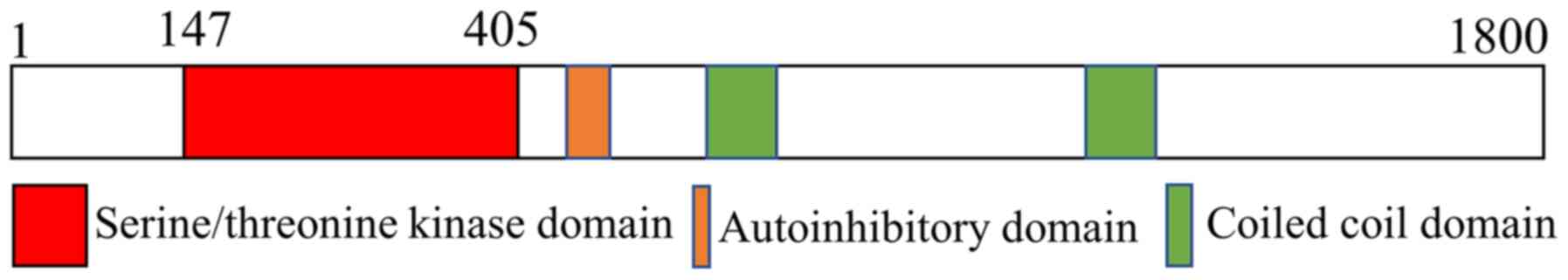
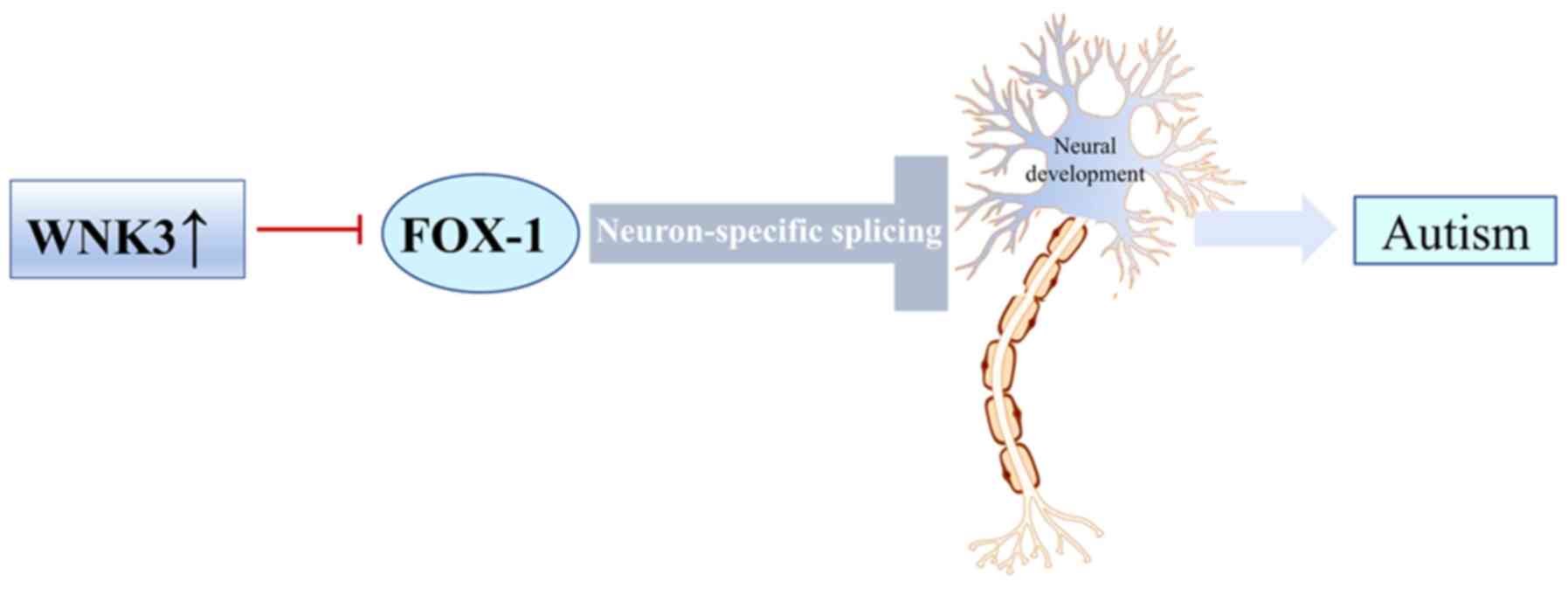
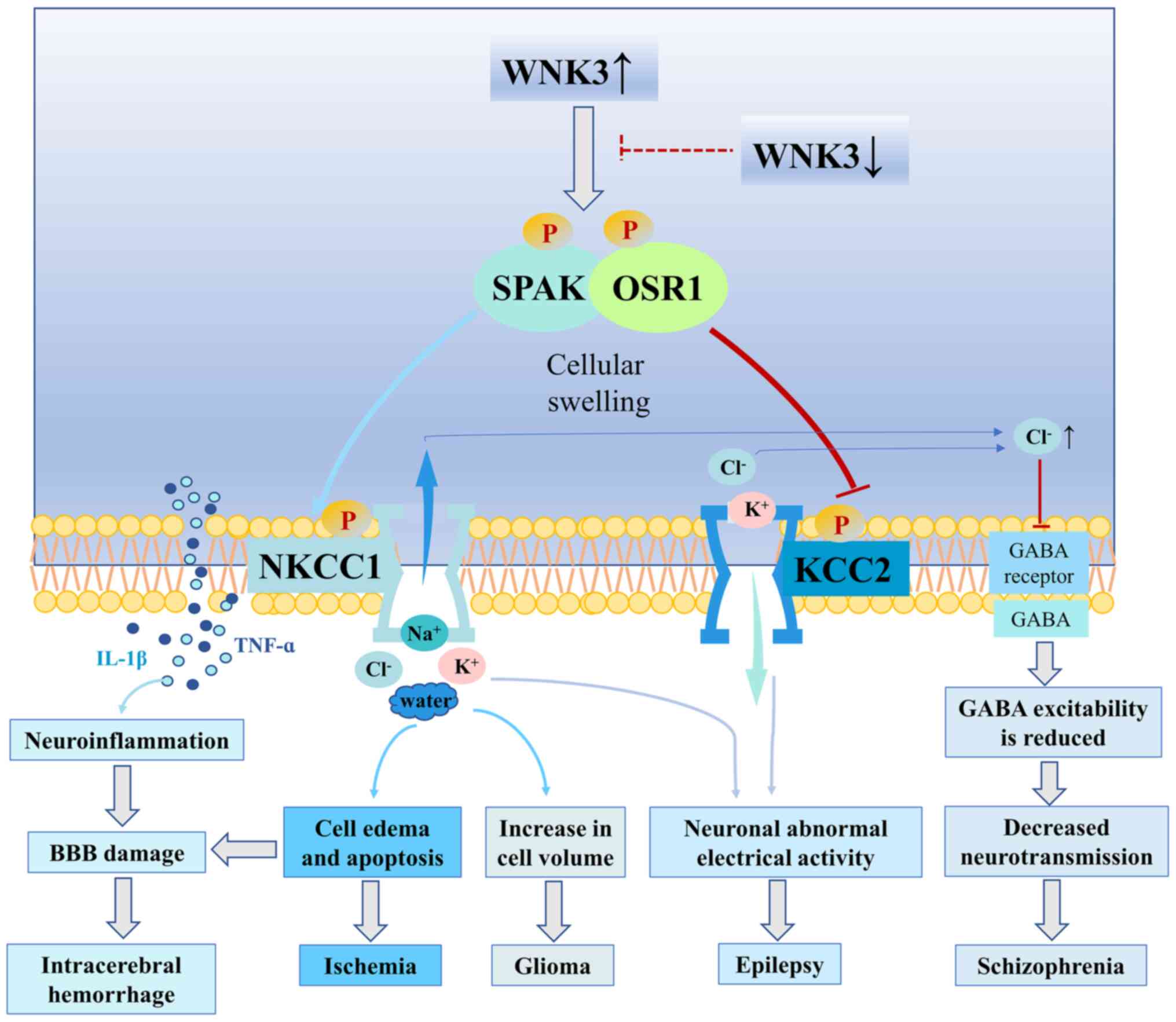
|
1
|
Siew K and O'Shaughnessy KM: Extrarenal
roles of the with-no-lysine[K] kinases (WNKs). Clin Exp Pharmacol
Physiol. 40:885–894. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu B, English JM, Wilsbacher JL, Stippec
S, Goldsmith EJ and Cobb MH: WNK1, a novel mammalian
serine/threonine protein kinase lacking the catalytic lysine in
subdomain II. J Biol Chem. 275:16795–16801. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Akella R, Drozdz MA, Humphreys JM, Jiou J,
Durbacz MZ, Mohammed ZJ, He H, Liwocha J, Sekulski K and Goldsmith
EJ: A phosphorylated intermediate in the activation of WNK kinases.
