Introduction
Arsenic is a common constituent of the Earth's crust
and has been used in traditional Chinese medicine to treat a number
of diseases, such as leukemia, psoriasis, syphilis and tuberculosis
(1,2). Arsenic trioxide (ATO) has been the
cornerstone of the treatment of acute promyelocytic leukemia (APL)
and other types of hematopoietic malignancy since its first
application in the 1970s at Harbin Medical University; its efficacy
has been proved in clinical trials (3). The United States Food and Drug
Administration approved ATO for the treatment of APL in September
2000 (4). High-dose ATO is used as
an effective chemotherapy drug in the treatment of certain types of
cancers; however, toxic side effects are of concern (5). ATO has been shown to be the most
effective single agent for the treatment of APL, and following the
co-treatment of ATO and all-trans retinoic acid, long-term survival
of patients with APL has been improved to 80–90% (6–8). An
increasing number of studies have consistently shown that ATO is
effective against other cancer types, including hepatocellular
carcinoma, pancreatic cancer and lung cancer (9,10). In
past decades, clinical data have shown that excessive use of ATO
causes hepatotoxicity, nephrotoxicity and cardiotoxicity (11–15).
The liver removes toxins and drugs, but it can also
be destroyed by these harmful substances (11,16).
Arsenic induces hepatotoxicity by oxidative stress (17,18).
ATO generates reactive oxygen species (ROS), including hydroxyl
radicals and superoxide anions, which decrease equilibrium and
disturb natural oxidation via complex redox reactions with
endogenous oxidants (19).
Oxidative stress occurs when pro-oxidants overpower anti-oxidants
in the living organisms (20).
Pro-oxidants are chemicals that can either generate ROS or
compromise anti-oxidants in cells (21). A high concentration of redox
signaling of ROS is commonly observed with cell damage and
metabolic dysregulation, including lipid peroxidation, and
permanent protein and DNA degeneration (22). Thus, the liver is the primary organ
susceptible to pathological cascades of oxidative stress (23). Parenchymal cells are most vulnerable
in an oxidative environment (24).
In order to control the generation of ROS in the liver, both
enzymatic and non-enzymatic systems are involved in maintaining
redox homeostasis (25). The
imbalance between ROS production and the antioxidant system is
maintained by key enzymes, such as catalase (CAT), superoxide
dismutase (SOD) and glutathione (GSH) (26,27).
Malondialdehyde (MDA) is an end product of lipid peroxidation and
is used as an indicator of oxidative damage in vivo
(28). In addition to the
significant role of oxidative stress, many reports suggest that the
activity of inflammatory cytokines and apoptotic proteins increases
with the etiology of hepatotoxicity (29,30).
High levels of ROS act as mediators of inflammation and induce
peripheral inflammation, but other studies have shown that ATO
alters expression levels of pro-inflammatory cytokines, such as
TNF-α, IL-1β and IL-6 (31,32). When antioxidant responses are
overwhelmed, ROS damage to cells leads to necrosis or apoptosis,
which is manifested by oxidative stress and inflammation (33). Apoptosis is characterized by
well-defined features, including cellular morphological changes,
activation of caspase-3 and cleaved caspase-3 and imbalance of
Bax/Bcl-2 (34–36).
Nuclear factor erythroid 2-related factor 2 (Nrf2)
is a sensitive sensor that is key to cellular defense against
oxidative species and toxic damage; it inhibits oxidative stress by
upregulating Nrf2-driven antioxidants (37). Previous research has shown that
adropin protects against liver injury in nonalcoholic
steatohepatitis via Nrf2-mediated antioxidant capacity (38). Under normal conditions, cells
maintain low constitutive levels of Nrf2-target genes via the
Kelch-like ECH-associated protein 1 (Keap1)-dependent E3 ubiquitin
ligase complex, which directly leads to continual ubiquitination
and subsequent degradation of the transcription factor Nrf2 in the
cell cytoplasm (39,40). Under oxidative stress, Keap1 is
inactivated, leading to the release of Nrf2 from Keap1. The
switching on and off of Nrf2 protects cells from free radical
damage and promotes cell survival (41). Therefore, antioxidants may serve key
roles in preventing ATO-induced hepatotoxicity.
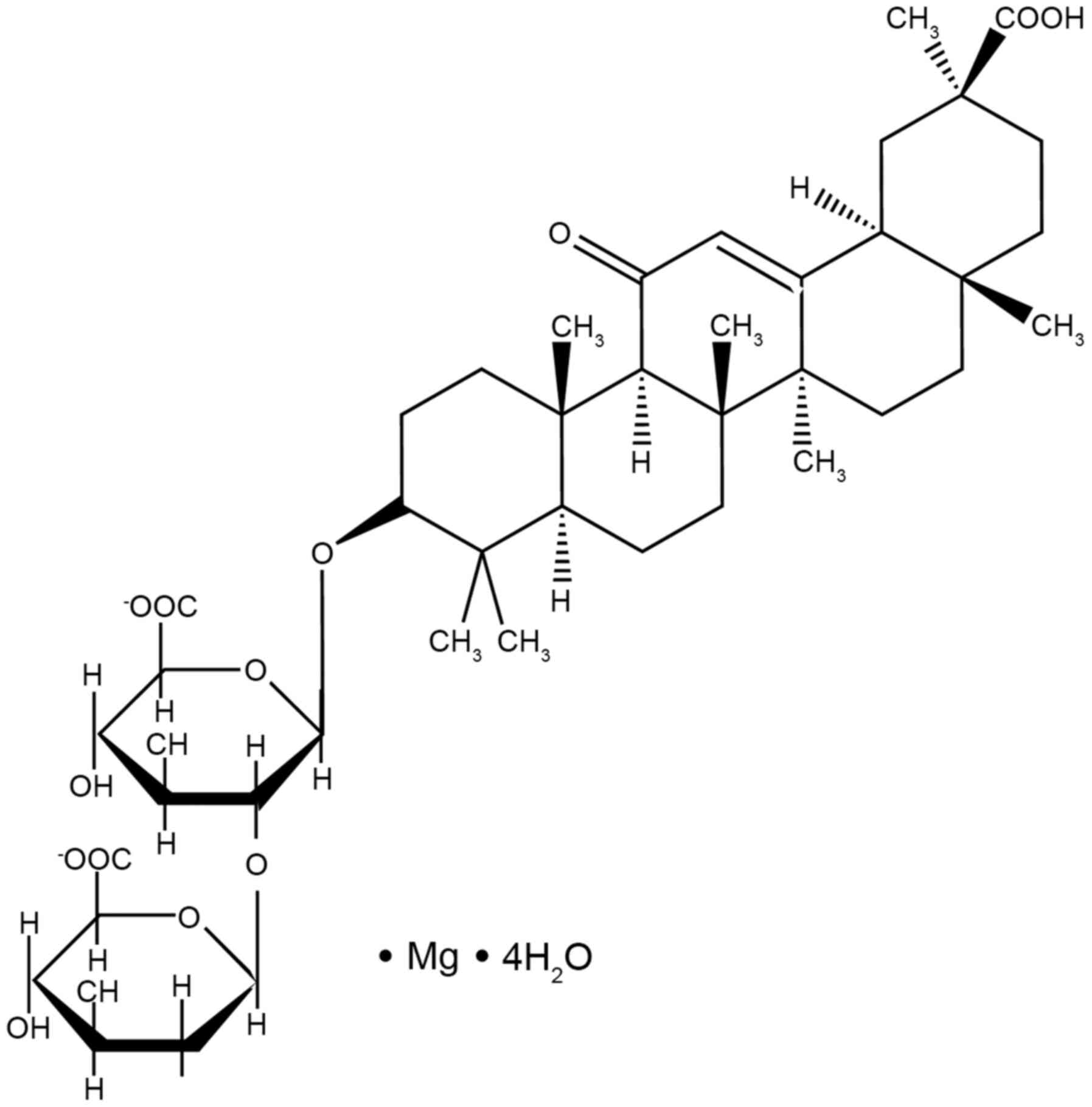
Magnesium isoglycyrrhizinate (MgIG; Fig. 1) is a magnesium salt of the 18-α
glycyrrhizic acid stereoisomer. It is a novel molecular compound
extracted from licorice root (42).
In China and Japan, it is used as a hepatoprotective agent and
inhibits inflammation, improves liver function and stabilizes cell
membranes (43). MgIG has been used
as a hepatoprotective and anti-inflammatory agent in the clinical
treatment of inflammatory liver disease due to its effective role
in hepatitis response and liver function recovery (44). According to our previous research,
MgIG ameliorates doxorubicin-induced cardiotoxicity and
hepatotoxicity via anti-oxidant and anti-apoptotic mechanisms
(42,45). To the best of our knowledge,
however, the potential effect and mechanism of MgIG on
hepatotoxicity caused by ATO has not yet been reported.
The present study aimed to evaluate the protective
effect and potential mechanism of MgIG on ATO-induced
hepatotoxicity, including oxidative stress, inflammatory responses,
apoptosis and activation of the Keap1-Nrf2 signaling pathway.
Materials and methods
Chemicals and reagents
MgIG (purity, 99.3%) was purchased from Chia Tai
Tianqing Pharmaceutical Group Co., Ltd. ATO parenteral solution was
purchased from Beijing SL Pharmaceutical Co., Ltd. All other
chemicals were purchased from Sigma-Aldrich (Merck KGaA) unless
otherwise specified.
Animals and treatment
A total of 50 adult male KunMing mice (age, 6–7
weeks; weight, 18–22 g) were provided by Experimental Animal Center
(Hebei Medical University, Shijiazhuang, China). All mice were
housed in plastic cages (n=10/cage) under standard conditions
(20–24°C) and 55±5% relative humidity with a 12-h dark-light cycle
environment and ad libitum access to pellet food and water.
All animal experiments were approved by the Ethics Committee for
Animal Experiments of Hebei University of Chinese Medicine
(approval no. DWLL2020005; approval date, 9 January, 2020).
Animals were randomly divided into the following
groups (n=10/group): Control [CON, normal saline, intraperitoneal
(i.p.) injection, 0.1 ml/kg/day]; ATO (i.p., 5 mg/kg/day);
MgIG-alone (MgIG, i.p., 50 mg/kg/day); high-MgIG + ATO (H-MgIG,
i.p., 50 mg/kg/day MgIG + 5 mg/kg ATO) and low-MgIG + ATO (L-MgIG,
i.p., 25 mg/kg/day MgIG + 5 mg/kg ATO). The hepatotoxicity model
was established via i.p. injection with ATO (5 mg/kg). The CON
group received isovolumic normal saline, as previously described
(46). The L-MgIG and H-MgIG groups
were given 25 and 50 mg/kg MgIG, respectively, followed by 5 mg/kg
ATO 6 h later. Dose selection of ATO and MgIG was determined
according to previous literature (47,48).
