Ghrelin was first discovered in 1999 and was
described as an endogenous ligand of the growth hormone
secretagogue receptor (GHSR) (1).
Ghrelin is produced in the fundic area of the stomach, where it is
secreted directly into the bloodstream (2). Initially thought to regulate growth
hormone (GH) secretion, ghrelin has been shown to serve various
biological functions. A main role of ghrelin is in the gut-brain
interaction (3), with expression in
the hypothalamus and pituitary (4).
This hormone is also known to serve a key role in appetite
stimulation (5), gastric motility
and acid secretion (6), stress and
anxiety (7), and regulation of the
circadian rhythm (8). Ghrelin is
typically released when the stomach is empty, suggestive of its
involvement in endocrine regulation of motility-dependent
interdigestive gastric secretion (9).
Ghrelin has long been considered to be involved in
tumorigenic proliferation and although its precise role is still
uncertain, it has received increasing attention in research
regarding gastrointestinal (GI) neoplasia. The immunohistochemical
(IHC) expression of ghrelin has been documented in a number of
endocrine and non-endocrine tumors (10,11),
but it is still unclear whether an autocrine/paracrine loop or
another type of interaction is involved. At present, it has not yet
been reported if the expression of ghrelin and its receptors in
tumor cells are protective against neoplasia or whether they
stimulate carcinogenesis. Therefore, this review provides an
assessment of the current literature, presenting the emerging
trends and highlighting potential gaps in current knowledge
regarding the implications of ghrelin from its production to the
interaction with its known receptors (known as the ghrelin axis) in
digestive malignancies.
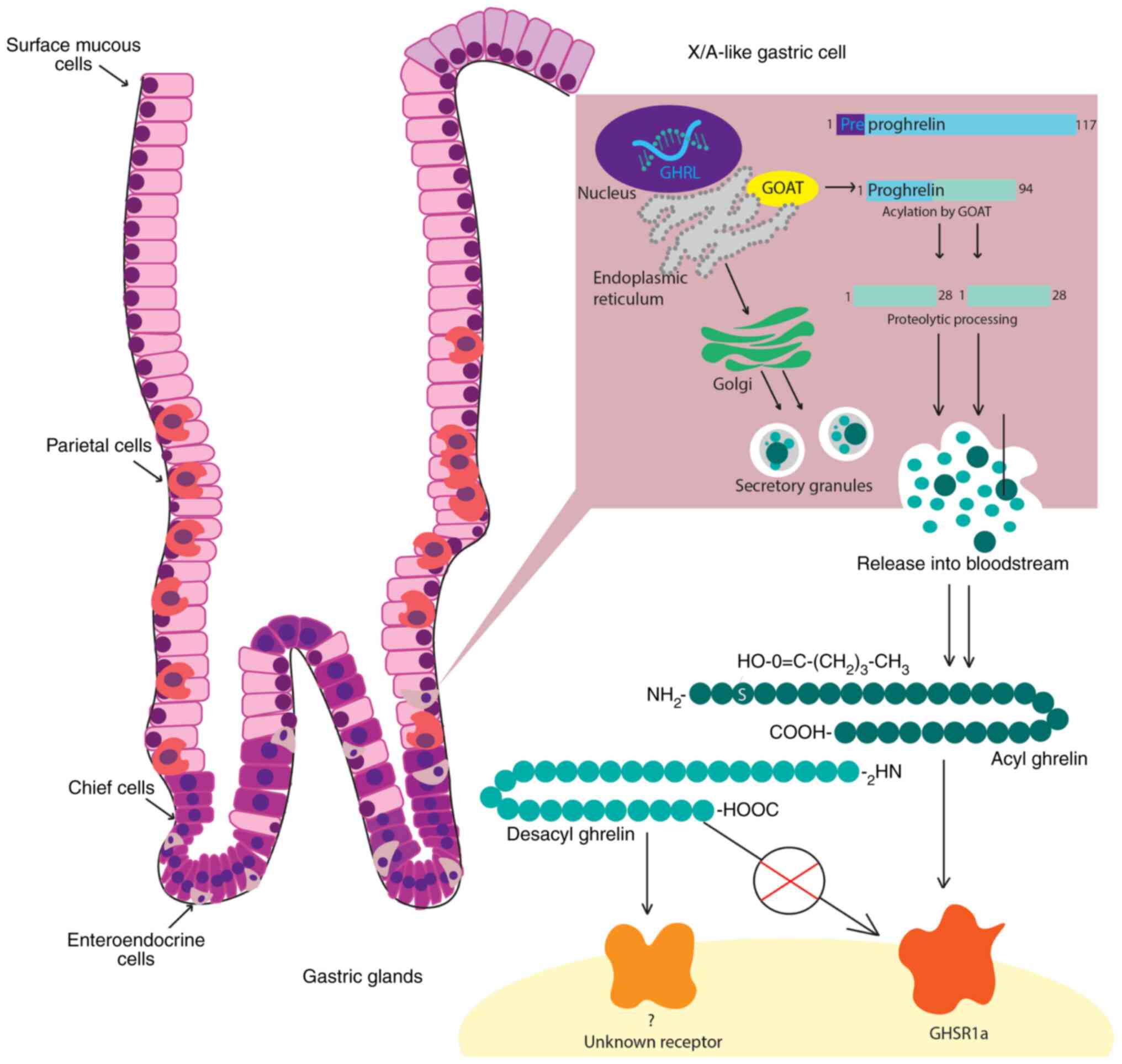
Genetic mapping has placed the ghrelin gene on the
short arm of chromosome 3 (3p25-26) (27), with an initial gene product known as
pre-proghrelin (29,30). Pre-proghrelin is a 117 amino acid
pre-protein, containing a 23-amino acid N-terminal signal peptide
that is cleaved to form the 94 amino acid peptide, proghrelin
(31). Proghrelin is then split by
a prohormone convertase to produce the 28 amino acid ghrelin
peptide and a 66 amino acid C-terminal fragment (known as
C-ghrelin), which gives rise to the hormone obestatin (32).
Acylated ghrelin, desacyl ghrelin and C-ghrelin,
have been found in ghrelin-secreting cells and in the circulation,
and have been previously described as peripheral forms of ghrelin
(33–36). Desacyl ghrelin shows a remarkable
predominance in peripheral blood (35,37),
mainly owing to it being the most secreted and more stable form of
ghrelin (34,38). Alternative ghrelin-related peptides
and mRNA splice variants were subsequently identified (31,39).
With multiple variants described to date, the intron 1
(In1)-ghrelin variant has an alternative C-terminal tail but
displays high areas of conservation to ghrelin that are essential
in the process of acylation and allow receptor binding (40). Ghrelin must be acylated with an
octanoyl group to activate its receptor, which is achieved by a
post-translational enzymatic change initiated by
ghrelin-o-acyltransferase (GOAT) (41). Although this process is necessary
for ghrelin to be highly physiologically active through its
receptor (42,43), certain studies also suggest a
metabolic role for desacyl ghrelin (44,45).
Acylated ghrelin preferentially binds to GHSR1a,
leading to GH secretion. Both the acylated and desacylated forms
have been identified as functionally active in local and systemic
processes, such as adipogenesis and lipid retention (52–55).
However, desacyl ghrelin does not bind to GHSR1a, suggesting its
effects are GHSR-independent, with its cognate receptor remaining
unknown (56,57). Desacyl ghrelin mainly exerts
nonendocrine activities, with involvement in vascular remodeling,
cardiovascular stability, muscle atrophy, cachexia and lipolysis
(38,58–62). A
schematic illustration of the main steps in the ghrelin circuit,
from production to interaction with its receptor, is presented in
Fig. 1.
