Introduction
One of the most common lethal malignancies in women
worldwide is ovarian cancer (1).
Ovarian cancer is ranked as the 7th most prevalent female cancer
type, as shown by a 2018 report, accounting for >180,000 deaths
in China (2). Moreover, ~70% of
ovarian cancer is diagnosed at an advanced stage due to silent
symptoms, the hidden growth of the tumor and the lack of
appropriate early diagnostic methods (3). Despite advancements in chemotherapy
and surgery, the prognosis of patients with ovarian cancer has not
changed significantly over the last few decades. Moreover, drug
resistance has become a new challenge for the therapy of ovarian
cancer (4). Thus, it is important
to illustrate the mechanism of drug resistance and overcome this
challenge for improving the therapy and prognosis of ovarian
cancer.
The standard treatment for ovarian cancer is surgery
combined with paclitaxel (PTX)- and platinum-based adjuvant
chemotherapy (5). However, the
first line obstruction in ovarian cancer treatment is PTX and
platinum resistance (6). As
indicated by the mechanism of the drug-resistant tumor, there may
be a pool of self-renewing malignant cells, namely ovarian cancer
stem-like cells, which could enhance the self-renewal, reproduction
and drug resistance of tumors, thereby serving key roles in the
initiation, diffusion, metastasis and recurrence of cancer
(7,8). As shown in previous studies, a
potential marker for ovarian stem-like cancer cells could be CD44,
along with CD117 (9,10). Increased expression levels of CD44,
CD117 and aldehyde dehydrogenase 1 family member A1 in SKOV3 cells
are significantly correlated with the enhancing features of
epithelial-mesenchymal transition (EMT), sphere formation,
metastasis and multidrug resistance abilities (11,12).
At present, the sensitivity of CD44+CD117+
cells to chemotherapy drugs, including PTX, remains unknown and
needs to be further investigated. Moreover, in the drug resistance
of ovarian cancer, the exact mechanism of cancer stem-like cells
are yet to be fully elucidated.
C-C motif chemokine ligand 20 (CCL20), as a ligand
of C-C motif chemokine receptor 6 (CCR6), serves a key role in the
development of numerous cancer types, such as lung cancer,
colorectal cancer and thyroid cancer (13,14).
Moreover, the CCL20/CCR6 axis serves an important role in the
process of drug resistance (15).
As shown in a recent study, cisplatin can stimulate macrophages to
secrete CCL20 and promote ovarian cancer cell migration via the
CCL20/CCR6 axis (16). However, in
ovarian cancer, the role of the CCL20/CCR6 axis and its mechanism
remain unclear and require further examination.
In embryonic development and numerous cellular
physiological processes, notch receptor (Notch) is involved in
regulating cell proliferation, apoptosis and differentiation
(17). As a member of the Notch
family, Notch1 is implicated in multiple cancer types, such as
breast cancer (especially triple-negative breast cancer), leukemias
and brain tumors, among others (18,19).
Notch1 is closely associated with numerous signaling pathways that
are therapeutically involved in tumorigenesis, such as TLR4/NF-κB
and PI3K/AKT (20). Together these
impact apoptosis, proliferation, chemosensitivity, immune response
and the population of cancer stem cells.
In the present study, a population of
CD44+CD117+ subgroup cells was isolated from
SKOV3 cells, followed by determining their stemness and drug
resistance. Furthermore, the underlying mechanism of
CD44+CD117+ cells in drug resistance and
stemness maintenance was examined. The current study also provides
novel insights into the mechanism and clinical therapy of PTX
resistance in ovarian cancer.
Materials and methods
Cell culture and isolation
The human ovarian cancer cell line, SKOV-3, was
purchased from The Shanghai Stem Cell Institute, and was cultured
in DMEM (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented
with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin mixture (Sigma-Aldrich; Merck KGaA) at 37°C
in a humidified atmosphere with 5% CO2.
CD44+CD117+ cells were then
separated from SKOV-3 cells using the magnetic-activated cell
sorting kit (Miltenyi Biotec GmbH) according to the manufacturer's
instructions. The kit included CD44+ and
CD117+ antibodies. CD44+ cells were sorted
using a mouse anti-human CD44+ antibody combined with
magnetic microbeads. After magnetic column depletion, among
CD44+ SKOV3 cells, CD117+ cells were isolated
using CD117+ antibody combined with magnetic microbeads.
Moreover, among CD44− cells, the CD117+
subset was isolated using CD117+ antibody combined with
magnetic microbeads. The CD44+CD117+
stem-like subsets and CD44−CD117− cells were
harvested after magnetic column depletion and maintained in a
DMEM/F12 medium containing 5 µg/ml insulin (Sigma-Aldrich; Merck
KGaA), 10 ng/ml basic fibroblast growth factor (bFGF;
Sigma-Aldrich; Merck KGaA), 20 ng/ml human recombinant epidermal
growth factor (EGF; Sigma-Aldrich; Merck KGaA) and 0.5% FBS.
Construction of transient small
interfering RNA (siRNA/si)Notch1 cell lines
According to GenBank (NM_017617.4), a siRNA sequence
of human Notch1 was designed. The sequence was as follows:
siNotch1-1 forward (F), 5′-AGUGGACAUCAGUACUGUA-3′ and reverse (R),
3′-UACAGUACUGAUGUCCACU-5′; siNotch1-2 F,
5′-AUGCGGGCAAGUGCAUCAACA-3′ and R, 3′-UGUUGAUGCACUUGCCCGCAU-5′; and
siNotch1-3 F, 5′-GCUACACAGGGAGCAUGUGUA-3′ and R,
3′-TACACATGCTCCCTGTGTAGC-5′. The negative control (siNC) was
designed as follows: F, 5′-UUCUCCGAACGUGUCACGUTT-3′ and R,
3′-ACGUGACACGUUCGGAGAATT-5′. All primers were synthesized and
recombined to the plasmids (pcDNA3.1) by Shanghai GenePharma Co.,
Ltd.. After cells were inoculated overnight in a 6-well plate with
60% confluency, 20 µl siNotch1 or siNC recombinant were transfected
into cells using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol
at 37° and 5% CO2 in an incubator and for the following
experiments 48 h later as indicated.
Xenograft and grouping
In total, 16 male nude mice (age, 6–8 weeks; weight,
25–30 g) were purchased from the Experimental Animal Center of the
Shandong University and raised at the Experimental Animal Center.
Mice were housed at 25±1°C with 12-h light/dark cycles, 50±5%
humidity and free access to food and water. The mice were randomly
divided into four groups: i) CD44+CD117+ +
PTX (Beijing Solarbio Science & Technology Co., Ltd.) group
(n=4); ii) CD44+CD117+ + PTX + CCL20 (Beijing
Solarbio Science & Technology Co., Ltd.) group (n=4); iii)
CD44−CD117− + PTX group (n=4); and iv)
CD44−CD117− + PTX + CCL20 group (n=4). For
xenografts, CD44+CD117+ (2×106)
subsets or CD44−CD117− subsets cells
(2×106) suspended in 0.5 ml PBS were subcutaneously
injected into the left flank of each nude mouse to establish
tumors. Then, PTX (10 mg/kg) and/or CCL20 (1 mg/kg) were injected
intraperitoneally simultaneously on days 1, 3 and 7. Tumor size was
monitored on the 3rd, 7th, 14th, 21st and 28th days (15). At the end of the experiment and
under continuous anesthesia, the animals were euthanized by an
overdose of pentobarbital (125 mg/kg). All experiments were
approved by The Animal Care and Use Committee of the Shandong
Provincial Hospital, Cheeloo College of Medicine, Shandong
University.
Tumor sphere formation assay
In a 24-well plate,
CD44+CD117+ or
CD44−CD117− cells were harvested, counted and
seeded with a low attachment (Corning, Inc.) at a density of
1.0×103 per well after isolation or treatment. Then, the
cells were maintained in a DMEM/F12 medium (Sigma-Aldrich; Merck
KGaA), without FBS, supplemented with 4 mg/ml heparin
(Sigma-Aldrich; Merck KGaA), 20 ng/ml EGF (Sigma-Aldrich; Merck
KGaA), 20 ng/ml bFGF (Sigma-Aldrich; Merck KGaA) and 2% B27
(Invitrogen; Thermo Fisher Scientific, Inc.). After 10–14 days,
spheres were analyzed and images were captured with an inverted
fluorescent microscope (Nikon Ni-U; Nikon Corporation;
magnification, ×100).
