Introduction
Chronic rhinosinusitis (CRS) is a chronic
inflammatory condition that affects the mucosa of the nasal and
paranasal sinuses for >12 weeks (1). CRS is a highly prevalent chronic
disease with a heavy socioeconomic burden, characterized by ≥2 of
the following symptoms: Blockage or discharge affecting the mucosa
of the nasal and/or paranasal sinuses, facial pain or pressure, and
problems with smell (1).
Clinically, endoscopy or CT scans are typically used as diagnostic
tools and according to the phenotypical differentiation of the
disease, CRS is divided into two types: i) CRS with nasal polyps
(CRSwNP); and ii) CRS without nasal polyps (2).
CRSwNP is the most common type of CRS, but the
mechanism underlying nasal polyp development is not completely
understood (3). However, the
differentiation of fibroblasts into myofibroblasts, which results
in an accumulation of extracellular matrix (ECM) proteins and
fibrosis, leads to nasal tissue remodeling, which has been
attributed to polyp development (4–6).
Myofibroblasts expressing α-smooth muscle actin (α-SMA) can promote
ECM protein secretion and collagen deposition, and α-SMA is a key
indicator of myofibroblast differentiation (7). TGF-β1 increases fibroblast collagen
secretion and promotes fibroblast differentiation into
myofibroblasts, leading to ECM accumulation and a significant
increase in nasal polyps (7). The
key components of TGF-β1 signaling include TGF-β1 receptor (TβR)-I,
TβR-II and the transcription factor Smad2/3 (8). As an important transcription factor in
TGF-β1 signaling, Smad2/3 serves an important role in
TGF-β1-induced myofibroblast differentiation and phosphorylated
(p)-Smad2/3 expression in nasal polyp fibroblasts (NPFs) (8). Matrix metallopeptidases (MMPs) are key
proteins involved in ECM remodeling that belong to the family of
zinc and calcium-dependent endopeptidases (9). MMP expression levels are increased in
chronic inflammatory diseases, including CRS (10). Several reports have demonstrated the
expression levels of MMP-2 and MMP-9 in CRSwNP are increased
compared with healthy individuals (11–13).
Moreover, MMP-2 and MMP-9 are involved in the pathophysiology of
CRSwNP via degrading the ECM and promoting inflammatory cell
migration to the ECM (8,14).
Salvia miltiorrhiza (Danshen), a traditional
Chinese medicine, has been widely used in the clinical setting due
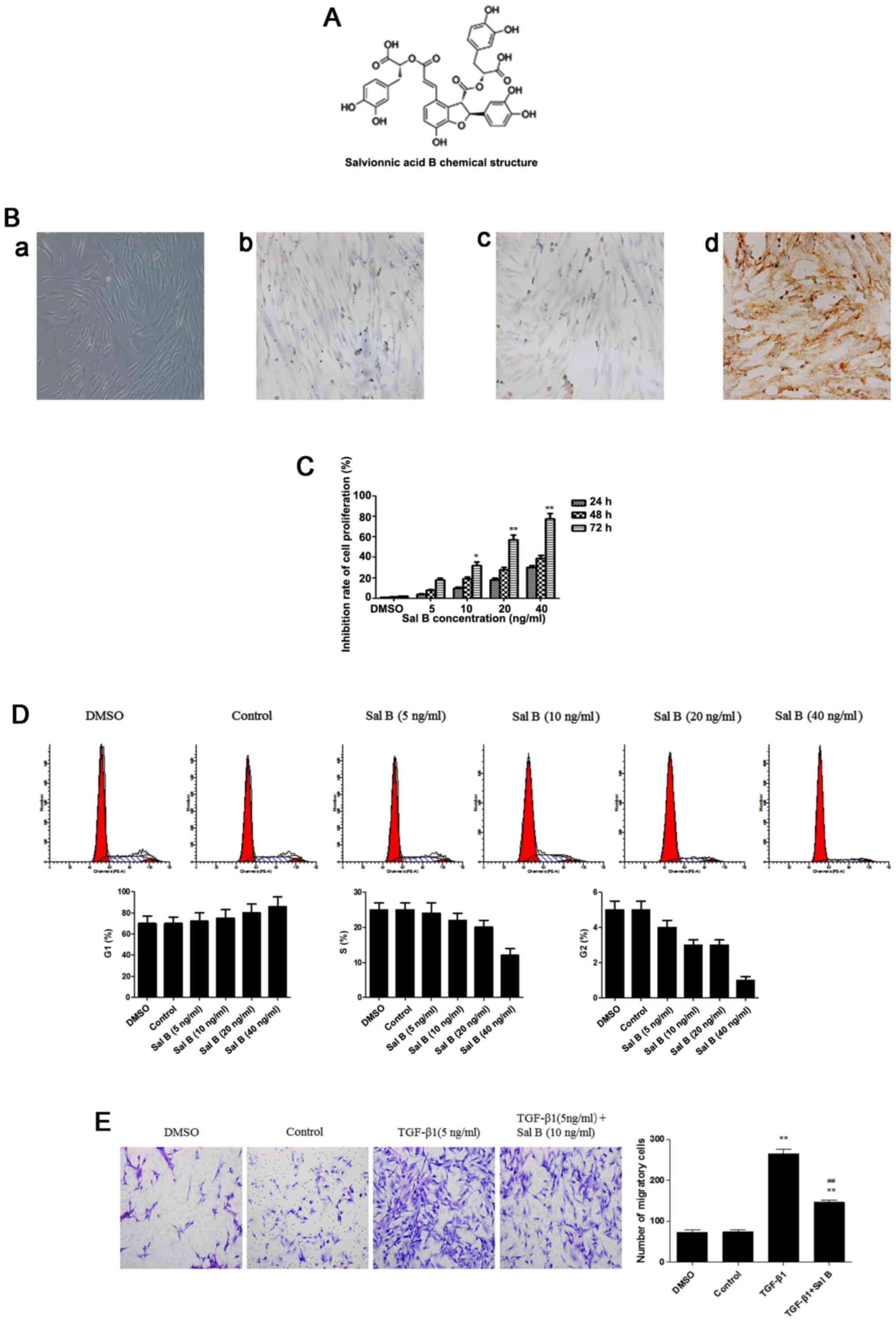
to its extensive reported therapeutic effects (15). Salvianolic acid B (Sal B; Fig. 1A) is the major water-soluble extract
of Salvia miltiorrhiza Bge. The key structural constituents
of Sal B are the trimolecular-3, 4-dihydroxybenyl lactic acids and
a molecule of caffeic acid (15,16).
