Introduction
Acute coronary syndrome (ACS) is characterized as a
group of clinical syndromes caused by acute myocardial ischemia.
ACS, which includes acute myocardial infarction and unstable
angina, endangers lives and health (1). Studies have found that activation of
platelets and impairment of endothelial function serve key roles in
ACS occurrence (2,3). Pathological changes caused by ACS are
characterized by coronary atheromatous plaque rupture, inflammatory
states, activated platelet adhesion/aggregation, vasospasm,
thrombus formation and disability or even death (4,5). Type
2 diabetes mellitus (T2DM), along with endothelial cell (EC) injury
and coagulation activation, is considered to be a key element
involved in the development of accelerated atherosclerosis
(6–8). However, silent myocardial ischemia is
a common feature in patients with ACS combined with T2DM due to
damage to the nervous system caused by hyperglycemia (9). Therefore, it is important to identify
indicator that may predict coronary artery damage in patients with
T2DM. Circulating microparticles (MPs), which were previously
considered to be cell waste, are primarily produced by injured ECs
and activated platelets (10).
However, a number of studies have revealed that MPs in pathological
conditions can induce activation of coagulation and neutrophils, as
well as vascular endothelial cell dysfunction or impairment
(10–12). Our previous study demonstrated that
the MP content increases in patients with ACS and that rising MP
levels impair endothelial-dependent vasodilatation by inhibiting
the AKT/endothelial nitric oxide synthase (eNOS)-heat shock protein
(Hsp)90 signaling pathway (12).
Thus, MPs are considered to be a marker of EC dysfunction and/or
injury (13). Whether MPs
participate in the influence of T2DM on coronary artery disease and
their biological function remains unclear. The present study aimed
to assess the effect of MPs from patients with ACS combined with
T2DM on vasodilatation and endothelial function in rat thoracic
aortas.
Materials and methods
Study population
Patients with ACS (without previous myocardial
infarction within 3 months) with (n=24; age, 42.73±8.46 years; 12
male patients and 12 female patients) or without T2DM (n=24; age,
46.64±10.25 years; 13 male patients and 11 female patients) and
patients with T2DM (n=20, 45.36±11.81 year, male/famale: 10/10)
were recruited (Xi'an, China). Patients were recruited between
March 2016 and September 2018. Patients with diseases that could
increase MPs were excluded, including hypertension, renal failure,
severe trauma, infectious disease, lupus anticoagulant, multiple
sclerosis and rheumatic disease. For their influence on
neurohumoral regulation, patients taking either
angiotensin-converting enzyme inhibitors or angiotensin receptor
blockers were excluded. Healthy subjects (n=20; age, 43.53±7.35
year; 11 male patients and 9 female patients), who were sex- and
age-matched, were enrolled between June 2016 and December 2016 as
volunteers. The present study was approved by The People's Hospital
of Shaanxi Province Ethics Review Board. All participants signed
informed consent forms.
Isolated MPs
Fasting peripheral blood from patients and healthy
subjects was obtained on the initial hospitalization day. After
centrifuging (11,000 × g; 2 min; 4°C) the blood, platelet-poor
plasma was obtained (50 µl was reserved for flow cytometric
analysis). Then, the platelet-poor plasma was further centrifuged
at 13,000 × g for 45 min at 4°C as previously described (14). MPs precipitated in the bottom of the
tube and were resuspended in 100 µl RPMI-1640 medium (HyClone;
Cytiva). A bicinchoninic acid protein assay (Merck Life Science UK,
Ltd.) was used to assess the MP concentration. Before further
analysis, MPs were stored at −80°C. Because limited blood samples
could be obtained from each patient, isolated MPs from each group
were mixed together for subsequent experiments. The time-points and
concentrations of MPs used in the present study were as described
in our previous study (15).
MP origin detection
The origin of MPs was detected by flow cytometric
analysis. Following incubation of platelet-poor plasma with
anti-CD31-PE (5 µl; cat. no. 555027; 1:400; BD Biosciences) and
anti-CD41-FITC (5 µl; cat. no. 561849; 1:400; BD Biosciences) for
30 min at 37°C as previously described (16), flow count calibrator beads (50 µl;
Beckman Coulter, Inc.) were added and incubated at 37°C for another
15 min. Platelet-derived MPs [PMPs; CD31(+)/CD41(+)] and
endothelial-derived MPs [EMPs; CD31(+)/CD41(−)] were counted and
analyzed using an MoFlo XDP Cell Sorter (Beckman Coulter, Inc.;
gate size, <1 µm).
Vasodilatation study (n=6)
Sprague-Dawley male rats (n=30; age, 6 weeks;
weight, 150±10 g; purchased from the Central Laboratory of Shaanxi
Provincial People's Hospital) were individually housed at 20±2°C
with 55±10% humidity, 12:1 dark-light cycles, and free access to
water and chow. Rats were decapitated following light anesthesia by
pentobarbital (10 mg/kg; Sigma-Aldrich; Merck KGaA). The thoracic
aorta was separated and cut into 3–5-mm segments [this process was
conducted in ice-cold Krebs solution (Sigma-Aldrich; Merck KGaA)].
Tissue surrounding the aorta was removed. The aortic rings were
then connected to an isometric force transducer (Emka Technologies)
as previously described (15).
Following equilibration in Krebs solution and continuous aeration
(95% O2 and 5% CO2) for 1 h at 37°C, aortic
ring stabilization was tested by exposure to 60 mmol/l KCl for 2
min at 37°C. Then, the rings were incubated with MPs (2.5 mg/ml)
from all groups for 30 min at 37°C. Phenylephrine (PE;
10−6 mol/l; Sigma-Aldrich; Merck KGaA) was added to
pre-constrict the rings. Rings were incubated with the eNOS
inhibitor NG-nitro-L-arginine methyl ester (L-NAME; 100 µmol/l,
Sigma-Aldrich; Merck KGaA) at 37°C for 30 min. Time- and
endothelium-dependent relaxation in response to acetylcholine
(10−8−10−4 mol/l; Sigma-Aldrich; Merck KGaA)
was measured following PE pre-constriction. Briefly, following
contraction to the maximum extent by PE, different concentrations
(10−8−10−4 mol/l) of acetylcholine were added
to dilate the blood vessels. The diastolic values of each
acetylcholine concentration were recorded and analyzed. Similar to
acetylcholine, endothelium-independent relaxation was tested using
the nitrovasodilator sodium nitroprusside (SNP;
10−8−10−4 mol/l; Sigma-Aldrich; Merck KGaA).
Experiments were approved by The People's Hospital of Shaanxi
Province Animal Ethics Committee. RPMI-1640 medium in the absence
MPs was set as a blank control.
