Introduction
In 2003, Zhao et al (1) confirmed that in the early stages of
ischemia-reperfusion (IR) injury, it is possible to achieve
myocardial protection by applying repeated ischemic treatments;
this process was termed ischemic postconditioning (IPO). IPO can
alleviate oxidative stress, maintain endothelial function, decrease
calcium accumulation and protect the heart muscle from damage
(2). The mechanism of IPO relies on
complex signaling pathways, involving G-protein-coupled (3) and membrane growth factor receptors and
mitochondrial ATP-sensitive potassium channels (KATPs) (4). These pathways act on terminal effector
mitochondrial permeability transition pores (mPTPs) to prevent pore
formation, and thus serve a key role in myocardial protection
(5). KATP regulates ATP levels via
intracellular alterations to potassium ions concentrations. There
are two types of independent KATPs in cardiomyocytes: Sarcolemmal
(sarcKATP) and mitochondrial (mitoKATP), both of which are
necessary for myocardial protection (6).
Pinacidil is a non-selective KATP channel opener
that exhibits cardioprotective effects (7). Pinacidil opens both sarcKATP and
mitoKATP channels, triggering cell membrane hyperpolarization and
mitochondrial membrane depolarization, thereby decreasing
myocardial ATP levels. This was confirmed by a previous study
(8) using isolated rat hearts as a
model to test the combination of histidine-tryptophan-ketoglutarate
solution and pinacidil as an improved strategy for donor heart
preservation. Our previous study confirmed that pinacidil
postconditioning (PPC) at different concentrations inhibits IR
injury in rats, primarily by upregulating nuclear factor erythroid
2-related factor 2 (Nrf2) and its downstream genes, such as
NADH-quinone oxidoreductase-1 (NQO1), heme oxygenase 1 (HO-1) and
superoxide dismutase 1 (SOD1), all of which serve important roles
in myocardial protection (9).
However, our study did not investigate the myocardial protective
effects of different pinacidil concentrations or the association
between pinacidil and reactive oxygen species (ROS), which are an
important component in myocardial protective signaling.
Furthermore, it was not determined whether different concentrations
of ROS alters Nrf2, NQO1, SOD1 and HO-1 expression levels to
alleviate myocardial IR injury (MIRI) (9).
Nrf2 is a member of the basic leucine zipper
transcription factor cap'n'collar family, which is constitutively
expressed in the cytoplasm. Its accumulation and activation in the
nucleus causes oxidative damage (10). In cancer cells, Nrf2 combines with
antioxidant response element (ARE) to decrease damage. Its
antioxidant defense enzymes (including HO-1 and NQO1) have
anti-inflammatory and anti-apoptotic functions, which can reduce
oxidative stress in cells. Nrf2 usually combines with the ARE,
which is the promoter region of HO-1, SOD, and NQO1, etc. (11). After combination, the enzyme complex
upregulates expression of endogenous protective antioxidant genes,
such as HO-1, NQO1 and SOD, in the tissue, thereby maintaining the
balance of oxidant and antioxidant levels in cells (12).
Studies have shown that HO-1 is the primary
endogenous protective gene regulated by the Nrf2-ARE signaling
pathway (13,14). HO-1 has multiple effects, which are
activated by Nrf2 and its metabolites; in particular, it can
scavenge hydroxyl-free radicals, singlet oxygen, and superoxide
anions to prevent oxidation of lipids. Therefore, HO-1 could play
an indispensable role in the prevention of apoptosis (14).
NQO1 is a two-electron reductase, which is widely
present in the cytoplasm and catalyzes the reduction of quinone
substrates (15). It has been
reported that NQO1 can protect cells from oxidative stress by
inhibiting the reduction of semiquinone free radicals and the
formation of ROS (16,17). During myocardial protection, NQO1 is
induced following activation of ARE and exhibits protective effects
against various metabolic oxidative stress responses (18).
SOD is one of the proteins regulated by the Nrf2-ARE
signaling pathway. SOD is the primary antioxidant enzyme in and
free radical scavenger in cells; thus SOD protects cells against
oxygen free radicals. The levels of SOD reflect the function of the
endogenous oxygen free radical scavenging system (19).
Research has confirmed that Nrf2 protect
cardiomyocytes from oxidative stress by increasing detoxification
pathways, enhancing antioxidant potential and alleviating oxidative
damage in various disease states (20).
N-(2-mercaptopropionyl)-glycine (MPG) is a class of
synthetic thiol compounds with ROS scavenging properties and exerts
protective effects in MIRI (21).
Studies have reported that MPG decreases heart hypertrophy of
spontaneously hypertensive rats due to IRI (22,23).
These effects may be associated with inhibited opening of mPTP and
decreased myocardial oxidative stress. MPG can be used for oral
prophylactic treatment and is approved by the United States Food
and Drug Administration (FDA) for human clinical use (24).
To the best of our knowledge, it remains unknown
whether ROS serve a role in PPC. Based on previous studies,
pinacidil-postconditioning is considered to be equivalent to
ischemic postconditioning in defeating cardiac IR injury in rats
(9,22); therefore, our present study aimed to
use the Langendorff device to establish an IR model in rats, and to
simulate the process of cardiac arrest-relapse during surgery.
Thereafter, changes in cardiac function, myocardial infarction and
myocardial ultrastructure were observed, and the expression levels
of Nrf2, HO1, SOD1 and other antioxidant proteins were detected. In
addition, the research assessed whether different doses of
pinacidil could generate different levels of ROS, activate the
Nrf2-ARE pathway to different degrees and exert different effects
on myocardial protection. The present study aimed to provide
evidence for the potential use of pinacidil in clinical practice
and the treatment of MIRI.
Materials and methods
Experimental animals
Healthy, specific-pathogen-free grade, 48 male
Sprague-Dawley rats (age, 16–20 weeks; weight, 250–300 g) were
purchased from the Animal Centre of DaPing Hospital, Army Military
Medical University in China [certificate no. SCXK (YU) 2007–0005].
The present experiment was performed in accordance with document
No. 36 of the Animal Ethics Review at The Affiliated Hospital of
Zunyi Medical University in 2016. The rats were housed in
individual ventilation cages and provided with food and water ad
libitum. Rats were housed under a 12-h light/dark cycle, at
20–25°C and 50–65% humidity. All animal experiments were performed
in accordance with the U.K Animals Act of 1986, and associated
guidelines EU Directive 2010/63/EU (25). Furthermore, the process of feeding
rats was compliant with the National Institutes of Health: Guide
for the Care and Use of Laboratory Animals (2011) (26).
Langendorff reperfusion protocol
The Langendorff reperfusion protocol was performed
as described previously (9). The
rats were intraperitoneally injected with 1% pentobarbital solution
(35 mg/kg) and heparin (250 U/kg). The xiphoid process was marked
and the upper abdomen was cut horizontally along both sides of the
costal margin to expose the diaphragm. The thoracic cavity was cut
on the cephalic side through the midline of both sides of the iliac
crest. The xiphoid process was lifted with tweezers to open the
thoracic cavity and expose the heart. Finally, the heart was
removed and separated from the roots along with the aorta and
quickly placed in pre-cooled (4°C) Krebs-Henseleit (K-H) buffer (in
mM: 118 NaCl, 25 NaHCO3, 4.8 KCl, 1.2
KH2PO4, 1.19 MgCl, 2.6 H2O, 1.2
MgSO4, 2.5 CaCl2, and 11.110 glucose; pH 7.4)
to clean and wash away the blood.
The aorta was fixed on a Langendorff system
perfusion needle (Panlab), which was prefilled with K-H solution
and retrogradely perfused. The K-H solution (gassed with 95%
O2 and 5% CO2) was heated to 37°C. The
solution was infused and connected to PowerLab physiological
experiment equipment (ADInstruments, Ltd.) to test the pressure.
The pulmonary artery root was incised to improve the right
ventricular flow. A small orifice was created at the left atrial
appendage and a latex balloon was placed into the mitral valve to
collect data on cardiac function. The balloon was adjusted to
maintain a left ventricular end-diastolic pressure (LVEDP) of 2–5
mmHg. Throughout the perfusion process, the K-H solution was
maintained at room temperature to sustain the heartbeat and
regulate the perfusion pressure, which was stabilized at 60–70
mmHg.
After K-H solution had equilibrated at 37°C for 20
min (time point T1), the left ventricular developed pressure (LVDP)
and heart rate (HR) were assessed. The experiment was allowed to
continue if LVDP >80 mmHg, HR >250 beats/min and ventricular
premature beat <2 times; if these conditions were not met, the
experiment was terminated.
At the end of balanced perfusion (T1), the isolated
hearts were randomly divided into the following six groups
(n=8/group): Normal (N) group, hearts were perfused with K-H
solution for a further 180 min; IR group, hearts underwent ischemia
for 40 min, followed by 120 min reperfusion; P10, P30 and P50
groups, hearts underwent ischemia for 40 min followed by 10, 30,
and 50 µmol/l pinacidil treatment, respectively, for 2 min prior to
reperfusion with K-H solution for 118 min; and M + P50 group,
hearts underwent ischemia for 40 min followed by treatment with 50
µmol/l pinacidil for 2 min, then treatment with K-H solution
containing 2 mmol/l ROS scavenger MPG (Sigma-Aldrich; Merck KGaA)
for 3 min and reperfusion with K-H solution for 115 min. The
experimental procedure for each group is presented in Fig. 1.
