Introduction
Diabetes mellitus (DM) is a group of metabolic
diseases characterized by hyperglycemia and divided into type 1 and
type 2 diabetes. The two types of DM can be caused by genetic and
environmental factors (1). The
typical symptoms of DM include polydipsia, polyuria, polyphagia,
weight loss, fatigue, weakness and obesity (2). In 2019, ~463 million adults aged
between 20 and 79 suffered from DM worldwide, with a prevalence
rate of about 9.3% (3). The major
long-term and chronic complications of diabetes mellitus (DM) are
vascular complications, which are the main causes of increased
morbidity and mortality in patients with DM (4,5).
Numerous associations between DM and atherosclerosis (AS) have been
identified, too much sugar in the blood is associated with
insufficient insulin production or a reduced body response to
insulin (6,7). Clinical studies have suggested that
there is an association between the risk of diabetic vascular
complications and poor glycemic control (8,9). AS
refers to the buildup of vascular wall lesions caused by chronic
inflammation, which result in hypertension, formation of blood
clot, strokes, heart attack (10).
Hyperglycemia and insulin resistance are important causes of
chronic inflammation in patients with DM, therefore accelerating
the development of AS (11). In
addition, inflammatory factors, such as IL-1β and IL-6, can promote
the migration and proliferation of smooth muscle cells to the
intima of blood vessels, leading to intima-media thickening and
promoting the occurrence and development of AS (12,13).
High mobility group box 1 (HMGB1) is an important
mediator of the inflammatory response, which can serve as a
pro-inflammatory factor that promotes the occurrence and
development of inflammation (14,15).
HMGB1 overexpression is associated with the pathogenesis of AS
(16), obesity (17) and metabolic syndromes (18). Glycyrrhizic acid (GA), an inhibitor
of HMGB1, may have a protective effect in AS. Ding et al
(19) reported that GA decreases
high-fat diet-induced AS in Apoe−/− mice by
significantly decreasing serum HMGB1 and lipid levels, and by
increasing the regulatory T-cell/T helper T-cell ratio. In
addition, it has been demonstrated that GA can prevent diabetic
nephropathy by activating the AMP-activated protein kinase/sirtuin
1/peroxisome proliferator-activated receptor γ co-activator 1
signaling pathway in db/db mice (20). GA can also decrease the vascular
complications induced by diabetic nephropathy (21). However, the effect of GA on
DM-associated AS remains unknown. The present study therefore aimed
to explore whether GA could decrease HMGB1 expression and reduce
the atherosclerotic damage caused by DM.
Materials and methods
Animal model and treatment
Male Sprague Dawley rats (n=40) weighing 180–200 g
from Changzhou Cavens Laboratory Animal Co. Ltd.) were divided into
the control group (n=10) and the diabetic AS group (DM-AS; n=30)
and were kept in an environment with a constant temperature of 25°C
and humidity of 30–70% with 12 h light/dark cycle, the mice were
fed normally and maintained in specific pathogen-free conditions.
All animal procedures and experimental methods were approved by the
Committee on the Ethics of Animal Experiments of the Fifth
Affiliated Hospital of Zhengzhou University (approval no.
2019A111501) and animal experiments were reported in accordance
with the ARRIVE guidelines. After 8 weeks of a high-fat diet, which
contained 45.6% fat, rats in the DM-AS group were injected with
low-dose streptozotocin (STZ; 30 mg/kg; Sigma-Aldrich; Merck KGaA)
to establish a diabetic AS model (22). The control group was given a normal
diet, which contained ~4.0% fat, and the same dose of citric acid
buffer containing STZ was injected intravenously at week 8. Rats
from the DM-AS group were subsequently divided into DM-AS, DM-AS +
GA (50 mg/kg) and DM-AS + GA (150 mg/kg) groups. Once the model was
established successfully, the rats were given 50 or 150 mg/kg/day
GA by intragastric administration for 16 weeks. The control and
experimental groups were given the same dose of normal saline by
gavage. Rats were sacrificed by cervical dislocation following
anesthesia with pentobarbital sodium (60 mg/kg intraperitoneal
injection). Peripheral blood (3 ml) was collected from the inner
canthus of each rat and serum was obtained following centrifugation
at 150 × g for 10 min at 4°C for biochemical assays. Furthermore,
aorta specimens were removed carefully and fixed in 4%
paraformaldehyde for hematoxylin and eosin (H&E) and
immunohistochemistry (IHC) staining. The other aorta tissues were
stored at −80°C for reverse transcription quantitative PCR
(RT-qPCR), western blotting and ELISA.
Biochemical assays
The levels of blood glucose, high-density
lipoprotein cholesterol (HDL-C), low-density lipoprotein
cholesterol (LDL-C), total cholesterol (TC), total triglycerides
(TG) and fasting insulin (FINS) were measured in the serum using an
automatic biochemical analyzer (MR-96A; Mindray Bio-Medical
Electronics Co., Ltd.) from the Hospital of Metabolic Disease of
Tianjin Medical University (Tianjin, China) according to the
manufacturers' instructions.
Histopathological examination
Once mice were euthanized, the chest was cut open
after the collection of blood. The aorta was collected quickly and
rinsed gently with saline. A 2- to 3-mm slice was cut from the
aortic arch and fixed with 4% paraformaldehyde at 4°C for 24 h and
embedded in paraffin. Samples were cut into 4-µm sections for
H&E staining for 10 min at room temperature. Aortic pathology
changes were observed using a microscope camera (BA400 Digital;
Mike Audi Industrial Group Co., Ltd.). The thickness of the intima
and the media of arterial tissue was measured using Image-Pro Plus
6.0 software (Media Cybernetics, Inc.).
IHC staining
Fixed and paraffin-embedded aorta tissue samples
were cut into 4-µm sections and were dewaxed. Endogenous peroxidase
activity and non-specific binding were blocked by incubation with
3% hydrogen peroxide and 100% non-immune serum (closed serum; cat.
no. G9023; Sigma-Aldrich; Merck KGaA) respectively for 30 min at
room temperature. Sections were incubated with anti-CD68 (EMD
Millipore; cat. no. 051050; 1:250) and anti-α-SMA (Abcam; cat. no.
ab32575; 1:250) antibodies at 4°C overnight, and with anti-rabbit
secondary antibody (1:5,000; cat. no. ab181658; Abcam) at room
temperature for 1 h. Diaminobenzidine hydrochloride (Dako; Agilent
Technologies, Inc.) was then added to localize positive staining
that was acquired using light microscopy. The sections were
counterstained with hematoxylin for 10 min at room temperature. The
data were analyzed via densitometry using ImageJ software (version
146; National Institutes of Health).
