Introduction
Coronary artery disease (CAD) is the most common
clinical manifestation of ischaemic heart disease and a leading
cause of mortality worldwide (1,2). A
characteristic feature of CAD is the formation of atherosclerotic
plaques, leading to stenosis or obstruction of the coronary artery.
Endothelial dysfunction has been implicated as the initiating
factor and key event in the pathogenesis of atherosclerosis
(3). Therefore, restoration of
endothelial activity may prevent the progression of CAD and
decrease cardiovascular events.
Circulating endothelial progenitor cells (EPCs)
promote neovascularization, repair endothelial injury and improve
endothelial function (4,5). Circulating EPCs are impaired in
patients with CAD (5) and studies
have reported an inverse correlation between the number of
circulating EPCs and Syntax score (6,7).
Derived from coronary anatomy and lesion characteristics, the
Syntax score was devised to grade the anatomical complexity and
severity of coronary lesions in patients with CAD (8–11).
Moreover, it has been reported as an independent predictor of
long-term major adverse cardiac and cerebrovascular events in
patients treated with percutaneous coronary intervention (8,12,13).
However, the association between the activity of EPCs, endothelial
function and Syntax score in patients with CAD is still not
clear.
Nitric oxide (NO) serves an important role in the
regulation of EPC function and can promote the mobilization,
proliferation and migratory activities of circulating EPCs
(14–16). Previous studies have shown that the
level of NO in plasma or secreted by EPCs were correlated to the
activity of circulating EPCs and FMD (17,18).
Based on the aforementioned studies, it was hypothesized that
circulating EPC and endothelial function may be negatively
correlated with Syntax score in patients with CAD and may be
associated with decreased NO production. Therefore, the present
study detected the number and function of circulating EPCs,
measured endothelial function and evaluated levels of NO in plasma
or culture medium in patients with different Syntax scores.
Moreover, the present study detected the correlation between
flow-mediated dilatation (FMD) or circulating EPCs and Syntax
score.
Materials and methods
Characteristics of patients
A total of 60 patients with CAD were enrolled in 3
groups according to syntax score: Low-(Syntax score <22 points;
n=20; 10 male patients and 10 female patients; age, 45–75 years),
middle-(Syntax score, 22–32 points; n=20; 10 male patients and 10
female patients; age, 45–75 years) and high-risk (Syntax score
>32 points; n=20; 10 male patients and 10 female patients; age,
45–75 years) and 20 healthy subjects (10 male patients and 10
female patients; age, 45–75 years) were recruited as a control
group. Patients and healthy subjected were recruited between
January 2018 and January 2019. In addition, according to the number
of lesioned coronary arteries (anterior descending branch,
circumflex branch and right coronary artery), the patients were
divided into single, double and triple lesion vessel groups. The
patients with CAD were diagnosed by coronary angiography because of
stable angina pectoris admitted to our hospital (Jiangmen Central
Hospital). In consideration of the factors that influence the
number of EPCs, patients were excluded on the basis of smoking,
chronic renal insufficiency (serum creatinine ≥1.4 mmol/l),
abnormal liver function, infection, inflammation, diabetes,
myocardial infarction, elevated troponin-I or creatine kinase-MB
levels and malignant or autoimmune disease. Patients taking
antiplatelet, anti-inflammatory, or hypolipidaemic agents were also
excluded. Blood samples (50 ml) were taken for routine biochemistry
characteristics measurements and EPCs were isolated. Written
informed consent was obtained from all patients and healthy
subjects and the protocol conformed to the ethical guidelines of
the 1975 Declaration of Helsinki and was approved by the Jiangmen
Central Hospital Ethics Committee on Research on Humans.
Isolation of circulating EPCs
Blood samples (35 ml) were diluted with sterile PBS.
A total of 15 ml human lymphocyte separation solution (cat. no.
HY2015; Tianjin Haoyang Biological Products Technology Co., Ltd.)
was added into a 50-ml centrifuge tube (cat. no. 430829; Corning,
Inc.). A total of 25 ml diluted peripheral blood was added along
the tube wall before centrifugation at 811.7 × g at 4°C for 25 min.
A capillary pipette was used to transfer the clouded mononuclear
cell layer into another sterile 50-ml centrifuge tube. Then, 5 ml
erythrocyte lysate (cat. no. C3702-120 ml; Beyotime Institute of
Biotechnology) was added and left to stand for 10 min at 4°C. The
mixture was diluted with pre-cooled PBS buffer and mixed before
centrifugation at 377.3 × g at 4°C for 10 min. The upper layer was
removed using a capillary pipette and discarded. Following the
addition of 20 ml pre-cooled PBS buffer to the original centrifuge
tube, the mixture was centrifuged again at 377.3 × g at 4°C for 10
min and a capillary pipette was used to remove the upper layer.
Then, 10 ml pre-cooled PBS was added to the original centrifuge
tube and mixed well. The mixture was transferred to another sterile
50-ml centrifuge tube before centrifugation at 4°C at 377.3 × g for
10 min, and the upper layer was discarded. Isolated circulating
EPCs were then cultured in EGM-2 (cat. no. CC-3162; Beijing Bitab
Biotech Co., Ltd.) containing 20% high-quality fetal bovine serum
at 37°C for 7 days, 100 U/ml penicillin/streptomycin and 50 ng/ml
VEGF 50 ng/ml.
Identification and evaluation of
circulating EPCs
Circulating EPCs were identified via flow cytometry
analysis (FCA) and immunofluorescence microscopy.
CD45−(FITC)/CD34+(PE-Cy7)/KDR+(APC)
(cat. nos. FHF045-025, FHN034-025 and FHK309-025, respectively; all
Beijing 4A Biotech Co., Ltd.) were defined as EPCs by FCA.
Dil-acLDL+/FITC-lectin+ were defined as EPCs
by immunofluorescence microscopy. FCA and immunofluorescence
microscopy were performed as previously described (19–26).
Migration and proliferation ability of
EPCs
EPC migration was evaluated as previously described
(19–22). Proliferation activity was assessed
via MTT assay (cat. no. ab211091; Abcam) according to the
manufacturer's instructions and our previous studies (19,20,22,27,28).
Evaluation of EPC adhesive
activity
EPCs were digested with Parenzyme (cat. no. CCS001;
Tetra-n-Bo Biotechnology Co., Ltd.) at 37°C for 1 min and
resuspended in culture medium to a final density of
2×105/ml. A total of 500 µl (2×105/ml per
well) cell suspension was inoculated in 24-well plates precoated
with fibronectin (cat. no. 5050-1MG; Tetra-n-Bo Biotechnology Co.,
Ltd.) and incubated at 37°C for 30 min. After being washed twice
with PBS, EPCs were fixed with 4% polyformic acid for 10 min at
37°C. After two further washes with PBS, adherent EPCs were
observed under an inverted immunofluorescence microscope
(magnification, ×100). Adherent cells were counted in three
randomly selected fields of view.
Measurement of plasma NO levels and NO
secretion by EPCs
NO levels were measured using the NO levels test kit
(cat. no. A012-1-2; Nanjing Jiancheng Bioengineering Institute)
according to the manufacturer's instructions.
FMD and nitroglycerin-mediated
dilation (NMD)
FMD was measured as previously described (29,30).
For NMD, pressure in an upper-forearm sphygmomanometer cuff was
raised to 250 mmHg for 5 min. FMD was calculated as the percentage
increase in mean diastolic diameter after reactive hyperaemia at
55–65 sec after deflation to baseline. NMD was calculated as
follows: at 15 min after FMD measurement, 400 µg sublingual
nitroglycerin was administered and after a further 5 min, the
diastolic diameter was remeasured to assess endothelial-independent
dilatation.
Western blot analysis
Western blot analysis was performed as previously
described (18). Briefly, protein
concentrations were determined using the BCA method. Proteins (50
µg) were separated via 5% SDS-PAGE. Following blocking with 5%
skimmed milk powder was used at 4°C for 12 h, the membranes were
incubated at 4°C for 12 h with the following primary antibodies:
β-actin (1:2,000; cat. no. 3700T; Cell Signaling Technology Inc.),
endothelial NO synthase (eNOS; 1:1,000; cat. no. 9575; Cell
Signaling Technology, Inc.) and phosphorylated (p)-eNOS (1:1,000;
cat. no. 9571; Cell Signaling Technology, Inc.). Subsequently, the
membranes were incubated with a HRP-conjugated secondary antibody
(1:3,000; cat. no. 7074; Cell Signaling Technology, Inc.) at room
temperature for 2 h. Protein expression was semi-quantified using
ImageJ software (version 1.0; National Institutes of Health) with
β-actin as the loading control.