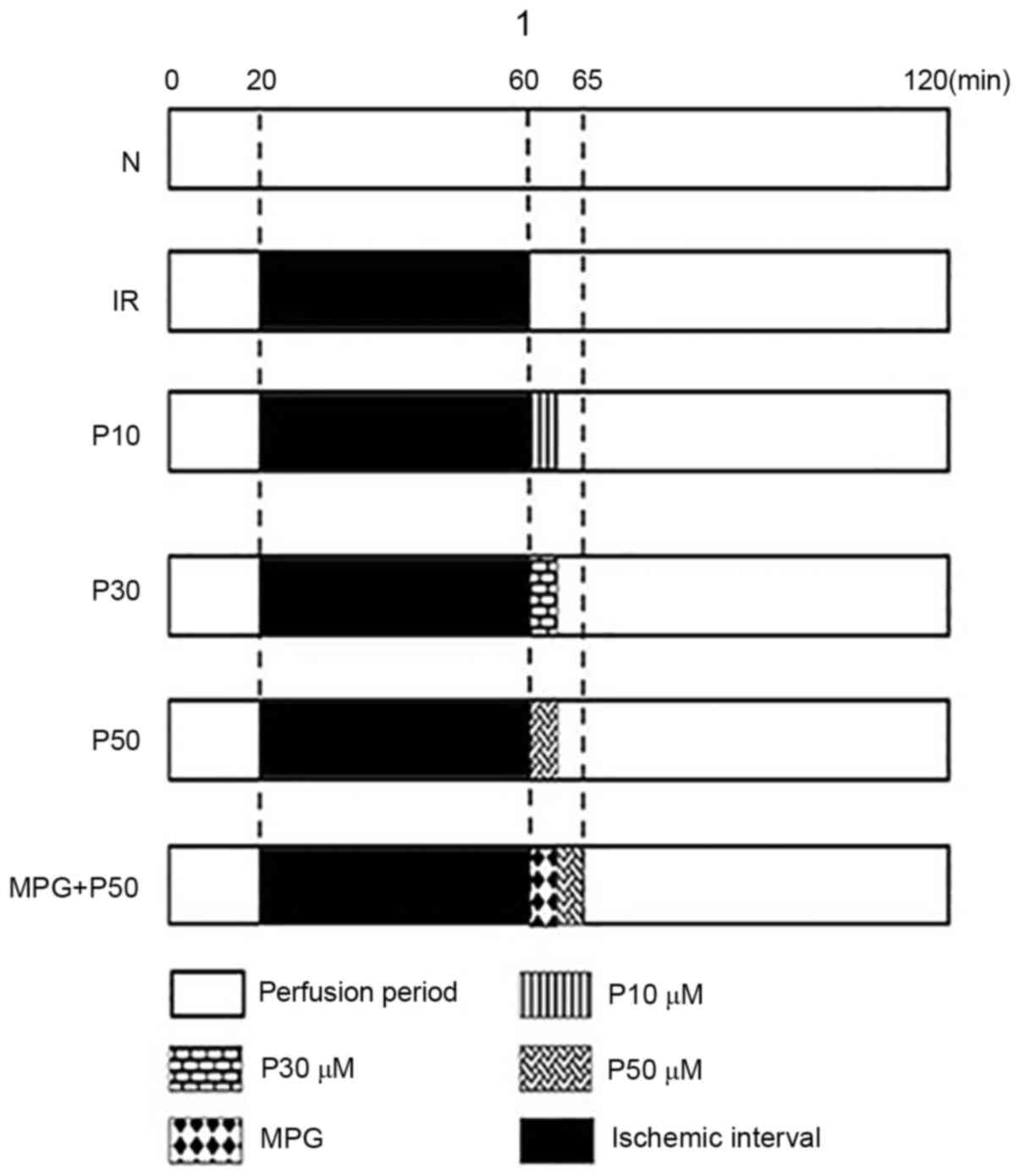 | Figure 1.Grouping strategy and perfusion
measures in rats. Rats were allocated to six groups (n=8/group): N,
IR, P10, P30, P50 and MPG + P50. In the N group, hearts were
perfused with K-H solution for 180 min. The other rat hearts were
equilibrated for ≥20 min (during which the flow was adjusted to a
mean perfusion pressure of 60–70 mmHg). In the IR group, hearts
underwent ischemia (perfusion was paused) for 40 min, followed by
120 min reperfusion. In the P10, P30 and P50 groups, hearts
underwent 40 min of ischemia followed by treatment with 10, 30, and
50 µmol/l pinacidil, respectively, for 2 min, and reperfusion for
118 min. In the M + P50 group, hearts underwent ischemia for 40 min
followed by treatment with 50 µmol/l pinacidil for 2 min and K-H
solution containing 2 mmol/l ROS scavenger MPG for 3 min, then
reperfusion with K-H solution for 115 min. N, normal; IR,
ischemia-reperfusion; MPG, N-(2-mercaptopropionyl)-glycine; K-H,
Krebs-Henseleit; P10, 10 µmol/l pinacidil; P30, 30 µmol/l
pinacidil; P50, 50 µmol/l pinacidil. |
T2 was defined as 5 min post-reperfusion and T3 was
defined as the end of reperfusion. At T1 and T3, PowerLab
physiological experiment equipment (ADInstruments Ltd.) was used to
collect data on cardiac function indicators, such as HR, LVDP,
LVEDP and the maximal rate of rise in blood pressure in the
ventricular chamber (+dP/dtmax).
Ultrastructural observation of
myocardial tissue and mitochondrial Flameng score
A pre-cooled (4°C) 2.5% glutaraldehyde electron
microscope fixative was prepared. Next, 0.1-cm2 pieces
of myocardial tissue were excised from the left ventricle at T3
(the entire process was completed in ≤1 min) and placed in the 4°C
precooled 2.5% glutaraldehyde fixative (for no more than 2 weeks).
The tissue samples were then rinsed three times with phosphate
buffer (every 2 h). Subsequently, the tissue samples were placed in
pre-cooled (4°C) 1% citric acid for 2 h. Finally, the tissue
samples were dehydrated using ethanol and acetone gradients and
embedded in epoxy resin at 45°C for 12 h and 60°C for 48 h, which
was allowed to polymerize, before double staining with uranyl
acetate at 20–25°C for 20 min and lead citrate at 20–25°C for 10
min. The ultrastructure of the myocardial tissue was then observed
using a transmission electron microscope (Hitachi H7500)
(magnification, ×20,000) and the mitochondrial Flameng score was
determined.
The specific criteria for determining the
mitochondrial Flameng score based on transmission electron
microscopy were as previously described (27). In brief, five fields of view (each
containing 20 mitochondria) were randomly selected for each tissue
slice. The mitochondrial Flameng score (range, 0–4) for each group
was expressed as the mean ± SD. Flameng score increases with damage
to the myocardial mitochondrial (28).
Reverse transcription-quantitative
(RT-q)PCR
At T3, total RNA was isolated from the myocardial
tissue using a Takara RNA Iso Plus kit (Takara Bio, Inc.). The RNA
concentration and purity were measured using a microplate reader to
assess the optical density (OD) at 260 and 280 nm of each sample.
RNA samples meeting the purity standards (OD260/OD280, 1.8–2.1)
were diluted and used to assess relative expression levels of Nrf2,
HO-1, SOD1 and NQO1. GAPDH was used as a control. The primer
sequences for each gene were as follows: Nrf2 forward,
5′-TTGGCAGAGACATTCCCATTTGTA-3′ and reverse,
5′-ATCAGTCATGGCCGTCTCCAG-3′; HO-1 forward,
5′-AGGTGCACATCCGTGCAGAG-3′ and reverse,
5′-TCCAGGGCCGTATAGATATGGTACA-3′; SOD1 forward,
5′-AATGTGTCCATTGAAGATCGTGTGA-3′ and reverse,
5′-GCTTCCAGCATTTCCAGTCTTTGTA-3′; GAPDH forward,
5′-GGCACAGTCAAGGCTGAGAATG-3′ and reverse
5′-ATGGTGGTGAAGACGCCAGTA-3′; and NQO1 forward,
5′-TGGAAGCTGCAGACCTGGTG-3′ and reverse,
5′-TTGTCATACATGGTGGCATACGTG-3′.
After preparing each pair of target gene primers,
the reaction system was configured and a Takara RT-qPCR kit was
used to perform the reaction according to the manufacturer's
instructions. If a single peak appeared in the dissolution curve of
the PCR target gene, the target gene results were considered
specific and were quantitatively analyzed, using the Cq value as a
comparative statistic (29).
Finally, the expression of each gene relative to the internal
reference GAPDH gene was determined.
Western blotting
At T3, left ventricular tissue with a net weight of
50 mg was obtained and added to a 1.5-ml Eppendorf tube containing
RIPA buffer (high efficiency) (Beijing Solarbio Science &
Technology Co., Ltd.) (sample:buffer, 50 mg: 1 ml. The total
protein level in the tissue was then quantified using a
bicinchoninic acid protein concentration assay kit (Beijing
Solarbio Science & Technology Co., Ltd.). The protein
concentrations were calculated according to a standard curve.
Following denaturation at 100°C for 5 min, the proteins were cooled
to room temperature and centrifuged (12,000 × g, 4°C, 2 min).
