Introduction
Patients presenting with age-related macular
degeneration (AMD) suffer from progressive visual degeneration due
to a damaged macular area (1). It
is estimated that ~300 million people will be diagnosed with AMD by
2040 (1). There are two prominent
pathological features associated with AMD, the formation and
accumulation of drusen, and damage to retinal pigment epithelial
(RPE) cells (2). Furthermore, the
decrease of neuronal nitric oxide synthase in RPE cell nuclei may
be associated with the redox status of the RPE in patients with AMD
(3). Therefore, protecting these
cells from injury is important to prevent AMD pathology.
As a critical part of the blood-retinal barrier, the
RPE plays an essential role in supporting the neural retina and
visual cycle, by protecting the fundus tissue from oxidation
(4). The RPE cell layer is easily
damaged by reactive oxygen species (ROS) compared with other cells,
due to the high oxygen consumption of the retina (5,6). In
addition, ROS-induced damage to RPE cells is an irreversible
process and is an early sign of AMD (7). Thus, therapies against oxidative
stress (OxS) should be effective in protecting the RPE and may help
prevent the development of AMD.
The antioxidant, nuclear factor erythroid 2-related
factor 2 (Nrf2) plays an essential role in the immune defense
system (8). For example, in the
cytoplasm, Nrf2 combines with the inhibitor epichlorohydrin-related
protein 1 (Keap1) like Kelch (9)
under physiological conditions. However, when the cell is damaged,
Nrf2 disassociates from Keap1 and translocates to the nucleus,
triggering the downstream gene expression of heme oxygenase-1
(HO-1) (10). Recent studies have
reported that Nrf2 and HO-1 participate in the etiology of AMD
(11), and that they are involved
in maintaining the dynamic balance of the retina under stress or
trauma (12). Therefore, the
activation of Nrf2 may represent a potentially useful therapy for
the treatment of AMD.
Lycium barbarum polysaccharide (LBP) has been
reported to exhibit several biological functions, including
immunomodulation, neuroprotection, anti-aging and antioxidative
capabilities (13). Furthermore,
LBP has been reported to reduce the levels of ROS and the extent of
apoptosis in human lens epithelial cells (14). In addition, ischemia-induced retinal
damage in diabetic rats is ameliorated by LBP (15). However, the protective effect of LBP
on AMD has not yet been studied; thus, the present study aimed to
investigate the inhibitory effect of LBP on
H2O2-induced OxS and apoptosis in RPE cells,
as well as to investigate its effect on the Nrf2/HO-1 pathway, and
employed an in vitro AMD model by exposing human retinal
epithelial cell lines, ARPE-19, to H2O2,
which may help to provide an alternative potential neoteric
strategy for AMD therapy.
Materials and methods
Materials and chemicals
LBP (purity >90%; cat. no. SP9311), RIPA lysis
buffer (cat. no. R0010) and Annexin V-FITC/PI double staining kit
(cat. no. CA1020) were purchased from Beijing Solarbio Science
& Technology Co., Ltd. DMEM/F12 medium (cat. no. PM150312) and
fetal bovine serum (FBS, cat. no. 164210) were purchased from
Procell Life Science & Technology Co., Ltd. Primary antibodies
against histone H3 (cat. no. ab1791), Bcl2 (cat. no. ab32124),
Caspase-3 (cat. no. ab13585), Cleaved caspase-3 (cat. no.
ab214430), Bax (cat. no. ab3191) and β-actin (cat. no. ab6276) were
purchased from Abcam (dilutions 1:500 or 1:1,000). Antibodies
against Nrf2 (cat. no. 12721) and HO-1 (cat. no. 5853) were
purchased from Cell Signaling Technology, Inc. (dilution 1:500).
Commercial kits for the detection of 2,7-dichlorodihydrofluorescein
diacetate (DCFH-DA, cat. no. S0033), malondialdehyde (MDA, cat. no.
