Introduction
Circular RNAs (circRNAs) are endogenous
single-stranded non-coding RNAs (ncRNAs) generated from
protein-coding genes without caps and tails at 5′ and 3′ ends of
their structure and shaped as covalently closed continuous loops
(1,2). Due to their circular structure,
circRNAs are more stable and resistant to degradation by
exonuclease RNase R compared with linear mRNAs (3). circRNAs were once considered a product
of mis-splicing or splicing noise of precursor mRNAs (pre-mRNAs)
(4). circRNAs are now an
increasingly popular subject of study in the field of scientific
research, particularly in regard to ncRNAs. Moreover, circRNAs are
attracting significant attention as functional regulators, and have
an important value in biomedical research, particularly with regard
to their clinical potential. circRNAs were first discovered in
1976, in a study of RNA viruses (5). Subsequently, Hsu and Coca-Prados
(6) first identified the existence
of circRNAs in the cytoplasm of eukaryotic cells using electron
microscopy in 1979. In the 21st century, benefiting from the
breakthroughs in next generation sequencing technologies and rapid
development of bioinformatics, numerous circRNAs have been
identified in various organisms (7–9).
Additionally, it has been reported that circRNAs are
widely expressed in a variety of living organisms and are
evolutionarily conserved amongst different species and often
display cell or tissue/developmental-stage-specific expression
(10–12). Furthermore, several studies have
revealed that circRNAs exhibit aberrant or differential expression
in different tissues and disease states, suggesting their important
functions in physiological and pathological processes (13–18).
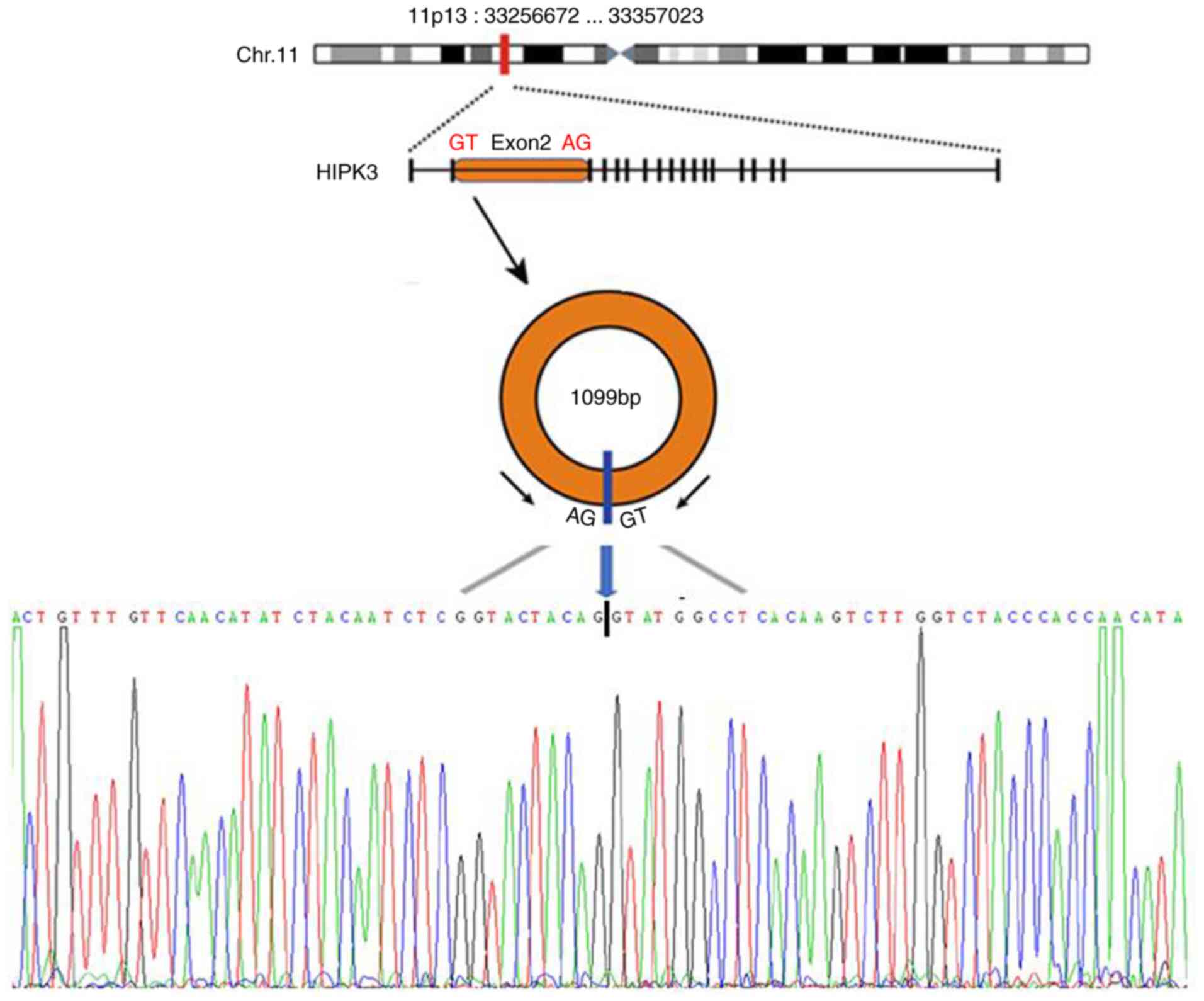
Homeodomain-interacting protein kinase 3 (HIPK3) is
a member of the HIPK gene family. circHIPK3 is a circular RNA
derived from exon2 of the HIPK3 gene, which is 1,099 nucleotides in
length (Fig. 1) (19–21).
An increasing number of studies have revealed that circHIPK3 is
strongly associated with the occurrence and development of several
human diseases, such as osteosarcoma, hepatocellular carcinoma and
colorectal cancer (22,23). circHIPK3 has a regulatory role as a
modulator of cellular behavior, as well as exhibiting oncogenic or
tumor suppressor functions, depending on the specific diseases
(24,25). Thus, circHIPK3 may serve as a novel
candidate of diagnosis and prognosis for disease biomarkers, as
well as a promising therapeutic molecular target. In the present
study, the biogenesis and possible functions of circRNAs are
discussed, with a focus on recent progress on the study of
circHIPK3 and its association with human diseases and cancer. The
aim of the present review was to broaden the knowledge on circHIPK3
and aid future studies assessing the regulatory function of
circHIPK3 in the development and progression of diseases.
Biogenesis of circRNAs
It is generally acknowledged that circRNAs are
transcribed from linear pre-mRNAs by RNA polymerase II (RNA Pol II)
and formed via back splicing, differing from canonical splicing to
form mRNAs (26). According to
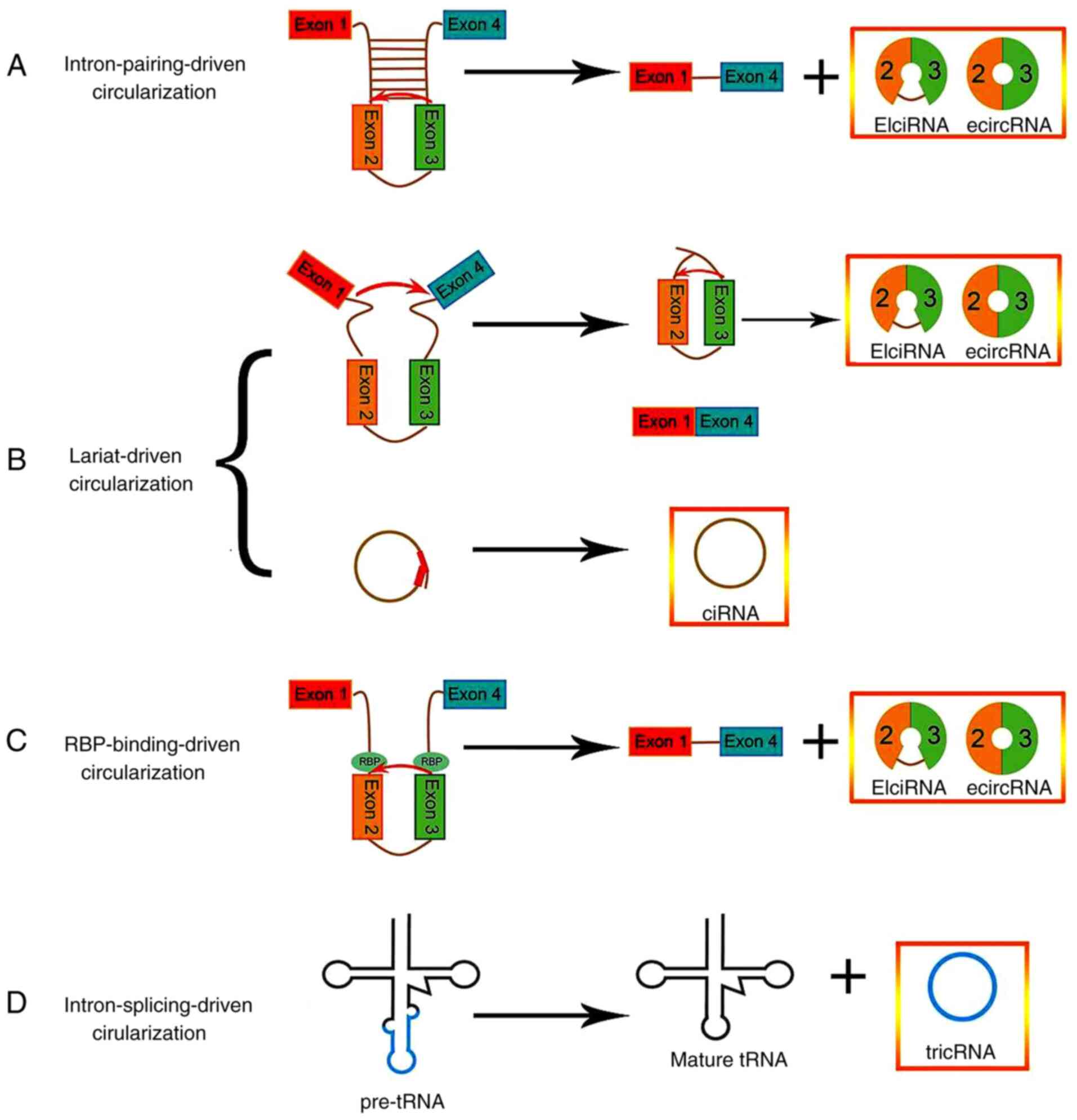
their genomic origin, circRNAs can be divided into four types:
Exonic circRNAs, circular intronic RNAs (ciRNAs), exon-intron
circRNAs and tRNA intronic circRNAs (tricRNAs) (1,27,28).
