Introduction
Type 2 diabetes mellitus (T2DM) is a severe and
lifelong metabolic disease that is often characterised by insulin
resistance (IR) and reduced insulin production, resulting in
abnormally elevated blood glucose levels (1). It is estimated that by 2040, the
number of individuals suffering from diabetes worldwide will reach
642 million, of which T2DM will account for ~90% (2). Previous studies have reported that
metabolic disorders associated with T2DM could lead to liver
injury, eventually resulting in a series of liver diseases such as
fatty liver, cirrhosis and hepatocellular carcinoma (3–5).
Diabetic liver injury is a major complication of T2DM caused by a
variety of factors, such as hyperglycaemia, IR, dyslipidaemia,
oxidative stress and inflammation (5). Among them, dyslipidaemia, oxidative
stress and inflammation are conceived as the most common factors
contributing to IR, which promotes the progression of T2DM
(6). However, the exact molecular
mechanism of diabetic liver injury in T2DM is not fully
understood.
Autophagy is the major degradation and dynamic
circulation system in the cell, via which cytoplasmic materials are
transferred to, and degraded in the lysosome for cellular
renovation and homeostasis (7). The
impairment of autophagy with IR can be observed in obesity-induced
diabetes, and the recovery of the autophagic flux can improve IR in
T2DM (8). Moreover, autophagy not
only regulates lipid metabolism but also reduces oxidative stress
and inflammation (9–11). Thus, autophagy may be closely
associated with the occurrence and development of diabetic liver
injury in T2DM. Adenosine monophosphate-activated protein kinase
(AMPK), a major cellular energy sensor, is a key factor in
maintaining metabolic homeostasis (12). Accumulating evidence has revealed
the close relationship between AMPK and autophagy, and AMPK can
promote autophagy by inhibiting the mTOR pathway, which directly
dephosphorylates and inhibits downstream 70-kD ribosomal protein S6
kinase (13–15). Therefore, AMPK/mTOR-mediated
autophagy may be an ideal target for the treatment of diabetic
liver injury in T2DM.
Astragaloside IV (AS-IV) is a natural saponin
isolated from the plant Astragalus membranaceus. Recent
studies have revealed that AS-IV has anti-oxidation,
anti-inflammation and anti-apoptosis effects, and can enhance
immunity (16–18). Our previous studies also showed that
AS-IV exerts protective effects on the endoplasmic reticulum
stress-induced apoptosis of renal tubular epithelial cells and
diabetic cardiomyopathy in T2DM rats (18,19).
However, it remains unknown whether AS-IV exerts a protective
effect on diabetic liver injury in T2DM, and whether autophagy is
involved in diabetic liver injury is yet to be elucidated.
Therefore, the present study was performed to investigate the
protective effect and molecular mechanism of AS-IV on diabetic
liver injury by regulating AMPK/mTOR-mediated autophagy in T2DM
rats. Importantly, this study may provide a potential therapy using
AS-IV in the prevention of diabetic liver injury in T2DM.
Materials and methods
Drug preparation
AS-IV (purity >98%; Nanjing Zelang Pharmaceutical
Technology Co., Ltd.) and metformin hydrochloride (Met; Shanghai
Shangyao Xinyi Pharmaceutical Co., Ltd.) were suspended in 0.5%
carboxyl methyl cellulose sodium (CMC-Na+) aqueous
solution as an administration vehicle.
Animals and treatment
Male Sprague-Dawley rats (age, 6–8 weeks; weight,
200±20 g; n=36) were purchased from the Shandong Experimental
Animal Center (Shandong, China) and housed in an environment at
24±2°C and 60±5% humidity with free access to water and standard
diet under a 12-h light/dark cycle. All animal experiments were
conducted under protocols approved by the Ethics Review Committee
of Anhui Medical University (approval no. LLSC20190302).
The rats were acclimatised for 1 week before the
experiment and randomly allocated to a normal control group (n=6)
and a diabetic group (n=30). In the diabetic group, rats were fed
with high-fat diets (HFD) containing 59.5% standard diet, 20%
sucrose, 10% egg yolk powder and 10% lard for 6 weeks, and were
then intraperitoneally injected with 35 mg/kg streptozotocin (STZ;
Shanghai Yuanye Biotechnology Co., Ltd.), which was dissolved in a
0.1 M citric acid-sodium citrate buffer (pH 4.4). Rats in the
normal control group were maintained on the standard diet for 6
weeks and were then given the same amount of vehicle (0.1 M citric
acid-sodium citrate buffer, pH 4.4) via an intraperitoneal
injection. After 72 h and 7 days, the levels of fasting blood
glucose (FBG) in the tail vein were measured with a portable
glucometer (GA-3 Sannuoyi accurate blood glucometer; Changsha
Sannuo Biosensing Co., Ltd.). The success of the diabetic rats was
based on FBG concentration ≥16.7 mmol/l (18,19).
Then, 1 week after STZ injection, diabetic rats were
randomly divided into three subgroups according to different
treatment methods (n=6/each subgroup): Diabetic model group, AS-IV
treatment group and Met treatment group, and were still fed a HFD.
Rats in the AS-IV and Met treatment groups were treated with an
intragastric administration of AS-IV (80 mg/kg) and Met (200 mg/kg)
daily for 8 weeks, respectively. The normal control group and model
group rats were treated with an equal volume of vehicle (0.5%
CMC-Na+ aqueous solution) daily for 8 weeks. After 8
weeks of intervention, rats were fasted overnight for 12 h and
anaesthetised with pentobarbital sodium (30 mg/kg). Blood samples
(8–10 ml/each rat) were collected via the abdominal aorta for
further analysis when the rats lost consciousness, and then the
rats were euthanized by exsanguination after anaesthesia. Liver
tissues were removed and weighed immediately for further
analysis.
Measurement of food intake, water
intake, urine volume, body weight and FBG
During the experiment, the general conditions of the
rats were observed, such as food intake, water intake, urine volume
and body weight. The food intake, water intake and urine volume of
rats within 24 h were measured every 4 weeks. Additionally, at a
fixed time (every Saturday at 8 am), the body weight of the rats
was measured 1 a week, and the levels of FBG from the tail vein
after fasting for 6 h in each group were measured every 2 weeks
from 0 to 8 weeks of administration using a portable
glucometer.
Oral glucose tolerance test
(OGTT)
The OGTT was performed on rats that were fasted
overnight for 12 h after the last administration (the normal
control group, diabetic model group, AS-IV treatment group and Met
treatment group were treated with CMC-Na+ aqueous
solution, CMC-Na+ aqueous solution, AS-IV and Met,
respectively). Rats in each group were given glucose at a dose of 2
g/kg by gavage. Blood glucose levels were measured using the
portable glucometer via the detection of blood samples collected
from the tail vein at 0 (before gavage), 30, 60 and 120 min after
gavage. The data were plotted curves of the blood glucose
concentrations over time, and the area under the curve (AUC) was
calculated.
Measurement of biochemical
analyses
Blood samples were centrifuged at 2,000 × g for 10
min at 4°C for analysis. Serum levels of alanine aminotransferase
(ALT; cat. no. C009-2-1), aspartate aminotransferase (AST; cat. no.
C010-2-1), total cholesterol (TC; cat. no. A111-1), triglyceride
(TG; cat. no. A110-1-1), high-density lipoprotein cholesterol
(HDL-C; cat. no. A112-1-1), low-density lipoprotein cholesterol
(LDL-C; cat. no. A113-1-1) and glycosylated serum protein (GSP;
cat. no. A037-2) were determined using the corresponding kits
(Nanjing Jiancheng Bioengineering Institute) according to the
manufacturer's instructions. The levels of TNF-α (cat. no.
MM-0180R1), IL-6 (cat. no. MM-0190R2) and fasting insulin (FINS;
cat. no. MM-0587R1) in serum were measured with their commercial
ELISA kits (Jiangsu Enzyme Free Experimental Co., Ltd.) according
to the manufacturer's instructions. The homeostasis model
assessment of insulin resistance (HOMA-IR), which indicates the
degree of IR, was calculated according to the following formula:
HOMA-IR=(FBGxFINS)/22.5 (20).
Detection of liver histology
Liver specimens were fixed in 4% paraformaldehyde at
room temperature for 24–48 h, dehydrated, embedded in paraffin
blocks and cut into 5-µm sections. Liver sections were dewaxed in
xylene, rehydrated in graded alcohol series and rinsed under
running water. Subsequently, sections were stained with H&E
(haematoxylin 3–5 min and eosin 30 sec, both at room temperature)
or Masson's trichrome staining (cat. no. G1340; Beijing Solarbio
Science & Technology Co., Ltd.; haematoxylin 3 min, ponceau
acid fuchsin 5–10 min, phosphomolybdic acid 1–3 min and aniline
blue 3–6 min, both at room temperature), and histopathological
changes were examined under a light microscope (Olympus IX71;
Olympus Corporation; magnification, ×400). The intensity of
positive areas (Masson's staining) was analysed using Image-Pro
Plus 6.0 analysis software (Media Cybernetics, Inc.) to assess
liver fibrosis.
Lipid deposition was assessed via Oil red O staining
in the liver. Briefly, liver specimens were embedded in optimal
cutting temperature compound and sliced with a frozen slicer (Leica
CM3050; Leica Microsystems GmbH) at a thickness of 10-µm. Liver
slices were fixed with 10% formalin at room temperature for 15 min,
stained with Oil red O solution at room temperature for 8–10 min
and observed under a light microscope (Olympus IX71; Olympus
Corporation). The intensity of positive areas from ≥5 random fields
(magnification, ×400) in each section was quantified using
Image-Pro Plus 6.0 analysis software to assess lipid
deposition.