Previous studies have demonstrated that Sal B displays a wide range
of pharmacological effects, including antioxidation,
antiatherosclerosis, antitumor, antifibrosis and protective effects
in the liver and heart (17). Sal B
has also been reported to be effective in attenuating cardiac,
hepatic, renal and lung fibrosis, and functions by degrading the
ECM and preventing myofibroblast differentiation (18–21).
To the best of our knowledge, whether Sal B alters tissue
remodeling in CRSwNP has not been previously reported. Therefore,
the present study assessed whether Sal B influenced NPF
myofibroblast differentiation and ECM production, and investigated
the mechanisms underlying the therapeutic effects of Sal B.
Materials and methods
Reagents
Sal B (98.0% purity; Sigma-Aldrich; Merck KGaA) and
human recombinant TGF-β1 (PeproTech, Inc.) were freshly prepared in
DMSO and diluted into working concentrations using DMEM (Thermo
Fisher Scientific, Inc.).
Cell culture
Primary nasal fibroblasts were isolated from nasal
polyps of eight patients with nasal polyposis (4 female patients
and 4 male patients; mean age, 40.2±3.4 years) from January 2019 to
January 2020 at the Department of Otorhinolaryngology, Shanghai
General Hospital of Shanghai Jiao Tong University (Shanghai,
China). The present study was approved by the ethics committee of
Shanghai First People's Hospital (approval no. 2018KY008). All
patients provided written informed consent. The following inclusion
criteria were used: i) No prior nasal surgery; ii) no active
inflammation, allergies or aspirin hypersensitivity; iii) no oral
or topical antibiotics, antihistamines, steroids or other
medications for at least 4 weeks prior to surgery; and iv) no oral
antiallergy medications for ≥2 weeks prior to surgery.
Polyp tissues were mechanically milled and
enzymatically digested in DMEM containing 500 U/ml collagenase, 30
U/ml hyaluronidase, 10 U/ml DNAse, 500 U/ml penicillin and 500
µg/ml streptomycin. At 80–90% confluence, the culture medium was
replaced to remove non-adherent cells. Subsequently, 0.05% EDTA
(Sigma Aldrich; Merck KGaA) was used to separate the cells from the
plate. Cells were subcultured in DMEM containing 10% FBS (Thermo
Fisher Scientific, Inc.), 500 U/ml penicillin and 500 µg/ml
streptomycin at 37°C with 5% CO2 with humidity. The purity of NPFs
was assessed by observing the characteristic spindle-shaped cell
morphology using an optical microscope and by performing
immunocytochemistry using vimentin as a positive marker and
pan-cytokeratin as a negative marker (Fig. 1B). Cells from passage 4–7 were used
for subsequent experiments.
Cells were divided into the following five groups:
i) Sal B group, cells cultured in DMEM containing 10 ng/ml Sal B;
ii) TGF-β1 group, cells cultured in DMEM containing 5 ng/ml TGF-β1;
iii) TGF-β1 + Sal B group, cells cultured in DMEM containing 5
ng/ml TGF-β1 and 10 ng/ml Sal B; iv) control group, cells cultured
in DMEM; and v) DMSO group, cells were cultured in DMEM containing
DMSO.
Cell proliferation and
cytotoxicity
The CCK-8 (Beyotime Institute of Biotechnology)
assay was performed to assess the effects of Sal B on NPF
proliferation. NPFs were seeded (5×103 cells/well) into
96-well plates. Cells were incubated with Sal B (0-40 ng/ml) or
DMSO (an equal volume to that used for 40 ng/ml Sal B) for 24, 48
or 72 h at 37°C. Subsequently, CCK-8 solution was added to each
well for 2 h. The optical density was measured at a wavelength of
450 nm using iMark™ Microplate Absorbance Reader (Bio-Rad
Laboratories, Inc.).
Cell migration assay
Cell migration was assessed by performing Transwell
migration assays. NPFs were seeded (1×105 cells/ml) into
the upper chamber with DMEM containing TGF-β1 (5 ng/ml), Sal B (10
ng/ml) or DMSO (an equal volume to that used for 10 ng/ml Sal B).
Subsequently, 600 µl DMEM containing 20% FBS was added to the lower
chamber. After incubation for 48 h at 37°C, migratory cells were
fixed with methanol at room temperature for 30 min, followed by
crystal violet staining for 10 min at room temperature. Stained
cells were observed using an inverted light microscope
(magnification, ×100).
Cell cycle analysis via flow
cytometry
Cells were seeded into 6-well culture plates and
incubated at 37°C with 5% CO2. The cell culture medium was replaced
with DMEM containing Sal B (0-40 ng/ml) or DMSO (an equal volume to
that used for 40 ng/ml Sal B). Following incubation for 48 h at
37°C, cells were collected, washed twice with PBS, resuspended in
250 µl PBS and vortexed. Subsequently, cells were added to 750 µl
pre-cooled absolute ethanol for fixation at 4°C overnight. Cells
were centrifuged twice at 1800 × g for 5 min at room temperature,
the supernatant was discarded and the pellet was resuspended in 500
µl PBS. The cells were then incubated with 20 µg/ml RNase and 50
µg/ml PI at room temperature for 30 min in the dark with gentle
agitation. Cells were analyzed via flow cytometry (Beckman Coulter
FC500 analyzer; Beckman Coulter, Inc.).
RNA isolation and reverse
transcription-quantitative PCR (qPCR)
Following treatment for 24 h at 37°C, total RNA was
isolated from fibroblasts using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol.
