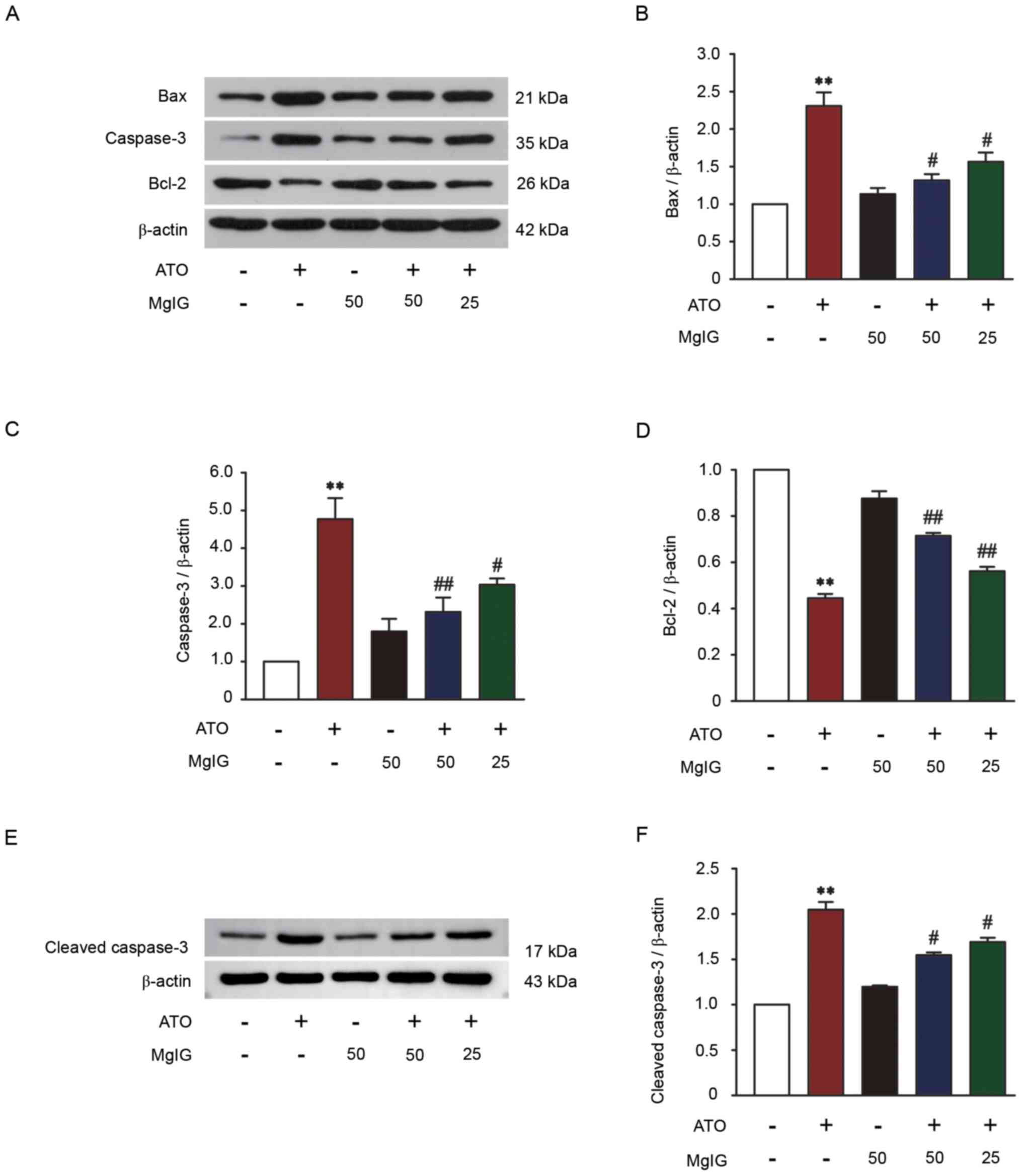
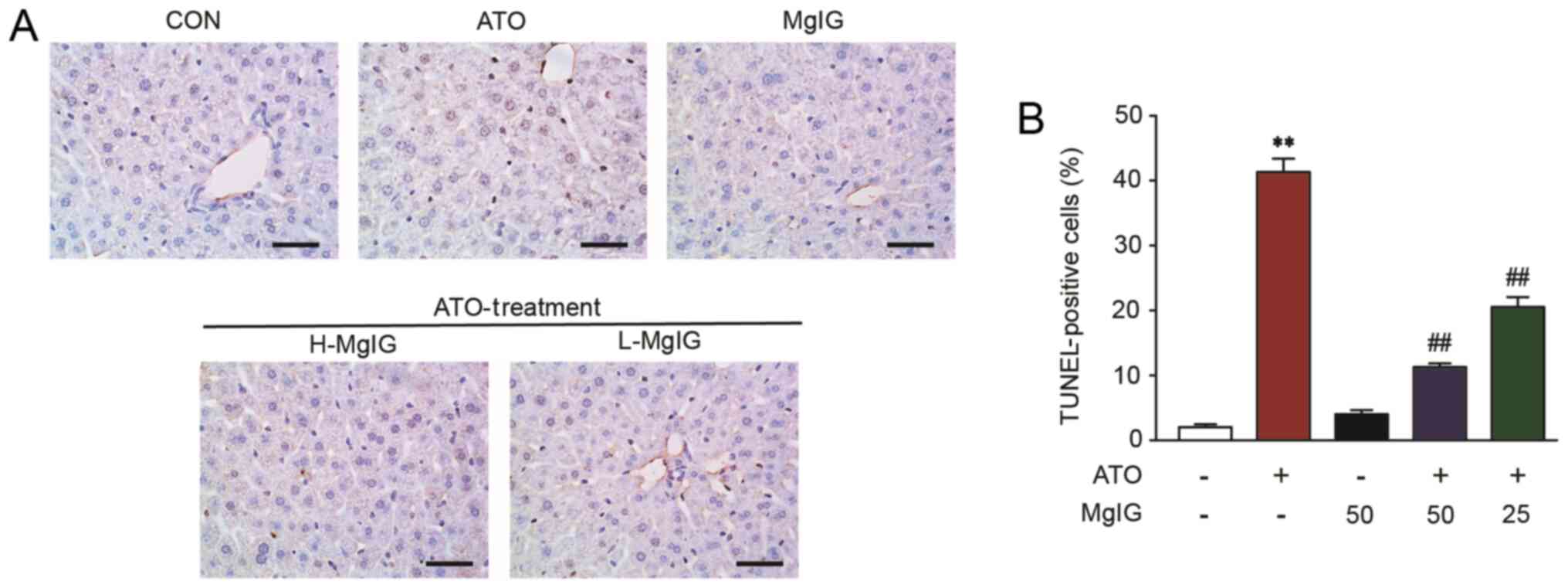
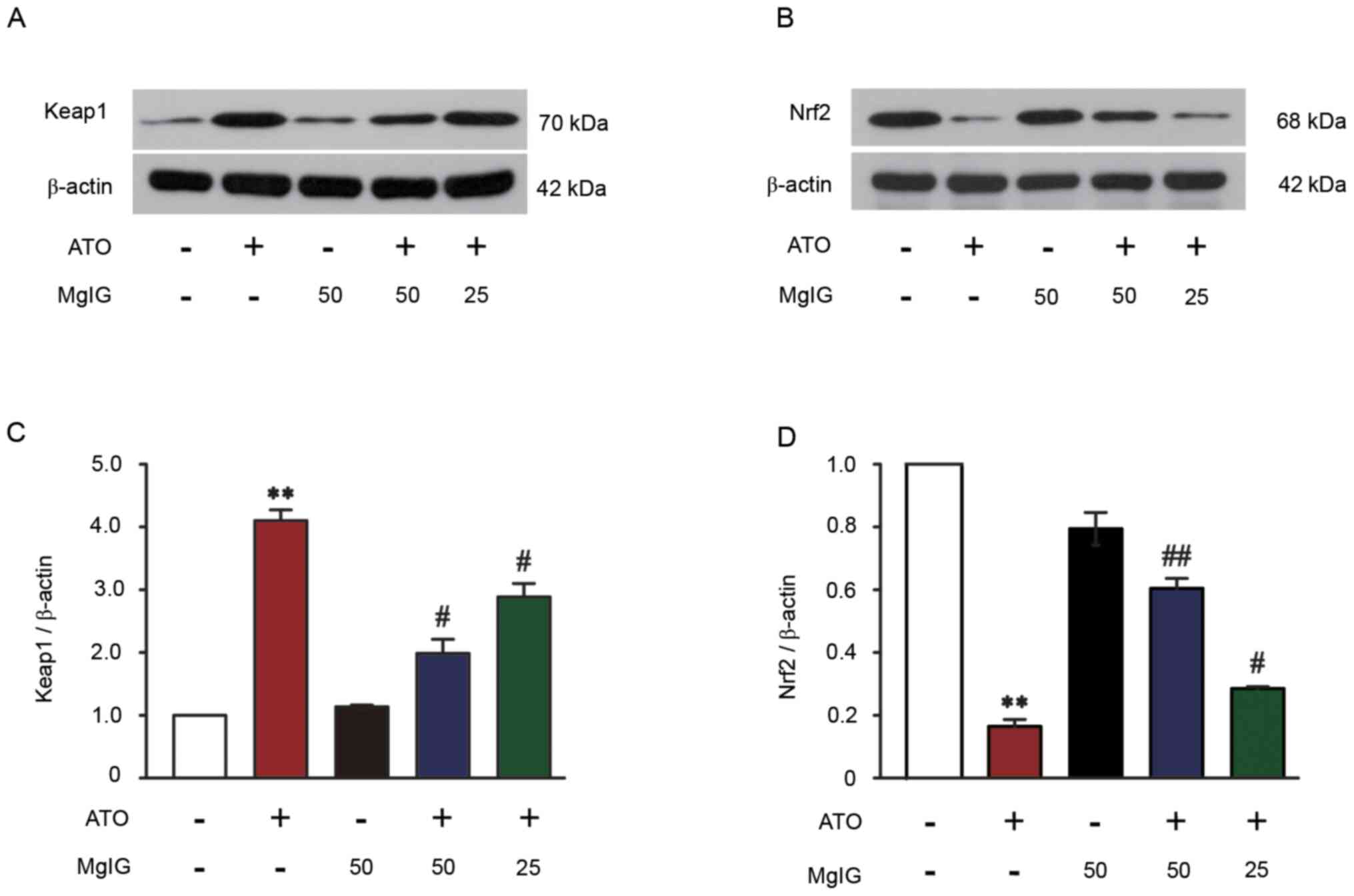
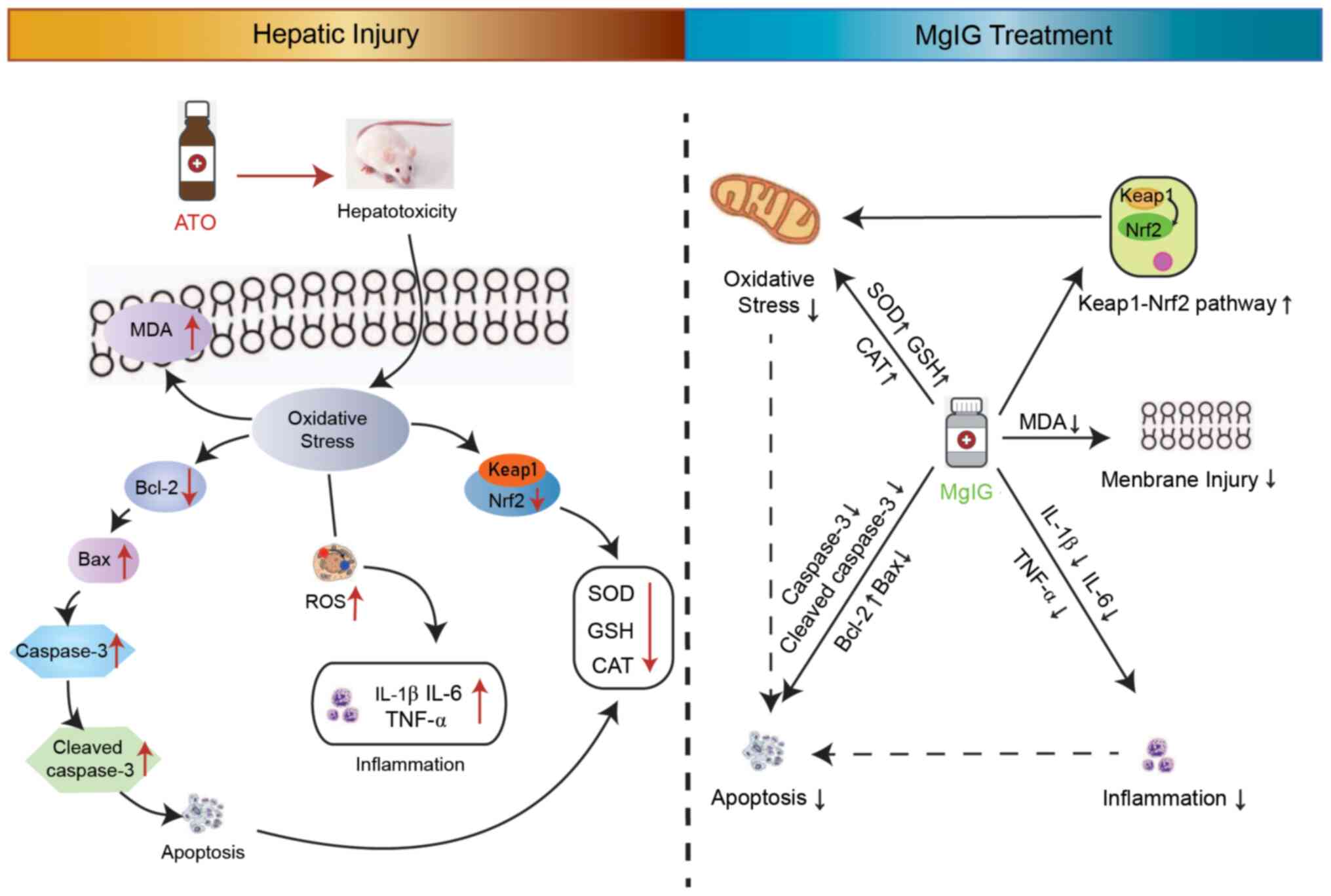
|
1
|
Centeno JA, Mullick FG, Martinez L, Page
NP, Gibb H, Longfellow D, Thompson C and Ladich ER: Pathology
related to chronic arsenic exposure. Environ Health Persp. 110
(Suppl 5):S883–S886. 2002. View Article : Google Scholar
|
|
2
|
Emadi A and Gore SD: Arsenic trioxide-An
old drug rediscovered. Blood Rev. 24:191–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gore SD, Gojo I, Sekeres MA, Morris L,
Devetten M, Jamieson K, Redner RL, Arceci R, Owoeye I, Dauses T, et
al: Single cycle of arsenic trioxide-based consolidation
chemotherapy spares anthracycline exposure in the primary
management of acute promyelocytic leukemia. J Clin Oncol.
