Trauma involves the regulation of immune
homeostasis, the production and release of various
damage-associated molecular patterns (DAMPs) and the activation of
the innate immune system (1). The
post-traumatic biological response is a complex physiological
phenomenon involving multiple inflammatory and thrombotic
mediators, including cytokines, chemokines, complement receptors,
oxygen free radicals, inflammatory cells (neutrophils, monocytes
and macrophages) and endothelial cells (2). Macrophages exist in mammalian tissues
and have essential functions (3).
Although its origin continues to be controversial, the embryonic
origin of the vital tissue that resides in macrophages is now
understood (4). Macrophages
residing in the majority of tissues are long-lived cells derived
from transient hematopoietic waves of erythro-myeloid progenitors
that emerge in the yolk sac (5).
Although macrophages were previously known for their properties of
host defense and scavenging of apoptotic cells, it is increasingly
recognized that macrophages play a variety of roles in tissue
development, homeostasis control and wound repair (6,7).
Inflammatory monocytes recruited by different mechanisms and
activated tissue-resident macrophages are critical regulatory cells
of tissue repair, regeneration and fibrosis (8). Several of these functional features
are essential for tissue injury and repair. These cells can not
only aggravate tissue damage by generating reactive oxygen species
and other toxic components, but also produce various growth
factors, including VEGF-α and TGF-β, to promote cell proliferation
and repair (9). In the early 1990s,
a type of selectively activated macrophage (type M2) was
identified, which was different from the classical activated
inflammatory macrophage (type M1) (10). M2 macrophages are usually described
by their anti-inflammatory and wound healing effects (11). Macrophages can affect the metabolic
tissue and alter the metabolic pathways. This phenotypic
transformation occurs in glucose and lipid metabolism (12). Macrophages are the principal
participants of the immune response after tissue injury, and their
phenotypes vary from pro-inflammatory (M1) to anti-inflammatory
(M2) macrophages, which can promote wound healing and scar repair
(13). In addition, macrophage
polarization is a highly dynamic process that is easily modulated
by its microenvironment (14).
Furthermore, the reversibility of macrophage polarization also has
an important therapeutic value, particularly in diseases caused by
an M1/M2 imbalance (15).
Acute kidney injury (AKI) has become a global public
health problem with high morbidity, and mortality rates, as well as
high healthcare costs (16). AKI is
a disease characterized by acutely decreased renal function, the
etiology of which can be multifactorial and is associated with
complex pathophysiological mechanisms (17). AKI has been associated with
mortality after traumatic war injuries (18). The pathogenesis of AKI varies with
different types of damage. In ischemia/reperfusion (I/R)-induced
AKI (I/R-AKI), the loss of the brush border of renal tubular
epithelial cells (RTECs) and cell polarization leads to tubular
obstruction, cell necrosis and apoptosis (19). In cisplatin-induced AKI, cisplatin
causes DNA damage and mitochondrial damage, leading to inflammation
and cell death (20). In
contrast-induced AKI (CI-AKI), the formation of vacuoles, swelling
of cells and oxidative stress lead to acute necrosis (21). In sepsis-induced AKI, sepsis may
lead to renal vasodilation caused by inducible nitric oxide
synthase release (19). Apart from
kidney dialysis, there is no treatment for AKI that can reliably
improve survival, reduce injury or accelerate recovery (22). AKI participates in the regulation of
immune system homeostasis, and numerous factors are involved,
including erythropoietin (EPO), glycoprotein non-metastatic
melanoma protein B (Gpnmb), retinoic acid (RA), colony stimulating
factor-1, myoglobin, dendritic cells, neutrophils and macrophages
(23–25). A previous study demonstrated that
macrophages are the principal effectors in AKI inflammatory
responses (26). The dynamic roles
and functional properties of macrophages in AKI are important for
the identification of effective therapeutic targets (27). The activation and functional state
of macrophages after renal injury are complex and diverse.
Macrophages damage or repair renal tubules, and their role in
interstitial fibrosis after renal injury varies with time and is
determined by the type of renal injury (28). Due to the functional plasticity of
macrophages (i.e., their ability to transform between the
pro-inflammatory M1 and anti-inflammatory M2 phenotypes),
macrophages play a complex role in the occurrence and development
of AKI (29). Importantly, the
polarization of macrophages may lead to the development of novel
treatments to promote AKI repair (29). At present, it is considered that M2
macrophages and regulatory T cells are critical cells controlling
inflammation, as well as tissue remodeling and repair following AKI
(30). Previous studies have shown
that M2 macrophage therapy can effectively reduce renal injury in
AKI mice (31,32). The increase in M2 macrophages may
play a pivotal role in the initial damage of human AKI and in the
transition from AKI to chronic kidney disease (CKD) (33). Notably, animal experiments have
shown that total or partial macrophage depletion in AKI may be
beneficial to kidney damage (34).
For example, M1 depletion has been demonstrated to have a universal
protective effect on AKI; however, the results caused by M2
depletion are controversial (34).
The latest research shows that nattokinase and hydrogen-rich
solution produce a therapeutic effect on the inflammatory response
of AKI by regulating the activity of macrophages (35,36).
In summary, at present, the multifaceted role of macrophages in the
occurrence and development of AKI remains open to further
investigation.
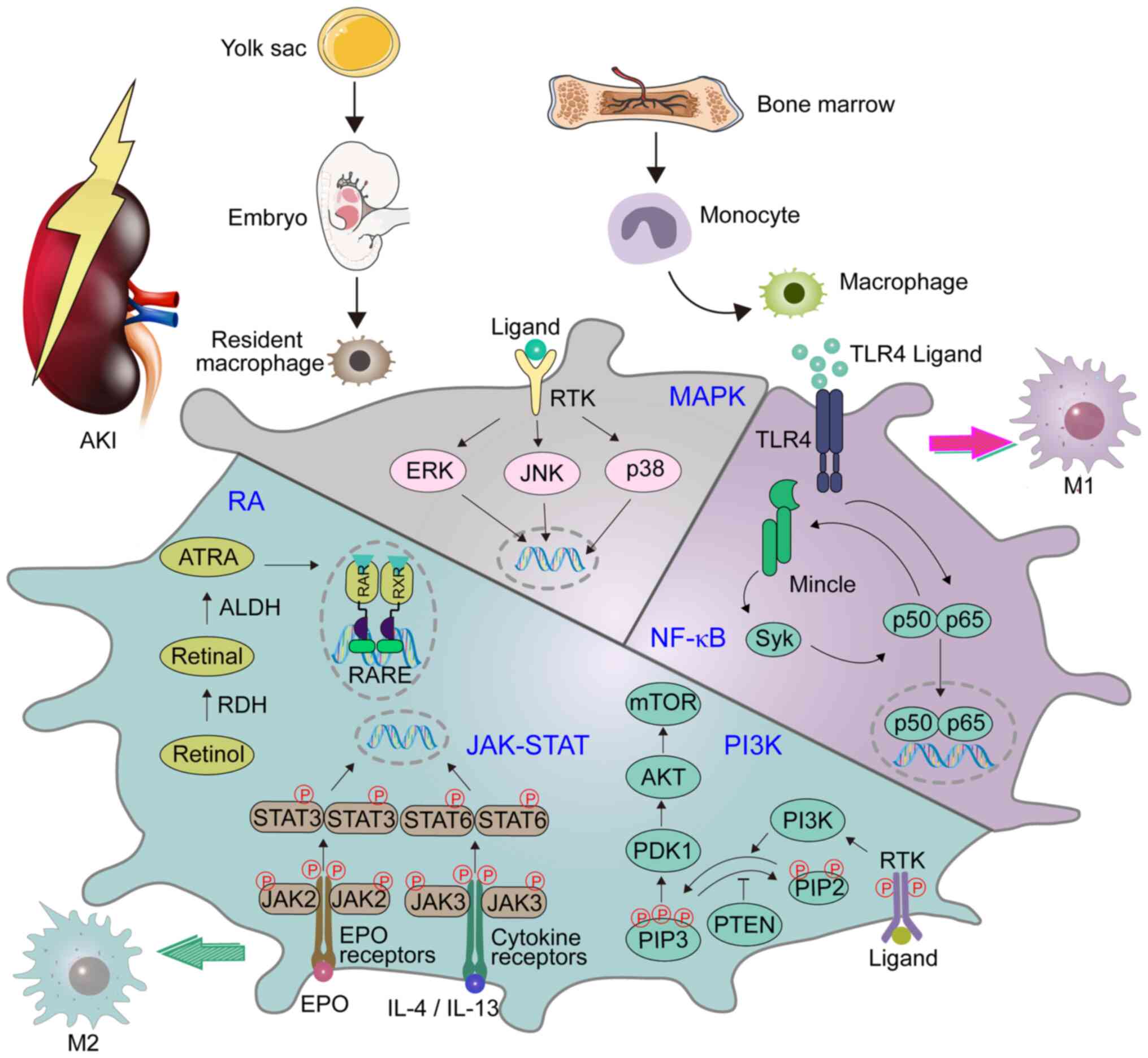
The PI3K pathway is one of the main signaling
pathways that regulates macrophages, and it controls the critical
switch between immune activation and inhibition in the process of
inflammation (37). In addition,
its contribution to macrophage polarization has gradually attracted
the attention of numerous researchers, and been demonstrated to
mediate the transformation of M2 macrophages (38,39).
