Introduction
According to the World Health Organization, among
the 56.9 million deaths recorded worldwide in 2016, 15.2 million
were caused by ischemic heart disease and stroke. Therefore,
cardiovascular diseases have consistently ranked first in the list
of global causes of death during the last 15 years (1). Atherosclerosis (AS) is an important
foundation for these two diseases. The accumulation of foam cells
and apoptotic residues, which are produced by foam cells, at the
lesions are landmark events of both heart disease and stroke
(2,3). In the early stage of AS, macrophages
reduce the lipids and apoptotic residues to limit plaque growth via
endocytosis. During disease progression, the function of
macrophages is decreased until it is lost, which is accompanied by
increased cell apoptosis, eventually leading to necrosis,
inflammation and plaque instability (4). The aforementioned findings suggested
that reducing macrophage apoptosis and maintaining their normal
function could be considered as a promising approach for the
prevention and treatment of AS.
Autophagy is a cellular self-defense mechanism that
has been widely studied in several organisms and is characterized
by the intracellular degradation of organelles, proteins and other
cytoplasmic components by lysosomes (5). When cells are exposed to external
stimuli, such as oxidative stress and starvation, the lipid
structure of the bilayer membrane encapsulates cytoplasmic
substances, such as damaged organelles and misfolded proteins, to
form autophagosomes. Subsequently, the autophagosome fuses with the
lysosome to form an autophagolysosome, which in turn catabolizes
cytoplasmic materials, including those internalized by endocytosis,
through its acidic environment and proteases, thereby maintaining
normal cell activity, morphology and structure, and delaying the
aging process (6). Autophagy is
also closely associated with the pathogenesis and prevention of
malignant tumors, as well as cardiovascular, neurodegenerative and
immune system diseases (7). For
instance, it has been reported that macrophage autophagy exhibits
an antiatherosclerotic effect via inhibiting inflammation and
promoting cholesterol efflux (8,9).
Recently, an increasing number of researchers have
turned their attention to the regulation of autophagy in apoptosis
(10,11). However, the intrinsic mechanisms
underlying the association between autophagy and apoptosis have not
yet been elucidated.
As a traditional Chinese medicine, Salvia
miltiorrhiza is widely used in Asia for the prevention and
treatment of cardiovascular diseases via promoting blood
circulation (12). Salvianolic acid
B (Sal B) is one of the main water-soluble components of Salvia
miltiorrhiza (13). It has been
reported that Sal B exerts anti-inflammatory, antioxidant and
antitumor effects (12). In
cardiovascular diseases, Sal B serves a significant role in
protecting against myocardial infarction, improving myocardial
ischemia-reperfusion injury and inhibiting the formation of
atherosclerotic plaques (13–15).
However, whether the antiatherosclerotic effects of Sal B are
associated with macrophage apoptosis is not completely
understood.
In the present study, RAW264.7 macrophages were
treated with cholesterol crystals (CHCs) to investigate the
intrinsic association between macrophage autophagy and apoptosis in
AS. Furthermore, the mechanism underlying the protective effects of
Sal B on macrophages in AS was explored.
Materials and methods
Cell culture
The RAW264.7 cell line was purchased from the
American Type Culture Collection and cultured in high glucose DMEM
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% (v/v)
FBS (Biological Industries) at 37°C in a 5% CO2
incubator.
Experimental design
In order to confirm whether CHCs can induce
macrophage apoptosis and autophagy dysfunction, different
concentrations of CHCs (0, 400, 800, 1,200 µg/ml) were used to
culture cells, followed by verification via Hoechst staining and
western blotting. Next, different concentrations of Sal B (0, 40,
80, 120, 160, 200, 240, 280, 320, 360 µM) were used to culture
cells and toxicity was evaluated via an MTT assay. A total of three
different concentrations (100, 150, 200 µM) were selected for
subsequent experiments. Afterwards, cells were divided into five
groups (untreated control; 800 µg/ml CHC; 800 µg/ml CHC + 100 µM
Sal B; 800 µg/ml CHC + 150 µM Sal B; and 800 µg/ml CHC + 200 µM Sal
B) and then it was evaluated whether Sal B could prevent
CHC-induced apoptosis, autophagy and inflammation via Hoechst, flow
cytometry, western blotting and ELISA. In addition, the effect of
Sal B on the autophagic flow of CHC-treated cells was detected via
an Ad-GFP-LC3B adenovirus infection experiment [experimental groups
were as follows: Untreated control; 800 µg/ml CHC; 800 µg/ml CHC +
200 µM Sal B; 20 nM BafA1 + 800 µg/ml CHC + 200 µM Sal B; and 20 nM
bafilomycin A1 (BafA1; Beijing Huamaike Biotechnology Co., Ltd.)].
Following which, cells were divided into four groups [experimental
groups were as follows: Untreated control; 800 µg/ml CHC; 800 µg/ml
CHC + 200 µM Sal B; and 800 µg/ml CHC + 200 µM Sal B + 5 mM
3-methyladenine (3-MA; Selleck Chemicals)] and the relationship
between the reduction in apoptosis and the improvement of autophagy
mediated by Sal B was explored via western blotting and flow
cytometry. Ultimately, cells were divided into four groups
[experimental groups were as follows: Untreated control; 800 µg/ml
CHC; 800 µg/ml CHC + 200 µM Sal B; and 800 µg/ml CHC + 200 µM Sal B
+ 1 µg/ml insulin (Beyotime Institute of Biotechnology)] and the
signaling pathway of Sal B-induced autophagy was evaluated via
western blotting. After each intervention drug was added, cells
were cultured at 37°C for 24 h before further experiments.
Preparation of CHCs
Cholesterol powder (Beyotime Institute of
Biotechnology) was diluted in 95% ethanol to a final concentration
of 12.5 mg/ml and then heated to 65°C. Once sufficiently dissolved,
the cholesterol solution was filtered through filter paper to
remove undissolved impurities. Subsequently, the solution was
cooled in an ice bath to precipitate crystals and then filtered
through filter paper to obtain crystals. The aforementioned steps
were repeated five times. Subsequently, all crystals were
collected, grounded using a mortar, dispersed in PBS, adjusted to a
final concentration of 5 mg/ml and stored at −20°C following
autoclaving.
Cell viability assay
RAW264.7 cells (5×104/well) were seeded
into 96-well plates and treated with different concentrations of
Sal B for 24 h. Then, the activity of cells was evaluated using an
MTT assay. Subsequently, each well was supplemented with 5 µl MTT
solution (5 mg/ml; Beyotime Biotechnology). Following incubation
for 3 h, the medium was discarded and 150 µl DMSO (Sangon Biotech
Co., Ltd.) was added to each well to dissolve the formed crystals.
Finally, the optical density of each well was measured using a
microplate reader (Molecular Devices, LLC) at a wavelength of 490
nm.