Cytotoxicity determination
The IC50 of cells exposed to PTX was
determined using a Cell Counting Kit (CCK)-8 assay (Dojindo
Molecular Technologies, Inc.) according to the manufacturer's
instructions. In brief, cells were inoculated at a concentration of
4×103/100 µl per well and cultured at 37°C for 12 h in a
96-well plate. The cells were then maintained at 37°C with 200 µl
fresh medium containing different concentrations of PTX (0–50 nM)
for 48 h. Following this, the cells were incubated with 20 µl CCK-8
solution at 37°C for 24 h. Then, the optical density value of each
well was detected using a microplate reader at 450 nm (Thermo
Fisher Scientific, Inc.).
Reactive oxygen species (ROS)
detection
For ROS detection, 5×105 cells were
seeded overnight in a 6-well plate and then treated with PTX (5 nM)
at 37°C for 48 h. Subsequently, 5 µM carboxy
2′-7′-dichlorofluorescein diacetate was added in Hanks' Balanced
Salt Solution and incubated with cells at room temperature for 0.5
h. Fresh tissues were harvested, followed by a single cell
suspension preparation. Tissue was digested with trypsin to obtain
single cell suspension. Cells were then inoculated in a 6-well
plate as aforementioned. The levels of intracellular peroxide were
measured using a flow cytometer (BD FACSAria; BD Biosciences) at
485 nm excitation and 520 nm emission to analyze the ROS
generation.
Analysis of apoptosis
The apoptosis of cells was determined using flow
cytometry and Hoechst assays. SKOV3 cells were seeded in a 6-well
plate for flow cytometry and treated with PTX (5 nM) at 37°C for 48
h. The adherent and floating cells were harvested, washed with cold
PBS and stained with FITC-conjugated Annexin V/PI (Beijing Solarbio
Science & Technology Co., Ltd.), according to the
manufacturer's instructions. The double-stained cells were then
detected via Accuri C6 plus flow cytometry (BD Bioscience).
For Hoechst staining, 5×105 cells were
seeded overnight in a 6-well plate and treated with PTX (5 nM) at
37°C for 48 h. Cells were then stained with 0.5 µg/ml Hoechst 33342
dye in a 5% CO2 incubator at 37°C for 25 min and were
analyzed using a Nikon Ni-U fluorescence microscope (Nikon
Corporation; magnification, ×100).
H&E staining
The tumors were removed and were fixed with 4%
paraformaldehyde at 37°C for >24 h. Specimens were then
dehydrated in isopropyl alcohol by xylene and embedded in paraffin.
According to the manufacturer's instructions, specimens were cut
into slices (thickness, 5 µm) and stained with H&E (Nanjing
Jiancheng Bioengineering Institute) at 37°C for 24 h. For
subsequent analysis, the slices were dehydrated and sealed with
neutral resins. Images were captured with an inverted fluorescent
microscope (Nikon Ni-U; Nikon Corporation; magnification, ×100). A
total of 3 field of view were observed by microscopy.
TUNEL assay
The apoptosis of tumor tissue slices was assessed
using the Clicl-iT Plus TUNEL assay (Thermo Fisher Scientific,
Inc.). The tumors were removed and were fixed with 4%
paraformaldehyde at 37°C for 24 h. According to the manufacturer's
instructions, specimens were sectioned at 5 µm and stained with
TUNEL reagent (Nanjing Jiancheng Bioengineering Institute) at 37°C
for 1 h. After staining, the sections were sealed with a Prolong
Gold antifade reagent with DAPI and images were captured with an
inverted fluorescent microscope (Nikon Ni-U; Nikon Corporation;
magnification, ×100). A total of 3 field of view were observed by
microscopy.
Immunohistochemistry (IHC)
IHC was used to detect the expression level of CCR6
in xenograft mice. The tumors were removed and were fixed with 4%
paraformaldehyde at 37°C for 48 h. To retrieve the antigen,
5-µm-thick slices from the center of paraffin-embedded tumor
samples were collected, deparaffinized in xylene, rehydrated with
gradient ethanol, immersed in a 10 mM citrate buffer (pH 6.0) and
then heated in a microwave oven for 10 min. Subsequently, slices
were incubated with 0.3% H2O2 at room
temperature for 30 min to block the endogenous peroxidase activity
and then with 1.5% normal goat serum at 37°C for 30 min (Takara
Biotechnology Co., Ltd.) for blocking non-specific protein binding.
Subsequently, the slices were immersed in the primary antibody in a
humidity chamber at 4°C overnight. Slices were then incubated with
a biotinylated goat anti-mouse IgG antibody (1:1,000; cat. no.
AP124; Sigma-Aldrich; Merck KGaA) at 37°C for 30 min and visualized
using the 3′3-diaminovbenzidine method. For further analysis,
slices were then counterstained with hematoxylin for 30 min at 37°C
and sealed with neutral resins. Images were captured with an
inverted fluorescent microscope (Nikon Ni-U; Nikon Corporation;
magnification, ×100).
Reverse transcription-quantitative
(RT-q) PCR
TRIzol® reagent (Takara Biotechnology
Co., Ltd.) was used to isolate total RNA samples from cells and
tissues according to the manufacturer's instructions. A RT-qPCR kit
(Takara Biotechnology Co., Ltd.) was used for cDNA synthesis,
according to the manufacturer's instructions. With cDNA as a
template, RT-qPCR was performed using SYBR Green (Takara
Biotechnology Co., Ltd.) in an ABI 6500 system (Applied
Biosciences; Thermo Fisher Scientific, Inc.) under the following
conditions: Initial denaturation at 95°C for 90 sec, followed by 40
cycles of 95°C for 15 sec, 58°C for 21 sec and 72°C for 24 sec. The
primers of genes are presented in Table
I. With GAPDH as the internal control, the relative gene
expression was calculated using the 2−ΔΔCq method
(21).
 | Table I.Primers for reverse
transcription-quantitative PCR detection. |
Table I.
Primers for reverse
transcription-quantitative PCR detection.
| Gene ID | Sequence
(5′-3′) | Product length
(bp) |
|---|
| OCT4-F |
ATCGAGAACCGAGTGAGAGG | 120 |
| OCT4-R |
CACTCGGACCACATCCTTCT |
|
| CCR6-F |
TTCAGCGATGTTTTCGACTCC | 134 |
| CCR6-R |
GAAATCGGTACAAATAGCCTGG |
|
| GAPDH-F |
TGTTCGTCATGGGTGTGAAC | 154 |
| GAPDH-R |
ATGGCATGGACTGGTCAT |
|
| Notch1-F |
GAGGCGTGGCAGACTATGC | 140 |
| Notch1-R |
CTTGTACTCCGTCAGCGTGA |
|
Western blotting
The cells and tissue samples were lysed with a RIPA
lysis buffer containing protease inhibitors (Sigma-Aldrich; Merck
KGaA). The protein suspension was then collected and quantified
using the BCA Protein Assay kit (Pierce; Thermo Fisher Scientific,
Inc.). Then, 5 µg protein was boiled with a load buffer for 10 min,
separated with 10% SDS-PAGE and transferred onto PVDF membranes
(MilliporeSigma). Then, 5% skimmed milk was used to block the
membrane at room temperature for 0.5 h, followed by primary
antibody incubation at 4°C overnight. After washing with TBS −0.05%
Tween-20 and polysorbate buffer, the membrane was incubated at room
temperature with the secondary antibodies for 1 h. The secondary
antibodies were horseradish peroxidase-labeled goat anti-rabbit IgG
(1:5,000; cat. no. AP156P; Sigma-Aldrich; Merck KGaA) or goat
anti-mouse IgG (1:5,000; cat. no. AP-308P; Sigma-Aldrich; Merck
KGaA). The protein bands were visualized using an enhanced
chemiluminescence kit (Beyotime Institute of Biotechnology)
according to the manufacturer's protocol and quantified using the
Gel-Pro-Analyzer 4.0 software (Media Cybernetics, Inc.). The
primary antibodies were: CCR6 (1:1,000; cat. no. GTX71397; GeneTex,
Inc.), ATP binding cassette subfamily G member 1 (ABCG1; cat. no.
ab201776; 1:1,000; Abcam), Oct4 (1:1,000; cat. no. ab200834;
Abcam), Notch1 (1:1,000; cat. no. 4380; Cell Signaling Technology,
Inc.) and GAPDH (1:5,000; cat. no. SAB2103104; Sigma-Aldrich; Merck
KGaA).
