Introduction
Sepsis is a pathological condition caused by a
number of factors, including severe infections, burn, trauma and
surgery (1–5). An imbalance between the secretion of
pro-inflammatory and anti-inflammatory factors that lead to
multiple organ failure appears in the pathophysiological process of
sepsis (6). Sepsis is one of the
leading causes of mortality in critical patients (7). Higher mortality in sepsis appears when
cardiac injury or acute kidney injury develops, especially in
intensive care unit patients (8,9).
Sepsis-induced cardiorenal syndrome is one of the multiple organ
dysfunctions observed in sepsis. It is determined by a primary
dysfunction in one organ that leads to secondary injury to another
organ (10). Its main clinical
manifestation is myocardial inhibition leading to decreased left
ventricular systolic function and cardiac output (10). Podocytes are differentiated cells
anchored on the basement membrane of the glomerulus. They maintain
the structure of glomerulus, which is an important part of the
glomerular filtration membrane (11). Toxic effects on podocytes that lead
to podocyte losing integrity or functional damage are the main
causes of proteinuria and glomerulosclerosis involved in the
occurrence and development of a number of kidney diseases (12).
MicroRNAs (miRs) are endogenous small molecules of
non-coding RNAs. Their biological effects are associated with gene
expression regulation by modifying mRNA protein synthesis. miRNAs
influence the protein synthesis at one or more mRNAs genes by
targeting the 3′ untranslated region (UTR), 5′UTR region and the
coding sequence of the gene (13).
Bioinformatics and research studies demonstrate that there are
thousands of target genes for human miRs and more >5,000 target
genes are regulated by miRs, indicating their involvement in
various physiological and pathological processes (14–20).
Environmental factors including diet, lifestyle, pollutants and
carcinogens can influence the expression of miRs (21–25)
and modulate important molecular pathways implicated in the
pathogenesis of chronic diseases. Studies have shown that the
profile of miRs has a great implication in the diagnosis and
prognosis of several pathologies, including types of cancer
(26–31). Disease-associated miRs can also
serve as targets for personalized therapy (32–34).
In the model of Dicer knockout mice, where miRs were knocked out,
proteinuria, serious renal damage and changes in podocyte
cytoskeleton proteins of podocytes are observed, showing the
association between miR and podocyte integrity (35).
Studies have associated miR-126 with multiple
functions in the organism, including the development of
cardiovascular pathologies, Parkinson's disease, diabetes, diabetic
nephropathy and types of cancer (36–40).
The present study hypothesized that miR-126 might be
involved in the podocyte damage associated with sepsis.
Lipopolysaccharide (LPS) is a part of the gram-negative bacterial
cell wall that mediates systemic inflammation leading to sepsis.
LPS induces podocyte injury by mediating the release of
pro-inflammatory factors (41). An
in vitro model of LPS-induced injury in conditioned
immortalized mouse podocytes was used to provide a theoretical
basis for further studies clarifying the pathogenesis of
sepsis-induced nephrotoxicity.
Materials and methods
Cell culture and transfection
Mouse podocyte cells (SV40 MES 13 cell line; cat.
no. CRL-1927; American Type Culture Collection) were cultured in
Roswell Park Memorial Institute-1640 medium (RPMI-1640; HyClone;
Cytiva) containing 10% fetal bovine serum (FBS; HyClone; Cytiva)
and 8×104 U/l recombinant mouse γ-interferon (Beijing
Solarbio Science & Technology Co., Ltd.) in 5% CO2
at 37°C in an incubator (Binder GmbH). The cells were cultured for
3–5 days and were then maintained in RPMI-1640 medium containing 5%
FBS.
Mouse podocyte cells with a density of
1.0×105 cells/well were inoculated in a 6-well plate
overnight. The podocyte injury model was established by stimulation
with 10 mg/l LPS for 12, 24 and 36 h. The normal control group was
treated with the same volume of phosphate-buffered saline (PBS).
The plates were incubated at 37°C for 12, 24 and 36 h. The
expression of nephrin protein was detected by western blotting.
According to the results of western blotting, the optimal time of
LPS stimulation was selected.
The cells in the miR-126 mimic group and miR-mimic
negative control (NC) group were treated separated with
miR-126-mimic (5′-ACCTCCAGCTGGGTCGTACCGTGAGTAATAATG-3′) and
miR-mimic NC (5′-CTCAACTGGTGTCCTGGA-3′), then mixed
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) until the final concentration in the RPMI-1640
medium was 20 nmol/l. Cells were incubated for 1 h then cultured in
complete medium for 24–48 h. All reagents were purchased from
Shanghai GenePharma Co., Ltd. and all experiments were performed in
triplicate.
Reverse transcription-quantitative
(RT-q) PCR
RT-PCR Eastrop™ Super total RNA Extraction kit
(Promega Corporation) was used to extract total RNA from samples.
RNA was reverse transcribed into cDNA using an RT kit (Thermo
Fisher Scientific, Inc.). RNA extraction, cDNA synthesis and qPCR
were performed according to the manufacturer's protocol. When cell
density reached 80%, culture medium was discarded, and 1 ml lysate
was added to each well of a 6-well plate for 10 min at room
temperature. The reaction system (20 µl) included: 1.0 cDNA, 10.0
SYBR-Green Master Mix (Thermo Fisher Scientific, Inc.), 0.5
upstream and downstream primers (SANGON Biotech Co., Ltd.; Table I) and 8 µl ddH2O (Milli-q
Academic A10; EMD Millipore). Each experiment was performed three
times and U6 was used as the internal reference. The reaction
conditions were as follows: 95°C pre denaturation for 10 min, 95°C
for 10 sec, 60°C for 20 sec and 72°C for 30 sec (40 cycles).
Quantification was performed via the 2−ΔΔCq method
(42).
 | Table I.Primer sequence information (Sangon
Biotech Co., Ltd.). |
Table I.