Cell apoptosis analysis
Following treatment with the corresponding
treatments for 24 h, RAW264.7 cell apoptosis was evaluated by
performing Hoechst staining. Briefly, cells (40×104
cells/well) were seeded into 6-well plates and cultured for 24 h,
then the medium was discarded. After washing with PBS, cells were
fixed with 4% paraformaldehyde (500 µl/well; Boster Biological
Technology) for 15 min at 4°C. Cells were washed again with PBS and
then stained with 500 µl Hoechst 33258 (Beyotime Institute of
Biotechnology) for 10 min at room temperature in the dark. Finally,
after washing with PBS, morphological changes were observed under a
fluorescence microscope (Thermo Fisher Scientific, Inc.).
In addition, RAW264.7 cells (50×104
cells/well) were seeded into 6-well plates and treated with CHCs
for 24 h. Then, the apoptosis rate was detected by conducting
Annexin V-FITC/PI double staining. Briefly, the medium was
aspirated, and cells were washed with PBS, digested with trypsin
(Beyotime Institute of Biotechnology), collected into a centrifuge
tube and centrifuged at 400 × g for 4 min at 4°C. Cells
(1×106 cells/ml) were resuspended in 1X Binding Buffer
(BD Biosciences). Subsequently, 100 µl cell suspension was
supplemented with 5 µl Annexin V-FITC and 5 µl PI (both from BD
Biosciences). Following staining for 15 min at room temperature in
the dark, 400 µl 1X Binding Buffer was added and the apoptotic rate
was measured within 30 min via flow cytometric analysis (BD
FACSymphony™ S6; Becton, Dickinson and Company). FlowJo software
version 10.5.3 (FlowJo LLC) was used for analysis.
Detection of the inflammatory factors
TNF-α and IL-6
Following treatment with the corresponding
intervention drugs for 24 h, the levels of the inflammatory factors
TNF-α and IL-6 were measured by performing ELISAs. Cells were
centrifuged at 400 × g for 20 min at 4°C. Subsequently, the levels
of TNF-α (cat. no. JM-02415M2) and IL-6 (cat. no. JM-02446M1) in
the supernatant were measured using the appropriate ELISA kits
(Jingmei Biotechnology) according to the manufacturer's
instructions.
Cell transfection with Ad-GFP-LC3B
adenovirus
To assess autophagy, RAW264.7 cells were infected
with Ad-GFP-LC3B adenovirus (cat. no. C3006; Beyotime Institute of
Biotechnology). The cells were inoculated into 12-well plates, then
the culture medium was changed and an appropriate volume of virus
solution was added 24 h later (MOI 20). After culturing at 37°C for
24 h, cells were treated with the corresponding intervention drugs
for 24 h. Cell proliferation and the expression of fluorescent
protein were observed under a fluorescence microscope.
Western blotting
Following treatment of RAW264.7 cells with the
corresponding intervention drugs, the protein expression levels of
cleaved-caspase 3 (1:500; cat. no. ab2302; Abcam), pro-caspase 3
(1:1,000; cat. no. ab32150; Abcam), Bax (1:2,000; cat. no.
ab182733; Abcam), Bcl-2 (1:2,000; cat. no. ab182858; Abcam), LC3-II
(1:2,000; cat. no. ab192890; Abcam), beclin-1, p62 (1:10,000; cat.
no. ab109012; Abcam), p-mTOR (1:1,000; cat. no. 5536; Cell
Signaling Technology, Inc.), mTOR (1:1,000; cat. no. 2972; Cell
Signaling Technology, Inc.), p-Akt (1:1,000; cat. no. 13038; Cell
Signaling Technology, Inc.) and Akt (1:1,000; cat. no. 9272; Cell
Signaling Technology, Inc.) were determined by performing western
blotting. Briefly, the cells were seeded into 6-well plates at
40×104 cells/well. After 24 h, intervention drugs were
added and cells were incubated for another 24 h. Afterwards, total
protein was extracted from cells using lysis buffer consisting of
RIPA lysate (Beyotime Institute of Biotechnology), 5X protease
inhibitor reagent (Roche Diagnostics) and 1% phenylmethylsulfonyl
fluoride (Beyotime Institute of Biotechnology) for 30 min on ice.
Subsequently, cells were centrifuged (400 × g, 4°C, 20 min) to
obtain the supernatant, the protein concentration was measured
using a BCA protein quantification kit (Beyotime Institute of
Biotechnology) and then the proteins were denatured. Proteins (10
µg) were separated via SDS-PAGE (15% gel for LC3 and 12% gel for
other proteins) and transferred onto a PVDF membrane (EMD
Millipore). Following blocking with 5% skimmed milk powder at room
temperature for 1 h, the membrane was incubated with the
aforementioned primary antibodies for 24 h at 4°C. Subsequently,
the membrane was incubated with corresponding secondary antibodies
(Goat anti-Rabbit; 1:10,000; cat. no. bs-0295Gs; BIOSS) for 1 h at
room temperature. Following washing with TBST (0.1% Tween-20;
Beijing Solarbio Science & Technology Co., Ltd.), protein bands
were visualized using an ECL developing solution (EMD Millipore)
and evaluated using a Bio-Rad gel imaging system (Bio-Rad
Laboratories, Inc.). β-actin (1:5,000; cat. no. bs-0061R; BIOSS)
was used as the loading control. ImageJ (v1.52; National Institutes
of Health) was used for semi-quantification.