28:1047–1053. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Antman KH: Introduction: The history of
arsenic trioxide in cancer therapy. Oncologist. 6 (Suppl 2):S1–S2.
2001. View Article : Google Scholar
|
|
5
|
Abaza Y, Kantarjian H, Garcia-Manero G,
Estey E, Borthakur G, Jabbour E, Faderl S, O'Brien S, Wierda W,
Pierce S, et al: Long-term outcome of acute promyelocytic leukemia
treated with all trans-retinoic acid, arsenic trioxide, and
gemtuzumab. Blood. 129:1275–1283. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Breccia M and Lo-Coco F: Arsenic trioxide
for management of acute promyelocytic leukemia: Current evidence on
its role in front-line therapy and recurrent disease. Expert Opin
Pharmacother. 13:1031–1043. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iland HJ, Bradstock K, Supple SG, Catalano
A, Collins M, Hertzberg M, Browett P, Grigg A, Firkin F, Hugman A,
et al: All-trans-retinoic acid, idarubicin, and IV arsenic trioxide
as initial therapy in acute promyelocytic leukemia (APML4). Blood.
120:1570–1580; quiz 1752. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lo-Coco F, Avvisati G, Vignetti M, Thiede
C, Orlando SM, Iacobelli S, Ferrara F, Fazi P, Cicconi L, Di Bona
E, et al: Retinoic acid and arsenic trioxide for acute
promyelocytic leukemia. N Engl J Med. 369:111–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang X, Jia S, Yang S and Yang Y, Yang T
and Yang Y: Arsenic trioxide induces G2/M arrest in hepatocellular
carcinoma cells by increasing the tumor suppressor PTEN expression.
J Cell Biochem. 113:3528–3535. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Walker AM, Stevens JJ, Ndebele K and
Tchounwou PB: Evaluation of arsenic trioxide potential for lung
cancer treatment: Assessment of apoptotic mechanisms and oxidative
damage. J Cancer Sci Ther. 8:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Messarah M, Klibet F, Boumendjel A,
Abdennour C, Bouzerna N, Boulakoud MS and El Feki A:
Hepatoprotective role and antioxidant capacity of selenium on
arsenic-induced liver injury in rats. Exp Toxicol Pathol.
64:167–174. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miller WH Jr, Schipper HM, Lee JS, Singer
J and Waxman S: Mechanisms of action of arsenic trioxide. Cancer
Res. 62:3893–3903. 2002.PubMed/NCBI
|
|
13
|
Jin W, Xue Y, Xue Y, Han X, Song Q, Zhang
J, Li Z, Cheng J, Guan S, Sun S and Chu L: Tannic acid ameliorates
arsenic trioxide-induced nephrotoxicity, contribution of NF-κB and
Nrf2 pathways. Biomed Pharmacother. 126:1100472020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Liang Y, Zheng B, Chu L, Ma D, Wang
H, Chu X and Zhang J: Protective effects of crocetin on arsenic
trioxide-induced hepatic injury: Involvement of suppression in
oxidative stress and inflammation through activation of Nrf2
signaling pathway in rats. Drug Des Dev Ther. 14:1921–1931. 2020.
View Article : Google Scholar
|
|
15
|
Li M, Liu P, Xue Y, Liang Y, Shi J, Han X,
Zhang J, Chu X and Chu L: Tannic acid attenuates hepatic oxidative
stress, apoptosis and inflammation by activating the Keap1Nrf2/ARE
signaling pathway in arsenic trioxide-toxicated rats. Oncol Rep.
44:2306–2316. 2020.PubMed/NCBI
|
|
16
|
Benramdane L, Accominotti M, Fanton L,
Malicier D and Vallon JJ: Arsenic speciation in human organs
following fatal arsenic trioxide poisoning-a case report. Clin
Chem. 45:301–306. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu M, Rui D, Yan Y, Xu S, Niu Q, Feng G,
Wang Y, Li S and Jing M: Oxidative damage induced by arsenic in
mice or rats: A systematic review and meta-analysis. Biol Trace
Elem Res. 176:154–175. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Valko M, Morris H and Cronin MT: Metals,
toxicity and oxidative stress. Curr Med Chem. 12:1161–1208. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alam MF, Khan G, Safhi MM, Alshahrani S,
Siddiqui R, Sivagurunathan Moni S and Anwer T: Thymoquinone
ameliorates doxorubicin-induced cardiotoxicity in swiss albino mice
by modulating oxidative damage and cellular inflammation. Cardiol
Res Pract. 2018:14830412018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim SY, Park C, Jang HJ, Kim BO, Bae HW,
Chung IY, Kim ES and Cho YH: Antibacterial strategies inspired by
the oxidative stress and response networks. J Microbiol.
