Introduction
Low back pain (LBP) is a common public health
problem; ~80% of the global population experiences LBP, which
represents a significant economic and societal burden (1,2).
Intervertebral disc degeneration (IDD) is a leading cause of LBP
and is characterized by progressive degradation of extracellular
matrix (ECM) (3). Current therapies
mainly focus on alleviating the clinical symptoms of patients with
IDD, and there are substantial barriers to targeting the potential
pathological changes in the disc. Therefore, a deeper understanding
of the pathogenesis of IDD may help to develop strategies for
improving clinical outcomes.
MicroRNAs (miRNAs/miRs) are small non-coding RNAs
with a length of 18–22 nucleotides (4). The biological function of miRNAs has
been reported in various diseases in recent years, including IDD
(5,6). For example, miR-139 has been reported
to promote the development of osteoarthritis by facilitating
apoptosis of chondrocytes (7).
miR-133 was shown to suppress cell proliferation and promote
apoptosis in lupus nephritis (8),
and it has been suggested that overexpression of miR-154 may
contribute to the degradation of ECM in IDD (9). Moreover, miR-654-5p has been reported
to be involved in several diseases, such as ovarian cancer, gastric
cancer and oral squamous cell carcinoma (10–12).
In addition, a recent study revealed that miR-654-5p is
significantly upregulated in NP cells from patients with IDD
(9). However, the specific role of
miR-654-5p in IDD pathology remains to be further investigated.
miRNAs participate in the development of diverse
diseases by targeting specific genes (13). For example, miR-128 has been
reported to targes PPARγ to promote the progression of Alzheimer's
disease (14). In addition, miR-373
has been reported to aggravate renal fibrosis by targeting Sirtuin
1 and regulating NF-κB/MMP-9 signaling (15). miR-129-5p inhibition may contribute
to IDD development by accelerating NP cell apoptosis by targeting
BMP2 (16). Moreover, increasing
evidence has indicated that miR-654-5p is involved in multiple
diseases in a similar manner. For example, miR-654-5p has been
shown to target epithelial stromal interaction 1 to suppress cell
growth and invasion in breast cancer development (17). miR-654-5p may promote cell
proliferation and migration by modulating the GRAP/Ras/MAPK
signaling pathway in oral squamous cell carcinoma (12). However, to the best of our
knowledge, the mechanism underlying the effects of miR-654-5p on
IDD development has not yet been revealed.
The present study aimed to investigate the key role
of miR-654-5p in IDD development. The results revealed that
miR-654-5p promoted ECM degradation by inhibiting autophagy via
suppression of autophagy-related gene 7 (ATG7) and activation of
the PI3K/AKT/mTOR signaling pathway. These findings may provide
information that aids in identifying novel therapeutic targets for
IDD.
Materials and methods
Sample collection
A total of 76 NP tissue samples were obtained from
patients with IDD who underwent discectomy and 20 healthy control
samples were donated by patients with lumbar vertebral fracture who
underwent anterior discectomy in Zhongda Hospital of Southeast
University between September 2016 and December 2019. Ages of the 76
patients with IDD (males 45; females 31) were between 31–53 years,
and the mean age was 42.3 years. Ages of the 20 control
participants (males 13; females 7) were between 24–45 years, and
the mean age was 33.6 years. Participants who had IDD were excluded
from the control group. The grade of IDD was estimated by
T2-weighted images following the Pfirrmann classification system
(18). The present study was
approved by the Ethics Committee of The Affiliated Zhongda Hospital
of Southeast University (Nanjing, China; approval no.
2015ZDKYSB014) and followed the guidelines of the Declaration of
Helsinki (19). All patients
provided written informed consent prior to the operation.
Isolation and culture of degenerated
NP cells
After washing twice with PBS, the degenerated NP
specimens from patients with IDD were cut into small fragments (1
mm3). Subsequently, the fragments were treated with
0.25% trypsin solution (Sigma-Aldrich; Merck KGaA) for 30 min at
37°C and centrifuged at 1,000 × g for 3 min at 4°C. After
centrifugation, the supernatant was discarded, and precipitates
were stimulated with 0.2% type II collagenase (Sigma-Aldrich; Merck
KGaA) at 37°C for 4 h. After filtration through a 0.45-µm nylon
filter membrane, the suspension was collected and the NP cells were
resuspended in DMEM/F12 supplemented with 15% FBS (both Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in an atmosphere containing
5% CO2. After digestion with 0.25% trypsin solution, the
cells were incubated. The culture medium was changed three times a
week, and the second-generation cells were collected and used in
subsequent experiments.
Cell transfection and treatment
Isolated NP cells were seeded into 6-well plates at
a density of 1×105 cells/well before transfection and 2
ml complete tissue culture medium was added to each well.
Subsequently, small interfering RNA (siRNA) targeting ATG7
(si-ATG7) was designed and synthesized by Shanghai GenePharma Co.,
Ltd., and was used to knockdown ATG7. The target sequence for
si-ATG7 was 5′-GGAGTCACAGCTCTTCCTT-3′. Scrambled siRNA-negative
control (si-NC; Shanghai GenePharma Co., Ltd.) served as the NC for
si-ATG7. The sequence for si-NC was 5′-GCACTGAGTAGCTCCTCTT-3′.
miR-654-5p mimics and NC mimics, miR-654-5p inhibitor and NC
inhibitor, and pcDNA3.1-ATG7 vector for overexpression of ATG7 and
its negative control (empty pcDNA3.1 vector) were obtained from
Shanghai GenePharma Co., Ltd. The sequence for miR-654-5p mimics
was 5′-UGGUGGGCCGCAGAACAUGUGC-3′, and that for scrambled NC mimics
was 5′-GAGUAGCCGUGGCUGCUAAGCG-3′. The sequence for miR-654-5p
inhibitor was 5′-GCACAUGUUCUGCGGCCCACCA-3′, and that for scrambled
NC inhibitor was 5′-CGGUCUCGACACACUCGAUCGC-3′.
miR-654-5p mimics (10 nM) and NC mimics (10 nM),
miR-654-5p inhibitor (10 nM), NC inhibitor (10 nM), si-ATG7 (20 nM)
and si-NC (20 nM) were transfected into NP cells (1×105)
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.) at 37°C for 48 h. The time between transfection and
subsequent experimentation was 48 h. Subsequently, transfected
cells were cultured in DMEM/F12 containing 10% FBS. Moreover, to
interfere with autophagy, cells were treated with rapamycin (Rap;
10 nM; autophagy activator; Sigma-Aldrich; Merck KGaA) or
3-methyladenine (3-MA; 10 mM; autophagy inhibitor; Sigma-Aldrich;
Merck KGaA) at 37°C for 6 h before transfection.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from NP tissues or cells
using TRIzol® (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. The RNA
concentration was measured using a NanoDrop ND-1000
spectrophotometer (NanoDrop; Thermo Fisher Scientific, Inc.) and
agarose gel electrophoresis was used to determine RNA integrity.
Subsequently, RT was conducted using a miRcute miRNA RT kit
(Tiangen Biotech Co., Ltd.) or a RevertAid RT kit (Thermo Fisher
Scientific). The RT reaction conditions were as follows: 37°C for
60 min, followed by 85°C for 5 min and 4°C. All qPCR amplification
reactions were performed in triplicate using the 7500 Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.) with a
final volume of 20 µl 2X SYBR Green mix (Thermo Fisher Scientific).
The thermocycling conditions were as follows: Initial denaturation
at 95°C for 2 min, followed by 40 cycles of denaturation at 95°C
for 15 sec, annealing at 59°C for 20 sec and elongation at 72°C for
20 sec; and a final extension at 72°C for 10 min. GAPDH served as
an internal control for mRNA analysis and U6 served as an internal
control for miR-654-5p analysis. The relative expression levels
were calculated using the 2−ΔΔCq method (20). Primer sequences used for PCR were
provided in Table I.
 | Table I.Relative primer sequences. |
Table I.
