Lung development initiates in utero and continues
until infancy, and involves a complex process regulated by
different types of cells, factors and mediators, such as
macrophages, dendritic cells and lymphocytes (1). Abnormal lung development can be
harmful to respiratory health, which may result in bronchopulmonary
dysplasia, neonatal respiratory distress syndrome, asthma and
chronic obstructive emphysema (2–4). Type
2 immune response is important for pulmonary development and
several types of pulmonary diseases, such as asthma, lung infection
and pulmonary fibrosis (5–7).
Interleukin (IL)-4, IL-5, IL-9 and IL-13 are
important cytokines that play key roles in type 2 immunity, and are
usually involved in allergic diseases or during helminthic
parasitic infections (8,9). Th2 cells and certain myeloid cells are
considered the primary source of these type 2 cytokines (10,11);
however, recent studies have reported that a rare subpopulation of
innate lymphocytes are the predominant source (12–14).
Type 2 innate lymphoid cells (ILC2s), which were first discovered
as non-T and B cells (15,16), play a defensive role in the initial
stage of helminthic infestation (17), and are considered a major component
of type 2 immunity in lungs (18,19).
Several types of cells, including eosinophils, mast
cells, basophils and alternative (M2) macrophages, activated by
IL-4 and IL-13 that are involved in type 2 immune response, also
regulate the repair response following tissue injury (20). M2 macrophages initiate different
responses to parasites, tissue remodeling, angiogenesis and
allergic diseases (21–23). Therefore, it may be hypothesized
that M2 macrophages can crosstalk with ILC2s during pulmonary
development and in different pulmonary diseases.
ILC2s are a subset of ILCs, and activation of these
produce several Th2 cytokines, including IL-4, IL-5, IL-9 and
IL-13, and/or dual-regulatory proteins, such as amphiregulin (AREG)
(27). ILC2s depend on
transcription factors, GATA binding protein 3 and retinoid acid
receptor related orphan receptor α, for their development and
function, but lack antigen-specific receptors (28,29).
ILC2s are distributed throughout the body and are abundant on
mucosal surfaces, such as the lungs, gastrointestinal tract and
skin, in both humans and mice (30). ILC2s account for a major proportion
in mouse pulmonary innate lymphocytes, and <3% of human lung
innate lymphocytes (31,32).
Lung ILC2s are rapidly activated when exposed to
epithelial-derived alarmin proteins and other inflammatory
mediators, including IL-33, IL-25 and thymic stromal lymphopoietin
(TSLP) (33). A previous study
demonstrated that IL-25 reactive lung ILC2s can change into IL-33
reactive lung ILC2s, both in vivo and in vitro
(34). IL-33 and IL-25 both promote
the enrichment of ILC2s in lung in vivo; however, only IL-33
can directly induce the migration of ILC2s in vitro
(35). Similar effects of IL-33 are
observed in skin (36), while TSLP
and IL-25 exhibit relatively poor chemotaxis, although they can be
detected at high concentrations in lungs (37,38).
Although ILC2s secrete IL-9, autocrine IL-9
maintains homeostasis of pulmonary ILC2s (37,38).
IL-2 was the first cytokine reported to promote the secretion of
IL-9 by ILC2s (39). IL-2 is also
important for activating and culturing ILC2s in vitro
(39,40). Another study demonstrated that IL-4
can increase IL-9 expression by stimulating ILC2s (41). Suppression of IL-9 production
inhibits IL-33-induced eosinophilic airway inflammation,
highlighting its role in effectively proliferating and activating
ILC2s (42). In addition, the
synergistic effects of TSLP and IL-33 markedly effect the
production of IL-9 via ILC2s (37).
ILC2s express corresponding receptors, including
suppression of tumorigenicity 2 (ST2), IL-25R (IL-17RB), TSLPR and
AREG receptor, as well as toll-like receptors (TLRs) 2 and 4
(28,29,43–45).
Upon activation, excluding Th2-type cytokines and/or AREG, ILC2s
also secrete other factors, including granulocyte-macrophage colony
stimulating factor (GM-CSF), IL-6 and IL-10 (46–48).
In addition to stimulators, there are also inhibitors of ILC2s. For
example, the neuropeptide calcitonin gene-related peptide and its
receptor can inhibit the secretion and enrichment of pulmonary
ILC2s and Th2 cytokines driven by alarmin, both in vitro and
in vivo (49).
Elevated numbers of ILC2s in patients with asthma
and chronic sinusitis suggest that ILC2s are detrimental to chronic
inflammation (50). However,
intrahepatic ILC2s can exacerbate fibrosis in liver diseases by
secreting AREG (51). Thus, the
roles of ILC2s vary in different tissues and diseases, and involve
complex molecular mechanisms.
Recently, ILC2s have become the research focus in
different tissue and organ diseases. It has been reported that
intestinal helminthic infection induces activation of ILC2s,
proliferation of IL-13 dependent goblet cells and increases mucin
production at distal sites, including the lungs (52). In severe cases, increased mucus
secretion via alveoli and the lungs inhibits lung metastasis
(52). This suggests that the
innate immunity of ILC2s is not only limited to certain tissues,
but also influences and interacts with different organs. According
to a previous study, aging influences innate immunity (53). ILC2s in elderly lungs are not
uniform in transcription and function, and cannot produce cytokines
during influenza infection and homeostasis in vivo (53). The transfer of ILC2s in the lungs of
young mice strengthens the immunity of old mice to influenza
infection (53). Notably, ILC2s in
neonatal lungs involve distinct pro-inflammatory and tissue repair
subgroups (54). Neonatal
endogenous IL-33 stimulates ILC2s in the pulmonary, which may
‘train’ ILC2s for implantation into the lungs following birth, thus
becoming resident cells that respond more effectively to future
challenges (55). Thus, by
secretion of a plethora of mediators, ILC2s play vital roles in
inducing and supporting type 2 immune responses in lung
tissues.
Macrophages, which act as myeloid cells, are among
the first cells that respond to pathogens and tissue damage
(56). They not only have innate
immune function, which acts by phagocytizing and killing pathogens
directly to exert innate immunity, but also initiate adaptive
immunity by presenting pathogens to T lymphocytes (57,58).
Tissue macrophages, which are important immune cells, are produced
by yolk sac or fetal liver and their function is guided by resident
tissues (59). Thus, it is
important to study the macrophages that reside in the lung to
understand the role of macrophages in lung diseases. There are two
subtypes based on anatomical position of pulmonary resident
macrophages, alveolar macrophages (AMs) and interstitial
macrophages (60).
AMs, which are the most important resident
macrophages in the lung, act as immune barriers in the alveoli
against several pathogens of the respiratory tract (61). Alveolar macrophages are highly
heterogeneous and exhibit unique phenotypes and functions in the
complex microenvironment of the body (62). They are non-polarized under normal
conditions (63). However,
macrophages are induced and polarized into classical activation
(M1) or alternate activation (M2) phenotypes under the stimulation
of inflammation or in different immune development stages (64,65).
These also play a role in producing different chemokines and
cytokines in the local microenvironment (66).
M2 macrophages are predominantly induced by
cytokines, including IL-4, IL-10 and IL-13, glucocorticoids and
immune complexes TLRs (67).
Similar to ILC2s, they can also induce typical Th2 cytokines to
decrease inflammatory response by promoting angiogenesis, tissue
repairing, remodeling and wound healing (68). In addition, excessive tissue repair
and remodeling results in fibrosis, which can aggravate the
condition (69). M2 macrophages
highly express type I arginase encoding genes (arginase-1, Arg1)
and mannose receptor (CD206), and thus the expression and activity
of Arg1 and CD206 are used to identify M2 macrophages (70). Under the induction of memory Th2
cells, M2 macrophages, which are important immune effector cells,
can scavenge pathogens, which is associated with Arg1 activity
(71). M2 macrophages have a weak
antigen-presenting capacity compared with M1 macrophages, and
downregulate the immune response by secreting inhibitory cytokines,
such as IL-10 and/or tumor growth factor β (TGF-β) (72). A different type of M2 macrophage
exists in the tumor site, which can be induced by IL-10 and is
affected by chemokines, including CCL2, M-CSF and vascular
endothelial growth factor (58).