Total RNA was reverse transcribed into cDNA using
the ReverTra Ace qPCR RT kit (Takara Bio, Inc.) according to the
manufacturer's protocol. Subsequently, qPCR was performed using a
MiniOpticon Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The following primers were used for qPCR: GAPDH
forward, 5′-GATGCCCCCATGTTCGTCAT-3′ and reverse,
5′-TCTTCTGGGTGGCAGTGATG-3′; α-SMA forward,
5′-TAGCACCCAGCACCATGAAG-3′ and reverse, 5′-TCTTCTGGGTGGCAGTGATG-3′;
TβR-I forward, 5′-GTGACAGATGGGCTCTGCTT-3′ and reverse,
5′-GCAATGGTCCTGATTGCAGC-3′; Smad2 forward,
5′-GAACTTCCGCCTCTGGATGA-3′ and reverse,
5′-CTGGAGAGCCTGTGTCCATAC-3′; Smad3 forward,
5′-CCATCTCCTACTACGAGCTGAA-3′ and reverse,
5′-CACTGCTGCATTCCTGTTGAC-3′; MMP-2 forward,
5′-GGTTCATTTGGCGGACTGTG-3′ and reverse, 5′-CACAGCCTTCTCCTCCTGTG-3′;
and MMP-9 forward, 5′-CCTGGGCAGATTCCAAACCT-3 and reverse,
5′-GTACACGCGAGTGAAGGTGA-3′; TβR-II forward,
5′-CGTGTGGAGGAAGAACGACA-3′ and reverse,
5′-CGTGGGAGAAGTGGCATCTT-3′.
The following thermocycling conditions were used for
qPCR: Initial denaturation at 95°C for 10 min; followed by 40
cycles of denaturation for 15 sec at 95°C and annealing for 30 sec
at 58°C. mRNA expression levels were normalized to the internal
reference gene GAPDH. Analysis of relative gene expression data was
carried out using the 2−ΔΔCt method (22).
Western blotting
Following culture in DMEM supplemented with 10% FBS
for 24 h at 37°C, NPFs were treated with Sal B (10 ng/ml) and/or
TGF-β1 (5 ng/ml) for 48 h at 37°C. Cells were collected and washed
twice with PBS. Total protein was isolated from cells using RIPA
lysis buffer (Thermo Fisher Scientific, Inc.) containing 1% PMSF
and 1% protease inhibitor. Proteins (30 µg) were quantitatively
analyzed by using BCA method and separated via SDS-PAGE on 10% gels
and transferred onto PVDF membranes. After blocking with 5% skimmed
milk for 1 h at room temperature, the membranes were incubated at
4°C overnight with primary antibodies targeted against: MMP-2 (cat.
no. 40994S; Cell Signaling Technology, Inc.; 1:1,000), MMP-9 (cat.
no. 13667S; Cell Signaling Technology, Inc.; 1:1,000), p-Smad2
(cat. no. 3104s; Cell Signaling Technology, Inc.; 1:5,00), p-Smad3
(cat. no. 9520S; Cell Signaling Technology, Inc.; 1:5,00), α-SMA
(cat. no. sc-53142; Santa Cruz Biotechnology, Inc.; 1:1,000), TβR-I
(cat. no. sc-518018; Santa Cruz Biotechnology, Inc.; 1:1,000),
TβR-II (cat. no. sc-17792; Santa Cruz Biotechnology, Inc.;
1:1,000), Smad2 (cat. no. sc-101153; Santa Cruz Biotechnology,
Inc.; 1:1,000) and Smad3 (cat. no. sc-101154; Santa Cruz
Biotechnology, Inc.;1:500). Following primary antibody incubation,
the membranes were incubated with HRP-linked anti-rabbit IgG (cat.
no. 7074P2; Cell Signaling Technology, Inc.; 1:3,000) or mouse IgGκ
light chain binding-protein (cat. no. sc-516102; Santa Cruz
Biotechnology, Inc.; 1:2,000) secondary antibodies for 1 h at room
temperature. Protein bands were visualized using an ECL system
(Thermo Fisher Scientific, Inc.). GAPDH (cat. no. 2118S; Cell
Signaling Technology, Inc.; 1:1,000) was used as the loading
control.
Immunofluorescence staining
NPFs were fixed with 4% paraformaldehyde for 30 min
at room temperature and soaked in 1% BSA (cat. no. A1933-25g;
Sigma-Aldrich; Merck KGaA) containing 0.2% Triton X-100 for 10 min.
After blocking with 3% BSA for 1 h at room temperature, NPFs were
incubated with an anti-α-SMA primary antibody (cat. no. sc-53142;
Santa Cruz Biotechnology, Inc.; 1:1,000) overnight at 4°C.
Subsequently, NPFs were incubated with a mouse IgGκ light chain
binding-protein secondary antibody (cat. no. sc-516102; Santa Cruz
Biotechnology, Inc.; 1:2,000) for 1 h at room temperature. Stained
NPFs were observed using a confocal laser scanning microscope.
Immunocytochemistry
The slides were placed in an oven for 30 min for
dewaxing, then hydrated with gradient alcohol. A total of 1 ml 30%
H2O2 and 9 ml methanol were used to remove endogenous catalase.
Subsequently, 5% BSA blocking solution (cat. no. A1933-25g;
Sigma-Aldrich; Merck KGaA) was added to block non-specific binding
in a 37°C incubator for 30 min. The slides were incubated with
primary antibody against vimentin (cat. no. 5741; Cell Signaling
Technology, Inc.; 1:200) or CK (cat. no. 13063; Cell Signaling
Technology, Inc.; 1:200) in 1% BSA overnight at 4°C. Subsequently,
NPFs were incubated with a HRP-conjugated rabbit secondary antibody
(SignalStain® Boost IHC Detection Reagent; cat. no.
8114; Cell Signaling Technology, Inc.) for 1 h at room temperature,
then wash three times in PBS for 2 min each. DAB substrate was used
to detect HRP activity. The results were observed using an inverted
microscope (Nikon Corporation; magnification, ×400).
ELISA
After culturing in DMEM containing 10% FBS for 24 h,
NPFs were treated with Sal B (10 ng/ml) and/or TGF-β1 (5 ng/ml) for
24 h at 37°C. Subsequently, the cells were collected following
centrifugation at 1,800 × g for 5 min at room temperature, and
phase-contrast microscopy and a hemocytometer were used to count
cells.
ELISA kits were used to measure the concentrations
of secreted type-III collagen (cat. no. CSI 007-01-02; Thermo
Fisher Scientific, Inc.) and fibronectin (cat. no. BMS2028TEN;
Thermo Fisher Scientific, Inc.) concentrations in the supernatants
were measured by performing ELISA, according to the manufacturer's
protocols.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 20.0; IBM Corp.). Data are presented as the mean
± SD of three independent experimental repeats. Comparisons among
multiple groups were analyzed using one-way ANOVA followed by
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Sal B inhibits cell viability and
TGF-β1-induced cell migration in NPFs
The CCK-8 assay was performed to examine the
cytotoxicity of Sal B in NPFs. Compared with in the control group,
fibroblast viability was significantly suppressed by treatment with
10 ng/ml Sal B for 72 h (Fig. 1C).