Relative primer sequences.
| Targets | Sequences |
|---|
| miR-654-5p | F:
5′-UGGUGGGCCGCAGAACAUGU-3′ |
|
| R:
5′-CTCTACAGCTATATTGCCAGCCAC-3′ |
| U6 | F:
5′-CTCGCTTCGGCAGCACA-3′ |
|
| R:
5′-AACGCTTCACGAATTTGCGT-3′ |
| Collagen I | F:
5′-AAAGATGGAGAGGCTGGAG-3′ |
|
| R:
5′-ATCACCCTTAGCACCATCG-3′ |
| Collagen II | F:
5′-CAAGGAGACAGAGGAGAAGC-3′ |
|
| R:
5′-CTTGAGGACCCTGGATTCC-3′ |
| SOX9 | F:
5′-CTCTGGAGACTTCTGAACGA-3′ |
|
| R:
5′-ACTTGTAATCCGGGTGGTC-3′ |
| MMP-3 | F:
5′-GGACAAATACTGGAGATTTGATGAG-3′ |
|
| R:
5′-CCCTGGAAAGTCTTCAGCT-3′ |
| MMP-9 | F:
5′-TACTGTGCCTTTGAGTCCG-3′ |
|
| R:
5′-GAATCGCCAGTACTTCCCA-3′ |
| MMP-13 | F:
5′-CTGGGCCAAATTATGGAGGA-3′ |
|
| R:
5′-GAAACAAGTTGTAGCCTTTGGA-3′ |
| ATG3 | F:
5′-TCACAACACAGGTATTACAGGA-3′ |
|
| R:
5′-GCTGAGCAATCTTGAAGCC-3′ |
| ATG5 | F:
5′-GGAAACTCATGGAATATCCTGC-3′ |
|
| R:
5′-GGTCTTTCAGTCGTTGTCTG-3′ |
| ATG7 | F:
5′-GGAGUCACAGCUCUUCCUUdTdT-3′ |
|
| R:
5′-AAGGAAGAGCUGUGACUCCTdTd-3′ |
| ATG16L1 | F:
5′-CCAATCGGCTTAATGCAGAG-3′ |
|
| R:
5′-TCATCATCCTGTTCGACTGG-3′ |
| Beclin 1 | F:
5′-GAACTACAAACGCTGTTTGGA-3′ |
|
| R:
5′-AGCTCCTTTAGCTCCATCTG-3′ |
| ULK1 | F:
5′-CCGAGAGGCTCATCTTCAG-3′ |
|
| R:
5′-CTGGAACATCTCGTCCAGG-3′ |
| GAPDH | F:
5′-GCATCCTGGGCTACACTG-3′ |
|
| R:
5′-TGGTCGTTGAGGGCAAT-3′ |
Luciferase reporter assay
The binding site of miR-654-5p on ATG7 was
identified using starBase (http://starbase.sysu.edu.cn/index.php). A wild-type
(Wt) or mutant (Mut) ATG7 fragment was subcloned into a pmirGLO
vector (Promega Corporation), The mutation gene sequence was
synthesized by Shanghai GenePharma Co., Ltd., and NP cells at a
density of 1×105 cells/well were then cotransfected with
miR-654-5p mimics (10 nM) or NC mimics (10 nM) and Wt or Mut
vectors (1 µg) using Lipofectamine 2000 at 37°C for 48 h. After 24
h, the Dual Luciferase Reporter Assay system (Promega Corporation)
was employed to detect luciferase activity. Relative luciferase
activity was calculated as the ratio of firefly luciferase activity
to Renilla luciferase activity.
RNA immunoprecipitation (RIP)
assay
The RIP assay was performed using an EZ-Magna RIP
Kit (EMD Millipore) according to the manufacturer's instructions.
NP cell lysates were lysed with RIPA buffer (Beyotime Institute of
Biotechnology) for 5 min at 4°C. Antibodies including
anti-Argonaute 2 (anti-Ago2; cat. no. ab186733; 1:50; Abcam) and
anti-Immunoglobulin G (anti-IgG; cat. no. 12-370; 1:100; EMD
Millipore) were incubated with protein A/G magnetic beads (Pierce;
Thermo Fisher Scientific, Inc.) for 1 h at 4°C. Then, cell lysate
was mixed with the beads to incubate for 4 h at 4°C. Beads were
washed twice using PBS buffer (Sangon Biotech Co., Ltd.), and the
mixture was centrifuged at 2,500 × g for 10 min at 4°C. RNA was
purified with 150 µl proteinase K buffer (Roche Diagnostics) and
extracted using TRIzol (Invitrogen; Thermo Fisher Scientific,
Inc.), and the immunoprecipitated RNA was detected via RT-qPCR as
aforementioned.
Western blot analysis
Total proteins were extracted from NP cells with
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology) containing phenylmethylsulfonyl fluoride. A
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology) was used to evaluate protein concentration.
Subsequently, equal amounts (50 µg per lane) of protein were
separated by SDS-PAGE on 10% gels. After electrophoresis, proteins
were transferred onto PVDF membranes (EMD Millipore), which were
blocked with 5% nonfat milk at room temperature for 2 h. Membranes
were then incubated with primary antibodies at 4°C overnight,
followed by further incubation with horseradish
peroxidase-conjugated goat-anti rabbit secondary antibody (cat. no.
ab205718; 1:2,000; Abcam) at room temperature for 1 h. The
following primary antibodies provided by Abcam were used: β-actin
(cat. no. ab8227; 1:1,000), collagen I (cat. no. ab34710; 1:1,000),
collagen II (cat. no. ab188570; 1:1,000), aggrecan (cat. no.
ab36861; 1:2,000), SOX9 (cat. no. ab185966; 1:1,000), MMP-3 (cat.
no. ab53015; 1:500), MMP-9 (cat. no. ab38898; 1:1,000), MMP-13
(cat. no. ab39012; 1:3,000), Beclin-1 (cat. no. ab62557; 1:2,000),
ATG7 (cat. no. ab53255; 1:2,000), ATG12 (cat. no. ab109491;
1:1,000), ATG13 (cat. no. ab105392; 1:2,000), LC3 (cat. no.
ab192890; 1:2,000), phosphorylated (p)-PI3K (cat. no. ab182651;
1:500), p-AKT (cat. no. ab38449; 1:500), p-mTOR (cat. no. ab109268;
1:1,000), PI3K (cat. no. ab191606; 1:1,000), AKT (cat. no.
ab182729; 1:5,000) and mTOR (cat. no. ab134903; 1:10,000). The
protein expression levels were normalized to those of β-actin.
Finally, the bands were visualized using an Enhanced
Chemiluminescence Substrate kit (EMD Millipore) and analyzed using
a Quantity One software (version 4.62; Bio-Rad Laboratories,
Inc.).
Monodansylcadaverine (MDC)
staining
Transfected NP cells at the concentration of
106/ml were exposed to MDC solution (0.1 mM; Cayman
Chemical Company) in 60-mm dishes at 37°C for 1 h. After washing
three times with PBS, cells were fixed with 4% paraformaldehyde at
room temperature for 15 min. To evaluate autophagic vacuoles
(acidic granular vacuoles), cells were observed using a
fluorescence microscope (Olympus Corporation) under an ultraviolet
filter.
Statistical analysis
All experiments were independently conducted three
times. Data are presented as the mean ± standard deviation. All
statistical analyses were conducted using SPSS 22.0 software (IBM
Corp.) and graphs were drawn with GraphPad software (version 8;
GraphPad Software, Inc.). All data were assessed for normal
distribution (Shapiro-Wilk test) and homogeneity of variance
(Bartlett's test). All results were corrected for multiple
comparisons using the false discovery rate method (21). Independent Student's t-test was used
to analyze the differences between two groups. One-way analysis of
variance followed by post hoc Dunnett's test (for comparisons with
one control) and Tukey's test (for comparisons among various
groups) was employed to analyze the differences among more than two
groups. Spearman's correlation analysis was applied to analyze the
correlation between miR-654-5p expression levels and Pfirrmann
scores of patients with IDD. P<0.05 was considered to indicate a
statistically significant difference.
