Introduction
Glioma tumors originate from the glial cells of the
brain or spine (1), with various
oncogenes serving a role in its development (2). Certain angiogenic blockers, including
bevacizumab, have been used in combination with conventional
chemotherapy for the successful treatment of recurrent high-grade
glioma (3).
A previous meta-analysis also compared the clinical
outcomes of surgical resection and biopsy, as these are the first
surgical interventions for patients with low-grade glioma (4). Another meta-analysis compared
radiotherapy against radiotherapy in combination with chemotherapy
for the treatment of patients with high-grade glioma. The results
of this analysis revealed that the latter strategy demonstrated a
small but clear improvement in the outcomes of treatments (5). While there are several treatment
options for patients, including surgery, radiation and
chemotherapy, the median overall survival of patients with
malignant glioma is 1–2 years (6).
Moreover, the poor prognosis of patients with glioma is largely a
result of rapid tumor growth and cell invasion and migration
(7). Therefore, suppressing cell
proliferation and migration may serve as a novel therapeutic
strategy for patients with glioma.
Anesthetics and anesthesia techniques impact the
migration of tumor cells (8).
Sevoflurane, a volatile anesthetic agent, inhibits the
proliferation of colonic (9) and
laryngeal cancer cells (10), and
inhibits the migration of lung cancer cells (11). Furthermore, sevoflurane has been
reported to modulate multiple microRNAs (miRNAs/miRs) in the brain
(12). However, the effect of
sevoflurane on the migration and proliferation of glioma cells is
unknown.
miRNAs are a group of small, non-coding RNAs
comprised of 21–23 nucleotides (13). miRNAs regulate gene expression by
binding to the 3′-untranslated regions of target mRNAs (14). Moreover, miRNAs serve crucial roles
in multiple oncogenic activities, including proliferation,
migration, invasion and angiogenesis (15). In addition, miRNAs are deregulated
in various types of cancer, including glioblastoma (16). miR-27b acts as an important tumor
suppressor in numerous cancer types and reduces tumor growth and
metastasis via targeting nuclear receptor subfamily 2 group F
member 2 in gastric cancer (17).
Moreover, miR-27b induces cell apoptosis and reduces cell viability
and survival by targeting frizzled class receptor 7 in lung cancer
(18), and reduces cell growth and
invasion via targeting Rab3D in colorectal cancer (19). In addition, miR-27b also serves a
tumor suppressor in glioma and miR-27b expression is significantly
lower in metastatic glioma tissues compared with non-metastatic
tissues (20). However, whether
sevoflurane regulates glioma cell migration and proliferation by
targeting miR-27b is yet to be elucidated.
Materials and methods
Cell lines
Normal human glial HEB cells and U251 (U251-MG; has
not been authenticated yet; glioma cells; Type culture Collection
of the Chinese Academy of Science) and U87 (cat. no.
ATCC®HTB-14; has not been authenticated yet;
glioblastoma of unknown origin; American Type Culture Collection)
glioma cell lines were cultured in DMEM (Invitrogen; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Invitrogen; Thermo
Fisher Scientific, Inc.), 1% penicillin and 1% streptomycin at 37°C
in 5% CO2.
miRNA transfection
miR-27b mimics (5′-CGTCTTGAATCGGTGACACTT-3′),
miR-27b inhibitors (5′-GGUAAUCCCUGGCAAUGUGAU-3′) and miR-negative
control (miR-NC; 5′-UUGUACUACACAAAAGUACUG-3′) were purchased from
Shanghai GenePharma Co., Ltd. Once cells reached a confluence of
50–70%, they were transfected with the aforementioned agents using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) in accordance with the manufacturer's protocol.
The final working concentration of the miR-27b mimic, miR-27b
inhibitor or miR-NC was 40 nmol/l. Following transfection for 24 h
at 37°C, the cells were collected for subsequent
experimentation.
Small interfering (si)RNA
transfection
Transfection with siRNA-NC (0.01 µM;
5′-ACGUGACACGUUCGGAGAATT-3′; Shanghai GenePharma Co., Ltd.) or
siRNA vascular epithelial growth factor (VEGF; 0.01 µM; siRNA-1
VEGF, 5′-GAUCUCAUCAGGGUACUCCdTdT-3′; siRNA-2 VEGF,
5′-GTGCTGGCCTTGGTGAGGTTT-3′; Shanghai GenePharma Co., Ltd.) was
performed using the TurboFect siRNA transfection reagent
(Fermentas; Thermo Fisher Scientific, Inc.) in accordance with the
manufacturer's protocol. At 48 h post-transfection, U251 and U87
cells were collected and used for further analyses.
Groups
U251 and U87 cells (1×105 cells/well) in
the exponential growth phase were seeded into 6-well plates and
incubated at 37°C overnight in DMEM (Thermo Fisher Scientific,
Inc.). Cell exposure to 3.4% sevoflurane (Maruishi Pharmaceutical
Co., Ltd.) for 6 h at 37°C was conducted according to a previous
report (11). Cell plates were put
into an airtight chamber that was connected to an anesthesia
machine (Cicero-EM 8060; Drägerwerk AG & Co. KGaA), attached to
which was an anesthetic vaporizer (Sevorane; Abbott Pharmaceutical
Co., Ltd.) that supplied sevoflurane and the sevoflurane
concentration (3.4%) was monitored using PM 8060 (Dräger KGaA).
Subsequently, cells were randomly divided into the following four
groups: Untreated control (95% air and 5% CO2),
sevoflurane (3.4% sevoflurane mixed with 95% air and 5%
CO2), sevoflurane + miR-27b inhibitor (administered 48 h
prior to sevoflurane) and sevoflurane + VEGF siRNA + miR-27b
inhibitor. The untreated control group acted as a negative control
group for the experiments. After cells were exposed to sevoflurane
for 6 h at 37°C, cells were grown at 37°C in a 5% CO2
incubator for an additional 24 h. Cells were then used for cell
proliferation assays, migration assays or molecular analyses.
RNA extraction and reverse
transcription-quantitative (RT-q) PCR
Total miRNA from U251 and U87 cells was extracted
using the mirVana miRNA Isolation kit (Ambion; Thermo Fisher
Scientific, Inc.) in accordance with the manufacturer's protocol.
Subsequently, cDNA was synthesized from 5 ng total RNA using the
TaqMan miRNA RT kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.) in accordance with the manufacturer's protocol. The following
RT protocol was used: 37°C for 45 min and 65°C for 10 min. The
expression of miR-27b was quantified using the miRNA-specific
TaqMan miRNA Assay kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) with the Applied Biosystems 7500 RT PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used for the qPCR: 95°C for 10 min;
followed by 40 cycles of 95°C for 10 sec and 60°C for 1 min. The
relative quantification of miR-27b was normalized to that of U6.
The primer sequences were as listed: miR-27b forward,
5′-CGGCGGTTCACAGTGGCTAA-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′; and
U6 forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′.