Primer sequence information (Sangon
Biotech Co., Ltd.).
| Name | Primer sequence
(5–3) |
|---|
| Nephrin | Upstream
primers |
CCCTCCGGGACCCTACTG |
|
| Downstream
primers |
TCTGGGAGGATGGGATTGG |
| miR-126 | Upstream
primers |
CGGCAGGAACCTCCTTACTC |
|
| Downstream
primers |
TGTGCCCTAGGGACGAAGGA |
| EGFL6 | Upstream
primers |
TCTGTTTGCTCTTTGATTACCG |
|
| Downstream
primers |
TTCCCTGTCTTCCACTTTTCAT |
| DKC1 | Upstream
primers |
GCTAAGTTGGACACGTCTCAG |
|
| Downstream
primers |
TGCAAGAGGTGTATAGTGTGTTG |
| GAPDH | Upstream
primers |
TGTGTCCGTCGTGGATCTGA |
|
| Downstream
primers |
TTGCTGTTGAAGTCGCAGGAG |
Western blotting
Mouse podocyte cells (5×105) were
collected, rinsed with PBS, mixed with 250 µl RIPA Lysis Buffer
(Thermo Fisher Scientific, Inc.) and left on ice for 5 min. Then
the mixture was transferred into a new EP tube and centrifuged at
3,000 × g, 4°C for 10 min. The supernatant obtained was used to
determine the total protein concentration of the cells by the BCA
Protein Assay kit (Thermo Fisher Scientific, Inc.). The protein
samples were diluted with 5X loading buffer (Thermo Fisher
Scientific, Inc.) and PBS, boiled in water bath for 5 min and
denatured. The protein liquid after denaturation was added to 12%
SDS-PAGE gel (Beijing Solarbio Science & Technology Co., Ltd.)
and underwent 80 V constant pressure electrophoresis for 60–120
min, each well was loaded with 20 µg protein. The SDS-PAGE gel was
placed onto a nitrocellulose membrane to perform the constant
current transfer. Bovine serum albumin (BSA; 3%; HyClone; Cytiva)
was used for blocking at 4°C for 1 h. Following blocking, the
nitrocellulose membrane was washed with PBS and incubated with
monoclonal Anti-nephrin (1:1,000; rabbit antibody (Y17-R); cat no.
ab136894, Abcam), anti-epidermal growth factor-like domain multiple
6 (EGFL6; 1:1,000; rabbit polyclonal mouse antibody 51182-T16,
Abcam), anti-dyskeratosis congenita 1 (DKC1; 1:1,000; rabbit
monoclonal antibody, EPR10398; Abcam) and anti-GAPDH (1:1,000;
mouse monoclonal antibody; cat. no. K106390M, Beijing Solarbio
Science & Technology Co., Ltd.), respectively. The membrane was
incubated overnight at 4°C. Then the nitrocellulose membrane was
washed with PBS buffer and incubated with Anti-mouse IgG for IP
(HRP; 1:5,000; cat no. ab131368, Abcam) labeled with horseradish
peroxidase at room temperature for 30 min. Then, the nitrocellulose
membrane was washed with PBS buffer and Chemistar ECL Western
Blotting Substrate (Ultra-sensitive ECL luminescent solution;
Hanbio Biotechnology Co., Ltd.) was used for chemiluminescence.
Kodak X (Kodak) was used to expose the nitrocellulose membrane for
~3 min. The X-ray film was scanned (Oxford Instruments plc) and the
grey analysis of the strip was processed by Gel-Pro Analyzer
software 4.0 (Media Cybernetics, Inc.). The grey value of each band
was measured by software in three independent repeated experiments
and the ratio of the grey value of the target gene product to that
of the β-actin product was used as the relative expression of the
protein.
Cell viability assay using cell
counting kit-8 (CCK8)
The treated cells of each group were inoculated into
96-well plates at a density of 1×104 cells/well. The
final volume of each well was 100 µl. Each experiment was performed
in triplicate. After 24 h, when the cells adhere to the wall, the
original culture medium was replaced and the cells were incubated
with the treatment according to their time points. Following
incubation, the cell viability was determined using a CCK-8 kit
(Thermo Fisher Scientific, Inc.). A volume of 10 µl CCK8 solution
was added to each well and cultured in 5% CO2 at 37°C
for 2 h. The absorbance (OD) was determined at 450 nm using a
microplate reader. Cell survival rate was calculated using the
following formula: Cell survival rate: OD value of experimental
group-OD value of blank group/OD value of the control group-OD
value of blank group ×100%.
Terminal deoxynucleotidyl transferase
dUTP nick labeling (TUNEL) assay
Cell apoptosis was determined using
TransDetect® In Situ Fluorescein TUNEL Cell
Apoptosis Detection kit (TransGen Biotech Co., Ltd.), according to
the manufacturer's instructions. Briefly, the cells were washed
with PBS and fixed with 4% paraformaldehyde or biyuntian (p0098)
for 30–60 min. Then the cells were washed with PBS or Hank's
balanced salt solution, followed by the addition of PBS containing
0.1% Triton X-100 and incubation on an ice bath for 2 min. To each
sample, 50 µl TUNEL detection solution was added and incubated for
60 min at 37°C, washed again with PBS and incubated with DAPI in
PBS for 30 min at 30°C. After incubation, the samples were washed
with PBS for three successive times. The samples were observed
under the IX73 fluorescence microscope (Olympus Corporation; ×400
magnification). The excitation wavelength range was 450–500 nm and
the emission wavelength range was 515–565 nm (green
fluorescence).
Dual-luciferase reporter assay
For the dual-luciferase reporter assay, the
TransDetect® Double-Luciferase Reporter Assay kit
(Firefly Luciferase and Renilla Luciferase and plasmids;
TransGen Biotech Co., Ltd.) was used. The cells were cultured and
transfected for 48 h with EGFL6 3UTR wild-type (Wt)/mutant (Mut)
reporter plasmids and DKC1 3UTR Wt/Mut reporter plasmids with or
without miR-126 mimic/control precursor plasmids using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Each experiment was performed in triplicate.
Following transfection, the medium was discarded and the cells were
lysed with cell lysis buffer. The luciferase activity was measured
using a GloMax 96 microplate luminescent detector (Promega
Corporation). Luciferase activity was normalized to that of
Renilla.
Statistical analysis
SPSS 22.0 statistical software (IBM Corp.) was used
for data analysis. The data were analyzed by t-test for the
differences between 2 groups and one-way analysis of variance
(ANOVA) for the comparison of >2 groups. ANOVA with Dunnett's
post hoc test was used to compare the data. P<0.05 was
considered to indicate a statistically significant difference.