Statistical analysis
Data are presented as the mean ± SD from three
independent experiments. Data were analyzed using GraphPad Prism
6.0 software (GraphPad Software, Inc.). Comparisons between
multiple groups were analyzed by one-way ANOVA and post hoc Tukey's
test. The persons who analyzed the data were blinded to group
assignment. Univariate correlations were calculated using Pearson's
coefficient (r). P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
Patient characteristics are presented in Table I. Age, BMI, levels of cholesterol,
high- and low-density lipoprotein, triglycerides, plasma glucose,
and high-sensitivity C-reactive protein were not significantly
different between groups.
 | Table I.Clinical and biochemical
characteristics. |
Table I.
Clinical and biochemical
characteristics.
| Characteristic | Control (n=20) | Low-risk
(n=20) | Middle-risk
(n=20) | High-risk
(n=20) |
|---|
| Age, years | 55.30±12.90 | 55.90±13.70 | 56.70±13.50 | 57.40±14.50 |
| Weight, kg | 70.40±5.90 | 70.80±6.40 | 71.40±6.20 | 70.90±6.50 |
| AST, mmol/l | 70.80±8.40 | 71.00±7.90 | 71.80±7.50 | 72.40±7.10 |
| ALT, mmol/l | 25.60±6.40 | 26.90±6.10 | 26.70±6.10 | 25.40±6.90 |
| BUN, mmol/l | 23.50±8.90 | 24.90±6.80 | 23.40±7.10 | 24.00±7.30 |
| Cr, mmol/l | 5.60±1.30 | 5.40±1.70 | 5.60±1.80 | 5.70±1.50 |
| LDL, mmol/l | 70.30±13.50 | 71.30±13.90 | 72.80±11.60 | 72.90±16.90 |
| TC, mmol/l | 2.80±0.56 | 2.90±0.58 | 2.95±0.59 | 3.15±0.43 |
| HDL, mmol/l | 4.83±0.52 | 5.02±0.51 | 4.97±0.54 | 5.07±0.50 |
| TG, mmol/l | 1.36±0.28 | 1.42±0.27 | 1.43±0.24 | 1.44±0.21 |
| CRP, mmol/l | 102.10±20.70 | 103.00±23.90 | 107.20±22.60 | 104.70±23.40 |
| HR, beats/min | 72.30±8.50 | 71.80±9.20 | 73.30±7.80 | 73.10±9.30 |
| BMI | 23.10±2.30 | 23.50±2.20 | 23.30±2.50 | 23.40±2.70 |
| SBP, mmHg | 119.20±10.50 | 118.90±11.10 | 119.80±10.70 | 120.30±9.90 |
| DBP, mmHg | 69.50±5.00 | 70.90±4.80 | 71.90±3.80 | 70.50±5.50 |
Levels and activity of circulating
EPCs
EPCs were evaluated by fluorescence activated cell
sorting analysis (Fig. S1A) and
phase-contrast fluorescent microscope labeled by Dil-LDL and
FITC-lectin (Fig. S1B).
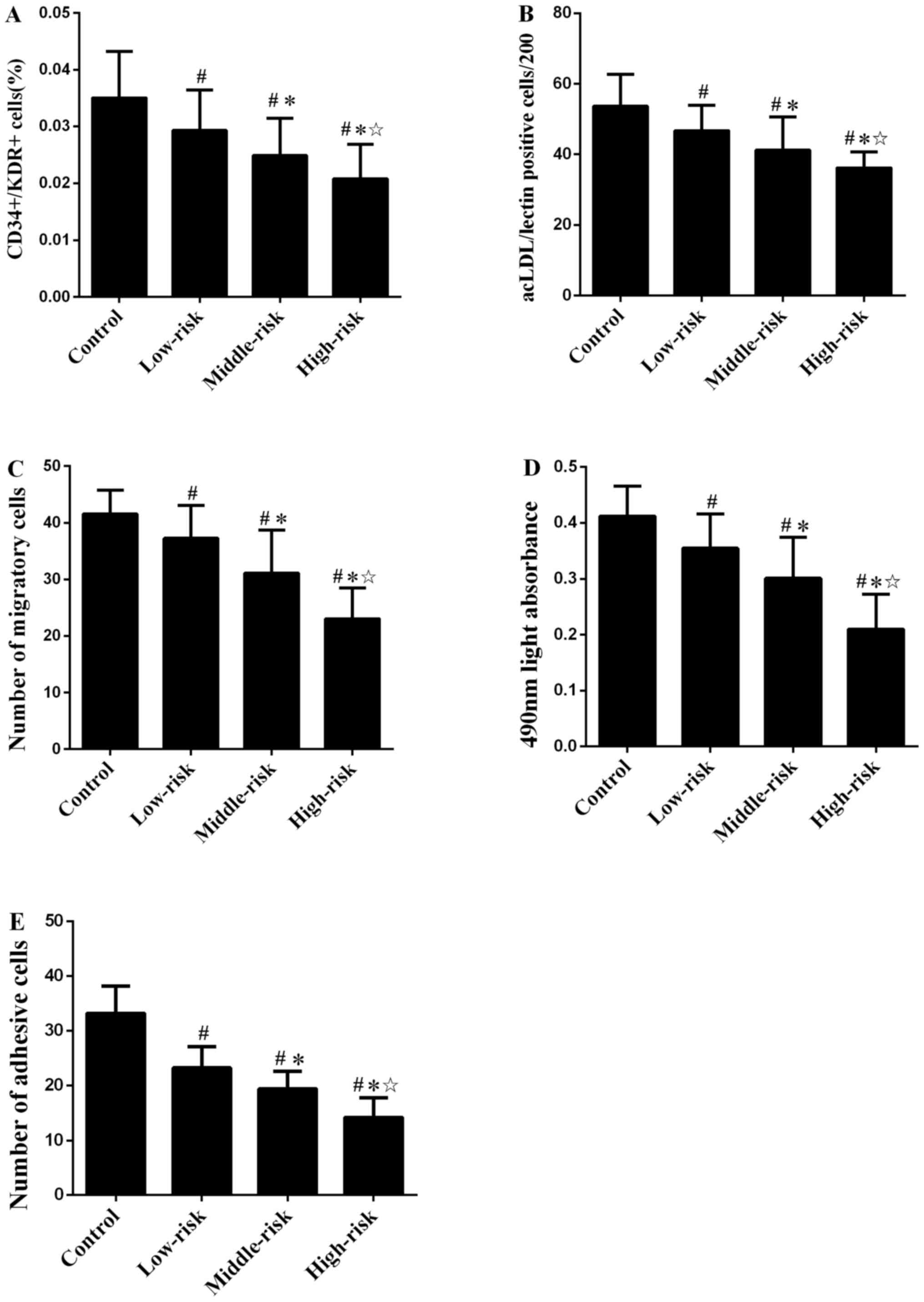
Number of circulating EPCs is shown in Fig. 1A and B. The levels of circulating
EPCs evaluated by FCA (Fig. S2)
and immunofluorescence microscopy in patients with CAD were
significantly lower compared with the control group. The number of
circulating EPCs in middle- and high-risk groups were decreased
compared with the low-risk group. The difference between middle-
and high-risk groups was also significant.
Migratory activity of EPCs in patients with CAD was
decreased compared with the control group (P<0.01; Figs. 1C and S3). Similarly, the proliferative and
adhesive activity (Fig. S4) of
circulating EPCs in patients with CAD were significantly impaired
compared with control group (both P<0.01; Fig. 1D and E). The migratory activity of
circulating EPCs in middle- and high-risk group were lower than in
the low-risk group. The migratory activity of circulating EPCs was
also significantly different between middle- and high-risk groups.
Similar results were observed for the proliferative and adhesive
function of circulating EPCs (Fig. 1D
and E).