NO detection (n=6)
Vessel rings were opened longitudinally. Following
equilibration at 37°C for 30 min in 48-well plates with Krebs
solution (Sigma-Aldrich; Merck KGaA), rings were incubated in the
presence or absence of MPs (2.5 mg/ml) from each group for 1 h at
37°C. Control groups were treated with an equal volume of
RPMI-1640. Then, the rings were treated with diaminofluorescein
diacetate (10 µM; Sigma-Aldrich; Merck KGaA) at 37°C for 30 min.
Vascular endothelial growth factor (50 ng/ml; Sigma-Aldrich; Merck
KGaA) was added as a positive control. Laser scanning confocal
microscopy (magnification, ×200, emission, 515 nm; excitation, 495
nm) was used to obtain fluorescence images. Fluorescence intensity
was assessed using ImageJ software (version 1.52; National
Institutes of Health) as previously described (16). RPMI-1640 medium in the absence of
MPs was set as a blank control.
Superoxide (O2˙-) detection
(n=6)
First, the vessel rings were opened longitudinally.
Following equilibration within Krebs solution for 30 min at 37°C,
the rings were treated in the presence or absence of L-NAME (1
mmol/l; Sigma-Aldrich; Merck KGaA) at 37°C for 30 min. Then, the
rings were incubated in the presence or absence of MPs from all
groups at 37°C for 30 min. Next, the rings were incubated with
hydroethidine (10 µmol/l; AnaSpec, Inc.) at 37°C for 30 min.
Fluorescence images were obtained by laser scanning confocal
microscopy (magnification, ×200; emission, 530 nm; excitation, 488
nm). H2O2 (0.5 mol/l; Sigma-Aldrich; Merck
KGaA) was added as a positive control. Fluorescence intensity was
assessed by ImageJ softwareas previously described (15). RPMI-1640 medium in the absence of
MPs was set as a blank control.
Western blot analysis (n=6)
Following treatment in the presence or absence MPs
from all groups at 37°C for 12 h, the rat aortic proteins were
obtained using RIPA lysis solution (Sigma-Aldrich; Merck KGaA).
Protein concentrations were determined using a bicinchoninic acid
protein assay. Proteins (20 µg) were separated via SPS-PAGE (5%
concentrated gel and 8% separation gel) and transferred to PVDF
membranes (Sigma-Aldrich; Merck KGaA). Following blocking with 5%
skimmed milk powder (Sigma-Aldrich; Merck KGaA) at 37°C for 1 h,
the membranes were incubated with the following primary antibodies:
eNOS (cat. no. sc-654; 1:1,000; Santa Cruz Biotechnology, Inc.),
phosphorylated eNOS at T495 (cat. no. 9574; 1:800; Cell Signaling
Technology, Inc.), phosphorylated eNOS at Ser1177 (cat. no. 9571;
1:800; Cell Signaling Technology, Inc.), caveolin-1 (cat. no. 3267;
1:10,000; Cell Signaling Technology, Inc.) and GAPDH (cat. no.
2118; 1:5,000, Cell Signaling Technology, Inc.) at 4°C for 12 h.
Subsequently, the membranes were incubated with horseradish
peroxidase-conjugated secondary antibodies (cat. no. 7074S; Cell
Signaling Technology, Inc.; equivalent dilution ratio to primary
antibodies) at 37°C for 1 h. Protein bands were visualized using
enhanced chemiluminescence solution (cat. no. sc-2048; Santa Cruz
Biotechnology, Inc.). Protein expression levels were
semi-quantified using ImageJ software. RPMI-1640 medium in the
absence of MPs was set as a blank control.
Immunoprecipitation (n=6)
Following treatment in the presence or absence of
MPs from all groups for 12 h at 37°C, aortic protein was harvested
using RIPA lysis solution (Sigma-Aldrich; Merck KGaA). Protein
concentrations were determined using a bicinchoninic acid protein
assay. Subsequently, samples were treated with anti-eNOS antibody
(cat. no. sc-136977; 1:1,000; Santa Cruz Biotechnology, Inc.) at
37°C for 24 h to immunoprecipitate eNOS. Then, the immunocomplex
was mixed with Laemmli buffer (Sigma-Aldrich; Merck KGaA) and
denatured (95°C for 5 min). After cooling on ice for ≥2 min,
protein was obtained by centrifugation (680 × g, 2 min, 4°C). Then,
immunoprecipitation of eNOS (cat. no. sc-654; 1:1,000; Santa Cruz
Biotechnology, Inc.) and Hsp90 (cat. no. sc-13119; 1:1,000; Santa
Cruz Biotechnology, Inc.) was tested as previously described
(10). RPMI-1640 in the absence of
MPs was set as a blank control.
Statistical analysis
Data are presented as the mean ± SD. Data were
analyzed using GraphPad Prism software (version 7.0; GraphPad
Software, Inc.). Sex, smoking and medicine were compared by
Chi-square test and Killip class was analyzed with Kruskal-Wallis
test. Multigroup comparisons were tested by one-way analysis of
variance followed by Bonferroni's multiple comparison post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical data
There were no differences in characteristics (sex
and age) between controls and patients except for lipids and
medications (Table I).
 | Table I.Demographic, clinical and therapeutic
characteristics of patients. |
Table I.
Demographic, clinical and therapeutic
characteristics of patients.
| Characteristic | Control (n=20) | ACS (n=24) | T2DM (n=20) | ACS with T2DM
(n=24) |
|---|
| Age, years | 43.53±7.35 | 46.64±10.25 | 45.36±11.81 | 42.73±8.46 |
| Sex,
male/female | 11/9 | 13/11 | 10/10 | 12/12 |
| Smoking | 6 | 11 | 10 | 11 |
| Total cholesterol,
mmol/l | 4.45±0.76 |
5.96±0.95a |
5.77±0.58a |
6.12±0.89a |
| Triglyceride,
mmol/l | 0.97±0.42 |
1.63±0.52a |
1.59±0.64a |
1.71±0.48a |
| High density
lipoprotein, mmol/l | 1.15±0.37 |
0.93±0.26a |
0.99±0.33a |
1.01±0.32a |
| Low density
lipoprotein, mmol/l | 2.89±0.65 |
3.67±0.61a |
3.48±0.75a |
3.89±0.58a |
| Killip class,
I/II/III/IV | 0/0/0/0 |
18/6/0/0a | 0/0/0/0 |
16/8/0/0a |
| Medication |
|
|
|
|
|
β-receptor blockers | NA | 24a | 20a | 24a |
| Nitrate
esters | NA | 10a | 0 | 12a |
|
Anticoagulants | NA | 24a | 20a | 24a |
|
Hypoglycemics | NA | 0 | 20a | 24a,b |
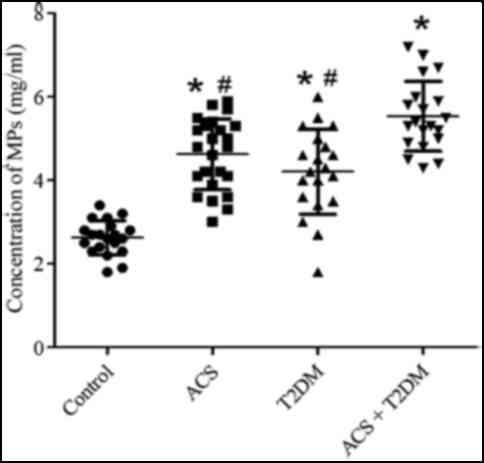
MP concentrations
Compared with the control (2.84±0.69 mg/ml), MPs
concentrations were increased in patients with ACS (4.63±0.86
mg/ml) and T2DM (4.21±0.77 mg/ml). MP levels (5.54±0.73 mg/ml) were
further increased in patients with both ACS and T2DM (Fig. 1).
