Introduction
Osteonecrosis of the femoral head (ONFH) is one of
the most common diseases affecting the hip joint, resulting in
severe pain or joint disability (1,2).
Steroid-induced avascular necrosis of the femoral head (SANFH) is
the most common type of ONFH (3).
If patients with SANFH are not treated properly, the femoral head
may collapse, resulting in hip function damage and disability
(4,5). Although conservative treatment and
surgery have been used as SANFH treatment, there is no recognized
effective therapy thus far. Therefore, it is important to explore
the exact pathogenesis of SANFH and find an ideal treatment for
SANFH.
Medicinal herbs are used to treat various diseases
in Japan, Korea, China, other Asian areas and now all over the
world (6). Chinese herb-derived
active components provide valuable compounds for new drug candidate
development (7). Rhizoma
drynariae is one of the herbs from Davallia (L.) Sm.,
which comprises phenolic acids, triterpenes and flavonoids
(8). Total flavonoids of Rhizoma
drynariae (TFRD) is a herbaceous component extracted from the
drained root of Rhizoma drynariae and the effective monomer
of TFRD includes neoeriocitrin, naringenin and naringin (9,10).
This compound stimulates bone formation or inhibits bone resorption
by modulating signalling in pathways involving bone morphogenetic
protein in bone metabolism, Wnt/β-catenin, receptor activator of
nuclear factor κB ligand (RANKL)/RANK and cathepsin K (a cysteine
protease) (8). In addition,
naringin serves as an active compound of TFRD to inhibit bone loss
and promote bone repair in rats with glucocorticoid-induced ONFH
via the AKT/Bad pathway (11).
However, the effects of TFRD on SANFH have not been reported.
The phosphoinositide 3-kinase (PI3K)/AKT pathway
serves an important role in several basic cellular processes
including survival, proliferation, growth and differentiation
(12,13). A study has shown that the
phosphatase and tensin homolog regulate angiogenesis in human
pancreatic cancer cells through the PI3K/AKT/VEGF pathway (14). Zhang et al (15) reported that exosomes could enhance
bone regeneration by activating the PI3K/AKT pathway. Hu et
al (16) found that ginsenoside
Rg1 protected rat bone marrow stem cell apoptosis induced by
hydrogen peroxide through the PI3K/AKT pathway. Hence, the present
study hypothesized that the PI3K/AKT pathway might be associated
with the pathogenesis of SANFH. However, there is little research
on the role of this pathway in SANFH.
The present study investigated the function of TFRD
on rats with SANFH, including pathological changes of the femoral
head, apoptosis of bone cells and the PI3K/AKT pathway. In
addition, the function of TFRD in osteoblasts was investigated. The
present study might provide useful information on the pathogenesis
of SANFH and support the further clinical application of TFRD.
Materials and methods
Animals
A total of 24 male Sprague-Dawley rats (12 weeks
old; 250–300 g; Liaoning Changsheng Biotechnology Co., Ltd.) were
used in the present study. All rats were housed under standard
laboratory conditions (12-h light/dark cycle, 24–25°C and 50–55%
humidity) and provided free access to food and water. The
experiments were performed in accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals (8th edition) (17) and
approved by the Institutional Animal Care and Use Committee of the
Affiliated Hospital of Shandong University of Traditional Chinese
Medicine (approval no. 2019-58).
Grouping and animal model
establishment
All rats were randomized into control, SANFH, or
SANFH+TFRD groups (n=8 each). TFRD was purchased from Beijing
Qihuang Pharmaceutical Manufacturing Co., Ltd. (trade name: Qiang
gu capsuled, drug approval number: Z20030007). Based on the
instructions of Qiang gu capsule, clinical usage (0.75 g·day for
adults) and the Meeh-Rubner equation (18) of dose conversion, 20 mg/kg dosage
was chosen for administration to rats by intraperitoneal injection
daily. A previous study reported that rats were administered with
Rhizoma drynariae extract at dose of 20 mg/kg body weight
once daily (19). The SANFH model
was induced by intramuscular injection of lipopolysaccharides (LPS)
and methylprednisolone (MPS). Briefly, rats in the SANFH and
SANFH+TFRD groups were given two doses of 20 µg/kg LPS
(Escherichia coli 055: B5; Sigma-Aldrich; Merck KGaA) on
days 0 and 1 (twice at 24 h intervals) and administered 40 mg/kg
MPS (Pfizer, Inc.) on days 3, 4 and 5 (three times after a 24-h
interval). Rats in the control group were injected with normal
saline. Additionally, rats in the SANFH+TFRD group were
intraperitoneally injected with 20 mg/kg/day TFRD every day, while
the remainder of the rats were injected with normal saline. The
time interval for all injections was 24 h. All rats were weighed
weekly. Subsequently, four weeks after the final MPS injection, all
rats were sacrificed by intraperitoneal overdose anaesthesia with
pentobarbital sodium (240 mg/kg, Sigma-Aldrich; Merck KGaA) and
femoral heads were harvested for subsequent tests.
Haematoxylin and eosin staining
The femur specimens were fixed in 4%
paraformaldehyde for 48 h at 4°C and then decalcified with 10%
ethylenediaminetetraacetic acid for 4–6 weeks at room temperature.
After complete decalcification, the specimens were dehydrated in
graded ethanol, embedded in paraffin and cut into 4-µm sections.
Thereafter, the sections were dewaxed with xylene and then
gradually dehydrated via gradient ethanol. The sections were
stained with haematoxylin solution for 5 min at 37°C followed by
eosin solution for 2 min at 37°C. Finally, the sections were
dehydrated with gradient ethanol, washed three times using xylene
and then mounted in neutral gum. The histopathological changes were
examined under a light microscope (Leica Microsystems GmbH) by two
independent authors. Three sections were obtained from each femoral
head and the percentage of empty lacunae was assessed at ×100
magnification in five randomly selected fields per section. The
diagnosis of ONFH was established on the basis of the pathological
features of empty bone lacuna numbers and surrounding necrotic bone
marrow tissues.
Terminal deoxynucleotidyl transferase
deoxyuridine triphosphate nick end labelling (TUNEL) assay
TUNEL assay was used to detect the apoptosis rate of
osteoblasts using a Situ Cell Death Detection kit (Nanjing
Jiancheng Bioengineering Institute) according to the manufacturer's
protocol. Following routine dewaxing and dehydration, the sections
were digested with pepsin (~0.25%-0.5% hydrochloric acid solution)
for 25 min at room temperature, incubated with 50 µl TUNEL reaction
mixture for 1 h at 37°C and then incubated with 50 µl of peroxidase
for 30 min at 37°C. Thereafter, the sections were dyed with
3,3-diaminobenzidine reagent and stained with hematoxylin for 2 min
at room temperature. Cells with brown nuclei were considered as
TUNEL-positive cells and were further assessed under a fluorescence
microscope (Olympus Corporation). The apoptosis rate of osteoblasts
was defined as the proportion of the number of TUNEL-positive cells
to the total number of cells.
Western blot analysis
Total protein was extracted from the osteoblasts and
tissues of the femoral head using radioimmunoprecipitation assay
buffer containing protease and phosphatase inhibitors (Abcam). A
bicinchoninic acid (BCA) kit (Thermo Scientific, Inc.) was used to
measure the total protein concentration. Equal amounts of protein
(30 µg/lane) were loaded on 10% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis and then transferred
onto polyvinylidene fluoride membranes (Pall Life Sciences).
