in paraffin blocks at room
temperature. Multiple 4-µm thick sections were deparaffinized with
xylene, stained with hematoxylin and eosin (H&E;1:5) for 5 min
at 25°C and representative samples were analyzed by light
microscopy (magnification, ×200). The number of follicular
epithelial cells and the diameter of the follicular cavity were
measured using Image-Pro Plus 6.0 image analysis software (Media
Cybernetics, Inc.) to quantitatively assess histological changes in
glands.
Total RNA extraction and reverse
transcription-quantitative (RT-q) PCR
Total RNA of tissues was extracted using
TRIzol® reagent (Thermo Fisher Scientific, Inc.) and
treated with genomic DNA wiper. cDNA was synthesized from 1 g total
RNA (65°C for 5 min and rapid cooling on ice) using ReverTra Ace
qPCR RT kit (cat. no. FSQ-201; Toyobo Life Science). RT was
performed at 37°C for 15 min and 98°C for 5 min. RT-qPCR was
performed using THUNDERBIRD SYBR® qPCR Mix with 50X ROX
reference dye (cat. no. QPS-201T; Toyobo Life Science) according to
the manufacturer's instructions. Expression levels were measured
for NIS, PAX-8, Tg, TSHr, TTF-1, TTF-2, PDS, TPO, TSH-β, TRHr, D1,
D2 and D3. The relative expression levels of target genes were
calculated using 2–ΔΔCq method (46). The primer sequences (Table I) were designed according to the
cDNA sequence from GenBank. All reactions were run in triplicate. A
melting curve was generated during amplification to verify the
absence of primer dimers or incorrectly paired products. All
primers were synthesized by Sangon Biotech, Co., Ltd.
 | Table I.Primers used in the present
study. |
Table I.
Primers used in the present
study.
| Primers | Type | Primer
sequence | GenBank |
|---|
| NIS | Forward |
5′-CAGTTCTGGAATGGACACGG-3′ | NM_052983.2 |
|
| Reverse |
5′-TCTTGGTCACAGCAGGGATG-3′ |
|
| PAX-8 | Forward |
5′-AGCAGCAGTAGTGGTCCTCG-3′ | NM_031141.2 |
|
| Reverse |
5′-TTTATGGCGTAGGGTGAATGA-3′ |
|
| Tg | Forward |
5′-GCCCTAACTCATCCGTCCA-3′ | NM_030988.2 |
|
| Reverse |
5′-TGTTGATAAGCCCATCGTCCT-3′ |
|
| TTF-1 | Forward |
5′-GCACTTGGAGTAAGGCAGAAA-3′ | XM_006224320.2 |
|
| Reverse |
5′-ACCCCACGATACACGAACC-3′ |
|
| TTF-2 | Forward |
5′-CGAGTGAAGCCATTGACGA-3′ | NM_001106454.2 |
|
| Reverse |
5′-AAGCGGGGCAGACGATT-3′ |
|
| PDS | Forward |
5′-TCCCAAAATACCGAGTCAAGG-3′ | NM_019214.1 |
|
| Reverse |
5′-TCAGAACAACGGACCCCAC-3′ |
|
| TPO | Forward |
5′-ATGAGGCTGTGACTGAAGATGA-3′ | NM_019353.2 |
|
| Reverse |
5′-GTGGTCCGTGAGGAGTTTGA-3′ |
|
| TSH-β | Forward |
5′-TACTGCCTGACCATCAACACC-3′ | NM_013116.2 |
|
| Reverse |
5′-GGTAGGAGAAATAAGGAGCAACAT-3′ |
|
| TSHr | Forward |
5′-GTGGGAATAAGCAGCTACGC-3′ | NM_012888.1 |
|
| Reverse |
5′-GGATTTCGGACGGTGATGT-3′ |
|
| TRHr | Forward |
5′-AGGAGTCAGACCGCTTTAGCA-3′ | NM_013047.3 |
|
| Reverse |
5′-GAACTGGGTCCATTCTTCTCG-3′ |
|
| D1 | Forward |
5′-GTGGTGGTGGACACAATGCAG-3′ | NM_021653.3 |
|
| Reverse |
5′-TTGTAGTTCCAAGGGCCAGGTTTA-3 |
|
| D2 | Forward |
5′-GCTCTATGACTCGGTCATTCTGCTC-3′ | NM_031720.3 |
|
| Reverse |
5′-GACACGTGCACCACACTGGA-3 |
|
| D3 | Forward |
5′-CGTGTCAGCGCAGCAAGAGTA-3′ | NM_017210.3 |
|
| Reverse |
5′-TGCCGCTCTGGATGACGTAG-3 |
|
| β-actin | Forward |
5′-CCGTAAAGACCTCTATGCCAACA-3′ | NM_013116.2 |
|
| Reverse |
5′-GGGGCCGGACTCATCGTA-3′ |
|
Western blot analysis
Samples were processed to determine tissue (thyroid,
liver, kidney and testis) protein concentrations as previously
described (47). The protein
concentrations were measured by the Bicinchoninic Acid Protein
Assay kit (Beyotime Institute of Biotechnology.). Equal amounts of
protein (20 µl per lane) were separated by electrophoresis on a 10%
SDS polyacrylamide gel under reducing conditions and the separated
proteins were transferred onto a polyvinylidene fluoride membrane.
The polyvinylidene fluoride membranes were then blocked with 1%
bovine serum albumin (cat. no. V900933; Sigma-Aldrich; Merck KGaA)
in TRIS-buffered saline with 0.05% Tween-20 (TBST) at room
temperature for 1 h, then incubated with NIS (1:1,000 dilution),
TSHr (1:1,000 dilution), Tg (1:1,000 dilution), TPO (1:1,000
dilution), TTF-1 (1:1,000 dilution), PAX-8 (1:1,000 dilution),
TSH-β (1:500 dilution) and GAPDH (1:2,000 dilution) overnight at
4°C. The next day, membranes were rinsed three times (10 min each)
with 1% TBST and incubated with AP-conjugated goat anti-rabbit IgG
secondary antibody (1:1,000 dilution), AP-conjugated rabbit
anti-goat IgG (1:1,000 dilution) or AP-conjugated rabbit anti-mouse
IgG (1:1,000 dilution) for 1–2 h at room temperature. After washing
six times in TBST buffer, a chemiluminescence detection system
(Tanon Science and Technology Co., Ltd.) was used to detect the
resultant signals. The individual protein bands were quantified by
using ImageJ software (v1.50; National Institutes of Health). Each
western blot analysis was repeated ≥3 times.
Statistical analysis
Each experiment was performed ≥3 times. Statistical
analysis was performed using SPSS version 20.0 (IBM Corp.). All
data are expressed as the mean ± standard error of the mean.
Differences among groups were analyzed using one-way analysis of
variance (ANOVA), followed by Bonferroni's test. Graphs presenting
the results were produced using GraphPad Prism 5.0 (GraphPad
Software, Inc.) and P<0.05 was considered to indicate a
statistically significant difference.
Results
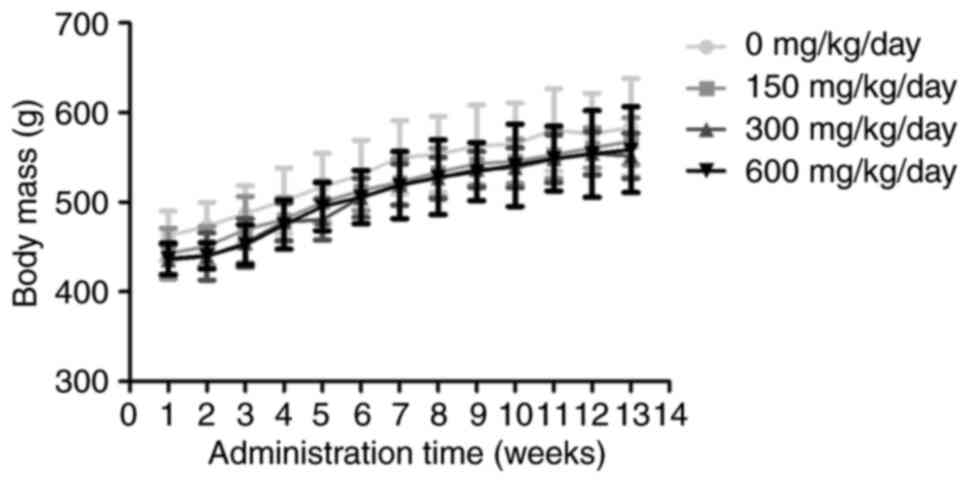
Changes in bodyweight and general
physical status
No rats succumbed during the treatments and rats in
each group gained body mass normally over time. There was no
difference in the physiological parameters of the rats in each
group compared with time-matched control groups at all time-points
(Fig. 1).