Biochemistry. 59:1747–1755. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thomson MN, Cuevas CA, Bewarder TM,
Dittmayer C, Miller LN, Si J, Cornelius RJ, Su XT, Yang CL,
McCormick JA, et al: WNK bodies cluster WNK4 and SPAK/OSR1 to
promote NCC activation in hypokalemia. Am J Physiol Renal Physiol.
318:F216–F228. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao JL, Peng K, Shen MW, Hou YH, Qian XB,
Meng XW, Ji FH, Wang LN and Yang JP: Suppression of WNK1-SPAK/OSR1
attenuates bone cancer pain by regulating NKCC1 and KCC2. J Pain.
20:1416–1428. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bergaya S, Vidal-Petiot E, Jeunemaitre X
and Hadchouel J: Pathogenesis of pseudohypoaldosteronism type 2 by
WNK1 mutations. Curr Opin Nephrol Hypertens. 21:39–45. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naray-Fejes-Toth A, Snyder PM and
Fejes-Toth G: The kidney-specific WNK1 isoform is induced by
aldosterone and stimulates epithelial sodium channel-mediated
Na+ transport. Proc Natl Acad Sci USA. 101:17434–17439.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang CL, Jian X and Yuh CH:
Wnk1-Osr1/spak kinase cascade is important for angiogenesis. Trans
Am Clin Climatol Assoc. 131:140–146. 2020.PubMed/NCBI
|
|
9
|
Liu Z, Yoon J, Wichaidit C, Jaykumar AB,
Dbouk HA, Embry AE, Liu L, Henderson JM, Chang AN, Cobb MH and
Miller RT: Control of podocyte and glomerular capillary wall
structure and elasticity by WNK1 kinase. Front Cell Dev Biol.
8:6188982020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chi RA, Wang T, Huang CL, Wu SP, Young SL,
Lydon JP and DeMayo FJ: WNK1 regulates uterine homeostasis and its
ability to support pregnancy. JCI Insight. 5:e1418322020.
View Article : Google Scholar
|
|
11
|
Zhao X, Lai G, Tu J, Liu S and Zhao Y:
Crosstalk between phosphorylation and ubiquitination is involved in
high salt-induced WNK4 expression. Exp Ther Med. 21:1332021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sie ZL, Li RY, Sampurna BP, Hsu PJ, Liu
SC, Wang HD, Huang CL and Yuh CH: WNK1 kinase stimulates
angiogenesis to promote tumor growth and metastasis. Cancers
(Basel). 12:5752020. View Article : Google Scholar
|
|
13
|
Rafael C, Chavez-Canales M and Hadchouel
J: New perspective on the role of WNK1 and WNK4 in the regulation
of NaCl reabsorption and K(+) secretion by the distal nephron. Med
Sci (Paris). 32:274–280. 2016.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Delaloy C, Lu J, Houot AM, Disse-Nicodeme
S, Gasc JM, Corvol P and Jeunemaitre X: Multiple promoters in the
WNK1 gene: One controls expression of a kidney-specific
kinase-defective isoform. Mol Cell Biol. 23:9208–9221. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Furusho T, Uchida S and Sohara E: The WNK
signaling pathway and salt-sensitive hypertension. Hypertens Res.
43:733–743. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Anderegg MA, Albano G, Hanke D, Deisl C,
Uehlinger DE, Brandt S, Bhardwaj R, Hediger MA and Fuster DG: The
sodium/proton exchanger NHA2 regulates blood pressure through a
WNK4-NCC dependent pathway in the kidney. Kidney Int. 99:350–363.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Klebe D, Iniaghe L, Burchell S, Reis C,
Akyol O, Tang J and Zhang JH: Intracerebral hemorrhage in mice.