Statistical analysis
Experiments were repeated in triplicate. Statistical
analyses were performed using SPSS 17.0 (SPSS, Inc.), GraphPad
Prism 8.0.2 (GraphPad Software, Inc.) software. Data are presented
as the mean ± SD. Comparisons among multiple groups were analyzed
using one-way ANOVA followed by Tukey's post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Treatment with CHCs induces apoptosis
and autophagic dysfunction in RAW264.7 macrophages
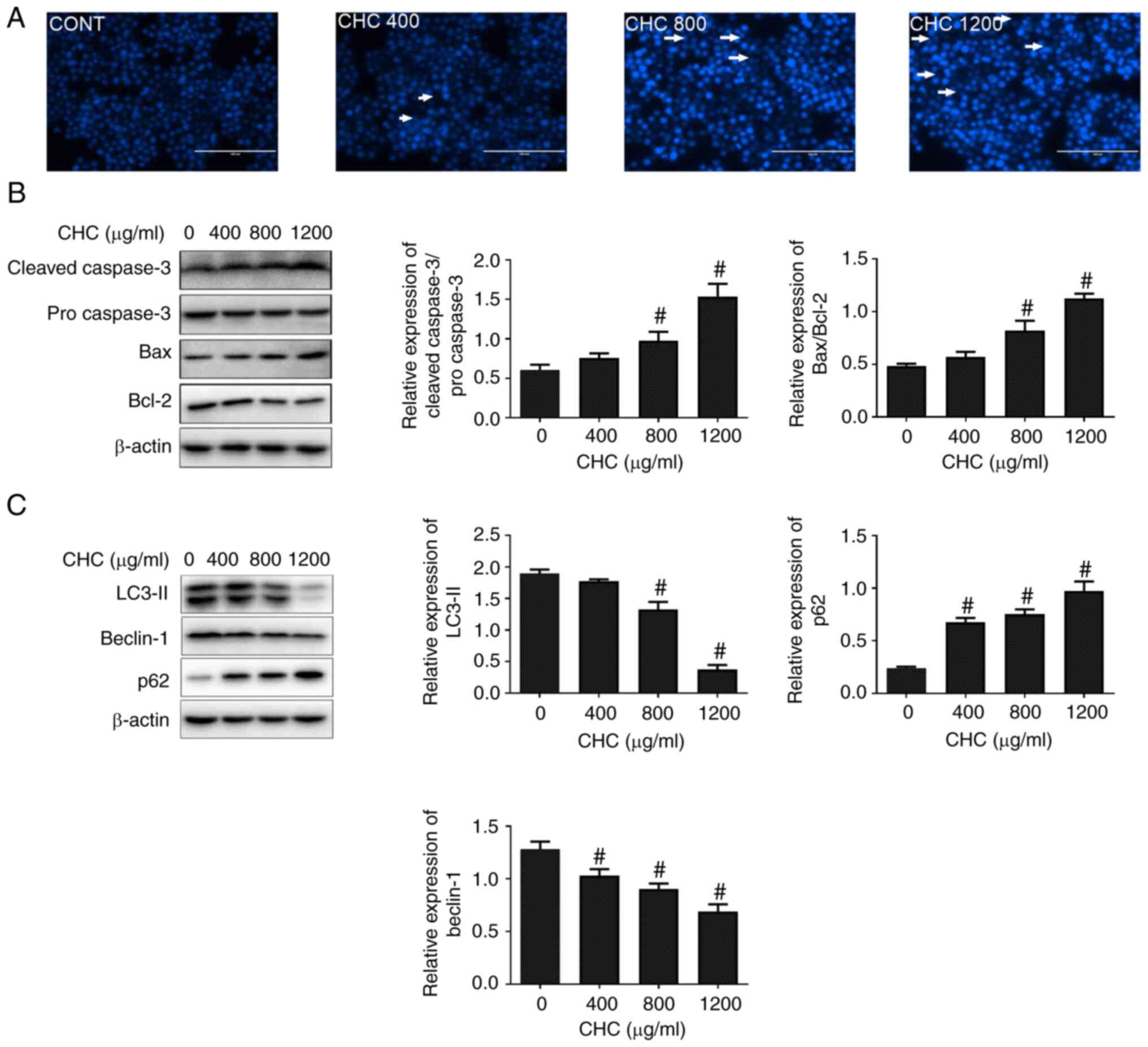
To investigate whether CHCs could induce macrophage
apoptosis, Hoechst 33258 staining assays were conducted to observe
the morphological alterations of cell nuclei. Following treatment
of RAW264.7 cells with different concentrations of CHCs for 24 h,
apoptosis-related morphological changes were markedly increased in
the cell nuclei, including chromatin condensation and nuclear
fragmentation, compared with the control group (Fig. 1A). Subsequently, western blotting
was performed to evaluate the effect of CHCs on the expression of
key apoptotic factors. The results revealed that, compared with the
untreated control group, the ratios of cleaved-caspase
3/pro-caspase 3 and Bax/Bcl-2 were significantly increased in
CHC-treated (800 and 1,200 µg/ml) RAW264.7 macrophages (Fig. 1B).
Furthermore, to determine whether CHCs could induce
autophagic dysfunction in RAW264.7 macrophages, their effect on the
expression of autophagy-related proteins was evaluated. The
expression levels of LC3-II and beclin-1 were significantly
downregulated, whereas that of p62 was significantly upregulated in
CHC-induced RAW264.7 cells at doses of 800 and 1,200 µg/ml compared
with those in the untreated control group (Fig. 1C). The aforementioned findings
suggested that CHCs (800 µg/ml) promoted apoptosis and autophagic
dysfunction in RAW264.7 macrophages.
Sal B reduces CHC-induced apoptosis
and the expression of proinflammatory cytokines in RAW264.7
macrophages
To evaluate the toxicity of Sal B, an MTT viability
assay was performed. Following treatment of RAW264.7 macrophages
with different concentrations of Sal B for 24 h, the MTT assay
results revealed that Sal B (>240 µM) significantly attenuated
the viability of macrophages compared with the untreated control
group (Fig. 2A). Therefore, the
concentrations of 100, 150 and 200 µM Sal B were selected for
subsequent experiments. The western blotting results demonstrated
that the expression levels of autophagy-related proteins were
significantly altered by Sal B treatment at the aforementioned
concentrations compared with those in the untreated control group
(Fig. 2B).
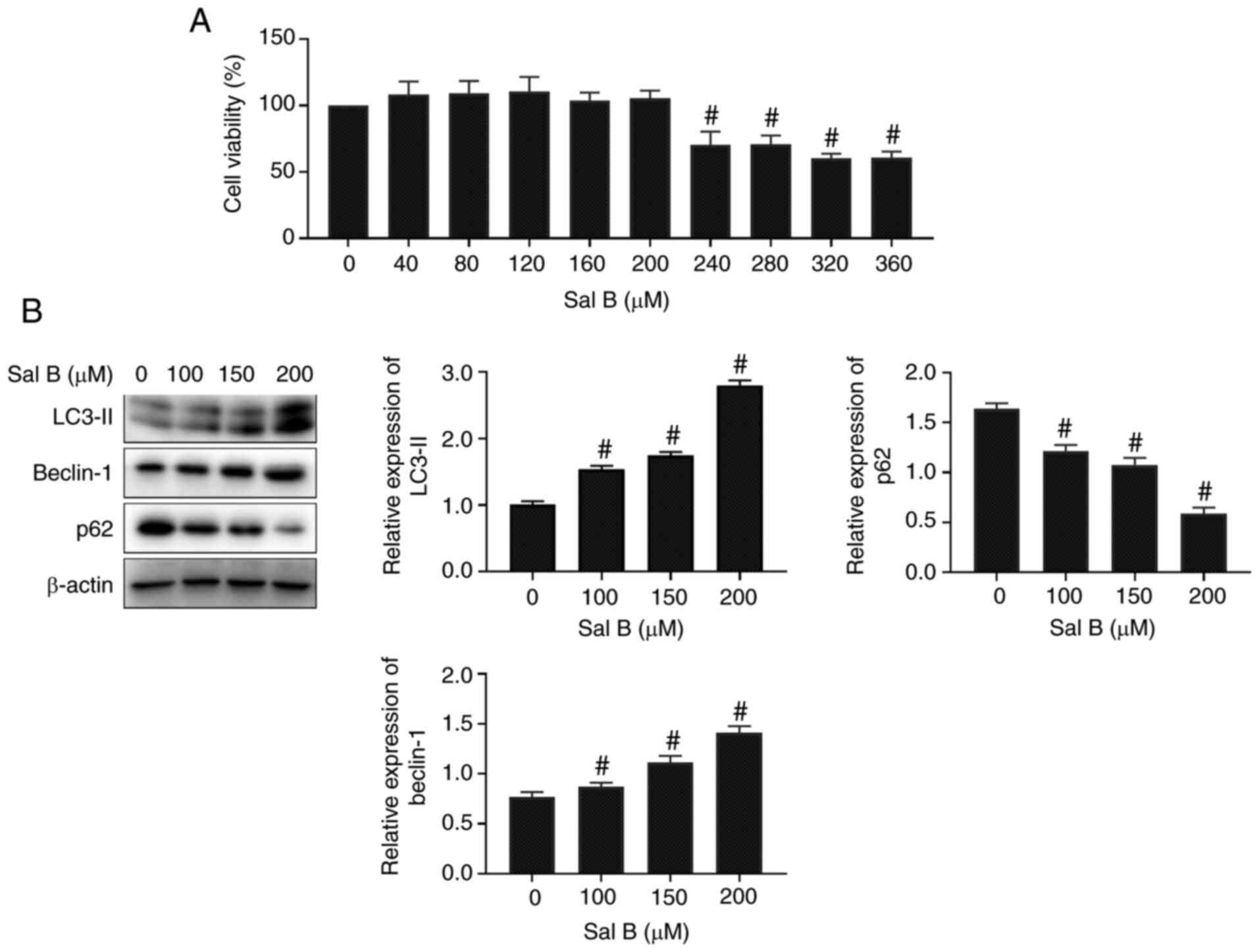 | Figure 2.Sal B activates autophagy in RAW264.7
macrophages. (A) Effect of Sal B on RAW264.7 macrophage viability
(n=6). The following groups are presented: Sal B 0, 40, 80, 120,
160, 200, 240, 280, 320, 360 µM. (B) Western blotting was performed
to determine the expression levels of the autophagy-related
proteins in Sal B-induced RAW264.7 macrophages (n=3). The following
groups are presented: Sal B 0, 100, 150, 200 µM.
