Introduction
Hemorrhagic shock (HS) is a condition characterized
by reduced tissue perfusion, which results in the delivery of
oxygen and nutrients being inadequate for cellular function
(1). As modern society has
developed, the incidence of HS resulting from traffic accidents and
natural disasters has increased, and HS has become one of the main
causes of mortality worldwide (2).
The induction of hepatic ischemic injury by HS is a common
pathophysiological occurrence in the clinical setting, and is
characterized by the release of inflammatory mediators and the
activation and recruitment of neutrophils (3). The pathological mechanisms underlying
hepatic ischemic injury remain unclear, but they may involve the
apoptosis of hepatocytes, accumulation of reactive oxygen species,
changes in mitochondrial permeability and endoplasmic reticulum
stress (4). Current research also
indicates that the main mechanism underlying hepatic ischemic
injury is an excessive and uncontrolled inflammatory response in
the liver (5).
Nuclear factor (NF)-κB is a nuclear transcription
factor that participates in a number of signal transduction
pathways in the process of inflammation, and NF-κB signaling plays
an important role in the development of ischemic injury in organs
(6). A recent study suggested that
the suppression of activation of the NF-κB signaling pathway is the
key to inhibiting the inflammatory response, and that NF-κB is a
potential target for the alleviation of ischemic injury (7).
Melatonin (MT) is an indoleamine hormone synthesized
by the pineal gland of vertebrates, which has a variety of strong
direct and indirect antioxidant and anti-inflammatory properties
(8). MT can counteract the ischemic
injury of multiple organs through its antioxidant effects and
strong free radical-scavenging capacity at the molecular level
(9). Furthermore, MT has been
demonstrated to prevent the development of ischemic injury by
reducing oxidative stress-induced activation of the NF-κB signaling
pathway (10). The aim of the
present study was to investigate the protective effects of
exogenous MT against HS-induced hepatic ischemic injury in rats and
its association with the NF-κB pathway.
Materials and methods
Reagents
MT (cat. no. M5250) and TRIzol® were
purchased from Invitrogen (Thermo Fisher Scientific, Inc.). Alanine
aminotransferase (ALT; cat. no. ml063179), aspartate
aminotransferase (AST; cat. no. ml058577) lactate dehydrogenase
(LDH; cat. no. ml037243) and glutamate dehydrogenase (GDH; cat. no.
ml037964) assay kits were purchased from Shanghai Enzyme-linked
Biotechnology Co., Ltd. ELISA kits for the detection of tumor
necrosis factor (TNF)-α (cat. no. ml037211), interferon (IFN)-γ
(cat. no. ml063095), interleukin (IL)-6 (cat. no. ml002293) and
IL-1β (cat. no. ml063132) were purchased from Shanghai
Enzyme-linked Biotechnology Co., Ltd. Hematoxylin and eosin
(H&E) staining assay kit was purchased from Beijing Solarbio
Science & Technology Co., Ltd. DeadEnd™ Fluorometric TUNEL
System kit was purchased from Promega Corporation. Nuclear and
Cytoplasmic Protein Extraction kit (cat. no. P0028) was bought from
Beyotime Institute of Biotechnology.
Animal grouping and HS model
preparation
A total of 45 healthy, clean-grade male Sprague
Dawley rats, weighing 250–280 and aged 10–12 weeks, were provided
and fed by the experimental animal center of Taizhou First People's
Hospital. All animals underwent adaptive feeding for 7 days before
the experiment, and were kept at a temperature between 20 and 25°C
and humidity between 40 and 70%. The animals were allowed to eat
and drink freely. All the animals, reagents and treatment methods
used in the experiments were approved by the animal experiment
ethics committee of Taizhou First People's Hospital, and all
experimental procedures were performed in a manner that minimized
suffering and reduced the number of animals used according to the
Animal Research Reporting In Vivo Experiments (ARRIVE)
Guidelines (11). The 45 Sprague
Dawley rats were randomly divided into three groups: Sham group, HS
model group and MT treatment group (n=15/group). Each rat was
anesthetized with 5% pentobarbital sodium (30 mg/kg body weight) by
intraperitoneal injection. The trachea was orally intubated, and
spontaneous breathing was maintained. The right carotid artery and
left jugular vein were separated, and polyethylene catheters were
inserted into them for the withdrawal and transfusion of blood.
Approximately 40% of the total blood volume was withdrawn in 30 min
through a two-way automatic infusion pump to establish the HS
model. After 30 min, fluid resuscitation was performed according to
Advanced Trauma Life Support guidelines (12). All animals were injected with
penicillin to avoid infection. The sham group was subjected to
tracheal intubation without trauma modeling. In the HS and MT
groups, a pressure-controlled traumatic HS model was established
according to the standard protocol (13). Following traumatic HS for 1 h, rats
in the MT group were injected intravenously with MT (10 mg/kg),
whereas the sham and HS group rats received an equal volume of PBS.
A 10 mg/kg dose of MT was used because 10 mg/kg MT showed a
satisfactory anti-hepatic ischemia injury effect in the HS model in
a preliminary study. Also, a 10 mg/kg dose is supported by previous
studies (12–14). Following surgery, the animals were
monitored by the laboratory group every 4 h. During the experiment,
12 Sprague Dawley rats (8 in the HS group and 4 in the MT group)
died of shock. The 33 Sprague Dawley rats (15 in the sham group, 7
in the HS group and 11 in the MT group) that remained alive 24 h
after surgery were euthanized by anesthesia with intraperitoneally
injected pentobarbital (30 mg/kg) followed by cervical dislocation.
Death was confirmed by lack of heartbeat, lack of respiration, lack
of corneal reflex and the presence of rigor mortis. Livers were
excised for pathological observation using a light microscope. The
study established specific criteria, i.e., humane endpoints, to
determine when animals should be euthanized according to ARRIVE
Guidelines. The humane endpoints were a reduction of 4–6°C in body
temperature, a weight loss of >10%, decreased activity
(lethargy) and alertness, a rough coat and hunched posture, which
are direct signs of illness, pain or distress.
Automatic biochemical analysis
Following traumatic HS for 6, 12, 18 and 24 h, blood
samples were collected from the inferior vena cava of the rats and
centrifuged at 4°C, 500 × g for 5 min. The upper layer of serum was
collected and stored at −80°C until use. The levels of ALT, AST,
LDH and GDH in the serum and hepatic tissue homogenate, were
measured using the aforementioned kits in an automatic biochemical
analyzer according to the manufacturer's instructions.
ELISA
Serum was prepared from blood samples as
aforementioned. The hepatic tissues were removed from the rats when
sacrificed, 24 h after surgery. After washing with normal saline,
hepatic tissue homogenate was prepared using an automatic
homogenizer, and centrifuged at 4°C and 500 × g for 5 min. A total
of 1 ml cell suspension was obtained from each group and placed
into 96-well ELISA plate, and the absorbance of each well at a
wavelength of 490 nm was detected using an ELISA reader. The
concentrations of TNF-α, IFN-γ, IL-6 and IL-1β in the serum and
tissue homogenate were measured and expressed as pg/ml in each
sample.
H&E staining
Hepatic tissue samples were fixed in 4%
paraformaldehyde at 37°C for 30 min and then dehydrated,
transparentized, embedded in paraffin and cut into 4-µm sections.