Western blotting
Fresh tissues were lysed using RIPA buffer
(Sigma-Aldrich; Merck KGaA). Protein concentration was determined
using bicinchoninic acid assay protein assay kit. Proteins (25
µg/lane) were separated by 10% SDS-PAGE and transferred onto PVDF
membranes and blocked in 5% non-fat milk at room temperature for 1
h. Membranes were subsequently incubated with primary antibodies
overnight at 4°C against fatty acid synthetase (FAS; 1:1,000; cat.
no. ab133619; Abcam), sterol regulatory element binding protein 1C
(SREBP-1c; 1:1,000; cat. no. PA1-337; Thermo Fisher Scientific,
Inc.), HMGB1 (1:1,000; cat. no. ab18256; Abcam), receptor for
advanced glycation end products (RAGE; 1:1,000; cat. no. ab216329;
Abcam) and GAPDH (1:1,000; cat. no. ab181602; Abcam). Membranes
were then incubated with goat anti-rabbit horseradish
peroxidase-conjugated secondary antibody (1:5,000; cat. no.
ab181658; Abcam) at room temperature for 2 h. Enhanced
chemiluminescence reagent (Amersham Pharmacia Biotech Inc.; Cytiva)
was used to detect the signal on the membrane. The data were
analyzed via densitometry using ImageJ software (version 1.46;
National Institutes of Health) and normalized to the expression of
the internal control GAPDH.
ELISA
Expression of inflammatory cytokines in serum and
tissues were detected by ELISA kits. The levels of IL-6 (cat. no.
506-RL-010), IL-1β (cat. no. 501-RL-010), and TNF-α; cat. no.
5035-TG-025) were measured using ELISA kits (R&D Systems, Inc.)
according to the manufacturers' instructions.
RT-qPCR
Total RNA was isolated from tissues using
TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the suppliers' instructions. cDNA was synthesized
using a SuperScript III First-Strand Synthesis SuperMix for qRT-PCR
(Thermo Fisher Scientific, Inc.) according to the manufacturers'
instructions. SYBR Green PCR Master mix (Applied Biosystems; Thermo
Fisher Scientific, Inc.) was used to detect mRNA expression level.
The thermocycling conditions were as follows: 95°C for 10 min, 40
cycles of 95°C for 10 sec, 55°C for 10 sec and 72°C for 30 sec.
GAPDH was used as an internal control. The sequences of the primers
were as follows: HMGB1 forward, 5′-GGGATGGCAAAGTTTTTCCCTTTA-3′ and
reverse 5′-CACTAACCCTGCTGTTCGCT-3′; RAGE forward,
5′-ACAGAAACCGGTGATGAAGG-3′ and reverse,
5′-ATTCAGCTCTGCACGTTCCCT-3′; and GAPDH forward,
5′-ATTGTCAGCAATGCATCCTG-3′ and reverse 5′-GTAGGCCATGAGGTCCACdCA-3′.
The relative levels of gene expression were quantified by using the
comparative CT method (23).
Statistical analysis
SPSS 18.0 (SPSS, Inc.) was used to analyze the
results. Data are expressed as the mean ± standard deviation.
Comparisons among multiple groups were analyzed using one-way ANOVA
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of GA on the body weight and
serum blood glucose level of DM-AS rats
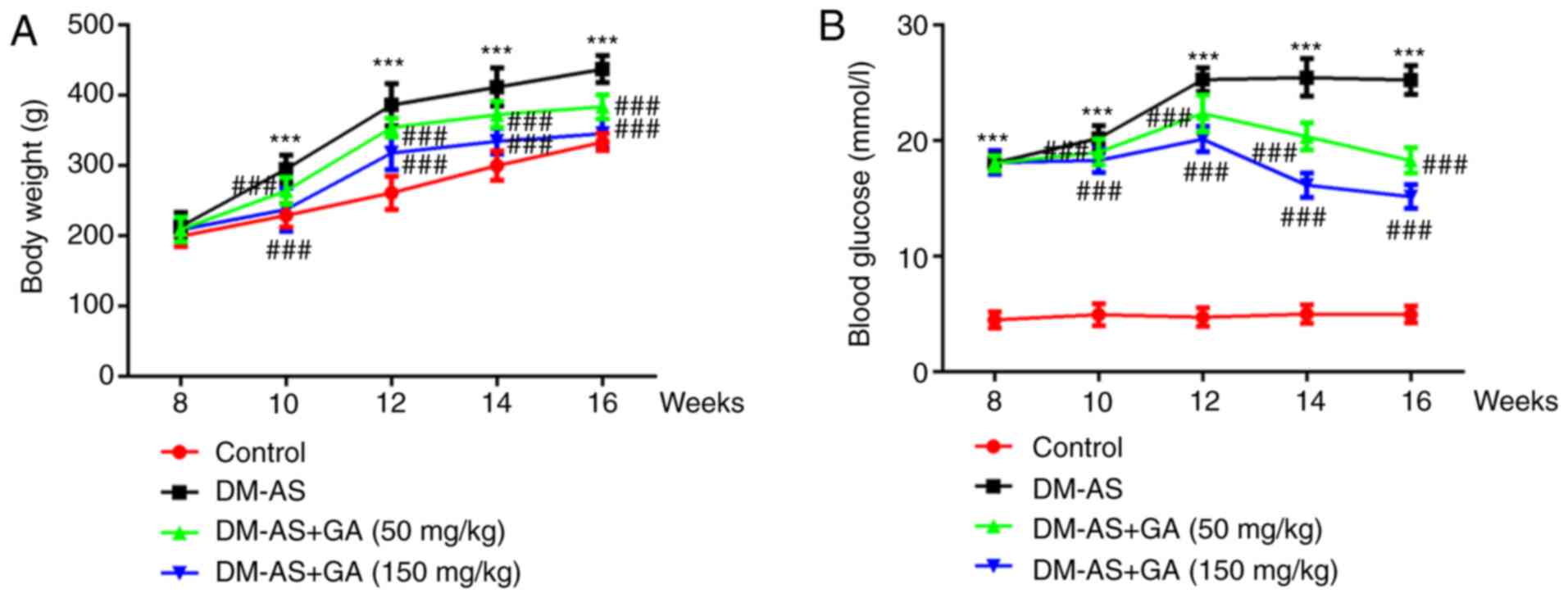
The weight of the rats was recorded week 8 and every
2 weeks up to week 16. The results demonstrated that, compared with
the control group, the weight of rats in the DM-AS group was
significantly increased; however, treatment with GA could decrease
rat body weight in a dose-dependent manner (Fig. 1A). During the same period, the serum
blood glucose level of rats was assessed, and the results
demonstrated that glycemia was significantly increased in the DM-AS
group compared with that in the control group. In addition,
compared with the DM-AS group, the blood glucose level of rats in
the DM-AS + GA group significant decreased from the second week
following administration of GA, and this decrease was time- and
concentration-dependent (Fig. 1B).