A003-1-2), superoxide dismutase (SOD, cat. no. A001-1-2),
GSH-peroxidase (GSH-Px, cat. no. A005-1-2) and catalase (CAT, cat.
no. A007-1-1) were purchased from Nanjing Jiancheng Bioengineering
Institute. Horseradish peroxidase secondary antibody (1:500, cat.
no. A0216), PBS (cat. no. ST447-5L) and enhanced chemiluminescence
(ECL, cat. no. P0018S) reagent were purchased from Beyotime
Institute of Biotechnology, and all other chemicals were purchased
from Sigma-Aldrich; Merck KGaA.
Cell culture and treatments
ARPE-19 cells (Procell Life Science & Technology
Co., Ltd., certified by STR) were maintained in DMEM/F-12
supplemented with 10% FBS, streptomycin (100 mg/ml) and penicillin
(100 U/ml), at 37°C with 5% CO2. All treatments were
performed when the cells reached ~80% confluence.
Cell viability assay
ARPE-19 cells were seeded into 96-well plates at a
density of 1×104 cells/well, with six replicates for
each group. Following incubation overnight at 37°C, the cells were
incubated with different concentrations of
H2O2 (0, 125, 250, 500 and 1,000 µM) for 2 h
at 37°C to determine the optimal concentration. ARPE-19 cells were
also pretreated with different concentrations of LBP (0, 0.25, 0.5,
1 and 2 mg/ml) for 24 h at 37°C to optimize the dose of LBP to be
used in the present study. Pretreatment with LBP was preceded by
co-incubation with H2O2 (500 µM) for 2 h at
37°C to assess the protective effect of LBP on
H2O2-triggered cell death.
Cell viability was assessed via the Cell Counting
Kit-8 (CCK-8, cat. no. HY-K0301; MedChemExpress) assay. Briefly,
CCK-8 solution was added into each well and incubated for 2 h at
37°C, in the dark. Cell viability was determined using a microplate
reader (BioTek Instruments, Inc.) and calculated as follows: Cell
viability (%)=[(absorbance of the test sample-absorbance of the
control sample)/mean absorbance of the control] ×100.
Measurement of intracellular ROS
The DCFH-DA method was used to measure ROS levels.
Briefly, ARPE-19 cells (1×106 cells/well) were cultured
in the presence or absence of different concentrations of LBP in
6-well plates for 24 h at 37°C, prior to treatment with 500 µM
H2O2. Cells were subsequently cultured in the
presence of DCFH-DA (10 mM) at room temperature in the dark for 20
min. Cells were washed three times with cold PBS and the
fluorescence intensity of the harvested cells was determined using
a FACSCalibur flow cytometer (Beckman Coulter, Inc.). All
experimental results are presented as percentages relative to that
of the control sample.
Measurement of MDA, SOD, CAT and
GSH-Px
Following the different treatments, ARPE-19 cells
(1×106 cells/well) in 1.5 ml Eppendorf tubes (Thermo
Fisher Scientific, Inc.) were co-incubated with 100 µl RIPA lysis
buffer and 10% protease inhibitor (cat. no. HY-K0010;
MedChemExpress) at 4°C for 30 min. Following lysis and
centrifugation at 12,000 × g for 15 min at 4°C, the proteins in the
lysate were quantified using the BCA kit (cat. no. P0009; Beyotime
Institute of Biotechnology). The intracellular activities of MDA
and SOD, and levels of CAT and GSH-Px were determined
spectrophotometrically using the relevant commercial kits. SOD, CAT
and GPX-Px activities are presented as units/mg protein, while MDA
levels are presented as nmol/g of protein. All experimental results
are presented as percentages of the control value.
Quantification of apoptosis
After collecting cells from different treatment
groups, the centrifuged ARPE-19 cells were resuspended in 100 µl
binding buffer at a density of 1×106 cells/ml.
Subsequently, 5 µl Annexin V-FITC and 5 µl of PI were added and
gently mixed into the cell suspension. Following incubation at room
temperature for 15 min in the dark, FACSVerse flow cytometer (BD
Biosciences) and FACSuite software (version 1.0.4.2650; BD
Biosciences) were used for the quantitation of apoptotic cells. The
non-apoptotic, early and late apoptotic cells are presented as
Annexin−/PI−, Annexin
V−FITC+/PI− and
Annexin+/PI+ cell populations,
respectively.