Several studies have demonstrated that the biogenesis of circRNAs
occurs via four primary models: Lariat-driven circularization path,
intron-pairing driven circularization path, RNA-binding protein
(RBP)-binding-driven circularization path and intron-splicing
driven circularization path (Fig.
2) (29,30). The first model is the lariat-driven
circularization path, which requires an upstream 3′ splice site to
be joined to a downstream 5′ splice site, resulting in exon
skipping to form an RNA lariat consisting of several exons and
introns (31).
Intron-pairing-driven circularization is the second model of
circRNA biogenesis. circRNA formation is dependent on ALU elements
or flanking inverted repetitive sequences to promote
circularization by base-pairing across different introns (32). Notably, ciRNA biogenesis requires a
key motif consisting of both a 7-nt GU-rich element near the 5′
splice site and an 11-nt C-rich element near the branch point site
(33). The third model is called
RBP-binding driven circularization, as RBP has an important
function in initiating circRNA formation via regulation of adjacent
splice site (34). Finally, the
fourth model is known as intron-splicing-driven circularization;
the pre-tRNA is identified and spliced by the tRNA splicing
nuclease complex to remove the excised tRNA introns, which then
release and ligate the intron termini to form a tRNA and a tricRNA
(35,36).
Biological functions of circRNAs
To date, the majority of biological functions
attributed to circRNAs remain largely incompletely understood.
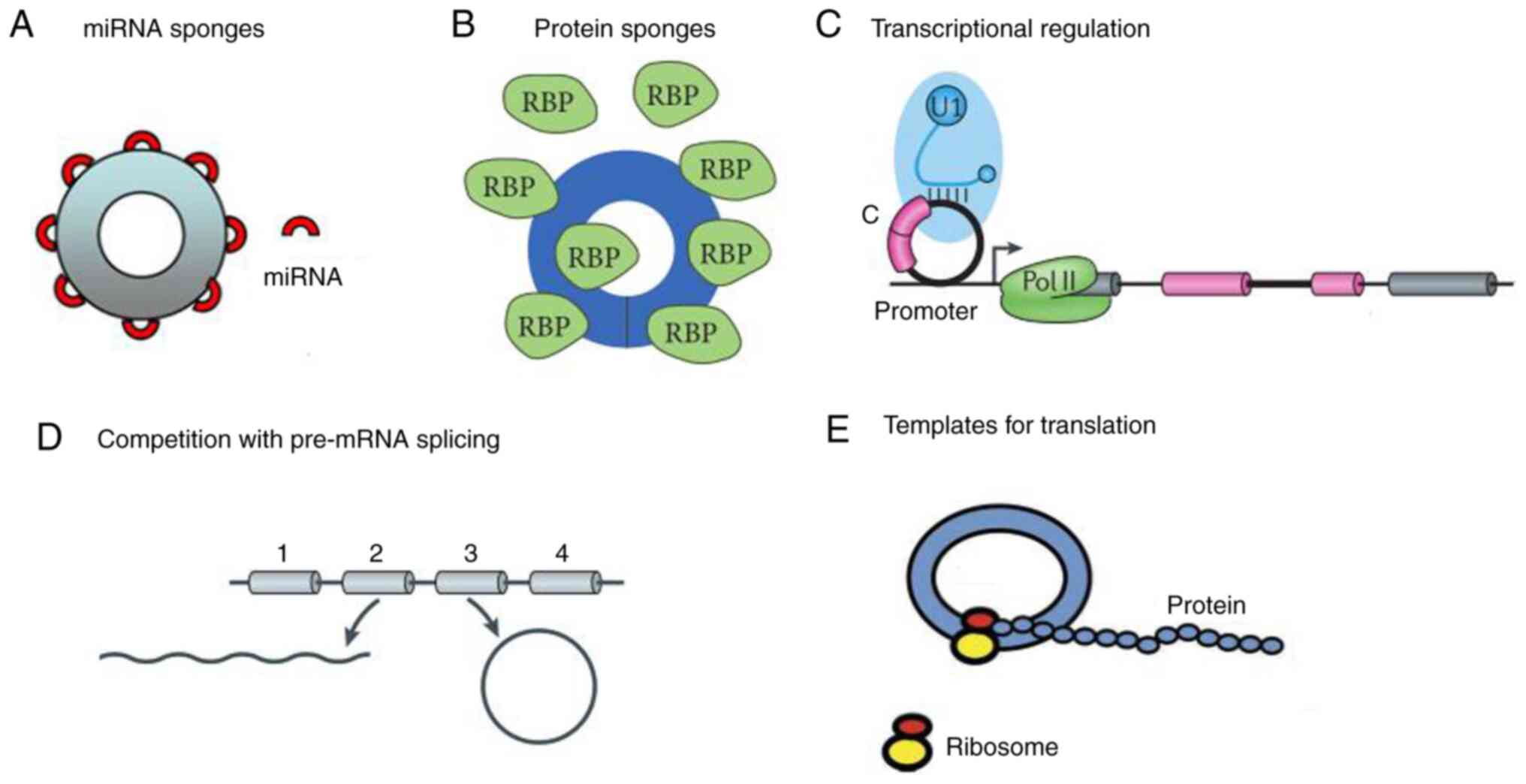
circRNA has a multitude of reported functions, which include
serving as a miRNA and protein sponge and transcriptional
regulator, and can be translated into protein (Fig. 3).
MicroRNA (miRNA/miR) and protein
sponges
circRNAs can bind miRNAs to inhibit their function
as competitive endogenous RNAs or miRNA sponges (37). An example of this is circRNA Cdr1as
(also known as ciRS-7), which was first reported to function as a
sponge of miR-7, containing >70 conserved miR-7 binding sites,
and thereby significantly decreasing miR-7 levels when its
expression is increased (3,38). Similarly, another established
example is circSRY, which is derived from the sex-determining
region of the Y chromosome and is composed of 16 binding sites for
miR-138 and specifically expressed in the testis (39). Furthermore, numerous studies have
demonstrated that circRNAs can function as miRNA sponges. For
example, circHIPK3 can regulate cell proliferation by functioning
as a sponge of miR-124, inhibiting its expression (20). circNT5E can directly bind miR-422a,
acting as a sponge to regulate cell proliferation, migration and
invasion in glioblastoma (40).
Yang et al (41) has
reported that circAmotl1 can bind with c-myc to induce c-myc
nuclear translocation and prevent c-myc mRNA degradation,
ultimately promoting tumorigenesis.
Studies have shown that circRNAs can also act as a
protein or RBP sponge. For example, circMbl, which is derived from
the second exon of muscleblind (Mbl), contains the binding sites
for the Mbl protein; circMb1 is able to directly bind with Mbl to
regulate Mbl expression by competing with conventional pre-mRNA
splicing, thereby decreasing the levels of Mbl and in turn
affecting the formation of circMbl (26,42).
The human antigen R (HuR) protein is an RBP, which is indispensable
for promoting the translation of the mRNAs (43).
circPABPN1 has been found to compete with poly(A)
binding protein nuclear 1 (PABPN1) mRNA to prevent HuR from binding
with PABPN1 mRNA, suppressing its translation (44). circFoxo3, which has recently
received increasing attention, has been found to be primarily
located in the cytoplasm, where it has been demonstrated to serve
as an adaptor bridging p21 and cyclin-dependent kinase 2 (CDK2)
(45). Moreover, circFoxo3 can
repress cell cycle progression via interaction with p21 and CDK2 to
establish a circFoxo3/p21/CDK2 ternary complex inducing cell cycle
regulation (45). Additionally, a
recent study has revealed that circCcnb1 can interact with both
CCNB1 and CDK1 proteins to suppress migration, invasion and
proliferation of HTB126 cells (46).
circRNAs as transcriptional
regulators
Zhang et al (28) has found that both circRNAs
ci-ankrd52 and ci-sirt7 can combine with RNA Pol II in cis, and
positively enhance the transcriptional activity of the host genes.