Methods Mol Biol. 1717:83–91. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rinehart J, Vazquez N, Kahle KT, Hodson
CA, Ring AM, Gulcicek EE, Louvi A, Bobadilla NA, Gamba G and Lifton
RP: WNK2 kinase is a novel regulator of essential neuronal
cation-chloride cotransporters. J Biol Chem. 286:30171–30180. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Costa AM, Pinto F, Martinho O, Oliveira
MJ, Jordan P and Reis RM: Silencing of the tumor suppressor gene
WNK2 is associated with upregulation of MMP2 and JNK in gliomas.
Oncotarget. 6:1422–1434. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alves ALV, Costa AM, Martinho O, da Silva
VD, Jordan P, Silva VAO and Reis RM: WNK2 inhibits autophagic flux
in human glioblastoma cell line. Cells. 9:4852020. View Article : Google Scholar
|
|
21
|
Moniz S, Martinho O, Pinto F, Sousa B,
Loureiro C, Oliveira MJ, Moita LF, Honavar M, Pinheiro C, Pires M,
et al: Loss of WNK2 expression by promoter gene methylation occurs
in adult gliomas and triggers Rac1-mediated tumour cell
invasiveness. Hum Mol Genet. 22:84–95. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Holden S, Cox J and Raymond FL: Cloning,
genomic organization, alternative splicing and expression analysis
of the human gene WNK3 (PRKWNK3). Gene. 335:109–119. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moniz S and Jordan P: Emerging roles for
WNK kinases in cancer. Cell Mol Life Sci. 67:1265–1276. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kahle KT, Ring AM and Lifton RP: Molecular
physiology of the WNK kinases. Annu Rev Physiol. 70:329–355. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Verissimo F, Silva E, Morris JD, Pepperkok
R and Jordan P: Protein kinase WNK3 increases cell survival in a
caspase-3-dependent pathway. Oncogene. 25:4172–4182. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
de Los Heros P, Pacheco-Alvarez D and
Gamba G: Role of WNK kinases in the modulation of cell volume. Curr
Top Membr. 81:207–235. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu D, Lai N, Deng R, Liang T, Pan P, Yuan
G, Li X, Li H, Shen H, Wang Z and Chen G: Activated WNK3 induced by
intracerebral hemorrhage deteriorates brain injury maybe via
WNK3/SPAK/NKCC1 pathway. Exp Neurol. 332:1133862020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pacheco-Alvarez D and Gamba G: WNK3 is a
putative chloride-sensing kinase. Cell Physiol Biochem.
28:1123–1134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Begum G, Yuan H, Kahle KT, Li L, Wang S,
Shi Y, Shmukler BE, Yang SS, Lin SH, Alper SL and Sun D: Inhibition
of WNK3 kinase signaling reduces brain damage and accelerates
neurological recovery after stroke. Stroke. 46:1956–1965. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang BL: (WNK)ing at death: With-no-lysine
(Wnk) kinases in neuropathies and neuronal survival. Brain Res
Bull. 125:92–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Haas BR, Cuddapah VA, Watkins S, Rohn KJ,
Dy TE and Sontheimer H: With-no-lysine kinase 3 (WNK3) stimulates
glioma invasion by regulating cell volume. Am J Physiol Cell
Physiol. 301:C1150–C1160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shekarabi M, Zhang J, Khanna AR, Ellison
DH, Delpire E and Kahle KT: WNK kinase signaling in ion homeostasis
and human disease. Cell Metab. 25:285–299. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schreck KA and Richdale AL: Sleep
problems, behavior, and psychopathology in autism:
inter-relationships across the lifespan. Curr Opin Psychol.
