Introduction
Ginseng (Panax ginseng), affiliated with the
Araliaceae family, is a perennial plant that is widely distributed
in Northeast Asia and North America (1). In particular, among various ginseng
species, Panax ginseng is known globally as Korean ginseng.
Korean ginseng has been generally used globally as a component of
traditional medicine and functional foods because it possesses many
beneficial health effects, such as antioxidant, anticancer,
anti-diabetes, anti-inflammation and neuroprotection (2–4). The
beneficial health effects of Korean ginseng are closely associated
with its bioactive components including ginsenosides, phenolic
acids, flavonoids and polysaccharides (2,4).
Korean ginseng, which grows in mountains without any artificial
manipulation after sowing, is called mountain ginseng in Northeast
Asia, and ethanolic extracts of mountain ginseng (MGE) have been
used as a herbal drug in pharmacopuncture treatment for cancer
patients in Korea (5). Recently, we
found that MGE, which is rich in ginsenosides, possessed anticancer
activity against human breast cancer in a xenograft model (6).
Angiogenesis is closely associated with various
physiological and pathological states, such as fetal development,
wound-healing, inflammation and vascular diseases (7). In addition, cancer cells easily form
tumor mass because of their rapid growth compared with the growth
of normal cells, and then the inside of the tumor mass becomes
hypoxic (8). As a result, cancer
cells themselves promote the formation of novel blood vessels under
hypoxic conditions by producing and secreting angiogenetic factors,
such as VEGF (8). Consequently,
cancer cells grow quickly and metastasize to other organs through
the neo blood vessels (8).
Therefore, the inhibition of neo blood vessel formation may be an
important key factor for anticancer therapy. Although MGE has the
anticancer properties, the effect of MGE on angiogenesis remains to
be unclarified.
In the present study, we investigated whether MGE
and an ethanolic extract of farm-cultivated ginseng (FGE) could
inhibit angiogenesis in both in vitro and in vivo
models. We found that both MGE and FGE suppressed angiogenesis of
vascular endothelial cells stimulated by angiogenetic factors. In
addition, the anti-angiogenic action of MGE was stronger than that
of FGE in both in vitro and in vivo models.
Furthermore, the anti-angiogenic effect of MGE was associated with
inhibition of the activation of the VEGF-R2 signaling pathway in
vascular endothelial cells. These findings may provide novel
information for the clinical application of MGE as a cancer dietary
supplement, and they may be helpful for understanding the action of
MGE in a tumor microenvironment.
Materials and methods
Reagents
DMEM (cat. no. 30-2002) was obtained from the
American Type Culture Collection. Antibiotics, FBS and 1X PBS were
procured from GE Healthcare Life Sciences. Specific antibodies
against focal adhesion kinase (FAK, cat. no. 3285), p-FAK (cat. no.
3281), steroid receptor coactivator (Src, cat. no. 2109), p-Src
(cat. no. 6943), Akt (cat. no. 9272), p-Akt (cat. no. 9271), ERK
(cat. no. 4695), p-ERK (cat. no. 9101), VEGF-R2 (cat. no. 9698) and
p-VEGF-R2 (cat. no. 3817) were purchased from Cell Signaling
Technology, Inc. A specific antibody against β-actin (sc-1616) was
obtained from Santa Cruz Biotechnology, Inc. VEGF was procured from
R&D Systems. Matrigel was purchased from Becton Dickinson (BD
Biosciences). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT), hemoglobin assay kit, and all other chemicals were
obtained from Sigma-Aldrich (Merck KGaA). All other chemicals were
of analytical grade.
Preparation of mountain ginseng and
farm-cultivated ginseng extracts
The MGE and FGE were prepared following a previous
method (6). Briefly, voucher
specimens of mountain ginseng and farm-cultivated ginseng were
deposited in the National Institute for Korean Medicine Development
(NIKOM, Gyeongsan, Korea) after identification by Dr H. Lee, a
herbalist. Dried fragments of the herbs were boiled in 30% ethanol
solution for about 3 h, and deposited at 4°C after cooling. Next
day, the separated supernatant was filtered through a 0.45 µm
filter, and the filtrate was concentrated and then lyophilized. The
dried pellets were stored at −20°C until use. The lyophilized
powders of MGE and FGE were dissolved in 4% ethanol solution for
in vitro and in vivo studies.
High-performance liquid chromatography
analysis
Ginsenoside contents in MGE and FGE were analyzed
according to a previous method (6).
Animals
Male C57BL/6 mice (6 weeks and 19–21 g) were
obtained from Koatech Co., and housed in cages (5 mice per cage)
under specific pathogen-free conditions (21–24°C and 40–60%
relative humidity) with a 12 h light/dark cycle. They were given
free access to standard rodent food (Envigo) and water. All animal
experiments were approved by the Committee of Animal Care and
Experiment of NIKOM with a reference number (NIKOM-2020-5). Animal
studies were performed according to the guidelines of the Animal
Care and Use Committee at NIKOM.