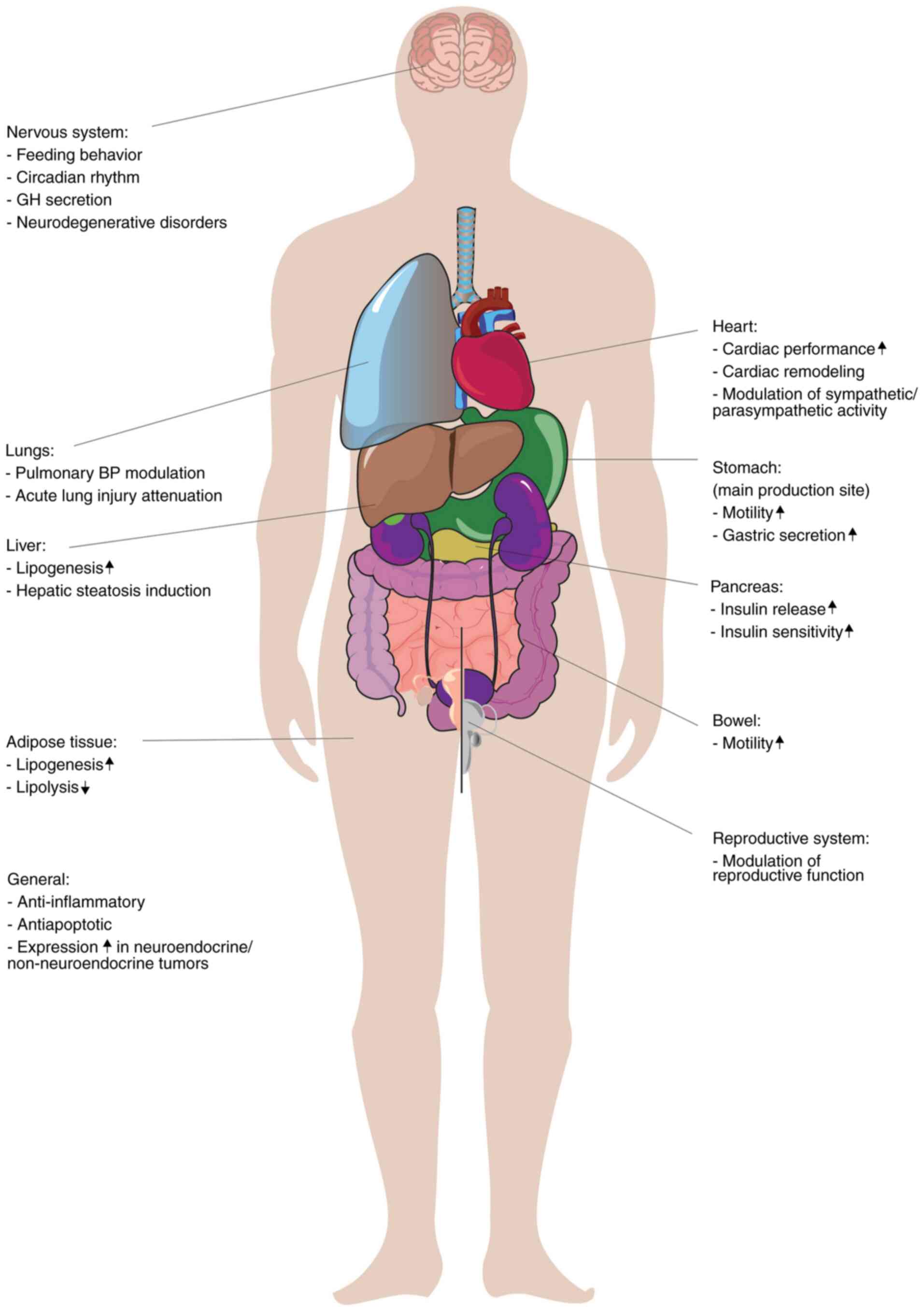
Ghrelin is a hormone involved in a number of
physiological processes outside of the GI tract; it is a key
participant in the interaction between the enteric and the central
nervous systems, with implications in physical, mental and
behavioral changes owing to the regulation of the circadian rhythm,
and stress and anxiety levels (3,7,60). In
the GI tract, ghrelin interacts with all organs, stimulating
gastric acid secretion, increasing GI motility (6) and modulating glucose levels through an
increase of pancreatic insulin release and through insulin
sensitivity (63). In the liver,
ghrelin promotes gluconeogenesis and lipogenesis (64,65),
the latter also being its main action in adipose tissue (66).
Even though the physiological roles of ghrelin are
yet to be fully revealed, it has been demonstrated that ghrelin
contributes to the pathology of a multitude of diseases. Its
orexigenic role is well established (5,67,68);
however, ghrelin also serves a function in inflammatory processes
(69). The presence of ghrelin and
its receptors on human leucocytes (T lymphocytes and macrophages)
was noted as early as 2004 (70),
with proven anti-inflammatory effects in both animal and human
studies, in acute (71–74) and chronic settings (75–78),
as well as in a neoplastic context (79). Ghrelin receptors have also been
identified in cardiac tissue (80),
where they modulate cardiac function and healing by influencing
myocardial contraction (81),
perfusion (82) and post-myocardial
infarction-changes (41,72,83).
In the lungs, ghrelin can attenuate pulmonary blood pressure
through vascular remodeling and mitigate the changes induced by
acute lung injury (84,85). Ghrelin also serves a role in the
intricate pathologies of anorexia and cachexia (86,87) as
well as neurodegenerative disorders, such as dementia (88), multiple sclerosis (89) and amyotrophic lateral sclerosis
(90). The pleiotropic effects of
the ghrelin axis are summarized in Fig.
2.
Present in both endocrine and non-endocrine tumors,
such as breast, prostate, testicular, GI, pancreatic, renal,
thyroid, lung and adrenal tumors (11,91–94),
ghrelin and its receptors may give rise to a complex set of
interactions that contribute to neoplastic development at various
stages. Although the precise role of ghrelin is still uncertain,
numerous studies have used different experimental approaches to
document the involvement of the ghrelin axis in neoplasia.
Experimental studies have investigated the
connection between ghrelin and neoplasia, in attempts to uncover a
novel prognostic factor or to find missing links in the processes
of tumorigenesis, invasion and metastasis. The majority of in
vitro studies have focused on ghrelin and GHSR splice variants
expression in various tumor cell lines, investigating the effects
of ghrelin on tumor development. A summary of these findings
regarding GI tumor cells and highlighting the diversity of results
published on this topic can be found in Table I. Ghrelin has also been reported to
be expressed in experimental studies using cell lines of leukemia
(95), breast carcinoma (96), pulmonary adenocarcinoma (97), pancreatic ductal adenocarcinoma
(98), prostate carcinoma (99), gastric adenocarcinoma (100) and colorectal adenocarcinoma
(101,102). and it has shown pleiotropic
effects in the vast majority of these tumor cell lines (11).
Ghrelin expression is less variable compared with
the expression of its receptors, which is highly variable between
different types of cancer. GHSR1a and GHSR1b are most often
co-expressed, with GHSR1a expression levels demonstrated to be
higher compared with those for GHSR1b in oral squamous cell
carcinoma (SCC) and gastric cancer (GC) cell lines (100,103). For breast cancer cell lines, an
initial study reported that lines MDA-MB-231, MCF-7, MDA-MB-435 and
T47D have positive IHC expression of the ghrelin/GHSR axis
(27), whereas GHSR1a mRNA could
not be detected in lines MDA-MB231, MCF7 and T47D, in spite of
specific binding of ghrelin in these cells, implying that an
unidentified interaction or binding site modulates these effects
(96). High GHSR1b expression was
identified in breast cancer cell line MDA-MB-231 (39). A similar difference in expression
was demonstrated in colon cancer cell lines (101).
The effects of ghrelin can vary according to the
type of cancer. Previous studies have found ghrelin can have a
proliferative effect on a number of cancer cell lines including
breast (27), prostate (28), gastric (100), colorectal (101,102,104), oral (103), pancreatic (98), adrenocortical (105) and endometrial (106). Conversely, D-Lys-growth hormone
releasing peptide 6 or other ghrelin-specific antibodies can
antagonize ghrelin and inhibit this proliferative effect (101). Certain studies attribute this
proliferative effect to the desacyl form (27,91,95),
suggesting that in these cell lines ghrelin can stimulate cell
proliferation via an autocrine pathway independent of GHSR1a
(95). However, ghrelin has also
been shown to inhibit proliferation and exhibit an antiapoptotic
effect in cell lines of the same cancer type, as is the case for
breast carcinoma cell lines MCF7 and MDA-MB231 (96) and prostate carcinoma cell lines PC-3
and DU-145 (91). Both acylated and
deacylated ghrelin inhibit DU-145 cell proliferation (91,99,107).
A recent study on chemosensitive ovarian cells demonstrated that
acylated ghrelin promotes cell proliferation and survival, and
inhibit apoptosis through its interaction with GHRS1a via the
PI3K/Akt signaling pathway, thereby rendering the cells resistant
to chemotherapy, even at very low levels (108).
The ghrelin splice variant, In1-ghrelin, can
increase the proliferation rate of the MDA-MB-231 breast cancer
cell line (39) and stimulate
proliferation and migration of MCF-7 and MDA-MB-231 cells (109). Expression of this variant was also
found in pancreatic adenocarcinoma cell lines, which do not express
ghrelin, wherein it enhanced the proliferation and migration of
tumor cells (110).
To better document and understand these effects, the
activation pathway of ghrelin has been examined extensively, and
numerous pathway activation mechanisms have been identified.
Through the interaction of ghrelin and GHRS the PI3-K/Akt/mTOR
signaling pathway is able to mediate the migration and invasiveness
of pancreatic adenocarcinoma cells (98). One study demonstrated that NF-κB
signaling pathway activation can contribute to ghrelin-induced cell
migration in glioma cells (111).
Furthermore, Lin et al (94)
determined ghrelin has diverse implications in tumorigenesis and
tumor spread, with the ability to promote metastasis in the case of
renal cell carcinoma (RCC) via Snail, a transcriptional repressor
of E-cadherin, and invasion through the upregulation of the kinase
Aurora A, an activating mechanism which was also associated with a
poor prognosis (112). Similarly,
invasion in GC was determined to be a result of GHSR/NF-κB
signaling pathway activation (100).
In the esophagus, expression of ghrelin was first
demonstrated in SCC by IHC, with tissue ghrelin levels correlating
with degree of differentiation, depth of tumor invasion,
lymphovascular invasion and tumor stage (115). However, ghrelin expression levels
showed no correlation with patient survival (115). IHC detection of ghrelin expression
in esophageal adenocarcinoma was undetectable (116). Ghrelin serum levels have an
inverse relationship with the risk of developing esophageal
malignancies, especially SCC (93,117),
and patients with a low ghrelin level are seven-times more likely
to develop this histological subtype (117). This unexpected relationship
between ghrelin levels and the risk of malignancy has been
validated in a larger cohort study (118).
Compared with esophageal SCC, ghrelin serum levels
did not correlate with the risk of developing esophageal
adenocarcinoma (93). High serum
concentrations of ghrelin (>3,200 pg/ml) were associated with a
lower risk of developing esophageal adenocarcinoma in overweight
patients with a body mass index (BMI) >25 (119). With well-known orexigenic effects,
ghrelin may prove to be the basis for the association between
abdominal obesity and Barrett's esophagus (BE) (120). A case-control study involving 886
patients with a diagnosis of BE demonstrated that higher ghrelin
serum concentrations were positively associated with an increased
risk of BE, irrespective of Helicobacter pylori infection
and BMI (120). A positive
association between ghrelin and BE development was also supported
in another study (121).
Upregulated expression of GHSR in BE compared with normal
esophageal mucosa has also been reported (122). However, no correlation between
ghrelin levels and the risk of BE was found in a recent
meta-analysis compared with other adipokines, but only two studies
approaching this interaction were considered (123).