Results
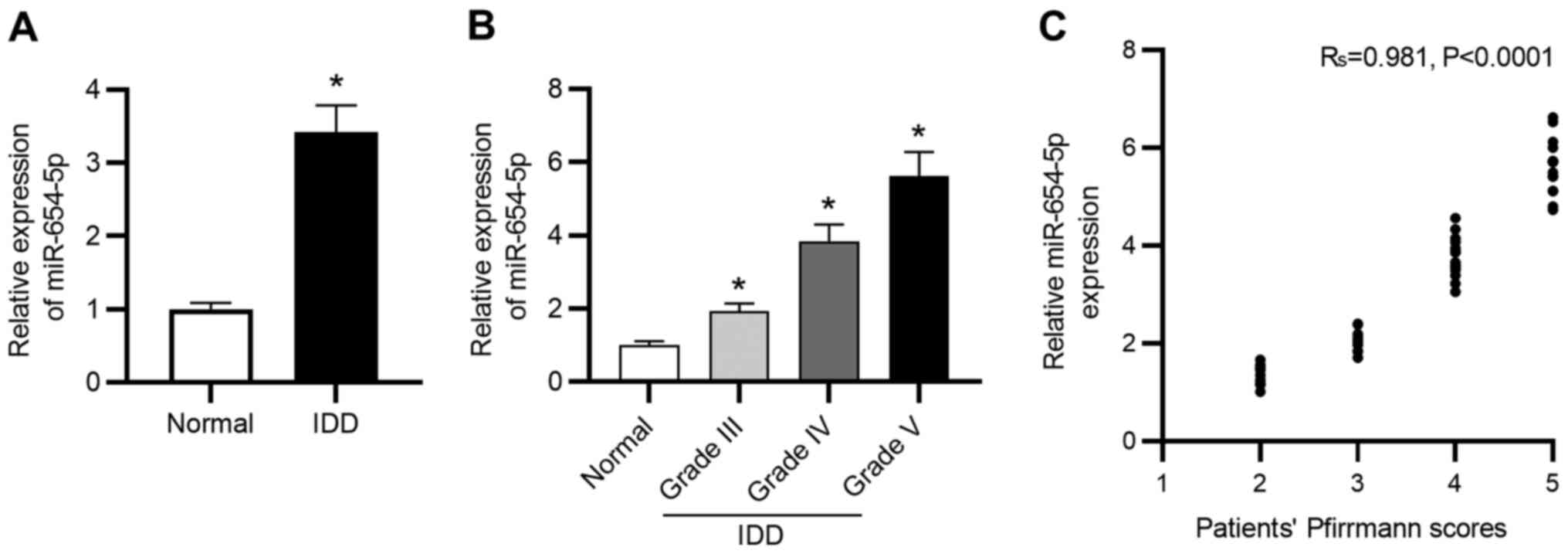
miR-654-5p is upregulated in IDD
To explore the role of miR-654-5p in the progression
of IDD, RT-qPCR was conducted to measure miR-654-5p expression in
NP tissues. As shown in Fig. 1A,
miR-654-5p expression levels were significantly elevated in
degenerated NP tissues compared with those in healthy control
tissues. Moreover, the expression levels of miR-654-5p were
gradually elevated with an increased degree of disc degeneration
(Fig. 1B). In addition, as shown in
Fig. 1C, miR-654-5p expression was
positively correlated with Pfirrmann scores in patients with
IDD.
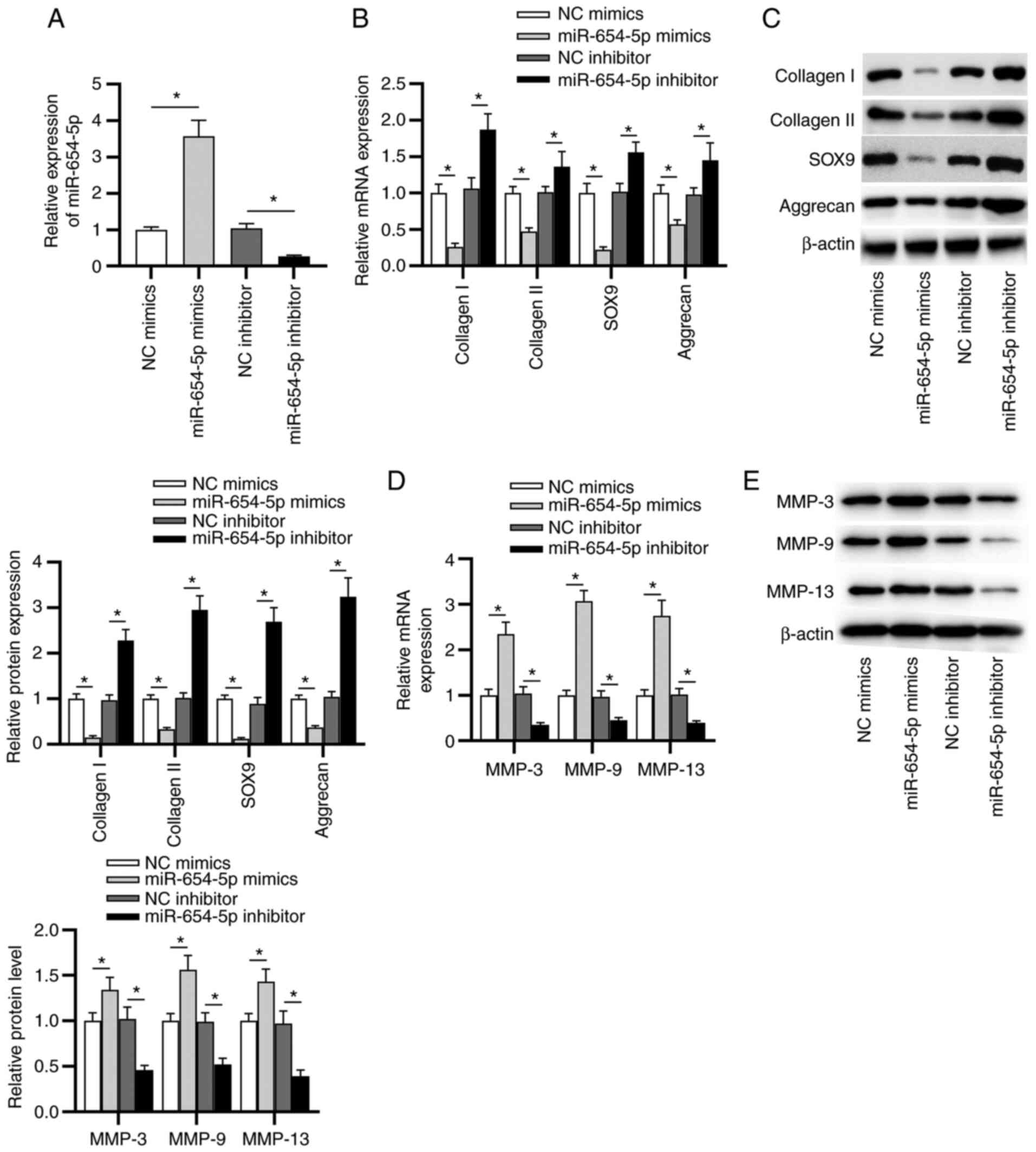
miR-654-5p contributes to ECM
degradation in NP cells
To further investigate the effects of miR-654-5p on
the progression of IDD, the subsequent experiments were performed.
As shown in Fig. 2A, the
transfection efficiency of miR-654-5p mimics or the miR-654-5p
inhibitor was confirmed by RT-qPCR. Next, the expression levels of
type I/II collagen, SOX9 and aggrecan were detected in transfected
NP cells. The results suggested that the mRNA and protein
expression levels of collagen I, collagen II, SOX9 and aggrecan
were significantly reduced by miR-654-5p mimics, and were elevated
by the miR-654-5p inhibitor (Fig. 2B
and C). In addition, the expression levels of MMP-3, MMP-9 and
MMP-13 were detected in transfected NP cells. As shown in Fig. 2D and E, the mRNA and protein
expression levels of MMP-3, MMP-9 and MMP-13 were significantly
increased in NP cells transfected with miR-654-5p mimics, whereas
an opposite trend was revealed in miR-654-5p inhibitor-transfected
NP cells.