Results
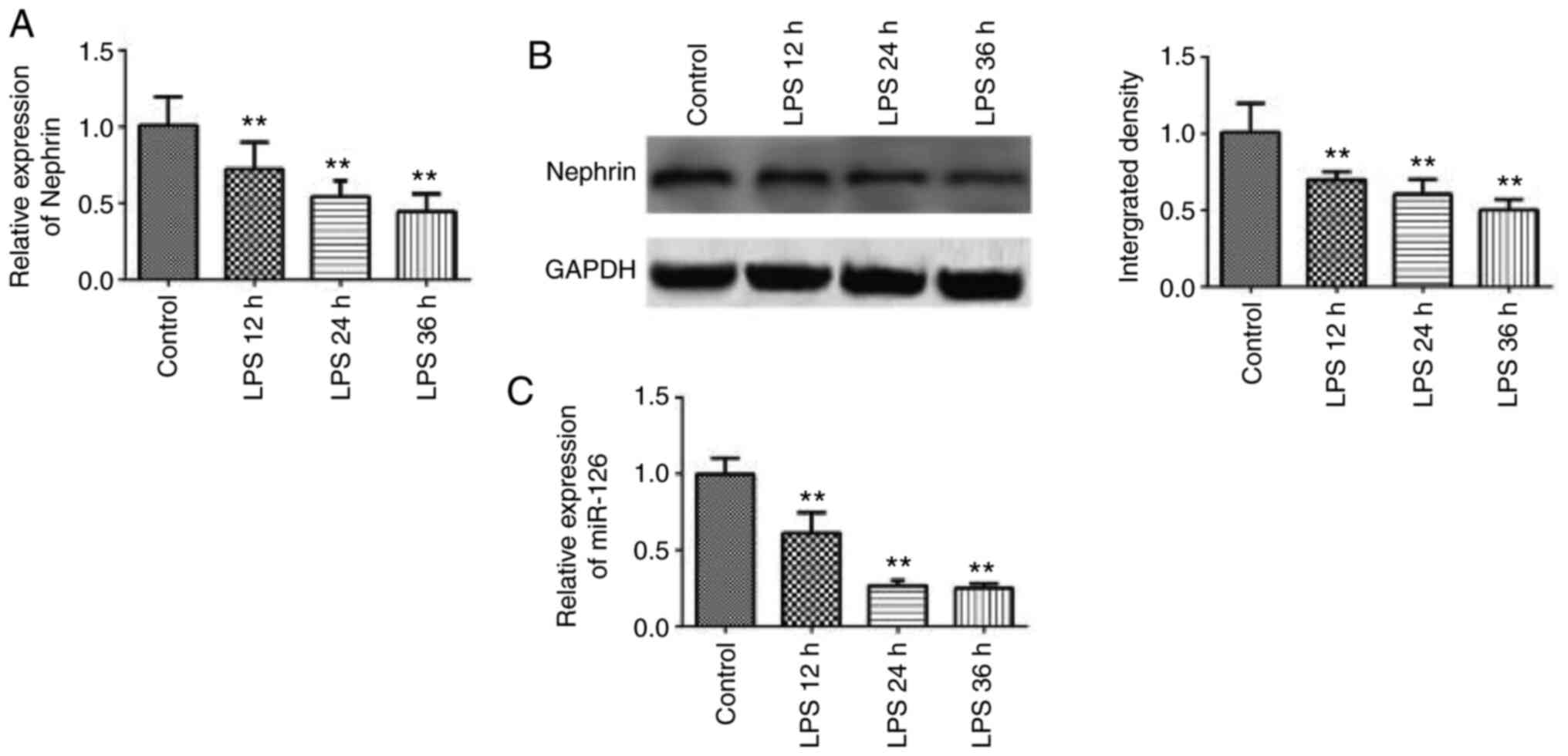
Podocyte injury model
LPS (10 µg/ml) was used to treat mouse immortalized
podocytes for sepsis model establishment. RT-qPCR demonstrated that
the mRNA expression of nephrin was significantly downregulated with
the prolongation of LPS treatment, reaching the lowest level after
36 h of culture (Fig. 1A), showing
a time-dependent effect. The results of western blotting
demonstrated that the protein expression of nephrin also
significantly decreased 36 h after LPS stimulation (Fig. 1B). In addition, the expression level
of miR-126 was downregulated with the prolongation of LPS treatment
(Fig. 1C). These results confirmed
the successful establishment of podocyte injury model and suggested
that miR-126 served a role in podocyte function.
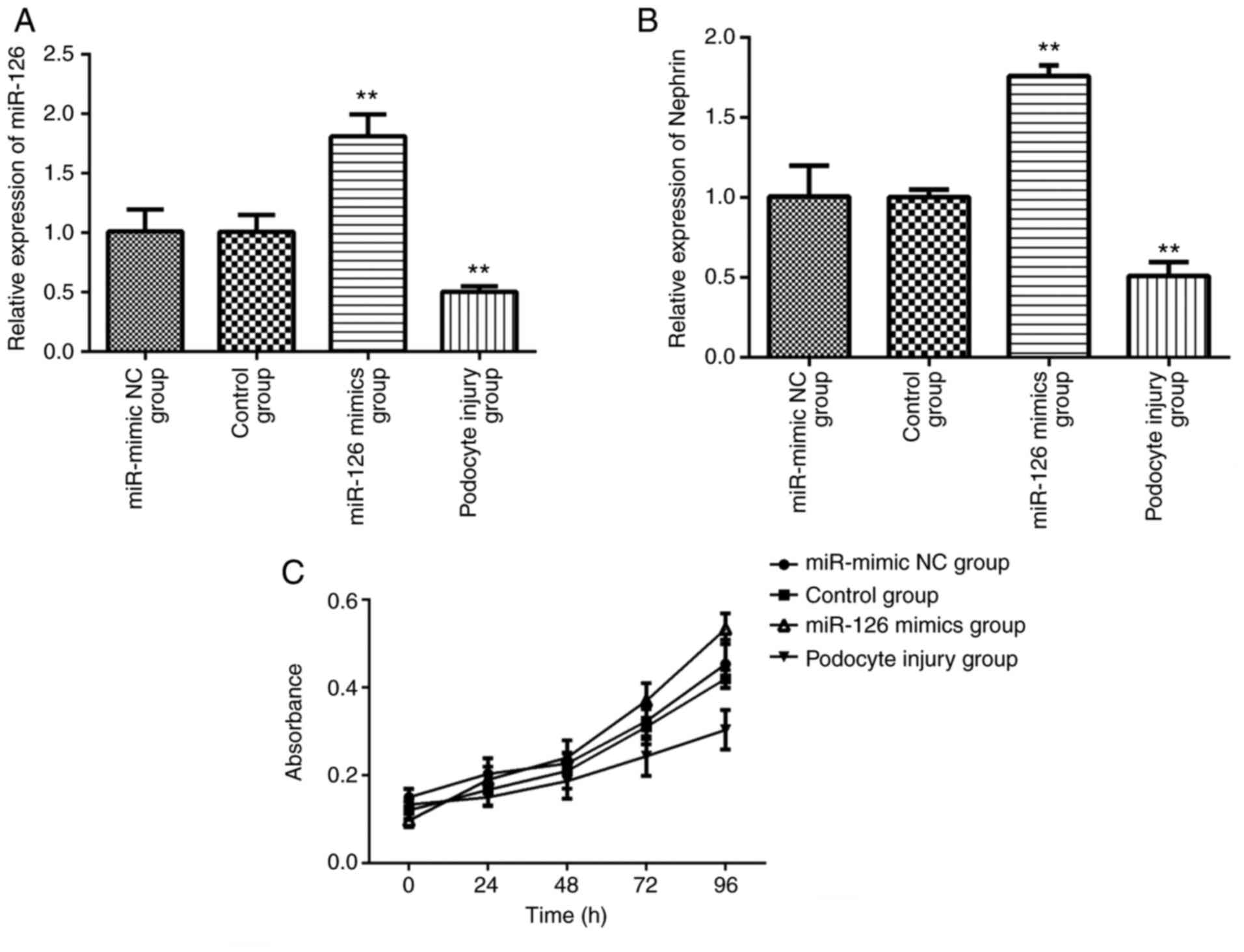
Increased miR-126 inhibited the
podocyte injury
To investigate the specific role of differentially
expressed miR-126 in septic podocyte injury, miR-126 mimics and
miR-mimic NC were transfected into podocytes. Following
transfection with miR-126 mimics and miR-mimic NC, RT-qPCR
demonstrated that the expressions of miR-126 in the miR-mimic NC
group and the control group were in the same level (Fig. 2A). Following transfection with
miR-126 mimics, RT-qPCR demonstrated that the expression of miR-126
(Fig. 2A) and nephrin (Fig. 2B) in podocytes was promoted,
indicating that upregulated miR-126 might mediate inhibition of
podocyte injury. CCK-8 assay was used to detect the effect of
miR-126 on the proliferation of podocytes. Results demonstrated
that the growth of podocyte cells transfected with miR-126 was
significantly promoted compared with podocyte injury group
(Fig. 2C). Following transfection
with miR-126 mimics, the growth of podocytes was promoted and the
proliferation ability was increased.