Considering the various factors that influence
ghrelin production, recent studies have looked at digestive tract
malignancies from a broader perspective. For example, serum levels
of ghrelin are correlated with nutritional status (124), with similar levels of active
(acylated) and desacyl ghrelin in a balanced (well-nourished vs.
malnourished) population of patients with esophageal cancer; it has
been argued that active ghrelin is positively correlated with
energy metabolism. Together, with a positive correlation with IL-6
levels, these findings may suggest the existence of a form of
ghrelin resistance (125). Ghrelin
serum levels also correlate with inflammatory responses and high
levels have been observed in a high-inflammation group of patients
with GI neoplasms (124), a
finding which could be further explored and integrated into the
context of inflammation as a prognostic factor for these
malignancies (126,127).
Ghrelin axis expression determined by IHC
examination shows undetectable levels in gastric adenocarcinoma
(116). However, previous studies
have a limited number of patients which may make it difficult to
draw conclusions. In a study of 10 patients, none tested
ghrelin-positive (116), these
findings were supported by a similar study analyzing 9 patients
with gastric adenocarcinoma (128).
The fluctuation of ghrelin levels over time
highlights the potential role of this peptide as a biomarker in
gastric malignancies (118). A
study on a small cohort of Korean patients with GC showed a 10-fold
increase in plasma ghrelin levels of these patients (129). However, ghrelin levels of the
tumor tissue were lower compared with those of the normal gastric
mucosa, suggesting that gastric tumorigenesis can inhibit ghrelin
production in the adjacent mucosa (129). Furthermore, certain studies have
demonstrated a significant increase in the risk of non-cardia GC
and esophago-gastric junction cancer in individuals with lower
baseline serum ghrelin concentrations, changes which occur early in
the carcinogenic process (93,130).
The risk of these diseases remains increased, irrespective of H.
pylori infection (130).
Ghrelin levels can fluctuate depending upon lesions
associated with the gastric mucosae with certain studies indicating
a reduction in ghrelin plasma levels in cases of H. pylori
infection, which steadily increase following the eradication of
infection (129,131,132). In addition, a histologically
higher degree of gastric atrophy seems to correlate with a lower
plasma ghrelin concentration (132).
Different surgical approaches seem to radically
influence ghrelin levels in patients with GC. Ghrelin levels were
higher in patients who had a distal gastrectomy compared with those
who had a total gastrectomy, and the physiological regulation of
ghrelin secretion and plasma levels was unaffected due to
preservation of the gastric fundus (133). In patients with proximal
resection, postoperative ghrelin increased more slowly compared
with that in patients after fundus-preserving resection (134). Takachi et al (135) demonstrated that there was a
significant decrease in the concentration of ghrelin following
total gastrectomy, with very low levels observed in the long-term
follow-up. Furthermore, Zub-Pokrowiecka et al (133) demonstrated that plasma levels of
ghrelin were lower in patients with GC and in those who formerly
had GC and undergone surgery 4–5 years previously, compared with
the healthy control group.
Ghrelin alone may not be a useful biomarker in
evaluating the risk of gastric malignancies, but when coupled with
other early detection biomarkers, such as pepsinogen I and
pepsinogen I/II ratio, as part of a complex panel it may provide
higher accuracy (118). Recent
advances in the field point towards the potential role of the
ghrelin gene, growth hormone secretagogue receptor ligand (GHRL),
as a poor prognosis biomarker in GC (136). A study involving 295 patients with
GC and data from 4 gene expression microarrays, found 12
upregulated and 59 downregulated differentially expressed genes,
with high expression of GHRL associated with poor overall survival
of patients with GC (136).
The interaction between ghrelin and its receptors,
as demonstrated through IHC and molecular studies, is indicative of
the presence of an autocrine/paracrine mechanism involved in
colorectal carcinogenesis (101,137). In CRC, the ghrelin axis serves a
role in the initial stages of carcinogenesis, with positive
expression of ghrelin and its receptors in low-grade tumors, as
opposed to almost complete loss of expression in high-grade tumors
(101). Axis upregulation has also
been demonstrated by Liu et al (138) in well-differentiated and
moderately differentiated adenocarcinomas, whereby the role of
promoting cell growth in CRC was attributed to the interaction of
ghrelin with the GHSR1a receptor. However, in spite of this Waseem
et al (101) demonstrated
that the expression levels of ghrelin and its orphan GHSR1b
receptor were increased in patients with CRC, contrary to the
decrease of GHSR1a with advancing tumor stage. Although tissue
expression of ghrelin seems to have a positive correlation with
tumor stage, a study on 110 patients with CRC failed to correlate
ghrelin plasma levels with any CRC clinicopathological features
(101).
Increased tissue expression of ghrelin and its
receptors in CRC has initiated the idea of its role as a potential
biomarker. However, even though this research area has attracted
the interest of numerous research groups it is not without
controversy. An initial study on a small sample of 20 patients
found no difference in circulating ghrelin levels in patients with
CRC compared with a control group and no correlation with tumor
clinicopathological characteristics (139). A similar study on a group of 29
patients with lower GI tract malignancies, found a statistically
significant difference in ghrelin levels between patients with CRC
and the control group and noticed a decrease in ghrelin levels with
tumor progression (140). This
study therefore advanced the idea of ghrelin serum levels as being
inversely correlated with tumor aggressiveness and their potential
use as a prognostic parameter (140).
The role of circulating ghrelin in the evaluation of
patients with CRC was demonstrated by Nikolopoulos et al
(141), indicating a significant
positive correlation between ghrelin levels and tumor size. Ghrelin
plasma levels were increased in end-stage disease and were
correlated with the degree of differentiation, being higher in
poorly differentiated CRC. However, no significance was detected in
using ghrelin as a predictor of CRC survival (141). A similar study found no difference
in ghrelin peripheral blood expression between patients with CRC
compared with the control group (142). Perioperative serum levels were
reported by Zhu et al (124) as being higher in patients with
cancer compared with patients in the control group, a finding that
contrasted with previous studies (139). The aforementioned study also noted
a dramatic decrease of ghrelin levels following surgical removal of
the tumors, advancing the possibility of a new early-warning marker
of CRC and other GI tract malignancies (124).
A large case-control study published in 2018
demonstrated a positive correlation between decreased levels of
ghrelin and the risk of developing CRC, spanning over a 10-year
period (143). Furthermore, a
validation study comparing plasma ghrelin levels in 60 patients
with CRC in a perioperative context and a 5-year interval preceding
the diagnosis of CRC prior to surgery, demonstrated that ghrelin
was not associated with an increased risk of malignancy, with
ghrelin levels remaining stable over time (144).
Current data indicates that there are no significant
changes in GHRL methylation levels (145). Furthermore, GHRL methylation is
unlikely to become a biomarker of CRC, as no significant
differences in hypermethylation of GHSR were observed in CRC
compared with normal mucosa (145). This process is present in
adenomas, irrespective of the degree of dysplasia, and results have
not demonstrated any increase in hypermethylation correlated with
tumor progression (145).
As in the case of esophageal cancer, it has been
suggested that ghrelin plasma levels should not be interpreted in
the absence of nutritional status and BMI (146). It is well documented that ghrelin
levels increase in the case of cancer cachexia and decrease with
obesity (147).
Expression of ghrelin has been detected in NETs of
gastropancreatic origin. Rindi et al (148) detected varying levels of ghrelin
expression in digestive NETs, particularly in gastric tumors; these
findings were supported by prior detection of ghrelin mRNA and were
reported in other studies (11,149).
A study investigating NETs of the GI tract in patients with
multiple endocrine neoplasia type 1, determined that only 25% of
cases were reported as ghrelin-positive and no clinicopathological
correlation was established (150). The proportion of ghrelin-positive
cells varied between 1 and 20% (148,150).
Pancreatic NETs express ghrelin in up to 95% of
cases, validated by quantitative PCR (qPCR), whereas up to 67% of
cases can be detected by IHC, with some tumors having only a small
proportion of cells staining positively and others with >80%
positive cells (148,151,152). Ghrelin expression has been
documented in both functioning and non-functioning tumors of the
pancreas (62 vs. 69% of cases, respectively) (153). Papotti et al (149) demonstrated ghrelin expression in
intestinal NETs; however, others did not find any ghrelin-positive
intestinal NET cells (148,153).
Furthermore, the expression of ghrelin in neuroendocrine carcinoma
was initially reported to be absent (148), whereas subsequent studies found
ghrelin expression in scattered cells or focally in up to 10% of
the tumor cells, which was associated with hyperghrelinemia
(154,155).
Plasma ghrelin levels in patients with
gastroenteropancreatic NETs were initially demonstrated to not be
significantly different between patients with tumors and healthy
controls, neither initially, nor during progression of the disease
(153,156). A small subset of patients had high
plasma ghrelin levels (>1280 ng/dl); however, two out of five
such patients had tumors that had complete lack of ghrelin protein
expression (153). In patients
with metastatic disease, the levels of ghrelin were elevated,
compared with the control group, in up to 85% of cases (157). A later study focusing on the
active peripheral forms of ghrelin (acylated ghrelin; desacyl
ghrelin; acylated ghrelin/desacyl ghrelin ratio) found no
statistical difference between patients with NETs and the control
group (158).
Although they are more rare than epithelial tumors,
mesenchymal tumors of the GI tract contribute to the overall cancer
burden of the region and may exhibit variable clinical behavior
(161,162). Among the most frequent are GI
stromal tumors (GISTs) (161).
Ghrelin expression in GISTs has only been reported by a single
study, which found both IHC and qPCR-detectable expression, with
77% of analyzed tumors showing immunoreactivity for ghrelin and all
tumors displaying detectable levels of ghrelin mRNA, albeit this
analysis was performed on only about a third of the 22 cases
(151). However, the study did not
find any statistical correlation between ghrelin levels and tumor
location, size, morphological parameters or clinical behavior
(151), the value of these results
being reiterated by another recent paper recognizing the potential
role of the ghrelin axis in GISTs and supporting further research
on this topic (163). Studies of
large patient cohorts need to be implemented to better understand
the role of the ghrelin axis in the pathogenesis of such tumors.
