Introduction
Colorectal cancer (CRC) is a type of malignant
tumour that arises from the epithelial cells of the mucosal lining
of the colon or rectum, and ranks third in incidence and second in
mortality among all cancers worldwide, with its occurrence, growth
and metastasis being affected by irregular diet, lack of exercise
and genetic factors (1,2). It has been predicted that the number
of new cases of CRC worldwide will increase to >2.2 million by
2030 (2). With the advances in
surgical resection, radiotherapy, chemotherapy, adjuvant
chemotherapy, receptor-targeted therapy and other therapeutic
measures, significant progress has been achieved in the clinical
treatment of CRC (3). Surgical
resection remains the preferred treatment method for local CRC,
whereas the applicability of postoperative chemotherapy is limited
by side effects and the development of drug resistance, which may
significantly reduce patient compliance and quality of life
(4–6).
Herbal medicines and natural products are known
sources of anti-tumour drugs, such as paclitaxel derived from the
bark of the Pacific yew Taxus brevifolia, camptothecin
derived from the root bark of Camptotheca acuminate and
podophyllotoxin derived from the rhizome of Diphylleia
sinensis (7–9). Saponins, a class of ingredients
abundant in plants, have been recognised for their anti-tumour
properties, with ginsenosides derived from Panax ginseng
(P. ginseng), a species in the Panax genus of the
Araliaceae family, considered as the most active components
(10,11). Ginsenosides include the
20(S)-protopanaxadiol and 20(S)-protopanaxatriol saponins (12). Among the hundreds of compounds
included in P. ginseng, ginsenoside Rg3 has been authorized
for use as a clinical drug by the National Medicinal Products
Administration of China (13).
Panax japonicus var. Major is another
species in the Panax genus, and its root, named Rhizoma
Panacis Majoris, has been found to predominantly contain saponins,
polysaccharides and organic acids (14). Previous studies have demonstrated
that the decoction of Rhizoma Panacis Majoris can inhibit
proliferation and induce apoptosis of human liver cancer SMMC-7721
cells and human promyelocytic leukaemia HL-60 cells, whereas the
polysaccharides of Rhizoma Panacis Majoris can inhibit tumour
growth and prolong survival in a H22 hepatoma mouse model through
enhancing immunity (15,16). However, the effect of saponins from
Rhizoma Panacis Majoris on CRC has not been investigated to
date.
In the present study, total saponins from Rhizoma
Panacis Majoris (RPMTG) were prepared, the effects of RPMTG on the
proliferation, cell cycle and apoptosis of HCT116 and SW620 cells
were investigated, and the underlying molecular mechanism was
elucidated.
Materials and methods
Plant material
Panax japonicus var. major was collected from
the Taibai region of Qinling Mountains in the Shaanxi Province of
China, and was identified morphologically and histologically by
Professor Wei Wang (TCM Identification Department of Shaanxi
University of Chinese Medicine, Xianyang, China). The roots of the
plant were processed by the TCM resource laboratory at the Shaanxi
University of Chinese Medicine (Xianyang, China) and authenticated
as Rhizoma Panacis Majoris, and a voucher specimen (herbarium no.
20180920) has been deposited in the Medicinal Plants Herbarium,
Shaanxi University of Chinese Medicine (Xianyang, China).
Preparation of RPMTG
The dried crude materials were extracted with 70%
ethanol three times. After removing the solvent under reduced
pressure, the ethanol extract was suspended in H2O,
subjected to macroporous resin (DIAION® HP20 gel,
Mitsubishi Gas Chemical Company, Inc.) column chromatography and
eluted with a gradient solvent system of ethanol (i.e., 0, 20, 60
and 95% ethanol) to yield four fractions. The fractions were
detected by UV spectrophotometry (UV-2600i; Shimadzu Corporation)
and high-performance liquid chromatography-electrospray
ionization-tandem mass spectrometry [Agilent 1260 HPLC (Agilent
Technologies, Inc.) equipped with QTRAP® 4500 MS system
(SCIEX)]. A SunFire C18 column (150×4.6 mm, 5 µm; Waters
Corporation) was used with 30°C column temperature. The mobile
phase consisted of A (H2O + 0.05% HCOOH) and B
(CH3CN + 0.05% HCOOH) using a gradient elution of 0–5
min, 10–23% B; 5–15 min, 23–35% B; 15–20 min, 35–48% B; 20–25 min,
48–96% B; 25–28 min, 96–10% B; 28–30 min, 10% B. The volume flow
was 0.8 ml/min, and the injection volume was 5 µl.
The 60% ethanol fraction was identified as the total
saponins site with 73.09% RPMTG content, and 15 saponins were
determined as ginsenosides Rb2, Re, Ro, Rd,
Rg1, Rb3, Rb1, Rc, Rh2,
F2, Rg3 and Rf, Panax notoginseng saponin
R1, Panax zingiberensis saponin R1 and
chikusetsusaponin IVa. Finally, RPMTG powder was obtained after
freeze-drying and used in mechanistic studies.
Cell culture
The human CRC cell lines HCT116 and SW620 were
purchased from The Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences. HCT116 and SW620 cells were cultured
in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.), supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin.
The HCT116 (1×104 cells/well) and SW620
(2×104 cells/well) cells were pretreated with JNK
inhibitor (SP600125, 10 µM; Beyotime Institute of Biotechnology)
and p38 inhibitor (SB203580, 10 µM; Beyotime Institute of
Biotechnology) for 2 h, and then treated with 250 µg/ml RPMTG for
24 h in a 37°C incubator containing 5% CO2. In
subsequent experiments, the effects of RPMTG combined with JNK
inhibitors or p38 inhibitors on proliferation and apoptosis were
evaluated.
MTT assay
Cell viability was detected by the MTT assay
(Sigma-Aldrich; Merck KGaA). HCT116 and SW620 cells were digested,
dispersed and inoculated in 96-well plates at a density of
1×104 cells/well and 2×104 cells/well,
respectively. The cells were cultured in complete DMEM and
incubated in a 37°C incubator containing 5% CO2. After
cells were allowed to proliferate for 24 h, RPMTG at different
concentrations (0, 100, 200, 250, 300, 400 and 500 µg/ml) was
added; after 12 and 24 h, 15 µl MTT was added into each well of the
blank group and the RPMTG treatment group; after a further 4 h,
DMSO was added, and the plates were analysed at 490 nm on a
microplate reader (Thermo Fisher Scientific, Inc.). The ratio of
cell survival was calculated against that of the untreated CRC cell
group.
Cell cycle assay
Cell cycle progression was assessed by flow
cytometry (FACSCanto II; BD Biosciences). Cells were treated with
different concentrations (0, 100 and 200 µg/ml) of RPMTG for 24 h,
washed with PBS, and fixed with absolute ethanol at −20°C
overnight. Then, cells were collected, and 100 µl RNase A was added
to resuspend the cells. Propidium iodide (PI; 400 µl, 50 µg/ml;
Beyotime Institute of Biotechnology) was added and allowed to act
in the dark for 30 min. The content of PI-labelled DNA in the cells
was then quantified by flow cytometry. FlowJo version 10 software
(FlowJo LLC) was used for analysis. The experiments were performed
three times.
DAPI staining
DAPI staining was used to evaluate the specific
morphological changes in the nucleus that induced apoptosis. HCT116
and SW620 cells were seeded into 6-well plates at a density of
1×105 cells/ml and 2×105 cells/ml (2 ml per
well), respectively. Cells were treated with different
concentrations (0, 100, 200 and 250 µg/ml) of RPMTG for 24 h, then
washed with PBS, fixed with 4% paraformaldehyde for 15 min in a
37°C incubator containing 5% CO2 and stained with 5
µg/ml DAPI (Beyotime Institute of Biotechnology) in the dark for 15
min at room temperature. Finally, cells were examined with an
inverted fluorescence microscope (magnification, ×20),
representative digital images were acquired for analysis. The mean
number of nuclear apoptotic cells and cell count of 10 selected
fields (500×500 pixel2) in the images were
calculated.