The sections were then stained with H&E for 4 h at room
temperature. The stained tissue was placed in 1% hydrochloric acid
ethanol (Merck KGaA) for differentiation at room temperature for 10
sec and sealed using neutral gum. The degree of fatty degeneration,
inflammation and necrosis of the H&E stained liver samples were
then evaluated under a light microscope. Hepatic pathology was
scored by Suzuki's criteria (14).
TUNEL assay
Hepatic tissue samples were fixed in 4%
paraformaldehyde for 30 min at room temperature and then
dehydrated, transparentized, embedded in paraffin and cut into 4-µm
sections. Subsequently, the sections were treated with 100 µl TUNEL
reaction mixture (cat. no. C1086; Beyotime Institute of
Biotechnology) at 37°C for 1 h, followed by incubation with 100 µl
DNase at room temperature for 5 min. Following washing with PBS,
the sections were treated with 100 µl DAB for at room temperature
10 min in the dark. Apoptotic cells were observed using a
fluorescence microscope (Olympus Corporation) at ×400
magnification.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA of hepatic tissue was extracted from using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocols. cDNA was
synthesized using a PrimeScript™ One Step RT-PCR kit (cat. no.
AM1558; Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. The primer sequences for NF-κB p65 and
NF-κB inhibitor α (IκBα) were designed using Primer Premier 5
(premierbiosoft.com). The sequences are
as follows: NF-κB p65, forward, 5′-AGGCAAGGAATAATGCTGTCCTG-3′ and
reverse, 5′-ATCATTCTCTAGTGTCTGGTTGG-3′; IκBα, forward,
5′-CACTCCATCCTGAAGGCTACCAA-3′ and reverse,
5′-AAGGGCAGTCCGGCCATTA-3′; GAPDH, forward
5′-TGAGAGGGAAATCGTGCGTG-3′, and reverse,
5′-TGCTTGCTGATCCACATCTGC-3′. The thermocycling conditions were as
follows: 95°C for 15 min, followed by 40 cycles of 94°C for 15 sec,
at 55°C for 30 sec and 70°C for 30 sec for a total of 40 cycles.
After qPCR, the relative expression levels were calculated using
the 2−Δ∆Cq method (15).
Western blotting
Total protein was extracted from the tissues using
RIPA protein lysis buffer (Beyotime Institute of Biotechnology).
Nuclear and cytoplasmic proteins were separated using the Nuclear
and Cytoplasmic Protein Extraction Kit. The protein concentration
was determined using a BCA protein concentration kit (Beyotime
Institute of Biotechnology) Total protein (50 µg/lane) was
separated by SDS-PAGE on 10% gels and then transferred onto PVDF
membranes. After blocking with 5% non-fat milk for 2 h at room
temperature, the membranes were incubated overnight at 4°C with the