The mice were sacrificed after 7 days of continuous treatment.
After 7 days, sodium pentobarbital (50 mg/kg) was used to
anesthetize mice and mice were weighed on an electronic balance to
the nearest milligram. Then, blood (0.5–1.2 ml) was collected by
exsanguination from the abdominal aorta for biochemical analysis.
Euthanasia of mice was performed by overdose with i.p. injection of
sodium pentobarbital (200 mg/kg) and was confirmed by absence of
respiration and heartbeat. Liver was collected for further analysis
(49).
Blood collection and serum
preparation
The weight was recorded for each mouse, followed by
i.p. injection by sodium pentobarbital (50 mg/kg). The blood sample
was collected and centrifuged at 1,500 × g for 10 min at room
temperature. The serum was aspirated into clean, dry tubes and then
frozen at −20°C for analysis.
Liver tissue collection and homogenate
preparation
Liver was removed after sacrificing the mice. The
livers were weighed on an electronic balance to the nearest
milligram and the relative weight of the liver was calculated using
the following formula: Index weight=organ weight/body weight ×100%.
The livers were then washed with ice-cold saline and homogenized at
12,000 × g at 4°C for 10 min in PBS (pH 7.4; w/v; 1 g tissue with 9
ml PBS). The supernatant was stored at −20°C and different
parameters were assayed.
Evaluation of histopathology
Livers were excised and small pieces were carefully
removed from experimental animals. The tissue was dissected and
fixed with ice-cold 4% paraformaldehyde overnight at 4°C, embedded
in paraffin, cut into 4 µm-thick slices and stained with 0.1%
hematoxylin for 15 min and 0.5% eosin for 5 min, both at room
temperature, according to standard procedures. Pathohistological
changes were observed by light microscopy (magnification,
×400).
Measurement of levels of ROS
Dihydroethidium was used to monitor cellular
production of ROS. Fresh liver tissue was embedded and sliced, as
aforementioned. Liver tissue was incubated at 37°C in the dark for
1 h. A fluorescence microscope was used to measure the nucleus of
liver following staining with 5 mg/ml DAPI for 20 min at room
temperature with an excitation source of 510–560 nm and an emission
wavelength of 590 nm. ROS generation was visualized and analyzed
using a high-content screening system (Leica DM4000B; Leica
Microsystems GmbH). ROS production was quantified using Image-Pro
Plus 6.0 software (Media Cybernetics, Inc.). All experiments were
repeated at least three times.
Detection of biochemical indices in
serum
Aspartate aminotransferase (AST) and alanine
aminotransferase (ALT) are significant predictors of liver injury
(50). The serum activity of ALT
(cat. no. C010-3-1), AST (cat. no. C009-3-1), SOD (cat. no.
C001-3-2) and CAT (cat. no. C007-1-1), GSH (cat. no. C006-2-1), as
well as the level of MDA (cat. no. C003-1-2) were detected by
commercial kits according to the manufacturer's instructions (all
Nanjing Jiancheng Bioengineering Institute).
Quantification of levels of IL-1β,
IL-6 and TNF-α
The levels of IL-1β (cat. no. 88-7013-88), IL-6
(cat. no. 88-7064-88) and TNF-α (cat. no. 88-7324-88) were measured
and calculated using ELISA kits, according to the manufacturers'
instructions (all Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from liver using
TRIzol® reagent (cat. no. G3013; Wuhan Servicebio
Technology Co., Ltd.). RT was performed using a TIANScript RT kit
(cat. no. G3330-100; Wuhan Servicebio Technology Co., Ltd.)
according to the manufacturer's instructions. The gene expression
levels of IL-1, IL-6 and TNF-α in liver tissue were assessed via
RT-qPCR using SYBR Green (cat. no. G3320; Wuhan Servicebio
Technology Co., Ltd.). The PCR conditions were as follows: Initial
denaturation at 95°C for 15 min, denaturation at 95°C for 10 sec,
followed by annealing at 58°C for 30 sec and extension at 72°C for
30 sec for 40 cycles. Relative gene expression profiles were
determined by normalization of expression to that of the
housekeeping gene (β actin) using the 2−ΔΔCq method
(51). Mouse primer sequences were
as follows: IL-1β forward, 5′-GGTCAAAGGTTTGGAAGCAG-3′ and reverse,
5′-TGTGAAATGCCACCTTTTGA-3′; IL-6 forward,
5′-ACCAGAGGAAATTTTCAATAGGC-3′ and reverse,
5′-TGATGCACTTGCAGAAAACA-3′; TNF-α forward,
5′-AGGGTCTGGGCCATAGAACT-3′ and reverse,
5′-CCACCACGCTCTTCTGTCTAC-3′; and β actin forward,
5′-CCTAGACTTCGAGCAAGAGA-3′ and 5′-reverseGGAAGGAAGGCTGGAAGA-3′.
Evaluation of expression levels of
Bax, Bcl-2, caspase-3, cleaved-caspase-3, Keap1 and Nrf2
In order to extract total protein, liver tissue was
homogenized in RIPA lysis buffer (Wuhan Servicebio Technology Co.,
Ltd.) and then centrifuged 12,000 × g for 10 min at 4°C to prepare
a supernatant. The proteins were quantified using the bicinchoninic
acid method. Equivalent amounts of protein (50 µg) were resolved
via 12% SDS-PAGE and transferred onto PVDF membranes and blocked in
5% skimmed milk at room temperature for 1 h. Membranes were
incubated with the following primary antibodies (all 1:1,000) at
4°C for 12 h: Anti-Bax (cat. no. GB11690; Wuhan Servicebio
Technology Co., Ltd.), anti-Bcl-2 (cat. no. PAA778Mu01; Wuhan
Servicebio Technology Co., Ltd.), anti-caspase-3 (cat. no.
66470-2-lg; ProteinTech Group, Inc.), anti-cleaved caspase-3 (cat.
no. 9664; Cell Signaling Technology, Inc.), anti-Keap1 (cat. no.
10503-2-ap; ProteinTech Group, Inc.), anti-Nrf2 (cat. no.
16396-1-ap; ProteinTech Group, Inc.) and anti β-actin (cat. no.
66470-2-lg; Wuhan Servicebio Technology Co., Ltd.). Next, the
membranes were washed three times with TBS-0.1% Tween-20, followed
by incubation at room temperature for 1 h with horseradish
peroxidase-conjugated secondary antibody (1:3,000; cat. no.
GB23303; Wuhan Servicebio Technology Co., Ltd.), and washing three
times. The proteins were visualized using an ECL Detection reagent
(TransGen Biotech Co., Ltd.) and imaged using a Tanon-1600 Gel
Image Analysis system (Tanon Science and Technology Co., Ltd.).
Densitometry was performed using Tanon Gis 1D software (4.00; Tanon
Science and Technology Co., Ltd.). All experiments were repeated
three times.
TUNEL assay
TUNEL staining was performed using an in-situ
cell death detection kit (cat. no. WLA029; Wanleibio Co., Ltd.)
according to the manufacturer's protocol. After the liver was
removed, tissues were fixed in 4% paraformaldehyde for 24 h at room
temperature, embedded in paraffin and cut into 5-µm thick sections.
Liver sections were deparaffinized, dehydrated using increasing
concentrations of alcohol, washed in distilled water followed by
PBS and deproteinized using proteinase K (20 µg/ml) at 37°C for 30
min. Subsequently, sections were rinsed and incubated with the
TUNEL reagent at 37°C for 1 h. Following rinsing, the sections were
visualized using peroxidase-conjugated anti-fluorescein antibody
(contained in the TUNEL kit) with 0.02% 3,3-diaminobenzidine at
room temperature for 5 min and then counterstained with 0.5%
hematoxylin (cat. no. H8070; Beijing Solarbio Science &
Technology Co., Ltd.) at room temperature for 10–30 sec. Neutral
balsam was used to bond the slides and cover glass together. A
total of five randomly selected microscopic fields of view were
selected in order to analyze the staining results, and micrographs
were scanned at ×400 magnification with a digital light microscope
system (Olympus Corporation; DP73).
Statistical analysis
Statistical analysis was performed using Origin Pro
version 9.1 software (OriginLab Corporation.). Data are presented
as the mean ± SEM. Each experiment was repeated more than three
times. Comparisons were analyzed via one-way ANOVA followed by post
hoc Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of MgIG on ALT and AST
levels
ATO group exhibited markedly higher liver weight and
index and ALT and AST activity compared with the CON and MgIG
groups (P<0.01; Fig. 2A;
Table I). MgIG treatment decreased
the liver index and activity of ALT and AST compared with the ATO
group (P<0.05 or P<0.01).