In vivo Matrigel plug assay
Matrigel plug assay was performed following a
modification of a previous protocol (9). Matrigel including VEGF (400 ng/ml) was
mixed with MGE (0–200 µg/ml) or FGE (200 µg/ml). After adaptation,
200 µl of the Matrigel mixtures was subcutaneously injected into
the flank of mice. After 2 weeks, mice were euthanized by
CO2 (in 30%/min). Matrigel plugs were isolated from
sacrificed mice, and then washed with cold 1X PBS twice. The washed
Matrigel plugs were used for hemoglobin content assay and
histological analysis.
Hemoglobin content assay
Hemoglobin content in Matrigel plugs was detected by
a hemoglobin assay kit in accordance with the manufacturer's
instructions.
Histological analysis
Histological analysis was carried out according to a
modified method, previously reported (10). To observe aspect of red blood cells
in Matrigel plugs, briefly, deparafinized Matrigel plug on slices
was stained with hematoxylin-eosin. The stained tissue slices were
embedded with the Mounting solution. Histological features in
Matrigel plugs were observed under a light microscope with 100×
magnification.
Cell culture
SVEC4-10 cells, a murine endothelial cell line
(11), were purchased from the
American Type Culture Collection. The cells were cultured in DMEM
medium containing 10% (v/v) FBS and antibiotics at 37°C in a
humidified atmosphere of 5% CO2. All in vitro
tests contained a vehicle control group (0.016% ethanol).
Cell viability assay
Cell viability was determined following a
modification of a method previously reported (12). Briefly, SVEC4-10 cells were seeded
on a 96-well plate (1×104 cells/well). Next day, the
cells were incubated with MGE or FGE (0–200 µg/ml) for 24 h, and
then further incubated with 100 µl culture media containing 300
µg/ml MTT reagent for 2 h. Next 100 µl of dimethyl sulfoxide was
added to the plate after removal of the supernatant, and then the
plate was incubated for 15 min. Cell viability was determined at
570 nm using a microplate reader (Tecan Sunrise).
Cell migration
Wound healing assay and Transwell migration assay
were performed in accordance with a modified method, previously
reported (13). SVEC4-10 cells were
seeded on a 6-well plate (1×105 cells/well), and then
the cells were incubated until 95% confluence in 10% FBS medium.
Next, the cells were wounded with a p20 pipette tip under serum
free condition, and then incubated with MGE (0–200 µg/ml) or FGE
(200 µg/ml) containing FBS overnight. Wound closure was observed
under a light microscope with 100× magnification. To confirm cell
migration using the Transwell migration assay, the cells were
seeded on a 24-well Transwell insert (1×103
cells/insert) in serum-free DMEM, and then incubated with MGE
containing FBS (lower chamber) overnight. The insert insides were
swabbed and then stained with 0.2% crystal violet in a 20% methanol
solution. The stained cells were observed under a light microscope
with 100× magnification. Wound closure and migrated cells were
measured using ImageJ software (version 1.51j8 for Windows;
National Institutes of Health).
Tube formation assay
Matrigel was added to a 96-well plate (50 µl/well),
which was then incubated for 30 min at 37°C. SVEC4-10 cells,
pretreated with MGE or FGE for 30 min, were seeded on the
Matrigel-coated 96-well plate. After 3 h, the cell morphology was
observed under a light microscope with 100× magnification. The tube
lengths and areas were measured using ImageJ software (National
Institutes of Health).
Immunoblotting analysis
Immunoblotting analysis was evaluated following a
method previously reported (14).
Briefly, the blotted proteins on PVDF membrane were visualized
using a chemiluminescent reaction (Immnobilon Western; Millipore
Corporation) with an Imaging system (ImageQuant LAS 4000, GE
Healthcare Life Sciences). The level of target proteins was
compared to that of a loading control (non-phosphorylated proteins
or β-actin), and the results were expressed as a ratio of density
of each protein identified by a protein standard size marker
(BIOFACT Co., Ltd.). The density of each inverted band was measured
using ImageJ software (National Institutes of Health).
Statistical analyses
The experimental results were listed as means ± SD
for in vitro studies or SEM for in vivo studies
One-way analysis of variance (ANOVA) was used for multiple
comparisons (GraphPad Prism version 5.03 for Windows; GraphPad
Software, Inc.). We applied the Dunnetts test or Tukey's test for
one-way ANOVA for significant variations between treated groups.
Differences at the *P<0.05 and **P<0.01 levels were
considered statistically significant.