In addition, the cell cycle analysis results indicated that Sal B
induced cell cycle arrest in G1 phase in a concentration-dependent
manner, indicating that Sal B mediated cell viability by altering
cell proliferation (Fig. 1D).
Therefore, 10 ng/ml Sal B was identified as the optimum
concentration for subsequent experiments.
Myofibroblasts serve an important role in wound
tissue repair by altering cell migration (8). Therefore, a Transwell assay was
performed to assess the effect of Sal B on myofibroblast migration.
Sal B significantly decreased TGF-β1-induced NPF migration
(Fig. 1E). These results indicated
that Sal B inhibited myofibroblast biological activity by
regulating cell viability and migration.
Sal B suppresses NPF myofibroblast
differentiation
To investigate whether Sal B inhibited myofibroblast
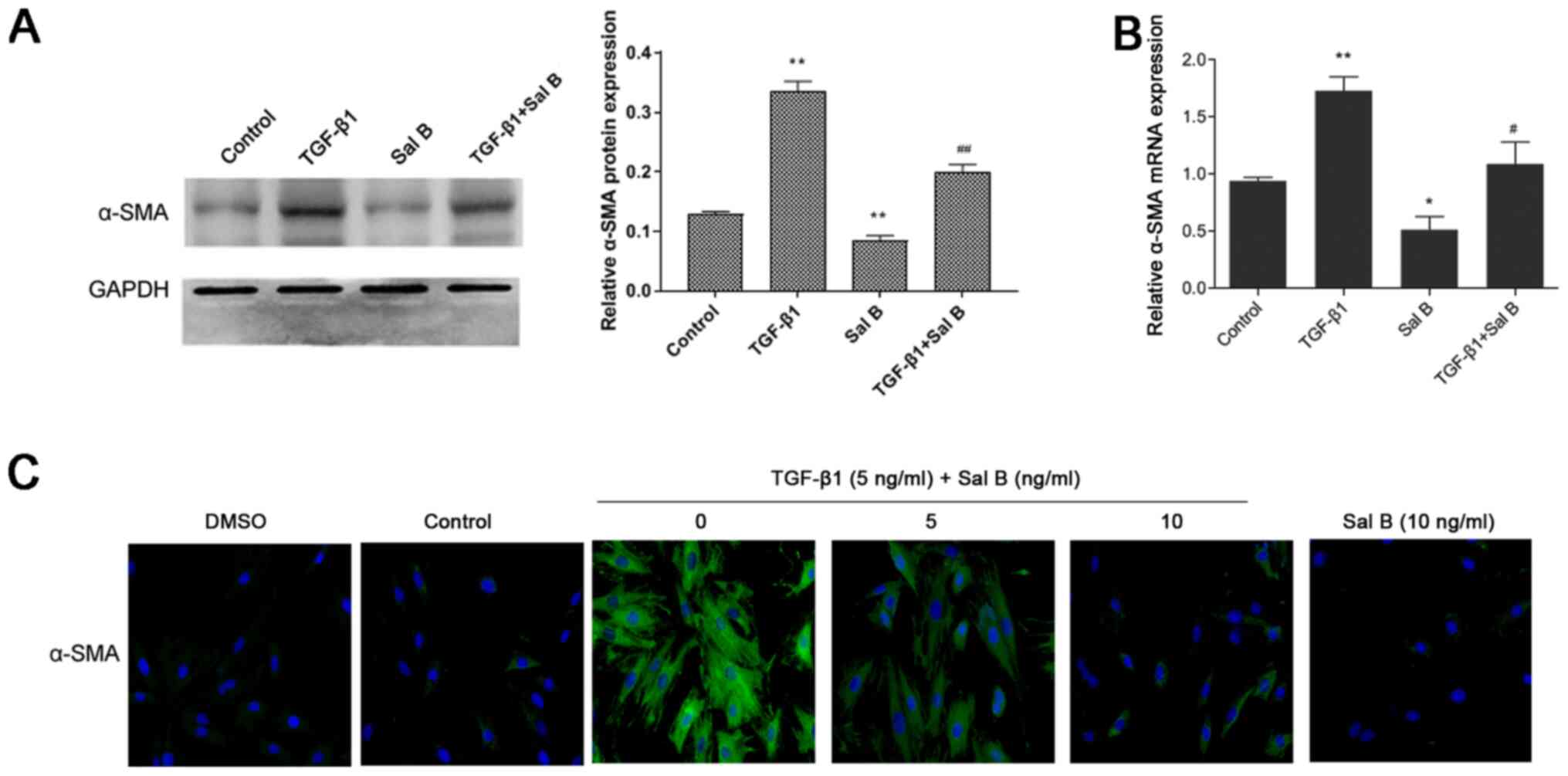
differentiation, the present study assessed α-SMA expression in
NPFs treated with Sal B and/or TGF-β1. The results demonstrated
that compared with in the control group, TGF-β1 treatment
significantly increased α-SMA mRNA and protein expression levels,
which were significantly downregulated by Sal B (Fig. 2A and B). In addition, the
immunofluorescence staining results indicated that α-SMA was
abundantly expressed in the cytoplasm of TGF-β1-stimulated NPFs,
whereas Sal B markedly inhibited α-SMA expression in
TGF-β1-stimulated NPFs (Fig. 2C).
These results suggested that Sal B inhibited TGF-β1-induced NPF
myofibroblast differentiation.