Total RNA was isolated from U251 and U87 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). cDNA was then reverse transcribed from total RNA using a
SuperScript II RT kit (Invitrogen; Thermo Fisher Scientific, Inc.).
The following RT temperature protocol was used: 50°C for 10 min and
80°C for 10 min. qPCR was performed using the SYBR Green PCR Master
Mix (Thermo Fisher Scientific, Inc.) and the Applied Biosystems
7500 RT PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The following thermocycling conditions were used for the
qPCR: 95°C for 30 sec; followed by 40 cycles of 95°C for 5 sec and
60°C for 30 sec. The expression of target genes was normalized to
that of GAPDH. The primer sequences were as listed: Matrix
metalloproteinase (MMP)-2 forward, 5′-GCCCCAGACAGGTGATCTTG-3′ and
reverse, 5′-GCTTGCGAGGGAAGAAGTTGT-3′; MMP-9 forward,
5′-AGACGGGTATCCCTTCGACG-3′ and reverse,
5′-AAACCGAGTTGGAACCACGAC-3′; and GAPDH forward,
5′-GGTCTCCTCTGACTTCAACA-3′ and reverse, 5′-GTGAGGGTCTCTCTCTTCCT-3′.
Expression levels were quantified using the 2−ΔΔCq
method (21).
Cell proliferation assay
The proliferation of U251 and U87 cells was analyzed
using a Cell Counting Kit-8 (CCK-8) assay (Beyotime Institute of
Biotechnology), according to the manufacturer's protocol. In total,
3×103 cells (100 µl) were seeded in 96-well plates and
incubated for 24 and 48 h. Subsequently, 10 µl CCK-8 solution was
added into each well and incubated for a further 1 h at 37°C.
Absorbance at 450 nm was then measured using an ELX-800
spectrometer reader (BioTek Instruments, Inc.).
Wound healing assays
U251 and U87 cells (1×105 cells/well)
were seeded into 6-well plates and cultured to 100% confluence,
after which a wound was created by manually scraping the cell
monolayer with a 10 µl pipette tip. Cells were washed with serum
free medium to remove floating cells and then incubated in DMEM
supplemented with 1% FBS at 37°C. Cell migration into the wound was
observed at two time points (0 and 24 h) in six randomly selected
fields of view. Images were acquired using a phase-contrast Leitz
light microscope (magnification, ×100). The migratory distance of
U251 and U87 cells was determined by subtracting the wound width at
24 h from the wound width at 0 h and analyzed using ImageJ software
(version 1.8.0; National Institutes of Health). The obtained values
were expressed as migration percentages, setting the gap width at 0
h as 0%.
Western blot analysis
Total protein was extracted from cells using RIPA
lysis buffer (Beyotime Institute of Biotechnology), according to
the manufacturer's protocol. Total protein was quantified using a
bicinchoninic acid assay kit (Beyotime Institute of Biotechnology).
Subsequently, 15 µg protein/lane was separated using 8–10% SDS-PAGE
and transferred onto a PVDF membrane, which was subsequently
blocked with 5% skimmed milk diluted with TBS-1% Tween (TBST) for 2
h at room temperature. The PVDF membrane was then incubated
overnight at 4°C with polyclonal antibodies for MMP-2 (cat. no.
ab215986; 1:1,000; Abcam) and MMP-9 (cat. no. ab219372; 1:1,000;
Abcam), with GAPDH antibody (cat. no. ab8245; 1:10,000; Abcam)
serving as an internal control. The membrane was further incubated
with horseradish peroxidase-conjugated anti-rabbit (cat. no.
sc-2030) or anti-mouse (cat. no. sc-2005) immunoglobulin-G
secondary antibodies (1:5,000; Santa Cruz Biotechnology, Inc.)
diluted with TBST for 1 h at room temperature. The resulting
protein signals were detected using an enhanced chemiluminescence
reaction system (EMD Millipore) and quantified using ImageJ
software (version 1.8.0; National Institutes of Health).
Luciferase assay
TargetScan release 7.1 (http://www.targetscan.org/vert_71/) was used in the
present study for the prediction of binding site between miR-27b
and VEGF. The 3′UTR of VEGF mRNA was amplified from the cDNA of HEB
cells and inserted into a pGL3-basic plasmid (Promega Corporation).
The pGL3-VEGF 3′UTR-mutant (Mut) was created by introducing VEGF
3′UTR site mutations using a Quick Site-Directed Mutation kit
(Agilent Technologies, Inc.). U251 and U87 cells were
co-transfected with miR-27b mimics (20 nM) or miR-NC (20 nM),
pGL3-VEGF 3′UTR-wild type (WT; 0.4 mg) or pGL3-VEGF 3′UTR-Mut (0.4
mg) and Renilla luciferase vectors using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. A dual
luciferase assay was performed at 48 h after transfection using a
Dual Luciferase kit (Promega Corporation). Activities were
normalized to that of Renilla luciferase.
Statistical analysis
Each experiment was performed three times and data
were analyzed using SPSS 16.0 software (IBM Corp.). Differences
between two groups were analyzed using a two-tailed Student's
t-test, while differences among ≥3 groups were evaluated using
one-way ANOVA followed by a Tukey's multiple comparison post hoc
test. Data are presented as the mean ± SEM. P<0.05 was
considered to indicate a statistically significant difference.
