Introduction
Glioma is the most common type of malignant primary
brain cancer (1), and is
characterized by rapid proliferation, high invasiveness, rapid
recurrence and chemo- and radiotherapeutic resistance (2). In spite of the advances in
microsurgery and combined treatments, the prognosis of patients
with glioma remains unsatisfactory (3). This has driven research to investigate
the potential mechanisms underlying the tumorigenesis and
development of glioma in order to identify novel therapeutic
targets.
A number of studies have demonstrated that the
non-coding RNAs [microRNAs (miRNAs/miRs), pseudogenes, long
non-coding RNAs (lncRNAs) and circular RNAs] play important roles
not only in normal biological processes, but also in the
development of tumors (4,5). Complex regulatory networks exist
between non-coding RNAs, mRNAs and proteins. A commonly accepted
theory is the competing endogenous RNA (ceRNA) hypothesis, through
which endogenous RNAs containing certain miRNA binding sites
competitively bind the same miRNAs to reduce the suppression of
targeted mRNAs, thus retaining the expression of target genes
(6). Increasing evidence suggests
that the ceRNA mechanism is also involved in tumorigenesis
(7–9).
lncRNAs, a group of non-coding RNAs >200
nucleotides in length, are involved in various disease processes,
including tumorigenesis (10,11).
An increasing number of studies have indicated that the aberrant
expression of specific lncRNAs is associated with the development
of glioma (12). lncRNAs exert
their functions through various mechanisms in an extensive array of
biological processes, such as the maintenance of stemness,
regulation of cell proliferation, tumor angiogenesis and drug
resistance (13). In glioma, the
functions of lncRNAs include regulating genome activity,
posttranscriptional protein modification and location, as well as
encoding functional micro-peptides and acting as intercellular
communicators (14). However, the
mechanisms by which lncRNAs regulate the biological functions of
glioma remain to be elucidated. The present study aimed to
demonstrate the role of LINC01116 in glioma and its underlying
mechanism. The expression levels of LINC01116 in glioma tissue and
cell lines were determined by reverse transcription-quantitative
(RT-q)PCR. The effect of LINC01116 on cell proliferation, migration
and invasion was assessed by cell viability, Transwell and colony
formation assays and tumor xenografts in nude mice. In order to
identify the underlying mechanism by which LINC01116 exerts its
role, bioinformatics analysis and dual-luciferase reporter assay
were performed.
Materials and methods
Cell culture and glioma samples
Glioma (U87MG, A172 and Shg44) and 293T cell lines
were purchased from the Cell Resource Center of Shanghai Institutes
for Biological Sciences. The U87MG cell line was a version from
American Type Culture Collection (ATCC; RRID:CVCL_0022), the origin
of which is unknown. The cell line was authenticated by STR
profiling, with a 96.55% match to the ATCC database profile. The
HEB normal human astrocyte cell line was purchased from Cobioer;
Nanjing Kebai Biotechnology Co., Ltd. All cells were cultured in
DMEM (Gibco; Thermo Fisher Scientific, Inc.) with 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Beijing Solarbio Science & Technology
Co., Ltd.) at 37°C (5% CO2 and 95% air). A total of 46
fresh glioma samples and 4 normal brain tissue samples (excised
from patients with intracerebral hemorrhage) were collected from
the Hangzhou First People's Hospital (all specimens were
pathologically diagnosed as glioma between January 2010 and October
2019), and stored in liquid nitrogen. The patients of the present
study consisted of 27 male patients and 19 female patients. The age
range of patients was between 28 and 74 years. Informed written
consent was obtained from the patients, agreeing to use their
samples for scientific research. All protocols associated with
animals or human tissues were approved by the Ethics Committee of
Hangzhou First People's Hospital [Hangzhou, China; approval no.
2020(106)-01].
Online cancer database analysis
The expression of LINC01116 in different grades of
glioma, as well as their association with overall survival time and
the expression of p53 target genes [BAK1, BAX, cyclin-dependent
kinase inhibitor 1 (CDKN1A) and growth arrest and DNA
damage-inducible protein GADD45 α (GADD45A)] were analyzed using
the Gene Expression Profiling Interactive Analysis (GEPIA) platform
(http://gepia.cancer-pku.cn/detail.php) (15).
Matrigel invasion assay
Invasion assays were performed using Transwell
plates (diameter, 6.5 mm; aperture, 8.0 µm; Corning, Inc.)
according to the manufacturer's protocols. Briefly, the filters of
the upper chamber were precoated with Matrigel (Becton, Dickinson
and Company) at 37°C for 30 min, and 1×104 glioma cells
resuspended in 200 µl serum-free medium were added into the
chambers. A total of 650 µl culture medium was added to the lower
compartments with 10% FBS as a chemoattractant. Following culture
at 37°C for 24–48 h, the cells in the upper chambers were removed,
and cells that had migrated to the lower membrane were fixed with
4% paraformaldehyde (Beijing Solarbio Science & Technology Co.,
Ltd.) for 20 min and stained with 0.5% crystal violet solution for
30 min at room temperature. The images were scanned by Invitrogen
EVOS FL AUTO (Thermo Fisher Scientific, Inc.).
Cell proliferation and colony
formation assays
A Cell Counting Kit-8 (CCK-8; cat. no. HY-K0301;
MedChem Express) assay was used to assess cell viability according
to the manufacturer's protocols. Briefly, the indicated cells were
seeded into a 96-well plate at a density of 70–80% cells per well.
The culture medium was replaced by serum-free medium and 10 µl
CCK-8 solution was added at the indicated times. After incubation
for 1 h, the OD values were determined at 450 nm. For the colony
formation assay, 1×103 cells per well were seeded into
6-well plates (Corning, Inc.). After 14 days of culture, the
colonies (>10 cells) were fixed with 4% paraformaldehyde for 20
min and stained with 0.5% crystal violet solution for 30 min at
room temperature. The images were scanned by Scan Wizard EZ
(Microtek) and the number of clones was counted using Image J
software (National Institutes of Health).
RNA extraction and RT-qPCR
Briefly, total RNA was extracted from tissues and
the indicated cells using TRIzol® reagent (cat. no.
15596026; Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocols. The RNA was reverse transcribed into
cDNA using the PrimeScript™ RT reagent kit (cat. no. RR047A; Takara
Bio, Inc.), and qPCR analyses were conducted according to the
manufacturer's protocol using a TB Green® Premix Ex Taq™
II (cat. no. RR820A; Takara Bio, Inc.) with GAPDH and U6 as the
internal controls. The relative expression levels were calculated
via the 2−ΔΔcq method (16). The sequences of the qPCR primers
were as follows: LINC01116 forward, 5′-GTTCAAGTGCGTCCGGGTTT-3′ and
reverse, 5′-CGGACTTCTTTTCCAGGCGG-3′; miR-744-5p forward,
5′-AATGCGGGGCTAGGGCTA−3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′; BAK1
forward, 5′-GTCAGAAAGTAGTGTCGCCA-3′ and reverse,
5′-ACTTGTAGCGTCAGGACAGC-3′; GADD45A forward,
5′-CTGGGAATTTGGCGACGTAA-3′ and reverse,
5′-ATGGATGTAGTCTGGGTGCAG−3′; TP53 forward,
5′-CCAAATACTCCACACGCAAAT−3′ and reverse,
5′-CCTTCCCAGAAAACCTACCAG−3′; MDM2 forward,
5′-GGCTCTGTGTGTAATAAGGGAGA3′ and reverse,
5′-GGACTGCCAGGACTAGACTTTG-3′, GAPDH forward,
5′-AGGAGCGAGATCCCGCCAACA−3′ and reverse,
5′-CGGCCGTCACGCCACATCTT-3′; and U6 forward, 5′-CTCGCTTCGGCAGCACA-3′
and reverse, 5′-AACGCTTCACGAATTTGCGT-3′.