Results
Inhibitory effects of MGE and FGE on
cell migration and capillary-like network in SVEC4-10 cells
Comparison between MGE and FGE. To compare the
effects of MGE and FGE on cell migration and tube formation in
vascular endothelial cells, we investigated the effects of MGE and
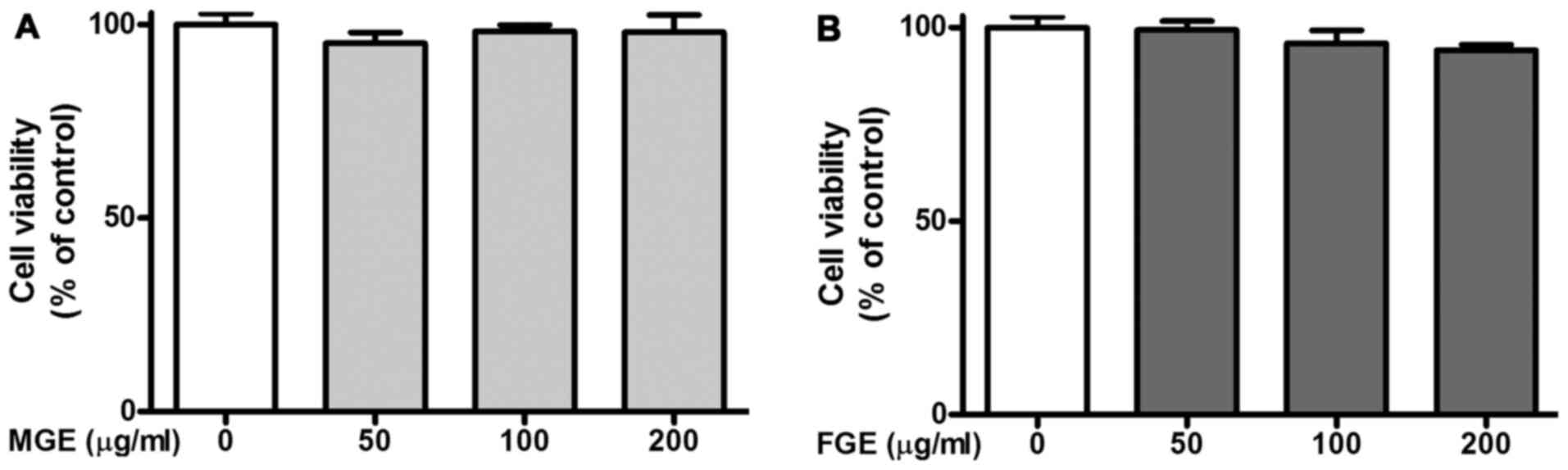
FGE on cell migration of SVEC4-10 cells. As presented in Fig. 1, MGE and FGE did not show
cytotoxicity within the concentration ranges (0–200 μg/ml).
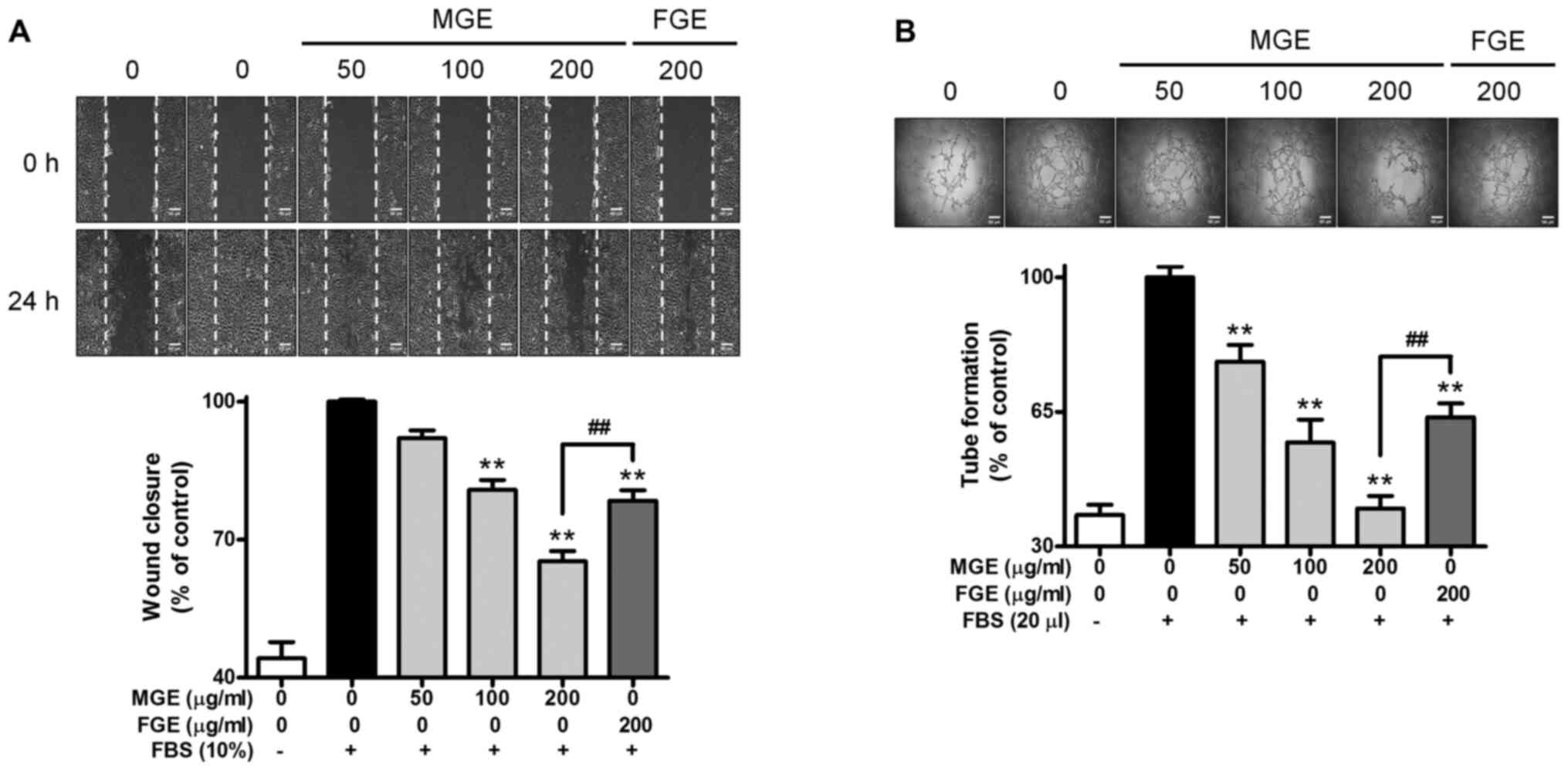
Subsequently, when SVEC4-10 cells were stimulated by FBS, the cells
completely covered a wound area (Fig.