Results
Sevoflurane induces the expression of
miR-27b in glioma cells
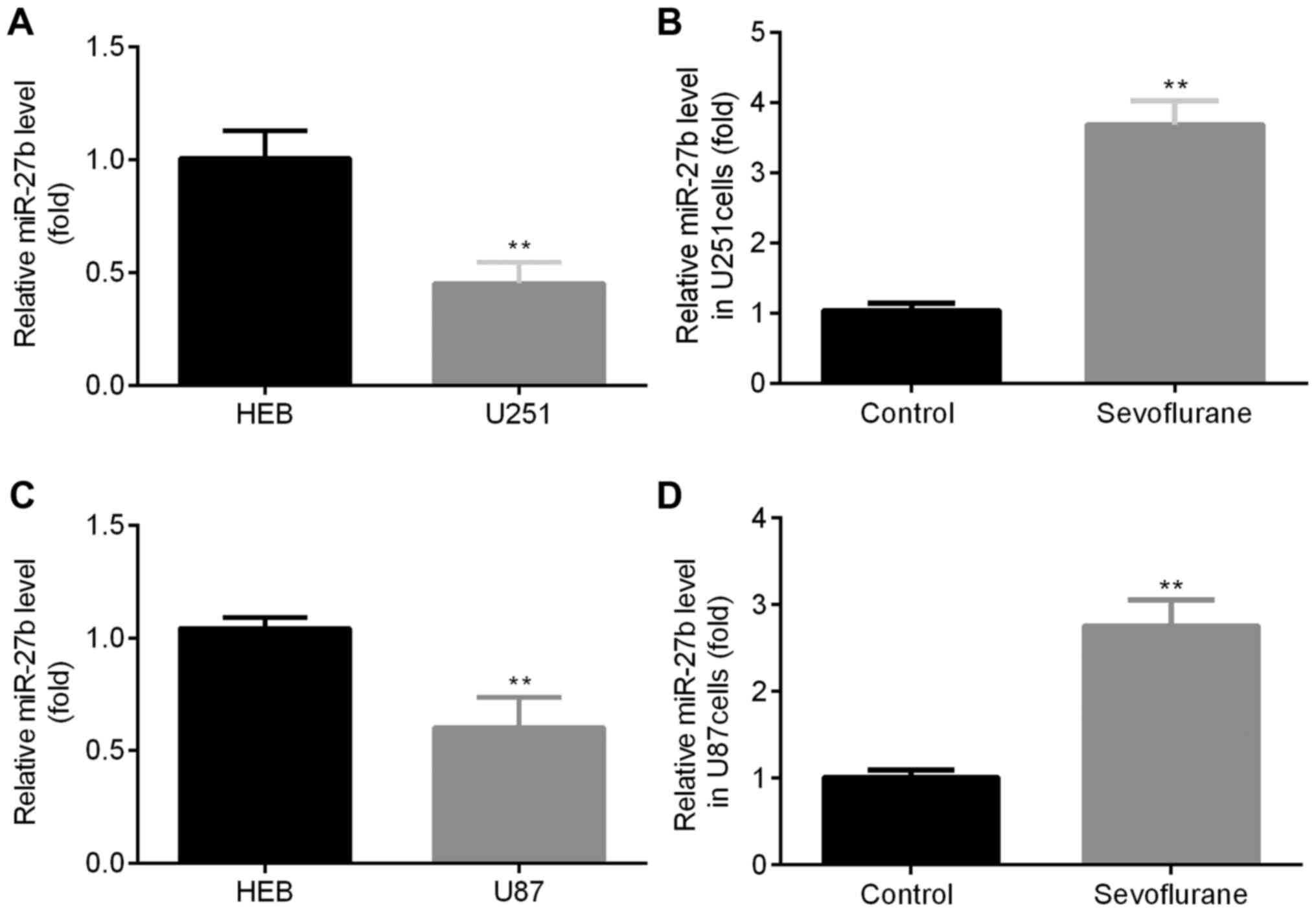
To determine the expression of miR-27b in glioma
cells compared with normal cells, RT-qPCR was performed. It was
found that U251 cells had significantly decreased miR-27b
expression compared with HEB cells (Fig. 1A). RT-qPCR was also performed to
determine whether sevoflurane effects the expression of miR-27b in
glioma cells. It was demonstrated that U251 cells treated with
sevoflurane had significantly increased miR-27b expression compared
with the control group (Fig. 1B).
Furthermore, in U87 cells, a decreased expression of miR-27b was
identified compared with HEB cells (Fig. 1C). In addition, sevoflurane
treatment also significantly increased miR-27b expression compared
with the control group (Fig.
1D).
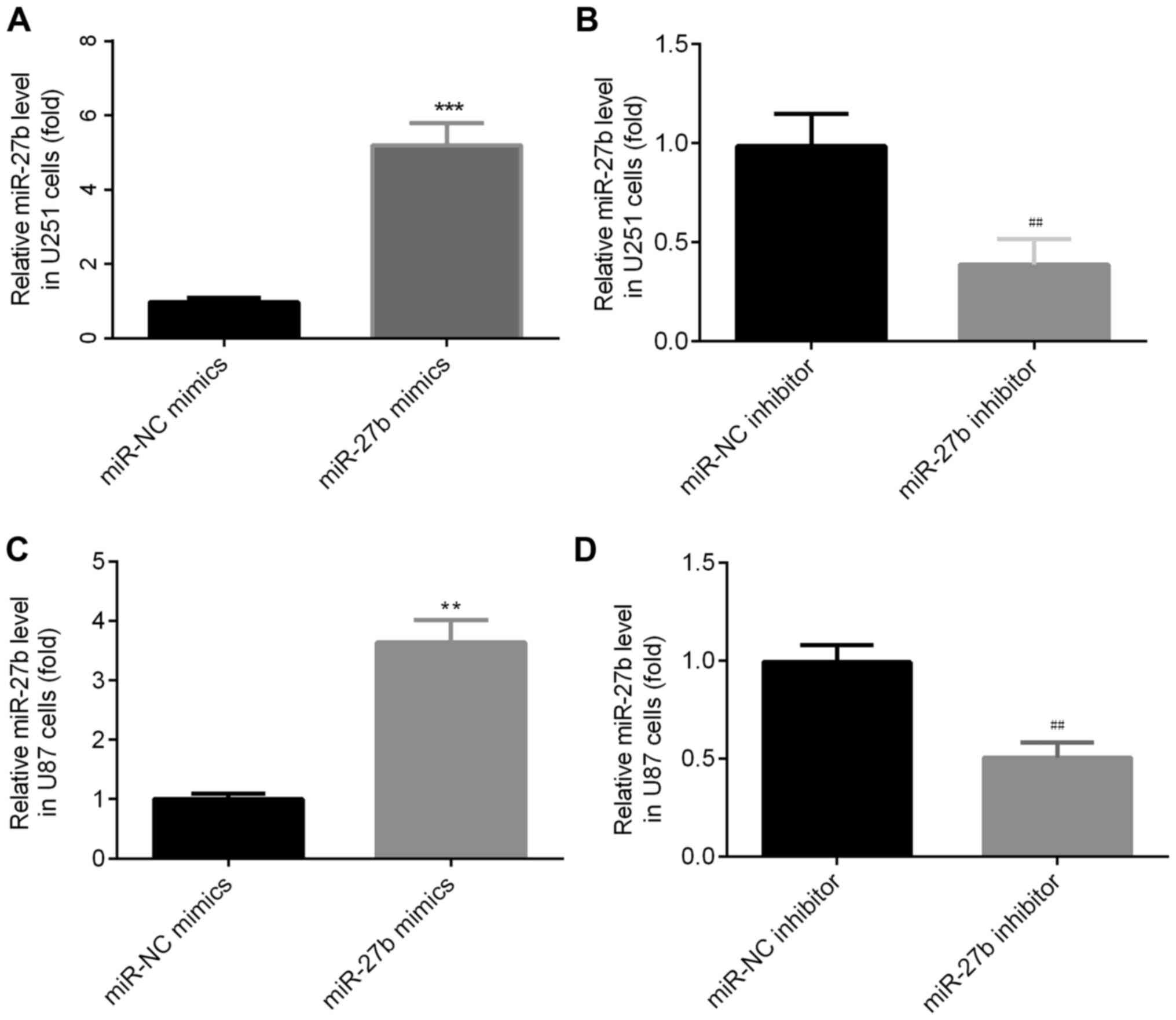
miR-27b mimics and inhibitors affect
the expression of miR-27b
Following transfection with the miR-27b mimic,
miR-27b was significantly increased in U251 cells (Fig. 2A). Moreover, after transfection with
miR-27b inhibitors, miR-27b expression was significantly decreased
in U251 cells (Fig. 2B). The same
effects were observed in U87 cells (Fig. 2C and D).