Vector construction and
transduction
Small hairpin RNA (shRNA) against human LINC01116
(01116 KD; 5′-CCAAAGGCCCTGAAGTACACAGTTT-3′) and corresponding
negative control (NC) sequences were purchased from Shanghai
GeneChem Co., Ltd., and the sequence was inserted into a G248
lentiviral vector (Shanghai GeneChem Co., Ltd.). For LINC01116
overexpression (OE) experiments, the indicated cells
(1×104) were infected with lentivirus containing
LINC01116-GV358 plasmids (MOI, 10) at 37°C for 12 h, which were
synthesized by Shanghai GeneChem Co., Ltd. All transfections were
conducted following the manufacturer's protocols. MDM2 small
interfering (si)RNA (MDM2 KD) (cat. no. #AM16708) was acquired from
Thermo Fisher Scientific, Inc., and the miR-744-5p mimics
(5′-UGCGGGGCUAGGGCUAACAGCA-3′) and inhibitor
(5′-UGCUGUUAGCCCUAGCCCCCA−3′) were designed and synthesized by
Shanghai GenePharma Co., Ltd. All transfections were performed
using Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocols. The
amount of mimics/inhibitor was 2 µg/2×106 cells.
Western blotting
Briefly, total protein was extracted from cells or
tissues by incubation with RIPA lysis buffer (Beyotime Institute of
Biotechnology) and the protein concentration was measured using a
BCA Protein Assay kit (Thermo Fisher Scientific, Inc.). Equal
amounts of protein sample (20 µg/lane) were separated via 10%
SDS-PAGE, and subsequently transferred onto PVDF membranes (Roche
Diagnostics). After blocking with skimmed milk (5%) for 1 h at room
temperature, the membranes were incubated with the following
primary antibodies overnight at 4°C and secondary antibodies for 1
h at RT: Anti-p53 (1:1,000; cat. no. 2527), anti-MDM2 (1:1,000;
cat. no. 86934), anti-BAK1 (1:1,000; cat. no. 12105), anti-GADD45A
(1:1,000; cat. no. 4632), anti-β-actin (1:1,000; cat. no. 3700),
anti-mouse IgG (1:5,000; cat. no. 7076) and anti-rabbit IgG
(1:5,000; cat. no. 7074) from Cell Signaling Technology, Inc. After
washing with TBS-Tween-20 (0.05%), bound HRP-conjugated antibodies
were detected using Western Lightning Plus-ECL reagents
(PerkinElmer, Inc.). The protein bands were measured by FluorChem Q
(ProteinSimple).
Online cancer database analysis
StarBase (starbase.sysu.edu.cn/) (17) was used to identify the ceRNA network
between LINC01116, microRNA-744-5p and MDM2.
Dual-luciferase reporter assay
Fragments of LINC01116 and the MDM2 mRNA 3′
untranslated region (UTR) containing miR-744-5p binding sites, and
mutated variants of these fragments, were amplified and subcloned
into the pGL3 promoter vector (Promega Corporation). The mutated
variants were generated using QuickMutation™ Site-Directed
Mutagenesis kit (cat. no. D0206; Beyotime Institute of
Biotechnology), according to the manufacturer's protocol. The
indicated plasmids (1 µg) and miRNA mimics or inhibitors (1 µg)
were transfected into 293T cells (2×106) using
Lipofectamine 3000. After 48 h, luciferase activity was measured
using a Dual-Luciferase Reporter Assay Kit (Promega Corporation)
according to the manufacturer's protocols. Renilla
luciferase was used as the control.
RNA immunoprecipitation (RIP)
assay
RIP assays were conducted using the Magna RIP™
RNA-Binding Protein Immunoprecipitation kit (EMD Millipore),
following the manufacturer's protocols. The indicated cells
(2×107) were incubated with 500 µl RIP lysis buffer
(Beyotime Institute of Biotechnology). Extracts were incubated with
5 µg antibodies against Ago2 (cat. no. 2897; Cell Signaling
Technology, Inc.) at 4°C overnight, followed by incubating with 30
µl Magnetic beads (MedChemExpress; cat. no. HY-K0205) for 6–8 h at
4°C. Argonaute proteins are involved in the various steps of
miRNA-mediated gene silencing by facilitating the formation of
micro-ribonucleoproteins complexes with miRNAs (18,19).
Rabbit IgG (cat. no. ab172730; Abcam) was used as a negative
control. Completes were washed with washing buffer and incubated
with proteinase K at 55°C for 30 min to isolate the RNA- protein
complexes from beads. RNA was isolated using TRIzol and then
reverse-transcribed into cDNA by PrimeScript™ RT reagent kit
(Takara Biotechnology Co., Ltd.) The levels of precipitated RNA
were then determined by RT-qPCR as aforementioned.
Tumor xenografts in nude mice
A total of 30 six-week-old female BALB/c nude mice
(weight, 14–20 g) were purchased from The Shanghai Laboratory
Animal Center of the Chinese Academy of Sciences. All mice were
kept in a temperature-controlled pathogen-free environment (21°C)
on a 12-h light–dark cycle in accordance with the Guide for the
Care and Use of Laboratory Animals. A total of 10 nude mice were
randomly divided into two groups for tumor xenografts. The
indicated Shg44 cells (5×106/100 µl in primary DMEM)
were subcutaneously injected into the flank of each nude mouse, and
tumor volume was recorded every 6 days. The tumor volumes were
calculated using the following formula: Tumor volume
(mm3) = (length × width2)/2. After 3 weeks,
the mice were sacrificed by cervical dislocation under anesthesia
(1% pentobarbital sodium was used for anesthesia at a dose of 50
mg/kg, intraperitoneal injection), then, the tumor samples were
collected for further analysis. All animal care and handling
procedures were in accordance with the National Institutes of
Health Guide for the Care and Use of Laboratory Animals (20) and were approved by the Ethics
Committee of Hangzhou First People's Hospital.
TUNEL staining
TUNEL staining was used to detect apoptosis in the
resected tissue samples. Frozen tumor tissue sections (3–5 µm;
stored at −20°C) from the indicated nude mice were processed for
TUNEL staining (Beyotime Institute of Biotechnology), according to
the manufacturer's instructions. Briefly, following fixation by 4%
paraformaldehyde for 20–30 min at room temperature, the frozen
sections were washed three times with PBS at room temperature (5
min each time). Then, sections were immersed in PBS containing 1%
Triton X-100 for 20 min at room temperature. Each sample was
incubated with 50 µl TdT enzyme reaction solution (45 µl
equilibration buffer, 1 µl biotin-11-dUTP and 4 µl TdT enzyme) for
60 min at RT. Following PBS washing, 50 µl streptavidin-TRITC
labeling buffer was added and incubated for 30 min at room
temperature. Then, sections were covered with DAPI (1:1,000) for 10
min at RT. Finally, the sections were placed under a cover slip and
scanned by Invitrogen EVOS FL AUTO (Thermo Fisher Scientific,
Inc.).