Immunohistochemistry
Liver tissues were fixed in 4% paraformaldehyde for
24 h at room temperature and embedded in liquid paraffin at room
temperature, and after waiting to solidify, these were cut into
5-µm sections. After dewaxing and rehydration, the liver sections
were placed in sodium citrate buffer (pH 6.0) in a microwave oven
for antigen retrieval (boiling for 5–10 min, fire ceased for 3 min,
boiling for 5–10 min). After natural cooling, the sections were
incubated in 3% H2O2 for 25 min to inhibit
endogenous peroxidase activity and were then blocked with 3% BSA
(cat. no. 4240GR100; BioFroxx GmbH) at 37°C for 30 min.
Immunohistochemistry was performed using Beclin1 (cat. no. AF5128;
1:100; Affinity Biosciences), P62 (cat. no. AF5384; 1:100; Affinity
Biosciences) and LC3 (cat. no. AF5402; 1:100; Affinity Biosciences)
primary antibodies at 4°C overnight. The sections were then treated
with HRP-labelled goat anti-rabbit secondary antibodies (cat. no.
S0001; 1:500; Affinity Biosciences) at 37°C for 50 min. Finally,
DAB chromogen (cat. no. G1211; Wuhan Servicebio Technology Co.,
Ltd.) was added before counterstaining with haematoxylin at room
temperature for 3 min. The intensity of positive areas from ≥5
random fields (magnification, ×400) in each section was quantified
using Image-Pro Plus 6.0 analysis software.
Western blotting
Liver tissues were collected, homogenised and then
lysed in ice-cold RIPA lysis buffer (cat. no. P0013B; Beyotime
Institute of Biotechnology) containing protease inhibitors and
phosphatase inhibitors to extract the total proteins. The
concentration of total proteins was determined using the enhanced
BCA protein assay kit (cat. no. P0010S; Shanghai Beyotime
Biotechnology Co., Ltd.) according to the manufacturer's
instructions. The same amount of protein (30 µg) was separated by
8–15% SDS-PAGE and transferred to PVDF membranes (EMD Millipore).
Then, the membranes were blocked with 5% fat-free milk in TBS-0.05%
Tween-20 (TBST) buffer at room temperature for 2 h and incubated
overnight at 4°C with primary antibodies against AMPK (cat. no.
AF6423; 1:1,000; Affinity Biosciences), phosphorylated (p)-AMPK
(cat. no. BS4010; 1:1,000; Bioworld Technology, Inc.), mTOR (cat.
no. 66888–1-lg; 1:5,000; ProteinTech Group, Inc.), p-mTOR (cat. no.
BS4706; 1:1,000; Bioworld Technology, Inc.), Beclin1 (cat. no.
WL02508; 1:1,000; Wanleibio Co., Ltd.), P62 (cat. no. WL02385;
1:500; Wanleibio Co., Ltd.), LC3 (cat. no. 14600-1-AP; 1:1,000;
ProteinTech Group, Inc.), TNF-α (cat. no. AF7014; 1:500; Affinity
Biosciences), IL-6 (cat. no. BS6419; 1:1,000; Bioworld Technology,
Inc.), heme oxygenase (HO)-1 (cat. no. ab13243; 1:2,000; Abcam) and
β-actin (cat. no. AF7019; 1:5,000; Affinity Biosciences).
Subsequently, the membranes were washed with TBST buffer three
times for 10 min and then incubated for 1 h at room temperature
with the corresponding HRP-conjugated secondary antibody (cat. no.
S0001; 1:10,000; Affinity Biosciences). An ECL reagent (Bridgen
Co., Ltd.: http://www.bridgen.cn) was used to
visualise the blotted proteins using a Chemi Doc TMMP Imaging
system (Bio-Rad Laboratories, Inc.). The density of the target
bands was analysed semi-quantitatively using ImageJ 1.45s software
(National Institutes of Health) and normalised with β-actin as the
internal control.
Transmission electron microscopy
detection
Liver specimens were cut into small pieces (1
mm3), and immediately fixed with 2.5% glutaraldehyde for
2 h at 4°C. After washing with PBS, the tissues were post-fixed in
1% osmium tetroxide for 2 h at room temperature, dehydrated and
then embedded in Epon 812. Ultrathin sections (thickness, 70 nm)
were cut with an ultramicrotome (Leica UC7; Leica Microsystems
GmbH) and double-stained with uranyl acetate and lead citrate at
room temperature for 15 min. Ultrastructural images were viewed and
imaged using a transmission electron microscope (HT 7700; Hitachi,
Ltd.; magnification, ×5,000).
Statistical analysis
Data are presented as the mean ± SD of ≥3
independent experiments. SPSS 16.0 statistical software (SPSS,
Inc.) was used to compare differences among groups via a one-way
ANOVA followed by Tukey's test. Mixed two-way ANOVA and Bonferroni
tests were used to measure differences over time and
between-subject comparisons of treatment groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
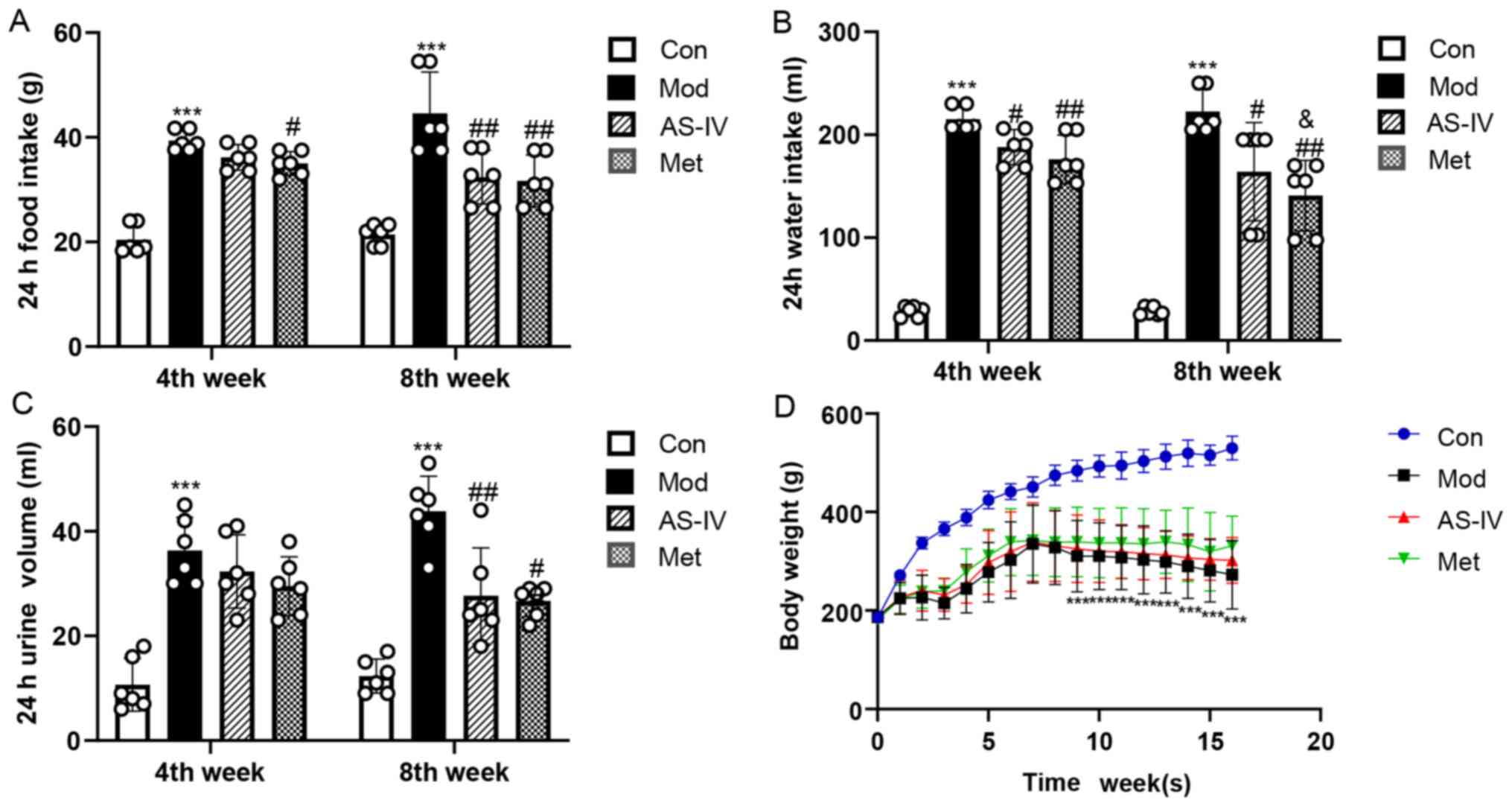
Effects of AS-IV on food intake, water
intake, urine volume and body weight in T2DM rats
As shown in Fig.
1A-C, at the end of the 4 and 8th weeks of the experiment, the
food intake, water intake and urine volume were significantly
increased in the model group compared with the control group
(P<0.001), while AS-IV and Met treatment significantly decreased
these indicators compared with the model group (P<0.05 or
P<0.01), especially at the end of the 8th week. Furthermore,
during the experiment, the body weight of the model group was
significantly lower compared that of the control group (P<0.001;
Fig. 1D), while AS-IV and Met
treatment notably inhibited the weight loss of the T2DM rats, but
the difference was not statistically significant (P>0.05;
Fig. 1D). These results support a
feasible role for AS-IV to improve food intake, water intake, urine
volume and body weight in T2DM rats.