Sal B blocks TGF-β1-induced ECM and
collagen production in NPFs
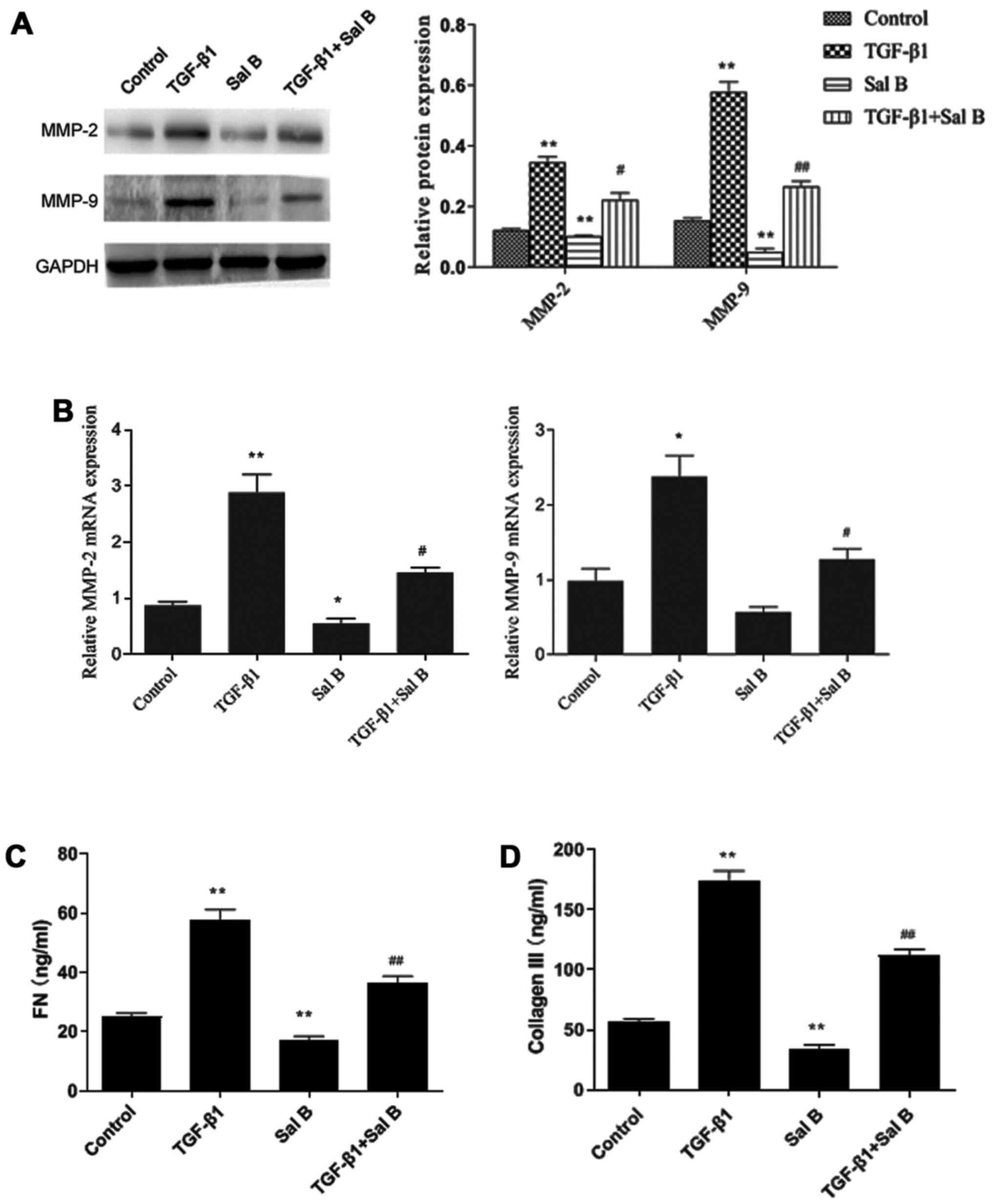
The present study investigated whether Sal B blocked
ECM production in NPFs. TGF-β1 treatment significantly increased
MMP-2 and MMP-9 expression levels compared with in the control
group. Sal B treatment significantly decreased MMP-2 and MMP-9 mRNA
and protein expression levels in TGF-β1-treated NPFs, with a larger
inhibitory effect detected on MMP-9 expression compared with MMP-2
expression (Fig. 3A and B). In
addition, the ELISA results demonstrated that compared with in the
control group, collagen III and fibronectin levels were
significantly increased by TGF-β1, and Sal B significantly
inhibited TGF-β1-induced effects (Fig.
3C and D). These results suggested that Sal B inhibited
TGF-β1-induced ECM accumulation and collagen production in
NPFs.
Sal B inhibits NPF myofibroblast
differentiation via the TGF-β1 signaling pathway
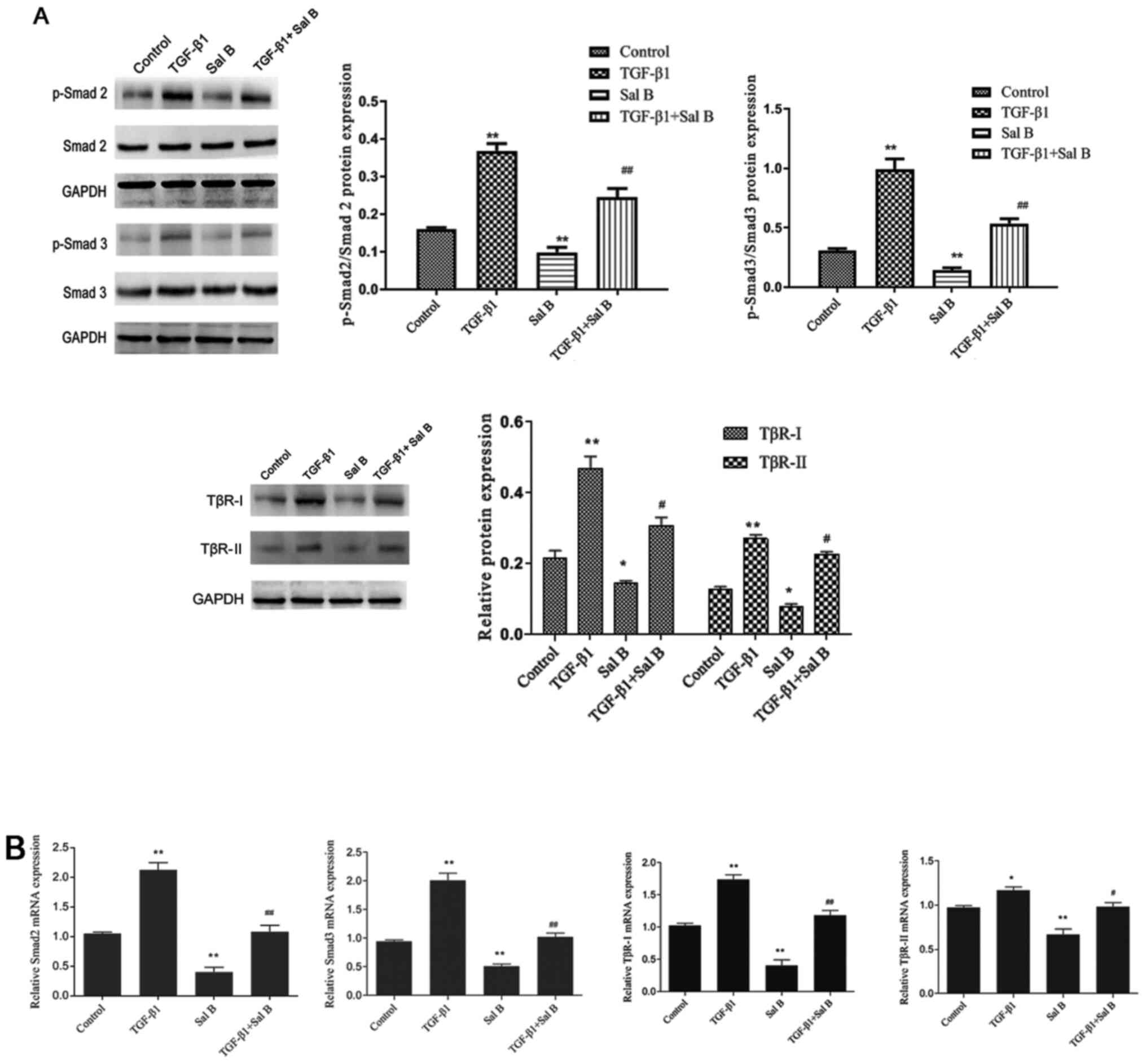
Smad2/3 is one of the primary transcription factors
in the TGF-β1 signaling pathway (8). To determine the mechanism underlying
the inhibitory effects of Sal B on myofibroblast differentiation
and ECM accumulation, the present study measured TβR-I, TβR-II and
Smad2/3 expression levels (Fig. 4).
After stimulation with TGF-β1, p-Smad2/3 protein expression levels
were significantly increased in NPFs compared with in control