Thereafter, the membranes were blocked with 5% non-fat milk
(Sigma-Aldrich; Merck KGaA) in Tris-buffer saline containing 0.1%
Tween-20 for 1 h at room temperature and then incubated with the
following primary antibodies overnight at 4°C: Anti-caspase-3 (cat.
no. 14220; 1:1,000), anti-Bax (cat. no. 14796; 1:1,000), anti-RUNX2
(cat. no. 12556; 1:1,000), anti-phosphorylated (p-)PI3K (cat. no.
17366; 1:1,000), anti-p-AKT (cat. no. 4060; 1:1,000), anti-AKT
(cat. no. 4691; 1:1,000) and anti-glyceraldehyde 3-phosphate
dehydrogenase (internal parameter; cat. no. 5174; 1:1,000 all from
Cell Signaling Technology, Inc.; anti-Bcl-2 (ab196495; 1:1,000) and
anti-OCN (ab13418; 1:1,000), from Abcam; and anti-VEGF (sc-7269;
1:1,000), anti-OPG (sc-390518; 1:1,000), anti-RANKL (sc-377079;
1:1,000) and anti-PI3K (sc-1637; 1:1,000) from Santa Cruz
Biotechnology, Inc. The membranes were then incubated with
corresponding horseradish peroxidase-conjugated secondary
antibodies (1:1,000, Wuhan Boster Biological Technology Co., Ltd.)
for 1 h at room temperature. Finally, the labelled proteins were
treated with enhanced chemiluminescence (ECL) solution (Pierce;
Thermo Fisher Scientific, Inc.) and the protein bands were analysed
using ImageJ software, version 1.8.0 (National Institutes of
Health).
Cell culture and treatment
Osteoblasts were isolated from calvariae of neonatal
rats as previously described (20).
Briefly, heads of 2-day-old neonatal rats were cut off, soaked in
phosphate-buffered saline (PBS; HyClone; Cytiva) and the calvariae
were then dissected out. Thereafter, the calvariae were cut into
pieces, incubated with 1% trypsin (Gibco; Thermo Fisher Scientific,
Inc.) for 15 min at 37°C and then incubated with PBS containing
0.25% collagenase (Type I; Sigma-Aldrich; Merck KGaA) for 60 min at
37°C. Following centrifugation at 300 × g for 10 min at 37°C, the
osteoblasts were obtained and cultured in α-minimal essential
medium containing 12% foetal bovine serum and antibiotics (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C with 5% CO2.
Osteoblasts were treated with different
concentrations of dexamethasone (Dex; 0, 10−6,
10−7 and 10−8 M; Sigma-Aldrich; Merck KGaA)
for 0, 24, 48 and 72 h at 37°C or TFRD (0, 0.1, 0.5, 2.5, 12.5 and
62.5 µg/ml) for 48 h at 37°C to estimate the dosage effects on cell
proliferation. Osteoblasts were divided into control (without
treatment), Dex (osteoblasts treated with 10−6 M Dex for
48 h at 37°C), Dex+TFRD [osteoblasts pre-treated with 12.5 µg/ml
TFRD (the maximum concentration of TFRD without cytotoxicity on
osteoblasts) for 2 h at 37°C and then incubated with
10−6 M Dex for 48 h at 37°C], Dex+TFRD+LY294002 group
(osteoblasts pre-treated with 12.5 µg/ml TFRD for 2 h at 37°C and
10 µM LY294002 [an inhibitor of PI3K/AKT pathway; Beyotime
Institute of Biotechnology] for 1 h at 37°C and then incubated with
10−6 M Dex for 48 h at 37°C). Following treatment,
osteoblasts were collected for further experiments.
MTT assay
Osteoblast proliferation was assessed using the MTT
assay. Briefly, osteoblasts (2.0×104 cells/ml) were
seeded onto 96-well plates and exposed to TFRD, Dex and LY294002 as
detailed in the previous experiment, respectively. Thereafter,
osteoblasts were incubated with 20 µl MTT solution (5 mg/ml,
Beyotime Institute of Biotechnology) at 37°C for 4 h and then
incubated with 150 µl dimethyl sulfoxide. Finally, optical density
(OD) was measured at 490 nm with a spectrophotometric plate reader
(Bio-Rad Laboratories, Inc.). The experiments were repeated three
times.
Flow cytometry
The Annexin V-fluorescein isothiocyanate (FITC)
early apoptosis detection kit (Cell Signaling Technology, Inc.) was
used to analyse osteoblast apoptosis. Briefly, osteoblasts were
incubated with Annexin V-FITC (10 µl) and propidium iodide (5 µl)
for 30 min without light at room temperature. The apoptosis rate of
osteoblasts (including early and late apoptotic cells) was measured
using a BD FACSCanto II flow cytometer (Becton, Dickinson and
Company).
Reactive oxygen species (ROS)
production assay
The ROS level in osteoblasts was measured using a
dichlorofluorescein-diacetate (DCFH-DA) dye (Sigma-Aldrich; Merck
KGaA). Briefly, osteoblasts (3×103 cells per well) were
seeded into 24-well plates and exposed to different treatments as
detailed in the previous experiments. The osteoblasts were then
collected, washed with PBS and incubated with 10 µM DCFH-DA for 30
min at 37°C without light. Fluorescence intensity was measured via
flow cytometry.
Statistical analysis
All data were presented as the mean ± standard
deviation and analysed using GraphPad Prism 7.0 software (GraphPad
Software, Inc.). Significant differences among three or more groups
were assessed by one-way analysis of variance (ANOVA) with Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
TFRD reduces the pathological changes
of the femoral head and inhibits apoptosis of the necrotic zone in
SANFH rats
In the present study, LPS and MPS were used to
establish a rat model of SANFH. To investigate the influence of
TFRD on SANFH rats, rats were intraperitoneally injected with TFRD.
