Introduction
Pulmonary arterial hypertension (PAH) typically
originates from hyperplasia of pulmonary arterial smooth muscle
cells (PASMCs) (1,2). Under normal physiological conditions,
PASMCs are quiescent and contractile (3). However, under hypoxia, the phenotype
of PASMCs can switch, leading to excessive proliferation and
migration (4,5), eventually resulting in pulmonary
vasoconstriction and vascular remodelling (6). Therefore, exploring the mechanism by
which hypoxia induces the proliferation and migration of PASMCs is
vital for treating PAH in the clinic.
Various long non-coding RNAs (lncRNAs) have been
revealed to promote the development of PAH (7–9). For
example, Zhang et al reported that HOXA-AS3 was
overexpressed in both the lung vasculature of monocrotaline
(MCT)-treated mice and PASMCs from patients with PAH (7). In addition, Wang et al detected
high MALAT1 expression in PAH tissues and PASMCs (8). Yang et al demonstrated that
hypoxia increased the expression of taurine upregulated gene 1
(TUG1) in the pulmonary artery (PA) of a PAH mouse model, whereas
silencing of TUG1 markedly decreased the hyperproliferation of
PASMCs (9). Additionally,
inhibition of NEAT1 has been reported to suppress the proliferation
and migration of numerous human cancers, including pancreatic
(10), breast (11), non-small cell lung (12) and colorectal cancers (13). More relevant to the present study,
Ahmed et al (14)
demonstrated that knockout of NEAT1 increased the levels of
contractile proteins in vascular smooth muscle (VSM) cells (VSMCs),
allowing them to maintain their contractile status and ultimately
inhibit vascular hyperplasia. However, the possible role of NEAT1
in hypoxia-treated PASMCs has yet to be elucidated.
Notably, the expression levels of some microRNAs
(miRNAs) are reduced in lung vascular tissues and/or PASMCs, which
are likely involved in the progression of PAH (15–17).
For instance, downregulation of miR-98 was revealed to enhance the
proliferation-promoting effect of hypoxia on PASMCs (15). In an MCT-induced rat model, low
miR-140-5p expression was observed, which suppressed PAH by
targeting TNF-α (16). MiR-182-3p
was downregulated in patients with PAH as well as in
hypoxia-stimulated model mice/rats, and miR-182-3p upregulation had
a distinct antiproliferative effect on PASMCs (17). Notably, a recent study revealed that
miR-34a was expressed at low levels in the PA of rats, and it
inhibited the proliferation of PASMCs by targeting PDGFRA (18). lncRNAs can modulate the function of
miRNAs by sponging them and thus acting as competing endogenous
RNAs. Whether miR-34a-5p interacts with NEAT1 and whether this
interaction is involved in the pathogenesis of PAH needs to be
further investigated.
Krüppel-like factor 4 (KLF4), a member of the KLF
family of zinc-finger transcription factors (19), was reported to be associated with
the progression of PAH (20,21).
Sun et al (20) revealed
that KLF4 expression was significantly increased in remodelled
pulmonary arteries from a rat model of pulmonary vascular
remodelling, and downregulation of KLF4 suppressed the
proliferation and migration of PASMCs. Liang et al (21) demonstrated that increased KLF4
expression promoted the migration and cell cycle progression of
pulmonary artery endothelial cells. In addition, KLF4 was revealed
to be an oncogene that is regulated by miRNAs to promote tumour
progression in various human cancers, such as miR-148-3p/miR-152-3p
in prostate cancer (22), miR-32 in
gastric cancer (23), and miR-543
in colorectal cancer (24). Most
relevant to our research, a recent study revealed that miR-182-3p
mediated vascular remodelling in PAH by targeting KLF4 (17). However, whether regulation of KLF4
by miR-34a-5p is involved in PAH and the underlying mechanism have
yet to be elucidated.
In the present study, the possible roles of NEAT1,
miR-34a-5p, and KLF4 in the pathogenesis of PAH in vitro
were investigated, which will provide novel insights into the
diagnosis and treatment of PAH.
Materials and methods
Serum samples
From January 2018 to December 2019, serum samples
were collected from 25 patients (19 females and 6 males; age range,
21–48 years old; mean age, 35.04±8.44 years old) with PAH and 25
healthy volunteers (19 females and 6 males; age range, 21–50 years
old; mean age, 34.96±8.58 years old) in The People's Hospital of
Rizhao (Shandong, China). Patients with ≥1 of the following
conditions were excluded: i) Other types of PAH, including familial
PAH; ii) heart diseases, including left ventricular diseases and
acute heart failure; iii) chronic respiratory disorders, including
chronic obstructive pulmonary disease; iv) diabetes mellitus; and
v) prior targeted therapy. No patients had received prior medical
treatment. The inclusion criteria for healthy controls were that
subjects must be age- and sex-matched with patients and absent of
any diseases when enrolled. The study was approved by the Ethics
Committee of The People's Hospital of Rizhao, and written informed
consent was obtained from all study participants. The collected
serum samples were centrifuged at 450 × g for 20 min at 20°C and
immediately stored at −80°C until use.
Cell culture, hypoxia stimulation, and
transfection
Human PASMCs (BioVector NTCC, Inc.) were cultured in
Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc.) containing 10% foetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) and 0.1% penicillin/streptomycin
(ScienCell Research Laboratories, Inc.) at 37°C with 5%
CO2. The cells were divided into two groups: The hypoxia
group, which was treated with 5% CO2 and 95%
N2 at 37°C for 48 h, and the normoxia group, which was
treated with 5% CO2, 21% O2, and 74%
N2 at 37°C for 48 h. The short hairpin (sh) RNA
targeting NEAT1 (sh-NEAT1-1, 5′-GCCAUCAGCUUUGAAUAAAUU-3′;
shNEAT1-2, 5′-GGUGUUAUCAAGUGAAUUAUU-3′) and negative control
(sh-NC, 5′-UUCUCCGAACGUGUCACGU-3′) were obtained from Shanghai
Transheep Bio-Tech Co., Ltd. The KLF4 overexpression vector
(pcDNA-KLF4), overexpression negative control (pcDNA-NC),
miR-34a-5p mimics (5′-GAUGGACGUGCUUGUCGUGAAAC-3′) and non-targeting
mimics control (miR-NC, 5′-UUCUCCGAACGUGUCACGUTT-3′), miR-34a-5p
inhibitor (5′-CUACCUGCACCAACAGCACUU-3′), and non-targeting
inhibitor control (inhibitor NC, 5′-CAGUACUUUUGUGUAGUACAA-3′) were
obtained from Beina Biology. All the above agents (all at 20 nM)
were co-transfected into hypoxia-treated PASMCs using the
Lipofectamine® RNAiMAX kit (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C. Subsequently, 48 h after transfection,
PASMCs were harvested to perform further experiments.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from the serum of patients
with PAH, healthy volunteers, and PASMCs using the Total RNA
Extraction Kit (Beijing Solarbio Science & Technology Co.,
Ltd.). According to the manufacturer's instructions, cDNA was
synthesized using the First-Strand cDNA Synthesis Kit (APExBIO
Technology), and RT-qPCR was performed with SYBR Green FAST
Mastermix (Qiagen GmbH). The following thermocycling conditions
were used for the qPCR: Initial denaturation at 95°C for 3 min
followed by 40 cycles at 95°C for 15 sec, annealing at 60°C for 30
sec, elongation at 72°C for 1 min, and a final extension at 72°C
for 5 min. Experimental results were quantified by the
2−ΔΔCq method (25). The
expression levels of NEAT1 and KLF4 were normalised to
GAPDH, and the expression level of miR-34a-5p was normalised
to U6. The respective sequences of primers were as follows:
NEAT1 forward, 5′-GGGGCCACATTAATCACAAC-3′ and reverse,
5′-CAGGGTGTCCTCCACCTTTA-3′; miR-34a-5p forward,
5′-GGGGTGGCAGTGTCTTAGC-3′ and reverse, 5′-CAGTGCGTGTCGTGGAGT-3′;
KLF4 forward, 5′-TTCCCATCTCAAGGCACACC-3′ and reverse,
5′-CATGTGTAAGGCGAGGTGGT-3′; GAPDH forward, 5′-CCAGGTGGTCTCCTCTGA-3′
and reverse, 5′-GCTGTAGCCAAATCGTTGT-3′; and U6 forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′.
