Introduction
Platelet integrin αIIbβ3, also known as glycoprotein
IIb/IIIa or CD41/CD61, is the most abundant membrane receptor on
platelets (1,2) and serves an important role in platelet
function. There are ~80,000-100,000 receptors present on the
surface of a resting platelet (3).
An additional 20,000-40,000 receptors are found inside platelets,
mainly in α-granule membranes, but are also present in dense
granules and the membranes lining the open canalicular system;
platelet integrins are transported to the plasma membrane when
platelets are activated and undergo release reaction (4–6).
Allosteric changes in the αIIbβ3 ectodomain are regulated by
agonist-induced intracellular signals (known as inside-out
signaling/activation), which enable platelets to bind to fibrinogen
with high affinity (7). Upon
binding to fibrinogen, αIIbβ3 transduces signals in an outside-in
direction, mediating platelet spreading and stable adhesion
(8). Some agonists, such as
adenosine diphosphate (ADP), thrombin and collagen, can also
activate αIIbβ3 by binding to their respective receptors (9). During this process, intracellular
αIIbβ3 on α-granule membrane is transferred to the membrane and its
expression on the platelet surface will increase by 20–50%
(10).
Qualitative or quantitative abnormalities of
platelet integrin Itga2b (αIIb) and/or Itgb3 (β3) can cause
Glanzmann thrombasthenia (GT), which is an inherited autosomal
recessive hemorrhagic disorder characterized by a severe reduction
in platelet aggregation in response to multiple physiologic
agonists (11,12). Itgb3 knockout homozygous
(Itgb3−/−) mice have been reported as a GT model and
used for platelet research in past decades (11,13),
but their hematological characteristics and whether they can fully
simulate patients with GT have not been fully elucidated. The
present study aimed to answer these questions.
Materials and methods
Reagents
Phycoerythrin (PE)-conjugated hamster anti-CD61
(Itgb3) monoclonal antibody (cat. no. 553347) and PE/cyanine7
(CY7)-conjugated mouse anti-CD41 (Itga2b) antibody (cat. no.
133916) were purchased from BD Pharmingen (BD Biosciences) and
Biolegend, Inc., respectively. Rabbit anti-CD3 monoclonal antibody
(cat. no. MAB4841) was purchased from R&D Systems China Co.,
Ltd. and rat anti-transferrin R (CD71) monoclonal antibody (8D3;
cat. no. NB 100-64979-0.05mg) was purchased from Novus Biologicals,
LLC. Rabbit anti-CD3ε monoclonal antibody (cat. no. 99940S) was
purchased from Cell Signaling Technology, Inc. Rat anti-CD19
antibody (1D3; cat. no. ARG55048) was purchased from Arigo
Biolaboratories. Goat anti-rat IgG (H+L) HRP (cat. no. FMS-Rt01)
for immunohistochemistry was purchased from Fcmacs Biotech Co.,
Ltd. Goat anti-rabbit IgG (H+L) HRP and DAB buffer kit (cat. no.
RQ7100) for immunohistochemistry was purchased from Quanhui Imp and
Exp Int'l Co., Ltd. Alexa-Fluor 647-conjugated human fibrinogen
(cat. no. F35200) was purchased from Molecular Probes (Thermo
Fisher Scientific, Inc.). Tetramethyl rhodamine isothiocyanate
(TRITC)-conjugated phalloidin (cat. no. P1951) was purchased from
Sigma-Aldrich (Merck KGaA). Rabbit anti-Itgb3 polyclonal antibody
(cat. no. 4702) for western blotting was purchased from Cell
Signaling Technology, Inc. Rabbit anti-β-actin polyclonal antibody
(cat. no. 4968) for western blot was purchased from Cell Signaling
Technology, Inc. HRP-conjugated goat anti-rabbit IgG secondary
antibodies (cat. no. 5196-2504) was purchased from Bio-Rad,
Laboratories, Inc. RIPA buffer (cat. no. 89900) and protease
inhibitor mixture (cat. no. 87786) were purchased from Thermo
Fisher Scientific, Inc. The 2X SDS Laemmli sample buffer (cat. no.
S3401) was purchased from Sigma-Aldrich. ECL (cat. no. 1705070) was
purchased from Bio-Rad, Laboratories, Inc. Collagen type I (cat.
no. 385) and ADP (cat. no. 384) were purchased from Chrono-Log
Corporation. Purified human fibrinogen (cat. no. FIB1) was
purchased from Enzyme Research Labs Inc. Multimer purified human
von Willebrand Factor (vWF; cat. no. HCVWF-0190) was purchased from
Hematologic Technologies, Inc. Calcein AM (cat. no. C1430) was
purchased from Thermo Fisher Scientific, Inc.
D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone
dihydrochloride (PPACK; cat. no. BML-P1117-0025) was purchased from
Enzo Life Sciences, Inc. Arg-Gly-Asp-Ser (RGDS) peptide and the
protease activated receptors 4 (PAR4)-thrombin receptor activating
peptide, AF [Ala-Tyr-Pro-Gly-Lys-Phe (AYPGKF)], were synthesized at
GL Biochem (Shanghai) Ltd. Mouse plasma ferritin (cat. no. JL11908)
and mouse fecal occult blood test ELISA test kits (cat. no.
JL50073) were purchased from Shanghai Jianglai Biological
Technology Co., Ltd. All other biochemical reagents were obtained
from Sigma-Aldrich (Merck KGaA).
Experimental animals
The Itgb3−/− C57BL/6 mice were received
as a generous gift from Professor J. Liu (Shanghai Jiao Tong
University School of Medicine, Shanghai, China). Wild-type
(Itgb3+/+) C57BL/6 mice were obtained from the Animal
Experiment Center of Jiangsu University (Jiangsu, China). A total
of 2 Itgb3−/− male mice were mated with 2
Itgb3+/+ female mice to obtain Itgb3 heterozygous
(Itgb3+/−) mice. Subsequently, Itgb3−/− male
mice were mated with Itgb3+/− female mice to obtain
continuous offspring Itgb3−/− mice. A total of 50 mice
per group were used. The mice were housed (5 mice per cage) under a
12-h light/dark cycle at 23°C in a specific-pathogen-free
environment with ad libitum access to autoclaved food and
water. The cages were changed each week. Routine sanitation and
environmental controls, including temperature, humidity (40–70%),
ventilation, illumination and light schedule and noise abatement,
were performed by the animal care staff. Mice (age, 6–8 weeks; 1:1
male/female ratio) were used for each procedure with 3–10
mice/group. The animal study protocol was approved by the Jiangsu
University Institutional Animal Care & Use Committee (approval
no. UJS-IACUC-AP-20190307021).
Blood collection and platelet
preparation
Itgb3−/− mice (offspring) were identified
by PCR according to previously published methods (11,14).
Whole blood containing the anticoagulant sodium citrate was
collected by cardiac puncture from Itgb3−/− mice
following anesthetization with an intraperitoneal injection of 60
mg/kg pentobarbital sodium. Subsequently, 20 µl of the blood was
put aside for measurement of whole-blood count. The anesthetized
mice were sacrificed by cervical dislocation after whole blood
collection. Platelets were separated from whole blood by sequential
centrifugation as previously described (14). The platelet suspensions were rested
at room temperature for 1 h before being used for spreading assay,
fibrinogen binding assay, adhesion assay and other experiments.
Itgb3+/+ and/or Itgb3+/− mice served as
controls.