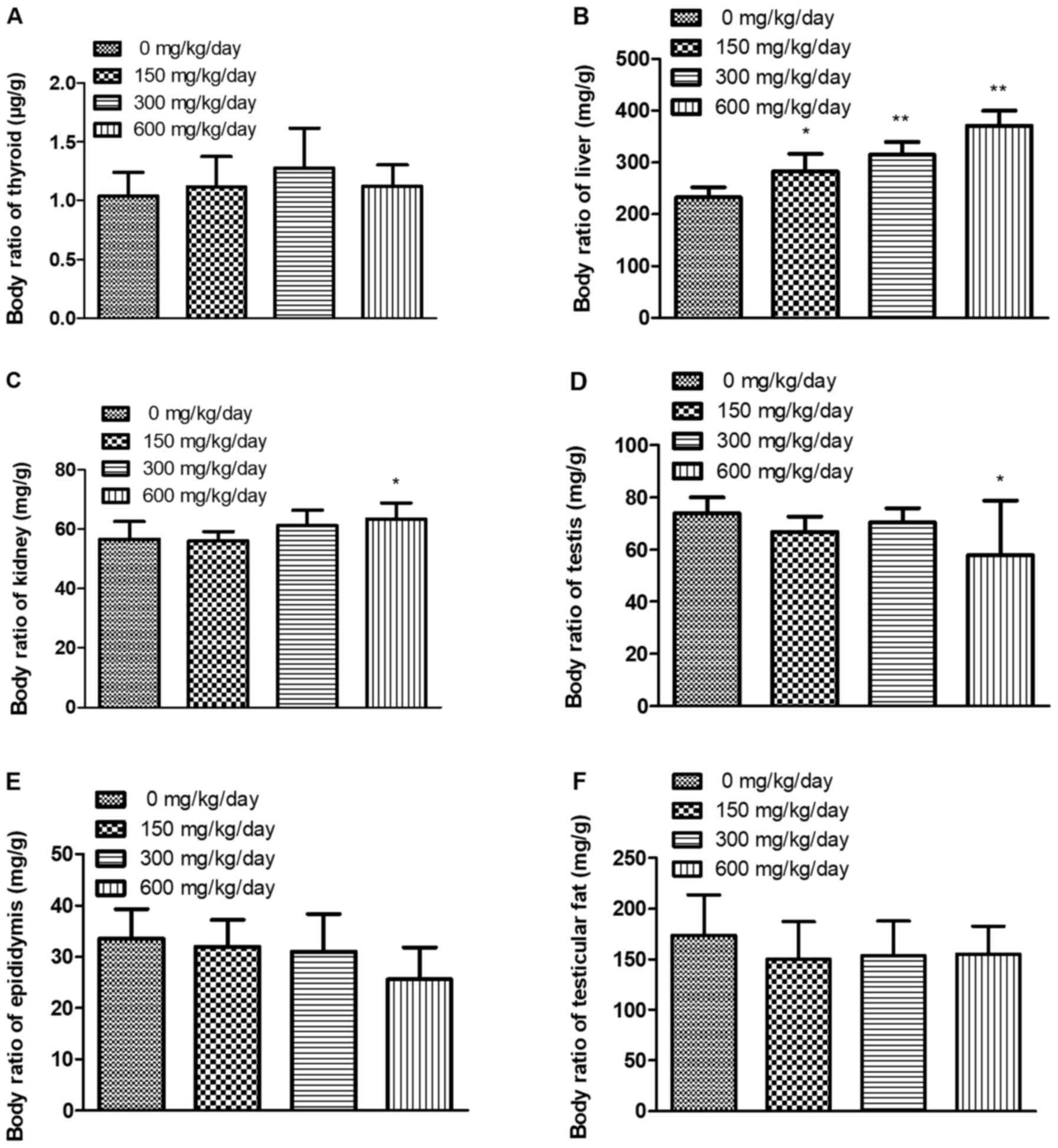
Effects of exposure to DEHP on
relative organ weight
To validate the role of DEHP in organs, relative
organ weight [(organ weight/body weight) × 10,000] were calculated
(Fig. 2). Relative organ weight for
thyroid, epididymis and testicular fat did not significantly differ
among treatment and control groups (P<0.05). However, relative
organ weights for liver, kidney were increased in DEHP treatment
groups compared with the control group; relative organ weights for
testis were decreased in DEHP 600 mg/kg/day treatment group
compared with the control group.
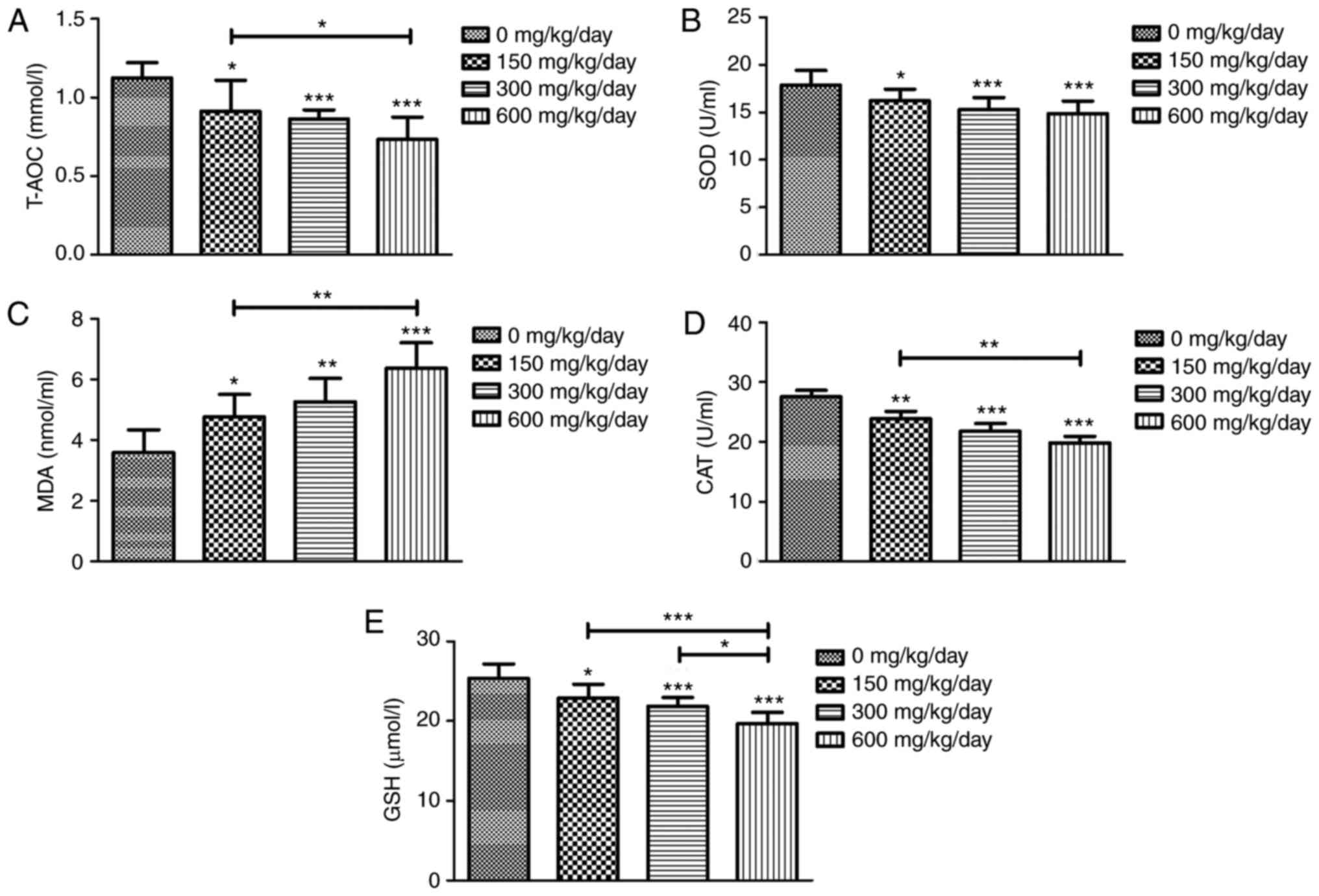
Effects of exposure to DEHP on
oxidative stress
To further clarify the potential effects of
DEHP-induced oxidative stress, several oxidative stress-associated
parameters were measured. The findings demonstrated that MDA levels
were significantly increased following DEHP treatment, while the
activities of T-AOC, SOD, CAT and GSH in DEHP-exposed rats were
decreased compared with the control group (Fig. 3).