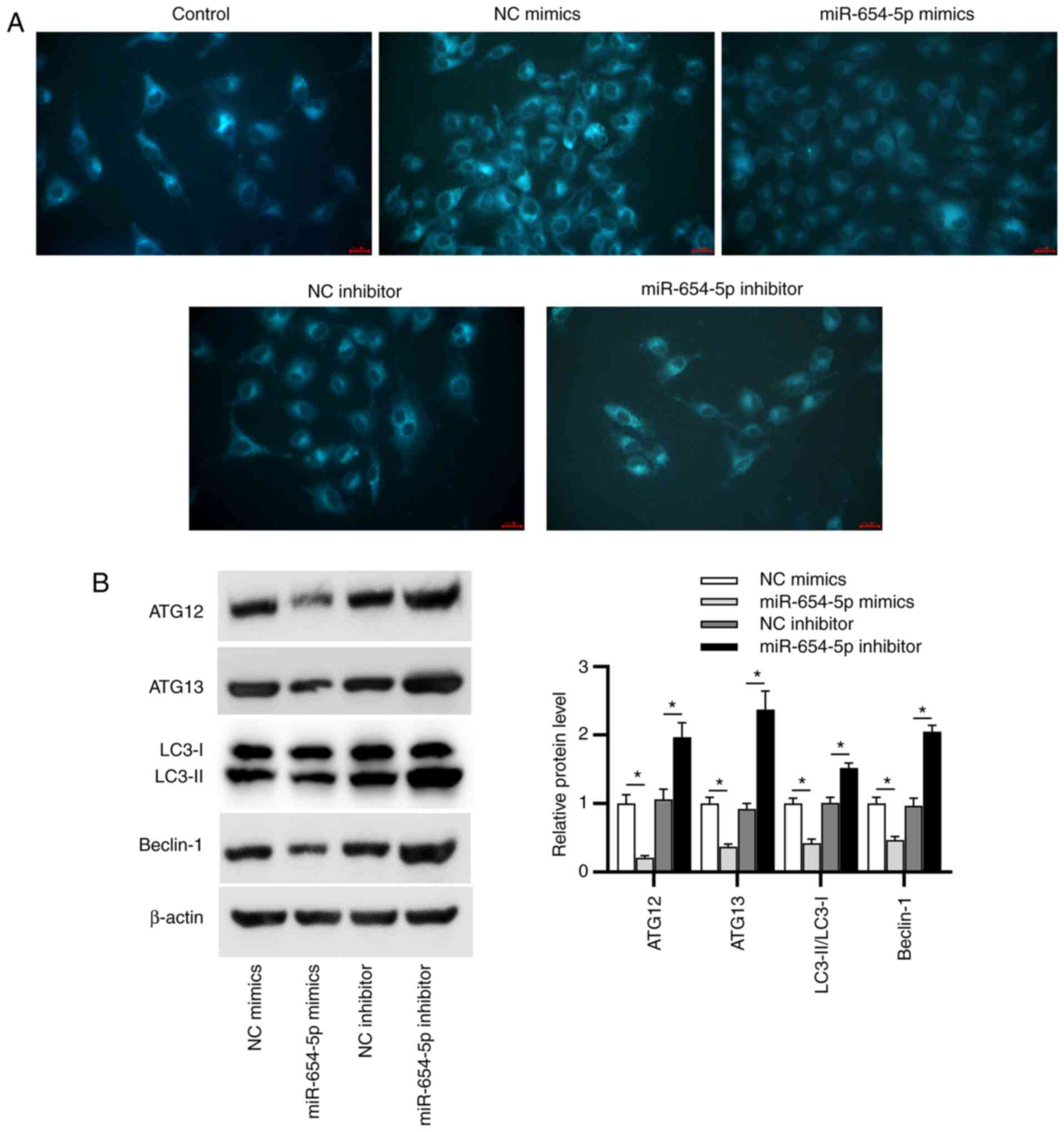
miR-654-5p suppresses autophagy in
IDD
First, MDC staining was employed to detect
autophagic vacuoles. As displayed in Fig. 3A, the accumulation of MDC-labeled
vacuoles in the cytoplasm of NP cells was inhibited by miR-654-5p
mimics and promoted by miR-654-5p inhibitor. Beclin-1, ATG12, ATG13
and LC3 (subtypes: LC3-I and LC3-II) have been widely identified as
autophagy markers; therefore, western blot analysis was performed
to examine the expression levels of these proteins in transfected
NP cells. It was revealed that miR-654-5p mimics reduced the
protein expression levels of Beclin-1, ATG12, ATG13 and the
LC3-II/LC3-I ratio; however, silencing miR-654-5p exerted the
opposite effects (Fig. 3B). These
data indicated that miR-654-5p may inhibit autophagy in IDD.
Autophagy is involved in
miR-654-5p-induced ECM degradation in NP cells
As autophagy is a crucial mechanism regulating ECM
metabolism, it was hypothesized that miR-654-5p may enhance the
degradation of ECM by inhibiting autophagy. To verify this
hypothesis, NP cells were cultured with Rap or 3-MA to activate or
antagonize autophagy, and were then transfected with miR-654-5p
mimics or miR-654-5p inhibitor, respectively. RT-qPCR analysis
revealed that the decreased expression levels of collagen I,
collagen II, SOX9 and aggrecan, and the increased expression levels
of MMP-3, MMP-9 and MMP-13 induced by miR-654-5p mimics
transfection were reversed by Rap pretreatment. Moreover, 3-MA
abrogated the increase in collagen I, collagen II, SOX9 and
aggrecan expression, as well as the decline in MMP-3, MMP-9 and
MMP-13 expression induced by the miR-654-5p inhibitor (Fig. 4A and B). Furthermore, similar
results were obtained by western blot analysis (Fig. 4C and D). Overall, miR-654-5p-induced
ECM degradation may be sustained by the inhibition of autophagy in
IDD.
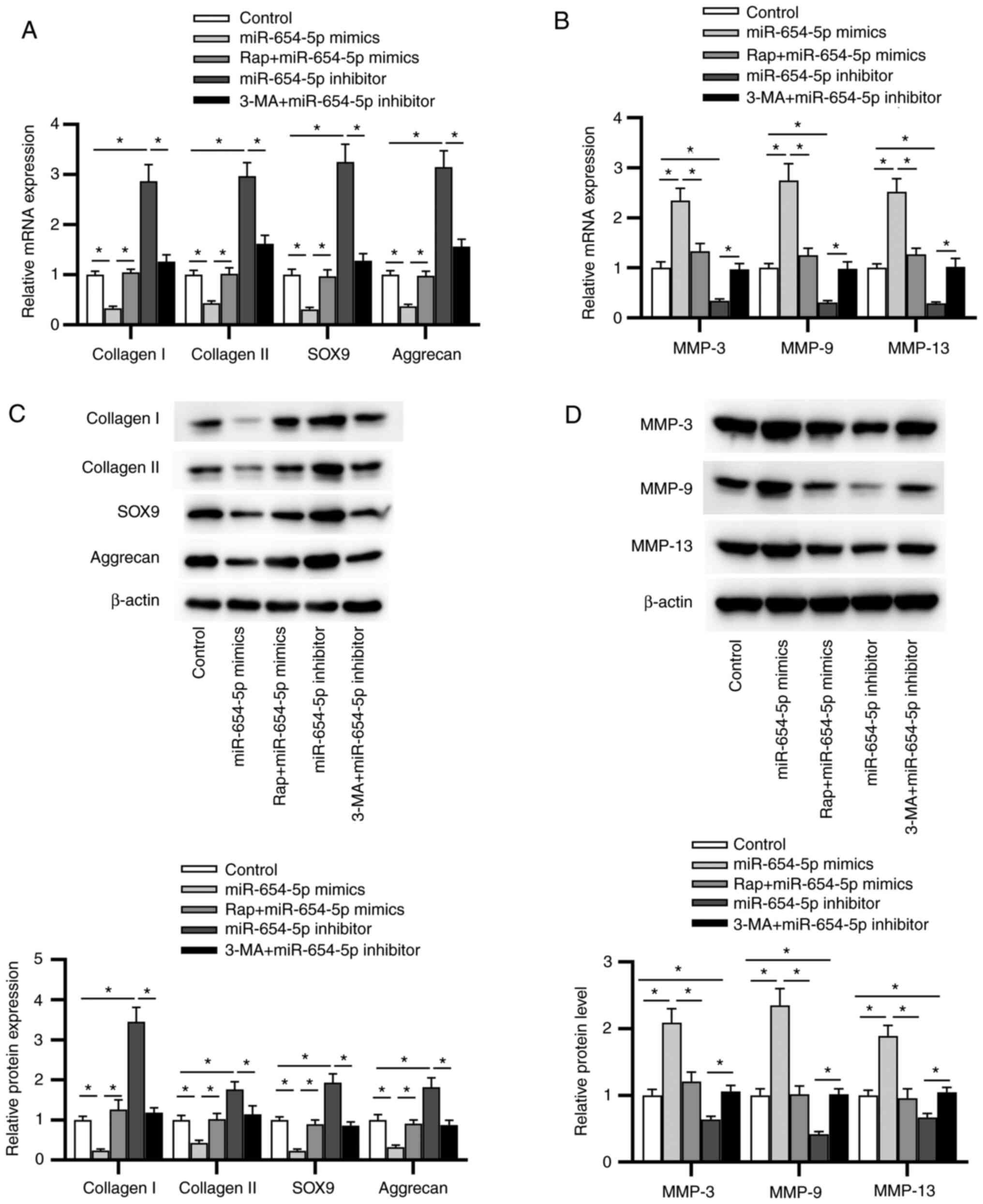 | Figure 4.Autophagy is involved in
miR-654-5p-induced extracellular matrix degradation in NP cells. NP
cells were treated with Rap or 3-MA, followed by transfection with
miR-654-5p mimics or an miR-654-5p inhibitor. mRNA expression of
levels of (A) collagen I, collagen II, SOX9 and aggrecan, and (B)
MMP-3, MMP-9 and MMP-13 were detected by reverse
transcription-quantitative PCR. Western blot analysis was used to
measure the protein expression levels of (C) collagen I, collagen
II, SOX9 and aggrecan, and (D) MMP-3, MMP-9 and MMP-13. *P<0.05.