Apoptosis assay
Early and late (lower and top right corner of the
flow dot plot, respectively) stage apoptosis were detected by the
Annexin V/PI detection kit (Absin Bioscience, Inc.) according to
the manufacturer's instructions. HCT116 and SW620 cells were
treated with different concentrations (0, 100, 200 and 250 µg/ml)
of RPMTG for 24 h, and the cells were collected, washed with
pre-cooled PBS, and resuspended in 300 µl 1X Binding Buffer (BD
Biosciences). The cells were stained with 5 µl Annexin V-FITC and 5
µl PI for 15 min in the dark. Then, apoptotic cells were detected
using flow cytometry (FACSCanto II; BD Biosciences), and FlowJo 7.6
software (FlowJo LLC) was used to determine the proportions (%) of
each subpopulation of cells.
Western blot analysis
Western blotting was used to determine changes in
protein expression that were associated with RPMTG treatment. After
treatment with RPMTG, the cells were collected and lysed in RIPA
lysis buffer (Beyotime Institute of Biotechnology) containing
protease and phosphatase inhibitors in an ice bath. Subsequently,
the lysates were removed and the supernatant was collected by
centrifugation at 10,000 × g for 10 min at 4°C. The total protein
concentration in each sample was determined using a BCA protein
analysis kit (Tiangen Biotech Co., Ltd.). The protein samples (10
µg/lane) were loaded, separated via 10% SDS-PAGE, and subsequently
transferred onto PVDF membranes (EMD Millipore). The membrane was
incubated with blocking solution (5% skimmed milk) at room
temperature for 1 h, and incubated with the corresponding primary
antibodies overnight at 4°C. The following primary antibodies were
used: Bcl-2 (cat. no. 15071; Cell Signaling Technology, Inc.),
Bcl-xL (cat. no. 10783-1-AP; ProteinTech Group, Inc.), induced
myeloid leukaemia cell differentiation protein Mcl-1 (Mcl-1; cat.
no. 16225-1-AP; ProteinTech Group, Inc.), cytochrome c (cat.
no. 11940; Cell Signaling Technology, Inc.), Bax (cat. no. 5023;
Cell Signaling Technology, Inc.), Bad (cat. no. 10435-1-AP;
ProteinTech Group, Inc.), cleaved caspase-9 (cat. no. 9509; Cell
Signaling Technology, Inc.), cleaved caspase-3 (cat. no. 9661; Cell
Signaling Technology, Inc.), cleaved poly(ADP)-ribose polymerase-1
(cleaved PARP-1; cat. no. 13371-1-AP; ProteinTech Group, Inc.),
extracellular signal-regulated kinase (ERK; cat. no. 4696; Cell
Signaling Technology, Inc.), phosphorylated (p-)ERK (cat. no. 4370;
Cell Signaling Technology, Inc.), c-Jun n-terminal kinase (JNK;
cat. no. 9252; Cell Signaling Technology, Inc.), p-JNK (cat. no.
4668; Cell Signaling Technology, Inc.), p38 (cat. no. 8690; Cell
Signaling Technology, Inc.), p-p38 (cat. no. 4511; Cell Signaling
Technology, Inc.), cyclin A (cat. no. 18202-1-AP; ProteinTech
Group, Inc.), cyclin D (cat. no. 60186-1-AP; ProteinTech Group,
Inc.), cyclin-dependent kinase 2 (CDK2; cat. no. 10122-1-AP;
ProteinTech Group, Inc.), p21 (cat. no. 103551-AP; ProteinTech
Group, Inc.) and β-actin (cat. no. 3700; Cell Signaling Technology,
Inc.). These primary antibodies were used at 1:1,000 dilution.
After washing three times with TBS with Tween-20 (0.05%), the
membranes were subsequently incubated with anti-mouse IgG,
HRP-conjugated antibody (cat. no. 7076; Cell Signaling Technology,
Inc.) or anti-rabbit IgG HRP-conjugated antibody (cat. no. 7074;
Cell Signaling Technology, Inc.) for 1 h at room temperature. The
secondary antibodies were used at 1:5,000 dilution. Positive
antibody binding was then visualized by ECL detection (Wuhan Boster
Biological Technology Co., Ltd.). Finally, ImageJ software (version
1.8.0; National Institutes of Health) was used for
densitometry.
Statistical analysis
All data are expressed as the mean ± SD of at least
three independent experiments. Statistical analysis was performed
using SPSS 22.0 software (IBM Corp.) and GraphPad Prism 8.0
software (GraphPad Software, Inc.). One way ANOVA was used for
statistical analysis, followed by Bonferroni's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
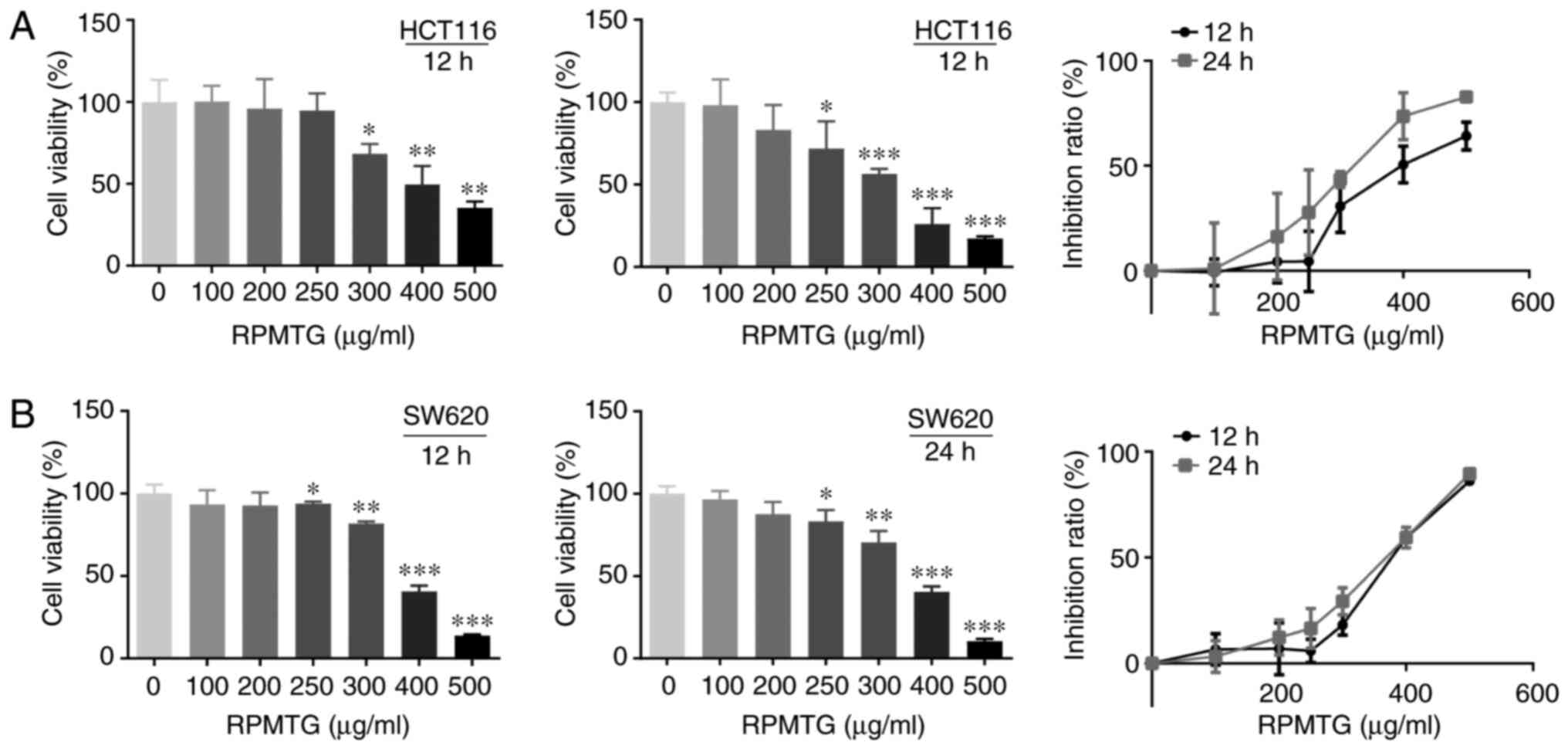
RPMTG inhibits the proliferation of
HCT116 and SW620 cells
HCT116 and SW620 cells were treated with different
concentrations of RPMTG (100–500 µg/ml) for 12 and 24 h, and cell
viability was determined by the MTT assay. As shown in Fig. 1A and B, cell viability decreased
with increasing RPMTG concentrations, and the proliferation
inhibition rate increased gradually. The IC50 values of
HCT116 cells at 12 and 24 h were 424.4 and 315.8 µg/ml,
respectively; the IC50 values of SW620 cells at 12 and
24 h were 386.2 and 355.1 µg/ml, respectively.