Effects of exposure to DEHP on
biochemical indices
The effects of exposure to DEHP on biochemical
indices are presented in Fig. 4.
The results of routine blood and urine metabolic indicators ALT,
AST, TG and UI revealed similarities among the four groups.
Unexpectedly, compared with the 300 mg/kg/day dose group, there
were significant differences in TC levels after treatment with 150
and 600 mg/kg/day.
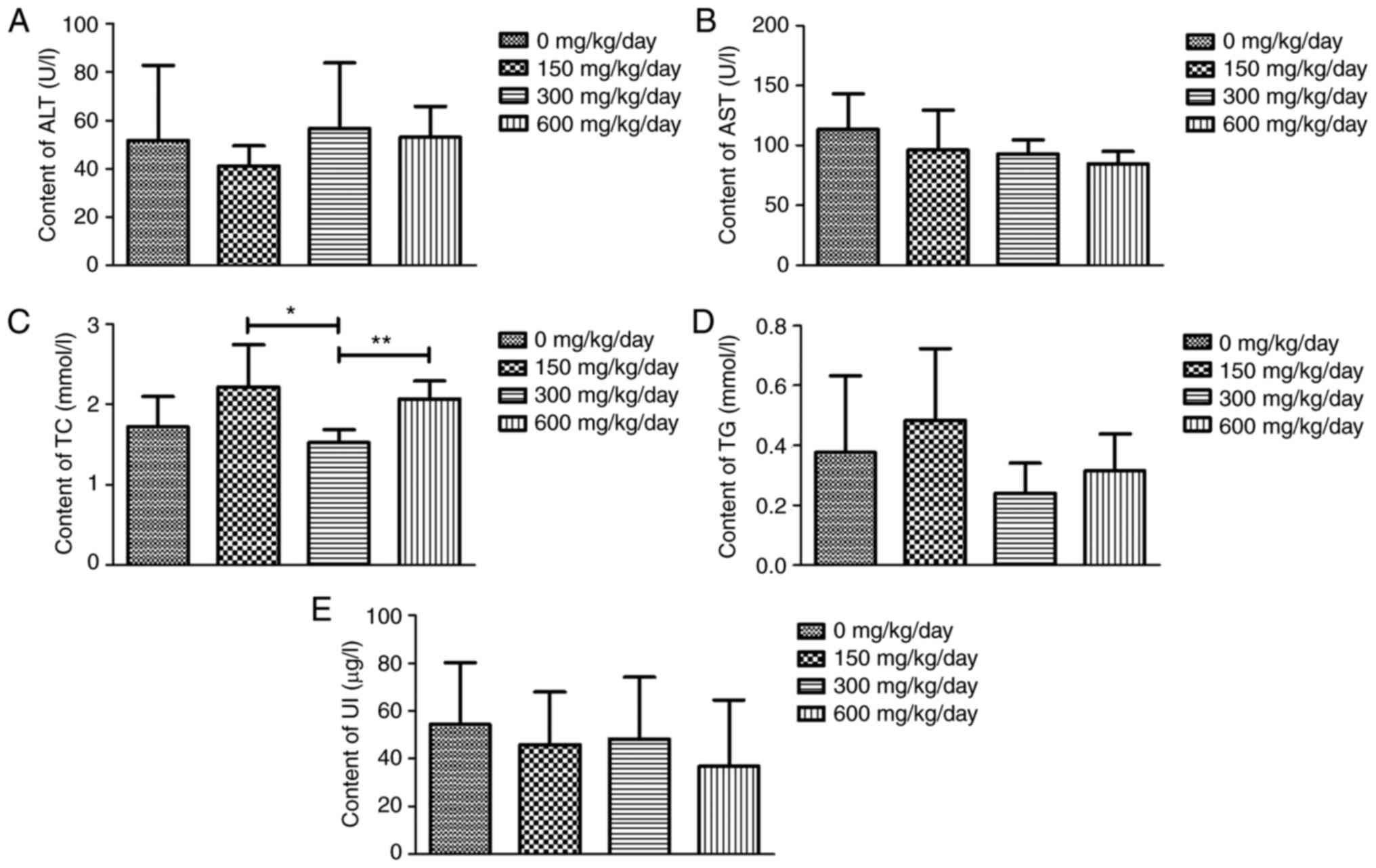 | Figure 4.Effects of DEHP exposure on
biochemical indices. (A) ALT, (B) AST, (C) TC, (D) TG and (E) UI.
*P<0.05, **P<0.01 vs. the control group; n=10 rats per group.
DEHP, di (2-ethylhexyl) phthalate; ALT, alanine aminotransferase;
AST, aspartate aminotransferase; TC, total cholesterol; TG,
triglyceride; UI, urine iodine. |
Effects of exposure to DEHP on
THs
As revealed in Fig.
5, no significant difference was observed in levels of THs
(FT4, TRH, TTR and TSAb) in DEHP treatment compared with the
control group (P>0.05). No changes were observed with 150
mg/kg/day DEHP (a no effect level) and that for FT3 and T4, the
effects were not dose-dependent. However, levels of T4 and FT3 in
the 300 mg/kg/day dose group were significantly decreased compared
with that of the control group (P<0.05). Levels of T3 and TSH in
the 600 mg/kg/day dose group were also significantly different
compared with the control group (P<0.05).
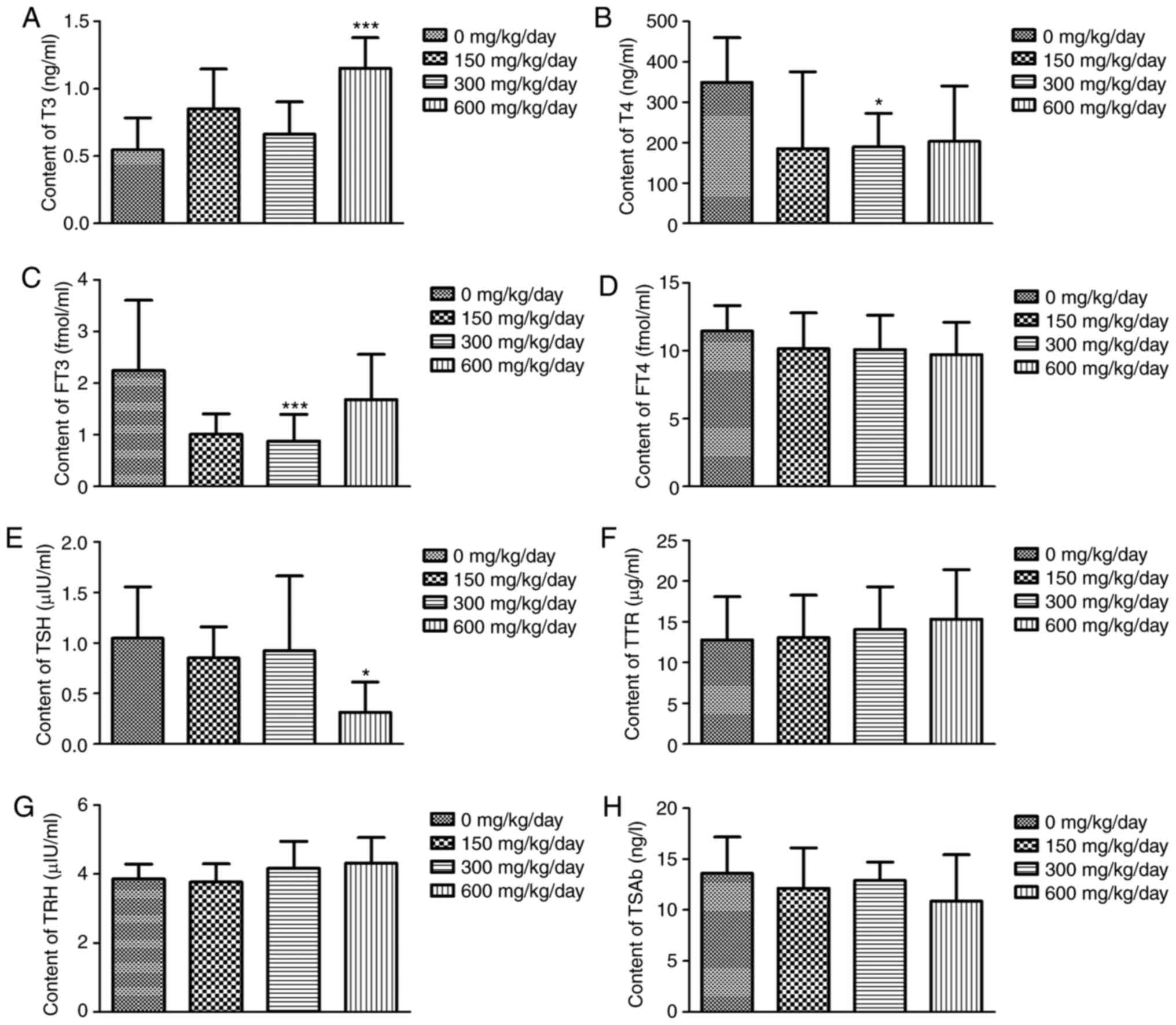 | Figure 5.Changes in THs in different groups.