Analysis of Itgb3 expression of
Itgb3−/− platelets using flow cytometry
Briefly, 10 µl whole blood containing the
anticoagulant sodium citrate was collected from the
Itgb3+/+, Itgb3+/− and Itgb3−/−
mice by tail cutting. The whole blood diluted in PBS was incubated
with PE-conjugated hamster anti-CD61 (Itgb3) monoclonal antibody
(1:100) and PE/CY7-conjugated mouse anti-CD41 (Itga2b) antibody
(1:50) at room temperature for 30 min. The expressions of Itga2b
and Itgb3 in platelets were tested using flow cytometry (n=3
mice/group).
Analysis of Itgb3 expression of
Itgb3−/− platelets using western blotting
Itgb3−/− platelets (1×108)
were lysed for 30 min at 4°C in ice-cold using RIPA buffer
containing the protease inhibitor mixture to obtain proteins.
Following centrifugation of the platelet lysate at 13,800 × g for
15 min at 4°C, the supernatant was added to an equal volume of 2X
SDS Laemmli sample buffer. The proteins from 1×108
platelets per mouse were dissolved in 200 µl buffer, 10 µl of which
was loaded per lane. The proteins, after being boiled at 100°C for
5 min, were separated by SDS-PAGE using 12% gels and transferred to
PVDF membranes. Membranes were blocked with 3% BSA for 1 h at room
temperature, then incubated with rabbit β3 polyclonal antibody
(1:1,000) or rabbit anti-β-actin polyclonal antibody (1:1,000)
overnight at 4°C followed by HRP-conjugated goat anti-rabbit IgG
secondary antibodies (1:10,000). Immunoreactive bands were detected
by ECL. The Itgb3+/− and Itgb3+/+ mice served
as controls (n=3 mice/group).
Analysis of soluble fibrinogen binding
of Itgb3−/− platelets using flow cytometry
Soluble fibrinogen binding assay was performed as
previously described (14).
Briefly, (1×106/ml) washed platelets from
Itgb3−/− mice resuspended in HEPES-Tyrode's buffer
(137.0 mM NaCl, 2.0 mM KCl, 12.0 mM NaHCO3, 0.3 mM
NaH2PO4, 1.0 mM CaCl2, 1.0 mM
MgCl2, 5.5 mM glucose, 5.0 mM HEPES, 0.1% BSA, pH 7.4)
were divided into several groups and treated at 37°C for 30 min
with agonists alone or co-treated with antagonists as follow:
Mn2+ (presented by formation of MnCl2),
Mn2+ + RGDS, ADP, ADP + RGDS, AF and AF + RGDS. The
final concentration was 0.5 mM for Mn2+, 50 µM for ADP,
0.5 mM for AF peptide and 2 mM for RGDS. Following stimulation, the
platelets were incubated with 100 µg/ml of Alexa-Fluor
647-conjugated fibrinogen for 30 min at 37°C in the dark. The
reaction was then stopped by fixation with 4% formaldehyde for 15
min at room temperature. Then the platelets were washed with PBS
and centrifuged at 600 × g for 5 min at room temperature. The
Itgb3+/+ and Itgb3+/− platelets were used as
controls. Fibrinogen binding of platelets was tested using a flow
cytometer (Beckman Coulter, Inc.) and the data were analyzed with
FlowJo 7.6 software (FlowJo LLC). Specific fibrinogen binding was
calculated by total binding minus nonspecific binding in the
absence of any agonists (n=3 mice/group).
Aggregation of Itgb3−/−
platelets
The assay was performed according to the instruction
manual of the Chrono-log whole blood lumi-aggregometer (Chrono-log
Corporation). Briefly, blood containing 3.8% sodium citrate from
Itgb3−/− mice by cardiac puncture was collected and
diluted 1:1 in physiological saline. Platelet aggregation was
measured at 37°C in whole blood lumi-aggregometer (Chrono-log
Corporation) using electrical impedance following the addition of 2
U/ml thrombin, 6 µg/ml collagen or 12 µg/ml collagen. An AC voltage
in the millivolt range is applied to the probe circuit. During a
brief period of equilibration, a monolayer of platelets forms on
the exposed portions of the wires, resulting in a stable baseline
of impedance which is assigned a value of zero ohms of resistance.
Then, 20.0±0.2 ohms were added to the impedance baseline and the
gain was adjusted, so that 20 ohms equals 50% of scale. Aggregation
was recorded for 14 min. Itgb3+/− and
Itgb3+/+ mice served as controls (n=3 mice/group).
Thrombus formation of
Itgb3−/− platelets under flow
Thrombus formation of Itgb3−/− platelets
under flow was performed as previously described (14). Briefly, 100 µg/ml collagen was
perfused at a shear rate of 125 s−1 (5
dynes/cm2 for shear stress) for 2 min through the
Bioflux 200 microfluidic channels (Fluxion Biosciences, Inc.) to
coat the channels at 4°C overnight, followed by blocking with 2%
BSA at room temperature for 1 h. The PPACK- anticoagulant whole
blood (without sodium citrate) collected from Itgb3−/−
mice through cardiac puncture was labeled with the fluorescence
dye, Calcein AM, and perfused at a shear rate of 1,500
s−1 for 10 min through the microfluidic channels. The
process of thrombus formation was recorded by a video using NIS D
image software version 3.1 (Nikon Corporation) and Bioflux 200
software. Itgb3+/− and Itgb3+/+ mice served
as controls (n=3 mice/group).
Adhesion and spreading of
Itgb3−/− platelets
For the adhesion assays, a 96-well plate was coated
with 20 µg/ml fibrinogen, 4 µg/ml collagen, or 4 µg/ml vWF at 4°C
overnight and then blocked with 2% BSA at room temperature for 1 h.
Washed platelets (1×106/ml) from Itgb3−/−
mice were allowed to adhere for 1 h at 37°C. Next, the wells were
rinsed with PBS to remove non-adherent or unstable adherent
platelets. 4-Nitrophenylphosphate (PNPP) was used to quantify the
adherent platelets. In detail, the buffer (1% Triton X-100, 3 mg/ml
PNPP, 100 mM sodium acetate, pH 5.0) was added and incubated at
37°C for 1 h. Then, sodium hydroxide (0.5 M) was added to stop the
reaction. The optical density (OD) value was read at a wavelength
of 405 nm using a microplate reader. Washed Itgb3+/+
platelets were used as a control.
Platelet spreading assays were performed as
previously described (14).
Briefly, the chamber slides were coated with 20 µg/ml fibrinogen
overnight at 4°C and then blocked with 2% BSA. Washed platelets
(2×105/ml) resuspended in HEPES-Tyrode's buffer were
allowed to adhere and spread on fibrinogen-coated slides at 37°C
for 2 h. After washing three times with PBS, the attached platelets
were fixed with 4% paraformaldehyde for 15 min at room temperature,
permeabilized with 0.2% Triton X-100 and stained with
TRITC-conjugated phalloidin as previously described (15). Itgb3+/+ platelets were
used as a control. The coverage area of spreading was measured
using ImageJ software version 1.4.3.67 (National Institutes of
Health). A total of five randomly selected fields from different
tests were used for statistical analysis (n=3 mice analyzed from
each group).
Tail bleeding time
Itgb3−/− mice were anesthetized with
intraperitoneal injection of 60 mg/kg pentobarbital sodium. A 3-mm
section was cut from the tail tip in 6-week-old mice and then the
bleeding time was counted. Every 30 sec the wounded tails were
touched to a filter paper to confirm whether the bleeding had
stopped or not. The maximum time for counting was 30 min.
Itgb3+/− and Itgb3+/+ mice served as
controls. Simultaneously, blood smear slides were made from the cut
tail followed by Wright's staining at room temperature for 15 min
(n=10 mice/group).