following primary antibodies: NF-κB p65 (1:1,000; cat. no. ab16502;
Abcam), NF-κB inhibitor α (IκBα; 1:1,000; cat. no. ab32518; Abcam),
phosphorylated (p)-IκBα (1:1,000; cat. no. ab133462; Abcam) and
β-actin (1:5,000; cat. no. ab8226; Abcam) at 4°C overnight,
followed by incubation with horseradish peroxidase-conjugated goat
anti-rabbit IgG (H+L) secondary antibody (1:5,000; cat. no. AS014;
ABclonal Biotech Co., Ltd.) at room temperature for 1 h. Lamin B1
antibody (cat. no. ab16048; Abcam) was used to detect lamin B1 as a
housekeeping protein in the nuclear fraction. Protein bands were
visualized using an enhanced chemiluminescence detection kit (ECL
Plus; EMD Millipore) and measured using Image J 1.47 software
(National Institutes of Health).
Statistical analysis
SPSS 17.0 statistical software (SPSS, Inc.) was used
to carry out the statistical analysis. Each experiment was
performed three times, and the data are expressed as the mean ± SD.
Comparisons among groups were analyzed by one-way analysis of
variance followed by Tukey's multiple comparison tests. Survival
rates were analyzed by the Kaplan-Meier method and log-rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Serum levels of ALT, AST, LDH and
GDH
An automatic biochemical analyzer was used to
measure the serum levels of ALT, AST, LDH and GDH every 6 h. The
results revealed that, compared with the sham group, the serum
levels of ALT, AST, LDH and GDH in the HS group gradually increased
over time (P<0.01; Fig. 1). In
addition, although the serum levels of ALT, AST, LDH and GDH also
increased in the MT group, the increase was significantly reduced
compared with that in the HS group (P<0.01; Fig. 1).
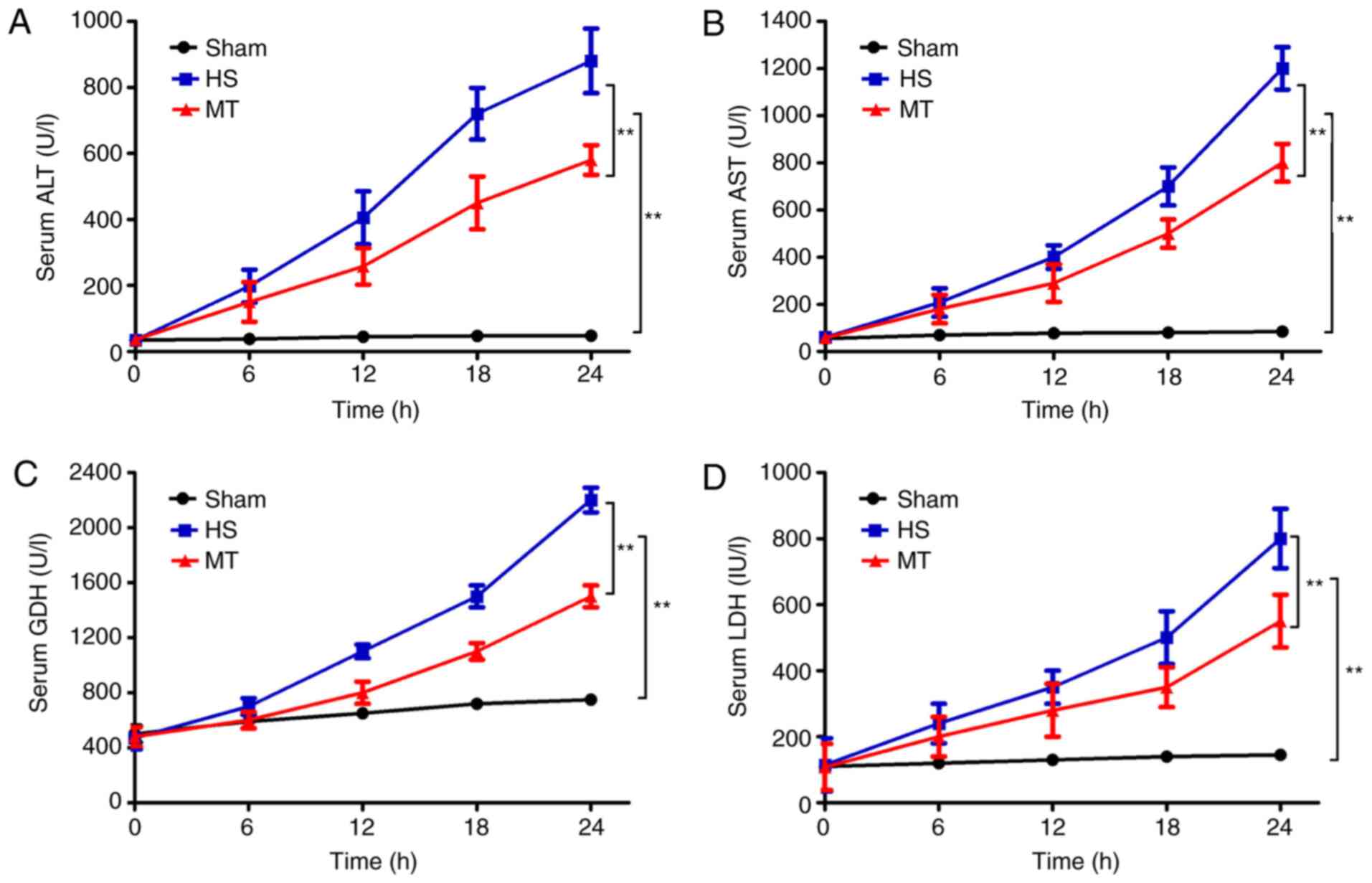 | Figure 1.Serum levels of ALT, AST, LDH and GDH
in each group at 6-h intervals as measured using an automatic
biochemical analyzer. Serum (A) ALT, (B) AST, (C) GDH and (D) LDH
levels. **P<0.01 as indicated. ALT, alanine aminotransferase;
AST, aspartate aminotransferase; LDH, lactate dehydrogenase; GDH,
glutamate dehydrogenase; HS, hemorrhagic shock; MT, melatonin. |
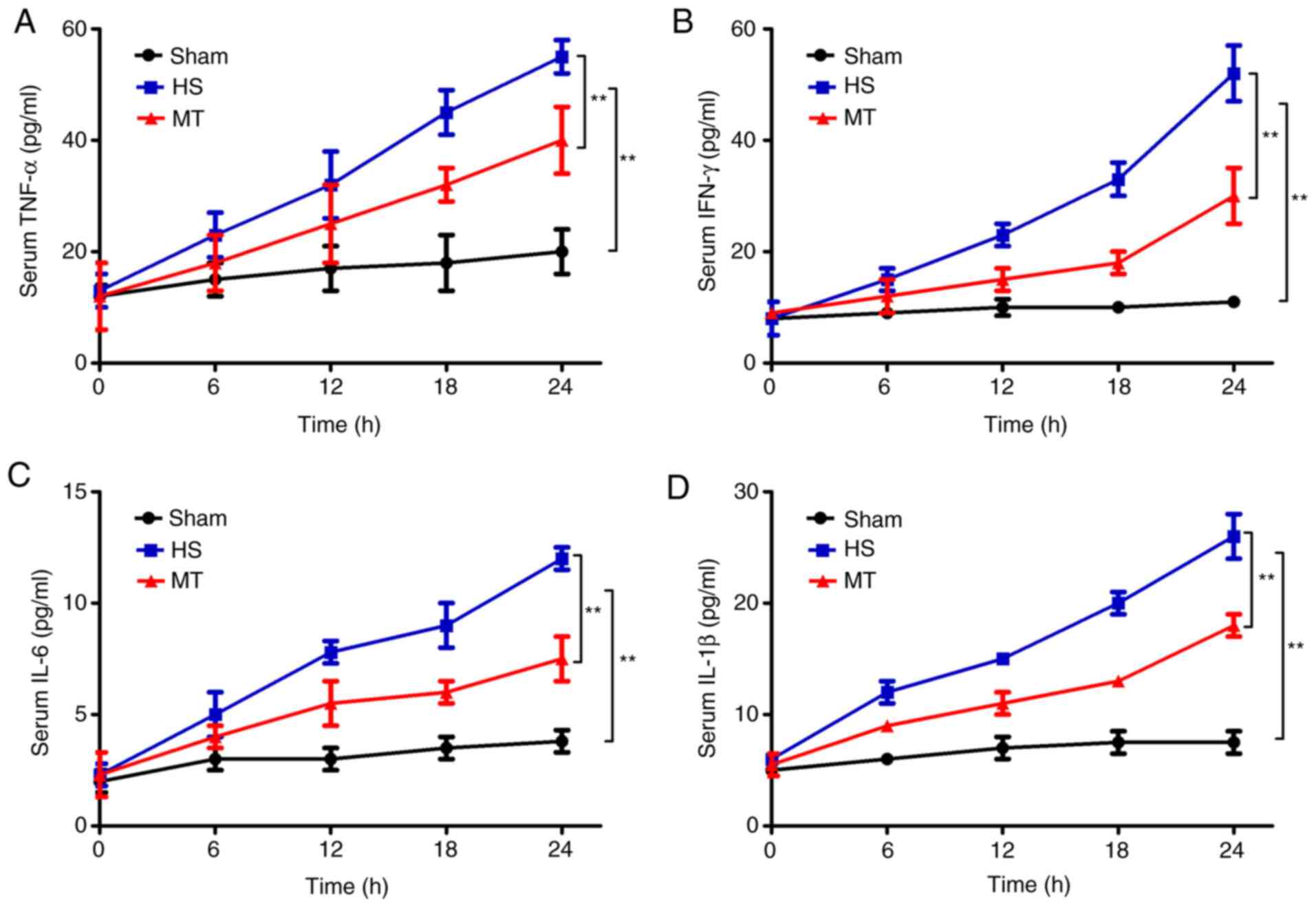
Serum levels of TNF-α, IFN-γ, IL-6 and
IL-1β
ELISAs were used to detect the serum levels of
TNF-α, IFN-γ, IL-6 and IL-1β every 6 h. The results revealed that
the serum levels of TNF-α, IFN-γ, IL-6 and IL-1β in the HS group
were significantly increased compared with those in the sham group
(P<0.01; Fig. 2). In addition,
the serum levels of TNF-α, IFN-γ, IL-6 and IL-1β were also
increased in the MT group, but the increase was significantly
reduced compared with that in the HS group (P<0.01; Fig. 2).