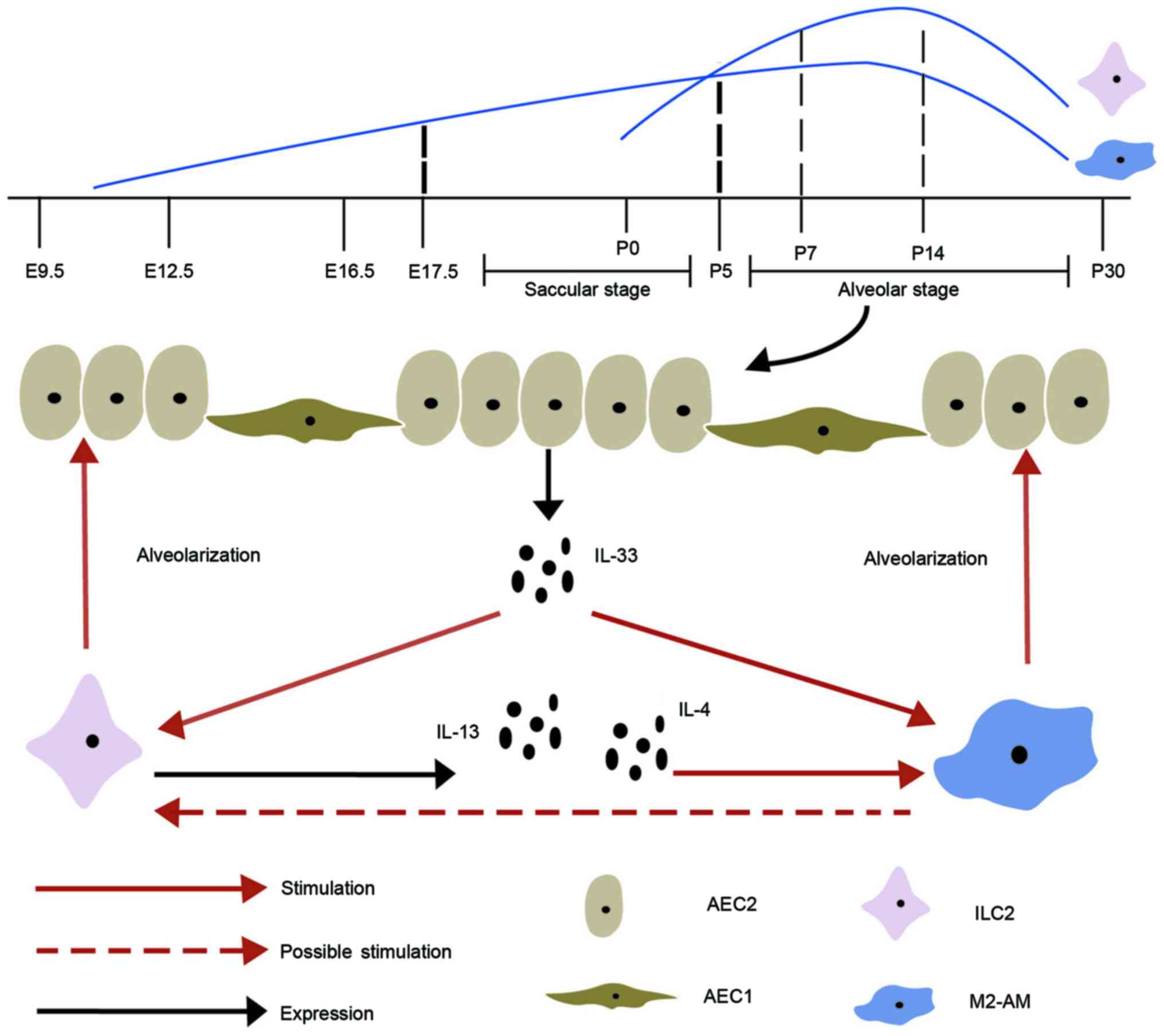
The developmental process of lungs involves complex
steps in humans/mice, and is subdivided into five stages,
embryonic, pseudoglandular, canalicular, saccular and alveolar
(73). Among these, the vesicle
[Embryo day(E)16-E266/E17.5-Postnatal day (P)5] and alveolar
(E252-2 years/P5-P30) stages are important as they affect the
development and maturity of lungs (Fig.
1) (73). Macrophages first
appear on day 10 of pregnancy and can be continuously detected
during fetal lung development (74), which then increases with
alveolarization (75,76). The perinatal period is a critical
window for transferring and distributing congenital immune cells to
all the tissues and organs during lung development (77). ILC2s, which are similar to tissue
macrophages, also appear during pregnancy, but at a later stage,
and most of the peripheral ILC2 pools are produced de novo
following birth (77). Several
studies have confirmed that rapid amplification and activation of
ILC2s in pulmonary occur during the early postnatal period
(78–80). Pulmonary resident ILC2s are minimal
at birth, increase during alveolarization, reach peak at 7–14 days
and subsequently decrease in adulthood, similar to AMs (76,81–83).
Thus, the interactions between ILC2s and macrophages most likely
occur during the vesicle and alveolar stages. Gradually, fewer
ILC2s in lung tissues are replaced by newly generated ILC2s, but
the expanded ILC2s during the early postnatal period account for
the majority of adult lung ILC2s (77).
A previous study revealed that M2 macrophages are
enriched in lung tissues and AEC2 proliferated rapidly following
pneumonectomy (91). ILC2s increase
and become the main source of IL-13, which induces AMs to
differentiate into M2 phenotype (91). Both IL-4R α-expressing ILC2s and M2
macrophages, which are necessary for optimal lung regeneration,
promote the regeneration of lung tissues by stimulating the growth
of AEC2 (91). Rindler et al
(92) reported that M2 macrophages
are clustered together and localized in the site of AEC2
multiplication during regeneration.
It has been reported that activation of IL-33 can
promote type 2 immunity in pulmonary development by amplifying and
activating ILC2s during the perinatal period (81). IL-4, IL-5 and IL-13 exhibit
upregulation after activation of ILC2s, which constitutively
express ST2. In addition to activating ILC2s, IL-33 also stimulate
the expression and polarization of AMs by basophils during alveolar
formation (93,94). Thus, it is hypothesized that IL-33
promotes proliferation and activation of ILC2s and M2 macrophages
during lung development, and crosstalk between ILC2s and M2
macrophages promotes alveolarization. This is consistent with the
IL-33-ST2 axis regulating regeneration of epithelial through
activation of monocyte differentiation into reparative M2
macrophages and ILC2s-mediated M2 macrophages (95). In summary, ILC2s promote the
polarization of M2 macrophages via IL-4/13. In addition to IL-4/13,
there may be other associations between ILC2s and M2 macrophages in
the complex process of embryonic development, which have not been
fully investigated. Thus, future studies are required to determine
how M2 macrophages directly affect ILC2s, and how their crosstalk
promotes fetal and preterm lung development.
The arrest of alveolar development or disruption of
alveolar structure is not only associated with neonatal respiratory
distress syndrome, bronchopulmonary dysplasia and persistent
pulmonary hypertension, but also chronic lung diseases, such as
asthma, allergic diseases and chronic obstructive pulmonary
emphysema (2–4). Pulmonary epithelial barrier
dysfunction is an important pathological component of lung injury,
which is mainly caused by damage of epithelial cell migration
(96). ILC2s participate in the
regulation of AEC2 and different lung diseases (37). M2 macrophages are a subgroup of
macrophages whose polarization is important for AEC2 regulation and
inflammatory response (97). Thus,
the crosstalk between increased ILC2s and upregulated M2
macrophages may regulate lung development, and modulate the
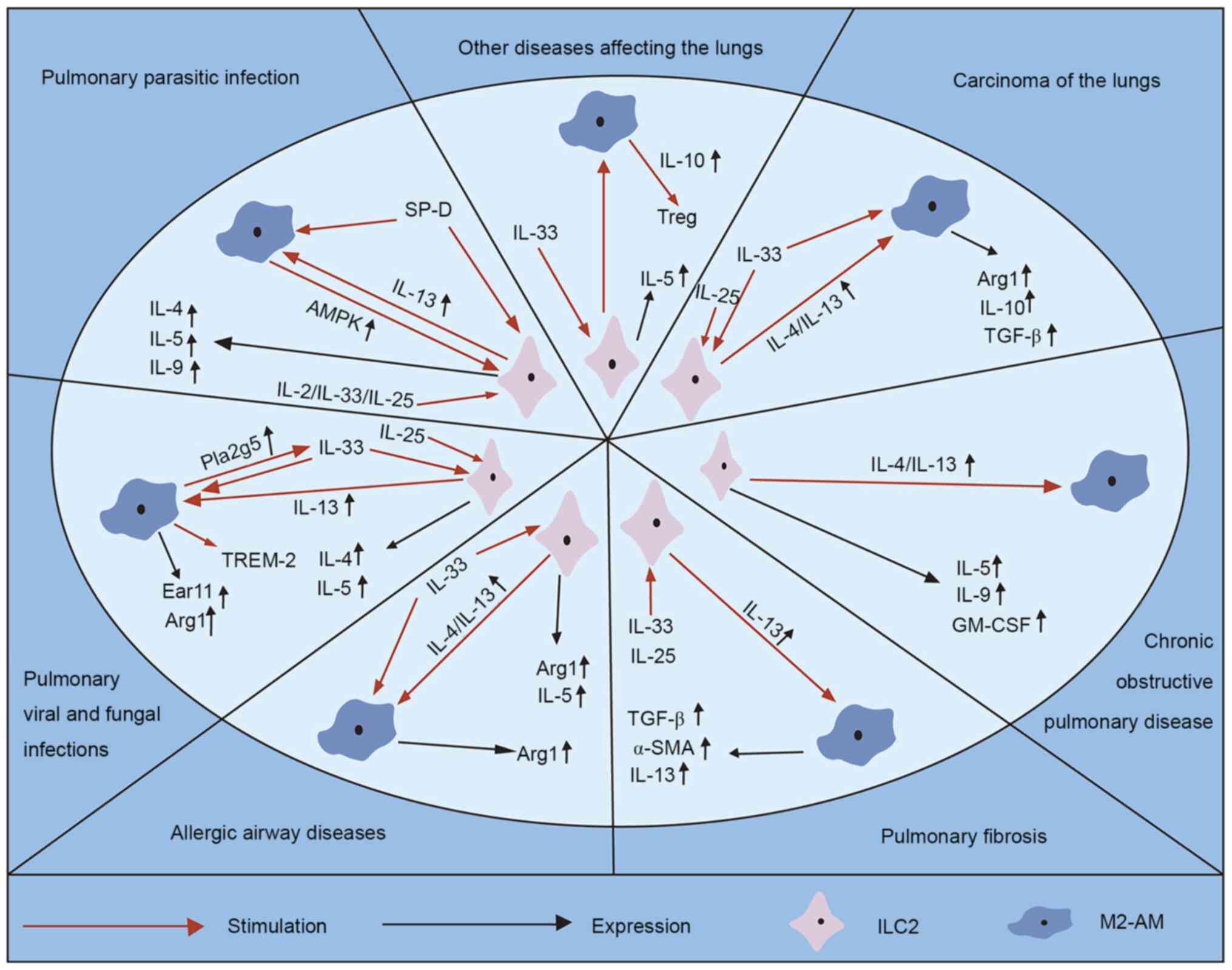
processes of several lung diseases (Fig. 2).