However, limitations arise as a result of their low incidence and
variability.
As previously mentioned, ghrelin and its receptors
are expressed in numerous endocrine and non-endocrine tumor cell
types, such as pituitary, prostate, breast and lung (10,96,99,164).
Since ghrelin acts not only as a local, but also as a systemic
hormone, its potential role in cancer development in other organs
has been investigated.
Oral SCC demonstrates the expression of the ghrelin
axis, with ghrelin levels being negatively correlated with tumor
invasiveness (165). Furthermore,
decreasing ghrelin levels appear to be correlated with lower
degrees of differentiation, indicating the potential value of
tissue ghrelin expression as a prognostic marker (165). Another study demonstrated that
expression of ghrelin and GHSR were gradually increased in oral
tumors as benign cells developed cytological and architectural
features of dysplasia and further progressed to becoming malignant
(166).
An initial study on kidney tumors reported low to
absent expression of ghrelin in RCC, with all 21 cases of
conventional-subtype analyzed having no IHC ghrelin expression and
partial-to-total loss of ghrelin expression when assessed through
radioimmunoassays (167). However,
certain kidney tumors, such as oncocytomas, did express ghrelin,
albeit less than normal kidney tissue (167). Furthermore, Lin et al
(94) assessed ghrelin expression
levels in a cohort of 562 clear-cell type RCC cases and
demonstrated that ghrelin levels were high and indicated a poor
prognosis, with high ghrelin expression being associated with lymph
node and distant metastases.
Ghrelin may also serve a role in endometrial
carcinogenesis through coupling with its GHSR1a receptor, with
hormone and receptor expression detectable in benign and malignant
tissue specimens (171).
Expression has also been identified in various histological
subtypes of lung cancer, accompanied by the expression of GHSR1a
(172,173).
The role of ghrelin in GI tract malignancies is
still incompletely characterized. The complexity of the ghrelin
axis and the interaction between this hormone and cancer cells is
still unclear. Studies on cancer cell lines show a spectrum of
ghrelin and ghrelin receptor expression, and new splice variants of
ghrelin involved in carcinogenesis are emerging. The role of IHC to
detect expression is unclear, as most papers do not focus on tumor
histological subtype or other factors such as tumor heterogeneity.
The use of ghrelin alone as a potential serological biomarker is
debatable, as it is regulated by various metabolic factors and is
linked with cancer-associated inflammation. Although certain of the
effects of ghrelin can be quantified, there are still large
discrepancies in this field owing to the variety of peripheral,
circulating forms of ghrelin incompletely matched with a
corresponding receptor. Further investigation is required to
identify a potential desacyl ghrelin receptor that will facilitate
the accurate identification of sites of action for this peptide and
to elucidate its underlying mechanisms of action. The present
review has demonstrated that our current understanding of the
ghrelin system does not provide sufficient evidence to justify its
role as a useful biomarker in GI malignancies. Therefore, large
cohort studies with well-defined exclusion criteria and a complex
framework, factoring in plasma levels, tissue expression and
genetic alterations, are needed to establish direct correlations
between ghrelin levels and GI malignancies.
Not applicable.
No funding was received.
Not applicable.
IAS conceived the paper, designed the overall
concept and created the figures. IDC supervised the project. IAS,
IDC, DGAC and SEG contributed equally to collecting the data and in
editing and shaping the manuscript. Data authentication is not
applicable. All authors read and approved the final version of the
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Kojima M, Hosoda H, Date Y, Nakazato M,
Matsuo H and Kangawa K: Ghrelin is a growth-hormone-releasing
acylated peptide from stomach. Nature. 402:656–660. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stengel A, Goebel M, Wang L and Taché Y:
Ghrelin, des-acyl ghrelin and nesfatin-1 in gastric X/A-like cells:
Role as regulators of food intake and body weight. Peptides.
31:357–369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davis EA, Wald HS, Suarez AN, Zubcevic J,
Liu CM, Cortella AM, Kamitakahara AK, Polson JW, Arnold M, Grill
HJ, et al: Ghrelin signaling affects feeding behavior, metabolism,
and memory through the vagus nerve. Curr Biol. 30:4510–4518.e6.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cowley MA, Smith RG, Diano S, Tschöp M,
Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M,
Heiman ML, et al: The distribution and mechanism of action of
ghrelin in the CNS demonstrates a novel hypothalamic circuit
regulating energy homeostasis. Neuron. 37:649–661. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wren AM, Seal LJ, Cohen MA, Brynes AE,
Frost GS, Murphy KG, Dhillo WS, Ghatei MA and Bloom SR: Ghrelin
enhances appetite and increases food intake in humans. J Clin
Endocrinol Metab. 86:59922001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Masuda Y, Tanaka T, Inomata N, Ohnuma N,
Tanaka S, Itoh Z, Hosoda H, Kojima M and Kangawa K: Ghrelin
stimulates gastric acid secretion and motility in rats. Biochem
Biophys Res Commun. 276:905–908. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carlini VP, Monzón ME, Varas MM,
Cragnolini AB, Schiöth HB, Scimonelli TN and de Barioglio SR:
Ghrelin increases anxiety-like behavior and memory retention in
rats. Biochem Biophys Res Commun. 299:739–743. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Steiger A: Ghrelin and sleep-wake
regulation. Am J Physiol Regul Integr Comp Physiol. 292:R573–R574.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Engevik AC, Kaji I and Goldenring JR: The
physiology of the gastric parietal cell. Physiol Rev. 100:573–602.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Korbonits M, Kojima M, Kangawa K and
Grossman AB: Presence of ghrelin in normal and adenomatous human
pituitary. Endocrine. 14:101–104. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Papotti M, Duregon E and Volante M:
Ghrelin and tumors. Endocr Dev. 25:122–134. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kojima M and Kangawa K: Ghrelin: Structure
and function. Physiol Rev. 85:495–522. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fakhry J, Stebbing MJ, Hunne B, Bayguinov
Y, Ward SM, Sasse KC, Callaghan B, McQuade RM and Furness JB:
Relationships of endocrine cells to each other and to other cell
types in the human gastric fundus and corpus. Cell Tissue Res.
376:37–49. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gribble FM and Reimann F: Enteroendocrine
cells: Chemosensors in the intestinal epithelium. Annu Rev Physiol.
78:277–299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi E, Roland JT, Barlow BJ, O'Neal R,
Rich AE, Nam KT, Shi C and Goldenring JR: Cell lineage distribution
atlas of the human stomach reveals heterogeneous gland populations
in the gastric antrum. Gut. 63:1711–1720. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sykaras AG, Demenis C, Cheng L, Pisitkun
T, Mclaughlin JT, Fenton RA and Smith CP: Duodenal CCK cells from
male mice express multiple hormones including ghrelin.
Endocrinology. 155:3339–3351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reynaud Y, Fakhry J, Fothergill L,
Callaghan B, Ringuet M, Hunne B, Bravo DM and Furness JB: The
chemical coding of 5-hydroxytryptamine containing enteroendocrine
cells in the mouse gastrointestinal tract. Cell Tissue Res.
364:489–449. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fothergill LJ, Callaghan B, Hunne B, Bravo
DM and Furness JB: Costorage of enteroendocrine hormones evaluated
at the cell and subcellular levels in male mice. Endocrinology.
158:2113–2123. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Glass LL, Calero-Nieto FJ, Jawaid W,
Larraufie P, Kay RG, Göttgens B, Reimann F and Gribble FM:
Single-cell RNA-sequencing reveals a distinct population of
proglucagon-expressing cells specific to the mouse upper small
intestine. Mol Metab. 6:1296–1303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fothergill LJ and Furness JB: Diversity of
enteroendocrine cells investigated at cellular and subcellular
levels: The need for a new classification scheme. Histochem Cell
Biol. 150:693–702. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grönberg M, Tsolakis AV, Magnusson L,
Janson ET and Saras J: Distribution of obestatin and ghrelin in
human tissues: Immunoreactive cells in the gastrointestinal tract,
pancreas, and mammary glands. J Histochem Cytochem. 56:793–801.
2008. View Article : Google Scholar
|
|
22
|
Zhao Z and Sakai T: Characteristic
features of ghrelin cells in the gastrointestinal tract and the
regulation of stomach ghrelin expression and production. World J
Gastroenterol. 14:6306–6311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Teive MB, Russi RF, Vieira DS, Teive AM,
Costa A and d'Acampora AJ: Quantitative immunohistochemical
analysis of duodenal ghrelin cells after sleeve gastrectomy in
Wistar rats. Acta Cir Bras. 27:595–599. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mehdar KM: The distribution of ghrelin
cells in the human and animal gastrointestinal tract: A review of
the evidence. Folia Morphol (Warsz). 80:225–236. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gnanapavan S, Kola B, Bustin SA, Morris
DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB
and Korbonits M: The tissue distribution of the mRNA of ghrelin and
subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol
Metab. 87:29882002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Santos M, Bastos P, Gonzaga S, Roriz JM,
Baptista MJ, Nogueira-Silva C, Melo-Rocha G, Henriques-Coelho T,
Roncon-Albuquerque R Jr, Leite-Moreira AF, et al: Ghrelin
expression in human and rat fetal lungs and the effect of ghrelin
administration in nitrofen-induced congenital diaphragmatic hernia.