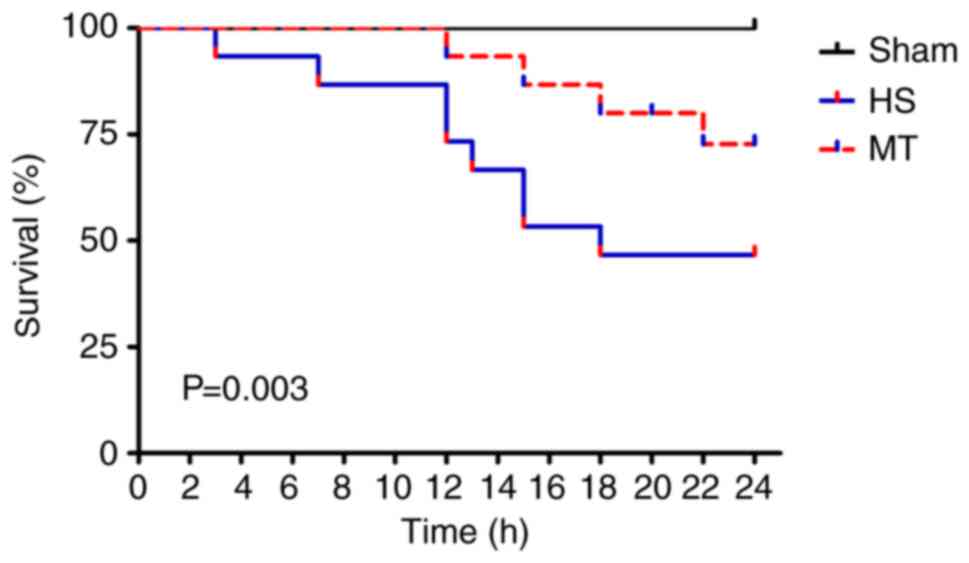
Survival rate
The survival rate of the rats in each group was
analyzed by the Kaplan-Meier method and log-rank test. A total of
12 rats (8 in the HS group and 4 in the MT group) died of shock
during the experiment, and the remaining 33 rats (15 in sham group,
7 in HS group and 11 in MT group) survived for 24 h. The results
show that the 24-h survival rate of the sham group was 100%,
whereas the 24-h survival rate of the MT group was significantly
higher compared with that of the HS group [73.33% (11/15) vs.
46.67% (7/15), respectively; P=0.003; Fig. 3].
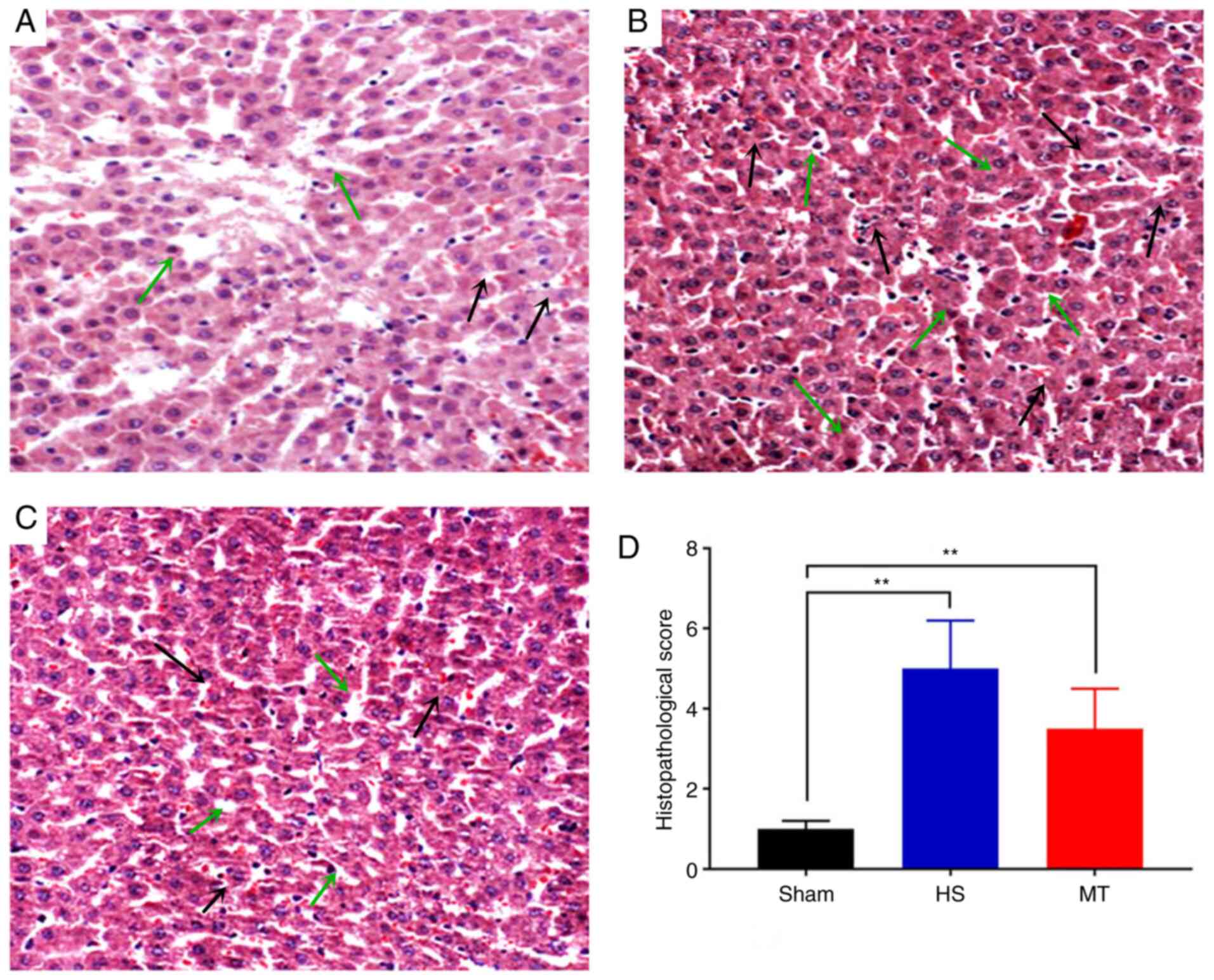
Pathological changes
Following sacrifice of the rats 24 h after surgery,
hepatic tissues were removed and stained with H&E. The results
revealed that the hepatic lobule structure of the sham group was
complete, the hepatocytes were arranged linearly and no cell
denaturation or inflammatory cell infiltration was present
(Fig. 4A). In the HS group,
moderate edema of the hepatocytes was observed, with fatty
degeneration, marked sinusoidal expansion, central venous
congestion, expansion of the portal area interlobular veins and
notable inflammatory cell infiltration (Fig. 4B). In the MT group, the hepatic
lobule structure was well-defined, with only a scattered
infiltration of fat droplets. Furthermore, the hepatocytes
exhibited only diffuse mild edema, with a small number of
lymphocytes visible in the hepatic cords and the portal area
(Fig. 4C). The HS group displayed
significant exacerbation of hepatic pathological injury compared
with the sham group (Suzuki score, 5.12±1.23 vs. 1.05±0.45,
respectively; P<0.01; Fig. 4D)
and the MT group (Suzuki score, 5.12±1.23 vs. 3.53±1.12,
respectively; P<0.01; Fig.
4D).