Effects of AS-IV on liver function in
T2DM rats
ALT, AST and the liver index of liver weight to body
weight were detected to investigate the effect of AS-IV on liver
function in T2DM rats. ALT and AST, which are sensitive markers of
liver function, are released into the blood due to the increased
permeability of the cell membrane when liver cells are damaged,
resulting in increased levels of ALT and AST in serum (21). The serum levels of ALT and AST in
the model group were significantly higher compared with those in
the control group, but were reversed by treatment with AS-IV and
Met (P<0.01 or P<0.001; Fig. 2D
and E). It has been reported that the liver index, another
indicator to evaluate liver function, is abnormally increased in
diabetes (22). It was found that
the liver index in the model group was significantly increased
compared with that of the control group, but was significantly
reduced by treatment with AS-IV and Met (P<0.01 or P<0.001;
Fig. 2F).
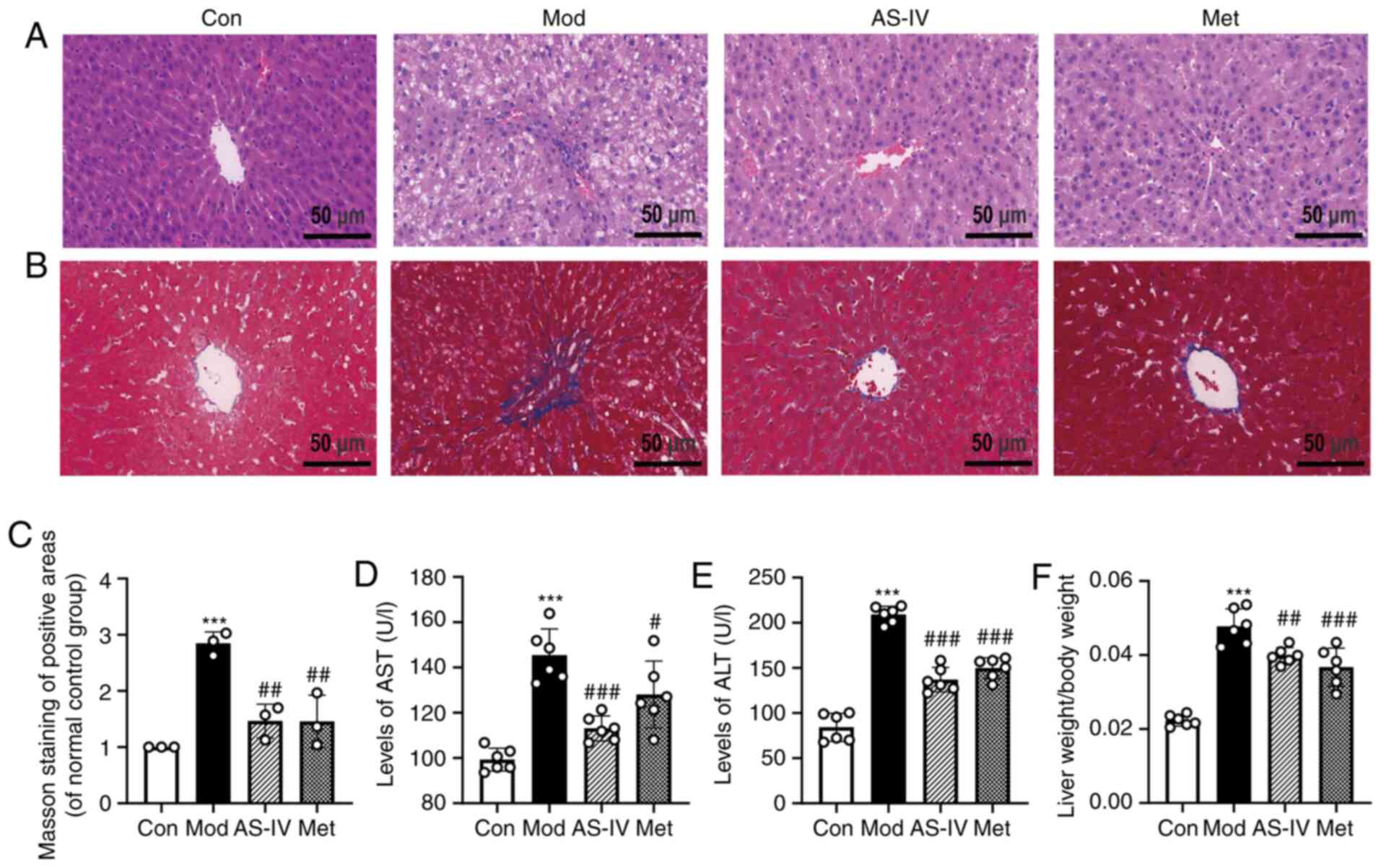 | Figure 2.AS-IV attenuates liver function in
T2DM rats. (A) H&E staining of liver tissues (magnification,
×400). (B) Masson staining of liver tissues (magnification, ×400).
(C) Quantitative analysis of positive areas of Masson staining was
normalised to the normal control group. (D) AST and (E) ALT levels
in serum. (F) Ratio of liver weight to body weight. Data are
presented as the mean ± SD, n=6. ***P<0.001 vs. control group;
#P<0.05, ##P<0.01,
###P<0.001 vs. model group. Con, Control; Mod, Model;
AS-IV, Astragaloside IV (80 mg/kg); Met, Metformin (200 mg/kg);
AST, aspartate aminotransferase; ALT, alanine aminotransferase. |
To further observe liver histopathology, H&E
staining was performed. As presented in Fig. 2A, neatly arranged hepatocytes
without steatosis were observed in the control group. In the model
group, disorganised hepatocytes and lipid droplets were notably
increased. However, AS-IV and Met treatment showed marked
improvements, such as reduced liver lipid droplets and hepatic
steatosis. Additionally, hepatic fibrosis was detected using Masson
staining. Compared with the control group, the model group showed
significant hepatic fibrosis with enlarged blue areas (P<0.001;
Fig. 2B and C), suggesting collagen
deposition in the liver of T2DM rats. By contrast, compared with
the model group, the collagen deposition was significantly improved
by the administration of AS-IV and Met (P<0.01; Fig. 2B and C). These data suggest that
AS-IV treatment improves liver function in T2DM rats.
Effects of AS-IV on glucose
homeostasis in T2DM rats
To determine whether AS-IV improves glucose
homeostasis in T2DM rats, GSP, FBG, FINS and HOMA-IR in serum were
examined. In the model group, the levels of FBG, FINS, HOMA-IR and
GSP were significantly increased, but AS-IV and Met treatment
reversed the elevated levels of these indexes (P<0.05, P<0.01
or P<0.001; Fig. 3A-D).
Moreover, FBG and OGTT levels were examined by taking blood from
the tail vein. As presented in Fig.
3E, a significant increase in FBG level was observed in the
model group, especially at 6–8 weeks (P<0.01 or P<0.001). It
was found that AS-IV treatment resulted in no hypoglycaemic
activity in the first 2 weeks, whereas long-term treatment with
AS-IV (4–8 weeks), especially at 6–8 weeks, had a hypoglycaemic
activity compared with the model group (P<0.001). In addition,
the AUC of the OGTT in the model group was higher compared with
that in the control group, but the elevations were significantly
reversed by AS-IV and Met treatment (P<0.001; Fig. 3F). Collectively, these results
indicated that AS-IV could improve glucose homeostasis in T2DM
rats.
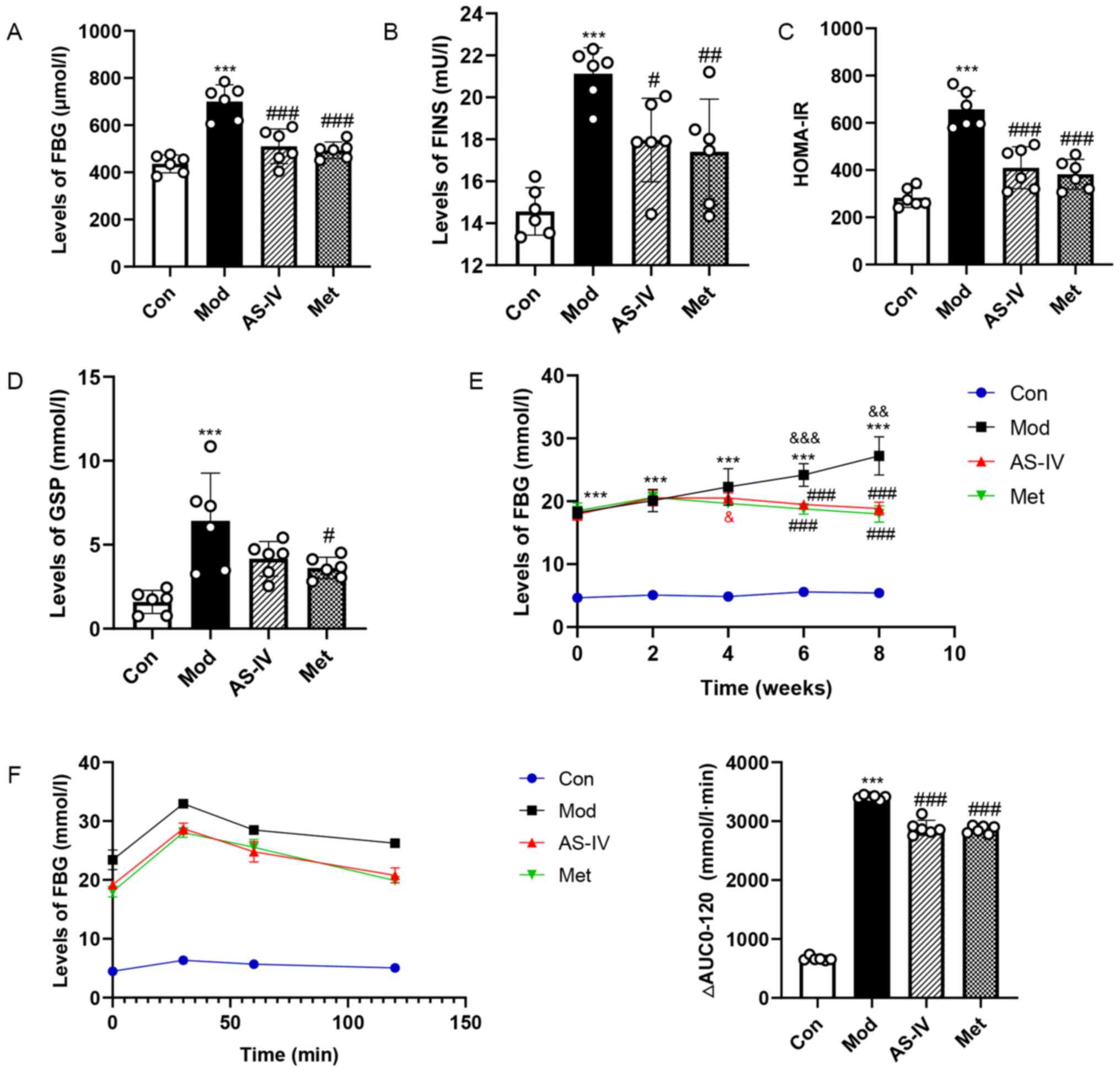 | Figure 3.AS-IV maintains glucose homeostasis
in T2DM rats. (A) FBG and (B) FINS levels in serum. (C) HOMA-IR.