3-(4,5-Dimethyl-2-thiazolyl)-2,
5-diphenyl-2-h-tetrazolium bromide (MTT) assay
Anoxic and transfected PASMCs (2×103)
were cultured in 96-well plates for 48 h at 37°C. Then, MTT
(Nanjing Keygen Biotech Co., Ltd.) was added, and the cells were
incubated for 2 h at 37°C. Thereafter, 100 µl dimethyl sulfoxide
was added to dissolve the formazan. Cell viability was assessed by
measuring the absorbance at an optical density of 450 nm using a
microplate reader (Molecular Devices, LLC).
Transwell migration assay
The hypoxia-treated and transfected PASMCs
(2×104) were resuspended in 200 µl of serum-free medium
and then plated into the upper chambers of each Transwell apparatus
(8 µm pore size; BD Biosciences). A total of 600 µl of medium
containing 10% FBS was added to the lower chambers followed by
incubation at 37°C for 48 h. Subsequently, the cells in the upper
chambers were wiped off using a cotton swab and those adhering to
the lower chambers were fixed with 4% paraformaldehyde for 1 h and
stained with 0.5% crystal violet [TCI (Shanghai) Development Co.,
Ltd.] at 37°C for 30 min. Stained cells were imaged using an
inverted light microscope (magnification, ×400; Olympus
Corporation) and analysed with ImageJ software (version 1.46,
National Institutes of Health).
Western blotting
Hypoxia-treated and transfected PASMCs were lysed
with RIPA buffer (Beyotime Institute of Biotechnology) to extract
total protein. The protein concentration was detected by the BCA
Protein Assay kit. Then, a total of 50 µg of protein/lane was
separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and then transferred onto polyvinylidene fluoride
membranes. Following blocking with 5% skimmed milk for 2 h at 25°C,
the membranes were incubated with the following primary antibodies:
PCNA (1:1,000; Abcam; cat. no. ab92552), MMP2 (1:1,000; Abcam; cat.
no. ab92536), α-smooth muscle actin (α-SMA; 1:1,000; Abcam; cat.
no. ab5694), total caspase-3 (t-caspase-3; 1:1,000; Abcam; cat. no.
ab32351), cleaved caspase-3 (c-caspase-3; 1:1,000; Abcam; cat. no.
ab2302), KLF4 (1:1,000; Abcam; cat. no. ab215036), and GAPDH
(1:1,000; Abcam; cat. no. ab215036) at 4°C overnight, and then with
the secondary antibody, HRP-conjugated anti-rabbit IgG (1:3,000;
Abcam; cat. no. ab6721) for 1 h at 37°C. The immunoblots were
visualised using an ECL detection kit (Thermo Fisher Scientific,
Inc.) under Gel-Pro analyzer (version 4.0; Media Cybernetics,
Inc.).
Dual luciferase reporter (DLR)
assay
The targeting relationship between NEAT1 and
miR-34a-5p was analysed using the StarBase software (version 2.0;
http://starbase.sysu.edu.cn).
Additionally, the targeting relationship between miR-34a-5p and
KLF4 was predicted using TargetScan software (release 7.2;
http://www.targetscan.org/vert_72/).
Fragments of NEAT1/KLF4 containing the miR-34a-5p binding site
[wild-type (wt) and mutated (mut)] were inserted into the pGL3
vector (Promega Corporation) to generate recombinant luciferase
reporter plasmids. PASMCs in 96-well plates (2,000 cells/well) were
co-transfected with either miR-34a-5p mimics or miR-NC and either
wt or mut NEAT1/KLF4 using Lipofectamine 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.). Following transfection for 48 h at 37°C,
luciferase activity was analysed using the DLR gene assay system
(Promega Corporation). The activity of firefly luciferase was
normalized to that of Renilla luciferase.
Statistical analysis
All experiments were performed in triplicate, and
each experiment was repeated three times. Data were analysed using
SPSS 23.0 software (IBM Corp.) and presented as the mean ± SD.
Comparisons between two groups were performed using unpaired
t-test, and comparisons among multiple groups were performed using
ANOVA, followed by Tukey's multiple comparison test. Pearson's
correlation analysis was used to assess the linear correlation of
serum concentrations in patients with PAH. P<0.05 was considered
to indicate a statistically significant difference.
Results
Low expression of lncRNA NEAT1
protects against PAH in vitro
It was initially observed that the expression of
NEAT1 was significantly increased in hypoxia-treated PASMCs when
compared to its expression levels in normoxic PASMCs (Fig. 1A; P<0.01). Given that NEAT1 was
overexpressed in hypoxia-treated PASMCs, NEAT1 was knocked down in
hypoxia-treated PASMCs by transfecting sh-NEAT1-1/-2 to further
explore the effects of NEAT1 on PAH in vitro. An analysis of
transfection efficiency revealed a decrease in NEAT1 expression in
transfected cells (Fig. 1B;
P<0.01). sh-NEAT1-1-transfected cells were selected for further
study due to their relatively low NEAT1 expression. An MTT assay
was performed to measure cell viability, which indicated that
transfection of sh-NEAT1 reversed the positive effect of hypoxia on
the viability of PASMCs (Fig. 1C;
P<0.01). The Transwell assay produced similar data, revealing
that hypoxia treatment significantly promoted the migration of
PASMCs, which was reversed by transfection with sh-NEAT1 (Fig. 1D; P<0.01). To further verify the
effects of sh-NEAT1 on the proliferation and migration of
hypoxia-treated PASMCs, a western blot assay was performed, which
demonstrated that knock down of NEAT1 expression markedly reduced
the hypoxia-induced increases in the protein levels of PCNA (a
proliferation-related protein) (26) and MMP2 (a migration-related protein)
(27) (Fig. 1E; P<0.01). It was also determined
that hypoxia treatment significantly reduced the level of α-SMA (a
marker of VSM-specific contraction) (28), and the ratio of
c-caspase-3/t-caspase-3 (a pro-apoptosis-related protein) (29) compared to the normoxic group,
indicating that more contractile PASMCs were converted to a
proliferative phenotype. However, these inhibitory effects were
reversed by transfection with sh-NEAT1-1 (Fig. 1F; P<0.01).