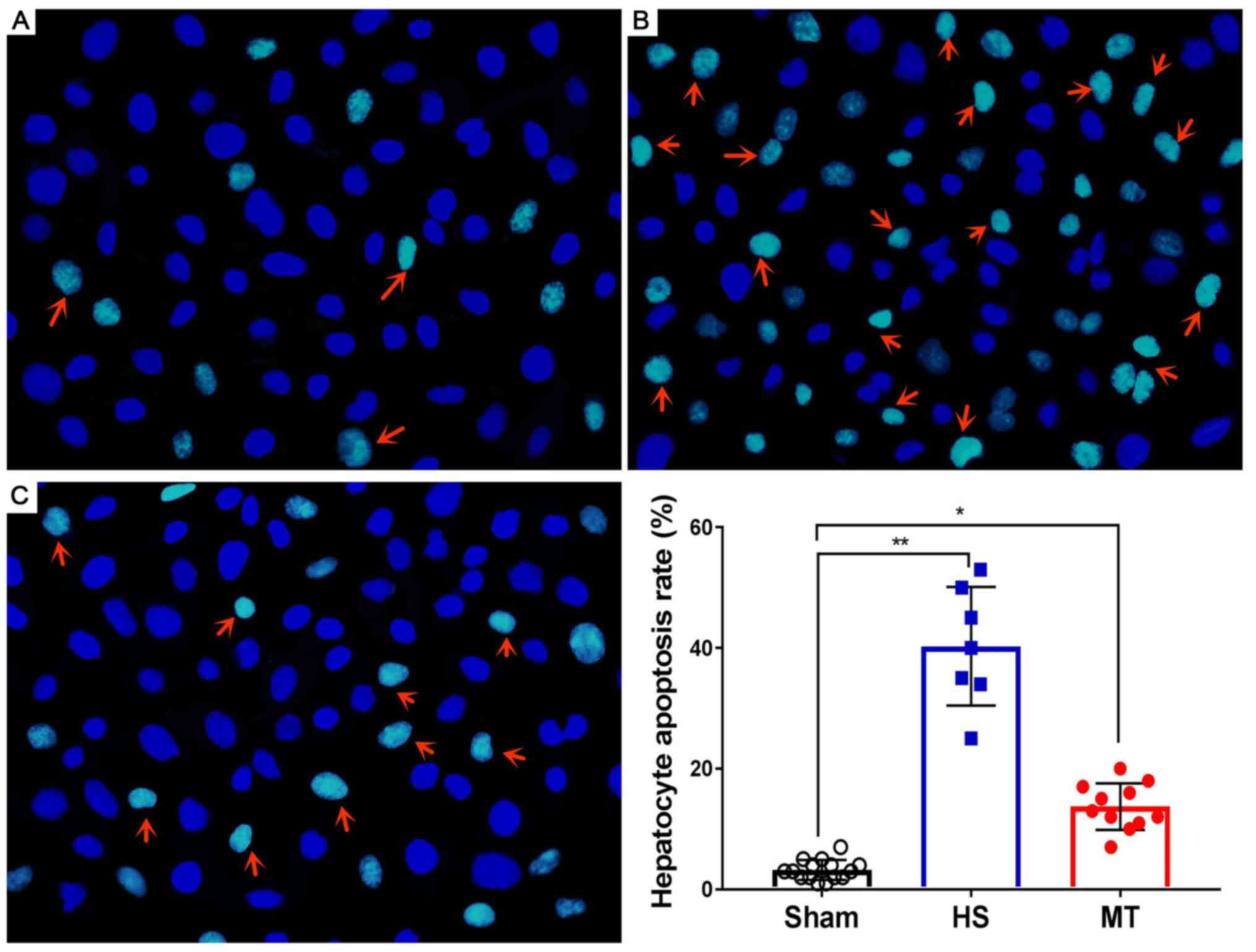
Hepatocyte apoptosis rate
After the rats were sacrificed, hepatic tissues were
removed and subjected to analysis of the hepatocyte apoptosis rate
using the DeadEnd™ Fluorometric TUNEL System. The results
demonstrated that the hepatocyte apoptosis rate was very low in the
sham group (Fig. 5A) and
significantly higher in the HS group (P<0.01; Fig. 5B); however, the hepatocyte apoptosis
rate was significantly decreased following treatment with MT
(Fig. 5C), and the difference
between the HS and MT groups was statistically significant
(P<0.05; Fig. 5D).
Hepatic tissue levels of TNF-α, IFN-γ,
IL-6 and IL-1β
When the rats were sacrificed 24 h after surgery,
hepatic tissues were removed and a hepatic tissue homogenate was
prepared. Analysis of the homogenate revealed that, the levels of
TNF-α, IFN-γ, IL-6 and IL-1β in the hepatic tissue homogenate were
significantly increased in the HS group compared with those in the
sham group; however, these levels were significantly decreased
following treatment with MT compared with those in the HS group
(P<0.05; Fig. 6).
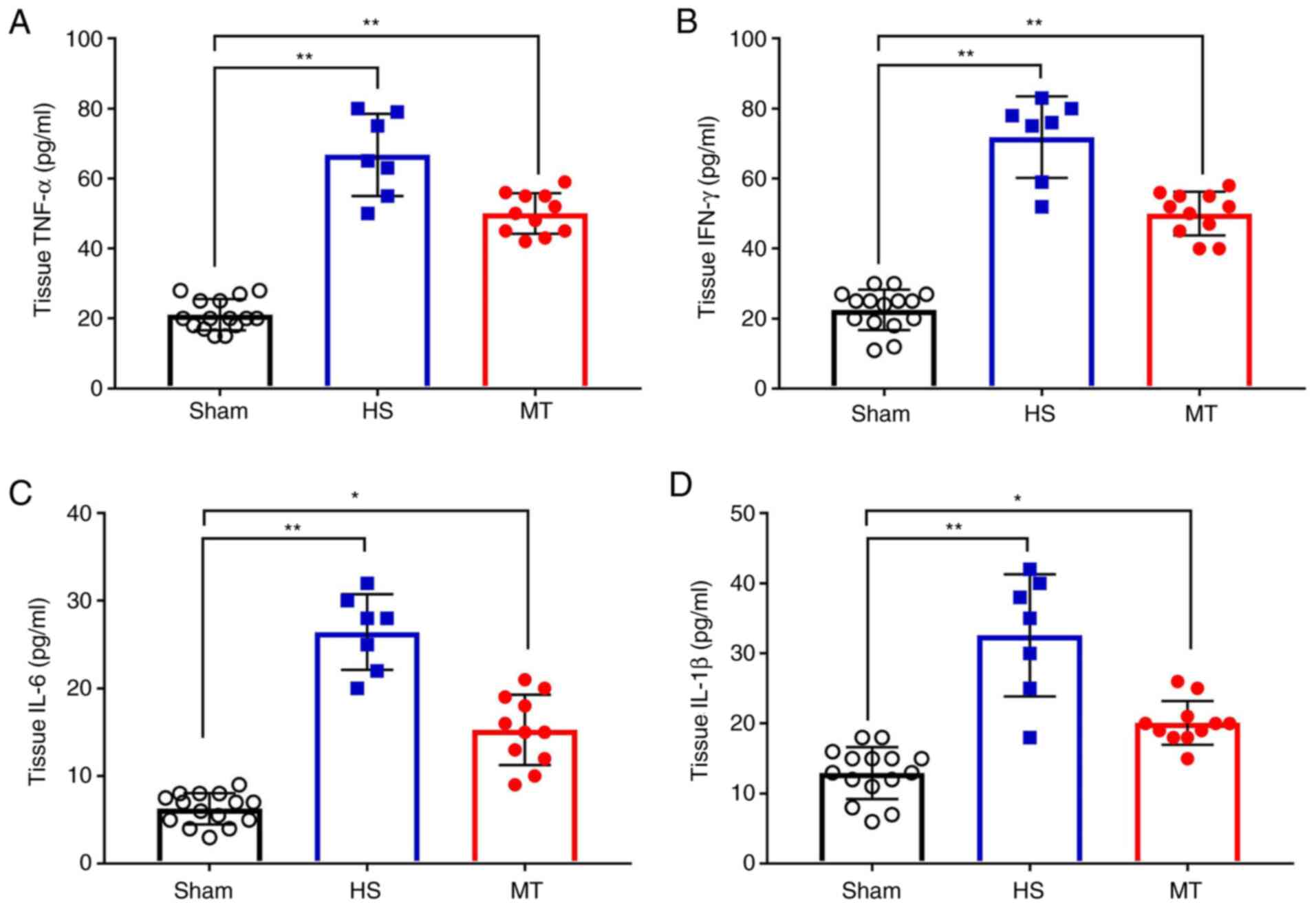 | Figure 6.Hepatic tissue levels of TNF-α, IFN-γ,