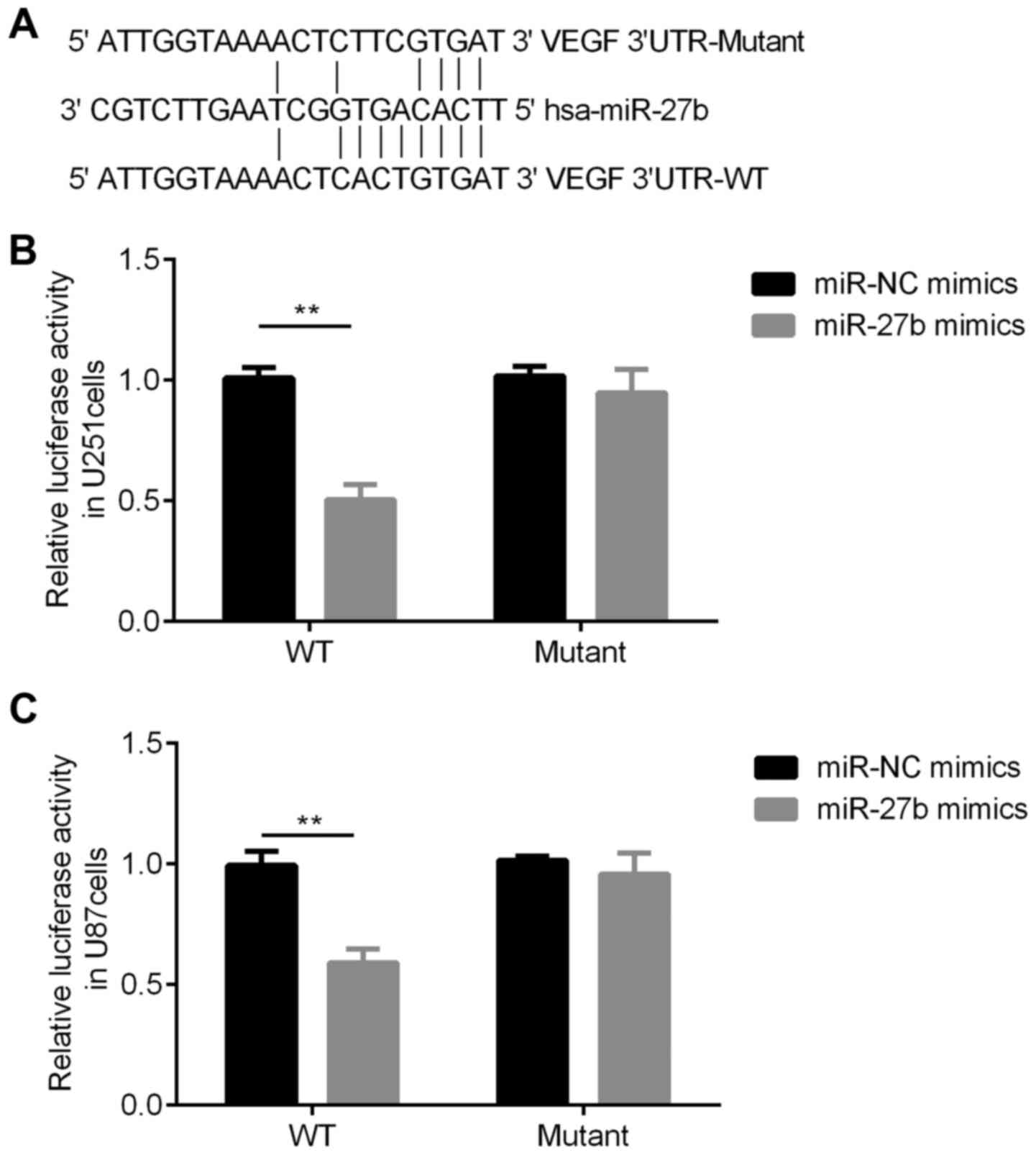
VEGF is a target of miR-27b
TargetScan was used to search for the potential
targets of miR-27b in humans and a conserved binding site for
miR-27b in the 3′UTR region of the VEGF gene was identified
(Fig. 3A).
To further assess whether miR-27b alters the
expression of VEGF by post-transcriptionally regulating its 3′UTR,
a luciferase reporter plasmid containing the 3′UTR of VEGF was
constructed. It was found that the luciferase activity of miR-27b
mimic + pGL3-VEGF 3′UTR-WT-transfected U251 and U87 cells was
significantly decreased compared with miR-NC + pGL3-VEGF
3′UTR-WT-transfected cells (Fig. 3B and
C).
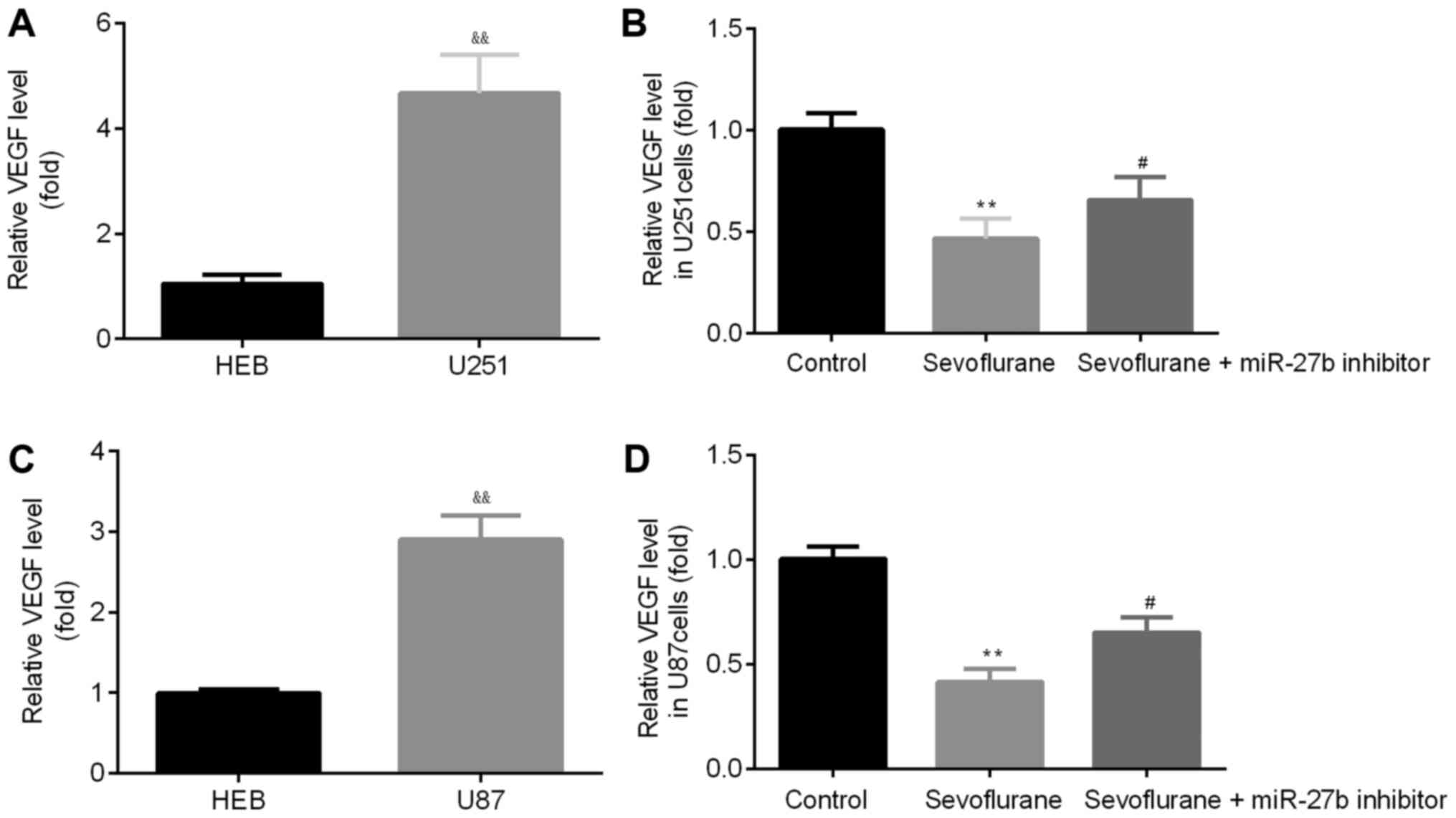
Sevoflurane reduces the expression of
VEGF in glioma cells by targeting miR-27b
To determine the expression of VEGF in glioma cells
compared with normal cells, VEGF expression was determined via
RT-qPCR. It was found that U251 cells had significantly increased
expression levels of VEGF compared with HEB cells (Fig. 4A).