34:105–111. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fakhoury M: Autistic spectrum disorders: A
review of clinical features, theories and diagnosis. Int J Dev
Neurosci. 43:70–77. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Richdale AL and Schreck KA: Sleep problems
in autism spectrum disorders: Prevalence, nature, and possible
biopsychosocial aetiologies. Sleep Med Rev. 13:403–411. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Horvath GA, Stowe RM, Ferreira CR and Blau
N: Clinical and biochemical footprints of inherited metabolic
diseases. III. Psychiatric presentations. Mol Genet Metab. 130:1–6.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fogel BL, Wexler E, Wahnich A, Friedrich
T, Vijayendran C, Gao F, Parikshak N, Konopka G and Geschwind DH:
RBFOX1 regulates both splicing and transcriptional networks in
human neuronal development. Hum Mol Genet. 21:4171–4186. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wen M, Yan Y, Yan N, Chen XS, Liu SY and
Feng ZH: Upregulation of RBFOX1 in the malformed cortex of patients
with intractable epilepsy and in cultured rat neurons. Int J Mol
Med. 35:597–606. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Voineagu I, Wang X, Johnston P, Lowe JK,
Tian Y, Horvath S, Mill J, Cantor RM, Blencowe BJ and Geschwind DH:
Transcriptomic analysis of autistic brain reveals convergent
molecular pathology. Nature. 474:380–384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sebat J, Lakshmi B, Malhotra D, Troge J,
Lese-Martin C, Walsh T, Yamrom B, Yoon S, Krasnitz A, Kendall J, et
al: Strong association of de novo copy number mutations with
autism. Science. 316:445–449. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Qiao Y, Liu X, Harvard C, Hildebrand MJ,
Rajcan-Separovic E, Holden JJ and Lewis ME: Autism-associated
familial microdeletion of Xp11.22. Clin Genet. 74:134–144. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Edens AC, Lyons MJ, Duron RM, Dupont BR
and Holden KR: Autism in two females with duplications involving
Xp11.22-p11.23. Dev Med Child Neurol. 53:463–466. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee AY, Chen W, Stippec S, Self J, Yang F,
Ding X, Chen S, Juang YC and Cobb MH: Protein kinase WNK3 regulates
the neuronal splicing factor Fox-1. Proc Natl Acad Sci USA.
109:16841–16846. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chung BH, Drmic I, Marshall CR,
Grafodatskaya D, Carter M, Fernandez BA, Weksberg R, Roberts W and
Scherer SW: Phenotypic spectrum associated with duplication of
Xp11.22-p11.23 includes autism spectrum disorder. Eur J Med Genet.
54:e516–e520. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gehman LT, Stoilov P, Maguire J, Damianov
A, Lin CH, Shiue L, Ares M Jr, Mody I and Black DL: The splicing
regulator Rbfox1 (A2BP1) controls neuronal excitation in the
mammalian brain. Nat Genet. 43:706–711. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Piton A, Gauthier J, Hamdan FF, Lafrenière
RG, Yang Y, Henrion E, Laurent S, Noreau A, Thibodeau P, Karemera
L, et al: Systematic resequencing of X-chromosome synaptic genes in
autism spectrum disorder and schizophrenia. Mol Psychiatry.
16:867–880. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Navidhamidi M, Ghasemi M and Mehranfard N:
Epilepsy- associated alterations in hippocampal excitability. Rev
Neurosci. 28:307–334. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhou Y, Liu M and Liang WN: Progress on
the epidemiological study of epilepsy. Zhonghua Liu Xing Bing Xue
Za Zhi. 28:92–94. 2007.(In Chinese). PubMed/NCBI
|
|
49
|
Thurman DJ, Hesdorffer DC and French JA:
Sudden unexpected death in epilepsy: Assessing the public health
burden. Epilepsia. 55:1479–1485. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Devinsky O, Vezzani A, O'Brien TJ, Jette
N, Scheffer IE, de Curtis M and Perucca P: Epilepsy. Nat Rev Dis
Primers. 4:180242018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen Z, Brodie MJ, Liew D and Kwan P:
Treatment outcomes in patients with newly diagnosed epilepsy
treated with established and new antiepileptic drugs: A 30-year
longitudinal cohort study. JAMA Neurol. 75:279–286. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shima A, Nitta N, Suzuki F, Laharie AM,
Nozaki K and Depaulis A: Activation of mTOR signaling pathway is
secondary to neuronal excitability in a mouse model of
mesio-temporal lobe epilepsy. Eur J Neurosci. 41:976–988. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Schmeiser B, Zentner J, Prinz M, Brandt A
and Freiman TM: Extent of mossy fiber sprouting in patients with
mesiotemporal lobe epilepsy correlates with neuronal cell loss and
granule cell dispersion. Epilepsy Res. 129:51–58. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jeong KH, Kim SH, Choi YH, Cho I and Kim
WJ: Increased expression of WNK3 in dispersed granule cells in
hippocampal sclerosis of mesial temporal lobe epilepsy patients.
Epilepsy Res. 147:58–61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kahle KT, Rinehart J, de Los Heros P,
Louvi A, Meade P, Vazquez N, Hebert SC, Gamba G, Gimenez I and
Lifton RP: WNK3 modulates transport of Cl- in and out of cells:
Implications for control of cell volume and neuronal excitability.