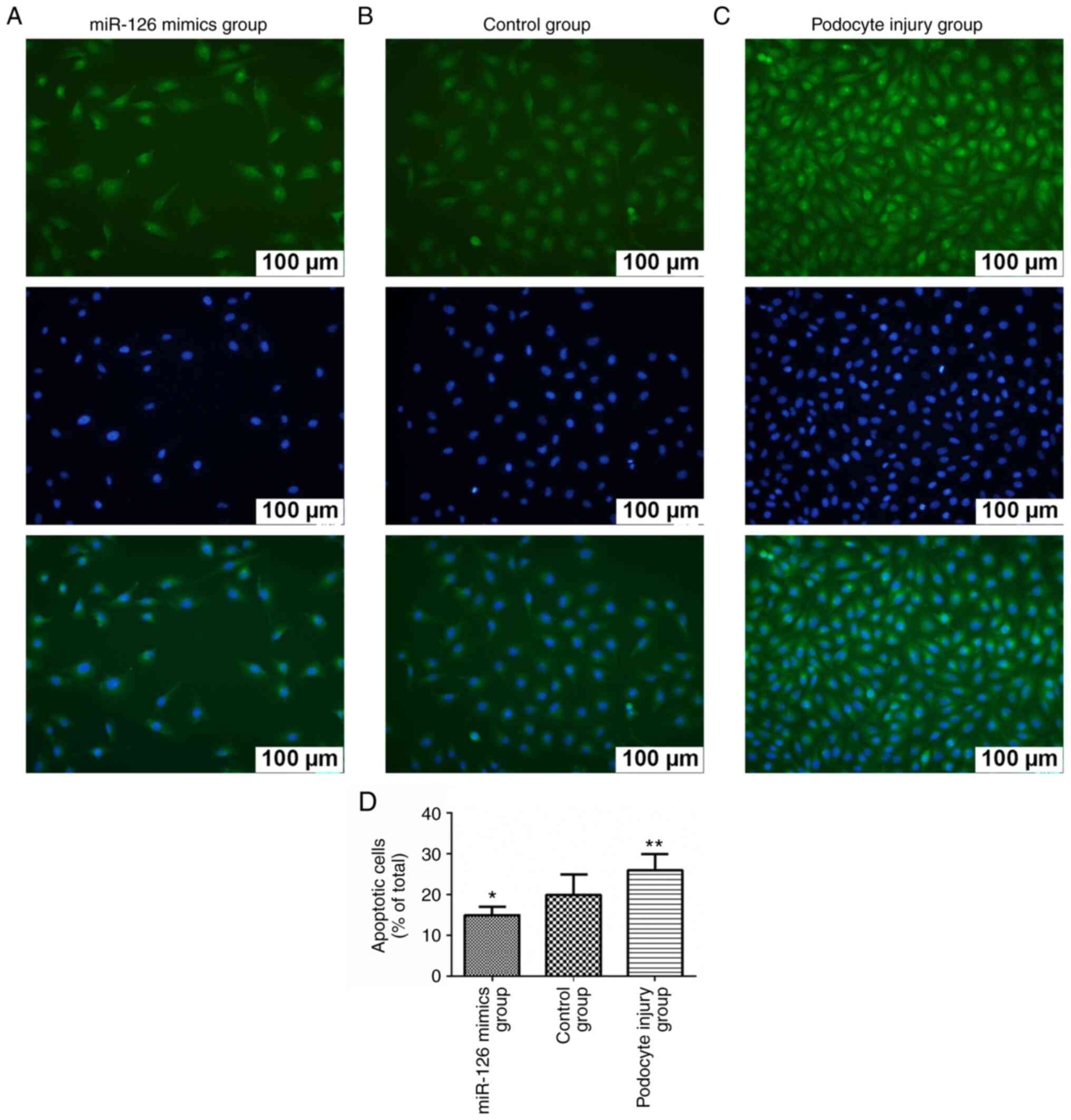
Increased miR-126 inhibited the
podocyte apoptosis
TUNEL assay results demonstrated that in the
podocyte injury group the number of apoptotic cells was
significantly increased compared with the control group, while the
overexpression of miR-126 can alleviate injury and reduce the
number of apoptotic cells (Fig.
3).