2A). In contrast, MGE dose-dependently inhibited the cell
migration of FBS-activated SVEC4-10 cells, and the inhibitory
effect of MGE at 200 µg/ml was more potent than that of FGE at the
same concentration (Fig. 2A). Based
on the cell migration results, we examined whether MGE and FGE
could suppress the formation of capillary-like network of
FBS-activated SVEC4-10 cells. The inclusion of MGE significantly
reduced the formation of capillary-like network in the cells, and
the inhibitory effect of MGE at maximum dose was stronger than that
of FGE at the same concentration (Fig.
2B). The results suggest that MGE and FGE have anti-angiogenic
properties within non-cytotoxic ranges in vascular endothelial
cells. In addition, the anti-angiogenic effect of MGE is stronger
than that of FGE.
Inhibitory effect of MGE on Transwell
cell migration of SVEC4-10 cells
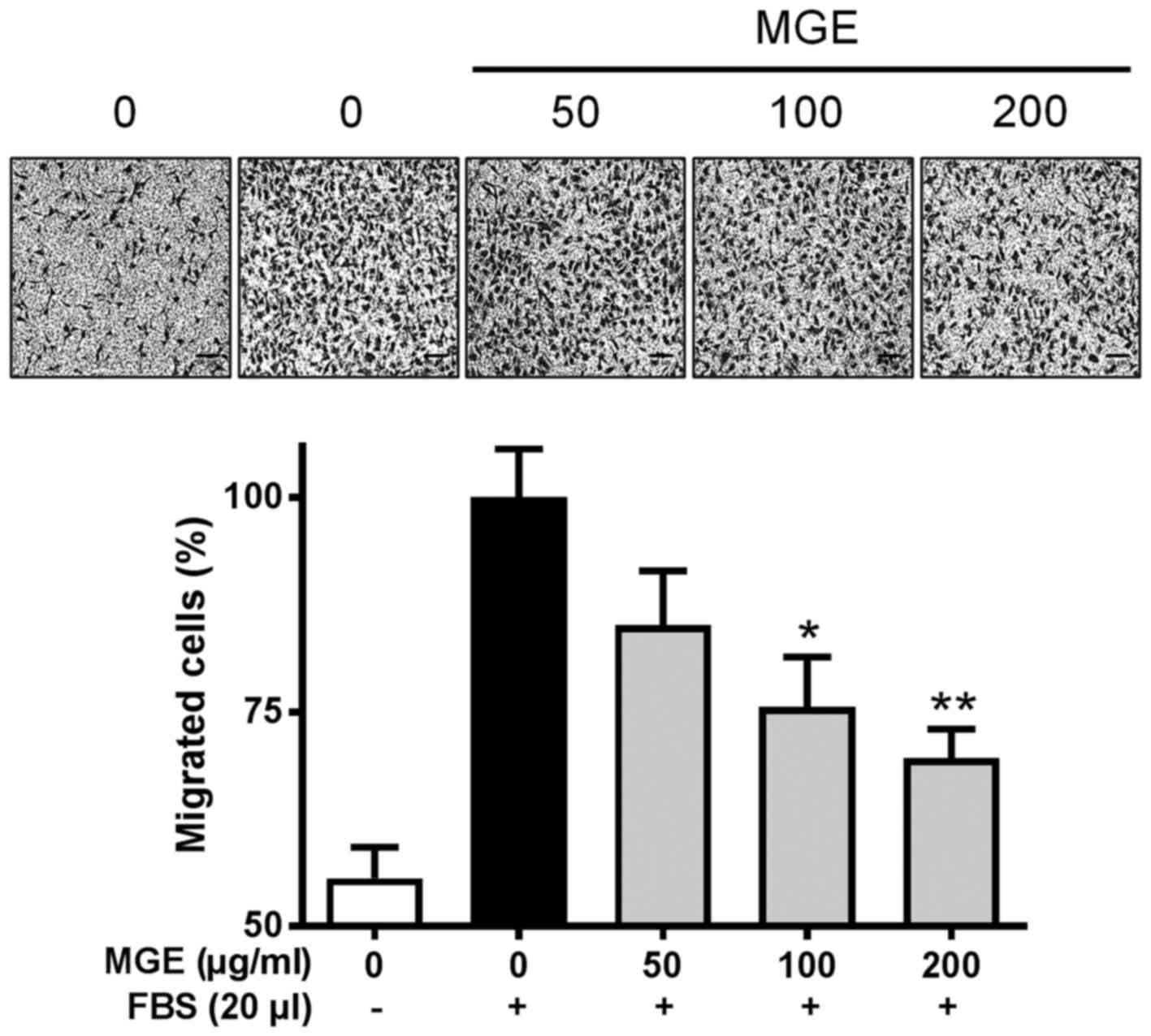
As we found that MGE was able to inhibit the cell
migration and capillary-like network formation of vascular
endothelial cells on a 2-D structure, we tried to confirm whether
MGE was capable of suppressing the cell migration of FBS-stimulated
SVEC4-10 cells on a 3-D structure. Consistent with the cell
migration and capillary-like network formation on the 2-D
structure, MGE concentration-dependently attenuated Transwell cell
migration of the cells (Fig. 3).