Data are presented as the mean ± standard error of the mean of
three independent experiments. (A) T3, (B) T4, (C) FT3, (D) FT4,
(E) TSH, (F) TTR, (G) TRH and (H) TSAb. *P<0.05, ***P<0.001
vs. the control group; n=10 rats per group. THs, thyroid hormones;
T3, triiodothyronine; T4, tetraiodothyronine; FT3, free
triiodothyronine; FT4, serum free thyroxine; TSH,-stimulating
hormone; TRH, thyrotropin-releasing hormone; TTR, serum
transthyretin protein; TSAb, thyroid-stimulating antibody. |
DEHP exposure leads to histological
changes
Analysis of histological changes revealed altered TH
levels following DEHP exposure. H&E staining demonstrated that
the follicular epithelium was cube-shaped in the control group,
colloids were uniform, most of the thyroid gland was normal, only
some epithelium was shed and inflammatory cell infiltration was
evident (Fig. 6A). In the low-dose
group, the follicular epithelium was also cube-shaped, part of the
epithelial cytoplasm was loose and epithelial shedding was evident
(Fig. 6B). In the medium dose
group, part of the follicular epithelium exhibited signs of
necrosis, follicular collapse was observed along with inflammatory
cell infiltration and residual follicular epithelial vacuolar
degeneration was apparent. In addition, the cytoplasm appeared
foamy and vacuolated and enlarged follicular epithelial cells were
noticeable (Fig. 6C). In the 600
mg/kg/day dose group, most of the thyroid follicles were collapsed
and disappeared, inflammatory cell infiltration was advanced, part
of the filter follicle epithelium was shed and follicle epithelium
vacuolar degeneration was even more pronounced. Additionally, the
nucleus was deformed while the nuclear membrane was shrunken and
chromatin was aggregated. The expansion of the rough endoplasmic
reticulum was also evident, along with the appearance of vacuoles
(Fig. 6D).
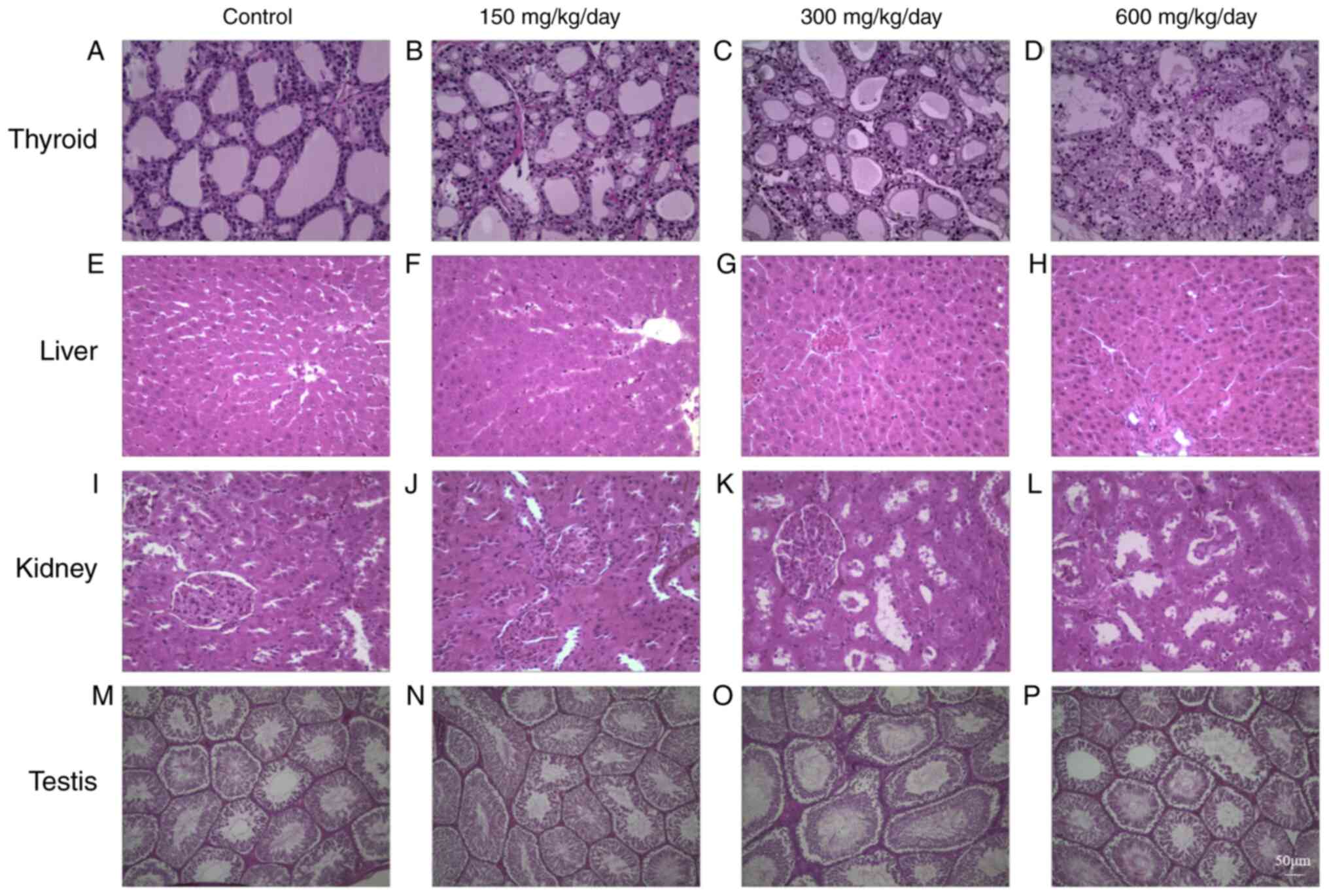 | Figure 6.Effects of DEHP on the histology of
(A-D) thyroid, (E-H) liver, (I-L) kidney and (M-P) testis. (A, E, I
and M) 0 mg/kg/day DEHP; (B, F, J and N) 150 mg/kg/day DEHP; (C, G,
K and O) 300 mg/kg/day DEHP; (D, H, L and P) 600 mg/kg/day DEHP.
Scale bar, 50 µm; magnification, ×200. DEHP, di (2-ethylhexyl)
phthalate. |
In Fig. 6E the liver
tissue of the control group is presented. In the 150 mg/kg/day dose
group, liver lobular structure is clear, hepatocytes are arranged
neatly, but partly bulked (Fig.
6F). In 300 mg/kg/day dose, a small amount of fatty liver cells
and punctate necrosis of the hepatocytes occasionally occurred
(Fig. 6G). In the 600 mg/kg/day
dose group, focal necrosis of hepatocytes, vacuum, hepatic
sinusoidal dilation, obvious hydrodegeneration and occasional
apoptotic hepatocytes were observed (Fig. 6H).
Histological studies of the kidneys in the control
group revealed normal glomeruli and tubules (Fig. 6I). In the 150 mg/kg/day group, the
glomeruli were contracted slightly and the tubular epithelial cells
expanded (Fig. 6J). The glomerular
epithelium of the 300 and 600 mg/kg/day group swelled, some of the
renal tubules disappeared and protein components were visible in
the tubes (Fig. 6K and L).