Pediatr Res. 59:531–537. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jeffery PL, Murray RE, Yeh AH, McNamara
JF, Duncan RP, Francis GD, Herington AC and Chopin LK: Expression
and function of the ghrelin axis, including a novel preproghrelin
isoform, in human breast cancer tissues and cell lines. Endocr
Relat Cancer. 12:839–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yeh AH, Jeffery PL, Duncan RP, Herington
AC and Chopin LK: Ghrelin and a novel preproghrelin isoform are
highly expressed in prostate cancer and ghrelin activates
mitogen-activated protein kinase in prostate cancer. Clin Cancer
Res. 11:8295–8303. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sato T, Nakamura Y, Shiimura Y, Ohgusu H,
Kangawa K and Kojima M: Structure, regulation and function of
ghrelin. J Biochem. 151:119–128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang JV, Ren PG, Avsian-Kretchmer O, Luo
CW, Rauch R, Klein C and Hsueh AJ: Obestatin, a peptide encoded by
the ghrelin gene, opposes ghrelin's effects on food intake.
Science. 310:996–999. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nishi Y, Yoh J, Hiejima H and Kojima M:
Structures and molecular forms of the ghrelin-family peptides.
Peptides. 32:2175–2182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hosoda H, Kojima M, Mizushima T, Shimizu S
and Kangawa K: Structural divergence of human ghrelin.
Identification of multiple ghrelin-derived molecules produced by
post-translational processing. J Biol Chem. 278:64–70. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pemberton C, Wimalasena P, Yandle T, Soule
S and Richards M: C-terminal pro-ghrelin peptides are present in
the human circulation. Biochem Biophys Res Commun. 310:567–573.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hosoda H, Doi K, Nagaya N, Okumura H,
Nakagawa E, Enomoto M, Ono F and Kangawa K: Optimum collection and
storage conditions for ghrelin measurements: Octanoyl modification
of ghrelin is rapidly hydrolyzed to desacyl ghrelin in blood
samples. Clin Chem. 50:1077–1080. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Patterson M, Murphy KG, le Roux CW, Ghatei
MA and Bloom SR: Characterization of ghrelin-like immunoreactivity
in human plasma. J Clin Endocrinol Metab. 90:2205–2211. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ibrahim Abdalla MM: Ghrelin-physiological
functions and regulation. Eur Endocrinol. 11:90–95. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Delhanty PJ, Neggers SJ and van der Lely
AJ: Mechanisms in endocrinology: Ghrelin: The differences between
acyl- and des-acyl ghrelin. Eur J Endocrino. 167:601–608. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lanfranco F, Baldi M, Cassoni P, Bosco M,
Ghé C and Muccioli G: Ghrelin and prostate cancer. Vitam Horm.
77:301–324. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gahete MD, Córdoba-Chacón J,
Hergueta-Redondo M, Martínez-Fuentes AJ, Kineman RD, Moreno-Bueno
G, Luque RM and Castaño JP: A novel human ghrelin variant
(In1-ghrelin) and ghrelin-O-acyltransferase are overexpressed in
breast cancer: Potential pathophysiological relevance. PLoS One.
6:e233022011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gahete MD, Rincón-Fernández D, Villa-Osaba
A, Hormaechea-Agulla D, Ibáñez-Costa A, Martínez-Fuentes AJ,
Gracia-Navarro F, Castaño JP and Luque RM: Ghrelin gene products,
receptors, and GOAT enzyme: Biological and pathophysiological
insight. J Endocrinol. 220:R1–R24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang J, Brown MS, Liang G, Grishin NV and
Goldstein JL: Identification of the acyltransferase that
octanoylates ghrelin, an appetite-stimulating peptide hormone.
Cell. 132:387–396. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Delporte C: Structure and physiological
actions of ghrelin. Scientifica (Cairo). 2013:5189092013.PubMed/NCBI
|
|
43
|
Ferré G, Louet M, Saurel O, Delort B,
Czaplicki G, M'Kadmi C, Damian M, Renault P, Cantel S, Gavara L, et
al: Structure and dynamics of G protein-coupled receptor-bound
ghrelin reveal the critical role of the octanoyl chain. Proc Natl
Acad Sci USA. 116:17525–17530. 2019. View Article : Google Scholar
|
|
44
|
Rodríguez A, Gómez-Ambrosi J, Catalán V,
Gil MJ, Becerril S, Sáinz N, Silva C, Salvador J, Colina I and
Frühbeck G: Acylated and desacyl ghrelin stimulate lipid
accumulation in human visceral adipocytes. Int J Obes (Lond).
33:541–552. 2009. View Article : Google Scholar
|
|
45
|
Heppner KM, Piechowski CL, Müller A,
Ottaway N, Sisley S, Smiley DL, Habegger KM, Pfluger PT, Dimarchi
R, Biebermann H, et al: Both acyl and des-acyl ghrelin regulate
adiposity and glucose metabolism via central nervous system ghrelin
receptors. Diabetes. 63:122–131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Howard AD, Feighner SD, Cully DF, Arena
JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC,
Anderson J, et al: A receptor in pituitary and hypothalamus that
functions in growth hormone release. Science. 273:974–977. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Volante M, Fulcheri E, Allìa E, Cerrato M,
Pucci A and Papotti M: Ghrelin expression in fetal, infant, and
adult human lung. J Histochem Cytochem. 50:1013–1021. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Muccioli G, Baragli A, Granata R, Papotti
M and Ghigo E: Heterogeneity of ghrelin/growth hormone secretagogue
receptors. Toward the understanding of the molecular identity of
novel ghrelin/GHS receptors. Neuroendocrinology. 86:147–164. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ueberberg B, Unger N, Saeger W, Mann K and
Petersenn S: Expression of ghrelin and its receptor in human
tissues. Horm Metab Res. 41:814–821. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xiao X, Bi M, Jiao Q, Chen X, Du X and
Jiang H: A new understanding of GHSR1a-independent of ghrelin
activation. Ageing Res Rev. 64:1011872020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Albarrán-Zeckler RG and Smith RG: The
ghrelin receptors (GHS-R1a and GHS-R1b). Endocr Dev. 25:5–15. 2013.
View Article : Google Scholar
|
|
52
|
Callaghan B and Furness JB: Novel and
conventional receptors for ghrelin, desacyl-ghrelin, and
pharmacologically related compounds. Pharmacol Rev. 66:984–1001.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Drucker DJ: Evolving concepts and
translational relevance of enteroendocrine cell biology. J Clin
Endocrinol Metab. 101:778–786. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Thompson NM, Gill DA, Davies R, Loveridge
N, Houston PA, Robinson IC and Wells T: Ghrelin and des-octanoyl
ghrelin promote adipogenesis directly in vivo by a mechanism
independent of the type 1a growth hormone secretagogue receptor.
Endocrinology. 145:234–242. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Churm R, Davies JS, Stephens JW and Prior
SL: Ghrelin function in human obesity and type 2 diabetes: A
concise review. Obes Rev. 18:140–148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Toshinai K, Yamaguchi H, Sun Y, Smith RG,
Yamanaka A, Sakurai T, Date Y, Mondal MS, Shimbara T, Kawagoe T, et
al: Des-acyl ghrelin induces food intake by a mechanism independent
of the growth hormone secretagogue receptor. Endocrinology.
147:2306–2314. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Au CC, Furness JB and Brown KA: Ghrelin
and breast cancer: Emerging roles in obesity, estrogen regulation,
and cancer. Front Oncol. 6:2652017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rodríguez A, Gómez-Ambrosi J, Catalán V,
Rotellar F, Valentí V, Silva C, Mugueta C, Pulido MR, Vázquez R,
Salvador J, et al: The ghrelin O-acyltransferase-ghrelin system
reduces TNF-α-induced apoptosis and autophagy in human visceral
adipocytes. Diabetologia. 55:3038–3050. 2012. View Article : Google Scholar
|
|
59
|
Sheriff S, Kadeer N, Joshi R, Friend LA,
James JH and Balasubramaniam A: Des-acyl ghrelin exhibits
pro-anabolic and anti-catabolic effects on C2C12 myotubes exposed
to cytokines and reduces burn-induced muscle proteolysis in rats.
Mol Cell Endocrinol. 351:286–295. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wu R, Chaung WW, Dong W, Ji Y, Barrera R,
Nicastro J, Molmenti EP, Coppa GF and Wang P: Ghrelin maintains the
cardiovascular stability in severe sepsis. J Surg Res. 178:370–377.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Porporato PE, Filigheddu N, Reano S,
Ferrara M, Angelino E, Gnocchi VF, Prodam F, Ronchi G, Fagoonee S,
Fornaro M, et al: Acylated and unacylated ghrelin impair skeletal
muscle atrophy in mice. J Clin Invest. 123:611–622. 2013.PubMed/NCBI
|
|
62
|
Bouillon-Minois JB, Trousselard M, Thivel
D, Gordon BA, Schmidt J, Moustafa F, Oris C and Dutheil F: Ghrelin
as a biomarker of stress: A systematic review and meta-analysis.
Nutrients. 13:7842021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Gray SM, Page LC and Tong J: Ghrelin
regulation of glucose metabolism. J Neuroendocrinol. 31:e127052019.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Nass RM, Gaylinn BD, Rogol AD and Thorner
MO: Ghrelin and growth hormone: Story in reverse. Proc Natl Acad
Sci USA. 107:8501–8502. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Quiñones M, Fernø J and Al-Massadi O:
Ghrelin and liver disease. Rev Endocr Metab Disord. 21:45–56. 2020.
View Article : Google Scholar
|
|
66
|
Akalu Y, Molla MD, Dessie G and Ayelign B:
Physiological effect of ghrelin on body systems. Int J Endocrinol.
2020:13851382020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tschöp M, Smiley DL and Heiman ML: Ghrelin
induces adiposity in rodents. Nature. 407:908–913. 2000. View Article : Google Scholar
|
|
68
|
Mihalache L, Arhire LI, Giuşcă SE,
Gherasim A, Niţă O, Constantinescu D, Constantinescu RN, Pădureanu
SS and Danciu M: Ghrelin-producing cells distribution in the
stomach and the relation with Helicobacter pylori in obese
patients. Rom J Morphol Embryol. 60:219–225. 2019.PubMed/NCBI
|
|
69
|
Chowen JA and Argente J: Ghrelin: A link
between energy homeostasis and the immune system. Endocrinology.