Certain studies have revealed that PI3Kγ plays a pivotal role in
the polarization of macrophages and in the development of renal
disease. Specifically, a lack of PI3Kγ leads to the polarization of
M1 macrophages, resulting in an inflammatory environment (40,41). A
previous study raised concerns regarding the inhibition of
PI3K/AKT/mTOR in the treatment of AKI (41). Furthermore, previous studies found
that aquaporin 1 (AQP1) can prevent renal tissue damage in AKI
induced by bacterial lipopolysaccharide (LPS) by mediating the
immune response. AQP1 alleviates sepsis-induced AKI by successfully
activating PI3K, eventually leading to macrophages polarization to
the M2 phenotype (Fig. 1) (42). Therefore, targeting PI3K-dependent
M2 macrophage polarization may constitute a novel therapeutic
approach to reducing sepsis-induced AKI.
MAPKs are serine-threonine protein kinases. In
mammals, MAPKs include c-Jun N-terminal kinase, p38 MAPK and
extracellular signal-regulated kinase. Each type exists in
different subtypes of the enzyme and can regulate a variety of
cellular activities, including proliferation, differentiation and
apoptosis (Fig. 1) (58). MAPK participates in the process of
sepsis-induced AKI (59,60). A previous study demonstrated that
the expression of kidney injury molecule-1 (KIM-1) is significantly
increased in both AKI and CKD (61). It has been reported that the MAPK
signaling pathway may play a pivotal role in KIM-1-mediated
macrophage phenotypic transition and migration (62). Li et al (63) demonstrated that AQP1 exerts a
protective effect in the regulation of AKI and attenuates
macrophage-mediated inflammation by downregulating the activity of
p38 MAPK induced by LPS in RAW264.7 cells. Consequently,
pharmacological inhibitors targeting the AQP1-mediated p38 MAPK
signaling pathway may be potential treatments for AKI.
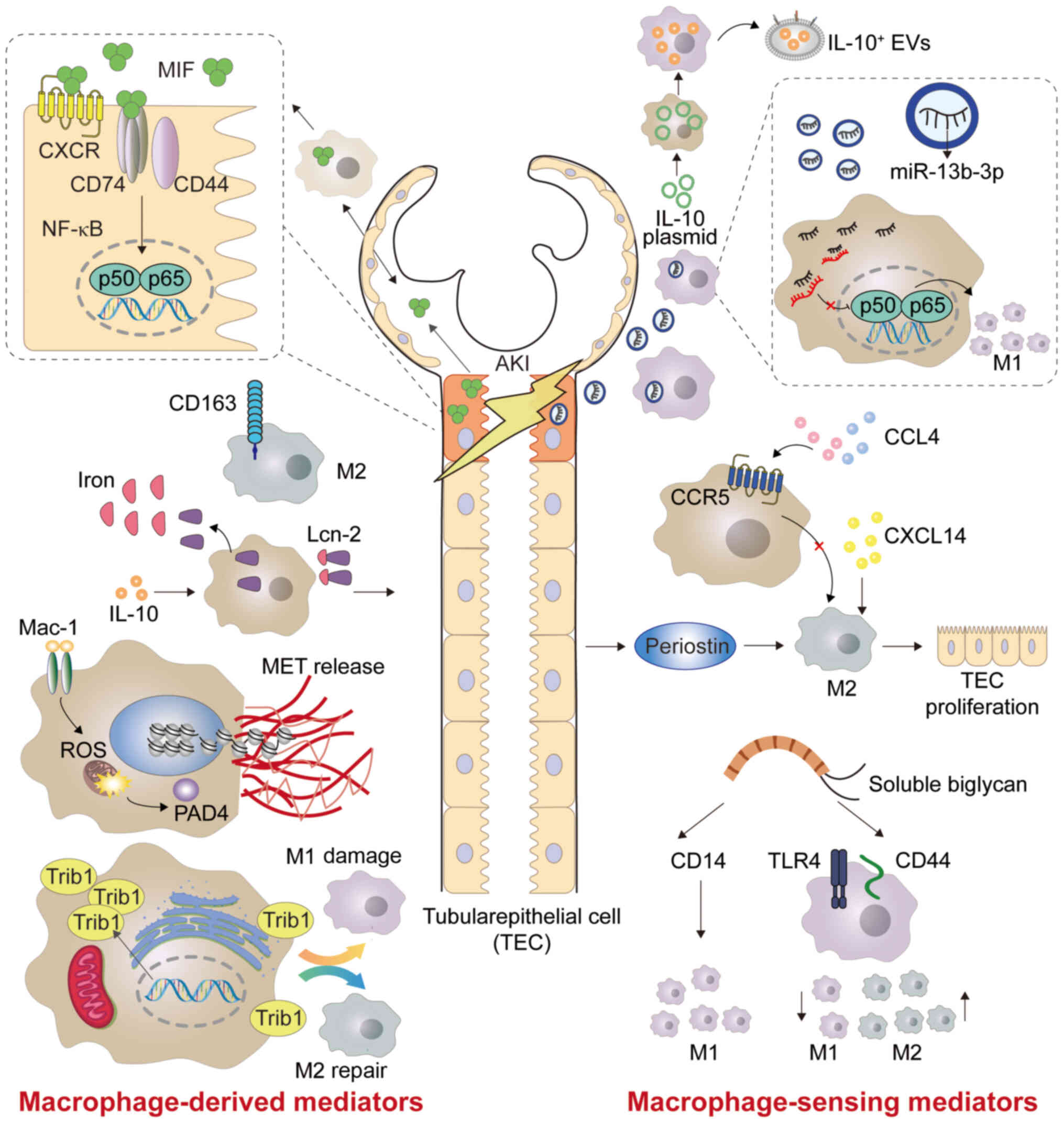
CD163 is a 130 kDa transmembrane receptor protein,
which is mainly expressed by M2c macrophages (83). Urinary soluble CD163 (sCD163) may be
used as a biomarker in certain renal inflammatory diseases
(Fig. 2) (84). Sun et al (85) quantified sCD163 in the urine, and
measured macrophage subtypes in the urine and renal biopsies
(Table I). The authors found that
urinary M1 is associated with interstitial M1 infiltration, while
urinary sCD163 level and M2 subtype are positively correlated with
infiltrating M2 in the glomeruli. The study by Sun et al
(85) also revealed that urinary
sCD163 has an improved diagnostic ability in distinguishing the
disease etiology than that of traditional AKI urinary [Lipocalin-2
(Lcn-2)/neutropil gelatinase-associated lipocalin (NGAL) and
KIM-1], myeloid cell (CD11b) and pan-macrophage (CD68) markers.