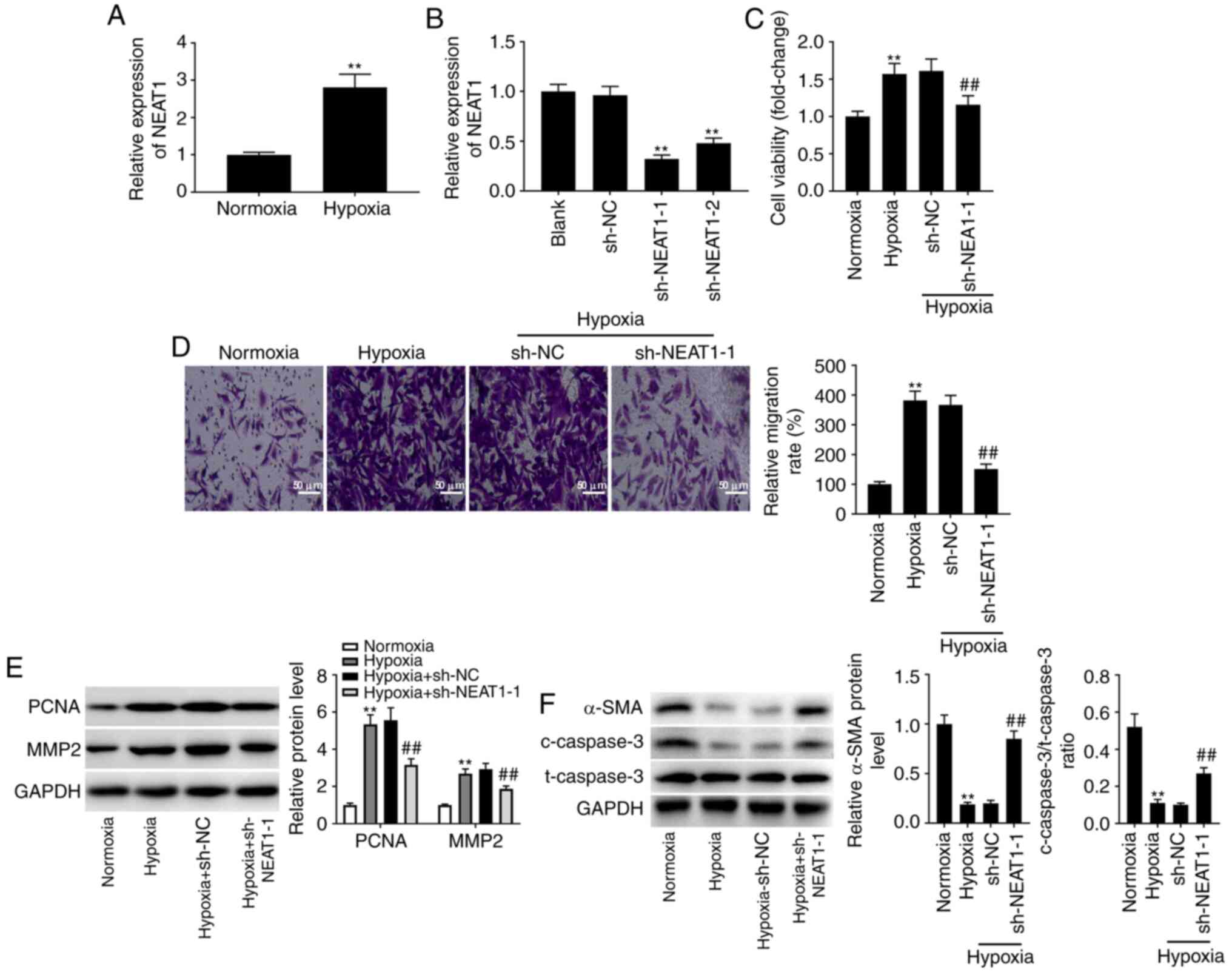 | Figure 1.Low expression of lncRNA NEAT1
protects against PAH in vitro. (A) The expression of NEAT1
in hypoxia-induced PASMCs was detected by RT-qPCR. **P<0.01 vs.
the normoxia PASMC group. (B) The expression of NEAT1 after
transfection of sh-NEAT1-1/-2/NC in hypoxia-induced PASMCs was
detected by RT-qPCR. **P<0.01 vs. the sh-NC group. (C) The
viability (OD450) of hypoxia-induced PASMCs was measured
by MTT assay. (D) The migration of hypoxia-induced PASMCs was
measured by Transwell assay. Scale bar, 50 µm. (E) The protein
levels of PCNA and MMP2 in hypoxia-induced PASMCs after
transfection of sh-NEAT1-1/NC were detected by western blotting.
(F) The protein levels of α-SMA, and c-caspase-3/t-caspase-3 ratio
in hypoxia-induced PASMCs after transfection of sh-NEAT1-1/NC was
detected by western blotting. **P<0.01 vs. the normoxia PASMC
group; ##P<0.01 vs. the hypoxia + sh-NC group.
lncRNA, long non-coding RNA; NEAT1, nuclear paraspeckle assembly
transcript 1; PAH, pulmonary arterial hypertension; PASMCs,
pulmonary arterial smooth muscle cells; sh-, short hairpin; NC,
negative control; RT-qPCR, reverse transcription-quantitative PCR;
MTT, 3-(4,5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2-h-tetrazolium
bromide. |
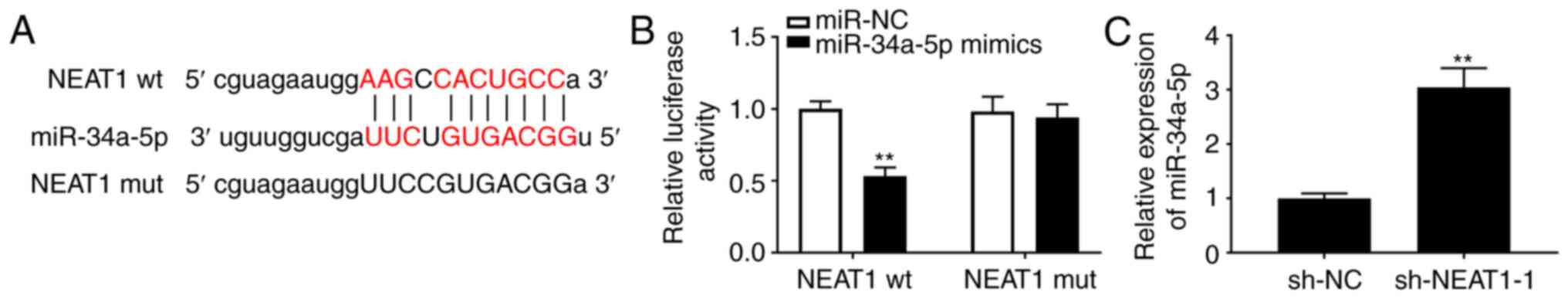
NEAT1 sponges miR-34a-5p
An underlying binding site between NEAT1 and
miR-34a-5p was revealed using StarBase (Fig. 2A). Then a DLR assay was conducted to
assess the role of this binding site and miR-34a-5p on NEAT1
expression, which yielded an interesting result; the luciferase
activity of the wt NEAT1 reporter was decreased by transfection of
miR-34a-5p mimics, whereas the luciferase activity of the NEAT1 mut
reporter was unaffected (Fig. 2B;
P<0.01). This result confirmed the relationship between NEAT1
and miR-34a-5p. Additionally, the expression of miR-34a-5p in
PASMCs was increased by transfection of sh-NEAT1 (Fig. 2C; P<0.01), indicating an inverse
relationship between them.
High expression of miR-34a-5p has
suppressive effects on PAH in vitro
The expression of miR-34a-5p in PASMCs under hypoxia
was evaluated by RT-qPCR. The results of RT-qPCR revealed that,
when compared to PASMCs under normoxia, the expression of
miR-34a-5p was reduced in hypoxia-treated PASMCs (Fig. 3A; P<0.01). To explore the
possible functions of miR-34a-5p in the pathogenesis of PAH in
vitro, miR-34a-5p mimics or inhibitor was transfected into
hypoxia-treated PASMCs. As anticipated, miR-34a-5p expression was
increased by miR-34a-5p mimics, whereas it was reduced by the
miR-34a-5p inhibitor (Fig. 3B;
P<0.01). The increases in proliferation (Fig. 3C; P<0.01) and migration (Fig. 3D; P<0.01) of PASMCs induced by
hypoxia were partly reversed by overexpression of miR-34a-5p.