IL-6 and IL-1β in each group after sacrifice were measured by
ELISA. Hepatic tissue levels of (A) TNF-α, (B) IFN-γ, (C) IL-6 and
(D) IL-1β. *P<0.05, **P<0.01 as indicated. TNF, tumor
necrosis factor; IFN, interferon; IL, interleukin; HS, hemorrhagic
shock; MT, melatonin. |
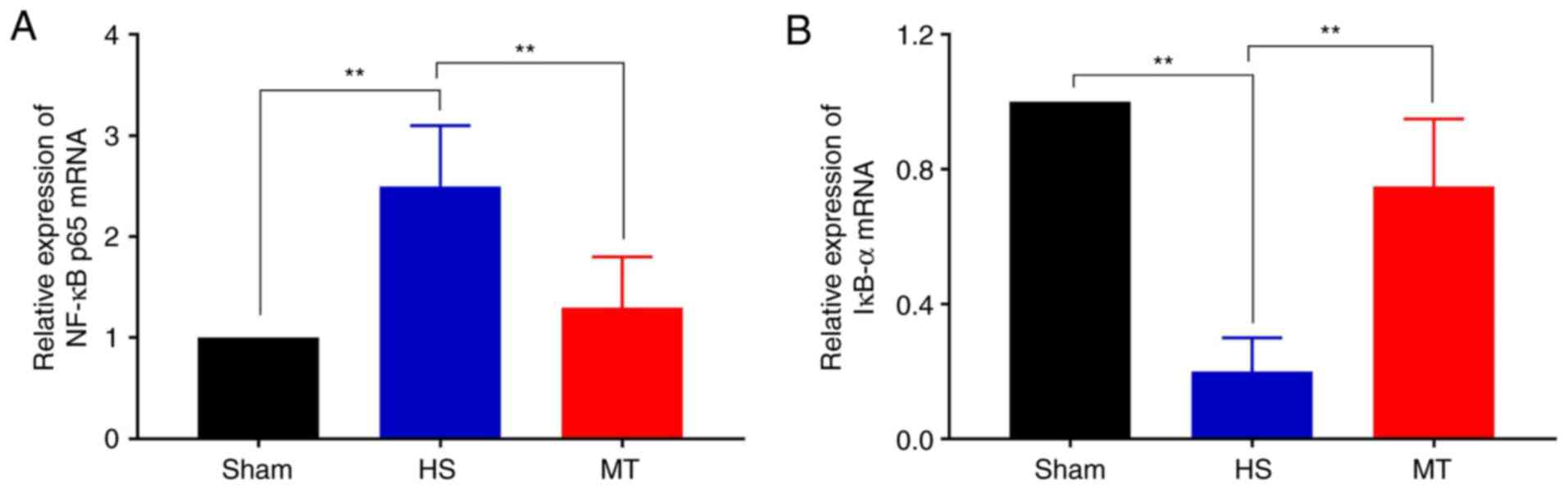
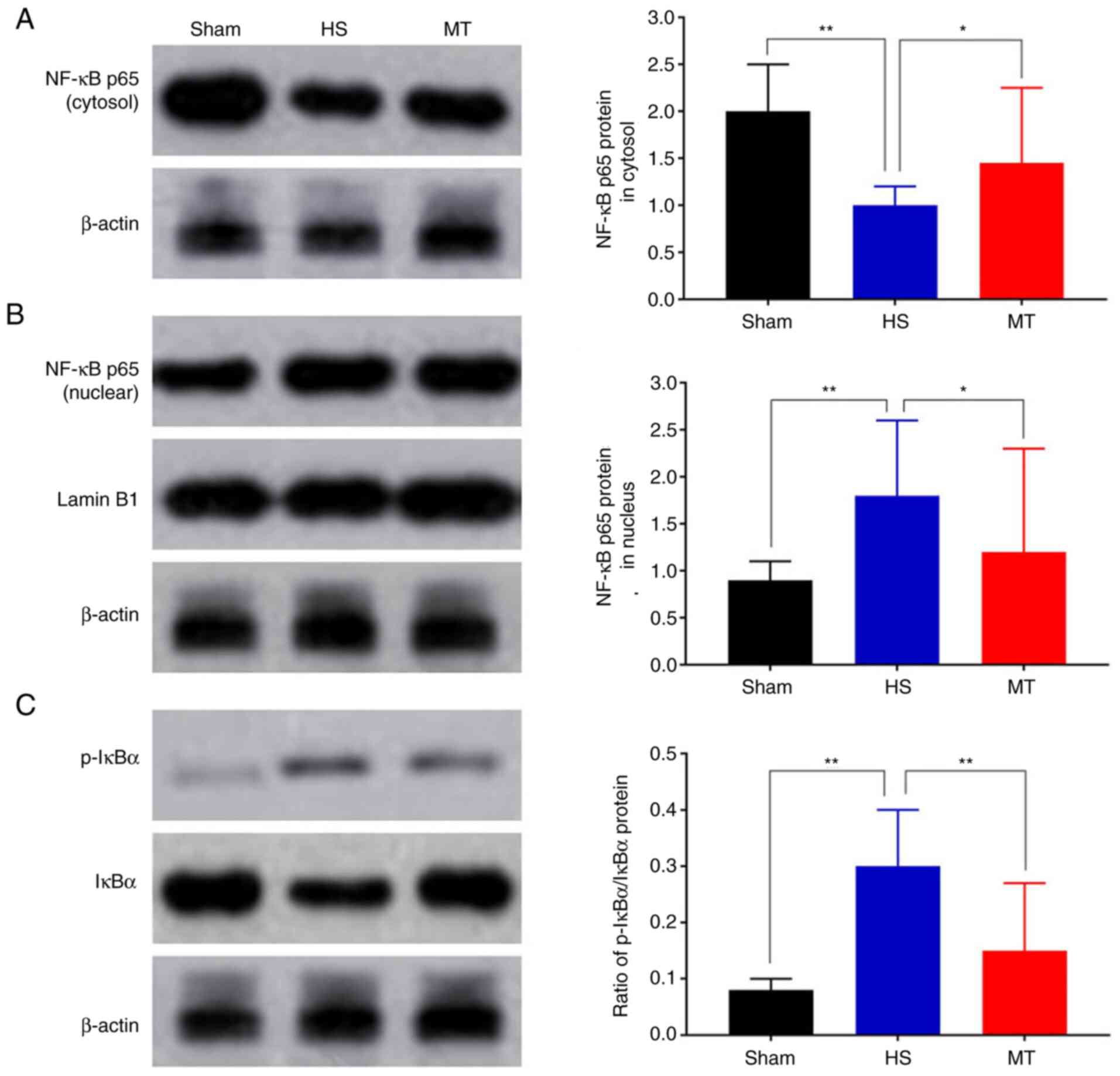
Activity of the NF-κB/IκBα
pathway
The expression levels of NF-κB p65 and IκBα in the
hepatic tissues of the rats were analyzed by RT-qPCR and western
blotting. The RT-qPCR results demonstrated that the mRNA expression
of NF-κB p65 was significantly decreased whereas that of IκBα was
increased in the MT group compared with the HS group (P<0.01;
Fig. 7). Furthermore, the western
blotting results revealed that the protein level of NF-κB p65 in
the HS group was significantly decreased in the cytosol but
increased in the nucleus compared with that in the control group,
but these HS-induced changes were significantly attenuated
following treatment with MT; also, the p-IκBα/IκBα ratio was
significantly increased in HS group compared with the control, but
the increase was significantly attenuated following treatment with
MT (P<0.05; Fig. 8).