Rubio-Navarro et al (86)
developed a targeted probe for CD163 by magnetic resonance imaging
in vivo, thus confirming the presence of CD163-positive
macrophages in human RI-AKI. Myoglobin activates early inflammatory
M1 reaction and partial transformation to the M2 phenotype at a
later stage, in which the high expression of CD163 is due to
activation of heme oxygenase 1 and release of IL-10 (86). Furthermore, the aforementioned study
developed gold-coated iron oxide nanoparticles with anti-CD163
antibody as a carrier, which specifically targeted CD163 in the
kidneys of mice injected with glycerol (86). Using probes targeting CD163
macrophages by magnetic resonance imaging could provide valuable
information on the cellular composition of kidney lesions in
rhabdomyolysis (86).
Lcn-2/ NGAL, is a member of the lipocalin family,
which shares a tight tertiary structure, and ligand binding and
post-translational modifications in Lcn-2 leads to diverse Lcn-2
functions (87). Lcn-2 is
considered a therapeutic target for AKI, CKD and numerous types of
cancer such as breast, esophagus and liver cancer (87–89).
Lcn-2 plays a pivotal role in various kidney diseases, such as
sepsis and IR (90). After renal
injury, Lcn-2 increases rapidly in macrophages, and has highly
stable iron binding and transport capacity (Fig. 2). A study by Urbschat et al
(91) showed that mouse tubular
epithelial cells (TECs) promoted the proliferation of epithelial
cells by taking macrophage-derived iron-loaded Lcn-2. Mertens et
al (92) concluded that the
cellular source of Lcn-2 (TECs or macrophages) and its iron loading
determine the biological function of Lcn-2 in cecal ligation and
puncture (CLP)-induced kidney injury. At 24 h after CLP-induced
kidney injury, elevated levels of iron-free Lcn-2 in TECs are
primarily considered as markers of kidney injury, whereas elevated
levels of iron-loaded Lcn-2 from macrophage sources are associated
with markers of recovery (92)
(Fig. 2; Table I). In summary, Lcn-2 is involved in
renal injury and renal function recovery.
TRIB family members are pseudokinase proteins that
are conserved among species and are associated with various human
diseases such as leukemia and metabolic disorders (99). Members of the TRIB family are basic
regulators of the cell cycle, proliferation, differentiation,
metabolism and cellular stress (100). Among them, TRIB1 can control the
differentiation of M2 macrophages (101), and its deficiency results in a
marked reduction of M2 macrophages not only in the bone marrow, but
also in adipose, lung and spleen tissue (102). TRIB1 may widely control the
polarization of M1/M2 macrophages via the JAK/STAT signaling
pathway (Figs. 2 and 3) (103).
It was previously demonstrated that, during the period of moderate
AKI adaptive recovery induced by I/R, TRIB1 regulates the
proliferation of renal tubular cells by affecting the polarization
of macrophages, thus playing a role in renal recovery and
regeneration (104) (Table I). Therefore, TRIB1 may be a
promising target for improving adaptive renal repair after I/R
injury.
Chemokines are a large family of small, secreted
proteins that play a fundamental role in the development and
dynamic balance of the immune system. Chemokine signals are sent
through cell surface G-protein coupled heptahelical chemokine
receptors (105). Chemokines play
a long-term role in physiological and pathological regulation by
inducing macrophage differentiation and polarization (106). C-X-C motif chemokine ligand 14
(CXCL14) is a relatively new CXC type of chemokine, which is
constitutively expressed in breast, kidney and other epithelial
tissues (107). A previous study
indicated that its overexpression inhibits M1 polarization and
increases M2 polarization. This suggested that the overexpression
of CXCL14 may reduce AKI caused by sepsis by downregulating the
production of macrophage-derived cytokines (Fig. 2) (108). Chemokine receptor 5 (CCR5) is a
pivotal regulator of the inflammatory cascade response of
macrophages in the kidney, and its deficiency contributes to the
activation of M2 macrophages (Fig.
2; Table I) (109). Therefore, blocking CCR5 may be
helpful in the treatment of I/R-AKI. It has been observed that the
deficiency of C-C motif CCR2 can prevent the migration of
Ly6C+ macrophages from the bone marrow to the injured
site, thus reducing ischemic-AKI in mice (110). In the activation of the
renin-angiotensin system, C-C motif chemokine ligand 5 (CCL5)
paradoxically limits the accumulation of macrophages in the injured
kidney by inhibiting the pro-inflammatory effect of CCL2 (111).
Periostin is a type of cellular-matrix protein of 90
kDa in size, which is highly expressed in bone and tooth tissues
(112). As a recently identified
biomarker of renal disease, periostin is mainly associated with CKD
(113). However, Kormann et
al (114) demonstrated that
periostin can increase the production of M2 macrophages in the
stage of AKI repair, as well as assist the repair of RTECs by
promoting the proliferation of macrophages and the secretion of
regenerating factors in the kidney (Table I).
Biglycan is a small proteoglycan, abundant in
leucine, which acts as a danger signal derived from the
extracellular matrix in a soluble form, and it is a high-affinity
ligand of CD14 in macrophages (115). The lack of CD14 prevents
biglycan-mediated cytokine expression, macrophage recruitment and
polarization to M1 macrophages. It also leads to a decrease in the
activation of the biglycan-TLR2/4 signaling pathway, thus improving
renal function (Figs. 2 and
3) (116). Poluzzi et al (117) showed that CD44 is a novel biglycan
signal coreceptor, and its interaction with biglycan is necessary
for TLR4/CD44-dependent autophagy in macrophages. Interfering with
the interaction between biglycan and specific TLR coreceptors may
constitute a novel therapeutic intervention to reduce renal
inflammation and injury. Specifically, the role of biglycan in
inflammation exceeds its function as a typical danger signal, and
it is the first ligand, among numerous types of DAMPs, that has
been reported to promote the polarization of M1 and M2 macrophages
simultaneously through the transduction of different coreceptor
signals (118) (Table I). Biglycan binds to CD14 to promote
tissue inflammation, while it binds to CD44 to induce autophagy in
M1 macrophages and then promote tissue remodeling (119).
Extracellular vesicles (EVs) refer to the membrane
structures released by all types of cells through different
biogenic pathways (120). EVs are
intercellular messengers that are involved in a wide range of
physiological and pathological processes (121). EVs have the advantages of
exhibiting stable physical and chemical properties (122). Therefore, EVs have been used as
natural carriers in drug delivery systems in recent years (123). It was previously shown that
macrophage-derived EVs have a pro-inflammatory effect (124). By contrast, Tang et al
(125) demonstrated that
macrophages secrete IL-10+ EVs under dexamethasone
stimulation, which can promote the transformation of macrophages
into the M2 phenotype (Fig. 2;
Table I).
Exosomes are nanoscale EVs that play a key role in
intercellular communication and signal transduction. They also play
a pivotal role in the processes of inflammation and immune response
(126). In recent years, a growing
body of evidence has found that exosomes participate in the
pathogenesis of renal disease (127,128). Among them, exosomal microRNA
(miR)-19b-3p may be a promising therapeutic target for renal
disease (129). Tubulointerstitial
inflammation is an important pathological feature of AKI. Lv et
al (130) found that exosomal
miR-19b-3p mediates the interaction between injured RTECs and
macrophages, which leads to the activation of M1 macrophages and
indicates that the exosome/miR-19b-3p/suppressor of the cytokine
signaling axis plays a pivotal role in the development of renal
tubulointerstitial inflammation (Fig.