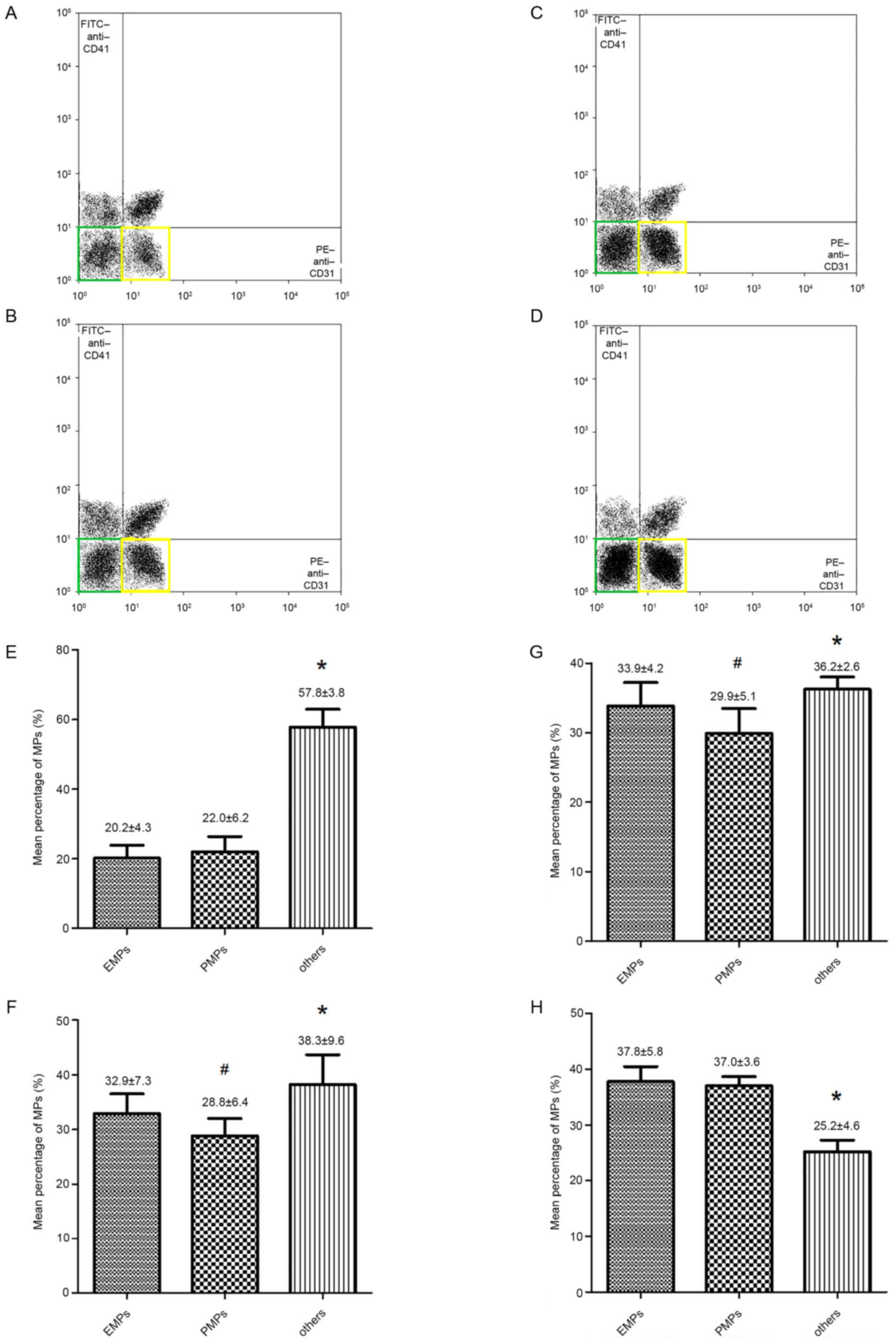
MP origins
Compared with the control, patients with ACS or T2DM
exhibited higher proportions of EMPs (32.9±7.3 and 33.9±4.2 vs.
20.2±4.3%, respectively) and PMPs (28.8±6.4 and 29.9±5.1 vs.
22.0±6.2%, respectively; Fig. 2).
The proportions of EMPs (37.8±5.8%) and PMPs (37.0±3.6%) in
patients with ACS concurrent with T2DM were significantly increased
compared with all other groups (Fig. 2D
and H).