3-MA, 3-methyladenine; miR-654-5p, microRNA-654-5p; NC, negative
control; NP, nucleus pulposus; Rap, rapamycin. |
ATG7 is a direct downstream target
gene of miR-654-5p
The present study conducted RT-qPCR to assess the
expression of autophagy-related genes in NP cells transfected with
miR-654-5p mimics. The results revealed that the miR-654-5p mimics
significantly reduced the expression levels of ATG7, ATG16L1 and
unc-51 like autophagy activating kinase 1, and ATG7 expression
exhibited the most obvious decline (Fig. 5A). Moreover, ATG7 was markedly
downregulated in NP tissues obtained from patients with IDD
compared with that in tissues obtained from the normal control
group (Fig. 5B). Therefore, ATG7
was selected for further study and it was hypothesized that
miR-654-5p might interact with ATG7 in IDD development. To test
this hypothesis, starBase was used to predict the potential targets
of miR-654-5p and it was revealed that a binding site exists
between miR-654-5p and the ATG7 3′ untranslated region.
Subsequently, a luciferase reporter assay revealed that miR-654-5p
significantly attenuated the luciferase activity of pmirGLO-ATG7-Wt
vectors but not pmirGLO-ATG7-Mut vectors (Fig. 5C). In addition, miR-654-5p and ATG7
were immunoprecipitated by anti-Ago2 antibodies but not anti-IgG
antibodies (Fig. 5D). Furthermore,
miR-654-5p mimics significantly reduced the mRNA and protein
expression levels of ATG7 (Fig.
5E). Collectively, these data indicated that miR-654-5p may
directly target ATG7.
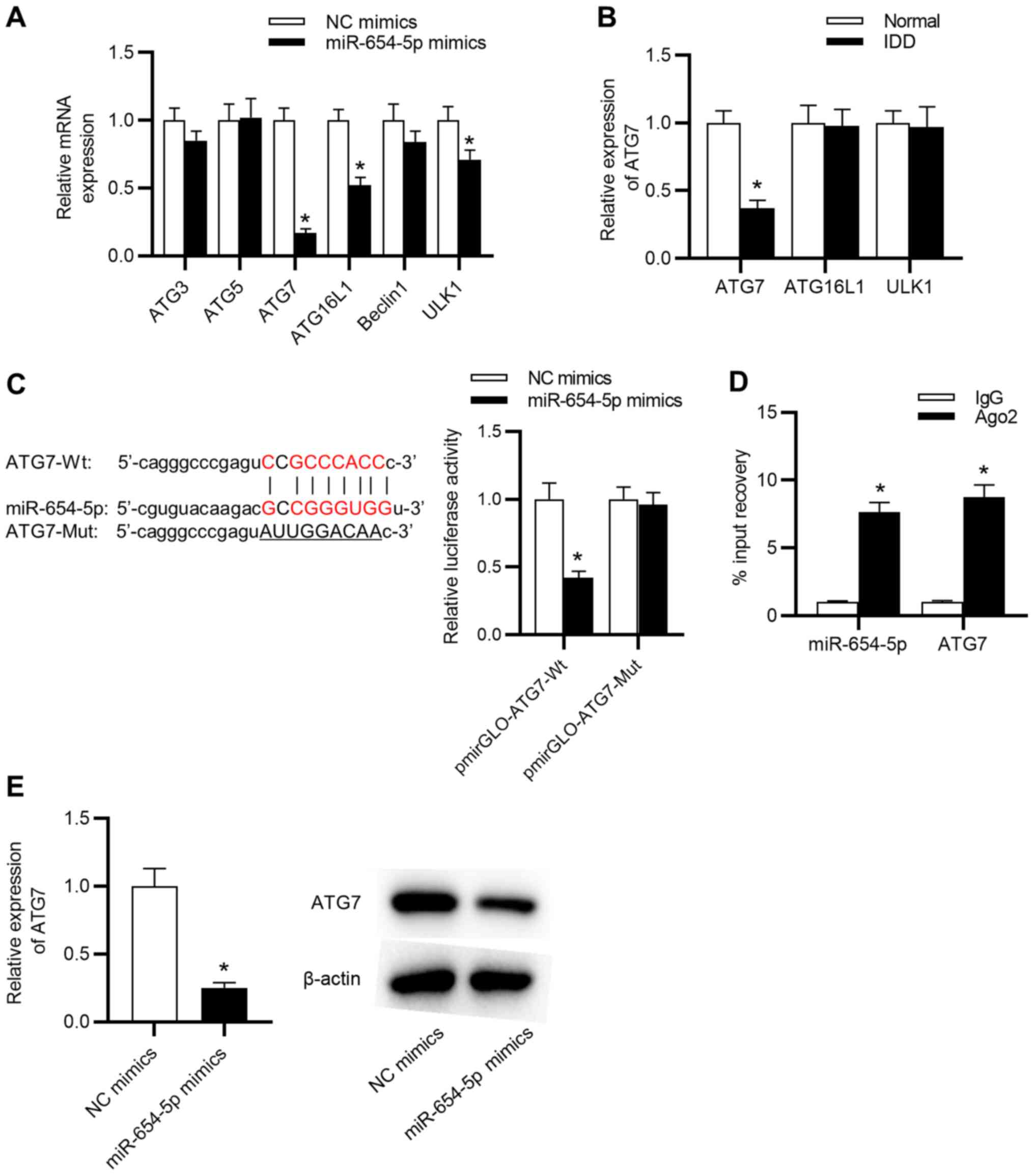 | Figure 5.ATG7 is a direct downstream target
gene of miR-654-5p. (A) mRNA expression levels of autophagy-related
proteins in NP cells transfected with miR-654-5p mimics. *P<0.05
vs. NC mimics. (B) RT-qPCR was carried out to detect the expression
levels of ATG7, ATG16L1 and ULK1. *P<0.05 vs. Normal. (C) A
luciferase reporter assay was performed in NP cells cotransfected
with miR-654-5p mimics and pmirGLO-ATG7-Wt vectors or
pmirGLO-ATG7-Mut vectors. *P<0.05 vs. NC mimics. (D) RNA
immunoprecipitation assays were used to further confirm the
interaction between miR-654-5p and ATG7. *P<0.05 vs. IgG. (E)
mRNA and protein expression levels of ATG7 were detected by RT-qPCR
and western blotting in miR-654-5p mimic-transfected NP cells,
respectively. *P<0.05 vs. NC mimics. ATG, autophagy-related
gene; IDD, intervertebral disc degeneration; miR-654-5p,
microRNA-654-5p; Mut, mutant; NC, negative control; NP, nucleus
pulposus; RT-qPCR, reverse transcription-quantitative PCR; Wt,
wild-type; ULK1, unc-51 like autophagy activating kinase 1. |
miR-654-5p inhibits autophagy by
targeting ATG7
To determine whether miR-654-5p generates its
effects on IDD by targeting ATG7, follow-up rescue assays were
performed. First, the knockdown efficiency of ATG7 was assessed by
RT-qPCR. The results indicated that the mRNA and protein expression
levels of ATG7 were significantly decreased in NP cells transfected
with si-ATG7 (Fig. 6A). Notably,
ATG7 knockdown counteracted the inhibitory effects of the
miR-654-5p inhibitor on ECM degradation, including the reduced
protein expression levels of MMP-3, MMP-9 and MMP-13, as well as
the elevated protein expression levels of collagen I, collagen II,
SOX9 and aggrecan (Fig. 6B and C).