57:203–212. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sies H and Jones DP: Reactive oxygen
species (ROS) as pleiotropic physiological signalling agents. Nat
Rev Mol Cell Biol. 21:363–383. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mortezaee K and Khanlarkhani N: Melatonin
application in targeting oxidative-induced liver injuries: A
review. J Cell Physiol. 233:4015–4032. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grossini E, Bellofatto K, Farruggio S,
Sigaudo L, Marotta P, Raina G, De Giuli V, Mary D, Pollesello P,
Minisini R, et al: Levosimendan inhibits peroxidation in
hepatocytes by modulating apoptosis/autophagy interplay. PLoS One.
10:e01247422015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weiskirchen R and Tacke F: Relevance of
autophagy in parenchymal and non-parenchymal liver cells for health
and disease. Cells. 8:162019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barrera C, Valenzuela R, Rincon MA,
Espinosa A, Echeverria F, Romero N, Gonzalez-Mañan D and Videla LA:
Molecular mechanisms related to the hepatoprotective effects of
antioxidant-rich extra virgin olive oil supplementation in rats
subjected to short-term iron administration. Free Radic Biol Med.
126:313–321. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chayapong J, Madhyastha H, Madhyastha R,
Nurrahmah QI, Nakajima Y, Choijookhuu N, Hishikawa Y and Maruyama
M: Arsenic trioxide induces ROS activity and DNA damage, leading to
G0/G1 extension in skin fibroblasts through
the ATM-ATR-associated Chk pathway. Environ Sci Pollut Res Int.
24:5316–5325. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nili-Ahmadabadi A, Alibolandi P, Ranjbar
A, Mousavi L, Nili-Ahmadabadi H, Larki-Harchegani A,
Ahmadimoghaddam D and Omidifar N: Thymoquinone attenuates
hepatotoxicity and oxidative damage caused by diazinon: An in vivo
study. Res Pharm Sci. 13:500–508. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu JP, Zhao XP, Ma XZ, Wang Y and Zheng
LJ: Effects of cigarette smoke on aerobic capacity and serum MDA
content and SOD activity of animal. Int J Clin Exp Med.
7:4461–4465. 2014.PubMed/NCBI
|
|
29
|
Shafik NM and El Batsh MM: Protective
effects of combined selenium and punica granatum treatment on some
inflammatory and oxidative stress markers in arsenic-induced
hepatotoxicity in rats. Bio Trace Elem Res. 169:121–128. 2016.
View Article : Google Scholar
|
|
30
|
Sharanek A, Burban A, Ciriacim N and
Guillouzo A: Pro-inflammatory cytokines enhance dilatation of bile
canaliculi caused by cholestatic antibiotics. Toxicol In Vitro.
58:51–59. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tu C, Gao D, Li XF, Li CY, Li RS, Zhao YL,
Li N, Jia GL, Pang JY, Cui HR, et al: Inflammatory stress
potentiates emodin-induced liver injury in rats. Front Pharmacol.
6:2332015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang J, Zhang Y, Wang W, Li C and Zhang
Z: Double-sided personality: Effects of arsenic trioxide on
inflammation. Inflammation. 41:1128–1134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dugo EB, Yedjou CG, Stevens JJ and
Tchounwou PB: Therapeutic potential of arsenic trioxide (ATO) in
treatment of hepatocellular carcinoma: Role of oxidative stress in
ATO-induced apoptosis. Ann Clin Pathol. 5:11012017.PubMed/NCBI
|
|
34
|
Abouzied MM, Eltahir HM, Abdel Aziz MA,
Ahmed NS, Abd El-Ghany AA, Abd El-Aziz EA and Abd El-Aziz HO:
Curcumin ameliorate DENA-induced HCC via modulating TGF-beta, AKT,
and caspase-3 expression in experimental rat model. Tumor Bio.
36:1763–1771. 2015. View Article : Google Scholar
|
|
35
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Carthy CM, Yanagawa B, Luo H, Granville
DJ, Yang D, Cheung P, Cheung C, Esfandiarei M, Rudin CM, Thompson
CB, et al: Bcl-2 and Bcl-xL overexpression inhibits cytochrome c
release, activation of multiple caspases, and virus release
following coxsackievirus B3 infection. Virology. 313:147–157. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miltonprabu S, Sumedha NC and Senthilraja
P: Diallyl trisulfide, a garlic polysulfide protects against
As-induced renal oxidative nephrotoxicity, apoptosis and
inflammation in rats by activating the Nrf2/ARE signaling pathway.