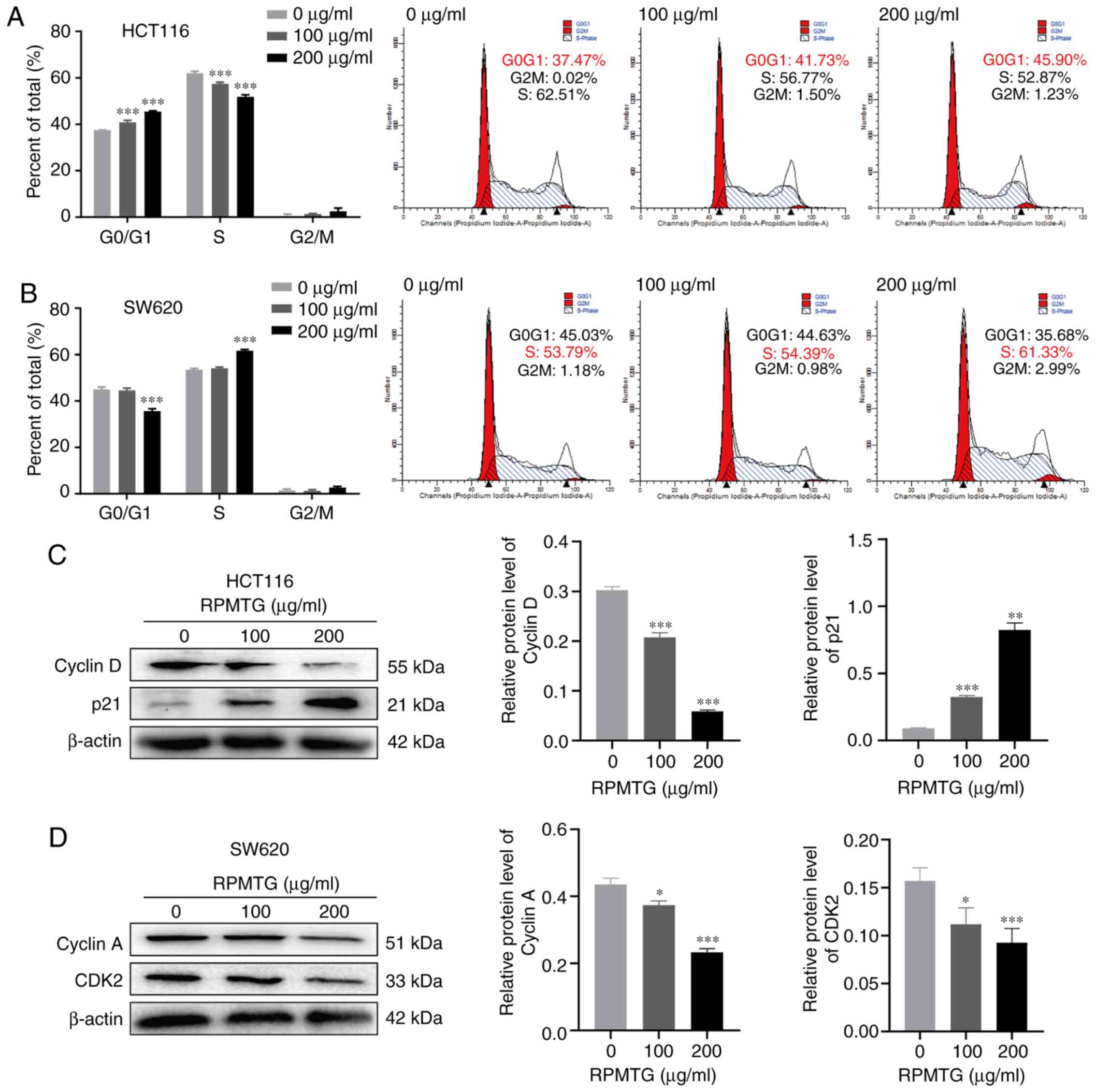
RPMTG induces cell cycle arrest in
HCT116 and SW620 cells
To determine whether RPMTG-induced inhibition of
cell proliferation was mediated via cell cycle arrest, flow
cytometry analysis was performed (Fig.
2). HCT116 and SW620 cells were treated with RPMTG (0, 100 and
200 µg/ml). As shown in Fig. 2,
compared with the control group, HCT116 and SW620 cells were
arrested at the G0/G1 and S phase, respectively; the
percentage of HCT116 cells in the G0/G1 phase
increased from 37.47 to 45.90% (Fig.
2A), and the percentage of SW620 cells in the S phase increased
from 53.79 to 66.89% (Fig. 2B). In
addition, western blotting demonstrated that the expression levels
of the G0/G1 phase-associated proteins cyclin
D and p21 were decreased and increased, respectively, by RPMTG
treatment in HCT116 cells (Fig.
2C). However, the expression levels of the S phase-associated
proteins cyclin A and CDK2 were decreased in SW620 cells (Fig. 2D). These results revealed that the
inhibition of the proliferation of HCT116 and SW620 cells by RPMTG
was mediated via cell cycle arrest at the
G0/G1 and S phases.
RPMTG induces morphological changes
and apoptosis in HCT116 and SW620 cells
DAPI staining was used to evaluate the specific
morphological changes. The results are shown in Fig. 3A and B. It was observed that the
nuclear structure of control cells was not altered, whereas, after
RPMTG (0, 100, 200 and 250 µg/ml) treatment for 24 h, the cells
exhibited changes in the nuclear structure, such as shrunk,
fragmented nuclei. The proportion of cells with nuclear apoptosis
has increased in a dose-dependent manner. Furthermore, the mean
cell count of 10 selected fields (500×500 pixel2) in the
images was calculated (Fig. 3A and
B). The results indicated that RPMTG treatment induced cell
apoptosis.