Moreover, knockdown of ATG7 rescued the miR-654-5p
inhibitor-mediated promotion of autophagy (Fig. 6D). In summary, miR-654-5p
facilitated ECM degradation by inhibiting autophagy by modulating
ATG7.
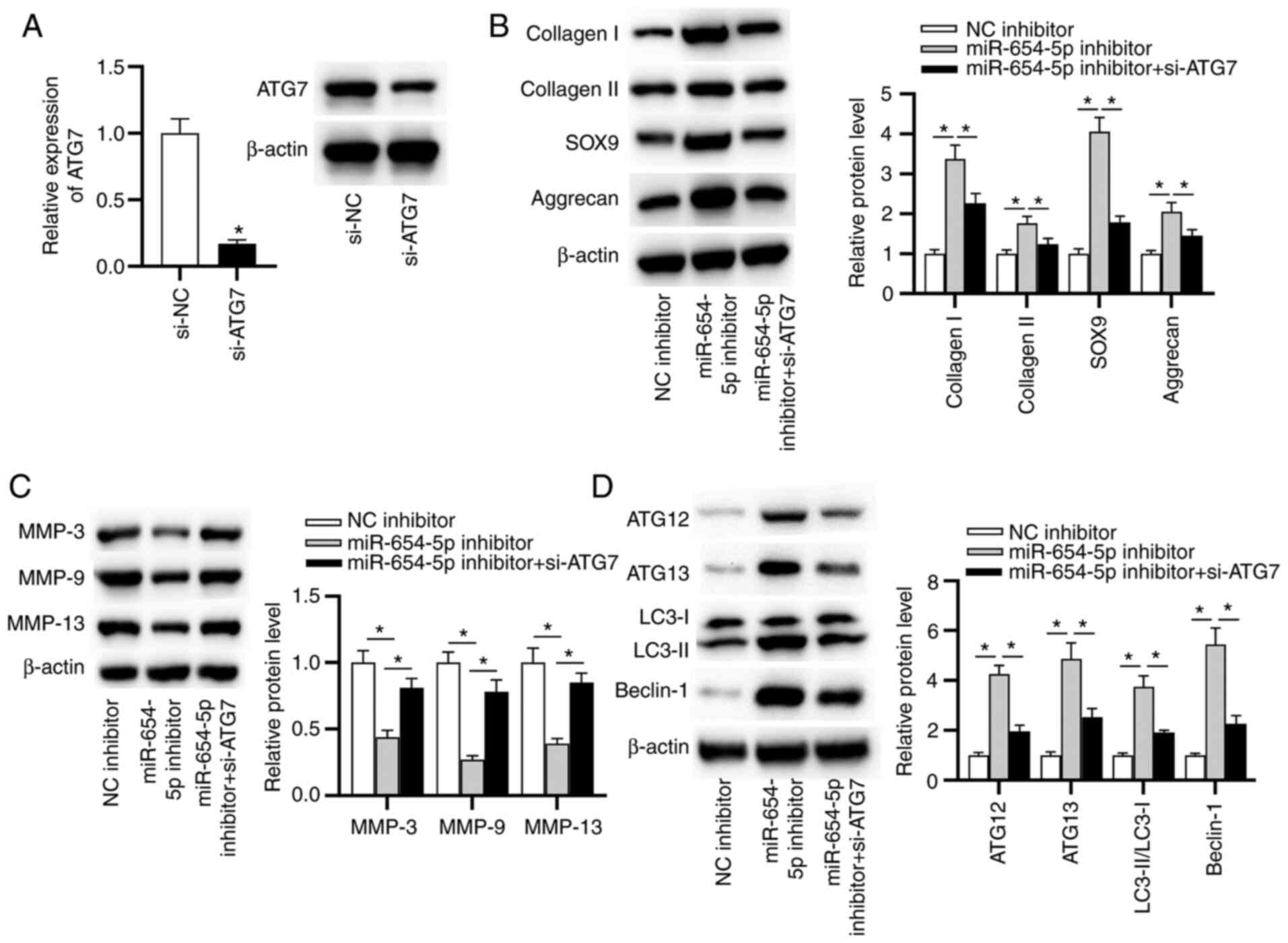 | Figure 6.miR-654-5p inhibits autophagy by
targeting ATG7. (A) Knockdown efficiency of ATG7 was determined by
reverse transcription-quantitative PCR. *P<0.05 vs. si-NC.
Western blot analysis was used to measure the protein expression
levels of (B) collagen I, collagen II, SOX9 and aggrecan, (C)
MMP-3, MMP-9 and MMP-13, and (D) LC3-II/LC3-I, ATG12, ATG13 and
Beclin-1 in transfected nucleus pulposus cells. *P<0.05. ATG,
autophagy-related gene; miR-654-5p, microRNA-654-5p; NC, negative
control; si, small interfering. |
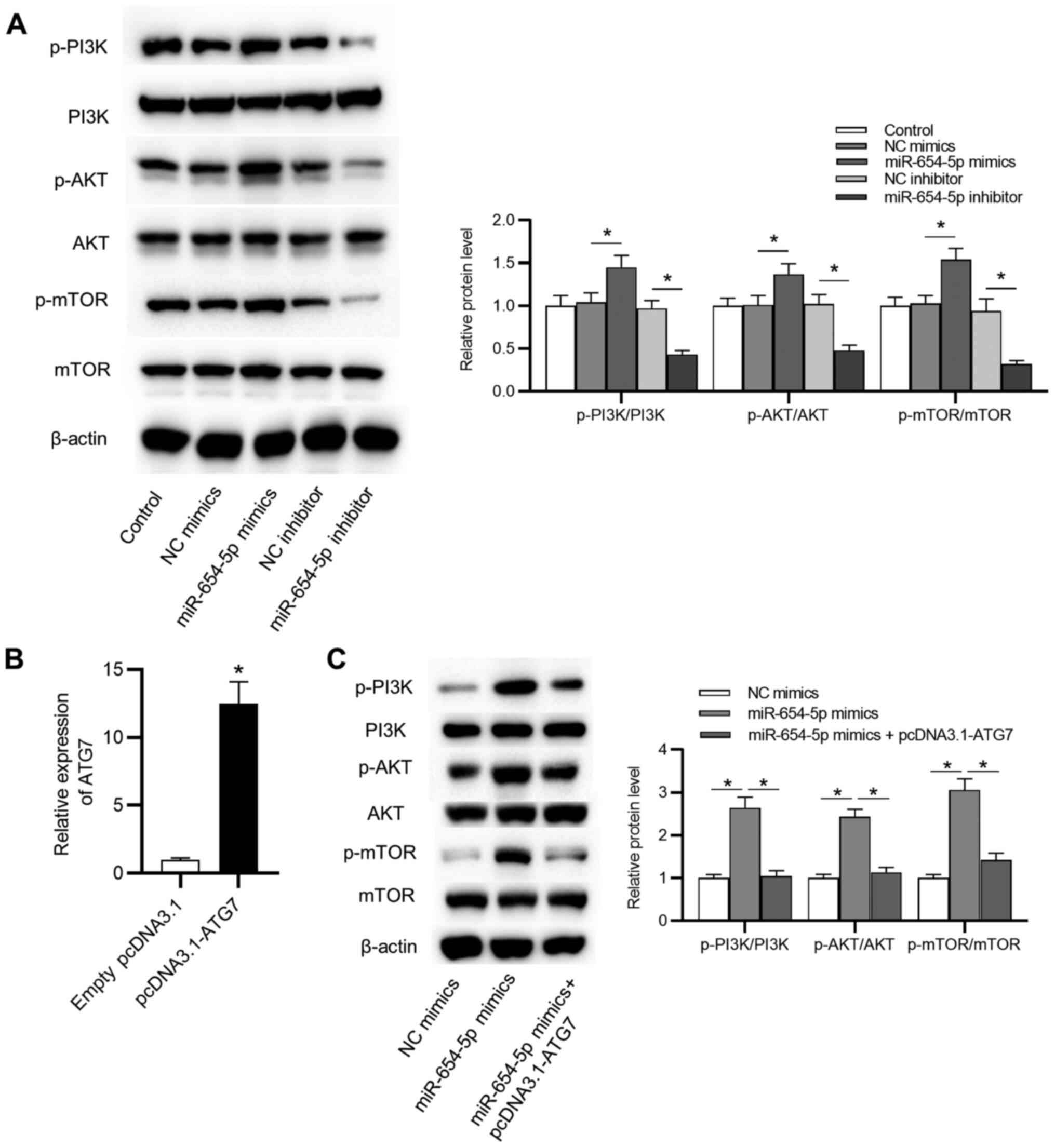
miR-654-5p activates the PI3K/AKT/mTOR
signaling pathway via ATG7 in IDD
As shown in Fig. 7A,
the protein expression levels of p-PI3K, p-AKT and p-mTOR were
significantly elevated by miR-654-5p mimics, and significantly
reduced by the miR-654-5p inhibitor. However, no significant
difference was detected in the total amount of PI3K, AKT and mTOR
protein among the control group and other groups. Moreover,
transfection with a pcDNA3.1-ATG7 vector effectively increased ATG7
expression in NP cells (Fig. 7B).