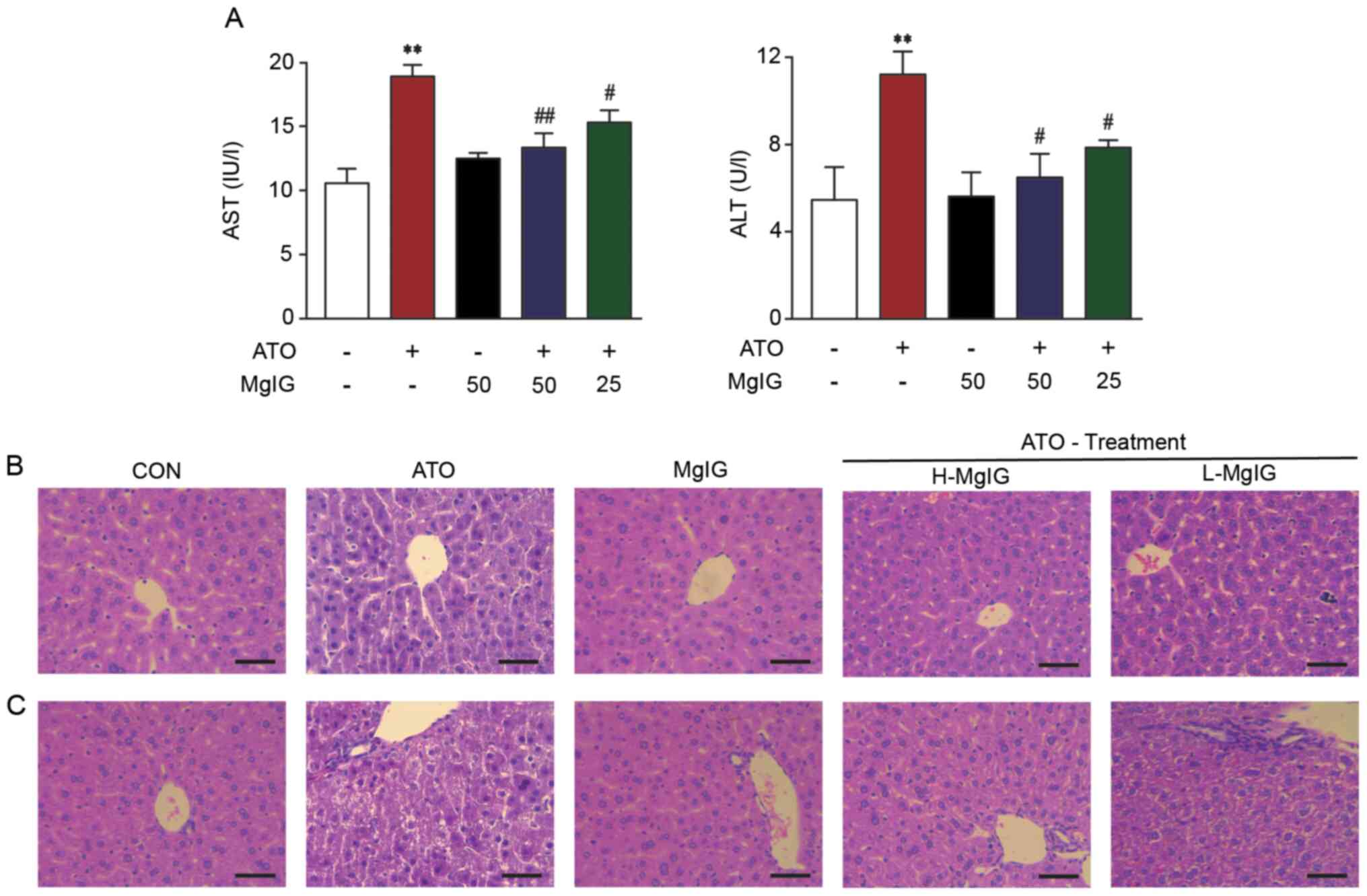 | Figure 2.Effect of MgIG on liver injury. (A)
AST and ALT activity. Representative sections of hematoxylin-eosin
staining in the hepatic (B) central vein and (C) duct area
(magnification, ×400). Scale bar, 50 µm. CON showed normal
hepatocyte architecture; ATO showed inflammation, hepatocyte
vacuolation and necrosis/disorganization of the parenchyma. These
symptoms decreased following MgIG treatment. Data are presented as
the mean ± SEM (n=10). **P<0.01 vs. CON, #P<0.05
and ##P<0.01 vs. ATO. AST, aspartate
aminotransferase; ALT, alanine aminotransferase; MgIG, magnesium
isoglycyrrhizinate; CON, control; ATO, arsenic trioxide; H-, high;
L-, low. |
 | Table I.Effect of MgIG on body weight and
relative liver weight in mice. |
Table I.
Effect of MgIG on body weight and
relative liver weight in mice.
| Parameter | CON | ATO | MgIG | H-MgIG | L-MgIG |
|---|
| Final body weight,
g | 30.78±0.72 | 29.71±0.91 | 30.05±0.63 | 29.13±1.05 | 29.02±0.78 |
| Liver weight,
g | 1.31±0.05 |
3.43±0.09a |
1.19±0.04b |
1.78±0.08b |
2.36±0.03b |
| Liver index, % | 4.27±0.20 |
11.54±0.43a |
3.94±0.10b |
6.13±0.35b | 8.18±
0.30b |
Effect of MgIG on liver
histopathology
Histopathological data are shown in Fig. 2B and C. Liver tissue of mice in the
CON and MgIG groups exhibited a normal structure. Severe
pathological changes were observed in liver sections in the ATO
group, including extensive hepatocyte vacuolation, disorganization
of the parenchyma and dilatation of intrahepatocyte spaces.
Furthermore, masses of acidophilic material were observed next to
the central vein and microvesicular vacuolization was observed near
the portal triad. Comparison of the histological features in mice
from the ATO, H-MgIG and L-MgIG groups showed that pretreatment
with MgIG diminished infiltration of inflammatory cells and
vacuolation of hepatocytes.
Effect of MgIG on ROS production
The effect of MgIG on ATO-induced liver injury was
evaluated via fluorescence of a dihydroethidium probe to assessing
liver tissue production of ROS. ATO group exhibited significantly
increased production of ROS compared with CON (P<0.01; Fig. 3A and B). However, the levels of ROS
in H-MgIG and L-MgIG groups decreased significantly compared with
ATO group (P<0.05 or P<0.01). These data indicated that MgIG
suppressed ATO-induced hepatic ROS overproduction.
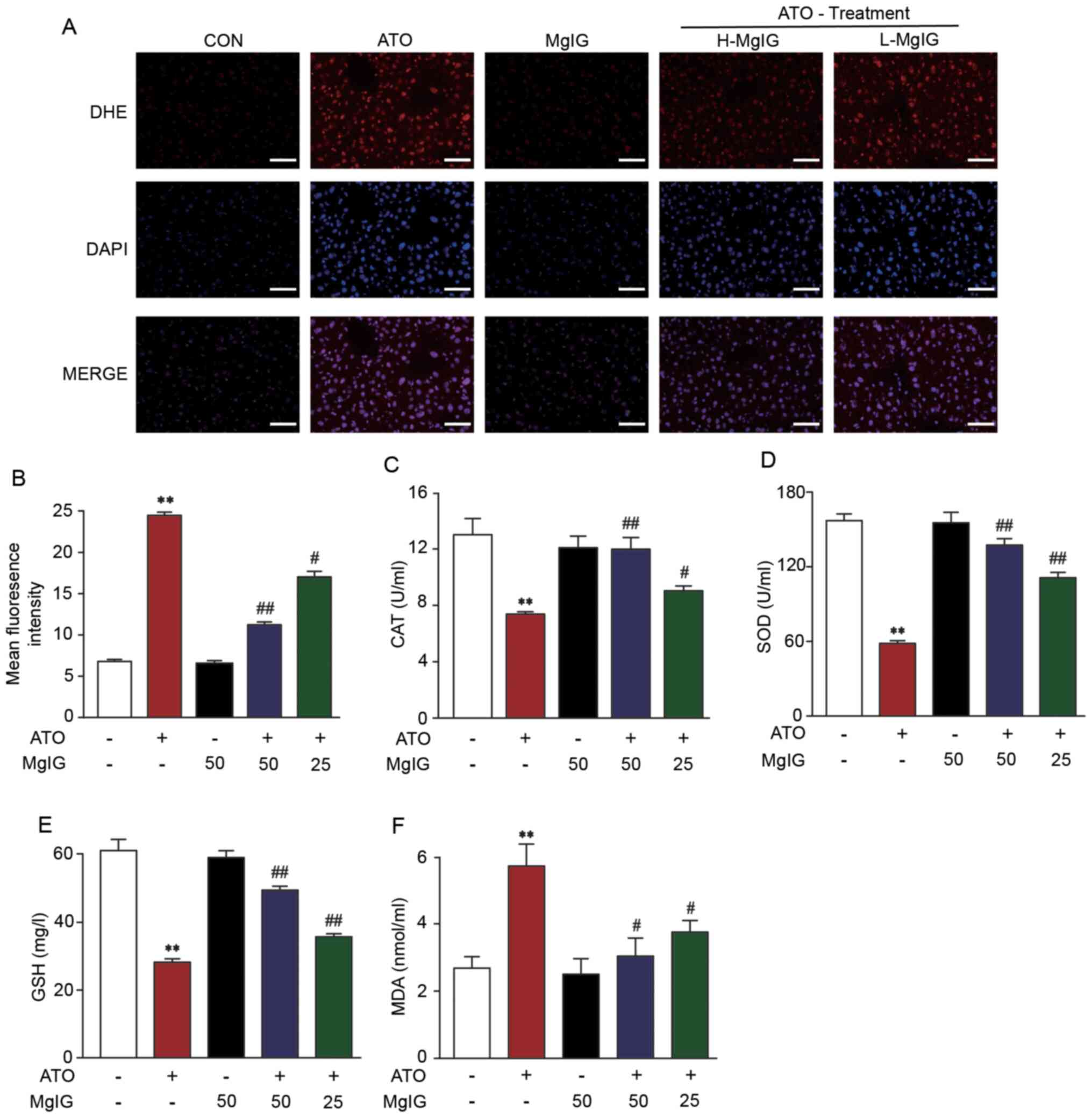 | Figure 3.Effect of MgIG on ATO-induced
oxidative stress. (A) Representative images and (B) quantitative
analysis of reactive oxygen species levels. Activity of (C) CAT,
(D) SOD, (E) GSH and (F) MDA. Data are presented the mean ± SEM
(n=10). **P<0.01 vs. CON, #P<0.05 and
##P<0.01 vs. ATO. MgIG, magnesium isoglycyrrhizinate;
ATO, arsenic trioxide; CAT, catalase; SOD, superoxide dismutase;
GSH, glutathione; MDA, malonaldehyde; H-, high; L-, low; DHE,
dihydroethidium. |
Effects of MgIG on activity of
antioxidant enzymes
ATO caused a prominent decrease in CAT, GSH and SOD
activity compared with the CON and MgIG groups. Treatment with
H-MgIG and L-MgIG (25 or 50 mg/kg) elevated the activity of GSH,
SOD and CAT in serum compared with the ATO group (P<0.01;
Fig. 3C-F). Additionally, treatment
with ATO increased MDA concentration, whereas MgIG significantly
reversed this effect (P<0.05 or P<0.01).