#P<0.05 vs. untreated control. Sal B, salvianolic
acid B. |
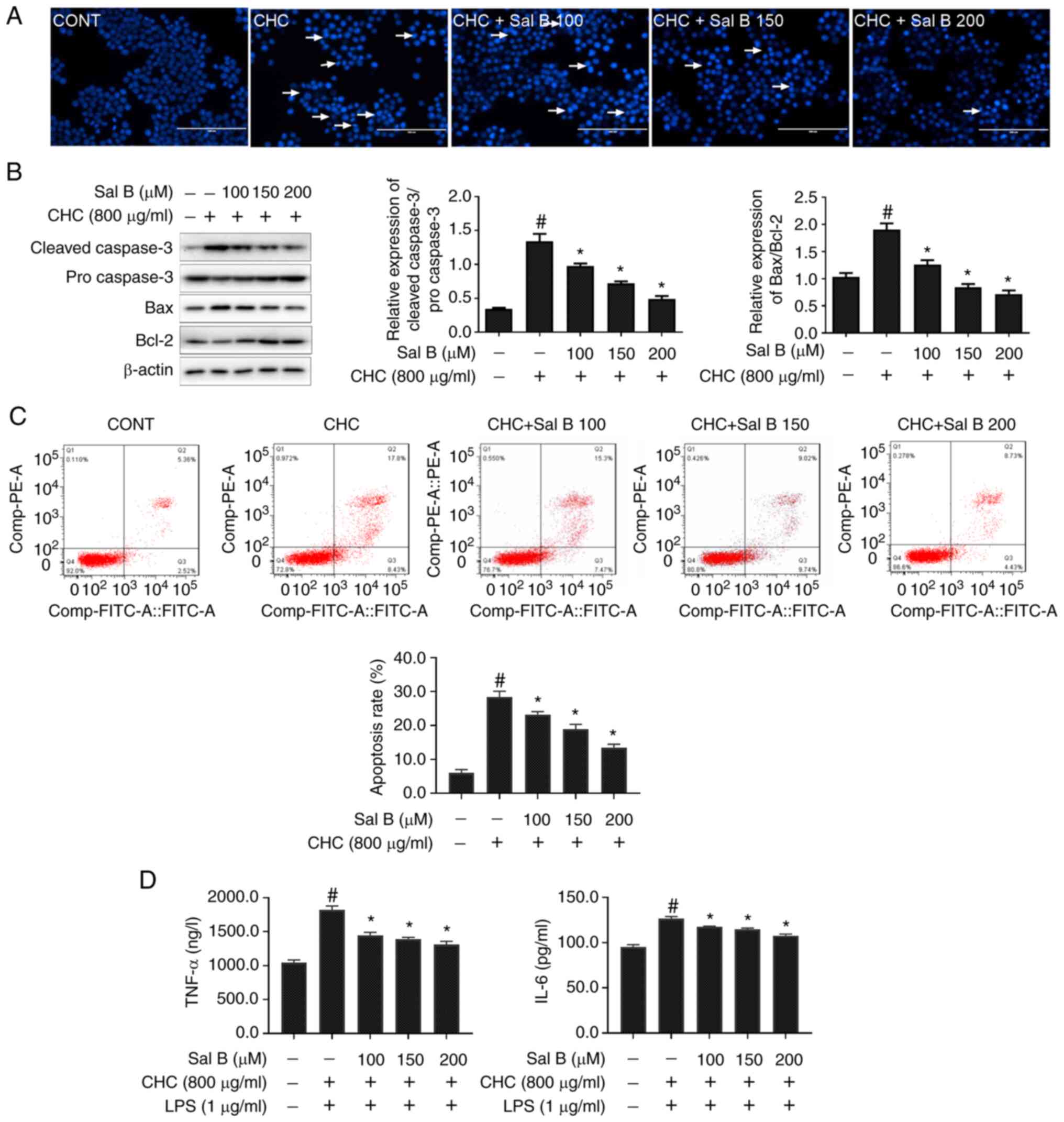
In addition, the effect of Sal B on CHC-induced
apoptosis in RAW264.7 macrophages was evaluated using Hoechst 33258
staining. Treatment of RAW264.7 macrophages with Sal B notably
reduced CHC-induced nuclear apoptosis-related morphological changes
in cell nuclei (Fig. 3A).
Additionally, the western blotting results demonstrated that,
compared with the CHC group, the cleaved-caspase 3/pro-caspase 3
and Bax/Bcl-2 ratios were significantly decreased in CHC-induced
cells treated with Sal B for 24 h (Fig.
3B).
To acquire quantification of cell apoptosis, an
Annexin V-FITC/PI dual staining assay was performed. As shown in
Fig. 3C, the apoptotic rate in the
untreated control group was 7.76±0.38%. Compared with the untreated
control group, the apoptotic rate in CHC-induced cells was
significantly increased (26.42±0.59%). Additionally, treatment of
CHC-induced RAW264.7 macrophages with 100, 150 and 200 µM Sal B
significantly decreased the apoptotic rate to 23.20±0.71,
18.95±1.13 and 13.33±0.51%, respectively, compared with cells
treated CHC alone. Furthermore, the secretion levels of
proinflammatory cytokines (TNF-α and IL-6) were significantly
reduced in Sal B-treated, CHC-induced RAW264.7 macrophages compared
with those in the CHC + LPS group (Fig.