2; Table I). Furthermore,
previous studies have demonstrated that the release of
hypoxia-inducible factor-1α-dependent miR-23a-enriched exosomes
from hypoxic TECs can activate macrophages and promote
tubulointerstitial inflammation (131) (Table
I). In addition, blocking the exosome-mediated transfer of
miR-23a between RTECs and macrophages may be an innovative method
for enhancing renal tubulointerstitial inflammation. In summary,
exosomes are involved in adjusting the routine function of the
kidney, as well as in promoting and inhibiting various
pathophysiological reactions (121).
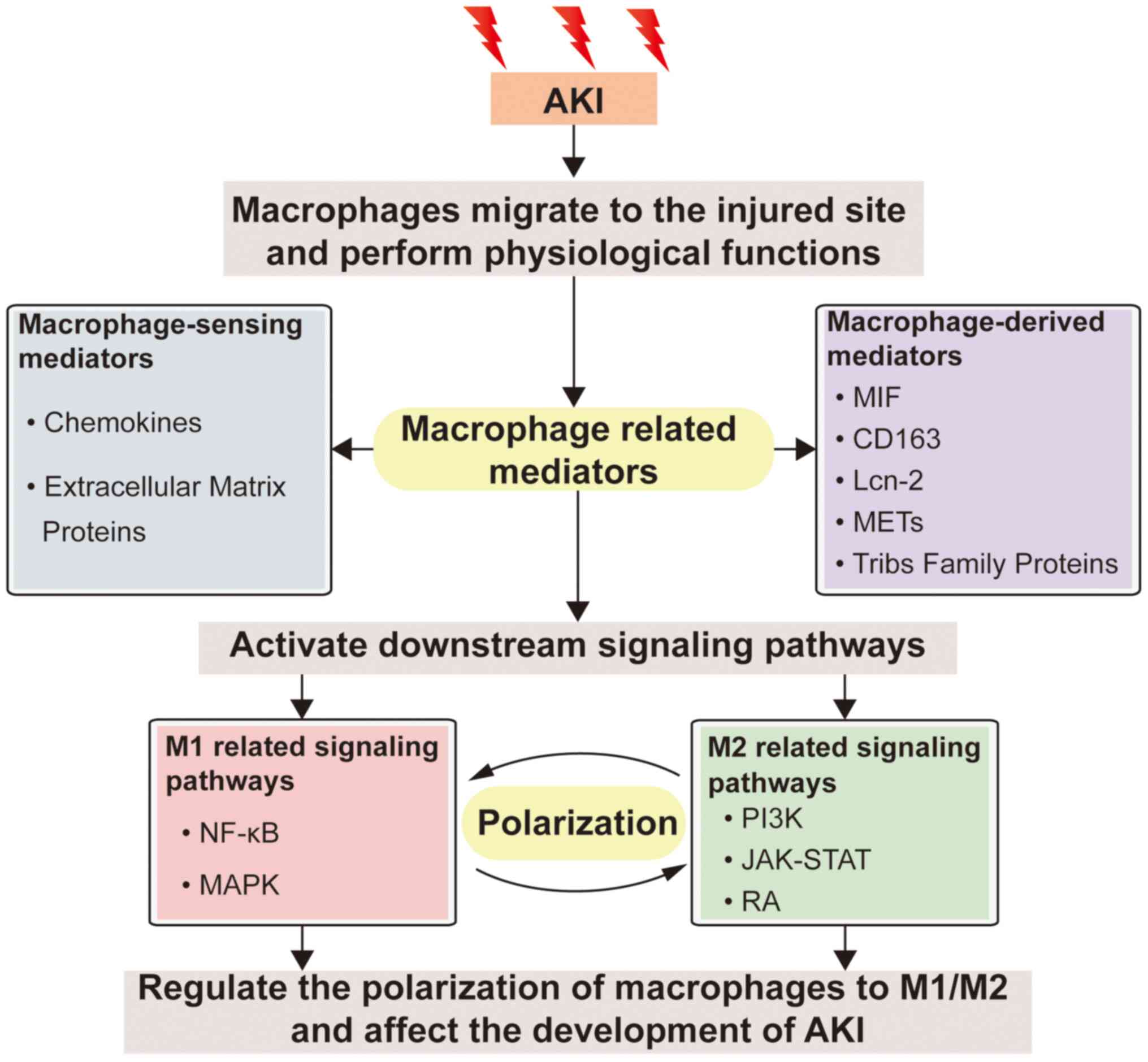
AKI may be caused by a variety of factors and
injuries, with a high incidence among critically ill patients
(132). When AKI occurs,
macrophages, as one of the most significant types of immune cells,
are recruited to the injury site to perform their physiological
functions. M1 macrophages can aggravate the inflammatory response
in order to eliminate potential pathogens. However, excessive
inflammatory response aggravates cell and tissue damage. M2
macrophages promote immune suppression and tissue regeneration
(28,133). Therefore, the application of M2
macrophages and macrophage-modulating agents is a current research
hotspot in the treatment of AKI (11,23).
It was previously demonstrated that M2 macrophages can cause the
transition from AKI to CKD, which increases the risk of CKD among
AKI survivors (34). Recent studies
on the role of macrophages in the pathogenesis of AKI suggest the
complication and diversity of macrophage activation and the
functional status after renal injury (134,135). The multifaceted role of
macrophages in AKI is mainly mediated by PI3K, JAK/STAT, RA,
NF-κB, MAPK and other signaling pathways (Fig. 1), which may regulate macrophage
polarization or oxidative stress (42,44,56,59,68).
Among them, the RA signaling pathway is considered to be the
current research hotspot, because it not only has an
immunosuppressive effect, but also participates in the inflammatory
response. Elucidating the mechanism of the RA signaling pathway in
AKI and its association with macrophages has great clinical
application prospects (136).
Macrophage-derived and -sensing mediators such as MIF, CD163,
Lcn-2, METs, TRIB, and biglycan (Fig.
2; Table I) also play an
essential role in AKI. Fig. 3 shows
the association between macrophage-associated mediators and
downstream signaling pathways after AKI. However, the signaling
pathway that macrophage mediators participate in may be a complex
network, and further research is needed to verify whether there is
interaction between different pathways. A thorough understanding of
the biological role of macrophages in the process of injury and
repair is a necessary condition for understanding the limitations
and further applicability of macrophages in therapy. Therefore,
future studies should elucidate the mechanism and timing of
macrophage polarization, as well as the precise regulatory
mechanisms of macrophages in the occurrence and development of AKI,
which will contribute to the understanding and identification of
novel therapeutic targets for AKI.
Not applicable.
This research was supported by grants from The Open
Fund of State Key Laboratory of Medicinal Chemical Biology (Nankai
University; grant no. 2020010) and The Tianjin University ‘Double
first class’ construction talent start-up fund.
Not applicable.
NL and JC conceived and designed the review,
researched the literature and wrote the manuscript. PW contributed
to literature collection and figure preparation. SH, HF and YG
designed the study, critically revised and supervised the
manuscript. Data authentication is not applicable. All authors read
and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Messerer DAC, Halbgebauer R, Nilsson B,
Pavenstädt H, Radermacher P and Huber-Lang M: Immunopathophysiology
of trauma-related acute kidney injury. Nat Rev Nephrol. 17:91–111.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eppensteiner J, Davis RP, Barbas AS, Kwun
J and Lee J: Immunothrombotic activity of damage-associated
molecular patterns and extracellular vesicles in secondary organ
failure induced by trauma and sterile insults. Front Immunol.
9:1902018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wynn TA, Chawla A and Pollard JW:
Macrophage biology in development, homeostasis and disease. Nature.
496:445–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ginhoux F and Guilliams M: Tissue-resident
macrophage ontogeny and homeostasis. Immunity. 44:439–449. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gomez Perdiguero E, Klapproth K, Schulz C,
Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF,
Geissmann F and Rodewald HR: Tissue-resident macrophages originate
from yolk-sac-derived erythro-myeloid progenitors. Nature.