Several parasites, namely pulmonary parasitic
diseases, spread to other parts of the human body via blood
circulation, and often reside in the lungs, causing pathological
changes (98). The host cells of
helminth mega parasites are involved in type 2 immune response,
including Th2 cells and type 2 cytokines (IL-4, IL-5, IL-9 and
IL-13), which are required to fight these pathogens (99,100).
Recently, it has been reported that the relative abundance of these
macrophages and the rare ILC2s have a swift and strong response to
helminth antigen and helminth induced injury, activating damaged
epithelial cells and recruiting other effector factors (101). Immunocompromised larvae of
helminths have a significant morphological defect, which is
affected by aggregation of IL-13-secreting ILC2s and
CD4+ T cells, and the polarization of M2 macrophages
(102). Application of IL-2 or
IL-33 can bypass the requirement of T cells, resulting in
proliferation of IL-13 and secretion of ILC2s and death of larvae,
and exhaustion of ILC2s inhibits larval death in mice by
transferring IL-2 (102). Thus, it
is not surprising that ILC2s are the key factor during infection
and are maintained by CD4+ T cells, which not only
ensure rapid activation of IL-13 dependent M2 macrophages, but also
maintain their immune function in lung tissues (102).
Amp activated protein kinase (AMPK) is a significant
driving factor of cellular energy, which exists in AMs (103). Deletion of AMPK decreases the
secretion of IL-13 and impairs the expansion of ILC2s in lung
tissues from mice that are selectively deprived of α 1 subunit,
thereby exacerbating lung injury following ancylostoma infection
(103). Surfactant protein D
(SP-D) is an important epithelial product (104). Increased levels of pulmonary SP-D
before infection can enhance parasite excretion and type 2 immune
response, including the increase of IL-13-producing ILC2s, M2
macrophages and the cytokines, IL-4 and IL-13 (104). Thus, it is speculated that AMs and
ILC2s assist in coordinating the regulation of mucosal tissue
damage through metabolic enzyme function (103,104).
Several studies have confirmed that the intensity of
infection is affected by type 1 immune response and polarization of
M1 macrophages, while type 2 immunity and polarization of M2
macrophages are closely associated with disease progression and
adverse outcomes (105–107). In infected lungs, the number of
ILC2s significantly increase following induction of type 2 response
(108). ILC2-deficient mice
exhibit a notable declination in type 2 immune response 14 days
after infection, which is characterized by decreased expression
levels of IL-4, IL-5 and IL-13, as well as the number of M2
macrophages (108).
The change in polarization of pulmonary macrophages
in ILC2-deficient mice is frequently associated with better control
of fungi and prolongation of survival time of infected mice
(108). Rhinovirus (RV) infection
also induces IL-25, IL-33, IL-4, IL-5, IL-13 and ILC2s expansion,
mucus metaplasia and airway hyperresponsiveness (109). IL-1 β of pulmonary macrophages
inhibits type 2 inflammation and mucus metaplasia following RV
infection by decreasing ILC2s and cytokines (109).
Group V phospholipase A2 (Pla2g5) is a
lipid-producing enzyme that is required for macrophage functioning
in lung inflammation (110).
Macrophages also assist in regulating IL-33 induction and free
fatty acids (FFAs)-driven ILC2s activation via Pla2g5,
significantly contributing to type-2 immunity (110). In addition, mass spectrometry
analysis demonstrated significant reduction of FFAs in Pla2g5
deficient lung tissues and BM-macrophages in Alternaria-exposed
wild-type mice (110).
Another study reported that type 2 immunoregulatory
neutrophil infiltration is influenced by mouse eosinophil
associated ribonuclease 11, and is secreted by M2 macrophages
downstream of ILC2s that are stimulated by IL-25 (111). Furthermore, neutrophils can
promote type 2 immune response without aggravating inflammation
(111).
Chronic post viral disease is characterized by
excessive airway mucus formation and multiplication of M2
differentiated pulmonary macrophages, requiring expression of
macrophages for triggering receptors on myeloid cell 2 (TREM-2)
(112). With increasing levels of
IL-13, virus replication increases the levels of macrophages and
TREM-2 in the lung tissues, preventing macrophage apoptosis in
acute diseases (112). Following
infection clearance, IL-13 promotes cleavage of TREM-2 into the
soluble form, STREM-2, which prevents macrophage apoptosis
(112). These results may explain
how crosstalk between ILC2s with M2 macrophages in acute infection
results in chronic inflammatory diseases.
Recruitment of neutrophils, eosinophils and
inflammatory chemokines (KC, eotaxin-1, MIP1a and MIP1b), Th2
cytokines (IL-4/5), arginase-1 (M2 macrophage marker) and IL-33R+
ILC2s cells are significantly elevated in adenovirus Oncostatin M
(OSM) mice, while these responses are significantly attenuated in
IL-33-/- mice (113). In
vitro, IL-33 upregulates OSM expression in RAW264.7 macrophage
cells and bone marrow-derived macrophages (113). Thus, IL-33 is a key mediator of
OSM-driven lung inflammation, induction of type 2 immune responses
and M2 macrophages in mice, which contributes via activation of
ILC2s (113).
In addition to the common tissue tropism, AAD also
have obvious inflammatory patterns, including eosinophils, M2
macrophages, ILC2s, IgE secreting B cells and Th2 cells, and
cytokines, including IL-33, IL-4, IL-5 and IL-13 (114,115). Reduction of Th2 cytokines (IL-4,
IL-5 and IL-13), macrophages, ILC2s and other cells in lung
tissues, and alveolar lavage fluid, can improve allergic airway
inflammation in mice, which may be a potential way to treat
allergic asthma (58,116).
Arg1, produced by M2 macrophages, can regulate
asthma and allergic inflammation (117). A study demonstrated that compared
with M2 macrophages expressing Arg1 after activation of STAT6
mediated by IL-4/13, ILC2s constitutively express Arg1 in a
STAT6-independent manner (117).
IL-33 can affect Arg1 in lung tissues by promoting the
proliferation of ILC2s and indirectly activating macrophages via
STAT6 (117). These results
further highlight that ILC2s and M2 macrophages have a synergistic
regulatory effect on asthma and allergic inflammation via Arg1.
During allergic response, the selective depletion of
E3 ligase VHL in innate lymphoid progenitor cells increases hypoxia
inducible factor-1α (HIF-1α) expression, which in turn decreases
ST2 and inhibits the development of ILC2s induced by IL-33 via
epigenetic modification (118).
HIF-1α affects glycolysis and phenotype of macrophages (119), suggesting that HIF-1α acts through
the regulation of ILC2s and macrophages during allergic
reaction.
Idiopathic pulmonary fibrosis is characterized by
fibroblast aggregation, collagen deposition and extracellular
matrix remodeling, in which myofibroblasts are considered effector
cells (72). In the pulmonary
fibrosis model, AMs were recruited into the alveoli, and the
phenotype involves M2 macrophages, which upregulates CD206 on the
cell surface (72). In
vitro, 264.7 cells treated with IL-4 were used as M2
macrophages, and the TGF-β levels in the supernatant significantly
increased. α-SMA expression increased following co-culturing of
lung epithelial cells (MLE-12) with M2 macrophages, suggesting that
M2 macrophages regulate pulmonary fibrosis by inducing
epithelial-to-mesenchymal transition (72).