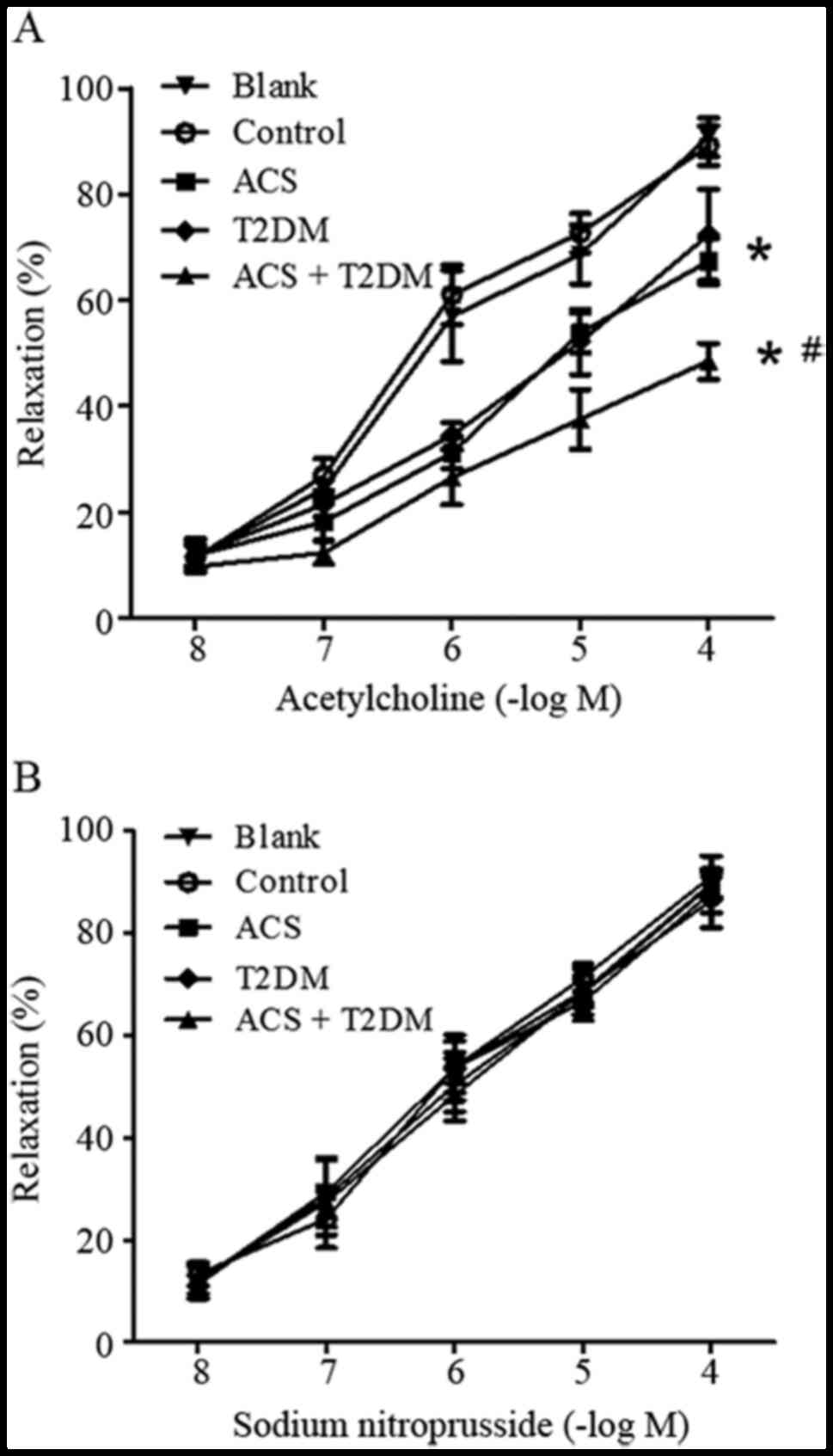
Effect of MPs on vasodilatation
Compared with the control, MPs from patients with
ACS or T2DM impaired endothelium-dependent relaxation (Fig. 3A). This effect was strengthened by
MPs from patients with ACS concurrent with T2DM (Fig. 3A). However, endothelium-independent
vasodilatation response to SNP was unaltered by MPs from all groups
(Fig. 3B).
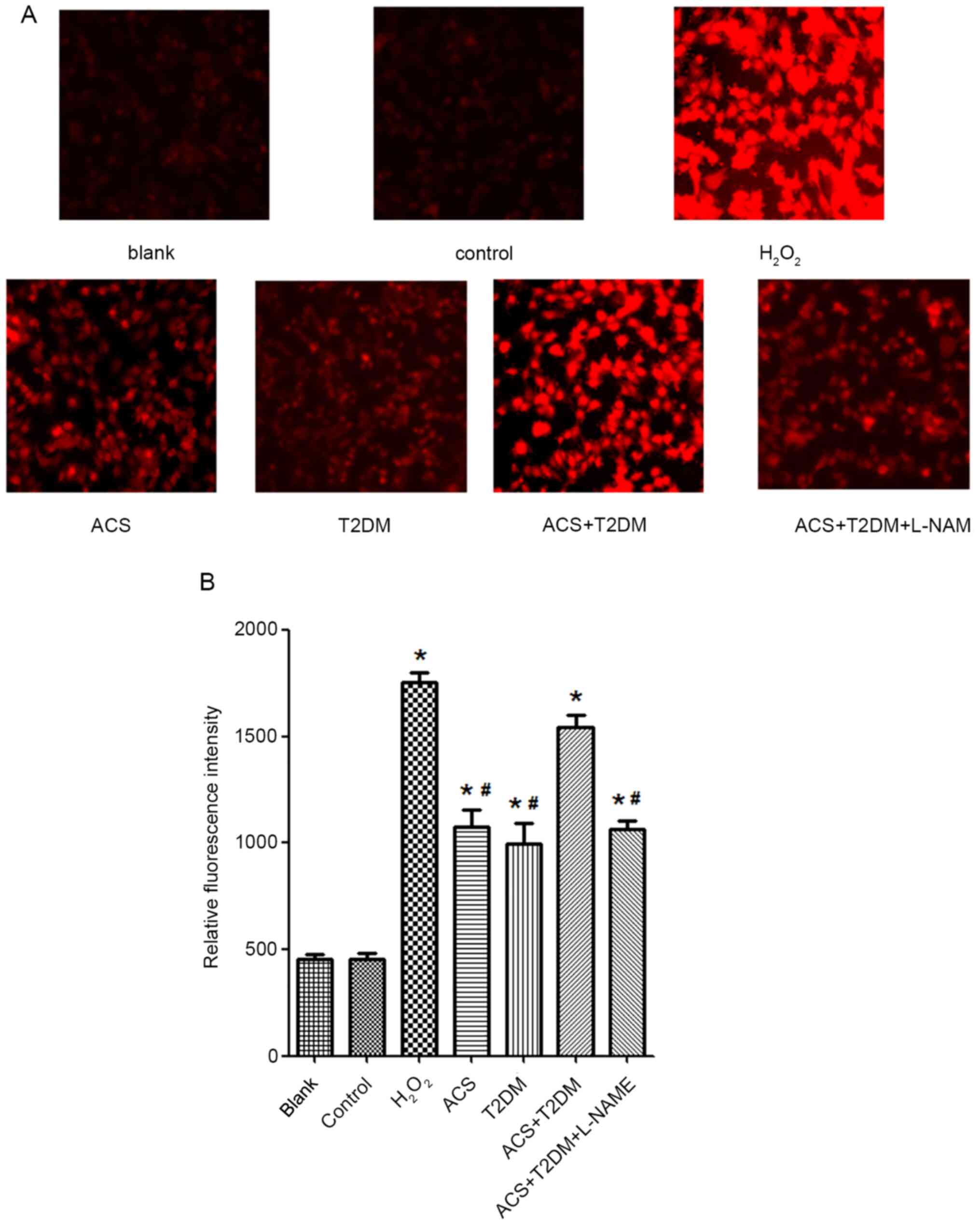
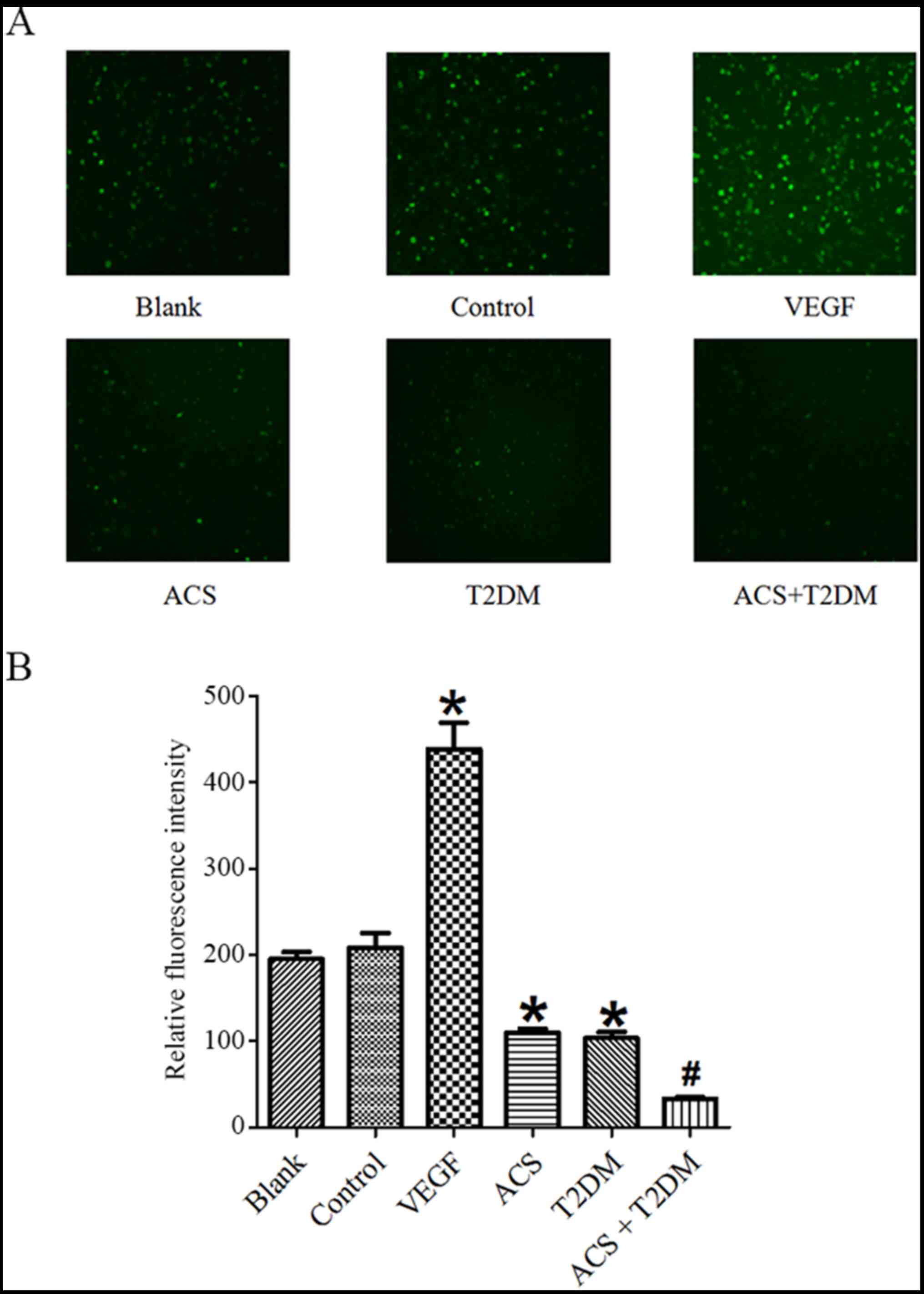
Effects of MPs on NO and
O2˙- generation
Compared with the control group, MPs from patients
with ACS or T2DM increased O2˙- generation (Fig. 4) but decreased NO (Fig. 5) production. The influence on both
NO and O2˙- generation was enhanced by MPs from patients