Statistical analysis
GraphPad Prism 7.0 (GraphPad Software, Inc.) was
used for statistical analysis. All experiments were conducted in
triplicate, and the data are presented as the mean ± SD.
Comparisons between groups were conducted using paired Student's
t-test, and multiple comparisons were made using a one-way ANOVA,
followed by Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
CD44+CD117+
subgroup cells present higher stemness and PTX resistance than
CD44−CD117−cells
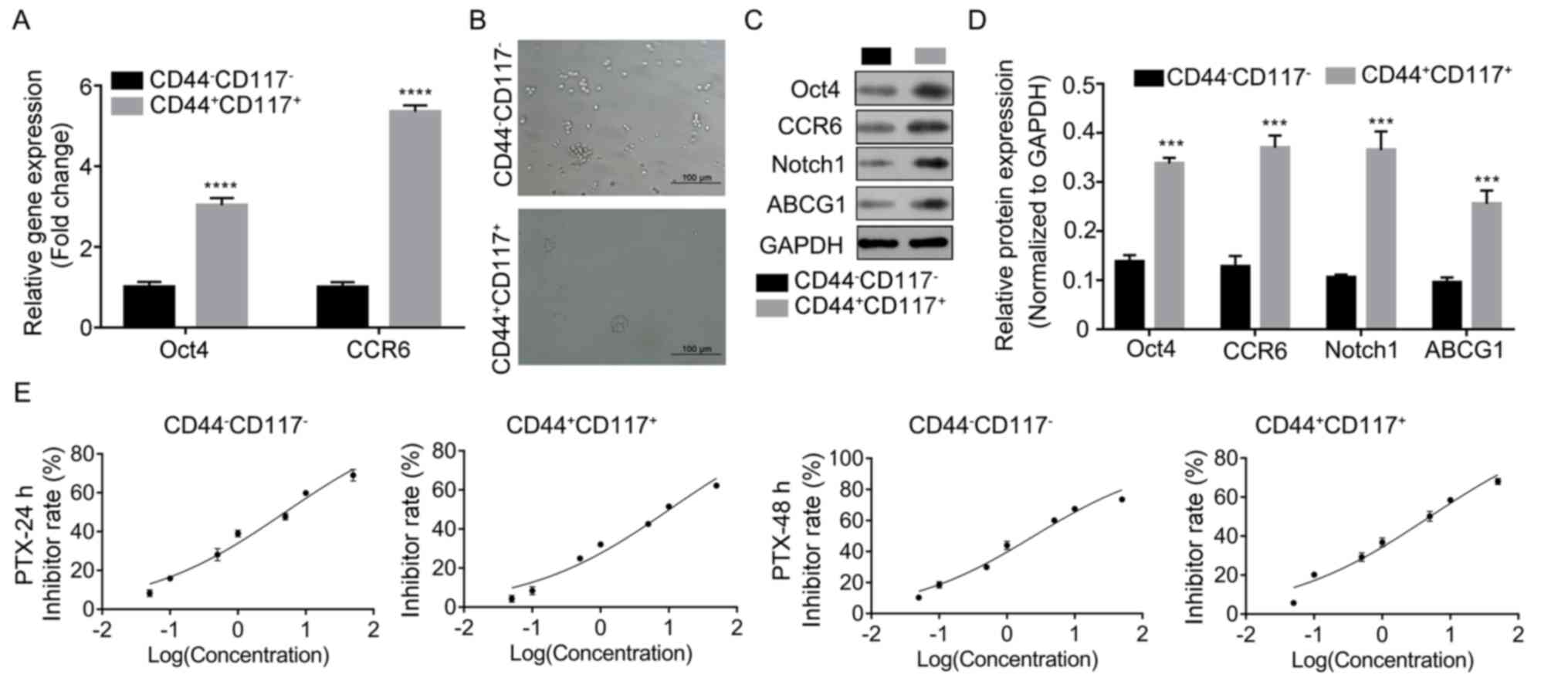
The results demonstrated that the expression levels
of CD44 and CD117 in the total subclones was significantly
increased (P<0.05; Fig. S1).
Once cells were isolated, CCR6 and Oct4 expression was determined
using RT-qPCR. It was found that both Oct4 and CCR6 expression
levels were significantly increased in
CD44+CD117+ cells compared with
CD44−CD117− cells (P<0.0001 and
P<0.001; Fig. 1A).
CD44+CD117+ cells also showed a higher sphere
formation ability compared with CD44−CD117−
subgroup cells, as detected in the sphere formation assay (Fig. 1B). Moreover,
CD44+CD117+ subgroup cells exhibited
significant higher protein expression levels of Oct4, CCR6, Notch1
and ABCG1 than CD44−CD117− cells (P<0.001;
Fig. 1C and D).
Considering these findings, the PTX resistance of
cells was determined using CCK-8 assay. The results indicated that
the IC50 of CD44+CD117+ subgroup
cells for PTX at 24 h (10.22 nM) and 48 h (5.04 nM) was higher
compared with that in CD44−CD117− subgroups
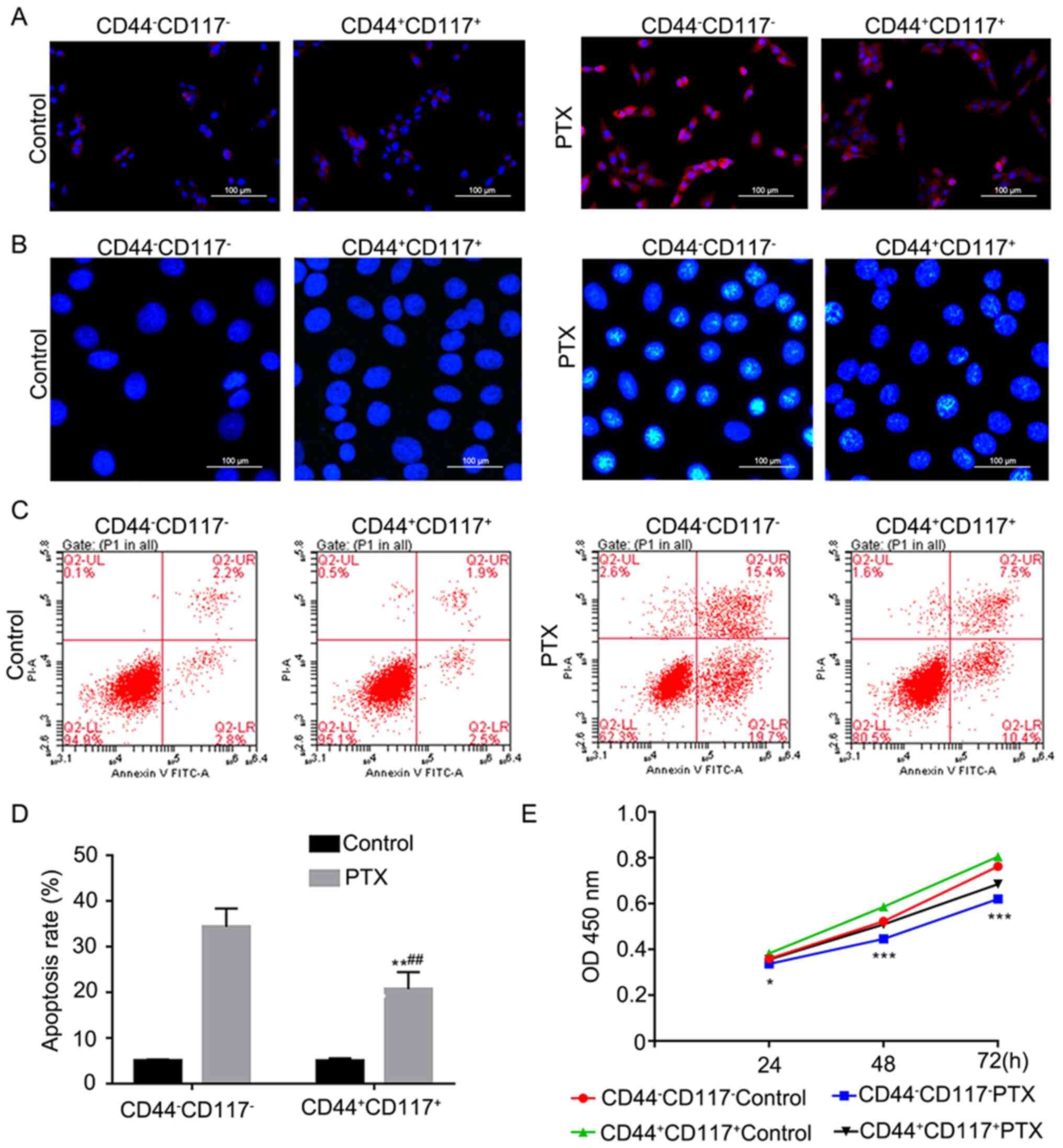
at 24 h (4.99 nM) and 48 h (2.51 nM), respectively (Fig. 1E). Moreover, the ROS level was lower
in CD44+CD117 cells compared with
CD44−CD117− cells after treatment with PTX
(Fig. 2A and B). Furthermore, a
lower apoptotic rate was observed in
CD44+CD117+ subgroup cells compared with that
in CD44−CD117− subgroup cells after treatment
with PTX (P<0.01, Fig. 2C and
D). The CCK-8 assay also indicated that
CD44+CD117+ cells showed increased
proliferation compared with CD44−CD117− cells
after treatment with PTX (P<0.05 and P<0.001; Fig. 2E). These findings suggest that
increased stemness and PTX resistance were observed in
CD44+CD117+ subgroup cells than in
CD44−CD117− cells, which could be attributed
to the involvement of the Notch1 signaling pathway.