In addition to the increase of M2 macrophages, the
increase of IL-33, IL-13, TGF-β1 and inflammatory chemokines are
also observed during pulmonary fibrosis (122). IL-13 and TGF-β1 are produced by M2
macrophages, and IL-13 is secreted by ILC2s, both in vivo
and in vitro, and induced by IL-33 (122). As IL-13 can induce the
polarization of M2 macrophages (123), a cycle where IL-13 can be produced
by M2 macrophages and promotes polarization of M2 macrophages is
formed. IL-33 sends signals through ST2, and recruits and guides
inflammatory cell function in ST2- and macrophage-dependent
manners, and enhances the generation of pro-fibrosis cytokines,
thus promoting the occurrence and development of pulmonary fibrosis
(122).
In human COPD, ILCs accumulate in lung tissues, with
increasing signature cytokines, such as IL-5 and GM-CSF (126). The levels of neutrophil elastase
and IL-5 increase in patients with acute exacerbation of COPD
(127), and the levels of IL-13
mRNA in eosinophils and endothelial cells in the sputum also
increase to about 30 times (128).
In addition, Th2 cytokine IL-9 can also aggravate lung injury by
activating STAT3 in COPD mice and increasing inflammation and
oxidative stress (129).
For the interaction of STIP1 homology and U-box-1
(STUB1), IL-4R α is used as the target, which prevents IL-4 or
IL-13 signal transduction via ubiquitination mediated proteasome
degradation (130). In
STUB1-deficient mice, spontaneous airway inflammation increases
IL-4R α expression, STAT6 is continuously activated, M2 macrophages
are activated and serum IgE increases (130). The level of STUB1 in the airway of
patients with asthma or COPD increases, suggesting that
upregulation of STUB1 may be an important feedback mechanism for
inhibiting IL-4R signal transduction in airway inflammation
(130).
In different tumors, type 2 immune responses induce
polarization of M2 macrophages, which in turn enhances the invasion
and migration of tumor cells by secreting Arg1, IL-10 and TGF-β
(107,131,132). The progression of lung cancer is
associated with poor patient prognosis and high mortality (133). The survival rate of tumor-bearing
mice with vitamin A deficiency diet is low, and the tumor size
increases with increasing number of type 2 cytokines, ILC2s and M2
macrophages in BALF of mice, suggesting that ILC2s and polarized M2
macrophages play a synergistic role in promoting cancer progression
(133). This synergistic effect
may be accomplished via two pathways, the co-promotion of ILC2s and
M2-type macrophages by IL-33 (134–136), and the promotion of M2 macrophage
polarization by type 2 cytokines (123,137), such as IL-4 and IL-13, secreted by
ILC2s (138). This is consistent
with the fact that both M2 subtype macrophages (M2a and M2b) and
IL-25-stimulated ILC2s favor cancer progression (139). Notably, other substances that
inhibit the polarization of M2 macrophages by IL-4/13 can change
the tumor microenvironment (140).
However, further studies are required to understand the crosstalk
between ILC2s and M2 macrophages in lung cancer and determine their
underlying molecular mechanisms.
Sepsis is defined as life-threatening organ
dysfunction caused by a dysregulated host response to infection
(141). The lung is an extremely
fragile organ that is prone to sepsis (142). In sepsis model with cecal ligation
and puncture, IL-33 upregulates IL-5 in ILC2s, whereas IL-5
inhibits neutrophil and monocyte infiltration, suggesting that this
axis is involved in lung injury early after sepsis (142). Survivors of sepsis will have
chronically low immune functions (143). IL-33, which is produced following
sepsis, activates ILC2s and promotes the polarization of M2
macrophages, thus accelerating the proliferation of Treg cells
through IL-10 (143).
Subsequently, increased ILC2s, M2 macrophages, IL-10 and Treg cells
result in immunosuppression (143).
Lung resident ILC2s are important immunoregulatory
cells that are involved in metabolism, tissue repair and multiple
organ remodeling, outlining a previously unanticipated role of type
2 immunity in regulating basal homeostasis. Similarly, macrophages
are a group of pluripotent and plasticity immune cells, that also
regulate type 2 immune response. In lungs, AMs and interstitial
macrophages differentiate into different cell phenotypes at
different stages of development, including M1 and M2
macrophages.
The proliferation and activation of ILC2s and M2
macrophages are consistent, and are not only involved in lung
development, but also in lung diseases. In addition, ILC2s and M2
macrophages interact to regulate the lung microenvironment, which
is effective in pulmonary development and pulmonary diseases. The
crosstalk between IL-4R α-expressing ILC2s and upregulated M2
macrophages produces remarkable effects in lung inflammation,
allergy, tumor and fibrosis responses. Further studies are required
to better understand the development, activation, turnover and
interaction between ILC2s and M2 macrophages in lung tissues.
Targeting the IL-33/ILC2s/M2-macrophage axis may be an effective
novel approach for the treatment of several lung diseases.
Not applicable.
The present review was financially supported by the
Natural Science Foundation of Jiangsu Province (grant no.
BK20201226) and the Social Development Foundation of Zhenjiang,
China (grant no. SH2020037).
Not applicable.
LLM and HYL conceived the present study and
performed the literature review. LLM and YZ collected and reviewed
the literature, and drafted the initial manuscript. All authors
confirm the authenticity of all the raw data and critically revised
the manuscript for important intellectual content. LLM and YZ
produced the figures. All authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Domingo-Gonzalez R, Zanini F, Che X, Liu
M, Jones RC, Swift MA, Quake SR, Cornfield DN and Alvira CM:
Diverse homeostatic and immunomodulatory roles of immune cells in
the developing mouse lung at single cell resolution. Elife.
9:e568902020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martinez FD: Early-life origins of chronic
obstructive pulmonary disease. N Engl J Med. 375:871–878. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lange P, Celli B, Agusti A, Boje Jensen G,
Divo M, Faner R, Guerra S, Marott JL, Martinez FD, Martinez-Camblor
P, et al: Lung-function trajectories leading to chronic obstructive
pulmonary disease. N Engl J Med. 373:111–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McGeachie MJ, Yates KP, Zhou X, Guo F,
Sternberg AL, Van Natta ML, Wise RA, Szefler SJ, Sharma S, Kho AT,
et al: Patterns of growth and decline in lung function in
persistent childhood asthma. N Engl J Med. 374:1842–1852. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Loering S, Cameron GJM, Bhatt NP, Belz GT,
Foster PS, Hansbro PM and Starkey MR: Differences in pulmonary
group 2 innate lymphoid cells are dependent on mouse age, sex and
strain. Immunol Cell Biol. Dec 8–2020.(Epub ahead of print). doi:
10.1111/imcb.12430. PubMed/NCBI
|
|
6
|
Lan F, Zhang N, Holtappels G, De Ruyck N,
Krysko O, Van Crombruggen K, Braun H, Johnston SL, Papadopoulos NG,
Zhang L and Bachert C: Staphylococcus aureus induces a mucosal type
2 immune response via epithelial cell-derived cytokines. Am J
Respir Crit Care Med. 198:452–463. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sciurba JC, Gieseck RL, Jiwrajka N, White
SD, Karmele EP, Redes J, Vannella KM, Henderson NC, Wynn TA and
Hart KM: Fibroblast-specific integrin-alpha V differentially
regulates type 17 and type 2 driven inflammation and fibrosis. J
Pathol. 248:16–29. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hajimohammadi B, Athari SM, Abdollahi M,
Vahedi G and Athari SS: Oral administration of acrylamide worsens
the inflammatory responses in the airways of asthmatic mice through
agitation of oxidative stress in the lungs. Front Immunol.
11:19402020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ryan NM and Oghumu S: Role of mast cells
in the generation of a T-helper type 2 dominated anti-helminthic
immune response. Biosci Rep. 39:BSR201817712019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choi JP, Kim YM, Choi HI, Choi SJ, Park
HT, Lee WH, Gho YS, Jee YK, Jeon SG and Kim YK: An important role
of tumor necrosis factor receptor-2 on natural killer T cells on
the development of dsRNA-enhanced Th2 cell response to inhaled
allergens. Allergy. 69:186–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun L, Cornell TT, LeVine A, Berlin AA,
Hinkovska-Galcheva V, Fleszar AJ, Lukacs NW and Shanley TP: Dual
role of interleukin-10 in the regulation of respiratory syncitial
virus (RSV)-induced lung inflammation. Clin Exp Immunol.
172:263–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Helou DG, Shafiei-Jahani P, Lo R, Howard
E, Hurrell BP, Galle-Treger L, Painter JD, Lewis G, Soroosh P,
Sharpe AH and Akbari O: PD-1 pathway regulates ILC2 metabolism and
PD-1 agonist treatment ameliorates airway hyperreactivity. Nat
Commun. 11:39982020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leyva-Castillo JM, Galand C, Mashiko S,
Bissonnette R, McGurk A, Ziegler SF, Dong C, McKenzie ANJ, Sarfati
M and Geha RS: ILC2 activation by keratinocyte-derived IL-25 drives
IL-13 production at sites of allergic skin inflammation. J Allergy
Clin Immunol. 145:1606–1614.e4. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miller MM, Patel PS, Bao K, Danhorn T,
O'Connor BP and Reinhardt RL: BATF acts as an essential regulator
of IL-25-responsive migratory ILC2 cell fate and function. Sci
Immunol. 5:eaay39942020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fort MM, Cheung J, Yen D, Li J, Zurawski
SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, et al: IL-25
induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in
vivo. Immunity. 15:985–995. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hurst SD, Muchamuel T, Gorman DM, Gilbert
JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, et
al: New IL-17 family members promote Th1 or Th2 responses in the
lung: In vivo function of the novel cytokine IL-25. J Immunol.