Equivalent amounts of protein (40 µg/lane) from each group were
separated by SDS-PAGE (10% separating gel; 4% stacking gel),
transferred onto polyvinylidene fluoride (PVDF) membranes and
blocked with western blocking buffer (Beijing Solarbio Science
& Technology Co., Ltd.) at room temperature for 2 h. The
membranes were then incubated at 4°C overnight with primary
antibodies (Nrf2, 1:1,000, cat. no. ab62352; NQO1, 1:1,000, cat.
no. ab79694; HO-1, 1:250, cat. no. ab137749; SOD1, 1:1,000, cat.
no. ab13498; all Abcam), using GAPDH (1:3,000, cat. no. ab181602,
Abcam) as the internal reference. Membranes were incubated with a
secondary antibody (1:3,000, cat. no. SE134; Beijing Solarbio
Science & Technology Co., Ltd.) for 1 h at room temperature and
then washed twice in TBS-0.1% Tween (Abcam). Finally, each membrane
was scanned using an infrared fluorescence imaging system with ECL
Western Blotting Substrate (Beijing Solarbio Science &
Technology Co., Ltd.). The PVDF membranes were scanned and detected
using ChemiDoc Imaging system (Bio-Rad Laboratories, Inc.).
ROS level detection
Samples of left ventricular muscle tissue (100 mg)
were taken at T2 and T3. The Fluorometric Intracellular ROS Kit
(cat. no. MAK144-1KT; Sigma-Aldrich, Inc.) was then used to
calculate the concentration of ROS in each sample based on a
standard curve.
Statistical analysis
Statistical analysis was performed using SPSS 18.0
statistical software for Windows (IBM Corp.). For myocardial
morphology and Flameng score, the Kruskal-Wallis test was used to
assess myocardial damage, with Dunn's post hoc test. Gene and
protein expression levels in isolated rat hearts at T3 with a
normal distribution are presented as the mean ± SD (n=3).
Significance of differences between the six groups was determined
by one-way ANOVA followed by post hoc Bonferroni's correction.
Welch's ANOVA was used when variance was not uniform. Scatter plots
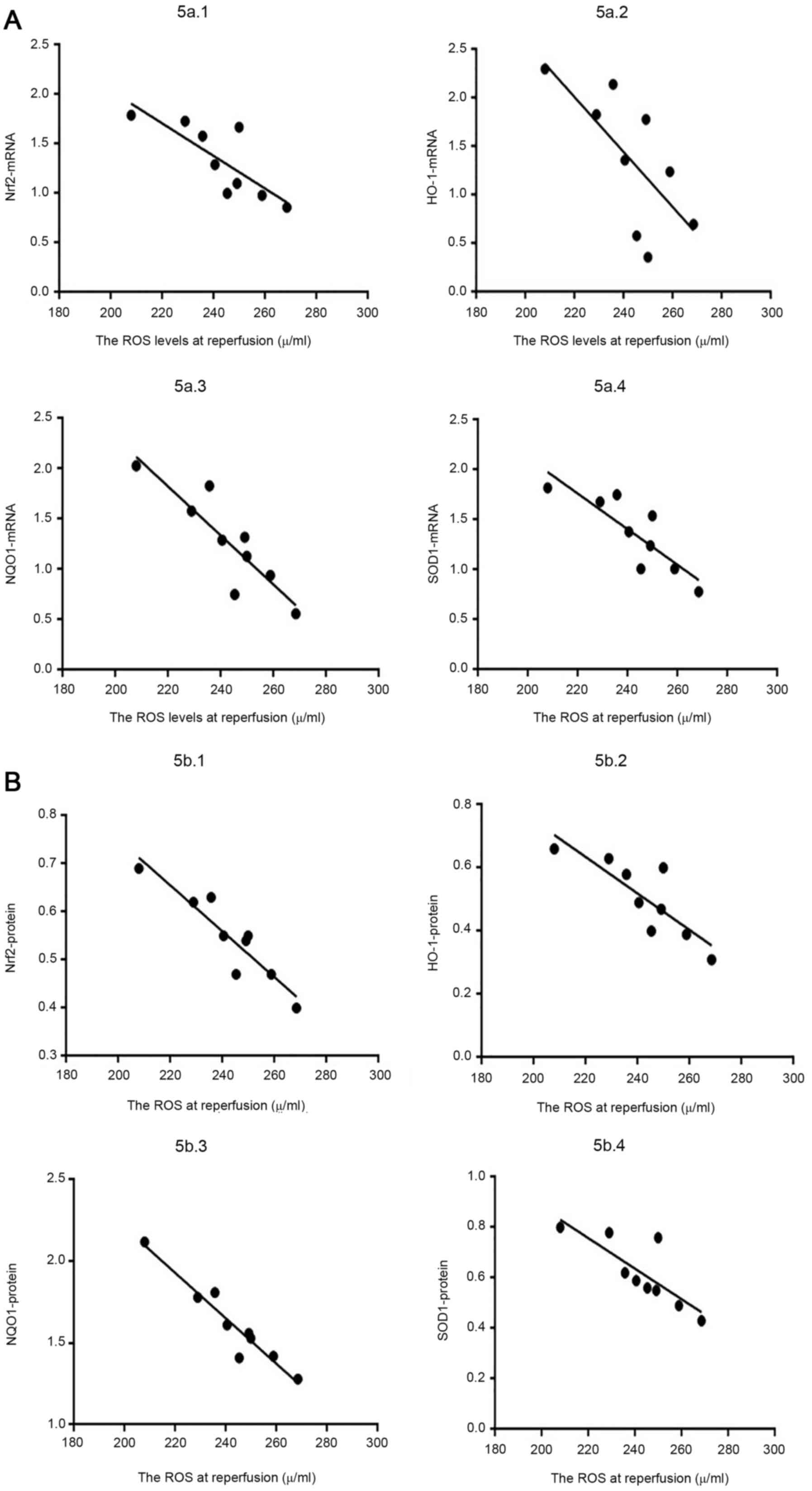
of ROS and Nrf2 gene/protein levels at T3 were assessed by Pearson
correlation analysis. For ROS levels at T2 and T3 in each group,
two-way ANOVA was used, with time as the independent factor.
Bonferroni's correction was performed as a post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Different concentrations of PPC
decrease MIRI to varying degrees
At T3 (end of reperfusion), the cardiac function of
the IR group was significantly decreased (HR, LVDP and +dp/dtmax
were decreased, whereas LVEDP was increased), suggesting impaired
left ventricular function.
The LVDP, LVEDP and +dp/dtmax of the IR myocardium
were restored in the P10, P30 and P50 groups, and recovery in the
P50 group was the most notable. The HR, LVDP and +dp/dtmax of the M
+ P50 group were decreased and LVEDP was increased compared with
the P50 group (Table I).
 | Table I.Changes in HR, LVEDP, LVDP and
+dP/dtmax in each group at T1 and T3. Compared with the N group,
the cardiac function of the IR group was significantly
impaired. |
Table I.
Changes in HR, LVEDP, LVDP and
+dP/dtmax in each group at T1 and T3. Compared with the N group,
the cardiac function of the IR group was significantly
impaired.
|
| HR, beats/min | LVEDP, mmHg | LVDP, mmHg | +dp/dtmax,
mmHg |
|---|
|
|
|
|
|
|
|---|
| Group | T1 | T3 | T1 | T3 | T1 | T3 | T1 | T3 |
|---|
| N | 318±13 | 311±17 | 5±1 | 5±1 | 108±8 | 99±4 | 4,589±257 | 3,779±387 |
| IR | 295±17 | 225±59a | 5±1 | 20±3a | 103±23 | 53±16a | 4,455±149 |
2,172±480a |
| P10 | 298±19 | 250±41b | 4±2 | 8±6b | 115±16 | 70±17a | 4,996±678 |
2,680±899b |
| P30 | 297±27 | 289±35b | 5±1 | 8±4b | 119±11 | 76±11a | 5,017±504 |
3,105±987b |
| P50 | 295±13 | 289±26b | 6±1 | 7±2b | 105±3 | 86±15a,b | 4,629±215 |
3,515±337b |
| M + P50 | 297±222 | 262±18a,b | 6±1 | 13±2b | 102±16 | 59±12c | 4,989±250 |
2,448±187c |
Compared with T1, there was a significant difference
in HR, LVDP, LVEDP and +dp/dtmax in the P10 and P30 groups at T3.
However, the P50 group showed no significant difference (Table I).
Different concentrations of PPC
decrease myocardial ultrastructural damage in IR (Fig. 2A and B)
The ultrastructure of cardiomyocytes was normal at
the end of reperfusion in the N group. The IR group exhibited the
most severe damage, cell edema, disordered myofilaments and
ruptured nuclear membrane. Mitochondria were notably swollen with
vacuolar degeneration. In the P10 and P30 groups, the myofilaments
were arranged neatly, the sarcoplasmic reticulum was dilated, part
of the mitochondria was swollen and the structure was clear but
intermittently dissolved and broken. In the P50 group, the
myocardial cell structure was more complete compared with the IR
group, the myofilaments were neatly arranged, the Z line and the
sarcomere were clearly visible, the mitochondria were structurally
intact and arranged neatly and the sarcoplasmic reticulum was
slightly expanded. In the M + P50 group, the myocardial cell
structure was more severely damaged compared with in the P50 group,
the myofilaments and Z-line were broken, the mitochondrial
structure was intact but slightly swollen and the sarcoplasmic
reticulum was slightly expanded, as compared with in the P50
group.