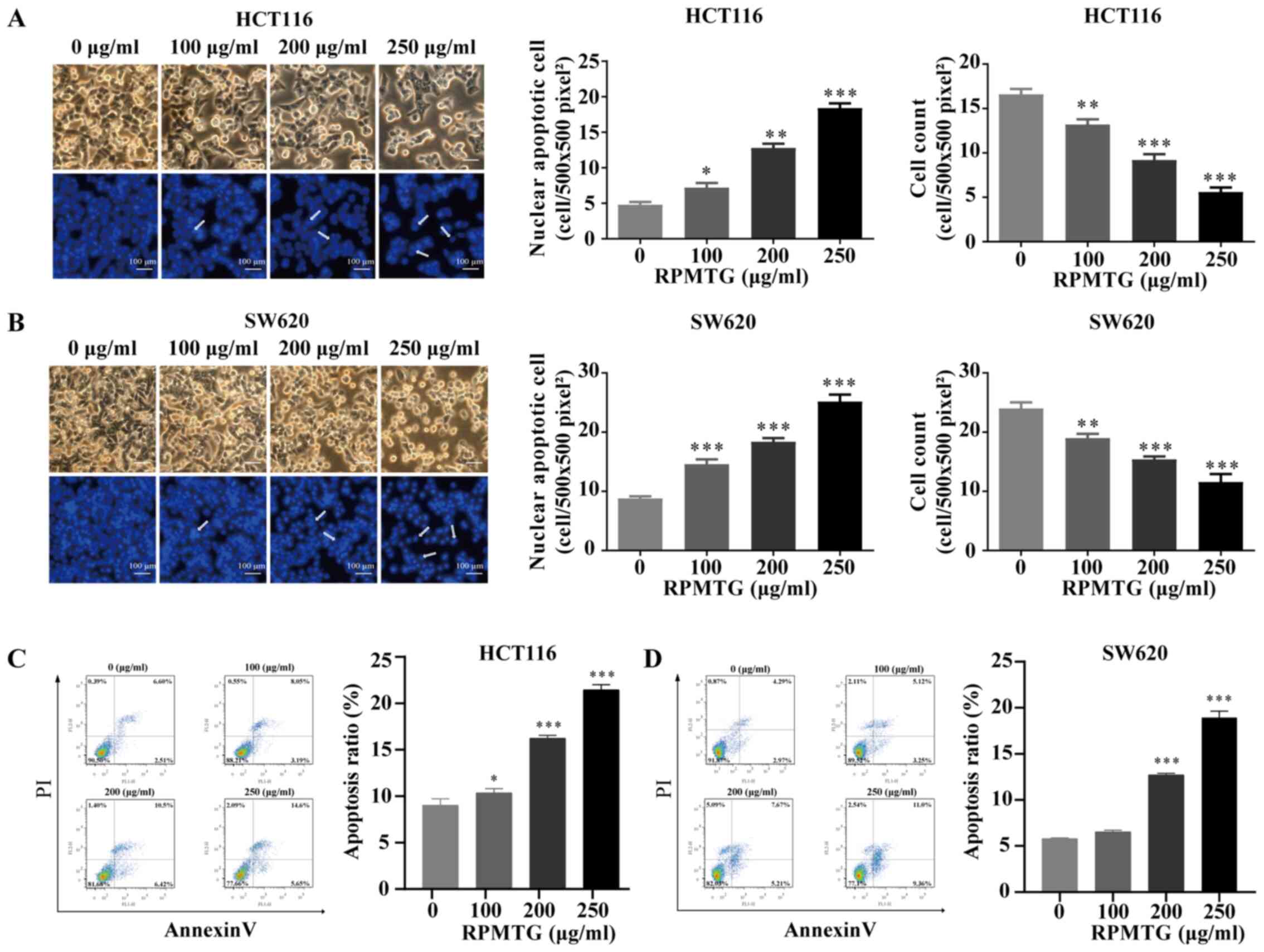 | Figure 3.RPMTG induces morphological changes
and induces apoptosis in HCT116 and SW620 cells. (A) HCT116 and (B)
SW620 cells were treated with RPMTG at increasing concentrations
(0, 100, 200 and 250 µg/ml) for 24 h, following which, shrinkage
was observed, with condensation of nuclear chromatin,
marginalization and chromatin DNA breaks. Scale bar, 100 µm. (C)
HCT116 and (D) SW620 cells were treated with RPMTG (0, 100, 200 and
250 µg/ml) for 24 h and analysed by Annexin V/PI flow cytometry.
The lower right quadrant indicates the percentage of early
apoptotic cells. Data are expressed as the mean ± SD from three
independent experiments. *P<0.05, **P<0.01 and ***P<0.001
vs. control group. RPMTG, total saponins from Rhizoma Panacis
Majoris. |
To further verify whether RPMTG induced cell
apoptosis, RPMTG (0, 100, 200 and 250 µg/ml) was used to treat
HCT116 and SW620 cells for 24 h, and then flow cytometry was
performed to detect the proportion of apoptotic cells. As shown in
Fig. 3C and D, the total percentage
of apoptotic cells among HCT116 and SW620 cells treated with 250
µg/ml RPMTG was 21.40 and 18.63%, respectively. These results
demonstrated that RPMTG treatment induced apoptosis of HCT116 and
SW620 cells.
RPMTG affects the expression of
mitochondrial pathway-related proteins in HCT116 and SW620
cells
The effects of RPMTG on the expression levels of
mitochondrial apoptosis-related proteins (Bcl-2, Bcl-xL, Mcl-1,
cytochrome c, Bax, Bad, cleaved caspase-9, cleaved caspase-3
and cleaved PARP-1) in HCT116 and SW620 cells were detected. As
shown in Fig. 4A and B, following
RPMTG treatment, the expression levels of the anti-apoptotic Bcl-2,
Bcl-xL and Mcl-1 proteins were reduced, whereas the expression
levels of cytochrome c, Bax, Bad, cleaved caspase-9, cleaved
caspase-3 and cleaved PARP-1 were increased. Thus suggesting that
RPMTG could induce the release of cytochrome c into the
cytoplasm and then activate the downstream caspase cascade
involving caspase-9 and caspase-3. These results indicated that the
apoptosis of CRC cells induced by RPMTG was associated with the
mitochondrial pathway.
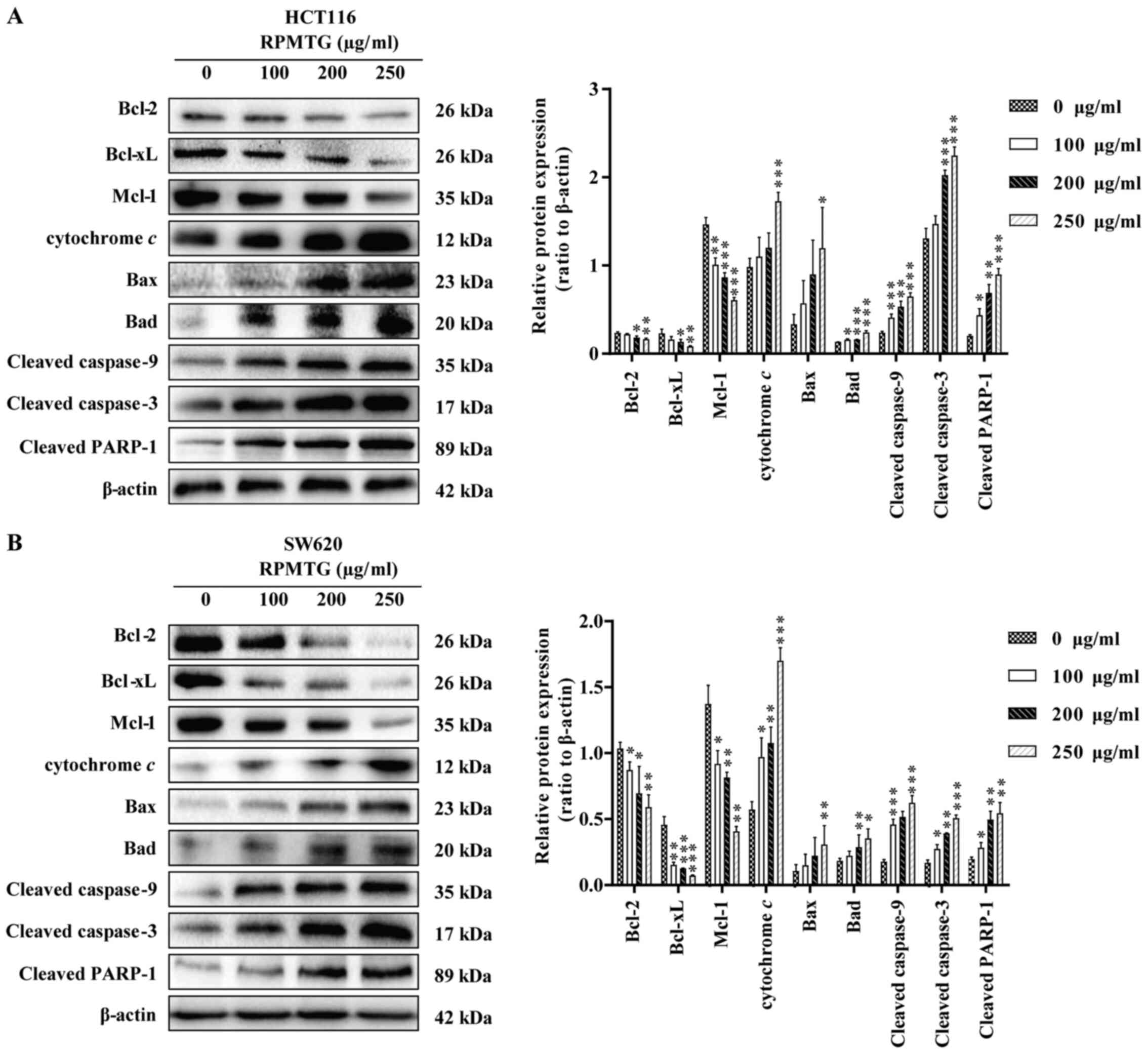 | Figure 4.RPMTG affects the expression of
mitochondrial pathway-related proteins in HCT116 and SW620 cells.