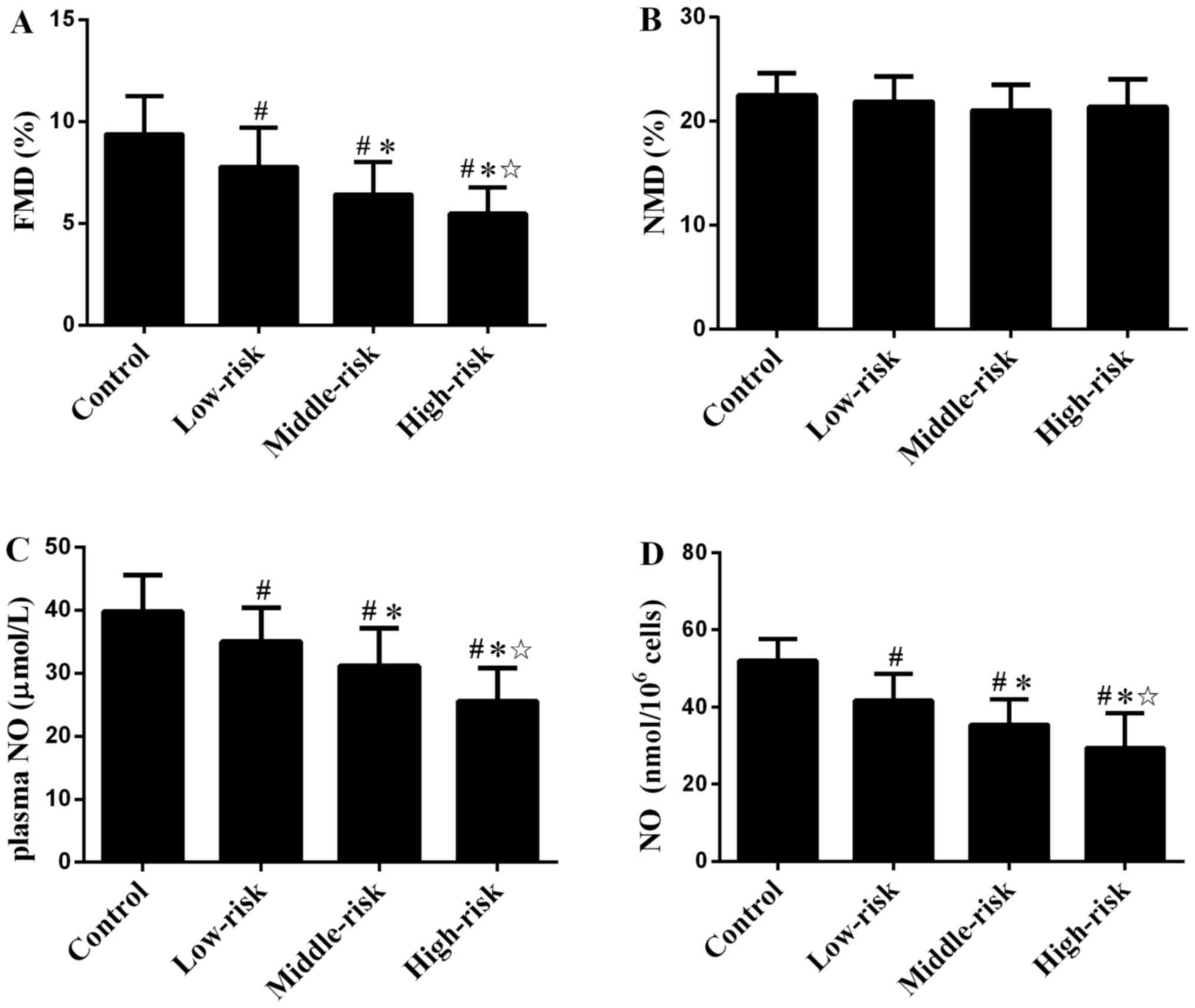
FMD and NMD
FMD in patients with CAD decreased compared with the
control group (Fig. 2A). The FMD in
middle- and high-risk group was lower than in the low-risk group.
FMD was also significantly different between the middle- and
high-risk groups. NMD (Fig. 2B)
showed no significant difference between groups.
Plasma NO levels and secretion by
EPCs
Plasma NO levels in patients with CAD were
significantly lower than in the Control group (Fig. 2C). The plasma NO levels in the
middle- and high-risk groups were lower than in the low-risk group.
The plasma NO levels were also significantly different between the
middle- and high-risk groups. Similar results were detected for NO
secretion by EPCs (Fig. 2D).
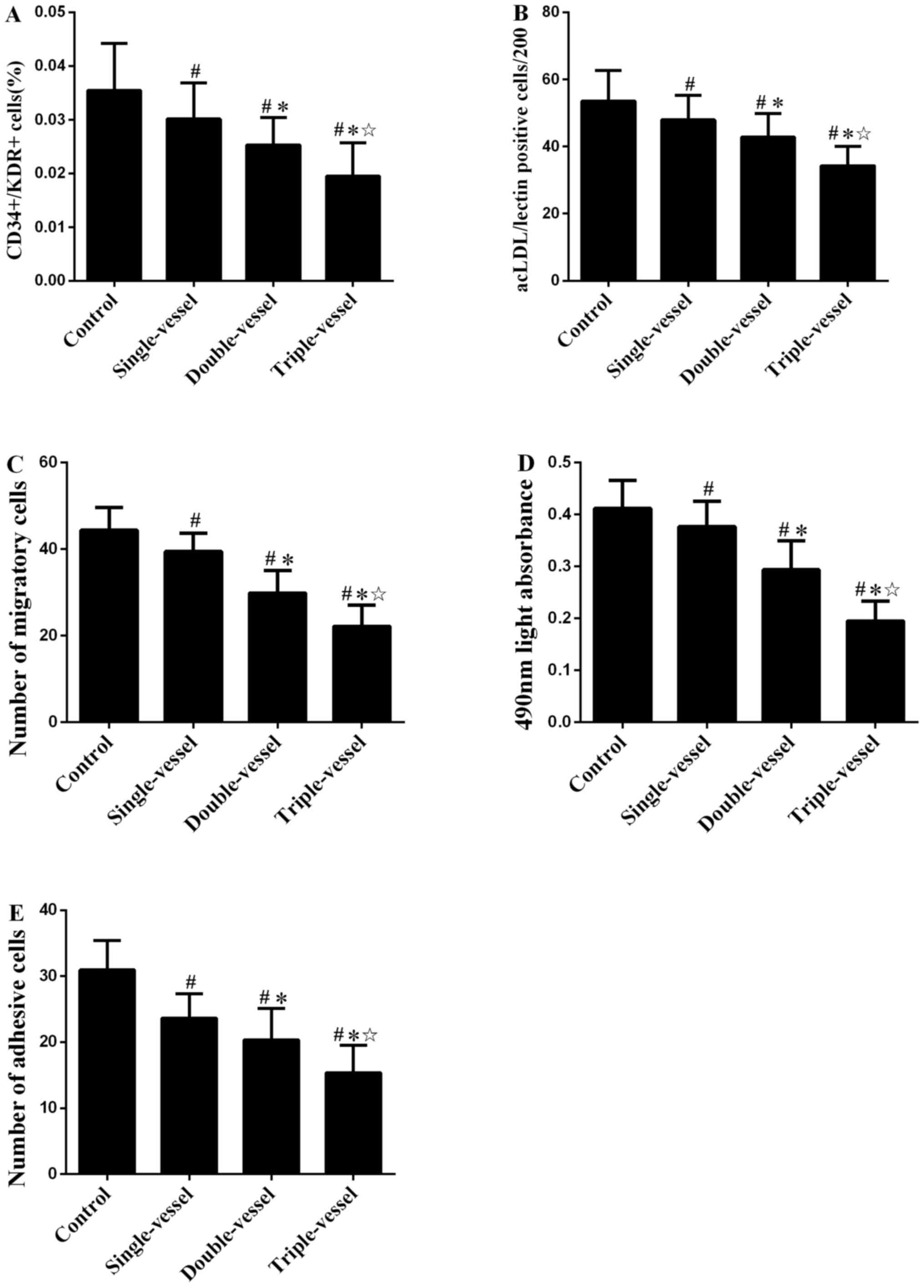
Number and activity of circulating
EPCs in groups with different numbers of coronary artery
lesions
The number of circulating EPCs evaluated by FCA
(Figs. 3A and S5) and immunofluorescence microscopy
(Figs. 3B and S1B) in patients with CAD was
significantly decreased compared with the Control group. The number
of circulating EPCs in multiple (double or triple) vessel groups
was decreased compared with the single vessel group. There was also
a significant difference in the number of circulating EPCs between
the double and triple vessel groups. Similar numbers of migratory,
proliferative and adhesive (Fig.
3C-E) circulating EPCs were observed in groups with different
number coronary artery lesions.