During the experimental period, all rats survived and there were no
significant differences in the mean body weights among the control,
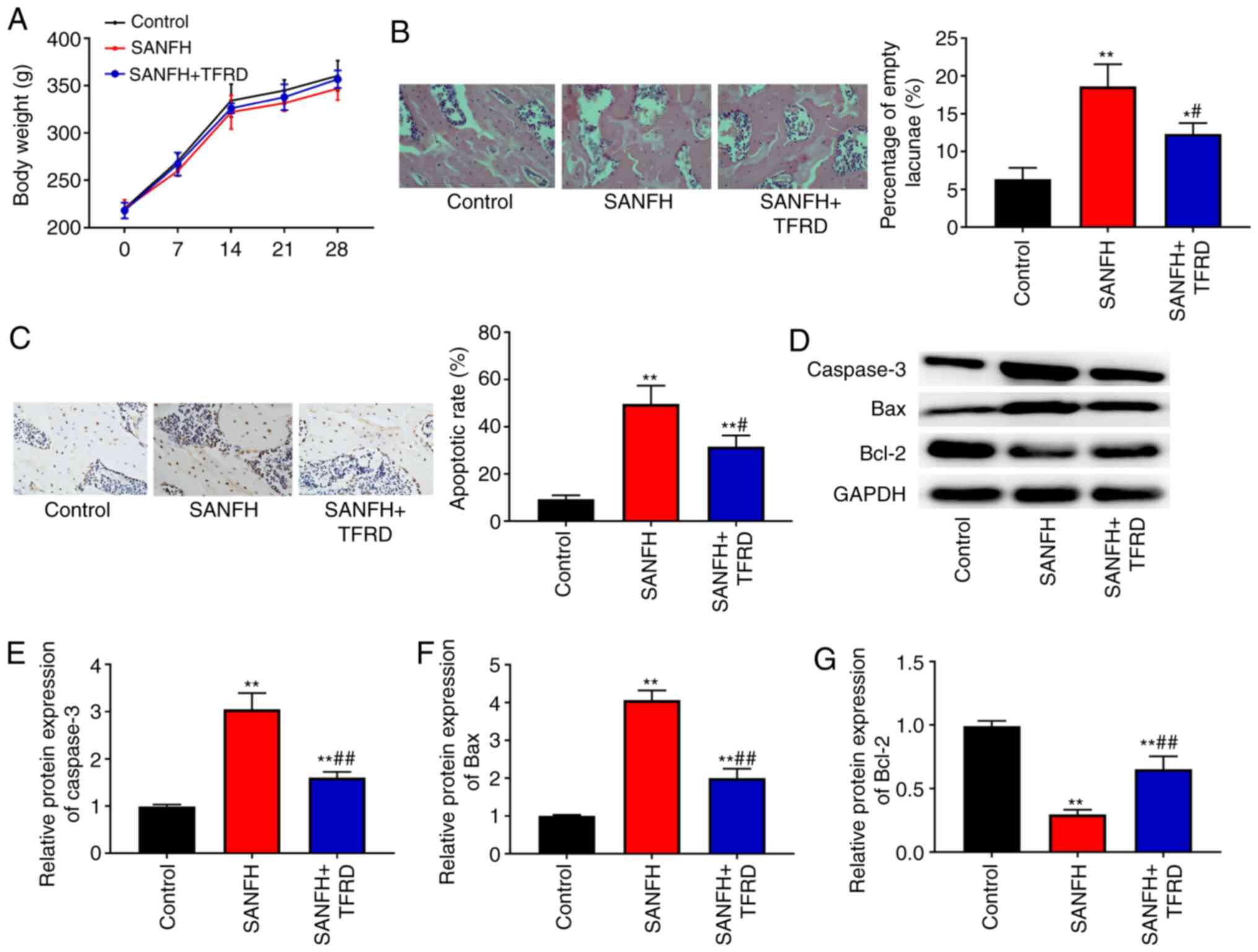
SANFH and SANFH+TFRD groups (P>0.05; Fig. 1A). Haematoxylin and eosin staining
was used to assess the pathological changes in the femoral head. As
shown in Fig. 1B, bone structure
was intact in the control group, while there were many empty bone
lacunae and broken trabeculae in the SANFH group. In addition, the
percentage of empty lacuna in the SANFH+TFRD group was lower
compared with the SANFH group (P<0.05). TUNEL assay was
conducted to detect apoptosis of the bone cells of the femoral
head. As presented in Fig. 1C, the
apoptosis rate in the SANFH and SANFH+TFRD groups was higher
compared with the control group (P<0.01), however treatment with
TFRD reduced the apoptosis rate compared with the SANFH group
(P<0.05). In addition, the expression of apoptosis-related
proteins (caspase-3, Bax and Bcl-2) was detected via western
blotting. The results showed that the expression of caspase-3 and
Bax in the SANFH and SANFH+TFRD groups was increased whereas the
expression of Bcl-2 was decreased in comparison with the control
group (Fig. 1D-G; P<0.01), while
treatment with TFRD increased the expression of Bcl-2 and decreased
the expression of Bax and caspase-3 compared with that in the SANFH
group (Fig. 1D-G; P<0.01).
TFRD increases the protein expression
of VEGF, RUNX2, OPG and OCN and decreases the protein expression of
RANKL in the femoral head of SANFH rats
Protein expression in the femoral head of rats was
detected via western blotting. As shown in Fig. 2, Compared with the control group,
the expression of VEGF, RUNX2, OPG and OCN in the SANFH and
SANFH+TFRD groups was decreased (P<0.01), whereas the expression
of RANKL was increased (P<0.01). Compared with the SANFH group,
treatment with TFRD increased the expression of VEGF, RUNX2, OPG
and OCN and reduced the expression of RANKL (P<0.05).
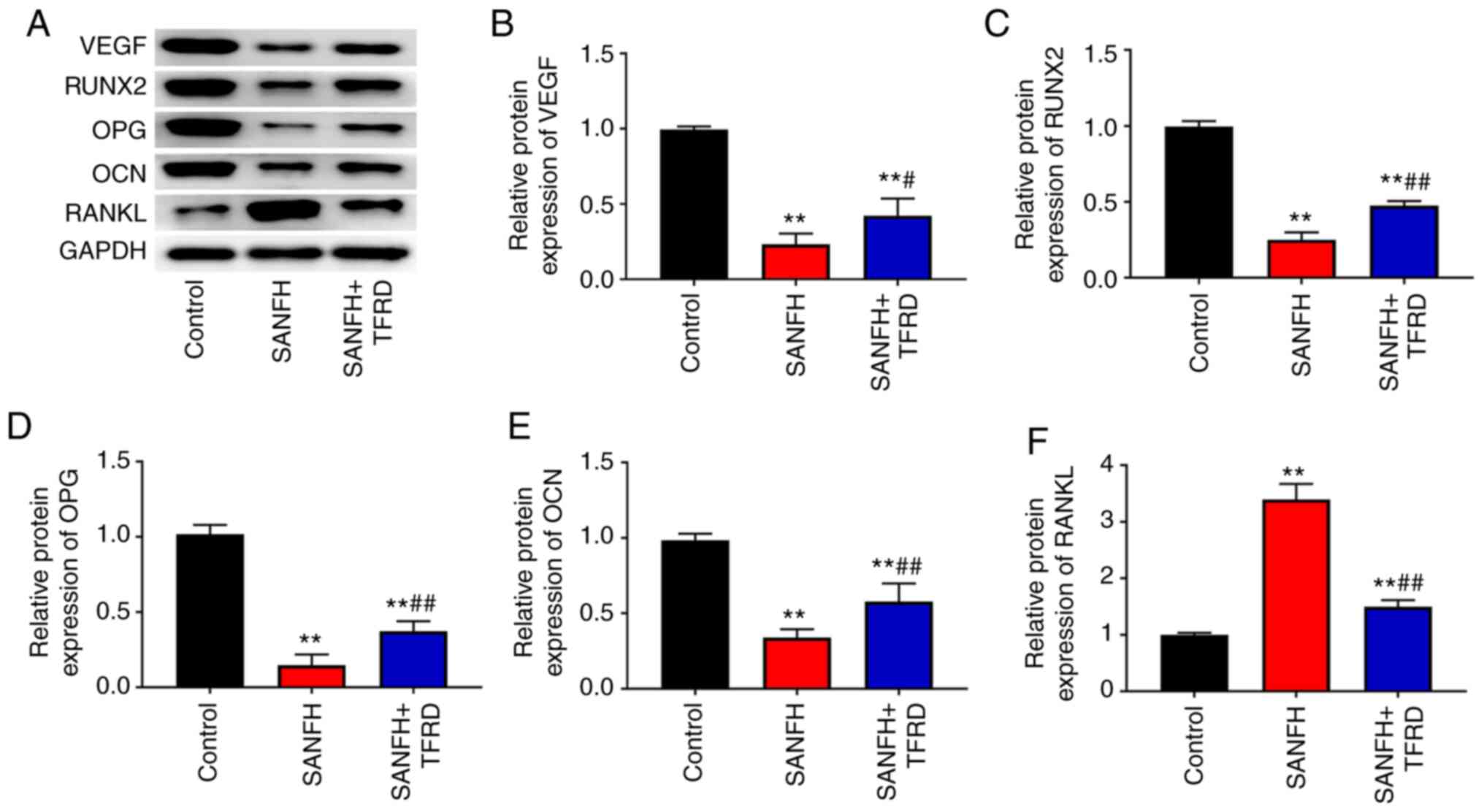 | Figure 2.TFRD increased the expression of
VEGF, RUNX2, OPG and OCN and decreased the expression of RANKL in
the femoral head of SANFH rats. (A) Protein bands of VEGF, RUNX2,