Discussion
Trauma accounts for 10% of fatalities and 16% of
disabilities worldwide (16). HS is
the most common preventable cause of mortality subsequent to
trauma. HS can stimulate the body to release excessive amounts of
inflammatory mediators and produce a systemic inflammatory
response, which may eventually lead to multiple organ dysfunction
syndrome (17). HS-induced hepatic
ischemic injury is the most common pathophysiological process in
the clinical setting. Recent studies (18,19)
report that an excessive and uncontrolled inflammatory response is
the key mechanism by which hepatic ischemic injury is mediated
during HS, with TNF-α, IFN-γ, IL-6 and IL-1β being the most
important inflammatory cytokines in this process.
MT is an indoleamine hormone synthesized by the
pineal gland of vertebrates, which has a variety of strong direct
and indirect antioxidant and anti-inflammatory effects (20). Recent studies (21,22)
demonstrated that the early administration of MT to rats is able to
attenuate oxidative and inflammatory ischemic organ injury. In the
present study, a rat HS model was successfully established and MT
treatment was administered. It was observed that the hepatic
function of the rats gradually worsened as time progressed, and the
levels of inflammatory factors also gradually increased, but these
effects were reversed by MT treatment. In addition, the survival
rate of the rats was significantly increased, and the hepatic
pathological injury score and hepatocyte apoptosis rate were
significantly decreased in the rats treated with MT compared with
those in the untreated HS group. To the best of our knowledge, the
present study is the first to report that exogenous MT is able to
alleviate HS-induced hepatic ischemic injury in rats.
NF-κB is a transcription factor that is involved in
the transcription and modulation of several genes that serve as
inflammatory mediators, including TNF-α, IFN-γ, IL-6 and IL-1β, and
plays an important role in the inflammatory immune response,
oxidation and cell apoptosis (23).
NF-κB consists of 5 subunits (24):
Rel (cRel), p65 (RelA, NF-κB3), RelB, p50 (NF-κB1) and p52
(NF-κB2), of which NF-κB p65 (25)
is the most important subunit in the NF-κB signaling pathway. IκBα
is the main inhibitory regulatory protein of NF-κB, and the
phosphorylation of IκBα is an important pathway mediating NF-κB
activation (26,27). In unstimulated cells, IκBα inhibits
NF-κB signaling by sequestering NF-κB in an inactive state in the
cytoplasm and reducing its nuclear localization (28). However, various extracellular
stimuli may cause the phosphorylation and degradation of IκBα. The
detachment of IκBα from the NF-κB complex leads to the activation
of NF-κB and its entry into the nucleus, thereby increasing the
transcriptional activity of inflammatory mediator genes (29). The balance of NF-κB p65 and IκBα
expression is key to activation of the NF-κB signaling pathway
(30).
Recent studies have demonstrated that MT plays a
regulatory role in a variety of diseases via the inhibition of
NF-κB pathways. Gu et al (31) reported that MT inhibits the
migration and invasion of TE-1 esophageal cancer cells by
suppressing the NF-κB signaling pathway and decreasing the
expression of matrix metalloproteinase-9. Chen et al
(32) observed that MT alleviates
intervertebral disc degeneration by disrupting the IL-1β/NF-κB-NLR
pyrin domain containing 3 inflammasome positive feedback loop.
Furthermore, Tiong et al (33) demonstrated that MT prevents
oxidative stress-induced mitochondrial dysfunction and apoptosis in
high-glucose-treated Schwann cells via the upregulation of Bcl2,
NF-κB, mammalian target of rapamycin and Wnt signaling pathways. In
the present study, RT-qPCR and western blotting assays were
employed to assess the activity of the NF-κB signaling pathway. The
results demonstrated that MT exerted a marked inhibitory effect on
the transcriptional activity of NF-κB p65, whereas it enhanced IκBα
activity. Furthermore, MT inhibited the transfer of NF-κB p65 into
the nucleus and the phosphorylation of IκBα.
There were several limitations to the present study.
One was that only treatment with 10 mg/kg MT was conducted; the
effects of MT may be dose-dependent and various treatment groups
using different doses of MT should be investigated in the future.
Also, the specific location of NF-κB p65 in the nucleus and
cytoplasm has not been explored in depth and merits further
study.
In summary, the present study was the first to
determine that exogenous MT alleviates HS-induced hepatic ischemic
injury in rats. The underlying mechanism may involve the inhibition
of NF-κB activation and IκBα phosphorylation, which could reduce
inflammation. Although the exact mechanism requires further
elucidation, to the best of our knowledge, the present study was
the first to provide evidence on the efficacy of MT against
HS-induced hepatic ischemic injury.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Public
Welfare Basic Research Project of Zhejiang Province (grant no.
LGF19H150001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XLW was responsible for overall planning of the
research. HWL and PY were responsible for most of the experiments
and manuscript preparation. HWL and QQC were involved in data
analysis and manuscript preparation. ZHY participated in
acquisition, analysis and interpretation of data. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Taizhou First People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cannon JW: Hemorrhagic shock. N Engl J
Med. 378:370–379. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kezelman C: Trauma-informed care and
practice in nursing. Aust Nurs Midwifery J. 24:282016.PubMed/NCBI
|
|
3
|
Slim C, Zaouali MA, Nassrallah H, Ammar
HH, Majdoub H, Bouraoui A and Abdennebi HB: Protective potential
effects of fucoidan in hepatic cold ischemia-reperfusion injury in
rats. Int J Biol Macromol. 155:498–507. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y, Gao M, Xu LN, Yin LH, Qi Y and Peng
JY: MicroRNA-142-3p attenuates hepatic ischemia/reperfusion injury
via targeting of myristoylated alanine-rich C-kinase substrate.
Pharmacol Res. 156:1047832020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ibrahim SG, El-Emam SZ, Mohamed EA and Abd
Ellah MF: Dimethyl fumarate and curcumin attenuate hepatic
ischemia/reperfusion injury via Nrf2/HO-1 activation and
anti-inflammatory properties. Int Immunopharmacol. 80:1061312020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fliegauf M and Grimbacher B: Nuclear
factor κB mutations in human subjects: The devil is in the details.