(D) GSP levels in serum. (E) FBG levels from 0–8 weeks after
intervention. (F) Oral glucose tolerance test results and AUC. Data
are presented as the mean ± SD, n=6. ***P<0.001 vs. control
group; #P<0.05, ##P<0.01,
###P<0.001 vs. model group;
&P<0.05, &&P<0.01,
&&&P<0.001 vs. week 0. Con, Control; Mod,
Model; AS-IV, Astragaloside IV (80 mg/kg); Met, Metformin (200
mg/kg); FBG, fasting blood glucose; GSP, glycosylated serum
protein; FINS, fasting insulin; HOMA-IR, homeostasis model
assessment of insulin resistance; AUC, area under the curve. |
Effects of AS-IV on dyslipidaemia and
liver lipid deposition in T2DM rats
To determine whether AS-IV ameliorates dyslipidaemia
in T2DM rats, the levels of TG, TC, LDL-C and HDL-C were detected
in serum. Compared with the control group, the TG and TC levels
were significantly increased in the model group, but the HDL-C
level was markedly decreased (P<0.001; Fig. 4C, D and F), indicating that T2DM may
cause dyslipidaemia. By contrast, AS-IV and Met treatment
significantly lowered TG and TC levels, and increased HDL-C level
(P<0.05 or P<0.001; Fig. 4C, D
and F). However, there was no significant change in LDL-C
levels (Fig. 4E).
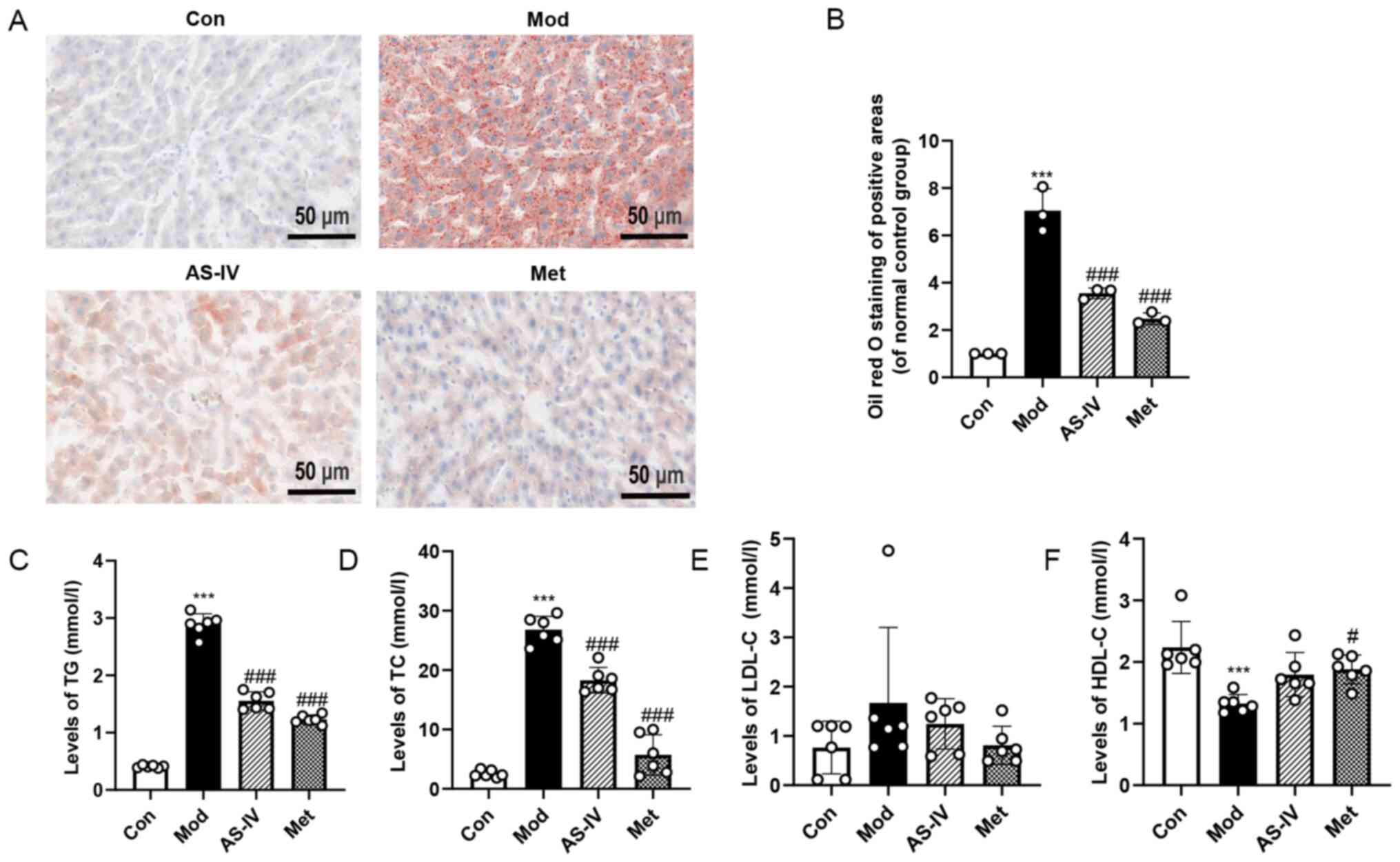 | Figure 4.AS-IV improves dyslipidaemia and
liver lipid deposition in T2DM rats. (A) Oil red O staining of
liver tissues (magnification, ×400). (B) Quantitative analysis of
positive areas of Oil red O staining was normalised to the normal
control group. (C) TG, (D) TC, (E) HDL-C and (F) LDL-C levels in
serum. Data are presented as the mean ± SD, n=3-6. ***P<0.001
vs. control group; #P<0.05, ###P<0.001
vs. model group. Con, Control; Mod, Model; AS-IV, Astragaloside IV
(80 mg/kg); Met, Metformin (200 mg/kg); TC, total cholesterol; TG,
triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C,
low-density lipoprotein cholesterol. |
H&E staining revealed a large number of vacuoles
in the liver of the model group, while AS-IV and Met treatment
induced significant improvements with reduced vacuoles (Fig. 2A). To further confirm these
findings, Oil red O staining was performed in the liver to assess
lipid deposition. As illustrated in Fig. 4A and B, the lipid deposition in the
model group was significantly increased compared with the control
group (P<0.001), while the degree of lipid deposition was
significantly improved after AS-IV and Met administration
(P<0.001). These results demonstrate that AS-IV treatment
improves dyslipidaemia and liver lipid deposition in T2DM rats.
Effects of AS-IV on inflammation and
oxidative stress in the liver of T2DM rats
To examine the level of inflammation in the liver,
the levels of pro-inflammatory cytokines TNF-α and IL-6 were
measured. The serum levels of TNF-α and IL-6 in the model group
were significantly higher compared with those in the control group
(P<0.001; Fig. 5A and B). After
the administration of AS-IV and Met, the serum levels of
inflammatory cytokines were significantly reduced (P<0.001;
Fig. 5A and B). Similarly, the
western blotting results demonstrated that the expression levels of
TNF-α and IL-6 in the liver of the model group were increased
significantly compared with the control group (P<0.001; Fig. 5C-E). Moreover, AS-IV and Met
treatment significantly decreased these indexes compared with the
model group (P<0.05 or P<0.01; Fig. 5C-E).