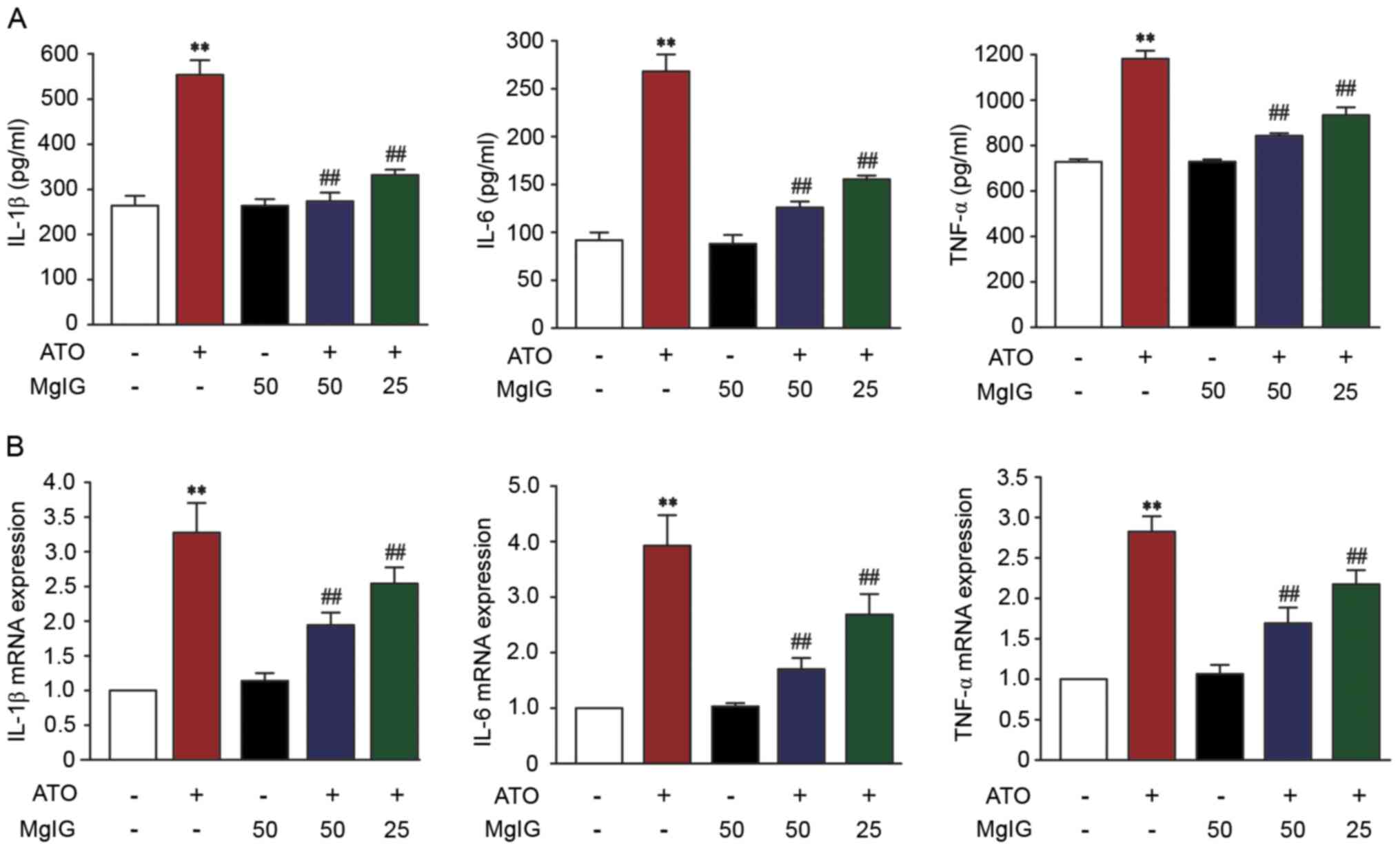
Effect of MgIG on IL-1β, IL-6 and
TNF-α expression levels
In order to assess the protective effect of MgIG,
the levels of IL-1 β, IL-6, and TNF-α were measured. Levels of IL-1
β, IL-6, and TNF-α in the ATO group were significantly higher than
those in the CON group (P<0.01; Fig.
4A). Treatment with MgIG (25 or 50 mg/kg) inhibited IL-1β, IL-6
and TNF-α expression (P<0.01). These results revealed that MgIG
attenuated IL-1β, IL-6 and TNF-α expression levels.
mRNA expression levels of proinflammatory cytokines,
such as IL-6, IL-1β and TNF-α, significantly increased in the liver
of ATO mice compared with the CON group (P<0.01; Fig. 4B). Compared with the ATO group, the
levels of IL-6, IL-1β and TNF-α in H-MgIG and L-MgIG groups was
significantly decreased (P<0.01).
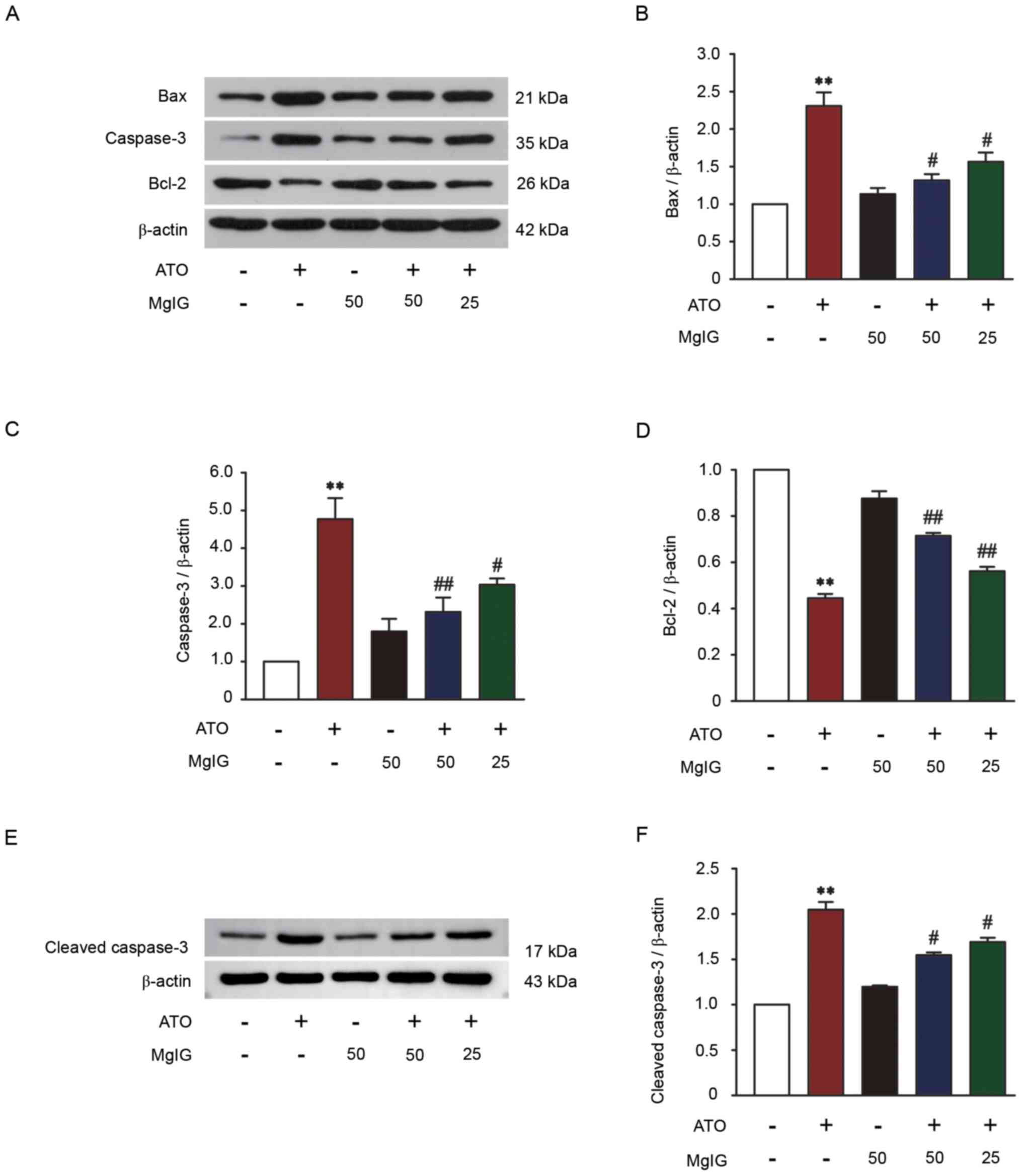
Effect of MgIG on Bax, Bcl-2,
caspase-3 and cleaved-caspase-3 expression levels
Western blotting was performed on liver tissue to
detect the expression levels of Bax, Bcl-2, caspase-3 and
cleaved-caspase-3 to assess the effect of MgIG against ATO-induced
hepatotoxicity. Apoptosis induced by ATO was significantly
increased compared with the CON group (P<0.01; Fig. 5). Compared with the CON group,
caspase-3, cleaved-caspase-3 and Bax expression levels were
increased and Bcl-2 expression was significantly decreased in the
ATO group (P<0.01). Compared with the ATO group, treatment with
MgIG (25 or 50 mg/kg) inhibited Bax, caspase-3 and
cleaved-caspase-3 expression and increased Bcl-2 expression
(P<0.05 or P<0.01). These results revealed that MgIG could
reduce apoptosis in ATO-induced liver injury.
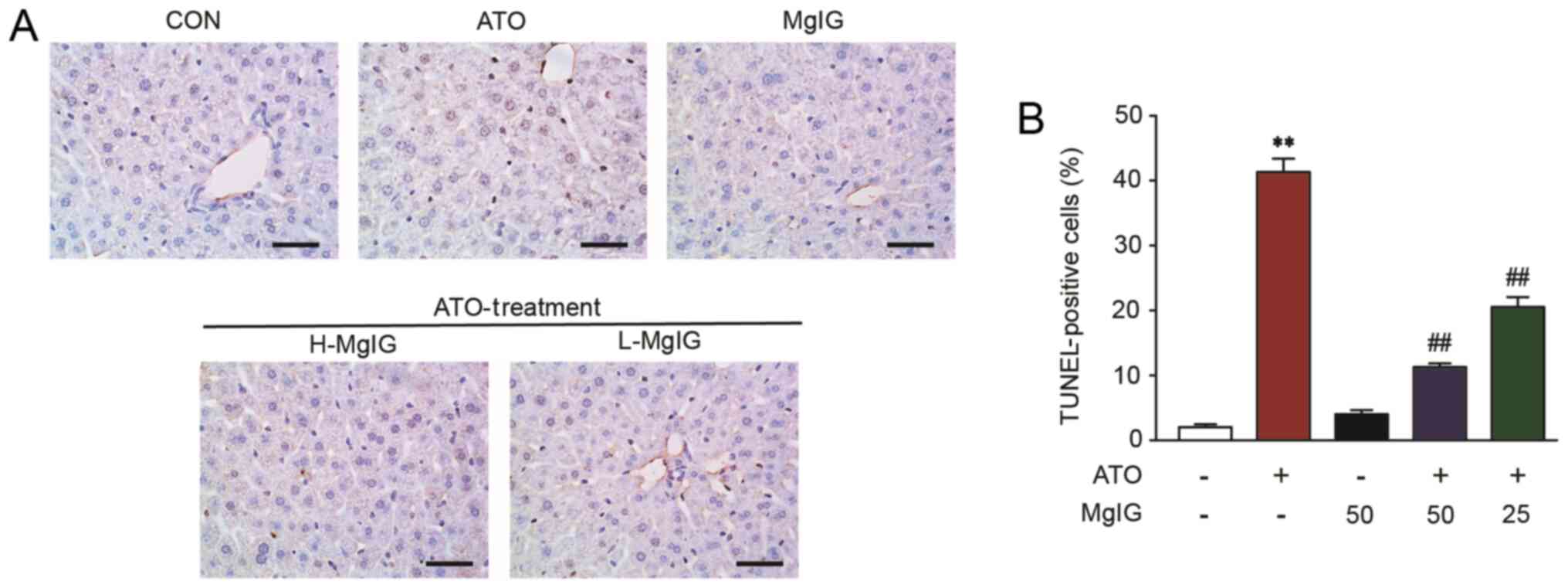
Effect of MgIG on TUNEL staining
Apoptosis in hepatic cells remained at consistently
low levels in the CON group (Fig.