Establishment of chronic hemorrhagic
model (CHM) mice
A total of 3 8-week-old male Itgb3+/+
mice were anesthetized with intraperitoneal injection of 60 mg/kg
pentobarbital sodium in accordance with animal welfare standards.
Mice were restrained and the neck gently pressed to cause head vein
congestion. A sterilized capillary pipette was gently pressed into
the posterior orbital venous plexus. The blood was naturally sucked
into the tube. After the required blood had been obtained, the
pressure on the neck was removed and the blood collection tube was
pulled out at the same time to prevent postoperative bleeding from
the puncture hole and sterile gauze or cotton ball was used to
compress the eyeball to stop bleeding. During the operation, a
veterinarian was nearby to observe the breathing and palpate heart
rate. Following the operation, 0.4 ml of 0.9% sodium chloride was
given through gavage twice to add fluid to prevent shock in
accordance with animal welfare standards. The animal was covered
with insulation blanket to keep it warm. The animal was placed in a
cage in warm environment after awakening. Blood (0.5 ml) was
collected every time and twice a week. After 1 month, CHM mice were
established successfully, and three CHM mouse models were
established. A total of 3 8-week-old male Itgb3+/+ mice
without treatment served as controls. The animals were kept
individually, as aforementioned, and monitored daily during the CHM
induction for animal welfare.
Bone marrow smear and spleen biopsy of
Itgb3−/− mice
Itgb3−/− mice were sacrificed after
anesthetization with pentobarbital sodium injection by cervical
dislocation and their organs, including the spleen, heart, kidneys
and liver were collected and weighed. Mouse femurs were dissected
with a scissor. Muscles and connective tissues were removed as
thoroughly as possible. The cartilages on both end of the femur
were removed. The marrow of femurs was leaked out and prepared for
bone marrow smear. Wright staining and iron staining (Prussian
Blue) at room temperature for 20 min were performed on bone marrow
smears. Itgb3+/+, Itgb3+/− and CHM mice
served as controls (n=10 mice/group).
Immunohistochemistry of spleen
biopsies from Itgb3−/− mice
Half of the spleens collected from
Itgb3+/+ and Itgb3−/− mice were fixed in 10%
formalin overnight at room temperature and subsequently embedded in
paraffin. The other half of the spleens were embedded in optimal
cutting temperature compound and sectioned (1 mm) at low
temperatures and fixed with precooled acetone to make the frozen
section. The spleen tissue sections were stained sequentially with
hematoxylin and eosin at room temperature for 2–3 min. The
immunohistochemical procedure was performed on paraffin sections or
frozen sections according to a previous study (16). The slides were incubated with
monoclonal anti-CD3 (1:50), anti-CD19 (1:200) or anti-CD71 (1:100)
antibodies at 4°C overnight followed by goat anti-rat IgG (H+L) HRP
(1:1,000) or goat anti-rabbit IgG (H+L) HRP (1:1,000) at 37°C for 1
h and diaminobenzidine substrate with hematoxylin counterstaining
(n=3 mice/group). The slides were observed under a light microscope
(Olympus BX53F; Olympus Corporation) with Smart V digital camera
and analyzed using JD-801 Image analysis system (JEDA
Science-Technology Development Co., Ltd.).
The concentration of plasma ferritin
from Itgb3−/− mice
Whole blood containing the anticoagulant sodium
citrate was collected by cardiac puncture from Itgb3−/−
mice after anesthetization as aforementioned. Then plasma was
obtained by centrifugation at 1,000 × g for 30 min at room
temperature. Plasma ferritin was tested using a ferritin ELISA test
kit following the manufacturer's protocol. Itgb3+/+ mice
served as controls (n=8 mice/group).
Fecal occult blood test of
Itgb3−/− mice
Feces from Itgb3−/− mice were collected
then were tested using fecal occult blood ELISA test kit (Shanghai
Jianglai Biological Technology Co., Ltd.) following the
manufacturer's protocol. Itgb3+/+ mice served as
controls (n=8 mice/group).
Statistical analysis
SPSS version 18 (SPSS, Inc.) was used for
statistical analysis. Quantitative data were expressed as the mean
± standard deviation. Differences between two groups were assessed
using Student's t-test. For datasets containing three or more
groups, one-way ANOVA followed by Tukey's test was used. P<0.05
was considered to indicate a statistically significant
difference.
Results
Genotyping and platelet Itgb3
expression of Itgb3−/− mice
DNA was extracted from the tail tissue of
Itgb3+/+, Itgb3+/− and Itgb3−/−
mice and then genotyped by PCR. A band of 538 bp was observed for
Itgb3−/− mice, 446 bp for Itgb3+/+ mice and
double bands for Itgb3+/− mice (Fig. S1A). Western blotting results showed
no Itgb3 protein expression in washed Itgb3−/− platelets
(Fig. S1B). Whole-blood from
Itgb3+/+, Itgb3+/− and Itgb3−/−
mice were incubated with PE-conjugated anti-CD61 (Itgb3) antibody
and PE/CY7-conjugated anti-CD41 (Itga2b) antibody and then
subjected to flow cytometry. Almost no Itga2b and Itgb3 was
expressed on the Itgb3−/− platelet surface, compared
with that on the Itgb3+/− and Itgb3−/−
platelet surface (Fig. S1C-E).
Soluble fibrinogen binding of
Itgb3−/− platelets
The washed Itgb3+/+ platelets can bind to
soluble fibrinogen under the stimulation of Mn2+, ADP or
AF peptide, which can be partially inhibited by co-treatment with
the antagonist RGDS peptide (Fig.
1A). The fibrinogen binding of Itgb3+/− platelets
was similar to that of Itgb3+/+ platelets. However, the
Itgb3−/− platelets did not bind to soluble fibrinogen
under the stimulation of any agonists.