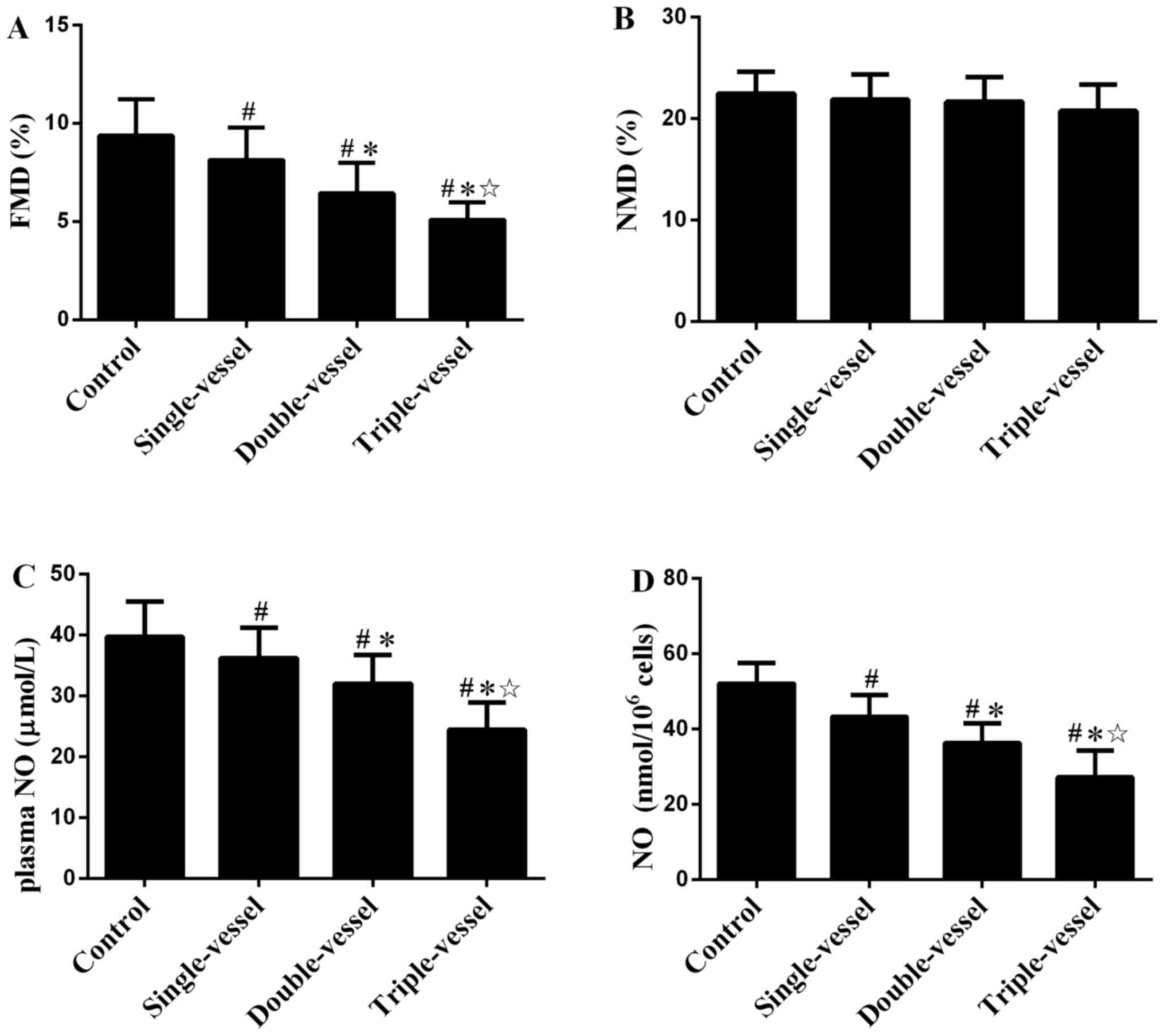
FMD and NMD in groups with different
numbers of coronary artery lesions
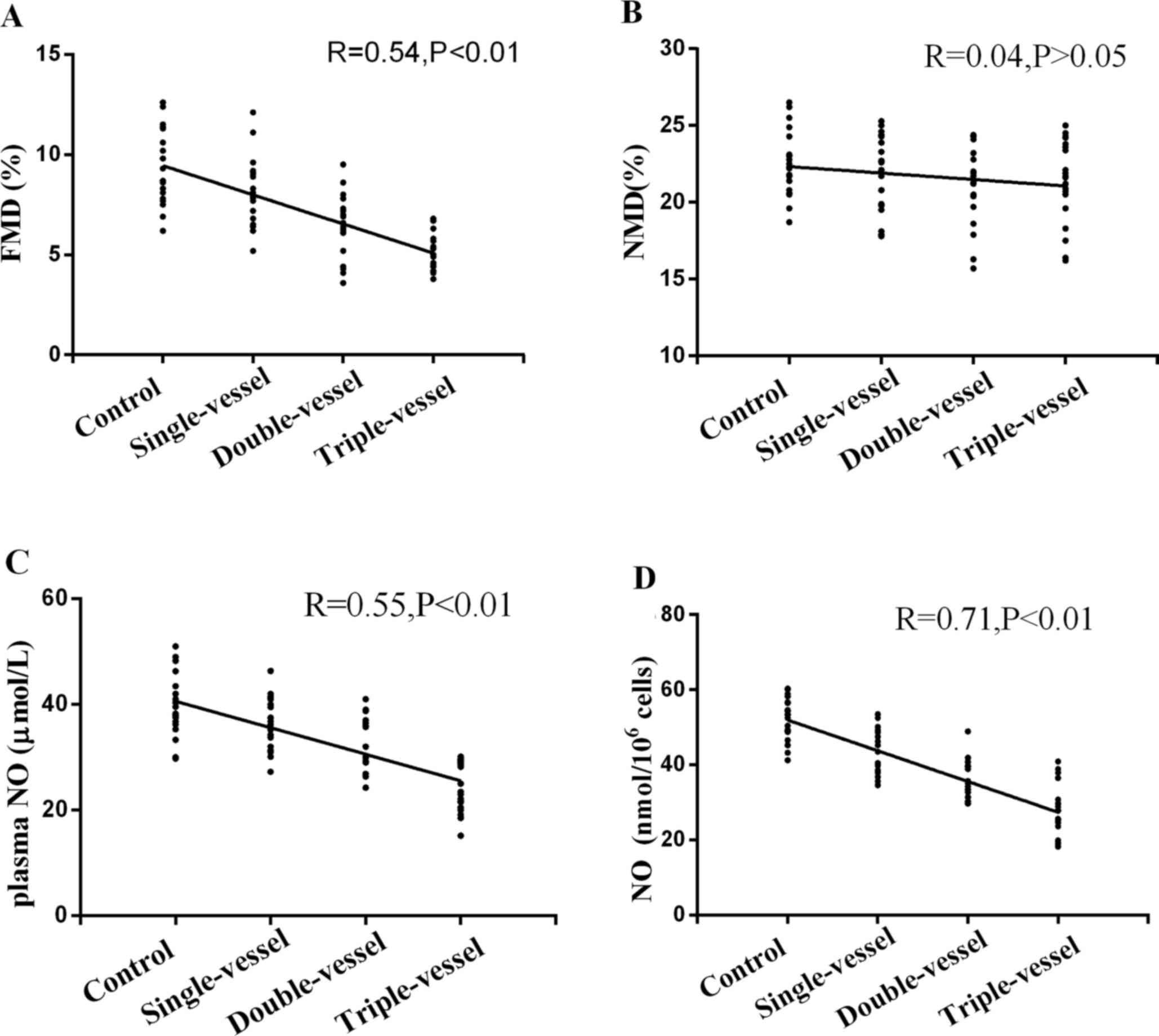
FMD in patients with CAD decreased compared with
that in the Control group (Fig.
4A). FMD in multiple (double or triple) vessels group was
decreased compared with single vessel group. There was also a
significant difference in FMD between the double and triple
vessels. NMD showed no significant difference between groups with
different numbers of coronary artery lesions (Fig. 4B).
NO plasma levels and secretion by EPCs
in groups with different numbers of coronary artery lesions
Plasma NO levels in patients with CAD were
significantly lower than in the Control group (Fig. 4C). The plasma NO levels in multiple
(double or triple) vessel groups were decreased compared with the
single vessel group. There was also a significant difference in
plasma NO levels between the double and triple vessel groups.
Similar results were observed in NO secretion by EPCs in groups
with different number of coronary artery lesions (Fig. 4D).