Silencing Notch1 reverses the effect
of CCL20 on enhancing the stemness and PTX resistance of
CD44+CD117+ cells
In order to investigate the underlying pathway
involved in the regulation of stemness and PTX resistance, Notch1
was silenced in CD44+CD117+ cells that were
treated with CCL20, a ligand of CCR6, followed by stemness and PTX
resistance analyses. It was found that transfection of siNotch1
significantly inhibited the mRNA and protein expression levels of
Notch1 (P<0.05, P<0.01 and P<0.001; Fig. S1).
The RT-qPCR analysis revealed that CCL20 treatment
could significantly increase the expression level of Oct4, but
silencing Notch1 could markedly reverse this upregulation in
CD44+CD117+ cells (P<0.001; Fig. 3A). Moreover, via western blot
analysis, it was identified that the protein expression levels of
Oct4, ABCG1 and CCR6 in CD44+CD117+ cells
were significantly be increased by CCL20, but that siNotch1 could
markedly reverse the effect of CCL20 in elevating Oct4, ABCG1 and
CCR6 expression in CD44+CD117+ cells
(P<0.001 and P<0.001; Fig. 3B and
C). Sphere formation assays also demonstrated that CCL20 could
increase the sphere formation capacity of
CD44+CD117+ cells, while siNotch1 could
notably impair the promotive effect of CCL20 (Fig. 3D). Thus, it was suggested that the
CCL20/CCR6 axis may increase the stemness of
CD44+CD117+ cells via the Notch1 pathway.
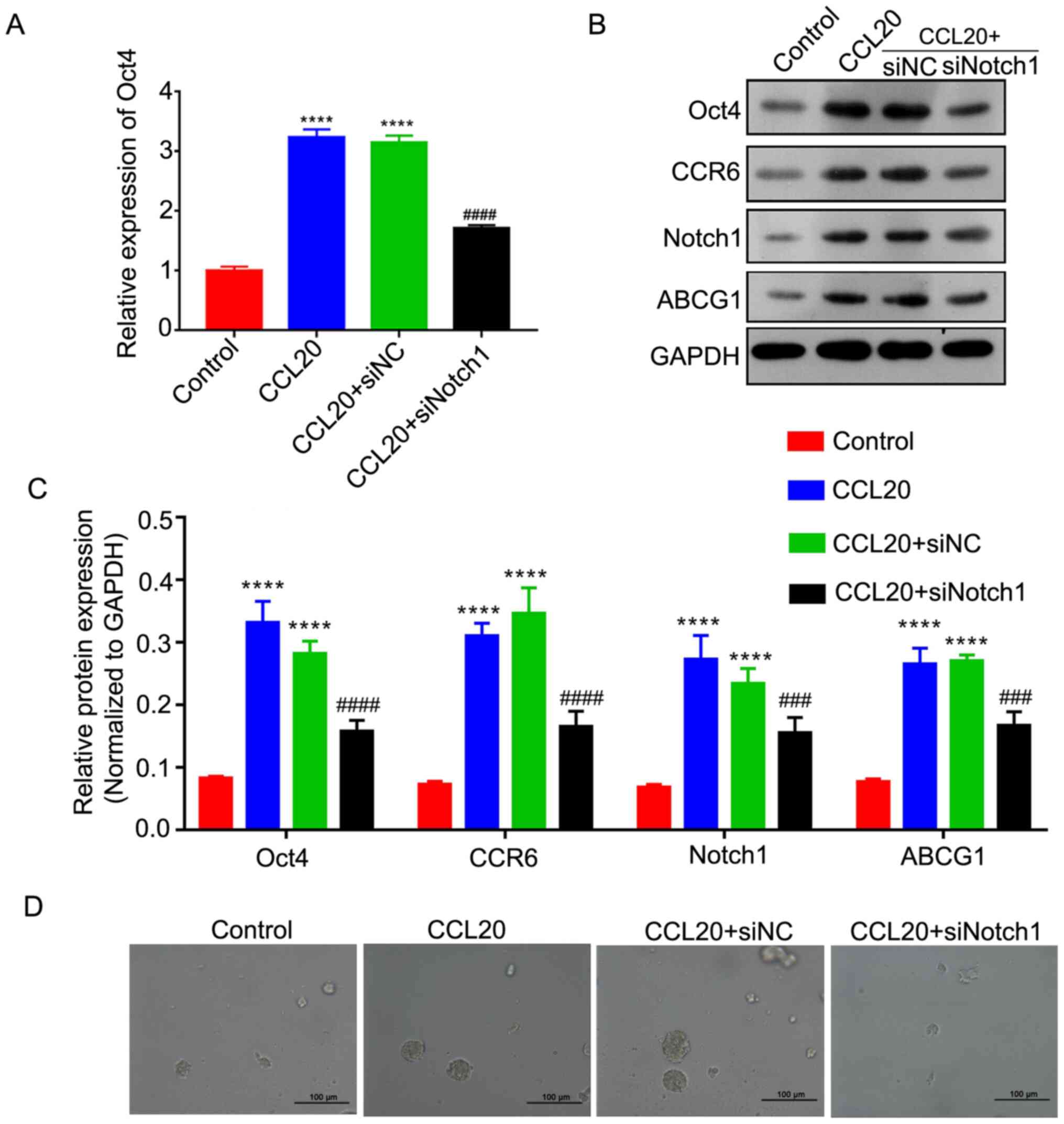 | Figure 3.siNotch1 reverses the effect of the
CCL20/CCR6 axis in enhancing the stemness of
CD44+CD117+ cells. (A) Expression level of
Oct4, as determined by reverse transcription-quantitative PCR. (B)
Protein expression levels of Oct4, CCR6, Notch1 and ABCG1 were
determined via western blotting. (C) Semi-quantitative analysis of
Oct4, CCR6, Notch1 and ABCG1 protein expression. (D) Sphere
formation analysis of CD44+CD117+ cells.
Scale bar, 100 µm. ****P<0.0001 vs. Control group;
###P<0.001 and ####P<0.0001 vs. CCL20
group. NC, negative control; si, small interfering RNA; CCR6, CCR6,
C-C motif chemokine receptor 6; CCL20, C-C motif chemokine ligand
20; ABCG1, ATP binding cassette subfamily G member 1; Notch1, notch
receptor 1. |
Next, the PTX resistance of
CD44+CD117+ cells after transfection with
siNotch1 and CCL20 treatment was determined. The results
demonstrated that CCL20 could markedly increase IC50 of
CD44+CD117+ cells (IC50=17.51 nM,
8.41 nM) to PTX compared with the control group
(IC50=10.98 nM, 4.86 nM) at 24 and 48 h, while siNotch1
could significantly reverse the effect of CCL20 on PTX resistance
of CD44+CD117+ at 24 h (IC50=7.92
nM, 4.22 nM; Fig. 4A),
respectively. After further analysis, it was observed that CCL20
could also markedly ameliorate the ROS level in
CD44+CD117+ cells induced by PTX, while
siNotch1 could attenuate the effect of CCL20 in alleviating ROS
levels in CD44+CD117+ cells after treatment
with PTX (Fig. 4B). Moreover, the
apoptosis of CD44+CD117+ cells was
significantly decreased by CCL20 after treatment with PTX
(P<0.0001), but siNotch1 could partially reverse the effect of
CCL20 in suppressing apoptosis of CD44+CD117+
cells induced by PTX (P<0.001; Fig.