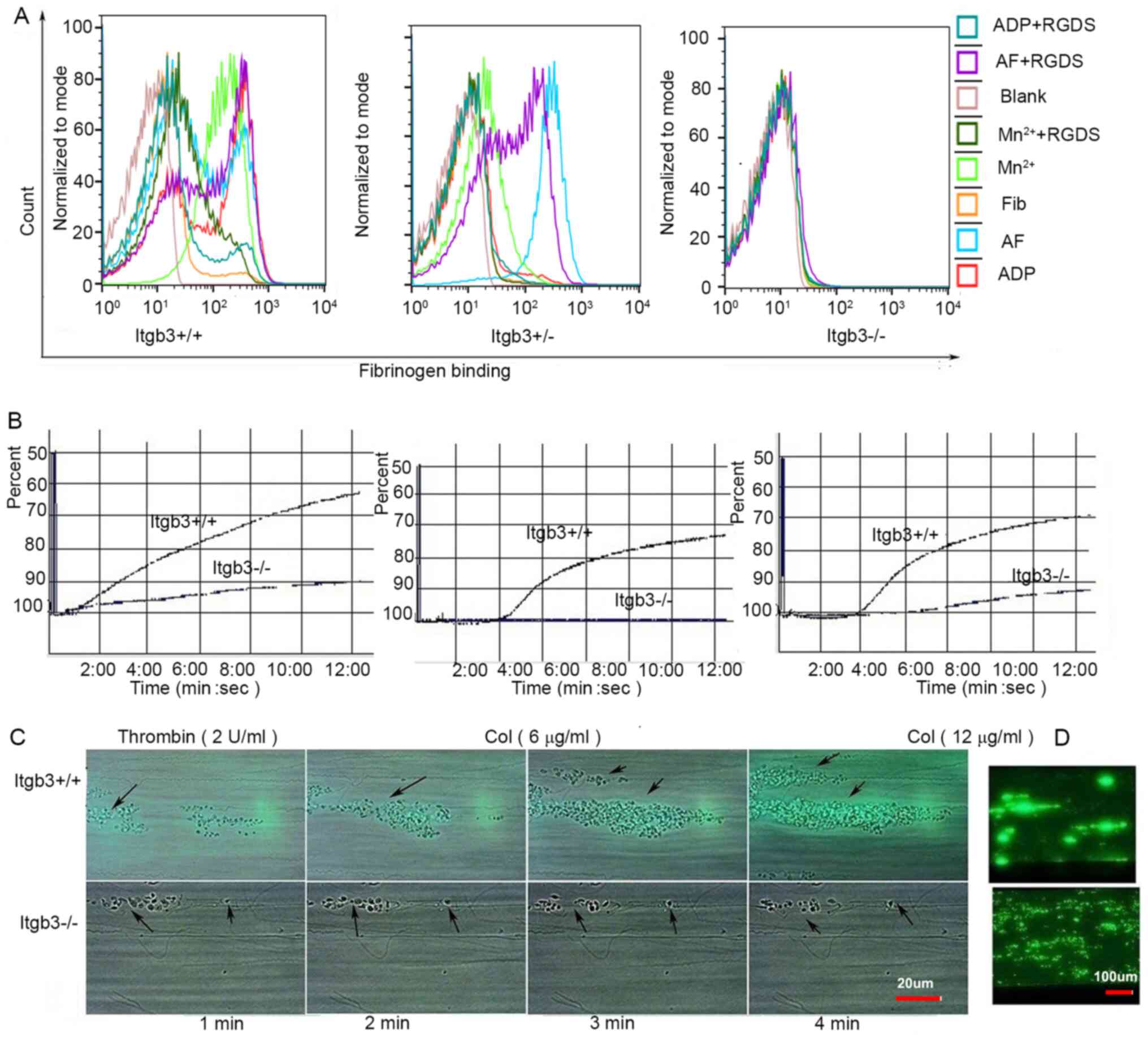 | Figure 1.Fibrinogen binding and aggregation of
Itgb3−/− platelets. (A) Fibrinogen binding of washed
platelets from three types of mice (Itgb3+/+,
Itgb3+/− and Itgb3−/− mice) under stimulation
by agonists, including ADP, AF, or Mn2+, with or without
the inhibitor RGDS. (B) Whole blood aggregation of
Itgb3+/+ and Itgb3−/− mice under stimulation
of 2 µg/ml thrombin, 6 µg/ml collagen or 12 µg/ml collagen. Calcein
AM-labeled whole blood from Itgb3+/+ mice and
Itgb3−/− mice was perfused over a collagen-coated
surface at shear rates of 1,500 s−1 and images were
captured under (C) bright-field at the indicated time points and
(D) fluorescence microscope at 4.5 min. Arrows indicated the
adhered platelets. ADP, adenosine diphosphate; AF,
Ala-Tyr-Pro-Gly-Lys-Phe (AYPGKF); Itgb3, integrin β3; RGDS,
Arg-Gly-Asp-Ser. |
Aggregation of Itgb3−/−
platelets
Electronic resistance mediated by platelet
aggregation from Itgb3+/+ mice was 8 ohms induced by 2
U/ml thrombin, 10 ohms induced by 6 µg/ml collagen and 11 ohms
induced by 12 µg/ml collagen, whereas that from Itgb3−/−
mice was 2, 0 and 2 ohms, respectively (Fig. 1B).
Thrombus formation of
Itgb3−/− platelets under flow
On a collagen-coated surface at shear rates of 1,500
s−1, the thrombus formed from Itgb3+/+ mice
was large and thick, showing strip-shaped distribution along the
direction of blood flow (Fig. 1C and
D). By contrast, the thrombus formed from Itgb3−/−
mice was scattered, thin and easily washed off.
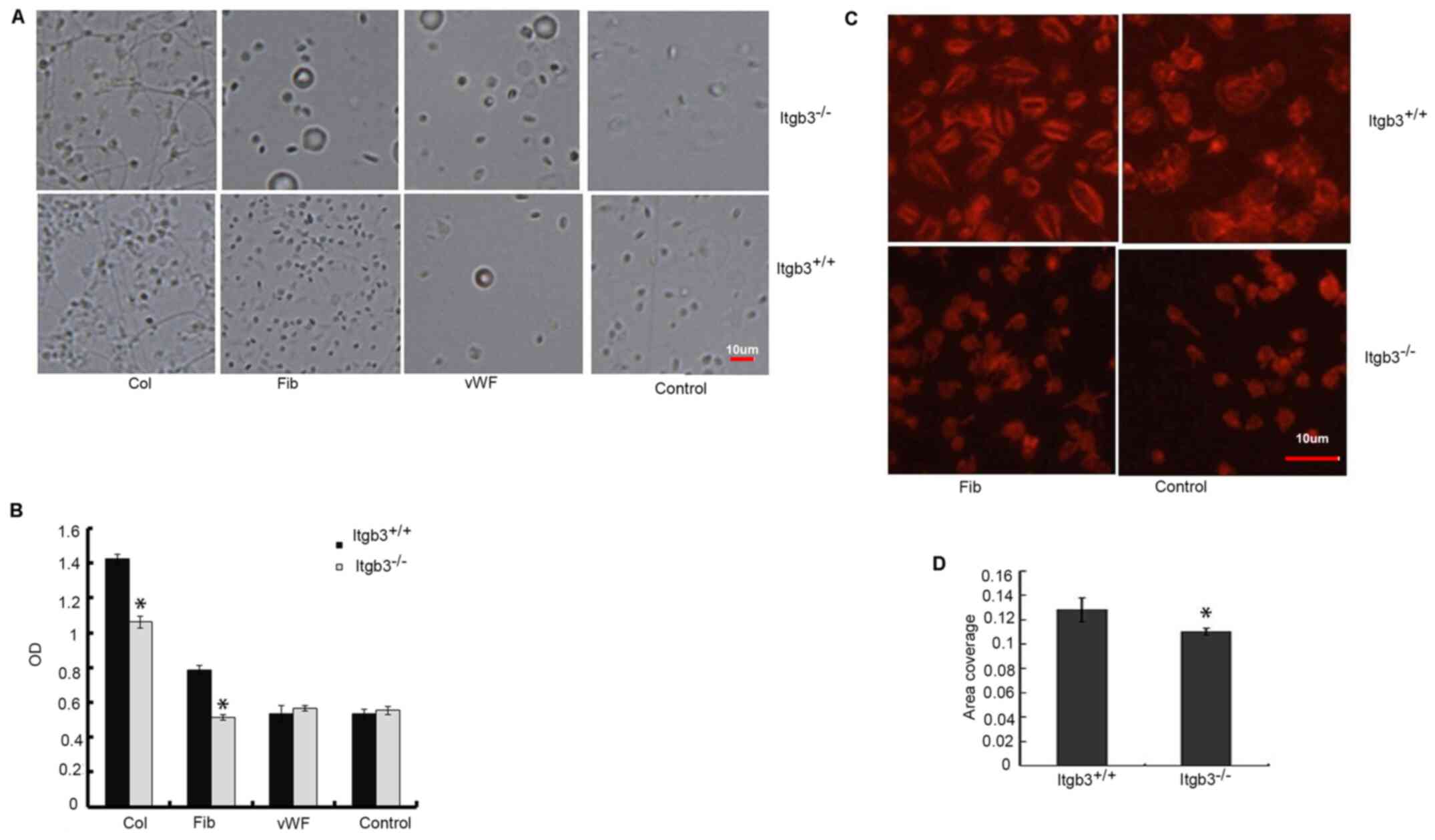
Adhesion and spreading of
Itgb3−/− platelets
The adhesion of washed Itgb3−/− platelets
was notably lower on the fibrinogen or collagen-coated surface
compared with that of washed Itgb3+/+ platelets, no
difference was observed in the adhesion on the vWF-coated surface
(Fig. 2A and B). The
Itgb3+/+ platelets spread well on the fibrinogen-coated
surface with the regular actin arrangement. On the surface without
any matrix (control), Itgb3+/+ platelets also spread,
but the actin arrangement was disordered and diffuse. However, the
spreading of Itgb3−/− platelets on fibrinogen-coated
surface and control was notably impaired (Fig. 2C and D).
