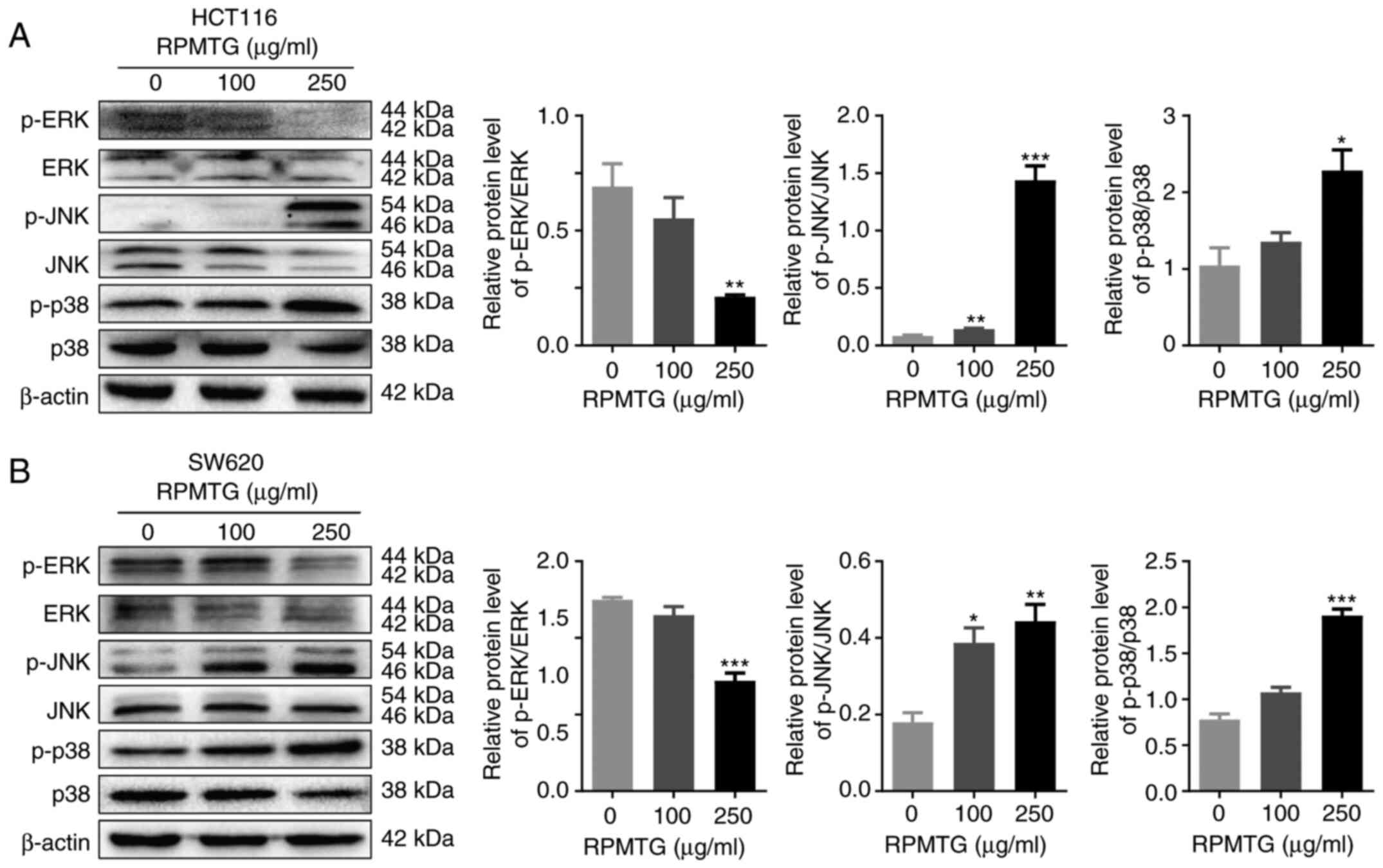
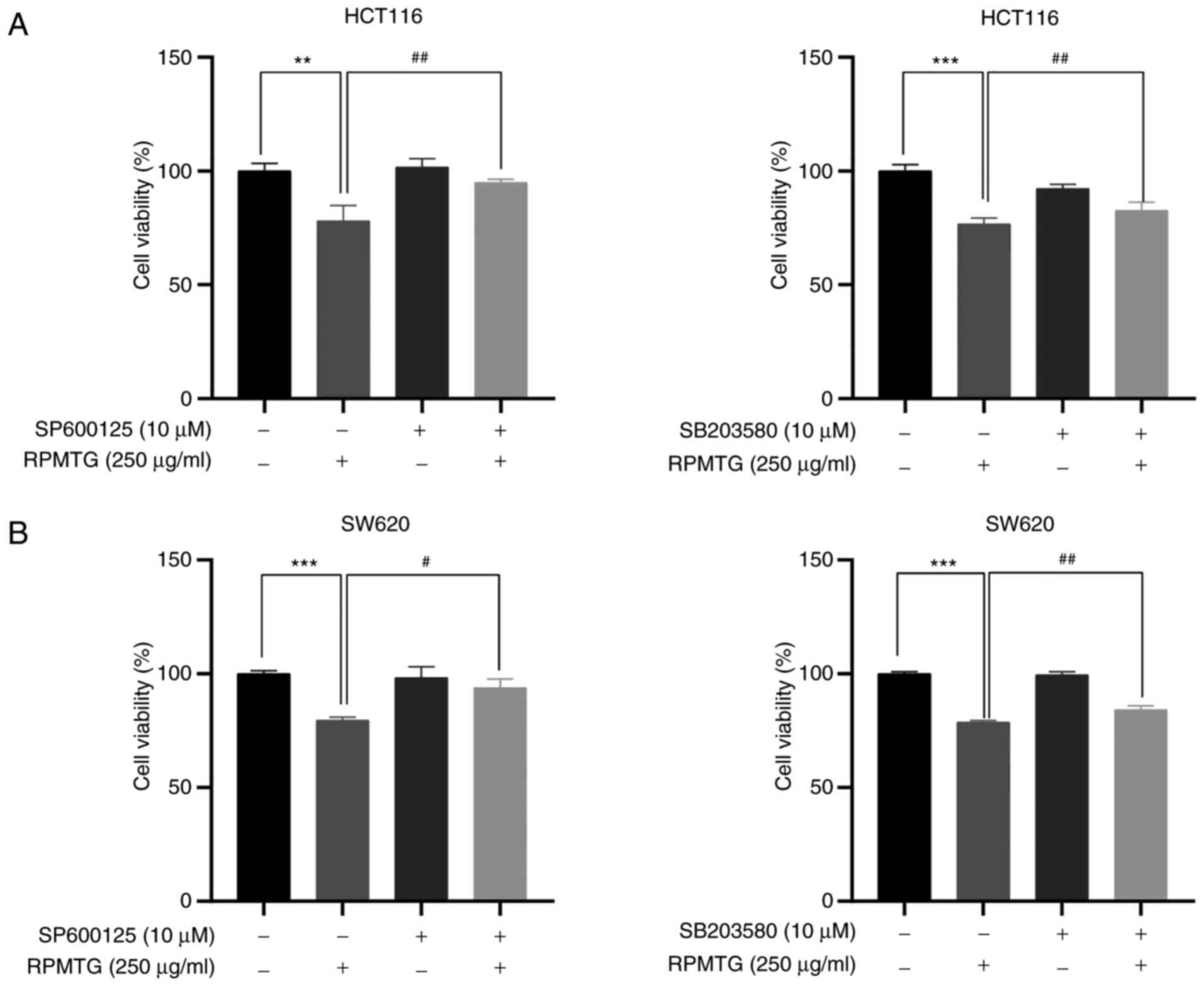
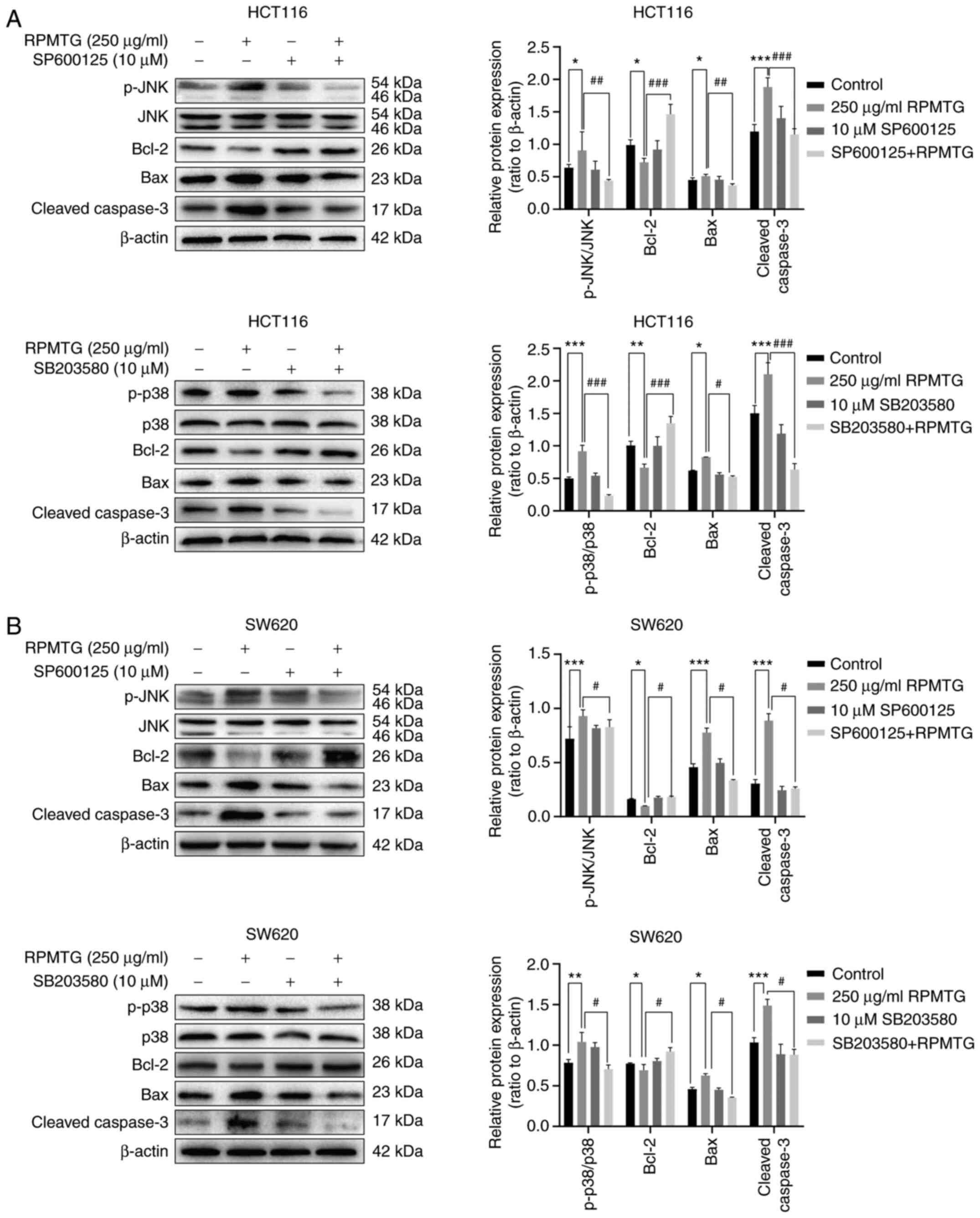
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ma M, Wang X, Liu N, Shan F and Feng Y:
Low-dose naltrexone inhibits colorectal cancer progression and
promotes apoptosis by increasing M1-type macrophages and activating
the Bax/Bcl-2/caspase-3/PARP pathway. Int Immunopharmacol.