Western blotting
Following the different treatments, ARPE-19 cells
(1×106 cells/well) in 1.5 ml Eppendorf tubes were
co-incubated with 100 µl RIPA lysis buffer and 10% protease
inhibitor at 4°C for 30 min. Following lysis and centrifugation at
12,000 × g for 15 min at 4°C, the proteins in the lysate were
quantified using the BCA kit. Following protein quantitation, 10 or
12% SDS-PAGE was used to resolve the proteins (30 µg/lane), which
were transferred onto PVDF membranes (MilliporeSigma) and
subsequently blocked with 5% skimmed milk for 2 h at room
temperature. The membranes were incubated with primary antibodies
overnight at 4°C. After washing three times with PBS, the membranes
were incubated with secondary antibodies for 2 h at room
temperature. Protein bands were visualized using ECL reagent and
analyzed using Image Lab software (version 4.0, Bio-Rad
Laboratories, Inc.). β-actin and histone H3 were used as the
internal controls.
Small interfering (si)RNA
ARPE-19 cells (1×105 cells/well) were
transfected with 100 µM negative control (NC,
5′-CACACTGGATGGCCTAGGAGGATAT-3′) siRNA or 100 µM siRNA Nrf2
(5′-CACACTGGATCAGACAGGAGGATAT-3′) (Shanghai GenePharma Co., Ltd.),
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After 12 h of transfection at 37°C, ARPE-19
cells were pretreated with LBP for 24 h and then exposed to 500 µM
H2O2 for 2 h. Next, western blotting and the
CCK-8 assay were performed to assess the effect of LBP on the
protection of ARPE-19 cells, and determine its underlying molecular
mechanism.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 8.0 (GraphPad Software, Inc.). All experiments were
performed in triplicate and data are presented as the mean ± SEM.
Unpaired Student's t-test was used to compare differences between
two groups, while one-way ANOVA and Tukey's post hoc test was used
to compare differences between multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
LBP reduces
H2O2-induced cell damage
The toxicity of LBP against ARPE-19 cells was
assessed. Following 24 h of pretreatment with LBP (0, 0.25, 0.5, 1
or 2 mg/ml), cell viability was assessed via the CCK-8 assay. The
results demonstrated that cell viability was retained before and
after 24 h of pretreatment, suggesting that the assessed
concentrations of LBP were safe and did not affect the cells
(Fig. 1A). To evaluate the
potential impact of H2O2, ARPE-19 cells were
incubated with 0, 125, 250, 500 and 1,000 µM
H2O2 for 2 h, and the resulting cell toxicity
was assessed. As expected, cell viability significantly decreased
following treatment with H2O2 compared with
the control group, in a dose-dependent manner (Fig. 1B). Notably, cell viability
significantly decreased by 53.6% (P<0.01) at a concentration of
500 µM H2O2. Thus, this concentration was
selected for subsequent experimentation. The antioxidant effect of
LBP pretreatment was subsequently evaluated. ARPE-19 cells were
treated with different concentrations of LBP (0.5, 1 or 2 mg/ml)
and 500 µM H2O2, and cell viability was
assessed via the CCK-8 assay. The results demonstrated that ARPE-19
cell viability increased up to 90.33% following pretreatment with 2
mg/ml LBP (P<0.001; Fig. 1C).
Taken together, these results suggested that 24 h of pretreatment
with LBP (0.5–2 mg/ml) effectively reduced the
H2O2-induced damage in these cells.