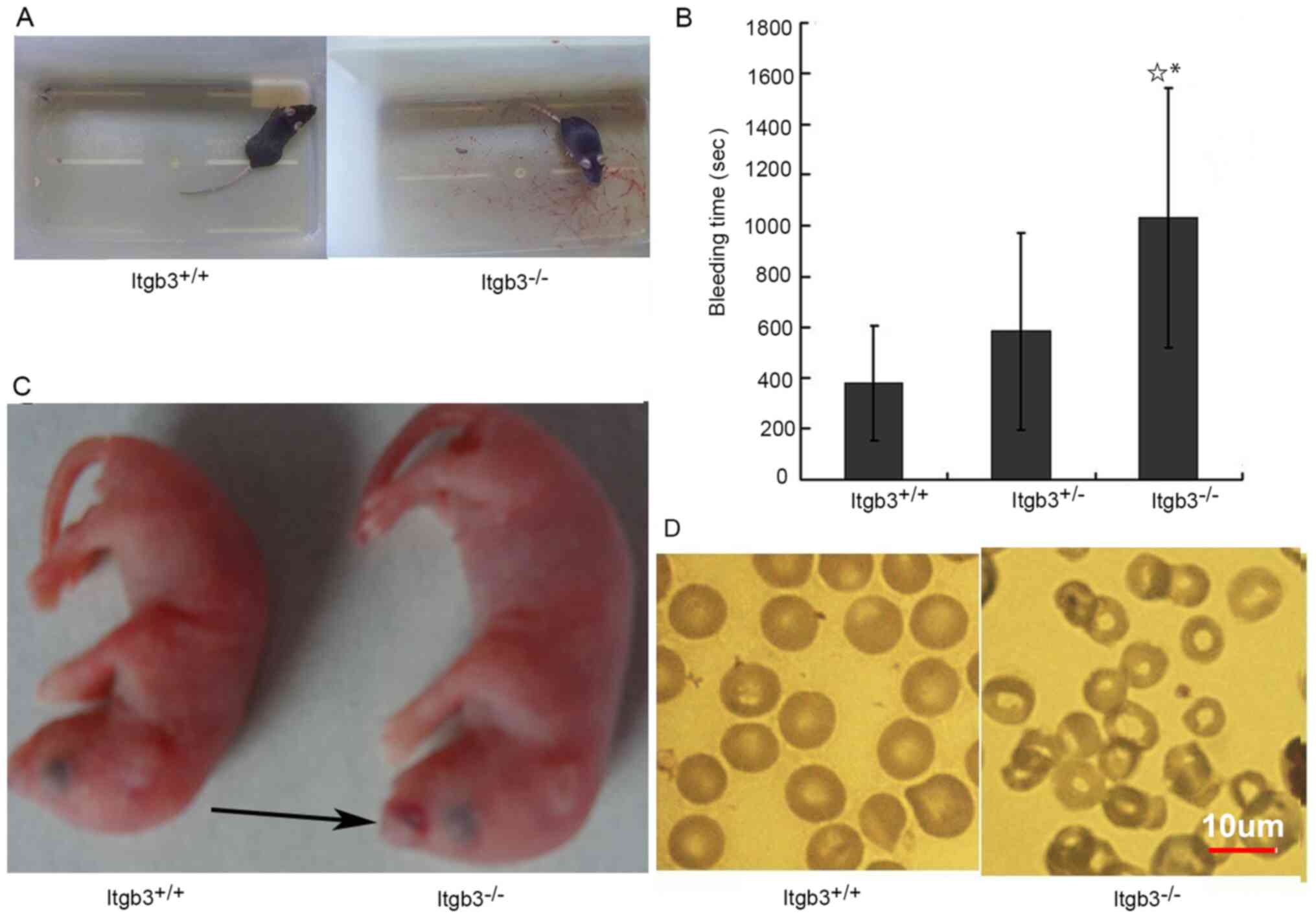
Spontaneous hemorrhage and anemia in
Itgb3−/− mice
Itgb3−/− mice exhibited prolonged
bleeding time (Fig. 3A and B) after
tail cutting and they were more prone to subcutaneous hemorrhage
(Fig. 3C) at birth compared with
Itgb3+/+ mice. Itgb3−/− mice had a mean red
blood cell count of 6.38±2.02×1012 cells/l, hemoglobin
of 89.38±25.73 g/l and a platelet count of
536.18±195.58×109 cells/l, which were lower compared
with Itgb3+/+ mice (Table
I). Wright staining showed increased microcytic hypochromic
erythrocytes in peripheral blood smear from Itgb3−/−
mice compared with that from Itgb3+/+ mice (Fig. 3D).
 | Table I.Whole blood count of different groups
of mice. |
Table I.
Whole blood count of different groups
of mice.