6). However, TUNEL-positive hepatic cells showed a significant
increase in the ATO group (P<0.01). Both H-MgIG and L-MgIG
inhibited this effect (P<0.01).
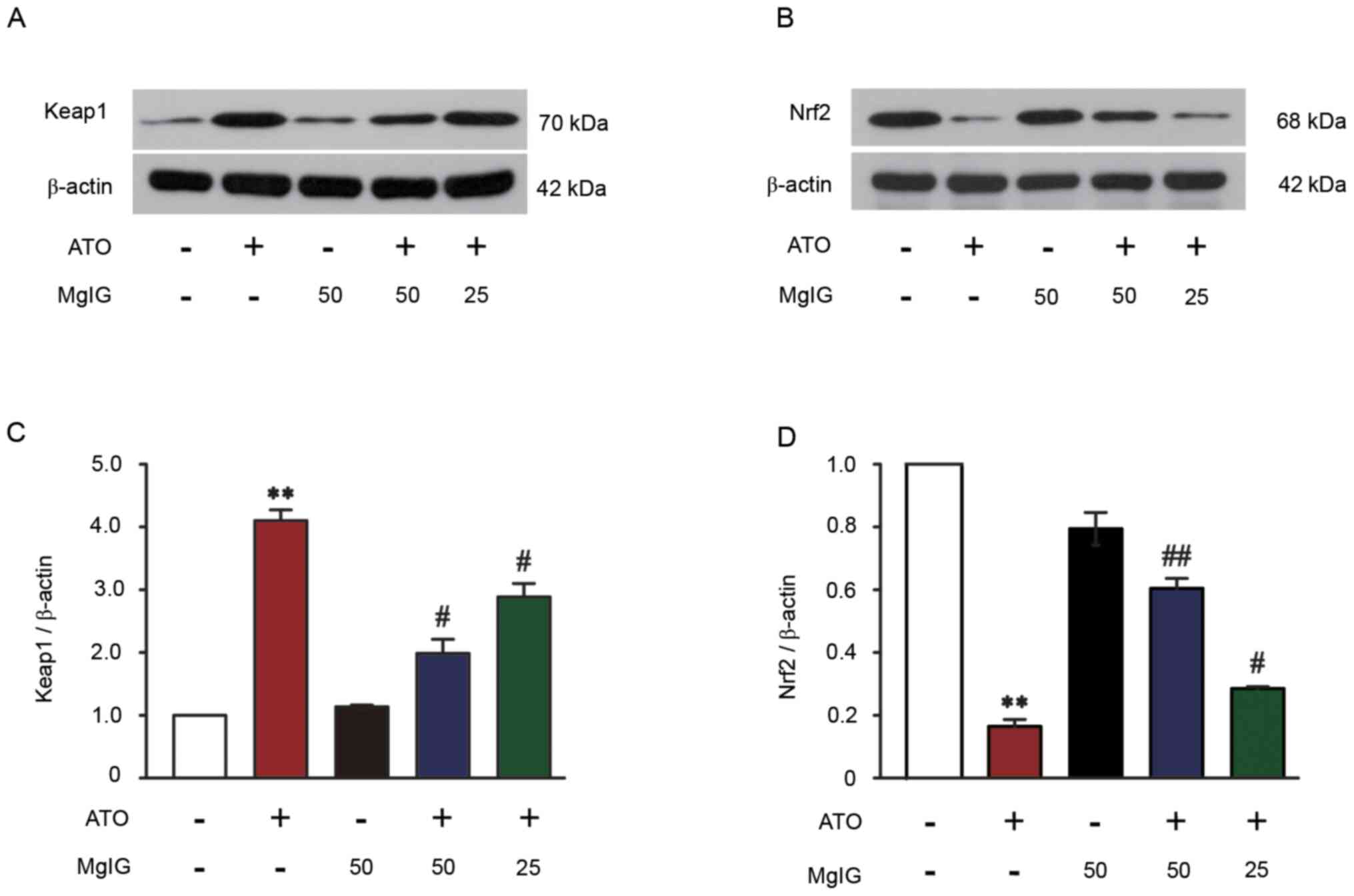
Effect of MgIG on Keap1-Nrf2
expression
The protein expression of Keap1 in ATO-induced mice
significantly increased compared with the CON group (P<0.01;
Fig. 7A and C). Treatment with MgIG
(25 or 50 mg/kg) significantly decreased expression levels of Keap1
in the liver of ATO-induced mice (P<0.05). ATO decreased Nrf2
expression compared with the CON group (P<0.01; Fig. 7B and D), but there were no
significant changes in the MgIG-alone group compared with the CON
group. Nrf2 expression was increased following treatment with 25 or
50 mg/kg MgIG, which suggested that Nrf2 expression was upregulated
(P<0.05 or P<0.01).
Discussion
Current cancer treatment methods include
chemotherapy, surgery and radiotherapy. Traditional Chinese
medicine and its components also show strong anti-tumor effects
(52). ATO is a primary component
in traditional Chinese medicine that is effective in the treatment
of both newly diagnosed and relapsed patients with APL (53). However, it can also lead to liver
toxicity, which limits its clinical application in cancer treatment
(54,55). Thus, it is important to find a
suitable ATO antidote.
Licorice is an herbal medicine and natural sweetener
that is widely used in China and contains numerous active
ingredients; its extracts exhibit anti-viral, anti-bacterial,
anti-inflammatory, anti-cancer and anti-oxidant activity (56). MgIG is a 4th-generation glycyrrhizic
acid agent extracted from licorice by alkali isomerization
catalysis; it is currently used as a liver-protecting agent in
clinical treatment (57). The
therapeutic effects of MgIG in the treatment of liver disease has
been confirmed in liver disease models, including drug-induced and
immune-mediated liver injury and fatty liver disease (58) The aim of the present study was to
investigate the protective effect and potential mechanism of MgIG
on liver injury induced by ATO.
Hepatotoxicity is caused by increased inflammatory
cytokines, oxidative stress and apoptosis. This is a major drawback
of adverse reactions of various anti-cancer and anti-tubercular
drugs (59). Here, the activity of
hepatic marker enzymes in serum significantly increased following
treatment with ATO, which may be due to leakage of enzymes into the
blood stream (60). MgIG
significantly reverses increased ALT and AST activity in serum,
indicating that MgIG restores ATO-induced liver injury (61). These results are associated with
hepatotoxicity induced by ATO, which was confirmed here by
histopathological examination. The histological sections of the
liver were normal in the CON and MgIG groups, but ATO caused
serious liver disease, including cellular necrosis, inflammatory
cell infiltration, pyknotic nuclei and vacuolated hepatocytes.
Slight hepatocyte swelling and bleeding were observed in the H-MgIG
and L-MgIG groups, suggesting that pretreatment with MgIG
diminished liver injury.
Oxidative stress is a result of the imbalance
between ROS and antioxidants in the body and leads to oxidative
damage of macromolecules; this has been implicated in the
pathogenesis of numerous types of disease, including chronic kidney
disease, atherosclerosis and Alzheimer's disease (62–65).
The production of mitochondrial ROS is increased upon exposure to
xenobiotics, especially ATO, which can overwhelm the antioxidant
defense mechanism and damage cellular ingredients such as DNA,
proteins and lipids (66,67). Mitochondria are the central site for
ROS generation and energy metabolism (68). Thus, uncontrolled overproduction of
ROS can overwhelm the cellular antioxidant capacity and impair the
mitochondria (69). Antioxidants
inhibit oxidative stress via inhibiting production of ROS and
improve the function of mitochondria (70). Therefore, antioxidants are a good
therapeutic strategy for treatment of liver disorders in light of
the key role of oxidative stress in liver disease (71).
The present study showed that exposure to ATO
significantly increased ROS generation in hepatocytes compared with
the CON group. The level of ROS in the ATO was notably attenuated
by MgIG at both high and low doses. Antioxidant enzymes, such as
SOD, CAT and GSH, are second line cellular defenses against
oxidative liver injury (72–74).
Changes in levels of these enzymes is an indirect method to
evaluate the antioxidant-prooxidant condition in ATO (75). Their inactivation leads to further
oxidative damage. In the present study, MDA content increased
gradually upon aggravation of liver damage. In recent years, the
powerful antioxidant and free radical scavenging activity of MgIG
have been extensively reported in in vitro experiments
(76,77). Here, the antioxidant effect of MgIG
was demonstrated using an animal model. There were notably
increased levels of SOD, GSH and CAT following treatment with MgIG,
along with decreased MDA content, which demonstrated that MgIG
alleviated ATO-induced oxidative damage. These data suggested that
MgIG suppressed ATO-induced hepatic ROS overproduction.
Activation of the antioxidant system requires
inhibition of the inflammatory response and cell apoptosis
(78). Inflammatory factors are
activated in response to toxic damage via ATO-induced
hepatotoxicity (79). One of the
marks of the inflammatory response is the production of
pro-inflammatory mediators, which are needed to repair injured
tissue. IL-1β, IL-6 and TNF-α are key pro-inflammatory factors of
the immune response; they primarily serve pro-inflammatory roles
that aggravate further tissue damage (80). Pro-inflammatory cytokines, such as
IL-1β, IL-6 and TNF-α, exhibited enhanced expression in the liver,
which confirmed ATO-induced hepatotoxicity. MgIG inhibited the
expression levels of inflammatory cytokines. Oxidative stress is
positively associated with cell apoptosis (81).