The expression of apoptosis-related proteins was detected by
western blotting. (A) HCT116 and (B) SW620 cells were treated with
different concentrations of RPMTG (0, 100, 200 and 250 µg/ml) for
24 h, and then the expression levels of Bcl-2, Bcl-xL, Mcl-1,
cytochrome c, Bax, Bad, cleaved caspase-9, cleaved caspase-3
and cleaved PARP-1 were detected by western blotting. ImageJ
software was used to determine the density ratio of each protein
band to the β-actin band. Data are expressed as the mean ± SD of
three independent experiments. *P<0.05, **P<0.01 and
***P<0.001 vs. control group. RPMTG, total saponins from Rhizoma
Panacis Majoris; PARP-1, poly(ADP)-ribose polymerase-1; Mcl-1,
induced myeloid leukaemia cell differentiation protein Mcl-1. |
RPMTG regulates mitogen-activated
protein kinase (MAPK) signalling in HCT116 and SW620 cells
Subsequently, the effect of RPMTG on the MAPK
signalling pathway was investigated, as this pathway has been
demonstrated to be important in cancer cell apoptosis, ERK
regulates cell proliferation and differentiation, whereas JNK and
p38 activation are related to apoptosis (17,18).
As shown in Fig. 5A and B, the
expression ratio of p-ERK/ERK was decreased, whereas p-JNK/JNK and
p-p38/p38 ratios were increased. The results demonstrated that the
induction of CRC cell apoptosis by RPMTG may be mediated via the
MAPK signalling pathway.
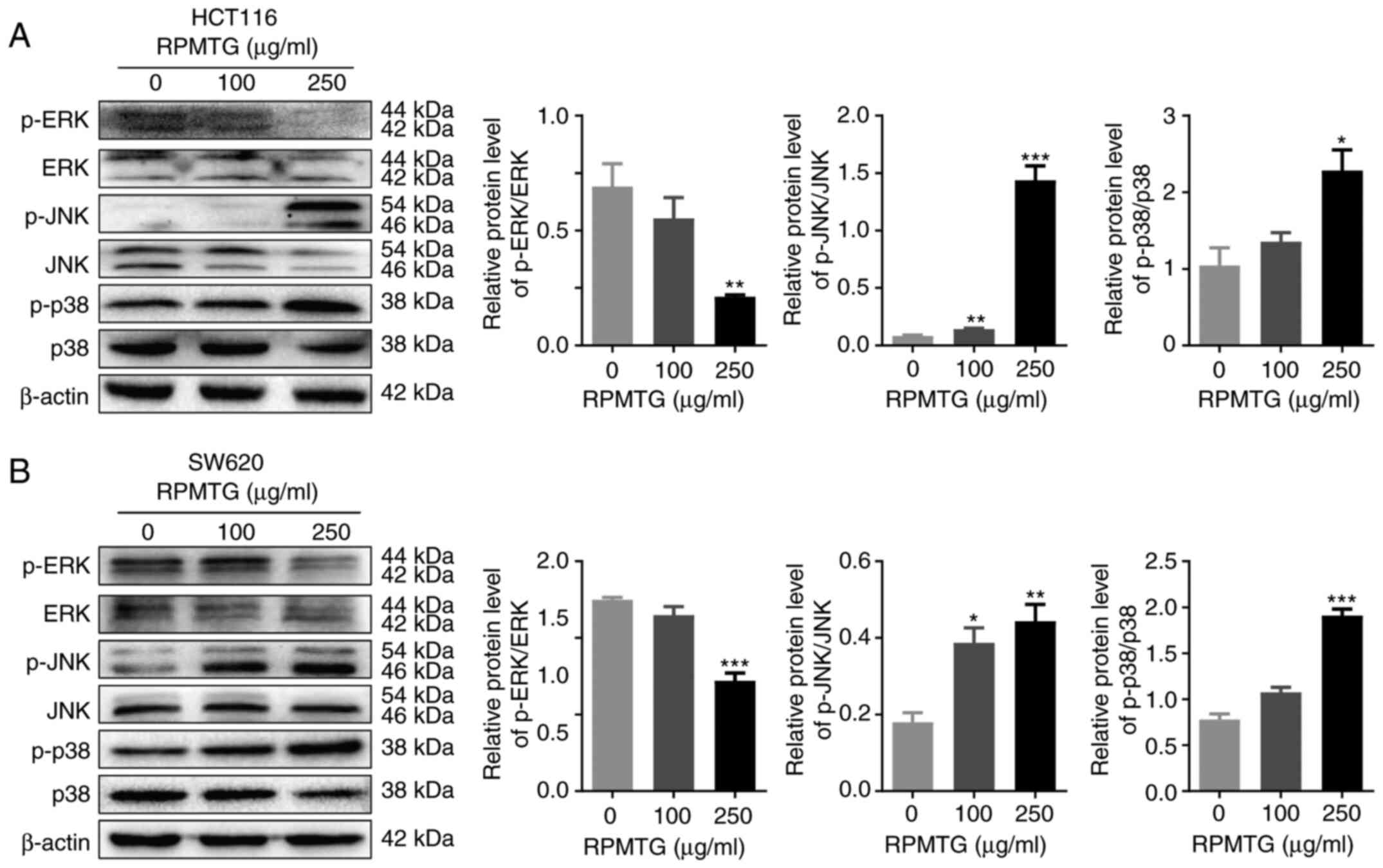 | Figure 5.RPMTG regulates MAPK signalling in
HCT116 and SW620 cells. (A) HCT116 and (B) SW620 cells were treated
with different concentrations of RPMTG (0, 100 and 250 µg/ml) for
24 h, and the protein expression levels of the MAPK signalling
pathway-related proteins p-ERK, ERK, p-JNK, JNK, p-p38 and p38 were
evaluated by western blotting. ImageJ software was used to
determine the density ratio of each phosphorylated protein band to
its total protein counterpart. Data are expressed as the mean ± SD