169:443–453. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oliphant CJ, Hwang YY, Walker JA, Salimi
M, Wong SH, Brewer JM, Englezakis A, Barlow JL, Hams E, Scanlon ST,
et al: MHCII-mediated dialog between group 2 innate lymphoid cells
and CD4(+) T cells potentiates type 2 immunity and promotes
parasitic helminth expulsion. Immunity. 41:283–295. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
She L, Alanazi HH, Yan L, Brooks EG, Dube
PH, Xiang Y, Zhang F, Sun Y, Liu Y, Zhang X and Li XD: Sensing and
signaling of immunogenic extracellular RNAs restrain group 2 innate
lymphoid cell-driven acute lung inflammation and airway
hyperresponsiveness. PLoS One. 15:e02367442020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Entwistle LJ, Gregory LG, Oliver RA,
Branchett WJ, Puttur F and Lloyd CM: Pulmonary group 2 innate
lymphoid cell phenotype is context specific: Determining the effect
of strain, location, and stimuli. Front Immunol. 10:31142019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gieseck RL III, Wilson MS and Wynn TA:
Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol.
18:62–76. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Katsura Y, Harada N, Harada S, Ishimori A,
Makino F, Ito J, Kamachi F, Okumura K, Akiba H, Atsuta R and
Takahashi K: Characteristics of alveolar macrophages from murine
models of OVA-induced allergic airway inflammation and LPS-induced
acute airway inflammation. Exp Lung Res. 41:370–382. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fang SB, Zhang HY, Meng XC, Wang C, He BX,
Peng YQ, Xu ZB, Fan XL, Wu ZJ, Wu ZC, et al: Small extracellular
vesicles derived from human MSCs prevent allergic airway
inflammation via immunomodulation on pulmonary macrophages. Cell
Death Dis. 11:4092020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Su B, Han H, Gong Y, Li X, Ji C, Yao J,
Yang J, Hu W, Zhao W, Li J, et al: Let-7d inhibits intratumoral
macrophage M2 polarization and subsequent tumor angiogenesis by
targeting IL-13 and IL-10. Cancer Immunol Immunother. Nov
25–2020.(Epub ahead of print). doi: 10.1007/s00262-020-02791-6.
View Article : Google Scholar
|
|
24
|
De Salvo C, Buela KA and Pizarro TT:
Cytokine-mediated regulation of innate lymphoid cell plasticity in
gut mucosal immunity. Front Immunol. 11:5853192020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Silver J, Humbles AA and Ohne Y:
Isolation, culture, and induction of plasticity in ILC2s. Methods
Mol Biol. 2121:115–127. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vacca P, Chiossone L, Mingari MC and
Moretta L: Heterogeneity of NK cells and other innate lymphoid
cells in human and murine decidua. Front Immunol. 10:1702019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li S, Bostick JW, Ye J, Qiu J, Zhang B,
Urban JF Jr, Avram D and Zhou L: Aryl hydrocarbon receptor
signaling cell intrinsically inhibits intestinal group 2 innate
lymphoid cell function. Immunity. 49:915–928.e5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Klose CS and Artis D: Innate lymphoid
cells as regulators of immunity, inflammation and tissue
homeostasis. Nat Immunol. 17:765–774. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kabata H, Moro K and Koyasu S: The group 2
innate lymphoid cell (ILC2) regulatory network and its underlying
mechanisms. Immunol Rev. 286:37–52. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pasha MA, Patel G, Hopp R and Yang Q: Role
of innate lymphoid cells in allergic diseases. Allergy Asthma Proc.
40:138–145. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Monticelli LA, Sonnenberg GF, Abt MC,
Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ,
Yang CY, Sathaliyawala T, et al: Innate lymphoid cells promote
lung-tissue homeostasis after infection with influenza virus. Nat
Immunol. 12:1045–1054. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Simoni Y, Fehlings M, Kloverpris HN,
McGovern N, Koo SL, Loh CY, Lim S, Kurioka A, Fergusson JR, Tang
CL, et al: Human innate lymphoid cell subsets possess tissue-type
based heterogeneity in phenotype and frequency. Immunity.
48:10602018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Camelo A, Rosignoli G, Ohne Y, Stewart RA,
Overed-Sayer C, Sleeman MA and May RD: IL-33, IL-25, and TSLP
induce a distinct phenotypic and activation profile in human type 2
innate lymphoid cells. Blood Adv. 1:577–589. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang Y, Guo L, Qiu J, Chen X, Hu-Li J,
Siebenlist U, Williamson PR, Urban JF Jr and Paul WE:
IL-25-responsive, lineage-negative KLRG1(hi) cells are
multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat
Immunol. 16:161–169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Chen S, Chi Y, Yang Y, Chen X, Wang
H, Lv Z, Wang J, Yuan L, Huang P, et al: Kinetics of the
accumulation of group 2 innate lymphoid cells in IL-33-induced and
IL-25-induced murine models of asthma: A potential role for the
chemokine CXCL16. Cell Mol Immunol. 16:75–86. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Salimi M, Barlow JL, Saunders SP, Xue L,
Gutowska-Owsiak D, Wang X, Huang LC, Johnson D, Scanlon ST,
McKenzie AN, et al: A role for IL-25 and IL-33-driven type-2 innate
lymphoid cells in atopic dermatitis. J Exp Med. 210:2939–2950.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mohapatra A, Van Dyken SJ, Schneider C,
Nussbaum JC, Liang HE and Locksley RM: Group 2 innate lymphoid
cells utilize the IRF4-IL-9 module to coordinate epithelial cell
maintenance of lung homeostasis. Mucosal Immunol. 9:275–286. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Moretti S, Renga G, Oikonomou V, Galosi C,
Pariano M, Iannitti RG, Borghi M, Puccetti M, De Zuani M, Pucillo
CE, et al: A mast cell-ILC2-Th9 pathway promotes lung inflammation
in cystic fibrosis. Nat Commun. 8:140172017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wilhelm C, Hirota K, Stieglitz B, Van
Snick J, Tolaini M, Lahl K, Sparwasser T, Helmby H and Stockinger
B: An IL-9 fate reporter demonstrates the induction of an innate
IL-9 response in lung inflammation. Nat Immunol. 12:1071–1077.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bartemes KR, Kephart GM, Fox SJ and Kita
H: Enhanced innate type 2 immune response in peripheral blood from
patients with asthma. J Allergy Clin Immunol. 134:671–678.e4. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Motomura Y, Morita H, Moro K, Nakae S,
Artis D, Endo TA, Kuroki Y, Ohara O, Koyasu S and Kubo M:
Basophil-derived interleukin-4 controls the function of natural
helper cells, a member of ILC2s, in lung inflammation. Immunity.
40:758–771. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Matsuki A, Takatori H, Makita S, Yokota M,
Tamachi T, Suto A, Suzuki K, Hirose K and Nakajima H: T-bet
inhibits innate lymphoid cell-mediated eosinophilic airway
inflammation by suppressing IL-9 production. J Allergy Clin
Immunol. 139:1355–1367.e6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang K, Jin Y, Lai D, Wang J, Wang Y, Wu
X, Scott M, Li Y, Hou J, Billiar T, et al: RAGE-induced ILC2
expansion in acute lung injury due to haemorrhagic shock. Thorax.
75:209–219. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ishii T, Muroi M, Horiguchi K, Tanamoto
KI, Nagase T and Yamashita N: Activation through toll-like receptor
2 on group 2 innate lymphoid cells can induce asthmatic
characteristics. Clin Exp Allergy. 49:1624–1632. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Maggi L, Montaini G, Mazzoni A, Rossettini
B, Capone M, Rossi MC, Santarlasci V, Liotta F, Rossi O, Gallo O,
et al: Human circulating group 2 innate lymphoid cells can express
CD154 and promote IgE production. J Allergy Clin Immunol.
139:964–976.e4. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gury-BenAri M, Thaiss CA, Serafini N,
Winter DR, Giladi A, Lara-Astiaso D, Levy M, Salame TM, Weiner A,
David E, et al: The spectrum and regulatory landscape of intestinal
innate lymphoid cells are shaped by the microbiome. Cell.
166:1231–1246.e13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Robinette ML, Fuchs A, Cortez VS, Lee JS,
Wang Y, Durum SK, Gilfillan S and Colonna M; Immunological Genome
Consortium, : Transcriptional programs define molecular
characteristics of innate lymphoid cell classes and subsets. Nat
Immunol. 16:306–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim HS, Jang JH, Lee MB, Jung ID, Kim YM,
Park YM and Choi WS: A novel IL-10-producing innate lymphoid cells
(ILC10) in a contact hypersensitivity mouse model. BMB Rep.