Statistical analysis
The results are presented as the mean ± SD and all
experiments were repeated at least three times. Statistical
analysis was performed using SPSS 16.0 (SPSS, Inc.). Differences
between two groups were analyzed using a Student's t-test, and
comparisons between two groups were analyzed by a oneway ANOVA,
followed by Tukey's post hoc test. Correlations between two sets of
data were analyzed using a Pearson's correlation analysis. A
log-rank test was used for Kaplan-Meier curves. GraphPad Prism 5.0
software (GraphPad Software, Inc.) was used for data presentation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
LINC01116 is highly expressed in
glioma
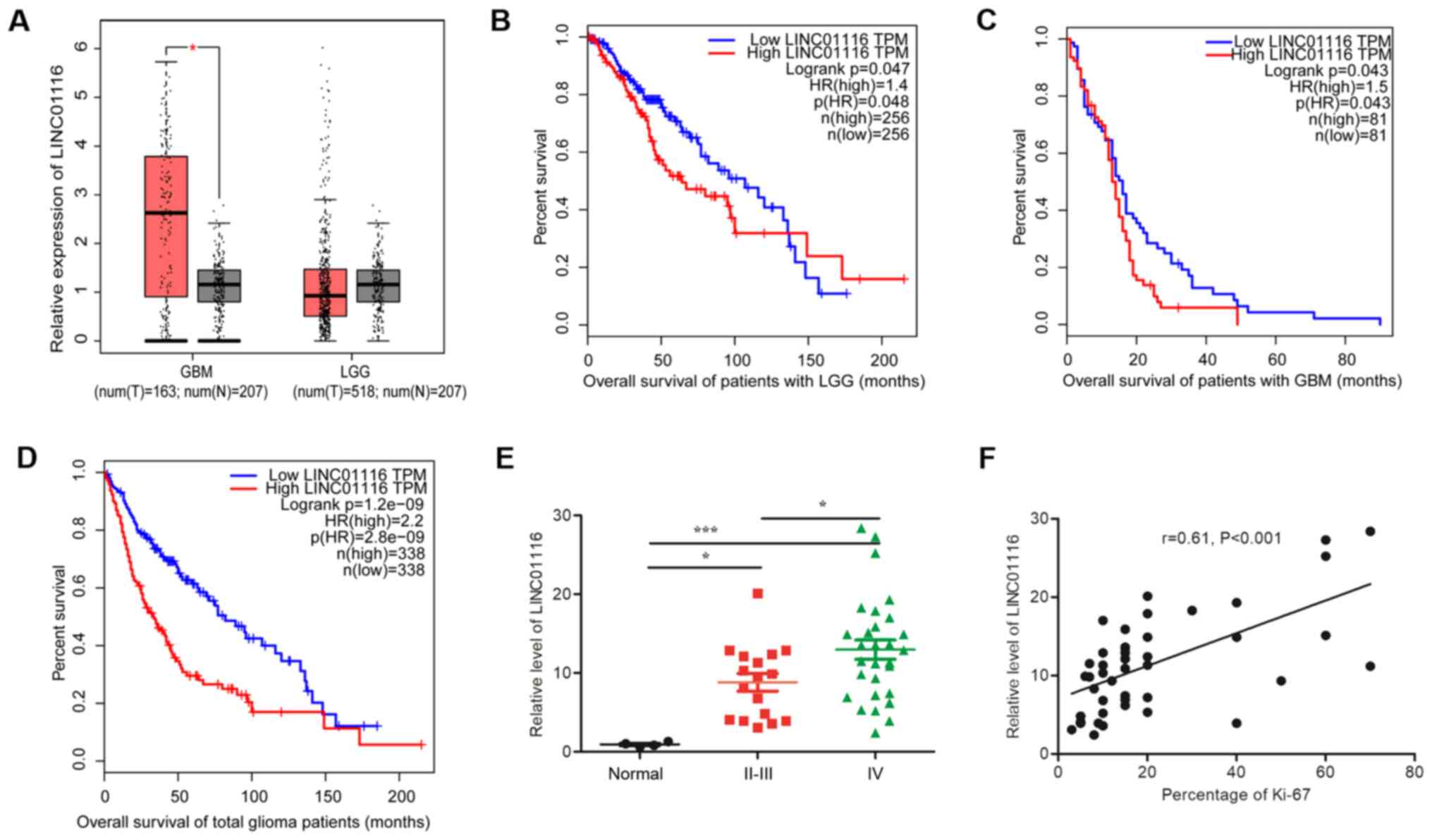
To determine the significance of LINC01116 in
glioma, its expression and prognostic value were assessed in
different grades of glioma [glioblastoma (GBM) and low-grade glioma
(LGG)] using the GEPIA website. As shown in Fig. 1A, LINC01116 expression was
significantly higher in GBM than in normal brain tissues. Although
a similar result was not observed in LGG, there were still a number
of these tissues with high levels of LINC01116 expression. In
survival analysis, high levels of LINC01116 were found to predict
poor prognosis, regardless of the glioma grade (Fig. 1B-D). To confirm whether LINC01116 is
associated with a malignant glioma phenotype, its expression was
assessed in 46 fresh glioma and 4 normal brain tissue samples. With
a deviation from the results of GEPIA analysis, higher expression
levels of LINC01116 were observed in low and high grade gliomas
than in normal brain tissues (Fig.
1E), and LINC01116 expression was positively correlated with
tumor grade and Ki-67 percentage positivity (Fig. 1F).
LINC01116 promotes the proliferation
and invasion of glioma
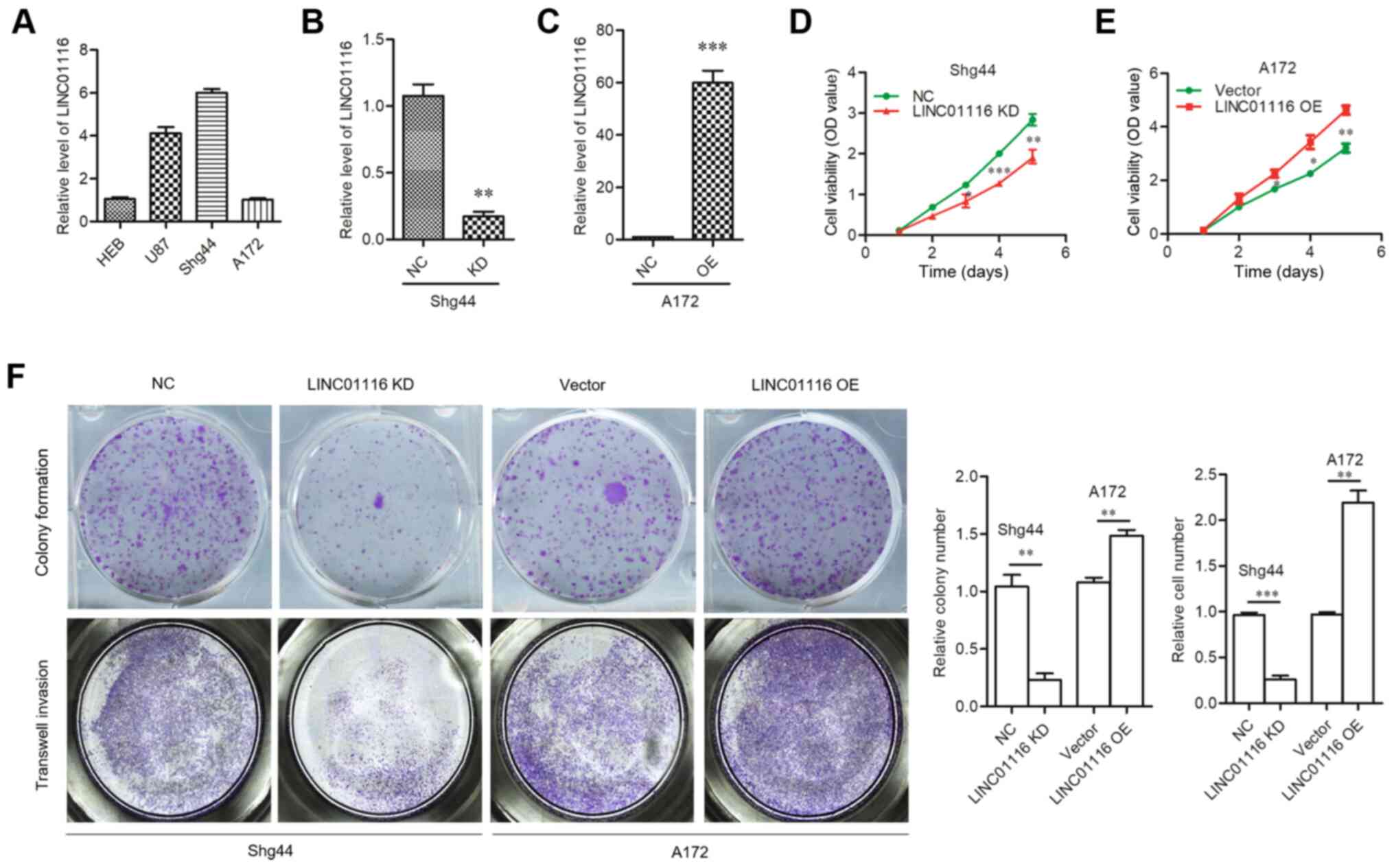
To determine its function in glioma, RT-qPCR was
used to assess the expression levels of LINC01116 in three glioma
cell lines (U87, A172 and Shg44), compared with normal human
astrocyte cells (HEB) (Fig. 2A).
According to the expression of LINC01116 in glioma cells, a stable
LINC01116 KD cell line was successfully constructed using
lentiviral shRNA delivery into Shg44 cells with relative high
expression of LINC01116, and stable OE cells were generated by
introducing expression plasmids into A172 cells with relative low
expression of LINC01116 (Fig. 2B and
C). Functional experiments were then performed. Colony
formation and cell viability assays revealed that the proliferative
capacity of A172 cells was promoted by LINC01116 OE, and attenuated
by LINC01116 KD in Shg44 cells (Fig.
