Introduction
Pulmonary artery hypertension (PAH) is a disease
characterized by pulmonary vascular remodeling and a progressive
increase of pulmonary vascular resistance (1). The main manifestations of PAH are
pulmonary vasoconstriction, intimal hyperplasia of pulmonary
artery, proliferation and hypertrophy of smooth muscle cells
(SMCs), which lead to a thickened pulmonary artery wall, narrow
lumen and increased pulmonary artery pressure (2,3). It
has been reported that PAH could be induced by various pulmonary
diseases that cause airflow restriction, destruction of the
pulmonary capillary bed, injury of the pulmonary vascular
endothelium and infiltration of inflammatory cells in the airways
(4,5). PAH is a clinical syndrome with high
morbidity and mortality (6).
Although the treatment of PAH has made remarkable progress in the
past 10 years, its prognosis remains poor (7). Since the exact pathogenesis of PAH is
not yet clear, there is no cure for PAH at present. Therefore, it
is of great significance to explore an effective treatment for the
prevention or alleviation of PAH.
Previous studies have reported that inflammatory
responses (8), oxidative stress
(9), proliferation of SMCs and
endothelial cells (10), and
vasodilation (11) are the possible
pathological basis of PAH. In a study by Liu et al (12), elevated levels of inflammatory
factors, such as interleukin-6 (IL-6) and tumor necrosis factor-α
(TNF-α), were found in PAH animal models (12). Increased levels of IL-6 are also
detected in patients with PAH (13). Oxidative stress may be the main
cause of endothelial injury and vascular wall remodeling. Zhang
et al (14) reported there
is increased malondialdehyde (MDA) and decreased superoxide
dismutase (SOD) activity in PAH rats. Therefore, inhibition of
chronic pulmonary vascular remodeling induced by inflammation,
oxidative stress and cell proliferation is essential to improve the
survival rates of patients with PAH.
There is a close association between the formation
and development of PAH and members of the transforming growth
factor-β (TGF-β) superfamily (15).
Abnormally high levels of TGF-β1 have been found in patients with
PAH (16). TGF-β1 is a
multifunctional channel protein for growth regulation (15). It is one of the most important
smooth muscle proliferative cytokines, and exerts physiological
effects by activating the p38 mitogen-activated protein kinase
(MAPK) signaling pathway (16). The
p38 MAPK pathway is the common pathway of proliferation and
migration of vascular smooth muscle cells (VSMCs) (17). The p38 MAPK signaling pathway can
also mediate inflammation and apoptosis, which is the key target of
anti-inflammatory drugs (17).
Inhibition of p38 MAPK activation can effectively prevent vascular
remodeling of monocrotaline (MCT)-induced PAH (18).
Anthocyanins (ACNs), a type of flavonoids, are
widely found in numerous dark-colored foods, such as mulberry and
black rice (19). ACNs have a
variety of biological effects, which includes anti-inflammatory and
anti-oxidative stress properties, thus they can play an inhibitory
role in the progression of several chronic diseases (20,21).
The anti-inflammatory effects of ACNs have attracted great
attention. Herath et al (22) revealed that ACNs inhibited the
secretion of the inflammatory factors IL-6 and TNF-α. Previous
studies reported the anti-atherosclerotic effect of ACNs, which is
associated with their anti-inflammatory and anti-oxidative stress
abilities (23–25). In addition, it has also been
demonstrated that ACNs have the capacity to inhibit the
proliferation of SMCs (26) and
prevent vascular endothelial cell injury (27). However, few studies have
investigated whether ACNs display a protective effect in the
development of PAH. In the present study, a rat PAH model was
established with the intervention of Cyanidin-3-O-β-glucoside
(Cy-3-g; a typical monomer ACN) to investigate whether Cy-3-g could
protect against PAH and whether Cy-3-g exerts its inhibitory effect
by regulating the TGF-β1 and p38 MAPK signaling pathways.
Materials and methods
Animal model
A total of 60 male Sprague-Dawley rats (age, 8
weeks; weight, 281.3±21.7 g) were obtained from the Animal Center
of the University of South China. After 1-week acclimation, rats
were randomly assigned to four groups (n=15): A control group
(control), a model group (MCT), a low-dose Cy-3-g group (Cy-3-g-L)
and a high-dose Cy-3-g group (Cy-3-g-H). On the first day of the
experiments, the rats in the control group were injected
intraperitoneally with 0.9% normal saline; rats in the MCT,
Cy-3-g-L and Cy-3-g-H groups were injected intraperitoneally with
MCT (60 mg/kg of body weight). On days 2–28, the rats in the
Cy-3-g-L and Cy-3-g-H groups received a gavage of 200 and 400 mg
Cy-3-g per kg of body weight, respectively, while the rats in the
control and MCT groups received a gavage of normal saline. During
the 28 days of animal experiments, rats were housed in a
temperature of 21–24°C and a humidity of 45–50°C with 12 h
light/dark cycles. All rats were allowed to drink water and eat a
normal chow diet (5% fat w/w; Animal Center of the University of
South China) ad libitum. The bedding and cages of rats were
changed every 2 days, and the weight and food intake of rats was
recorded every week. After 28-day treatment, rats were
anaesthetized by intraperitoneal injection of 1% pentobarbital
sodium (at the dose of 40 mg/kg of body weight; Beyotime Institute
of Biotechnology), and then were euthanized using cervical
dislocation, with the endpoint of cervical fracture and mydriasis.
The procedures used in the present animal experiments were approved
by the Animal Care Committee of the University of South China
[approval no. SYXK (Xiang): 2005-0001].
Hemodynamic measurements
Right catheterization was performed according to a
previous study (10). Briefly, rats
were anesthetized with an intraperitoneal injection of 1% sodium
pentobarbital, and their right external jugular vein was exposed,
which was cut longitudinally and a catheter was inserted into it.
The catheter was slowly inserted into the right atrium, right
ventricle and pulmonary artery. The catheter was filled with
heparin saline and connected to the pressure sensor of PowerLab
physiological recorder (ADInstruments). The mean pulmonary artery
pressure (mPAP) and right ventricular systolic pressure (RVSP) were
recorded.
After being dissected, the hearts of rats were
cleared and isolated, and then separated into right ventricle (RV),
left ventricle (LV) and septum (S). The weights of the RV, LV and S
were recorded and used for the calculation of the right ventricular
hypertrophy index (RVHI) according to the formula RVHI = W (RV)/[W
(LV)+W (S)].
Morphological analysis of pulmonary
arterioles
The upper lobe of the left lung was fixed with 4%
paraformaldehyde at 4°C overnight, and cut into 5-µm-thick paraffin
sections, which were subjected to hematoxylin and eosin (H&E)
staining at room temperature for 5 min. Images were acquired and
analyzed by microscopy (Leica Microsystem, Inc.) at magnification,
×200. The medial wall thickness (MT) and medial wall area (MA) were
calculated according to the formulae: MT (%) = wall thickness/total
vessel thickness ×100%; and MA (%) = wall area/total vessel area
×100%, respectively.
Blood gas analysis
After anesthetization with 1% pentobarbital sodium,
the epidermis of the rats was cut off, and blood was collected from
the left intercostal space between the third and fourth ribs. In
total, 0.5 ml of the collected blood was analyzed with a blood gas
analyzer (Instrumentation Laboratory). Partial pressure of arterial
oxygen (PaO2), partial pressure of arterial carbon
dioxide (PaCO2) and PH values were recorded.
ELISA determination of IL-6, TNF-α and
IL-10
According to the manufacturer's protocols, rat ELISA
kits of IL-6 (cat. no. EK0412), TNF-α (cat. no. EK0526) and IL-10
(cat. no. EK0418; all purchased from Boster Biological Technology),
standards and samples (tissue homogenate) were added to the plate
(100 µl/well), and a blank was set as the control. The plate was
covered with a membrane and incubated at 37°C for 1 h. After
discarding the liquid, buffer A (100 µl/well) was added to the
plate, and incubated at 37°C for 1 h. Next, the plate was washed 3
times with PBS with 1% Tween-20 and then incubated with buffer B
(100 µl/well) at 37°C for 30 min. After rinsing 5 times, the plate
was incubated with the chromogenic substrate
3,3′,5,5′-Tetramethylbenzidine for 10 min in the dark. After
terminating the reaction with a terminating solution, the optical
density (OD) of each well was measured at 450 nm using a microplate
reader (Tecan Spark 10M; Tecan Group, Ltd.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from lung tissues using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). After
measuring the concentration, cDNA was synthesized by PrimeScript RT
Reagent kit (Takara Bio, Inc.) at 37°C for 15 min. Then, RT-qPCR
was conducted with a ViiA™ 7 real-time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) with the SYBR Premix Ex
Taq II (Tli RNaseH Plus) kit (Takara Bio, Inc.). The thermocycling
conditions were as follows: 95°C for 30 sec; then, 95°C for 5 sec
and 60°C for 30 sec (40 cycles). The expression folds were
calculated with the 2−ΔΔCq method (28). All primers are listed in Table SI.