158:2077–2081. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Dixit VD, Schaffer EM, Pyle RS, Collins
GD, Sakthivel SK, Palaniappan R, Lillard JW Jr and Taub DD: Ghrelin
inhibits leptin- and activation-induced proinflammatory cytokine
expression by human monocytes and T cells. J Clin Invest.
114:57–66. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kasımay O, Işeri SO, Barlas A, Bangir D,
Yeğen C, Arbak S and Yeğen BC: Ghrelin ameliorates
pancreaticobiliary inflammation and associated remote organ injury
in rats. Hepatol Res. 36:11–19. 2006. View Article : Google Scholar
|
|
72
|
Huang CX, Yuan MJ, Huang H, Wu G, Liu Y,
Yu SB, Li HT and Wang T: Ghrelin inhibits post-infarct myocardial
remodeling and improves cardiac function through anti-inflammation
effect. Peptides. 30:2286–2291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chang RJ, Wang HL, Qin MB, Liang ZH, He
JP, Wei YL, Fu HZ and Tang GD: Ghrelin inhibits IKKβ/NF-κB
activation and reduces pro-inflammatory cytokine production in
pancreatic acinar AR42J cells treated with cerulein. Hepatobiliary
Pancreat Dis Int. Jun 2–2020.(Online ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mao Y, Wang J, Yu F, Cheng J, Li H, Guo C
and Fan X: Ghrelin reduces liver impairment in a model of
concanavalin A-induced acute hepatitis in mice. Drug Des Devel
Ther. 9:5385–5396. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Karmiris K, Koutroubakis IE, Xidakis C,
Polychronaki M, Voudouri T and Kouroumalis EA: Circulating levels
of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel
disease. Inflamm Bowel Dis. 12:100–105. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Theil MM, Miyake S, Mizuno M, Tomi C,
Croxford JL, Hosoda H, Theil J, von Hörsten S, Yokote H, Chiba A,
et al: Suppression of experimental autoimmune encephalomyelitis by
ghrelin. J Immunol. 183:2859–2866. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Granado M, Priego T, Martín AI, Villanúa
MA and López-Calderón A: Anti-inflammatory effect of the ghrelin
agonist growth hormone-releasing peptide-2 (GHRP-2) in arthritic
rats. Am J Physiol Endocrinol Metab. 288:E486–E492. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Gonzalez-Rey E, Chorny A and Delgado M:
Therapeutic action of ghrelin in a mouse model of colitis.
Gastroenterology. 130:1707–1720. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Liu H, Luo J, Guillory B, Chen JA, Zang P,
Yoeli JK, Hernandez Y, Lee II, Anderson B, Storie M, et al: Ghrelin
ameliorates tumor-induced adipose tissue atrophy and inflammation
via Ghrelin receptor-dependent and -independent pathways.
Oncotarget. 11:3286–3302. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lacerda-Miranda G, Soares VM, Vieira AK,
Lessa JG, Rodrigues-Cunha AC, Cortez E, Garcia-Souza EP and Moura
AS: Ghrelin signaling in heart remodeling of adult obese mice.
Peptides. 35:65–73. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Sullivan R, McGirr R, Hu S, Tan A, Wu D,
Charron C, Lalonde T, Arany E, Chakrabarti S, Luyt L and
Dhanvantari S: Changes in the cardiac GHSR1a-ghrelin system
correlate with myocardial dysfunction in diabetic cardiomyopathy in
mice. J Endocr Soc. 2:178–189. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Grossini E, Raina G, Farruggio S, Camillo
L, Molinari C, Mary D, Walker GE, Bona G, Vacca G, Moia S, et al:
Intracoronary des-acyl ghrelin acutely increases cardiac perfusion
through a nitric oxide-related mechanism in female anesthetized
pigs. Endocrinology. 157:2403–2415. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Soeki T, Kishimoto I, Schwenke DO,
Tokudome T, Horio T, Yoshida M, Hosoda H and Kangawa K: Ghrelin
suppresses cardiac sympathetic activity and prevents early left
ventricular remodeling in rats with myocardial infarction. Am J
Physiol Heart Circ Physiol. 294:H426–H432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Henriques-Coelho T, Correia-Pinto J,
Roncon-Albuquerque R Jr, Baptista MJ, Lourenço AP, Oliveira SM,
Brandão-Nogueira A, Teles A, Fortunato JM and Leite-Moreira AF:
Endogenous production of ghrelin and beneficial effects of its
exogenous administration in monocrotaline-induced pulmonary
hypertension. Am J Physiol Heart Circ Physiol. 287:H2885–H2890.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wu R, Dong W, Zhou M, Zhang F, Marini CP,
Ravikumar TS and Wang P: Ghrelin attenuates sepsis-induced acute
lung injury and mortality in rats. Am J Respir Crit Care Med.
176:805–813. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
DeBoer MD, Zhu XX, Levasseur P, Meguid MM,
Suzuki S, Inui A, Taylor JE, Halem HA, Dong JZ, Datta R, et al:
Ghrelin treatment causes increased food intake and retention of
lean body mass in a rat model of cancer cachexia. Endocrinology.
148:3004–3012. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang W, Andersson M, Iresjö BM, Lönnroth C
and Lundholm K: Effects of ghrelin on anorexia in tumor-bearing
mice with eicosanoid-related cachexia. Int J Oncol. 28:1393–1400.
2006.PubMed/NCBI
|
|
88
|
Bayliss JA, Lemus MB, Stark R, Santos VV,
Thompson A, Rees DJ, Galic S, Elsworth JD, Kemp BE, Davies JS and
Andrews ZB: Ghrelin-AMPK signaling mediates the neuroprotective
effects of calorie restriction in Parkinson's disease. J Neurosci.
36:3049–3063. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Unger MM, Oertel WH and Tackenberg B:
Cerebrospinal fluid concentrations of ghrelin in patients with
multiple sclerosis. Neuro Endocrinol Lett. 34:14–17.
2013.PubMed/NCBI
|
|
90
|
Ngo ST, Steyn FJ, Huang L, Mantovani S,
Pfluger CM, Woodruff TM, O'Sullivan JD, Henderson RD and McCombe
PA: Altered expression of metabolic proteins and adipokines in
patients with amyotrophic lateral sclerosis. J Neurol Sci.
357:22–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Cassoni P, Ghé C, Marrocco T, Tarabra E,
Allia E, Catapano F, Deghenghi R, Ghigo E, Papotti M and Muccioli
G: Expression of ghrelin and biological activity of specific
receptors for ghrelin and des-acyl ghrelin in human prostate
neoplasms and related cell lines. Eur J Endocrinol. 150:173–184.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Grönberg M, Fjällskog ML, Jirström K and
Janson ET: Expression of ghrelin is correlated to a favorable
outcome in invasive breast cancer. Acta Oncol. 51:386–393. 2012.
View Article : Google Scholar
|
|
93
|
Sadjadi A, Yazdanbod A, Lee YY, Boreiri M,
Samadi F, Alizadeh BZ, Islami F, Fyfe V, Babaei M, Namazi MJ, et
al: Serum ghrelin; a new surrogate marker of gastric mucosal
alterations in upper gastrointestinal carcinogenesis. PLoS One.
8:e744402013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Lin TC, Liu YP, Chan YC, Su CY, Lin YF,
Hsu SL, Yang CS and Hsiao M: Ghrelin promotes renal cell carcinoma
metastasis via Snail activation and is associated with poor
prognosis. J Pathol. 237:50–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
De Vriese C and Delporte C: Autocrine
proliferative effect of ghrelin on leukemic HL-60 and THP-1 cells.
J Endocrinol. 192:199–205. 2007. View Article : Google Scholar
|
|
96
|
Cassoni P, Papotti M, Ghè C, Catapano F,
Sapino A, Graziani A, Deghenghi R, Reissmann T, Ghigo E and
Muccioli G: Identification, characterization, and biological
activity of specific receptors for natural (ghrelin) and synthetic
growth hormone secretagogues and analogs in human breast carcinomas
and cell lines. J Clin Endocrinol Metab. 86:1738–1745. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Tsubouchi H, Onomura H, Saito Y, Yanagi S,
Miura A, Matsuo A, Matsumoto N and Nakazato M: Ghrelin does not
influence cancer progression in a lung adenocarcinoma cell line.
Endocr J. 64 (Suppl 1):S41–S46. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Duxbury MS, Waseem T, Ito H, Robinson MK,
Zinner MJ, Ashley SW and Whang EE: Ghrelin promotes pancreatic
adenocarcinoma cellular proliferation and invasiveness. Biochem
Biophys Res Commun. 309:464–468. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Jeffery PL, Herington AC and Chopin LK:
Expression and action of the growth hormone releasing peptide
ghrelin and its receptor in prostate cancer cell lines. J
Endocrinol. 172:R7–R11. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Tian C, Zhang L, Hu D and Ji J: Ghrelin
induces gastric cancer cell proliferation, migration, and invasion
through GHS-R/NF-κB signaling pathway. Mol Cell Biochem.