Upregulation of ATG7 reversed the effects of miR-654-5p mimics on
the P13K/AKT/mTOR signaling pathway (Fig. 7C). These findings suggested that
miR-654-5p may suppress autophagy by activation of the
P13K/AKT/mTOR signaling pathway.
Discussion
IDD is a common cause of LBP (22). It has been demonstrated that the
etiology of IDD involves several complex mechanisms, including
genetic, developmental and biochemical aspects (23). Although IDD therapy has been
improved in recent decades, there is still much work to be done to
achieve effective treatment.
It has been reported that miR-654-5p may have an
important role in several diseases, including ovarian cancer,
gastric cancer and oral squamous cell carcinoma (11,12,24).
In particular, miR-654-5p has been identified to be upregulated in
NP cells from patients with IDD (9). Consistent with a previous study, the
present study revealed that miR-654-5p exhibited high expression
levels in degenerated NP tissues and that elevated miR-654-5p
levels were closely associated with exacerbation of IDD.
Increasing evidence has indicated that early
degenerative changes suggestive of IDD appear in NP cells, and the
main characteristic of IDD is progressive degradation of ECM
macromolecules, of which collagen II and aggrecan show
significantly decreased expression levels (25). MMPs are the main enzymes that
promote the cleavage of collagen II and aggrecan (3). MMP-3, MMP-9 and MMP-13 are members of
the MMP family proteins and are well known to be highly expressed
in IDD (26). Moreover, reduced
MMP-3, MMP-9 and MMP-13 expression can facilitate ECM repair and
disc regeneration (26). Therefore,
a deeper understanding of ECM homeostasis is helpful for developing
novel therapeutic approaches for IDD. The present study revealed
that miR-654-5p contributed to ECM degradation by decreasing
collagen II and aggrecan levels, as well as increasing the
expression levels of MMP-3, MMP-9 and MMP-13 in NP cells.
Autophagy is a homeostatic process that participates
in the degradation and digestion of intracellular components by
lysosomes (27). Compared with that
in healthy controls, the number of autophagosomes has been shown to
be significantly decreased in human degenerative NP cells (28). In addition, the activation of
autophagy has been found to suppress MMP-3 expression in NP cells
treated with TNF-α, suggesting that autophagy may promote disc ECM
anabolism (29,30). Furthermore, previous studies have
reported that miRNAs may function as regulators of autophagy in
various diseases, including IDD (31). For example, inhibition of miR-20
promoted chondrocyte proliferation and autophagy in osteoarthritis
(32). In addition, miR-889
maintained mycobacterial survival by suppressing autophagy in
patients with latent tuberculosis infection (33). miR-21 may contribute to ECM
degradation by inhibiting autophagy via the PTEN/AKT/mTOR signaling
pathway in IDD (34). Similarly,
the present findings indicated that autophagy is involved in
miR-654-5p-induced ECM degradation in IDD.
ATG7, in conjunction with various signaling
pathways, serves an important role in autophagosome formation and
vesicle progression (35).
Recently, a great number of miRNAs have been shown to suppress the
autophagic process by targeting ATG7. For example, miR-96-5p
suppressed autophagy to inhibit hepatic stellate cell activation by
targeting ATG7 (36). Furthermore,
miR-210 inhibited autophagy by targeting ATG7 in IDD (37). Notably, in the present study, ATG7
was found to be directly targeted by miR-654-5p. Moreover, the
present findings revealed that ATG7 knockdown significantly
reversed the effects of the miR-654-5p inhibitor on ECM degradation
and autophagy.
The PI3K/AKT/mTOR signaling pathway has been
reported to play a crucial role in regulating autophagy in various
diseases. For example, miR-129-5p inhibited autophagy by targeting
ATG14 and activated the PI3K/AKT/mTOR signaling pathway in ischemic
heart disease (38). miR-20
suppressed chondrocyte proliferation and autophagy by regulating
the PI3K/AKT/mTOR pathway in osteoarthritis (39). In addition, PTEN has been considered
a negative regulator of the PI3K/AKT/mTOR pathway (40). It has been reported that silencing
ATG7 may promote AKT phosphorylation via the c-JUN/PTEN axis to
promote the AKT pathway (41).
Moreover, direct targets of miR-654-5p are associated with the AKT
pathway in ovarian cancer (11).
The results of the present study indicated that miR-654-5p promoted
the activation of the PI3K/AKT/mTOR signaling pathway by
downregulating ATG7 in NP cells.
In conclusion, the results of the present study
revealed that miR-654-5p may participate in IDD development by
directly targeting ATG7 and promoting activation of the
PI3K/AKT/mTOR signaling pathway. The present study may provide
valuable information for future explorations into the beneficial
effects of targeting miR-654-5p as a potential therapeutic strategy
for IDD. However, the sample size in the present study was limited
and further studies are required to investigate the role of
miR-654-5p in animal models of intervertebral disc NP.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant no. 81572188).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SW designed the study. SW, YG, XZ and CW performed
the experiments. SW and CW wrote the paper. SW and CW contributed
to data analysis. SW, YG, XZ and CW confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The Ethics Committee of The Affiliated Zhongda
Hospital of Southeast University (Nanjing, China) approved the
present study (approval no. 2015ZDKYSB014). All patients provided
written informed consent prior to the operation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Clouet J, Fusellier M, Camus A, Le Visage
C and Guicheux J: Intervertebral disc regeneration: From cell
therapy to the development of novel bioinspired endogenous repair
strategies. Adv Drug Deliv Rev. 146:306–324. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Karran EL, McAuley JH, Traeger AC, Hillier
SL, Grabherr L, Russek LN and Moseley GL: Can screening instruments
accurately determine poor outcome risk in adults with recent onset
low back pain? A systematic review and meta-analysis. BMC Med.
15:132017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang WJ, Yu XH, Wang C, Yang W, He WS,
Zhang SJ, Yan YG and Zhang J: MMPs and ADAMTSs in intervertebral
disc degeneration. Clin Chim Acta. 448:238–246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Evangelatos G, Fragoulis GE, Koulouri V
and Lambrou GI: MicroRNAs in rheumatoid arthritis: From
pathogenesis to clinical impact. Autoimmun Rev. 18:1023912019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beermann J, Piccoli MT, Viereck J and Thum
T: Non-coding RNAs in development and disease: Background,
mechanisms, and therapeutic approaches. Physiol Rev. 96:1297–1325.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou X, Chen L, Grad S, Alini M, Pan H,
Yang D, Zhen W, Li Z, Huang S and Peng S: The roles and
perspectives of microRNAs as biomarkers for intervertebral disc
degeneration. J Tissue Eng Regen Med. 11:3481–3487. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Makki MS and Haqqi TM: MiR-139 modulates
MCPIP1/IL-6 expression and induces apoptosis in human OA
chondrocytes. Exp Mol Med. 47:e1892015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang Z, Pang G, Huang YG and Li C:
MiR-133 inhibits proliferation and promotes apoptosis by targeting
LASP1 in lupus nephritis. Exp Mol Pathol. 114:1043842020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Liu X, Sun B, Du W, Zheng Y and
Sun Y: Upregulated miR-154 promotes ECM degradation in
intervertebral disc degeneration. J Cell Biochem. 2019.(Epub ahead
of print).
|
|
10
|
Zhu W, Li L and Li D: Rs11655237
polymorphism of LINC00673 affects the prognosis of cervical cancer
by interfering with the interaction between LINC00673 and
microRNA-1231. J Cell Physiol. 235:8155–8166. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Majem B, Parrilla A, Jiménez C,
Suárez-Cabrera L, Barber M, Marín A, Castellví J, Tamayo G,
Moreno-Bueno G, Ponce J, et al: MicroRNA-654-5p suppresses ovarian
cancer development impacting on MYC, WNT and AKT pathways.