Measurement of SOD activity and MDA
content
The activity of SOD was measured with a SOD assay
kit (cat. no. A001-3-2; Nanjing Jiancheng Bioengineering Institute)
using the Water-Soluble Tetrazolium-1 method (29). The OD values were recorded at 450
nm. The content of MDA was detected with a MDA assay kit (cat. no.
A003-1-2; Nanjing Jiancheng Bioengineering Institute) using the
Thiobarbituric Acid method, as described previously (29), and the OD values were analyzed at
532 nm.
Cell culture and identification
Human pulmonary artery SMCs (HPASMCs) were purchased
from The Cell Bank of Type Culture Collection of the Chinese
Academy of Sciences, and grown in 20% fetal bovine serum-RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc.) at 37°C, 5%
CO2 and 95% humidity. Cells (3–5 passages) were used in
subsequent experiments. HPASMCs were pretreated with Cy-3-g (10 or
20 µm) at 37°C for 24 h, followed by treatment with TGF-β1 (8
ng/ml) or P79350 (0.2 µg/kg), an agonist of p38 MAPK, for an
additional 24 h at 37°.
HPASMCs were seeded at a density of 1×104
and fixed with 4% paraformaldehyde at 4°C overnight. After blocking
with 5% not-fat milk at room temperature for 1 h, the slides were
stained with a primary antibody against a-smooth muscle actin
(α-SMA; 1:100; cat. no. 19245S; Cell Signaling Technology, Inc.)
for 8–12 h at 4°C, followed by incubation with FITC-conjugated goat
anti-rabbit IgG (1:200; cat. no. SA00003-2; ProteinTech Group,
Inc.) for 1 h at room temperature. Micrographs were captured with a
Leica TSC SP5 confocal microscope (Leica Microsystems, Inc.) at
magnification, ×200.
Cell viability assay
HPASMCs were seeded in 96-well plates
(4×103 cells in 100 µl/well). After treatment with
Cy-3-g for 24 h at 37°C, cell viability was detected by a CCK8
assay (Beyotime Institute of Biotechnology), according to the
manufacturer's protocols. CCK-8 was added to the cells (10 µl/well)
and incubated for 1 h at 37°C. Next, the OD values were observed by
a versatile microplate reader (Leica Microsystems, Inc.) at 450
nm.
Western blotting
Briefly, total protein was extracted from lung
tissues using RIPA buffer (Beyotime Institute of Biotechnology) and
quantified using a BCA protein assay kit (Beyotime Institute of
Biotechnology). Next, the extracted proteins (50 µg) were loaded
and separated via SDS-PAGE on a 8% gel, and transferred onto PVDF
membranes. After blocking with 5% BSA (Shanghai Yeasen
Biotechnology Co., Ltd.) at room temperature for 1 h, the membrane
was stained with the following primary antibodies: Rabbit
anti-α-SMA (1:1,000; cat. no. ab32575), anti-von Willebrand factor
(vWF; 1:1,000; cat. no. ab6994), anti-intercellular adhesion
molecule-1 (ICAM-1; 1:1,000; cat. no. ab33894), anti-vascular
adhesion molecule-1 (VCAM-1; 1:1,000; cat. no. ab134047),
anti-smooth muscle 22 (SM22; 1:1,000; cat. no. ab14106),
anti-vascular endothelial growth factor (VEGF; 1:1,000; cat. no.
ab32152), anti-platelet-derived growth factor (PDGF)-BB (1:1,000;
cat. no. ab178409), anti-TGF-β1 (1:1,000; cat. no. ab92486),
anti-p38 MAPK (1:1,000; cat. no. ab170099), anti-phosphorylated
(p)-p38 MAKP (1:1,000; cat. no. ab4822), anti-cAMP-response element
binding protein (CREB; 1:1,000; cat. no. ab32515), anti-p-CREB
(1:1,000; cat. no. ab220798) and anti-β-actin (1:2,000; cat. no.
3700S; Cell Signaling Technology, Inc.) overnight at 4°C. Then, the
membrane was incubated with goat anti-rabbit IgG horseradish
peroxidase-conjugated secondary antibody (1:2,000; cat. no.
SC-2004; Santa Cruz Biotechnology, Inc.) at 37°C for 1 h. Then, an
ECL kit (Thermo Fisher Scientific, Inc.) was used to detect protein
expression. Image-Pro Plus 6.0 software (Media Cybernetics, Inc.)
was used for densitometry.
Statistical analysis
Data are presented as the mean ± SD. One-way ANOVA
was performed for comparison of statistical differences between
groups, followed by Tukey's post hoc test, using SPSS version 22.0
software (IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Inhibition of Cy-3-g on hemodynamics
and morphological characteristics of PAH
After injection of MCT and intervention with Cy-3-g,
the PAH animal model was verified by detecting hemodynamic
parameters. As expected, compared with those of normal rats, the
mPAP, RVSP and RVHI of MCT-induced rats markedly increased (from
25.00±2.582 to 64.25±5.909 mmHg; from 17.50±3.416 to 54.25±5.901
mmHg; and from 0.1975±0.028 to 0.4450±0.040 mmHg, respectively), as
shown in Fig. 1A-C. A low dose
Cy-3-g (200 mg/kg of body weight) significantly decreased RVSP in
the MCT-induced rats, and a high dose of Cy-3-g (400 mg/kg of body
weight) reduced mPAP, RVSP and RVHI significantly in the
MCT-induced rats (all P<0.05; Fig.
1A-C).
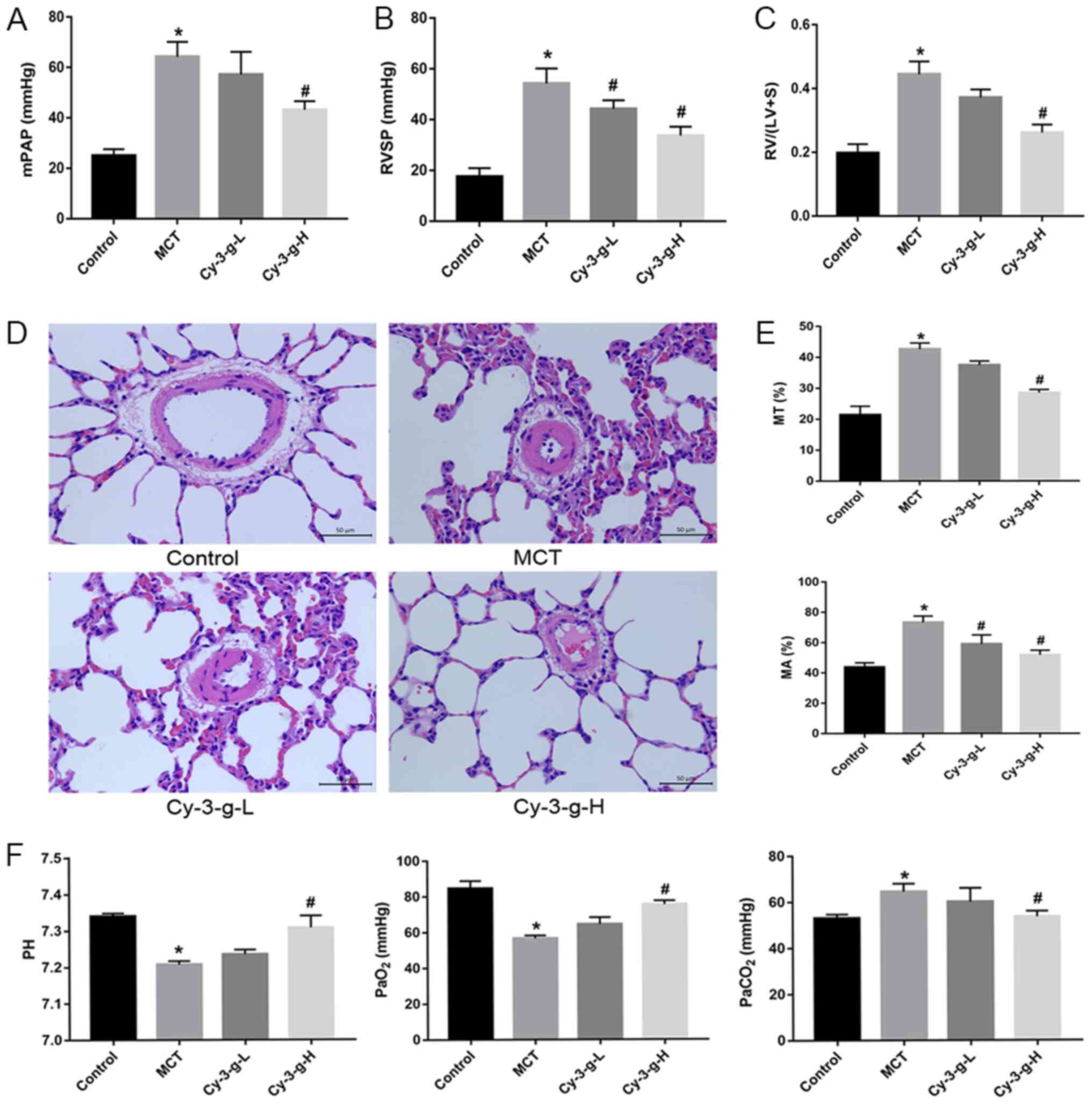 | Figure 1.Protective effect of Cy-3-g on
hemodynamics in rats with pulmonary artery hypertension induced by
MCT. Rats were randomly assigned into four groups (n=15): Control
group, model group (MCT), Cy-3-g-L group and Cy-3-g-H group. On the
first day of the experiments, rats in the MCT, Cy-3-g-L and
Cy-3-g-H groups were injected with MCT (60 mg/kg). On days 2–28,
the rats in the Cy-3-g-L and Cy-3-g-H groups received a gavage of
200 and 400 mg/kg body weight, respectively. Changes in: (A) Mean
mPAP, (B) RVSP and (C) RV hypertrophy index. (D) Representative
images of hematoxylin and eosin staining of lung tissue. (E)
Quantitative data of percentage of MT and MA of the pulmonary
artery. (F) Changes in pH and partial pressure of PaO2
and PaCO2. Data are shown as the mean ± SD. *P<0.05
vs. control; #P<0.05 vs. MCT. MCT, monocrotaline;
Cy-3-g, cyanidin-3-O-β-glucoside; L, low-dose; H, high-dose; mPAP,
pulmonary artery pressure; RVSP, right ventricular systolic
pressure; RV, right ventricular; MT, medial wall thickness; MA,
medial wall area; PaO2, arterial oxygen;
PaCO2, arterial carbon dioxide. |
The vascular remodeling parameters were then
detected by H&E staining. As depicted in Fig. 1D and E, MCT increased the percentage
of MT and MA from 21.39±2.849 to 42.66±1.961% and from 43.75±2.986
to 73.25±4.272%, respectively. The consumption of Cy-3-g exerted an
inhibitory effect on the MCT-induced increase in MT and MA, which
was most significant in the high dose group.