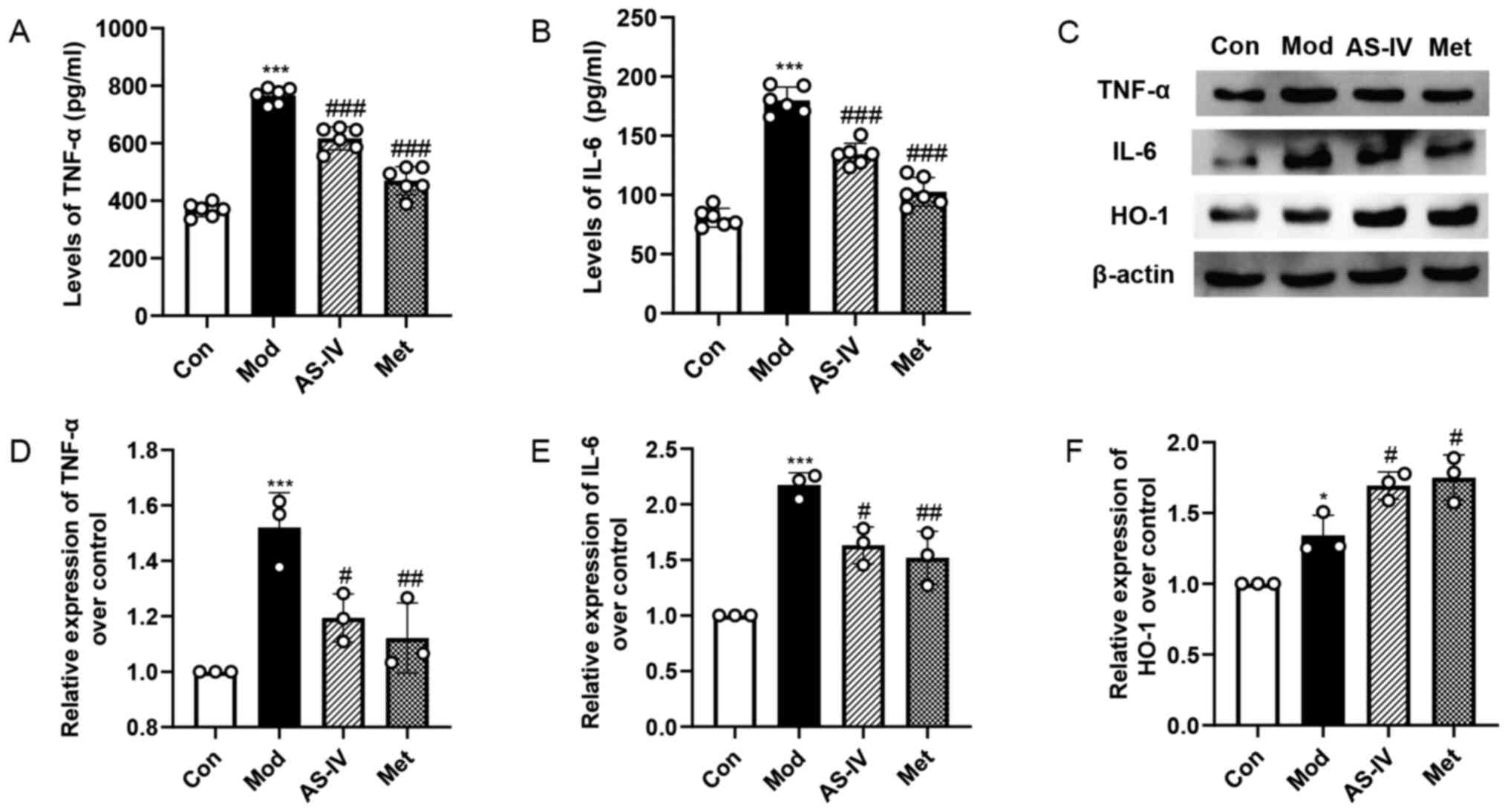 | Figure 5.AS-IV ameliorates inflammation and
oxidative stress in the liver of T2DM rats. (A) TNF-α and (B) IL-6
levels in serum. (C) Protein expression levels of TNF-α, IL-6 and
HO1 in the liver tissues were detected via western blotting.
Semi-quantitative analysis of (D) TNF-α, (E) IL-6 and (F) HO-1 was
normalised to β-actin. Data are presented as the mean ± SD, n=3.
***P<0.001 vs. control group; #P<0.05,
##P<0.01, ###P<0.001 vs. model group.
Con, Control; Mod, Model; AS-IV, Astragaloside IV (80 mg/kg); Met,
Metformin (200 mg/kg); HO-1, heme oxygenase-1. |
HO-1 is an important antioxidant enzyme involved in
numerous biological processes. After oxidative stress and cell
damage, HO-1 expression can be upregulated (23,24).
It has been reported that the protein expression level of HO-1 was
increased under the conditions of diabetes and other diseases, and
its expression was further enhanced when the administration of
chlorogenic acid and biochanin A treatment improved oxidative
stress (23,24). The current study further examined
the expression level of the oxidative stress protein HO-1 in the
liver via western blotting. As shown in Fig. 5C and F, the expression level of HO-1
was increased in the model group compared with the control group
(P<0.05). However, compared with the model group, AS-IV and Met
treatment significantly upregulated the expression level of HO-1
(P<0.05). The results suggest that AS-IV suppresses the
inflammation and oxidative stress in the liver of T2DM rats.
AS-IV activates autophagy in the liver
of T2DM rats
Previous studies have revealed that autophagy can
ameliorate IR, dyslipidaemia, oxidative stress and inflammation
(8–11). Based on previous research, the
current study focused on autophagy. To analyse the effect of AS-IV
on autophagy under diabetic liver injury conditions, liver
autophagy was examined using western blotting and
immunohistochemistry to detect the protein levels of Beclin1, LC3
and P62 in the liver. As presented in Fig. 6A, B, E and F, the accumulation of
Beclin1, an essential autophagic protein in the initial stages of
autophagy (25), was decreased in
the model group (P<0.001), but it was significantly increased in
the AS-IV and Met treatment groups (P<0.01 or P<0.001).
Moreover, the transformation from LC3I to LC3II, a marker of
autophagy (26), was decreased in
the model group, but was significantly increased by AS-IV and Met
treatment (P<0.05 or P<0.001; Fig. 6A, C, E and G). P62 is involved in
the degradation of the proteasome and can reflect autophagy
activity (27). It was found that
P62 expression was elevated in the model group, but it was
significantly decreased after treatment with AS-IV and Met
(P<0.05 or P<0.001; Fig. 6A, D, E
and H).
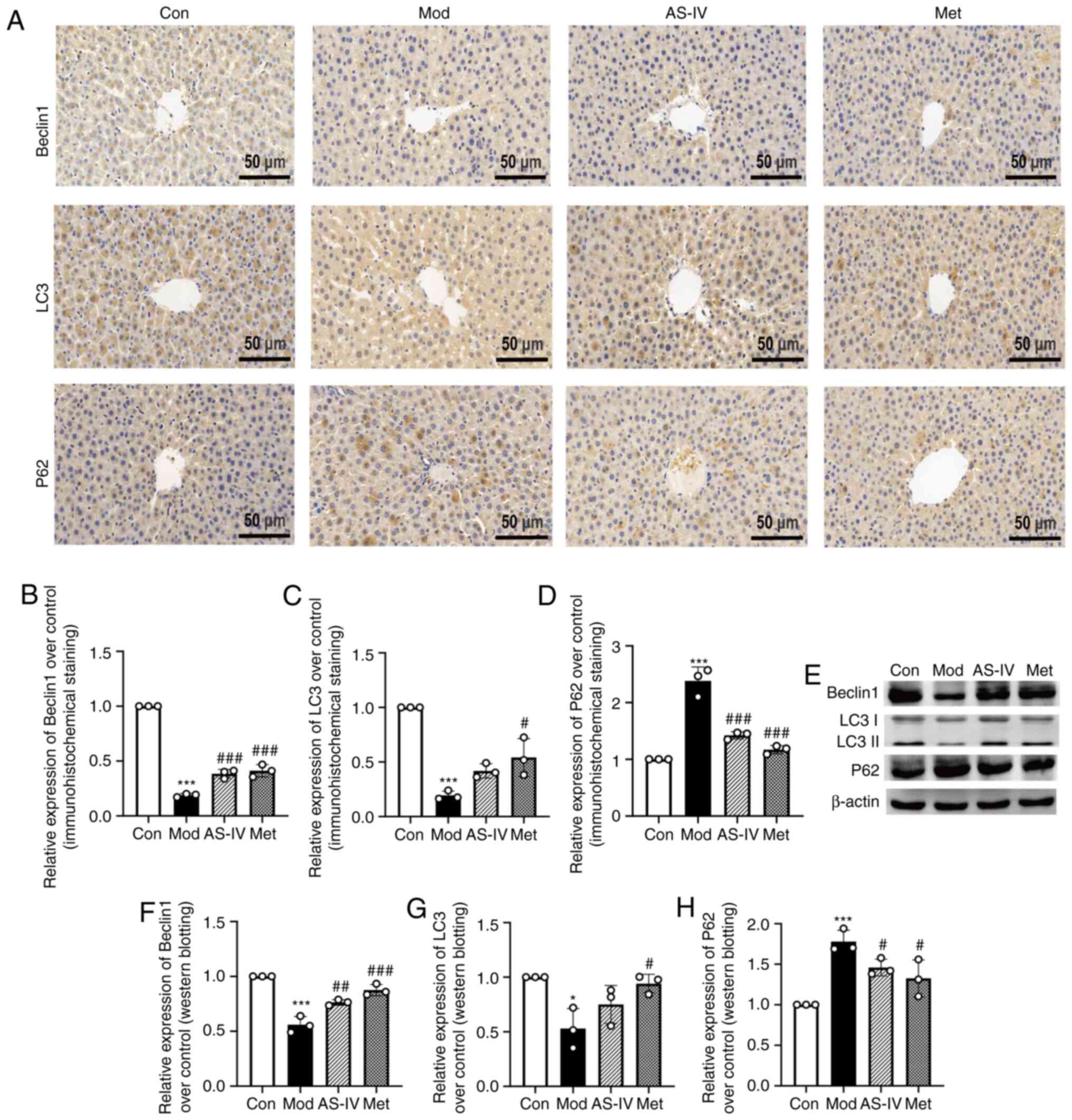 | Figure 6.AS-IV activates the suppressed
autophagy in the liver of T2DM rats. (A) Representative images of
Beclin1, LC3 and P62 (immunohistochemical staining; magnification,
×400). Quantitative analysis of (B) Beclin1, (C) LC3 and (D) P62
were normalized to the normal control group. (E) Protein expression
levels of Beclin1, LC3 and P62 in the liver tissues were detected
via western blotting. Semi-quantitative analysis of (F) Beclin1,
(G) LC3 and (H) P62 was normalised to β-actin. Data are presented
as the mean ± SD, n=3. *P<0.05, ***P<0.001 vs. control group;
#P<0.05, ##P<0.01,
###P<0.001 vs. model group. Con, Control; Mod, Model;
AS-IV, Astragaloside IV (80 mg/kg); Met, Metformin (200 mg/kg). |
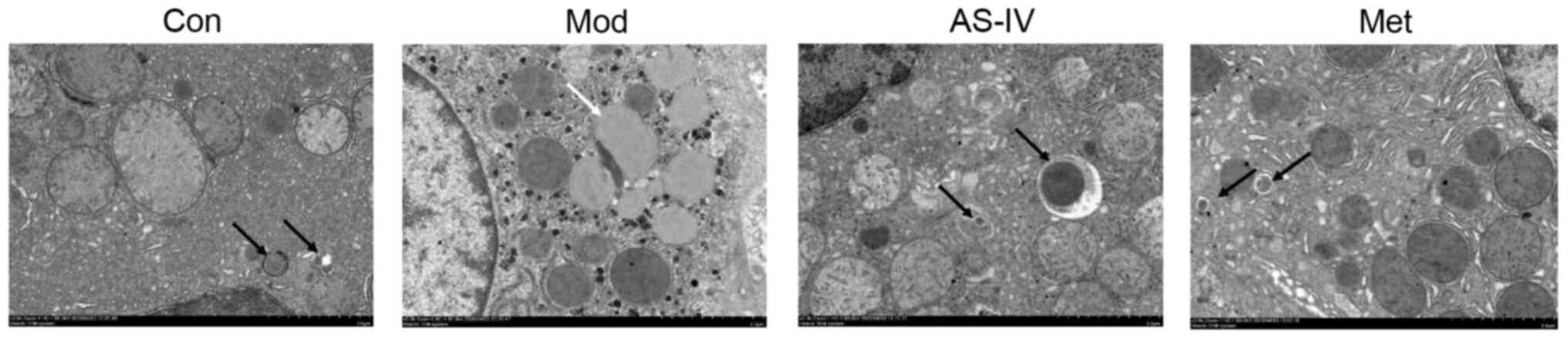
The autophagosomes in the liver were examined using
a transmission electron microscope to determine the occurrence of
autophagy. Compared with the control group, the autophagosomes in
the model group were inhibited, while AS-IV and Met treatment
increased autophagosome formation (Fig.