Proc Natl Acad Sci USA. 102:16783–16788. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Huberfeld G, Blauwblomme T and Miles R:
Hippocampus and epilepsy: Findings from human tissues. Rev Neurol
(Paris). 171:236–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Eftekhari S, Mehvari Habibabadi J, Najafi
Ziarani M, Hashemi Fesharaki SS, Gharakhani M, Mostafavi H,
Joghataei MT, Beladimoghadam N, Rahimian E and Hadjighassem MR:
Bumetanide reduces seizure frequency in patients with temporal lobe
epilepsy. Epilepsia. 54:e9–e12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Loscher W, Puskarjov M and Kaila K:
Cation-chloride cotransporters NKCC1 and KCC2 as potential targets
for novel antiepileptic and antiepileptogenic treatments.
Neuropharmacology. 69:62–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Silayeva L, Deeb TZ, Hines RM, Kelley MR,
Munoz MB, Lee HH, Brandon NJ, Dunlop J, Maguire J, Davies PA and
Moss SJ: KCC2 activity is critical in limiting the onset and
severity of status epilepticus. Proc Natl Acad Sci USA.
112:3523–3528. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen Y, Zhou H, Jin T, Ye T and Xie W:
Clinical observation of the phased acupuncture for ischemic stroke
hemiplegia. Zhongguo Zhen Jiu. 38:1027–1034. 2018.(In Chinese).
PubMed/NCBI
|
|
61
|
Hu YY, Li L, Xian XH, Zhang M, Sun XC, Li
SQ, Cui X, Qi J and Li WB: GLT-1 upregulation as a potential
therapeutic target for ischemic brain injury. Curr Pharm Des.
23:5045–5055. 2017.PubMed/NCBI
|
|
62
|
Tuttolomondo A, Puleo MG, Velardo MC,
Corpora F, Daidone M and Pinto A: Molecular biology of
atherosclerotic ischemic strokes. Int J Mol Sci. 21:93722020.
View Article : Google Scholar
|
|
63
|
Zhao H, Nepomuceno R, Gao X, Foley LM,
Wang S, Begum G, Zhu W, Pigott VM, Falgoust LM, Kahle KT, et al:
Deletion of the WNK3-SPAK kinase complex in mice improves
radiographic and clinical outcomes in malignant cerebral edema
after ischemic stroke. J Cereb Blood Flow Metab. 37:550–563. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Demian WL, Persaud A, Jiang C, Coyaud É,
Liu S, Kapus A, Kafri R, Raught B and Rotin D: The ion transporter
NKCC1 links cell volume to cell mass regulation by suppressing
mTORC1. Cell Rep. 27:1886–1896.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yan Y, Dempsey RJ, Flemmer A, Forbush B
and Sun D: Inhibition of Na(+)-K(+)-Cl(−) cotransporter during
focal cerebral ischemia decreases edema and neuronal damage. Brain
Res. 961:22–31. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chen H, Luo J, Kintner DB, Shull GE and
Sun D: Na(+)-dependent chloride transporter (NKCC1)-null mice
exhibit less gray and white matter damage after focal cerebral
ischemia. J Cereb Blood Flow Metab. 25:54–66. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Krueger M, Hartig W, Reichenbach A,
Bechmann I and Michalski D: Blood-brain barrier breakdown after
embolic stroke in rats occurs without ultrastructural evidence for
disrupting tight junctions. PLoS One. 8:e564192013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chen H, Kintner DB, Jones M, Matsuda T,
Baba A, Kiedrowski L and Sun D: AMPA-mediated excitotoxicity in
oligodendrocytes: Role for Na(+)-K(+)-Cl(−) co-transport and
reversal of Na(+)/Ca(2+) exchanger. J Neurochem. 102:1783–1795.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hossain Khan MZ, Sohara E, Ohta A, Chiga
M, Inoue Y, Isobe K, Wakabayashi M, Oi K, Rai T, Sasaki S and
Uchida S: Phosphorylation of Na-Cl cotransporter by OSR1 and SPAK
kinases regulates its ubiquitination. Biochem Biophys Res Commun.