3D). These results indicated that Sal B attenuated CHC-induced
RAW264.7 macrophage apoptosis and the production of proinflammatory
cytokines.
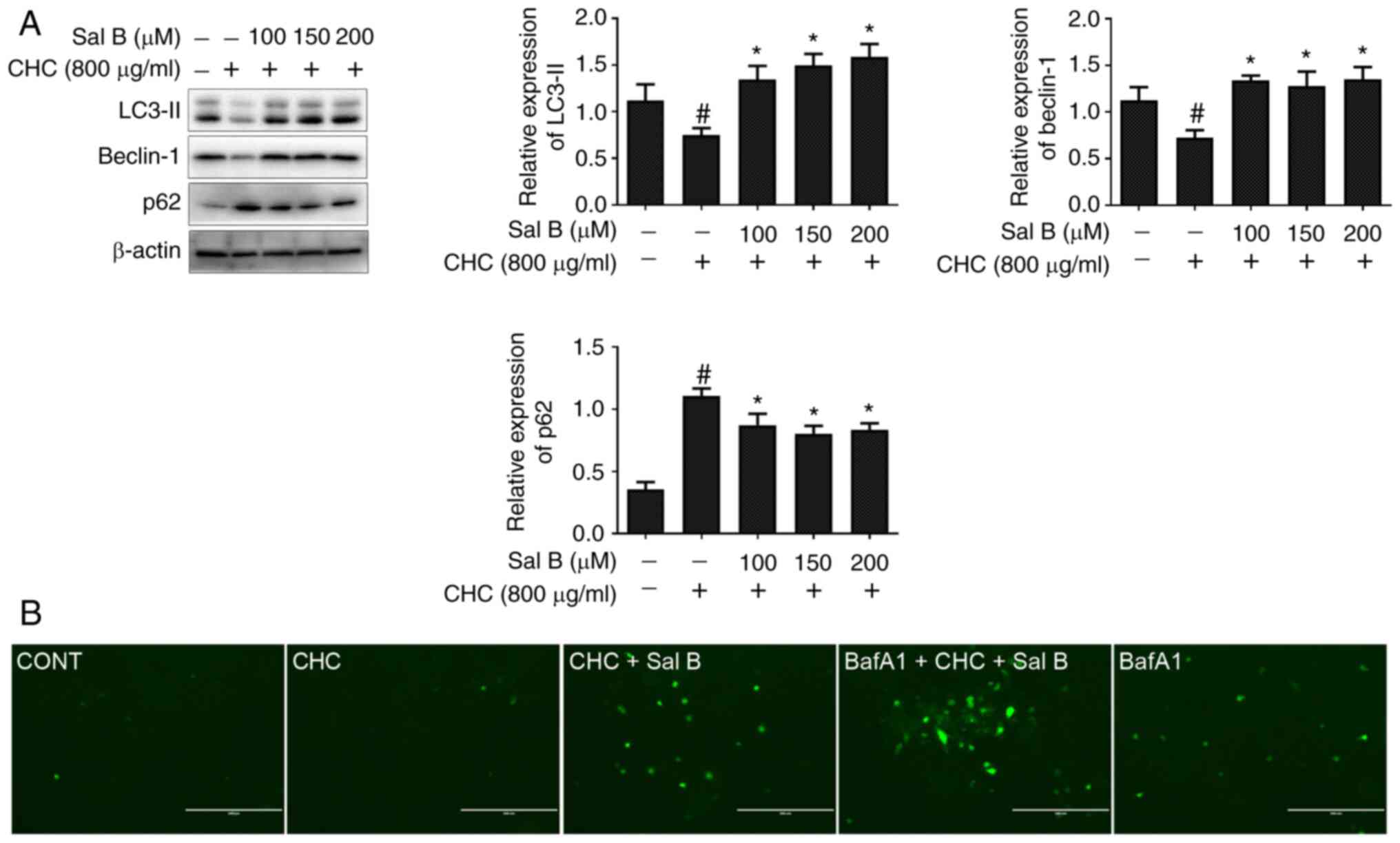
Sal B improves CHC-induced autophagic
dysfunction in RAW264.7 macrophages
To assess whether Sal B could ameliorate CHC-induced
autophagy dysfunction in RAW264.7 macrophages, western blotting was
performed to detect the expression levels of autophagy-related
proteins. Compared with the CHC group, the results demonstrated
that the expression levels of LC3-II and beclin-1 were
significantly increased, whereas those of p62 were significantly
decreased in RAW264.7 macrophages co-treated with Sal B and CHCs
for 24 h (Fig. 4A).
In addition, to further verify the Sal B-mediated
autophagic flux, RAW264.7 macrophages were infected with
Ad-GFP-LC3B for 24 h. Following cell treatment with BafA1, an
autophagosome-lysosome degradation inhibitor, the specific green
fluorescent point distribution of LC3B was observed under a
microscope. As shown in Fig. 4B, a
markedly enhanced accumulation of green fluorescent puncta was
observed in CHC-induced and Sal B-treated RAW264.7 macrophages
treated with 20 nM BafA1 for 4 h compared with the CHC + Sal B
group. These findings suggested that Sal B improved CHC-induced
autophagic dysfunction in RAW264.7 macrophages.