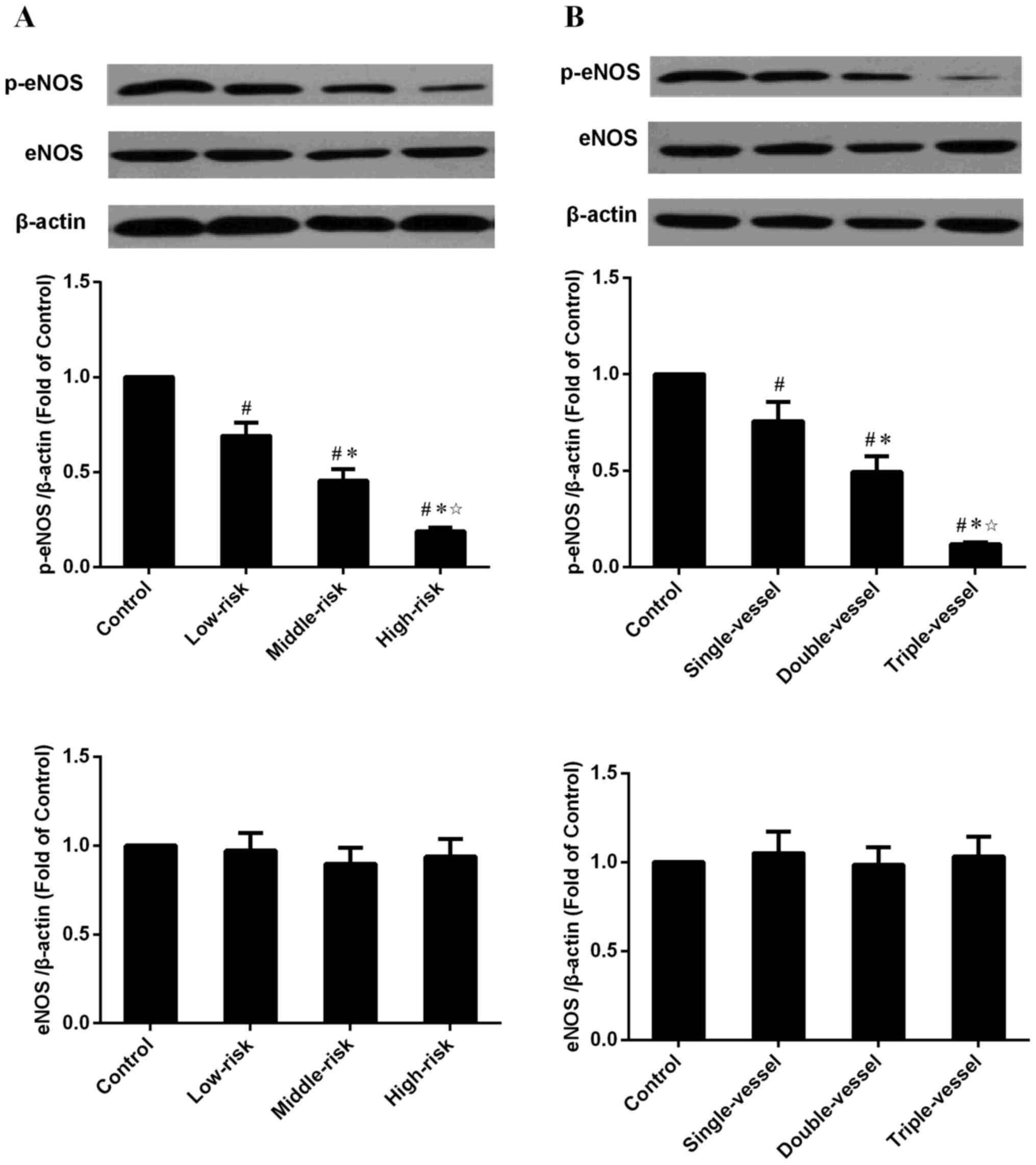
Western blot results
eNOS and p-eNOS protein expression levels of
circulating EPCs were determined in groups with different Syntax
scores and numbers of coronary lesion (Fig. 5A and B). eNOS expression levels were
not significantly different, whereas p-eNOS protein expression
levels were decreased in the CADs group compared with the Control
group. p-eNOS protein expression levels in the middle- and
high-risk groups were decreased compared with the low-risk group;
there were also significant differences between the high- and
middle-risk groups. eNOS expression were not significantly
different between groups with different numbers of coronary artery
lesions, whereas p-eNOS protein expression levels were decreased in
the CAD groups compared with the Control group (Fig. 5B). p-eNOS protein expression levels
in the multiple vessel groups were decreased compared with the
single vessel group and there was a significant difference between
the triple and double vessel groups.
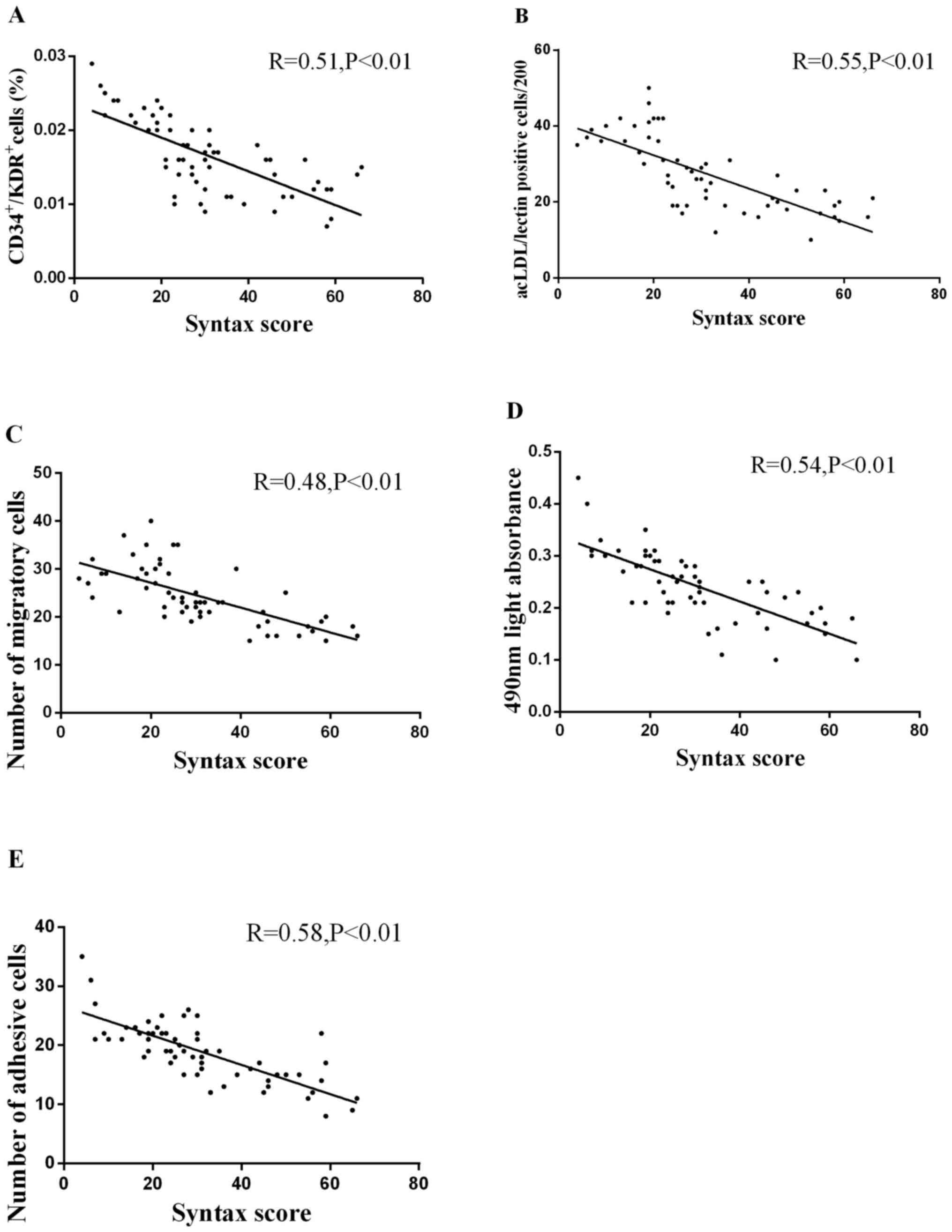
Correlation between circulating EPC
levels and activity and Syntax score
A negative univariate correlation between the number
of circulating EPCs and Syntax score (Fig. 6A and B) was detected. Both were
evaluated by FCA (Fig. 6A; R=0.51;
P<0.01) and cell culture (Fig.
6B; R=0.55; P<0.01). The numbers of migratory (Fig. 6C; R=0.48), proliferative (Fig. 6D; R=0.54) and adhesive EPCs
(Fig. 6E; R=0.58) was significantly
inversely correlated with the Syntax score (all P<0.01).