83:1063882020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun J, Feng Y, Wang Y, Ji Q, Cai G, Shi L,
Wang Y, Huang Y, Zhang J and Li Q: α-hederin induces autophagic
cell death in colorectal cancer cells through reactive oxygen
species dependent AMPK/mTOR signaling pathway activation. Int J
Oncol. 54:1601–1612. 2019.PubMed/NCBI
|
|
5
|
Liu Z, Xiong L, Ouyang G, Ma L, Sahi S,
Wang K, Lin L, Huang H, Miao X, Chen W, et al: Investigation of
copper cysteamine nanoparticles as a new type of radiosensitiers
for colorectal carcinoma Treatment. Sci Rep. 7:92902017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu M, Yu Z, Mei P, Li J, Luo D, Zhang H,
Zhou M, Liang F and Chen R: Correction for: Lycorine induces
autophagy-associated apoptosis by targeting MEK2 and enhances
vemurafenib activity in colorectal cancer. Aging (Albany NY).
12:6488–6489. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weaver BA: How Taxol/paclitaxel kills
cancer cells. Mol Biol Cell. 25:2677–2681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
GLB: L. Gatti, ; Perego P and Zaffaroni N:
Camptothecin resistance in cancer: Insights into the molecular
mecha-nisms of a DNA-damaging drug. Curr Med Chem. 20:1541–1565.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paidakula S, Nerella S, Vadde R, Kamal A
and Kankala S: Design and synthesis of
4β-acetamidobenzofuranone-podophyllotoxin hybrids and their
anti-cancer evaluation. Bioorg Med Chem Lett. 29:2153–2156. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Man S, Gao W, Zhang Y, Huang L and Liu C:
Chemical study and medical application of saponins as anti-cancer
agents. Fitoterapia. 81:703–714. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi JS, Chun KS, Kundu J and Kundu JK:
Biochemical basis of cancer chemoprevention and/or chemotherapy
with ginsenosides (Review). Int J Mol Med. 32:1227–1238. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee DG, Jang SI, Kim YR, Yang KE, Yoon SJ,
Lee ZW, An HJ, Jang IS, Choi JS and Yoo HS: Anti-proliferative
effects of ginsenosides extracted from mountain ginseng on lung
cancer. Chin J Integr Med. 22:344–352. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun M, Ye Y, Xiao L, Duan X, Zhang Y and
Zhang H: Anticancer effects of ginsenoside Rg3 (Review). Int J Mol
Med. 39:507–518. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song X, Wang W, Zhang X, Jiang Y, Yang X,
Deng C, Yue Z and Tang Z: Deglucose chikusetsusaponin IVa isolated
from Rhizoma Panacis Majoris induces apoptosis in human HepG2
hepatoma cells. Mol Med Rep. 12:5494–5500. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen T, Chen LF, Jin GQ and Li D: Panax
japlcus var induced apoptosis of human hepatocarcinoma cells in
vitro. Tumor. 26:144–147. 2006.(In Chinese). PubMed/NCBI
|
|
16
|
Chen T, Chen M, Hu Y, Gong Z and Deng L:
Extraction and anticancer activity of polysaccharide from Rhizoma
Panacis Majoris. Chinese Journal of Traditional Chinese Medicine.
35:912–914. 2010.(In Chinese).
|
|
17
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wagner EF and Nebreda ÁR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pagès PB, Le Pimpec-Barthes F and Bernard
A: Surgery for pulmonary metastases from colorectal cancer:
Predictive factors for survival. Rev Mal Respir. 33:838–852.