with ACS concurrent with T2DM (Figs.
4 and 5). However, the
increased O2˙- in patients with ACS concurrent with T2DM
was partly blocked by L-NAME (Fig.
4).
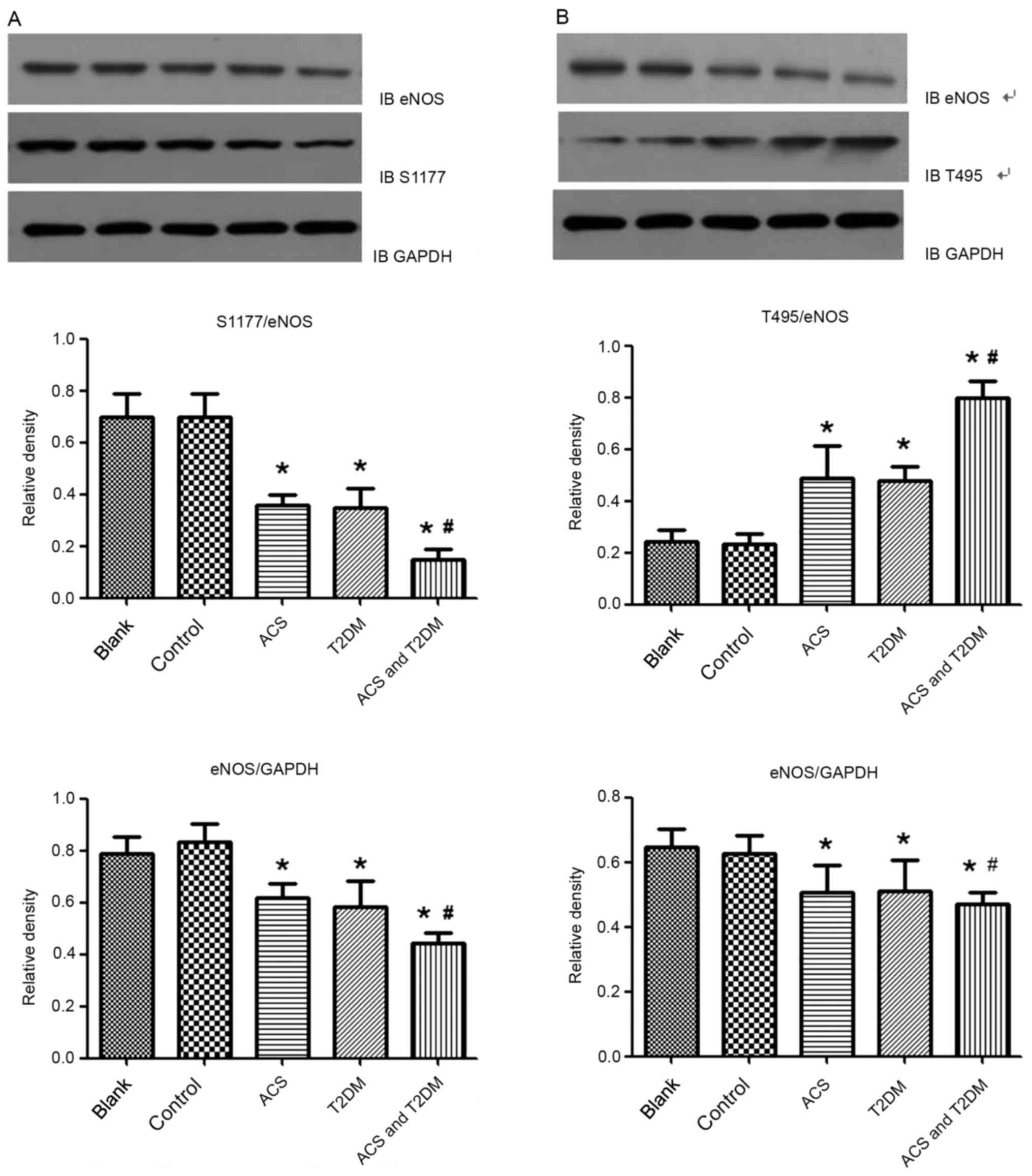
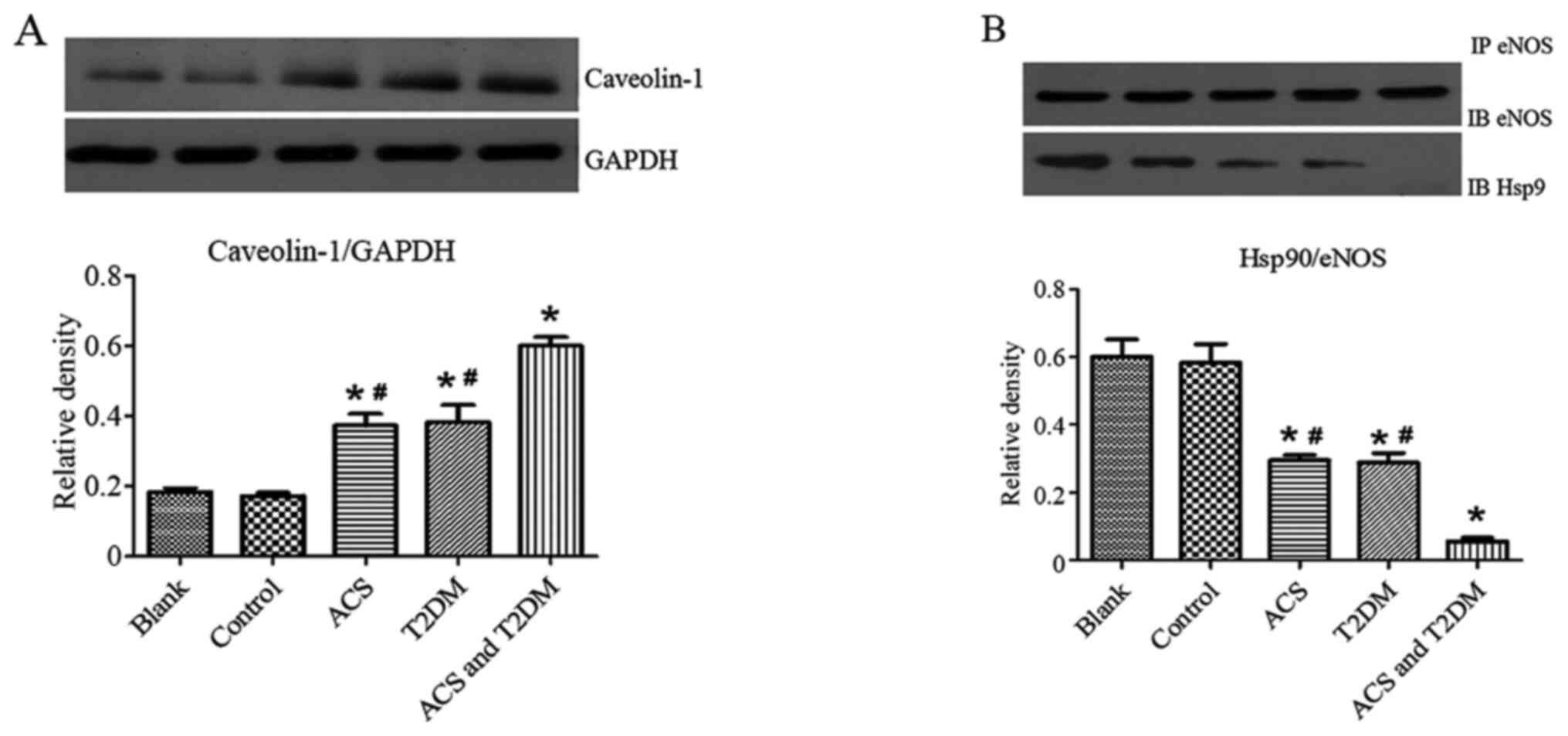
Effects of MP on eNOS and caveolin-1
expression levels
Western blotting was used to investigate the
mechanism by which MPs affect endothelial function. Compared with
the control group, MPs from patients with ACS or T2DM decreased
eNOS and its phosphorylation at the Ser1177 site (Fig. 6A) but increased caveolin-1 (Fig. 7A) and eNOS phosphorylation at the
T495 site (Fig. 6B). These effects
were strengthened by MPs from patients with ACS concurrent with
T2DM (Figs. 6 and 7A).
Effects of MPs on
immunoprecipitation
Immunoprecipitation was performed to detect the
effect of MPs on the association of eNOS with Hsp90. MPs from
patients with ACS or T2DM decreased the association of eNOS with
Hsp90; MPs from patients with ACS concurrent with T2DM further
enhanced these effects (Fig.
7B).
Discussion
The present study indicated that MPs increased in
patients with ACS with or without T2DM. MPs from patients with ACS,
particularly those with concurrent T2DM, impaired
endothelium-dependent vasodilatation, increased O2˙-
production, caveolin-1 expression and eNOS phosphorylation at T495
but decreased NO generation, eNOS and its phosphorylation at
Ser1177, and uncoupled the association between eNOS and Hsp90 in
the rat aorta.