Similarly, Li et al (47)
has revealed that circEIF3J and circPAIP2 can interact with U1
small nuclear ribonucleic proteins to form a complex, and then bind
to Pol II at the promoter region of the host genes to regulate
transcription of their host genes. It has been suggested that
circRNAs are involved in the regulation of alternative splicing. In
1998, Chao et al (48)
reported that the Fmn gene could produce a circRNA via back
splicing. In another example, a circRNA from SEPALLATA3 (SEP3) gene
regulates splicing of its host SEP3 mRNA via formation of the
R-loop, resulting in the suspension of host gene transcription
(49). Similar observations have
also been attributed to circITCH, which is involved in the
regulation of the expression of its host gene (ITCH) indirectly,
via sponging its targets miR-1, miR-17 and miR-214 (50).
Translation
As circRNAs lack a 5′-cap and a 3′-end, they were
initially shown to not possess protein encoding capacity. However,
emerging evidence has indicated that circRNAs may function as a
template for translation or synthesis of proteins or peptides
(51).
circRNAs were first suggested to possess protein
encoding capacity if the sequence contained an internal ribosome
entry site (IRES), in a study published in 1995 (52). A subsequent study revealed that
circZNF609 could translate proteins in murine myoblasts when driven
by IRES in a splicing-dependent and cap-independent manner, which
indicated that circRNAs exhibited protein-coding capacity (53). Yang et al (54) demonstrated that circFBXW7 could be
translated into a novel 21-kDa functional protein [FBXW7-185 amino
acids (aa) and its expression was negatively associated with
glioblastoma. Another study reported that circSHPRH contained an
open reading frame and encoded a 17-kDa protein (SHPRH-146aa)
driven by IRES (55). Furthermore,
both circSHPRH and SHPRH-146aa expression was decreased in
glioblastoma, thereby suppressing cell proliferation and
tumorigenesis (55). In addition, a
more recent study by Yang et al (56) demonstrated that N-methyladenosine,
the most frequent RNA modification, could promote efficient
initiation of protein translation from circRNAs. circPINT, which is
derived from exon 2 of long intergenic non-protein coding RNA
p53-induced transcript (LINC-PINT), can translate proteins, termed
PINT87aa encoded by the circular, but not the linear, form of
LINC-PINT (57). Whether these
translatable circRNAs serve a physiological function remains to be
experimentally investigated and confirmed.
circHIPK3 and human diseases
circHIPK3 and prostate cancer
(PC)
PC is a common malignancy of the urinary system and
is a leading cause of cancer-associated death in men (58). Cai et al (59) revealed that circHIPK3 expression was
upregulated in PC tissues and cells. Overexpression of circHIPK3
accelerated the proliferation and invasiveness of PC cells by
sponging miR-338-3p to regulate ADAM17 expression (59). This result provides a potentially
novel preventative and therapeutic target for the management of PC.
Similarly, Chen et al (60)
illustrated that circHIPK3 mediates miR-193a-3p/MCL1 signaling to
promote cell proliferation and invasion of PC.
circHIPK3 and lung cancer (LC)
LC is a major health threat and the largest cause of
cancer-associated death worldwide (61). Studies have revealed that circHIPK3
exerts oncogenic properties in LC. Yu et al (62) reported that circHIPK3 regulated the
expression levels of sphingosine kinase 1, CDK4 and STAT3 by acting
as a miR-124 sponge in LC. Recently, Lu et al (63) assessed the clinical significance of
circHIPK3 in patients with primary non-small cell LC (NSCLC).
Further study (63) demonstrated
that circHIPK3 induced cell proliferation and inhibited apoptosis
in NSCLC by sponging miR-149, indirectly increasing FOXM1
expression. Similarly, Hong et al (64) confirmed that circHIPK3 promoted
NSCLC progression via a circHIPK3/miR-107/brain-derived
neurotrophic factor (BDNF) axis, highlighting potential markers for
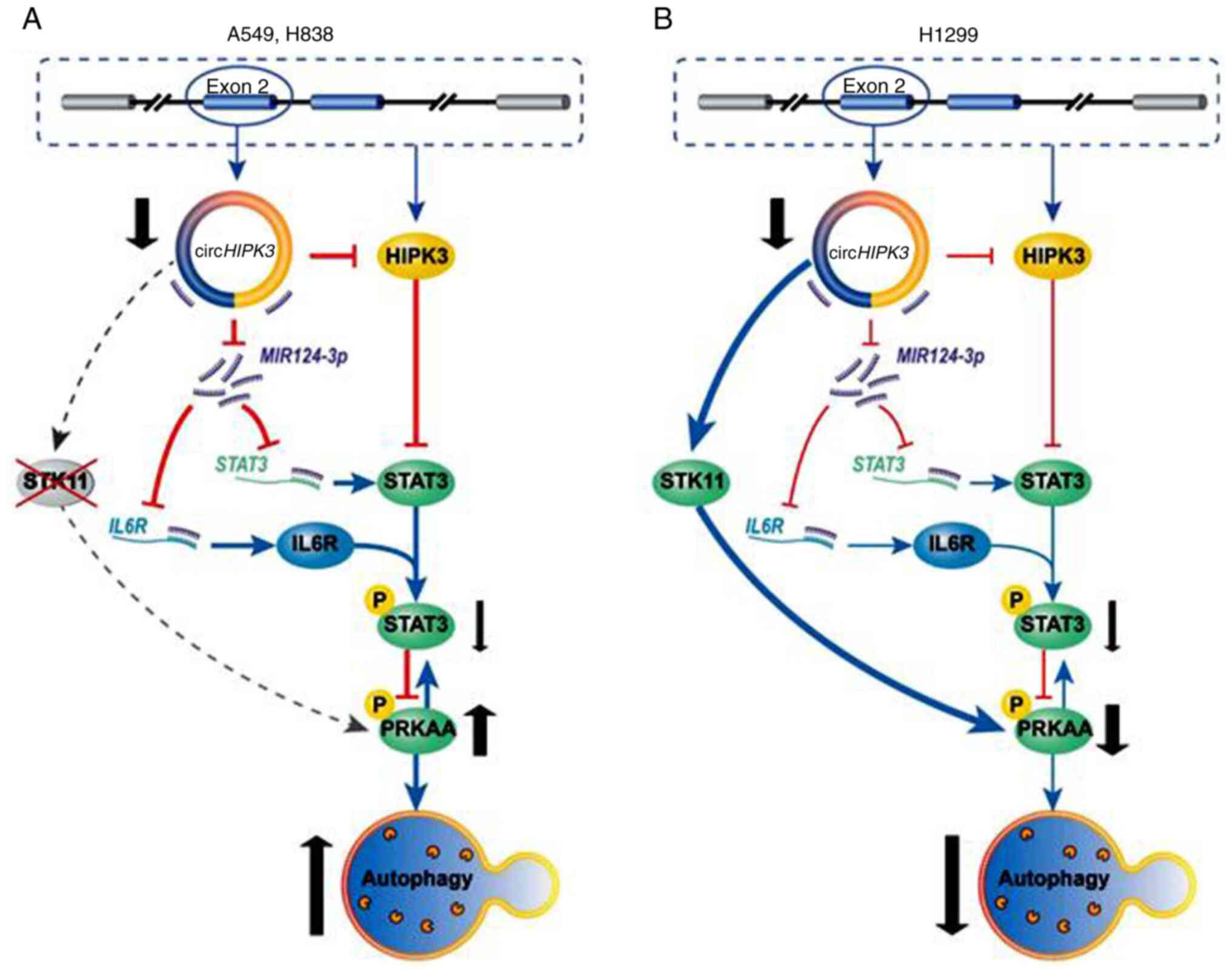
NSCLC screening. A study by Chen et al (65) demonstrated that circHIPK3 functioned
as an oncogene, and loss of circHIPK3 significantly impaired cell
proliferation, migration and invasion, and induced protective
autophagy via a miR-124-3p/STAT3/protein kinase AMP-activated
catalytic subunit a2/AMPKa axis in serine/threonine kinase
11-mutant LC cell lines (A549 and H838; Fig. 4). Overall, the aforementioned
results highlight the potential prognostic and therapeutic value of
circHIPK3 in LC.
circHIPK3 and colorectal cancer
(CRC)
CRC is the most common gastrointestinal malignant
disease, which ranks third in incidence and second in mortality
amongst all types of cancer (66).