Western blotting revealed that transfection of miR-34a-5p mimics
significantly reduced the hypoxia-induced increases in PCNA and
MMP2 protein levels (Fig. 3E;
P<0.01), which further confirmed the results of the MTT and
Transwell assays. Concurrently, overexpression of miR-34a-5p
increased the protein levels of α-SMA, and the
c-caspase-3/t-caspase-3 ratio (Fig.
3F; P<0.01).
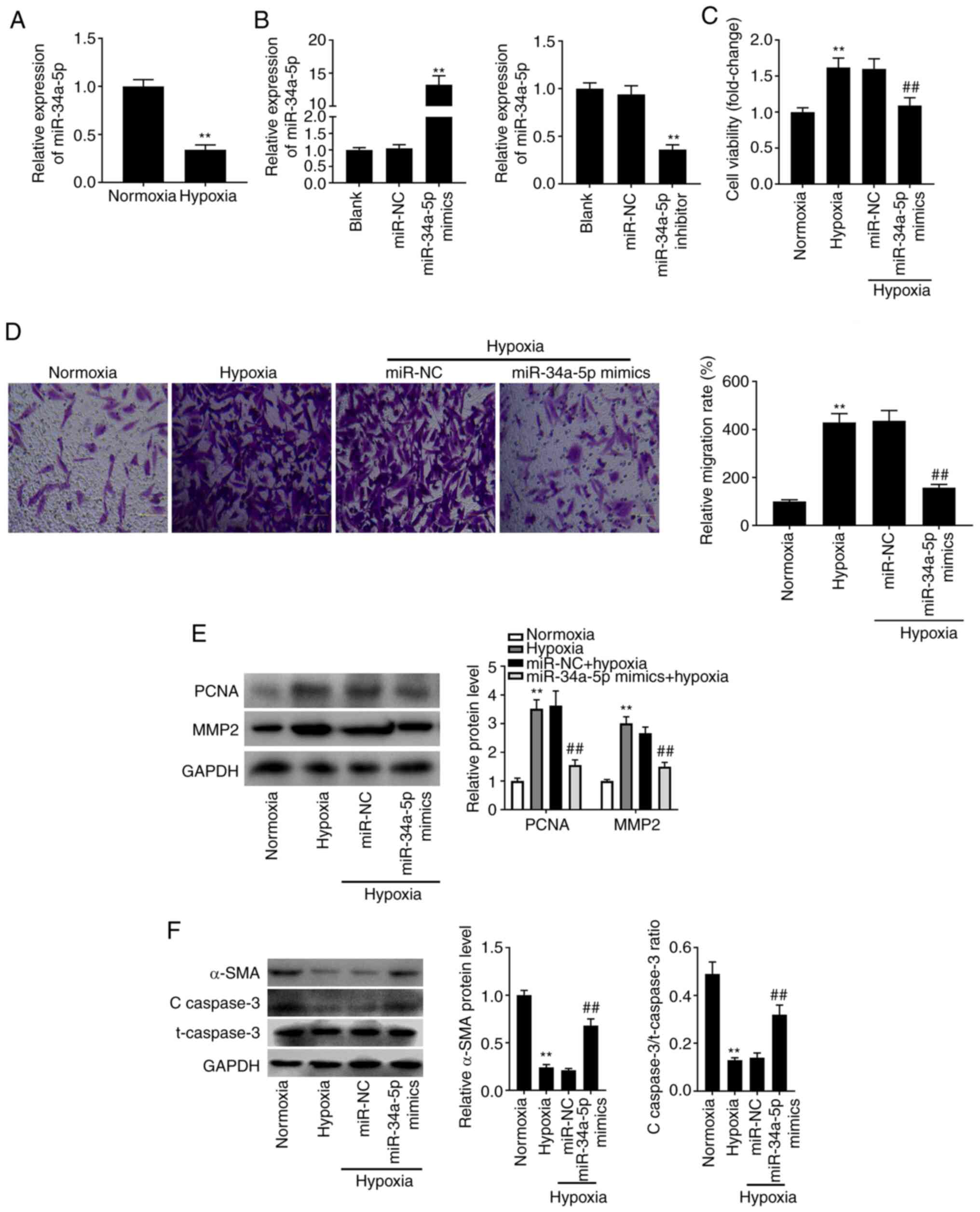 | Figure 3.High expression of miR-34a-5p plays a
suppressive role in PAH in vitro. (A) The expression of
miR-34a-5p in hypoxia-induced PASMCs was detected by RT-qPCR.
**P<0.01 vs. the normoxia PASMC group. (B) The expression of
miR-34a-5p after transfection of miR-34a-5p mimics/inhibitor in
hypoxia-induced PASMCs was detected by RT-qPCR. **P<0.01 vs. the
miR-NC group. (C) The viability (OD450) of
hypoxia-induced PASMCs was measured by MTT assay. (D) The migration
of hypoxia-induced PASMCs was measured by Transwell assay. Scale
bar, 50 µm. (E) The protein levels of PCNA and MMP2 in
hypoxia-induced PASMCs after transfection of miR-34a-5p mimics/NC
were detected by western blotting. (F) The protein levels of α-SMA,
and c-caspase-3/t-caspase-3 ratio in hypoxia-induced PASMCs after
transfection of miR-34a-5p mimics/NC were detected by western
blotting. **P<0.01 vs. the normoxia PASMC group;
##P<0.01 vs. the hypoxia + miR-NC group. miR-34a-5p,
microRNA-34a-5p; PAH, pulmonary arterial hypertension; PASMCs,
pulmonary arterial smooth muscle cells; RT-qPCR, reverse
transcription-quantitative PCR; NC, negative control; MTT,
3-(4,5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2-h-tetrazolium bromide;
c-, cleaved; t-, total. |
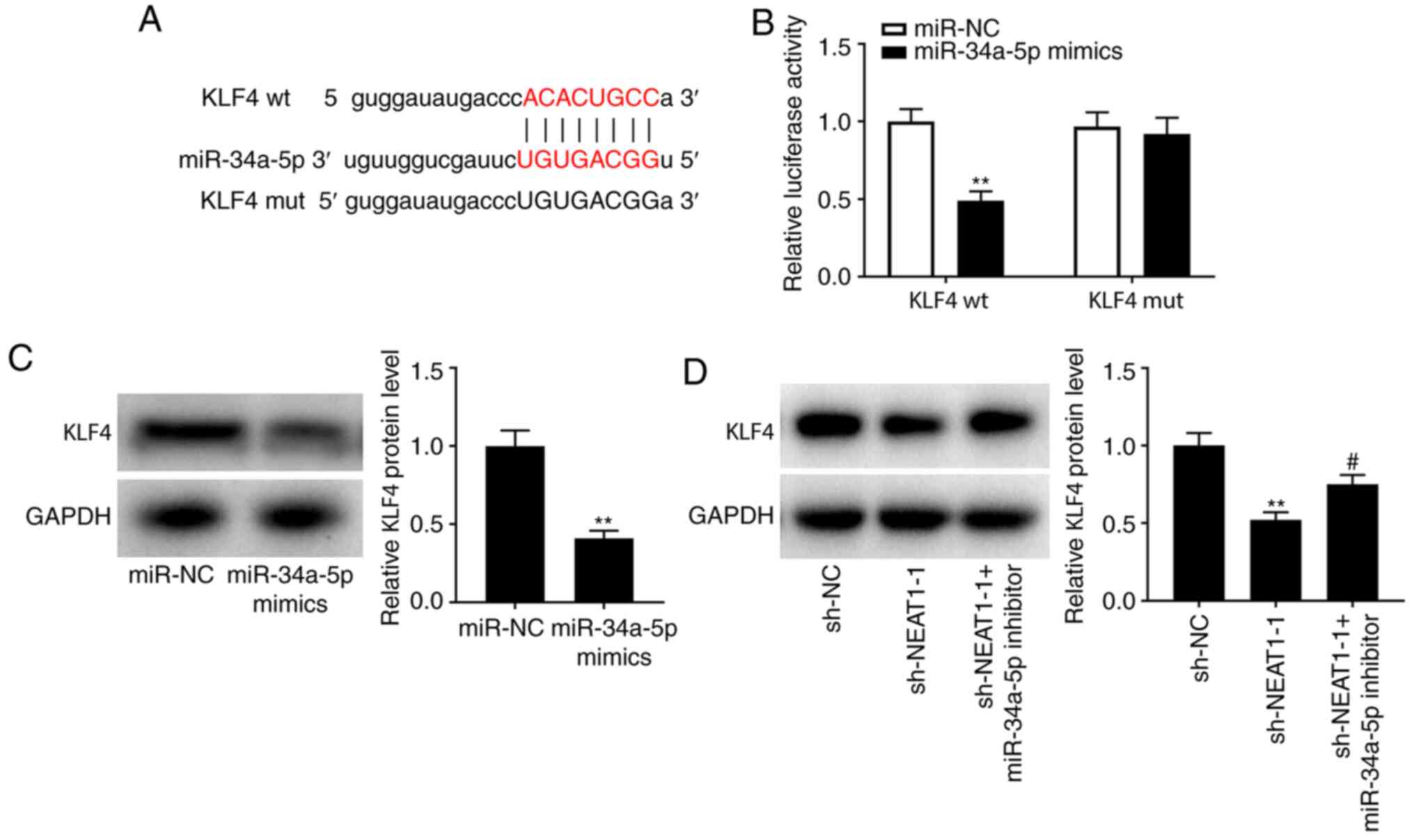
MiR-34a-5p targets KLF4
TargetScan predicted a potential binding site for
miR-34a-5p in KLF4 (Fig. 4A;
P<0.01). In addition, a DLR assay revealed lower luciferase
activity in KLF4-wt/miR-34a-5p-mimic-transfected cells than in
KLF4-wt/miR-NC-transfected cells (Fig.