7). These results indicate that AS-IV enhances liver autophagy
in T2DM rats.
AS-IV activates the AMPK/mTOR pathway
in the liver of T2DM rats
To evaluate the potential mechanism of AS-IV
activation of autophagy, the AMPK/mTOR pathway, a major pathway
that regulates liver autophagy (28), was examined in the liver. As
presented in Fig. 8A-C, the
expression level of p-AMPK was significantly downregulated and the
expression level of p-mTOR was significantly upregulated in the
model group compared with the control group (P<0.01 or
P<0.001); there was no significant change in AMPK and mTOR
expression. However, the suppressed AMPK/mTOR pathway in the model
group was significantly reversed by AS-IV and Met treatment, as
evidenced by the significantly elevated p-AMPK/AMPK ratio
(P<0.01 or P<0.001) and decreased p-mTOR/mTOR (P<0.05 or
P<0.01) ratio in the AS-IV and Met treatment groups. These data
suggest that AS-IV may enhance autophagy via the activation of the
AMPK/mTOR pathway in the liver of T2DM rats.
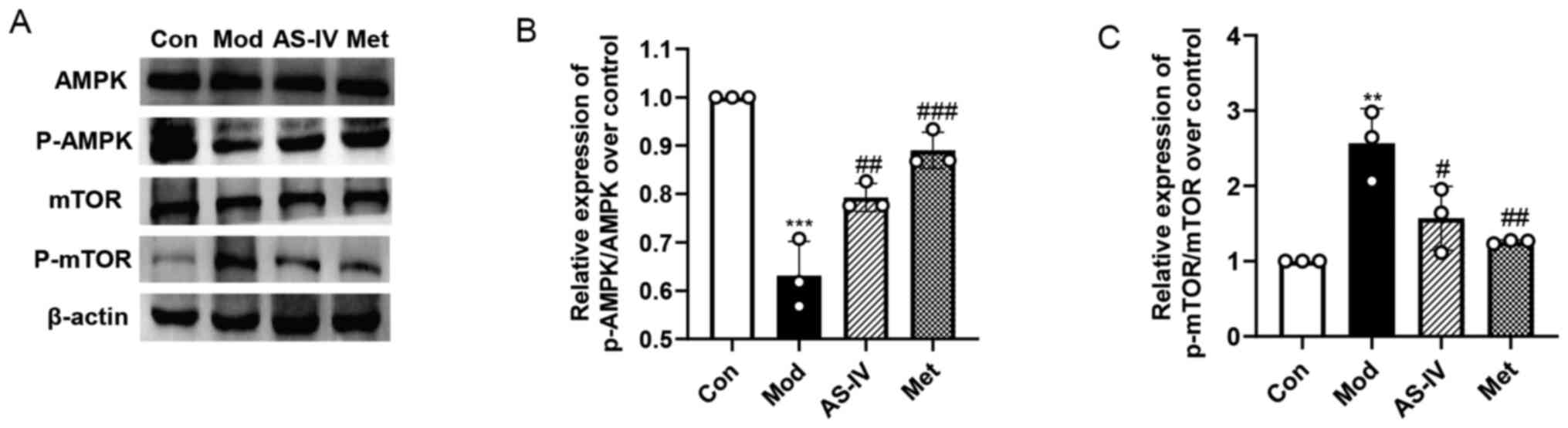 | Figure 8.AS-IV activates autophagy via the
AMPK/mTOR pathway in the liver of T2DM rats. (A) Protein expression
levels of AMPK, p-AMPK, mTOR and p-mTOR in liver tissues were
detected via western blotting. (B and C) Semi-quantitative analysis
of p-AMPK/AMPK ratio and p-mTOR/mTOR ratio. Data are presented as
the mean ± SD, n=3. **P<0.01, ***P<0.001 vs. control group;
#P<0.05, ##P<0.01,
###P<0.001 vs. model group. Con, Control; Mod, Model;
AS-IV, Astragaloside IV (80 mg/kg); Met, Metformin (200 mg/kg); p-,
phosphorylated; AMPK, adenosine monophosphate-activated protein
kinase. |
Discussion
The present study provided evidence that AS-IV
treatment ameliorated diabetic liver injury in T2DM rats.
Furthermore, it was confirmed that autophagy served a crucial role
in diabetic liver injury in T2DM rats, which is also closely
associated with the AMPK/mTOR signalling pathway. Based on the
protective effect of AS-IV on diabetic liver injury by promoting
autophagy, the current research indicated that AS-IV administration
may provide a potentially effective treatment strategy for diabetic
liver injury in T2DM.
Diabetic liver injury is one of the complications in
patients with T2DM (5). At present,
there is no effective treatment method for diabetic liver injury,
and so the effective functional ingredients from plants have become
a new treatment method (29–31).
AS-IV is the main active ingredient of medicinal plant
Astragalus membranaceus, which has been reported to possess
comprehensive pharmacological effects, especially in diabetes
(32). Previous research has
confirmed that AS-IV at a dose of 80 mg/kg is widely used in the
study of liver diseases and other diseases in Sprague-Dawley rats
(33,34). Moreover, in our previous studies, a
dose of 80 mg/kg AS-IV could significantly improve the
complications of diabetic rats, such as diabetic nephropathy and
cardiomyopathy (18,19). Therefore, in the current study, a
concentration of AS-IV 80 mg/kg was used to evaluate the protective
effect on diabetic liver injury in T2DM.
In the present study, HFD combined with low-dose STZ
was used to induce a diabetic liver injury model in T2DM rats
(18,19). It was found that the common
manifestations of food intake, water intake and urine volume in
T2DM were significantly increased, while body weight was
significantly decreased in the T2DM model group. In addition, the
levels of ALT, AST, FBG, FINS and GSP in serum and the liver index
of liver weight to body weight were significantly increased. It was
also observed that the pathological changes in the liver, including
hepatocyte disorder, lipid deposition and collagen expression, were
significantly increased in T2DM rats. Importantly, IR and
dyslipidaemia appeared, and oxidative stress and inflammation
levels were significantly increased in the liver. This confirmed
the success of modelling in T2DM rats and the occurrence of
diabetic liver injury. However, AS-IV treatment relieved the
aforementioned symptoms, indicating that AS-IV exerted a protective
effect on diabetic liver injury in T2DM rats. Additionally, the
present findings revealed that AS-IV could promote liver autophagy
in T2DM rats by modulating the AMPK/mTOR pathway.
Autophagy is a key self-repair mechanism and an
important regulator of cellular homeostasis (7). The basal level of autophagy can adapt
to various stresses and is essential for maintaining normal liver
function (35). Accumulating
evidence suggests that abnormal autophagy function is closely
associated with diabetic liver injury (36,37).
The present study observed that the liver autophagy ability was
impaired in T2DM rats. These results are consistent with previous
studies, and provide further support for the role of autophagy in
the occurrence of diabetic liver injury in T2DM (36,37).
The recovery or activation of autophagy has a protective effect on
target organs (10,38). Similarly, the present study found
that AS-IV treatment normalised the autophagic activity in the
liver of T2DM rats, suggesting that AS-IV activated the suppressed
autophagy in the liver of T2DM rats. Furthermore, it has been
reported that autophagy can ameliorate IR, dyslipidaemia, oxidative
stress and inflammation (8–11). In summary, these findings indicate
that autophagy can participate in the occurrence and development of
diabetic liver injury in T2DM by improving IR, dyslipidaemia,
oxidative stress and inflammation.