425:456–461. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhang J, Gao G, Begum G, Wang J, Khanna
AR, Shmukler BE, Daubner GM, de Los Heros P, Davies P, Varghese J,
et al: Functional kinomics establishes a critical node of
volume-sensitive cation-Cl-cotransporter regulation in the
mammalian brain. Sci Rep. 6:359862016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang P, Wang T, Zhang D, Zhang Z, Yuan S,
Zhang J, Cao J, Li H, Li X, Shen H and Chen G: Exploration of
MST1-mediated secondary brain injury induced by intracerebral
hemorrhage in rats via hippo signaling pathway. Transl Stroke Res.
10:729–743. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kamel H and Hemphill JC III:
Characteristics and sequelae of intracranial hypertension after
intracerebral hemorrhage. Neurocrit Care. 17:172–176. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Honner SK, Singh A, Cheung PT, Alter HJ,
Dutaret CG, Patel AK and Acharya A: Emergency department control of
blood pressure in intracerebral hemorrhage. J Emerg Med.
41:355–361. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zheng H, Chen C, Zhang J and Hu Z:
Mechanism and therapy of brain edema after intracerebral
hemorrhage. Cerebrovasc Dis. 42:155–169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Dang G, Yang Y, Wu G, Hua Y, Keep RF and
Xi G: Early erythrolysis in the hematoma after experimental
intracerebral hemorrhage. Transl Stroke Res. 8:174–182. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hemphill JC III, Greenberg SM, Anderson
CS, Becker K, Bendok BR, Cushman M, Fung GL, Goldstein JN,
Macdonald RL, Mitchell PH, et al: Guidelines for the management of
spontaneous intracerebral hemorrhage: A guideline for healthcare
professionals from the American Heart Association/American Stroke
Association. Stroke. 46:2032–2060. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang X, Gu Y, Li P, Jiang A, Sheng X, Jin
X, Shi Y and Li G: Matrix metalloproteases-mediated cleavage on
β-dystroglycan may play a key role in the blood-brain barrier after
intracerebral hemorrhage in rats. Med Sci Monit. 25:794–800. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Mracsko E and Veltkamp R:
Neuroinflammation after intracerebral hemorrhage. Front Cell
Neurosci. 8:3882014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wu X, Fu S, Liu Y, Luo H, Li F, Wang Y,
Gao M, Cheng Y and Xie Z: NDP-MSH binding melanocortin-1 receptor
ameliorates neuroinflammation and BBB disruption through
CREB/Nr4a1/NF-κB pathway after intracerebral hemorrhage in mice. J
Neuroinflammation. 16:1922019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Tian Y, Guo SX, Li JR, Du HG, Wang CH,
Zhang JM and Wu Q: Topiramate attenuates early brain injury
following subarachnoid haemorrhage in rats via duplex protection
against inflammation and neuronal cell death. Brain Res.
1622:174–185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Digregorio M, Lombard A, Lumapat PN,
Scholtes F, Rogister B and Coppieters N: Relevance of translation
initiation in diffuse glioma biology and its therapeutic potential.
Cells. 8:15422019. View Article : Google Scholar
|
|
82
|
Giese A and Westphal M: Glioma invasion in
the central nervous system. Neurosurgery. 39:232–250. 1996.
|
|
83
|
de Paula LB, Primo FL and Tedesco AC:
Nanomedicine associated with photodynamic therapy for glioblastoma
treatment. Biophys Rev. 9:761–773. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sontheimer H: Ion channels and amino acid
transporters support the growth and invasion of primary brain
tumors. Mol Neurobiol. 29:61–71. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sontheimer H: An unexpected role for ion
channels in brain tumor metastasis. Exp Biol Med (Maywood).
233:779–791. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Garzon-Muvdi T, Schiapparelli P, ap Rhys
C, Guerrero-Cazares H, Smith C, Kim DH, Kone L, Farber H, Lee DY,
An SS, et al: Regulation of brain tumor dispersal by NKCC1 through
a novel role in focal adhesion regulation. PLoS Biol.
10:e10013202012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhou B, Lu X, Hao Y and Yang P: Real-time
monitoring of the regulatory volume decrease of cancer cells: A
model for the evaluation of cell migration. Anal Chem.