In 2018, a study by Zeng et al (21) was the first study to reveal an
association between circHIPK3 and CRC. circHIPK3 was shown to
function as an oncogene, resulting in the proliferation, migration
and invasion of CRC cells, whilst decreasing apoptosis (21). Furthermore, circHIPK3 expression was
upregulated in CRC by sponging miR-7 to sequester and inhibit miR-7
activity, suggesting that circHIPK3 may have value as a prognostic
biomarker in CRC (21). Similarly,
another study demonstrated that circPIK3 expression was upregulated
in CRC cells and further promoted migration, invasion and
proliferation of CRC cells by sponging miR-1207-5p, which directly
targeted formin-like 2 (FMNL2) (67). These results suggest that circHIPK3
may modulate a miR-1207-5p/FMNL2 axis, highlighting a potentially
novel strategy in the management of CRC.
circHIPK3 and gastric cancer (GC)
GC is the leading cause of malignant
tumor-associated mortality worldwide and is the most common type of
digestive tract cancer, accounting for a third of tumor-associated
deaths (68). circHIPK3 has been
reported to be involved in GC progression by sponging miR-124 and
miR-29b to regulate its target genes collagen type I α1 chain
(COL1A1), COL4A1 and CDK6 (69).
circHIPK3-knockdown inhibits GC cell proliferation (69). Subsequently, Wei et al
(70) confirmed that circHIPK3 was
upregulated in GC and associated with clinical stage and grade of
GC. Furthermore, circHIPK3-knockdown suppressed the proliferation
and migration of GC cells via a circHIPK3/miR-107/BDNF axis.
Upregulation of circHIPK3 is associated with a poor prognosis, and
thus it may serve as a potential target for GC treatment (70).
circHIPK3 and bladder cancer (BC)
BC is the most common type of tumor of the urinary
system and the 9th most frequently diagnosed malignant cancer in
the world (71). Recently, Li et
al (72) identified thousands
of differentially expressed circRNAs between human BC tissues and
normal control bladder tissues using RNA sequencing. circHIPK3 is
significantly downregulated in BC tissues and cell lines by
sponging miR-558 and further suppressing the expression of
heparanase. Furthermore, overexpression of circHIPK3 significantly
decreased invasion, metastasis and angiogenesis of BC cells,
suggesting that circHIPK3 was negatively associated with BC grade
(72). circHIPK3 may thus function
as a tumor suppressor. Additionally, Xie et al (73) found that circHIPK3 expression was
low in BC tissue and its overexpression promoted gemcitabine
sensitivity in patients with BC.
circHIPK3 and nasopharyngeal carcinoma
(NPC)
NPC is a common malignant tumor in humans, which
occurs in the head region (74). In
2018, Ke et al (75)
revealed that circHIPK3 expression was higher in NPC tissues and
cell lines compared with in normal tissues and cells. circHIPK3
overexpression enhanced tumor cell proliferation, and when
circHIPK3 expression was silenced or depleted, proliferation,
migration and invasion were significantly decreased in vitro
(75). Thus, circHIPK3 seemed to
possess an oncogenic role in NPC. Further analyses revealed that
circHIPK3 acted as a sponge to negatively regulate miR-4288
expression, which in turn targeted E74-like ETS transcription
factor 3 (ELF3), thereby increasing ELF3 expression (75).
circHIPK3 and gallbladder cancer
(GBC)
GBC is an aggressive and lethal malignancy of the
bile duct, and patients often have a poor prognosis (76). In 2018, Kai et al (77) found that circHIPK3 expression in
human GBC cells was higher than that in gallbladder epithelial
cells. Apoptosis was induced when circHIPK3 expression was knocked
down using a small interfering RNA, which potently inhibited
survival and proliferation of established and primary human GBC
cells. It was further demonstrated that circHIPK3 acted as a sponge
of miR-124, ultimately increasing the expression of miR-124 targets
(Rho-associated coiled-coil containing protein kinase 1 and CDK6)
and decreasing the activity of miR-124 in GBC cells (77).
circHIPK3 and hepatocellular carcinoma
(HCC)
HCC is the 5th leading type of cancer and the most
lethal type of carcinoma worldwide (78). circHIPK3 is significantly
upregulated in HCC, where it promotes cell proliferation,
highlighting its role as an oncogene (79). Chen et al (79) demonstrated that circHIPK3 regulated
HCC via a circHIPK3/miR124/aquaporin 3 (AQP3) axis. Notably,
circHIPK3 was upregulated in HCC cells and promoted cell
proliferation and migration by sponging miR-124 and regulating AQP3
expression (79).
circHIPK3 and osteosarcoma (OS)
OS is the most common type of primary bone cancer,
and is more likely to occur in children and adolescents (80). A recent study by Xiao-Long et
al (81) revealed that
circHIPK3 expression was significantly downregulated in OS cell
lines and tissues, and its low expression was associated with a
poor prognosis. Functional analysis revealed that downregulated
circHIPK3 expression resulted in a shorter OS cell survival time,
whereas overexpression of circHIPK3 suppressed proliferation,
migration and invasion of OS cells (81). These results suggest that circHIPK3
expression levels may negatively regulate cell behavior and thus
that circHIPK3 may be used as a biomarker for OS detection.
circHIPK3 and glioma
Glioma is the most malignant tumor of the adult
brain (82). Emerging evidence has
revealed that circHIPK3 is associated with glioma, where it
functions as an oncogene. For example, circHIPK3 accelerates tumor
growth in glioma by sponging miR-124-3p, resulting in upregulation
of STAT3 expression (83). Jin
et al (84) demonstrated
that circHIPK3 overexpression significantly promoted the malignant
behaviors of glioma cells, increasing proliferation and invasion by
sponging miR-654 via a circHIPK3/miR-654/IGF2BP3insulin-like growth
factor 2 mRNA binding protein 3 axis, suggesting circHIPK3 may
serve as a potential therapeutic target for the treatment of
patients with glioma.
circHIPK3 and epithelial ovarian
cancer (EOC)
OC is the most fatal gynecological cancer amongst
women and has a death rate of 60% in Asia (85). Teng et al (86) investigated the expression profiles
of circRNAs between EOC and normal ovarian tissues using RNA
sequencing, revealing that circHIPK3 expression was significantly
downregulated in EOC. Moreover, silencing of circHIPK3 promoted the
proliferation, migration and invasion of OC cells, suggesting that
circHIPK3 may be an important regulator of OC progression, where it
exerts a tumor-suppressive function (86).
circHIPK3 and oral squamous cell
carcinoma (OSCC)
OSCC is one of the top 10 most common types of
cancer of the head and neck worldwide (87). Wang et al (88) analyzed circHIPK3 expression and its
clinical significance in OSCC, and its association with miR-124
expression. circHIPK3 expression in OSCC tissues was significantly
higher compared with that of the adjacent non-cancerous tissues.
Moreover, miR-124 expression in OSCC tissues was significantly
lower compared with that in precancerous tissues. Further
correlation analysis revealed that circHIPK3 expression negatively
regulated miR-124 expression, and silencing of circHIPK3 expression
decreased the proliferation of OSCC cells (88). The aforementioned findings suggest
that circHIPK3 may contribute to the occurrence and development of
OSCC by regulating miR-124 expression.
circHIPK3 and chronic myeloid leukemia
(CML)
CML is caused by a reciprocal translocation in
chromosomes, and accounts for 15% of reported cases of leukemia
(89). Feng et al (90) investigated the expression profile of
circHIPK3 in CML, revealing that its expression was significantly
upregulated in peripheral blood mononuclear cells and serum samples
from CML compared with in healthy normal samples. Further
experiments demonstrated that loss-function of circHIPK3 promoted
CML progression, indicating that circHIPK3 may serve as a
prognostic biomarker (90).
circHIPK3 and age-related cataracts
(ARC)
ARC is the leading cause of visual impairment and
blindness worldwide (91).
Recently, Liu et al (92)
reported that circHIPK3 regulated human lens epithelial cell (HLEC)
function via a circHIPK3/miR-193a/crystallin αA regulatory network.
Furthermore, knockdown of circHIPK3 affected the viability,
apoptosis and proliferation of HLECs, suggesting a potential role
of circHIPK3 in ARC formation (92). These findings highlight a
potentially novel targeted method for the prevention and treatment
of ARC.
circHIPK3 and preeclampsia
Preeclampsia, a devastating multisystem syndrome, is
becoming an increasingly common disease worldwide, and is
associated with a high rate of pregnancy-associated morbidity and
mortality (93). Zhang et al
(94) first explored the possible
role of dysregulated circHIPK3 expression and its potential
contribution to the pathogenesis of preeclampsia. It was revealed
that circHIPK3 was significantly downregulated in preeclampsia
compared with healthy pregnant controls. circHIPK3 silencing
inhibited the migration, invasion, proliferation and tube formation
capacities of HTR8/SVneo cells, and circHIPK3 overexpression
significantly promoted these capacities, excluding
proliferation.
circHIPK3 and pancreatic cancer
(PCa)
PCa is a malignant tumor of the digestive system
with a low probability of incidence (95). circHIPK3 is expressed in pancreatic
tissues (96). Liu et al
(97) revealed that circHIPK3
expression was upregulated in PCa tissues and was associated with
GEM-resistant PCa tissues and cells. Moreover, circHIPK3 exerted
its function by enhancing GEM resistance in PCa cells by sponging
miR-330-5p, resulting in upregulation of Ras association domain
family member 1, ultimately regulating PCa cell proliferation,
invasion, migration, epithelial-mesenchymal transition (EMT) and
apoptosis (97).