518:547–551. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Okabe Y and Medzhitov R: Tissue biology
perspective on macrophages. Nat Immunol. 17:9–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim SY and Nair MG: Macrophages in wound
healing: Activation and plasticity. Immunol Cell Biol. 97:258–267.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wynn TA and Vannella KM: Macrophages in
tissue repair, regeneration, and fibrosis. Immunity. 44:450–462.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vannella KM and Wynn TA: Mechanisms of
organ injury and repair by macrophages. Annu Rev Physiol.
79:593–617. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stein M, Keshav S, Harris N and Gordon S:
Interleukin 4 potently enhances murine macrophage mannose receptor
activity: A marker of alternative immunologic macrophage
activation. J Exp Med. 176:287–292. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen T, Cao Q, Wang Y and Harris DCH: M2
macrophages in kidney disease: Biology, therapies, and
perspectives. Kidney Int. 95:760–773. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Verdeguer F and Aouadi M: Macrophage
heterogeneity and energy metabolism. Exp Cell Res. 360:35–40. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Smigiel KS and Parks WC: Macrophages,
wound healing, and fibrosis: Recent insights. Curr Rheumatol Rep.
20:172018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shapouri-Moghaddam A, Mohammadian S,
Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi
A, Afshari JT and Sahebkar A: Macrophage plasticity, polarization,
and function in health and disease. J Cell Physiol. 233:6425–6440.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Funes SC, Rios M, Escobar-Vera J and
Kalergis AM: Implications of macrophage polarization in
autoimmunity. Immunology. 154:186–195. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Levey AS and James MT: Acute kidney
injury. Ann Intern Med. 167:ITC66–ITC80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gameiro J, Fonseca JA, Outerelo C and
Lopes JA: Acute kidney injury: From diagnosis to prevention and
treatment strategies. J Clin Med. 9:17042020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stewart IJ, Sosnov JA, Howard JT and Chung
KK: Acute kidney injury in critically injured combat veterans: A
retrospective cohort study. Am J Kidney Dis. 68:564–570. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rahbar Saadat Y, Hosseiniyan Khatibi SM,
Ardalan M, Barzegari A and Zununi Vahed S: Molecular
pathophysiology of acute kidney injury: The role of sirtuins and
their interactions with other macromolecular players. J Cell
Physiol. 236:3257–3274. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maekawa H, Inoue T, Ouchi H, Jao TM, Inoue
R, Nishi H, Fujii R, Ishidate F, Tanaka T, Tanaka Y, et al:
Mitochondrial damage causes inflammation via cGAS-STING signaling
in acute kidney injury. Cell Rep. 29:1261–1273.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ward DB and Valentovic MA: Contrast
induced acute kidney injury and direct cytotoxicity of iodinated
radiocontrast media on renal proximal tubule cells. J Pharmacol Exp
Ther. 370:160–171. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bellomo R, Kellum JA and Ronco C: Acute
kidney injury. Lancet. 380:756–766. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang S, Zhang C, Li J, Niyazi S, Zheng L,
Xu M, Rong R, Yang C and Zhu T: Erythropoietin protects against
rhabdomyolysis-induced acute kidney injury by modulating macrophage
polarization. Cell Death Dis. 8:e27252017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou L, Zhuo H, Ouyang H, Liu Y, Yuan F,
Sun L, Liu F and Liu H: Glycoprotein non-metastatic melanoma
protein b (Gpnmb) is highly expressed in macrophages of acute
injured kidney and promotes M2 macrophages polarization. Cell
Immunol. 316:53–60. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu J, Wan X, Zhang H, Li W, Ma M, Pan B,
Liang X and Cao C: Retinoic acid attenuates contrast-induced acute
kidney injury in a miniature pig model. Biochem Biophys Res Commun.
512:163–169. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huen SC and Cantley LG:
Macrophage-mediated injury and repair after ischemic kidney injury.
Pediatr Nephrol. 30:199–209. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jang HR and Rabb H: Immune cells in
experimental acute kidney injury. Nat Rev Nephrol. 11:88–101. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huen SC and Cantley LG: Macrophages in
renal injury and repair. Annu Rev Physiol. 79:449–469. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Han HI, Skvarca LB, Espiritu EB, Davidson
AJ and Hukriede NA: The role of macrophages during acute kidney
injury: Destruction and repair. Pediatr Nephrol. 34:561–569. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Singbartl K, Formeck CL and Kellum JA:
Kidney-immune system crosstalk in AKI. Semin Nephrol. 39:96–106.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li X, Mu G, Song C, Zhou L, He L, Jin Q
and Lu Z: Role of M2 Macrophages in Sepsis-induced acute kidney
injury. Shock. 50:233–239. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mao R, Wang C, Zhang F, Zhao M, Liu S,
Liao G, Li L, Chen Y, Cheng J, Liu J and Lu Y: Peritoneal M2
macrophage transplantation as a potential cell therapy for
enhancing renal repair in acute kidney injury. J Cell Mol Med.
24:3314–3327. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim MG, Lim K, Lee YJ, Yang J, Oh SW, Cho
WY and Jo SK: M2 macrophages predict worse long-term outcomes in
human acute tubular necrosis. Sci Rep. 10:21222020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baek JH: The impact of versatile
macrophage functions on acute kidney injury and its outcomes. Front
Physiol. 10:10162019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu H, Wang Y, Zhang Y, Xu F, Chen J, Duan
L, Zhang T, Wang J and Zhang F: Breaking the vicious loop between
inflammation, oxidative stress and coagulation, a novel
anti-thrombus insight of nattokinase by inhibiting LPS-induced
inflammation and oxidative stress. Redox Biol. 32:1015002020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yao W, Guo A, Han X, Wu S, Chen C, Luo C,
Li H, Li S and Hei Z: Aerosol inhalation of a hydrogen-rich
solution restored septic renal function. Aging (Albany NY).
11:12097–12113. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kaneda MM, Messer KS, Ralainirina N, Li H,
Leem CJ, Gorjestani S, Woo G, Nguyen AV, Figueiredo CC, Foubert P,
et al: PI3Kγ is a molecular switch that controls immune
suppression. Nature. 539:437–442. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qin S, Li J, Zhou C, Privratsky B,
Schettler J, Deng X, Xia Z, Zeng Y, Wu H and Wu M: SHIP-1 regulates
phagocytosis and M2 polarization through the PI3K/Akt-STAT5-Trib1
circuit in pseudomonas aeruginosa infection. Front Immunol.
11:3072020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Duan Y, Zheng H, Li Z, Yao Y, Ding J, Wang
X, Nakkala JR, Zhang D, Wang Z, Zuo X, et al: Unsaturated
polyurethane films grafted with enantiomeric polylysine promotes
macrophage polarization to a M2 phenotype through PI3K/Akt1/mTOR
axis. Biomaterials. 246:1200122020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
An C, Wen J, Hu Z, Mitch WE and Wang Y:
Phosphoinositide 3-kinase γ deficiency attenuates kidney injury and
fibrosis in angiotensin II-induced hypertension. Nephrol Dial
Transplant. 35:1491–1500. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Amano MT, Castoldi A, Andrade-Oliveira V,
Latancia MT, Terra FF, Correa-Costa M, Breda CNS, Felizardo RJF,
Pereira WO, da Silva MB, et al: The lack of PI3Kγ favors M1
macrophage polarization and does not prevent kidney diseases
progression. Int Immunopharmacol. 64:151–161. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu C, Li B, Tang K, Dong X, Xue L, Su G
and Jin Y: Aquaporin 1 alleviates acute kidney injury via
PI3K-mediated macrophage M2 polarization. Inflamm Res. 69:509–521.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Markó L, Vigolo E, Hinze C, Park JK, Roël
G, Balogh A, Choi M, Wübken A, Cording J, Blasig IE, et al: Tubular
epithelial NF-kappaB activity regulates ischemic AKI. J Am Soc
Nephrol. 27:2658–2669. 2016. View Article : Google Scholar
|
|
44
|
Huang RS, Zhou JJ, Feng YY, Shi M, Guo F,
Gou SJ, Salerno S, Ma L and Fu P: Pharmacological inhibition of
macrophage toll-like receptor 4/Nuclear Factor-kappa B alleviates
rhabdomyolysis-induced acute kidney injury. Chin Med J (Engl).