382:163–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Waseem T, Javaid-Ur-Rehman, Ahmad F, Azam
M and Qureshi MA: Role of ghrelin axis in colorectal cancer: A
novel association. Peptides. 29:1369–1376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Lien GS, Lin CH, Yang YL, Wu MS and Chen
BC: Ghrelin induces colon cancer cell proliferation through the
GHS-R, Ras, PI3K, Akt, and mTOR signaling pathways. Eur J
Pharmacol. 776:124–131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Kraus D, Reckenbeil J, Wenghoefer M, Stark
H, Frentzen M, Allam JP, Novak N, Frede S, Götz W, Probstmeier R,
et al: Ghrelin promotes oral tumor cell proliferation by modifying
GLUT1 expression. Cell Mol Life Sci. 73:1287–1299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Waseem T, Duxbury M, Ashley SW and
Robinson MK: Ghrelin promotes intestinal epithelial cell
proliferation through PI3K/Akt pathway and EGFR trans-activation
both converging to ERK 1/2 phosphorylatio. Peptides. 52:113–121.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Barzon L, Pacenti M, Masi G, Stefani AL,
Fincati K and Palù G: Loss of growth hormone secretagogue receptor
1a and overexpression of type 1b receptor transcripts in human
adrenocortical tumors. Oncology. 68:414–421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Fung JN, Seim I, Wang D, Obermair A,
Chopin LK and Chen C: Expression and in vitro functions of the
ghrelin axis in endometrial cancer. Horm Cancer. 1:245–255. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Lawnicka H, Mełeń-Mucha G, Motylewska E,
Mucha S and Stępień H: Modulation of ghrelin axis influences the
growth of colonic and prostatic cancer cells in vitro. Pharmacol
Rep. 64:951–959. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
El-Kott AF, Shati AA, Al-Kahtani MA and
Alqahtani S: Acylated ghrelin renders chemosensitive ovarian cancer
cells resistant to cisplatin chemotherapy via activation of the
PI3K/Akt/mTOR survival pathway. Anal Cell Pathol (Amst).
2019:96278102019.PubMed/NCBI
|
|
109
|
Rincón-Fernández D, Culler MD, Tsomaia N,
Moreno-Bueno G, Luque RM, Gahete MD and Castaño JP: In1-ghrelin
splicing variant is associated with reduced disease-free survival
of breast cancer patients and increases malignancy of breast cancer
cells lines. Carcinogenesis. 39:447–457. 2018. View Article : Google Scholar
|
|
110
|
Hormaechea-Agulla D, Gahete MD,
Jiménez-Vacas JM, Gómez-Gómez E, Ibáñez-Costa A, L-López F,
Rivero-Cortés E, Sarmento-Cabral A, Valero-Rosa J,
Carrasco-Valiente J, et al: The oncogenic role of the In1-ghrelin
splicing variant in prostate cancer aggressiveness. Mol Cancer.
16:1462017. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Chen JH, Huang SM, Chen CC, Tsai CF, Yeh
WL, Chou SJ, Hsieh WT and Lu DY: Ghrelin induces cell migration
through GHS-R, CaMKII, AMPK, and NF-κB signaling pathway in glioma
cells. J Cell Biochem. 112:2931–2941. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Lin TC, Yeh YM, Fan WL, Chang YC, Lin WM,
Yang TY and Hsiao M: Ghrelin upregulates oncogenic Aurora A to
promote renal cell carcinoma invasion. Cancers (Basel). 11:3032019.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Tiaka EK, Manolakis AC, Kapsoritakis AN
and Potamianos SP: Unraveling the link between leptin, ghrelin and
different types of colitis. Ann Gastroenterol. 24:20–28.
2011.PubMed/NCBI
|
|
114
|
Ghomraoui FA, Alotaibi ST, Alharthi MA,
Asiri SS, Almadi MA, Alharbi OR, Azzam NA, Aljebreen AM, Saeed M,
Hajkhder B, et al: Plasma ghrelin and leptin in patients with
inflammatory bowel disease and its association with nutritional
status. Saudi J Gastroenterol. 23:199–205. 2017.PubMed/NCBI
|
|
115
|
Omoto I, Matsumoto M, Uchikado Y, Kita Y,
Sakurai T, Sasaki K, Setoyama T, Okumura H, Owaki T, Ishigami S and
Natsugoe S: Immunohistochemical evidence of association between
ghrelin expression and tumor growth in esophageal carcinoma.
Anticancer Res. 34:2727–2733. 2014.PubMed/NCBI
|
|
116
|
Mottershead M, Karteris E, Barclay JY,
Suortamo S, Newbold M, Randeva H and Nwokolo CU:
Immunohistochemical and quantitative mRNA assessment of ghrelin
expression in gastric and oesophageal adenocarcinoma. J Clin
Pathol. 60:405–409. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Murphy G, Kamangar F, Albanes D, Stanczyk
FZ, Weinstein SJ, Taylor PR, Virtamo J, Abnet CC, Dawsey SM and
Freedman ND: Serum ghrelin is inversely associated with risk of
subsequent oesophageal squamous cell carcinoma. Gut. 61:1533–1537.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Pritchett NR, Maziarz M, Shu XO, Kamangar
F, Dawsey SM, Fan JH, Ji BT, Gao YT, Xiang YB, Qiao YL, et al:
Serum ghrelin and esophageal and gastric cancer in two cohorts in
China. Int J Cancer. 146:2728–2735. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
de Martel C, Haggerty TD, Corley DA,
Vogelman JH, Orentreich N and Parsonnet J: Serum ghrelin levels and
risk of subsequent adenocarcinoma of the esophagus. Am J
Gastroenterol. 102:1166–1172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Thomas SJ, Almers L, Schneider J, Graham
JE, Havel PJ and Corley DA: Ghrelin and leptin have a complex
relationship with risk of Barrett's esophagus. Dig Dis Sci.
61:70–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Rubenstein JH, Morgenstern H, McConell D,
Scheiman JM, Schoenfeld P, Appelman H, McMahon LF Jr, Kao JY, Metko
V, Zhang M and Inadomi JM: Associations of diabetes mellitus,
insulin, leptin, and ghrelin with gastroesophageal reflux and
Barrett's esophagus. Gastroenterolog. 145:1237–1244.e1-e5. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Konturek PC, Burnat G, Rau T, Hahn EG and
Konturek S: Effect of adiponectin and ghrelin on apoptosis of
Barrett adenocarcinoma cell line. Dig Dis Sci. 53:597–605. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Xie SH, Rabbani S, Ness-Jensen E and
Lagergren J: Circulating levels of inflammatory and metabolic
biomarkers and risk of esophageal adenocarcinoma and Barrett
esophagus: Systematic review and meta-analysis. Cancer Epidemiol
Biomarkers Prev. 29:2109–2118. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Zhu C, Liu Y, Kang W, Zhang Z, Zeng Z and
Liu D: Exploration of the role of serum ghrelin in the diagnosis
and treatment of digestive tract malignancies. J Int Med Res.
48:3000605209204412020. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Shinsyu A, Bamba S, Kurihara M, Matsumoto
H, Sonoda A, Inatomi O, Andoh A, Takebayashi K, Kojima M, Iida H,
et al: Inflammatory cytokines, appetite-regulating hormones, and
energy metabolism in patients with gastrointestinal cancer. Oncol
Lett. 20:1469–1479. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB,
Peng JJ, Chen CQ, He YL and Cai SR: Systemic immune-inflammation
index for predicting prognosis of colorectal cancer. World J
Gastroenterol. 23:6261–6272. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Chang WJ, Du Y, Zhao X, Ma LY and Cao GW:
Inflammation-related factors predicting prognosis of gastric
cancer. World J Gastroenterol. 20:4586–4596. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Aydin S, Ozercan IH, Dagli F, Aydin S,
Dogru O, Celebi S, Akin O and Guzel SP: Ghrelin
immunohistochemistry of gastric adenocarcinoma and mucoepidermoid
carcinoma of salivary gland. Biotech Histochem. 80:163–168. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
An JY, Choi MG, Noh JH, Sohn TS, Jin DK
and Kim S: Clinical significance of ghrelin concentration of plasma
and tumor tissue in patients with gastric cancer. J Surg Res.
143:344–349. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Murphy G, Kamangar F, Dawsey SM, Stanczyk
FZ, Weinstein SJ, Taylor PR, Virtamo J, Abnet CC, Albanes D and
Freedman ND: The relationship between serum ghrelin and the risk of
gastric and esophagogastric junctional adenocarcinomas. J Natl
Cancer Inst. 103:1123–1129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Nwokolo CU, Freshwater DA, O'Hare P and
Randeva HS: Plasma ghrelin following cure of Helicobacter
pylori. Gut. 52:637–640. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Osawa H, Nakazato M, Date Y, Kita H,
Ohnishi H, Ueno H, Shiiya T, Satoh K, Ishino Y and Sugano K:
Impaired production of gastric ghrelin in chronic gastritis
associated with Helicobacter pylori. J Clin Endocrinol
Metab. 90:10–16. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Zub-Pokrowiecka A, Rembiasz K, Konturek
PC, Budzyński A, Konturek SJ, Winiarski M and Bielański W: Ghrelin
and gastrin in advanced gastric cancer before and after
gastrectomy. World J Gastroenterol. 17:449–458. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Jeon TY, Lee S, Kim HH, Kim YJ, Son HC,
Kim DH and Sim MS: Changes in plasma ghrelin concentration
immediately after gastrectomy in patients with early gastric
cancer. J Clin Endocrinol Metab. 89:5392–5396. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Takachi K, Doki Y, Ishikawa O, Miyashiro
I, Sasaki Y, Ohigashi H, Murata K, Nakajima H, Hosoda H, Kangawa K,
et al: Postoperative ghrelin levels and delayed recovery from body
weight loss after distal or total gastrectomy. J Surg Res. 130:1–7.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Wu X, Wu Y, Ye B, Wu F and Wang P: High
expression of ghrelin and obestatin prepropeptide in tumor tissues
predicted adverse overall survival in gastric carcinoma patients.
Medicine (Baltimore). 99:e206352020. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Wolf I, Sadetzki S, Kanety H, Kundel Y,
Pariente C, Epstein N, Oberman B, Catane R, Kaufman B and Shimon I:
Adiponectin, ghrelin, and leptin in cancer cachexia in breast and
colon cancer patients. Cancer. 106:966–973. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Liu A, Huang C, Xu J and Cai X:
Lentivirus-mediated shRNA interference of ghrelin receptor blocks
proliferation in the colorectal cancer cells. Cancer Med.