To examine whether sevoflurane effects the
expression of VEGF in glioma cells, RT-qPCR was performed following
sevoflurane treatment in U251 cells. The untreated control group
acted as a negative control group for the experiments. Decreased
VEGF expression was identified in the sevoflurane group compared
with the control group, which was subsequently reversed following
treatment with the miR-27b inhibitor (Fig. 4B). Moreover, the same results were
obtained in U87 cells (Fig. 4C and
D).
Sevoflurane-induced inhibition of
glioma cell proliferation is mediated by the miR-27b/VEGF axis
To further assess the effects of sevoflurane on
glioma cell proliferation, U251 and U87 cells were treated with
miR-27b inhibitors or VEGF siRNA + miR-27b inhibitor prior to
sevoflurane exposure.
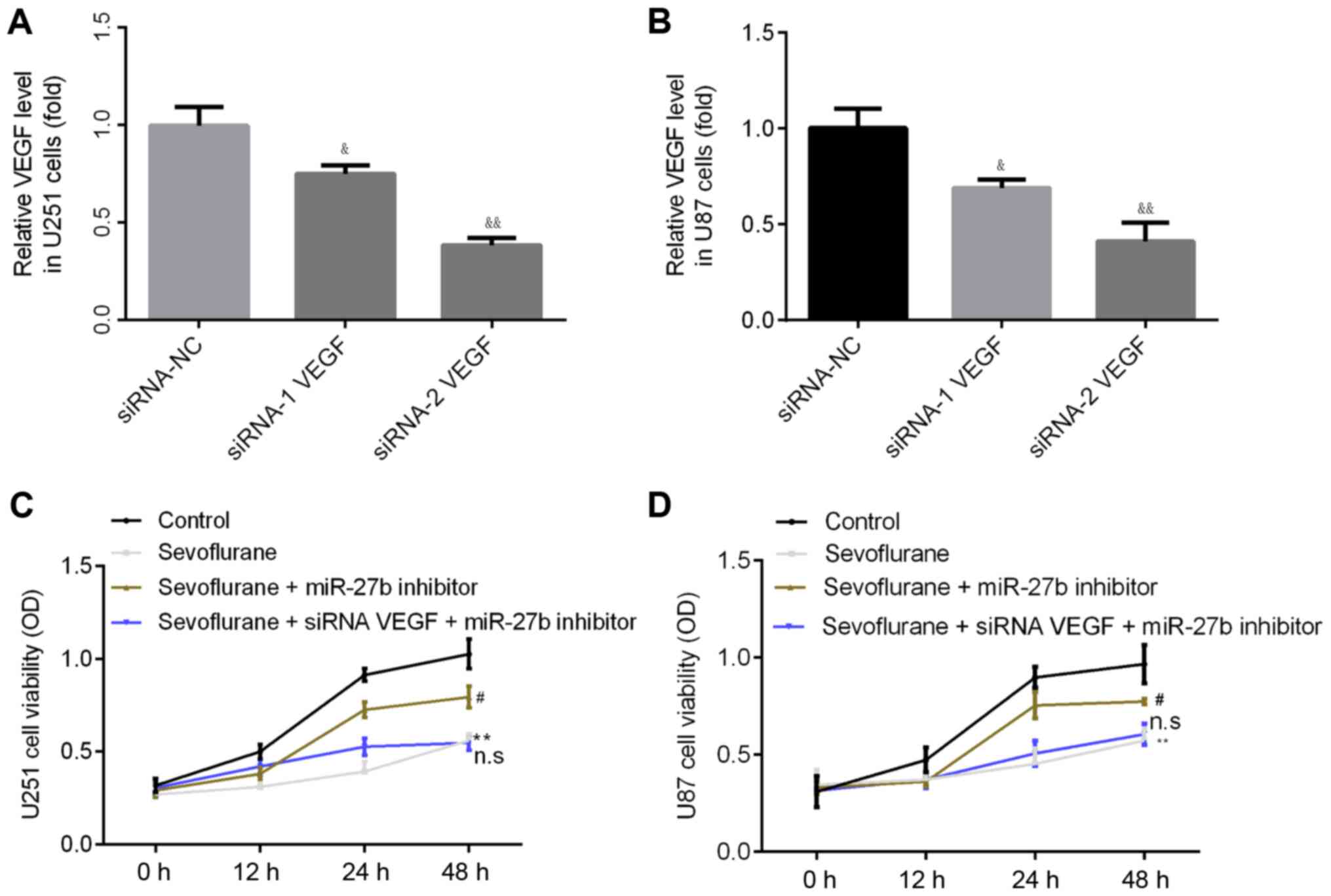
The effect of VEGF siRNA on the expression of VEGF
in U251 and U87 cells was determined via RT-qPCR. The present
results suggested that, compared with the siRNA-NC group, VEGF
siRNA-1 and VEGF siRNA-2 significantly decreased the expression of
VEGF, with siRNA-2 demonstrating a more significant effect
(Fig. 5A and B). Therefore, VEGF
siRNA-2 was used for subsequent experimentation.
It was demonstrated that the inhibitory effects of
sevoflurane on U251 and U87 cell proliferation were abolished by
pre-treatment with the miR-27b inhibitor (Fig. 5C and D). However, no effect was
identified when cells were treated with VEGF siRNA + miR-27b
inhibitor (Fig. 5C and D).