OPG, OCN and RANKL. The expression of (B) VEGF, (C) RUNX2, (D) OPG,
(E) OCN and (F) RANKL in the femoral head of rats was detected
using western blotting. **P<0.01 vs. the control group;
#P<0.05 and ##P<0.01 vs. the SANFH
group. TFRD, total flavonoids from Rhizoma drynariae; RUNX2,
runt-related transcription factor 2; OPG, osteoprotegerin; OCN,
osteocalcin; RANKL, receptor activator of nuclear factor kappa B
ligand; SANFH, steroid-induced avascular necrosis of the femoral
head. |
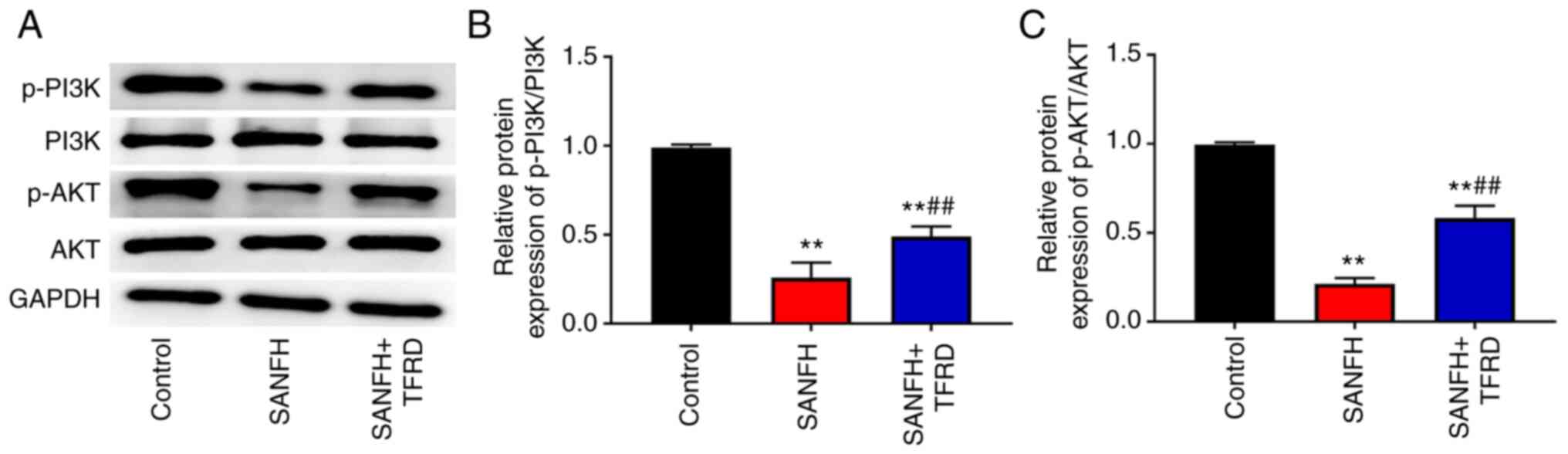
TFRD activates the PI3K/AKT pathway in
the SANFH rats
To explore the protective mechanism of TFRD in SANFH
rats, the PI3K/AKT pathway-related proteins were detected by
western blotting. The results showed that the SANFH and SANFH+TFRD
groups had lower expression of p-P13K/P13K and p-AKT/AKT than that
in the control group (P<0.01; Fig.
3), while treatment with TFRD increased the protein expression
of p-P13K/P13K and p-AKT/AKT compared with the SANFH group
(P<0.01; Fig. 3).
TFRD promotes proliferation, inhibits
apoptosis, reduces ROS levels and activates the PI3K/AKT pathway in
osteoblasts
The effect of different concentrations of Dex at
different time points on osteoblast proliferation was investigated
using MTT assay. The results showed that Dex inhibited osteoblast
proliferation in a dose- and time-dependent manner and that
10−6 M Dex inhibited osteoblast proliferation
(P<0.05; Fig. 4A). Additionally,
MTT assay was used to evaluate the cytotoxic effect of TFRD on
osteoblasts. As shown in Fig. 4B,
when the concentration of TFRD was less than 12.5 µg/ml, TFRD had
no cytotoxic effect on osteoblasts. Therefore, 10−6 M
Dex and 12.5 µg/ml TFRD were selected for subsequent experiments.
As can be seen in Fig. 4C-E,
treatment with Dex reduced the OD490 value, increased
apoptotic rate and ROS production in osteoblasts compared with the
control group (P<0.01), while the opposite effects of TFRD in
osteoblasts were observed. Compared with the control group, Dex
increased the expression of caspase-3 and Bax and decreased the
expression of Bcl-2, p-P13K/P13K and p-AKT/AKT (P<0.01; Fig. 4F), but this effect was weakened by
TFRD.