Apoptosis is programmed cell death that leads to
death or morphological changes in the cell to replace older cells
with newer cells. Apoptosis involves a series of active death
processes and is regulated by multiple genes associated with the
pro-apoptotic Bax subfamily and the anti-apoptotic Bcl-2 subfamily
(82,83). Caspase-3 is the ‘effector’ protease
in the apoptosis cascade and is one of the primary executors of
apoptosis (84). One of the
best-known markers of apoptosis is proteolytic cleavage of
pro-caspase-3 into its active form, caspase-3 (85). When cells undergo apoptosis,
caspase-3 is activated to cleaved caspase-3, which promotes
apoptosis (86). Here, ATO caused
excessive ROS production and promoted apoptosis. ATO is cytotoxic
and causes liver injury; it also decreases cell activity and
increases apoptosis (35,87). The increase in expression levels of
Bax in the ATO group, concomitant with a decrease in the expression
levels of Bcl-2, demonstrated apoptotic events in ATO mice;
however, there were significant improvements following treatment
with MgIG. Compared with the ATO-induced hepatotoxicity group, MgIG
notably decreased the expression levels of caspase-3 and cleaved
caspase-3 and the number of TUNEL-positive cells in the liver
tissue. These biological indices suggested that MgIG treatment
ameliorated liver injury induced by ATO.
The exact mechanism of ATO-induced hepatotoxicity
remains unclear, but it has been reported that reactive metabolites
decrease Nrf2 levels, induce ROS production and inhibit an
endogenous antioxidant defense system that results in severe
oxidative stress (88,89). Nrf2 is a key antioxidant
transcription factor that is recognized as a primary
transcriptional regulation pathway involved in the metabolism and
detoxification of toxic substances (90,91).
The mechanism by which ATO inhibits Nrf2 expression may involve
accelerating Nrf2 degradation by promoting Keap1 protein expression
levels (92). Zhao et al
(41) demonstrated that activating
Nrf2 and expression levels of Nrf2-regulated antioxidant enzymes
and detoxification protects HaCaT cells from ATO-induced
cytotoxicity and apoptosis. In the present study, the decrease in
Nrf2 content induced by ATO indicates that excessive oxidative
stress consumes a large amount of Nrf2 or disrupts homeostasis
between Nrf2 production and degradation. This damages the
antioxidant defense system and constantly aggravates injury unless
alleviated. However, MgIG maintained high levels of Nrf2 in the
liver and protected the antioxidative defense system to attenuate
oxidative stress and prevent ATO-induced liver injury.
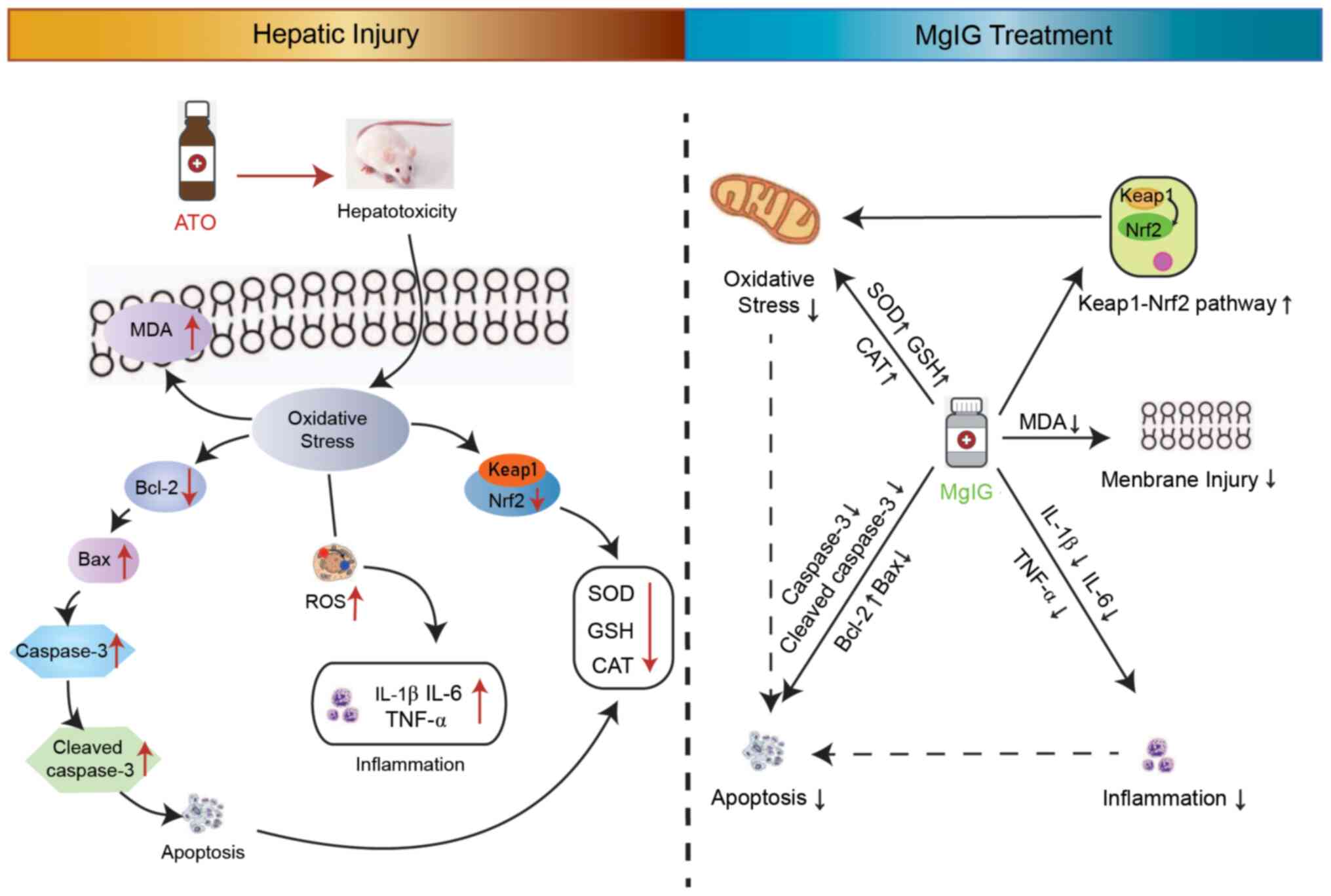
Here, MgIG restrained ATO-mediated hepatotoxicity by
inhibiting oxidative stress, inflammation and apoptosis (Fig. 8). The potential molecular mechanism
of MgIG' hepatoprotection may be due to activation of the
Keap1-Nrf2 signaling pathway. Overall, MgIG exhibited a protective
potential effect on ATO-induced hepatotoxicity. Future experiments
are needed to delineate the specific mechanism involved in this
protective function.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Research
Foundation of Administration of Traditional Chinese Medicine of
Hebei Province, China (grant no. 2020188), Research Foundation of
Hebei University of Chinese Medicine (grant no. KTZ2019041) and the
open projects of Hebei Key Laboratory of Integrative Medicine on
Liver-kidney Patterns (grant no. B 201907).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XH, LC and JS contributed to the design of the
experiments. ML, BZ, YaL, JZ and CD performed experiments and
obtained the data. YiL, XC and PL analyzed the data. ML and PL
wrote the manuscript. ML, LC and JZ revised the manuscript. ML and
XH confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All operating procedures regarding experimental
animals were approved by the Ethics Committee for Animal
Experiments of Hebei University of Chinese Medicine (approval no.
DWLL2020005).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Centeno JA, Mullick FG, Martinez L, Page
NP, Gibb H, Longfellow D, Thompson C and Ladich ER: Pathology
related to chronic arsenic exposure. Environ Health Persp. 110
(Suppl 5):S883–S886. 2002. View Article : Google Scholar
|
|
2
|
Emadi A and Gore SD: Arsenic trioxide-An
old drug rediscovered. Blood Rev. 24:191–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gore SD, Gojo I, Sekeres MA, Morris L,
Devetten M, Jamieson K, Redner RL, Arceci R, Owoeye I, Dauses T, et
al: Single cycle of arsenic trioxide-based consolidation
chemotherapy spares anthracycline exposure in the primary
management of acute promyelocytic leukemia. J Clin Oncol.
28:1047–1053. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Antman KH: Introduction: The history of
arsenic trioxide in cancer therapy. Oncologist. 6 (Suppl 2):S1–S2.
2001. View Article : Google Scholar
|
|
5
|
Abaza Y, Kantarjian H, Garcia-Manero G,
Estey E, Borthakur G, Jabbour E, Faderl S, O'Brien S, Wierda W,
Pierce S, et al: Long-term outcome of acute promyelocytic leukemia
treated with all trans-retinoic acid, arsenic trioxide, and
gemtuzumab. Blood. 129:1275–1283. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Breccia M and Lo-Coco F: Arsenic trioxide
for management of acute promyelocytic leukemia: Current evidence on
its role in front-line therapy and recurrent disease. Expert Opin
Pharmacother. 13:1031–1043. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iland HJ, Bradstock K, Supple SG, Catalano
A, Collins M, Hertzberg M, Browett P, Grigg A, Firkin F, Hugman A,
et al: All-trans-retinoic acid, idarubicin, and IV arsenic trioxide
as initial therapy in acute promyelocytic leukemia (APML4). Blood.
120:1570–1580; quiz 1752. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lo-Coco F, Avvisati G, Vignetti M, Thiede
C, Orlando SM, Iacobelli S, Ferrara F, Fazi P, Cicconi L, Di Bona
E, et al: Retinoic acid and arsenic trioxide for acute
promyelocytic leukemia. N Engl J Med. 369:111–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang X, Jia S, Yang S and Yang Y, Yang T
and Yang Y: Arsenic trioxide induces G2/M arrest in hepatocellular
carcinoma cells by increasing the tumor suppressor PTEN expression.
J Cell Biochem. 113:3528–3535. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Walker AM, Stevens JJ, Ndebele K and
Tchounwou PB: Evaluation of arsenic trioxide potential for lung
cancer treatment: Assessment of apoptotic mechanisms and oxidative
damage. J Cancer Sci Ther. 8:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Messarah M, Klibet F, Boumendjel A,
Abdennour C, Bouzerna N, Boulakoud MS and El Feki A:
Hepatoprotective role and antioxidant capacity of selenium on
arsenic-induced liver injury in rats. Exp Toxicol Pathol.