J Allergy Clin Immunol. 142:1062–1065. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiao Y, Huang J, Xu J, Zeng L, Tian J, Lou
Y, Liu Y, Hu B, Tong F and Shen R: Targeted delivery of
puerarin/glycyrrhetinic acid-PEG-PBLA complex attenuated liver
ischemia/reperfusion injury via modulating Toll-like receptor
4/nuclear factor-κB pathway. Ther Deliv. 9:245–255. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Amaral FGD and Cipolla-Neto J: A brief
review about melatonin, a pineal hormone. Arch Endocrinol Metab.
62:472–479. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carrascal L, Nunez-Abades P, Ayala A and
Cano M: Role of melatonin in the inflammatory process and its
therapeutic potential. Curr Pharm Des. 24:1563–1588. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang YL, Sun X, Huang LB, Liu XJ, Qin G,
Wang LN, Zhang XL, Ke ZY, Luo JS, Liang C, et al: Melatonin
inhibits MLL-rearranged leukemia via RBFOX3/hTERT and NF-κB/COX-2
signaling pathways. Cancer Lett. 443:167–178. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
https://www.nc3rs.org.uk/arrive-guidelines
|
|
12
|
ATLS Subcommittee: American College of
Surgeons' Committee on Trauma; International ATLS working group, .
Advanced trauma life support (ATLS(R)): The ninth edition. J Trauma
Acute Care Surg. 74:1363–1366. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang H, Liu J, Xu Z and Zheng C: Efficacy
of different fluid resuscitation methods on coagulation function of
rats with traumatic hemorrhagic shock. J Surg Res. 260:259–266.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suzuki S, Nakamura S, Koizumi T, Sakaguchi
S, Baba S, Muro H and Fujise Y: The beneficial effect of a
prostaglandin I2 analog on ischemic rat liver. Transplantation.
52:979–983. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Behrends M, Martinez-Palli G, Niemann CU,
Cohen S, Ramachandran R and Hirose R: Acute hyperglycemia worsens
hepatic ischemia/reperfusion injury in rats. J Gastrointest Surg.
14:528–535. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sommer JL, El-Gabalawy R, Taillieu T,
Afifi TO and Carleton RN: Associations between trauma exposure and
physical conditions among public safety personnel. Can J
Psychiatry. 12:548–558. 2020. View Article : Google Scholar
|
|
18
|
Türedi S, Şahin A, Akça M, Demir S, Köse
GDR, Çekiç AB, Yıldırım M, Yuluğ E, Menteşe A, Türkmen S and Acar
S: Ischemia-modified albumin and the IMA/albumin ratio in the
diagnosis and staging of hemorrhagic shock: A randomized controlled
experimental study. Ulus Travma Acil Cerrahi Derg. 26:153–162.
2020.PubMed/NCBI
|
|
19
|
Kyriakopoulos G, Tsaroucha AK, Valsami G,
Lambropoulou M, Kostomitsopoulos N, Christodoulou E, Kakazanis Z,
Anagnostopoulos C, Tsalikidis C and Simopoulos CE: Silibinin
improves TNF-α and M30 expression and histological parameters in
rat kidneys after hepatic ischemia/reperfusion. J Invest Surg.
31:201–209. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ye Y, Wang W, Zhang W, Peng Y, Liu Y, Yu
S, Chen Q, Geng L, Zhou L, Xie H, et al: Galectin-1 attenuates
hepatic ischemia reperfusion injury in mice. Int Immunopharmacol.
77:1059972019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Yan X, Tian Y, Li W, Wang H, Li
Q, Li Y, Li Z and Wu T: Synthesis of a new water-soluble melatonin
derivative with low toxicity and a strong effect on sleep aid. ACS
Omega. 5:6494–6499. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pranil T, Moongngarm A and Loypimai P:
Influence of pH, temperature, and light on the stability of
melatonin in aqueous solutions and fruit juices. Heliyon.
6:e036482020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ullah U, Badshah H, Malik Z, Uddin Z, Alam
M, Sarwar S, Aman A, Khan AU and Shah FA: Hepatoprotective effects
of melatonin and celecoxib against ethanol-induced hepatotoxicity
in rats. Immunopharmacol Immunotoxicol. 42:255–263. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu HY, Meng LF, Lu XH, Liu LH, Ci X and
Zhuo Z: Protective effect of miR-146 against kidney injury in
diabetic nephropathy rats through mediating the NF-κB signaling
pathway. Eur Rev Med Pharmacol Sci. 24:3215–3222. 2020.PubMed/NCBI
|
|
25
|
Jia Y, He W, Zhang H, He L, Wang Y, Zhang
T, Peng J, Sun P and Qian Y: Morusin ameliorates IL-1β-induced
chondrocyte inflammation and osteoarthritis via NF-κB signal
pathway. Drug Des Devel Ther. 14:1227–1240. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feng Y, Duan T, Du Y, Jin S, Wang M, Cui J
and Wang RF: LRRC25 functions as an inhibitor of NF-κB signaling
pathway by promoting p65/RelA for autophagic degradation. Sci Rep.
7:134482017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boisson B, Puel A, Picard C and Casanova
JL: Human IκBα gain of function: A severe and syndromic
immunodeficiency. J Clin Immunol. 37:397–412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rahimova N, Babazada H, Higuchi Y,
Yamashita F and Hashida M: Development of mKO2 fusion proteins for
real-time imaging and mechanistic investigation of the degradation
kinetics of human IκBα in living cells. Biochim Biophys Acta Mol
Cell Res. 1866:190–198. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kanan T, Kanan D, Erol I, Yazdi S, Stein M
and Durdagi S: Targeting the NF-κB/IκBα complex via fragment-based
E-Pharmacophore virtual screening and binary QSAR models. J Mol
Graph Model. 86:264–277. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi CX, Qi QH, Xu J and Zhao WW:
Protective effect of magnesium sulfate on cranial nerves in
preeclampsia rats through NF-κB/ICAM-1 pathway. Eur Rev Med
Pharmacol Sci. 24:2785–2794. 2020.PubMed/NCBI
|
|
31
|
Gu H, Shen Q, Mei D, Yang Y, Wei R and Ni
M: Melatonin inhibits TE-1 esophageal cancer cells metastasis by
suppressing the NF-κB signaling pathway and decreasing MMP-9. Ann
Clin Lab Sci. 50:65–72. 2020.PubMed/NCBI
|
|
32
|
Chen F, Jiang G, Liu H, Li Z, Pei Y, Wang
H, Pan H, Cui H, Long J, Wang J and Zheng Z: Melatonin alleviates
intervertebral disc degeneration by disrupting the
IL-1β/NF-κB-NLRP3 inflammasome positive feedback loop. Bone Res.
8:102020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tiong YL, Ng KY, Koh RY, Ponnudurai G and
Chye SM: Melatonin prevents oxidative stress-induced mitochondrial
dysfunction and apoptosis in high glucose-treated schwann cells via
upregulation of Bcl2, NF-κB, mTOR, wnt signalling pathways.
Antioxidants (Basel). 8:1982019. View Article : Google Scholar
|