49:293–296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wallrapp A, Burkett PR, Riesenfeld SJ, Kim
SJ, Christian E, Abdulnour RE, Thakore PI, Schnell A, Lambden C,
Herbst RH, et al: Calcitonin gene-related peptide negatively
regulates alarmin-driven type 2 innate lymphoid cell responses.
Immunity. 51:709–723.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ho J, Bailey M, Zaunders J, Mrad N, Sacks
R, Sewell W and Harvey RJ: Group 2 innate lymphoid cells (ILC2s)
are increased in chronic rhinosinusitis with nasal polyps or
eosinophilia. Clin Exp Allergy. 45:394–403. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jeffery HC, McDowell P, Lutz P, Wawman RE,
Roberts S, Bagnall C, Birtwistle J, Adams DH and Oo YH: Human
intrahepatic ILC2 are IL-13positive amphiregulinpositive and their
frequency correlates with model of end stage liver disease score.
PLoS One. 12:e01886492017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Campbell L, Hepworth MR, Whittingham-Dowd
J, Thompson S, Bancroft AJ, Hayes KS, Shaw TN, Dickey BF, Flamar
AL, Artis D, et al: ILC2s mediate systemic innate protection by
priming mucus production at distal mucosal sites. J Exp Med.
216:2714–2723. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
D'Souza SS, Shen X, Fung ITH, Ye L,
Kuentzel M, Chittur SV, Furuya Y, Siebel CW, Maillard IP, Metzger
DW and Yang Q: Compartmentalized effects of aging on group 2 innate
lymphoid cell development and function. Aging Cell. 18:e130192019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ghaedi M, Shen ZY, Orangi M,
Martinez-Gonzalez I, Wei L, Lu X, Das A, Heravi-Moussavi A, Marra
MA, Bhandoola A and Takei F: Single-cell analysis of RORα tracer
mouse lung reveals ILC progenitors and effector ILC2 subsets. J Exp
Med. 217:jem.20182293. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Steer CA, Matha L, Shim H and Takei F:
Lung group 2 innate lymphoid cells are trained by endogenous IL-33
in the neonatal period. JCI Insight. 5:e1359612020. View Article : Google Scholar
|
|
56
|
Lindquist RL, Bayat-Sarmadi J, Leben R,
Niesner R and Hauser AE: NAD(P)H oxidase activity in the small
intestine is predominantly found in enterocytes, not professional
phagocytes. Int J Mol Sci. 19:13652018. View Article : Google Scholar
|
|
57
|
Vellozo NS, Pereira-Marques ST,
Cabral-Piccin MP, Filardy AA, Ribeiro-Gomes FL, Rigoni TS, DosReis
GA and Lopes MF: All-trans retinoic acid promotes an M1- to
M2-phenotype shift and inhibits macrophage-mediated immunity to
leishmania major. Front Immunol. 8:15602017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Moreira AP, Cavassani KA, Hullinger R,
Rosada RS, Fong DJ, Murray L, Hesson DP and Hogaboam CM: Serum
amyloid P attenuates M2 macrophage activation and protects against
fungal spore-induced allergic airway disease. J Allergy Clin
Immunol. 126:712–721.e7. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wu Y and Hirschi KK: Tissue-resident
macrophage development and function. Front Cell Dev Biol.
8:6178792020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu G, Zhai H, Zhang T, Li S, Li N, Chen
J, Gu M, Qin Z and Liu X: New therapeutic strategies for IPF: Based
on the ‘phagocytosis-secretion-immunization’ network regulation
mechanism of pulmonary macrophages. Biomed Pharmacother.
118:1092302019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li R, Shang Y, Hu X, Yu Y, Zhou T, Xiong W
and Zou X: ATP/P2X7r axis mediates the pathological process of
allergic asthma by inducing M2 polarization of alveolar
macrophages. Exp Cell Res. 386:1117082020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ke X, Chen C, Song Y, Cai Q, Li J, Tang Y,
Han X, Qu W, Chen A, Wang H, et al: Hypoxia modifies the
polarization of macrophages and their inflammatory
microenvironment, and inhibits malignant behavior in cancer cells.
Oncol Lett. 18:5871–5878. 2019.PubMed/NCBI
|
|
63
|
Bazzan E, Turato G, Tine M, Radu CM,
Balestro E, Rigobello C, Biondini D, Schiavon M, Lunardi F, Baraldo
S, et al: Dual polarization of human alveolar macrophages
progressively increases with smoking and COPD severity. Respir Res.
18:402017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lin F, Song C, Zeng Y, Li Y, Li H, Liu B,
Dai M and Pan P: Canagliflozin alleviates LPS-induced acute lung
injury by modulating alveolar macrophage polarization. Int
Immunopharmacol. 88:1069692020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Soliman E, Elhassanny AE, Malur A, McPeek
M, Bell A, Leffler N, Van Dross R, Jones JL, Malur AG and Thomassen
MJ: Impaired mitochondrial function of alveolar macrophages in
carbon nanotube-induced chronic pulmonary granulomatous disease.
Toxicology. 445:1525982020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Nenasheva T, Gerasimova T, Serdyuk Y,
Grigor'eva E, Kosmiadi G, Nikolaev A, Dashinimaev E and Lyadova I:
Macrophages derived from human induced pluripotent stem cells are
low-activated ‘Naive-Like’ cells capable of restricting
mycobacteria growth. Front Immunol. 11:10162020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang L, Wang Y, Wu G, Xiong W, Gu W and
Wang CY: Macrophages: Friend or foe in idiopathic pulmonary
fibrosis? Respir Res. 19:1702018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bronte V and Zanovello P: Regulation of
immune responses by L-arginine metabolism. Nat Rev Immunol.
5:641–654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Grabarz F, Aguiar CF, Correa-Costa M,
Braga TT, Hyane MI, Andrade-Oliveira V, Landgraf MA and Camara NOS:
Protective role of NKT cells and macrophage M2-driven phenotype in
bleomycin-induced pulmonary fibrosis. Inflammopharmacology.
26:491–504. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
de Campos GY, Oliveira RA, Oliveira-Brito
PK, Roque-Barreira MC and da Silva TA: Pro-inflammatory response
ensured by LPS and Pam3CSK4 in RAW 264.7 cells did not improve a
fungistatic effect on Cryptococcus gattii infection. PeerJ.
8:e102952020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Anthony RM, Urban JF Jr, Alem F, Hamed HA,
Rozo CT, Boucher JL, Van Rooijen N and Gause WC: Memory T(H)2 cells
induce alternatively activated macrophages to mediate protection
against nematode parasites. Nat Med. 12:955–960. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhu L, Fu X, Chen X, Han X and Dong P: M2
macrophages induce EMT through the TGF-beta/Smad2 signaling
pathway. Cell Biol Int. 41:960–968. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Loering S, Cameron GJ, Starkey MR and
Hansbro PM: Lung development and emerging roles for type 2
immunity. J Pathol. 247:686–696. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Blackwell TS, Hipps AN, Yamamoto Y, Han W,
Barham WJ, Ostrowski MC, Yull FE and Prince LS: NF-kappaB signaling
in fetal lung macrophages disrupts airway morphogenesis. J Immunol.
187:2740–2747. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Jones CV, Williams TM, Walker KA,
Dickinson H, Sakkal S, Rumballe BA, Little MH, Jenkin G and Ricardo
SD: M2 macrophage polarisation is associated with alveolar
formation during postnatal lung development. Respir Res. 14:412013.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Saluzzo S, Gorki AD, Rana BMJ, Martins R,
Scanlon S, Starkl P, Lakovits K, Hladik A, Korosec A, Sharif O, et
al: First-breath-induced type 2 pathways shape the lung immune
environment. Cell Rep. 18:1893–1905. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Schneider C, Lee J, Koga S,
Ricardo-Gonzalez RR, Nussbaum JC, Smith LK, Villeda SA, Liang HE
and Locksley RM: Tissue-resident group 2 innate lymphoid cells
differentiate by layered ontogeny and in situ perinatal priming.
Immunity. 50:1425–1438.e5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Huang Y, Mao K, Chen X, Sun MA, Kawabe T,
Li W, Usher N, Zhu J, Urban JF Jr, Paul WE and Germain RN:
S1P-dependent interorgan trafficking of group 2 innate lymphoid
cells supports host defense. Science. 359:114–119. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Steer CA, Martinez-Gonzalez I, Ghaedi M,
Allinger P, Matha L and Takei F: Group 2 innate lymphoid cell
activation in the neonatal lung drives type 2 immunity and allergen
sensitization. J Allergy Clin Immunol. 140:593–595.e3. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Nussbaum JC, Van Dyken SJ, von Moltke J,
Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla
A, Liang HE and Locksley RM: Type 2 innate lymphoid cells control
eosinophil homeostasis. Nature. 502:245–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
de Kleer IM, Kool M, de Bruijn MJ, Willart
M, van Moorleghem J, Schuijs MJ, Plantinga M, Beyaert R, Hams E,
Fallon PG, et al: Perinatal activation of the interleukin-33
pathway promotes type 2 immunity in the developing lung. Immunity.