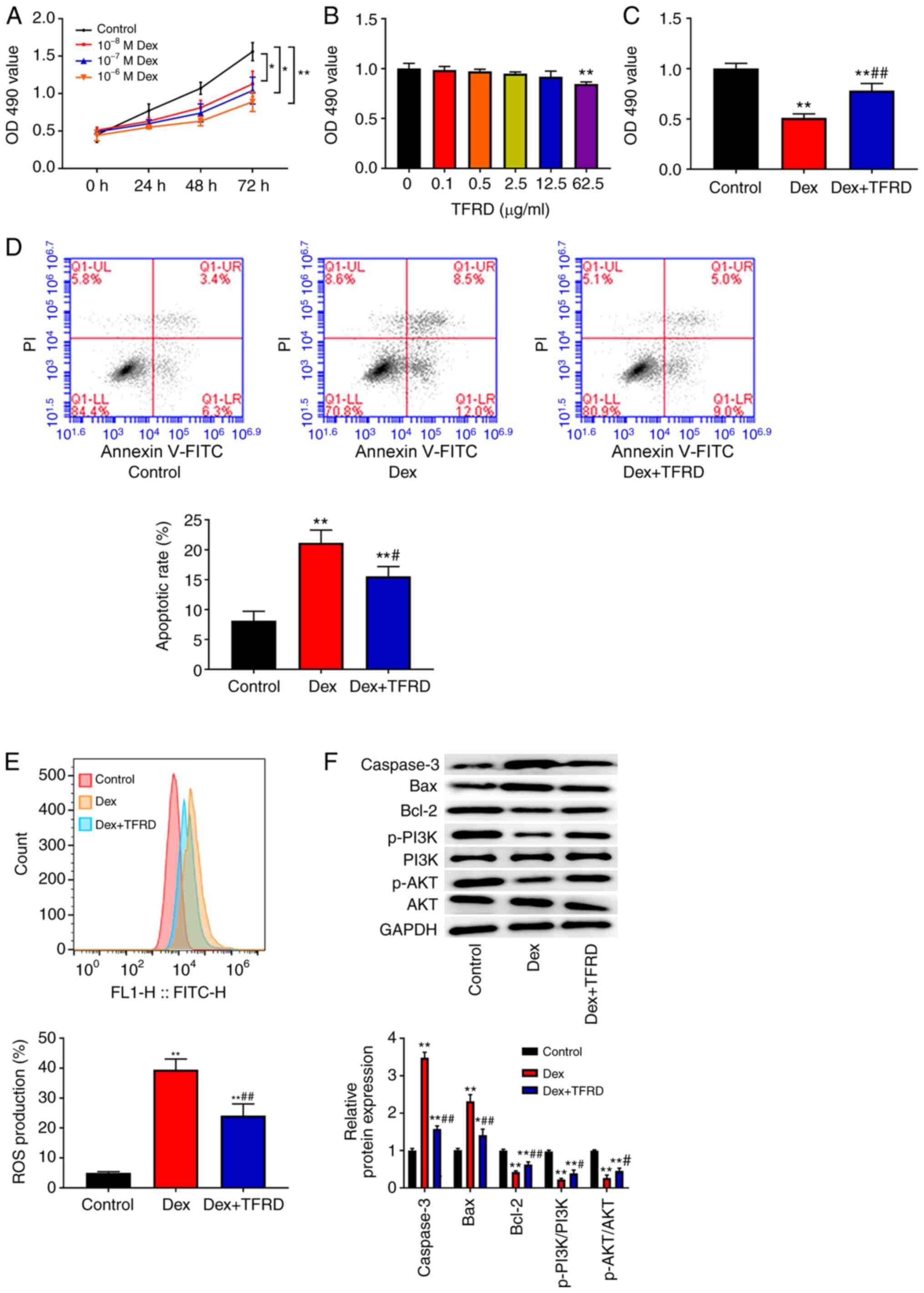 | Figure 4.TFRD promoted proliferation,
inhibited apoptosis, reduced ROS levels and activated the PI3K/AKT
pathway in osteoblast. (A-C) MTT assay was used to detect
osteoblast proliferation. (D) Flow cytometry was used to analyse
osteoblast apoptosis. (E) DCFH-DA staining was used to detect the
ROS level in osteoblasts. (F) The expression of caspase-3, Bax,
Bcl-2, p-PI3K/PI3K and p-AKT/AKT in osteoblasts was detected using
western blotting. *P<0.05 and **P<0.01 vs. the control group;
#P<0.05 and ##P<0.01 vs. the Dex group.
TFRD, total flavonoids from Rhizoma drynariae; ROS, reactive
oxygen species; PI3K, phosphoinositide 3-kinase; p-,
phosphorylated; DCFH-DA, dichlorofluorescein-diacetate; Dex,
dexamethasone; OD, optical density; FITC, fluorescein
isothiocyanate. |
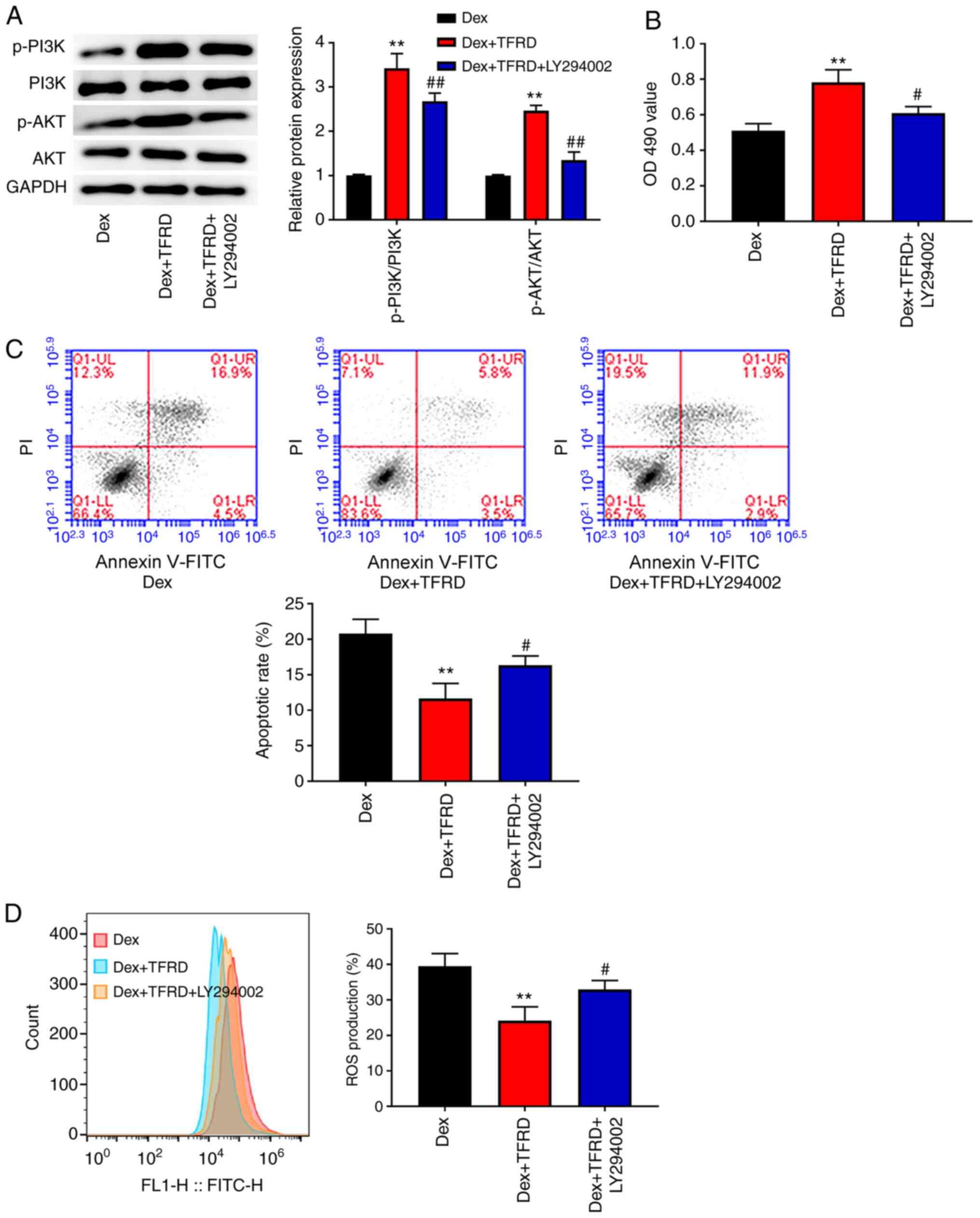
TFRD protects osteoblasts from
Dex-induced damage through the PI3K/AKT pathway
In order to elucidate the mechanism of TFRD-induced
protection of osteoblasts from Dex-induced damage, the PI3K/AKT
pathway inhibitor (LY294002) was used to treat the osteoblasts in
the Dex+TFRD group. As shown in Fig.