4B; P<0.01), indicating a strong association between KLF4
and miR-34a-5p. Western blotting was performed to further examine
the interactions among NEAT1, miR-34a, and KLF4 in hypoxia-treated
PASMCs. It was determined that KLF4 protein levels were reduced by
transfection of miR-34a-5p mimics (Fig.
4C; P<0.01) as well as by transfection of sh-NEAT1-1,
whereas transfection of a miR-34a-5p inhibitor reversed the
suppressive effect of sh-NEAT-1 on KLF4 levels (Fig. 4D; P<0.05).
Knockdown of NEAT1 slows the process
of PAH by sponging miR-34a-5p and downregulating KLF4
As presented in Fig.
5A, KLF4 protein levels were increased in hypoxia-treated
PASMCs but not in cells under normoxia (P<0.01). pcDNA-KLF4 was
transfected into hypoxia-treated PASMCs and the transfection
efficiency was first evaluated. Transfection of pcDNA-KLF4 was
determined to be successful due to the significant increase in KLF4
levels (Fig. 5B; P<0.01). Using
these transfected cells, feedback verification experiments were
performed to further explore the regulatory mechanism involving
NEAT1, miR-34a-5p, and KLF4 in PAH progression in vitro. The
MTT, Transwell, and western blot assays revealed that under
hypoxia, transfection of miR-34a-5p inhibitor and pcDNA-KLF4 partly
eliminated the inhibitory effects of sh-NEAT1-1 on the
proliferation and migration of PASMCs (Fig. 5C-E; P<0.05). The role of the
NEAT1/miR-34a-5p/KLF4 axis in PASMC phenotype conversion and
apoptosis was then further explored. As revealed in Fig. 5F, both low miR-34a-5p expression and
high KLF4 expression reversed the promoting effects of NEAT1
downregulation on α-SMA level and the ratio of
c-caspase-3/t-caspase-3 (P<0.01).
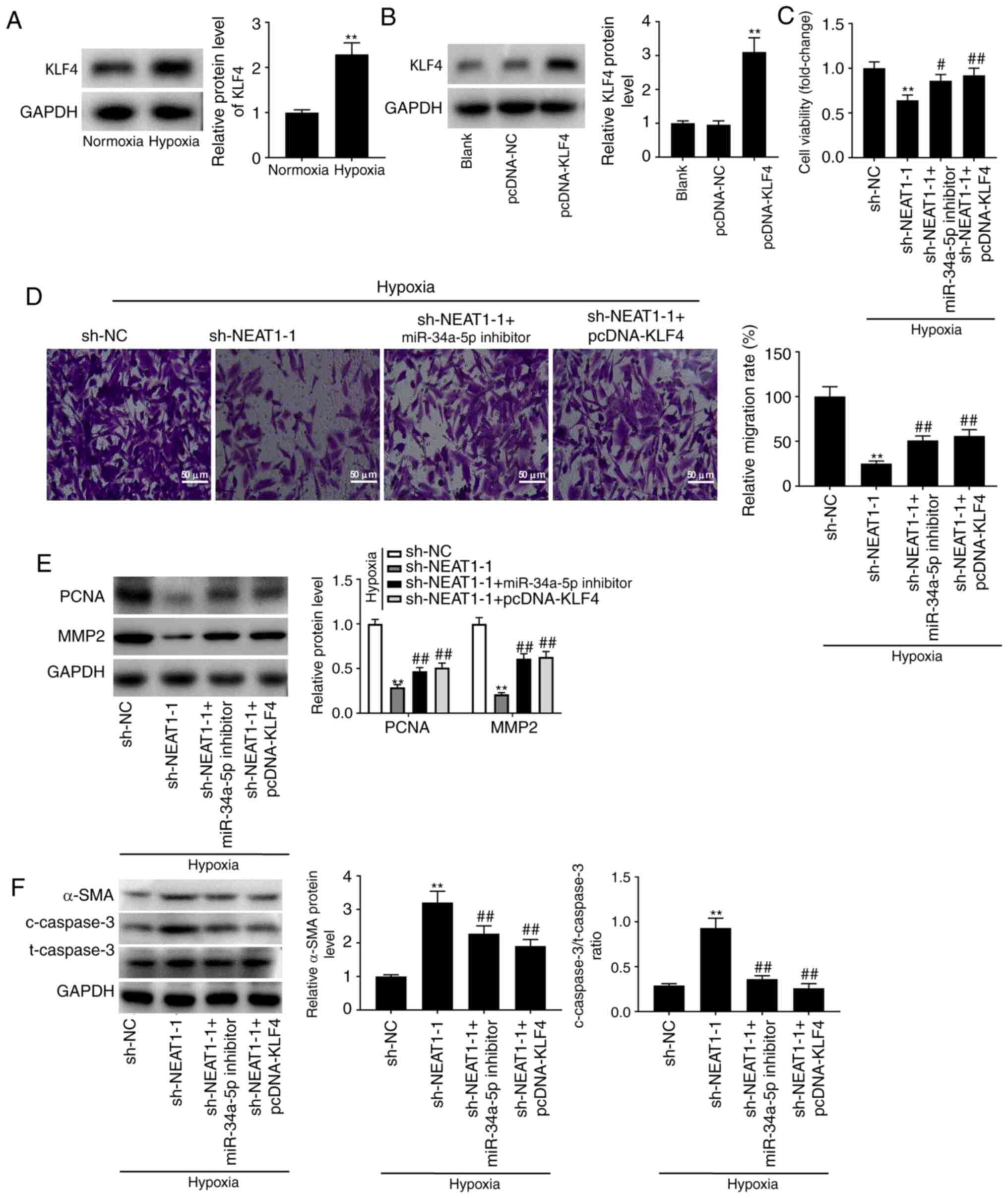 | Figure 5.Knockdown of NEAT1 slows the process
of PAH via sponging miR-34a-5p and downregulating KLF4. (A) The
protein level of KLF4 in hypoxia-induced PASMCs was determined by
western blotting. **P<0.01 vs. the normoxia PASMC group. (B) The
protein level of KLF4 in hypoxia-induced PASMCs after transfection
of pcDNA-KLF4/NC was determined by western blotting. **P<0.01
vs. the pcDNA-NC group. (C) The viability (OD450) of
hypoxia-induced PASMCs was measured by MTT assay. (D) The migration
of hypoxia-induced PASMCs was measured by Transwell assay. Scale
bar, 50 µm. (E) The protein levels of PCNA and MMP2 in
hypoxia-induced PASMCs after transfection of sh-NEAT1-1/sh-NEAT1-1
+ miR-34a-5p inhibitor/sh-NEAT1-1 + pcDNA-KLF4 were detected by