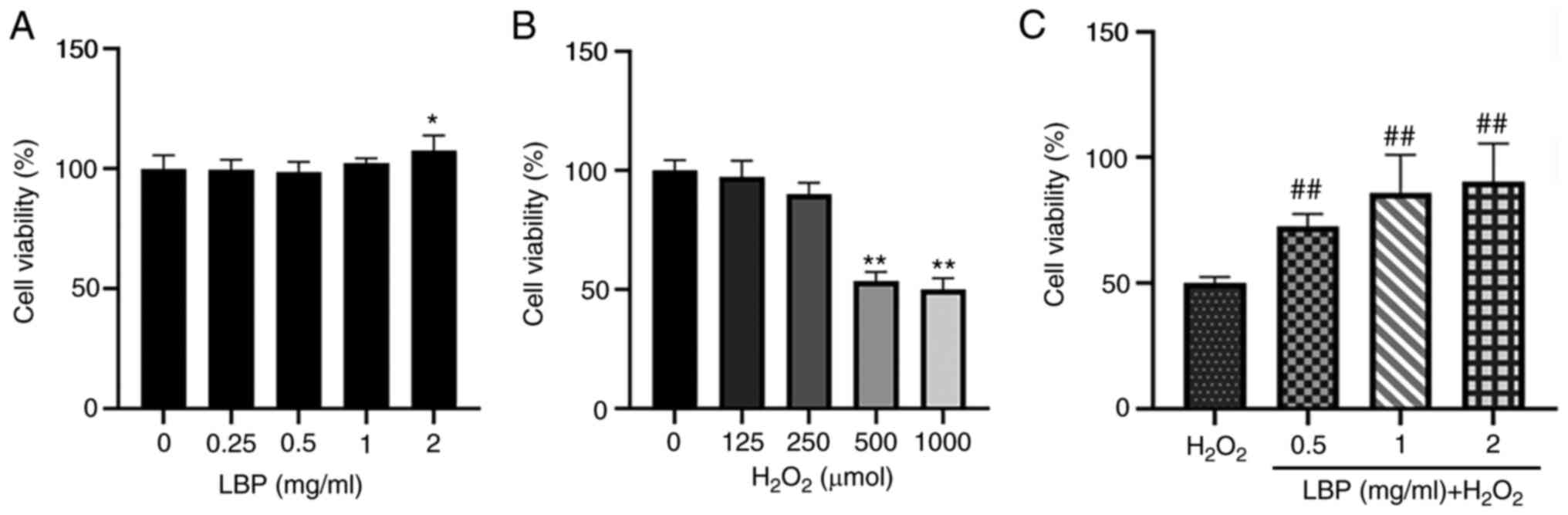 | Figure 1.LBP reduces
H2O2-induced cytotoxicity. (A) Effect of LBP
on the viability of ARPE-19 cells treated with different
concentrations of LBP (0, 0.25, 0.5, 1 and 2 mg/ml) for 24 h. (B)
Effect of H2O2 on the viability of ARPE-19
cells. ARPE-19 cells were treated with different concentrations of
H2O2 (0, 125, 250, 500 and 1,000 µM) for 2 h.
(C) Effect of LBP on H2O2-induced
cytotoxicity in ARPE-19 cells. ARPE-19 cells were pretreated with
different concentrations of LBP (0.5, 1 and 2 mg/ml) for 24 h
followed by 500 µM H2O2 for 2 h. Data are
presented as the mean ± SEM (n=3). *P<0.05, **P<0.01 vs.
control; ##P<0.001 vs.
H2O2-treated cells with no LBP pretreatment.