5:2417–2426. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Huang Q, Fan YZ, Ge BJ, Zhu Q and Tu ZY:
Circulating ghrelin in patients with gastric or colorectal cancer.
Dig Dis Sci. 52:803–809. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
D'Onghia V, Leoncini R, Carli R, Santoro
A, Giglioni S, Sorbellini F, Marzocca G, Bernini A, Campagna S,
Marinello E and Vannoni D: Circulating gastrin and ghrelin levels
in patients with colorectal cancer: Correlation with tumour stage,
Helicobacter pylori infection and BMI. Biomed Pharmacother.
61:137–141. 2007. View Article : Google Scholar
|
|
141
|
Nikolopoulos D, Theocharis S,
Moutsios-Rentzos A, Kouraklis G and Kostakis A: The role of serum
total ghrelin level elevation in colon cancer patients. J BUON.
19:388–393. 2014.PubMed/NCBI
|
|
142
|
Kemik O, Sumer A, Kemik AS, Hasirci I,
Purisa S, Dulger AC, Demiriz B and Tuzun S: The relationship among
acute-phase response proteins, cytokines and hormones in cachectic
patients with colon cancer. World J Surg Oncol. 8:852010.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Murphy G, Cross AJ, Dawsey SM, Stanczyk
FZ, Kamangar F, Weinstein SJ, Taylor PR, Männistö S, Albanes D,
Abnet CC and Freedman ND: Serum ghrelin is associated with risk of
colorectal adenocarcinomas in the ATBC study. Gut. 67:1646–1651.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Sundkvist A, Myte R, Palmqvist R, Harlid S
and Van Guelpen B: Plasma ghrelin is probably not a useful
biomarker for risk prediction or early detection of colorectal
cancer. Gut. 68:373–374. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Coppedè F, Stoccoro A, Lazzarotti A,
Spisni R and Migliore L: Investigation of GHSR and GHRL methylation
in colorectal cancer. Epigenomics. 10:1525–1539. 2018. View Article : Google Scholar
|
|
146
|
Chopin LK, Seim I, Walpole CM and
Herington AC: The ghrelin axis-does it have an appetite for cancer
progression? Endocr Rev. 33:849–891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Molfino A, Formiconi A, Rossi Fanelli F
and Muscaritoli M: Ghrelin: From discovery to cancer cachexia
therapy. Curr Opin Clin Nutr Metab Care. 17:471–476. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Rindi G, Savio A, Torsello A, Zoli M,
Locatelli V, Cocchi D, Paolotti D and Solcia E: Ghrelin expression
in gut endocrine growths. Histochem Cell Biol. 117:521–525. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Papotti M, Cassoni P, Volante M, Deghenghi
R, Muccioli G and Ghigo E: Ghrelin-producing endocrine tumors of
the stomach and intestine. J Clin Endocrinol Metab. 86:5052–5059.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Raffel A, Krausch M, Cupisti K, Gerharz
CD, Eisenberger CF and Knoefel WT: Ghrelin expression in
neuroendocrine tumours of the gastrointestinal tract with multiple
endocrine neoplasia type 1. Horm Metab Res. 37:653–655. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Ekeblad S, Nilsson B, Lejonklou MH,
Johansson T, Stålberg P, Nilsson O, Ahlman H and Skogseid B:
Gastrointestinal stromal tumors express the orexigen ghrelin.
Endocr Relat Cancer. 13:963–970. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Volante M, Allìa E, Gugliotta P, Funaro A,
Broglio F, Deghenghi R, Muccioli G, Ghigo E and Papotti M:
Expression of ghrelin and of the GH secretagogue receptor by
pancreatic islet cells and related endocrine tumors. J Clin
Endocrinol Metab. 87:1300–1308. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Ekeblad S, Lejonklou MH, Grimfjärd P,
Johansson T, Eriksson B, Grimelius L, Stridsberg M, Stålberg P and
Skogseid B: Co-expression of ghrelin and its receptor in pancreatic
endocrine tumours. Clin Endocrinol (Oxf). 66:115–122.
2007.PubMed/NCBI
|
|
154
|
Tsolakis AV, Stridsberg M, Grimelius L,
Portela-Gomes GM, Falkmer SE, Waldum HL and Janson ET: Ghrelin
immunoreactive cells in gastric endocrine tumors and their relation
to plasma ghrelin concentration. J Clin Gastroenterol. 42:381–388.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Walter T, Chardon L, Hervieu V, Cohen R,
Chayvialle JA, Scoazec JY and Lombard-Bohas C: Major
hyperghrelinemia in advanced well-differentiated neuroendocrine
carcinomas: Report of three cases. Eur J Endocrino. 161:639–645.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Corbetta S, Peracchi M, Cappiello V, Lania
A, Lauri E, Vago L, Beck-Peccoz P and Spada A: Circulating ghrelin
levels in patients with pancreatic and gastrointestinal
neuroendocrine tumors: Identification of one pancreatic ghrelinoma.
J Clin Endocrinol Metab. 88:3117–3120. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Wang HS, Oh DS, Ohning GV and Pisegna JR:
Elevated serum ghrelin exerts an orexigenic effect that may
maintain body mass index in patients with metastatic neuroendocrine
tumors. J Mol Neurosci. 33:225–231. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
van Adrichem RC, van der Lely AJ, Huisman
M, Kramer P, Feelders RA, Delhanty PJ and de Herder WW: Plasma
acylated and plasma unacylated ghrelin: Useful new biomarkers in
patients with neuroendocrine tumors? Endocr Connect. 5:143–151.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Luque RM, Sampedro-Nuñez M, Gahete MD,
Ramos-Levi A, Ibáñez-Costa A, Rivero-Cortés E, Serrano-Somavilla A,
Adrados M, Culler MD, Castaño JP and Marazuela M: In1-ghrelin, a
splice variant of ghrelin gene, is associated with the evolution
and aggressiveness of human neuroendocrine tumors: Evidence from
clinical, cellular and molecular parameters. Oncotarget.
6:19619–19633. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Herrera-Martínez AD, Gahete MD,
Sánchez-Sánchez R, Alors-Perez E, Pedraza-Arevalo S, Serrano-Blanch
R, Martínez-Fuentes AJ, Gálvez-Moreno MA, Castaño JP and Luque RM:
Ghrelin-O-acyltransferase (GOAT) enzyme as a novel potential
biomarker in gastroenteropancreatic neuroendocrine tumors. Clin
Transl Gastroenterol. 9:1962018. View Article : Google Scholar
|
|
161
|
Hirota S: Differential diagnosis of
gastrointestinal stromal tumor by histopathology and
immunohistochemistry. Transl Gastroenterol Hepatol. 3:272018.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Doyle LA and Hornick JL: Gastrointestinal
stromal tumours: From KIT to succinate dehydrogenase.
Histopathology. 64:53–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Zhu CZ, Liu D, Kang WM, Yu JC, Ma ZQ, Ye X
and Li K: Ghrelin and gastrointestinal stromal tumors. World J
Gastroenterol. 23:1758–1763. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Ghè C, Cassoni P, Catapano F, Marrocco T,
Deghenghi R, Ghigo E, Muccioli G and Papotti M: The
antiproliferative effect of synthetic peptidyl GH secretagogues in
human CALU-1 lung carcinoma cells. Endocrinology. 143:484–491.
2002. View Article : Google Scholar
|
|
165
|
Alnema MM, Aydin S, Ozkan Y, Dagli AF,
Ozercan HI, Yildirim N, Sahin I, Karaoglu A, Kilic N, Yilmaz M, et
al: Ghrelin and obestatin expression in oral squamous cell
carcinoma: An immunohistochemical and biochemical study. Mol Cell
Biochem. 339:173–179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Lou J, Liu L, Zhang W, Zhou Z and Fan Y:
Differential expression of ghrelin and GHSR via the mTOR pathway
during the dynamic carcinogenic process involving oral, potentially
malignant disorders. Biosci Rep. 39:BSR201921022019. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Dagli AF, Aydin S, Karaoglu A, Akpolat N,
Ozercan IH and Ozercan MR: Ghrelin expression in normal kidney
tissue and renal carcinomas. Pathol Res Pract. 205:165–173. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Grönberg M, Amini RM, Stridsberg M, Janson
ET and Saras J: Neuroendocrine markers are expressed in human
mammary glands. Regul Pept. 160:68–74. 2010. View Article : Google Scholar
|
|
169
|
Grönberg M, Nilsson C, Markholm I,
Hedenfalk I, Blomqvist C, Holmberg L, Tiensuu Janson E and
Fjällskog ML: Ghrelin expression is associated with a favorable
outcome in male breast cancer. Sci Rep. 8:135862018. View Article : Google Scholar
|
|
170
|
Malendowicz W, Ziolkowska A, Szyszka M and
Kwias Z: Elevated blood active ghrelin and unaltered total ghrelin
and obestatin concentrations in prostate carcinoma. Urol Int.
83:471–475. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Fung JN, Jeffery PL, Lee JD, Seim I, Roche
D, Obermair A, Chopin LK and Chen C: Silencing of ghrelin receptor
expression inhibits endometrial cancer cell growth in vitro and in
vivo. Am J Physiol Endocrinol Metab. 305:E305–E313. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Kerenidi T, Lada M, Tsaroucha A,
Georgoulias P, Mystridou P and Gourgoulianis KI: Clinical
significance of serum adipokines levels in lung cancer. Med Oncol.
30:5072013. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Li X, Zhao X, Li C, Liu S, Yan F, Teng Y,
Feng J and Miao D: Inhibitor of ghrelin receptor reverses gefitinib
resistance in lung cancer. Hum Cell. 32:360–366. 2019. View Article : Google Scholar : PubMed/NCBI
|