western blotting. (F) The protein levels of α-SMA, and
c-caspase-3/t-caspase-3 ratio in hypoxia-induced PASMCs after
transfection of sh-NEAT1-1/sh-NEAT1-1 + miR-34a-5p
inhibitor/sh-NEAT1-1 + pcDNA-KLF4 were detected by western
blotting. **P<0.01 vs. the hypoxia + sh-NC group;
#P<0.05, ##P<0.01 vs. the hypoxia +
sh-NEAT1-1 group. NEAT1, nuclear paraspeckle assembly transcript 1;
PAH, pulmonary arterial hypertension; KLF4, Krüppel-like factor 4;
PASMCs, pulmonary arterial smooth muscle cells; NC, negative
control; MTT, 3-(4,5-dimethyl-2-thiazolyl)-2,
5-diphenyl-2-h-tetrazolium bromide; sh-, short hairpin; miR-34a-5p,
microRNA-34a-5p; c-, cleaved; t-, total. |
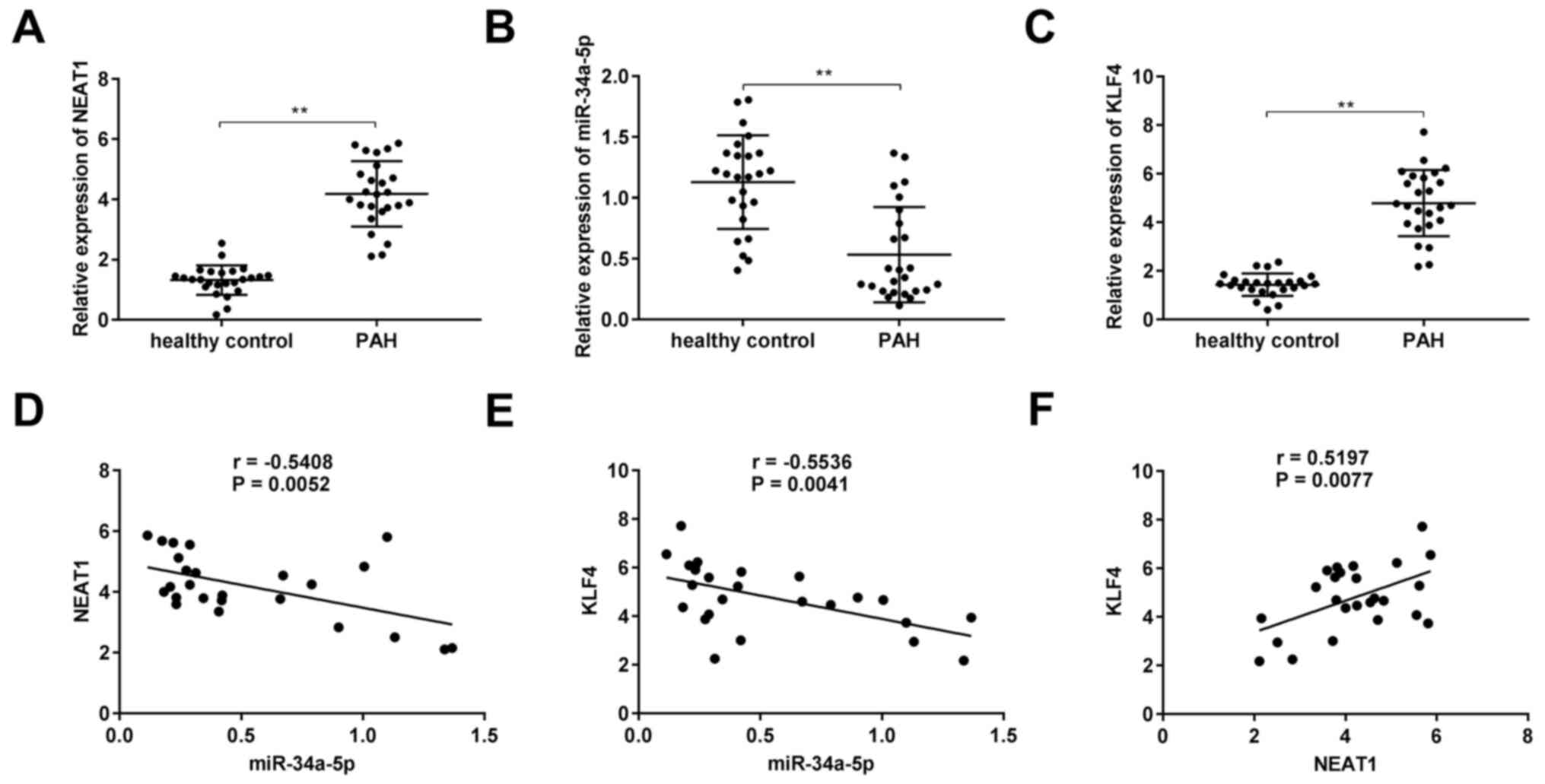
NEAT1 expression is upregulated in the
serum of patients with PAH
To investigate the roles of NEAT1, miR-34a-5p, and
KLF4 in PAH, their expression levels were assessed in the serum of
patients with PAH. As revealed in Fig.
6A-C, in comparison to healthy volunteers, the expression
levels of NEAT1 and KLF4 were significantly increased in the serum
of patients with PAH, whereas miR-34a-5p expression was decreased
(P<0.01). Additionally, there was a significant negative
correlation between miR-34a-5p and NEAT1 (Fig. 6D; P=0.0052, r=−0.5408) as well as
between miR-34a-5p and KLF4 (Fig.
6E; P=0.0041, r=−0.5536), whereas a significant positive
correlation was observed between NEAT1 and KLF4 (Fig. 6F; P=0.0077, r=0.5197).
Discussion
PAH is a cardiovascular disease with a high
mortality rate that is characterised by aberrant function of PASMCs
(18,30). Sun et al analysed a
microarray of lncRNAs in a rat model of PAH, and revealed that
several lncRNAs were overexpressed (31). In addition, numerous previous
studies have revealed several lncRNAs that are highly expressed in
PAH, such as HOXA-AS3 (7), MALAT1
(8) and TUG1 (9). Consistently, in the present study it
was revealed that NEAT1 expression was increased not only in
hypoxia-treated PASMCs but also in the serum of patients with PAH.
This result suggested that NEAT1 may be a pathogenetic lncRNA
involved in the progression of PAH.
sh-NEAT1 was transfected into hypoxia-treated PASMCs
to explore the possible role of NEAT1 in PAH progression in
vitro. Notably, it was revealed that under hypoxic conditions,
downregulation of NEAT1 markedly inhibited the proliferation and
migration of PASMCs. Our results are in line with data from
previous studies showing that aberrantly expressed NEAT1 had
significant effects on lung-related diseases (32–36).