4C-E). In addition, the CCK-8 assays identified that CCL20
could increase the proliferation of
CD44+CD117+ cells, and the promotive effect
of CCL20 after treatment with PTX could be impaired by siNotch1
(Fig. 4F). This evidence suggested
that the CCL20/CCR6 axis nay increase the stemness and PTX
resistance of CD44+CD117+ cells via the
Notch1 signaling pathway.
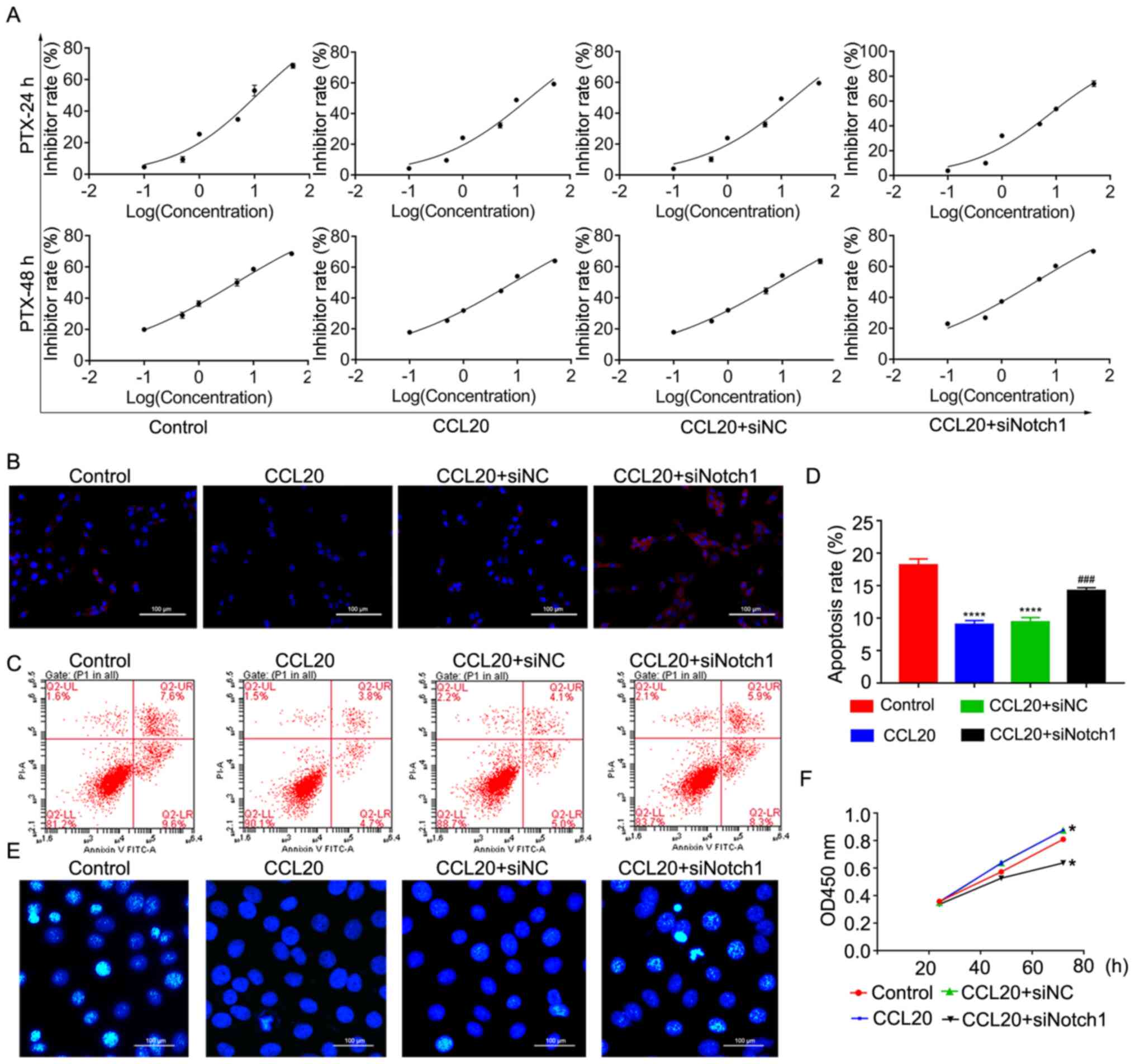 | Figure 4.siNotch1 reverses the effect of the
CCL20/CCR6 axis in enhancing the drug resistance of
CD44+CD117+ cells. (A) IC50 of
CD44+CD117+ cells with different treatments
of PTX at 24 and 48 h. (B) Reactive oxygen species levels of
CD44+CD117+ cells were detected using the
dichlorofluorescein diacetate method. Scale bar, 100 µm. (C)
Apoptosis of CD44+CD117+ cells, as determined
via flow cytometry. (D) Quantitative analysis of apoptosis, as
determined by flow cytometry. (E) Apoptosis of
CD44+CD117+ cells was detected using a
Hoechst assay. Scale bar, 100 µm. (F) Proliferation of
CD44+CD117+ cells was determined using a Cell
Counting Kit-8 assay. *P<0.05 vs. the control and CCL20+siNC
groups; ****P<0.0001 vs. control group; ###P<0.001
vs. CCL20 group. PTX, paclitaxel; OD, optical density; NC, negative
control; si, small interfering RNA; CCL20, C-C motif chemokine
ligand 20; Notch1, notch receptor 1. |
A CCL20/CCR6 axis promotes
tumorigenicity and PTX resistance of
CD44+CD117+ cells in ovarian cancer via the
Notch1 signaling pathway
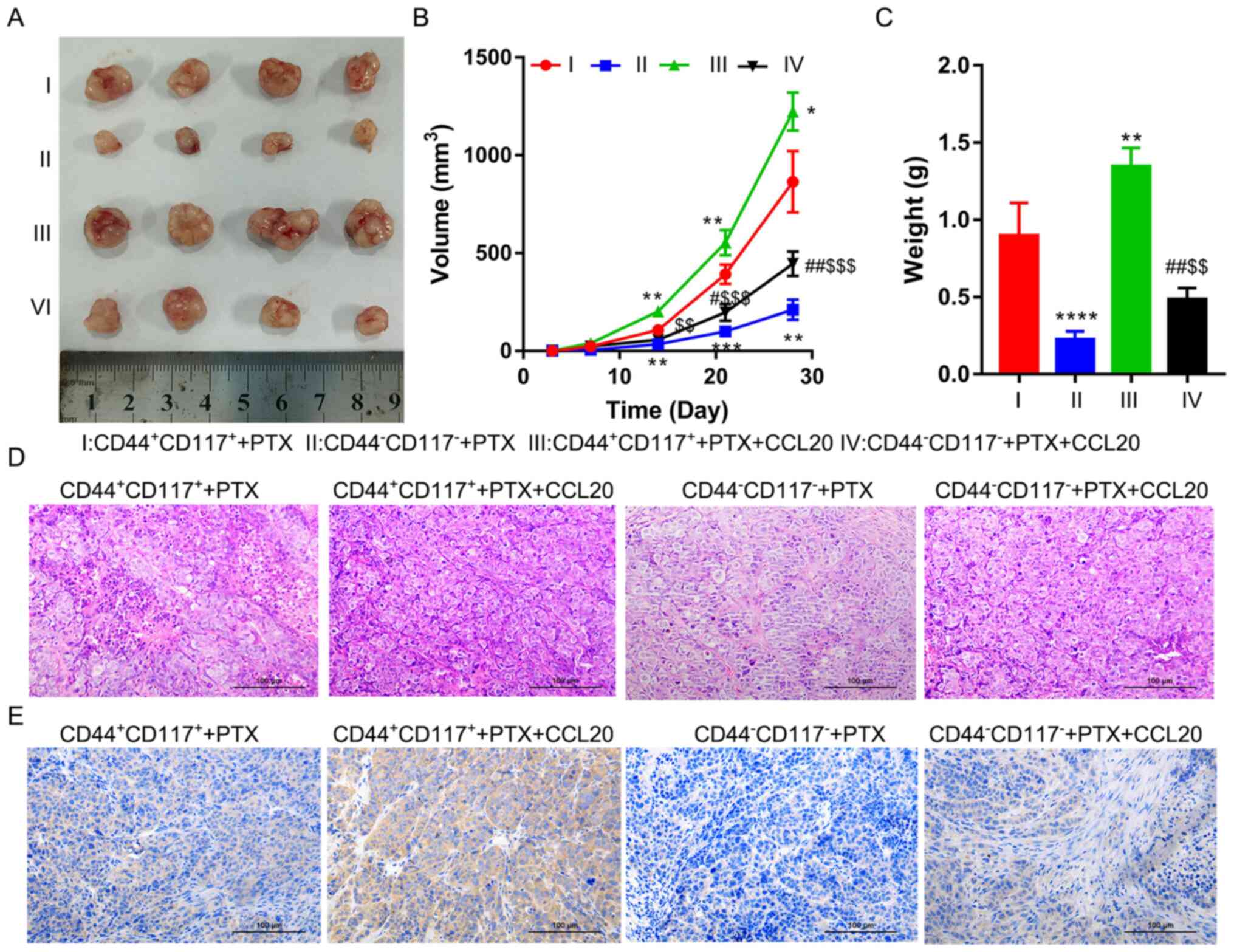
To confirm the role of the CCL20/CCR6 axis in PTX
resistance, SKOV3 CD44+CD117+ and
CD44−CD117− subgroup cells were subjected to
the xenograft assay, followed by CCL20 and/or PTX treatment. The
results demonstrated that CCL20 could significantly increase the
tumor volume of CD44+CD117+ and
CD44−CD117− cells in nude mice compared with
the respective PTX treatment alone groups (Fig. 5A and B). From the tumor growth curve
analysis, it was found that tumors formed by
CD44+CD117+ cells grew more quickly than
those formed by CD44−CD117− (P<0.001;
Fig. 5B). The growth of all tumors
could be significantly increased by treating with PTX, but the
volume of tumor-derived from CD44+CD117+
cells was significantly higher compared with that from
CD44−CD117− cells (P<0.01; Fig. 5B). Moreover, the weight of tumors
derived from CD44+CD117+ cells was
significantly greater than that derived from
CD44−CD117− cells treated with PTX
(P<0.0001; Fig. 5C). The tumor
weight was significantly increased by CCL20 treatment in
CD44+CD117+ and
CD44−CD117− cells treated with PTX. However,
tumor weight in the CD44+CD117+ group was
significantly higher than that in the
CD44−CD117− group (P<0.001; Fig. 5C).
H&E staining analysis tumors of the