5A, the expression of p-P13K/P13K and p-AKT/AKT in the
Dex+TFRD+LY294002 group was reduced compared with that in the
Dex+TFRD group (P<0.01), indicating that the activation of the
PI3K/AKT pathway induced by TFRD was blocked via LY294002.
Subsequently, the effects of LY294002 on proliferation, apoptosis
and ROS production was detected in osteoblasts. As presented in
Fig. 5B-D, following LY294002
treatment, the OD490 value was decreased, while the
apoptotic rate and ROS production were increased in the osteoblasts
(P<0.05).
Discussion
ONFH is a common disease, mainly caused by the abuse
of steroid hormones (21). SANFH is
characterized by difficult recovery and poor prognosis, which has a
serious impact on the health and quality of life of patients
(22). The main chemical
constituents of the Rhizoma drynariae extract include
phenolic acids, flavonoids and triterpenes, as well as their
glycosides (8). Among these,
studies have mainly focused on total flavonoids (23–25).
As TFRD has antioxidant properties and anti-osteoporosis activity,
it has been widely used in clinic and has great value in research
and development (26). The present
study found that TFRD reduced the pathological changes, inhibited
cell apoptosis, increased the expression of VEGF, RUNX2, OPG and
OCN, decreased RANKL expression and activated the PI3K/AKT pathway
in SANFH rats. In addition, TFRD promoted proliferation, inhibited
apoptosis and reduced ROS levels by activating the PI3K/AKT pathway
in osteoblasts.
In the treatment of ONFH, restoring the bone blood
and nutrient supply is important for bone healing and bone
formation (27,28). When the blood supply is obstructed,
it causes ischemia and anoxia of the femoral head, leading to the
apoptosis of osteoblasts and ONFH (29,30).
VEGF is a crucial angiogenic factor that regulates the
proliferation and migration of vascular endothelial cells and
vascularization and serves a key role in bone formation and healing
(31–33). In addition, VEGF and endothelial
cells can induce osteogenic differentiation of bone marrow-derived
mesenchymal stem cells (34). The
present study found that VEGF expression in rats with SANFH was
decreased, while treatment with TFRD increased VEGF expression.
These findings indicated that TFRD increases VEGF expression, which
may promote bone formation and osteoblast differentiation.
Bone cell damage and the imbalance between
osteogenesis and bone exfoliation activities ultimately lead to
bone structure destruction and collapse (35). RUNX2, a transcription activator of
osteoblasts, serves an important role in the regulation of gene
expression during osteogenic differentiation (36). The degree of cell deposition and
mineralization can be reflected by the expression level of OCN,
which is involved in the late stage of osteogenic differentiation
(37). OPG produced by osteoblasts
can bind to osteoblast membranes, block the differentiation of
osteoclast precursors and promote osteoclast apoptosis, thus
preventing the development of ONFH (38,39).
The present study detected the expression of RUNX2, OPG, OCN and
RANKL in the femoral head of rats. Treatment with TFRD was found to
increase the expression of RUNX2, OPG and OCN and reduce the
expression of RANKL. The above results indicate that TFRD promotes
the differentiation of osteoblasts.
Ischaemic necrosis of the femoral head is the main
process resulting in bone cell death; after ischaemia and hypoxia
occur, cell death (apoptosis) is activated (40). Previous studies have shown that Dex
can induce osteoblast apoptosis and SANFH in rat or human specimens
by upregulating caspase-3 (20,41).
Bcl-2 is an anti-apoptotic gene, whereas Bax is a pro-apoptotic
gene (3) and studies have reported
that the cause of Dex-induced osteoblast apoptosis is the imbalance
between Bcl-2 and Bax (mitochondrial dysfunction) (42,43).
Additionally, Dex treatment can increase ROS production and
excessive ROS may cause cell apoptosis through the
mitochondrial-mediated caspase apoptosis pathway (44). In the present study, SANFH modelling
and Dex treatment promoted cell apoptosis, increased the expression
of caspase-3 and Bax and decreased the protein expression of Bcl-2
in vivo and in vitro. In addition, compared with the
SANFH and Dex groups, TFRD treatment inhibited cell apoptosis,
decreased the protein expression of caspase-3 and Bax and increased
the protein expression of Bcl-2. TFRD treatment reduced ROS
production and promoted Dex-induced osteoblast proliferation. These
findings indicate that TFRD serves an anti-apoptosis role in SANFH
partly by inhibiting the mitochondrial pathway.
At present, the pathogenesis of ONFH is still
unclear, although apoptosis is one of its most studied mechanisms
(45). Previous studies have
reported apoptotic signals transduced by the
PI3K/AKT-Bax/Bcl-2/caspase-3 pathway in some orthopaedic diseases
(46,47). In addition, Cakir et al
(48) have found that the
accumulation of ROS causes oxidative stress and activates the c-Jun
N-terminal kinase pathway, which may inhibit the activation of the
AKT pathway and promote osteoblast apoptosis. The present study
found that SANFH modelling and Dex treatment reduced the expression
of p-P13K/P13K and p-AKT/AKT in vivo and in vitro,
while treatment with TFRD increased the protein expression of
p-P13K/P13K and p-AKT/AKT compared with expression in SANFH and Dex
groups. The addition of PI3K inhibitor (LY294002) reversed the
promotion of the PI3K/AKT pathway in osteoblasts induced by TFRD,
as well as the promotion of proliferation and inhibition of
apoptosis and ROS production in osteoblasts caused by TFRD. These
findings suggested that TFRD protects osteoblasts from Dex-induced
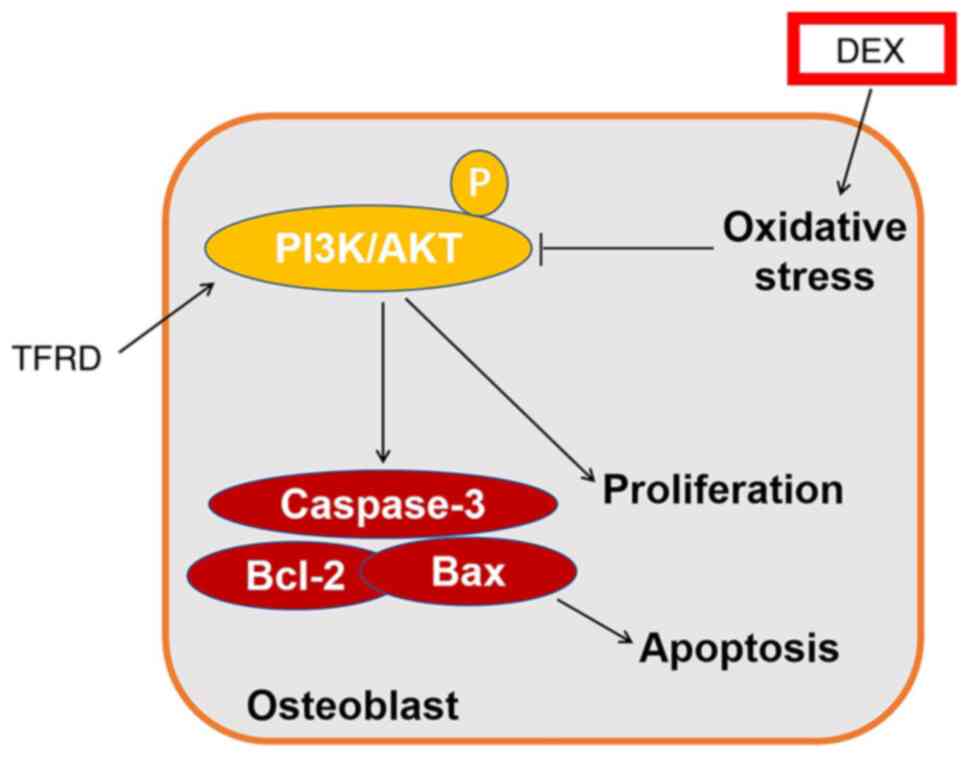
damage through the PI3K/AKT pathway (Fig. 6).