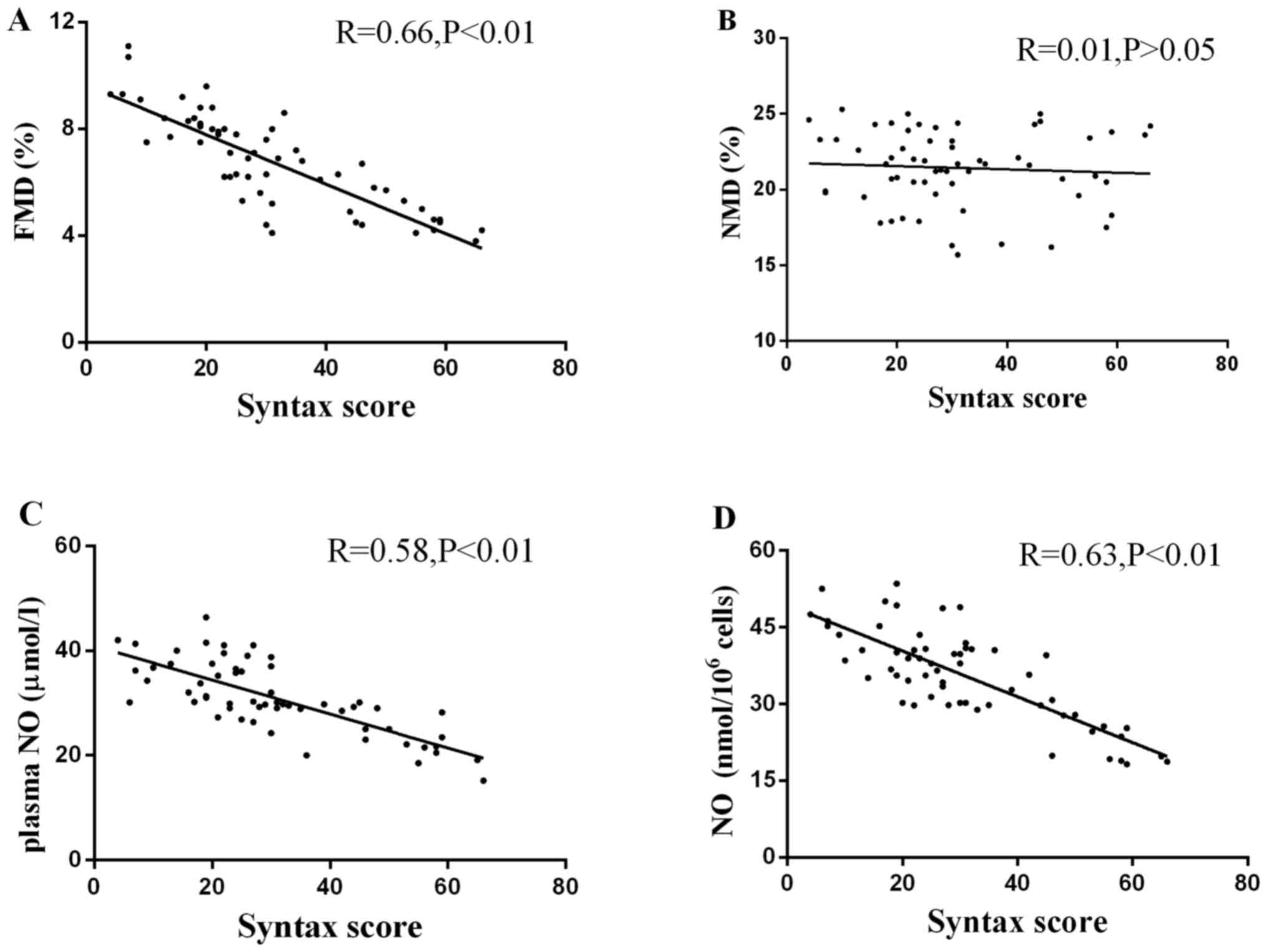
Correlation between FMD, NMD and
Syntax score
There was a significant inverse correlation between
FMD and Syntax score (Fig. 7A;
R=0.66; P<0.01). There was no significant correlation between
NMD and Syntax score (Fig. 7B;
R=0.01; P>0.05).
Correlation between NO plasma levels
or secretion by EPCs and Syntax score
Both NO plasma levels and secretion by EPCs were
negatively correlated with Syntax score (Fig. 7C and D; R=0.58 and R=0.63,
respectively; both P<0.01).
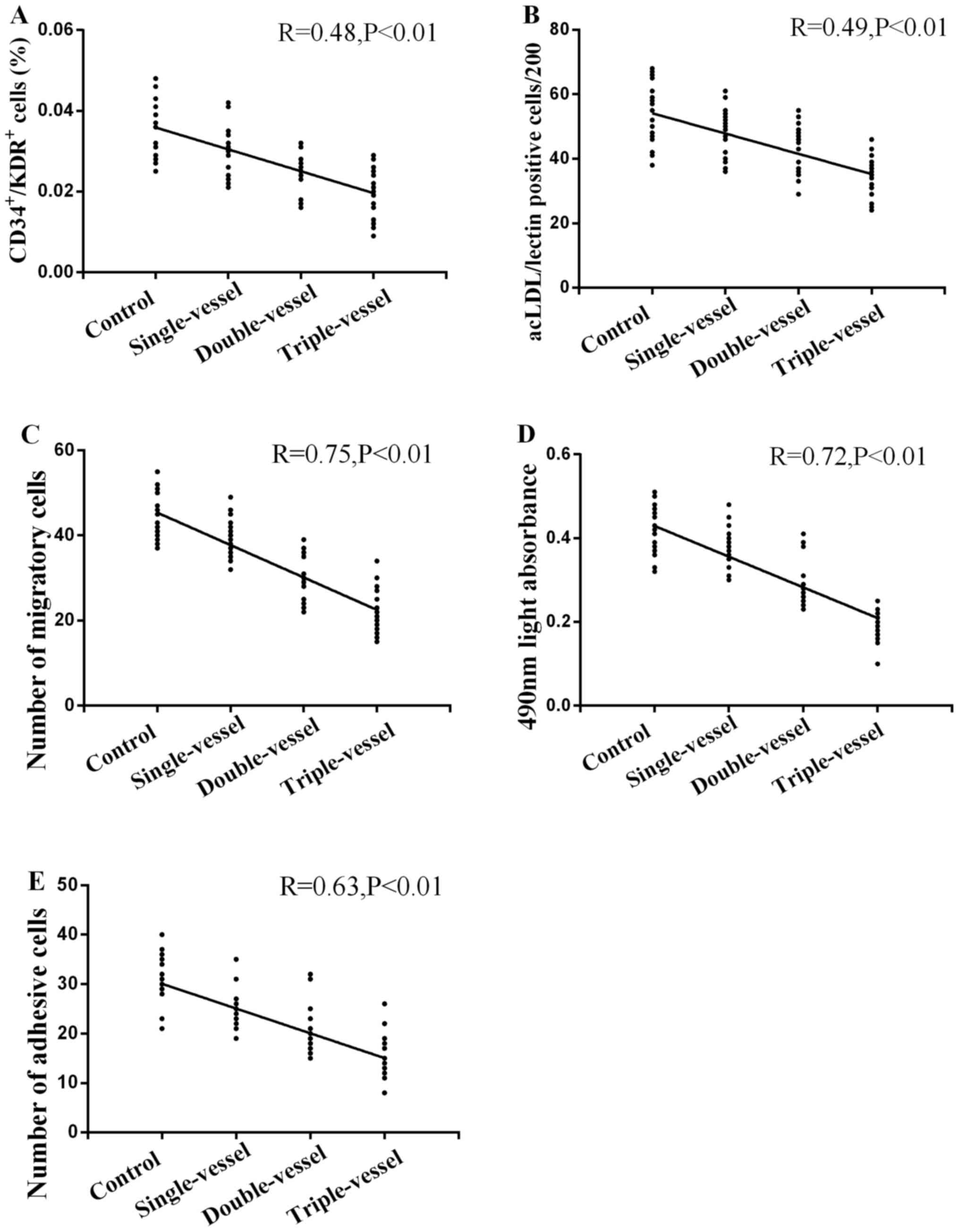
Correlation between circulating EPCs
and the number of coronary lesions
A strong negative univariate correlation was
detected between circulating EPCs number and number of coronary
lesions, as evaluated by FCA (Fig.
8A; R=0.48; P<0.01) and immunofluorescence microscopy
(Fig. 8B; R=0.49; P<0.01). The
migratory (Fig. 8C; R=0.75),
proliferative (Fig. 8D; R=0.72) and
adhesive activity (Fig. 8E; R=0.63)
exhibited a significant inverse correlation with the number of
coronary lesions (all P<0.01).
Correlation between FMD, NMD and the
number of coronary lesions
There was a significant inverse correlation between
FMD and the number of coronary lesions (Fig. 9A; R=0.54; P<0.01). By contrast,
there was no significant correlation between NMD and the number of
coronary lesions (Fig. 9B; R=0.04;
P>0.05).