2D-F). The results of the Transwell assay revealed that
LINC01116 OE increased the invasive ability of A172 cells, while
LINC01116 KD decreased that of Shg44 cells (Fig. 2F). These results indicated that
LINC01116 may act as an oncogene in glioma.
LINC01116 inhibits the p53 pathway by
upregulating MDM2
To investigate the potential tumor promoting
mechanisms of LINC01116, its association with the expression of
proliferation- and invasion-related genes was investigated using
the GEPIA platform. As shown in Fig.
3A, the mRNA expression levels of BAX, BAK1, CDKN1A and GADD45A
were negatively associated with LINC01116 expression in glioma
(Fig. 3A). Notably, these genes are
all associated with the p53 pathway (21,22).
Thus, it was hypothesized that LINC01116 may promote the
development of glioma by inhibiting the p53 pathway. To confirm
these findings, the two p53-targeted-genes (BAK1 and
GADD45A) with the most significant association with
LINC01116 were chosen for further assays, combined with MDM2
and TP53. Then, the mRNA expression levels of BAK1,
GADD45A, MDM2 and TP53 in LINC01116 OE cells (A172) and
LINC01116 KD cells (Shg44) were determined. As shown in Fig. 3B and C, LINC01116 negatively
regulated BAK1 and GADD45A, and positively regulated
MDM2 mRNA expression in glioma cells, but had no effect on
TP53 mRNA levels. The protein expression levels of these
genes were also determined, and those of BAK1, GADD45A and MDM2
were in accordance with their mRNA expression. However, p53
expression was decreased in LINC01116 OE cells and increased in
LINC01116 KD cells (Fig. 3D and E).
It was therefore speculated that LINC01116 may inhibit the p53
pathway by upregulating MDM2 in glioma. To verify this hypothesis,
p53 protein expression was determined in LINC0111 OE cells
following MDM2 KD. As shown in Fig. 3F
and G, LINC01116 failed to repress the expression of p53, BAK1
and GADD45A protein in the absence of MDM2. These results indicated
that LINC01116 inhibited the p53 pathway in glioma by upregulating
MDM2.
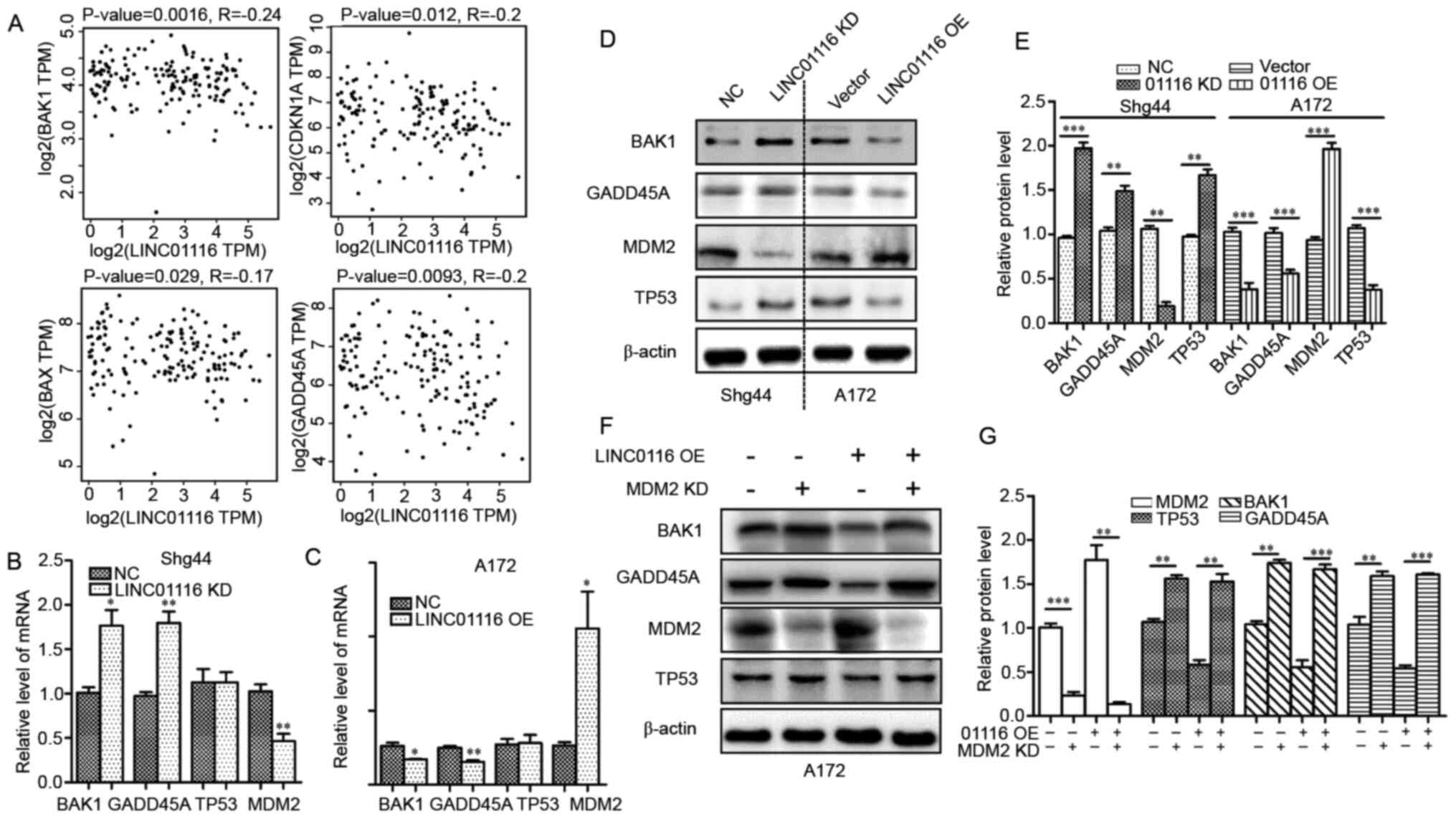 | Figure 3.LINC01116 mediates the MDM2-p53
pathway in glioma. (A) Gene Expression Profiling Interactive
Analysis revealed that LINC01116 levels negatively correlate with
the mRNA levels of p53 target genes BAK1, BAX, CDKN1A and
GADD45A in GBM tissues (n=81). (B and C) LINC01116 regulated
the mRNA expression of MDM2, BAK1 and GADD45A, but
not TP53 in glioma cell lines. (D) Protein expression of
MDM2, p53, BAK1 and GADD45A were regulated by LINC01116 in glioma
cells. (E) Histogram indicating the relative protein levels in (D).
(F) LINC01116 regulated the p53 pathway in A172 cells, which was
dependent on the presence of MDM2. (G) Histogram indicating the
relative protein levels in (F). *P<0.05, **P<0.01 and
***P<0.001 vs NC. LINC01116, long intergenic non-protein coding
RNA 01116; MDM2, E3 ubiquitin-protein ligase Mdm2; KD, knockdown;
OE, overexpression; NC, negative control; CDKN1A, cyclin-dependent
kinase inhibitor 1; GADD45A, growth arrest and DNA damage-inducible
protein GADD45 α. |
LINC01116 upregulates MDM2 by sponging
miR-744-5p
One of the mechanisms by which lncRNAs exert their
biological functions is by competitively sponging miRNAs, acting as
ceRNAs. To investigate whether a ceRNA mechanism exists between
LINC01116 and MDM2, the ENCORI platform (17) was used to identify miRNAs that
potentially bound both LINC01116 and the 3′UTR of MDM2 mRNA. The
miRNAs that could interact with LINC01116 and 3′UTR of MDM2 mRNA
simultaneously were analyzed, which led to the identification of
miR −744-5p. To determine the association between LINC01116,
miR-744-5p and MDM2, miR-744-5p expression was evaluated in A172
LINC01116 OE cells and Shg44 LINC01116 KD cells. LINC01116 was
found to negatively regulate the expression of miR-744-5p in A172
and Shg44 cells (Fig. 4A). Next,
Shg44 cells were transfected with miR-744-5p mimics and A172 cells
were treated with a miR-744-5p inhibitor, RT-qPCR results showed
that the relative miR-744-5p expression levels were significantly
increased in Shg44 cells treated with miR-744-5p mimics and
significantly reduced in A172 cells treated with miR-744-5p
inhibitor (Fig. S1A and B). Then,
the relative expression of LINC01116 and MDM2 mRNA was determined.