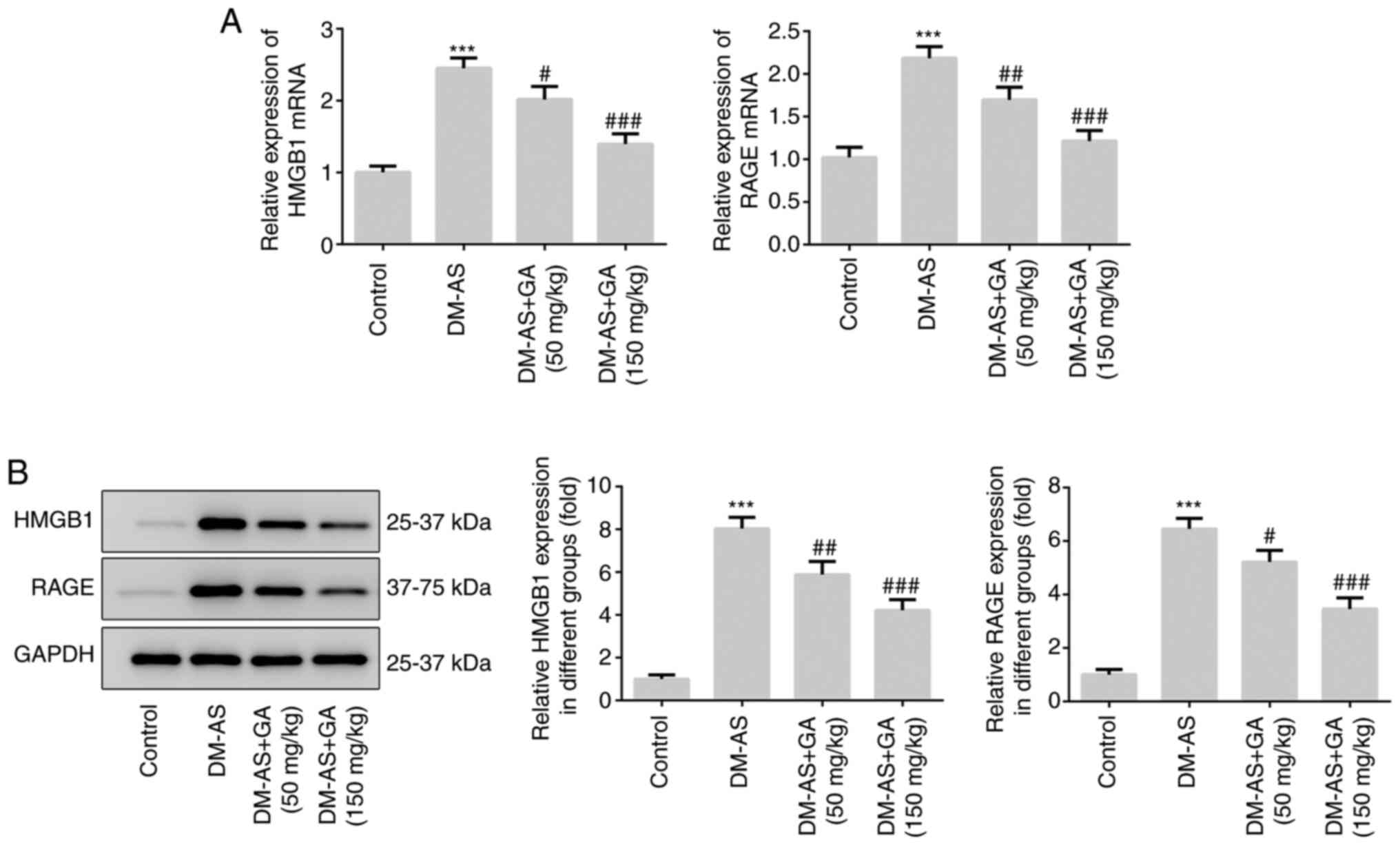
Furthermore, the expression levels of HMGB1 and RAGE were
significantly increased in the DM-AS group compared with the
control group, which was reversed following treatment with GA in a
dose-dependent manner (Fig. 2A).
These results were similar to those obtained following western
blotting (Fig. 2B).
Effects of GA on the biochemical
parameters of DM-AS rats
The results demonstrated that, compared with the
control group, the levels of FINS, TG, TC and LDL-C (Fig. 3A-C) were significantly increased in
the DM-AS group, whereas HDL-C level was significantly decreased
(Fig. 3C). However, following DM-AS
rat treatment with 50 and 150 mg/kg GA, the levels of FINS, TG, TC
and LDL-C (Fig. 3A-C) were
significantly decreased in a dose-dependent manner compared with
that in the control group, whereas HDL-C level was significantly
increased (Fig. 3C).