| Group | Wbc,
×1012/l | Rbc,
×1012/l | Hb, g/l | Plt,
×109/l |
|---|
|
Itgb3+/+ | 5.86±5.17 | 9.38±1.54 | 123.1±24.86 | 1031.27±418.26 |
|
Itgb3+/− | 7.73±7.80 | 10.44±2.40 | 140.35±33.69 | 1112.58±356.91 |
|
Itgb3−/− | 4.40±3.58 |
6.38±2.02a,b |
89.38±25.73a,b |
536.18±195.28a,b |
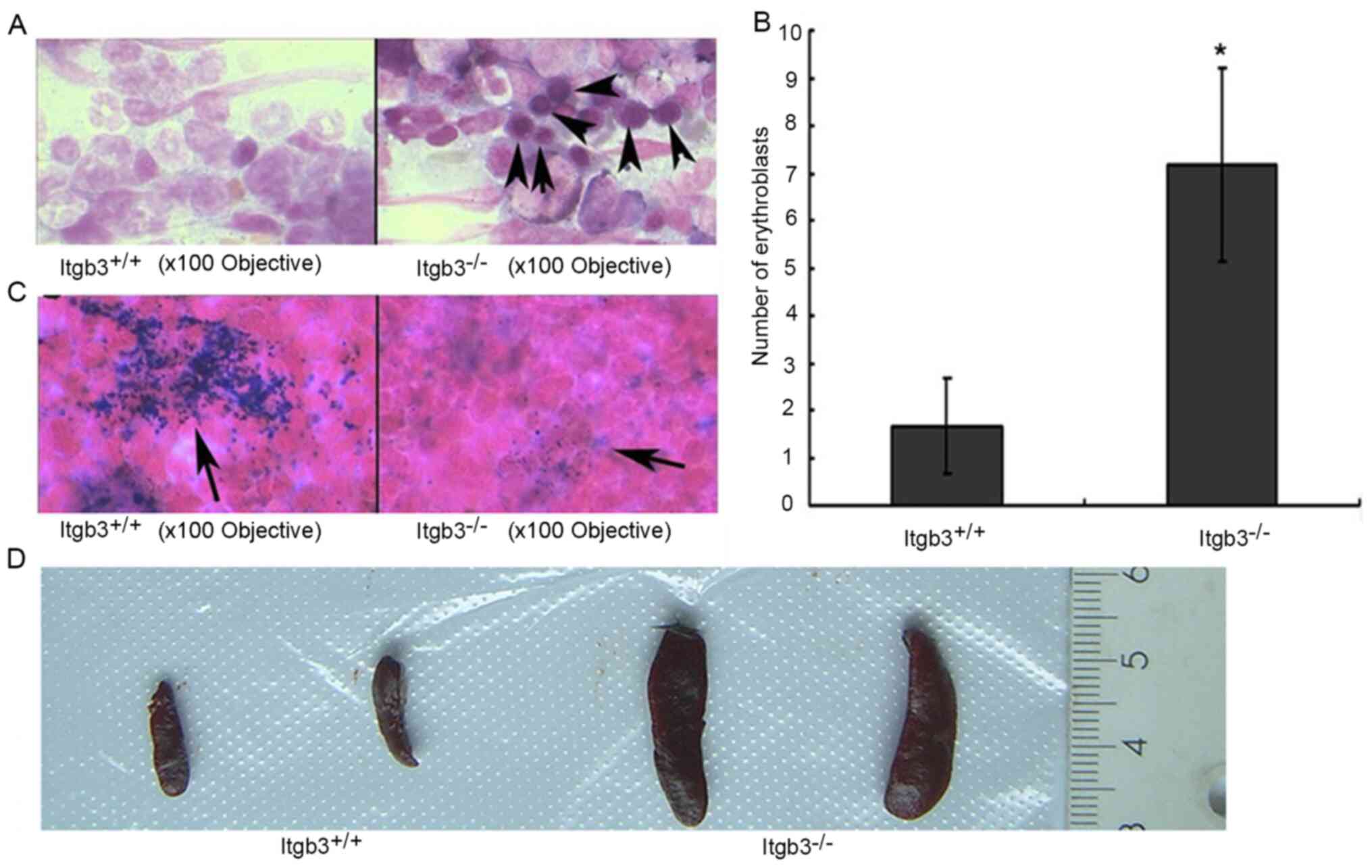
Increased erythroblasts and
extramedullary hematopoiesis in Itgb3−/− mice
The bone marrow smears of Itgb3−/− mice
exhibited significantly more early erythroblasts compared with
Itgb3+/+ mice (Fig. 4A and
B), and the Itgb3−/− mice exhibited a relatively
lower amounts of iron granules compared with Itgb3+/+
mice, indicating iron deficiency in Itgb3−/− mice
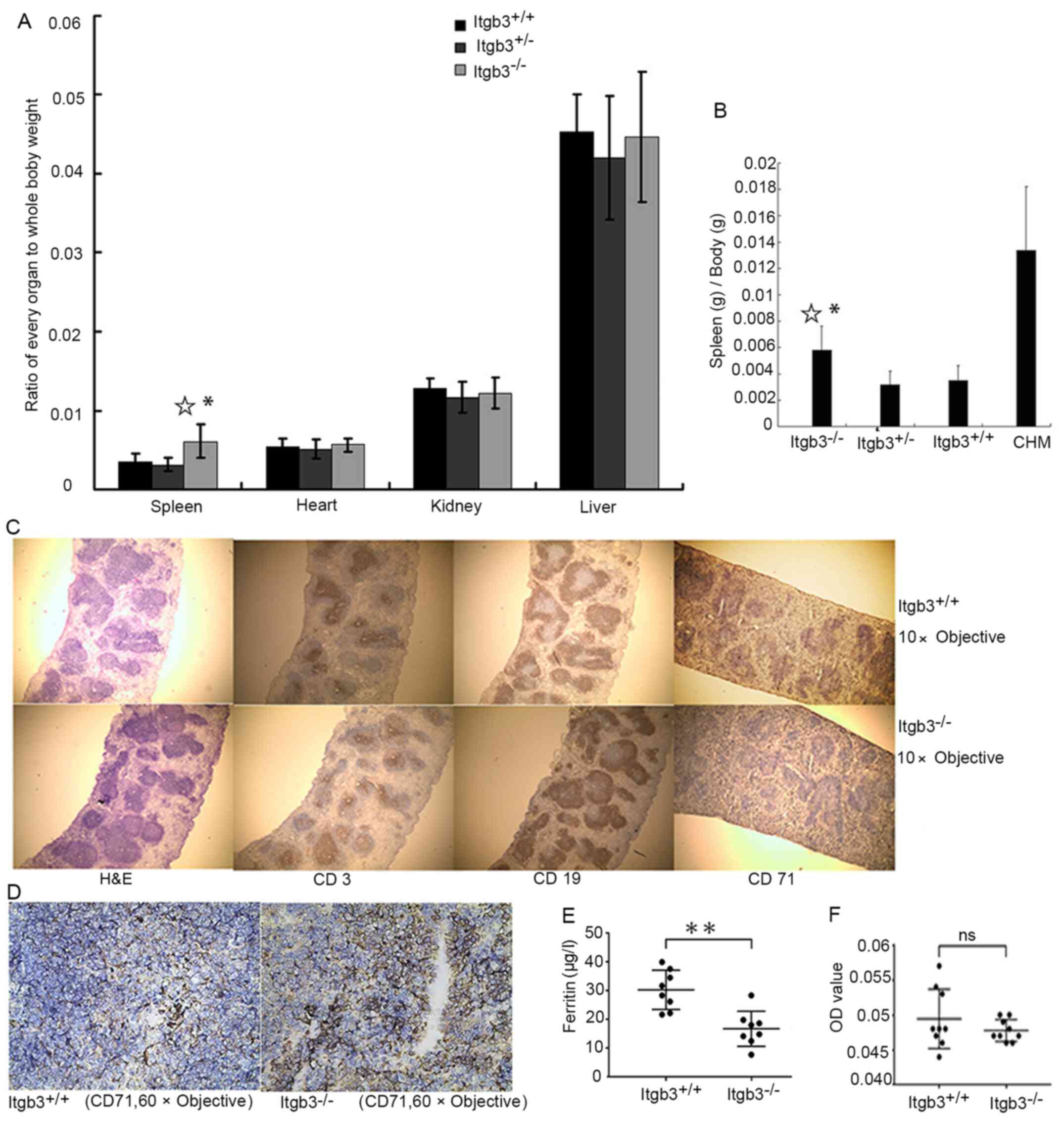
(Fig. 4C). Enlarged spleens were
observed in Itgb3−/− mice (Fig. 4D). The ratio of spleen to whole body
weight was higher in Itgb3−/− mice than in
Itgb3+/+ mice suggesting a splenomegaly in
Itgb3−/− mice (Fig. 5A).
CHM mice were established to confirm whether CHM also exhibits
splenomegaly and to determine whether the splenomegaly in
Itgb3−/− mice is a result of chronic bleeding (Fig. 5B). Immunohistochemical staining of
spleen biopsies from Itgb3−/− mice showed no change in
the expression of CD3 and CD19, which are the markers of T
lymphocytes and B lymphocytes, respectively, whereas the expression
of CD71, which is a marker of erythrocytes, increased compared with
Itgb3+/+ mice (Fig. 5C and
D).
Decreased level of plasma ferritin in
in Itgb3−/− mice
Itgb3−/− mice had significantly lower
level of plasma ferritin compared with Itgb3−/− mice
(Fig. 5E). There was no difference
of fecal occult blood between Itgb3+/+ mice and
Itgb3−/− mice (Fig.