of three independent experiments. *P<0.05, **P<0.01 and
***P<0.001 vs. control group. RPMTG, total saponins from Rhizoma
Panacis Majoris; ERK, extracellular signal-regulated kinase; JNK,
c-Jun N-terminal kinase; p-, phosphorylated; MAPK,
mitogen-activated protein kinase. |
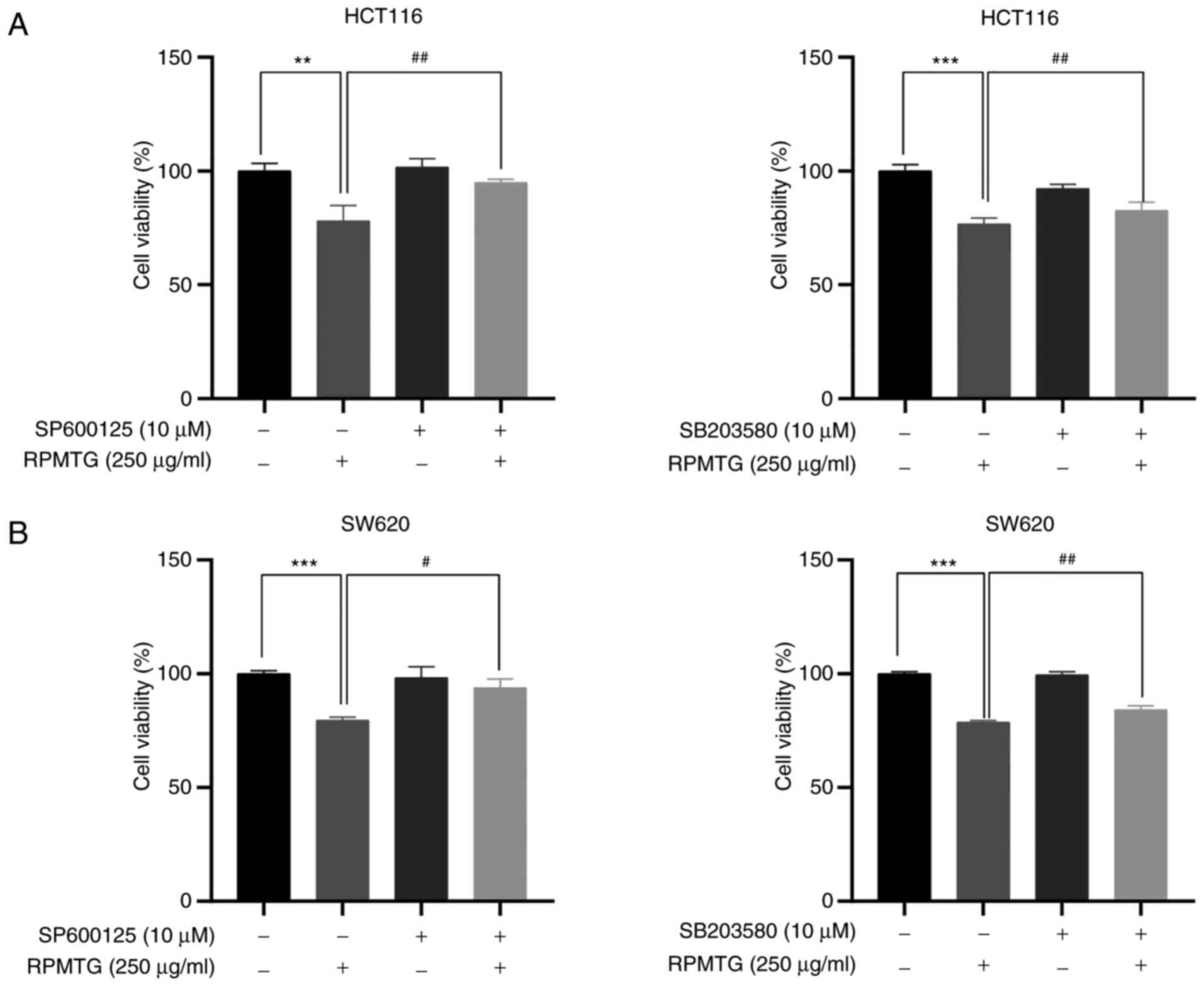
To further confirm whether the apoptosis induced by
RPMTG was dependent on the JNK and p38 MAPK signalling pathways,
HCT116 and SW620 cells were pretreated with JNK and p38 inhibitors
(SP600125 and SB203580, respectively), and then treated with RPMTG
(250 µg/ml). The MTT assay demonstrated that the combination of
RPMTG with SP600125 or SB203580 could markedly reverse the
inhibitory effect of RPMTG on cell viability (Fig. 6A and B). In addition, the western
blotting results revealed that the combination of RPMTG with
SP600125 or SB203580 could reduce the expression levels of p-JNK,
p-p38, the pro-apoptotic proteins Bax and cleaved caspase-3, and
increase the expression of the anti-apoptotic protein Bcl-2
(Fig. 7A and B). These results
suggested that RPMTG-induced apoptosis was associated with the
activation of the JNK and p38 MAPK signalling pathways.
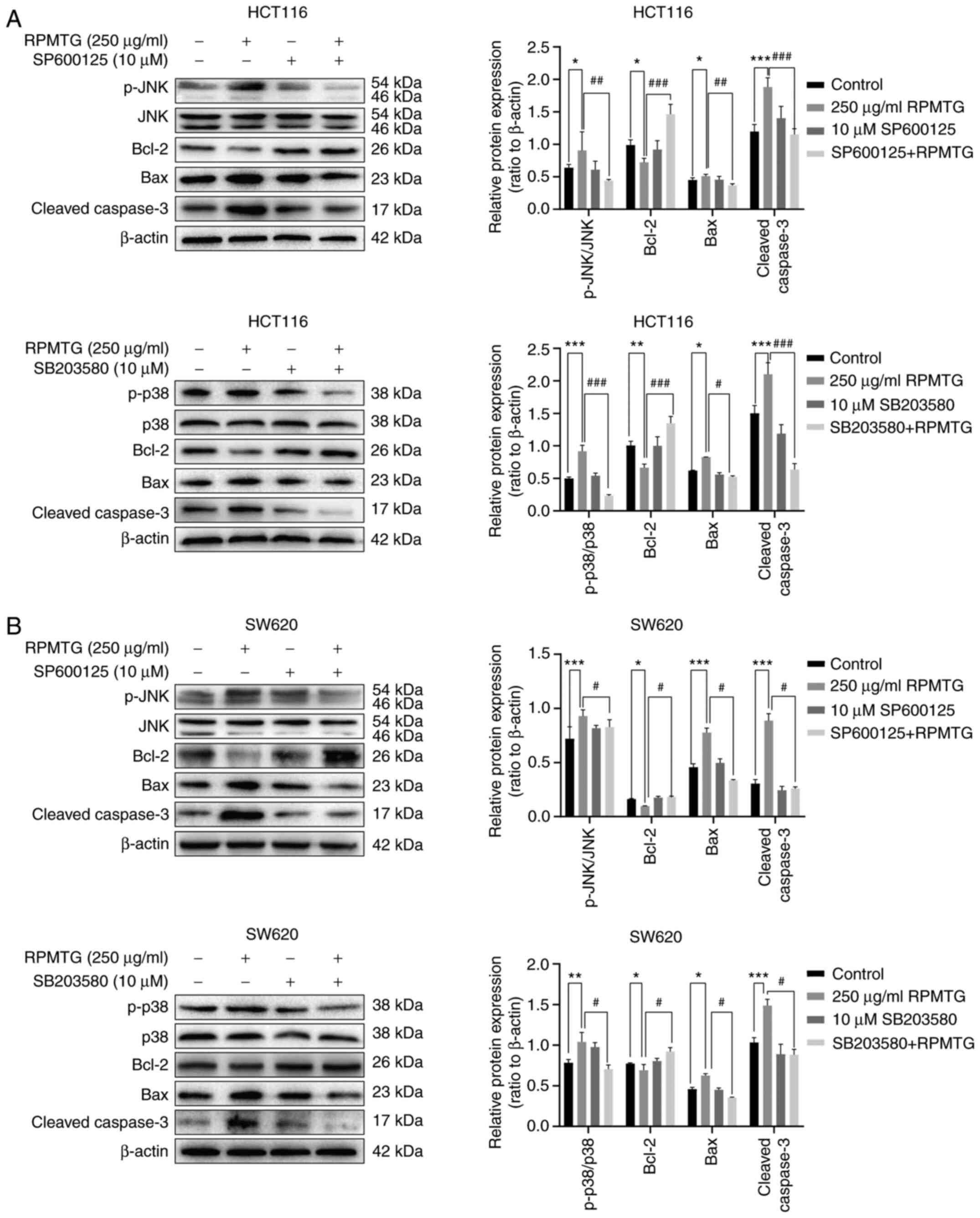 | Figure 7.Effect of JNK inhibitor and p38
inhibitor on the apoptosis of HCT116 and SW620 cells. The two cell
types were pretreated with JNK inhibitor (SP600125) and p38
inhibitor (SB203580) for 2 h, and then treated with 250 µg/ml RPMTG
for 24 h. Western blot analysis of the expression of p-JNK, total
JNK, p-p38, total p38, Bcl-2, Bax and cleaved caspase-3 in (A)
HCT116 and (B) SW620 cells. ImageJ software was used to determine
the density ratio of the p-JNK and p-p38 bands relative to total
protein content in SW620 cells, and the density ratio of the Bcl-2,
Bax and cleaved caspase-3 bands relative to the β-actin band. Data
are expressed as the mean ± SD of three independent experiments.