In addition, blood gas analysis was used for the
evaluation of hypoxic status in rats. MCT led to an increase in
PaCO2, and induced a reduction in pH and
PaO2, whereas Cy-3-g-H consumption inhibited the effects
of MCT on PaCO2, pH and PaO2 (all P<0.05;
Fig. 1F).
Protective effects of Cy-3-g on
inflammatory and oxidative stress conditions in MCT-induced PAH
rats
Next, the possible underlying mechanism of Cy-3-g in
the inhibition of PAH development was explored. Immunoinflammatory
factors play a key role in the formation and vascular remodeling of
PAH (30). Therefore, the
expression of inflammatory factors was measured by RT-qPCR and
ELISA. The results of Fig. 2A
demonstrated that MCT significantly elevated the mRNA levels of
IL-6 and TNF-α, and decreased the levels of IL-10. However, Cy-3-g
treatment reversed MCT-induced changes. Similar effects of MCT and
Cy-3-g could be observed on the plasma levels of IL-6, TNF-α and
IL-10 (Fig. 2B).
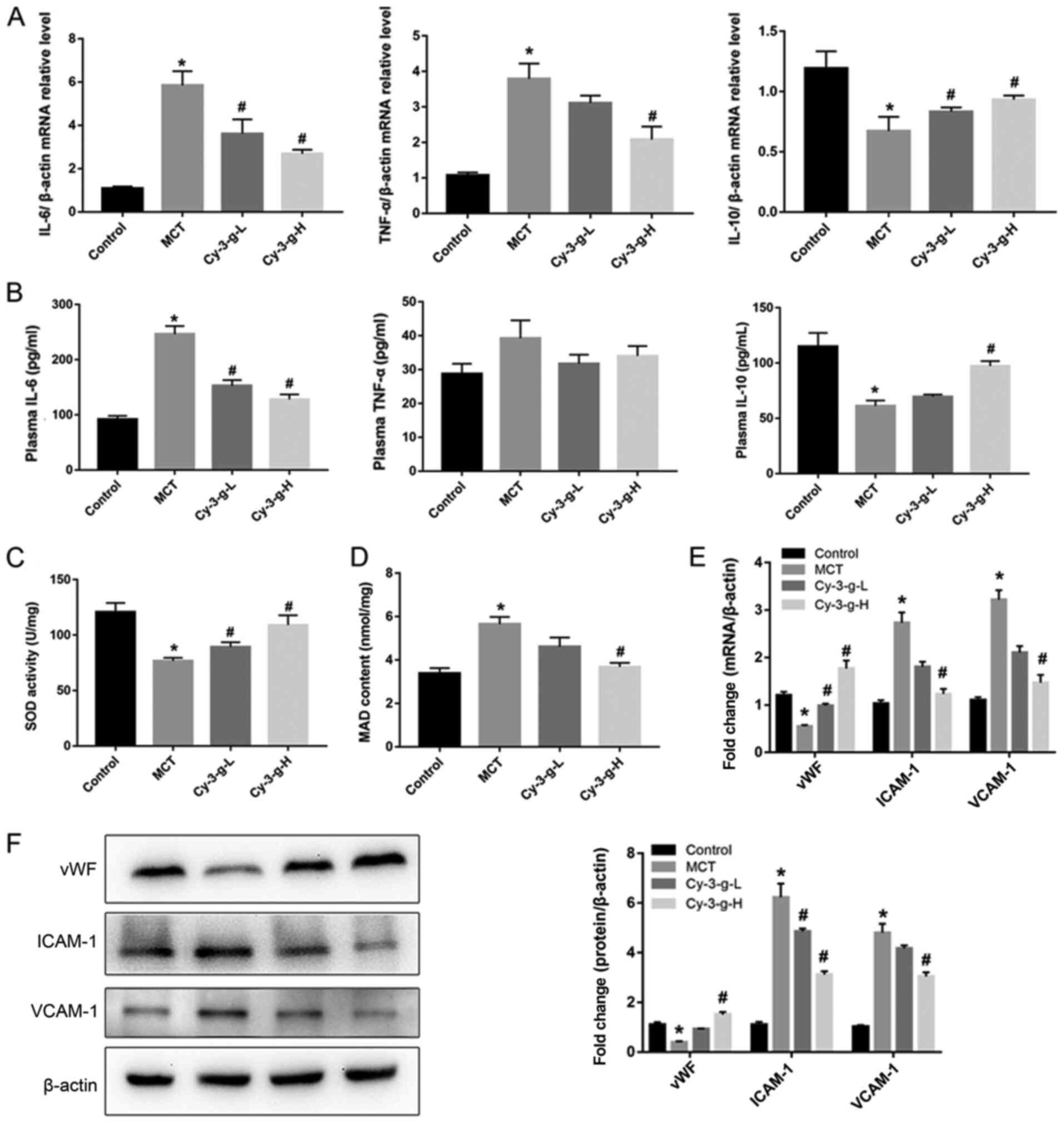 | Figure 2.Effect of Cy-3-g on inflammatory
responses and oxidative stress in rats with pulmonary artery
hypertension. Rats were randomly assigned into four groups (n=15):
Control group, model group (MCT), Cy-3-g-L group and Cy-3-g-H
group. On the first day of the experiments, the rats in the MCT,
Cy-3-g-L and Cy-3-g-H groups were injected with MCT (60 mg/kg). On
days 2–28, the rats in the Cy-3-g-L and Cy-3-g-H groups received a
gavage of 200 and 400 mg/kg body weight, respectively. (A) mRNA
level of IL-6, TNF-α and IL-10 in the lung tissue of rats. (B)
ELISA analysis of protein levels of IL-6, TNF-α and IL-10 in the
plasma of rats. Changes in (C) SOD activity and (D) MAD content in
the plasma of rats. (E) mRNA and (F) protein expression of vWF,
ICAM-1 and VCAM-1 in the lung tissue of rats. Data are shown as the
mean ± SD. *P<0.05 vs. control; #P<0.05 vs. MCT.
MCT, monocrotaline; Cy-3-g, cyanidin-3-O-β-glucoside; L, low-dose;
H, high-dose; IL, interleukin; TNF, tumor necrosis factor; SOD,
superoxide dismutase; MAD, malondialdehyde; vWF, von Willebrand
factor; ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular
adhesion molecule-1. |
Excessive oxidative stress leads to injury of
vascular endothelial cells, which is a risk factor for PAH
(8). Thus, the present study
evaluated the effects of Cy-3-g on oxidative stress. As shown in
Fig. 2C and D, the SOD activity and
MAD content of the MCT group increased 0.64- and 1.67-fold than
those of the control group, respectively. Compared with that of the
MCT group, the SOD activity of the Cy-3-g-L and Cy-3-g-H groups
increased by 0.16- and 0.42-fold, respectively, while the MAD
content of the Cy-3-g-L and Cy-3-g-H groups decreased by 0.18- and
0.39-fold, respectively. Considering the harmful effects of
oxidative stress on vascular function, the expression levels of
vWF, ICAM-1 and VCAM-1 were evaluated. The results of Fig. 2E and F indicated that the expression
of vWF was reduced, while the levels of ICAM-1 and VCAM-1 were
increased significantly after treatment with MCT. Cy-3-g
intervention reversed the above changes significantly.
Effects of Cy-3-g on cell
proliferation in MCT-induced PAH rats
Vascular remodeling induced by PASMC proliferation
is also one of the pathological mechanisms of PAH (10). The present study evaluated the
effects of Cy-3-g on PASMC proliferation by detecting the
expression of α-SMA and SM22. Fig.