91:8078–8084. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Algharabil J, Kintner DB, Wang Q, Begum G,
Clark PA, Yang SS, Lin SH, Kahle KT, Kuo JS and Sun D: Inhibition
of Na(+)-K(+)-2Cl(−) cotransporter isoform 1 accelerates
temozolomide-mediated apoptosis in glioblastoma cancer cells. Cell
Physiol Biochem. 30:33–48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ernest NJ and Sontheimer H: Extracellular
glutamine is a critical modulator for regulatory volume increase in
human glioma cells. Brain Res. 1144:231–238. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Haas BR and Sontheimer H: Inhibition of
the sodium-potassium-chloride cotransporter isoform-1 reduces
glioma invasion. Cancer Res. 70:5597–5606. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Hamann S, Herrera-Perez JJ, Zeuthen T and
Alvarez-Leefmans FJ: Cotransport of water by the
Na+-K+−2Cl(−) cotransporter NKCC1 in
mammalian epithelial cells. J Physiol. 588:4089–4101. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Mach C and Dollfus S: Scale for assessing
negative symptoms in schizophrenia: A systematic review. Encephale.
42:165–171. 2016.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Tandon R, Gaebel W, Barch DM, Bustillo J,
Gur RE, Heckers S, Malaspina D, Owen MJ, Schultz S, Tsuang M, et
al: Definition and description of schizophrenia in the DSM-5.
Schizophr Res. 150:3–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Guessoum SB, Le Strat Y, Dubertret C and
Mallet J: A transnosographic approach of negative symptoms
pathophysiology in schizophrenia and depressive disorders. Prog
Neuropsychopharmacol Biol Psychiatry. 99:1098622020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Gonzalez-Burgos G and Lewis DA: GABA
neurons and the mechanisms of network oscillations: implications
for understanding cortical dysfunction in schizophrenia. Schizophr
Bull. 34:944–961. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Lewis DA, Hashimoto T and Volk DW:
Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci.
6:312–324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Arion D and Lewis DA: Altered expression
of regulators of the cortical chloride transporters NKCC1 and KCC2
in schizophrenia. Arch Gen Psychiatry. 68:21–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Blanquie O, Liebmann L, Hubner CA, Luhmann
HJ and Sinning A: NKCC1-mediated GABAergic signaling promotes
postnatal cell death in neocortical cajal-retzius cells. Cereb
Cortex. 27:1644–1659. 2017.PubMed/NCBI
|
|
99
|
Lewis DA and Sweet RA: Schizophrenia from
a neural circuitry perspective: Advancing toward rational
pharmacological therapies. J Clin Invest. 119:706–716. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
de Los Heros P, Kahle KT, Rinehart J,
Bobadilla NA, Vázquez N, San Cristobal P, Mount DB, Lifton RP,
Hebert SC and Gamba G: WNK3 bypasses the tonicity requirement for
K-Cl cotransporter activation via a phosphatase-dependent pathway.
Proc Natl Acad Sci USA. 103:1976–1981. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
de Los Heros P, Alessi DR, Gourlay R,
Campbell DG, Deak M, Macartney TJ, Kahle KT and Zhang J: The
WNK-regulated SPAK/OSR1 kinases directly phosphorylate and inhibit
the K+-Cl− co-transporters. Biochem J.
458:559–573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Vorontsova I, Donaldson PJ, Kong Z,
Wickremesinghe C, Lam L and Lim JC: The modulation of the
phosphorylation status of NKCC1 in organ cultured bovine lenses:
Implications for the regulation of fiber cell and overall lens
volume. Exp Eye Res. 165:164–174. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Alessi DR, Zhang J, Khanna A, Hochdorfer
T, Shang Y and Kahle KT: The WNK-SPAK/OSR1 pathway: Master
regulator of cation-chloride cotransporters. Sci Signal. 7:re32014.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Conway LC, Cardarelli RA, Moore YE, Jones
K, McWilliams LJ, Baker DJ, Burnham MP, Bürli RW, Wang Q, Brandon
NJ, et al: N-Ethylmaleimide increases KCC2 cotransporter activity
by modulating transporter phosphorylation. J Biol Chem.
292:21253–21263. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Glover M, Zuber AM and O'Shaughnessy KM:
Renal and brain isoforms of WNK3 have opposite effects on NCCT
expression. J Am Soc Nephrol. 20:1314–1322. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Lu DC, Hannemann A, Wadud R, Rees DC,
Brewin JN, Low PS and Gibson JS: The role of WNK in modulation of
KCl cotransport activity in red cells from normal individuals and
patients with sickle cell anaemia. Pflugers Arch. 471:1539–1549.
2019. View Article : Google Scholar : PubMed/NCBI
|