*P<0.05, **P<0.01 and ***P<0.001 vs. control group;
#P<0.05, ##P<0.01 and
###P<0.001 vs. RPMTG group. RPMTG, total saponins
from Rhizoma Panacis Majoris; JNK, c-JunN-terminal kinase; p-,
phosphorylated. |
Discussion
CRC clinical chemotherapy is restricted by systemic
toxicity, such as moderate myelosuppression, nausea, vomiting,
diarrhoea and other adverse reactions (4). Adjuvant chemotherapy is only effective
in 25% of patients, which indicates that >70% of patients suffer
more from the toxic effects, rather than experiencing the
therapeutic benefits (3–6). Furthermore, the 5-year survival rate
for CRC is <10% (19).
Therefore, new drugs for CRC with high efficiency and low toxicity
are urgently needed. Saponins in natural medicines with a broad
range of pharmacological activities have been attracting increasing
attention, among which ginsenosides derived from P. ginseng
have been found to play an important role in the prevention and
treatment of cancer (10). For
example, total ginsenosides can induce human non-small cell lung
cancer cell death (20), whereas
ginsenoside Rh2 has been reported to inhibit human
prostate and breast cancer cell proliferation (21,22).
In particular, ginsenoside Rg3 has been approved for
clinical use, either alone, which has been shown to inhibit the
proliferation of CRC and breast cancer cells (23,24),
or as a supplement to chemotherapy drugs, such as Adriamycin in
liver cancer and cyclophosphamide in lung cancer (25,26).
RPMTG was extracted from the roots of P. japonicus var.
Major, a species in the Panax genus of the Araliaceae
family as P. ginseng. After being processed by macroporous
resin column chromatography, the content of saponins in RPMTG
reached 73.09% as detected by UV spectrophotometry, and 15
ginsenosides were determined by HPLC-ESI-MS/MS, which indicated
that RPMTG may be effective against tumour cells. Therefore, the
present study was undertaken to investigate the effects of RPMTG
against CRC cells.
The results demonstrated that RPMTG exerted an
anti-CRC effect by inhibiting cell proliferation, inducing cell
cycle arrest and promoting apoptosis of HCT116 and SW620 cells. The
MTT assay revealed that the proliferation of HCT116 and SW620 cells
treated with RPMTG was significantly reduced in a dose-dependent
manner, suggesting that RPMTG exerted an antiproliferative effect
on CRC cells (Fig. 1). It is worth
noting that the IC50 screening results of MTT test were
between 300–400, but in the process of the experiment, it was found
that its efficacy was too significant. In order to improve the
detection of protein, the maximum concentration of 250 µg/ml was
chosen. The cell cycle is crucial for the control of cell
proliferation. It is commonly known that the expression of the
cyclin/CDK complex plays a pivotal role in
G0/G1 and S phase progression. During the
G0/G1 phase, cyclin D binds with CDK2 or CDK4
to promote cell cycle progression, and is highly expressed
(27). The CDK inhibitor p21 acts
as a cell cycle inhibitor by inhibiting the binding process,
thereby leading to cell cycle arrest in the
G0/G1 phase (28). During the S phase, cyclin A partners
with CDK2 to initiate DNA replication from preformed replication
initiation complexes, accelerating the process of cell division
from the S to the G2 phase (29). The results of the present study
demonstrated that RPMTG treatment inhibited the expression of
cyclins A and D and CDK2 activity, causing
G0/G1 and S phase arrest of HCT116 and SW620
cells, respectively (Fig. 2).
Apoptosis is an orderly and closely regulated cell
death process, and the change in cell morphology is an important
mechanism underlying drug-induced apoptosis (30). Accordingly, the induction of
apoptosis in CRC cells may be beneficial for controlling the
progression of CRC (31,32). In the present study, RPMTG treatment
induced cell atrophy and typical apoptotic characteristics, such as
chromatin condensation, apoptotic body formation, nuclear
condensation and fragmentation (Fig. 3A
and B). Moreover, apoptosis in the early and late stages was
observed by flow cytometry analysis (Fig. 3C and D). Mitochondrial apoptosis
mediated by the increase of mitochondrial outer membrane
permeability is an important cell apoptotic pathway (30). This pathway is mainly regulated by
the balance between the expression levels of pro-apoptotic and
anti-apoptotic proteins of the Bcl-2 family (33). The balance between anti-apoptotic
(such as Bcl-2 and Bcl-xL) and pro-apoptotic (such as Bax and Bad)
members may be disrupted when apoptosis occurs, which may lead to
the increased permeability of the mitochondrial membrane and the
release of cytochrome c into the cytoplasm, causing activation of
the downstream caspase family (34,35).
In the present study, RPMTG treatment decreased the expression of
the anti-apoptotic Bcl-2 and Bcl-xL proteins, whereas it increased
the expression of the pro-apoptotic Bax and Bad proteins. In
addition, the levels of cleaved caspase-9 and cleaved caspase-3 in
the cytoplasm were increased, along with the increased levels of
cytochrome c in the cytoplasm (Fig. 4). These results suggested that RPMTG
may induce apoptosis of HCT116 and SW620 cells through
mitochondrial-related pathways.
The MAPK signalling pathways regulate cell
proliferation, differentiation, apoptosis, migration and other cell
processes, in response to environmental signals associated with
cell proliferation and metabolism (36,37).
MAPK family members expressed in CRC mainly include ERK, JNK and
p38 (38). It has been reported
that ERK is a positive regulator of cell survival (17). Furthermore, JNK and p38 MAPK are
responsible for a variety of stress responses and participate in
the regulation of apoptosis (18).
In the present study, the expression ratio of p-ERK/ERK was
decreased, while p-JNK/JNK and p-p38/p38 were significantly
increased in a dose-dependent manner following RPMTG treatment
(Fig. 5). Of note, compared with
RPMTG treatment, the addition of the JNK inhibitor SP600125 and the
p38 inhibitor SB203580 markedly reversed the inhibitory effect on
cell proliferation and the induction of apoptosis (Figs. 6 and 7), suggesting that RPMTG may induce
apoptosis of HCT116 and SW620 cells via the MAPK signalling
pathways.
While the present study suggested that RPMTG may
exert a therapeutic effect in CRC, future in vivo
experimental models are required to substantiate the results
obtained. Additionally, as the effects of saponins possess
surface-active characteristics due to the amphiphilic nature of
their chemical structure, and saponin treatment has been found to
increase cell membrane permeability (39,40),
further investigation is warranted, and thus these are the main
areas of interest for our next study.
In conclusion, the present study demonstrated that
RPMTG inhibited cell proliferation, caused cell cycle arrest in the
G0/G1 and S phase and induced apoptosis of
CRC cells by regulating the activation of mitochondrial-related and
MAPK signalling pathways. These findings uncovered the molecular
mechanism through which RPMTG induces cancer cell apoptosis and
indicated that RPMTG may be valuable as a supplementary drug for
the treatment of CRC.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National
Natural Science Foundation (grant nos. 81803946 and 81773919), the
Key Scientific Research Plan of Shaanxi Shaanxi Education
Department 2020 (grant no. 20JY010) and Subject Innovation Team of
Shaanxi University of Chinese Medicine (grant no. 2019-YS01), Key
Industry Innovation Chain (group) Project of Shaanxi Provincial
Science and Technology Department (grant no. 2020ZDLSF05-08),
Subsidy Project of Xianyang Comprehensive Experimental Station of
National Technical System of Chinese Medicinal Materials Industry
(grant no. 2019JCW-06).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RZ, YH and ZY conceived and designed the present
study. ZT and ZY provided administrative support. Learning
materials were provided by YL and YP. LC, YP, YH and MM conducted
the experiment. LC, ZT, YL and JH conducted data analysis and
interpretation. LC, ZY and YH confirm the authenticity of all the
raw data. LC, RZ and ZY wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ma M, Wang X, Liu N, Shan F and Feng Y:
Low-dose naltrexone inhibits colorectal cancer progression and
promotes apoptosis by increasing M1-type macrophages and activating
the Bax/Bcl-2/caspase-3/PARP pathway. Int Immunopharmacol.