3A showed that, compared with that of the control group, the
protein expression of α-SMA and SM22 was enhanced by 5.53- and
7.03-fold, respectively, in the MCT group. As shown in Fig. 3B, the mRNA levels of α-SMA and SM22
were significantly higher in MCT-induced rats than in normal rats
(11.67±1.022- and 7.915±0.458-fold, respectively). After Cy-3-g
consumption, the protein and mRNA expression of α-SMA and SM22 was
reduced significantly. In addition, the expression of the
pro-apoptotic protein Bax and the anti-apoptotic protein Bcl2 was
measured. Cy-3-g consumption significantly increased the expression
of Bax and decreased the expression of Bcl2 (Fig. 3A). VEGF and PDGF are important
indicators for evaluating the migration and proliferation of
vascular endothelial cells (31).
MCT stimulation elevated the expression of VEGF and PDGF-BB, which
could be an indicator of migration, proliferation and angiogenesis
of vascular endothelial cells. Consumption of Cy-3-g inhibited the
MCT-induced increase in VEGF and PDGF-BB expression, which further
suggested that Cy-3-g can prevent cell proliferation in MCT-induced
PAH rats.
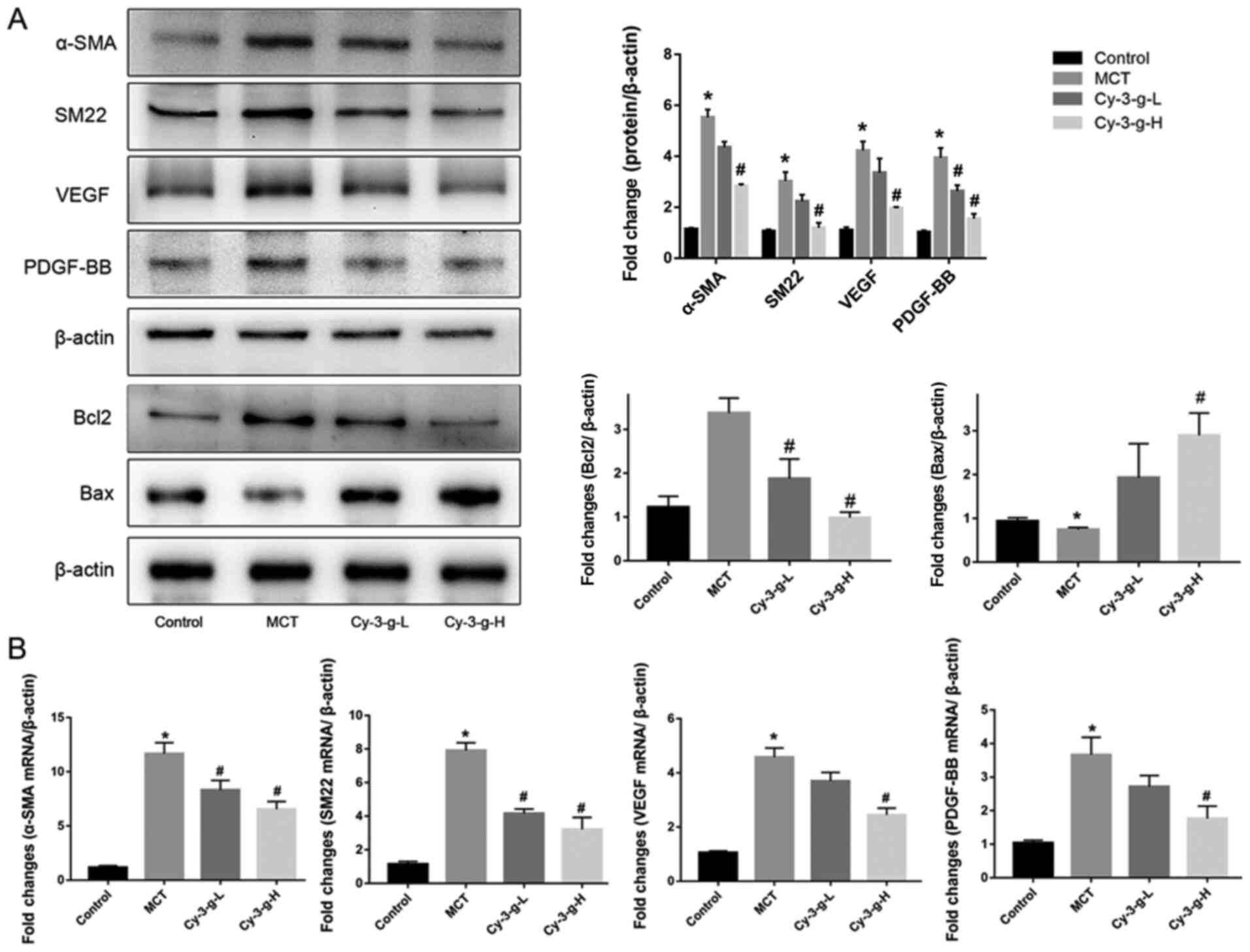 | Figure 3.Effect of Cy-3-g on cell
proliferation in rats with pulmonary artery hypertension. Rats were
randomly assigned into four groups (n=15): Control group, model
group (MCT), Cy-3-g-L group and Cy-3-g-H group. On the first day of
the experiments, the rats in the MCT, Cy-3-g-L and Cy-3-g-H groups
were injected with MCT (60 mg/kg). On days 2–28, the rats in the
Cy-3-g-L and Cy-3-g-H groups received a gavage of 200 and 400 mg/kg
body weight, respectively. (A) Protein expression of α-SMA, SM22,
VEGF, PDGF-BB, Bcl2 and Bax in the lung tissue of rats. (B) mRNA
level of α-SMA, SM22, VEGF and PDGF-BB in the lung tissue of rats.
Data are shown as the mean ± SD. *P<0.05 vs. control;
#P<0.05 vs. MCT. MCT, monocrotaline; Cy-3-g,
cyanidin-3-O-β-glucoside; L, low-dose; H, high-dose; VEGF, vascular
endothelial growth factor; PDGF, platelet-derived growth factor;
α-SMA, α-smooth muscle actin; SM22, smooth muscle 22. |
Involvement of the TGF-β1-p38
MAPK-CREB signaling pathway in the Cy-3-g-mediated inhibition of
PAH
Since Cy-3-g suppressed the inflammation, oxidative
stress and cell proliferation induced by MCT treatment, the
possible molecular mechanisms were investigated. The p38 MAPK
signaling pathway is a common pathway for the regulation of
vascular remodeling (18). Lungs of
rats were collected to detect the expression of p38 MAPK and its
phosphorylation, as well as the expression of the upstream and
downstream factors TGF-β1 and CREB. As depicted in Fig. 4, MCT treatment increased the
expression of TGF-β1, which activated the p38 MAPK signaling
pathway and promoted the level of p-p38 MAPK, followed by an
increase in the phosphorylation of CREB. The activation of p38 MAPK
was inhibited significantly by the consumption of Cy-3-g, followed
by a decrease in the expression of p-CREB. These findings suggested
that Cy-3-g prevented PAH induced by MCT, possibly via the
regulation of the TGF-β1-p38 MAPK-CREB signaling pathway.