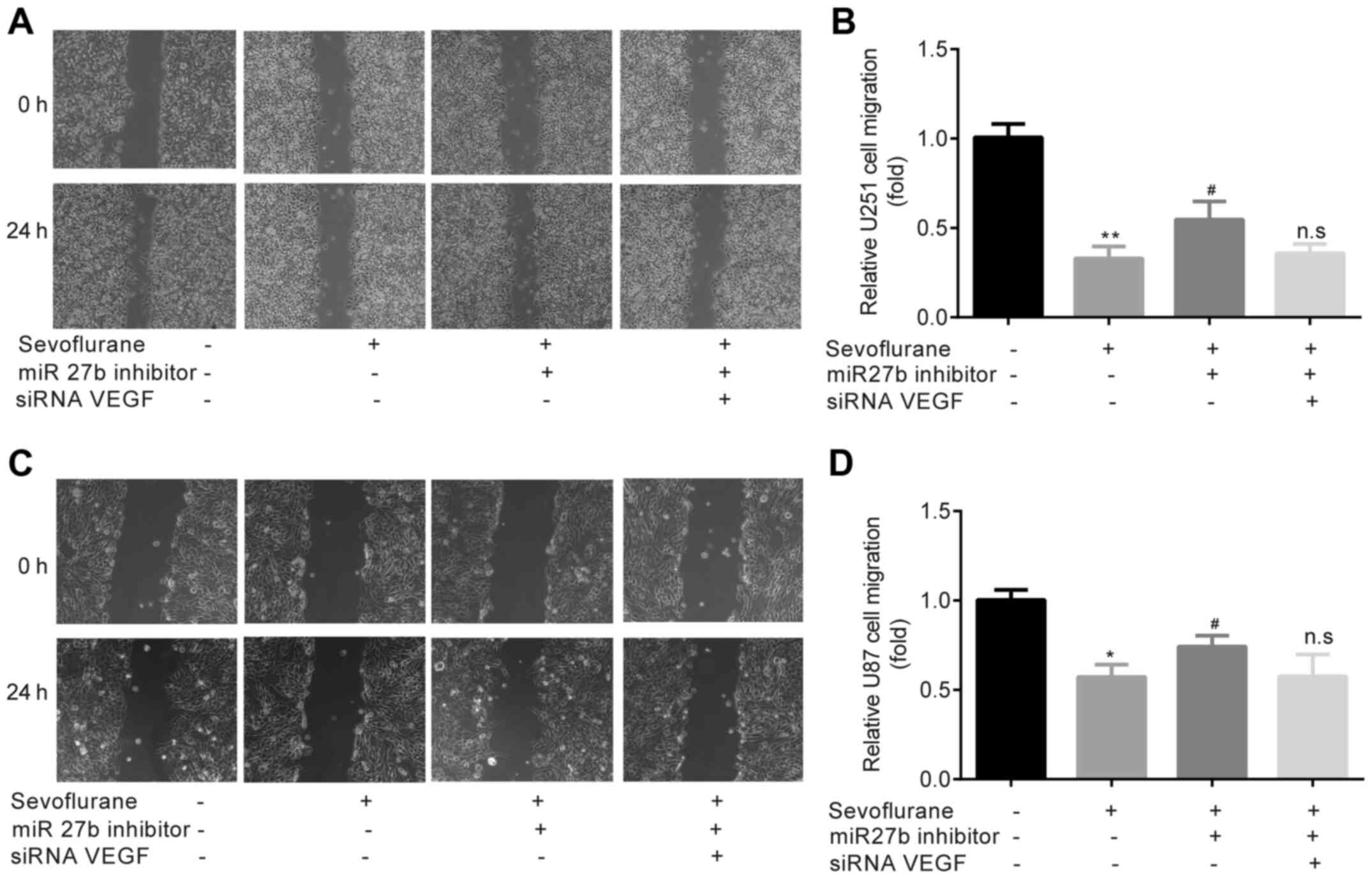
Sevoflurane-induced inhibition of
glioma cell migration is mediated by the miR-27b/VEGF axis
To further investigate the effects of sevoflurane on
glioma cell migration, U251 cells were treated with miR-27b
inhibitor or siRNA VEGF + miR-27b inhibitor prior to sevoflurane
exposure. It was found that the inhibitory effect of sevoflurane on
U251 cell migration was partially reversed by the pre-treatment
with the miR-27b inhibitor; however, no effect was observed when
cells were treated with siRNA VEGF + miR-27b inhibitor (Fig. 6A and B). Furthermore, similar
results were obtained in U87 cells (Fig. 6C and D). The present results
indicated that there were no significant pathological morphology
changes in U251 or U87 cells (Fig.
6A-D).
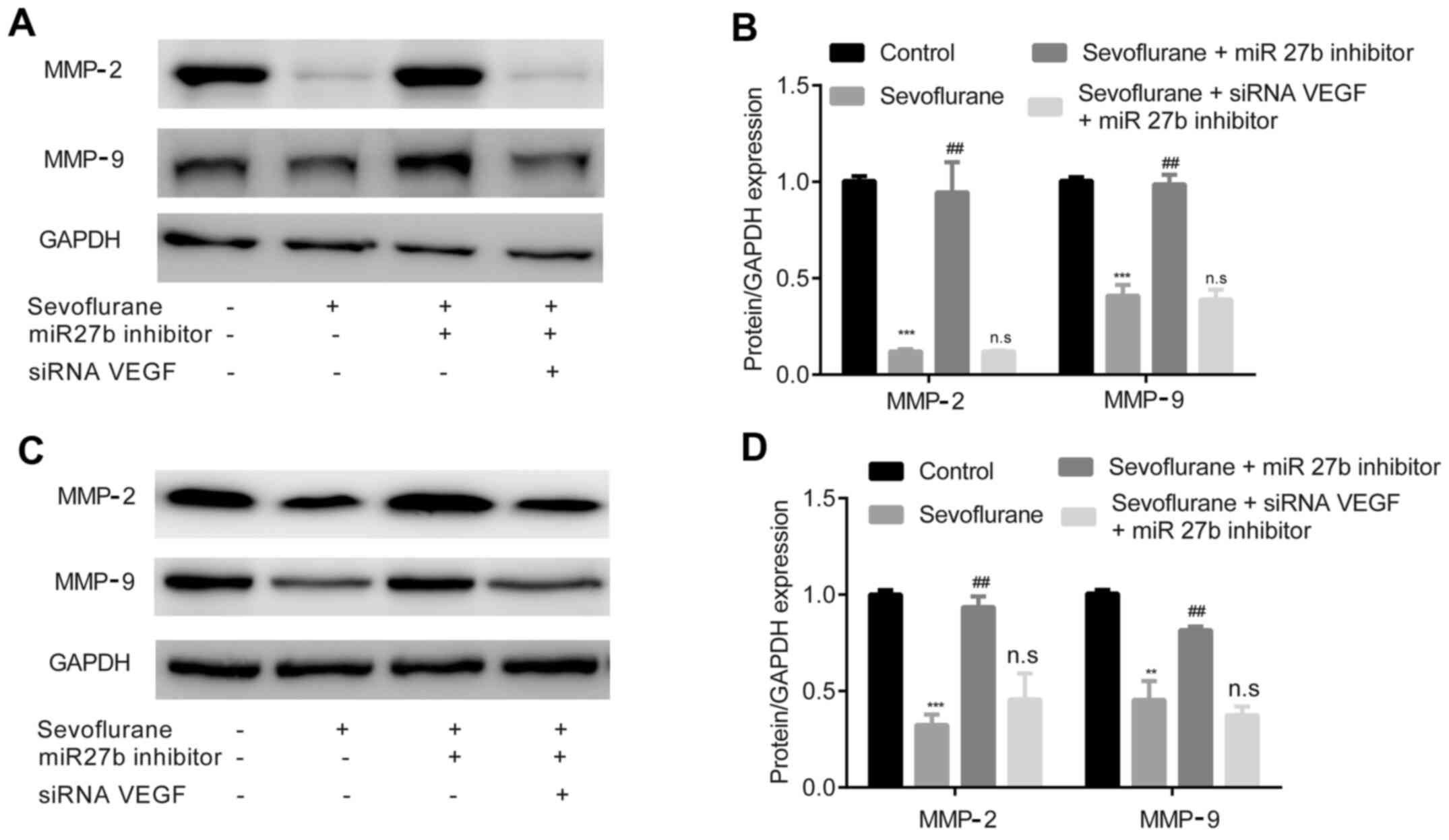
Sevoflurane inhibits the expression
levels of MMP-2 and MMP-9
To determine whether sevoflurane inhibits the
expression levels of MMP-2 and MMP-9, U251 and U87 cells were
pre-treated with miR-27b inhibitors or VEGF siRNA + miR-27b
inhibitors, and these proteins were assessed following sevoflurane
exposure. The untreated control group acted as a negative control
group for the experiments.