130:2163–2169. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shu B, Feng Y, Gui Y, Lu Q, Wei W, Xue X,
Sun X, He W, Yang J and Dai C: Blockade of CD38 diminishes
lipopolysaccharide-induced macrophage classical activation and
acute kidney injury involving NF-κB signaling suppression. Cell
Signal. 42:249–258. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Y, Quan F, Cao Q, Lin Y, Yue C, Bi R,
Cui X, Yang H, Yang Y, Birnbaumer L, et al: Quercetin alleviates
acute kidney injury by inhibiting ferroptosis. J Adv Res.
28:231–243. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lu H, Wu L, Liu L, Ruan Q, Zhang X, Hong
W, Wu S, Jin G and Bai Y: Quercetin ameliorates kidney injury and
fibrosis by modulating M1/M2 macrophage polarization. Biochem
Pharmacol. 154:203–212. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tan RZ, Wang C, Deng C, Zhong X, Yan Y,
Luo Y, Lan HY, He T and Wang L: Quercetin protects against
cisplatin-induced acute kidney injury by inhibiting
Mincle/Syk/NF-κB signaling maintained macrophage inflammation.
Phytother Res. 34:139–152. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tan RZ, Liu J, Zhang YY, Wang HL, Li JC,
Liu YH, Zhong X, Zhang YW, Yan Y, Lan HY and Wang L: Curcumin
relieved cisplatin-induced kidney inflammation through inhibiting
Mincle-maintained M1 macrophage phenotype. Phytomedicine.
52:284–294. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hui D, Rui-Zhi T, Jian-Chun L, Xia Z, Dan
W, Jun-Ming F and Li W: Astragalus propinquus Schischkin and
Panax notoginseng (A&P) compound relieved
cisplatin-induced acute kidney injury through inhibiting the mincle
maintained macrophage inflammation. J Ethnopharmacol.
252:1126372020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhou J, Bai Y, Jiang Y, Tarun P, Feng Y,
Huang R and Fu P: Immunomodulatory role of recombinant human
erythropoietin in acute kidney injury induced by crush syndrome via
inhibition of the TLR4/NF-κB signaling pathway in macrophages.
Immunopharmacol Immunotoxicol. 42:37–47. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu P, Shen P, Yu B, Xu X, Ge R, Cheng X,
Chen Q, Bian J, Li Z and Wang J: Janus kinases (JAKs): The
efficient therapeutic targets for autoimmune diseases and
myeloproliferative disorders. Eur J Med Chem. 192:1121552020.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bousoik E and Montazeri Aliabadi H: ‘Do We
Know Jack’ About JAK? A closer Look at JAK/STAT signaling pathway.
Front Oncol. 8:2872018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Banerjee S, Biehl A, Gadina M, Hasni S and
Schwartz DM: JAK-STAT signaling as a target for inflammatory and
autoimmune diseases: Current and future prospects. Drugs.
77:521–546. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhu M, Wang L, Yang J, Xie K, Zhu M, Liu
S, Xu C, Wang J, Gu L, Ni Z, et al: Erythropoietin ameliorates lung
injury by accelerating pulmonary endothelium cell proliferation via
janus kinase-signal transducer and activator of transcription 3
pathway after kidney ischemia and reperfusion injury. Transplant
Proc. 51:972–978. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang MZ, Wang X, Wang Y, Niu A, Wang S,
Zou C and Harris RC: IL-4/IL-13-mediated polarization of renal
macrophages/dendritic cells to an M2a phenotype is essential for
recovery from acute kidney injury. Kidney Int. 91:375–386. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
van der Lienden MJC, Gaspar P, Boot R,
Aerts JMFG and van Eijk M: Glycoprotein non-metastatic protein B:
An emerging biomarker for lysosomal dysfunction in macrophages. Int
J Mol Sci. 20:662018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kim EK and Choi EJ: Compromised MAPK
signaling in human diseases: An update. Arch Toxicol. 89:867–882.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ren Q, Guo F, Tao S, Huang R, Ma L and Fu
P: Flavonoid fisetin alleviates kidney inflammation and apoptosis
via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways
in septic AKI mice. Biomed Pharmacother. 122:1097722020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sun S, Wang J, Wang J, Wang F, Yao S and
Xia H: Maresin 1 mitigates sepsis-associated acute kidney injury in
mice via inhibition of the NF-κB/STAT3/MAPK pathways. Front
Pharmacol. 10:13232019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wen Y and Parikh CR: Current concepts and
advances in biomarkers of acute kidney injury. Crit Rev Clin Lab
Sci. 1–24. 2021.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tian L, Shao X, Xie Y, Wang Q, Che X,
Zhang M, Xu W, Xu Y, Mou S and Ni Z: Kidney injury molecule-1 is
elevated in nephropathy and mediates macrophage activation via the
mapk signalling pathway. Cell Physiol Biochem. 41:769–783. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Li B, Liu C, Tang K, Dong X, Xue L, Su G,
Zhang W and Jin Y: Aquaporin-1 attenuates macrophage-mediated
inflammatory responses by inhibiting p38 mitogen-activated protein
kinase activation in lipopolysaccharide-induced acute kidney
injury. Inflamm Res. 68:1035–1047. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Conserva MR, Anelli L, Zagaria A, Specchia
G and Albano F: The pleiotropic role of retinoic acid/retinoic acid
receptors signaling: From vitamin A metabolism to gene
rearrangements in acute promyelocytic leukemia. Int J Mol Sci.
20:29212019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Cunningham TJ and Duester G: Mechanisms of
retinoic acid signalling and its roles in organ and limb
development. Nat Rev Mol Cell Biol. 16:110–123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ghyselinck NB and Duester G: Retinoic acid
signaling pathways. Development. 146:dev1675022019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Vellozo NS, Pereira-Marques ST,
Cabral-Piccin MP, Filardy AA, Ribeiro-Gomes FL, Rigoni TS, DosReis
GA and Lopes MF: All-Trans Retinoic Acid Promotes an M1- to
M2-Phenotype shift and inhibits macrophage-mediated immunity to
leishmania major. Front Immunol. 8:15602017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chiba T, Skrypnyk NI, Skvarca LB, Penchev
R, Zhang KX, Rochon ER, Fall JL, Paueksakon P, Yang H, Alford CE,
et al: Retinoic acid signaling coordinates macrophage-dependent
injury and repair after AKI. J Am Soc Nephrol. 27:495–508. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Brilli Skvarca L, Han HI, Espiritu EB,
Missinato MA, Rochon ER, McDaniels MD, Bais AS, Roman BL, Waxman
JS, Watkins SC, et al: Enhancing regeneration after acute kidney
injury by promoting cellular dedifferentiation in zebrafish. Dis
Model Mech. 12:dmm0373902019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Harris J, VanPatten S, Deen NS, Al-Abed Y
and Morand EF: Rediscovering MIF: New tricks for an old cytokine.