This finding suggests that MGE may be able to attenuate the
formation of neo blood vessels in a whole body system.
Inhibitory effects of MGE on the
phosphorylation of proteins related to the VEGF-R2 signaling
cascade
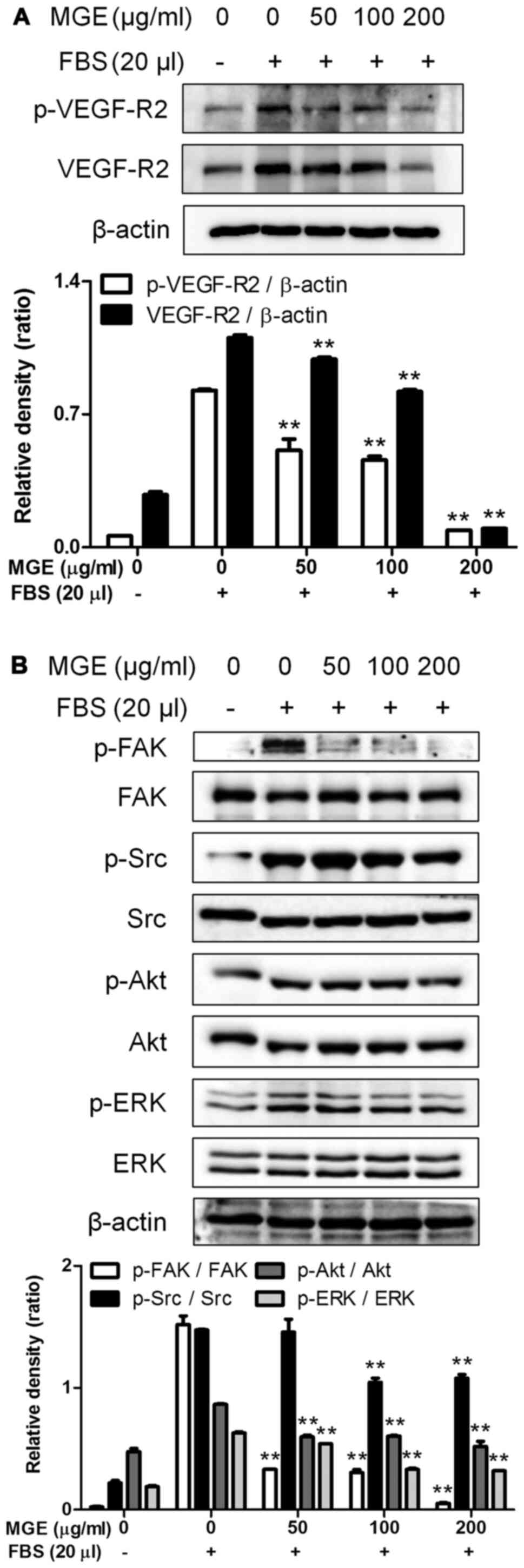
After we found that MGE was able to reduce the cell
migration and tube formation of vascular endothelial cells, we were
interested in the effect of MGE on the VEGF-R2 signaling pathway
because angiogenic factor-exposed vascular endothelial cells are
capable of formatting neo blood vessels through activation of the
VEGF-R2 signaling pathway (15).
MGE not only reduced both phosphorylation and expression of VEGF-R2
(Fig. 4A) but also attenuated the
phosphorylation of FAK, Src, Akt and ERK, intermediate proteins in
the VEGF-R2 signaling pathway (16), in SVEC4-10 cells activated by FBS
(Fig. 4B). Also, MGE at 50 µg/ml
almost blocked the phosphorylation of FAK, one of the intermediate
proteins of the VEGF-R2 signaling pathway (Fig. 4B). These results suggest that MGE
has anti-angiogenic properties through inhibition of activation of
the VEGF-R2 signaling cascade in vascular endothelial cells.