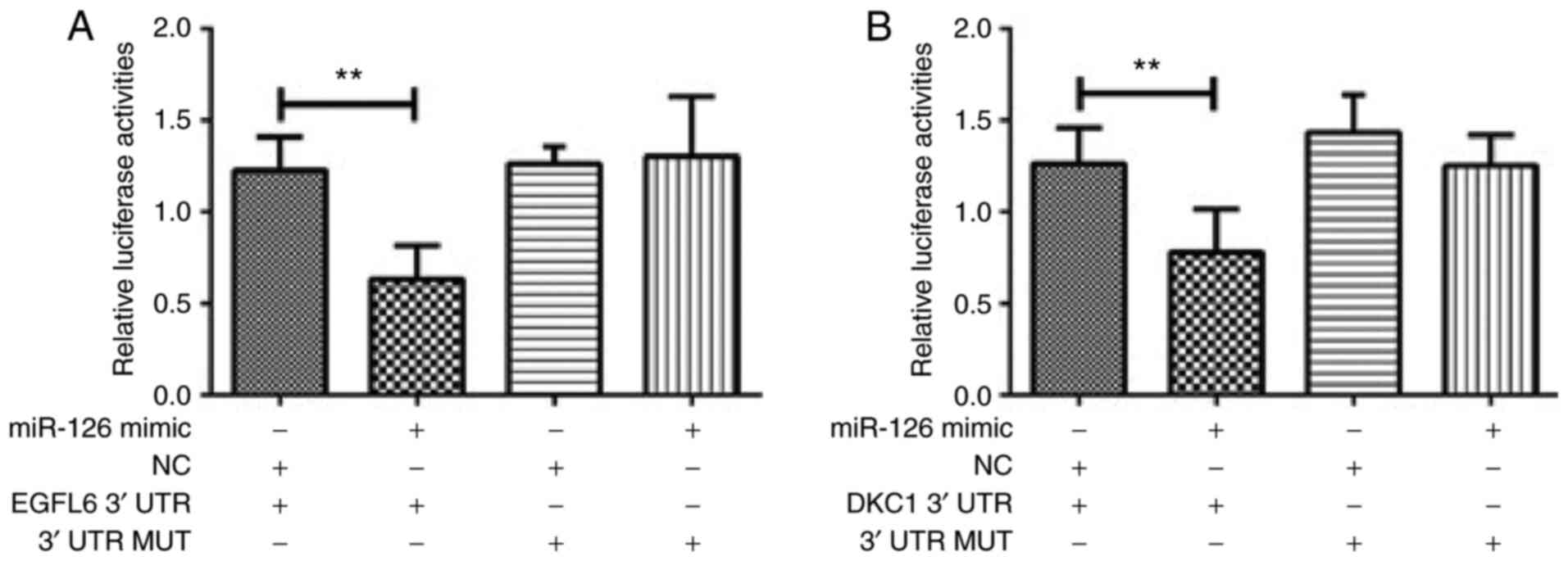
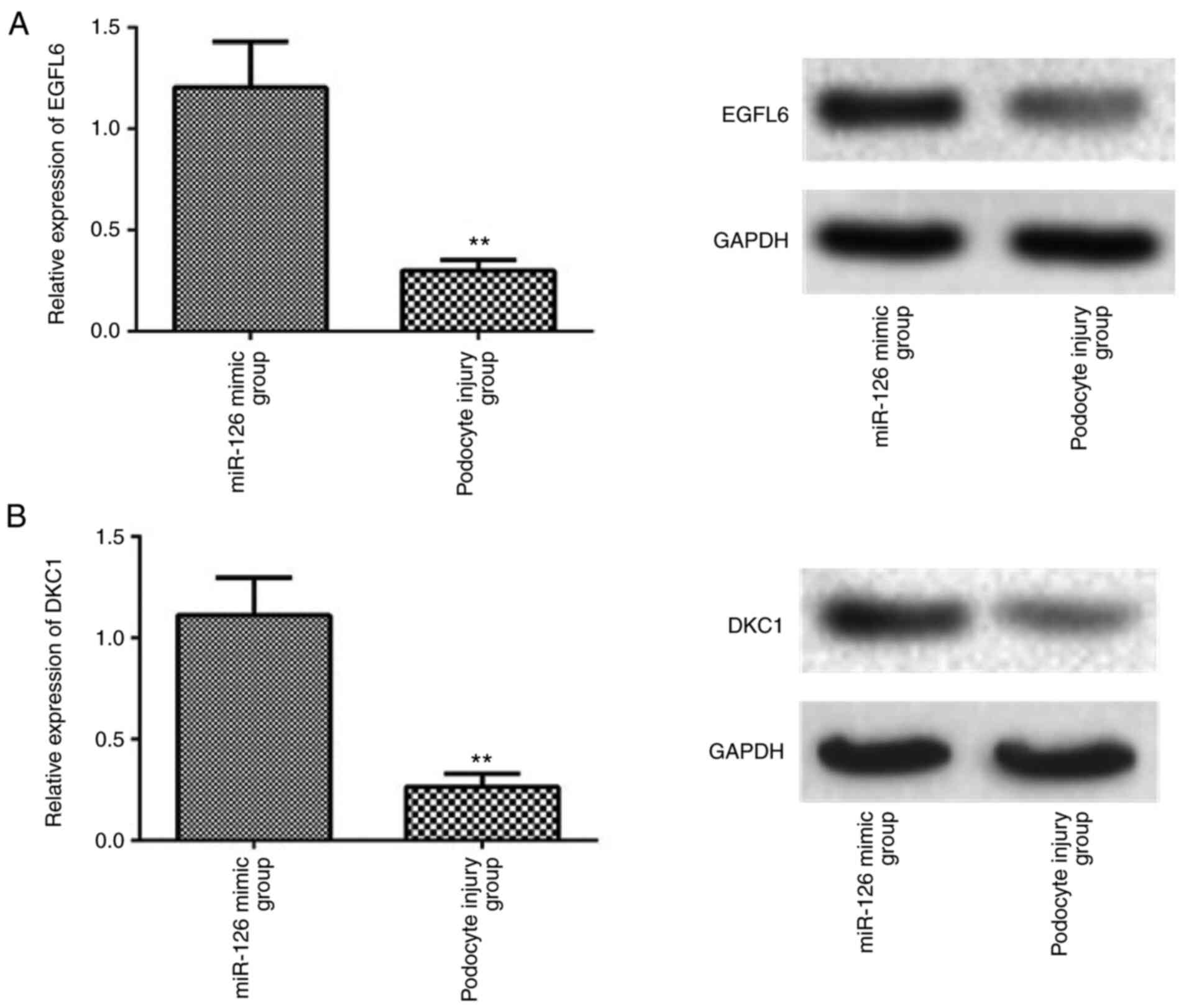
miR-126 target 3UTR binding sites of
EGFL6/DKC1 mRNA to regulate their expression and inhibit podocyte
injury
Following co-transfection of miR-126 and Wt 3′UTR of
EGFL6, luciferase activity was significantly reduced (P<0.001;
Fig. 4A). Similarly, as shown in
Fig. 4B, luciferase activity was
significantly reduced compared with the control group (P<0.001)
after co-transfection of miR-126 and Wt 3′UTR of DKC1. When miR-126
was upregulated, the protein levels of both EGFL6 and DKC1 as
determined by western blotting were increased (Fig. 5).
Discussion
Glomerular podocytes are differentiated terminal
cells with a weak capability of division and regeneration.
Podocytes, endothelial cells and basement membrane act together as
glomerular filtration barrier. Negative charge of podocytes can
prevent loss (or alternatively deprivation) of albumin and other
macromolecules (11,12). Inflammation is an important factor
in promoting damage of podocytes and proteinuria formation
(43). The loss of podocyte
structure and function is closely associated with inflammatory
factors (11) and its mechanism has
remains to be elucidated. The implication of inflammatory factors
in podocyte damage has attracted the attention of a number of
researchers aiming to find new therapeutic targets which can
provide a fundamental theoretical basis for the diagnosis and
treatment of kidney diseases.