In conclusion, TFRD protected against SANFH in a rat
model. In addition, TFRD protected osteoblasts from steroidal
damage via PI3K/AKT pathway. The findings of the present study may
have implications for future treatments of SANFH.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL and QY designed the study; MY, PK and BY
performed the experiments, analysed the data and wrote the paper.
WL and QY confirm the authenticity of all the raw data. All authors
reviewed and approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocol of the present study was
performed in accordance with the Guide for the Care and Use of
Laboratory Animals and approved by the Affiliated Hospital of
Shandong University of Traditional Chinese Medicine (approval no.
2019-58).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SANFH
|
steroid-induced avascular necrosis of
the femoral head
|
|
Dex
|
dexamethasone
|
|
TFRD
|
total flavonoids from Rhizoma
drynariae
|
|
ROS
|
reactive oxygen species
|
|
RUNX2
|
runt-related transcription factor
2
|
|
OPG
|
osteoprotegerin
|
|
OCN
|
osteocalcin
|
|
RANKL
|
receptor activator of nuclear factor
kappa B ligand
|
|
PI3K
|
phosphoinositide 3-kinase
|
References
|
1
|
Xiang S, Li Z and Weng X: The role of
lncRNA RP11-154D6 in steroid-induced osteonecrosis of the femoral
head through BMSC regulation. J Cell Biochem. 120:18435–18445.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yan YQ, Pang QJ and Xu RJ: Effects of
erythropoietin for precaution of steroid-induced femoral head
necrosis in rats. BMC Musculoskelet Disord. 19:2822018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xue XH, Feng ZH, Li ZX and Pan XY:
Salidroside inhibits steroid-induced avascular necrosis of the
femoral head via the PI3K/Akt signaling pathway: In vitro and in
vivo studies. Mol Med Rep. 17:3751–3757. 2018.PubMed/NCBI
|
|
4
|
Ren X, Fan W, Shao Z, Chen K, Yu X and
Liang Q: A metabolomic study on early detection of steroid-induced
avascular necrosis of the femoral head. Oncotarget. 9:7984–7995.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wong GKC, Poon WS and Chiu KH:
Steroid-induced avascular necrosis of the hip in neurosurgical
patients: Epidemiological study. ANZ J Surg. 75:409–410. 2015.
View Article : Google Scholar
|
|
6
|
Bae WY, Kim HY, Choi KS, Chang KH, Hong
YH, Eun J, Lee NK and Paik HD: Investigation of Brassica juncea,
Forsythia suspensa, and Inula britannica: Phytochemical properties,
antiviral effects, and safety. BMC Complement Altern Med.
19:2532019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li L, Hou X, Xu R, Liu C and Tu M:
Research review on the pharmacological effects of astragaloside IV.
Fundam Clin Pharmacol. 31:17–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Jiang J, Shen H, Chai Y, Wei X
and Xie Y: Total flavonoids from Rhizoma Drynariae (Gusuibu) for
treating osteoporotic fractures: Implication in clinical practice.
Drug Des Devel Ther. 11:1881–1890. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin FX, Du SX, Liu DZ, Hu QX, Yu GY, Wu
CC, Zheng GZ, Xie D, Li XD and Chang B: Naringin promotes
osteogenic differentiation of bone marrow stromal cells by
up-regulating Foxc2 expression via the IHH signaling pathway. Am J
Transl Res. 8:5098–5107. 2016.PubMed/NCBI
|
|
10
|
Wong RW, Rabie B, Bendeus M and Hägg U:
The effects of rhizoma curculiginis and rhizoma drynariae extracts
on bones. Chin Med. 2:132007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuang MJ, Zhang WH, He WW, Sun L, Ma JX,
Wang D and Ma XL: Naringin regulates bone metabolism in
glucocorticoid-induced osteonecrosis of the femoral head via the
Akt/Bad signal cascades. Chem Biol Interact. 304:97–105. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guntur AR and Rosen CJ: The skeleton: A
multi-functional complex organ. New insights into osteoblasts and
their role in bone formation: The central role of PI3Kinase. J
Endocrinol. 211:123–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gu Y, Du J, Si M, Mo J, Qiao S and Lai H:
The roles of PI3K/Akt signaling pathway in regulating MC3T3-E1
preosteoblast proliferation and differentiation on SLA and SLActive
titanium surfaces. J Biomed Mater Res A. 101:748–754. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma J, Sawai H, Ochi N, Matsuo Y, Xu D,
Yasuda A, Takahashi H, Wakasugi T and Takeyama H: PTEN regulates
angiogenesis through PI3K/Akt/VEGF signaling pathway in human
pancreatic cancer cells. Mol Cell Biochem. 331:161–171. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang J, Liu X, Li H, Chen C, Hu B, Niu X,
Li Q, Zhao B, Xie Z and Wang Y: Exosomes/tricalcium phosphate
combination scaffolds can enhance bone regeneration by activating
the PI3K/Akt signaling pathway. Stem Cell Res Ther. 7:1362016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu J, Gu Y and Fan W: Rg1 protects rat
bone marrow stem cells against hydrogen peroxide-induced cell
apoptosis through the PI3K/Akt pathway. Mol Med Rep. 14:406–412.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
National Research Council: Guide for the
Care and Use of Laboratory Animals: Eighth edition. The National
Academies Press; Washington, DC: 2011
|
|
18
|
Spiers DE and Candas V: Relationship of
skin surface area to body mass in the immature rat: A
reexamination. J Appl Physiol Respir Environ Exerc Physiol.