5F).
Discussion
Platelet integrin Itgb3 subunit is a cell-surface
receptor that mediates cell-cell and cell-matrix interactions
(17). Integrin Itgb3 (β3) subunit
binds with Itga2b (αIIb) to form integrin αIIbβ3, which is the most
abundant receptor on the platelet surface (2). Qualitative or quantitative
abnormalities of the platelet integrin αIIb and Itgb3 can cause GT
(18), an inherited hemorrhagic
disorder (19). The incidence is ~1
in 1,000,000; the highest incidence rate is 6 in 700 in the
Iraqi-Jewish population where consanguineous mating is common
(20). Patients with GT show
defects in platelet aggregation and clot retraction, as well as
prolonged bleeding times (21,22).
Thus far, ~50 specific gene mutations in Itgb3 or αIIb are known
(23). Itgb3−/− mice
were generated as a GT model in 1999 (11) and have been used widely in recent
decades on platelet research. However, it is unclear whether this
mouse model can fully simulate patients with GT; moreover, the
hematological characteristics of this model have not been fully
described. Therefore, the present study investigated the
characteristics of Itgb3−/− mice.
Platelets from Itgb3−/− mice did not
express Itgb3 and αIIb. Integrin Itgb3 and αIIb can form a complex
that protects glycoproteins from proteolytic digestion; thus, if
either integrin αIIb or Itgb3 is absent or unable to form a normal
complex, the other subunit will be rapidly degraded (24). The inability of Itgb3−/−
platelets to bind to fibrinogen under the stimulation of agonists,
including Mn2+ (which directly acts on the extracellular
segment of αIIbβ3), ADP (which binds to P2Y12 or P2Y1 receptors),
or AF peptide (which binds to thrombin receptor) (25–27),
confirmed the important role of αIIbβ3 in fibrinogen binding. The
aggregation of platelets is the connection of a platelet to another
platelet through its αIIbβ3 receptor binding to fibrinogen.
Therefore, Itgb3−/− platelets rarely aggregate under the
stimulation of collagen or thrombin, nor on a collagen-coated
surface under flow. The adhesion of Itgb3−/− platelets
was impaired on the fibrinogen-coated surface and partially
inhibited on the collagen-coated surface. The adhesion of platelets
to collagen is complex. α2β1 and GPVI on the surface of platelets
can bind to collagen, which triggers the inside out signaling
pathway leading to the activation of αIIbβ3 (28,29).
This may occur because adhesion on fibrinogen wholly depends on
αIIbβ3, whereas adhesion on collagen depends on not only αIIbβ3 but
also α2β1 and GPIV. There was no significant difference in platelet
adhesion to the vWF-coated surface between Itgb3−/− mice
and Itgb3+/+ mice, probably because vWF mainly binds to
GPIbα (30). The bleeding time of
Itgb3−/− mice was prolonged and subcutaneous bleeding
was found in newborn pups
The whole blood test showed that the number of red
blood cells and hemoglobin in blood from Itgb3−/− mice
decreased compared with that in blood from Itgb3+/+ mice
or Itgb3+/− mice, in accordance with a previous finding
(11). The peripheral blood smear
from Itgb3−/− mice showed microcytic hypochromic
erythrocytes. Further bone marrow smear and iron staining confirmed
the proliferation of erythroblasts and iron deficiency, suggesting
occurrence of iron deficiency anemia (IDA). In addition to
morphology and iron staining of bone marrow, IDA has several
diagnostic criteria (31),
including the decreased level of ferritin and/or gastrointestinal
bleeding. The plasma ferritin was tested, and it was found that the
concentration of plasma ferritin in Itgb3−/− mice was
lower compared with Itgb3+/+ mice, which fulfilled the
diagnostic criteria of IDA. However, fecal occult blood test
demonstrated no difference between Itgb3−/− mice and
Itgb3+/+ mice, probably due to the intermittent
characteristics of gastrointestinal bleeding of Itgb3−/−
mice. In patients with GT, gastrointestinal bleeding is usually
intermittent (21).
Platelet count in Itgb3−/− mice was
decreased compared with Itgb3+/+ mice or
Itgb3+/− mice, although a previous study reported no
significant difference in platelet count between
Itgb3−/− and Itgb3+/+ mice (11). The hemorrhagic diathesis may be due
to not only the damaged platelet function but also to the decreased
platelet count. Patients with GT have normal platelet counts
(32). However, although
Itgb3−/− mice are regarded as a model for GT, the
present study found that platelet count was decreased in
Itgb3−/− mice. This finding suggested that
Itgb3−/− mice differ markedly from patients with GT.
Another difference that the present study found was
splenomegaly, which is absent in patients with GT, even in those
with IDA (32). At first, it was
hypothesized that there might be an abnormality in the lymphatic
immune system of Itgb3−/− compared with that of
Itgb3+/+ mice. However, immunohistochemical analysis of
spleen biopsy showed that the expression of CD3, a marker on the T
cell surface and CD19, a marker on the B cell surface, did not
differ between Itgb3−/− and Itgb3+/+ mice.
Furthermore, immunohistochemical analysis of spleen biopsy showed
increased expression of the erythrocyte marker, CD71, suggesting
that the splenomegaly in Itgb3−/− mice may be associated
with extramedullary hematopoiesis. Furthermore, to identify whether
extramedullary hematopoiesis is a result of bleeding-induced
anemia, CHM mice were established by regular phlebotomy of
Itgb3+/+ mice. Evident enlargement of the spleen was
observed in CHM mice suggesting that the splenomegaly was a result
of compensatory extramedullary hematopoiesis due to chronic blood
loss (33,34). These discrepancies following blood
loss between humans and mice may result from species-specific
differences. Additionally, there is a wide spectrum of phenotypes
of patients with GT and environmental factors also contribute to
these variations (32), whereas the
genotype of Itgb3−/− mice is homogeneous and the
breeding environment of mice was also unified and standardized.
Despite possessing a number of characteristics that
mimic GT, such as the absence of Itgb3 and αIIb, damage of platelet
function, bleeding trend and IDA, Itgb3−/− mice also
displayed a number of characteristics that are absent in patients
with GT, such as decreased platelet count, splenomegaly and
extramedullary hematopoiesis. Therefore, as Itgb3−/−
mice possessed special characteristics that differed from those
found in human patients with GT, and they cannot completely
simulate patients with GT.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by The National
Natural Science Foundation of China (grant no. 81700130), Natural
Science Foundation of Jiangsu Province of China (grant no.
BK20150474), The Science and Technology Commission of Zhenjiang
Municipality (grant no. SH2017006), Novo Nordisk Haemophilia
Research Fund in China and Youth Medical Talents Project of ‘Ke
Jiao Qiang Wei’ project of Jiangsu province (grant no.
QNRC201684).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
XS conceived and designed the experiments. DL, JP
and TL performed the experiments. YL and MC analyzed the data. XS
and DL wrote the manuscript. XS and DL were responsible for
confirming the authenticity of the data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal procedures were approved by The Animal
Ethics Committee of Jiangsu University (approval no.
UJS-IACUCAP-20190307021).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ADP
|
adenosine diphosphate
|
|
AF
|
Ala-Tyr-Pro-Gly-Lys-Phe (AYPGKF)
|
|
CHM
|
chronic hemorrhagic model
|
|
GT
|
Glanzmann thrombasthenia
|
|
Itgb3−/−
|
Itgb3-integrin-deficient
|
|
IDA
|
iron deficiency anemia
|
References
|
1
|
Coller BS: αIIbβ3: Structure and function.