The relationship between autophagy, IR and
dyslipidaemia is important in the treatment of diabetic liver
injury in T2DM (8,9,11). It
has been reported that IR and dyslipidaemia are important risk
factors of diabetic liver injury in T2DM (5). IR, a core feature of T2DM, is defined
as the reduced efficiency of insulin in promoting glucose uptake
and utilization, which is particularly critical for maintaining
blood glucose stability (39).
Moreover, IR can develop into obvious T2DM, accompanied by the loss
of β-cell function, and eventually leading to the loss of β-cells
(40). Dyslipidaemia usually exists
in patients with T2DM, and becomes an important predictor of the
development of T2DM (41). The
accumulation of liver fat caused by dyslipidaemia leads to the
weakening of the liver's detoxification ability (5). Additionally, the excessive
accumulation of liver fat may aggravate IR and cause severe
metabolic dysfunction (5,6). Therefore, improving IR and
dyslipidaemia has become an important method for the treatment of
diabetic liver injury in T2DM.
Accumulating evidence has indicated that the
enhancement of autophagy has been shown to improve IR and
dyslipidaemia (36,42–44).
Moreover, autophagy can maintain the function of pancreatic β-cells
during the occurrence and development of diabetic liver injury in
T2DM (45,46). AS-IV has a protective effect on
hepatic steatosis by attenuating IR and lipid deposition in HepG2
cells (47). In the present study,
it was found that IR was significantly increased, and dyslipidaemia
appeared in T2DM rats, while AS-IV treatment improved IR and
dyslipidaemia. These current findings suggested that AS-IV
treatment may improve the IR and dyslipidaemia in T2DM rats by
enhancing liver autophagy.
The interaction of autophagy, oxidative stress and
inflammation is crucial in the occurrence and development of
diabetic liver injury in T2DM. Oxidative stress and inflammation
are considered to be the main factors in the progression of
diabetes complications (6,48). Hyperglycaemia can induce acute
oxidative stress, which stimulates tissue-specific inflammation and
participates in the pathogenesis of T2DM (48,49).
Therefore, improving oxidative stress and inflammation has become
an important strategy for treating diabetic liver injury in T2DM.
It has been reported that autophagy improves cell damage by
inhibiting oxidative stress and inflammation (50). Furthermore, AS-IV can protect the
kidney from damage by inhibiting oxidative stress and inflammation
in T2DM (51). The present study
demonstrated that oxidative stress and inflammation levels were
significantly increased in the liver of T2DM rats, and AS-IV
treatment significantly improved oxidative stress and inflammation
levels. These observations are consistent with other studies
(23,24). These data suggested that AS-IV
treatment may attenuate oxidative stress and inflammation levels in
the liver of T2DM rats by enhancing autophagy.
AMPK/mTOR-mediated autophagy has been reported to be
involved in diabetes complications (52). Previous studies have revealed that
AS-IV prevented podocyte injury by activating AMPK-mediated
autophagy in diabetic nephropathy and promoted functional recovery
after acute spinal cord injury by activating mTOR-mediated
autophagy (53,54). To identify the underlying mechanism
of autophagy in diabetic liver injury in T2DM, the present study
focused on the AMPK/mTOR pathway. It was found that the AMPK
activity in the liver was decreased in T2DM rats, and was improved
by AS-IV treatment. Furthermore, it was demonstrated that AS-IV
suppressed the increase of mTOR activity in T2DM rats. The results
suggested that AS-IV treatment may regulate liver autophagy via the
AMPK/mTOR pathway in diabetic liver injury in T2DM rats.
There are several limitations to the present study.
First, whether autophagy was caused by the activation of the
AMPK/mTOR pathway requires further research. Second, the specific
relationship between autophagy and IR, dyslipidaemia, oxidative
stress and inflammation needs to be further investigated.
In conclusion, the present study demonstrated that
AS-IV alleviated diabetic liver injury in T2DM rats, and its
mechanism may be associated with the promotion of
AMPK/mTOR-mediated autophagy, which improved IR, dyslipidaemia,
oxidative stress and inflammation. The present results provide
evidence for the protective effect of AS-IV in diabetic liver
injury in T2DM. Furthermore, regulating autophagy may be an
effective strategy to improve diabetic liver injury in T2DM.
Acknowledgements
The authors would like to thank Mrs. Zhirui Fang in
the Department of Pharmacology, and Mr. Dake Huang in the Synthetic
Laboratory of Basic Medicine College for their technical
assistance.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 81970630) and Major
Science and Technology Projects in Anhui Province (grant no.
201903a07020025).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding authors on reasonable
request.
Authors' contributions
YFZ performed the experiments, analyzed data and was
a major contributor in writing the manuscript. YS and JZ assessed
the authenticity of the raw data and ensured its legitimacy. YS and
JZ helped with the statistical analysis. YHZ, YL, YH and XD helped
to establish the animal model and revised the manuscript. WZL and
WPL designed the study and critically revised the manuscript. All
authors read and approved the final submitted manuscript.
Ethics approval and consent to
participate
All animal experiments were conducted under
protocols approved by the Ethics Review Committee of Anhui Medical
University (approval no. LLSC20190302).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AS-IV
|
Astragaloside IV
|
|
T2DM
|
type 2 diabetes mellitus
|
|
HFD
|
high-fat diets
|
|
STZ
|
streptozotocin
|
|
IR
|
insulin resistance
|
|
AMPK
|
adenosine monophosphate-activated
protein kinase
|
|
CMC-Na+
|
carboxyl methyl cellulose sodium
|
|
FBG
|
fasting blood glucose
|
|
Met
|
Metformin
|
|
OGTT
|
oral glucose tolerance test
|
|
AUC
|
area under the curve
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
TC
|
total cholesterol
|
|
TG
|
triglyceride
|
|
HDL-C
|
high-density lipoprotein
cholesterol
|
|
LDL-C
|
low-density lipoprotein
cholesterol
|
|
GSP
|
glycosylated serum protein
|
|
FINS
|
fasting insulin
|
|
HOMA-IR
|
homeostasis model assessment of
insulin resistance
|
|
HO-1
|
heme oxygenase-1
|
References
|
1
|
Tan SY, Mei Wong JL, Sim YJ, Wong SS,
Mohamed Elhassan SA, Tan SH, Ling Lim GP, Rong Tay NW, Annan NC,
Bhattamisra SK and Candasamy M: Type 1 and 2 diabetes mellitus: A
review on current treatment approach and gene therapy as potential
intervention. Diabetes Metab Syndr. 13:364–372. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng Y, Ley SH and Hu FB: Global
aetiology and epidemiology of type 2 diabetes mellitus and its
complications. Nat Rev Endocrinol. 14:88–98. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shima T, Uto H, Ueki K, Takamura T, Kohgo
Y, Kawata S, Yasui K, Park H, Nakamura N, Nakatou T, et al:
Clinicopathological features of liver injury in patients with type
2 diabetes mellitus and comparative study of histologically proven
nonalcoholic fatty liver diseases with or without type 2 diabetes
mellitus. J Gastroenterol. 48:515–525. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hsiang JC, Gane EJ, Bai WW and Gerred SJ:
Type 2 diabetes: A risk factor for liver mortality and
complications in hepatitis B cirrhosis patients. J Gastroenterol
Hepatol. 30:591–599. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bedi O, Aggarwal S, Trehanpati N,
Ramakrishna G and Krishan P: Molecular and pathological events
involved in the pathogenesis of diabetes-associated nonalcoholic
fatty liver disease. J Clin Exp Hepatol. 9:607–618. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Akash MS, Rehman K and Chen S: Role of
inflammatory mechanisms in pathogenesis of type 2 diabetes
mellitus. J Cell Biochem. 114:525–531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu H, Javaheri A, Godar RJ, Murphy J, Ma
X, Rohatgi N, Mahadevan J, Hyrc K, Saftig P, Marshall C, et al:
Intermittent fasting preserves beta-cell mass in obesity-induced
diabetes via the autophagy-lysosome pathway. Autophagy.
13:1952–1968. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chatterjee T, Pattanayak R, Ukil A,
Chowdhury S and Bhattacharyya M: Autophagy protects peripheral
blood mononuclear cells against inflammation, oxidative and
nitrosative stress in diabetic dyslipidemia. Free Radic Biol Med.
143:309–323. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fan W, Han D, Sun Z, Ma S, Gao L, Chen J,
Li X, Li X, Fan M, Li C, et al: Endothelial deletion of mTORC1
protects against hindlimb ischemia in diabetic mice via activation
of autophagy, attenuation of oxidative stress and alleviation of
inflammation. Free Radic Biol Med. 108:725–740. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Martinez-Lopez N and Singh R: Autophagy
and lipid droplets in the liver. Annu Rev Nutr. 35:215–237. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang BB, Zhou G and Li C: AMPK: An
emerging drug target for diabetes and the metabolic syndrome. Cell
Metab. 9:407–416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu TY, Xiong XQ, Ren XS, Zhao MX, Shi CX,
Wang JJ, Zhou YB, Zhang F, Han Y, Gao XY, et al: FNDC5 alleviates
hepatosteatosis by restoring AMPK/mTOR-mediated autophagy, fatty
acid oxidation, and lipogenesis in mice. Diabetes. 65:3262–3275.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang Y, Gao J, Zhang Y, Xu W, Hao Y, Xu Z
and Tao L: Natural pyrethrins induce autophagy of HepG2 cells
through the activation of AMPK/mTOR pathway. Environ Pollut.
241:1091–1097. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang E, Wang L, Ding R, Zhai M, Ge R, Zhou
P, Wang T, Fang H, Wang J and Huang J: Astragaloside IV acts
through multi-scale mechanisms to effectively reduce diabetic
nephropathy. Pharmacol Res. 157:1048312020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song MT, Ruan J, Zhang RY, Deng J, Ma ZQ
and Ma SP: Astragaloside IV ameliorates neuroinflammation-induced
depressive-like behaviors in mice via the PPARγ/NF-κB/NLRP3
inflammasome axis. Acta Pharmacol Sin. 39:1559–1570. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ju Y, Su Y, Chen Q, Ma K, Ji T, Wang Z and
Li W and Li W: Protective effects of Astragaloside IV on
endoplasmic reticulum stress-induced renal tubular epithelial cells
apoptosis in type 2 diabetic nephropathy rats. Biomed Pharmacother.