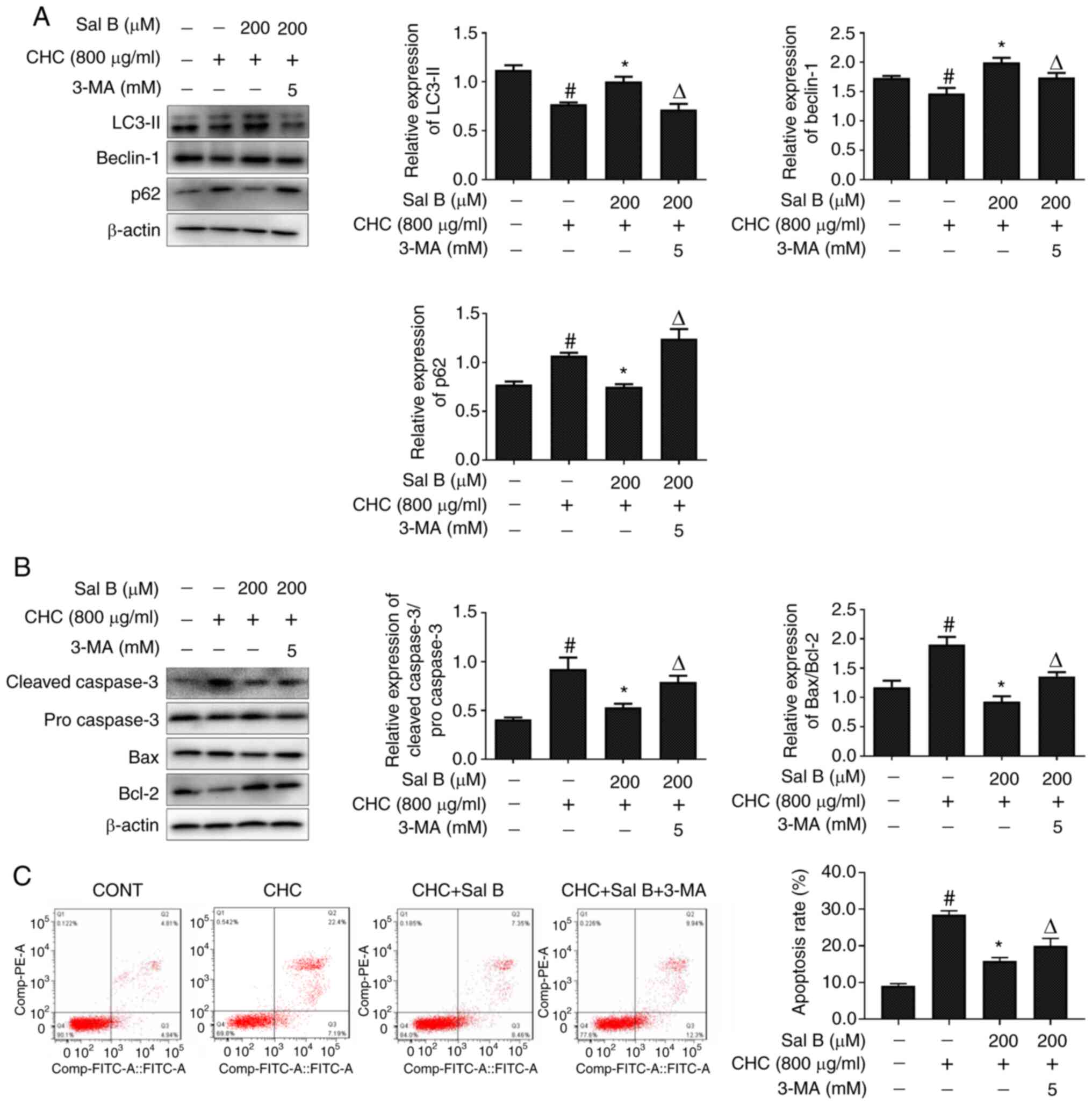
Sal B attenuates CHC-induced RAW264.7
macrophage apoptosis partially via improving autophagic
dysfunction
The inhibitor 3-MA is widely used to selectively
inhibit the activity of type III PI3K, thereby blocking the
formation of autophagosomes (16).
To further explore the association between Sal B-mediated decreases
in apoptosis and improvements in autophagy, RAW264.7 macrophages
were co-treated with Sal B and 3-MA for 24 h. Subsequently, western
blotting was performed to detect alterations in the expression
levels of autophagy- and apoptosis-related proteins. As shown in
Fig. 5A, compared with the Sal B +
CHC group, LC3-II and beclin-1 expression levels were significantly
downregulated, but p62 was significantly upregulated in CHC-induced
RAW264.7 macrophages co-treated with Sal B and 3-MA.
More importantly, 3-MA-mediated autophagy inhibition
significantly increased the cleaved caspase 3/pro-caspase 3 and
Bax/Bcl-2 ratios in Sal B-treated CHC-induced RAW264.7 macrophages
(Fig. 5B). Consistent with the
western blotting results, the Annexin V-FITC/PI dual staining assay
results revealed that the apoptotic rate of RAW264.7 macrophages
treated with Sal B and 3-MA was also significantly enhanced
compared with that in the Sal B + CHC group (Fig. 5C). Overall, the aforementioned
results indicated that Sal B attenuated CHC-induced apoptosis in
RAW264.7 macrophages partially via improving autophagic
dysfunction.
Sal B improves CHC-induced autophagic
dysfunction in RAW264.7 macrophages partially via inhibiting the
Akt/mTOR signaling pathway
The Akt/mTOR signaling pathway serves an important
role in the regulation of autophagy (17). Therefore, the present study aimed to
investigate whether the Akt/mTOR signaling pathway was involved in
Sal B-induced autophagy. The western blotting results demonstrated
that, compared with the untreated control group, the p-mTOR/mTOR
and p-Akt/Akt ratios were significantly elevated in the CHC group
(Fig. 6A). However, the p-mTOR/mTOR
and p-Akt/Akt ratios were significantly decreased in the Sal B
groups compared with the CHC group. These findings revealed that
Sal B exerted an effective inhibitory effect on the Akt/mTOR
signaling pathway, thus improving CHC-induced autophagy dysfunction
in RAW264.7 macrophages.
 | Figure 6.Sal B improves CHC-induced autophagic
dysfunction in RAW264.7 macrophages partially via inhibiting the
Akt/mTOR signaling pathway. (A) Western blotting was performed to
assess the effect of Sal B on the expression of Akt/mTOR signaling
pathway-related proteins in RAW264.7 macrophages (n=3). The
following groups are presented: CONT, untreated control; CHC, 800
µg/ml CHC; CHC + Sal B 100, 800 µg/ml CHC + 100 µM Sal B; CHC + Sal
B 150, 800 µg/ml CHC + 150 µM Sal B; and CHC + Sal B 200, 800 µg/ml
CHC + 200 µM Sal B. (B) Western blotting was performed to assess
the effect of Sal B combined with insulin on the expression of
Akt/mTOR signaling pathway- and autophagy-related proteins in
CHC-induced RAW264.7 macrophages (n=3). The following groups are
presented: CONT, untreated control; CHC, 800 µg/ml CHC; CHC + Sal
B, 800 µg/ml CHC + 200 µM Sal B; and CHC + Sal B + insulin, 800
µg/ml CHC + 200 µM Sal B + 1 µg/ml insulin. #P<0.05
vs. untreated control; *P<0.05 vs. CHC; ΔP<0.05
vs. CHC + Sal B. Sal B, salvianolic acid B; CHC, cholesterol
crystal; p, phosphorylated. |
Subsequently, RAW264.7 macrophages were co-treated
with Sal B and insulin, an activator of the PI3K/Akt/mTOR signaling
pathway, for 24 h. As shown in Fig.
6B, compared with the Sal B + CHC group, the p-mTOR/mTOR and
p-Akt/Akt ratios were significantly increased in Sal B +
insulin-treated RAW264.7 macrophages, whereas the expression levels
of LC3-II and beclin-1 were significantly downregulated. In
conclusion, these findings revealed that insulin partially restored
Sal B-induced autophagy via activating the Akt/mTOR signaling
pathway, which suggested that Sal B partially inhibited the
Akt/mTOR signaling pathway to improve CHC-induced autophagy
dysfunction in RAW264.7 macrophages.