45:1285–1298. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ghaedi M, Steer CA, Martinez-Gonzalez I,
Halim TYF, Abraham N and Takei F:
Common-lymphoid-progenitor-independent pathways of innate and T
lymphocyte development. Cell Rep. 15:471–480. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Sahoo D, Zaramela LS, Hernandez GE, Mai U,
Taheri S, Dang D, Stouch AN, Medal RM, McCoy AM, Aschner JL, et al:
Transcriptional profiling of lung macrophages identifies a
predictive signature for inflammatory lung disease in preterm
infants. Commun Biol. 3:2592020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ubags NDJ, Alejandre Alcazar MA, Kallapur
SG, Knapp S, Lanone S, Lloyd CM, Morty RE, Pattaroni C, Reynaert
NL, Rottier RJ, et al: Early origins of lung disease: Towards an
interdisciplinary approach. Eur Respir Rev. 29:2001912020.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Obata-Ninomiya K, Ishiwata K, Tsutsui H,
Nei Y, Yoshikawa S, Kawano Y, Minegishi Y, Ohta N, Watanabe N,
Kanuka H and Karasuyama H: The skin is an important bulwark of
acquired immunity against intestinal helminths. J Exp Med.
210:2583–2595. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Minutti CM, Jackson-Jones LH,
Garcia-Fojeda B, Knipper JA, Sutherland TE, Logan N, Ringqvist E,
Guillamat-Prats R, Ferenbach DA, Artigas A, et al: Local amplifiers
of IL-4Rα-mediated macrophage activation promote repair in lung and
liver. Science. 356:1076–1080. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Chen S, Kammerl IE, Vosyka O, Baumann T,
Yu Y, Wu Y, Irmler M, Overkleeft HS, Beckers J, Eickelberg O, et
al: Immunoproteasome dysfunction augments alternative polarization
of alveolar macrophages. Cell Death Differ. 23:1026–1037. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Kim J, Chang Y, Bae B, Sohn KH, Cho SH,
Chung DH, Kang HR and Kim HY: Innate immune crosstalk in asthmatic
airways: Innate lymphoid cells coordinate polarization of lung
macrophages. J Allergy Clin Immunol. 143:1769–1782.e11. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
King SD and Chen SY: Recent progress on
surfactant protein A: cellular function in lung and kidney disease
development. Am J Physiol Cell Physiol. 319:C316–C320. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Buckley S, Bui KC, Hussain M and Warburton
D: Dynamics of TGF-beta 3 peptide activity during rat alveolar
epithelial cell proliferative recovery from acute hyperoxia. Am J
Physiol. 271:L54–L60. 1996.PubMed/NCBI
|
|
91
|
Lechner AJ, Driver IH, Lee J, Conroy CM,
Nagle A, Locksley RM and Rock JR: Recruited monocytes and type 2
immunity promote lung regeneration following pneumonectomy. Cell
Stem Cell. 21:120–134.e7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Rindler TN, Stockman CA, Filuta AL, Brown
KM, Snowball JM, Zhou W, Veldhuizen R, Zink EM, Dautel SE, Clair G,
et al: Alveolar injury and regeneration following deletion of
ABCA3. JCI Insight. 2:e973812017. View Article : Google Scholar
|
|
93
|
Kurowska-Stolarska M, Stolarski B, Kewin
P, Murphy G, Corrigan CJ, Ying S, Pitman N, Mirchandani A, Rana B,
van Rooijen N, et al: IL-33 amplifies the polarization of
alternatively activated macrophages that contribute to airway
inflammation. J Immunol. 183:6469–6477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Cohen M, Giladi A, Gorki AD, Solodkin DG,
Zada M, Hladik A, Miklosi A, Salame TM, Halpern KB, David E, et al:
Lung single-cell signaling interaction map reveals basophil role in
macrophage imprinting. Cell. 175:1031–1044.e18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Dagher R, Copenhaver AM, Besnard V, Berlin
A, Hamidi F, Maret M, Wang J, Qu X, Shrestha Y, Wu J, et al:
IL-33-ST2 axis regulates myeloid cell differentiation and
activation enabling effective club cell regeneration. Nat Commun.
11:47862020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Silva JD, Su Y, Calfee CS, Delucchi KL,
Weiss D, McAuley DF, O'Kane C and Krasnodembskaya AD: MSC
extracellular vesicles rescue mitochondrial dysfunction and improve
barrier integrity in clinically relevant models of ARDS. Eur Respir
J. Dec 17–2020.(Epub ahead of print). doi:
10.1183/13993003.02978-2020. View Article : Google Scholar
|
|
97
|
Duan F, Guo L, Yang L, Han Y, Thakur A,
Nilsson-Payant BE, Wang P, Zhang Z, Ma CY, Zhou X, et al: Modeling
COVID-19 with human pluripotent stem cell-derived cells reveals
synergistic effects of anti-inflammatory macrophages with ACE2
inhibition against SARS-CoV-2. Res Sq. Aug 20–2020.(Epub ahead of
print). doi: 10.21203/rs.3.rs-62758/v1. PubMed/NCBI
|
|
98
|
Sersar SI, Elnahas HA, Saleh AB, Moussa SA
and Ghafar WA: Pulmonary parasitosis: Applied clinical and
therapeutic issues. Heart Lung Circ. 15:24–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Miller MM and Reinhardt RL: The
heterogeneity, origins, and impact of migratory iILC2 cells in
anti-helminth immunity. Front Immunol. 11:15942020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Meiners J, Reitz M, Rudiger N, Turner JE,
Heepmann L, Rudolf L, Hartmann W, McSorley HJ and Breloer M: IL-33
facilitates rapid expulsion of the parasitic nematode Strongyloides
ratti from the intestine via ILC2- and IL-9-driven mast cell
activation. PLoS Pathog. 16:e10091212020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Webb LM and Tait Wojno ED: The role of
rare innate immune cells in Type 2 immune activation against
parasitic helminths. Parasitology. 144:1288–1301. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Bouchery T, Kyle R, Camberis M, Shepherd
A, Filbey K, Smith A, Harvie M, Painter G, Johnston K, Ferguson P,
et al: ILC2s and T cells cooperate to ensure maintenance of M2
macrophages for lung immunity against hookworms. Nat Commun.
6:69702015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Nieves W, Hung LY, Oniskey TK, Boon L,
Foretz M, Viollet B and Herbert DR: Myeloid-restricted AMPKα1
promotes host immunity and protects against IL-12/23p40-dependent
lung injury during hookworm infection. J Immunol. 196:4632–4640.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Thawer S, Auret J, Schnoeller C, Chetty A,
Smith K, Darby M, Roberts L, Mackay RM, Whitwell HJ, Timms JF, et
al: Surfactant protein-D is essential for immunity to helminth
infection. PLoS Pathog. 12:e10054612016. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Snietura M, Brewczynski A, Kopec A and
Rutkowski T: Infiltrates of M2-like tumour-associated macrophages
are adverse prognostic factor in patients with human
papillomavirus-negative but not in human papillomavirus-positive
oropharyngeal squamous cell carcinoma. Pathobiology. 87:75–86.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Yan C, Wu J, Xu N, Li J, Zhou QY, Yang HM,
Cheng XD, Liu JX, Dong X, Koda S, et al: TLR4 deficiency
exacerbates biliary injuries and peribiliary fibrosis caused by
clonorchis sinensis in a resistant mouse strain. Front Cell Infect
Microbiol. 10:5269972021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wang H, Zhang CS, Fang BB, Hou J, Li WD,
Li ZD, Li L, Bi XJ, Li L, Abulizi A, et al: Dual role of hepatic
macrophages in the establishment of the echinococcus multilocularis
metacestode in mice. Front Immunol. 11:6006352021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Kindermann M, Knipfer L, Obermeyer S,
Muller U, Alber G, Bogdan C, Schleicher U, Neurath MF and Wirtz S:
Group 2 innate lymphoid cells (ILC2) suppress beneficial type 1
immune responses during pulmonary cryptococcosis. Front Immunol.
11:2092020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Han M, Ishikawa T, Bermick JR, Rajput C,
Lei J, Goldsmith AM, Jarman CR, Lee J, Bentley JK and Hershenson
MB: IL-1β prevents ILC2 expansion, type 2 cytokine secretion, and
mucus metaplasia in response to early-life rhinovirus infection in
mice. Allergy. 75:2005–2019. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Yamaguchi M, Samuchiwal SK, Quehenberger
O, Boyce JA and Balestrieri B: Macrophages regulate lung ILC2
activation via Pla2g5-dependent mechanisms. Mucosal Immunol.
11:615–626. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Panova V, Gogoi M, Rodriguez-Rodriguez N,
Sivasubramaniam M, Jolin HE, Heycock MWD, Walker JA, Rana BM,
Drynan LF, Hodskinson M, et al: Group-2 innate lymphoid
cell-dependent regulation of tissue neutrophil migration by
alternatively activated macrophage-secreted Ear11. Mucosal Immunol.