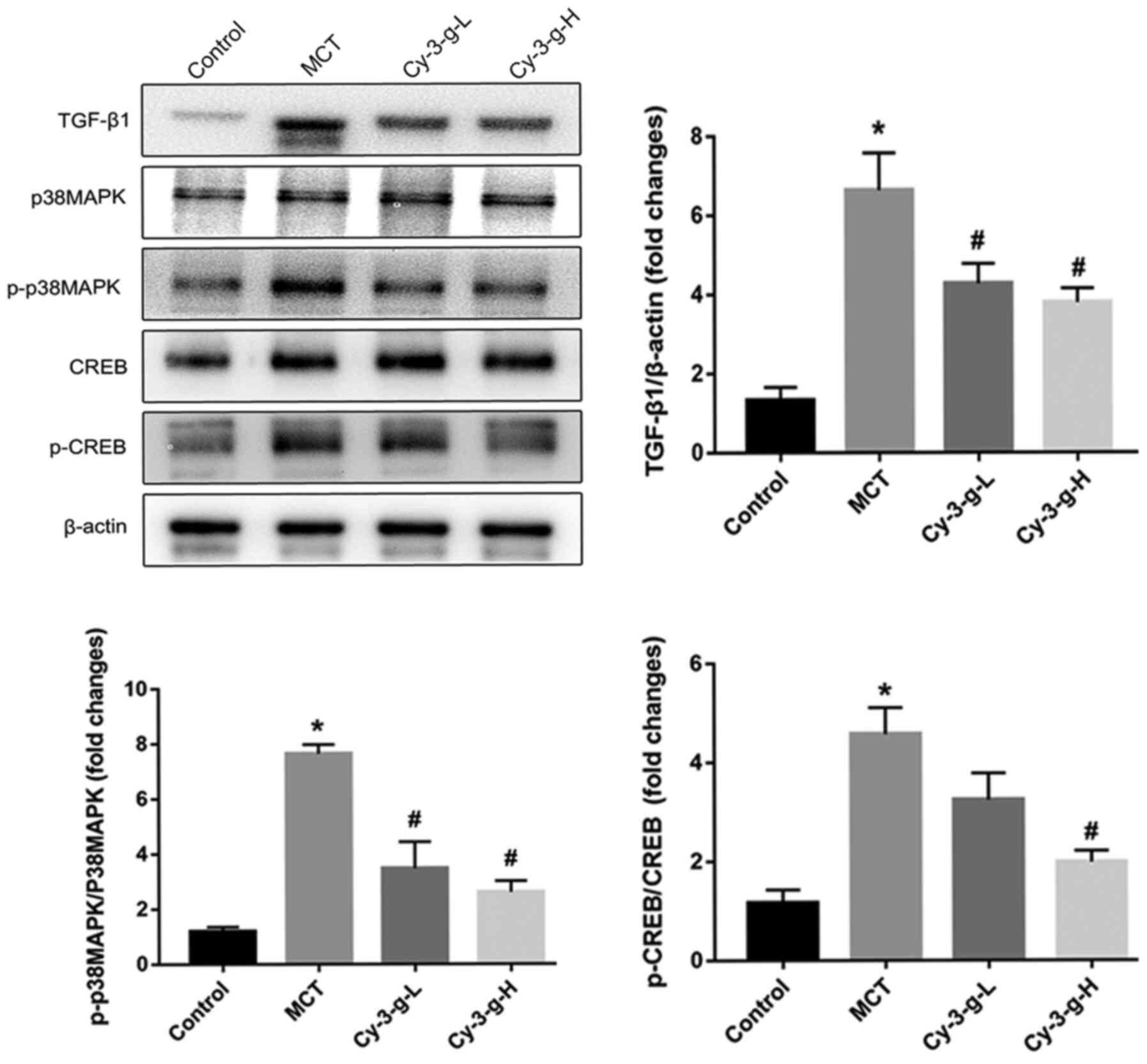 | Figure 4.Cy-3-g inhibits vascular remodeling
in rats with pulmonary artery hypertension via the TGF-β1/p38
MAPK/CREB signaling pathway. Rats were randomly assigned into four
groups (n=15): Control group, model group (MCT), Cy-3-g-L group and
Cy-3-g-H group. On the first day of the experiments, the rats in
the MCT, Cy-3-g-L and Cy-3-g-H groups were injected with MCT (60
mg/kg). On days 2–28, the rats in the Cy-3-g-L and Cy-3-g-H groups
received a gavage of 200 and 400 mg/kg body weight, respectively.
The protein expression of TGF-β1, p-p38 MAPK and p-CREB in the lung
tissue of rats was analyzed. Data are shown as the mean ± SD.
*P<0.05 vs. control; #P<0.05 vs. MCT. MCT,
monocrotaline; Cy-3-g, cyanidin-3-O-β-glucoside; L, low-dose; H,
high-dose; p-, phosphorylated; TGF, transforming growth factor;
MAPK, mitogen-activated protein kinase; CREB, cAMP-response element
binding protein. |
To confirm the role of the TGF-β1-p38 MAPK-CREB
signaling pathway on the inhibition of Cy-3-g on PAH, further in
vitro experiments using HPASMCs were performed. The cells were
induced by TGF-β1, and the expression of α-SMA was then detected
(Fig. 5B). Cy-3-g had no effect on
cell viability, as detected by the CCK-8 assay, but had a
significant effect on cell proliferation, as shown by the decrease
in α-SMA (Fig. 5A). The results of
western blotting showed that TGF-β1 stimulation elevated the
expression of p-p38 MAPK and p-CREB, which could be blocked by
Cy-3-g treatment (Fig. 5C). In
order to further validate the aforementioned results, the cells
were pretreated with P79350, a common agonist of p38 MAPK. After
treatment with P79350, the expression of Bcl2 increased and Bax
decreased, thus indicating that the protective effect of Cy-3-g on
cell proliferation was reversed by P79350 (Fig. 5D). Moreover, the inhibitory effects
of Cy-3-g on the phosphorylation of p38 MAPK and CREB were
significantly suppressed. These results indicated that the
TGF-β1-p38 MAPK-CREB signaling pathway is involved in the
inhibitory action of Cy-3-g in the development of PAH.
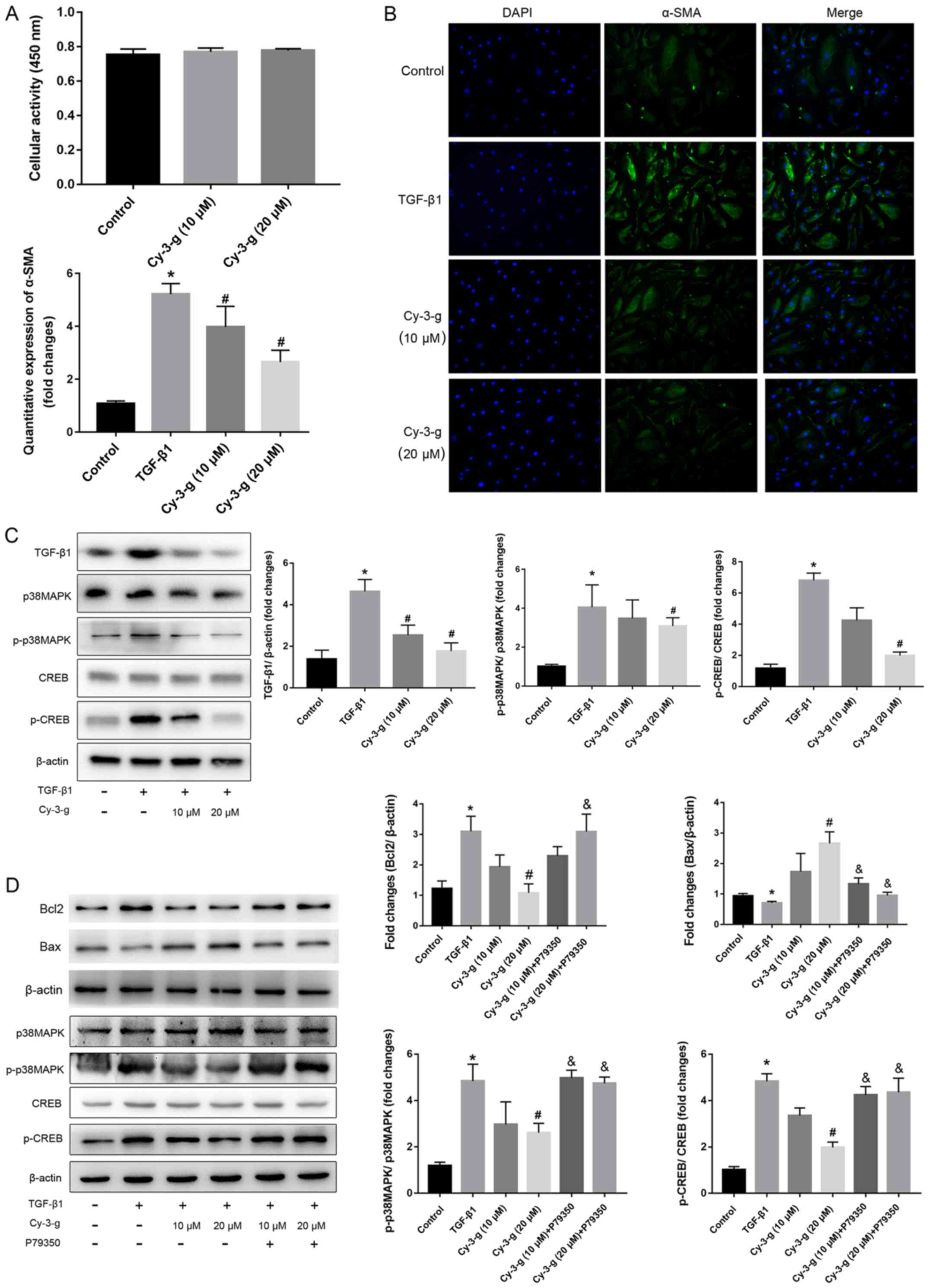 | Figure 5.Cy-3-g decreases TGF-β1-mediated cell
proliferation of HPASMCs via the p38 MAPK/CREB signaling pathway.
HPASMCs were pretreated with Cy-3-g (10 or 20 µm) for 24 h,
followed by treatment with TGF-β1 (8 ng/ml) or P79350 (0.2 µg/kg),
an agonist of p38 MAPK, for another 24 h. (A) Cell viability of
HPACMCs after treatment with Cy-3-g (10 or 20 µm) for 24 h. (B)
Representative photographs of immunofluorescence for α-SMA. (C)
Protein expression of TGF-β1, p-p38 MAPK and p-CREB in HPACMCs
after treatment with TGF-β1 and Cy-3-g. (D) Protein expression of
Bcl2, Bax, p-p38 MAPK and p-CREB in HPACMCs with the addition of
P79350. Data are shown as the mean ± SD. *P<0.05 vs. control;
#P<0.05 vs. TGF-β1; &P<0.05 vs.