Trends Immunol. 40:447–462. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kang I and Bucala R: The immunobiology of
MIF: Function, genetics and prospects for precision medicine. Nat
Rev Rheumatol. 15:427–437. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Calandra T and Roger T: Macrophage
migration inhibitory factor: A regulator of innate immunity. Nat
Rev Immunol. 3:791–800. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Averdunk L, Bernhagen J, Fehnle K, Surowy
H, Lüdecke HJ, Mucha S, Meybohm P, Wieczorek D, Leng L, Marx G, et
al: The macrophage migration inhibitory factor (MIF) promoter
polymorphisms (rs3063368, rs755622) predict acute kidney injury and
death after cardiac surgery. J Clin Med. 9:29362020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hong MY, Tseng CC, Chuang CC, Chen CL, Lin
SH and Lin CF: Urinary macrophage migration inhibitory factor
serves as a potential biomarker for acute kidney injury in patients
with acute pyelonephritis. Mediators Inflamm. 2012:3813582012.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wu B, Chen J and Yang Y: Biomarkers of
acute kidney injury after cardiac surgery: A narrative review.
Biomed Res Int. 2019:72986352019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Fuhrman DY and Kellum JA: Epidemiology and
pathophysiology of cardiac surgery-associated acute kidney injury.
Curr Opin Anaesthesiol. 30:60–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Stoppe C, Averdunk L, Goetzenich A,
Soppert J, Marlier A, Kraemer S, Vieten J, Coburn M, Kowark A, Kim
BS, et al: The protective role of macrophage migration inhibitory
factor in acute kidney injury after cardiac surgery. Sci Transl
Med. 10:eaan48862018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Leaver SK, MacCallum NS, Pingle V, Hacking
MB, Quinlan GJ, Evans TW and Burke-Gaffney A: Increased plasma
thioredoxin levels in patients with sepsis: Positive association
with macrophage migration inhibitory factor. Intensive Care Med.
36:336–341. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Nishida K, Watanabe H, Ogaki S, Kodama A,
Tanaka R, Imafuku T, Ishima Y, Chuang VT, Toyoda M, Kondoh M, et
al: Renoprotective effect of long acting thioredoxin by modulating
oxidative stress and macrophage migration inhibitory factor against
rhabdomyolysis-associated acute kidney injury. Sci Rep.
5:144712015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Li J, Tang Y, Tang PMK, Lv J, Huang XR,
Carlsson-Skwirut C, Da Costa L, Aspesi A, Fröhlich S, Szczęśniak P,
et al: Blocking macrophage migration inhibitory factor protects
against cisplatin-induced acute kidney injury in mice. Mol Ther.
26:2523–2532. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Li JH, Tang Y, Lv J, Wang XH, Yang H, Tang
PMK, Huang XR, He ZJ, Zhou ZJ, Huang QY, et al: Macrophage
migration inhibitory factor promotes renal injury induced by
ischemic reperfusion. J Cell Mol Med. 23:3867–3877. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Lv J, Huang XR, Klug J, Fröhlich S, Lacher
P, Xu A, Meinhardt A and Lan HY: Ribosomal protein S19 is a novel
therapeutic agent in inflammatory kidney disease. Clin Sci (Lond).
124:627–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Matsushita T and Takehara K: Soluble CD163
is a potential biomarker in systemic sclerosis. Expert Rev Mol
Diagn. 19:197–199. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Mejia-Vilet JM, Zhang XL, Cruz C,
Cano-Verduzco ML, Shapiro JP, Nagaraja HN, Morales-Buenrostro LE
and Rovin BH: Urinary soluble CD163: A novel noninvasive biomarker
of activity for lupus nephritis. J Am Soc Nephrol. 31:1335–1347.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sun PP, Zhou XJ, Su JQ, Wang C, Yu XJ, Su
T, Liu G, Wang SX, Nie J and Yang L: Urine macrophages reflect
kidney macrophage content during acute tubular interstitial and
glomerular injury. Clin Immunol. 205:65–74. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Rubio-Navarro A, Carril M, Padro D,
Guerrero-Hue M, Tarín C, Samaniego R, Cannata P, Cano A, Villalobos
JM, Sevillano ÁM, et al: CD163-Macrophages are involved in
rhabdomyolysis-induced kidney injury and may be detected by MRI
with targeted gold-coated iron oxide nanoparticles. Theranostics.
6:896–914. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Li D, Yan Sun W, Fu B, Xu A and Wang Y:
Lipocalin-2-The myth of its expression and function. Basic Clin
Pharmacol Toxicol. 127:142–151. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Rahimi S, Roushandeh AM, Ahmadzadeh E,
Jahanian-Najafabadi A and Roudkenar MH: Implication and role of
neutrophil gelatinase-associated lipocalin in cancer: Lipocalin-2
as a potential novel emerging comprehensive therapeutic target for
a variety of cancer types. Mol Biol Rep. 47:2327–2346. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Santiago-Sánchez GS, Pita-Grisanti V,
Quiñones-Díaz B, Gumpper K, Cruz-Monserrate Z and Vivas-Mejía PE:
Biological functions and therapeutic potential of lipocalin 2 in
cancer. Int J Mol Sci. 21:43652020. View Article : Google Scholar
|
|
90
|
Desanti De Oliveira B, Xu K, Shen TH,
Callahan M, Kiryluk K, D'Agati VD, Tatonetti NP, Barasch J and
Devarajan P: Molecular nephrology: Types of acute tubular injury.
Nat Rev Nephrol. 15:599–612. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Urbschat A, Thiemens AK, Mertens C,
Rehwald C, Meier JK, Baer PC and Jung M: Macrophage-secreted
Lipocalin-2 promotes regeneration of injured primary murine renal
tubular epithelial cells. Int J Mol Sci. 21:20382020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Mertens C, Kuchler L, Sola A, Guiteras R,
Grein S, Brüne B, von Knethen A and Jung M: Macrophage-derived
iron-bound lipocalin-2 correlates with renal recovery markers
following sepsis-induced kidney damage. Int J Mol Sci. 21:75272020.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Brinkmann V, Reichard U, Goosmann C,
Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y and Zychlinsky A:
Neutrophil extracellular traps kill bacteria. Science.
303:1532–1535. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Doster RS, Rogers LM, Gaddy JA and Aronoff
DM: Macrophage extracellular traps: A scoping review. J Innate
Immun. 10:3–13. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Nakazawa D, Marschner JA, Platen L and
Anders HJ: Extracellular traps in kidney disease. Kidney Int.