The present results suggested that the decreased
protein expression levels of MMP-2 and MMP-9 induced by sevoflurane
in U251 cells were reversed following pre-treatment with the
miR-27b inhibitor. However, no effect was identified following VEGF
siRNA + miR-27b inhibitor treatment compared with sevoflurane
treatment (Fig. 7A and B).
Moreover, similar effects were demonstrated in U87 cells (Fig. 7C and D).
Discussion
Sevoflurane has been shown to exert
anti-proliferative effects in colon cancer (9) and laryngeal cancer cells (10). Furthermore, sevoflurane prevents the
migration of lung cancer cells (11) and regulates various miRNAs of the
brain (12). Moreover, sevoflurane
was reported to inhibit the migration and invasion of glioma cells
by upregulating miR-637 (22).
Various miRNAs have also been revealed to inhibit glioma cell
proliferation and migration, including miR-10b (23), miR-218 (24) and miR-29c (25). The inhibitory effects of miR-27b on
the progression of cancer have been identified in different cancer
types: miR-27b inhibits cell proliferation and migration by
targeting PI3K p110α in colorectal cancer (26), and long non-coding RNA DLX6-AS1
induces the progression of lung cancer by targeting miR-27b-3p
(27). With regard to glioma, the
role of miR-27b is controversial. It has been shown that miR-27b
promotes cell invasion in glioma cells (28), and that upregulation of miR-27b
contributes to the malignancy of glioma (29), which indicates that miR-27b has an
oncogenic role. However, miR-27b expression is significantly lower
in metastatic glioma tissues compared with non-metastatic tissues
(20), and miR-27b is decreased in
metastatic tumors of glioma (20),
which indicates that miR-27b acts as a tumor suppressor. Yet,
whether sevoflurane inhibits glioma cell migration and
proliferation by regulating miR-27b is not fully understood.
The present results indicated that there were
significantly decreased expression levels of miR-27b in U251 and
U87 cells compared with HEB cells. Furthermore, sevoflurane
treatment significantly increased miR-27b expression in cells
compared with the control group. Thus, the present results
suggested that miR-27b may serve as a tumor suppressor in glioma,
which is consistent with previous studies (20). Furthermore, it was speculated that
sevoflurane may serve a pivotal role in the progression of glioma
by upregulating miR-27b.
miRNAs negatively regulate gene expression by
inhibiting translation or inducing the degradation of target mRNA
(30). Therefore, future studies
should focus on mRNAs that function as oncogenes.
Using computational methods, the present study
predicted that miR-27b targeted the 3′UTR of VEGF. Adequate blood
supply is indispensable for tumor growth and metastasis. As
neoplasms grow larger, adequate blood supply is obtained via
angiogenesis (31). Moreover, tumor
cells produce various inducers of angiogenesis, including VEGF,
which has important roles in endothelial survival, proliferation
and new vessel formation (32). To
assess whether miR-27b targeted the 3′UTR of VEGF, a
dual-luciferase reporter assay was performed. The present results
indicated that there was increased VEGF expression in U251 and U87
cells compared with HEB cells. In addition, compared with the
control group, decreased VEGF expression was demonstrated in the
sevoflurane group, which was subsequently rescued following
treatment with the miR-27b inhibitor.
The effects of miR-27b on the sevoflurane-induced
proliferation and migration of U251 and U87 cells were examined in
the present study. It was found that the inhibitory effects of
sevoflurane on U251 and U87 cell proliferation and migration were
abolished following pre-treatment with the miR-27b inhibitor.
However, no effects were identified following treatment with VEGF
siRNA + miR-27b inhibitor.
MMPs are a family of zinc-dependent endopeptidases
that degrade components of the extracellular matrix (ECM).
Furthermore, MMP activation induces cell migration and invasion
(33). MMP-2 is a key enzyme that
degrades certain components of the ECM, including type IV (34). Moreover, the present results
suggested that the sevoflurane-induced decrease of MMP-2 and MMP-9
protein expression levels in U251 and U87 cells was reversed
following pre-treatment with the miR-27b inhibitor. However, no
effects were demonstrated following treatment with VEGF siRNA +
miR-27b inhibitor.
However, there are several limitations in the
present study. Firstly, there are several mRNAs that could be
targeted by miR-27b and the present study only investigated the
role of VEGF. Thus, future studies will investigate these other
potential targets. Secondly, the culture medium for the wound
healing assay contained 1% FBS, which could influence the
migration, therefore it will be replaced by serum-free culture
medium in the future.
In conclusion, the present results indicated that
miR-27b may serve as a tumor suppressor and that VEGF acts as an
oncogene in glioma. Therefore, miR-27b may be a novel target for
sevoflurane in glioma. Moreover, it was found that sevoflurane
inhibited glioma cell proliferation and migration, which may be
related to miR-27b and its target VEGF. Collectively, the present
results indicated that targeting the miR-27b/VEGF axis may be a
potential treatment for glioma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ and CL performed the experiments and analyzed the
data. LY conceived the study, analyzed the data and prepared the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mamelak AN and Jacoby DB: Targeted
delivery of antitumoral therapy to Glioma and other malignancies
with synthetic chlorotoxin (TM-601). Expert Opin Drug Deliv.