Inhibitory effect of MGE on neo blood
vessels in mice
Finally, because we found that MGE possesses
anti-angiogenic properties through inhibiting the phosphorylation
of proteins related to the VEGF-R2 signaling cascade in vascular
endothelial cells, we tried to confirm the anti-angiogenic action
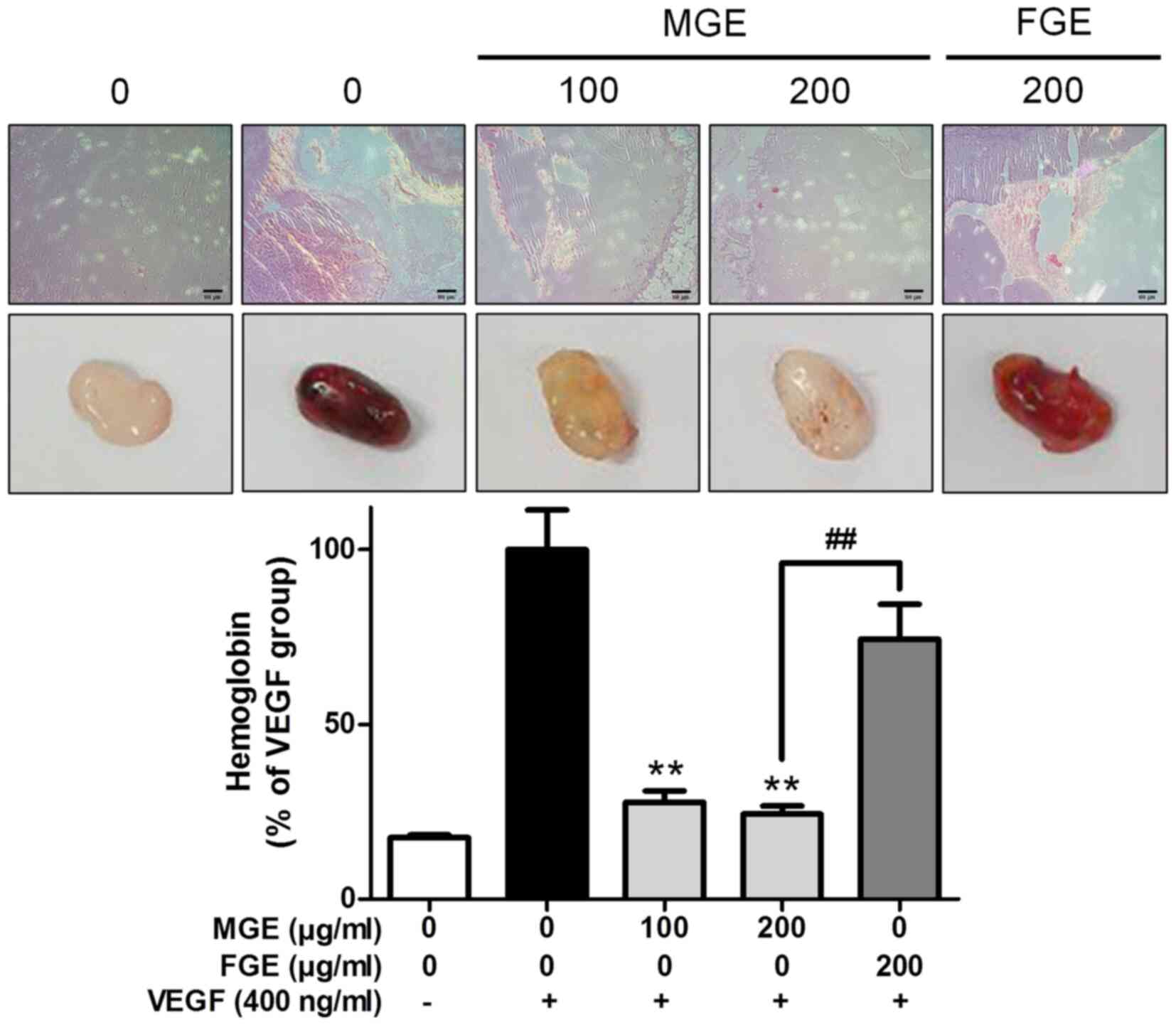
of MGE on a whole body system. In histological observation of a
Matrigel plug assay in vivo, MGE dose-dependently attenuated
accumulation of erythrocytes induced by VEGF in Matrigels. Also,
the inhibitory effect of MGE at 200 µg/ml was stronger than that of
FGE (Fig. 5). Consistent with the
histological results, MGE dramatically reduced the level of
hemoglobin, a biomarker of blood vessels, in Matrigel including
VEGF (400 ng/ml) in mice (Fig. 5).
Surprisingly, MGE at 200 µg/ml almost suppressed the level of
hemoglobin in Matrigels to a level similar to that in Matrigels of
the control group. In addition, the inhibitory effect of MGE was
more potent than that of FGE at the same concentration (Fig. 5). These findings suggest that MGE is
able to inhibit neo blood vessels in a whole body system. Overall,
MGE may be a possible herbal drug candidate or adjuvant for therapy
for cancer patients.