circHIPK3 and acute pancreatitis
(AP)
AP is a dangerous disease with a high mortality rate
(98). Wang et al (99) revealed that circHIPK3 expression was
closely associated with inflammation in AP. Silencing its
expression inhibited the release of IL-1β and TNF-α (99). Additionally, a recent study
demonstrated that circHIPK3 expression was upregulated in the serum
of patients compared with AP and in caerulein-stimulated AR42J
cells (100). Furthermore, it was
revealed that circHIPK3 sponged miR-193a-5p to negatively regulate
its expression. Gasdermin D (GSDMD) is a target gene of
miR-193a-5p, and is a key gene involved in pyroptosis. Thus,
silencing miR-193a-5p reversed the effects of GSDMD. These findings
suggest that circHIPK3 may promote pyroptosis and inflammation via
regulation of the miR-193a-5p/GSDMD axis in AR42J cells (100).
circHIPK3 and cardiac fibrosis
(CF)
CF is a common pathological process that often
results in death (101). Ni et
al (102) revealed that
circHIPK3 promoted the proliferation and migration of cardiac
fibroblasts in CF. Furthermore, circHIPK3 expression was markedly
increased in CF and heart tissues following treatment with
angiotensin II (Ang II). Inhibition of circHIPK3 prevented Ang
II-induced CF by sponging miR-29b-3p. The silencing of circHIPK3
effectively reversed miR-29b-3p-induced promotion of CF function
and influenced the expression levels of genes targeted by
miR-29b-3p (α-smooth muscle actin, COL1A1 and COL3A1), suggesting
that circHIPK3 may exhibit potential as a targeted therapy for the
management of CF (102).
circHIPK3 and allergic rhinitis
(AR)
AR is the most common allergic disease affecting
individuals of various demographics worldwide (103). circHIPK3 and long non-coding RNA
(lncGAS5) were shown to promote differentiation of T helper 2 cells
and aggravate AR via modulating their common target miR-495
(104). Moreover, intranasal
administration of circHIPK3/lncGAS5 knockdown lentivirus resulted
in a decrease in AR symptoms by downregulating GATA binding protein
3 expression, highlighting a potential therapeutic means for AR
management (104).
circHIPK3 and renal cancer (RC)
RC is one of the most common malignant tumors of the
urinary system, and the incidence of RC is increasing worldwide
(105). Recently, Lai et al
(106) revealed that circHIPK3
exerted an oncogenic role, and its expression was upregulated in RC
tissues and cells. Additionally, it was shown to promote
proliferation and migration, and inhibit the apoptosis of RC cells
by competitively binding with miR-485-3p, in-turn indirectly
increasing the expression levels of Bcl-2, N-cadherin, vimentin and
Ki-67 (106). circHIPK3-knockdown
inhibited the proliferation, migration and invasion of RC cells
(106). These results provide a
promising basis for the molecular-targeted therapy of patients with
RC. circHIPK3 may serve as a tumor suppressor in RC progression. Li
et al (107) found that the
overexpression of circHIPK3 significantly inhibited RC cell
invasion and migration in vitro, and it repressed
proliferation of RC cells in vitro and in vivo.
Additionally, another study by Han et al (108) demonstrated that circHIPK3 promoted
clear cell RC cell proliferation and migration by altering
miR-5083p/CXCL13 signaling, highlighting a potentially novel target
for the molecular treatment of clear cell RC.
circHIPK3 and cervical cancer
(CC)
CC is the most common malignancy in women worldwide
with >400,000 new cases of CC diagnosed each year (109). Recently, Qian et al
(110) investigated the function
of circHIPK3 and its clinical application in CC. circHIPK3
functioned as a sponge of miR-338-3p, resulting in upregulation of
hypoxia-inducible factor (HIF)-1α expression, and thus promoting CC
cell proliferation and EMT, resulting in tumorigenesis (110). miR-338-3p silencing or HIF-1α
overexpression rescued circHIPK3-knockdown-mediated inhibition or
induction of apoptosis in CC cells (110). These findings may serve as a basis
in the search for a promising treatment strategy by highlighting
the role of the circHIPK3/miR-338-3p/HIF-1α axis in CC.
circHIPK3 and thyroid cancer (TC)
TC is one of the most common endocrine malignancies
globally, and it originates from follicular or parafollicular
thyroid cells (111). Recently,
Shu et al (112)
investigated the effects of circHIPK3 on the proliferation of TC
cells and migration of TC. It was revealed that circHIPK3 acted as
an oncogenic circRNA in TC, promoting tumorigenesis and
invasiveness of TC by sponging miR-338-3p, in turn upregulating the
expression of its target gene RAB23. Knockdown of circHIPK3
significantly decreased the migration, invasion and proliferation
of TC cells (112). These results
suggest that circHIPK3 may serve as a novel biomarker for the
diagnosis and prognosis of TC.
circHIPK3 and breast cancer (BRC)
BRC is one of the most common malignant types of
cancer amongst women and is the leading cause of cancer-associated
death worldwide (113). BRC is a
major disease threatening female health, affecting >7% of women
in >100 countries (114). Chen
et al (115) revealed that
circHIPK3 expression was upregulated in BRC, where it facilitated
cell proliferation, migration and invasion by targeting miR-193a.
Furthermore, overexpression of circHIPK3 enhanced high mobility
group box 1/PI3K/AKT signaling, highlighting circHIPK3 as a novel
potential therapeutic target for BRC management.
Conclusions and future perspectives
circRNAs have attracted increasing attention over
the last decade. In recent years, with the advancement of high
throughput RNA sequencing technologies and the rapid development of
bioinformatics tools, a large number of circRNAs have been
discovered and identified in various organisms. With the efforts of
scientists and the application of novel biological methods, the
potential role of circRNAs in biological functions is being
elucidated. Given the large number of different circRNAs and their
potential tissue-specific functions, an in-depth understanding of
the complex networks in which they participate and regulate should
be developed.
circHIPK3 has been reported to be involved in
various human diseases, including different types of cancer.
Dysregulation of circHIPK3 has been observed in a range of diseases
and cancerous tissues, where it has been shown to exhibit notable
effects on cell cycle progression, cell proliferation, apoptosis,
invasion and migration in cancer, suggesting that circHIPK3 may
possess significant value as a molecular biomarker for the
diagnosis, prognosis and monitoring of diseases. The vital
physiological and pathological functions of circHIPK3 in diseases
are listed in Table I. circHIPK3
has been described as both a tumor suppressor and an oncogene.
Currently, due to the technical limitations of available methods,
the role of circHIPK3 in cancer and various diseases remains to be
further elucidated. miRNA sponging is one of the primary mechanisms
by which circHIPK3 exerts its different functions in various
diseases. Thus, further studies are required to extensively explore
the interaction network of circHIPK3 in cancer and diseases,
including the involvement of miRNAs, lncRNAs, mRNAs and protein
degrading pathways. Whether there are other regulatory mechanisms
or if they possess other functions should be further studied.
 | Table I.Functional roles of circHIPK3 in
different types of cancer and diseases. |
Table I.
Functional roles of circHIPK3 in
different types of cancer and diseases.
| First author,
year | Disease | Expression | Functional
roles | Target miRs and
genes | Refs. |
|---|
| Cai et al,
2019; Chen et al, 2019 | Prostate
cancer | Up | Proliferation and
invasion | miR-338-3p/ADAM17;
miR-193a-3p/MCL1 | (59,60) |
| Yu et al,
2018; Lu et al, 2020; Hong et al 2020; Chen et
al, 2020 | Lung cancer | Up | Viability,
proliferation and apoptosis | miR-124/SphK1,
STAT3, CDK4; miR-149/FOXM1 miR-107/BDNF miR-124-3p/STAT3, PRKAA,
AMPKa | (62–65) |
| Yan et al,
2020 | Colorectal
cancer | Up | Proliferation,
migration, invasion and apoptosis |
miR-1207-5p/FMNL2 | (67) |
| Cheng et al,
2018; Wei et al, 2020 | Gastric cancer | Down | Unknown | miR-124,
miR-29b/COL1A1, COL4A1, CDK6, WNT1 | (69,70) |
|
|
| Up | Proliferation and
migration | miR-107/BDNF TCF4,
β-catenin |
|
| Li et al,
2017 | Bladder cancer | Down | Migration,
invasion, angiogenesis and metastasis | miR-558/HPSE,
VEGF | (72) |
| Ke et al,
2019 | Nasopharyngeal
carcinoma | Up | Proliferation,
migration and invasion | miR-4288/ELF3 | (75) |
| Kai et al,
2018 | Gallbladder
cancer | Up | Viability,
proliferation and apoptosis | miR-124/ROCK1,
CDK6, miR-29b | (77) |
| Chen et al,
2018 | Hepatocellular
carcinoma | Up | Proliferation and
migration | miR-124/AQP3 | (79) |
| Xiao-Long et
al, 2018 | Osteosarcoma | Down | Proliferation,
migration and invasion | miR-7/miR-124 | (81) |
| Hu and
Zhang, 2019; Jin et al, 2018 | Glioma | Up | Proliferation,
migration and invasion | miR-654/IGF2BP3,
miR-124-3p/STAT3 | (83,84) |
| Teng et al,
2019 | Epithelial ovarian
cancer | Up | Unknown | Unknown | (86) |
| Wang et al,
2018 | Oral squamous cell
carcinoma | Up | Proliferation | miR-124 | (88) |
| Feng et al,
2020 | Chronic myeloid
leukemia | Up | Unknown | Unknown | (90) |
| Liu et al,
2018 | Age-related
cataract | Down | Viability,
proliferation and apoptosis | miR-193a/CRYAA | (92) |
| Zhang et al,
2019) | Preeclampsia | Down | Migration, invasion
and proliferation | Unknown | (94) |
| Liu et al,
2020 | Pancreatic
cancer | Up | Proliferation,
invasion, migration and apoptosis |
miR-330-5p/RASSF1 | (97) |
| Wang et al,
2020 | Acute
pancreatitis | Up | Infiltration |
miR-193a-5p/GSDMD | (100) |
| Ni et al,
2019 | Cardiac
fibrosis | Up | Proliferation and
migration | miR-29b-3p/a-SMA,
COL1A1, COL3A1 | (102) |
| Zhu et al,
2020 | Allergic
rhinitis | Up | Unknown | miR-495 | (104) |
| Lai et al,
2020; Li et al, 2020; Han et al, 2020 | Renal cancer | Up | Proliferation and
migration | miR-485-3p/Bcl-2,
N-cadherin, vimentin, Ki-67, miR-5083P/CXCL13 | (106–108) |
| Qian et al,
2020 | Cervical
cancer | Up | Proliferation |
miR-338-3p/HIF-1α | (110) |
| Shu et al,
2020 | Thyroid cancer | Up | Proliferation,
migration and invasion |
miR-338-3p/RAB23 | (112) |
| Chen et al,
2020 | Breast cancer | Up | Proliferation,
migration and invasion |
miR-193a/HMGB1/PI3K/AKT | (115) |
The exact mechanisms by which circHIPK3 regulates
pathological processes remain unclear in certain diseases, such as
EOC, CML, AR and preeclampsia. Future studies should explore any
other potential mechanisms and functions of circHIPK3. To the best
of our knowledge, there is no preclinical evidence for the
application of circHIPK3 as a target or molecular targeted
therapeutic tool for cancer treatment. Based on the findings
described in the present review, it may be suggested that circHIPK3
may serve as a predictive biomarker and therapeutic target with
clinical promise in the future.