4:175–186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Radner H, El-Shabrawi Y, Eibl RH, Brüstle
O, Kenner L, Kleihues P and Wiestler OD: Tumor induction by ras and
myc oncogenes in fetal and neonatal brain: Modulating effects of
developmental stage and retroviral dose. Acta Neuropathol.
86:456–465. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wong ET and Brem S: Taming glioblastoma:
Targeting angiogenesis. J Clin Oncol. 25:4705–4706. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang B, Chaichana K, Veeravagu A, Chang
SD, Black KL and Patil CG: Biopsy versus resection for the
management of low-grade gliomas. The Cochrane Database of
Systematic Reviews. 4:CD0093192017.PubMed/NCBI
|
|
5
|
Stewart L and Burdett S; Glioma
Meta-analysis Trialists Group (GMT), : Chemotherapy for high-grade
glioma. Cochrane Database Syst Rev. CD0039132012.
|
|
6
|
Davis FG and McCarthy BJ: Current
epidemiological trends and surveillance issues in brain tumors.
Expert Rev Anticancer Ther. 1:395–401. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giese A, Bjerkvig R, Berens ME and
Westphal M: Cost of migration: Invasion of malignant gliomas and
implications for treatment. J Clin Oncol. 21:1624–1636. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Snyder GL and Greenberg S: Effect of
anaesthetic technique and other perioperative factors on cancer
recurrence. Br J Anaesth. 105:106–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kvolik S, Glavas-Obrovac L, Bares V and
Karner I: Effects of inhalation anesthetics halothane, sevoflurane,
and isoflurane on human cell lines. Life Sci. 77:2369–2383. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kvolik S, Dobrosevic B, Marczi S, Prlic L
and Glavas-Obrovac L: Different apoptosis ratios and gene
expressions in two human cell lines after sevoflurane anaesthesia.
Acta Anaesthesiol Scand. 53:1192–1199. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang H, Gu M, Yang C, Wang H, Wen X and
Zhou Q: Sevoflurane inhibits invasion and migration of lung cancer
cells by inactivating the p38 MAPK signaling pathway. J Anesth.
26:381–392. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goto G, Hori Y, Ishikawa M, Tanaka S and
Sakamoto A: Changes in the gene expression levels of microRNAs in
the rat hippocampus by sevoflurane and propofol anesthesia. Mol Med
Rep. 9:1715–1722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ambros V: microRNAs: Tiny regulators with
great potential. Cell. 107:823–826. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ciafre SA, Galardi S, Mangiola A, Ferracin
M, Liu CG, Sabatino G, Negrini M, Maira G, Croce CM and Farace MG:
Extensive modulation of a set of microRNAs in primary glioblastoma.
Biochem Biophys Res Commun. 334:1351–1358. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feng Q, Wu X, Li F, Ning B, Lu X, Zhang Y,
Pan Y and Guan W: miR-27b inhibits gastric cancer metastasis by
targeting NR2F2. Protein Cell. 8:114–122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun Y, Xu T, Cao YW and Ding XQ: Antitumor
effect of miR-27b-3p on lung cancer cells via targeting Fzd7. Eur
Rev Med Pharmacol Sci. 21:4113–4123. 2017.PubMed/NCBI
|
|
19
|
Luo Y, Yu SY, Chen JJ, Qin J, Qiu YE,
Zhong M and Chen M: MiR-27b directly targets Rab3D to inhibit the
malignant phenotype in colorectal cancer. Oncotarget. 9:3830–3841.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khan FH, Pandian V, Ramraj S, Aravindan S,
Herman TS and Aravindan N: Reorganization of metastamiRs in the
evolution of metastatic aggressive neuroblastoma cells. BMC
Genomics. 16:5012015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yi W, Li D, Guo Y, Zhang Y, Huang B and Li
X: Sevoflurane inhibits the migration and invasion of glioma cells
by upregulating microRNA-637. Int J Mol Med. 38:1857–1863. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin J, Teo S, Lam DH, Jeyaseelan K and
Wang S: MicroRNA-10b pleiotropically regulates invasion,
angiogenicity and apoptosis of tumor cells resembling mesenchymal
subtype of glioblastoma multiforme. Cell Death Dis. 3:e3982012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song L, Huang Q, Chen K, Liu L, Lin C, Dai
T, Yu C, Wu Z and Li J: miR-218 inhibits the invasive ability of
glioma cells by direct downregulation of IKK-β. Biochem Biophys Res
Commun. 402:135–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fan YC, Mei PJ, Chen C, Miao FA, Zhang H
and Li ZL: MiR-29c inhibits glioma cell proliferation, migration,
invasion and angiogenesis. J Neurooncol. 115:179–188. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen Y, Zhang B, Jin Y, Wu Q and Cao L:
MiR-27b targets PI3K p110α to inhibit proliferation and migration
in colorectal cancer stem cell. Am J Transl Res. 11:5988–5997.
2019.PubMed/NCBI
|
|
27
|
Sun W, Zhang L, Yan R, Yang Y and Meng X:
LncRNA DLX6-AS1 promotes the proliferation, invasion, and migration
of non-small cell lung cancer cells by targeting the
miR-27b-3p/GSPT1 axis. Onco Targets Ther. 12:3945–3954. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu C, Liang S, Xiao S, Lin Q, Chen X, Wu
Y and Fu J: MicroRNA-27b inhibits Spry2 expression and promotes
cell invasion in glioma U251 cells. Oncol Lett. 9:1393–1397. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen L, Li H, Han L, Zhang K, Wang G, Wang
Y, Liu Y, Zheng Y, Jiang T, Pu P, et al: Expression and function of
miR-27b in human glioma. Oncol Rep. 26:1617–1621. 2011.PubMed/NCBI
|
|
30
|
Wen MM: Getting miRNA therapeutics into
the target cells for neurodegenerative diseases: A mini-review.
Front Mol Neurosci. 9:1292016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dhup S, Dadhich RK, Porporato PE and
Sonveaux P: Multiple biological activities of lactic acid in
cancer: Influences on tumor growth, angiogenesis and metastasis.
Curr Pharm Des. 18:1319–1330. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yancopoulos GD: Clinical application of
therapies targeting VEGF. Cell. 143:13–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stetler-Stevenson WG and Seo DW: TIMP-2:
An endogenous inhibitor of angiogenesis. Trends M ol Med.
11:97–103. 2005. View Article : Google Scholar
|
|
34
|
Tapia A, Salamonsen LA, Manuelpillai U and
Dimitriadis E: Leukemia inhibitory factor promotes human first
trimester extravillous trophoblast adhesion to extracellular matrix
and secretion of tissue inhibitor of metalloproteinases-1 and −2.
Hum Reprod. 23:1724–1732. 2008. View Article : Google Scholar : PubMed/NCBI
|