56:240–243. 1984.PubMed/NCBI
|
|
19
|
Lu X, Xiong Z, Li J, Zheng S, Huo T and Li
F: Metabonomic study on ‘Kidney-Yang Deficiency syndrome’ and
intervention effects of Rhizoma Drynariae extracts in rats using
ultra performance liquid chromatography coupled with mass
spectrometry. Talanta. 83:700–708. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng Z, Zheng W, Tang Q, Cheng L, Li H, Ni
W and Pan X: Fludarabine inhibits STAT1-mediated up-regulation of
caspase-3 expression in dexamethasone-induced osteoblasts apoptosis
and slows the progression of steroid-induced avascular necrosis of
the femoral head in rats. Apoptosis. 22:1001–1012. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang A, Ren M and Wang J: The pathogenesis
of steroid-induced osteonecrosis of the femoral head: A systematic
review of the literature. Gene. 671:103–109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song HM, Wei YC, Li N, Wu B, Xie N, Zhang
KM, Wang SZ and Wang HM: Effects of Wenyangbushen formula on the
expression of VEGF, OPG, RANK and RANKL in rabbits with
steroid-induced femoral head avascular necrosis. Mol Med Rep.
12:8155–8161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu L, Huang G, Guo X, Zhou Q and He S:
Total flavonoids, extracted from Polygonum knotweed L, exert
beneficial hepatoprotection against liver injury. J Cell Biochem.
120:12677–12683. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng Y, Yang Z, Shi J, Yang J, Zhao J, He
Y and Qi M: Total flavonoids of Epimedium ameliorates testicular
damage in streptozotocin-induced diabetic rats by suppressing
inflammation and oxidative stress. Environ Toxicol. 35:268–276.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo Y, Xing L, Chen N, Gao C, Ding Z and
Jin B: Total flavonoids from the Carya cathayensis Sarg. leaves
inhibit HUVEC senescence through the miR-34a/SIRT1 pathway. J Cell
Biochem. 120:17240–17249. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kang SN, Lee JS, Park JH, Cho JH, Park JH,
Cho KK, Lee OH and Kim IS: In vitro anti-osteoporosis properties of
diverse Korean Drynariae rhizoma phenolic extracts. Nutrients.
6:1737–1751. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weinstein RS: Glucocorticoid-induced
osteonecrosis. Endocrine. 41:183–190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Assouline-Dayan Y, Chang C, Greenspan A,
Shoenfeld Y and Gershwin ME: Pathogenesis and natural history of
osteonecrosis. Semin Arthritis Rheum. 32:94–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Inoue A and Ono K: A histological study of
idiopathic avascular necrosis of the head of the femur. J Bone
Joint Surg Br. 61-B:138–143. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ichiseki T, Ueda S, Ueda Y, Tuchiya M,
Kaneuji A and Kawahara N: Involvement of necroptosis, a newly
recognized cell death type, in steroid-induced osteonecrosis in a
rabbit model. Int J Med Sci. 14:110–114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Varoga D, Drescher W, Pufe M, Groth G and
Pufe T: Differential expression of vascular endothelial growth
factor in glucocorticoid-related osteonecrosis of the femoral head.
Clin Orthop Relat Res. 467:3273–3282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carlevaro MF, Cermelli S, Cancedda R and
Cancedda FD: Vascular endothelial growth factor (VEGF) in cartilage
neovascularization and chondrocyte differentiation: Auto-paracrine
role during endochondral bone formation. J Cell Sci. 113:59–69.
2000.PubMed/NCBI
|
|
33
|
Carmeliet P, Ferreira V, Breier G,
Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A,
Harpal K, Eberhardt C, et al: Abnormal blood vessel development and
lethality in embryos lacking a single VEGF allele. Nature.
380:435–439. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng LZ, Cao HJ, Chen SH, Tang T, Fu WM,
Huang L, Chow DHK, Wang YX, Griffith JF, He W, et al: Blockage of
src by specific siRNA as a novel therapeutic strategy to prevent
destructive repair in steroid-associated osteonecrosis in rabbits.
J Bone Miner Res. 30:2044–2057. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Piuzzi NS, Anis HK and Muschler GF:
Osteonecrosis of the femoral head with subchondral collapse. Cleve
Clin J Med. 86:511–512. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu C, Lv Y, Qian C, Qian H, Jiao T, Wang
L and Zhang F: Proliferation and osteogenic differentiation of rat
BMSCs on a novel Ti/SiC metal matrix nanocomposite modified by
friction stir processing. Sci Rep. 6:388752016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qian C, Zhu C, Yu W, Jiang X and Zhang F:
High-fat diet/low-dose streptozotocin-induced type 2 diabetes in
rats impacts osteogenesis and wnt signaling in bone marrow stromal
cells. PLoS One. 10:e01363902015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Croucher PI, Mcdonald MM and Martin TJ:
Bone metastasis: The importance of the neighbourhood. Nat Rev
Cancer. 16:373–386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Peng WX, Ye C, Dong WT, Yang LL, Wang CQ,
Wei ZA, Wu JH, Li Q, Deng J and Zhang J: MicroRNA-34a alleviates
steroid-induced avascular necrosis of femoral head by targeting
Tgif2 through OPG/RANK/RANKL signaling pathway. Exp Biol Med
(Maywood). 242:1234–1243. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang H, Zhou F, Pan Z, Bu X, Wang Y and
Chen F: 11β-hydroxysteroid dehydrogenases-2 decreases the apoptosis
of MC3T3/MLO-Y4 cells induced by glucocorticoids. Biochem Biophys
Res Commun. 490:1399–1406. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xu X, Wen H, Hu Y, Yu H, Zhang Y, Chen C
and Pan X: STAT1-caspase 3 pathway in the apoptotic process
associated with steroid-induced necrosis of the femoral head. J Mol
Histol. 45:473–485. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li DW, Liu ZQ, Chen W, Yao M and Li GR:
Association of glycogen synthase kinase-3β with Parkinson's disease
(Review). Mol Med Rep. 9:2043–2050. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chao DT and Korsmeyer SJ: BCL-2 family:
Regulators of cell death. Annu Rev Immunol. 16:395–419. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ravindran J, Gupta N, Agrawal M, Asb B and
Rao PVL: Modulation of ROS/MAPK signaling pathways by okadaic acid
leads to cell death via, mitochondrial mediated caspase-dependent
mechanism. Apoptosis. 16:145–161. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Deng S, Zhou JL, Fang HS, Nie ZG, Chen S
and Peng H: Sesamin protects the femoral head from osteonecrosis by
inhibiting ROS-induced osteoblast apoptosis in rat model. Front
Physiol. 9:17872018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sato A, Tu X, McAndrews K, Plotkin L and
Bellido T: Prevention of glucocorticoid induced-apoptosis of
osteoblasts and osteocytes by protecting against endoplasmic
reticulum (ER) stress in vitro and in vivo in female mice. Bone.
73:60–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li J, He C, Tong W, Zou Y, Li D, Zhang C
and Xu W: Tanshinone IIA blocks dexamethasone-induced apoptosis in
osteoblasts through inhibiting Nox4-derived ROS production. Int J
Clin Exp Pathol. 8:13695–13706. 2015.PubMed/NCBI
|
|
48
|
Cakir E, Yilmaz A, Demirag F, Oguztuzun S,
Sahin S, Yazici UE and Aydin M: Prognostic significance of
micropapillary pattern in lung adenocarcinoma and expression of
apoptosis-related markers: Caspase-3, bcl-2, and p53. APMIS.
119:574–580. 2011. View Article : Google Scholar : PubMed/NCBI
|