Int Immunopharmacol. 50:107–120. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen X, Xue H, Fang W, Chen K, Chen S,
Yang W, Shen T, Chen X, Zhang P and Ling W: Adropin protects
against liver injury in nonalcoholic steatohepatitis via the Nrf2
mediated antioxidant capacity. Redox Biol. 21:1010682019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mitsuishi Y, Motohashi H and Yamamoto M:
The Keap1-Nrf2 system in cancers: Stress response and anabolic
metabolism. Front Oncol. 2:2002012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sabouny R, Fraunberger E, Geoffrion M, Ng
AC, Baird SD, Screaton RA, Milne R, McBride HM and Shutt TE: The
Keap1-Nrf2 stress response pathway promotes mitochondrial
hyperfusion through degradation of the mitochondrial fission
protein drp1. Antioxid Redox Sign. 27:1447–1459. 2017. View Article : Google Scholar
|
|
41
|
Zhao R, Yang B, Wang L, Xue P, Deng B,
Zhang G, Jiang S, Zhang M, Liu M, Pi J and Guan D: Curcumin
protects human keratinocytes against inorganic arsenite-induced
acute cytotoxicity through an NRF2-dependent mechanism. Oxid Med
Cell Longev. 2013:4125762013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu Z, Zhang Y, Song T, Song Q, Zhang Y,
Zhang X, Han X, Zhang J and Chu L: Magnesium isoglycyrrhizinate
ameliorates doxorubicin-induced acute cardiac and hepatic toxicity
via anti-oxidant and anti-apoptotic mechanisms in mice. Exp Ther
Med. 15:1005–1012. 2018.PubMed/NCBI
|
|
43
|
Zhang JC, Zheng GF, Wu MX, Wu JW, Ouyang
LY and Liu XQ: Effect of magnesium isoglycyrrhizinate on PLA2
during liver tissue injury following limb ischemia/reperfusion in
rats. Zhonghua Gan Zang Bing Za Zhi. 20:537–541. 2012.(In Chinese).
PubMed/NCBI
|
|
44
|
Sun L, Shen J, Pang X, Lu L, Mao Y and
Zeng M: Phase I safety and pharmacokinetic study of magnesium
isoglycyrrhizinate after single and multiple intravenous doses in
chinese healthy volunteers. J Clin Pharmacol. 47:767–773. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ma D, Zhang J, Zhang Y, Zhang X, Han X,
Song T, Zhang Y and Chu L: Inhibition of myocardial hypertrophy by
magnesium isoglycyrrhizinate through the TLR4/NF-κB signaling
pathway in mice. Int Immunopharmacol. 55:237–244. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xue Y, Li M, Xue Y, Jin W, Han X, Zhang J,
Chu X, Li Z and Chu L: Mechanisms underlying the protective effect
of tannic acid against arsenic trioxideinduced cardiotoxicity in
rats: Potential involvement of mitochondrial apoptosis. Mol Med
Rep. 22:4663–4674. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jiang W, Chen Q, Li P, Lu Q, Pei X, Sun Y,
Wang G and Hao K: Magnesium Isoglycyrrhizinate attenuates
lipopolysaccharide-induced depressive-like behavior in mice. Biomed
Pharmacother. 86:177–184. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Birari LA, Mahajan UB, Patil KR, Patil DD,
Bagul NA, Belemkar S, Goyal SN, Ojha S and Patil CR: Aloin protects
against arsenic trioxide-induced myocardial membrane damage and
release of inflammatory cytokines. Naunyn Schmiedebergs Arch
Pharmacol. 393:1365–1372. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Knuckles TL, Buntz JG, Paffett M, Channell
M, Harmon M, Cherng T, Lucas SN, McDonald JD, Kanagy NL and Campen
MJ: Formation of vascular S-nitrosothiols and plasma
nitrates/nitrites following inhalation of diesel emissions. J
Toxicol Environ Health A. 74:828–837. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Thapa BR and Walia A: Liver function tests
and their interpretation. Indian J Pediatr. 74:663–671. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expres-sion data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hede K: Chinese folk treatment reveals
power of arsenic to treat cancer, new studies under way. J Natl
Cancer Inst. 99:667–668. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Qu W, Cheng L, Dill AL, Saavedra JE, Hong
SY, Keefer LK and Waalkes MP: Nitric oxide donor, V-PROLI/NO,
provides protection against arsenical induced toxicity in rat liver
cells: Requirement for Cyp1a1. Chem Biol Interact. 193:88–96. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mathews V, Desire S, George B, Lakshmi KM,
Rao JG, Viswabandya A, Bajel A, Srivastava VM, Srivastava A and
Chandy M: Hepatotoxicity profile of single agent arsenic trioxide
in the treatment of newly diagnosed acute promyelocytic leukemia,
its impact on clinical outcome and the effect of genetic
polymorphisms on the incidence of hepatotoxicity. Leukemia.