Relatively low levels of MPs exist in healthy
subjects. However, numerous studies have reported that increased
MPs are found in a number of diseases associated with the vascular
system (17–20). Our previous study (12) revealed that MPs are increased in
patients with ACS; this impairs vasodilatation by uncoupling the
correlation of eNOS with Hsp90, inhibiting the eNOS-Hsp90 pathway
and Hsp90 and increasing oxidative stress. Biasucci et al
(21) reported that MPs are further
increased in ACS compared with stable angina (SA) but MP levels are
not positively correlated with the atherosclerotic burden of
patients with SA. Bernal-Mizrachi et al (22) determined that a high number of EMPs
was associated with lesions with thrombi, multiple irregular
lesions and eccentric type II lesions. Mild to moderate (but not
severe) stenosis is associated with increasing EMP levels, which
indicates that EMP may be a useful marker of endothelial
dysfunction. The present study demonstrated that increasing MPs are
further increased in patients with both ACS and T2DM compared with
ACS-alone.
Oxidative stress, inflammation and endothelial
dysfunction have been reported in T2DM (23,24).
As a key etiological factor and therapeutic target of vascular
complications in T2DM, endothelial dysfunction may also be
associated with diseases with vascular complications (25–28).
In order to investigate the overlap of T2DM and MPs in the
circulatory system, the present study examined whether T2DM could
further enhance the effects of MPs on endothelial dysfunction.
Vasodilatation, which is primarily regulated by NO,
serves a key role in hemoperfusion regulation (29). Wong et al (26) reported that specific therapies
targeting increased eNOS activity may decrease morbidity and
mortality ofcardiovascular diseases related to diabetes and
hypertension by reversing endothelial dysfunction. Our previous
study (12) indicated that MPs from
patients with ACS impaired endothelial-dependent vasodilatation via
the AKT/eNOS-Hsp90 pathway. In the present study, MPs from patients
with both ACS and T2DM induced greater impairment of
endothelium-dependent vasodilatation by blocking eNOS and its
phosphorylation at Ser1177, thus strengthening the expression of
eNOS phosphorylation at T495.
As a structural caveolin protein, caveolin-1
decreases NO generation by interacting with eNOS (30,31).
Meye et al (32) revealed
that homocysteine affected the association of eNOS with caveolin-1,
which results in endothelial dysfunction and decreased NO
generation. Qin et al (33)
reported that hypercholesterolemia impairs NO production and may
participate in atherosclerosis by promoting eNOS association with
caveolin-1. Zhao et al (34)
revealed that mice deficient in the caveolin-1 gene exhibited
significantly higher NO levels. The present study indicated that
MPs from patients with ACS, particularly those with concurrent
T2DM, upregulated expression of caveolin-1. This may participate in
the impairment of both vasodilatation and NO production.
An imbalance between the production of
vasoconstricting and vasodilating (e.g. NO) factors plays a key
role in atherosclerosis. NO offsets reactive oxygen species
(35). eNOS-derived NO serves a key
role in the regulation of vessel inflammatory status and tone
(36). The primary mechanism
underlying the imbalance between NO and O2˙- is eNOS
uncoupling (12). Thus, the present
study determined whether MPs from patients with ACS and/or T2DM
could affect oxidative stress and the association of eNOS with
Hsp90. MPs from patients with ACS, particularly those with
concurrent T2DM, decreased NO generation and the amount of eNOS
that associated with Hsp90 but increased O2˙-
production. In addition, the increased O2˙- was blocked
by L-NAME. These data indicated that MPs increased oxidative stress
by eNOS uncoupling.
The present study used a mixture of MPs of different
origins, as it was not possible to isolate EMPs and PMPs from human
blood. Rats are often used in cardiovascular pharmacology research
and screening of novel drugs due to their responsiveness (indicated
by changes in blood pressure and vascular resistance). However,
differences between the aorta of SD rats and humans means
experiments must be performed on human aortic endothelial cells to
verify the present results. In addition, future research should use
a larger sample size and include classification of ACS (e.g.
unstable angina, acute ST/non-ST segment elevation myocardial
infarction).
In summary, MPs from patients with both ACS and T2DM
further enhanced the effects of MPs from patients with ACS on
endothelial-dependent vasodilatation by decreasing eNOS, its
phosphorylation at Ser1177 and association with Hsp90 and NO
production and increasing caveolin-1 expression, O2˙-
generation and eNOS phosphorylation at T495. These results
confirmed that MPs may be a useful marker of endothelial
dysfunction.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FJC conceptualized and designed the study. XLW and
FJC performed the experiments, and drafted and revised the
manuscript. WZ, ZL and HYW collected, analyzed and interpreted
data. WQH, QRW and GC performed the experiments, and drafted and
revised the manuscript. XHL, KX and GC conceived the study and
provided final approval of the version to be published. FJC, XLW
and GC confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The People's
Hospital of Shaanxi Province Ethics Review Board. All participants
signed informed consent forms. Experiments involving animals were
approved by The People's Hospital of Shaanxi Province Animal Ethics
Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gach O, El HZ and Lancellotti P: Acute
coronary syndrome. Rev Med Liege. 73:243–250. 2018.(In French).
PubMed/NCBI
|
|
2
|
Wang R, Wang M, Ye J, Sun G and Sun X:
Mechanism overview and target mining of atherosclerosis:
Endothelial cell injury in atherosclerosis is regulated by
glycolysis (Review). Int J Mol Med. 47:65–76. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Libby P, Ridker PM and Maseri A:
Inflammation and atherosclerosis. Circulation. 105:1135–1143. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kristensen SD, Ravn HB and Falk E:
Insights into the pathophysiology of unstable coronary artery
disease. Am J Cardiol. 80:5E–9E. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Freedman JE and Loscalzo J: Nitric oxide
and its relationship to thrombotic disorders. J Thromb Haemost.
1:1183–1188. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McGuire DK, Emanuelsson H, Granger CB, E
Magnus Ohman, D J Moliterno, H D White, D Ardissino, J W Box, R M
Califf and E J Topol: Influence of diabetes mellitus on clinical
outcomes across the spectrum of acute coronary syndromes. Findings
from the GUSTO-IIb study. GUSTO IIb Investigators. Eur Heart J.
21:1750–1758. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hildebrandt P: Diabetic patients and acute
coronary syndromes. Eur Heart J. 22:887–888. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han D, Rozanski A, Gransar H, Sharir T,
Einstein AJ, Fish MB, Ruddy TD, Kaufmann PA, Sinusas AJ, Miller EJ,
et al: Myocardial ischemic burden and differences in prognosis
among patients with and without diabetes: Results from the
multicenter international REFINE SPECT registry. Diabetes Care.