83:1063882020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun J, Feng Y, Wang Y, Ji Q, Cai G, Shi L,
Wang Y, Huang Y, Zhang J and Li Q: α-hederin induces autophagic
cell death in colorectal cancer cells through reactive oxygen
species dependent AMPK/mTOR signaling pathway activation. Int J
Oncol. 54:1601–1612. 2019.PubMed/NCBI
|
|
5
|
Liu Z, Xiong L, Ouyang G, Ma L, Sahi S,
Wang K, Lin L, Huang H, Miao X, Chen W, et al: Investigation of
copper cysteamine nanoparticles as a new type of radiosensitiers
for colorectal carcinoma Treatment. Sci Rep. 7:92902017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu M, Yu Z, Mei P, Li J, Luo D, Zhang H,
Zhou M, Liang F and Chen R: Correction for: Lycorine induces
autophagy-associated apoptosis by targeting MEK2 and enhances
vemurafenib activity in colorectal cancer. Aging (Albany NY).
12:6488–6489. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weaver BA: How Taxol/paclitaxel kills
cancer cells. Mol Biol Cell. 25:2677–2681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
GLB: L. Gatti, ; Perego P and Zaffaroni N:
Camptothecin resistance in cancer: Insights into the molecular
mecha-nisms of a DNA-damaging drug. Curr Med Chem. 20:1541–1565.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paidakula S, Nerella S, Vadde R, Kamal A
and Kankala S: Design and synthesis of
4β-acetamidobenzofuranone-podophyllotoxin hybrids and their
anti-cancer evaluation. Bioorg Med Chem Lett. 29:2153–2156. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Man S, Gao W, Zhang Y, Huang L and Liu C:
Chemical study and medical application of saponins as anti-cancer
agents. Fitoterapia. 81:703–714. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi JS, Chun KS, Kundu J and Kundu JK:
Biochemical basis of cancer chemoprevention and/or chemotherapy
with ginsenosides (Review). Int J Mol Med. 32:1227–1238. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee DG, Jang SI, Kim YR, Yang KE, Yoon SJ,
Lee ZW, An HJ, Jang IS, Choi JS and Yoo HS: Anti-proliferative
effects of ginsenosides extracted from mountain ginseng on lung
cancer. Chin J Integr Med. 22:344–352. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun M, Ye Y, Xiao L, Duan X, Zhang Y and
Zhang H: Anticancer effects of ginsenoside Rg3 (Review). Int J Mol
Med. 39:507–518. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song X, Wang W, Zhang X, Jiang Y, Yang X,
Deng C, Yue Z and Tang Z: Deglucose chikusetsusaponin IVa isolated
from Rhizoma Panacis Majoris induces apoptosis in human HepG2
hepatoma cells. Mol Med Rep. 12:5494–5500. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen T, Chen LF, Jin GQ and Li D: Panax
japlcus var induced apoptosis of human hepatocarcinoma cells in
vitro. Tumor. 26:144–147. 2006.(In Chinese). PubMed/NCBI
|
|
16
|
Chen T, Chen M, Hu Y, Gong Z and Deng L:
Extraction and anticancer activity of polysaccharide from Rhizoma
Panacis Majoris. Chinese Journal of Traditional Chinese Medicine.
35:912–914. 2010.(In Chinese).
|
|
17
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wagner EF and Nebreda ÁR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pagès PB, Le Pimpec-Barthes F and Bernard
A: Surgery for pulmonary metastases from colorectal cancer:
Predictive factors for survival. Rev Mal Respir. 33:838–852.
2016.(In French). View Article : Google Scholar
|
|
20
|
Zhao M, Chen Q, Xu W, Wang H, Che Y, Wu M,
Wang L, Lijuan C and Hao H: Total ginsenosides extract induce
autophagic cell death in NSCLC cells through activation of
endoplasmic reticulum stress. J Ethnopharmacol. 243:1120932019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang Y, Huang H, Han Z, Li W, Mai Z and
Yuan R: Ginsenoside Rh2 inhibits angiogenesis in prostate cancer by
targeting CNNM1. J Nanosci Nanotechnol. 19:1942–1950. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeong D, Ham J, Park S, Kim HW, Kim H, Ji
HW and Kim SJ: Ginsenoside Rh2 suppresses breast cancer cell
proliferation by epigenetically regulating the long noncoding RNA
C3orf67-AS1. Am J Chin Med. 47:1643–1658. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang YC, Zhang Y, Zhou J, Zhi Q, Wu MY,
Gong FR, Shen M, Liu L, Tao M, Shen B, et al: Ginsenoside Rg3
targets cancer stem cells and tumor angiogenesis to inhibit
colorectal cancer progression in vivo. Int J Oncol. 52:127–138.
2018.PubMed/NCBI
|
|
24
|
Nakhjavani M, Hardingham JE, Palethorpe
HM, Tomita Y, Smith E, Price TJ and Townsend AR: Ginsenoside Rg3:
Potential Molecular Targets and Therapeutic Indication in
Metastatic Breast Cancer. Medicines (Basel). 6(17): 62019
|
|
25
|
Kim DG, Jung KH, Lee DG, Yoon JH, Choi KS,
Kwon SW, Shen HM, Morgan MJ, Hong SS and Kim YS: 20(S)-Ginsenoside
Rg3 is a novel inhibitor of autophagy and sensitizes hepatocellular
carcinoma to doxorubicin. Oncotarget. 5:4438–4451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Q, Kang X and Zhao W: Antiangiogenic
effect of low-dose cyclophosphamide combined with ginsenoside Rg3
on Lewis lung carcinoma. Biochem Biophys Res Commun. 342:824–828.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Coffman JA: Cell cycle development. Dev
Cell. 6:321–327. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park M-T and Lee S-J: Cell cycle and
cancer. J Biochem Mol Biol. 36:60–65. 2003.PubMed/NCBI
|
|
29
|
Schafer KA: The cell cycle: A review. Vet
Pathol. 35:461–478. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kaczanowski S: Apoptosis: its origin,
history, maintenance and the medical implications for cancer and
aging. Phys Biol. 13:32016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arumugam A and Razis AF: Apoptosis as a
mechanism of the cancer chemopreventive activity of glucosinolates:
A Review. Asian Pac J Cancer Prev. 19:1439–1448. 2018.PubMed/NCBI
|
|
33
|
Pistritto G, Trisciuoglio D, Ceci C,
Garufi A and D'Orazi G: Apoptosis as anticancer mechanism: function
and dysfunction of its modulators and targeted therapeutic
strategies. Aging (Albany NY). 8:603–619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu T, Wu Z, He Y, Xiao Y and Xia C:
Single and dual target inhibitors based on Bcl-2: Promising
anti-tumor agents for cancer therapy. Eur J Med Chem.
201:1124462020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kalpage HA, Wan J, Morse PT, Zurek MP,
Turner AA, Khobeir A, Yazdi N, Hakim L, Liu J, Vaishnav A, et al:
Cytochrome c phosphorylation: Control of mitochondrial
electron transport chain flux and apoptosis. Int J Biochem Cell
Biol. 121:1057042020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun J and Nan G: The mitogen-activated
protein kinase (MAPK) signaling pathway as a discovery target in
stroke. J Mol Neurosci. 59:90–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Raman M, Chen W and Cobb MH: Differential
regulation and properties of MAPKs. Oncogene. 26:3100–3112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fang JY and Richardson BC: The MAPK
signalling pathways and colorectal cancer. Lancet Oncol. 6:322–327.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Berlowska J, Dudkiewicz M, Kregiel D,
Czyzowska A and Witonska I: Cell lysis induced by membrane-damaging
detergent saponins from Quillaja saponaria. Enzyme Microb Technol.
75-76:44–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rao AV and Sung MK: Saponins as
anticarcinogens. J Nutr. 125 (Suppl 3):717S–724S. 1995.PubMed/NCBI
|