20:881–883. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ducas RA, Seftel MD, Ducas J and Seifer C:
Monomorphic ventricular tachycardia caused by arsenic trioxide
therapy for acute promyelocytic leukaemia. J R Coll Physicians
Edinb. 41:117–118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang ZF, Liu J, Yang YG and Zhu HL: A
review: The anti-inflammatory, anticancer and antibacterial
properties of four kinds of licorice flavonoids isolated from
licorice. Curr Med Chem. 27:1997–2011. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen KJ, Chen WY, Chen X, Jia YM, Peng GQ
and Chen L: Increased elimination of paclitaxel by magnesium
isoglycyrrhizinate in epithelial ovarian cancer patients treated
with paclitaxel plus cisplatin: A pilot clinical study. Eur J Drug
Metab Pharmacokinet. 39:25–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lv J, Xiao Q, Chen Y, Fan X, Liu X, Liu F,
Luo G, Zhang B and Wang S: Effects of magnesium isoglycyrrhizinate
on AST, ALT, and serum levels of Th1 cytokines in patients with
allo-HSCT. Int Immunopharmacol. 46:56–61. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cetin A, Kaynar L, Kocyigit I, Hacioglu
SK, Saraymen R, Ozturk A, Sari I and Sagdic O: Role of grape seed
extract on methotrexate induced oxidative stress in rat liver. Am J
Chin Med. 36:861–872. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kim WR, Flamm SL, Di Bisceglie AM and
Bodenheimer HC; Public Policy Committee of the American association
for the study of liver disease, : Serum activity of alanine
aminotransferase (ALT) as an indicator of health and disease.
Hepatology. 47:1363–1370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yang RZ, Park S, Reagan WJ, Goldstein R,
Zhong S, Lawton M, Rajamohan F, Qian K, Liu L and Gong DW: Alanine
aminotransferase isoenzymes: Molecular cloning and quantitative
analysis of tissue expression in rats and serum elevation in liver
toxicity. Hepatology. 49:598–607. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sifuentes-Franco S, Pacheco-Moises FP,
Rodriguez-Carrizalez AD and Miranda-Diaz AG: The role of oxidative
stress, mitochondrial function, and autophagy in diabetic
polyneuropathy. J Diabetes Res. 2017:16730812017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Daenen K, Andries A, Mekahli D, Van
Schepdael A, Jouret F and Bammens B: Oxidative stress in chronic
kidney disease. Pediatr Nephrol. 34:975–991. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Förstermann U, Xia N and Li H: Roles of
vascular oxidative stress and nitric oxide in the pathogenesis of
atherosclerosis. Circ Res. 120:713–735. 2017. View Article : Google Scholar
|
|
65
|
Butterfield DA and Halliwell B: Oxidative
stress, dysfunctional glucose metabolism and Alzheimer disease. Nat
Rev Neurosci. 20:148–160. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
He JL, Dong XH, Li ZH, Wang XY, Fu ZA and
Shen N: Pterostilbene inhibits reactive oxygen species production
and apoptosis in primary spinal cord neurons by activating
autophagy via the mechanistic target of rapamycin signaling
pathway. Mol Med Rep. 17:4406–4414. 2018.PubMed/NCBI
|
|
67
|
Haga N, Fujita N and Tsuruo T: Involvement
of mitochondrial aggregation in arsenic trioxide (As2O3)-induced
apoptosis in human glioblastoma cells. Cancer Sci. 96:825–833.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hosseini MJ, Shaki F, Ghazi-Khansari M and
Pourahmad J: Toxicity of copper on isolated liver mitochondria:
Impairment at complexes I, II, and IV leads to increased ROS
production. Cell Biochem Biophys. 70:367–381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ortiz M, Soto-Alarcon SA, Orellana P,
Espinosa A, Campos C, López-Arana S, Rincón MA, Illesca P,
Valenzuela R and Videla LA: Suppression of high-fat diet-induced
obesity-associated liver mitochondrial dysfunction by
docosahexaenoic acid and hydroxytyrosol co-administration. Dig
Liver Dis. 52:895–904. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hernandez-Rodas MC, Valenzuela R,
Echeverria F, Rincón-Cervera MÁ, Espinosa A, Illesca P, Muñoz P,
Corbari A, Romero N, Gonzalez-Mañan D and Videla LA:
Supplementation with docosahexaenoic acid and extra virgin olive
oil prevents liver steatosis induced by a high-fat diet in mice
through PPAR-α and Nrf2 upregulation with concomitant SREBP-1c and
NF-kB downregulation. Mol Nutr Food Res. 61:2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kim SH and Kim H: Inhibitory effect of
astaxanthin on oxidative stress-induced mitochondrial dysfunction-A
mini-review. Nutrients. 10:11372018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Santos C, Pires Mdos A, Santos D and
Payan-Carreira R: Distribution of superoxide dismutase 1 and
glutathione peroxidase 1 in the cyclic canine endometrium.