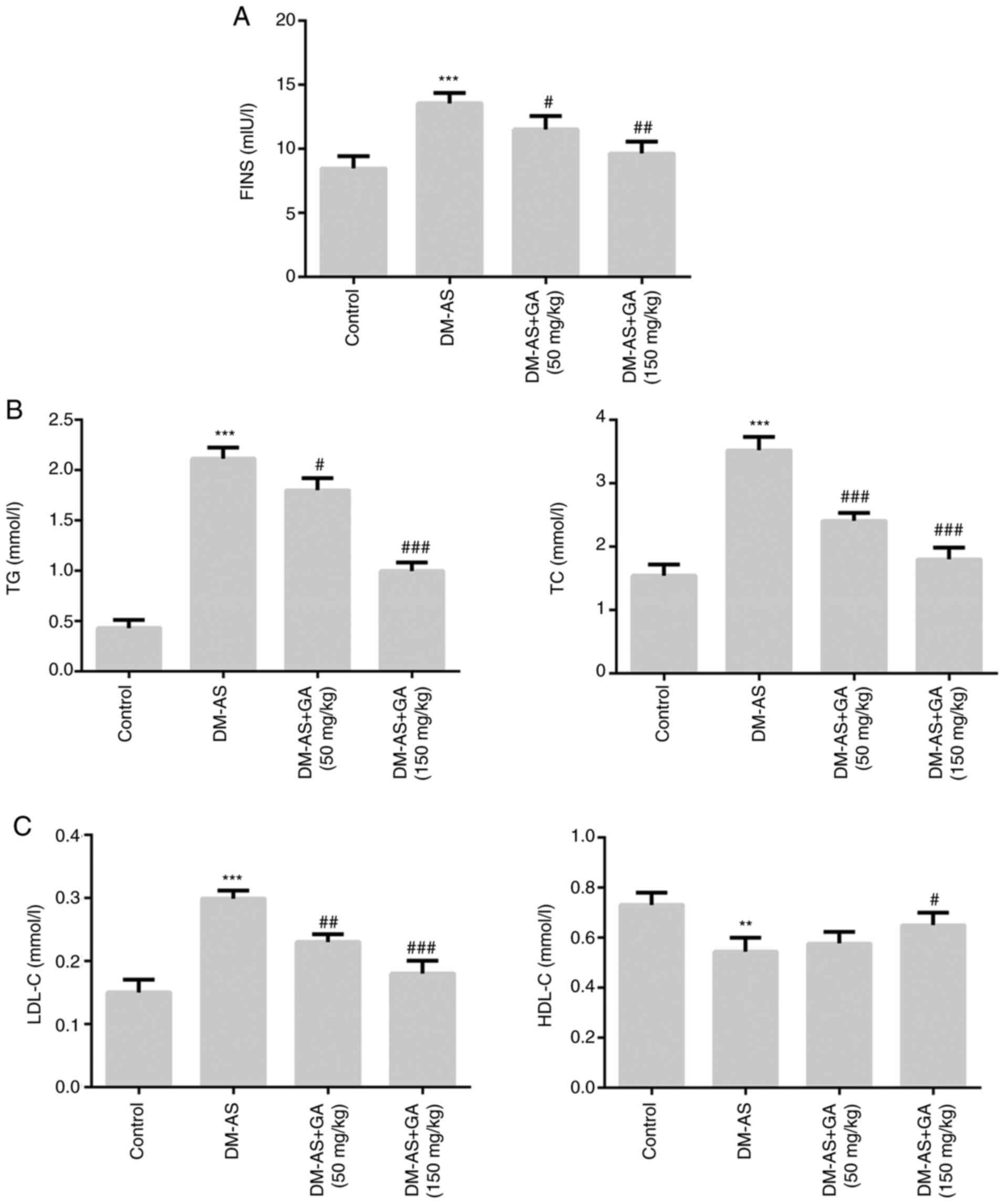 | Figure 3.Effects of GA on biochemical
parameters in DM-AS rats. Levels of (A) FINS, (B) TG and TC, (C)
LDL-C and HDL-C were measured using a biochemical analyzer.
**P<0.01, ***P<0.001 vs. control group;
#P<0.05, ##P<0.01 and
###P<0.001 vs. DM-AS group. AS, atherosclerosis. AS,
atherosclerosis; DM, diabetes mellitus; GA, gycyrrhizic acid; FINS,
fasting insulin; HDL-C, high density lipoprotein cholesterol;
LDL-C, low density lipoprotein cholesterol; TC, total cholesterol;
TG, total triglyceride. |
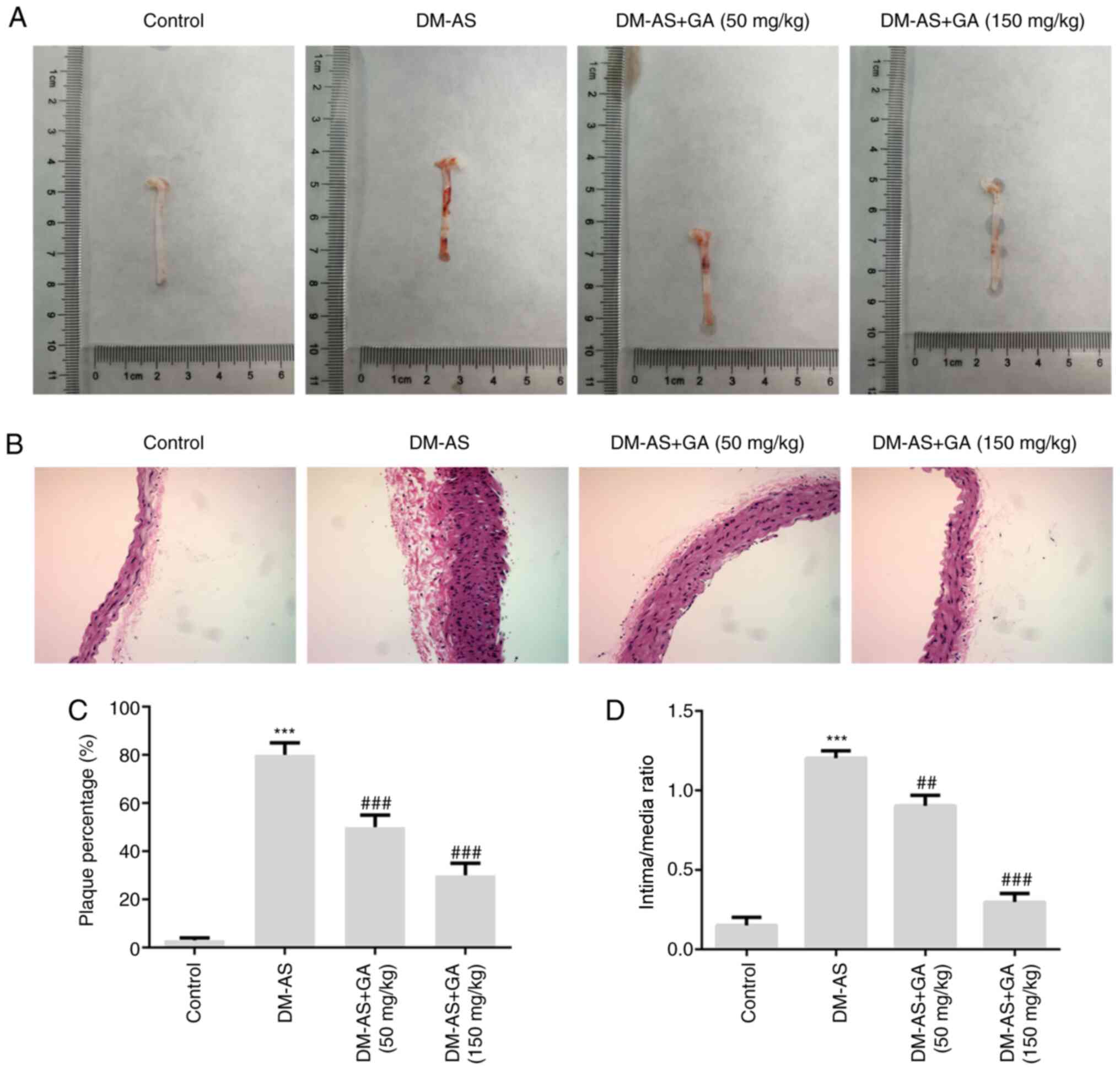
Effects of GA on aortic plaques in
DM-AS rats
Aortic plaques were observed in the rats and the
results demonstrated that plaque number was increased in rats from
the DM-AS group compared with that in rats in the control group.
Compared with that in the DM-AS group, the percentage of aortic
plaques in the DM-AS + GA (50 mg/kg) and DM-AS + GA (150 mg/kg)
groups was decreased in a dose-dependent manner (Fig. 4A and C). Furthermore, the intima
thickness of arterial tissues was observed by H&E staining, and
the results demonstrated that the intima thickness of arterial
tissues in the DM-AS group was significantly increased compared