cells. Moreover, Sal B significantly decreased p-Smad2/3 protein
levels in NPFs treated with or without TGF-β1. The western blotting
results indicated that compared with the control group, total
Smad2/3 expression levels were not notably altered by TGF-β1 or Sal
B. Compared with in the control group, TGF-β1 significantly
increased TβR-I and TβR-II expression levels, increasing TβR-I
expression to a higher level compared with TβR-II. Sal B
significantly decreased TβR-I and TβR-II expression levels in NPFs
treated with or without TGF-β1. These results suggested that Sal B
inhibited myofibroblast differentiation and ECM accumulation via
the TGF-β1 signaling pathway.
Discussion
CRSwNP develops due to abnormal growth of the mucous
membranes of the nasal cavity or paranasal sinuses (23), with the majority of research
indicating that airway remodeling and complex inflammatory
reactions are involved in the underlying mechanisms (24–27).
The current treatment for CRSwNP comprises short-term oral steroids
and long-term topical steroids (1).
Other forms of anti-inflammatory and antiproliferative agents,
including traditional Chinese extracts, have been studied as they
display low toxicity and minimal side effects (28). It was hypothesized that the
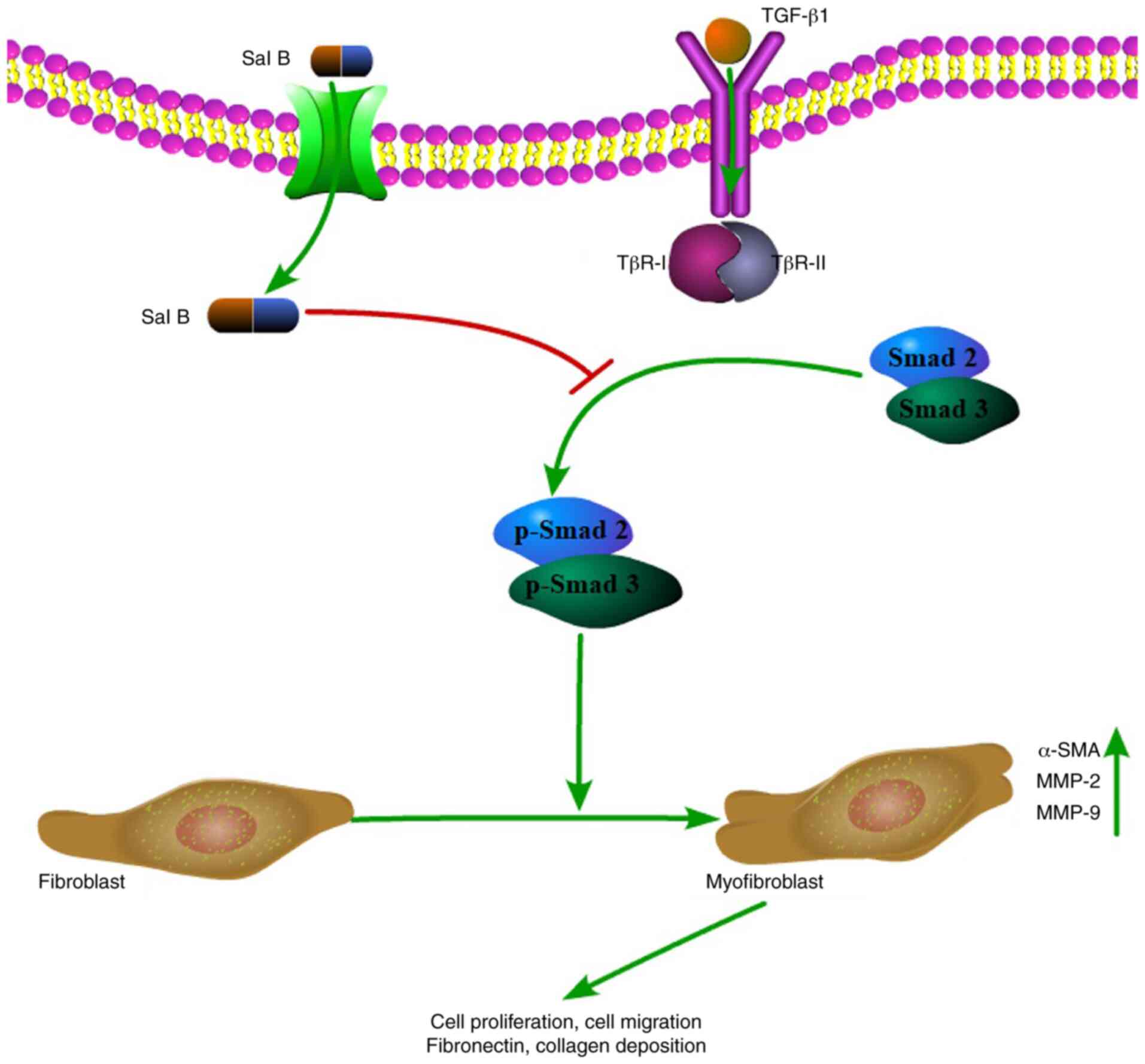
antifibrotic effect of Sal B occurred via inhibition of the TGF-β1
signaling pathway (17,19,20).