Cy-3-g. HPASMCs, human pulmonary artery smooth muscle cells;
Cy-3-g, cyanidin-3-O-β-glucoside; p-, phosphorylated; α-SMA,
α-Smooth muscle actin; TGF, transforming growth factor; MAPK,
mitogen-activated protein kinase; CREB, cAMP-response element
binding protein. |
Discussion
The model of a single injection of MCT in rats is
considered to be one of the most effective animal models for
studying the development of PAH. Similar to the development of
human PAH, MCT transforms into MCT-pyrrole in the liver of rats,
and then damages endothelial cells and increases the infiltration
of mononuclear cells, which promotes the development of PAH
(32,33). In the present study, after the
induction of MCT, significantly increased mPAH, RVSP and RVHI, and
thickened pulmonary artery membrane of rats were observed, which
indicated the successful establishment of the animal model of PAH.
Wang et al (34) has
reported that plasma Cy-3-g reached the maximum levels (160.4±46.7
nmol/l) at 0.5 h after the oral gavage of Cy-3-g with 25 mg/kg body
weight. Additionally, according to the intervention concentration
(range of 10–1,000 mg/kg body weight) in numerous animal studies
(35,36), 200 and 400 mg/kg body weight was
selected as the intervention concentration. As expected, the
inhibitory effects of Cy-3-g on mPAH, RVSP, RVHI and pulmonary
artery membrane thickening were observed in MCT-induced rats, which
suggested an anti-PAH role of Cy-3-g in MCT-exposed rats.
In the absence of hypoxia, inflammatory cell
infiltration and inflammatory destruction in the lung are important
factors for pulmonary vascular remodeling and hemodynamic changes
(37). During hypoxia, inflammatory
factors can further mediate and regulate pulmonary vascular
remodeling (37). Therefore,
chronic inflammatory response is one of the pathogenic mechanisms
of PAH. IL-6 stimulates antigens to promote the proliferation of T
cells and the maturation of B cells, and strengthens the local
immune response, thereby stimulating the release of systemic
inflammatory factors, which promotes the development of PAH
(8). Animal experiments have
confirmed that overexpression of TNF-α induces the formation and
aggravation of PAH, whereas TNF-α receptor-deficient mice failed to
induce PAH (38). In addition,
abnormal activity of the ras homolog gene/a Rho-associated coiled
coil-forming protein kinase signaling pathway promotes the
migration and infiltration of inflammatory factors, leading to
pulmonary vasoconstriction and formation of PAH (39).
Oxidative stress may be the main cause of
endothelial injury and vascular wall remodeling (40). Oxidative stress destroys vascular
homeostasis, increases intracellular Ca2+ concentration,
and damages proteins, lipids and DNA. In addition, SMCs are
vulnerable to superoxide damage, which leads to disorder of cell
regulation, resulting in vascular contraction and increased blood
pressure caused by smooth muscle contraction (41). Reactive oxygen species promote
increased pulmonary vascular responses in newborn piglets and lead
to the formation of PAH (42).
Zhang et al (14) reported
increased MAD content and decreased SOD activity in PAH rats. The
present study also observed similar phenomena in MAD content and
SOD activity, whereas Cy-3-g consumption reduced MAD content and
enhanced SOD activity, which suggested that Cy-3-g has
antioxidative effects in PAH model rats. Moreover, it was found
that Cy-3-g elevated the downregulated expression of vWF, and
reduced the upregulated expression of ICAM-1 and VCAM-1 induced by
MCT. Cy-3-g attenuated oxidative stress-induced vascular
endothelial damage.
Proliferation of PASMCs, which leads to pulmonary
artery contraction and chronic pulmonary artery remodeling, is one
of the pathological mechanisms of PAH (10). Therefore, exploring the underlying
mechanism of pulmonary artery contraction inhibition, reducing
PASMC proliferation and delaying pulmonary vascular remodeling are
the key factors to improve the survival rate of patients with PAH.
It has been found that inhibition of transmembrane protein 16A
expression in pulmonary vessels inhibits the proliferation of VSMCs
and thus prevents the development of PAH (43). Classical protein kinase C is closely
associated with the abnormal proliferation of PASMCs and the
progression of PAH (44). Fan et
al (45) indicated that YM155
(sepantronium bromide) inhibited the proliferation of PASMCs and
improved pulmonary vascular remodeling in PAH rats. The present
study observed the effect of Cy-3-g on the proliferation of PASMCs
by detecting the expression of α-SMA, SM22, pro-apoptotic protein
Bax and anti-apoptotic protein Bcl2. These findings demonstrated
that Cy-3-g notably reduced the expression of α-SMA, SM22 and Bcl2
but elevated the levels of Bax, suggesting the protective effect of
Cy-3-g on the proliferation of PASMCs.
Numerous studies have focused on the preventive
effects of ACNs on chronic diseases (46). Kong et al (21) demonstrated the properties of ACNs
and its metabolite protocatechuic acid, including
anti-inflammatory, anti-oxidative stress, inhibition of vascular
endothelial cell damage and promotion of cholesterol outflow
(17,47). In a study by Zhu et al
(48), ACNs increased nitric oxide
(NO) release, leading to improved endothelium-dependent
vasodilation. A previous epidemiological study also found an
increase of ACNs in serum NO secretion (49). Abnormal systolic and diastolic
function of pulmonary arterioles is one of the risk factors for PAH
(11). NO produced by endothelial
cells is a powerful vasodilator (50). It mainly causes relaxation of
vascular smooth muscles by activating guanylate cyclase on vascular
walls, and can reduce the sensitivity of blood vessels to
angiotensin, thereby reducing vascular tension and expanding blood
vessels (51). Endothelin has a
strong vasoconstrictive effect, and promotes cell proliferation and
inflammation (52). The level of
endothelin-1 (ET-1) in patients with COPD is significantly
increased, and ET-1 receptor antagonists are currently an important
drug target in the treatment of PAH (53). ET-1 and NO are two opposite factors
that regulate vasodilation. Their imbalance leads to changes in
pulmonary vascular endothelial function, especially in the early
stage of pulmonary vascular remodeling (54,55).
Some studies have reported on the inhibition of
other polyphenols in the progression of PAH. Numerous studies
focused on the preventative action of resveratrol on PAH via the
activation of silent information regulator 1 (56,57).
Rashid et al (58) indicated
that polyphenol-rich blackcurrant juice protects chronic bile duct
ligation-induced endothelial dysfunction in rats by promoting the
production of NO and inhibiting the expression of inflammatory
factors. Hua et al (59)
indicated that polyphenol extracted from apples decreased the
expression of caspase-3 and inducible NO synthase, and inhibited
the activation of cation channels, which attenuated the development
of pulmonary vasoconstriction. However, the molecular mechanism
involved has not been explored in these studies.
The progression of PAH is closely associated with
members of the TGF-β superfamily (15). Mutations of bone morphogenetic
protein receptor type 2 gene are highly prevalent in patients with
PAH, as well as the coding genes of some important molecules in the
pathway of the TGF-β superfamily (16). An increasing number of studies have
indicated that TGF-β1 is the key mediator of pathogenesis in PAH
(60). It has been demonstrated
that the increased synthesis and accumulation of TGF-β1 are
necessary for PAH progression. Moreover, abnormally elevated levels
of TGF-β1 have been found in patients with PAH (16,61).
Previous studies used TGF-β1 or BMP factors to interfere with the
proliferation of PASMCs (62).
TGF-β1 is a multifunctional channel protein, which plays a vital
role in growth regulation (16). It
promotes the proliferation of VSMCs by changing them from a static
and contractile state to a proliferative state via the activation
of the p38 MAPK signaling pathway (17). The p38 MAPK signaling pathway
regulates cell proliferation, apoptosis, extracellular matrix
metabolism and inflammation. Ren et al (63) observed that blocking the p38 MAPK
pathway could alleviate the inflammation induced by LPS. In
addition, activated p38 MAPK leads to the activation of NF-κB
signaling, thereby mediating the inflammatory response (64). CREB is a nuclear transcription
enhancer, which regulates cell proliferation, differentiation and
survival via phosphorylation (65).
The p38 MAPK-CREB signaling pathway can be activated rapidly in
hyperglycemia, oxidative stress and inflammatory reactions
(66). In the present study, MCT
promoted the expression of TGF-β1, and the phosphorylation of p38
MAPK and CREB, while Cy-3-g blocked the activation of the
TGF-β1-p38 MAPK-CREB signaling pathway.
In summary, the current study revealed the
inhibitory effect of Cy-3-g on hemodynamics and morphological
characteristics of PAH in MCT-induced rats. Furthermore, the
suppressive effect of Cy-3-g on PAH progression was exerted via the
inhibition of vascular remodeling, including inflammatory response,
oxidative stress and cell proliferation, possibly through
regulation of the TGF-β1-p38 MAPK-CREB signaling pathway. These
results demonstrated the protective effects of Cy-3-g on PAH.