Discussion
Korean ginseng has been commonly used worldwide as
an ingredient of functional foods and in traditional medicine
because Korean ginseng possesses various beneficial health effects
(2–4). Such beneficial health properties are
closely correlated with various bioactive compounds in Korean
ginseng (2,4). Especially, mountain ginseng is richer
in bioactive compounds than farm-cultivated ginseng (17). Currently, MGE has been used as an
element of pharmacopuncture for treatment of cancer patients in
Korea (5). In addition, we found in
a recent study that MGE exerted anticancer activity against human
breast cancer (6). Growth and
metastasis of cancer cells are closely related to angiogenesis
(8). Nevertheless, the effect of
MGE on angiogenesis has not been known.
In this study, we examined whether MGE was capable
of suppressing angiogenesis in vascular endothelial cells in both
in vitro and in vivo models, and we found that MGE
had more potent anti-angiogenic action than FGE. In addition, MGE
inhibited the phosphorylation and expression of VEGF-R2 as well as
the phosphorylation of FAK, Src, Akt and ERK, which are
intermediate proteins in the VEGF-R2 signaling cascade (16), in vascular endothelial cells.
Consonant with in vitro data, MGE showed stronger
anti-angiogenic action than FGE in the formation of neo blood
vessels in Matrigels including VEGF in mice. Such an
anti-angiogenic action of MGE may be closely associated with its
richness in ginsenosides because some ginsenosides possess
anti-angiogenic activities (18).
One possible mechanism for the anti-angiogenic
action of MGE may be associated with the inhibition of the VEGF-R2
signaling cascade in vascular endothelial cells. It is well known
that VEGF is a strong angiogenic activator among various angiogenic
activators, and VEGF exists in five isoforms (16). In addition, VEGF is produced from
various cells, such as cancer cells, fibroblasts, and inflammatory
cells (16). Most importantly, VEGF
is able to initiate angiogenesis through promoting the activation
of the VEGF-R2 signaling pathway in vascular endothelial cells
(16) because VEGF-R2 is expressed
in vascular endothelial cells alone (16). When VEGF-R2, which belongs to the
receptor tyrosine kinase superfamily, is activated by VEGF, VEGF-R2
can phosphorylate Akt, ERK, FAK, and Src (16). Then, the phosphorylated intermediate
proteins promote cell migration, vascular permeability,
proliferation, and survival of vascular endothelial cells (16). As a result, neo blood vessels are
formed. In particular, cancer cells easily make VEGF under hypoxic
conditions (8). Therefore,
inhibiting the activation of the VEGF-R2 signaling cascade is an
important point for the treatment of cancer patients. In support of
this, MGE inhibited cell migration and tube formation of vascular
endothelial cells. In addition, MGE reduced the phosphorylation of
FAK, Src, Akt and ERK during suppressing both phosphorylation and
expression of VEGF-R2 in the cells. Moreover, MGE dramatically
suppressed the formation of neo blood vessels in Matrigel plug in
mice. This indicates that MGE exerts anti-angiogenic properties
through inhibiting the activation of the VEGF-R2 signaling pathway
in vascular endothelial cells.
Another possible anti-angiogenic mechanism of MGE
may be correlated with the rich ginsenosides in MGE. It is well
known that some ginsenosides, such as ginsenoside Rb1, Rb2, Rg3,
Rh1 and Rh2, have anti-angiogenic activities (18). Recently, we found that MGE possessed
a stronger anticancer action than FGE in human breast cancer, and
MGE was richer in total ginsenosides than FGE (6). Remarkably, among the anti-angiogenetic
ginsenosides, MGE included higher concentrations of ginsenoside Rb1
and Rb2 than FGE (6). Overall, this
study may support the possibility of using MGE as a herbal drug for
the treatment of cancer patients in clinics. Nevertheless, this
study has some limits in representing a preclinical study for
clinical application.
In summary, this study demonstrates that MGE
possesses a stronger anti-angiogenic action than FGE in vascular
endothelial cells in both in vitro and in vivo
models. The anti-angiogenic mechanism of MGE is closely associated
with the activation of the VEGF-R2 signaling cascade. Thus, the
intracellular targets of MGE include VEGF-R2, FAK, Src, Akt and ERK
in vascular endothelial cells. In addition, such an anti-angiogenic
effect may be correlated with MGE being rich in ginsenosides. These
findings may provide novel information for understanding how MGE
attenuates the growth of human cancers by regulating the tumor
microenvironment. Furthermore, this study may provide support for
using MGE as a possible herbal drug for the treatment of cancer
patients in clinics. However, other preclinical studies are
necessary to confirm the anti-angiogenic action of MGE and to
provide complete support for any clinical application because this
study has some limits in representing a preclinical study for
clinical application. Furthermore, other studies, such as
toxicology, metabolism, pharmacokinetics, and phytochemical
studies, are necessary to ensure patient safety with MGE and to
identify the active phytochemicals of the anti-angiogenic action in
MGE.
Acknowledgements
Not applicable.
Funding
The present study was supported by the
Standardization Project of Korean Medicine Acupuncture funded by
the Korean Ministry of Health and Welfare (grant no. 3243-302).