To the best of our knowledge, the present study
reported the role and potential molecular mechanisms underlying Sal
B in blocking airway remodeling by inhibiting NPF myofibroblast
differentiation and ECM production for the first time. A previous
studies have demonstrated that TGF-β1 may be involved in the
production of α-SMA protein and the accumulation of ECM, and could
be considered an inducer of myofibroblast differentiation (29). Therefore, blocking TGF-β1 activity
could inhibit ECM deposition and regulate the fibrotic process of
nasal polyp formation (29). The
present study demonstrated that Sal B suppressed TGF-β1-induced
α-SMA expression levels, and fibronectin and collagen III levels.
Furthermore, Sal B significantly downregulated TGF-β1-induced
TβR-I, TβR-II and p-Smad2/3 expression levels. Collectively, the
results indicated that Sal B regulated the TGF-β1 signaling
pathway, which may serve as the mechanism underlying the
antifibrotic actions of Sal B (Fig.
5).
MMPs are key players in the process of airway
remodeling by restructuring basement membranes and regulating
extracellular components (30).
Several studies have suggested that when patients with CRSwNP are
infected with Staphylococcus aureus, MMP secretion increases
and nasal polyp formation may also be affected (6,30,31).
Thus, the present study demonstrated that Sal B decreased
TGF-β1-induced MMP-2 and MMP-9 mRNA and protein expression levels
in NPFs, which was consistent with the results of previous studies
(6,32,33).
Collectively, the results of the present study suggested that the
anti-inflammatory effect of Sal B might be associated with NPF
myofibroblast differentiation and ECM production.
In summary, the present study indicated that Sal B
mediated effects via multiple molecular mechanisms to inhibit the
development of nasal polyposis due to its anti-inflammatory
activity. Therefore, Sal B may serve as a novel therapeutic target
for nasal polyposis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81271067).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZS contributed to study design and wrote the
manuscript. DL, SW and RH performed the data analysis. PD
participated in drafting the work and data analysis and revised it
critically for important intellectual content. All authors read and
approved the final manuscript. ZS, DL, SW, RH and PD confirm the
authenticity of the data.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Shanghai First People's Hospital (approval no.
2018KY008). All patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fokkens WJ, Lund VJ, Mullol J, Bachert C,
Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, et
al: European Position Paper on Rhinosinusitis and Nasal Polyps
2012. Rhinol Suppl. 23:3 p preceding table of contents, 1. –298.
2012.PubMed/NCBI
|
|
2
|
Van Crombruggen K, Van Bruaene N,
Holtappels G and Bachert C: Chronic sinusitis and rhinitis:
Clinical terminology ‘Chronic Rhinosinusitis’ further supported.
Rhinology. 48:54–58. 2010.PubMed/NCBI
|
|
3
|
Van Zele T, Holtappels G, Gevaert P and
Bachert C: Differences in initial immunoprofiles between recurrent
and nonrecurrent chronic rhinosinusitis with nasal polyps. Am J
Rhinol Allergy. 28:192–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pawankar R: Nasal polyposis: an update:
editorial review. Curr Opin Allergy Clin Immunol. 3:1–6. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pezato R, Voegels RL, Pinto Bezerra TF,
Perez-Novo C, Stamm AC and Gregorio LC: Mechanical disfunction in
the mucosal oedema formation of patients with nasal polyps.
Rhinology. 52:162–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shin JM, Park JH, Kang B, Lee SA, Park IH
and Lee HM: Effect of doxycycline on transforming growth
factor-beta-1-induced matrix metalloproteinase 2 expression,
migration, and collagen contraction in nasal polyp-derived
fibroblasts. Am J Rhinol Allergy. 30:385–390. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang QP, Escudier E, Roudot-Thoraval F,
Abd-Al Samad I, Peynegre R and Coste A: Myofibroblast accumulation
induced by transforming growth factor-beta is involved in the
pathogenesis of nasal polyps. Laryngoscope. 107:926–931. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shin SH, Ye MK, Lee DW and Che MH: Effect
of Acacia Honey on Transforming Growth Factor-Beta-1-Induced
Myofibroblast Differentiation and Matrix Metalloproteinase-9
Production in Nasal Polyp Fibroblasts. Am J Rhinol Allergy.
33:483–489. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang L, Wu Z, Qin H, Chen W and Zhang G:
Changes of Transforming Growth Factor-β1 and Extracellular Matrix
in the Wound Healing Process of Rats Infected With Pseudomonas
aeruginosa. Wounds. 26:293–300. 2014.PubMed/NCBI
|
|
10
|
Wang LF, Chien CY, Chiang FY, Chai CY and
Tai CF: Corelationship between matrix metalloproteinase 2 and 9
expression and severity of chronic rhinosinusitis with nasal
polyposis. Am J Rhinol Allergy. 26:e1–e4. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Watelet JB, Bachert C, Claeys C and Van
Cauwenberge P: Matrix metalloproteinases MMP-7, MMP-9 and their
tissue inhibitor TIMP-1: Expression in chronic sinusitis vs nasal
polyposis. Allergy. 59:54–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eyibilen A, Cayli S, Aladag I, Koç S,
Gurbuzler L and Atay GA: Distribution of matrix metalloproteinases
MMP-1, MMP-2, MMP-8 and tissue inhibitor of matrix
metalloproteinases-2 in nasal polyposis and chronic rhinosinusitis.