43:453–459. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wackers FJ, Young LH, Inzucchi SE, Chyun
DA, Davey JA, Barrett EJ, Taillefer R, Wittlin SD, Heller GV,
Filipchuk N, et al: Detection of silent myocardial ischemia in
asymptomatic diabetic subjects: The DIAD study. Diabetes Care.
27:1954–1961. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martinez MC, Tesse A, Zobairi F and
Andriantsitohaina R: Shed membrane microparticles from circulating
and vascular cells in regulating vascular function. Am J Physiol
Heart Circ Physiol. 288:H1004–H1009. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zaldivia MTK, McFadyen JD, Lim B, Wang X
and Peter K: Platelet-derived microvesicles in cardiovascular
diseases. Front Cardiovasc Med. 4:742017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han WQ, Chang FJ, Wang QR and Pan JQ:
Microparticles from patients with acute coronary syndrome impair
vasodilatation by inhibiting the Akt/eNOS-Hsp90 signaling pathway.
Cardiology. 132:252–260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koga H, Sugiyama S, Kugiyama K, Watanabe
K, Fukushima H, Tanaka T, Sakamoto T, Yoshimura M, Jinnouchi H and
Ogawa H: Elevated levels of VE-cadherin-positive endothelial
microparticles in patients with type 2 diabetes mellitus and
coronary artery disease. J Am Coll Cardiol. 45:1622–1630. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boulanger CM, Scoazec A, Ebrahimian T,
Henry P, Mathieu E, Tedgui A and Mallat Z: Circulating
microparticles from patients with myocardial infarction cause
endothelial dysfunction. Circulation. 104:2649–2652. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng G, Shan XF, Wang XL, Dong WW, Li Z,
Liu XH, Zhang W, Xing K and Chang FJ: Endothelial damage effects of
circulating microparticles from patients with stable angina are
reduced by aspirin through ERK/p38 MAPKs pathways. Cardiovasc Ther.
35:2017. View Article : Google Scholar
|
|
16
|
Ci HB, Ou ZJ, Chang FJ, Liu DH, He GW, Xu
Z, Yuan HY, Wang ZP, Zhang X and Ou JS: Endothelial microparticles
increase in mitral valve disease and impair mitral valve
endothelial function. Am J Physiol Endocrinol Metab. 304:E695–E702.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wen B, Combes V, Bonhoure A, Weksler BB,
Couraud PO and Grau GE: Endotoxin-induced monocytic microparticles
have contrasting effects on endothelial inflammatory responses.
PLoS One. 9:e915972014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Colle IO, De Vriese AS, Van Vlierberghe
HR, Lameire NH and De Vos MM: Vascular hyporesponsiveness in the
mesenteric artery of anaesthetized rats with cirrhosis and portal
hypertension: An in-vivo study. Eur J Gastroenterol Hepatol.
16:139–145. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Erol A and Koşay S: Effects of
aminoguanidine administration on vascular hyporeactivity in
thoracic aorta from endotoxaemic rats. Eur J Pharmacol.
408:175–181. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martin S, Tesse A, Hugel B, Martínez MC,
Morel O, Freyssinet JM and Andriantsitohaina R: Shed membrane
particles from T lymphocytes impair endothelial function and
regulate endothelial protein expression. Circulation.
109:1653–1659. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Biasucci LM, Porto I, Di Vito L, De Maria
GL, Leone AM, Tinelli G, Tritarelli A, Di Rocco G, Snider F,
Capogrossi MC and Crea F: Differences in microparticle release in
patients with acute coronary syndrome and stable angina. Circ J.
76:2174–2182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bernal-Mizrachi L, Jy W, Fierro C,
Macdonough R, Velazques HA, Purow J, Jimenez JJ, Horstman LL,
Ferreira A, de Marchena E and Ahn YS: Endothelial microparticles
correlate with high-risk angiographic lesions in acute coronary
syndromes. Int J Cardiol. 97:439–446. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu FB and Stampfer MJ: Is type 2 diabetes
mellitus a vascular condition? Arterioscler Thromb Vasc Biol.
23:1715–1716. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ceriello A and Motz E: Is oxidative stress
the pathogenic mechanism underlying insulin resistance, diabetes,
and cardiovascular disease? The common soil hypothesis revisited.
Arterioscler Thromb Vasc Biol. 24:816–823. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang X, Luo YX, Chen HZ and Liu DP:
Mitochondria, endothelial cell function, and vascular diseases.
Front Physiol. 5:1752014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wong WT, Wong SL, Tian XY and Huang Y:
Endothelial dysfunction: The common consequence in diabetes and
hypertension. J Cardiovasc Pharmacol. 55:300–307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang F, Guo X, Shen X, Kream RM, Mantione
KJ and Stefano GB: Vascular dysfunction associated with type 2
diabetes and Alzheimer's disease: A potential etiological linkage.
Med Sci Monit Basic Res. 20:118–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Münzel T: Endothelial dysfunction:
Pathophysiology, diagnosis and prognosis. Dtsch Med Wochenschr.
133:2465–2470. 2008.(In German). View Article : Google Scholar
|
|
29
|
Tejero J, Shiva S and Gladwin MT: Sources
of vascular nitric oxide and reactive oxygen species and their
regulation. Physiol Rev. 99:311–379. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Voldstedlund M, Vinten J and Tranum-Jensen
J: Cav-p60 expression in rat muscle tissues. Distribution of
caveolar proteins. Cell Tissue Res. 306:265–276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mineo C and Shaul PW: Regulation of eNOS
in caveolae. Adv Exp Med Biol. 729:51–62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meye C, Schumann J, Wagner A and Gross P:
Effects of homocysteine on the levels of caveolin-1 and eNOS in
caveolae of human coronary artery endothelial cells.
Atherosclerosis. 190:256–263. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qin L, Zhu N, Ao BX, Liu C, Shi YN, Du K,
Chen JX, Zheng XL and Liao DF: Caveolae and caveolin-1 integrate
reverse cholesterol transport and inflammation in atherosclerosis.
Int J Mol Sci. 17:4292016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao YY, Liu Y, Stan RV, Fan L, Gu Y,
Dalton N, Chu PH, Peterson K, Ross J Jr and Chien KR: Defects in
caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension
in knockout mice. Proc Natl Acad Sci USA. 99:11375–11380. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pechánová O and Simko F: The role of
nitric oxide in the maintenance of vasoactive balance. Physiol Res.
2 (Suppl 56):S7–S16. 2007.
|
|
36
|
Yetik-Anacak G and Catravas JD: Nitric
oxide and the endothelium: History and impact on cardiovascular
disease. Vascul Pharmacol. 45:268–276. 2006. View Article : Google Scholar : PubMed/NCBI
|