Acknowledgements
Not applicable.
Funding
The present review was partly supported by the
Natural Science Foundation of China (grant nos. 1471971, 31402263
and 31872537).
Availability of data and materials
Not applicable.
Authors' contributions
QS conceptualized the review, performed the
literature search and drafted the manuscript. YH and CZ helped to
draft and revise the manuscript. XG and SG edited and revised the
paper. All authors have read and approved the final version of the
manuscript to be published.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu J, Liu T, Wang X and He A: Circles
reshaping the RNA world: From waste to treasure. Mol Cancer.
16:582017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jeck WR and Sharpless NE: Detecting and
characterizing circular RNAs. Nat Biotechnol. 32:453–461. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cocquerelle C, Mascrez B, Hétuin D and
Bailleul B: Mis-splicing yields circular RNA molecules. FASEB J.
7:155–160. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sanger HL, Klotz G, Riesner D, Gross HJ
and Kleinschmidt AK: Viroids are single-stranded covalently closed
circular RNA molecules existing as highly base-paired rod-like
structures. Proc Natl Acad Sci USA. 73:3852–3856. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hsu MT and Coca-Prados M: Electron
microscopic evidence for the circular form of RNA in the cytoplasm
of eukaryotic cells. Nature. 280:339–340. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang W, Qin P, Gong X, Huang L, Wang C,
Chen G, Chen J, Wang L and Lv Z: Identification of circRNAs in the
liver of whitespotted bamboo shark (Chiloscyllium
plagiosum). Front Genet. 11:5963082020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang J, Liu R, Zhu Y, Gong J, Yin S, Sun
P, Feng H, Wang Q, Zhao S, Wang Z and Li G: Identification and
characterization of circRNAs responsive to methyl jasmonate in
Arabidopsis thaliana. Int J Mol Sci. 21:7922020. View Article : Google Scholar
|
|
9
|
Li L, Sun D, Li X, Yang B and Zhang W:
Identification of key circRNAs in non-small cell lung cancer. Am J
Med Sci. 361:98–105. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen LL and Yang L: Regulation of circRNA
biogenesis. RNA Biol. 12:381–388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Geng X, Jia Y, Zhang Y, Shi L, Li Q, Zang
A and Wang H: Circular RNA: Biogenesis, degradation, functions and
potential roles in mediating resistance to anticarcinogens.
Epigenomics. 12:267–283. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Salzman J: Circular RNA expression: Its
potential regulation and function. Trends Genet. 32:309–316. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wan B, Liu B and Lv C: Progress of
research into circular RNAs in urinary neoplasms. PeerJ.
8:e86662020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jahani S, Nazeri E, Majidzadeh-A K, Jahani
M and Esmaeili R: Circular RNA; a new biomarker for breast cancer:
A systematic review. J Cell Physiol. 235:5501–5510. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang G, Li S, Yang N, Zou Y, Zheng D and
Xiao T: Recent progress in circular RNAs in human cancers. Cancer
Lett. 404:8–18. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee ECS, Elhassan SAM, Lim GPL, Kok WH,
Tan SW, Leong EN, Tan SH, Chan EWL, Bhattamisra SK, Rajendran R and
Candasamy M: The roles of circular RNAs in human development and
diseases. Biomed Pharmacother. 111:198–208. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lei K, Bai H, Wei Z, Xie C, Wang J, Li J
and Chen Q: The mechanism and function of circular RNAs in human
diseases. Exp Cell Res. 368:147–158. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Soghli N, Qujeq D, Yousefi T and Soghli N:
The regulatory functions of circular RNAs in osteosarcoma.
Genomics. 112:2845–2856. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Conte A and Pierantoni GM: Update on the
regulation of HIPK1, HIPK2 and HIPK3 protein kinases by microRNAs.
Microrna. 7:178–186. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng Q, Bao C, Guo W, Li S, Chen J, Chen
B, Luo Y, Lyu D, Li Y, Shi G, et al: Circular RNA profiling reveals
an abundant circHIPK3 that regulates cell growth by sponging
multiple miRNAs. Nat Commun. 7:112152016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeng K, Chen X, Xu M, Liu X, Hu X, Xu T,
Sun H, Pan Y, He B and Wang S: circHIPK3 promotes colorectal cancer
growth and metastasis by sponging miR-7. Cell Death Dis. 9:4172018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wen Y, Li B, He M, Teng S, Sun Y and Wang
G: circHIPK3 promotes proliferation and migration and invasion via
regulation of miR-637/HDAC4 signaling in osteosarcoma cells. Oncol
Rep. 45:169–179. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Liu Q and Liao Q: circHIPK3: A
promising cancer-related circular RNA. Am J Transl Res.
12:6694–6704. 2020.PubMed/NCBI
|
|
24
|
Wang J, Zhu M, Pan J, Chen C, Xia S and
Song Y: Circular RNAs: A rising star in respiratory diseases.
Respir Res. 20:32019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xie Y, Yuan X, Zhou W, Kosiba AA, Shi H,
Gu J and Qin Z: The circular RNA HIPK3 (circHIPK3) and its
regulation in cancer progression: Review. Life Sci. 254:1172522020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ashwal-Fluss R, Meyer M, Pamudurti NR,
Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N and
Kadener S: circRNA biogenesis competes with pre-mRNA splicing. Mol
Cell. 56:55–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ragan C, Goodall GJ, Shirokikh NE and
Preiss T: Insights into the biogenesis and potential functions of
exonic circular RNA. Sci Rep. 9:20482019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang C, Ma L, Niu Y, Wang Z, Xu X, Li Y
and Yu Y: Circular RNA in lung cancer research: Biogenesis,
functions, and roles. Int J Biol Sci. 16:803–814. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Panda AC, Grammatikakis I, Munk R, Gorospe
M and Abdelmohsen K: Emerging roles and context of circular RNAs.
Wiley Interdiscip Rev RNA. 8:10.1002/wrna.1386. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zang J, Lu D and Xu A: The interaction of
circRNAs and RNA binding proteins: An important part of circRNA
maintenance and function. J Neurosci Res. 98:87–97. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kelly S, Greenman C, Cook PR and
Papantonis A: Exon skipping is correlated with exon
circularization. J Mol Biol. 427:2414–2417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kristensen LS, Andersen MS, Stagsted LVW,
Ebbesen KK, Hansen TB and Kjems J: The biogenesis, biology and
characterization of circular RNAs. Nat Rev Genet. 20:675–691. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Y, Zhang XO, Chen T, Xiang JF, Yin
QF, Xing YH, Zhu S, Yang L and Chen LL: Circular intronic long
noncoding RNAs. Mol Cell. 51:792–806. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stagsted LVW, O'Leary ET, Ebbesen KK and
Hansen TB: The RNA-binding protein SFPQ preserves long-intron
splicing and regulates circRNA biogenesis in mammals. Elife.