Correlation between NO plasma levels
or secretion by EPCs and the number of coronary lesions
Both NO plasma levels and secretion by EPCs were
negatively correlated with the number of coronary lesions (Fig. 9C and D; R=0.55 and R=0.71,
respectively; both P<0.01).
Discussion
The present study showed that the number and
function of circulating EPCs, as well as FMD, were impaired in
patients with CAD with a higher Syntax score. Similar alterations
were observed in NO levels in plasma or secreted by EPCs into
culture medium. In addition, the number and activity of EPCs, FMD,
and NO levels in plasma or secreted by EPCs were attenuated in
multiple vessel groups compared with the single vessel group. There
were significant inverse correlations between the number and
activity of circulating EPCs and endothelial function and Syntax
score. The NO plasma levels and secretion by EPCs were also
negatively correlated with Syntax score.
Syntax score is a risk stratification tool for
patients with CAD based on the anatomy of the coronary artery,
providing a quantifiable objective evaluation of complexity and
severity for coronary artery lesions (31–34).
The primary cause of CAD is atherosclerosis, which is characterized
by defective endothelial function and plaque formation in the inner
wall of the artery (35). The
present study revealed that FMD decreased in patients with high
Syntax score, indicating that endothelial function was impaired and
was associated with greater severity and complexity of coronary
artery lesions.
A previous study showed that the number and activity
of circulating EPCs are decreased in patients with CAD (5), suggesting that attenuated endogenous
endothelial repair capacity is involved in coronary artery
abnormalities. Studies have reported that the number of circulating
EPCs is inversely correlated with Syntax score (6,7),
suggesting that the quantitative alteration in circulating EPCs may
be a cytobiological parameter to evaluate the severity and
complexity of CAD. However, the association between activity of
EPCs, as well as endothelial function, and Syntax score is not
clear.
Prior studies revealed a correlation between
endothelial function and the number and activity of circulating
EPCs (17,18). In light of the association between
impaired endothelial function and high Syntax score, it was
hypothesized that the activity of circulating EPCs is decreased in
patients with high Syntax score. Here, the migratory, proliferative
and adhesive function of circulating EPCs were shown to be
attenuated in patients with a higher Syntax score, indicating that
the quantitative and qualitative alteration in circulating EPCs is
a surrogate parameter to evaluate the severity and complexity of
CAD. These data provide evidence for the potential role of
endothelial function and capacity for endogenous repair of
endothelial injury in assessment of anatomical abnormalities of the
coronary artery. The migration, proliferation and adhesion
capability of EPCs, which are characteristic of morphological
vascular changes (4,5), were measured and analyzed. FMD is an
indicator of vascular functional change (36). Here, the characteristics of vascular
morphological and functional changes were assessed to investigate
the correlation between functional change and risk levels.
Endothelial function and circulating EPCs were also
correlated with the number of lesioned vessels. Compared with
single vessel lesions, multiple vessel lesions resulted in
decreased numbers or activity of circulating EPCs, FMD and NO
levels in plasma or secreted by EPCs. This is similar to the
correlations with Syntax score, which is primarily based on the
anatomy of the coronary artery and includes the number of vessel
lesions and severity of lesions.
NO serves an important role in regulating
endothelial function and the number and function of circulating
EPCs. eNOS is the NOS isoform responsible for the production of NO
and key regulators of mobilization and function of EPCs (17,18).
In the present study, levels of eNOS in circulating EPCs were
preserved in patients with CAD but levels of p-eNOS were decreased
compared with the Control group. These results indicate that the
decreased phosphorylation of eNOS, but not the alteration of eNOS
expression levels, contributed to decreased NO secretion by
circulating EPCs in patients with CAD. In addition, phosphorylation
of eNOS was lower in patients with high a Syntax score compared
with the low Syntax score group. Furthermore, patients with CAD
with multiple vessel lesions also exhibited notably decreased eNOS
phosphorylation. Therefore, it was inferred that decreased
phosphorylation of eNOS may be the mechanism underlying decreased
NO in circulating EPCs and FMD in patients with CAD.
In addition, the present study showed a negative
correlation between Syntax score and the number and activity of
circulating EPCs, as well as endothelial function, further
indicating that impaired repair capacity of endothelial injury and
subsequent decreased endothelial function is accompanied by serious
and complicated coronary artery anatomical abnormalities. NO serves
an important role in regulating endothelial function and the number
and function of circulating EPCs (37–39).
There was an inverse correlation between NO production and Syntax
score, suggesting that decreased NO bioavailability may be the
mechanism underlying decreased levels of circulating EPCs and
endothelial dysfunction in patients with CAD with a high Syntax
score.
The present study may have important implications.
First, the present data revealed that decreased FMD was associated
with high Syntax score, indicating endothelial function may be a
clinical indicator of coronary artery anatomical abnormalities.
Second, these results showed that decreased number or activity of
circulating EPCs was associated with a higher Syntax score,
demonstrating that circulating EPCs may serve as an important
surrogate biomarker for evaluation of the severity of CAD. Finally,
decreased NO biosynthesis may be an important mechanism underlying
impaired endothelial repair capacity and dysfunction in patients
with a high Syntax score. Therefore, strategies to increase NO
production, such as exercise and statin therapy, may be a novel
therapeutic approach for endothelial injury in patients with
CAD.
To the best of our knowledge, the present study is
the first to demonstrate that the number and activity of
circulating EPCs, as well as FMD, are negatively correlated with
Syntax score, indicating that circulating EPCs and endothelial
function may be key surrogate biomarkers for the evaluation of
severity and complexity of CAD. Enhancing the number or activity of
circulating EPCs and improving endothelial function may be
significant therapeutic approaches for serious and complicated
CAD.
Supplementary Material
Supporting Data
Acknowledgements
The abstract was presented at the 29th Great Wall
International Cardiology Conference Oct 11–14 in Beijing, China and
published as abstract in Journal of the American College of
Cardiology, 2018 Volume 72, Issue 16, Supplement, 16 October 2018,
Page C8.
Funding
The present study was financially supported by the
projects of the Fundamental Research Funds of Jiangmen Central
Hospital (grant no. D201901), the Outstanding Youth Fund Projects
of Jiangmen Central Hospital (grant no. J202003) and Jiangmen City
Science and Technology Plan (grant nos. 2020YLA100 and
2020YLA133).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BZ and DL performed the experiments, analyzed data
and wrote the manuscript. GL and WT performed statistical analysis
and discussion and investigated study subjects. GZ and JL designed
the study and reviewed the manuscript. BZ and GZ confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Jiangmen
Central Hospital Ethics Committee on Research on Humans (approval
no. 20170419). Written informed consent was obtained from all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
He Y, Kothari V and Bornfeldt KE:
High-density lipoprotein function in cardiovascular disease and
diabetes mellitus. Arterioscler Thromb Vasc Biol. 38:e10–e16. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Piccolo R, Giustino G, Mehran R and
Windecker S: Stable coronary artery disease: Revascularisation and
invasive strategies. Lancet. 386:702–713. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Calderón-Gerstein WS, López-Peña A,
Macha-Ramírez R, Bruno-Huamán A, Espejo-Ramos R, Vílchez-Bravo S,
Ramírez-Breña M, Damián-Mucha M and Matos-Mucha A: Endothelial
dysfunction assessment by flow-mediated dilation in a high-altitude
population. Vasc Health Risk Manag. 13:421–426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aicher A, Zeiher AM and Dimmeler S:
Mobilizing endothelial progenitor cells. Hypertension. 45:321–325.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hill JM, Zalos G, Halcox JP, Schenke WH,
Waclawiw MA, Quyyumi AA and Finkel T: Circulating endothelial
progenitor cells, vascular function, and cardiovascular risk. N
Engl J Med. 348:593–600. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ghem C, Dias LD, Sant'Anna RT, Kalil RAK,
Markoski M and Nardi NB: Combined analysis of endothelial,
hematopoietic, and mesenchymal stem cell compartments shows
simultaneous but independent effects of age and heart disease. Stem
Cells Int. 2017:52376342017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chi J, Hong X, Wang Y, Zhao J and Yang W:
Inverse correlation between circulating endothelial progenitor
cells with CD34+CD133+ and the severity of
coronary atherosclerosis assessed by Syntax score. Am J Med Sci.
347:457–462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kolh P and Windecker S: ESC/EACTS
myocardial revascularization guidelines 2014. Eur Heart J.