Cy-3-g may be used as a novel product for the prevention and
treatment of PAH.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Scientific
Research Projects of Health and Family Planning Commission in Hunan
Province of China (grant no. B20180057), the Science and Health
Joint Project of the Hunan Provincial Natural Science Foundation of
China (grant no. 2018JJ6069), the Scientific Research Projects of
Health Commission in Hunan Province of China (grant no. 20201945),
the Scientific Research Projects of the University of South China
in 2020 (grant no. 202006), the Key Laboratory of Heart Failure
Prevention and Treatment in Hengyang (grant no. 2019jh426001) and
the Youth Academic Leadership Project of The Second Affiliated
Hospital of the University of South China.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SO and WC developed the overall research plan and
oversaw the study. SO carried out all the experiments and wrote the
manuscript. ZG, LC and YM contributed to the experiments. TG, ZM
and LY analyzed the experimental data and revised manuscript. JL
constructed the animal model of pulmary heart disease and part of
the cytology experiment, and participated in the data analysis. The
manuscript is the original work of all authors, and the final
manuscript has been read and approved by all authors.
Ethics approval and consent to
participate
The procedures used in the animal experiments were
performed in line with the Guide for the Care and Use of Laboratory
Animals, and was approved by the Animal Care Committee at the
University of South China [approval no. SYXK (Xiang):
2005-0001].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vender RL: Chronic hypoxic pulmonary
hypertension. Chest. 106:236–243. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Greyson CR: Pathophysiology of right
ventricular failure. Crit Care Med. 36 (Suppl 1):S57–S65. 2018.
View Article : Google Scholar
|
|
3
|
Humbert M, Morrell NW, Archer SL, Stenmark
KP, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O,
Voelkel FN and Rabinovitch M: Cellular and molecular pathobiology
of pulmonary arterial hypertension. J Am Coll Cardiol. 43 (Suppl
12):S13–S24. 2014. View Article : Google Scholar
|
|
4
|
Morrell NW, Adnot S, Archer SL, Dupuis J,
Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA,
Weissmann N, et al: Cellular and molecular basis of pulmonary
arterial hypertension. J Am Coll Cardiol. 54 (Suppl 1):S20–S31.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tuder RM, Archer SL, Dorfmuller P, Erzurum
SC, Guignabert C, Michelakis E, Rabinovitch M, Schermuly R,
Stenmark KR and Morrell NW: Relevant issues in the pathology and
pathobiology of pulmonary hypertension. J Am Coll Cardiol. 62
(Suppl 25):D4–D12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peacock AJ, Murphy NF, McMurray JJ,
Caballero L and Stewart S: An epidemiological study of pulmonary
arterial hypertension. Eur Respir J. 30:104–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Montani D, Chaumais MC, Guignabert C,
Günther S, Girerd B, Jaïs X, Algalarrondo V, Price LC, Savale L,
Sitbon O, et al: Targeted therapies in pulmonary arterial
hypertension. Pharmacol Ther. 141:172–191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rabinovitch M, Guignabert C, Humbert M and
Nicolls MR: Inflammation and immunity in the pathogenesis of
pulmonary arterial hypertension. Circ Res. 115:165–175. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ahmed LA, Obaid AA, Zaki HF and Agha AM:
Role of oxidative stress, inflammation, nitric oxide and
transforming growth factor-beta in the protective effect of
diosgenin in monocrotaline-induced pulmonary hypertension in rats.
Eur J Pharmacol. 740:379–387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Satoh K, Satoh T, Kikuchi N, Omura J,
Kurosawa R, Suzuki K, Sugimura K, Aoki T, Nochioka K, Tatebe S, et
al: Basigin mediates pulmonary hypertension by promoting
inflammation and vascular smooth muscle cell proliferation. Circ
Res. 115:738–750. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y and Wu S: Effects of fasudil on
pulmonary hypertension in clinical practice. Pulm Pharmacol Ther.
46:54–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu C, Fang C, Cao G, Liu K, Wang B, Wan
Z, Li S and Wu S: Ethyl pyruvate ameliorates monocrotaline-induced
pulmonary arterial hypertension in rats. J Cardiovasc Pharmacol.
64:7–15. 2017. View Article : Google Scholar
|
|
13
|
Balabanian K, Foussat A, Dorfmüller P,
Durand-Gasselin I, Capel F, Bouchet-Delbos L, Portier A,
Marfaing-Koka A, Krzysiek R, Rimaniol AC, et al: CX(3)C chemokine
fractalkine in pulmonary arterial hypertension. Am J Respir Crit
Care Med. 165:1419–1425. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Q, Fan K, Wang P, Yu J, Liu R, Qi H,
Sun H and Cao Y: Carvacrol induces the apoptosis of pulmonary
artery smooth muscle cells under hypoxia. Eur J Pharmacol.
770:134–146. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y and Hu CN: Effects of Maher
BbeChatain on the expression of transforming growth factor-1 and
connective tissue growth factor in diabetic rats. Chin J Geriat
Med. 34:6691–6693. 2014.
|
|
16
|
Joshua DS, Andrew WH and Jackson R:
AMP-activated protein kinase inhibits transforming growth
factor-β-mediated vascular smooth muscle cell growth: Implications
for a Smad-3-dependent mechanism. Am J Physiol Heart Circ Physiol.
309:H1251–H1259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kwon IS, Yim JH, Lee HK and Pyo S: Lobaric
acid inhibits VCAM-1 expression in TNF-α-etimulated vascular smooth
muscle cells via modulation of NF-κB and MAPK signaling pathways.
Biomol Ther (Seoul). 24:25–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu Y, Gu Q and Qu C: Capsaicin
pretreatment reversed pulmonary arterial hypertension by
alleviating inflammation via p38MAPK pathway. Ex Lung Res. 43:8–18.
2017. View Article : Google Scholar
|
|
19
|
Ding M, Feng R, Wang SY, Bowman L, Lu Y,
Qian Y, Castranova V, Jiang BL and Shi X: Cyanidin-3-glucoside, a
natural product derived from blackberry, exhibits chemopreventive
and chemotherapeutic activity. J Biol Chem. 281:17359–17368. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fang J: Bioavailability of anthocyanins.
Drug Metab Rev. 46:508–520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kong JM, Chia LS, Goh NK, Chia TF and
Brouillard R: Analysis and biological activities of anthocyanins.
Phytochemistry. 64:923–933. 2008. View Article : Google Scholar
|
|
22
|
Herath HM, Takano-Ishikawa Y and Yamaki K:
Inhibitory effect of some flavonoids on tumor necrosis factor-alpha
production in lipopolysaccharide-stimulated mouse macrophage cell
line J774.1. J Med Food. 6:365–370. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xia XD, Ling WH, Ma J, Xia M, Hou MJ, Wang
Q, Zhu HL and Tang ZH: Anthocyanin-rich extract from black rice
enhances atherosclerotic slaque stabilization in apolipoprotein
E-deficient mice. J Nutr. 136:2220–2225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang DL, Wei X, Yan X, Jin T and Ling WH:
Protocatechuic acid, a metabolite of anthocyanins, inhibits
monocyte adhesion and reduces atherosclerosis in apolipoprotein
E-deficient mice. J Agric Food Chem. 58:12722–12728. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Wang X, Pang J, Zhang HY, Luo J,
Qian XY, Chen Q and Ling WH: Attenuation of atherosclerosis by
protocatechuic acid via inhibition of M1 and promotion of M2
macrophage polarization. J Agric Food Chem. 67:807–818. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dell'Agli M, Busciala A and Bosisio E:
Vascular effects of wine polyphenols. Cardiovasc Res. 63:593–602.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xia M, Ling WH, Zhu HL, Ma J, Wang Q, Hou
MJ, Tang ZH, Guo HH, Liu C and Ye QY: Anthocyanin attenuates
CD40-mediated endothelial cell activation and apoptosis by
inhibiting CD40-induced MAPK activation. Atherosclerosis.
202:41–47. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mao GX, Zheng LD, Cao YB, Chen ZM, Lv YD,
Wang YZ, Hu XL, Wang GF and Yan J: Antiaging effect of pine pollen
in human diploid fibroblasts and in a mouse model induced by
D-Galactose. Oxid Med Cell Longev. 2012:7509632012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kylhammar D, Hesselstrand R, Nielsen S,
Scheele C and Rådegran G: Angiogenic and inflammatory biomarkers
for screening and follow-up in patients with pulmonary arterial
hypertension. Scand J Rheumatol. 47:319–324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Appelmann I, Liersch R, Kessler T, Mesters
RM and Berdel WE: Angiogenesis inhibition in cancer therapy:
Platelet-derived growth factor (PDGF) and vascular endothelial
growth factor (VEGF) and their receptors: Biological functions and
role in malignancy. Recent Results Cancer Res. 180:51–81. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu N, Zhao X, Xiang Y, Ye S, Huang J, Hu
W, Lv L and Zeng C: Thymoquinone attenuates monocrotaline-induced
pulmonary artery hypertension via inhibiting pulmonary arterial
remodeling in rats. Int J Cardiol. 221:587–596. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maarman G, Lecour S, Butrous G, Thienemann
F and Sliwa K: A comprehensive review: The evolution of animal
models in pulmonary hypertension research; are we there yet? Pulm
Circ. 3:739–756. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang D, Zou T, Yang Y, Yan X and Ling W:
Cyanidin-3-O-β-glucoside with the aid of its metabolite
protocatechuic acid, reduces monocyte infiltration in
apolipoprotein E-deficient mice. Biochem. Pharmacol. 7:713–719.