The present study found that miR-126 was
downregulated in sepsis-induced podocyte injury. Overexpression of
miR-126 could protect LPS-induced podocytes damage through an
EGFL6/DKC1 signaling pathway. Bioinformatics analysis and
dual-luciferase reporter assay demonstrated that EGFL6 and DKC1 are
the key targets of miR-126.
miRs serve a key role in the post-transcriptional
regulation of gene expression and are involved in normal and
pathological renal function (44).
Upregulation of miR-27a, miR-21 and miR-370 and downregulation of
miR-15b-5p and miR-34c is associated with podocytes damage in
diabetic nephropathy (45–49). Downregulation of miR-120a-5p is
associated with increased expression of M-type phospholipase A2
receptor, which determines podocyte apoptosis and the progression
of membranous nephropathy (50).
Henique et al (51)
demonstrate that upregulation of miRNA-92a is associated with the
development and progression of glomerulonephritis. Sepsis-induced
acute kidney disease is associated with the miR-15a-5p-XIST-CUL3
regulatory axis (52). Upregulation
of miR-27b is associated with puromycin aminonucleoside-induced
podocytes damage by targeting adenosine receptor 2B (53). The present study focused attention
on miR-126 as one of the main regulators of sepsis-induced
podocytes injury, potentially by targeting EGFL6 and DKC1
proteins.
The EGFL6 gene is a member of the EGF superfamily.
The members of EGF superfamily are implicated in a wild spectrum of
functions in the organism including cell proliferation, cell cycle
and developmental processes (54).
EGFL6 has been associated with tumor angiogenic functions in
several types of cancers, including hepatocellular carcinoma,
ovarian and breast cancer (54–56).
Its role in preventing LPS-induced podocytes damage has yet to be
investigated. The present study is the first, to the best of the
authors' knowledge, that associates increased EGFL6 protein
expression with a protective kidney effect. Further studies are
required to elucidate these findings and potential utilization as a
therapeutic strategy.
DKC1 is a gene that provides instruction for
dyskerin protein involved in telomere integrity (57). A previous study demonstrated that
downregulation of miR-126 is associated with high glucose-induced
ageing to human glomerular mesangial cells and transfection with
miR-126 mimics can act as an inhibitor of telomere-p-53-p21-RB
signaling pathway and delay the effects (58). The current study supported these
findings, showing that the modulation of DKC1 could be involved in
the protective mechanism of miR-126 against podocytes damage.
The signal pathway regulated by various miRs could
be interpreted as the key pathophysiological mechanism of
nephropathy. These miR-related signal pathway inhibitors or
inducers are expected to become clinical therapeutic drugs in the
future (59). Understanding the key
mechanism of miRNA in the development and progression of renal
injury will help to identify new potential therapeutic targets and
design new therapeutic strategies. At present, it has been found
that these miRNAs serve a regulatory role in some specific
molecular pathways (59). It is
necessary to expand the interaction between different pathways of
miRNA and construct the miRNA interaction network in nephrotic
patients. This will contribute to the understanding of the specific
mechanism of miRNA in functional renal injury and structural renal
injury.
From the perspective of treatment, miR as a new
diagnostic and therapeutic marker of nephropathy is a new (or
recent) trend (51). However, it is
still necessary to fully understand the exact regulatory mechanism
and specific functions of each miR at the transcription and
translation level.
In conclusion, the present study found that miR-126
was downregulated in sepsis-induced podocyte injury and that
overexpression of miR-126 could protect podocytes through an
EGFL6/DKC1 signaling pathway. However, more research is needed to
clarify the exact function and mechanism of miRNAs as a specific
treatment for nephropathy.
Acknowledgements
Not applicable.
Funding
This work was supported by Zhejiang Province
Traditional Chinese Medicine Science and Technology Project (grant
no. 2015ZA089).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JS and LD performed the experiments, analyzed and
interpreted data and wrote the manuscript. Both authors were
responsible for confirming the authenticity of the data. Both
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zlatian O, Balasoiu AT, Balasoiu M,
Cristea O, Docea AO, Mitrut R, Spandidos DA, Tsatsakis AM, Bancescu
G and Calina D: Antimicrobial resistance in bacterial pathogens
among hospitalised patients with severe invasive infections. Exp
Ther Med. 16:4499–4510. 2018.PubMed/NCBI
|
|
2
|
Călina D, Docea AO, Rosu L, Zlatian O,
Rosu AF, Anghelina F, Rogoveanu O, Arsene AL, Nicolae AC, Drăgoi
CM, et al: Antimicrobial resistance development following surgical
site infections. Mol Med Rep. 15:681–688. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ungureanu A, Zlatian O, Mitroi G, Drocaş
A, Ţîrcă T, Călina D, Dehelean C, Docea AO, Izotov BN, Rakitskii
VN, et al: Staphylococcus aureus colonisation in patients
from a primary regional hospital. Mol Med Rep. 16:8771–8780. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Calina D, Rosu L, Rosu AF, Ianosi G,
Ianosi S, Zlatian O, Mitrut R, Oana DA, Rogoveanu O, Mitruţ P, et
al: Etiological diagnosis and pharmacotherapeutic management of
parapneumonic pleurisy. Farmacia. 64:946–952. 2016.
|
|
5
|
Tanase A, Colita A, Ianosi G, Neagoe D,
Branisteanu DE, Calina D, Docea AO, Tsatsakis A and Ianosi SL: Rare
case of disseminated fusariosis in a young patient with graft vs.
host disease following an allogeneic transplant. Exp Ther Med.
12:2078–2082. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seymour CW, Liu VX, Iwashyna TJ,
Brunkhorst FM, Rea TD, Scherag A, Rubenfeld G, Kahn JM,
Shankar-Hari M, Singer M, et al: Assessment of clinical criteria
for sepsis: For the third international consensus definitions for
sepsis and septic shock (Sepsis-3). JAMA. 315:762–774. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marik PE: Patterns of death in patients
with sepsis and the use of hydrocortisone, ascorbic acid, and
thiamine to prevent these deaths. Surg Infect (Larchmt).
19:812–820. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Genga KR and Russell JA: Update of sepsis
in the intensive care unit. J Innate Immun. 9:441–455. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi B, Shi F, Xu K, Shi L, Tang H, Wang N,
Wu Y, Gu J, Ding J and Huang Y: The prognostic performance of
Sepsis-3 and SIRS criteria for patients with
urolithiasis-associated sepsis transferred to ICU following
surgical interventions. Exp Ther Med. 18:4165–4172. 2019.PubMed/NCBI
|
|
10
|
Ronco C, Bellasi A and Di Lullo L:
Cardiorenal syndrome: An overview. Adv Chronic Kidney Dis.
25:382–390. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Greka A and Mundel P: Cell biology and
pathology of podocytes. Annu Rev Physiol. 74:299–323. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wharram BL, Goyal M, Wiggins JE, Sanden
SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman
LB, et al: Podocyte depletion causes glomerulosclerosis: Diphtheria
toxin-induced podocyte depletion in rats expressing human
diphtheria toxin receptor transgene. J Am Soc Nephrol.