Different concentrations of PPC
maintain myocardial mitochondrial structural integrity in IR
At T3, the mitochondrial structure of myocardial
tissue in the N group was normal and the Flameng score was the
lowest (Fig. 2B). The IR group
score was the highest, suggesting most severe mitochondrial damage.
The mitochondrial Flameng scores of the P10, P30 and P50 groups
were all lower compared with the IR group; the P50 group had the
lowest score. As the score of the M + P50 group was higher than
that of the P50 group, this was suggestive of aggravated damage
(Fig. 2B).
RT-qPCR analysis of relative
expression levels of Nrf2, HO-1, NQO1 and SOD-1 in myocardial
tissue at T3
At T3, the relative mRNA expression levels of Nrf2,
HO-1, NQO1 and SOD1 were significantly decreased in the IR group
compared with the N group (Fig. 3).
Compared with the IR group, the relative mRNA expression levels in
the P10, P30 and P50 groups were significantly increased. Among all
groups, the relative expression levels of Nrf2, HO-1, NQO1 and SOD1
were highest in the P50 group. The relative expression levels in
the M + P50 group were lower compared with in the P50 group.
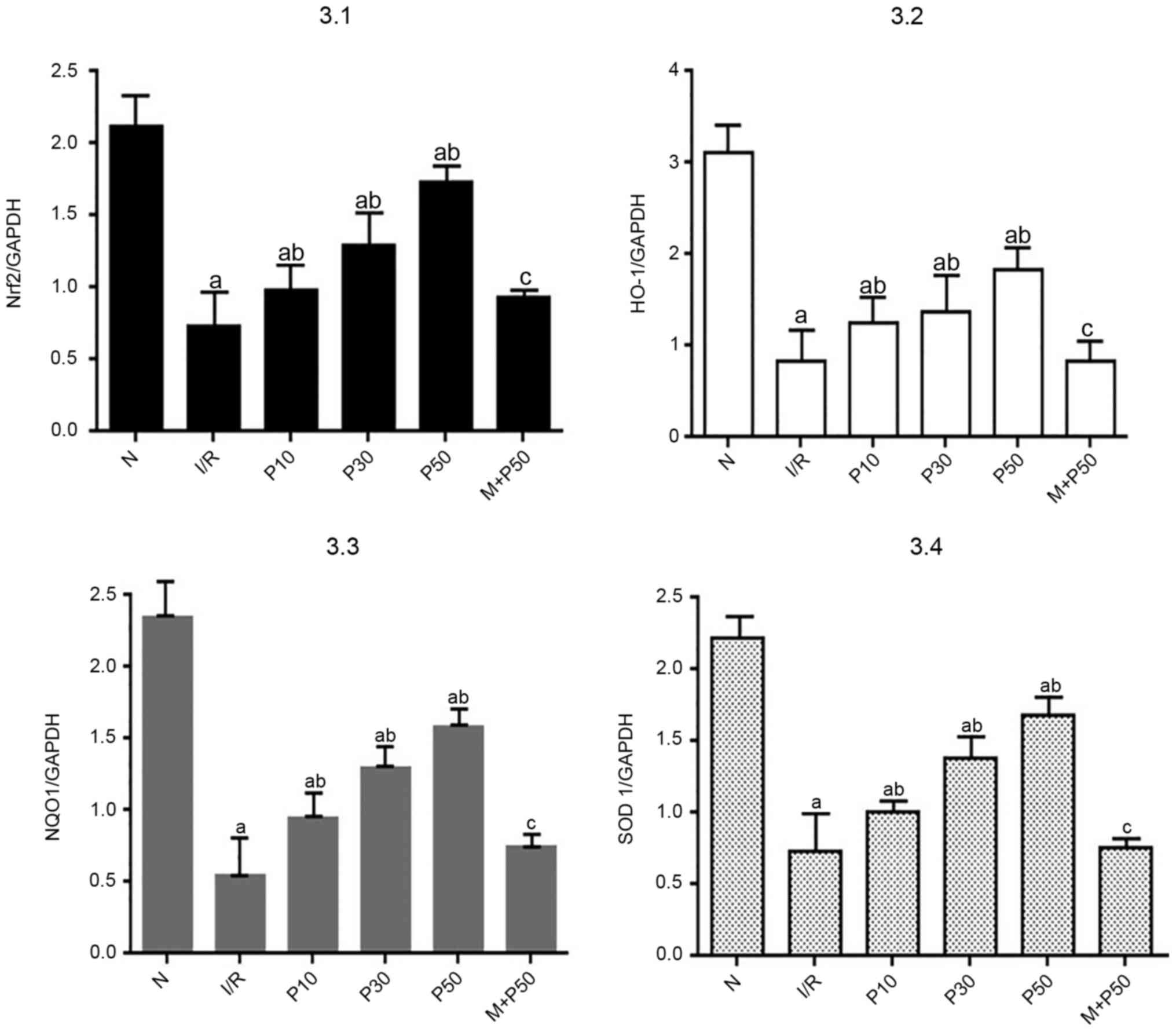 | Figure 3.Gene expression levels in isolated
rat hearts at T3. Nrf2 pathway-associated mRNA expression levels
were lowest in the IR group. The P50 group exhibited the highest
mRNA expression levels. aP<0.05 vs. N.
bP<0.05 vs. IR. cP<0.05 vs. P50. Data
are presented as the mean ± SD (n=3) and were analyzed by one-way
ANOVA followed by Bonferroni's correction. T3, end of reperfusion;
Nrf2, nuclear factor-E2 related factor 2; IR, ischemia-reperfusion;
P10, 10 µmol/l pinacidil; P30, 30 µmol/l pinacidil; P50, 50 µmol/l
pinacidil; N, normal; HO-1, heme oxygenase 1; NQO1, NADH-quinone
oxidoreductase-1; SOD1, superoxide dismutase 1. |
Western blot analysis of relative
protein expression levels of Nrf2, HO-1, NQO1 and SOD-1 in
myocardial tissue at T3
At T3, the expression levels of each protein in the
IR group were significantly lower compared with the N group
(Fig. 4). The relative expression
levels of Nrf2, HO-1, NQO1 and SOD-1 protein were higher in the
P10, P30 and P50 groups compared with the IR group. The protein
expression levels were highest in the P50 group.
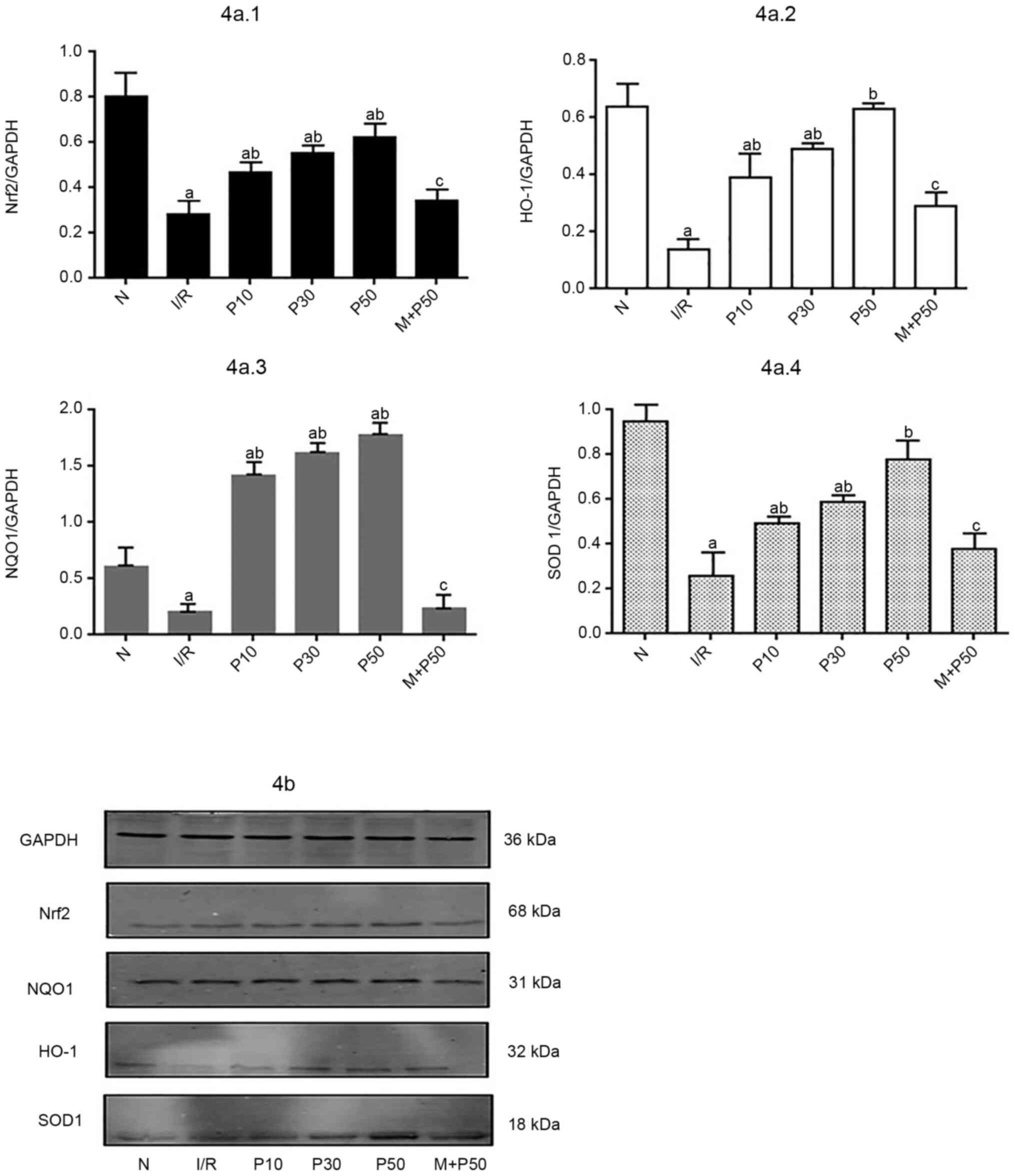 | Figure 4.Protein levels in isolated rat hearts
at T3. (A) Changes in relative expression levels of Nrf2, NQO1,
HO-1 and SOD1. (B) Western blotting was performed to evaluate the
protein expression levels of Nrf2, HO-1, NQO1 and SOD1. The IR
group exhibited the lowest protein expression levels of Nrf2, HO-1,
NQO1 and SOD1. The P50 group exhibited the highest protein
expression levels. aP<0.05 vs. N.