Oncogene. 38:6035–6050. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu M, Wang C, Chen W, Mao C and Wang J:
MiR-654-5p Targets GRAP to promote proliferation, metastasis, and
chemoresistance of oral squamous cell carcinoma through Ras/MAPK
Signaling. DNA Cell Biol. 37:381–388. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pu M, Chen J, Tao Z, Miao L, Qi X, Wang Y
and Ren J: Regulatory network of miRNA on its target: Coordination
between transcriptional and post-transcriptional regulation of gene
expression. Cell Mol Life Sci. 76:441–451. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Zhang Y, Liu P, Bai H, Li X, Xiao
J, Yuan Q, Geng S, Yin H, Zhang H, et al: MicroRNA-128 knockout
inhibits the development of Alzheimer's disease by targeting PPARγ
in mouse models. Eur J Pharmacol. 843:134–144. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang H, Liao D, Tong L, Zhong L and Wu K:
MiR-373 exacerbates renal injury and fibrosis via
NF-κB/MatrixMetalloproteinase-9 signaling by targeting Sirtuin1.
Genomics. 111:786–792. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang W and Sun P: Downregulation of
microRNA-129-5p increases the risk of intervertebral disc
degeneration by promoting the apoptosis of nucleus pulposus cells
via targeting BMP2. J Cell Biochem. 120:19684–19690. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tan YY, Xu XY, Wang JF, Zhang CW and Zhang
SC: MiR-654-5p attenuates breast cancer progression by targeting
EPSTI1. Am J Cancer Res. 6:522–532. 2016.PubMed/NCBI
|
|
18
|
Pfirrmann CW, Metzdorf A, Zanetti M,
Hodler J and Boos N: Magnetic resonance classification of lumbar
intervertebral disc degeneration. Spine (Phila Pa 1976).
26:1873–1878. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
World Medical Association: World medical
association declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194. 2013.
View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hochberg Y and Benjamini Y: More powerful
procedures for multiple significance testing. Stat Med. 9:811–818.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng C, Liu H, Yang Y, Huang B and Zhou Y:
Growth and differentiation factor-5 contributes to the structural
and functional maintenance of the intervertebral disc. Cell Physiol
Biochem. 35:1–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng C, Liu H, Yang M, Zhang Y, Huang B
and Zhou Y: Disc cell senescence in intervertebral disc
degeneration: Causes and molecular pathways. Cell Cycle.
15:1674–1684. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li ZY, Wang XL, Dang Y, Zhu XZ, Zhang YH,
Cai BX and Zheng L: Long non-coding RNA UCA1 promotes the
progression of paclitaxel resistance in ovarian cancer by
regulating the miR-654-5p/SIK2 axis. Eur Rev Med Pharmacol Sci.
24:591–603. 2020.PubMed/NCBI
|
|
25
|
Le Maitre CL, Pockert A, Buttle DJ,
Freemont AJ and Hoyland JA: Matrix synthesis and degradation in
human intervertebral disc degeneration. Biochem Soc Trans.
35:652–655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vo NV, Hartman RA, Yurube T, Jacobs LJ,
Sowa GA and Kang JD: Expression and regulation of
metalloproteinases and their inhibitors in intervertebral disc
aging and degeneration. Spine J. 13:331–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lippai M and Szatmari Z: Autophagy-from
molecular mechanisms to clinical relevance. Cell Biol Toxicol.
33:145–168. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang W, Zhang X, Hao J, Shen J, Fang J,
Dong W, Wang D, Zhang X, Shui W, Luo Y, et al: SIRT1 protects
against apoptosis by promoting autophagy in degenerative human disc
nucleus pulposus cells. Sci Rep. 4:74562014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang XH, Zhu L, Hong X, Wang YT, Wang F,
Bao JP, Xie XH, Liu L and Wu XT: Resveratrol attenuated
TNF-α-induced MMP-3 expression in human nucleus pulposus cells by
activating autophagy via AMPK/SIRT1 signaling pathway. Exp Biol Med
(Maywood). 241:848–853. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu K, Chen W, Wang X, Peng Y, Liang A,
Huang D, Li C and Ye W: Autophagy attenuates the catabolic effect
during inflammatory conditions in nucleus pulposus cells, as
sustained by NF-κB and JNK inhibition. Int J Mol Med. 36:661–668.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Akkoc Y and Gozuacik D: MicroRNAs as major
regulators of the autophagy pathway. Biochim Biophys Acta Mol Cell
Res. 1867:1186622020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gaviraghi M, Vivori C, Pareja Sanchez Y,
Invernizzi F, Cattaneo A, Santoliquido BM, Frenquelli M, Segalla S,
Bachi A, Doglioni C, et al: Tumor suppressor PNRC1 blocks rRNA
maturation by recruiting the decapping complex to the nucleolus.
EMBO J. 37:e991792018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen DY, Chen YM, Lin CF, Lo CM, Liu HJ
and Liao TL: MicroRNA-889 inhibits autophagy to maintain
mycobacterial survival in patients with latent tuberculosis
infection by targeting TWEAK. mBio. 11:e03045–19. 2020. View Article : Google Scholar
|
|
34
|
Wang WJ, Yang W, Ouyang ZH, Xue JB, Li XL,
Zhang J, He WS, Chen WK, Yan YG and Wang C: MiR-21 promotes ECM
degradation through inhibiting autophagy via the PTEN/akt/mTOR
signaling pathway in human degenerated NP cells. Biomed
Pharmacother. 99:725–734. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiong J: Atg7 in development and disease:
Panacea or Pandora's Box? Protein Cell. 6:722–734. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu K, Li N, Cheng Q, Zheng J, Zhu M, Bao
S, Chen M and Shi G: MiR-96-5p prevents hepatic stellate cell
activation by inhibiting autophagy via ATG7. J Mol Med (Berl).
96:65–74. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang C, Zhang ZZ, Yang W, Ouyang ZH, Xue
JB, Li XL, Zhang J, Chen WK, Yan YG and Wang WJ: MiR-210
facilitates ECM degradation by suppressing autophagy via silencing
of ATG7 in human degenerated NP cells. Biomed Pharmacother.
93:470–479. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang H, Zhang X and Zhang J: MiR-129-5p
inhibits autophagy and apoptosis of H9c2 cells induced by hydrogen
peroxide via the PI3K/AKT/mTOR signaling pathway by targeting
ATG14. Biochem Biophys Res Commun. 506:272–277. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
He W and Cheng Y: Inhibition of miR-20
promotes proliferation and autophagy in articular chondrocytes by
PI3K/AKT/mTOR signaling pathway. Biomed Pharmacother. 97:607–615.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang Y, Kwok-Shing Ng P, Kucherlapati M,
Chen F, Liu Y, Tsang YH, de Velasco G, Jeong KJ, Akbani R,
Hadjipanayis A, et al: A pan-cancer proteogenomic atlas of
PI3K/AKT/mTOR pathway alterations. Cancer Cell. 31:820–832.e3.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao D, Zhang S, Wang X, Gao D, Liu J, Cao
K, Chen L, Liu R, Liu J and Long J: ATG7 regulates hepatic Akt
phosphorylation through the c-JUN/PTEN pathway in high fat
diet-induced metabolic disorder. FASEB J. 33:14296–14306. 2019.
View Article : Google Scholar : PubMed/NCBI
|