In addition, it has been revealed that downregulation of NEAT1
suppressed cell proliferation, migration, and invasion in lung
cancer (32–34), and NEAT1 overexpression aggravated
inflammation and decreased lung function in asthma (35) and chronic obstructive pulmonary
disease (36). It was hypothesized
that knockdown of NEAT1 may also inhibit hyperproliferation of the
PA, thereby accelerating the progression of PAH. Transfection of
si-NEAT1 was shown to inhibit the proliferation and migration of
vascular smooth muscle cells (14).
Given the inhibitory effects of NEAT1 silencing on the
proliferation and migration of several types of human cancers
(10–13), it was concluded that NEAT1
downregulation attenuates the progression of PAH in
vitro.
Over the past decade, several researchers have
identified miRNAs with decreased expression levels in a rat model
of PAH and PASMCs, including miR-98 (16), miR-140-5p (16), miR-106b-5p (37) and miR-124-3p (8). Similarly, in the present study, a
reduction in the expression of miR-34a-5p in hypoxia-treated PASMCs
and PAH patient serum was also revealed. Aberrant expression
(especially overexpression) of miR-34a-5p has been shown to inhibit
the malignant behaviours of pulmonary cancers and acute lung injury
(38,39), and high miR-34a-5p expression
inhibited cell proliferation, migration, and invasion in lung
adenocarcinoma (38) and non-small
cell lung cancer (39). In the
present study, it was revealed that cell viability was reduced by
transfection with miR-34a-5p mimics. Data from a recent study
conducted by Wang et al are in line with our results: That
upregulation of miR-34a reduced the proliferation of PASMCs
(40). It was further demonstrated
that miR-34a-5p plays an important role in suppressing cell
migration and decreasing PCNA and MMP2 protein levels. Binding of
NEAT1 and miR-34a-5p was predicted and confirmed in the present
study. Therefore, it was predicted that the effect of miR-34a-5p on
hyperplasia of PASMCs is modulated by NEAT1. To verify this, it was
assessed whether NEAT1 can interact with miR-34a-5p, and the
results revealed that transfection of a miR-34a-5p inhibitor
reversed the suppressive effects of sh-NEAT1 on cell proliferation
and migration. It was also revealed that miR-34a-5p expression was
inversely correlated with NEAT1 expression. These results indicated
that NEAT1 knockdown alleviates the development of PAH by
upregulating miR-34a.
Deletion of KLF4 in smooth muscle protects against
vascular diseases, such as aortic aneurysm and atherosclerosis
(41,42). Notably, expression of KLF4 was shown
to be elevated in both PAH model rats and patients (43,44).
Consistently, it was determined that KLF4 was not only highly
expressed in the serum of PAH patients but also in hypoxia-treated
PASMCs. Given the positive correlation between KLF4 and NEAT1 in
the serum of PAH patients and the targeting relationship between
KLF4 and miR-34a-5p, it was hypothesized that KLF4 is also involved
in PAH. The feedback verification experiments demonstrated that in
hypoxia-treated PASMCs, transfection of pcDNA-KLF4 markedly
reversed the inhibitory effects of sh-NEAT1 on cell proliferation
and migration, which supported our hypothesis. In conclusion, the
present results indicated that NEAT1 silencing alleviates PAH by
regulating miR-34a-5p/KLF4.
However, there may be some limitations in this
study. First, the expression of NEAT1 was only determined in PAH
patients, and further detection of NEAT1 expression in patients
with remission of PAH may be more scientific. Second, this study
only focused on the cellular level, and more in vivo
experiments are required. These issues will be elucidated in future
studies.
In summary, the present results revealed that NEAT1
expression was increased in the serum of PAH patients and
hypoxia-treated PASMCs. NEAT1 silencing attenuated PAH progression
by upregulating miR-34a-5p and targeting KLF4. Our data provide a
potential novel target and biomarker for the treatment or diagnosis
of PAH.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XD and YM designed and analysed the data obtained
from the experiments and were major contributors in writing the
manuscript. XD and YM confirm the authenticity of all the raw data.
YQ and QD performed the experiments and helped draft the
manuscript. SZ, RT, and MP assisted in the experiments and revision
of the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The People's Hospital of Rizhao (Rizhao, China).
Written informed consent was obtained from all subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hu W and Huang Y: Targeting the
platelet-derived growth factor signalling in cardiovascular
disease. Clin Exp Pharmacol Physiol. 42:1221–1224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lai YC, Potoka KC, Champion HC, Mora AL
and Gladwin MT: Pulmonary arterial hypertension: The clinical
syndrome. Circ Res. 115:115–130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gomez D and Owens GK: Smooth muscle cell
phenotypic switching in atherosclerosis. Cardiovasc Res.
95:156–164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nie X, Chen Y, Tan J, Dai Y, Mao W, Qin G,
Ye S, Sun J, Yang Z and Chen J: MicroRNA-221-3p promotes pulmonary
artery smooth muscle cells proliferation by targeting AXIN2 during
pulmonary arterial hypertension. Vascul Pharmacol. 116:24–35. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu X, Murphy TC, Nanes MS and Hart CM:
PPAR{gamma} regulates hypoxia-induced Nox4 expression in human
pulmonary artery smooth muscle cells through NF-{kappa}B. Am J
Physiol Lung Cell Mol Physiol. 299:L559–L566. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shimoda LA and Laurie SS: Vascular
remodeling in pulmonary hypertension. J Mol Med (Berl). 91:297–309.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang H, Liu Y, Yan L, Wang S, Zhang M, Ma
C, Zheng X, Chen H and Zhu D: Long noncoding RNA Hoxaas3
contributes to hypoxia-induced pulmonary artery smooth muscle cell
proliferation. Cardiovasc Res. 115:647–657. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang D, Xu H, Wu B, Jiang S, Pan H, Wang R
and Chen J: Long noncoding RNA MALAT1 sponges miR1243p.1/KLF5 to
promote pulmonary vascular remodeling and cell cycle progression of
pulmonary artery hypertension. Int J Mol Med. 44:871–884.
2019.PubMed/NCBI
|
|
9
|
Yang L, Liang H, Shen L, Guan Z and Meng
X: lncRNA Tug1 involves in the pulmonary vascular remodeling in
mice with hypoxic pulmonary hypertension via the
microRNA-374c-mediated Foxc1. Life Sci. 237:1167692019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng Y, Gao L, Cui G and Cao Y: lncRNA
NEAT1 facilitates pancreatic cancer growth and metastasis through
stabilizing ELF3 mRNA. Am J Cancer Res. 10:237–248. 2020.PubMed/NCBI
|
|
11
|
Yan L, Zhang Z, Yin X and Li Y: lncRNA
NEAT1 facilitates cell proliferation, invasion and migration by
regulating CBX7 and RTCB in breast cancer. Onco Targets Ther.
13:2449–2458. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen LM, Niu YD, Xiao M, Li XJ and Lin H:
lncRNA NEAT1 regulated cell proliferation, invasion, migration and
apoptosis by targeting has-miR-376b-3p/SULF1 axis in non-small cell
lung cancer. Eur Rev Med Pharmacol Sci. 24:4810–4821.