As shown in Fig. 4B, miR-744-5p
mimics significantly inhibited the expression of LINC01116 and MDM2
mRNA in Shg44 cells, whereas the miR-744-5p inhibitor promoted the
expression of LINC01116 and MDM2 mRNA in A172 cells. Furthermore,
miR-744-5p mimics decreased the expression of MDM2 mRNA and protein
promoted by LINC01116 OE in A172 cells, yet the miR-744-5p
inhibitor increased the expression of MDM2 mRNA and protein
downregulated by LINC01116 KD in the Shg44 cell line (Fig. 4C and D). A dual-luciferase reporter
assay was then performed to determine whether miR-744-5p directly
binds LINC01116 and the 3′UTR of MDM2. As shown in Fig. 4E, wild-type reporter plasmids were
constructed containing the LINC01116 sequence and the 3′UTR of
MDM2, as well as the corresponding mutant-type plasmids. The assay
results showed that miR-744-5p mimics reduced the luciferase
activity of the wild-type, but not the mutant-type plasmids
(Fig. 4F and G). In addition,
LINC01116 OE reversed the attenuated luciferase activity of the
MDM2 wild-type plasmid caused by the miR-744-5p mimics (Fig. 4F). However, miR-744-5p inhibitor did
not significantly change the luciferase activity of LINC01116 wild
or mutant-type plasmids (Fig. 4G).
Finally, a RIP assay was performed to verify whether LINC01116 and
miR-744-5p could bind to the same Ago protein. As shown in Fig. 4H, both LINC01116 and miR-744-5p were
found to bind Ago2. These results indicated that LINC01116
upregulates MDM2 by sponging miR-744-5p.
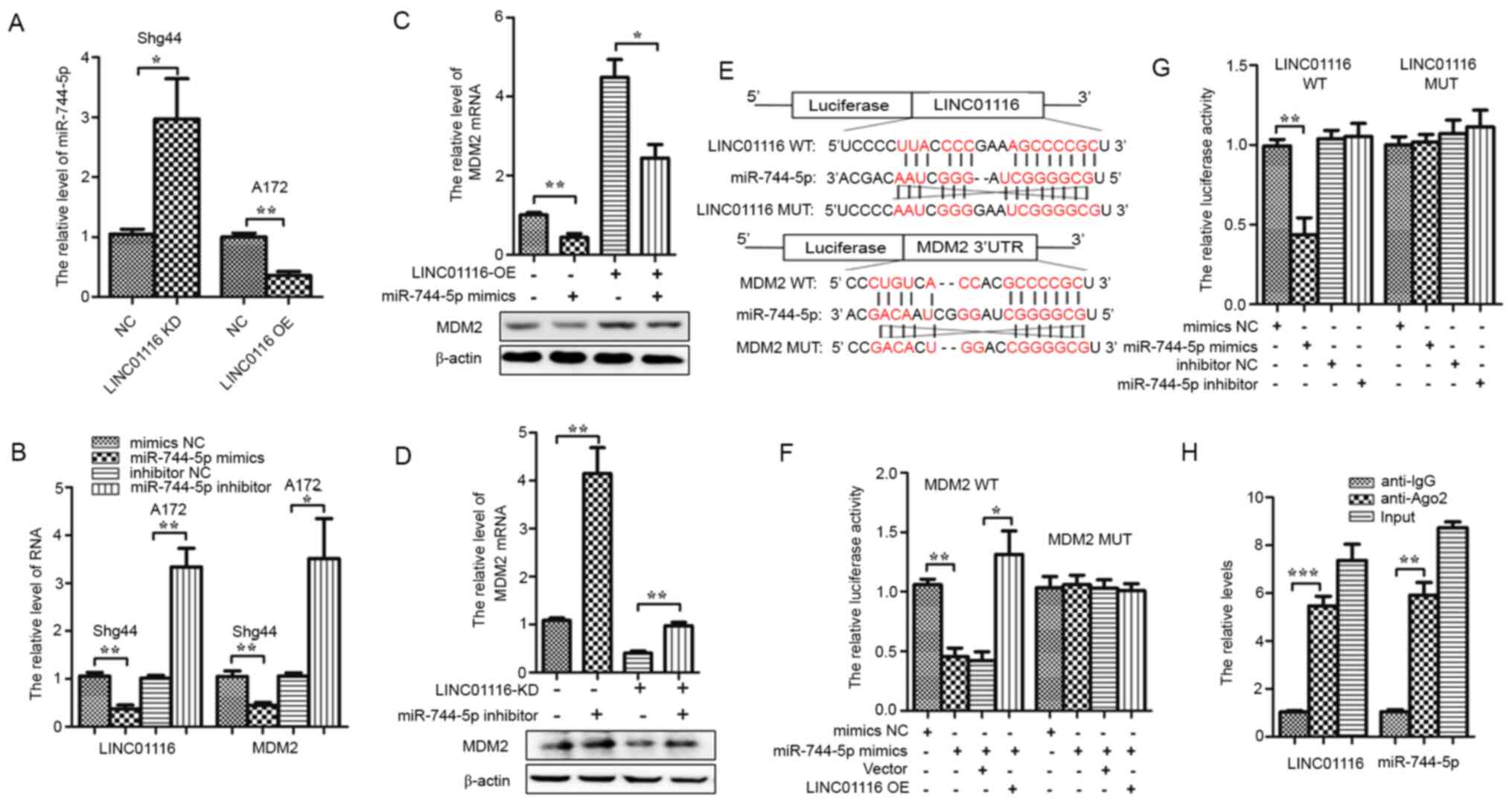 | Figure 4.LINC01116 increases MDM2 mRNA
levels by sponging miR-744-5p in glioma. (A) LINC01116 negatively
regulated the expression of miR-744-5p in Shg44 and A172 cells. (B)
mRNA expression of LINC01116 and MDM2 were downregulated by
miR-744-5p mimics in Shg44 cells, and upregulated by the miR-744-5p
inhibitor in A172 cells. (C) LINC01116 OE partially rescued the
expression of MDM2 mRNA and protein downregulated by
miR-744-5p mimics in A172 cells. (D) miR-744-5p inhibitors
partially rescued MDM2 mRNA and protein expression
downregulated by LINC01116 KD in Shg44 cells. (E) Predicted binding
sites between miR-744-5p and LINC01116 or the MDM2 mRNA
3′-UTR in WT and MUT sequences. (F) miR-744-5p significantly
reduced the luciferase activity of WT LINC01116 in 293T cells, but
not the MUT sequence, and the reduction in luciferase activity was
rescued by LINC01116 OE. (G) Luciferase activity of the WT
MDM2 3′-UTR, but not the MUT, was significantly reduced by
miR-744-5p mimics in 293T cells. However, miR-744-5p inhibitor did
not significantly change the luciferase activity of LINC01116 WT or
MUTplasmids. (H) RNA immunoprecipitation assays showed that both
LINC01116 and miR-744-5p could bind Ago2. *P<0.05, **P<0.01
and ***P<0.001. LINC01116, long intergenic non-protein coding
RNA 01116; MDM2, E3 ubiquitin-protein ligase Mdm2; miR, microRNA;
KD, knockdown; OE, overexpression; NC, negative control; UTR,
untranslated region; WT, wild-type; MUT, mutant. |
miR-744-5p partially reverses the
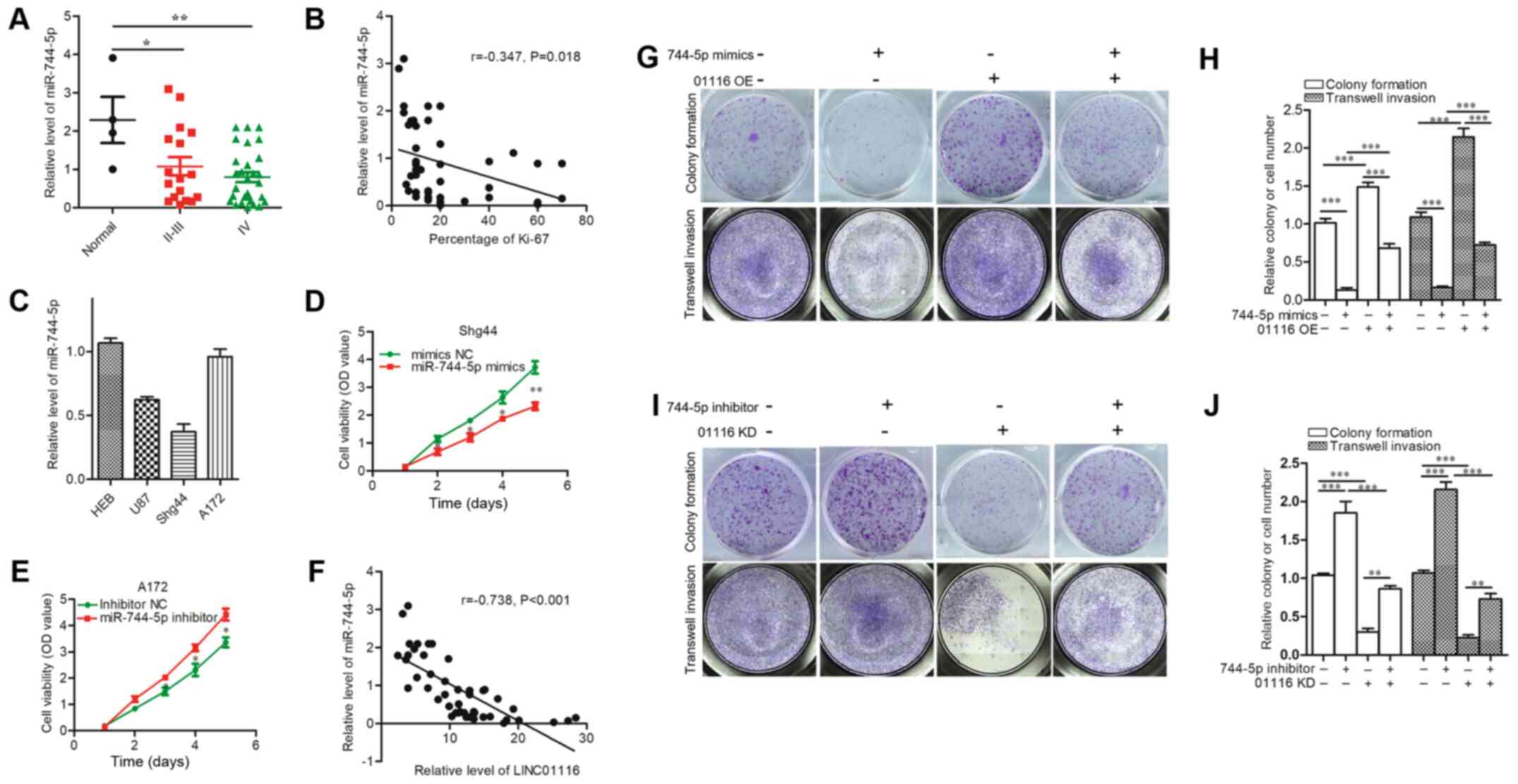
tumor-promoting ability of LINC01116 in glioma