Theriogenology. 86:738–748. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Durak I, Yurtarslanl Z, Canbolat O and
Akyol O: A methodological approach to superoxide dismutase (SOD)
activity assay based on inhibition of nitroblue tetrazolium (NBT)
reduction. Clin Chim Acta. 214:103–104. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mates JM: Effects of antioxidant enzymes
in the molecular control of reactive oxygen species toxicology.
Toxicology. 153:83–104. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Shen H, Niu Q, Xu M, Rui D, Xu S, Feng G,
Ding Y, Li S and Jing M: Factors affecting arsenic methylation in
arsenic-exposed humans: A systematic review and meta-analysis. Int
J Environ Res Public Health. 13:2052016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Xie C, Li X, Zhu J, Wu J, Geng S and Zhong
C: Magnesium isoglycyrrhizinate suppresses LPS-induced inflammation
and oxidative stress through inhibiting NF-κB and MAPK pathways in
RAW264.7 cells. Bioorg Med Chem. 27:516–524. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yang YZ, Liu ZH, Wang SC, Zhang XQ, Xu HJ,
Yang L and Kong LD: Magnesium isoglycyrrhizinate alleviates
fructose-induced liver oxidative stress and inflammatory injury
through suppressing NOXs. Eur J Pharmacol. 883:1733142020.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zheng Y, Tao S, Lian F, Chau BT, Chen J,
Sun G, Fang D, Lantz RC and Zhang DD: Sulforaphane prevents
pulmonary damage in response to inhaled arsenic by activating the
Nrf2-defense response. Toxicol Appl Pharm. 265:292–299. 2012.
View Article : Google Scholar
|
|
79
|
Islam LN, Nabi AH, Rahman MM and Zahid MS:
Association of respiratory complications and elevated serum
immunoglobulins with drinking water arsenic toxicity in human. J
Environ Sci Health A Tox Hazard Subst Environ Eng. 42:1807–1814.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhang JX, Xing JG, Wang LL, Jiang HL, Guo
SL and Liu R: Luteolin inhibits fibrillary
β-amyloid1-40-induced inflammation in a human
blood-brain barrier model by suppressing the p38 MAPK-Mediated
NF-κB signaling pathways. Molecules. 22:3342017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bai J and Meng Z: Effects of sulfur
dioxide on apoptosis-related gene expressions in lungs from rats.
Regul Toxicol Pharmacol. 43:272–279. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Robertson JD and Orrenius S: Molecular
mechanisms of apoptosis induced by cytotoxic chemicals. Crit Rev
Toxicol. 30:609–627. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yang LJ and Wang WL: Preparation of
monoclonal antibody against apoptosis-associated antigens of
hepatoma cells by subtractive immunization. World J Gastroenterol.
8:808–814. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Van Opdenbosch N and Lamkanfi M: Caspases
in cell death, inflammation, and disease. Immunity. 50:1352–1364.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Rotschafer SE, Allen-Sharpley MR and
Cramer KS: Axonal cleaved caspase-3 regulates axon targeting and
morphogenesis in the developing auditory brainstem. Front Neural
Circuits. 10:842016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Bernard A, Chevrier S, Beltjens F, Dosset
M, Viltard E, Lagrange A, Derangère V, Oudot A, Ghiringhelli F,
Collin B, et al: Cleaved caspase-3 transcriptionally regulates
angiogenesis-promoting chemotherapy resistance. Cancer Res.
79:5958–5970. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Han B, Zhou G, Zhang Q, Zhang J, Wang X,
Tang W and Kakudo K: Effect of arsenic trioxide (ATO) on human lung
carcinoma PG cell line: ATO induced apoptosis of PG cells and
decreased expression of Bcl-2, Pgp. J Exp Ther Oncol. 4:335–342.
2004.PubMed/NCBI
|
|
88
|
Yao P, Nussler A, Liu L, Hao L, Song F,
Schirmeier A and Nussler N: Quercetin protects human hepatocytes
from ethanol-derived oxidative stress by inducing heme oxygenase-1
via the MAPK/Nrf2 pathways. J Hepatol. 47:253–261. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wu KC, Liu J and Klaassen CD: Role of Nrf2
in preventing ethanol-induced oxidative stress and lipid
accumulation. Toxicol Appl Pharm. 262:321–329. 2012. View Article : Google Scholar
|
|
90
|
Lee CS, Ho DV and Chan JY: Nuclear
factor-erythroid 2-related factor 1 regulates expression of
proteasome genes in hepatocytes and protects against endoplasmic
reticulum stress and steatosis in mice. FEBS J. 280:3609–3620.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lee JM, Li J, Johnson DA, Stein TD, Kraft
AD, Calkins MJ, Jakel RJ and Johnson JA: Nrf2, a multi-organ
protector? FASEB J. 19:1061–1066. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Copple IM, Goldring CE, Kitteringham NR
and Park BK: The keap1-nrf2 cellular defense pathway: Mechanisms of
regulation and role in protection against drug-induced toxicity.
Handb Exp Pharmacol. 233–266. 2010. View Article : Google Scholar : PubMed/NCBI
|