with that in the control group. However, following treatment with
GA (50 or 150 mg/kg), intima thickness was decreased in a
concentration-dependent manner (Fig. 4B
and D). These findings suggested that GA may reduce the intimal
thickness of the aorta and the formation of plaques in DM-AS rats
in a dose-dependent manner.
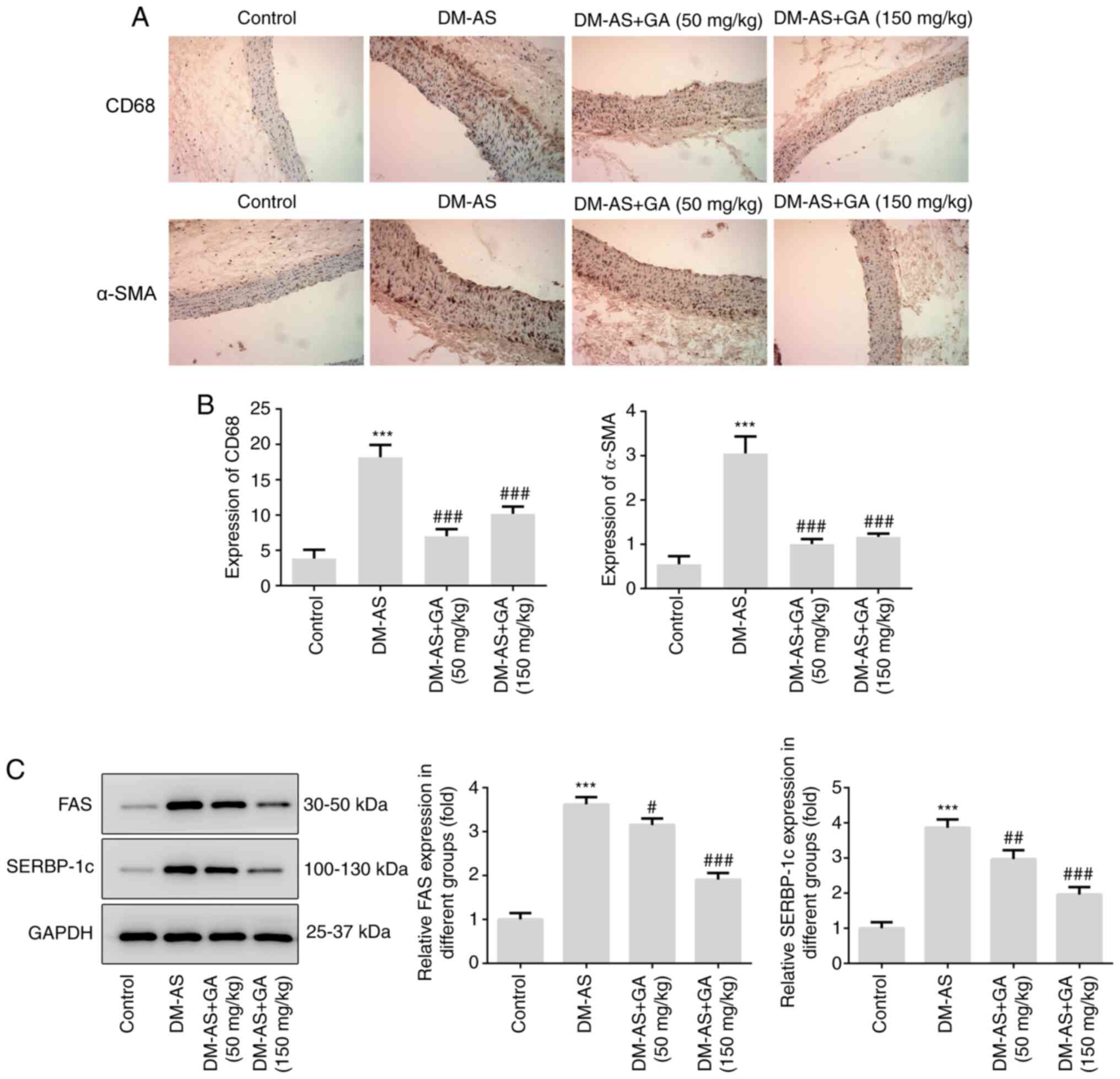
Effects of GA on macrophage
activation, α-SMA expression and lipid factors (FAS and SREBP-1c)
expression in DM-AS rats
IHC was performed to detect the expression of CD68
and α-SMA in aorta tissues from rats. As presented in Fig. 5, the expression of CD68 and α-SMA
was significantly increased in the DM-AS group compared with that
in the control group. In addition, following treatment with GA, the
expression of CD68 and α-SMA was significantly decreased (Fig. 5A and B). Subsequently, the
expression of lipid metabolism-related proteins FAS and SREBP-1c
was detected by western blotting. The expression of FAS and
SREBP-1c increased in the DM-AS group compared with that in the
control group. In addition, following treatment with GA, the
expression of FAS and SREBP-1c was also significantly decreased
(Fig. 5C).