After confirming the existence of a ceRNA mechanism
between LINC01116 and miR-744-5p, the expression of miR-744-5p and
its correlation with LINC01116 and Ki-67 in glioma were
investigated. As shown in Fig. 5A,
the expression of miR-744-5p was downregulated in glioma compared
with normal brain tissues. Correlation analysis between miR-744-5p
and Ki-67 showed that glioma tissues with low miR-744-5p expression
displayed higher proliferative ability, although the correlation
was weak (r=−0.347) (Fig. 5B).
Next, the expression of miR-744-5p was detected in glioma cell
lines (Fig. 5C); as a result, Shg44
cells were transfected with miR-744-5p mimics, and A172 cells were
treated with an miR-744-5p inhibitor accordingly. As shown in
Fig. 5D and E, cell proliferation
was inhibited by miR-744-5p mimics and promoted by the inhibitor.
Rescue experiments were performed to determine whether miR-744-5p
could reverse the tumor-promoting role of LINC01116 in glioma.
Firstly, the correlation between LINC01116 and miR-744-5p was
analyzed, and the relative expression of miR-744-5p was found to be
negatively correlated with that of LINC01116 (Fig. 5F). Rescue experiments revealed that
miR-744-5p mimics could reverse the promotion of LINC01116 OE on
the proliferative and invasive abilities of A172 cells (Fig. 5G and H). Similar results were
obtained in Shg44 cells. Furthermore, findings also suggested that
the LINC01116 KD-induced inhibition of proliferation and invasion
was reversed by the miR-744-5p inhibitor (Fig. 5I and J).
LINC01116 promotes glioma development
in vivo
After partially ascertaining the tumor-promoting
mechanisms of LINC01116 in glioma, its potential as a therapeutic
target was investigated. A tumor xenograft model was established by
subcutaneously injecting Shg44 LINC01116 KD cells into nude mice.
As shown in Fig. 6A, LINC01116 KD
impaired the growth of Shg44 cell tumors. To confirm whether
LINC01116 exerted a similar effect on the expression of p53 pathway
proteins, the mRNA and protein expression levels of these genes
were evaluated in the tumors of nude mice. As predicted, the
results were in accordance with those of the in vitro
cellular experiments (Fig. 6B and
C). In addition, TUNEL assay results revealed that LINC01116 KD
increased the percentage of apoptotic cells in glioma tissues
(Fig. 6D). Collectively, these
findings suggested that targeting LINC01116 may be a novel approach
for the treatment of glioma.
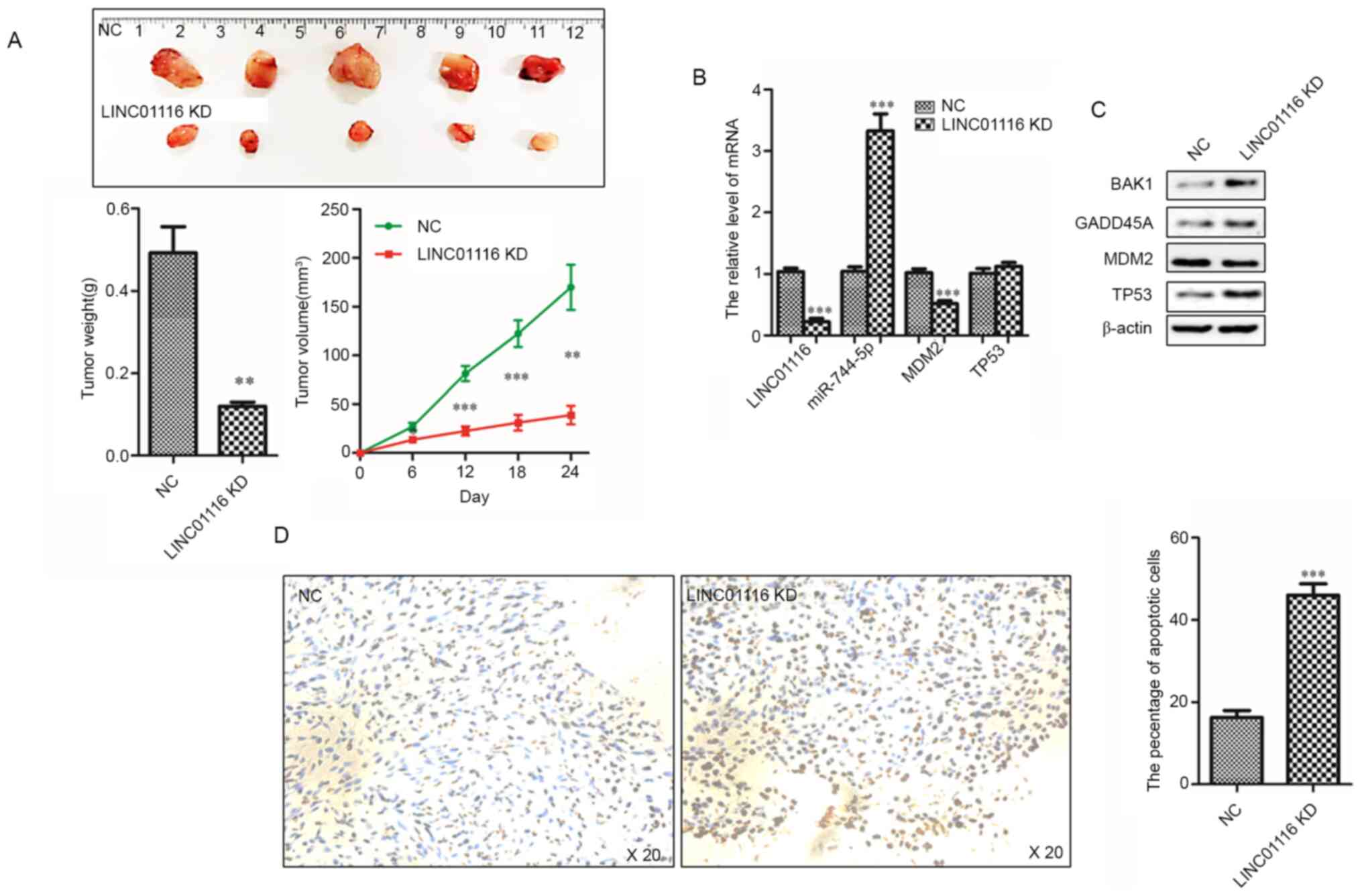 | Figure 6.LINC01116 promotes gliomagenesis
in vivo. (A) LINC01116 KD inhibited the growth of Shg44 cell
tumors. Histogram indicating the mean weight of the xenograft
tumors (n=5). Line chart of tumor growth curves (n=5). (B) mRNA
expression levels of LINC01116, miR-744-5p, MDM2 and
TP53 in the indicated xenograft tumors. (C) Protein
expression of BAK1, GADD45A, MDM2 and p53 in xenograft tumors. (D)
TUNEL assays were performed to detect glioma cell apoptosis in the
xenograft tumors. Histogram indicating the percentage of apoptotic
cells in the xenograft tumors (n=5). Magnification, ×20.