109:84–92. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Z, Zhu Y, Zhang Y, Zhang J, Ji T and
Li W and Li W: Protective effects of AS-IV on diabetic
cardiomyopathy by improving myocardial lipid metabolism in rat
models of T2DM. Biomed Pharmacother. 127:1100812020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Antuna-Puente B, Disse E, Rabasa-Lhoret R,
Laville M, Capeau J and Bastard JP: How can we measure insulin
sensitivity/resistance? Diabetes Metab. 37:179–188. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu L, Yu Y, Sang R, Li J, Ge B and Zhang
X: Protective effects of taraxasterol against ethanol-induced liver
injury by regulating CYP2E1/Nrf2/HO-1 and NF-κB signaling pathways
in mice. Oxid Med Cell Longev. 2018:82841072018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Michalopoulos GK: Hepatostat: Liver
regeneration and normal liver tissue maintenance. Hepatology.
65:1384–1392. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bao L, Li J, Zha D, Zhang L, Gao P, Yao T
and Wu X: Chlorogenic acid prevents diabetic nephropathy by
inhibiting oxidative stress and inflammation through modulation of
the Nrf2/HO-1 and NF-κB pathways. Int Immunopharmacol. 54:245–253.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo M, Lu H, Qin J, Qu S, Wang W, Guo Y,
Liao W, Song M, Chen J and Wang Y: Biochanin A provides
neuroprotection against cerebral Ischemia/Reperfusion Injury by
Nrf2-mediated inhibition of oxidative stress and inflammation
signaling pathway in rats. Med Sci Monit. 25:8975–8983. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ding GB, Sun J, Wu G, Li B, Yang P, Li Z
and Nie G: Robust anticancer efficacy of a biologically synthesized
tumor acidity-responsive and Autophagy-Inducing functional beclin
1. ACS Appl Mater Interfaces. 10:5227–5239. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hanada T, Noda NN, Satomi Y, Ichimura Y,
Fujioka Y, Takao T, Inagaki F and Ohsumi Y: The Atg12-Atg5
conjugate has a novel E3-like activity for protein lipidation in
autophagy. J Biol Chem. 282:37298–37302. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bardag-Gorce F, Francis T, Nan L, Li J, He
Lue Y, French BA and French SW: Modifications in P62 occur due to
proteasome inhibition in alcoholic liver disease. Life Sci.
77:2594–2602. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rao Z, Pan X, Zhang H, Sun J, Li J, Lu T,
Gao M, Liu S, Yu D and Ding Z: Isoflurane preconditioning
alleviated murine liver ischemia and reperfusion injury by
restoring AMPK/mTOR-mediated autophagy. Anesth Analg.
125:1355–1363. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Han D: Treatment with astragaloside IV
reduced blood glucose, regulated blood lipids, and protected liver
function in diabetic rats. J Int Med Res. 49:3000605198411652021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, Cao Y, Chen J, Qin H and Yang L:
A New possible mechanism by which punicalagin protects against
liver injury induced by type 2 diabetes mellitus: Upregulation of
autophagy via the Akt/FoxO3a signaling pathway. J Agric Food Chem.
67:13948–13959. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liang W, Zhang D, Kang J, Meng X, Yang J,
Yang L, Xue N, Gao Q, Han S and Gou X: Protective effects of rutin
on liver injury in type 2 diabetic db/db mice. Biomed Pharmacother.
107:721–728. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao K, Yang R, Zhang J, Wang Z, Jia C,
Zhang F, Li S, Wang J, Murtaza G, Xie H, et al: Effects of Qijian
mixture on type 2 diabetes assessed by metabonomics, gut microbiota
and network pharmacology. Pharmacol Res. 130:93–109. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qu X, Gao H, Tao L, Zhang Y, Zhai J, Sun
J, Song Y and Zhang S: Astragaloside IV protects against
cisplatin-induced liver and kidney injury via autophagy-mediated
inhibition of NLRP3 in rats. J Toxicol Sci. 44:167–175. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu W, Liu G and Liu J: Effects of
astragaloside IV on the pharmacokinetics of omeprazole in rats.
Pharm Biol. 57:449–452. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Q, Li Y, Liang T, Lu X, Zhang C, Liu
X, Jiang X, Martin RC, Cheng M and Cai L: ER stress and autophagy
dysfunction contribute to fatty liver in diabetic mice. Int J Biol
Sci. 11:559–568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin CW, Zhang H, Li M, Xiong X, Chen X,
Chen X, Dong XC and Yin XM: Pharmacological promotion of autophagy
alleviates steatosis and injury in alcoholic and non-alcoholic
fatty liver conditions in mice. J Hepatol. 58:993–999. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang F, Zhao S, Yan W, Xia Y, Chen X,
Wang W, Zhang J, Gao C, Peng C, Yan F, et al: Branched chain amino
acids cause liver injury in obese/diabetic mice by promoting
adipocyte lipolysis and inhibiting hepatic autophagy. EBioMedicine.
13:157–167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yao Q, Ke ZQ, Guo S, Yang XS, Zhang FX,
Liu XF, Chen X, Chen HG, Ke HY and Liu C: Curcumin protects against
diabetic cardiomyopathy by promoting autophagy and alleviating
apoptosis. J Mol Cell Cardiol. 124:26–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Artunc F, Schleicher E, Weigert C,
Fritsche A, Stefan N and Häring HU: The impact of insulin
resistance on the kidney and vasculature. Nat Revi Nephrol.
12:721–737. 2016. View Article : Google Scholar
|
|
40
|
Jourdan T, Godlewski G and Kunos G:
Endocannabinoid regulation of β-cell functions: Implications for
glycaemic control and diabetes. Diabetes Obes Metab. 18:549–557.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ahmadieh H and Azar ST: Liver disease and
diabetes: Association, pathophysiology, and management. Diabetes
Res Clin Pract. 104:53–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu HY, Han J, Cao SY, Hong T, Zhuo D, Shi
J, Liu Z and Cao W: Hepatic autophagy is suppressed in the presence
of insulin resistance and hyperinsulinemia: Inhibition of
FoxO1-dependent expression of key autophagy genes by insulin. J
Biol Chem. 284:31484–31492. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Codogno P and Meijer AJ: Autophagy: A
potential link between obesity and insulin resistance. Cell
Metabolism. 11:449–451. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li R, Guo E, Yang J, Li A, Yang Y, Liu S,
Liu A and Jiang X: 1,25(OH)2 D3 attenuates
hepatic steatosis by inducing autophagy in mice. Obesity (Silver
Spring). 25:561–571. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jung HS, Chung KW, Won Kim J, Kim J,
Komatsu M, Tanaka K, Nguyen YH, Kang TM, Yoon KH, Kim JW, et al:
Loss of autophagy diminishes pancreatic beta cell mass and function
with resultant hyperglycemia. Cell Metab. 8:318–324. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mir SU, George NM, Zahoor L, Harms R,
Guinn Z and Sarvetnick NE: Inhibition of autophagic turnover in
β-cells by fatty acids and glucose leads to apoptotic cell death. J
Biol Chem. 290:6071–6085. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang C, Li Y, Hao M and Li W:
Astragaloside IV inhibits triglyceride accumulation in
insulin-resistant HepG2 cells via AMPK-induced SREBP-1c
phosphorylation. Front Pharmacol. 9:3452018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wu MY, Yiang GT and Lai TT: The Oxidative
stress and mitochondrial dysfunction during the pathogenesis of
diabetic retinopathy. Oxid Med Cell Longev. 2018:34201872018.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang Y, Yuan D, Yao W, Zhu Q, Liu Y,
Huang F, Feng J, Chen X, Huang Y, Chi X and Hei Z: Hyperglycemia
aggravates hepatic ischemia reperfusion injury by inducing chronic
oxidative stress and inflammation. Oxid Med Cell Longev.
2016:39196272016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Meng L, Zhao X and Zhang H: HIPK1
interference attenuates inflammation and oxidative stress of acute
lung injury via autophagy. Med Sci Monit. 25:827–835. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
He KQ, Li WZ, Chai XQ, Yin YY, Jiang Y and
Li WP: Astragaloside IV prevents kidney injury caused by iatrogenic
hyperinsulinemia in a streptozotocin-induced diabetic rat model.
Int J Mol Med. 41:1078–1088. 2018.PubMed/NCBI
|
|
52
|
Yang F, Zhang L, Gao Z, Sun X, Yu M, Dong
S, Wu J, Zhao Y, Xu C, Zhang W and Lu F: Exogenous H2S protects
against diabetic cardiomyopathy by activating autophagy via the
AMPK/mTOR pathway. Cell Physiol Biochem. 43:1168–1187. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Guo H, Wang Y, Zhang X, Zang Y, Zhang Y,
Wang L, Wang H, Wang Y, Cao A and Peng W: Astragaloside IV protects
against podocyte injury via SERCA2-dependent ER stress reduction
and AMPKα-regulated autophagy induction in streptozotocin-induced
diabetic nephropathy. Sci Rep. 7:68522017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lin J, Pan X, Huang C, Gu M, Chen X, Zheng
X, Shao Z, Hu S, Wang B, Lin H, et al: Dual regulation of microglia
and neurons by Astragaloside IV-mediated mTORC1 suppression
promotes functional recovery after acute spinal cord injury. J Cell
Mol Med. 24:671–685. 2020. View Article : Google Scholar : PubMed/NCBI
|