64:167–174. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miller WH Jr, Schipper HM, Lee JS, Singer
J and Waxman S: Mechanisms of action of arsenic trioxide. Cancer
Res. 62:3893–3903. 2002.PubMed/NCBI
|
|
13
|
Jin W, Xue Y, Xue Y, Han X, Song Q, Zhang
J, Li Z, Cheng J, Guan S, Sun S and Chu L: Tannic acid ameliorates
arsenic trioxide-induced nephrotoxicity, contribution of NF-κB and
Nrf2 pathways. Biomed Pharmacother. 126:1100472020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Liang Y, Zheng B, Chu L, Ma D, Wang
H, Chu X and Zhang J: Protective effects of crocetin on arsenic
trioxide-induced hepatic injury: Involvement of suppression in
oxidative stress and inflammation through activation of Nrf2
signaling pathway in rats. Drug Des Dev Ther. 14:1921–1931. 2020.
View Article : Google Scholar
|
|
15
|
Li M, Liu P, Xue Y, Liang Y, Shi J, Han X,
Zhang J, Chu X and Chu L: Tannic acid attenuates hepatic oxidative
stress, apoptosis and inflammation by activating the Keap1Nrf2/ARE
signaling pathway in arsenic trioxide-toxicated rats. Oncol Rep.
44:2306–2316. 2020.PubMed/NCBI
|
|
16
|
Benramdane L, Accominotti M, Fanton L,
Malicier D and Vallon JJ: Arsenic speciation in human organs
following fatal arsenic trioxide poisoning-a case report. Clin
Chem. 45:301–306. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu M, Rui D, Yan Y, Xu S, Niu Q, Feng G,
Wang Y, Li S and Jing M: Oxidative damage induced by arsenic in
mice or rats: A systematic review and meta-analysis. Biol Trace
Elem Res. 176:154–175. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Valko M, Morris H and Cronin MT: Metals,
toxicity and oxidative stress. Curr Med Chem. 12:1161–1208. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alam MF, Khan G, Safhi MM, Alshahrani S,
Siddiqui R, Sivagurunathan Moni S and Anwer T: Thymoquinone
ameliorates doxorubicin-induced cardiotoxicity in swiss albino mice
by modulating oxidative damage and cellular inflammation. Cardiol
Res Pract. 2018:14830412018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim SY, Park C, Jang HJ, Kim BO, Bae HW,
Chung IY, Kim ES and Cho YH: Antibacterial strategies inspired by
the oxidative stress and response networks. J Microbiol.
57:203–212. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sies H and Jones DP: Reactive oxygen
species (ROS) as pleiotropic physiological signalling agents. Nat
Rev Mol Cell Biol. 21:363–383. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mortezaee K and Khanlarkhani N: Melatonin
application in targeting oxidative-induced liver injuries: A
review. J Cell Physiol. 233:4015–4032. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grossini E, Bellofatto K, Farruggio S,
Sigaudo L, Marotta P, Raina G, De Giuli V, Mary D, Pollesello P,
Minisini R, et al: Levosimendan inhibits peroxidation in
hepatocytes by modulating apoptosis/autophagy interplay. PLoS One.
10:e01247422015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weiskirchen R and Tacke F: Relevance of
autophagy in parenchymal and non-parenchymal liver cells for health
and disease. Cells. 8:162019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barrera C, Valenzuela R, Rincon MA,
Espinosa A, Echeverria F, Romero N, Gonzalez-Mañan D and Videla LA:
Molecular mechanisms related to the hepatoprotective effects of
antioxidant-rich extra virgin olive oil supplementation in rats
subjected to short-term iron administration. Free Radic Biol Med.
126:313–321. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chayapong J, Madhyastha H, Madhyastha R,
Nurrahmah QI, Nakajima Y, Choijookhuu N, Hishikawa Y and Maruyama
M: Arsenic trioxide induces ROS activity and DNA damage, leading to
G0/G1 extension in skin fibroblasts through
the ATM-ATR-associated Chk pathway. Environ Sci Pollut Res Int.
24:5316–5325. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nili-Ahmadabadi A, Alibolandi P, Ranjbar
A, Mousavi L, Nili-Ahmadabadi H, Larki-Harchegani A,
Ahmadimoghaddam D and Omidifar N: Thymoquinone attenuates
hepatotoxicity and oxidative damage caused by diazinon: An in vivo
study. Res Pharm Sci. 13:500–508. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu JP, Zhao XP, Ma XZ, Wang Y and Zheng
LJ: Effects of cigarette smoke on aerobic capacity and serum MDA
content and SOD activity of animal. Int J Clin Exp Med.
7:4461–4465. 2014.PubMed/NCBI
|
|
29
|
Shafik NM and El Batsh MM: Protective
effects of combined selenium and punica granatum treatment on some
inflammatory and oxidative stress markers in arsenic-induced
hepatotoxicity in rats. Bio Trace Elem Res. 169:121–128. 2016.
View Article : Google Scholar
|
|
30
|
Sharanek A, Burban A, Ciriacim N and
Guillouzo A: Pro-inflammatory cytokines enhance dilatation of bile
canaliculi caused by cholestatic antibiotics. Toxicol In Vitro.
58:51–59. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tu C, Gao D, Li XF, Li CY, Li RS, Zhao YL,
Li N, Jia GL, Pang JY, Cui HR, et al: Inflammatory stress
potentiates emodin-induced liver injury in rats. Front Pharmacol.
6:2332015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang J, Zhang Y, Wang W, Li C and Zhang
Z: Double-sided personality: Effects of arsenic trioxide on
inflammation. Inflammation. 41:1128–1134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dugo EB, Yedjou CG, Stevens JJ and
Tchounwou PB: Therapeutic potential of arsenic trioxide (ATO) in
treatment of hepatocellular carcinoma: Role of oxidative stress in
ATO-induced apoptosis. Ann Clin Pathol. 5:11012017.PubMed/NCBI
|
|
34
|
Abouzied MM, Eltahir HM, Abdel Aziz MA,
Ahmed NS, Abd El-Ghany AA, Abd El-Aziz EA and Abd El-Aziz HO:
Curcumin ameliorate DENA-induced HCC via modulating TGF-beta, AKT,
and caspase-3 expression in experimental rat model. Tumor Bio.
36:1763–1771. 2015. View Article : Google Scholar
|
|
35
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Carthy CM, Yanagawa B, Luo H, Granville
DJ, Yang D, Cheung P, Cheung C, Esfandiarei M, Rudin CM, Thompson
CB, et al: Bcl-2 and Bcl-xL overexpression inhibits cytochrome c
release, activation of multiple caspases, and virus release
following coxsackievirus B3 infection. Virology. 313:147–157. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miltonprabu S, Sumedha NC and Senthilraja
P: Diallyl trisulfide, a garlic polysulfide protects against
As-induced renal oxidative nephrotoxicity, apoptosis and
inflammation in rats by activating the Nrf2/ARE signaling pathway.
Int Immunopharmacol. 50:107–120. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen X, Xue H, Fang W, Chen K, Chen S,
Yang W, Shen T, Chen X, Zhang P and Ling W: Adropin protects
against liver injury in nonalcoholic steatohepatitis via the Nrf2
mediated antioxidant capacity. Redox Biol. 21:1010682019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mitsuishi Y, Motohashi H and Yamamoto M:
The Keap1-Nrf2 system in cancers: Stress response and anabolic
metabolism. Front Oncol. 2:2002012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sabouny R, Fraunberger E, Geoffrion M, Ng
AC, Baird SD, Screaton RA, Milne R, McBride HM and Shutt TE: The
Keap1-Nrf2 stress response pathway promotes mitochondrial
hyperfusion through degradation of the mitochondrial fission
protein drp1. Antioxid Redox Sign. 27:1447–1459. 2017. View Article : Google Scholar
|
|
41
|
Zhao R, Yang B, Wang L, Xue P, Deng B,
Zhang G, Jiang S, Zhang M, Liu M, Pi J and Guan D: Curcumin
protects human keratinocytes against inorganic arsenite-induced
acute cytotoxicity through an NRF2-dependent mechanism. Oxid Med
Cell Longev. 2013:4125762013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu Z, Zhang Y, Song T, Song Q, Zhang Y,
Zhang X, Han X, Zhang J and Chu L: Magnesium isoglycyrrhizinate
ameliorates doxorubicin-induced acute cardiac and hepatic toxicity
via anti-oxidant and anti-apoptotic mechanisms in mice. Exp Ther
Med. 15:1005–1012. 2018.PubMed/NCBI
|
|
43
|
Zhang JC, Zheng GF, Wu MX, Wu JW, Ouyang
LY and Liu XQ: Effect of magnesium isoglycyrrhizinate on PLA2
during liver tissue injury following limb ischemia/reperfusion in
rats. Zhonghua Gan Zang Bing Za Zhi. 20:537–541. 2012.(In Chinese).
PubMed/NCBI
|
|
44
|
Sun L, Shen J, Pang X, Lu L, Mao Y and
Zeng M: Phase I safety and pharmacokinetic study of magnesium
isoglycyrrhizinate after single and multiple intravenous doses in
chinese healthy volunteers. J Clin Pharmacol. 47:767–773. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ma D, Zhang J, Zhang Y, Zhang X, Han X,
Song T, Zhang Y and Chu L: Inhibition of myocardial hypertrophy by
magnesium isoglycyrrhizinate through the TLR4/NF-κB signaling
pathway in mice. Int Immunopharmacol. 55:237–244. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xue Y, Li M, Xue Y, Jin W, Han X, Zhang J,
Chu X, Li Z and Chu L: Mechanisms underlying the protective effect
of tannic acid against arsenic trioxideinduced cardiotoxicity in
rats: Potential involvement of mitochondrial apoptosis. Mol Med
Rep. 22:4663–4674. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jiang W, Chen Q, Li P, Lu Q, Pei X, Sun Y,
Wang G and Hao K: Magnesium Isoglycyrrhizinate attenuates
lipopolysaccharide-induced depressive-like behavior in mice. Biomed
Pharmacother. 86:177–184. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Birari LA, Mahajan UB, Patil KR, Patil DD,
Bagul NA, Belemkar S, Goyal SN, Ojha S and Patil CR: Aloin protects
against arsenic trioxide-induced myocardial membrane damage and
release of inflammatory cytokines. Naunyn Schmiedebergs Arch
Pharmacol. 393:1365–1372. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Knuckles TL, Buntz JG, Paffett M, Channell
M, Harmon M, Cherng T, Lucas SN, McDonald JD, Kanagy NL and Campen
MJ: Formation of vascular S-nitrosothiols and plasma
nitrates/nitrites following inhalation of diesel emissions. J
Toxicol Environ Health A. 74:828–837. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Thapa BR and Walia A: Liver function tests
and their interpretation. Indian J Pediatr. 74:663–671. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expres-sion data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hede K: Chinese folk treatment reveals
power of arsenic to treat cancer, new studies under way. J Natl
Cancer Inst. 99:667–668. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Qu W, Cheng L, Dill AL, Saavedra JE, Hong
SY, Keefer LK and Waalkes MP: Nitric oxide donor, V-PROLI/NO,
provides protection against arsenical induced toxicity in rat liver
cells: Requirement for Cyp1a1. Chem Biol Interact. 193:88–96. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mathews V, Desire S, George B, Lakshmi KM,
Rao JG, Viswabandya A, Bajel A, Srivastava VM, Srivastava A and
Chandy M: Hepatotoxicity profile of single agent arsenic trioxide
in the treatment of newly diagnosed acute promyelocytic leukemia,
its impact on clinical outcome and the effect of genetic
polymorphisms on the incidence of hepatotoxicity. Leukemia.