**P<0.01 and ***P<0.001 vs NC. LINC01116, long intergenic
non-protein coding RNA 01116; miR, microRNA; KD, knockdown; NC,
negative control; GADD45A, growth arrest and DNA damage-inducible
protein GADD45 α; MDM2, E3 ubiquitin-protein ligase Mdm2. |
Discussion
The high mortality rate of glioma is primarily due
to its infiltration and migration into a large area of adjacent
brain tissue (23). Therefore,
verifying the potential mechanisms involved in glioma development
is necessary for future therapeutic advancements (2). To date, numerous studies have
identified that lncRNAs possess pro- and/or antitumor functions in
glioma (24–27), and identifying the regulatory
effects of lncRNAs in glioma is a popular area of research.
Previous studies have demonstrated that LINC01116 is
highly expressed in several types of cancer (28–30),
and that it regulates cancer cell proliferation, migration and
chemoresistance via a number of different mechanisms (28,29,31–37).
In accordance with these studies, high LINC01116 expression was
also observed in glioma in the present study, and was found to be
positively associated with glioma grade and proliferative ability.
Furthermore, LINC01116 KD inhibited the proliferative and invasive
abilities of glioma cells, implying that LINC01116 is involved in
the development and progression of glioma. Thus, the potential
mechanisms through which LINC01116 is involved in gliomagenesis
were investigated.
In a variety of cancer types, including glioma, the
tumor suppressor p53 plays a crucial role in the development and
progression of tumors, and its mutation or functional inactivation
are found in the majority of human cancers (38). p53 acts as ‘guardian of the genome’
through surveillance and maintenance of genomic stability (39). As a key transcription factor, p53
triggers cell cycle arrest, senescence or apoptosis in response to
DNA damage and various oncogenic stimuli (40). However, mutation of the TP53 gene or
instability of the p53 protein contributes to tumor progression by
producing dysfunctional p53 variants or accelerating p53
degradation (41). Therefore,
maintaining the stability and function of p53 is important for
cellular homeostasis. In the present study, a negative association
between the expression of LINC01116 and p53 target genes was
identified, suggesting that LINC01116 may promote glioma
proliferation and invasion by inhibiting the p53 pathway. LINC01116
was also confirmed to inhibit the expression of BAK1 and GADD45A in
three glioma cell lines. Notably, LINC01116 regulated the
expression of p53 protein, but not p53 mRNA, which impelled the
present study to focus on MDM2, an upstream regulator of the p53
pathway. As predicted, LINC01116 positively regulated the protein
and mRNA expression of MDM2.
MDM2 functions as an E3 ubiquitin-ligase. It is
highly expressed in various malignant tumors and is considered to
be a proto-oncogene (42). MDM2
binds the transactivation domain of p53 and induces its degradation
through the proteasomal system (43). Additionally, a number of proteins
can repress the transcriptional activation of p53 by cooperating
with MDM2 (44). In the present
study, LINC01116 failed to regulate p53 without MDM2, which
suggested that the regulatory role of LINC01116 in the p53 pathway
was dependent on MDM2.
Various lncRNAs exert their biological functions via
the ceRNA mechanism (45). To
verify whether a ceRNA mechanism exists between LINC01116 and MDM2,
miRNAs that potentially bind both LINC01116 and the 3′UTR of MDM2
were predicted using the StarBase platform. miR-744-5p was found to
be a potential bridge between the regulation of LINC01116 and MDM2.
Through further investigation, LINC01116 was found to regulate the
mRNA expression of MDM2 by sponging miR-744-5p and promoting its
degradation. miRNAs are small endogenous RNAs that control cellular
and physiological processes by post-transcriptionally regulating
gene expression (46). As miRNAs
are crucial regulators of various tumorigenesis-associated genes,
research into the functions of miRNA in tumors is expanding
(47). Numerous miRNAs have been
identified as promising candidate biomarkers for different types of
cancer (48). In the present study,
miRNA-744-5p was found to be downregulated in glioma tissue
samples, and its expression was negatively associated with that of
LINC01116. miR-744-5p also inhibited the proliferative and invasive
abilities of glioma. Moreover, the pro-oncogenic functions of
LINC01116 were attenuated by miR-744-5p OE. Those findings
confirmed the presence of a ceRNA mechanism between LINC01116,
miR-744-5p and MDM2. Given that targeting non-coding RNAs may be a
potential approach to tumor treatment, LINC01116 KD was revealed to
inhibit the growth of tumors in a nude mouse xenograft model. This
finding suggested that LINC01116 may be a potential target for
glioma treatment. Although miR-744-5p was found to link LINC01116
and the MDM2-p53 pathway, there were several limitations of the
present study that need further study: i) The nature of the
relationship between LINC01116 and p53 target genes (direct or
undirect) was not determined; ii) only several p53-targeted genes
were tested, and more genes related to the p53 pathway need to be
explored in the future; iii) during the in vivo study, it
was not tested whether miR-744-5p mimics could restore
LINC01116-reduced tumor size; and iv) A172 cells with relatively
low expression of LINC01116 were chosen for overexpression and
Shg44 cells with relatively high expression were chosen for KD, but
it would have been more suitable to perform LINC01116
overexpression and KD in the same cell line.
In conclusion, these preliminary data demonstrated
that LINC01116 was highly expressed in glioma, which was associated
with a malignant phenotype. LINC01116 promoted the proliferation
and invasiveness of glioma by sponging miR-744-5p, thus preserving
MDM2 expression, which inhibited the antitumor functions of the p53
pathway. Therefore, targeting LINC01116 may be a potential future
therapeutic approach for patients with glioma.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LJ, CC, WJ, HW, QD and XD performed the experiments.
JS and WY designed the study and prepared the manuscript. JS and WY
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study received approval from the Ethics
Committee of Hangzhou First People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gusyatiner O and Hegi ME: Glioma
epigenetics: From ubclassification to novel treatment options.
Semin Cancer Biol. 51:50–58. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aldape K, Brindle KM, Chesler L, Chopra R,
Gajjar A, Gilbert MR, Gottardo N, Gutmann DH, Hargrave D, Holland
EC, et al: Challenges to curing primary brain tumours. Nat Rev Clin
Oncol. 16:509–520. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lapointe S, Perry A and Butowski NA:
Primary brain tumours in adults. Lancet. 392:432–446. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Post-transcriptional processing generates
a diversity of 5′-modified long and short RNAs. Nature.