CD44+CD117+ groups revealed poorly
differentiated morphology compared with the
CD44−CD117− groups, as characterized by the
number of small, round cells with hyperchromatic nuclei and scanty
cytoplasm, after treatment with PTX. CCL20 treatment could notably
decrease tumor differentiation in the
CD44+CD117+ group compared with in the
CD44−CD117− group (Fig. 5D). A higher expression level of CCR6
in the tumors was identified in the
CD44+CD117+ group compared with the
CD44−CD117− group via the IHC analysis.
Furthermore, CCL20 treatment could markedly increase the expression
level of CCR6 in tumors of the CD44+CD117+
group compared with in the CD44−CD117− group
(Fig. 5E).
As observed in further investigations, the
CD44+CD117+ group tissue presented with
higher proliferation but lower apoptosis compared with the tumor
tissue of the CD44−CD117− group after
treatment with PTX (Fig. 6A and B).
In the CD44+CD117+ and
CD44−CD117− groups, CCL20 treatment could
significantly accelerate proliferation but inhibit apoptosis after
treatment with PTX. However, these changes in the
CD44+CD117+ group were higher compared with
those in the CD44−CD117− group (Fig. 6A and B). Furthermore, ROS levels of
tumors in the CD44+CD117+ group were markedly
lower compared with those in the CD44−CD117−
group after treatment with PTX or CCL20 (Fig. 6C). The stemness and involved
signaling pathway were also determined in tumor tissue to further
confirm the underlying mechanism. The results indicated that the
expression levels of Oct4, CCR6, ABCG1 and Notch1 in tumor of
CD44+CD117+ and
CD44−CD117− groups could be significantly
accelerated by CCL20 treatment, while such facilitation was
increased in the CD44+CD117+ group compared
with the CD44−CD117− group (P<0.0001;
Fig. 6D and E). Taken together,
these findings suggested that CCL20/CCR6/Notch1 signaling could
markedly facilitate the stemness and proliferation of the
CD44+CD117+ subgroup cancer cells to promote
ovarian cancer resistance to PTX.
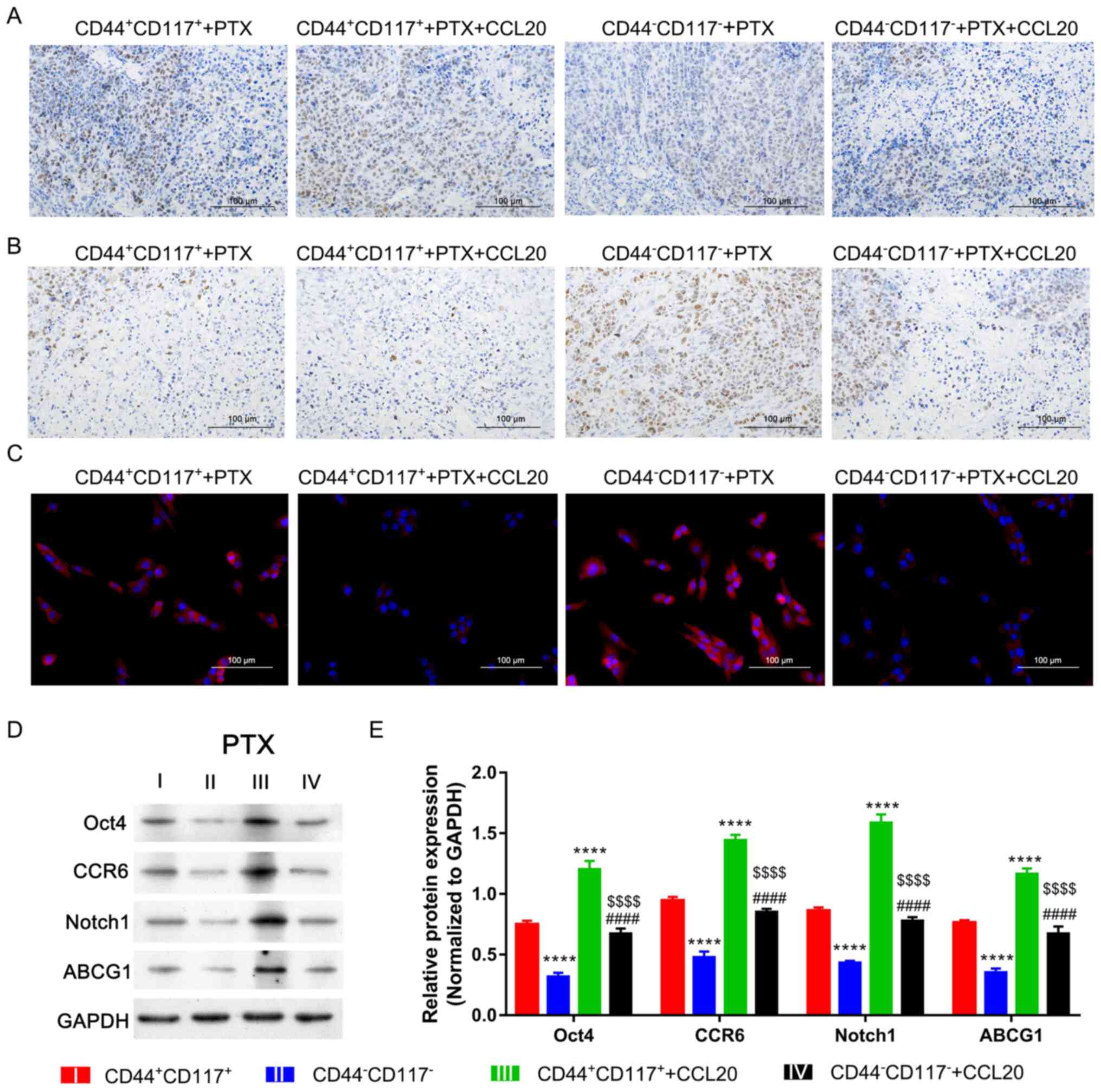 | Figure 6.CCL20/CCR6 enhances the stemness and
tumorigenicity of CD44+CD117+ cells treated
with PTX via the Notch1 signaling pathway. (A) Expression level of
Ki-67 in tumor was determined via immunohistochemistry. (B)
Apoptosis of tumor cells was determined using a TUNEL assay. (C)
Reactive oxygen species levels of tumors were determined by the
dichlorofluorescein diacetate method. Scale bar, 100 µm. (D)
Expression levels of Oct4, CCR6, Notch1 and ABCG1 in tumor tissue,
as determined via western blotting. (E) Semi-quantitative analysis
of Oct4, CCR6, Notch1 and ABCG1 protein expression. ****P<0.0001
vs. CD44+CD117+ group;
####P<0.0001 vs. CD44−CD117−
group; $$$$P<0.0001 vs.