2011.
|
|
35
|
Yan XR, Wu L, Li B, Meng XJ, Dai HP, Zheng
YN and Fu JF: Cyanidin-3-O-glucoside attenuates acute lung injury
in sepsis rats. J Surg Res. 199:592–600. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qian XY, Wang X, Luo J, Liu Y, Pang J,
Zhang HY, Xu ZL, Xie JW, Jiang XW and Ling WH: Hypouricemic and
nephroprotective roles of anthocyanins in hyperuricemic mice. Food
Func. 10:867–878. 2019. View Article : Google Scholar
|
|
37
|
Joppa P, Petrasova D, Stancak B and
Tkacova R: Systemic inflammation in patients with COPD and
pulmonary hypertension. Chest. 130:326–333. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Price LC, Wort SJ, Perros F, Dorfmuller P,
Huertas A, Montani D, Cohen-Kaminsky S and Humbert M: Inflammation
in pulmonary arterial hypertension. Chest. 141:210–221. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fukumoto Y: Role of the Rho-kinase pathway
in pulmonary arterial hypertension. Nihon Yakurigaku Zasshi.
143:178–181. 2014.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Irarrázaval S, Allard J, Campodónico J,
Perez D, Strobel P, Vasquez L, Urquiaga I, Echeverria G and
Leighton F: Oxidative stress in acute hypobaric hypoxia. High Alt
Med Biol. 18:128–134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen J, Wang YX, Dong MQ, Zhang B, Luo Y,
Niu W and Li ZC: Reoxygenation reverses hypoxic pulmonary arterial
remodeling by inducing smooth muscle cell apoptosis via reactive
oxygen species-mediated mitochondrial dysfunction. Am Heart Assoc.
6:e0056022017.
|
|
42
|
Suresh K, Servinsky L, Jiang HY, Bigham Z,
Yun X, Kliment C, Huetsch J, Damarla M and Shimoda LA: Reactive
oxygen species induced Ca2+ influx via TRPV4 and
microvascular endothelial dysfunction in the SU5416/hypoxia model
of pulmonary arterial hypertension. Am J Physiol Lung Cell Mol
Physiol. 314:L893–L907. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zeng JW, Chen BY, Lv XF, Sun L, Zeng XL,
Zheng HQ, Du YH, Wang GL, Ma MM and Guan YY: Transmembrane member
16A participates in hydrogen peroxide-induced apoptosis by
facilitating mitochondria-dependent pathway in vascular smooth
muscle cells. Br J Pharmacol. 175:3669–3684. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hisayama T, Inomoto M, Hioki Y and Fukui
H: Identification of PKC isozymes and effect of knockdown of PKC
alpha by antisense oligodeoxynucleotide on iNOS expression via
interleukin-1 receptor in vascular smooth muscle cells. Nihon
Yakurigaku Zasshi. 114 (Suppl 1):86P–91P. 1999.(In Japanese).
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fan Z, Liu B, Zhang S, Liu H, Li Y, Wang
D, Liu Y, Li J, Wang N, Liu Y and Zhang B: YM155, a selective
survivin inhibitor, reverses chronic hypoxic pulmonary hypertension
in rats via upregulating voltage-gated potassium channels. Clin Exp
Hypertens. 37:381–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Manach C, Mazur A and Scalbert A:
Polyphenols and prevention of cardiovascular diseases. Curr Opin
Lipidol. 16:77–84. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xia M, Hou M, Zhu H, Ma J, Tang Z, Wang Q,
Li Y, Chi D, Yu X, Zhao T, et al: Anthocyanins induce cholesterol
efflux from mouse peritoneal macrophages: The role of the
peroxisome proliferator-activated receptor {gamma}-liver X receptor
{alpha}-ABCA1 pathway. J Biol Chem. 280:36792–36801. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhu Y, Xia M, Yang Y, Liu F, Li Z, Hao YT,
Mi MT, Jin T and Ling WH: Purified anthocyanin supplementation
improves endothelial function via NO-cGMP activation in
hypercholesterolemic individuals. Clin Chem. 57:1524–1533. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Edirisinghe I, Banaszewski K, Cappozzo J,
McCarthy D and Burton-Freeman BM: Effect of black currant
anthocyanins on the activation of endothelial nitric oxide synthase
(eNOS) in vitro in human endothelial cells. J Agric Food Chem.
59:8616–8624. 2013. View Article : Google Scholar
|
|
50
|
Sun XZ, Tian XY, Wang DW and Li J: Effects
of fasudil on hypoxic pulmonary hypertension and pulmonary vascular
remodeling in rats. Eur Rev Med Pharmacol Sci. 18:959–964.
2014.PubMed/NCBI
|
|
51
|
Sim JY: Nitric oxide and pulmonary
hypertension. Korean J Anesthesiol. 58:4–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Polonio IB, Acencio MM, Pazetti R, Almeida
FM, Silva BS, Pereira KA and Souza R: Lodenafil treatment in the
monocrotaline model of pulmonary hypertension in rats. J Bras
Pneumol. 40:421–424. 2014.(In English, Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Satwiko MG, Ikeda K, Nakayama K, Yagi K,
Hocher B, Hirata K and Emoto N: Targeted activation of endothelin-1
exacerbates hypoxia-induced pulmonary hypertension. Biochem Biophys
Res Commun. 465:356–362. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wardle AJ, Seager MJ, Wardle R, Tulloh RM
and Gibbs JS: Guanylate cyclase stimulators for pulmonary
hypertension. Cochrane Database Syst Rev. 8:CD0112052016.
|
|
55
|
Hoeper MM, McLaughlin VV, Dalaan AM, Satoh
T and Galie N: Treatment of pulmonary hypertension. Lancet Respir
Med. 4:323–336. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yu L, Tu Y, Jia X, Fang K, Liu L, Wan L,
Xiang C, Wang Y, Sun X, Liu T, et al: Resveratrol protects against
pulmonary arterial hypertension in rats via activation of silent
information regulator 1. Cell Physiol Biochem. 42:55–67. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhou S, Li MT, Jia YY, Liu JJ, Wang Q,
Tian Z, Liu YT, Chen HZ, Liu DP and Zeng XF: Regulation of cell
cycle regulators by SIRT1 contributes to resveratrol-mediated
prevention of pulmonary arterial hypertension. Biomed Res Int.
2015:7623492015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rashid S, Idris-Khodja N, Auger C, Kevers
C, Pincemail J, Alhosin M, Boehm N, Oswald-Mammosser M and
Schini-Kerth VB: Polyphenol-rich blackcurrant juice prevents
endothelial dysfunction in the mesenteric artery of cirrhotic rats
with portal hypertension: Role of oxidative stress and the
angiotensin system. J Med Food. 21:390–399. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hua C, Zhao J, Wang H, Chen F, Meng H,
Chen L, Zhang Q, Yan J and Yuan L: Apple polyphenol relieves
hypoxia-induced pulmonary arterial hypertension via pulmonary
endothelium protection and smooth muscle relaxation: In vivo and in
vitro studies. Biomed Pharmacother. 107:937–944. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang N, Dong MQ, Luo Y, Zhao F and Li YJ:
Danshensu prevents hypoxic pulmonary hypertension in rats by
inhibiting the proliferation of pulmonary artery smooth muscle
cells via TGF-β-smad3-associated pathway. Eur J Pharmacol. 820:1–7.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Upton PD, Davies RJ, Tajsic T and Morrell
NW: Transforming growth factor-β(1) represses bone morphogenetic
protein-mediated Smad signaling in pulmonary artery smooth muscle
cells via Smad3. Am J Respir Cell Mol Biol. 49:1135–1145. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sheares KK, Jeffery TK, Long L and Morrell
NW: Differential effects of TGF-beta1 and BMP-4 on the hypoxic
induction of cyclooxygenase-2 in human pulmonary artery smooth
muscle cells. Am J Physiol Lung Cell Mol Physiol. 287:L919–L927.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ren X, Shi Y, Zhao D, Xu M, Li X, Dang Y
and Ye X: Naringin protects ultraviolet B-induced skin damage by
regulating p38MAPK signal pathway. J Dermatol Sci. 82:106–114.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chen X, Xiu M, Xing J, Yu S, Min D and Guo
F: Lanthanum chloride inhibits LPS mediated expressions of
pro-inflammatory cytokines and adhesion molecules in HUVECs:
Involvement of NF-κB-Jmjd3 signaling. Cell Physiol Biochem.
42:1713–1724. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ge J, Zhang Y and Zhang ZZ: Research
progress on CREB and the signal transduction by phosphorylation of
CREB at serine 133. Cell. 59:675–680. 2009.
|
|
66
|
Gonzallez GA and Montminy MR: Cyclic AMPs
timulatessomatostat in gene transcription by phosphorylation of
CREB at serine 133. Cell. 59:675–680. 2009. View Article : Google Scholar
|



