457:1028–1032. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arun G, Diermeier SD and Spector DL:
Therapeutic targeting of long non-coding RNAs in cancer. Trends Mol
Med. 24:257–277. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The rosetta stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qi X, Zhang DH, Wu N, Xiao JH, Wang X and
Ma W: ceRNA in cancer: Possible functions and clinical
implications. J Med Genet. 52:710–718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abdollahzadeh R, Daraei A, Mansoori Y,
Sepahvand M, Amoli MM and Tavakkoly-Bazzaz J: Competing endogenous
RNA (ceRNA) cross talk and language in ceRNA regulatory networks: A
new look at hallmarks of breast cancer. J Cell Physiol.
234:10080–10100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qu S, Liu Z, Yang X, Zhou J, Yu H, Zhang R
and Li H: The emerging functions and roles of circular RNAs in
cancer. Cancer Lett. 414:301–309. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang KC and Chang HY: Molecular mechanisms
of long noncoding RNAs. Mol Cell. 43:904–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peng Z, Liu C and Wu M: New insights into
long noncoding RNAs and their roles in glioma. Mol Cancer.
17:612018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin C and Yang L: Long Noncoding RNA in
cancer: Wiring signaling circuitry. Trends Cell Biol. 28:287–301.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kopp F and Mendell JT: Functional
classification and experimental dissection of long noncoding RNAs.
Cell. 172:393–407. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang Z, Li C, Kang B, Gao G and Zhang Z:
GEPIA: A web server for cancer and normal gene expression profiling
and interactive analyses. Nucleic Acids Res. 45:W98–W102. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
StarBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42:D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peters L and Meister G: Argonaute
proteins: Mediators of RNA silencing. Mol Cell. 26:611–623. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Filipowicz W, Bhattacharyya SN and
Sonenberg N: Mechanisms of post-transcriptional regulation by
microRNAs: Are the answers in sight? Nat Rev Genet. 9:102–114.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
National Research Council: Guide for the
Care and Use of Laboratory Animals. The National Academies Press;
Washington, DC: 1996
|
|
21
|
Alavi MV: Targeted OMA1 therapies for
cancer. Int J Cancer. 145:2330–2341. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thu HE, Hussain Z, Mohamed IN and Shuid
AN: Eurycoma longifolia, a potential phytomedicine for the
treatment of cancer: Evidence of p53-mediated apoptosis in
cancerous cells. Curr Drug Targets. 19:1109–1126. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jung E, Alfonso J, Osswald M, Monyer H,
Wick W and Winkler F: Emerging intersections between neuroscience
and glioma biology. Nat Neurosci. 22:1951–1960. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rynkeviciene R, Simiene J, Strainiene E,
Stankevicius V, Usinskiene J, Kaubriene EM, Meskinyte I, Cicenas J
and Suziedelis K: Non-coding RNAs in glioma. Cancers (Basel).
11:172018. View Article : Google Scholar
|
|
25
|
Zheng Y, Xie J, Xu X, Yang X, Zhou Y, Yao
Q and Xiong Y: lncRNA DDX11-AS1 exerts oncogenic roles in glioma
through regulating miR-499b-5p/RWDD4 axis. Onco Targets Ther.
14:157–164. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei Y, Zhou K, Wang C, Du X, Xiao Q and
Chen C: Adsorption of miR-218 by lncRNA HOTAIR regulates PDE7A and
affects glioma cell proliferation, invasion, and apoptosis. Int J
Clin Exp Pathol. 13:2973–2983. 2020.PubMed/NCBI
|
|
27
|
Wu YJ, Yang QS, Chen H, Wang JT, Wang WB
and Zhou L: Long non-coding RNA CASC19 promotes glioma progression
by modulating the miR-454-3p/RAB5A axis and is associated with
unfavorable MRI features. Oncol Rep. 45:728–737. 2021. View Article : Google Scholar
|
|
28
|
Wang H, Lu B, Ren S, Wu F, Wang X, Yan C
and Wang Z: Long noncoding RNA LINC01116 contributes to gefitinib
resistance in non-small cell lung cancer through regulating IFI44.
Mol Ther Nucleic Acids. 19:218–227. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu HB, Chen Q and Ding SQ: lncRNA
LINC01116 competes with miR-145 for the regulation of ESR1
expression in breast cancer. Eur Rev Med Pharmacol Sci.
22:1987–1993. 2018.PubMed/NCBI
|
|
30
|
Chen J, Yuan ZH, Hou XH, Shi MH and Jiang
R: LINC01116 promotes the proliferation and inhibits the apoptosis
of gastric cancer cells. Eur Rev Med Pharmacol Sci. 24:1807–1814.
2020.PubMed/NCBI
|
|
31
|
Xing H, Sun H and Du W: LINC01116
accelerates nasopharyngeal carcinoma progression based on its
enhancement on MYC transcription activity. Cancer Med. 9:267–277.
2020. View Article : Google Scholar
|
|
32
|
Zhang ZF, Xu HH, Hu WH, Hu TY and Wang XB:
LINC01116 promotes proliferation, invasion and migration of
osteosarcoma cells by silencing p53 and EZH2. Eur Rev Med Pharmacol
Sci. 23:6813–6823. 2019.PubMed/NCBI
|
|
33
|
Wu J, Chen Z, Zhang L, Cao J, Li X, Gong
Z, Bo H, Zhang S and He D: Knockdown of LINC01116 inhibits cell
migration and invasion in head and neck squamous cell carcinoma
through epithelial-mesenchymal transition pathway. J Cell Biochem.
121:867–875. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen Z, Tao Q, Qiao B and Zhang L:
Silencing of LINC01116 suppresses the development of oral squamous
cell carcinoma by up-regulating microRNA-136 to inhibit FN1. Cancer
Manag Res. 11:6043–6059. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ye J, Zhu J, Chen H, Qian J, Zhang L, Wan
Z, Chen F, Sun S, Li W and Luo C: A novel lncRNA-LINC01116
regulates tumorigenesis of glioma by targeting VEGFA. Int J Cancer.
146:248–261. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fang YN, Huang ZL, Li H, Tan WB, Zhang QG,
Wang L and Wu JL: LINC01116 promotes the progression of epithelial
ovarian cancer via regulating cell apoptosis. Eur Rev Med Pharmacol
Sci. 22:5127–5133. 2018.PubMed/NCBI
|
|
37
|
Zhang B, Yu L, Han N, Hu Z, Wang S, Ding L
and Jiang J: LINC01116 targets miR-520a-3p and affects IL6R to
promote the proliferation and migration of osteosarcoma cells
through the Jak-stat signaling pathway. Biomed Pharmacother.
107:270–282. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wade M, Li YC and Wahl GM: MDM2, MDMX and
p53 in oncogenesis and cancer therapy. Nat Rev Cancer. 13:83–96.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tiwari B, Jones AE and Abrams JM:
Transposons, p53 and genome security. Trends Genet. 34:846–855.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hafner A, Bulyk ML, Jambhekar A and Lahav
G: The multiple mechanisms that regulate p53 activity and cell
fate. Nat Rev Mol Cell Biol. 20:199–210. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Stiewe T and Haran TE: How mutations shape
p53 interactions with the genome to promote tumorigenesis and drug
resistance. Drug Resist Updat. 38:27–43. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Oliner JD, Saiki AY and Caenepeel S: The
role of MDM2 amplification and overexpression in tumorigenesis.
Cold Spring Harb Perspect Med. 6:a0263362016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu X, Bayle JH, Olson D and Levine AJ: The
p53-mdm-2 autoregulatory feedback loop. Genes Dev. 7:1126–1132.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hernandez-Monge J, Rousset-Roman AB,
Medina-Medina I and Olivares-Illana V: Dual function of MDM2 and
MDMX toward the tumor suppressors p53 and RB. Genes Cancer.
7:278–287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao J, Li L, Han ZY, Wang ZX and Qin LX:
Long noncoding RNAs, emerging and versatile regulators of
tumor-induced angiogenesis. Am J Cancer Res. 9:1367–1381.
2019.PubMed/NCBI
|
|
46
|
Adams BD, Parsons C, Walker L, Zhang WC
and Slack FJ: Targeting noncoding RNAs in disease. J Clin Invest.
127:761–771. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bracken CP, Scott HS and Goodall GJ: A
network-biology perspective of microRNA function and dysfunction in
cancer. Nat Rev Genet. 17:719–732. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Anfossi S, Babayan A, Pantel K and Calin
GA: Clinical utility of circulating non-coding RNAs - an update.
Nat Rev Clin Oncol. 15:541–563. 2018. View Article : Google Scholar : PubMed/NCBI
|