bP<0.05 vs. IR. cP<0.05 vs. P50. Data
are presented as the mean ± SD (n=3). T3, end of reperfusion; Nrf2,
nuclear factor-E2 related factor 2; HO-1, heme oxygenase 1; NQO1,
NADH-quinone oxidoreductase-1; SOD1, superoxide dismutase 1; IR,
ischemia-reperfusion; P10, 10 µmol/l pinacidil; P30, 30 µmol/l
pinacidil; P50, 50 µmol/l pinacidil; N, normal. |
Effects of different concentrations of
pinacidil on ROS in rat myocardial tissue
At T2, ROS content in the IR group was the highest.
The ROS content of the P10, P30 and P50 groups was significantly
lower compared with the IR group (Table II).
 | Table II.ROS levels at T2 and T3 in each
group. |
Table II.
ROS levels at T2 and T3 in each
group.
| Group | T2 | T3 |
|---|
| N | 150.20±25.97 | 155.20±30.81 |
| IR |
330.51±17.73a |
313.01±35.31a |
| P10 |
220.01±12.53a,b |
258.75±9.70a–c |
| P30 |
241.77±6.35a,b |
235.45±4.81a,b |
| P50 |
263.85±9.34a,b |
228.85±20.99a–c |
The P10 group had the lowest ROS level and the P50
group had the highest ROS level. Until the end of reperfusion, the
amount of ROS in the IR group was high but the ROS levels in the
P10, P30 and P50 groups decreased over time. The P10 and P50 groups
had the highest and lowest ROS contents, respectively. The P10, P30
and P50 groups exhibited lower ROS values at T3 compared with T2.
The P50 group exhibited the lowest ROS content at the end of
reperfusion (Table III; Fig. 5).
 | Table III.Association between ROS content and
relative expression levels of Nrf2 gene and protein at T3
(n=9). |
Table III.
Association between ROS content and
relative expression levels of Nrf2 gene and protein at T3
(n=9).
| Group (sample) | ROS levels at T3,
U/ml | Nrf2 mRNA | Nrf2 protein |
|---|
| P10 |
|
|
|
|
(1) | 249.05 | 1.10 | 0.54 |
|
(2) | 258.75 | 0.98 | 0.47 |
|
(3) | 268.45 | 0.86 | 0.40 |
| P30 |
|
|
|
|
(4) | 235.64 | 1.58 | 0.63 |
|
(5) | 240.45 | 1.29 | 0.55 |
|
(6) | 245.26 | 1.00 | 0.47 |
| P50 |
|
|
|
|
(7) | 207.86 | 1.79 | 0.69 |
|
(8) | 228.85 | 1.73 | 0.62 |
|
(9) | 249.84 | 1.67 | 0.55 |
In summary, each concentration of pinacidil was
positively associated with relative expression levels of Nrf2,
HO-1, NQO1 and SOD1 at T2 but negatively associated at T3. The ROS
levels in the P10 group were lowest and those in the P50 group were
highest at T2; however, at T3, ROS levels in the P10 group were
highest, while those in the P50 group were lowest.
Discussion
Timely and effective reperfusion measures are
necessary to save ischemic myocardial tissue (30). However, reperfusion can trigger
MIRI, which typically manifests in the early stages of reperfusion
and directly induces cardiomyocyte apoptosis or death. The
mechanism involves ROS production (31,32),
anaerobic metabolic accumulation (33), increased transmembrane osmotic
gradient loading, Na+-K+ ATPase activation
and mPTP opening. ROS production first occurs in the early stages
of reperfusion. The earliest stage of reperfusion results in ROS
production, which is derived from xanthine oxidase, NADPH oxidase
(Nox), mitochondria and unconjugated nitric oxide synthase
(34). The present study identified
that when the myocardium underwent IR, cardiac function, including
HR, +dp/dtmax and LVDP, decreased significantly at the end of
reperfusion. Transmission electron microscopy demonstrated cell
damage characterized by mitochondrial swelling and vacuolar-like
degeneration, with a high Flameng score of 3.19. These results
indicate that IR caused severe damage to the myocardium, as well as
changes in myocardial morphology and function.
Furthermore, the amount of ROS in the IR group were
highest in the early stage or at the end of reperfusion. A study by
Chen et al (22) confirmed
that at the end of reperfusion, MIRI induces significant ROS
production, leading to a chain reaction of membrane lipid
peroxidation, cell membrane fluidity changes and increased
production of the lipid peroxidation by-product malondialdehyde;
this increases cellular oxidative stress, leading to myocardial
cell death. Han et al (35)
reported that following IR-induced oxidative stress injury, action
potential duration is shortened by IR and ROS production is
triggered, leading to mPTP opening and MIRI. Another study
demonstrated that myocardial damage caused by IR may be due to
activation of mitoKATP, which promotes autophosphorylation of ROS
products (36). The
autophosphorylation of ROS products can result in sustained opening
of mitoKATP, which aggravates MIRI (37). These results are consistent with
those of the present study.
Pinacidil, a non-selective KATP opener, causes
mitochondrial membrane depolarization, decreases transmembrane
potential difference, weakens Ca2+ internal flow,
promotes fatty acid oxidation and maintains ATP levels in
cardiomyocytes. The integrity of cardiomyocytes and mitochondrial
membranes is maintained to exert myocardial protection (38,39).
According to Yang et al (9),
pinacidil could relieve the MIRI, not only in cardiac function,
mitochondrial respiratory function, ATP level and myocardial enzyme
content, but also caused myocardial ultrastructure by improving the
morphology of muscle fibers.
MPG is a thiol compound with ROS scavenging
properties and exerts a protective effect on irreversible and
reversible RI (40). In 2004, MPG
was approved by the FDA for clinical oral prophylactic treatment
(41). Fantinelli et al
(42) demonstrated that MPG
protects hypertrophic myocardium in rats, prevents MIRI, decreases
infarct size and oxidative stress, improves myocardium following
ischemia and promotes recovery of vascular function.
Ihnken et al (43) demonstrated that MPG, as an
antioxidant, provides the same protection as coenzyme Q10,
allopurinol and arachidonic acid; in dogs during cardiopulmonary
bypass, it removed nitrite and harmful substances and served a
necessary role in myocardial protection. MPG also decreases the
degree of mitochondrial swelling caused by 12.5 mM sodium lactate
and 1 mM phenoxide, removes free radicals generated by
Na+ overload in the cytoplasm, prevents free radical
attacks, and decreases MIRI. Our previous study (9) confirmed that PPC at a concentration of
50 mmol/l improves cardiac function, decreases myocardial infarct
size, maintains mitochondrial structure stability, promotes Nrf2,
SOD1, NQO1 and HO-1 expression levels, decreases MIRI and exerts
myocardial protection. However, the study did not investigate
whether the myocardial protective effects of pinacidil are
concentration-dependent or the association between pinacidil and
ROS. Therefore, the present study aimed to determine whether
different amounts of ROS could affect the expression levels of
Nrf2, NQO1, SOD1 and HO-1 to serve a role in alleviating MIRI.
The Nrf2/ARE signaling pathway, which includes HO-1,
NQO1 and SOD1, is the regulatory center of the endogenous
antioxidant system of the myocardium and serves an important role
in anti-MIRI (44). A previous
study confirmed that IPO activates Nrf2 and is associated with
antioxidant enzymes, such as SOD1, to decrease MIRI (45). Similar to SOD1, NQO1 is expressed at
low levels under normal conditions, but oxidative stress, such as
IR, promotes expression and increases ROS clearance of NQO1
(46). NQO1 also serves a
protective role in renal (47) and
cerebral ischemia (48). According
to Freixa et al (49),
β-naphthoflavone increases NQO1 expression, which attenuates
cardiomyocyte apoptosis induced by doxorubicin, and enhances the
protective effect of progesterone. HO-1 is an endogenous
antioxidant that plays a key role in maintaining mitochondrial
biochemical function. Previous studies have confirmed that IPO
(9,22,50) or
drug post-conditioning increases the relative mRNA and protein
expression levels of HO-1 to exert anti-MIRI effects. These
aforementioned studies confirmed that the expression levels of
Nrf2, NQO1, SOD1 and HO-1 increase following MIRI to decrease
myocardial oxidative stress and exert a myocardial protective
effect.