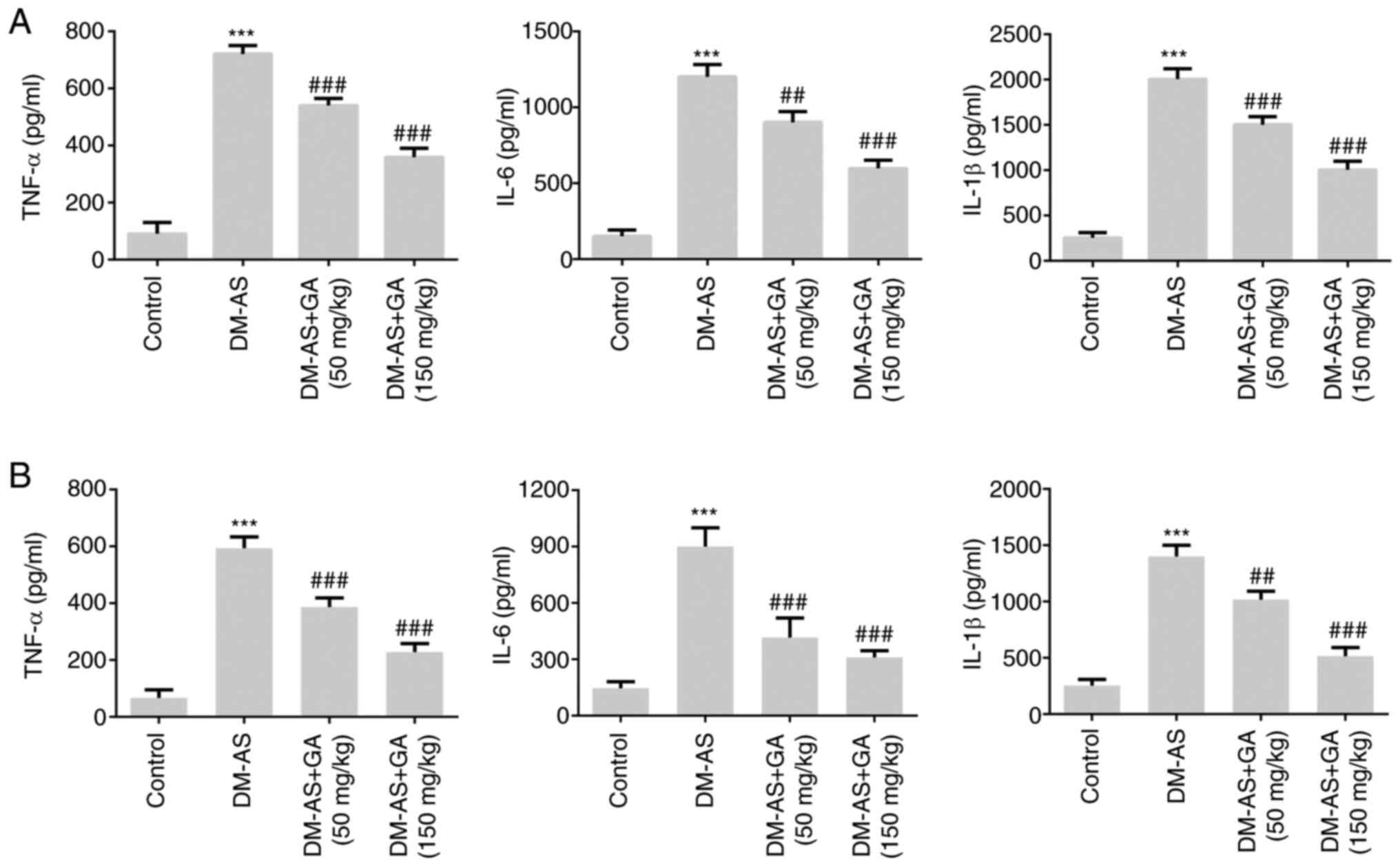
Effects of GA on the concentration of
inflammatory factors in serum and atherosclerotic tissue of DM-AS
rats
The concentrations of inflammatory cytokines were
measured in the serum (Fig. 6A) and
atherosclerotic tissue (Fig. 6B) of
rats. Compared with that of the control group, the expression of
TNF-α, IL-6 and IL-1β in the DM-AS group was significantly
increased. In addition, the expression of TNF-α, IL-6, and IL-1β in
the DM-AS + GA (50 mg/kg) and DM-AS + GA (150 mg/kg) groups was
significantly decreased in a dose-dependent manner compared with
that in the DM-AS group. These findings suggested that GA may
reduce inflammation in DM-AS rats.
Discussion
Cardiovascular disease is a common complication that
can be fatal in people with DM. Previous studies have demonstrated
that cardiovascular diseases account for >50% of mortality cases
in patients with DM, with ischemia, angina, myocardial infarction,
stroke and sudden cardiac death caused by AS accounting for a large
proportion of these cases (9,24). In
the diabetic population, the incidence of AS is higher, the disease
is more serious and the mortality rate is increased compared with
the non-diabetic population (25).