2016.(In French). View Article : Google Scholar
|
|
20
|
Zhao M, Chen Q, Xu W, Wang H, Che Y, Wu M,
Wang L, Lijuan C and Hao H: Total ginsenosides extract induce
autophagic cell death in NSCLC cells through activation of
endoplasmic reticulum stress. J Ethnopharmacol. 243:1120932019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang Y, Huang H, Han Z, Li W, Mai Z and
Yuan R: Ginsenoside Rh2 inhibits angiogenesis in prostate cancer by
targeting CNNM1. J Nanosci Nanotechnol. 19:1942–1950. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeong D, Ham J, Park S, Kim HW, Kim H, Ji
HW and Kim SJ: Ginsenoside Rh2 suppresses breast cancer cell
proliferation by epigenetically regulating the long noncoding RNA
C3orf67-AS1. Am J Chin Med. 47:1643–1658. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang YC, Zhang Y, Zhou J, Zhi Q, Wu MY,
Gong FR, Shen M, Liu L, Tao M, Shen B, et al: Ginsenoside Rg3
targets cancer stem cells and tumor angiogenesis to inhibit
colorectal cancer progression in vivo. Int J Oncol. 52:127–138.
2018.PubMed/NCBI
|
|
24
|
Nakhjavani M, Hardingham JE, Palethorpe
HM, Tomita Y, Smith E, Price TJ and Townsend AR: Ginsenoside Rg3:
Potential Molecular Targets and Therapeutic Indication in
Metastatic Breast Cancer. Medicines (Basel). 6(17): 62019
|
|
25
|
Kim DG, Jung KH, Lee DG, Yoon JH, Choi KS,
Kwon SW, Shen HM, Morgan MJ, Hong SS and Kim YS: 20(S)-Ginsenoside
Rg3 is a novel inhibitor of autophagy and sensitizes hepatocellular
carcinoma to doxorubicin. Oncotarget. 5:4438–4451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Q, Kang X and Zhao W: Antiangiogenic
effect of low-dose cyclophosphamide combined with ginsenoside Rg3
on Lewis lung carcinoma. Biochem Biophys Res Commun. 342:824–828.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Coffman JA: Cell cycle development. Dev
Cell. 6:321–327. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park M-T and Lee S-J: Cell cycle and
cancer. J Biochem Mol Biol. 36:60–65. 2003.PubMed/NCBI
|
|
29
|
Schafer KA: The cell cycle: A review. Vet
Pathol. 35:461–478. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kaczanowski S: Apoptosis: its origin,
history, maintenance and the medical implications for cancer and
aging. Phys Biol. 13:32016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arumugam A and Razis AF: Apoptosis as a
mechanism of the cancer chemopreventive activity of glucosinolates:
A Review. Asian Pac J Cancer Prev. 19:1439–1448. 2018.PubMed/NCBI
|
|
33
|
Pistritto G, Trisciuoglio D, Ceci C,
Garufi A and D'Orazi G: Apoptosis as anticancer mechanism: function
and dysfunction of its modulators and targeted therapeutic
strategies. Aging (Albany NY). 8:603–619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu T, Wu Z, He Y, Xiao Y and Xia C:
Single and dual target inhibitors based on Bcl-2: Promising
anti-tumor agents for cancer therapy. Eur J Med Chem.
201:1124462020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kalpage HA, Wan J, Morse PT, Zurek MP,
Turner AA, Khobeir A, Yazdi N, Hakim L, Liu J, Vaishnav A, et al:
Cytochrome c phosphorylation: Control of mitochondrial
electron transport chain flux and apoptosis. Int J Biochem Cell
Biol. 121:1057042020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun J and Nan G: The mitogen-activated
protein kinase (MAPK) signaling pathway as a discovery target in
stroke. J Mol Neurosci. 59:90–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Raman M, Chen W and Cobb MH: Differential
regulation and properties of MAPKs. Oncogene. 26:3100–3112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fang JY and Richardson BC: The MAPK
signalling pathways and colorectal cancer. Lancet Oncol. 6:322–327.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Berlowska J, Dudkiewicz M, Kregiel D,
Czyzowska A and Witonska I: Cell lysis induced by membrane-damaging
detergent saponins from Quillaja saponaria. Enzyme Microb Technol.
75-76:44–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rao AV and Sung MK: Saponins as
anticarcinogens. J Nutr. 125 (Suppl 3):717S–724S. 1995.PubMed/NCBI
|