In the present study, the production of ROS at T2
increased gradually with increased pinacidil concentration; ROS
production in the P10 group was the lowest, while that in the P50
group was the highest. In addition, owing to the difference in
levels of ROS, the relative mRNA and protein expression levels of
Nrf2, NQO1, SOD1 and HO-1 also differed. The P10 group exhibited
the lowest ROS content and protein expression levels of Nrf2 and
its associated antioxidants; thus, the anti-MIRI effect was weak.
Transmission electron microscopy revealed mitochondrial swelling
and Z line; a high Flameng score was also noted. By contrast, the
ROS content in the P50 group was the highest and the expression
levels of Nrf2, SOD1 and NQO1 were also significantly increased at
T2. The mitochondrial structure was intact, there was no
dissolution or rupture, and the Flameng score was significantly
decreased. Paul et al (51)
reported that by altering ROS levels to activate Nrf2, increased
expression of Nrf2 activates the Notch pathway to stimulate
self-renewal of human airway basal stem cells, which, in turn,
initiate antioxidant processes and eliminate high intracellular ROS
levels to maintain a stable environment. This regulation of ROS to
regulate Nrf2-pulmonary stem cell function mediated by
anti-oxidation is important for stem cell biology, lung injury
repair and cancer (51). High
expression levels of Nrf2 also induce rapid enzymatic modification,
excretion of chemical carcinogens and ROS quenching, and regulate
the expression levels of associated antioxidant proteins, such as
HO-1 and SOD1, to repair oxidative damage and prevent cancer
(52). Similar regulatory features
have also been reported in studies by Kensler et al
(53), Ma (54), Jaramillo and Zhang (55) and Harder et al (56).
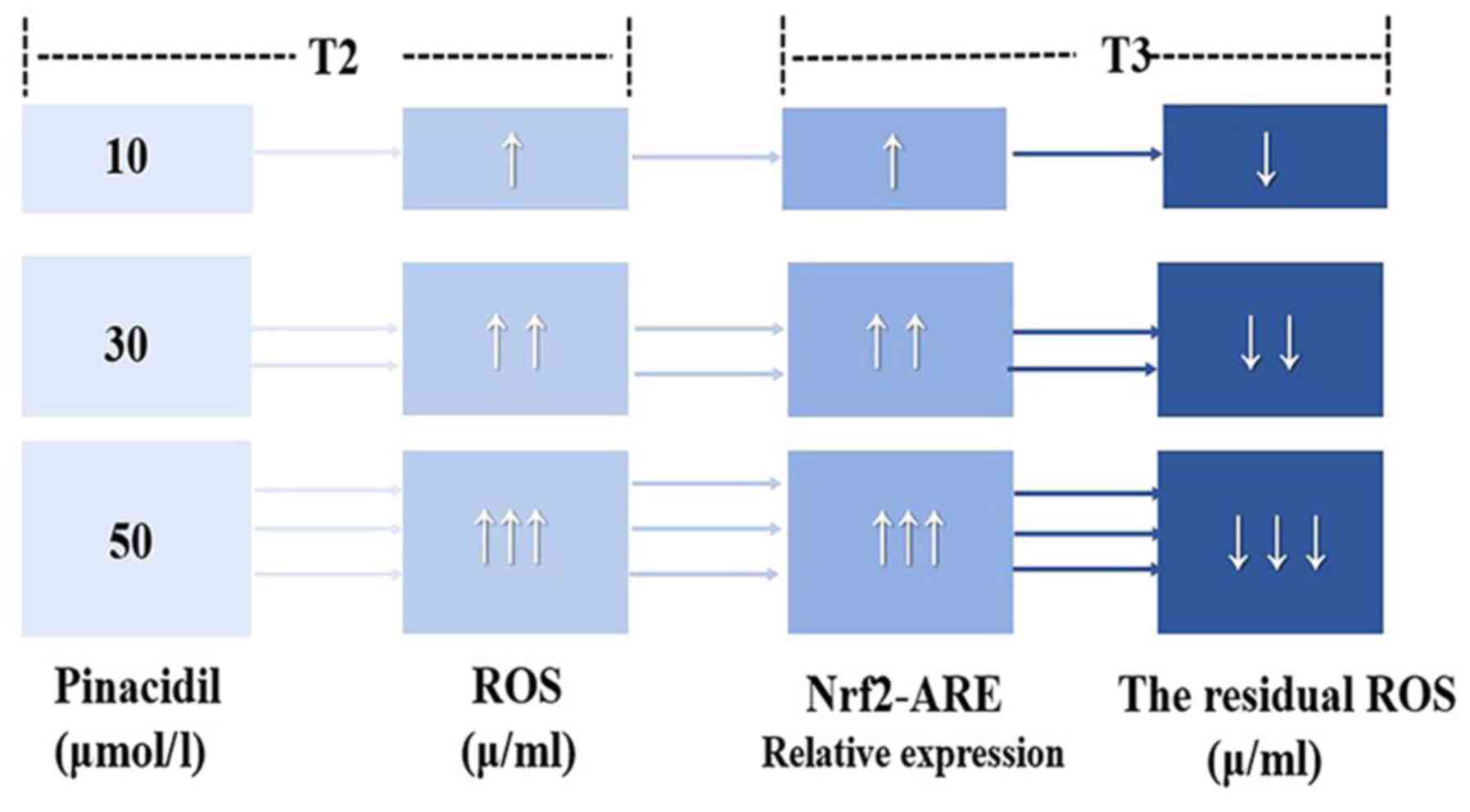
The present study investigated the effect of high
expression levels of Nrf2, HO-1, NQO1 and SOD1 on ROS. The present
study found that at T2, the amount of ROS in the P10 group was the
lowest, and the expressions of Nrf2, HO-1, NQO1 and SOD1 decreased
until T3, at which point the remaining ROS increased. ROS content
of the P50 group was highest at T2, although still lower than that
of the IR group, and the expression levels of Nrf2, HO-1, NQO1 and
SOD1 increased; the ROS content was the lowest at T3. Briefly, at
T3, the relative mRNA and protein expression levels of Nrf2, HO-1,
NQO1 and SOD1 were negatively linearly correlated with ROS content.
This may be because in the early stage of reperfusion, PPC with 10
µmol/l pinacidil resulted in less ROS generation and activated the
expression of antioxidant proteins via its phase II detoxification
enzyme downstream of the Nrf2 and Nrf2-ARE pathways to a lesser
extent (57). The ability to
eliminate ROS during reperfusion is limited, which may have
resulted in a higher ROS content in the P10 group at the time of
reperfusion. Low ROS concentration functions as a signaling
molecule that serves different biological roles and determines cell
fate by regulating transcription factors of different redox
reactions (58); the expression of
NQO1, HO-1 and SOD1 genes were all affected by low ROS
concentrations, and these genes could alleviate the effect of
oxidative stress and toxins (59).
This supports the conclusion that low-dose ROS is a signaling
molecule that activates the Nrf2-ARE pathway and participates in
PPC myocardial protection. The highest concentration of pinacidil
used in the present study (50 µmol/l) stimulated greater production
of ROS at T2 and activated Nrf2 and its downstream antioxidant
proteins, such as HO-1, NQO1 and SOD1, to a greater extent. High
expression levels of antioxidant proteins are associated with the
elimination of a large amount of ROS molecules produced during
reperfusion and therefore exert greater protective effects.
Therefore, the ROS concentration in the P50 group was lowest at T3
and the degree of myocardial damage was least severe. Furthermore,
the mitochondrial structure was found to be intact. This may be
because when ROS are released, the uncoupling of Nrf2 and
inhibitory protein kelch-like ECH-associated protein 1 causes Nrf2
to be transferred into the nucleus and bind to ARE to form a
heterodimer. The cis-reactive ARE is recognized, which encodes
downstream antioxidant protein and phase II detoxification enzyme
gene expression, such as glutathione S-transferases, glutathione
synthetase, HO-1, NQO1 and SOD1 (32). ARE enhances the antioxidant capacity
of tissue, protects tissue from toxic substances and serves an
important role in antitumor, anti-inflammatory and anti-apoptotic
responses (60).
In the present study, following the application of
the ROS scavenger MPG immediately after reperfusion, the degree of
myocardial ultrastructural damage and overall cardiac function
damage were more severe in the M + P50 group compared with the P50
group and the expression levels of Nrf2 and its downstream genes,
such as HO-1, SOD1 and NQO1, were significantly lower in the M +
P50 group compared with the P50 group. ROS scavenger may decrease
expression levels of genes and proteins, such as Nrf2, HO-1 and
SOD1, and downstream regulators, and regulate MIRI by antioxidant
proteins and phase II detoxification enzymes, thereby eliminating
PPC myocardial protection. This indicates that ROS is an important
signaling molecule that activates the Nrf2-ARE pathway and confers
protection in PPC. In addition, our previous study aimed to improve
the anti-MIRI effect of Nrf2-ARE by increasing pinacidil to 100
µmol/l in rat cardiomyocyte experiments (61). However, the relative expression
levels of the genes and proteins did not show a continued increase
and myocardial protection was not enhanced (61). The present study further
demonstrated that 50 µmol/l pinacidil is the optimal concentration
to activate the Nrf2-ARE pathway and decrease MIRI to exert a
protective effect.