CD44+CD117+ + CCL20 group. PTX, paclitaxel;
CCL20, C-C motif chemokine ligand 20; CCR6, CCR6, C-C motif
chemokine receptor 6; Notch1, notch receptor 1; ABCG1, ATP binding
cassette subfamily G member 1. |
Discussion
Cancer stem-like cells are widely accepted to be
responsible for drug resistance of ovarian cancer (21). Therefore, it is of great
significance to reveal the role of cancer stem-like cells in drug
resistance. It has been shown that two surface markers of cancer
cells in ovarian cancer are CD44 and CD117 (22). In the present study,
CD44+CD117+ subgroup cells were isolated from
SKOV3 cells and used for PTX resistance investigation.
Several studies have reported that CD44 and CD117
are two stem cell markers in ovarian cancer, and that
CD44+CD117+ cancer cells present with a
powerful survival ability and tumorigenic capability (23,24).
In ovarian cancer cells, the overexpression of miR-199a could
significantly inhibit CD44 expression and attenuate multidrug
resistance and tumorigenicity (25). Moreover, a clinical meta-analysis
revealed that a marker for the poor prognosis of ovarian cancer
could be the upregulation of CD117 (26). In the present study,
CD44+CD117+ cells were isolated from SKOV3
cells. IC50 analysis identified that
CD44+CD117+ cells had a higher
IC50 concentration of PTX compared with
CD44−CD117− cells, suggesting that
CD44+CD117+ cells showed a higher PTX
resistance. In addition, the expression levels of other cancer
stem-like cell markers, including Oct4 and ABCG1, were higher
compared with those in the CD44−CD117− cells,
indicating that CD44+CD117+ cells had a more
powerful stemness ability than CD44−CD117−
cells. After treatment with PTX in both in vitro and in
vivo, CD44+CD117+ cells also presented
higher sphere formation proliferation abilities, and lower
apoptosis and ROS levels. It is well known that oxidative stress is
directly assessed by measuring ROS. Moreover, ROS are involved in
the regulation of cell inventory and death (27). ROS is also reported to be closely
associated with inflammation, aging and chronic diseases, including
cancer (28). Collectively, the
present results suggested that CD44+CD117+
cells presented a powerful stemness and tumorigenic capability than
CD44−CD117− cells.
The only known chemokine ligand for CCR6 is CCL20,
and this axis has been reported to serve a critical role in the
development of numerous types of cancer, such as lung (29), colorectal (30) and thyroid cancers (31). In a recent study, cisplatin was
shown to stimulate macrophages to secrete CCL20 and promote ovarian
cancer cell migration via the CCL20/CCR6 axis (16). CCL20 can also enhance the
chemotherapy resistance of ovarian cancer by regulating ABCB1
expression (31). In the present
study, the expression levels of CCL20 and CCR6 were significantly
upregulated in CD44+CD117+ cells compared
with those in CD44−CD117− cells. Moreover,
CCL20 treatment could significantly increase the PTX resistance,
proliferation and tumorigenic abilities of
CD44+CD117+ cells, but decreased apoptosis
and ROS in vitro and in vivo.
Accumulating evidence has shown that the CCL20/CCR6
axis also served a critical role in regulating cancer stem cells
(15,32–34).
However, there are relatively limited associated reports on ovarian
cancer. In the current study, it was found that CCL20 treatment
could significantly increase the expression levels of stem cell
markers, including Oct4, and ABCG1. Previous studies have also
suggested that the CCL20/CCR6 axis may play a key role in
regulating ovarian cancer stem cells (16,35).
Collectively, these findings suggested that the CCL20/CCR6 axis may
act as a promotor in maintaining stemness and drug resistance of
ovarian cancer. As a ligand of CCR6, CCL20 promotes ovarian stem
cells and PTX resistance of ovarian stem cell-like cancer cells.
However, whether PTX resistance cancels the silencing of CCR6 needs
to be further examined.
A common dysregulated pathway in the development of
cancer is the Notch1 signaling pathway, which has been shown to
play a critical role in regulating stemness, proliferation,
metastasis and drug resistance of cancer (36–38).
As shown in a previous study, the hypoxia-induced Notch/SOX2 axis
was crucial for maintaining cancer stem-like cells in ovarian
cancer (39). It has also been
reported that galectin-3 could activate the intracellular domain of
Notch1 to maintain the stemness of ovarian cancer stem cells
(40). In the present study, it was
identified that siNotch1 could significantly decrease the
expression levels of Oct4, ABCG1 and CCR6 in
CD44+CD117+ cells treated with PTX.
Furthermore, the effects of CCL20 could be significantly reversed
by siNotch1 to enhance drug resistance and tumorigenic capability,
as well as reduce the apoptosis and ROS levels of
CD44+CD117+ cells treated with PTX in
vivo and in vitro. Such findings also suggested that the
CCL20/CCR6 axis may promote the stemness and tumorigenicity of
CD44+CD117+ cells by activating the Notch1
signaling pathway. However, the lack of research on the sensitivity
of CD44+CD117+ cells to platinum may be
considered a potential limitation of this study.
In conclusion, the present study demonstrated that
the CD44+CD117+ subgroup SKOV3 cells showed
increased stemness and drug resistance compared with
CD44−CD117− cells. The results indicated that
the stemness, tumorigenicity and PTX resistance of
CD44+CD117+ cells could significantly enhance
CCL20 by activating the Notch1 signaling pathway.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science
Foundation of Shandong Province (grant no. 2015GSF118140).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contribution
MC, YT and LZ conceived and designed the research.
MC, JS, CF and YL performed the experiments. MC, YT and LZ confirm
the authenticity of all the raw data. JS, CF and YL analyzed the
data and prepared the figures. MC and JS edited and revised the
manuscript. All authors approved the final version of the
manuscript.
Ethics approval and consent to
participate
The Animal Care and Use Committee of the Shandong
Provincial Hospital, Cheeloo College of Medicine, Shandong
University, authorized the present study. All methods were carried
out in accordance with relevant guidelines and regulations. The
animal study was carried out in compliance with the ARRIVE
guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vafadar A, Shabaninejad Z, Movahedpour A,
Fallahi F, Taghavipour M, Ghasemi Y, Akbari M, Shafiee A,
Hajighadimi S, Moradizarmehri S, et al: Quercetin and cancer: New
insights into its therapeutic effects on ovarian cancer cells. Cell
Biosci. 10:322020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Momenimovahed Z, Tiznobaik A, Taheri S and
Salehiniya H: Ovarian cancer in the world: Epidemiology and risk
factors. Int J Womens Health. 11:287–299. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stewart C, Ralyea C and Lockwood S:
Ovarian cancer: An integrated review. Semin Oncol Nurs. 35:151–156.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Norouzi-Barough L, Sarookhani MR, Sharifi
M, Moghbelinejad S, Jangjoo S and Salehi R: Molecular mechanisms of
drug resistance in ovarian cancer. J Cell Physiol. 233:4546–4562.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Damia G and Broggini M: Platinum
resistance in ovarian cancer: Role of DNA repair. Cancers (Basel).