20:881–883. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ducas RA, Seftel MD, Ducas J and Seifer C:
Monomorphic ventricular tachycardia caused by arsenic trioxide
therapy for acute promyelocytic leukaemia. J R Coll Physicians
Edinb. 41:117–118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang ZF, Liu J, Yang YG and Zhu HL: A
review: The anti-inflammatory, anticancer and antibacterial
properties of four kinds of licorice flavonoids isolated from
licorice. Curr Med Chem. 27:1997–2011. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen KJ, Chen WY, Chen X, Jia YM, Peng GQ
and Chen L: Increased elimination of paclitaxel by magnesium
isoglycyrrhizinate in epithelial ovarian cancer patients treated
with paclitaxel plus cisplatin: A pilot clinical study. Eur J Drug
Metab Pharmacokinet. 39:25–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lv J, Xiao Q, Chen Y, Fan X, Liu X, Liu F,
Luo G, Zhang B and Wang S: Effects of magnesium isoglycyrrhizinate
on AST, ALT, and serum levels of Th1 cytokines in patients with
allo-HSCT. Int Immunopharmacol. 46:56–61. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cetin A, Kaynar L, Kocyigit I, Hacioglu
SK, Saraymen R, Ozturk A, Sari I and Sagdic O: Role of grape seed
extract on methotrexate induced oxidative stress in rat liver. Am J
Chin Med. 36:861–872. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kim WR, Flamm SL, Di Bisceglie AM and
Bodenheimer HC; Public Policy Committee of the American association
for the study of liver disease, : Serum activity of alanine
aminotransferase (ALT) as an indicator of health and disease.
Hepatology. 47:1363–1370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yang RZ, Park S, Reagan WJ, Goldstein R,
Zhong S, Lawton M, Rajamohan F, Qian K, Liu L and Gong DW: Alanine
aminotransferase isoenzymes: Molecular cloning and quantitative
analysis of tissue expression in rats and serum elevation in liver
toxicity. Hepatology. 49:598–607. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sifuentes-Franco S, Pacheco-Moises FP,
Rodriguez-Carrizalez AD and Miranda-Diaz AG: The role of oxidative
stress, mitochondrial function, and autophagy in diabetic
polyneuropathy. J Diabetes Res. 2017:16730812017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Daenen K, Andries A, Mekahli D, Van
Schepdael A, Jouret F and Bammens B: Oxidative stress in chronic
kidney disease. Pediatr Nephrol. 34:975–991. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Förstermann U, Xia N and Li H: Roles of
vascular oxidative stress and nitric oxide in the pathogenesis of
atherosclerosis. Circ Res. 120:713–735. 2017. View Article : Google Scholar
|
|
65
|
Butterfield DA and Halliwell B: Oxidative
stress, dysfunctional glucose metabolism and Alzheimer disease. Nat
Rev Neurosci. 20:148–160. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
He JL, Dong XH, Li ZH, Wang XY, Fu ZA and
Shen N: Pterostilbene inhibits reactive oxygen species production
and apoptosis in primary spinal cord neurons by activating
autophagy via the mechanistic target of rapamycin signaling
pathway. Mol Med Rep. 17:4406–4414. 2018.PubMed/NCBI
|
|
67
|
Haga N, Fujita N and Tsuruo T: Involvement
of mitochondrial aggregation in arsenic trioxide (As2O3)-induced
apoptosis in human glioblastoma cells. Cancer Sci. 96:825–833.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hosseini MJ, Shaki F, Ghazi-Khansari M and
Pourahmad J: Toxicity of copper on isolated liver mitochondria:
Impairment at complexes I, II, and IV leads to increased ROS
production. Cell Biochem Biophys. 70:367–381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ortiz M, Soto-Alarcon SA, Orellana P,
Espinosa A, Campos C, López-Arana S, Rincón MA, Illesca P,
Valenzuela R and Videla LA: Suppression of high-fat diet-induced
obesity-associated liver mitochondrial dysfunction by
docosahexaenoic acid and hydroxytyrosol co-administration. Dig
Liver Dis. 52:895–904. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hernandez-Rodas MC, Valenzuela R,
Echeverria F, Rincón-Cervera MÁ, Espinosa A, Illesca P, Muñoz P,
Corbari A, Romero N, Gonzalez-Mañan D and Videla LA:
Supplementation with docosahexaenoic acid and extra virgin olive
oil prevents liver steatosis induced by a high-fat diet in mice
through PPAR-α and Nrf2 upregulation with concomitant SREBP-1c and
NF-kB downregulation. Mol Nutr Food Res. 61:2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kim SH and Kim H: Inhibitory effect of
astaxanthin on oxidative stress-induced mitochondrial dysfunction-A
mini-review. Nutrients. 10:11372018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Santos C, Pires Mdos A, Santos D and
Payan-Carreira R: Distribution of superoxide dismutase 1 and
glutathione peroxidase 1 in the cyclic canine endometrium.
Theriogenology. 86:738–748. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Durak I, Yurtarslanl Z, Canbolat O and
Akyol O: A methodological approach to superoxide dismutase (SOD)
activity assay based on inhibition of nitroblue tetrazolium (NBT)
reduction. Clin Chim Acta. 214:103–104. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mates JM: Effects of antioxidant enzymes
in the molecular control of reactive oxygen species toxicology.
Toxicology. 153:83–104. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Shen H, Niu Q, Xu M, Rui D, Xu S, Feng G,
Ding Y, Li S and Jing M: Factors affecting arsenic methylation in
arsenic-exposed humans: A systematic review and meta-analysis. Int
J Environ Res Public Health. 13:2052016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Xie C, Li X, Zhu J, Wu J, Geng S and Zhong
C: Magnesium isoglycyrrhizinate suppresses LPS-induced inflammation
and oxidative stress through inhibiting NF-κB and MAPK pathways in
RAW264.7 cells. Bioorg Med Chem. 27:516–524. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yang YZ, Liu ZH, Wang SC, Zhang XQ, Xu HJ,
Yang L and Kong LD: Magnesium isoglycyrrhizinate alleviates
fructose-induced liver oxidative stress and inflammatory injury
through suppressing NOXs. Eur J Pharmacol. 883:1733142020.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zheng Y, Tao S, Lian F, Chau BT, Chen J,
Sun G, Fang D, Lantz RC and Zhang DD: Sulforaphane prevents
pulmonary damage in response to inhaled arsenic by activating the
Nrf2-defense response. Toxicol Appl Pharm. 265:292–299. 2012.
View Article : Google Scholar
|
|
79
|
Islam LN, Nabi AH, Rahman MM and Zahid MS:
Association of respiratory complications and elevated serum
immunoglobulins with drinking water arsenic toxicity in human. J
Environ Sci Health A Tox Hazard Subst Environ Eng. 42:1807–1814.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhang JX, Xing JG, Wang LL, Jiang HL, Guo
SL and Liu R: Luteolin inhibits fibrillary
β-amyloid1-40-induced inflammation in a human
blood-brain barrier model by suppressing the p38 MAPK-Mediated
NF-κB signaling pathways. Molecules. 22:3342017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bai J and Meng Z: Effects of sulfur
dioxide on apoptosis-related gene expressions in lungs from rats.
Regul Toxicol Pharmacol. 43:272–279. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Robertson JD and Orrenius S: Molecular
mechanisms of apoptosis induced by cytotoxic chemicals. Crit Rev
Toxicol. 30:609–627. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yang LJ and Wang WL: Preparation of
monoclonal antibody against apoptosis-associated antigens of
hepatoma cells by subtractive immunization. World J Gastroenterol.
8:808–814. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Van Opdenbosch N and Lamkanfi M: Caspases
in cell death, inflammation, and disease. Immunity. 50:1352–1364.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Rotschafer SE, Allen-Sharpley MR and
Cramer KS: Axonal cleaved caspase-3 regulates axon targeting and
morphogenesis in the developing auditory brainstem. Front Neural
Circuits. 10:842016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Bernard A, Chevrier S, Beltjens F, Dosset
M, Viltard E, Lagrange A, Derangère V, Oudot A, Ghiringhelli F,
Collin B, et al: Cleaved caspase-3 transcriptionally regulates
angiogenesis-promoting chemotherapy resistance. Cancer Res.
79:5958–5970. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Han B, Zhou G, Zhang Q, Zhang J, Wang X,
Tang W and Kakudo K: Effect of arsenic trioxide (ATO) on human lung
carcinoma PG cell line: ATO induced apoptosis of PG cells and
decreased expression of Bcl-2, Pgp. J Exp Ther Oncol. 4:335–342.
2004.PubMed/NCBI
|
|
88
|
Yao P, Nussler A, Liu L, Hao L, Song F,
Schirmeier A and Nussler N: Quercetin protects human hepatocytes
from ethanol-derived oxidative stress by inducing heme oxygenase-1
via the MAPK/Nrf2 pathways. J Hepatol. 47:253–261. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wu KC, Liu J and Klaassen CD: Role of Nrf2
in preventing ethanol-induced oxidative stress and lipid
accumulation. Toxicol Appl Pharm. 262:321–329. 2012. View Article : Google Scholar
|
|
90
|
Lee CS, Ho DV and Chan JY: Nuclear
factor-erythroid 2-related factor 1 regulates expression of
proteasome genes in hepatocytes and protects against endoplasmic
reticulum stress and steatosis in mice. FEBS J. 280:3609–3620.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lee JM, Li J, Johnson DA, Stein TD, Kraft
AD, Calkins MJ, Jakel RJ and Johnson JA: Nrf2, a multi-organ
protector? FASEB J. 19:1061–1066. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Copple IM, Goldring CE, Kitteringham NR
and Park BK: The keap1-nrf2 cellular defense pathway: Mechanisms of
regulation and role in protection against drug-induced toxicity.
Handb Exp Pharmacol. 233–266. 2010. View Article : Google Scholar : PubMed/NCBI
|