At T2, the P10 group had the lowest ROS content and
the P50 group had the highest ROS content. At T3, the expression
levels of Nrf2 and its downstream antioxidant proteins in the P10
group were lowest and the ROS content was higher. The P50 group
exhibited the highest expression of Nrf2 and its downstream
antioxidant proteins, but lower ROS content. The reason may be
that, at T2, 10 µmol/l pinacidil produced less ROS and activated
the expression of antioxidant proteins and phase II detoxification
enzymes downstream of the Nrf2 and Nrf2-ARE pathways to a lesser
extent. The ability to remove ROS was also small ROS content in the
P10 group was still high at T3. Additionally, 50 µmol/l pinacidil
produced a higher concentration of ROS and activated expression of
antioxidant proteins and phase II detoxification enzymes downstream
of the Nrf2 and Nrf2-ARE pathway to a greater extent; the ability
to remove ROS also increased so, at T3, the P50 group exhibited
lower ROS content.
The higher the pinacidil concentration and
expression levels of Nrf2, HO-1, NQO1 and SOD1, the greater the
ability to resist MIRI at T3 and the lower the ROS content.
Conversely, lower Nrf2 expression levels are associated with
decreased ability to resist MIRI and higher ROS content at the end
of reperfusion (Fig. 6).
In conclusion, PPC (30–50 µM) promoted the formation
of ROS at T2, activated the Nrf2-ARE signaling pathway, regulated
the expression of downstream antioxidant proteins and alleviated
MIRI in rats. At 50 µM, pinacidil postconditioning produced the
most ROS and exhibited the best myocardial protection. The present
study may provide an experimental basis for the clinical
application of pinacidil in cardiac surgery, coronary artery bypass
or percutaneous coronary intervention surgery to decrease MIRI.
Acknowledgements
The authors would like to thank the Key Laboratory
of Anesthesia and Organ Protection in Guizhou Province for
providing the laboratory.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 30960366).
Availability of data and materials
All data used and/or analyzed during the study are
available from the corresponding author on reasonable request.
Authors' contributions
WC and HYW participated in the conception and design
of the experiments. MYD was involved in drafting the primary
manuscript and revising it. WC, MYD, YW and WJZ and TY made
substantial contributions to the acquisition of data and
interpretation of the data. HYW and TY were involved in revising
the manuscript critically for important intellectual content. WC,
MYD, HYW, YW, WJZ and TY confirm the authenticity of the raw data
in this study. Each author participated sufficiently in the work to
take public responsibility for appropriate portions of the content.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present experiment was approved by document No.
36 of the Animal Ethics Review at The Affiliated Hospital of Zunyi
Medical University in 2016. The use and processing of animals was
in accordance with the Guide for the Care and Use of Laboratory
Animals, published by the National Institute of Health (NIH
Publication 88.23, revised 1996).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhao ZQ, Corvera JS, Halkos ME, Kerendi F,
Wang NP, Guyton RA and Vinten-Johansen J: Inhibition of myocardial
injury by ischemic postconditioning during reperfusion: Comparison
with ischemic preconditioning. Am J Physiol Heart Circ Physiol.
285:H579–H588. 2003. View Article : Google Scholar
|
|
2
|
Hao M, Zhu S, Hu L, Zhu H, Wu X and Li Q:
Myocardial ischemic postconditioning promotes autophagy against
ischemia reperfusion injury via the Activation of the
nNOS/AMPK/mTOR Pathway. Int J Mol Sci. 18:6142017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang L, Cao S, Deng S, Yao G and Yu T:
Ischemic postconditioning and pinacidil suppress calcium overload
in anoxia-reoxygenation cardiomyocytes via down-regulation of the
calcium-sensing receptor. PeerJ. 4:e26122016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Foster MN and Coetzee WA: KATP channels in
the cardiovascular system. Physiol Rev. 96:177–252. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cohen MV and Downey JM: Signalling
pathways and mechanisms of protection in pre- and postconditioning:
Historical perspective and lessons for the future. Br J Pharmacol.
172:1913–1932. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang HQ, Foster MN, Jana K, Ho J, Rindler
MJ and Coetzee WA: Plasticity of sarcolemmal KATP channel surface
expression: Relevance during ischemia and ischemic preconditioning.
Am J Physiol Heart Circ Physiol. 310:H1558–H1566. 2016. View Article : Google Scholar
|
|
7
|
Yaşar S, Bozdoğan Ö, Kaya ST and Orallar
HS: The effects of ATP-dependent potassium channel opener;
pinacidil, and blocker; glibenclamide, on the ischemia induced
arrhythmia in partial and complete ligation of coronary artery in
rats. Iran J Basic Med Sci. 18:188–193. 2015.
|
|
8
|
Yang L and Yu T: Prolonged donor heart
preservation with pinacidil: The role of mitochondria and the
mitochondrial adenosine triphosphate-sensitive potassium channel. J
Thorac Cardiovasc Surg. 139:1057–1063. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang YH, Zhang Y, Chen W, Wang Y, Cao S,
Yu T and Wang H: Pinacidil-postconditioning is equivalent to
ischemic postconditioning in defeating cardiac ischemia-reperfusion
injury in rat. Eur J Pharmacol. 780:26–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen X, Han K, Zhang T, Qi G, Jiang Z and
Hu C: Grass carp (Ctenopharyngodon idella) NRF2 alleviates the
oxidative stress and enhances cell viability through upregulating
the expression of HO-1. Fish Physiol Biochem. 46:417–428. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moon EJ and Giaccia A: Dual roles of NRF2
in tumor prevention and progression: Possible implications in
cancer treatment. Free Radic Biol Med. 79:292–299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patwardhan J and Bhatt P: Flavonoids
derived from abelmoschus esculentus attenuates UV-B induced cell
damage in human dermal fibroblasts through Nrf2-ARE pathway.
Pharmacogn Mag. 12 (Suppl 2):S129–S138. 2016. View Article : Google Scholar
|
|
13
|
Loboda A, Damulewicz M, Pyza E, Jozkowicz
A and Dulak J: Role of Nrf2/HO-1 system in development, oxidative
stress response and diseases: An evolutionarily conserved
mechanism. Cell Mol Life Sci. 73:3221–3247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bao L, Li J, Zha D, Zhang L, Gao P, Yao T
and Wu X: Chlorogenic acid prevents diabetic nephropathy by
inhibiting oxidative stress and inflammation through modulation of
the Nrf2/HO-1 and NF-κB pathways. Int Immunopharmacol. 54:245–253.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oh ET and Park HJ: Implications of NQO1 in
cancer therapy. BMB Rep. 48:609–617. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schlager JJ and Powis G: Cytosolic
NAD(P)H: (quinone-acceptor)oxidoreductase in human normal and tumor
tissue: Effects of cigarette smoking and alcohol. Int J Cancer.
45:403–409. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng Q, Lu Y, Lao X, Chen Z, Li R, Sui J,
Qin X and Li S: The NQO1 Pro187Ser polymorphism and breast cancer
susceptibility: Evidence from an updated meta-analysis. Diagn
Pathol. 9:1002014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang CY, Ren XM, Li HB, Wei W, Wang KX,
Li YM, Hu JL and Li X: Simvastatin alleviates inflammation and
oxidative stress in rats with cerebral hemorrhage through Nrf2-ARE
signaling pathway. Eur Rev Med Pharmacol Sci. 23:6321–6329.
2019.PubMed/NCBI
|
|
19
|
Feng S, Xu Z, Wang F, Yang T, Liu W, Deng
Y and Xu B: Sulforaphane prevents methylmercury-induced oxidative
damage and excitotoxicity through activation of the Nrf2-ARE
pathway. Mol Neurobiol. 54:375–391. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao T, Chen S, Wang B and Cai D:
L-Carnitine reduces myocardial oxidative stress and alleviates
myocardial ischemia-reperfusion injury by activating nuclear
transcription-related factor 2 (Nrf2)/Heme Oxygenase-1 (HO-1)
signaling pathway. Med Sci Monit. 26:e9232512020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma W, Liu M, Liang F, Zhao L, Gao C, Jiang
X, Zhang X, Zhan H, Hu H and Zhao Z: Cardiotoxicity of sorafenib is
mediated through elevation of ROS level and CaMKII activity and
dysregulation of calcium homoeostasis. Basic Clin Pharmacol
Toxicol. 126:166–180. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen W, Chen XY, Wang Y, Wang HY, Zhou WJ
and Yu T: Mechanism of emulsified isoflurane
Postconditioning-induced activation of the Nrf2-antioxidant
response element signaling pathway during myocardial
ischemia-reperfusion: The relationship with reactive oxygen
species. J Cardiovasc Pharmacol. 73:265–271. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang SY, Chen YC, Kao YH, Hsieh MH, Lin
YK, Chen SA and Chen YJ: Redox and activation of protein kinase a
dysregulates calcium homeostasis in pulmonary vein cardiomyocytes
of chronic kidney disease. J Am Heart Assoc. 6:e0057012017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ivanova S, Batliwalla F, Mocco J, Kiss S,
Huang J, Mack W, Coon A, Eaton JW, Al-Abed Y, Gregersen PK, et al:
Neuroprotection in cerebral ischemia by neutralization of
3-aminopropanal. Proc Natl Acad Sci USA. 99:5579–5584. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hartung T: Comparative analysis of the
revised Directive 2010/63/EU for the protection of laboratory
animals with its predecessor 86/609/EEC-a t4 report. ALTEX.