10:e630882021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schmidt CA, Giusto JD, Bao A, Hopper AK
and Matera AG: Molecular determinants of metazoan tricRNA
biogenesis. Nucleic Acids Res. 47:6452–6465. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wen J, Liao J, Liang J, Chen XP, Zhang B
and Chu L: Circular RNA HIPK3: A key circular RNA in a variety of
human cancers. Front Oncol. 10:7732020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sumazin P, Yang X, Chiu HS, Chung WJ, Iyer
A, Llobet-Navas D, Rajbhandari P, Bansal M, Guarnieri P, Silva J
and Califano A: An extensive microRNA-mediated network of RNA-RNA
interactions regulates established oncogenic pathways in
glioblastoma. Cell. 147:370–381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Capel B, Swain A, Nicolis S, Hacker A,
Walter M, Koopman P, Goodfellow P and Lovell-Badge R: Circular
transcripts of the testis-determining gene Sry in adult mouse
testis. Cell. 73:1019–1030. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang R, Zhang S, Chen X, Li N, Li J, Jia
R, Pan Y and Liang H: CircNT5E acts as a sponge of miR-422a to
promote glioblastoma tumorigenesis. Cancer Res. 78:4812–4825. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang Q, Du WW, Wu N, Yang W, Awan FM, Fang
L, Ma J, Li X, Zeng Y, Yang Z, et al: A circular RNA promotes
tumorigenesis by inducing c-myc nuclear translocation. Cell Death
Differ. 24:1609–1620. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Barrett SP and Salzman J: Circular RNAs:
Analysis, expression and potential functions. Development.
143:1838–1847. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Joassard OR, Bélanger G, Karmouch J, Lunde
JA, Shukla AH, Chopard A, Legay C and Jasmin BJ: HuR mediates
changes in the stability of AChR β-subunit mRNAs after skeletal
muscle denervation. J Neurosci. 35:10949–10962. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Abdelmohsen K, Panda AC, Munk R,
Grammatikakis I, Dudekula DB, De S, Kim J, Noh JH, Kim KM,
Martindale JL and Gorospe M: Identification of HuR target circular
RNAs uncovers suppression of PABPN1 translation by circPABPN1. RNA
Biol. 14:361–369. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Du WW, Yang W, Liu E, Yang Z, Dhaliwal P
and Yang BB: Foxo3 circular RNA retards cell cycle progression via
forming ternary complexes with p21 and CDK2. Nucleic Acids Res.
44:2846–2858. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fang L, Du WW, Awan FM, Dong J and Yang
BB: The circular RNA circ-Ccnb1 dissociates Ccnb1/Cdk1 complex
suppressing cell invasion and tumorigenesis. Cancer Lett.
459:216–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li Z, Huang C, Bao C, Chen L, Lin M, Wang
X, Zhong G, Yu B, Hu W, Dai L, et al: Exon-intron circular RNAs
regulate transcription in the nucleus. Nat Struct Mol Biol.
22:256–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chao CW, Chan DC, Kuo A and Leder P: The
mouse formin (Fmn) gene: Abundant circular RNA transcripts and
gene-targeted deletion analysis. Mol Med. 4:614–628. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Conn VM, Hugouvieux V, Nayak A, Conos SA,
Capovilla G, Cildir G, Jourdain A, Tergaonkar V, Schmid M, Zubieta
C and Conn SJ: A circRNA from SEPALLATA3 regulates splicing of its
cognate mRNA through R-loop formation. Nat Plants. 3:170532017.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li Y, Ge YZ, Xu L and Jia R: Circular RNA
ITCH: A novel tumor suppressor in multiple cancers. Life Sci.
254:1171762020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pamudurti NR, Bartok O, Jens M,
Ashwal-Fluss R, Stottmeister C, Ruhe L, Hanan M, Wyler E,
Perez-Hernandez D, Ramberger E, et al: Translation of CircRNAs. Mol
Cell. 66:9–21.e7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen CY and Sarnow P: Initiation of
protein synthesis by the eukaryotic translational apparatus on
circular RNAs. Science. 268:415–417. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Legnini I, Di Timoteo G, Rossi F, Morlando
M, Briganti F, Sthandier O, Fatica A, Santini T, Andronache A, Wade
M, et al: Circ-ZNF609 is a circular RNA that can be translated and
functions in myogenesis. Mol Cell. 66:22–37.e9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang Y, Gao X, Zhang M, Yan S, Sun C, Xiao
F, Huang N, Yang X, Zhao K, Zhou H, et al: Novel role of FBXW7
circular RNA in repressing glioma tumorigenesis. J Natl Cancer
Inst. 110:304–315. 2018. View Article : Google Scholar
|
|
55
|
Zhang M, Huang N, Yang X, Luo J, Yan S,
Xiao F, Chen W, Gao X, Zhao K, Zhou H, et al: A novel protein
encoded by the circular form of the SHPRH gene suppresses glioma
tumorigenesis. Oncogene. 37:1805–1814. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang Y, Fan X, Mao M, Song X, Wu P, Zhang
Y, Jin Y, Yang Y, Chen LL, Wang Y, et al: Extensive translation of
circular RNAs driven by N6-methyladenosine. Cell Res.
27:626–641. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang M, Zhao K, Xu X, Yang Y, Yan S, Wei
P, Liu H, Xu J, Xiao F, Zhou H, et al: A peptide encoded by
circular form of LINC-PINT suppresses oncogenic transcriptional
elongation in glioblastoma. Nat Commun. 9:44752018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Paschalis A and de Bono JS: Prostate
cancer 2020: ‘The times they are a'changing’. Cancer Cell.
38:25–27. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cai C, Zhi Y, Wang K, Zhang P, Ji Z, Xie C
and Sun F: circHIPK3 overexpression accelerates the proliferation
and invasion of prostate cancer cells through regulating
miRNA-338-3p. Onco Targets Ther. 12:3363–3372. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen D, Lu X, Yang F and Xing N: Circular
RNA circHIPK3 promotes cell proliferation and invasion of prostate
cancer by sponging miR-193a-3p and regulating MCL1 expression.
Cancer Manag Res. 11:1415–1423. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yu H, Chen Y and Jiang P: Circular RNA
HIPK3 exerts oncogenic properties through suppression of miR-124 in
lung cancer. Biochem Biophys Res Commun. 506:455–462. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lu H, Han X, Ren J, Ren K, Li Z and Sun Z:
Circular RNA HIPK3 induces cell proliferation and inhibits
apoptosis in non-small cell lung cancer through sponging miR-149.
Cancer Biol Ther. 21:113–121. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hong W, Zhang Y, Ding J, Yang Q, Xie H and
Gao X: circHIPK3 acts as competing endogenous RNA and promotes
non-small-cell lung cancer progression through the miR-107/BDNF
signaling pathway. Biomed Res Int. 2020:60759022020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chen X, Mao R, Su W, Yang X, Geng Q, Guo
C, Wang Z, Wang J, Kresty LA, Beer DG, et al: Circular RNA
circHIPK3 modulates autophagy via MIR124-3p-STAT3-PRKAA/AMPKα
signaling in STK11 mutant lung cancer. Autophagy. 16:659–671. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Malvezzi M, Carioli G, Bertuccio P,
Boffetta P, Levi F, La Vecchia C and Negri E: European cancer
mortality predictions for the year 2018 with focus on colorectal
cancer. Ann Oncol. 29:1016–1022. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yan Y, Su M and Qin B: circHIPK3 promotes
colorectal cancer cells proliferation and metastasis via modulating
of miR-1207-5p/FMNL2 signal. Biochem Biophys Res Commun.
524:839–846. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ilson DH: Advances in the treatment of
gastric cancer: 2019. Curr Opin Gastroenterol. 35:551–554. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Cheng J, Zhuo H, Xu M, Wang L, Xu H, Peng
J, Hou J, Lin L and Cai J: Regulatory network of circRNA-miRNA-mRNA
contributes to the histological classification and disease
progression in gastric cancer. J Transl Med. 16:2162018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wei J, Xu H, Wei W, Wang Z, Zhang Q, De W
and Shu Y: circHIPK3 promotes cell proliferation and migration of
gastric cancer by sponging miR-107 and regulating BDNF expression.
Onco Targets Ther. 13:1613–1624. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Li Y, Zheng F, Xiao X, Xie F, Tao D, Huang
C, Liu D, Wang M, Wang L, Zeng F and Jiang G: circHIPK3 sponges
miR-558 to suppress heparanase expression in bladder cancer cells.
EMBO Rep. 18:1646–1659. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Xie F, Zhao N, Zhang H and Xie D: Circular
RNA circHIPK3 promotes gemcitabine sensitivity in bladder cancer. J
Cancer. 11:1907–1912. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Akcay M, Etiz D, Celik O and Ozen A:
Evaluation of prognosis in nasopharyngeal cancer using machine
learning. Technol Cancer Res Treat. 19:15330338209098292020.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ke Z, Xie F, Zheng C and Chen D: circHIPK3
promotes proliferation and invasion in nasopharyngeal carcinoma by
abrogating miR-4288-induced ELF3 inhibition. J Cell Physiol.
234:1699–1706. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Sharma A, Sharma KL, Gupta A, Yadav A and
Kumar A: Gallbladder cancer epidemiology, pathogenesis and
molecular genetics: Recent update. World J Gastroenterol.
23:3978–3998. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kai D, Yannian L, Yitian C, Dinghao G, Xin
Z and Wu J: Circular RNA HIPK3 promotes gallbladder cancer cell
growth by sponging microRNA-124. Biochem Biophys Res Commun.