Availability of data and materials
All data generated and analyzed during this study
are included in this published article.
Authors' contributions
JSK participated in the design of this study,
performed most experiments and acquired all the data. JMY
participated in the design of this study and interpretation of all
the experimental data, improved the design of this study, analyzed
statistics of all the experimental data, drafted this manuscript
and revised the manuscript. JEP prepared MGE and FGE. JK and SGK
participated in in vivo studies. YMS and JHS participated in
the study design and interpretation of the data. HJK designed this
study, interpreted all the experimental data and supervised all
experiments. JSK and HJK confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the
Committee of Animal Care and Experiment of NIKOM with a reference
number (NIKOM-2020-5).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
FAK
|
focal adhesion kinase
|
|
FGE
|
ethanolic extract of farm-cultivated
ginseng
|
|
MGE
|
ethanolic extract of mountain
ginseng
|
|
Src
|
steroid receptor coactivator
|
References
|
1
|
Baeg IH and So SH: The world ginseng
market and the ginseng (Korea). J Ginseng Res. 37:1–7. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Choi KT: Botanical characteristics,
pharmacological effects and medicinal components of Korean Panax
ginseng C A Meyer. Acta Pharmacol Sin. 29:1109–1118. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee JY, Yoo JM, Baek SY and Kim MR:
Anti-dermatitic effect of fermented ginseng extract including rich
compound K through inhibiting activation of macrophage. Food Sci
Biotechnol. 28:1845–1852. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim KH, Lee D, Lee HL, Kim CE, Jung K and
Kang KS: Beneficial effects of Panax ginseng for the
treatment and prevention of neurodegenerative diseases: Past
findings and future directions. J Ginseng Res. 42:239–247. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee KH, Cho YY, Kim S and Sun SH: History
of research on pharmacopuncture in Korea. J Pharmacopuncture.
19:101–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim J, Yoo JM, Kim JS, Kim SG, Park JE,
Seok YM, Son JH and Kim HJ: Anticancer effect of mountain ginseng
on human breast cancer: Comparison with farm-cultivated ginseng.
Evid Based Complement Alternat Med. 2020:25847832020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carmeliet P: Angiogenesis in health and
disease. Nat Med. 9:653–660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huber V, Camisaschi C, Berzi A, Ferro S,
Lugini L, Triulzi T, Tuccitto A, Tagliabue E, Castelli C and
Rivoltini L: Cancer acidity: An ultimate frontier of tumor immune
escape and a novel target of immunomodulation. Semin Cancer Biol.
43:74–89. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gatto C, Rieppi M, Borsotti P, Innocenti
S, Ceruti R, Drudis T, Scanziani E, Casazza AM, Taraboletti G and
Giavazzi R: BAY 12-9566, a novel inhibitor of matrix
metalloproteinases with antiangiogenic activity. Clin Cancer Res.
5:3603–3607. 1999.PubMed/NCBI
|
|
10
|
Yoo JM, Park KI and Ma JY: anticolitic
effect of viscum coloratum through suppression of mast cell
activation. Am J Chin Med. 47:203–221. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
O'Connell KA and Edidin M: A mouse
lymphoid endothelial cell line immortalized by simian virus 40
binds lymphocytes and retains functional characteristics of normal
endothelial cells. J Immunol. 144:521–525. 1990.
|
|
12
|
Yoo JM, Park KI, Yang JH, Cho WK, Lee B
and Ma JY: Anti-allergic actions of F-PASA, a novel herbal
cocktail, in IgE/antigen-mediated allergic responses in RBL-2H3
cells and passive cutaneous anaphylaxis in mice. Phytomedicine.
55:229–237. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoo JM, Yang JH, Kim YS, Yang HJ, Cho WK
and Ma JY: Inhibitory effects of viscum coloratum extract on
IgE/antigen-activated mast cells and mast cell-derived inflammatory
mediator-activated chondrocytes. Molecules. 22:372016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jeon HL, Yoo JM, Lee BD, Lee SJ, Sohn EJ
and Kim MR: Anti-inflammatory and antioxidant actions of
N-Arachidonoyl serotonin in RAW264.7 cells. Pharmacology.
97:195–206. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu X and Zhou W: The emerging regulation
of VEGFR-2 in triple-negative breast cancer. Front Endocrinol
(Lausanne). 6:1592015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clarke JM and Hurwitz HI: Targeted
inhibition of VEGF receptor 2: An update on ramucirumab. Expert
Opin Biol Ther. 13:1187–1196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu XF, Cheng XL, Lin QH, Li SS, Jia Z, Han
T, Lin RC, Wang D, Wei F and Li XR: Identification of
mountain-cultivated ginseng and cultivated ginseng using
UPLC/oa-TOF MSE with a multivariate statistical sample-profiling
strategy. J Ginseng Res. 40:344–350. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dai D, Zhang CF, Williams S, Yuan CS and
Wang CZ: Ginseng on cancer: Potential role in modulating
inflammation-mediated angiogenesis. Am J Chin Med. 45:13–22. 2017.
View Article : Google Scholar : PubMed/NCBI
|