Coronary heart disease, myocardial infarction and acute
cerebrovascular disease are the main causes of mortality in
patients with DM (26). Therefore,
whether cardiovascular diseases could be prevented by decreasing
blood glucose level has become a focus of interest.
In the present study, rats were fed with a high-fat
diet for 8 weeks and injected with STZ to establish a DM-AS model
(22). The blood glucose level and
DM-related indexes in DM-AS rats were significantly increased
compared with those of the control group. Furthermore, the number
of arterial plaques, intima thickness, macrophage activation and
α-SMA expression in DM-AS rats were increased compared with those
in the control rats, indicating the successful establishment of the
DM-AS model. It was found that the normal blood glucose level of
rats is ~4 mmol/l (27), similar to
the results observed in the present study .
The present study mainly evaluated whether GA could
decrease blood glucose level in patients with DM and improve renal
injury caused by DM (28). In the
present study, GA treatment decreased blood glucose, insulin, TC,
TG and LDL-C levels, and stimulated the formation of HDL-C in DM-AS
rats. The typical pathological features of AS are increased
arterial plaque number, a thickened arterial intima, activated
macrophages, increased lipid metabolism and aggravated inflammatory
reactions (29). In the present
study, GA treatment decreased the number of atherosclerotic artery
plaques in DM-AS rats in a dose-dependent manner, reduced the
intima thickness, inhibited the activation of macrophages, and
alleviated the inflammatory response and lipid metabolism. These
findings suggested that GA may have some therapeutic effects on
DM-AS. GA is a small inhibitor of HMGB1 that has a protective
effect on blood vessels in AS (19,30)
and can decrease renal complications caused by DM (21). In the present study, GA was found to
inhibit the expression of HMGB1 in DM-AS rats. Furthermore, RAGE is
one of the important receptors of HMGB1. When combined, the two
receptors activate the intracellular signal transduction pathway,
leading to pathological damage and affecting the pathogenesis of
inflammatory diseases, such as arthritis and AS (31). In the present study, GA was
demonstrated to also inhibit the expression of RAGE in DM-AS
rats.
SREBP-1c coordinates the synthesis of FA and
cholesterol, and FAS is its downstream transcription factor
(32). A previous study has
reported that GA can improve lipid metabolism in AS and inhibit
vascular inflammation in Apoe−/− mice (19). The results from the present study
demonstrated that GA could inhibit the expression of the
lipid-related proteins FAS and SREBP-1c in DM-AS rats. It has been
reported that GA has some inhibitory effects on neointimal
hyperplasia in rat models of common carotid artery injury (33). Similarly, the present study
demonstrated that GA could decrease the intima thickness of
arterial tissues in DM-AS rats. Furthermore, it is well known that
macrophages serve a crucial role in the formation of
atherosclerotic plaques, and CD68 is considered as a marker of
macrophage activation (34). In the
present study, a significant decrease in the expression of CD68 in
DM-AS rats treated with GA was observed.
In summary, the findings from this study suggested
that GA may improve atherosclerotic injury caused by DM and provide
a theoretical basis for the treatment of DM-AS.
Acknowledgements
Not applicable.
Funding
This study was funded by the Provincial Key Research
and Development Program (grant no. BE2018611) and the Science and
Technology Foundation of Henan Province (grant no.
112102310189).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DZ made substantial contributions to the conception
and design of the study, and the acquisition of data. YZ and WL
made substantial contributions to analysis and interpretation of
data. YZ and DZ confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of The Fifth Affiliated Hospital of Zhengzhou University.
All animal experiments complied with the ethical requirements of
the animal council.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
α-SMA
|
α-smooth muscle actin
|
|
AS
|
atherosclerosis
|
|
DM
|
diabetes mellitus
|
|
FINS
|
fasting insulin
|
|
FAS
|
fatty acid synthetase
|
|
GA
|
gycyrrhizic acid
|
|
HDL-C
|
high-density lipoprotein
cholesterol
|
|
HMGB1
|
high mobility group box 1
|
|
IL-1β
|
interleukin-1β
|
|
IL-6
|
interleukin-6
|
|
LDL-C
|
low-density lipoprotein
cholesterol
|
|
RAGE
|
receptor for advanced glycation end
products
|
|
SREBP-1c
|
sterol regulatory element binding
protein 1C
|
|
TC
|
total cholesterol
|
|
TG
|
total triglyceride
|
|
TNF-α
|
tumor necrosis factor-α
|
References
|
1
|
Guthrie RA and Guthrie DW: Pathophysiology
of diabetes mellitus. Crit Care Nurs Q. 27:113–125. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Unnikrishnan R, Anjana RM and Mohan V:
Diabetes mellitus and its complications in India. Nat Rev
Endocrinol. 12:357–370. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saeedi P, Petersohn I, Salpea P, Malanda
B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA,
Ogurtsova K, et al: Global and regional diabetes prevalence
estimates for 2019 and projections for 2030 and 2045: Results from
the International Diabetes Federation Diabetes Atlas. (9(th)
edition). Diabetes Res Clin Pract. 157:1078432019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Strain WD and Paldánius PM: Diabetes,
cardiovascular disease and the microcirculation. Cardiovasc
Diabetol. 17:572018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kannenkeril D, Bosch A, Harazny J, Karg M,
Jung S, Ott C and Schmieder RE: Early vascular parameters in the
micro- and macrocirculation in type 2 diabetes. Cardiovasc
Diabetol. 17:1282018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Di Pino A and DeFronzo RA: Insulin
resistance and atherosclerosis: Implications for
insulin-sensitizing agents. Endocr Rev. 40:1447–1467. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stabley JN and Towler DA: Arterial
calcification in diabetes mellitus: Preclinical models and
translational implications. Arterioscler Thromb Vasc Biol.