In the 0 and 150 mg/kg/day DEHP-induced group, the
testes tissue was well organized with an intact epithelium
(Fig. 6M and N). Exposure to DEHP
at 300 and 600 mg/kg/day caused the seminiferous tubules at all
levels of the seminiferous epithelium to be disorderly arranged
with few layers, disintegration of germinal epithelial and
reduction of round spermatozoa in the seminiferous tubules
(Fig. 6O and P).
DEHP influences the mRNA expression
levels of TSH/TSHR signaling pathway-related genes
Results for thyroid gene expression are presented in
Fig. 7. The mRNA levels of PDS,
NIS, PAX-8, TPO, TTF-1 and Tg were significantly increased compared
with the control group after DEHP treatment. mRNA levels of TSHr,
D1 and TTF-2 significantly decreased compared with the control
following DEHP treatment (P<0.05). Exposure to DEHP caused an
initial decrease in mRNA expression of pituitary TSH-β and
significant differences between 150 and 600 mg/kg/day groups
(P<0.05) and 150 and 300 mg/kg/day groups (P<0.05). The mRNA
levels of hypothalamus TRHr were increased after DEHP treatment,
with significant differences between 600 mg/kg/day and the control
group (P<0.01) and between 150 and 600 mg/kg/day groups
(P<0.01).
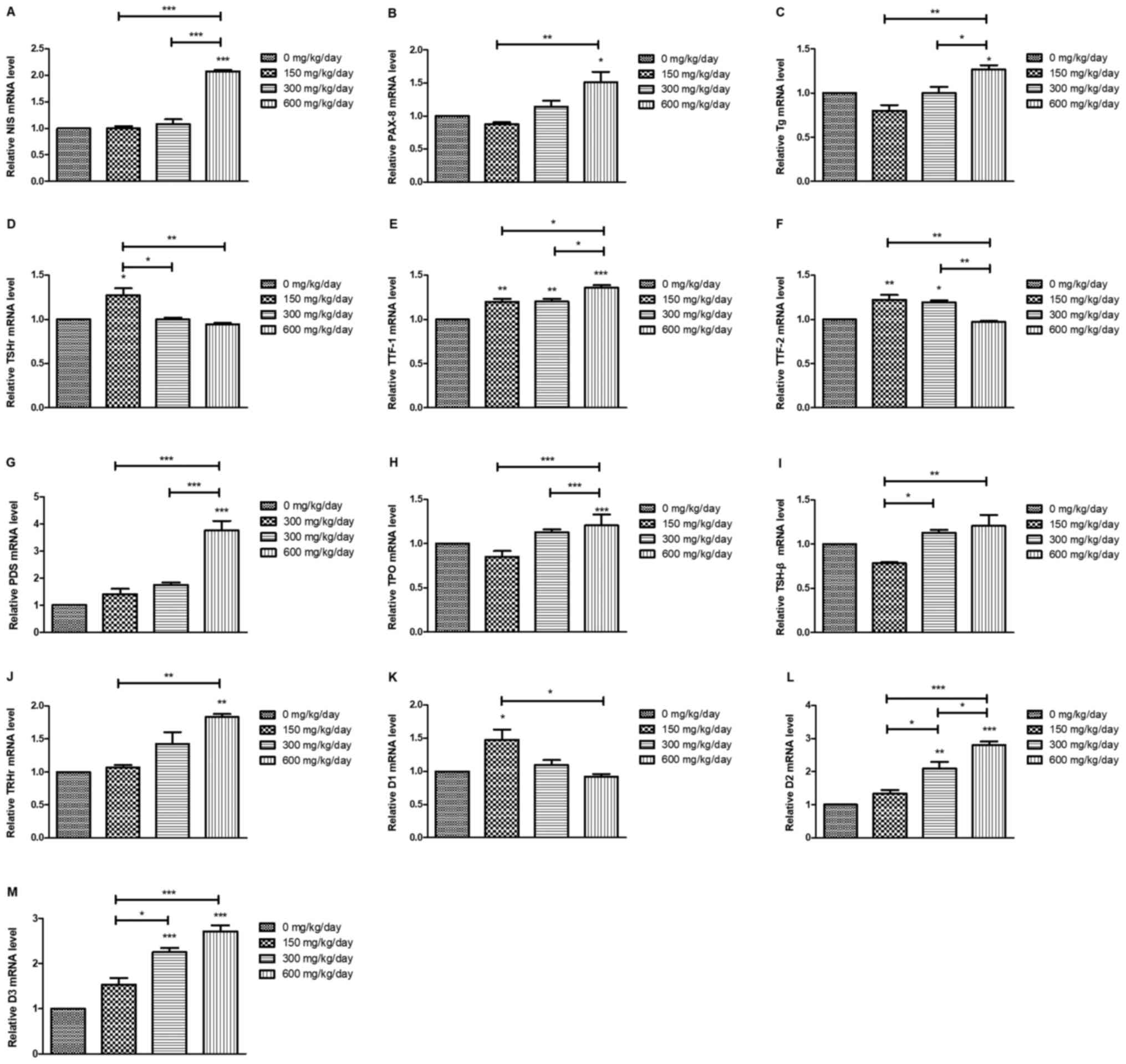 | Figure 7.Thyroid gene expression levels in
rats after DEHP treatment. (A) NIS, (B) PAX-8, (C) Tg, (D) TSHr,
(E) TTF-1, (F) TTF-2, (G) PDS, (H) TPO, (I) TSH-β, (J) TRHr, (K)
D1, (L) D2 and (M) D3. *P<0.05, **P<0.01, ***P<0.001 vs.
the control group. DEHP, di (2-ethylhexyl) phthalate; NIS, sodium
iodide symporter; PAX-8, paired box protein 8; Tg, thyroglobulin;
TSHr, stimulating hormone receptor; TTF-1, thyroid transcription
factor 1; TTF-2, thyroid transcription factor 2; PDS, pendrin
protein; TPO, thyroperoxidase; TSH-β, thyroid-stimulating hormone
β; TRHr, thyrotropin-releasing hormone receptor; D1, deiodinases 1;
D2, deiodinases 2; D3, deiodinases 3. |
DEHP influences the abundance of
proteins associated with TSH/TSHR signaling pathways
Compared with that of the control group, the protein
levels of NIS, PAX-8, Tg, TTF-1 and TPO were significantly
increased after DEHP treatment (Fig.
8). TSHr protein levels significantly decreased compared with
that of the control group following DEHP treatment (P<0.05). The
protein levels of pituitary TSH-β were increased after DEHP
treatment and significant differences between 150 and 600 mg/kg/day
groups (P<0.05) and between 300 and 600 mg/kg/day groups
(P<0.05) were observed.