16:2941–2952. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gambari R, Brognara E, Spandidos DA and
Fabbri E: Targeting oncomiRNAs and mimicking tumor suppressor
miRNAs: Νew trends in the development of miRNA therapeutic
strategies in oncology (Review). Int J Oncol. 49:5–32. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roberts JT and Borchert GM: Computational
prediction of MicroRNA target genes, target prediction databases,
and web resources. Methods Mol Biol. 1617:109–122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Candido S, Lupo G, Pennisi M, Basile MS,
Anfuso CD, Petralia MC, Gattuso G, Vivarelli S, Spandidos DA, Libra
M, et al: The analysis of miRNA expression profiling datasets
reveals inverse microRNA patterns in glioblastoma and Alzheimer's
disease. Oncol Rep. 42:911–922. 2019.PubMed/NCBI
|
|
16
|
Bibaki E, Tsitoura E, Vasarmidi E,
Margaritopoulos G, Trachalaki A, Koutoulaki C, Georgopoulou T,
Spandidos DA, Tzanakis N and Antoniou KM: miR-185 and miR-29a are
similarly expressed in the bronchoalveolar lavage cells in IPF and
lung cancer but common targets DNMT1 and COL1A1 show disease
specific patterns. Mol Med Rep. 17:7105–7112. 2018.PubMed/NCBI
|
|
17
|
Falzone L, Romano GL, Salemi R, Bucolo C,
Tomasello B, Lupo G, Anfuso CD, Spandidos DA, Libra M and Candido
S: Prognostic significance of deregulated microRNAs in uveal
melanomas. Mol Med Rep. 19:2599–2610. 2019.PubMed/NCBI
|
|
18
|
Doukas SG, Vageli DP, Lazopoulos G,
Spandidos DA, Sasaki CT and Tsatsakis A: The effect of NNK, a
tobacco smoke carcinogen, on the miRNA and mismatch DNA repair
expression profiles in lung and head and neck squamous cancer
cells. Cells. 9:10312020. View Article : Google Scholar
|
|
19
|
Koutsaki M, Libra M, Spandidos DA and
Zaravinos A: The miR-200 family in ovarian cancer. Oncotarget.
8:66629–66640. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sahu SC and Tsatsakis A: microRNAs:
Potential biomarkers of toxicity: A special issue of the Journal
Toxicology Reports. Toxicol Rep. 7:198–199. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Falzone L, Grimaldi M, Celentano E,
Augustin LSA and Libra M: Identification of modulated MicroRNAs
associated with breast cancer, diet, and physical activity. Cancers
(Basel). 12:25552020. View Article : Google Scholar
|
|
22
|
Polo A, Crispo A, Cerino P, Falzone L,
Candido S, Giudice A, De Petro G, Ciliberto G, Montella M, Budillon
A, et al: Environment and bladder cancer: Molecular analysis by
interaction networks. Oncotarget. 8:65240–65252. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Filetti V, Falzone L, Rapisarda V,
Caltabiano R, Eleonora Graziano AC, Ledda C and Loreto C:
Modulation of microRNA expression levels after naturally occurring
asbestiform fibers exposure as a diagnostic biomarker of
mesothelial neoplastic transformation. Ecotoxicol Environ Saf.
198:1106402020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sharifi-Rad J, Rodrigues CF, Sharopov F,
Docea AO, Can Karaca A, Sharifi-Rad M, Kahveci Karıncaoglu D,
Gülseren G, Şenol E, Demircan E, et al: Diet, lifestyle and
cardiovascular diseases: Linking pathophysiology to
cardioprotective effects of natural bioactive compounds. Int J
Environ Res Public Health. 17:23262020. View Article : Google Scholar
|
|
25
|
Sharifi-Rad M, Anil Kumar NV, Zucca P,
Varoni EM, Dini L, Panzarini E, Rajkovic J, Tsouh Fokou PV, Azzini
E, Peluso I, et al: Lifestyle, oxidative stress, and antioxidants:
Back and forth in the pathophysiology of chronic diseases. Front
Physiol. 11:6942020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Finotti A, Allegretti M, Gasparello J,
Giacomini P, Spandidos DA, Spoto G and Gambari R: Liquid biopsy and
PCR-free ultrasensitive detection systems in oncology (Review). Int
J Oncol. 53:1395–1434. 2018.PubMed/NCBI
|
|
27
|
Silantyev AS, Falzone L, Libra M, Gurina
OI, Kardashova KS, Nikolouzakis TK, Nosyrev AE, Sutton CW, Mitsias
PD and Tsatsakis A: Current and future trends on diagnosis and
prognosis of glioblastoma: From molecular biology to proteomics.
Cells. 8:8632019. View Article : Google Scholar
|
|
28
|
Nikolouzakis TK, Vassilopoulou L,
Fragkiadaki P, Mariolis Sapsakos T, Papadakis GZ, Spandidos DA,
Tsatsakis AM and Tsiaoussis J: Improving diagnosis, prognosis and
prediction by using biomarkers in CRC patients (Review). Oncol Rep.
39:2455–2472. 2018.PubMed/NCBI
|
|
29
|
Zaravinos A, Radojicic J, Lambrou GI,
Volanis D, Delakas D, Stathopoulos EN and Spandidos DA: Expression
of miRNAs involved in angiogenesis, tumor cell proliferation, tumor
suppressor inhibition, epithelial-mesenchymal transition and
activation of metastasis in bladder cancer. J Urol. 188:615–623.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rizos E, Siafakas N, Skourti E,
Papageorgiou C, Tsoporis J, Parker TH, Christodoulou DI, Spandidos
DA, Katsantoni E and Zoumpourlis V: miRNAs and their role in the
correlation between schizophrenia and cancer (Review). Mol Med Rep.
14:4942–4946. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Doukas SG, Vageli DP, Nikolouzakis TK,
Falzone L, Docea AO, Lazopoulos G, Kalbakis K and Tsatsakis A: Role
of DNA mismatch repair genes in lung and head and neck cancer
(Review). World Acad Sci J. 1:184–191. 2019.
|
|
32
|
Cheng Y, Yang M and Peng J: Correlation
the between the regulation of miRNA-1 in c-Met-induced EMT and
cervical cancer progression. Oncol Lett. 17:3341–3349.
2019.PubMed/NCBI
|
|
33
|
Xiong DD, Xu WQ, He RQ, Dang YW, Chen G
and Luo DZ: In silico analysis identified miRNA based therapeutic
agents against glioblastoma multiforme. Oncol Rep. 41:2194–2208.