37:205–217. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Poznyak A, Grechko AV, Poggio P,
Myasoedova VA, Alfieri V and Orekhov AN: The diabetes
mellitus-atherosclerosis connection: The role of lipid and glucose
metabolism and chronic inflammation. Int J Mol Sci. 21:18352020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ioachimescu AG: Diabetes and
atherosclerotic cardiovascular disease. Endocrinol Metab Clin North
Am. 47:xiii–xiv. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu Y, Xian X, Wang Z, Bi Y, Chen Q, Han
X, Tang D and Chen R: Research progress on the relationship between
atherosclerosis and inflammation. Biomolecules. 8:802018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Laakso M and Kuusisto J: Insulin
resistance and hyperglycaemia in cardiovascular disease
development. Nat Rev Endocrinol. 10:293–302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yahagi K, Kolodgie FD, Lutter C, Mori H,
Romero ME, Finn AV and Virmani R: Pathology of human coronary and
carotid artery atherosclerosis and vascular calcification in
diabetes mellitus. Arterioscler Thromb Vasc Biol. 37:191–204. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dryden M, Baguneid M, Eckmann C, Corman S,
Stephens J, Solem C, Li J, Charbonneau C, Baillon-Plot N and Haider
S: Pathophysiology and burden of infection in patients with
diabetes mellitus and peripheral vascular disease: Focus on skin
and soft-tissue infections. Clin Microbiol Infect. 21 (Suppl
2):S27–S32. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malahfji M and Mahmarian JJ: Imaging to
stratify coronary artery disease risk in asymptomatic patients with
diabetes. Methodist Debakey Cardiovasc J. 14:266–272.
2018.PubMed/NCBI
|
|
15
|
Andersson U and Tracey KJ: HMGB1 is a
therapeutic target for sterile inflammation and infection. Annu Rev
Immunol. 29:139–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng M, Scott MJ, Fan J and Billiar TR:
Location is the key to function: HMGB1 in sepsis and trauma-induced
inflammation. J Leukoc Biol. 106:161–169. 2019.PubMed/NCBI
|
|
17
|
Wang R, Wu W, Li W, Huang S, Li Z, Liu R,
Shan Z, Zhang C, Li W and Wang S: Activation of NLRP3 inflammasome
promotes foam cell formation in vascular smooth muscle cells and
atherogenesis Via HMGB1. J Am Heart Assoc. 7:e0085962018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang J, Zhang L, Zhang S, Yu Q, Xiong F,
Huang K, Wang CY and Yang P: HMGB1, an innate alarmin, plays a
critical role in chronic inflammation of adipose tissue in obesity.
Mol Cell Endocrinol. 454:103–111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ding JW, Luo CY, Wang XA, Zhou T, Zheng
XX, Zhang ZQ, Yu B, Zhang J and Tong XH: Glycyrrhizin, a
high-mobility group box 1 inhibitor, improves lipid metabolism and
suppresses vascular inflammation in apolipoprotein e knockout mice.
J Vasc Res. 55:365–377. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hou S, Zhang T, Li Y, Guo F and Jin X:
Glycyrrhizic acid prevents diabetic nephropathy by activating
AMPK/SIRT1/PGC-1α Signaling in db/db Mice. J Diabetes Res.
2017:28659122017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang H, Zhang R, Chen J, Shi M, Li W and
Zhang X: High Mobility Group Box1 inhibitor glycyrrhizic acid
attenuates kidney injury in streptozotocin-induced diabetic rats.
Kidney Blood Press Res. 42:894–904. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Liu X, Fang Q, Ding M and Li C:
Liraglutide attenuates atherosclerosis via inhibiting ER-induced
macrophage derived microvesicles production in T2DM rats. Diabetol
Metab Syndr. 9:942017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shah AD, Langenberg C, Rapsomaniki E,
Denaxas S, Pujades-Rodriguez M, Gale CP, Deanfield J, Smeeth L,
Timmis A and Hemingway H: Type 2 diabetes and incidence of
cardiovascular diseases: A cohort study in 1.9 million people.
Lancet Diabetes Endocrinol. 3:105–113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Haas AV and McDonnell ME: Pathogenesis of
cardiovascular disease in diabetes. Endocrinol Metab Clin North Am.
47:51–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Buszman PP, Bochenek A, Konkolewska M,
Trela B, Kiesz RS, Wilczyński M, Cisowski M, Krejca M,
Banasiewicz-Szkróbka I, Krol M, et al: Early and long-term outcomes
after surgical and percutaneous myocardial revascularization in
patients with non-ST-elevation acute coronary syndromes and
unprotected left main disease. J Invasive Cardiol. 21:564–569.
2009.PubMed/NCBI
|
|
27
|
Zhou J, Zhe R, Guo X, Chen Y, Zou Y, Zhou
L and Wang Z: The Role of PPARδ Agosnist GW501516 in rats with
gestational diabetes mellitus. Diabetes Metab Syndr Obes.
13:2307–2316. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rani R, Dahiya S, Dhingra D, Dilbaghi N,
Kaushik A, Kim KH and Kumar S: Antidiabetic activity enhancement in
streptozotocin + nicotinamide-induced diabetic rats through
combinational polymeric nanoformulation. Int J Nanomedicine.
14:4383–4395. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moroni F, Ammirati E, Norata GD, Magnoni M
and Camici PG: The role of monocytes and macrophages in human
atherosclerosis, plaque neoangiogenesis, and atherothrombosis.
Mediators Inflamm. 2019:74343762019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Palone F, Pasquali E, Giardullo P,
Stronati L, Vitali R and Mancuso M: Low dose of dipotassium
glycyrrhizate counteracts atherosclerosis progression in
apoe-/-female mice. J Vasc Res. 56:267–270. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Le Y, Zhao W, Lin Y, Wu Y, Yu C,
Xiong J, Zou F, Dong H, Cai S and Zhao H: Short thymic stromal
lymphopoietin attenuates toluene diisocyanate-induced airway
inflammation and inhibits high mobility group box 1-receptor for
advanced glycation end products and long thymic stromal
lymphopoietin expression. Toxicol Sci. 157:276–290. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brown MS and Goldstein JL: The SREBP
pathway: regulation of cholesterol metabolism by proteolysis of a
membrane-bound transcription factor. Cell. 89:331–340. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen J, Zhang J, Xu L, Xu C, Chen S, Yang
J and Jiang H: Inhibition of neointimal hyperplasia in the rat
carotid artery injury model by a HMGB1 inhibitor. Atherosclerosis.
224:332–339. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cybulsky MI, Cheong C and Robbins CS:
Macrophages and dendritic cells: Partners in atherogenesis. Circ
Res. 118:637–652. 2016. View Article : Google Scholar : PubMed/NCBI
|