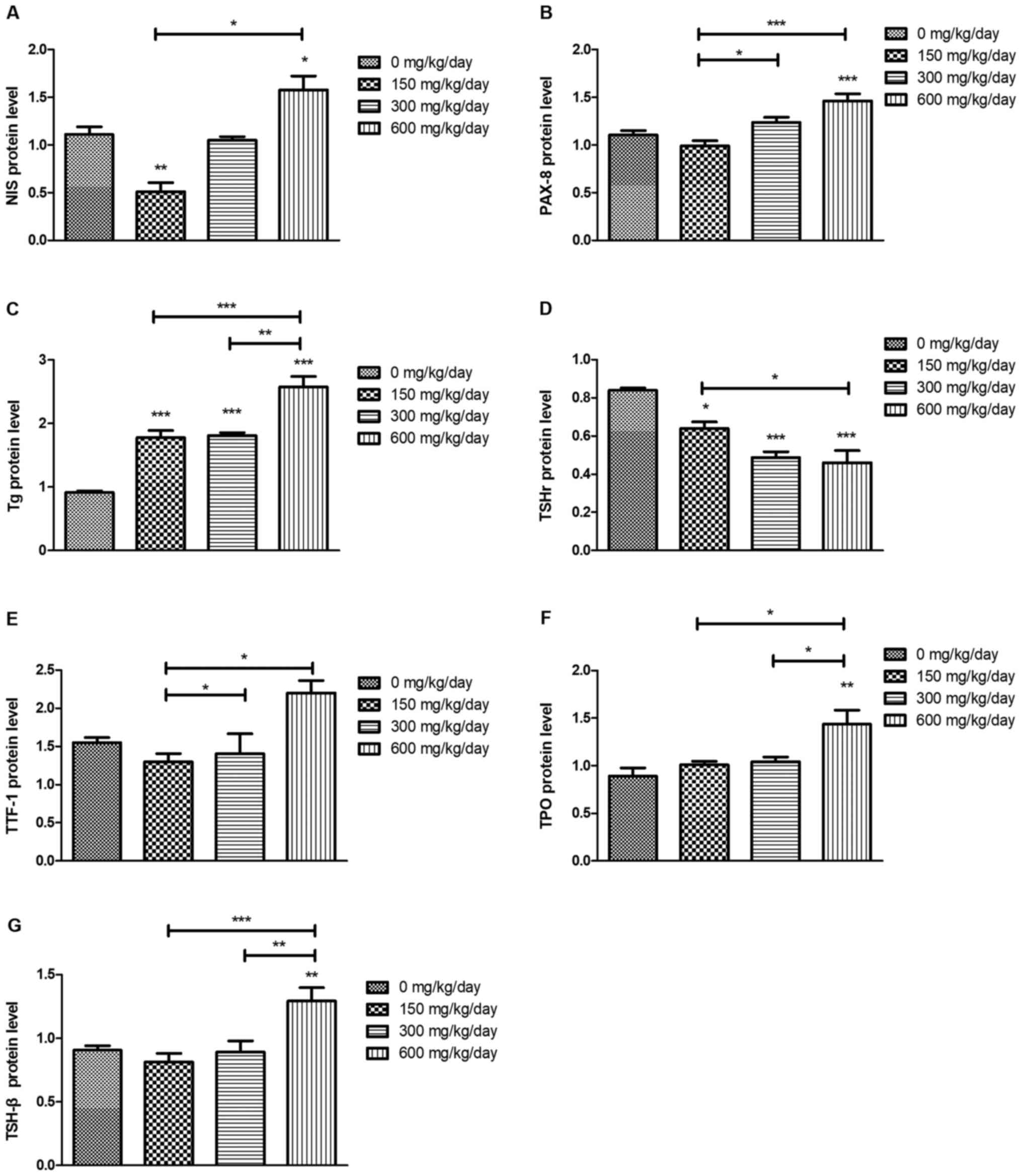 | Figure 8.Abundance of proteins associated with
the HPT axis following DEHP treatment. (A) NIS, (B) PAX-8, (C) Tg,
(D) TSHr, (E) TTF-1, (F) TPO and (G) TSH-β. *P<0.05,
**P<0.01, ***P<0.001 vs. the control group. HPT,
hypothalamic-pituitary-thyroid; DEHP, di (2-ethylhexyl) phthalate;
NIS, sodium iodide symporter; PAX-8, paired box protein 8; Tg,
thyroglobulin; TSHr, stimulating hormone receptor; TTF-1, thyroid
transcription factor 1; TPO, thyroperoxidase; TSH-β,
thyroid-stimulating hormone β. |
Discussion
The normal thyroid function of the human body is
able to maintain the function of the thyroid itself while
establishing a properly functioning HPT axis (48). Thyroid indicators are intricate and
interrelated and one or several indicators may be up- or
downregulated following interference by exogenous chemicals
(49). DEHP can interfere with the
HPT axis at varying levels and thereby alter thyroid function
through numerous potential mechanisms, including inducing thyroid
oxidative injury, disrupting TH homeostasis, damaging hormone
receptors and modifying the mechanisms of transporter proteins and
cellular uptake (50).
In view of the morphological and histological
changes, the results of the present study suggested that DEHP may
damage the follicles and nuclei. In addition, various organelles of
cells may also be damaged. The exposure to DEHP was dose-dependent
and under different doses, follicular cell division increased, the
nucleus became more confined and some functional organelles
gradually disappeared. These findings indicated the disintegration
of thyroid follicular cells, which may lead to reduced absorption
of iodide from the blood, phenolic coupling, iodination of proteins
and endocytosis, resulting in altered signal transduction and
disturbance to biosynthesis and bio-transportation of THs (51).
In vertebrates, numerous biological processes are
carefully regulated by THs and the HPT axis and serve an essential
role in hormone biosynthesis and release (52). For example, a potential interaction
between the thyroid and stress systems in the context of fetal
brain development has been previously reported (53). A biologically based
dose-response-HPT axis model is linked with physiologically based
pharmacokinetic models for thyroid-active chemicals in the adult
rat (54). TRH (secreted from the
hypothalamus) binds to the TRHr and is not only important in the TH
synthesis and stimulation of TSH (secreted from the pituitary)
release, but also serves an essential role in the TH feedback loop
of the HPT axis in animal models (55). During the oxidation of iodine in the
follicular lumen, tyrosine [3-monoiodotyrosine (MIT);
3,5-diiodotyrosine (DIT)] is iodized and iodotyrosine is coupled to
the tyrosine residues of Tg, eventually leading to the effects of
T3 and T4 synthesis (56). Changes
in deiodinase mRNA levels can be used as sensitive markers for
detecting TH disruptions (57). D1
has a considerable influence on iodine recovery and TH degradation;
D2 exclusively catalyzes outer-ring deiodination of T4 into active
T3, consequently controlling the intracellular concentration of T3
(58). D3 has different catalytic
properties, including inactivating enzymes, preventing T4
activation and terminating T3 action (48). Hence, changes of deiodinase mRNA
levels have been revealed to coincide with deiodinase enzyme
activities (59).
The present study measured gene expression and
protein abundance associated with the synthesis and secretion of
THs. Iodine is an essential constituent regulating THs (60). NIS, a plasma membrane glycoprotein,
is a special active transporter that mediates I−
accumulation and breakdown and serves a crucial function in the
initial biosynthesis of THs (61).
Since iodine is acted on by TPO, the results of the present study
revealed abnormal urine iodide following DEHP exposure (62). In the HPT axis, TSH-β serves an
important role and assessment of TSH-β gene transcription can be
used to evaluate DEHP-induced thyroid dysfunction (63). The present study measured both
protein and mRNA levels for TSH-β, TTF-1, NIS, TRHr and TSH
following DEHP exposure, but in some instances 150 mg/kg/day
appeared to exhibit no notable effect on the levels, which
suggested that the HPT axis was activated at higher concentrations
of DEHP (64). Hence, all
biosynthesis and release steps are stimulated, including Tg
iodination, with increased serum TSH levels (65). Additionally, TTF-1 can regulate Tg
iodination in the follicular cavity, leading to upregulation of
TSHr and PAX-8. These changes in thyroid tissue confirm that DEHP
alters the follicle sensitivity of TSH/TSHR signalling (66). Collectively, these results indicate
that DEHP induces morphological and physiological changes in the
thyroid. Thus, DEHP disrupts the bio-transportation of THs through
TSH/TSHR signaling.
The present study demonstrated that DEHP regulated
the redox status of biological systems, biotransformation,
biotransport and receptor levels of THs and thereby disrupted the
delicate balance of the HPT axis. In addition, the findings of the
present study contributed to an improved understanding of
thyrotoxicity caused by phthalates.
It is unclear whether certain endocrine disruptors
affect thyroid function by altering thyroid growth, or by
interacting with the production of anti-thyroid antibodies and
other substances that are important in thyroid metabolism (67). In addition, individuals are
simultaneously exposed to various endocrine disruptors, hence mixed
effects of certain endocrine disruptors may differ from effects of
exposure to DEHP alone on thyroid function (68). DEHP could contribute to an
environmental risk factor for changes of the endocrine system and
pathogenesis of thyroid dysfunction. Thus, further research is
required to elucidate the mechanisms by which DEHP disrupts hormone
homeostasis and to emphasize the importance of evaluating
DEHP-induced environmental risks.
Acknowledgements
The authors would like to thank Professor Kun Ma, Dr
Siqi Jia and Dr Mingzhe Zhao from Harbin Medical University
(Harbin, China) for editing the manuscript.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 81273079).
Availability of data and materials
The data sets that are used and/or analysed in the
current study can be reasonably obtained from the corresponding
authors.