14:26–37. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wu K, Byers DE, Jin X, Agapov E,
Alexander-Brett J, Patel AC, Cella M, Gilfilan S, Colonna M, Kober
DL, et al: TREM-2 promotes macrophage survival and lung disease
after respiratory viral infection. J Exp Med. 212:681–697. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Botelho F, Dubey A, Ayaub EA, Park R, Yip
A, Humbles A, Kolbeck R and Richards CD: IL-33 mediates lung
inflammation by the IL-6-type cytokine oncostatin M. Mediators
Inflamm. 2020:40873152020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Pei W, Zhang Y, Li X, Luo M, Chen T, Zhang
M, Zhong M and Lv K: LncRNA AK085865 depletion ameliorates
asthmatic airway inflammation by modulating macrophage
polarization. Int Immunopharmacol. 83:1064502020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Cai H, Wang J, Mo Y, Ye L, Zhu G, Song X,
Zhu M, Xue X, Yang C and Jin M: Salidroside suppresses group 2
innate lymphoid cell-mediated allergic airway inflammation by
targeting IL-33/ST2 axis. Int Immunopharmacol. 81:1062432020.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Nagashima R, Kosai H, Masuo M, Izumiyama
K, Noshikawaji T, Morimoto M, Kumaki S, Miyazaki Y, Motohashi H,
Yamamoto M and Tanaka N: Nrf2 suppresses allergic lung inflammation
by attenuating the type 2 innate lymphoid cell response. J Immunol.
202:1331–1339. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Bando JK, Nussbaum JC, Liang HE and
Locksley RM: Type 2 innate lymphoid cells constitutively express
arginase-I in the naive and inflamed lung. J Leukoc Biol.
94:877–884. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Li Q, Li D, Zhang X, Wan Q, Zhang W, Zheng
M, Zou L, Elly C, Lee JH and Liu YC: E3 Ligase VHL promotes group 2
innate lymphoid cell maturation and function via glycolysis
inhibition and induction of interleukin-33 receptor. Immunity.
48:258–270.e5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Liu J, Qiu P, Qin J, Wu X, Wang X, Yang X,
Li B, Zhang W, Ye K, Peng Z and Lu X: Allogeneic adipose-derived
stem cells promote ischemic muscle repair by inducing M2 macrophage
polarization via the HIF-1α/IL-10 pathway. Stem Cells.
38:1307–1320. 2020.PubMed/NCBI
|
|
120
|
Scoville DK, Nolin JD, Ogden HL, An D,
Afsharinejad Z, Johnson BW, Bammler TK, Gao X, Frevert CW,
Altemeier WA, et al: Quantum dots and mouse strain influence house
dust mite-induced allergic airway disease. Toxicol Appl Pharmacol.
368:55–62. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Schuijs MJ, Hammad H and Lambrecht BN:
Professional and ‘Amateur’ Antigen-presenting cells in type 2
immunity. Trends Immunol. 40:22–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Li D, Guabiraba R, Besnard AG, Komai-Koma
M, Jabir MS, Zhang L, Graham GJ, Kurowska-Stolarska M, Liew FY,
McSharry C and Xu D: IL-33 promotes ST2-dependent lung fibrosis by
the induction of alternatively activated macrophages and innate
lymphoid cells in mice. J Allergy Clin Immunol. 134:1422–1432.e11.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Park HJ, Chi GY, Choi YH and Park SH:
Lupeol suppresses plasminogen activator inhibitor-1-mediated
macrophage recruitment and attenuates M2 macrophage polarization.
Biochem Biophys Res Commun. 527:889–895. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Zhao Y, De Los Santos FG, Wu Z, Liu T and
Phan SH: An ST2-dependent role of bone marrow-derived group 2
innate lymphoid cells in pulmonary fibrosis. J Pathol. 245:399–409.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Hams E, Armstrong ME, Barlow JL, Saunders
SP, Schwartz C, Cooke G, Fahy RJ, Crotty TB, Hirani N, Flynn RJ, et
al: IL-25 and type 2 innate lymphoid cells induce pulmonary
fibrosis. Proc Natl Acad Sci USA. 111:367–372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
De Grove KC, Provoost S, Verhamme FM,
Bracke KR, Joos GF, Maes T and Brusselle GG: Characterization and
quantification of innate lymphoid cell subsets in human lung. PLoS
One. 11:e01459612016. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Pouwels SD, Zijlstra GJ, van der Toorn M,
Hesse L, Gras R, Ten Hacken NH, Krysko DV, Vandenabeele P, de Vries
M, van Oosterhout AJ, et al: Cigarette smoke-induced necroptosis
and DAMP release trigger neutrophilic airway inflammation in mice.
Am J Physiol Lung Cell Mol Physiol. 310:L377–L386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Hershenson MB: Rhinovirus-induced
exacerbations of asthma and COPD. Scientifica (Cairo).
2013:4058762013.PubMed/NCBI
|
|
129
|
Zou SC, Pang LL, Mao QS, Wu SY and Xiao
QF: IL-9 exacerbates the development of chronic obstructive
pulmonary disease through oxidative stress. Eur Rev Med Pharmacol
Sci. 22:8877–8884. 2018.PubMed/NCBI
|
|
130
|
Wei Q, Sha Y, Bhattacharya A, Abdel Fattah
E, Bonilla D, Jyothula SS, Pandit L, Khurana Hershey GK and Eissa
NT: Regulation of IL-4 receptor signaling by STUB1 in lung
inflammation. Am J Respir Crit Care Med. 189:16–29. 2014.PubMed/NCBI
|
|
131
|
Saha J, Sarkar D, Pramanik A, Mahanti K,
Adhikary A and Bhattacharyya S: PGE2-HIF1α reciprocal induction
regulates migration, phenotypic alteration and immunosuppressive
capacity of macrophages in tumor microenvironment. Life Sci.
253:1177312020. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Lu Q, Wang X, Zhu J, Fei X, Chen H and Li
C: Hypoxic tumor-derived exosomal Circ0048117 facilitates M2
macrophage polarization acting as miR-140 sponge in esophageal
squamous cell carcinoma. Onco Targets Ther. 13:11883–11897. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Cui W, Zhang W, Yuan X, Liu S, Li M, Niu
J, Zhang P and Li D: Vitamin A deficiency execrates Lewis lung
carcinoma via induction of type 2 innate lymphoid cells and
alternatively activates macrophages. Food Sci Nutr. 7:1288–1294.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Robbins SM and Senger DL: To promote or
inhibit glioma progression, that is the question for IL-33. Cell
Stress. 5:19–22. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Mai S, Liu L, Jiang J, Ren P, Diao D, Wang
H and Cai K: Oesophageal squamous cell carcinoma-associated IL-33
rewires macrophage polarization towards M2 via activating ornithine
decarboxylase. Cell Prolif. 54:e129602021. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Li J, Razumilava N, Gores GJ, Walters S,
Mizuochi T, Mourya R, Bessho K, Wang YH, Glaser SS, Shivakumar P
and Bezerra JA: Biliary repair and carcinogenesis are mediated by
IL-33-dependent cholangiocyte proliferation. J Clin Invest.
124:3241–3251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Yang Y, Xia S, Zhang L, Wang W, Chen L and
Zhan W: MiR-324-5p/PTPRD/CEBPD axis promotes papillary thyroid
carcinoma progression via microenvironment alteration. Cancer Biol
Ther. 21:522–532. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
You Y, Zhang X, Wang X, Yue D, Meng F, Zhu
J, Wang Y and Sun X: ILC2 Proliferated by IL-33 stimulation
alleviates acute colitis in Rag1(−/-) Mouse through promoting M2
macrophage polarization. J Immunol Res. 2020:50189752020.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Della Valle L, Gatta A, Farinelli A,
Scarano G, Lumaca A, Tinari N, Cipollone F, Paganelli R and Di
Gioacchino M: Allergooncology: An expanding research area. J Biol
Regul Homeost Agents. 34:319–326. 2020.PubMed/NCBI
|
|
140
|
Park HJ, Chi GY, Choi YH and Park SH: The
root bark of Morus alba L. regulates tumor-associated macrophages
by blocking recruitment and M2 polarization of macrophages.
Phytother Res. 34:3333–3344. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Esposito S, De Simone G, Boccia G, De Caro
F and Pagliano P: Sepsis and septic shock: New definitions, new
diagnostic and therapeutic approaches. J Glob Antimicrob Resist.
10:204–212. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Xu H, Xu J, Xu L, Jin S, Turnquist HR,
Hoffman R, Loughran P, Billiar TR and Deng M: Interleukin-33
contributes to ILC2 activation and early inflammation-associated
lung injury during abdominal sepsis. Immunol Cell Biol. 96:935–947.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Nascimento DC, Melo PH, Pineros AR,
Ferreira RG, Colon DF, Donate PB, Castanheira FV, Gozzi A,
Czaikoski PG, Niedbala W, et al: IL-33 contributes to
sepsis-induced long-term immunosuppression by expanding the
regulatory T cell population. Nat Commun. 8:149192017. View Article : Google Scholar : PubMed/NCBI
|