Discussion
The role of apoptosis in the pathological process of
AS has received increasing attention. Several factors further
promote macrophage inflammatory responses and apoptosis, secondary
inflammation and necrosis of other cells at the site of the plaque,
thereby leading to increased plaque instability, and eventually
thrombosis (18–20). Therefore, reducing apoptosis and
maintaining normal endocytic capacity in macrophages could be
considered as a potential therapeutic approach for advanced AS.
CHCs are commonly found in the necrotic core and atherosclerotic
subendothelium (21). Emerging
evidence has suggested that the massive accumulation of CHCs at the
lesions induces inflammatory responses and increases plaque
instability (22–25). Sergin et al (26) demonstrated that CHCs could increase
the expression of p62 and ubiquitinated proteins at the site of the
lesion. However, whether CHCs can promote macrophage apoptosis in
atherosclerotic lesions and their specific underlying mechanism
remains to be elucidated. The present study revealed that CHCs
induced macrophage apoptosis and autophagy dysfunction.
As a highly conserved and widespread physiological
and defense mechanism in organisms (27), the effects of autophagy on the
development of several diseases have been the focus of research.
Liao et al (8) suggested
that macrophage-mediated autophagy displayed an inhibitory effect
on the progression of AS. In addition, the role of traditional
Chinese medicine in provoking and suppressing autophagy for
treating different diseases has also received increasing attention.
In traditional Chinese medicine, Salvia miltiorrhiza has
been widely used in the treatment of cardiovascular diseases by
relieving pain and promoting blood circulation (28).
Sal B, one of the primary water-soluble components
in the Salvia miltiorrhiza extracts, also exerts protective
effects against various cardiovascular diseases (29–31).
However, no study has yet investigated the possible benefits of Sal
B in CHC-induced apoptosis. The present study showed that Sal B
offered an important protective effect to alleviate macrophage
autophagy dysfunction and apoptosis in advanced AS. The protective
effects of Sal B were determined in the current study via the
Hoechst 33258 staining assay, as demonstrated by the reduction in
the apoptosis of CHC-insulted macrophages and the decreased Annexin
V-FITC/PI staining. The western blotting results indicated that Sal
B significantly increased autophagy in CHC-induced macrophages,
which was consistent with the results obtained by Jing et al
(32). Surprisingly, the ELISA
results also demonstrated that Sal B significantly attenuated the
release of proinflammatory cytokines (TNF-α and IL-6) by
CHC-induced macrophages, and we hypothesized that the
aforementioned mechanism was closely associated with Sal B-mediated
improvements in macrophage autophagy. However, the specific
mechanism requires further investigation.
It has been suggested that autophagy is an upstream
response to apoptosis, indicating that apoptosis is mediated by the
activation of autophagy (33–35).
However, it has also been suggested that enhancing autophagy may
attenuate apoptosis (36). For
example, Wang et al (37)
demonstrated that apigenin could simultaneously induce autophagy
and apoptosis in macrophages, whereas suppressing autophagy could
increase macrophage apoptosis, thus negatively regulating AS. The
role of autophagy in apoptosis remains controversial. The present
results indicated that Sal B significantly decreased macrophage
apoptosis by improving autophagy. Macrophages were co-treated with
Sal B and the autophagy inhibitor, 3-MA. The western blotting
results suggested that 3-MA successfully attenuated the effect of
Sal B on autophagy, partially compensating for the protective
effect of Sal B on apoptosis. This finding indicated that the role
of Sal B in reducing the apoptotic rate of RAW264.7 macrophages was
partially due to its effect on relieving CHC-induced autophagy
dysfunction.
Autophagy is regulated by a variety of signal
transduction pathways. The adenylate-activated protein kinase/mTOR
and PI3K/Akt/mTOR signaling pathways are two common cellular
regulatory pathways of autophagy (38). The Akt/mTOR signal transduction
pathway is closely associated with the occurrence of autophagy and
regulation of apoptosis, and is considered as the only inhibitory
signal transduction pathway regulating autophagy (39). Zhai et al (40) demonstrated that specifically
inhibiting the Akt/mTOR signaling pathway notably enhanced
macrophage autophagy, eventually attenuating the infiltration of
macrophages and enhancing the stability of the atherosclerotic
plaque. To investigate the molecular mechanism underlying the
effect of Sal B on improving autophagy function, macrophages were
treated with Sal B and insulin, an agonist of the Akt/mTOR
signaling pathway. Insulin inhibited Sal B-mediated inactivation of
Akt/mTOR signaling in CHC-induced macrophages, which partially
restored Sal B-induced autophagy. The results of the present study
suggested that CHCs induced autophagy dysfunction in macrophages
via activating the Akt/mTOR signaling pathway, whereas Sal B
partially improved autophagy dysfunction in RAW264.7 macrophages
via blocking the Akt/mTOR signaling pathway.
In summary, the results of the present study
revealed that Sal B partially improved RAW264.7 macrophage
autophagy dysfunction via inhibiting the Akt/mTOR signaling pathway
to attenuate macrophage apoptosis. In addition, treatment of
macrophages with Sal B inhibited CHC-induced release of
proinflammatory cytokines (TNF-α and IL-6). Overall, the present
study may provide novel insight into the molecular mechanism
underlying the beneficial effects of Sal B on AS.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Technology
Bureau of Luzhou city (grant nos. 2015LZCYD-S03 and 2020LZXNYDJ18),
the Department of Science and Technology of the Sichuan Province
(grant no. 17YYJC0441).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MS, SW and YH contributed to designing the study.
MS, WY and ZY performed the experiments. YY analyzed the data. MS,
YY and YH drafted the manuscript. All authors read and approved the
final manuscript. MS and SW confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for participation
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hansson GK: Mechanisms of disease:
Inflammation, atherosclerosis, and coronary artery disease. N Engl
J Med. 352:1685–95. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schrijvers DM, De Meyer GR, Herman AG and
Martinet W: Phagocytosis in atherosclerosis: Molecular mechanisms
and implications for plaque progression and stability. Cardiovasc
Res. 73:470–480. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shao BZ, Han BZ, Zeng YX, Su DF and Liu C:
The roles of macrophage autophagy in atherosclerosis. Acta
Pharmacol Sin. 37:150–156. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yorimitsu T and Klionsky DJ: Autophagy:
Molecular machinery for self-eating. Cell Death Differ. 12 (Suppl
2):S1542–S1552. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liao XH, Sluimer JC, Wang Y, Subramanian
M, Brown K, Pattison JS, Robbins J, Martinez J and Tabas I:
Macrophage autophagy plays a protective role in advanced
atherosclerosis. Cell Metab. 15:545–553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grootaert MOJ, Lynn R, Schrijvers DM, De
Meyer GRY and Martinet W: Defective autophagy in atherosclerosis:
To die or to senesce? Oxid Med Cell Longev. 2018:76870832018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong Y, Chen H, Gao J, Liu Y, Li J and
Wang J: Molecular machinery and interplay of apoptosis and
autophagy in coronary heart disease. J Mol Cell Cardiol. 136:27–41.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang K: Autophagy and apoptosis in liver
injury. Cell Cycle. 14:1631–1642. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng TO: Cardiovascular effects of
Danshen. Int J Cardiol. 121:9–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xue L, Wu Z, Ji XP, Gao XQ and Guo YH:
Effect and mechanism of salvianolic acid B on the myocardial
ischemia-reperfusion injury in rats. Asian Pac J Trop Med.