2020.PubMed/NCBI
|
|
13
|
Wang S, Du H and Sun P: Long noncoding RNA
NEAT1 contributes to the tumorigenesis of colorectal cancer through
regulating SLC38A1 expression by sponging miR-138. Cancer Biother
Radiopharm. Jul 17–2020.(Epub ahead of print). doi:
10.1089/cbr.2020.3608. View Article : Google Scholar
|
|
14
|
Ahmed ASI, Dong K, Liu J, Wen T, Yu L, Xu
F, Kang X, Osman I, Hu G, Bunting KM, et al: Long noncoding RNA
NEAT1 (nuclear paraspeckle assembly transcript 1) is critical for
phenotypic switching of vascular smooth muscle cells. Proc Natl
Acad Sci USA. 115:E8660–E8667. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Q and Zhou X and Zhou X: Downregulation
of miR98 contributes to hypoxic pulmonary hypertension by targeting
ALK1. Mol Med Rep. 20:2167–2176. 2019.PubMed/NCBI
|
|
16
|
Zhu TT, Zhang WF, Yin YL, Liu YH, Song P,
Xu J, Zhang MX and Li P: MicroRNA-140-5p targeting tumor necrosis
factor-alpha prevents pulmonary arterial hypertension. J Cell
Physiol. 234:9535–9550. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun L, Lin P, Chen Y, Yu H, Ren S, Wang J,
Zhao L and Du G: miR-182-3p/Myadm contribute to pulmonary artery
hypertension vascular remodeling via a KLF4/p21-dependent
mechanism. Theranostics. 10:5581–5599. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Humbert M, Sitbon O and Simonneau G:
Treatment of pulmonary arterial hypertension. N Engl J Med.
351:1425–1436. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ghaleb AM and Yang VW: Kruppel-like factor
4 (KLF4): What we currently know. Gene. 611:27–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun D, Li Q, Ding D, Li X, Xie M, Xu Y and
Liu X: Role of Kruppel-like factor 4 in cigarette smoke-induced
pulmonary vascular remodeling. Am J Transl Res. 10:581–591.
2018.PubMed/NCBI
|
|
21
|
Liang S, Yu H, Chen X, Shen T, Cui Z, Si
G, Zhang JT, Cheng Y, Jia S, Song S, et al: PDGF-BB/KLF4/VEGF
signaling axis in pulmonary artery endothelial cell angiogenesis.
Cell Physiol Biochem. 41:2333–2349. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng F, Liu H, Chen A, Xia Q, Zhao Y, Jin
X and Huang J: miR-148-3p and miR-152-3p synergistically regulate
prostate cancer progression via repressing KLF4. J Cell Biochem.
120:17228–17239. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao L, Han T, Li Y, Sun J, Zhang S, Liu
Y, Shan B, Zheng D and Shi J: The lncRNA SNHG5/miR-32 axis
regulates gastric cancer cell proliferation and migration by
targeting KLF4. FASEB J. 31:893–903. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhai F, Cao C, Zhang L and Zhang J:
miR-543 promotes colorectal cancer proliferation and metastasis by
targeting KLF4. Oncotarget. 8:59246–59256. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cardano M, Tribioli C and Prosperi E:
Targeting proliferating cell nuclear antigen (PCNA) as an effective
strategy to inhibit tumor cell proliferation. Curr Cancer Drug
Targets. 20:240–252. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu DM, Deng SH, Liu T, Han R, Zhang T and
Xu Y: TGF-β-mediated exosomal lnc-MMP2-2 regulates migration and
invasion of lung cancer cells to the vasculature by promoting MMP2
expression. Cancer Med. 7:5118–5129. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu MM, Deng HY and Li HH: MicroRNA-27a
regulates angiotensin II-induced vascular smooth muscle cell
proliferation and migration by targeting α-smooth muscle-actin in
vitro. Biochem Biophys Res Commun. 509:973–977. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shi S, Liu XL and Li HB: Downregulation of
caspase-3 alleviates mycoplasma pneumoniae-induced apoptosis in
alveolar epithelial cells. Mol Med Rep. 16:9601–9606. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Archer SL, Weir EK and Wilkins MR: Basic
science of pulmonary arterial hypertension for clinicians: New
concepts and experimental therapies. Circulation. 121:2045–2066.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun Z, Liu Y, Yu F, Xu Y, Yanli L and Liu
N: Long non-coding RNA and mRNA profile analysis of metformin to
reverse the pulmonary hypertension vascular remodeling induced by
monocrotaline. Biomed Pharmacother. 115:1089332019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qi L, Liu F, Zhang F, Zhang S, Lv LY, Bi Y
and Yu Y: lncRNA NEAT1 competes against let-7a to contribute to
non-small cell lung cancer proliferation and metastasis. Biomed
Pharmacother. 103:1507–1515. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu PF, Wang Y, Lv W, Kou D, Hu HL, Guo SS
and Zhao YJ: lncRNA NEAT1/miR-1224/KLF3 contributes to cell
proliferation, apoptosis and invasion in lung cancer. Eur Rev Med
Pharmacol Sci. 23:8403–8410. 2019.PubMed/NCBI
|
|
34
|
Ma F, Lei YY, Ding MG, Luo LH, Xie YC and
Liu XL: lncRNA NEAT1 interacted with DNMT1 to regulate malignant
phenotype of cancer cell and cytotoxic T cell infiltration via
epigenetic inhibition of p53, cGAS, and STING in lung cancer. Front
Genet. 11:2502020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li X, Ye S and Lu Y: Long non-coding RNA
NEAT1 overexpression associates with increased exacerbation risk,
severity, and inflammation, as well as decreased lung function
through the interaction with microRNA-124 in asthma. J Clin Lab
Anal. 34:e230232020.PubMed/NCBI
|
|
36
|
Ming X, Duan W and Yi W: Long non-coding
RNA NEAT1 predicts elevated chronic obstructive pulmonary disease
(COPD) susceptibility and acute exacerbation risk, and correlates
with higher disease severity, inflammation, and lower miR-193a in
COPD patients. Int J Clin Exp Pathol. 12:2837–2848. 2019.PubMed/NCBI
|
|
37
|
Chen H, Ma Q, Zhang J, Meng Y, Pan L and
Tian H: miR-106b-5p modulates acute pulmonary embolism via NOR1 in
pulmonary artery smooth muscle cells. Int J Mol Med. 45:1525–1533.
2020.PubMed/NCBI
|
|
38
|
Li W, Pan T, Jiang W and Zhao H:
HCG18/miR-34a-5p/HMMR axis accelerates the progression of lung
adenocarcinoma. Biomed Pharmacother. 129:1102172020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Luo S, Shen M, Chen H, Li W and Chen C:
Long noncoding RNA TP73AS1 accelerates the progression and
cisplatin resistance of nonsmall cell lung cancer by upregulating
the expression of TRIM29 via competitively targeting microRNA34a5p.
Mol Med Rep. 22:3822–3832. 2020.PubMed/NCBI
|
|
40
|
Wang P, Xu J, Hou Z, Wang F, Song Y, Wang
J, Zhu H and Jin H: miRNA-34a promotes proliferation of human
pulmonary artery smooth muscle cells by targeting PDGFRA. Cell
Prolif. 49:484–493. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shankman LS, Gomez D, Cherepanova OA,
Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene
ES, Straub AC, et al: KLF4-dependent phenotypic modulation of
smooth muscle cells has a key role in atherosclerotic plaque
pathogenesis. Nat Med. 21:628–637. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Salmon M, Johnston WF, Woo A, Pope NH, Su
G, Upchurch GR Jr, Owens GK and Ailawadi G: KLF4 regulates
abdominal aortic aneurysm morphology and deletion attenuates
aneurysm formation. Circulation. 128 (11 Suppl 1):S163–S174. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sheikh AQ, Misra A, Rosas IO, Adams RH and
Greif DM: Smooth muscle cell progenitors are primed to muscularize
in pulmonary hypertension. Sci Transl Med. 7:308ra1592015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sheikh AQ, Saddouk FZ, Ntokou A, Mazurek R
and Greif DM: Cell autonomous and non-cell autonomous regulation of
SMC progenitors in pulmonary hypertension. Cell Rep. 23:1152–1165.
2018. View Article : Google Scholar : PubMed/NCBI
|