35:3235–3236. 2014.PubMed/NCBI
|
|
9
|
Sianos G, Morel MA, Kappetein AP, Morice
MC, Colombo A, Dawkins K, van den Brand M, Van Dyck N, Russell ME,
Mohr FW and Serruys PW: The Syntax score: An angiographic tool
grading the complexity of coronary artery disease.
EuroIntervention. 1:219–227. 2005.PubMed/NCBI
|
|
10
|
Tanaka T, Seto S, Yamamoto K, Kondo M and
Otomo T: An assessment of risk factors for the complexity of
coronary artery disease using the Syntax score. Cardiovasc Interv
Ther. 28:16–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wykrzykowska JJ, Garg S, Girasis C, de
Vries T, Morel MA, van Es GA, Buszman P, Linke A, Ischinger T,
Klauss V, et al: Value of the Syntax score for risk assessment in
the all-comers population of the randomized multicenter LEADERS
(limus eluted from A durable versus ERodable stent coating) trial.
J Am Coll Cardiol. 56:272–277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mohr FW, Morice MC, Kappetein AP, Feldman
TE, Ståhle E, Colombo A, Mack MJ, Holmes DR Jr, Morel MA, Van Dyck
N, et al: Coronary artery bypass graft surgery versus percutaneous
coronary intervention in patients with three-vessel disease and
left main coronary disease: 5-year follow-up of the randomised,
clinical Syntax trial. Lancet. 381:629–638. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mohr FW, Rastan AJ, Serruys PW, Kappetein
AP, Holmes DR, Pomar JL, Westaby S, Leadley K, Dawkins KD and Mack
MJ: Complex coronary anatomy in coronary artery bypass graft
surgery: Impact of complex coronary anatomy in modern bypass
surgery? Lessons learned from the Syntax trial after two years. J
Thorac Cardiovasc Surg. 141:130–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aicher A, Heeschen C, Mildner-Rihm C,
Urbich C, Ihling C, Technau-Ihling K, Zeiher AM and Dimmeler S:
Essential role of endothelial nitric oxide synthase for
mobilization of stem and progenitor cells. Nat Med. 9:1370–1376.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Duda DG, Fukumura D and Jain RK: Role of
eNOS in neovascularization: NO for endothelial progenitor cells.
Trends Mol Med. 10:143–145. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Urbich C and Dimmeler S: Endothelial
progenitor cells: characterization and role in vascular biology.
Circ Res. 95:343–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhen Y, Xiao S, Ren Z, Shen HW, Su H, Tang
YB and Zeng H: Increased endothelial progenitor cells and nitric
oxide in young prehypertensive women. J Clin Hypertens (Greenwich).
17:298–305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeng H, Jiang Y, Tang H, Ren Z, Zeng G and
Yang Z: Abnormal phosphorylation of Tie2/Akt/eNOS signaling pathway
and decreased number or function of circulating endothelial
progenitor cells in prehypertensive premenopausal women with
diabetes mellitus. BMC Endocr Disord. 16:132016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Z, Chen L, Su C, Xia WH, Wang Y, Wang
JM, Chen F, Zhang YY, Wu F, Xu SY, et al: Impaired endothelial
progenitor cell activity is associated with reduced arterial
elasticity in patients with essential hypertension. Clin Exp
Hypertens. 32:444–452. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang Z, Wang JM, Chen L, Luo CF, Tang AL
and Tao J: Acute exercise-induced nitric oxide production
contributes to upregulation of circulating endothelial progenitor
cells in healthy subjects. J Hum Hypertens. 21:452–460. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vasa M, Fichtlscherer S, Aicher A, Adler
K, Urbich C, Martin H, Zeiher AM and Dimmeler S: Number and
migratory activity of circulating endothelial progenitor cells
inversely correlate with risk factors for coronary artery disease.
Circ Res. 89:E1–E7. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Z, Xia WH, Su C, Wu F, Zhang YY, Xu
SY, Liu X, Zhang XY, Ou ZJ, Lai GH, et al: Regular exercise-induced
increased number and activity of circulating endothelial progenitor
cells attenuates age-related decline in arterial elasticity in
healthy men. Int J Cardiol. 165:247–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang Z, Xia WH, Zhang YY, Xu SY, Liu X,
Zhang XY, Yu BB, Qiu YX and Tao J: Shear stress-induced activation
of Tie2-dependent signaling pathway enhances reendothelialization
capacity of early endothelial progenitor cells. J Mol Cell Cardiol.
52:1155–1163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Z, Tao J, Wang JM, Tu C, Xu MG, Wang
Y and Pan SR: Shear stress contributes to t-PA mRNA expression in
human endothelial progenitor cells and nonthrombogenic potential of
small diameter artificial vessels. Biochem Biophys Res Commun.
342:577–584. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hirschi KK, Ingram DA and Yoder MC:
Assessing identity, phenotype, and fate of endothelial progenitor
cells. Arterioscler Thromb Vasc Biol. 28:1584–1595. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Walther C, Gaede L, Adams V, Gelbrich G,
Leichtle A, Erbs S, Sonnabend M, Fikenzer K, Korner A, Kiess W, et
al: Effect of increased exercise in school children on physical
fitness and endothelial progenitor cells: A prospective randomized
trial. Circulation. 120:2251–2259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zeng W, Yuan W, Li L, Mi J, Xu S, Wen C,
Zhou Z, Xiong J, Sun J, Ying D, et al: The promotion of endothelial
progenitor cells recruitment by nerve growth factors in
tissue-engineered blood vessels. Biomaterials. 31:1636–1645. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Heiss C, Keymel S, Niesler U, Ziemann J,
Kelm M and Kalka C: Impaired progenitor cell activity in
age-related endothelial dysfunction. J Am Coll Cardiol.
45:1441–1448. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Corretti MC, Anderson TJ, Benjamin EJ,
Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H,
Gerhard-Herman M, Herrington D, et al: Guidelines for the
ultrasound assessment of endothelial-dependent flow-mediated
vasodilation of the brachial artery: A report of the international
brachial artery reactivity task force. J Am Coll Cardiol.
39:257–265. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sibal L, Aldibbiat A, Agarwal SC, Mitchell
G, Oates C, Razvi S, Weaver JU, Shaw JA and Home PD: Circulating
endothelial progenitor cells, endothelial function, carotid
intima-media thickness and circulating markers of endothelial
dysfunction in people with type 1 diabetes without macrovascular
disease or microalbuminuria. Diabetologia. 52:1464–1473. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Farooq V, Head SJ, Kappetein AP and
Serruys PW: Widening clinical applications of the Syntax score.
Heart. 100:276–287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen SL, Chen JP, Mintz G, Xu B, Kan J, Ye
F, Zhang J, Sun X, Xu Y, Jiang Q, et al: Comparison between the
NERS (new risk stratification) score and the Syntax (synergy
between percutaneous coronary intervention with taxus and cardiac
surgery) score in outcome prediction for unprotected left main
stenting. JACC Cardiovasc Interv. 3:632–641. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yadav M, Palmerini T, Caixeta A, Madhavan
MV, Sanidas E, Kirtane AJ, Stone GW and Généreux P: Prediction of
coronary risk by Syntax and derived scores: Synergy between
percutaneous coronary intervention with taxus and cardiac surgery.
J Am Coll Cardiol. 62:1219–1230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cuenza L, Collado MP and Ho Khe Sui J:
Global risk score and clinical Syntax score as predictors of
clinical outcomes of patients undergoing unprotected left main
percutaneous catheter intervention. Cardiol Res. 8:312–318. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bentzon JF, Otsuka F, Virmani R and Falk
E: Mechanisms of plaque formation and rupture. Circ Res.
114:1852–1866. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Flammer AJ, Anderson T, Celermajer DS,
Creager MA, Deanfield J, Ganz P, Hamburg NM, Lüscher TF, Shechter
M, Taddei S, et al: The assessment of endothelial function: From
research into clinical practice. Circulation. 126:753–767. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shyu KG: Enhancement of new vessel
formation by angiopoietin-2/Tie2 signaling in endothelial
progenitor cells: A new hope for future therapy? Cardiovasc Res.
72:359–360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fulton D, Gratton JP, McCabe TJ, Fontana
J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A and Sessa WC:
Regulation of endothelium-derived nitric oxide production by the
protein kinase Akt. Nature. 399:597–601. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Thum T, Fraccarollo D, Schultheiss M,
Froese S, Galuppo P, Widder JD, Tsikas D, Ertl G and Bauersachs J:
Endothelial nitric oxide synthase uncoupling impairs endothelial
progenitor cell mobilization and function in diabetes. Diabetes.
56:666–674. 2007. View Article : Google Scholar : PubMed/NCBI
|