2019.PubMed/NCBI
|
|
34
|
Liu C, Tong Z, Tan J, Xin Z, Wang Z and
Tian L: MicroRNA-21-5p targeting PDCD4 suppresses apoptosis via
regulating the PI3K/AKT/FOXO1 signaling pathway in tongue squamous
cell carcinoma. Exp Ther Med. 18:3543–3551. 2019.PubMed/NCBI
|
|
35
|
Krill KT, Gurdziel K, Heaton JH, Simon DP
and Hammer GD: Dicer deficiency reveals microRNAs predicted to
control gene expression in the developing adrenal cortex. Mol
Endocrinol. 27:754–768. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Asgeirsdóttir SA, van Solingen C, Kurniati
NF, Zwiers PJ, Heeringa P, van Meurs M, Satchell SC, Saleem MA,
Mathieson PW, Banas B, et al: MicroRNA-126 contributes to renal
microvascular heterogeneity of VCAM-1 protein expression in acute
inflammation. Am J Physiol Renal Physiol. 302:F1630–F1639. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Metzinger-Le Meuth V, Andrianome S,
Chillon JM, Bengrine A, Massy ZA and Metzinger L: microRNAs are
dysregulated in the cerebral microvasculature of CKD mice. Front
Biosci (Elite Ed). 6:80–88. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zampetaki A, Kiechl S, Drozdov I, Willeit
P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E,
et al: Plasma microRNA profiling reveals loss of endothelial
miR-126 and other microRNAs in type 2 diabetes. Circ Res.
107:810–817. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang S, Aurora AB, Johnson BA, Qi X,
McAnally J, Hill JA, Richardson JA, Bassel-Duby R and Olson EN: The
endothelial-specific microRNA miR-126 governs vascular integrity
and angiogenesis. Dev Cell. 15:261–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Y, Gao G, Yang C, Zhou K, Shen B,
Liang H and Jiang X: Stability of miR-126 in Urine and Its
Potential as a Biomarker for Renal Endothelial Injury with Diabetic
Nephropathy. Int J Endocrinol. 2014:3931092014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sheng X, Zuo X, Liu X, Zhou Y and Sun X:
Crosstalk between TLR4 and Notch1 signaling in the IgA nephropathy
during inflammatory response. Int Urol Nephrol. 50:779–785. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Langham RG, Kelly DJ, Cox AJ, Thomson NM,
Holthöfer H, Zaoui P, Pinel N, Cordonnier DJ and Gilbert RE:
Proteinuria and the expression of the podocyte slit diaphragm
protein, nephrin, in diabetic nephropathy: Effects of angiotensin
converting enzyme inhibition. Diabetologia. 45:1572–1576. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wolenski FS, Shah P, Sano T, Shinozawa T,
Bernard H, Gallacher MJ, Wyllie SD, Varrone G, Cicia LA, Carsillo
ME, et al: Identification of microRNA biomarker candidates in urine
and plasma from rats with kidney or liver damage. J Appl Toxicol.
37:278–286. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou Z, Wan J, Hou X, Geng J, Li X and Bai
X: MicroRNA-27a promotes podocyte injury via PPARγ-mediated
β-catenin activation in diabetic nephropathy. Cell Death Dis.
8:e26582017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen X, Zhao L, Xing Y and Lin B:
Down-regulation of microRNA-21 reduces inflammation and podocyte
apoptosis in diabetic nephropathy by relieving the repression of
TIMP3 expression. Biomed Pharmacother. 108:7–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fu Y, Wang C, Zhang D, Chu X, Zhang Y and
Li J: miR-15b-5p ameliorated high glucose-induced podocyte injury
through repressing apoptosis, oxidative stress, and inflammatory
responses by targeting Sema3A. J Cell Physiol. 234:20869–20878.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xian Y, Dong L, Jia Y, Lin Y, Jiao W and
Wang Y: miR-370 promotes high glucose-induced podocyte injuries by
inhibiting angiotensin II type 1 receptor-associated protein. Cell
Biol Int. 42:1545–1555. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu XD, Zhang LY, Zhu TC, Zhang RF, Wang
SL and Bao Y: Overexpression of miR-34c inhibits high
glucose-induced apoptosis in podocytes by targeting Notch signaling
pathways. Int J Clin Exp Pathol. 8:4525–4534. 2015.PubMed/NCBI
|
|
50
|
Liu D, Liu F, Wang X, Qiao Y, Pan S, Yang
Y, Hu Y, Zhang Y, Tian F and Liu Z: MiR-130a-5p prevents
angiotensin II-induced podocyte apoptosis by modulating M-type
phospholipase A2 receptor. Cell Cycle. 17:2484–2495. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Henique C, Bollée G, Loyer X, Grahammer F,
Dhaun N, Camus M, Vernerey J, Guyonnet L, Gaillard F, Lazareth H,
et al: Genetic and pharmacological inhibition of microRNA-92a
maintains podocyte cell cycle quiescence and limits crescentic
glomerulonephritis. Nat Commun. 8:18292017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu G, Mo L, Wu C, Shen X, Dong H, Yu L,
Pan P and Pan K: The miR-15a-5p-XIST-CUL3 regulatory axis is
important for sepsis-induced acute kidney injury. Ren Fail.
41:955–966. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zheng Z, Hu H, Tong Y, Hu Z, Cao S, Shan
C, Lin W, Yin Y and Li Z: MiR-27b regulates podocyte survival
through targeting adenosine receptor 2B in podocytes from non-human
primate. Cell Death Dis. 9:11332018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Noh K, Mangala LS, Han H-D, Zhang N,
Pradeep S, Wu SY, Ma S, Mora E, Rupaimoole R, Jiang D, et al:
Differential effects of EGFL6 on tumor versus wound angiogenesis.
Cell Rep. 21:2785–2795. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Larimer BM and Deutscher SL:
Identification of a peptide from in vivo bacteriophage display with
homology to EGFL6: A candidate tumor vasculature ligand in breast
cancer. J Mol Biomark Diagn. 5:1782014.PubMed/NCBI
|
|
56
|
Mas VR, Maluf DG, Archer KJ, Yanek KC and
Fisher RA: Angiogenesis soluble factors as hepatocellular carcinoma
noninvasive markers for monitoring hepatitis C virus cirrhotic
patients awaiting liver transplantation. Transplantation.
84:1262–1271. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ballew BJ and Savage SA: Updates on the
biology and management of dyskeratosis congenita and related
telomere biology disorders. Expert Rev Hematol. 6:327–337. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cao DW, Jiang CM, Wan C, Zhang M, Zhang
QY, Zhao M, Yang B, Zhu DL and Han X: Upregulation of miR-126
delays the senescence of human glomerular mesangial cells Induced
by high glucose via telomere-p53-p21-Rb signaling pathway. Curr Med
Sci. 38:758–764. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yu X, Odenthal M and Fries JW: Exosomes as
miRNA carriers: Formation-function-future. Int J Mol Sci.
17:20282016. View Article : Google Scholar
|