Authors' contributions
XN, HW and WZ designed the experiments. HW and WZ
performed the experiments and the data analysis. YZ, ZK and XM
contributed to data collection and analysis. HW wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All protocols were performed in accordance with the
Regulations of the Ethical Committee for Research on Laboratory
Animals, as assessed and approved by the Ministry of Health of
China and the Institute of Zoology Animal and Medical Ethics
Committee of Harbin Medical University, which complies with
National Institutes of Health Guidelines. Subsequent experiments
were conducted under the strict principles of Good Laboratory
Practice to ensure good quality in vivo toxicology
studies.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
CAT
|
catalase
|
|
D1
|
deiodinases 1
|
|
D2
|
deiodinases 2
|
|
D3
|
deiodinases 3
|
|
FT3
|
free triiodothyronine
|
|
FT4
|
serum free thyroxine
|
|
GSH
|
glutathione
|
|
MDA
|
malondialdehyde
|
|
NIS
|
sodium iodide symporter
|
|
PAX-8
|
paired box protein 8
|
|
PDS
|
pendrin protein
|
|
SOD
|
superoxide dismutase
|
|
T3
|
triiodothyronine
|
|
T4
|
tetraiodothyronine
|
|
T-AOC
|
total antioxidant capacity
|
|
TC
|
total cholesterol
|
|
Tg
|
thyroglobulin
|
|
TG
|
triglyceride
|
|
TH
|
thyroid hormone
|
|
TPO
|
thyroperoxidase
|
|
TRH
|
thyrotropin-releasing hormone
|
|
TRHr
|
thyrotropin-releasing hormone
receptor
|
|
TSAb
|
thyroid-stimulating antibody
|
|
TSHr
|
thyroid-stimulating hormone
receptor
|
|
TSH-β
|
thyroid-stimulating hormone β
|
|
TTF-1
|
thyroid transcription factor 1
|
|
TTF-2
|
thyroid transcription factor 2
|
|
TTR
|
serum transthyretin protein
|
References
|
1
|
Jabbar A, Pingitore A, Pearce SH, Zaman A,
Iervasi G and Razvi S: Thyroid hormones and cardiovascular disease.
Nat Rev Cardiol. 14:39–55. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garmendia Madariaga A, Santos Palacios S,
Guillén-Grima F and Galofre JC: The incidence and prevalence of
thyroid dysfunction in Europe: A meta-analysis. J Clin Endocrinol
Metab. 99:923–931. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
UE: Regulation (EC) No 1907/2006 of the
european parliament and of the council of 18 december 2006
concerning the registration, evaluation, authorisation and
restriction of chemicals (REACH). 2006, https://eur-lex.europa.eu/eli/reg/2006/1907/2014-04-10
|
|
4
|
Chaker L, Bianco AC, Jonklaas J and
Peeters RP: Hypothyroidism. Lancet. 390:1550–1562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Colella M, Cuomo D, Giacco A, Mallardo M,
De Felice M and Ambrosino C: Thyroid hormones and functional
ovarian reserve: Systemic vs. Peripheral dysfunctions. J Clin Med.
9:16792020. View Article : Google Scholar
|
|
6
|
Tomer Y: Genetic susceptibility to
autoimmune thyroid disease: Past, present, and future. Thyroid.
20:715–725. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brent GA: Environmental exposures and
autoimmune thyroid disease. Thyroid. 20:755–761. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lamb JC IV, Boffetta P, Foster WG, Goodman
JE, Hentz KL, Rhomberg LR, Staveley J, Swaen G, Van Der Kraak G,
Williams AL; Comments on the opinions published by Bergman, ; et
al: (2015) on critical comments on the WHO-UNEP state of the
science of endocrine disrupting chemicals (Lamb et al.,
2014). Regul Toxicol Pharmacol. 73:754–757. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ema M and Miyawaki E: Adverse effects on
development of the reproductive system in male offspring of rats
given monobutyl phthalate, a metabolite of dibutyl phthalate,
during late pregnancy. Reprod Toxicol. 15:189–194. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pan G, Hanaoka T, Yoshimura M, Zhang S,
Wang P, Tsukino H, Inoue K, Nakazawa H, Tsugane S and Takahashi K:
Decreased serum free testosterone in workers exposed to high levels
of di-n-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP):
A cross-sectional study in China. Environ Health Perspect.
114:1643–1648. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Api AM: Toxicological profile of diethyl
phthalate: A vehicle for fragrance and cosmetic ingredients. Food
Chem Toxicol. 39:97–108. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kavlock R, Boekelheide K, Chapin R,
Cunningham M, Faustman E, Foster P, Golub M, Henderson R, Hinberg
I, Little R, et al: NTP center for the evaluation of risks to human
reproduction: Phthalates expert panel report on the reproductive
and developmental toxicity of di-n-butyl phthalate. Reprod Toxicol.
16:489–527. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Howarth JA, Price SC, Dobrota M, Kentish
PA and Hinton RH: Effects on male rats of di-(2-ethylhexyl)
phthalate and di-n-hexylphthalate administered alone or in
combination. Toxicol Lett. 121:35–43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boas M, Feldt-Rasmussen U and Main KM:
Thyroid effects of endocrine disrupting chemicals. Mol Cell
Endocrinol. 355:240–248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weuve J, Sánchez BN, Calafat AM, Schettler
T, Green RA, Hu H and Hauser R: Exposure to phthalates in neonatal
intensive care unit infants: Urinary concentrations of monoesters
and oxidative metabolites. Environ Health Perspect. 114:1424–1431.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
McLeod DS and Cooper DS: The incidence and
prevalence of thyroid autoimmunity. Endocrine. 42:252–265. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Earls AO, Axford IP and Braybrook JH: Gas
chromatography-mass spectrometry determination of the migration of
phthalate plasticisers from polyvinyl chloride toys and childcare
articles. J Chromatogr A. 983:237–246. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gimeno P, Thomas S, Bousquet C, Maggio AF,
Civade C, Brenier C and Bonnet PA: Identification and
quantification of 14 phthalates and 5 non-phthalate plasticizers in
PVC medical devices by GC-MS. J Chromatogr B Analyt Technol Biomed
Life Sci. 949-950:99–108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kambia K, Dine T, Gressier B, Dupin-Spriet
T, Luyckx M and Brunet C: Evaluation of the direct toxicity of
trioctyltrimellitate (TOTM), di(2-ethylhexyl) phthalate (DEHP) and
their hydrolysis products on isolated rat hepatocytes. Int J Artif
Organs. 27:971–978. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sampson J and de Korte D: DEHP-plasticised
PVC: Relevance to blood services. Transfus Med. 21:73–83. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heudorf U, Mersch-Sundermann V and Angerer
J: Phthalates: Toxicology and exposure. Int J Hyg Environ Health.
210:623–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ghisari M and Bonefeld-Jorgensen EC:
Effects of plasticizers and their mixtures on estrogen receptor and
thyroid hormone functions. Toxicol Lett. 189:67–77. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hauser R and Calafat AM: Phthalates and
human health. Occup Environ Med. 62:806–818. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meeker JD and Ferguson KK: Relationship
between urinary phthalate and bisphenol A concentrations and serum
thyroid measures in U.S. adults and adolescents from the national
health and nutrition examination survey (NHANES) 2007–2008. Environ
Health Perspect. 119:1396–1402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marotta V, Russo G, Gambardella C, Grasso
M, Sala DL, Chiofalo MG, D'Anna R, Puzziello A, Docimo G, Masone S,
et al: Human exposure to bisphenol AF and diethylhexylphthalate
increases susceptibility to develop differentiated thyroid cancer
in patients with thyroid nodules. Chemosphere. 218:885–894. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rais-Bahrami K, Nunez S, Revenis ME, Luban
NL and Short BL: Follow-up study of adolescents exposed to
di(2-ethylhexyl) phthalate (DEHP) as neonates on extracorporeal
membrane oxygenation (ECMO) support. Environ Health Perspect.
112:1339–1340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Engel SM, Villanger GD, Nethery RC,
Thomsen C, Sakhi AK, Drover SSM, Hoppin JA, Zeiner P, Knudsen GP,
Reichborn-Kjennerud T, et al: Prenatal phthalates, maternal thyroid
function, and risk of attention-deficit hyperactivity disorder in
the norwegian mother and child cohort. Environ Health Perspect.
126:0570042018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gore AC, Chappell VA, Fenton SE, Flaws JA,
Nadal A, Prins GS, Toppari J and Zoeller RT: EDC-2: The endocrine
society's second scientific statement on endocrine-disrupting
chemicals. Endocr Rev. 36:E1–E150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
O'Shea PJ and Williams GR: Insight into
the physiological actions of thyroid hormone receptors from
genetically modified mice. J Endocrinol. 175:553–570. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ravera S, Reyna-Neyra A, Ferrandino G,
Amzel LM and Carrasco N: The sodium/iodide symporter (NIS):
Molecular physiology and preclinical and clinical applications.