7:280–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee HJ, Seo M and Lee EJ: Salvianolic acid
B inhibits atherogenesis of vascular cells through induction of
Nrf2-dependent heme oxygenase-1. Curr Med Chem. 21:3095–3106. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han X, Liu JX and Li XZ: Salvianolic acid
B inhibits autophagy and protects starving cardiac myocytes. Acta
Pharmacol Sin. 32:38–44. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu YT, Tan HL, Shui G, Bauvy C, Huang Q,
Wenk MR, Ong CN, Codogno P and Shen HM: Dual role of
3-methyladenine in modulation of autophagy via different temporal
patterns of inhibition on class I and III phosphoinositide
3-kinase. Biol Chem. 2:10850–10861. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heras-Sandoval D, Pérez-Rojas JM,
Hernández-Damián J and Pedraza-Chaverri J: The role of
PI3K/AKT/mTOR pathway in the modulation of autophagy and the
clearance of protein aggregates in neurodegeneration. Cell Signal.
26:2694–2701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kojima Y, Weissman IL and Leeper NJ: The
role of efferocytosis in atherosclerosis. Circulation. 135:476–489.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Linton MRF, Babaev VR, Huang J, Linton EF,
Tao H and Yancey PG: Macrophage apoptosis and efferocytosis in the
pathogenesis of atherosclerosis. Circ J. 80:2259–2268. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Poon IKH, Lucas CD, Rossi AG and
Ravichandran KS: Apoptotic cell clearance: Basic biology and
therapeutic potential. Nat Rev Immunol. 14:166–180. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Manabu K, Linbo L, Chu KK, Sun CH, Tanaka
A, Gardecki JA and Tearney GJ: Feasibility of the assessment of
cholesterol crystals in human macrophages using micro optical
coherence tomography. PLoS One. 9:e1026692014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Whiting MJ and Watts JM: Cholesterol
crystal formation and growth in model bile solutions. J Lipid Res.
24:861–868. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duewell P, Kono H, Rayner KJ, Sirois CM,
Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr
M, et al: NLRP3 inflammasomes are required for atherogenesis and
activated by cholesterol crystals. Nature. 466:652. 2010.
View Article : Google Scholar
|
|
24
|
Rajamäki K, Lappalainen J, Öörni K,
Välimäki E, Matikainen S, Kovanen PT and Eklund KK: Cholesterol
crystals activate the NLRP3 inflammasome in human monocytes and
macrophages. Chem Physics of Lipids. 163 (Suppl):S27–S28. 2010.
View Article : Google Scholar
|
|
25
|
Zhou L, Jia Y and Li XH: LXRα agonist
inhibits activation of macrophage NLRP3 inflammatory corpuscles
through NF-κB pathway leading to increased mature IL-1β. Chin J
Arterioscler. 23:17–23. 2015.(In Chinese).
|
|
26
|
Sergin I, Bhattacharya S, Emanuel R, Esen
E, Stokes CJ, Evans TD, Arif B, Curci JA and Razani B: Inclusion
bodies enriched for p62 and polyubiquitinated proteins in
macrophages protect against atherosclerosis. Sci Signal. 9:ra22016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singh R, Kaushik S, Wang Y, Xiang Y, Novak
I, Komatsu M, Tanaka K, Cuervo AM and Czaja MJ: Autophagy regulates
lipid metabolism. Nature. 458:1131–1135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li ZM, Xu SW and Liu PQ: Salvia
miltiorrhizaBurge (Danshen): A golden herbal medicine in
cardiovascular therapeutics. Acta Pharmacol Sin. 39:802–824. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang SL, Lin Y and Tang ZY: Progress on
research of salviae and salvianolic acid B in treating myocardial
infarction with myocardial cell orientating differentiation of bone
marrow mesenchymal stem cell. Zhongguo Zhong Xi Yi Jie He Za Zhi.
30:1334–1337. 2010.(In Chinese). PubMed/NCBI
|
|
30
|
Wang L, Jiang H, Yang F, Du Y, Jia X, Si S
and Hong B: Role of salvianolic acid B in cholesterol efflux of
macrophages. China Medical Biotechnology. 8:100–106. 2013.(In
Chinese).
|
|
31
|
Gao F, Sun GB, Ren X, Nie Y, Sun J, Qin M
and Sun X: Protective effect of salvianolic acid B on isolated
heart ischemia/reperfusion injury in rats. Zhongguo Zhong Yao Za
Zhi. 37:358–361. 2012.(In Chinese). PubMed/NCBI
|
|
32
|
Jing Z, Fei W, Zhou J, Zhang L, Chen L,
Zhang X, Liang X, Xie J, Fang Y, Sui X, et al: Salvianolic acid B,
a novel autophagy inducer, exerts antitumor activity as a single
agent in colorectal cancer cells. Oncotarget. 7:61509–61519. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Furuya D, Tsuji N, Yagihashi A and
Watanabe N: Beclin 1 augmented cis-diamminedichloroplatinum induced
apoptosis via enhancing caspase-9 activity. Exp Cell Res.
307:26–40. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yee KS, Wilkinson S, James J, Ryan KM and
Vousden KH: PUMA- and Bax-induced autophagy contributes to
apoptosis. Cell Death Differ. 16:1135–1145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shang Y and Xian Lu: Relationship between
apoptosis and autophagy during tumor treatment. Advances in Modern
Biomedicine. 10:766–769. 2010.(In Chinese).
|
|
36
|
Mariño G, Niso-Santano M, Baehrecke EH and
Kroemer G: Self-consumption: The interplay of autophagy and
apoptosis. Nat Rev Mol Cell Biol. 15:81–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Q, Zeng P, Liu Y, Wen G, Fu X and Sun
X: Inhibition of autophagy ameliorates atherogenic inflammation by
augmenting apigenin-induced macrophage apoptosis. Int
Immunopharmacol. 27:24–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jung CH, Ro SH, Cao J, Otto NM and Kim DH:
mTOR regulation of autophagy. FEBS Lett. 584:1287–1295. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chang Z, Shi G, Jin J, Guo H, Guo X, Luo
F, Song Y and Jia X: Dual PI3K/mTOR inhibitor NVP-BEZ235-induced
apoptosis of hepatocellular carcinoma cell lines is enhanced by
inhibitors of autophagy. Int J Mol Med. 31:1449–1456. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhai CG, Cheng J, Mujahid H, Wang H, Kong
J, Yin Y, Li J, Zhang Y, Ji X and Chen W: Selective inhibition of
Akt/mTOR signaling pathway regulates autophagy of macrophage and
vulnerability of atherosclerotic plaque. PLoS One. 9:e905632014.
View Article : Google Scholar : PubMed/NCBI
|