Histol Histopathol. 26:615–621. 2011.PubMed/NCBI
|
|
13
|
Can IH, Ceylan K, Caydere M, Samim EE,
Ustun H and Karasoy DS: The expression of MMP-2, MMP-7, MMP-9, and
TIMP-1 in chronic rhinosinusitis and nasal polyposis. Otolaryngol
Head Neck Surg. 139:211–215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li X, Meng J, Qiao X, Liu Y, Liu F, Zhang
N, Zhang J, Holtappels G, Luo B, Zhou P, et al: Expression of TGF,
matrix metalloproteinases, and tissue inhibitors in Chinese chronic
rhinosinusitis. J Allergy Clin Immunol. 125:1061–1068. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kastrup J, Jørgensen E, Rück A, Tägil K,
Glogar D, Ruzyllo W, Bøtker HE, Dudek D, Drvota V, Hesse B, et al
Euroinject One Group, : Direct intramyocardial plasmid vascular
endothelial growth factor-A165 gene therapy in patients with stable
severe angina pectoris A randomized double-blind placebo-controlled
study: The Euroinject One trial. J Am Coll Cardiol. 45:982–988.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang XW, Zhang Y, Yang SK, Zhang H, Lu K
and Sun GL: Efficacy of salvianolic acid B combined with
triamcinolone acetonide in the treatment of oral submucous
fibrosis. Oral Surg Oral Med Oral Pathol Oral Radiol. 115:339–344.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin YL, Wu CH, Luo MH, Huang YJ, Wang CN,
Shiao MS and Huang YT: In vitro protective effects of salvianolic
acid B on primary hepatocytes and hepatic stellate cells. J
Ethnopharmacol. 105:215–222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lv Z and Xu L: Salvianolic Acid B Inhibits
ERK and p38 MAPK Signaling in TGF-β1-Stimulated Human Hepatic
Stellate Cell Line (LX-2) via Distinct Pathways. Evid Based
Complement Alternat Med. 2012:9601282012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu HY, Zhou J, Lu M, Liu YM, Wang F, Lin M
and Zhang Y: Protection and mechanisms of salvianolic-acid B on
experimental renal interstitial fibrosis in rats. Zhong Yao Cai.
33:1755–1759. 2010.(In Chinese). PubMed/NCBI
|
|
20
|
Liu Q, Chu H, Ma Y, Wu T, Qian F, Ren X,
Tu W, Zhou X, Jin L, Wu W, et al: Salvianolic Acid B Attenuates
Experimental Pulmonary Fibrosis through Inhibition of the TGF-β
Signaling Pathway. Sci Rep. 6:276102016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Wang L, Dong Z, Wang S, Qi L, Cho K,
Zhang Z, Li N, Hu Y and Jiang B: Cardioprotection of salvianolic
acid B and ginsenoside Rg1 combination on subacute myocardial
infarction and the underlying mechanism. Phytomedicine. 57:255–261.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−∆ ∆ C(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cervin A: The anti-inflammatory effect of
erythromycin and its derivatives, with special reference to nasal
polyposis and chronic sinusitis. Acta Otolaryngol. 121:83–92. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Katainen E, Kostamo K, Virkkula P, Sorsa
T, Tervahartiala T, Haapaniemi A and Toskala E: Local and systemic
proteolytic responses in chronic rhinosinusitis with nasal
polyposis and asthma. Int Forum Allergy Rhinol. 5:294–302. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uluyol S, Arslan IB, Demir A, Mercan GC,
Dogan O and Çukurova İ: The role of the uncinate process in
sinusitis aetiology: Isolated agenesis versus maxillary sinus
hypoplasia. J Laryngol Otol. 129:458–461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang LF, Tai CF, Chien CY, Chiang FY and
Chen JY: Vitamin D decreases the secretion of matrix
metalloproteinase-2 and matrix metalloproteinase-9 in fibroblasts
derived from Taiwanese patients with chronic rhinosinusitis with
nasal polyposis. Kaohsiung J Med Sci. 31:235–240. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park HH, Park IH, Cho JS, Lee YM and Lee
HM: The effect of macrolides on myofibroblast differentiation and
collagen production in nasal polyp-derived fibroblasts. Am J Rhinol
Allergy. 24:348–353. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mullol J, Roca-Ferrer J, Alobid I, Pujols
L, Valero A, Xaubet A, Bernal-Sprekelsen M and Picado C: Effect of
desloratadine on epithelial cell granulocyte-macrophage
colony-stimulating factor secretion and eosinophil survival. Clin
Exp Allergy. 36:52–58. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cho JS, Moon YM, Park IH, Um JY, Moon JH,
Park SJ, Lee SH, Kang HJ and Lee HM: Epigenetic regulation of
myofibroblast differentiation and extracellular matrix production
in nasal polyp-derived fibroblasts. Clin Exp Allergy. 42:872–882.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Um JY, Lee SA, Park JH, Shin JM, Park IH
and Lee HM: Role of adenosine monophosphate-activated protein
kinase on cell migration, matrix contraction, and matrix
metalloproteinase-1 and matrix metalloproteinase-2 production in
nasal polyp-derived fibroblasts. Am J Rhinol Allergy. 31:357–363.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Muluk NB, Arikan OK, Atasoy P, Kiliç R and
Yalçinozan ET: The role of MMP-2, MMP-9, and TIMP-1 in the
pathogenesis of nasal polyps: Immunohistochemical assessment at
eight different levels in the epithelial, subepithelial, and deep
layers of the mucosa. Ear Nose Throat J. 94:E1–E13. 2015.PubMed/NCBI
|
|
32
|
Suzuki M, Ramezanpour M, Cooksley C, Li J,
Nakamaru Y, Homma A, Psaltis A, Wormald PJ and Vreugde S: Sirtuin-1
Controls Poly (I:C)-Dependent Matrix Metalloproteinase 9 Activation
in Primary Human Nasal Epithelial Cells. Am J Respir Cell Mol Biol.
59:500–510. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li X, Tao Y and Li X: Expression of
MMP-9/TIMP-2 in nasal polyps and its functional implications. Int J
Clin Exp Pathol. 8:14556–14561. 2015.PubMed/NCBI
|