503:863–869. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chen G, Shi Y, Liu M and Sun J: circHIPK3
regulates cell proliferation and migration by sponging miR-124 and
regulating AQP3 expression in hepatocellular carcinoma. Cell Death
Dis. 9:1752018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wang Y, Zhang Y, Yang T, Zhao W, Wang N,
Li P, Zeng X and Zhang W: Long non-coding RNA MALAT1 for promoting
metastasis and proliferation by acting as a ceRNA of miR-144-3p in
osteosarcoma cells. Oncotarget. 8:59417–59434. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Xiao-Long M, Kun-Peng Z and Chun-Lin Z:
Circular RNA circ_HIPK3 is down-regulated and suppresses cell
proliferation, migration and invasion in osteosarcoma. J Cancer.
9:1856–1862. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Quartuccio N, Laudicella R, Vento A,
Pignata S, Mattoli MV, Filice R, Comis AD, Arnone A, Baldari S,
Cabria M and Cistaro A: The additional value of 18F-FDG
PET and MRI in patients with glioma: A review of the literature
from 2015 to 2020. Diagnostics (Basel). 10:3572020. View Article : Google Scholar
|
|
83
|
Hu D and Zhang Y: Circular RNA HIPK3
promotes glioma progression by binding to miR-124-3p. Gene.
690:81–89. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Jin P, Huang Y, Zhu P, Zou Y, Shao T and
Wang O: CircRNA circHIPK3 serves as a prognostic marker to promote
glioma progression by regulating miR-654/IGF2BP3 signaling. Biochem
Biophys Res Commun. 503:1570–1574. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Cai Z and Liu Q: Understanding the global
cancer statistics 2018: Implications for cancer control. Sci China
Life Sci. Aug 26–2019.(Epub ahead of print). doi:
10.1007/s11427-019-9816-1. View Article : Google Scholar
|
|
86
|
Teng F, Xu J, Zhang M, Liu S, Gu Y, Zhang
M, Wang X, Ni J, Qian B, Shen R and Jia X: Comprehensive circular
RNA expression profiles and the tumor-suppressive function of
circHIPK3 in ovarian cancer. Int J Biochem Cell Biol. 112:8–17.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liang S, Zhang S, Wang P, Yang C, Shang C,
Yang J and Wang J: LncRNA, TUG1 regulates the oral squamous cell
carcinoma progression possibly via interacting with Wnt/β-catenin
signaling. Gene. 608:49–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wang J, Zhao SY, Ouyang SS, Huang ZK, Luo
Q and Liao L: Circular RNA circHIPK3 acts as the sponge of
microRNA-124 to promote human oral squamous cell carcinoma cells
proliferation. Zhonghua Kou Qiang Yi Xue Za Zhi. 53:546–551.
2018.(In Chinese). PubMed/NCBI
|
|
89
|
Minciacchi VR, Kumar R and Krause DS:
Chronic myeloid leukemia: A model disease of the past, present and
future. Cells. 10:1172021. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Feng XQ, Nie SM, Huang JX, Li TL, Zhou JJ,
Wang W, Zhuang LK and Meng FJ: Circular RNA circHIPK3 serves as a
prognostic marker to promote chronic myeloid leukemia progression.
Neoplasma. 67:171–177. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Congdon NG, Friedman DS and Lietman T:
Important causes of visual impairment in the world today. JAMA.
290:2057–2060. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Liu X, Liu B, Zhou M, Fan F, Yu M, Gao C,
Lu Y and Luo Y: Circular RNA HIPK3 regulates human lens epithelial
cells proliferation and apoptosis by targeting the miR-193a/CRYAA
axis. Biochem Biophys Res Commun. 503:2277–2285. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Gathiram P and Moodley J: Pre-eclampsia:
Its pathogenesis and pathophysiolgy. Cardiovasc J Afr. 27:71–78.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zhang Y, Cao L, Jia J, Ye L, Wang Y, Zhou
B and Zhou R: circHIPK3 is decreased in preeclampsia and affects
migration, invasion, proliferation, and tube formation of human
trophoblast cells. Placenta. 85:1–8. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Vera R, Dotor E, Feliu J, González E,
Laquente B, Macarulla T, Martínez E, Maurel J, Salgado M and
Manzano JL: SEOM clinical guideline for the treatment of pancreatic
cancer (2016). Clin Transl Oncol. 18:1172–1178. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Xu T, Wu J, Han P, Zhao Z and Song X:
Circular RNA expression profiles and features in human tissues: A
study using RNA-seq data. BMC Genomics. 18 (Suppl 6):S6802017.
View Article : Google Scholar
|
|
97
|
Liu Y, Xia L, Dong L, Wang J, Xiao Q, Yu X
and Zhu H: circHIPK3 promotes gemcitabine (GEM) resistance in
pancreatic cancer cells by sponging miR-330-5p and targets RASSF1.
Cancer Manag Res. 12:921–929. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Rahman A, O'Connor DB, Gather F, Koscic S,
Gilgan J, Mockler D, Bashir Y, Memba R, Duggan SN and Conlon KC:
Clinical classification and severity scoring systems in chronic
pancreatitis: A systematic review. Dig Surg. 37:181–191. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wang L, Luo T, Bao Z, Li Y and Bu W:
Intrathecal circHIPK3 shRNA alleviates neuropathic pain in diabetic
rats. Biochem Biophys Res Commun. 505:644–650. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wang J, Li X, Liu Y, Peng C, Zhu H, Tu G,
Yu X and Li Z: circHIPK3 promotes pyroptosis in acinar cells
through regulation of the miR-193a-5p/GSDMD axis. Front Med
(Lausanne). 7:882020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Holmström L, Haukilahti A, Vähätalo J,
Kenttä T, Appel H, Kiviniemi A, Pakanen L, Huikuri HV, Myerburg RJ
and Junttila J: Electrocardiographic associations with myocardial
fibrosis among sudden cardiac death victims. Heart. 106:1001–1006.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ni H, Li W, Zhuge Y, Xu S, Wang Y, Chen Y,
Shen G and Wang F: Inhibition of circHIPK3 prevents angiotensin
II-induced cardiac fibrosis by sponging miR-29b-3p. Int J Cardiol.
292:188–196. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Gotua M, Gamkrelidze A, Rukhadze M,
Abramidze T, Bochorishvili E, Shengelidze G, Dolidze N,
Chkhartishvili E, Bachert C, Pfaar O, et al: 2020 Aria care
pathways for allergic rhinitis-georgia. Georgian Med News. 108–117.
2019.PubMed/NCBI
|
|
104
|
Zhu X, Wang X, Wang Y and Zhao Y: The
regulatory network among circHIPK3, LncGAS5, and miR-495 promotes
Th2 differentiation in allergic rhinitis. Cell Death Dis.
11:2162020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Taneja K and Williamson SR: Updates in
pathologic staging and histologic grading of renal cell carcinoma.
Surg Pathol Clin. 11:797–812. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Lai J, Xin J, Fu C and Zhang W: circHIPK3
promotes proliferation and metastasis and inhibits apoptosis of
renal cancer cells by inhibiting MiR-485-3p. Cancer Cell Int.
20:2482020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Li H, Heng B, Ouyang P, Xie X, Zhang T,
Chen G, Chen Z, Cheang K and Lai C: Comprehensive profiling of
circRNAs and the tumor suppressor function of circHIPK3 in clear
cell renal carcinoma. J Mol Histol. 51:317–327. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Han B, Shaolong E, Luan L, Li N and Liu X:
circHIPK3 promotes clear cell renal cell carcinoma (ccRCC) cells
proliferation and metastasis via altering of miR-508-3p/CXCL13
signal. Onco Targets Ther. 13:6051–6062. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Qian W, Huang T and Feng W: Circular RNA
HIPK3 promotes EMT of cervical cancer through sponging miR-338-3p
to up-regulate HIF-1α. Cancer Manag Res. 12:177–187. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Chen S, Fan X, Gu H, Zhang L and Zhao W:
Competing endogenous RNA regulatory network in papillary thyroid
carcinoma. Mol Med Rep. 18:695–704. 2018.PubMed/NCBI
|
|
112
|
Shu T, Yang L, Sun L, Lu J and Zhan X:
circHIPK3 promotes thyroid cancer tumorigenesis and invasion
through the Mirna-338-3p/RAB23 axis. Med Princ Pract. Oct
26–2020.(Epub ahead of print). doi: 10.1159/000512548. View Article : Google Scholar
|
|
113
|
Ji F, Yang CQ, Li XL, Zhang LL, Yang M, Li
JQ, Gao HF, Zhu T, Cheng MY, Li WP, et al: Risk of breast
cancer-related death in women with a prior cancer. Aging (Albany
NY). 12:5894–5906. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Howell A, Anderson AS, Clarke RB, Duffy
SW, Evans DG, Garcia-Closas M, Gescher AJ, Key TJ, Saxton JM and
Harvie MN: Risk determination and prevention of breast cancer.
Breast Cancer Res. 16:4462014. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Chen ZG, Zhao HJ, Lin L, Liu JB, Bai JZ
and Wang GS: Circular RNA CirCHIPK3 promotes cell proliferation and
invasion of breast cancer by sponging miR-193a/HMGB1/PI3K/AKT axis.
Thorac Cancer. 11:2660–2671. 2020. View Article : Google Scholar : PubMed/NCBI
|