J Thromb Haemost. 13 (Suppl 1):S17–S25. 2015. View Article : Google Scholar
|
|
2
|
Hynes RO: Integrins: Bidirectional,
allosteric signaling machines. Cell. 110:673–687. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wagner CL, Mascelli MA, Neblock DS,
Weisman HF, Coller BS and Jordan RE: Analysis of GPIIb/IIIa
receptor number by quantification of 7E3 binding to human
platelets. Blood. 88:907–914. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Woods VL Jr, Wolff LE and Keller DM:
Resting platelets contain a substantial centrally located pool of
glycoprotein IIb-IIIa complex which may be accessible to some but
not other extracellular proteins. J Biol Chem. 261:15242–15251.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wencel-Drake JD, Plow EF, Kunicki TJ,
Woods VL, Keller DM and Ginsberg MH: Localization of internal pools
of membrane glycoproteins involved in platelet adhesive responses.
Am J Pathol. 124:324–334. 1986.PubMed/NCBI
|
|
6
|
Cramer EM, Savidge GF, Vainchenker W,
Berndt MC, Pidard D, Caen JP, Massé JM and Breton-Gorius J:
Alpha-granule pool of glycoprotein IIb-IIIa in normal and
pathologic platelets and megakaryocytes. Blood. 75:1220–1227. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ginsberg MH, Du X and Plow EF: Inside-out
integrin signalling. Curr Opin Cell Biol. 4:766–771. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shattil SJ: Signaling through platelet
integrin alpha IIb beta 3: Inside-out, outside-in, and sideways.
Thromb Haemost. 82:318–325. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leclerc JR: Platelet glycoprotein IIb/IIIa
antagonists: Lessons learned from clinical trials and future
directions. Crit Care Med. 30 (Suppl 5):S332–S340. 2002. View Article : Google Scholar
|
|
10
|
Youssefian T, Massé JM, Rendu F, Guichard
J and Cramer EM: Platelet and megakaryocyte dense granules contain
glycoproteins Ib and IIb-IIIa. Blood. 89:4047–4057. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hodivala-Dilke KM, McHugh KP, Tsakiris DA,
Rayburn H, Crowley D, Ullman-Culleré M, Ross FP, Coller BS,
Teitelbaum S and Hynes RO: Beta3-integrin-deficient mice are a
model for Glanzmann thrombasthenia showing placental defects and
reduced survival. J Clin Invest. 103:229–238. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nurden AT: Glanzmann thrombasthenia.
Orphanet J Rare Dis. 1:102006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng X, Novack DV, Faccio R, Ory DS, Aya
K, Boyer MI, McHugh KP, Ross FP and Teitelbaum SL: A Glanzmann's
mutation in beta 3 integrin specifically impairs osteoclast
function. J Clin Invest. 107:1137–1144. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi X, Yang J, Cui X, Huang J, Long Z,
Zhou Y, Liu P, Tao L, Ruan Z, Xiao B, et al: Functional effect of
the mutations similar to the cleavage during platelet activation at
integrin β3 cytoplasmic tail when expressed in mouse platelets.
PLoS One. 11:e01661362016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yin H, Liu J, Li Z, Berndt MC, Lowell CA
and Du X: Src family tyrosine kinase Lyn mediates
VWF/GPIb-IX-induced platelet activation via the cGMP signaling
pathway. Blood. 112:1139–1146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kikuchi H, Higuchi T, Hashida Y, Taniguchi
A, Kamioka M, Taguchi T, Yokoyama A, Murakami I, Fujieda M and
Daibata M: Generation and characteristics of a novel ‘double-hit’
high grade B-cell lymphoma cell line DH-My6 with MYC/IGH and
BCL6/IGH gene arrangements and potential molecular targeted
therapies. Oncotarget. 9:33482–33499. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fullard JF: The role of the platelet
glycoprotein IIb/IIIa in thrombosis and haemostasis. Curr Pharm
Des. 10:1567–1576. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Coller BS and Shattil SJ: The GPIIb/IIIa
(integrin alphaIIbbeta3) odyssey: A technology-driven saga of a
receptor with twists, turns, and even a bend. Blood. 112:3011–3025.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wilcox DA, Olsen JC, Ishizawa L, Bray PF,
French DL, Steeber DA, Bell WR, Griffith M and White GC II:
Megakaryocyte-targeted synthesis of the integrin beta(3)-subunit
results in the phenotypic correction of Glanzmann thrombasthenia.
Blood. 95:3645–3651. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rosenberg N, Yatuv R, Orion Y, Zivelin A,
Dardik R, Peretz H and Seligsohn U: Glanzmann thrombasthenia caused
by an 11.2-kb deletion in the glycoprotein IIIa (beta3) is a second
mutation in Iraqi Jews that stemmed from a distinct founder. Blood.
89:3654–3662. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
George JN, Caen JP and Nurden AT:
Glanzmann's thrombasthenia: The spectrum of clinical disease.
Blood. 75:1383–1395. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mesquita R, Santos I and Monteiro H:
Severe intestinal bleeding in a woman with glanzmann
thrombasthenia. Eur J Case Rep Intern Med. 5:0007962018.PubMed/NCBI
|
|
23
|
Fang J, Hodivala-Dilke K, Johnson BD, Du
LM, Hynes RO, White GC II and Wilcox DA: Therapeutic expression of
the platelet-specific integrin, alphaIIbbeta3, in a murine model
for Glanzmann thrombasthenia. Blood. 106:2671–2679. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
O'Toole TE, Loftus JC, Plow EF, Glass AA,
Harper JR and Ginsberg MH: Efficient surface expression of platelet
GPIIb-IIIa requires both subunits. Blood. 74:14–18. 1989.
View Article : Google Scholar
|
|
25
|
Offermanns S: Activation of platelet
function through G protein-coupled receptors. Circ Res.
99:1293–1304. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ablooglu AJ, Kang J, Petrich BG, Ginsberg
MH and Shattil SJ: Antithrombotic effects of targeting
alphaIIbbeta3 signaling in platelets. Blood. 113:3585–3592. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Petrich BG, Fogelstrand P, Partridge AW,
Yousefi N, Ablooglu AJ, Shattil SJ and Ginsberg MH: The
antithrombotic potential of selective blockade of talin-dependent
integrin alpha IIb beta 3 (platelet GPIIb-IIIa) activation. J Clin
Invest. 117:2250–2259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gibbins JM, Okuma M, Farndale R, Barnes M
and Watson SP: Glycoprotein VI is the collagen receptor in
platelets which underlies tyrosine phosphorylation of the Fc
receptor gamma-chain. FEBS Lett. 413:255–259. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Emsley J, Knight CG, Farndale RW, Barnes
MJ and Liddington RC: Structural basis of collagen recognition by
integrin alpha2beta1. Cell. 101:47–56. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ruggeri ZM: Von Willebrand factor,
platelets and endothelial cell interactions. J Thromb Haemost.
1:1335–1342. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kaushansky K, Lichtman MA and Prchal JT:
Williams Hematology. 9th edition. McGraw Hill Education; New York,
NY: pp. 628–639. 2015
|
|
32
|
Kaushansky K, Lichtman MA and Prchal JT:
Williams Hematology. 9th edition. McGraw Hill Education; New York,
NY: pp. 20472015
|
|
33
|
Elmore SA: Enhanced histopathology of the
spleen. Toxicol Pathol. 34:648–655. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rui Z, Ya-Nan H, Jun Y, Wen-Yu W, Bei-Bei
W and Hui-Ming Z: Pathological research of splenic extramedullary
hematopoie sis in aged rats. J Exp Hematol. 26:268–272. 2018.
|