Annu Rev Physiol. 79:261–289. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Postiglione MP, Parlato R,
Rodriguez-Mallon A, Rosica A, Mithbaokar P, Maresca M, Marians RC,
Davies TF, Zannini MS, De Felice M and Di Lauro R: Role of the
thyroid-stimulating hormone receptor signaling in development and
differentiation of the thyroid gland. Proc Natl Acad Sci USA.
99:15462–15467. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dohán O, De la Vieja A, Paroder V, Riedel
C, Artani M, Reed M, Ginter CS and Carrasco N: The sodium/iodide
symporter (NIS): Characterization, regulation, and medical
significance. Endocr Rev. 24:48–77. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu C, Zhao L, Wei L and Li L: DEHP
reduces thyroid hormones via interacting with hormone
synthesis-related proteins, deiodinases, transthyretin, receptors,
and hepatic enzymes in rats. Environ Sci Pollut Res Int.
22:12711–12719. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Breous E, Wenzel A and Loos U: The
promoter of the human sodium/iodide symporter responds to certain
phthalate plasticisers. Mol Cell Endocrinol. 244:75–78. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shen O, Du G, Sun H, Wu W, Jiang Y, Song L
and Wang X: Comparison of in vitro hormone activities of selected
phthalates using reporter gene assays. Toxicol Lett. 191:9–14.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wenzel A, Franz C, Breous E and Loos U:
Modulation of iodide uptake by dialkyl phthalate plasticisers in
FRTL-5 rat thyroid follicular cells. Mol Cell Endocrinol.
244:63–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Johns LE, Ferguson KK, McElrath TF,
Mukherjee B and Meeker JD: Associations between repeated measures
of maternal urinary phthalate metabolites and thyroid hormone
parameters during pregnancy. Environ Health Perspect.
124:1808–1815. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yao HY, Han Y, Gao H, Huang K, Ge X, Xu
YY, Xu YQ, Jin ZX, Sheng J, Yan SQ, et al: Maternal phthalate
exposure during the first trimester and serum thyroid hormones in
pregnant women and their newborns. Chemosphere. 157:42–48. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang HB, Pan WH, Chang JW, Chiang HC, Guo
YL, Jaakkola JJK and Huang PC: Does exposure to phthalates
influence thyroid function and growth hormone homeostasis? The
Taiwan environmental survey for toxicants (TEST) 2013. Environ Res.
153:63–72. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Luongo C, Dentice M and Salvatore D:
Deiodinases and their intricate role in thyroid hormone
homeostasis. Nat Rev Endocrinol. 15:479–488. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
David RM, Moore MR, Finney DC and Guest D:
Chronic toxicity of di(2-ethylhexyl)phthalate in rats. Toxicol Sci.
55:433–443. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Reagan-Shaw S, Nihal M and Ahmad N: Dose
translation from animal to human studies revisited. FASEB J.
22:659–661. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pisoschi AM and Pop A: The role of
antioxidants in the chemistry of oxidative stress: A review. Eur J
Med Chem. 97:55–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yaping Z, Dongxing Y, Jixiang C, Tianshiu
L and Huiqin C: Spectrophotometric determination of urinary iodine
by flow-injection analysis with on-line catalytic digestion. Clin
Chem. 42:2021–2027. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang H, Wu M, Yang L, Wu J, Hu Y, Han J,
Gu Y, Li X, Wang H, Ma L and Yang X: Evaluation of median urinary
iodine concentration cut-off for defining iodine deficiency in
pregnant women after a long term USI in China. Nutr Metab (Lond).
16:622019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ma K, Wu H, Li P and Li B: LC3-II may
mediate ATR-induced mitophagy in dopaminergic neurons through
SQSTM1/p62 pathway. Acta Biochim Biophys Sin (Shanghai).
50:1047–1061. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ortiga-Carvalho TM, Chiamolera MI,
Pazos-Moura CC and Wondisford FE: Hypothalamus-pituitary-thyroid
axis. Compr Physiol. 6:1387–1428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yilmaz B, Terekeci H, Sandal S and
Kelestimur F: Endocrine disrupting chemicals: Exposure, effects on
human health, mechanism of action, models for testing and
strategies for prevention. Rev Endocr Metab Disord. 21:127–147.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Boas M, Feldt-Rasmussen U, Skakkebaek NE
and Main KM: Environmental chemicals and thyroid function. Eur J
Endocrinol. 154:599–611. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ye H, Ha M, Yang M, Yue P, Xie Z and Liu
C: Di2-ethylhexyl phthalate disrupts thyroid hormone homeostasis
through activating the Ras/Akt/TRHr pathway and inducing hepatic
enzymes. Sci Rep. 7:401532017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Vander JA, Luciano DS and Sherman JH:
Human physiology: The Mechanism of Body Function. William C Brown
Pub. Subsequent Edition (January 1, 1998).
|
|
53
|
Moog NK, Entringer S, Heim C, Wadhwa PD,
Kathmann N and Buss C: Influence of maternal thyroid hormones
during gestation on fetal brain development. Neuroscience.
342:68–100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
McLanahan ED, Andersen ME and Fisher JW: A
biologically based dose-response model for dietary iodide and the
hypothalamic-pituitary-thyroid axis in the adult rat: Evaluation of
iodide deficiency. Toxicol Sci. 102:241–253. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mondal S, Raja K, Schweizer U and Mugesh
G: Chemistry and biology in the biosynthesis and action of thyroid
hormones. Angew Chem Int Ed Engl. 55:7606–7630. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Citterio CE, Targovnik HM and Arvan P: The
role of thyroglobulin in thyroid hormonogenesis. Nat Rev
Endocrinol. 15:323–338. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yamauchi I, Sakane Y, Yamashita T, Hirota
K, Ueda Y, Kanai Y, Yamashita Y, Kondo E, Fujii T, Taura D, et al:
Effects of growth hormone on thyroid function are mediated by type
2 iodothyronine deiodinase in humans. Endocrine. 59:353–363. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Maia AL, Kim BW, Huang SA, Harney JW and
Larsen PR: Type 2 iodothyronine deiodinase is the major source of
plasma T3 in euthyroid humans. J Clin Invest. 115:2524–2533. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bianco AC, Salvatore D, Gereben B, Berry
MJ and Larsen PR: Biochemistry, cellular and molecular biology, and
physiological roles of the iodothyronine selenodeiodinases. Endocr
Rev. 23:38–89. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Salvatore D, Simonides WS, Dentice M,
Zavacki AM and Larsen PR: Thyroid hormones and skeletal muscle-new
insights and potential implications. Nat Rev Endocrinol.
10:206–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Portulano C, Paroder-Belenitsky M and
Carrasco N: The Na+/I-symporter (NIS): Mechanism and medical
impact. Endocr Rev. 35:106–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ferrandino G, Kaspari RR, Reyna-Neyra A,
Boutagy NE, Sinusas AJ and Carrasco N: An extremely high dietary
iodide supply forestalls severe hypothyroidism in Na(+)/I(−)
symporter (NIS) knockout mice. Sci Rep. 7:53292017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ji C, Jin X, He J and Yin Z: Use of
TSHβ:EGFP transgenic zebrafish as a rapid in vivo model for
assessing thyroid-disrupting chemicals. Toxicol Appl Pharmacol.
262:149–155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kim MJ, Moon S, Oh BC, Jung D, Choi K and
Park YJ: Association between diethylhexyl phthalate exposure and
thyroid function: A meta-analysis. Thyroid. 29:183–192. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chiamolera MI and Wondisford FE:
Minireview: Thyrotropin-releasing hormone and the thyroid hormone
feedback mechanism. Endocrinology. 150:1091–1096. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
De Felice M, Postiglione MP and Di Lauro
R: Minireview: Thyrotropin receptor signaling in development and
differentiation of the thyroid gland: Insights from mouse models
and human diseases. Endocrinology. 145:4062–4067. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Taylor PN, Albrecht D, Scholz A,
Gutierrez-Buey G, Lazarus JH, Dayan CM and Okosieme OE: Global
epidemiology of hyperthyroidism and hypothyroidism. Nat Rev
Endocrinol. 14:301–316. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kortenkamp A: Low dose mixture effects of
endocrine disrupters and their implications for regulatory
thresholds in chemical risk assessment. Curr Opin Pharmacol.
19:105–111. 2014. View Article : Google Scholar : PubMed/NCBI
|