11:1192019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pagotto A, Pilotto G, Mazzoldi EL,
Nicoletto MO, Frezzini S, Pastò A and Amadori A: Autophagy
inhibition reduces chemoresistance and tumorigenic potential of
human ovarian cancer stem cells. Cell Death Dis. 8:e29432017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pieterse Z, Amaya-Padilla MA, Singomat T,
Binju M, Madjid BD, Yu Y and Kaur P: Ovarian cancer stem cells and
their role in drug resistance. Int J Biochem Cell Biol.
106:117–126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klemba A, Purzycka-Olewiecka JK, Wcisło G,
Czarnecka AM, Lewicki S, Lesyng B, Szczylik C and Kieda C: Surface
markers of cancer stem-like cells of ovarian cancer and their
clinical relevance. Contemp Oncol (Pozn). 22:48–55. 2018.PubMed/NCBI
|
|
10
|
Chung H, Kim YH, Kwon M, Shin SJ, Kwon SH,
Cha SD and Cho CH: The effect of salinomycin on ovarian cancer
stem-like cells. Obstet Gynecol Sci. 59:261–268. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang S, Balch C, Chan MW, Lai HC, Matei
D, Schilder JM, Yan PS, Huang TH and Nephew KP: Identification and
characterization of ovarian cancer-initiating cells from primary
human tumors. Cancer Res. 68:4311–4320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wintzell M, Löfstedt L, Johansson J,
Pedersen AB, Fuxe J and Shoshan M: Repeated cisplatin treatment can
lead to a multiresistant tumor cell population with stem cell
features and sensitivity to 3-bromopyruvate. Cancer Biol Ther.
13:1454–1462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen W, Qin Y and Liu S: CCL20 signaling
in the tumor microenvironment. Adv Exp Med Biol. 1231:53–65. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su S, Sun X, Zhang Q, Zhang Z and Chen J:
CCL20 promotes ovarian cancer chemotherapy resistance by regulating
ABCB1 expression. Cell Struct Funct. 44:21–28. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kadomoto S, Izumi K and Mizokami A: The
CCL20-CCR6 axis in cancer progression. Int J Mol Sci. 21:51862020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu W, Wang W, Wang X, Xu C, Zhang N and
Di W: Cisplatin-stimulated macrophages promote ovarian cancer
migration via the CCL20-CCR6 axis. Cancer Lett. 472:59–69. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yi L, Zhou X, Li T, Liu P, Hai L, Tong L,
Ma H, Tao Z, Xie Y, Zhang C, et al: Notch1 signaling pathway
promotes invasion, self-renewal and growth of glioma initiating
cells via modulating chemokine system CXCL12/CXCR4. J Exp Clin
Cancer Res. 38:3392019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gharaibeh L, Elmadany N, Alwosaibai K and
Alshaer W: Notch1 in cancer therapy: Possible clinical implications
and challenges. Mol Pharmacol. 98:559–576. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sanchez-Martin M and Ferrando A: The
NOTCH1-MYC highway toward T-cell acute lymphoblastic leukemia.
Blood. 129:1124–1133. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo Y, Kishi S, Sasaki T, Ohmori H,
Fujiwara-Tani R, Mori S, Goto K, Nishiguchi Y, Mori T, Kawahara I,
et al: Targeting claudin-4 enhances chemosensitivity in breast
cancer. Cancer Sci. 111:1840–1850. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ning Y, Feng W, Cao X, Ren K, Quan M, Chen
A, Xu C, Qiu Y, Cao J, Li X and Luo X: Genistein inhibits stemness
of SKOV3 cells induced by macrophages co-cultured with ovarian
cancer stem-like cells through IL-8/STAT3 axis. J Exp Clin Cancer
Res. 38:192019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martincuks A, Li PC, Zhao Q, Zhang C, Li
YJ, Yu H and Rodriguez-Rodriguez L: CD44 in ovarian cancer
progression and therapy resistance-A critical role for STAT3. Front
Oncol. 10:5896012020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen J, Wang J, Chen D, Yang J, Yang C,
Zhang Y, Zhang H and Dou J: Evaluation of characteristics of
CD44+CD117+ ovarian cancer stem cells in
three dimensional basement membrane extract scaffold versus two
dimensional monocultures. BMC Cell Biol. 14:72013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang R, Zhang P, Wang H, Hou D, Li W,
Xiao G and Li C: Inhibitory effects of metformin at low
concentration on epithelial-mesenchymal transition of
CD44(+)CD117(+) ovarian cancer stem cells. Stem Cell Res Ther.
6:2622015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng W, Liu T, Wan X, Gao Y and Wang H:
MicroRNA-199a targets CD44 to suppress the tumorigenicity and
multidrug resistance of ovarian cancer-initiating cells. FEBS J.
279:2047–2059. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang B, Yan X, Liu L, Jiang C and Hou S:
Overexpression of the cancer stem cell marker CD117 predicts poor
prognosis in epithelial ovarian cancer patients: Evidence from
meta-analysis. Onco Targets Ther. 10:2951–2961. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kleih M, Böpple K, Dong M, Gaißler A,
Heine S, Olayioye MA, Aulitzky WE and Essmann F: Direct impact of
cisplatin on mitochondria induces ROS production that dictates cell
fate of ovarian cancer cells. Cell Death Dis. 10:8512019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moloney JN and Cotter TG: ROS signalling
in the biology of cancer. Semin Cell Dev Biol. 80:50–64. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang XP, Hu ZJ, Meng AH, Duan GC, Zhao QT
and Yang J: Role of CCL20/CCR6 and the ERK signaling pathway in
lung adenocarcinoma. Oncol Lett. 14:8183–8189. 2017.PubMed/NCBI
|
|
31
|
Frick VO, Rubie C, Keilholz U and Ghadjar
P: Chemokine/chemokine receptor pair CCL20/CCR6 in human colorectal
malignancy: An overview. World J Gastroenterol. 22:833–841. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zeng W, Chang H, Ma M and Li Y: CCL20/CCR6
promotes the invasion and migration of thyroid cancer cells via
NF-kappa B signaling-induced MMP-3 production. Exp Mol Pathol.
97:184–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thapa S and Bhusal K: SUN-545 A rare case
of resistance to thyroid hormone due to novel mutation in THRB gene
mistreated as hyperthyroidism. J Endocr Soc. 3 (Suppl 1):SUN–545.
2019. View Article : Google Scholar
|
|
34
|
Ranasinghe R and Eri R: Modulation of the
CCR6-CCL20 axis: A potential therapeutic target in inflammation and
cancer. Medicina (Kaunas). 54:882018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu W, Wang W, Zhang N and Di W: The role
of CCL20-CCR6 axis in ovarian cancer metastasis. Onco Targets Ther.
13:12739–12750. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang J, Yu K, Han X, Zhen L, Liu M, Zhang
X, Ren Y and Shi J: Paeoniflorin influences breast cancer cell
proliferation and invasion via inhibition of the Notch-1 signaling
pathway. Mol Med Rep. 17:1321–1325. 2018.PubMed/NCBI
|
|
37
|
Zhang Z, Han H, Rong Y, Zhu K, Zhu Z, Tang
Z, Xiong C and Tao J: Hypoxia potentiates gemcitabine-induced
stemness in pancreatic cancer cells through AKT/Notch1 signaling. J
Exp Clin Cancer Res. 37:2912018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang X, Wang R, Bai S, Xiong S, Li Y, Liu
M, Zhao Z, Wang Y, Zhao Y, Chen W, et al: Musashi2 contributes to
the maintenance of CD44v6+ liver cancer stem cells via notch1
signaling pathway. J Exp Clin Canc Res. 38:5052019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Seo EJ, Kim DK, Jang IH, Choi EJ, Shin SH,
Lee SI, Kwon SM, Kim KH, Suh DS and Kim JH: Hypoxia-NOTCH1-SOX2
signaling is important for maintaining cancer stem cells in ovarian
cancer. Oncotarget. 7:55624–55638. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kang HG, Kim DH, Kim SJ, Cho Y, Jung J,
Jang W and Chun KH: Galectin-3 supports stemness in ovarian cancer
stem cells by activation of the Notch1 intracellular domain.
Oncotarget. 7:682292016. View Article : Google Scholar : PubMed/NCBI
|