27:285–303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press (US); Washington, DC: 2011
|
|
27
|
Flameng W, Borgers M, Daenen W and
Stalpaert G: Ultrastructural and cytochemical correlates of
myocardial protection by cardiac hypothermia in man. J Thorac
Cardiovasc Surg. 79:413–424. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li J, Zhou W, Chen W, Wang H, Zhang Y and
Yu T: Mechanism of the hypoxia inducible factor 1/hypoxic response
element pathway in rat myocardial ischemia/diazoxide
post-conditioning. Mol Med Rep. 21:1527–1536. 2020.PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hausenloy DJ, Barrabes JA, Bøtker HE,
Davidson SM, Di Lisa F, Downey J, Engstrom T, Ferdinandy P,
Carbrera-Fuentes HA, Heusch G, et al: Ischaemic conditioning and
targeting reperfusion injury: A 30 year voyage of discovery. Basic
Res Cardiol. 111:702016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Neri M, Riezzo I, Pascale N, Pomara C and
Turillazzi E: Ischemia/Reperfusion injury following acute
myocardial infarction: A critical issue for clinicians and forensic
pathologists. Mediators Inflamm. 2017:70183932017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsutsumi YM, Yokoyama T, Horikawa Y, Roth
DM and Patel HH: Reactive oxygen species trigger ischemic and
pharmacological postconditioning: In vivo and in vitro
characterization. Life Sci. 81:1223–1227. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Granger DN and Kvietys PR: Reperfusion
injury and reactive oxygen species: The evolution of a concept.
Redox Biol. 6:524–551. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pisarenko O, Shulzhenko V, Studneva I,
Pelogeykina Y, Timoshin A, Anesia R, Valet P, Parini A and
Kunduzova O: Structural apelin analogues: Mitochondrial ROS
inhibition and cardiometabolic protection in myocardial ischaemia
reperfusion injury. Br J Pharmacol. 172:2933–2945. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han J, Kim N, Park J, Seog DH, Joo H and
Kim E: Opening of mitochondrial ATP-sensitive potassium channels
evokes oxygen radical generation in rabbit heart slices. J Biochem.
131:721–727. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zi C, Zhang C, Yang Y and Ma J:
Penehyclidine hydrochloride protects against anoxia/reoxygenation
injury in cardiomyocytes through ATP-sensitive potassium channels,
and the Akt/GSK-3β and Akt/mTOR signaling pathways. Cell Biol Int.
44:1353–1362. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fan Z, Wen T, Chen Y, Huang L, Lin W, Yin
C and Tan W: Isosteviol sensitizes sarcKATP channels towards
pinacidil and potentiates mitochondrial uncoupling of diazoxide in
guinea pig ventricular myocytes. Oxid Med Cell Longev.
2016:63628122016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liang W, Chen J, Mo L, Ke X, Zhang W,
Zheng D, Pan W, Wu S, Feng J, Song M and Liao X: ATP-sensitive K+
channels contribute to the protective effects of exogenous hydrogen
sulfide against high glucose-induced injury in H9c2 cardiac cells.
Int J Mol Med. 37:763–772. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Slove S, Lannoy M, Behmoaras J, Pezet M,
Sloboda N, Lacolley P, Escoubet B, Buján J and Jacob MP: Potassium
channel openers increase aortic elastic fiber formation and reverse
the genetically determined elastin deficit in the BN rat.
Hypertension. 62:794–801. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tanonaka K, Iwai T, Motegi K and Takeo S:
Effects of N-(2-mercaptopropionyl)-glycine on mitochondrial
function in ischemic-reperfused heart. Cardiovasc Res. 57:416–425.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Andreadou I, Iliodromitis EK, Souridis V,
Prokovas E, Kostidis S, Zoga A, Dagres N, Tsantili-Kakoulidou A,
Kremastinos DT, Mikros E and Anastasiou-Nana M: Investigating the
effect of antioxidant treatment on the protective effect of
preconditioning in anesthetized rabbits. J Cardiovasc Pharmacol.
58:609–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fantinelli JC, González ALF, Pérez NIA and
Mosca SM: Protective effects of N-(2-mercaptopropionyl)-glycine
against ischemia-reperfusion injury in hypertrophied hearts. Exp
Mol Pathol. 94:277–284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ihnken K, Morita K, Buckberg GD, Sherman
MP and Young HH: Studies of hypoxemic/reoxygenation injury: Without
aortic clamping. VI. Counteraction of oxidant damage by exogenous
antioxidants: N-(2-mercaptopropionyl)-glycine and catalase. J
Thorac Cardiovasc Surg. 110:1212–1220. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shanmugam G, Narasimhan M, Tamowski S,
Darley-Usmar V and Rajasekaran NS: Constitutive activation of Nrf2
induces a stable reductive state in the mouse myocardium. Redox
Biol. 12:937–945. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Buelna-Chontal M, Guevara-Chávez JG,
Silva-Palacios A, Medina-Campos ON, Pedraza-Chaverri J and Zazueta
C: Nrf2-regulated antioxidant response is activated by protein
kinase C in postconditioned rat hearts. Free Radic Biol Med.
74:145–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zai CC, Tiwari AK, Basile V, de Luca V,
Müller DJ, Voineskos AN, Remington G, Meltzer HY, Lieberman JA,
Potkin SG and Kennedy JL: Oxidative stress in tardive dyskinesia:
Genetic association study and meta-analysis of NADPH quinine
oxidoreductase 1 (NQO1) and Superoxide dismutase 2 (SOD2, MnSOD)
genes. Prog Neuropsychopharmacol Biol Psychiatry. 34:50–56. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gang GT, Hwang JH, Kim YH, Noh JR, Kim KS,
Jeong JY, Choi DE, Lee KW, Jung JY, Shong M and Lee CH: Protection
of NAD(P)H:quinone oxidoreductase 1 against renal
ischemia/reperfusion injury in mice. Free Radic Biol Med.
67:139–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen G, Fang Q, Zhang J, Zhou D and Wang
Z: Role of the Nrf2-ARE pathway in early brain injury after
experimental subarachnoid hemorrhage. J Neurosci Res. 89:515–523.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Freixa X, Bellera N, Ortiz-Pérez JT,
Jiménez M, Paré C, Bosch X, De Caralt TM, Betriu A and Masotti M:
Ischaemic postconditioning revisited: Lack of effects on infarct
size following primary percutaneous coronary intervention. Eur
Heart J. 33:103–112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Limalanathan S, Andersen GØ, Kløw NE,
Abdelnoor M, Hoffmann P and Eritsland J: Effect of ischemic
postconditioning on infarct size in patients with ST-elevation
myocardial infarction treated by primary PCI results of the POSTEMI
(POstconditioning in ST-Elevation Myocardial Infarction) randomized
trial. J Am Heart Assoc. 3:e0006792014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Paul MK, Bisht B, Darmawan DO, Chiou R, Ha
VL, Wallace WD, Chon AT, Hegab AE, Grogan T, Elashoff DA, et al:
Dynamic changes in intracellular ROS levels regulate airway basal
stem cell homeostasis through Nrf2-dependent Notch signaling. Cell
Stem Cell. 15:199–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rojo de la Vega M, Chapman E and Zhang DD:
NRF2 and the hallmarks of cancer. Cancer Cell. 34:21–43. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kensler TW, Wakabayashi N and Biswal S:
Cell survival responses to environmental stresses via the
Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 47:89–116.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ma Q: Role of nrf2 in oxidative stress and
toxicity. Annu Rev Pharmacol Toxicol. 53:401–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jaramillo MC and Zhang DD: The emerging
role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev.
27:2179–2191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Harder B, Jiang T, Wu T, Tao S, Rojo de la
Vega M, Tian W, Chapman E and Zhang DD: Molecular mechanisms of
Nrf2 regulation and how these influence chemical modulation for
disease intervention. Biochem Soc Trans. 43:680–686. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Iguchi K, Saotome M, Yamashita K, Hasan P,
Sasaki M, Maekawa Y and Watanabe Y: Pinacidil, a KATP channel
opener, stimulates cardiac Na+/Ca2+ exchanger
function through the NO/cGMP/PKG signaling pathway in guinea pig
cardiac ventricular myocytes. Naunyn Schmiedebergs Arch Pharmacol.
392:949–959. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Itoh K, Chiba T, Takahashi S, Ishii T,
Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et
al: An Nrf2/small Maf heterodimer mediates the induction of phase
II detoxifying enzyme genes through antioxidant response elements.
Biochem Biophys Res Commun. 236:313–322. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Satta S, Mahmoud AM, Wilkinson FL, Yvonne
AM and White SJ: The role of Nrf2 in cardiovascular function and
disease. Oxid Med Cell Longev. 2017:92372632017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Penna C, Rastaldo R, Mancardi D, Raimondo
S, Cappello S, Gattullo D, Losano G and Pagliaro P:
Post-conditioning induced cardioprotection requires signaling
through a redox-sensitive mechanism, mitochondrial ATP-sensitive K+
channel and protein kinase C activation. Basic Res Cardiol.
101:180–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shoujia Y, Haiying W, Tian Y and Xingkui
L: The role of Nrf2-ARE pathway in hypoxia/pinacidil post-treatment
in reducing hypoxia-reoxygenation injury of rat cardiomyocytes.
Chin J Pathophysiology. 1696–1699, +1703. 2013.
|