94:1087–1098. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Hartl D: Macrophages and platelets join
forces to release kidney-damaging DNA traps. Nat Med. 24:128–129.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Okubo K, Kurosawa M, Kamiya M, Urano Y,
Suzuki A, Yamamoto K, Hase K, Homma K, Sasaki J, Miyauchi H, et al:
Macrophage extracellular trap formation promoted by platelet
activation is a key mediator of rhabdomyolysis-induced acute kidney
injury. Nat Med. 24:232–238. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Lichtman A: The kidney gets caught in a
macrophage trap. Sci Immunol. 3:eaat37452018. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Satoh T, Kidoya H, Naito H, Yamamoto M,
Takemura N, Nakagawa K, Yoshioka Y, Morii E, Takakura N, Takeuchi O
and Akira S: Critical role of Trib1 in differentiation of
tissue-resident M2-like macrophages. Nature. 495:524–528. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Eyers PA, Keeshan K and Kannan N: Tribbles
in the 21st century: The evolving roles of tribbles pseudokinases
in biology and disease. Trends Cell Biol. 27:284–298. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Liu ZZ, Han ZD, Liang YK, Chen JX, Wan S,
Zhuo YJ, Cai ZD, Deng YL, Lin ZY, Mo RJ, et al: TRIB1 induces
macrophages to M2 phenotype by inhibiting IKB-zeta in prostate
cancer. Cell Signal. 59:152–162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Shiraishi M, Shintani Y, Shintani Y,
Ishida H, Saba R, Yamaguchi A, Adachi H, Yashiro K and Suzuki K:
Alternatively activated macrophages determine repair of the
infarcted adult murine heart. J Clin Invest. 126:2151–2166. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Arndt L, Dokas J, Gericke M, Kutzner CE,
Müller S, Jeromin F, Thiery J and Burkhardt R: Tribbles homolog 1
deficiency modulates function and polarization of murine bone
marrow-derived macrophages. J Biol Chem. 293:11527–11536. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Xie X, Yang X, Wu J, Ma J, Wei W, Fei X
and Wang M: Trib1 contributes to recovery from
ischemia/reperfusion-induced acute kidney injury by regulating the
polarization of renal macrophages. Front Immunol. 11:4732020.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Hughes CE and Nibbs RJB: A guide to
chemokines and their receptors. FEBS J. 285:2944–2971. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Ruytinx P, Proost P, Van Damme J and
Struyf S: Chemokine-induced macrophage polarization in inflammatory
conditions. Front Immunol. 9:19302018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Lu J, Chatterjee M, Schmid H, Beck S and
Gawaz M: CXCL14 as an emerging immune and inflammatory modulator. J
Inflamm (Lond). 13:12016. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Lv J, Wu ZL, Gan Z, Gui P and Yao SL:
CXCL14 overexpression attenuates sepsis-associated acute kidney
injury by inhibiting proinflammatory cytokine production. Mediators
Inflamm. 2020:24317052020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Yoo KD, Cha RH, Lee S, Kim JE, Kim KH, Lee
JS, Kim DK, Kim YS and Yang SH: Chemokine receptor 5 blockade
modulates macrophage trafficking in renal ischaemic-reperfusion
injury. J Cell Mol Med. 24:5515–5527. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Yang Q, Wang Y, Pei G, Deng X, Jiang H, Wu
J, Zhou C, Guo Y, Yao Y, Zeng R and Xu G: Bone marrow-derived
Ly6C(−) macrophages promote ischemia-induced chronic kidney
disease. Cell Death Dis. 10:2912019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Rudemiller NP, Patel MB, Zhang JD, Jeffs
AD, Karlovich NS, Griffiths R, Kan MJ, Buckley AF, Gunn MD and
Crowley SD: C-C Motif Chemokine 5 Attenuates Angiotensin
II-Dependent kidney injury by limiting renal macrophage
infiltration. Am J Pathol. 186:2846–2856. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Prakoura N and Chatziantoniou C: Periostin
in kidney diseases. Cell Mol Life Sci. 74:4315–4320. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Wallace DP: Periostin in the kidney. Adv
Exp Med Biol. 1132:99–112. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Kormann R, Kavvadas P, Placier S,
Vandermeersch S, Dorison A, Dussaule JC, Chadjichristos CE,
Prakoura N and Chatziantoniou C: Periostin promotes cell
proliferation and macrophage polarization to drive repair after
AKI. J Am Soc Nephrol. 31:85–100. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Roedig H, Nastase MV, Wygrecka M and
Schaefer L: Breaking down chronic inflammatory diseases: The role
of biglycan in promoting a switch between inflammation and
autophagy. FEBS J. 286:2965–2979. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Roedig H, Nastase MV, Frey H, Moreth K,
Zeng-Brouwers J, Poluzzi C, Hsieh LT, Brandts C, Fulda S, Wygrecka
M and Schaefer L: Biglycan is a new high-affinity ligand for CD14
in macrophages. Matrix Biol. 77:4–22. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Poluzzi C, Nastase MV, Zeng-Brouwers J,
Roedig H, Hsieh LT, Michaelis JB, Buhl EM, Rezende F, Manavski Y,
Bleich A, et al: Biglycan evokes autophagy in macrophages via a
novel CD44/Toll-like receptor 4 signaling axis in
ischemia/reperfusion injury. Kidney Int. 95:540–562. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Meissner M, Viehmann SF and Kurts C:
DAMPening sterile inflammation of the kidney. Kidney Int.
95:489–491. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Roedig H, Damiescu R, Zeng-Brouwers J,
Kutija I, Trebicka J, Wygrecka M and Schaefer L: Danger matrix
molecules orchestrate CD14/CD44 signaling in cancer development.
Semin Cancer Biol. 62:31–47. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zhang PL and Liu ML: Extracellular
vesicles mediate cellular interactions in renal diseases-Novel
views of intercellular communications in the kidney. J Cell
Physiol. 2021.(Epub ahead of print). View Article : Google Scholar
|
|
121
|
Rigalli JP, Barros ER, Sommers V, Bindels
RJM and Hoenderop JGJ: Novel aspects of extracellular vesicles in
the regulation of renal physiological and pathophysiological
processes. Front Cell Dev Biol. 8:2442020. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Wu P, Zhang B, Ocansey DKW, Xu W and Qian
H: Extracellular vesicles: A bright star of nanomedicine.
Biomaterials. 269:1204672020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zhang X, Zhang H, Gu J, Zhang J, Shi H,
Qian H, Wang D, Xu W, Pan J and Santos HA: Engineered extracellular
vesicles for cancer therapy. Adv Mater e2005709. 2021.(Epub ahead
of print). PubMed/NCBI
|
|
124
|
Quaglia M, Dellepiane S, Guglielmetti G,
Merlotti G, Castellano G and Cantaluppi V: Extracellular vesicles
as mediators of cellular crosstalk between immune system and kidney
graft. Front Immunol. 11:742020. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Tang TT, Wang B, Wu M, Li ZL, Feng Y, Cao
JY, Yin D, Liu H, Tang RN, Crowley SD, et al: Extracellular
vesicle-encapsulated IL-10 as novel nanotherapeutics against
ischemic AKI. Sci Adv. 6:eaaz07482020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Console L, Scalise M and Indiveri C:
Exosomes in inflammation and role as biomarkers. Clin Chim Acta.
488:165–171. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ståhl AL, Johansson K, Mossberg M, Kahn R
and Karpman D: Exosomes and microvesicles in normal physiology,
pathophysiology, and renal diseases. Pediatr Nephrol. 34:11–30.
2019. View Article : Google Scholar
|
|
128
|
Thongboonkerd V: Roles for exosome in
various kidney diseases and disorders. Front Pharmacol.
10:16552020. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Wang ZW and Zhu X: Exosomal miR-19b-3p
communicates tubular epithelial cells and M1 macrophage. Cell Death
Dis. 10:7622019. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Lv LL, Feng Y, Wu M, Wang B, Li ZL, Zhong
X, Wu WJ, Chen J, Ni HF, Tang TT, et al: Exosomal miRNA-19b-3p of
tubular epithelial cells promotes M1 macrophage activation in
kidney injury. Cell Death Differ. 27:210–226. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Li ZL, Lv LL, Tang TT, Wang B, Feng Y,
Zhou LT, Cao JY, Tang RN, Wu M, Liu H, et al: HIF-1α inducing
exosomal microRNA-23a expression mediates the cross-talk between
tubular epithelial cells and macrophages in tubulointerstitial
inflammation. Kidney Int. 95:388–404. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Harrois A, Soyer B, Gauss T, Hamada S,
Raux M and Duranteau J: Prevalence and risk factors for acute
kidney injury among trauma patients: A multicenter cohort study.
Crit Care. 22:3442018. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Zuk A and Bonventre JV: Acute kidney
injury. Annu Rev Med. 67:293–307. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Wen Y and Crowley SD: The varying roles of
macrophages in kidney injury and repair. Curr Opin Nephrol
Hypertens. 29:286–292. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Tang PM, Nikolic-Paterson DJ and Lan HY:
Macrophages: Versatile players in renal inflammation and fibrosis.
Nat Rev Nephrol. 15:144–158. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Poeck H, Bscheider M, Gross O, Finger K,
Roth S, Rebsamen M, Hannesschläger N, Schlee M, Rothenfusser S,
Barchet W, et al: Recognition of RNA virus by RIG-I results in
activation of CARD9 and inflammasome signaling for interleukin 1
beta production. Nat Immunol. 11:63–69. 2010. View Article : Google Scholar : PubMed/NCBI
|