LBP, Lycium barbarum polysaccharide. |
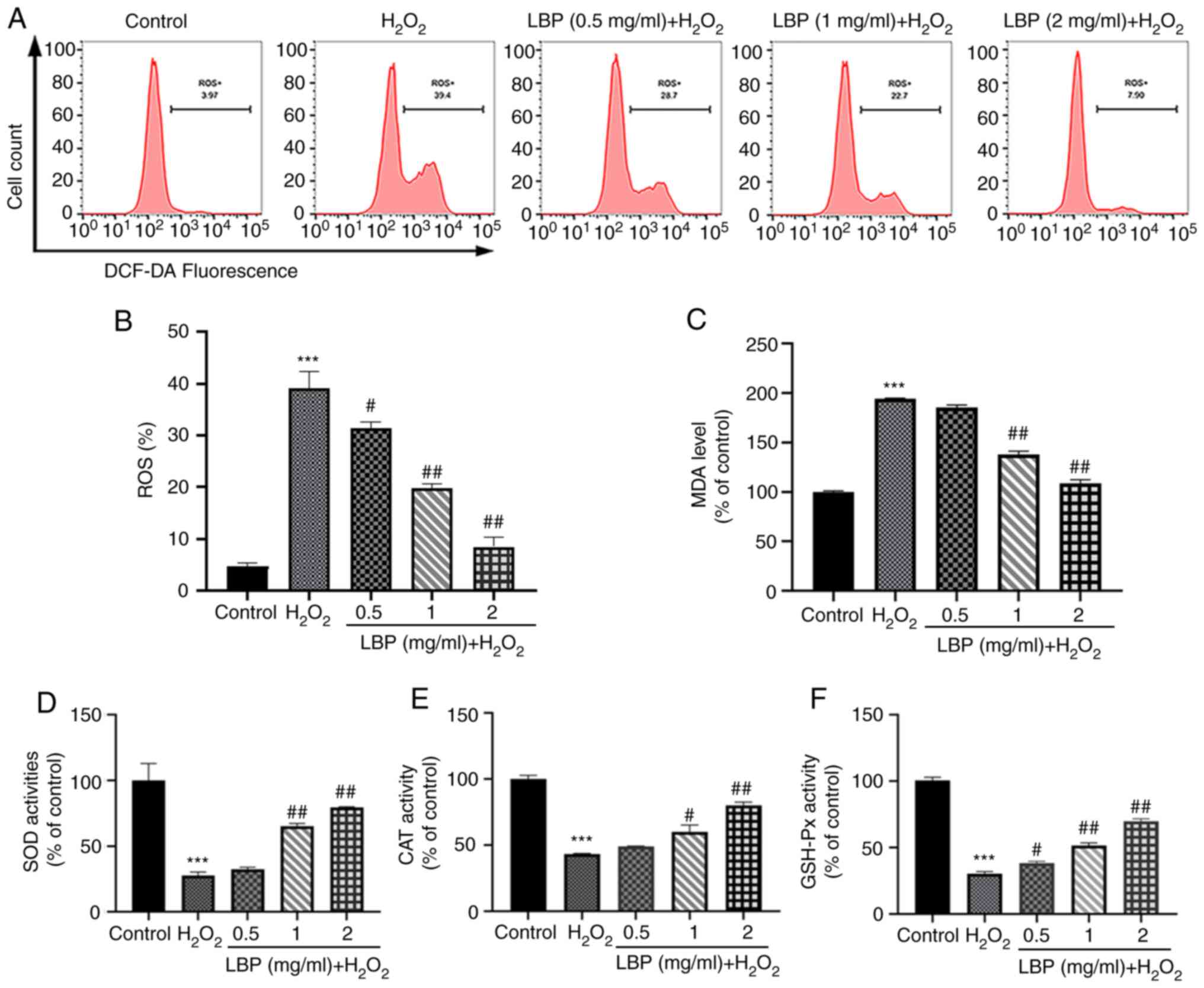
LBP ameliorates
H2O2-induced OxS
To determine the mechanism by which LBP exerts its
cellular protection, indicators of intracellular OxS levels were
used along with the evaluation of antioxidant enzyme levels. The
DCFH-DA assay was performed to measure ROS levels, as well as the
ability of LBP to scavenge H2O2-induced ROS.
As presented in Fig. 2A-C, in
comparison with the controls, both ROS and MDA levels in ARPE-19
cells significantly increased following treatment with
H2O2 (P<0.001). However, pretreatment with
LBP significantly decreased both ROS and MDA levels (P<0.01 or
P<0.001). These results suggest the critical influence of LBP on
the inhibition of H2O2-induced OxS in ARPE-19
cells. In addition, antioxidant stress markers (SOD, CAT and
GSH-Px) were monitored both in the presence and absence of LBP and
H2O2. As presented in Fig. 2D and E, H2O2
significantly decreased the activities of these antioxidant enzymes
(P<0.001), while LBP pretreatment effectively restored the
activities of SOD and CAT to normal levels (P<0.01 or
P<0.001). Similarly, the GSH-Px ratio significantly enhanced
following pretreatment with LBP compared with cells undergoing
H2O2 treatment alone (P<0.01 or
P<0.001; Fig. 2F), suggesting
that LBP pretreatment is a potent inhibitor of OxS damage.
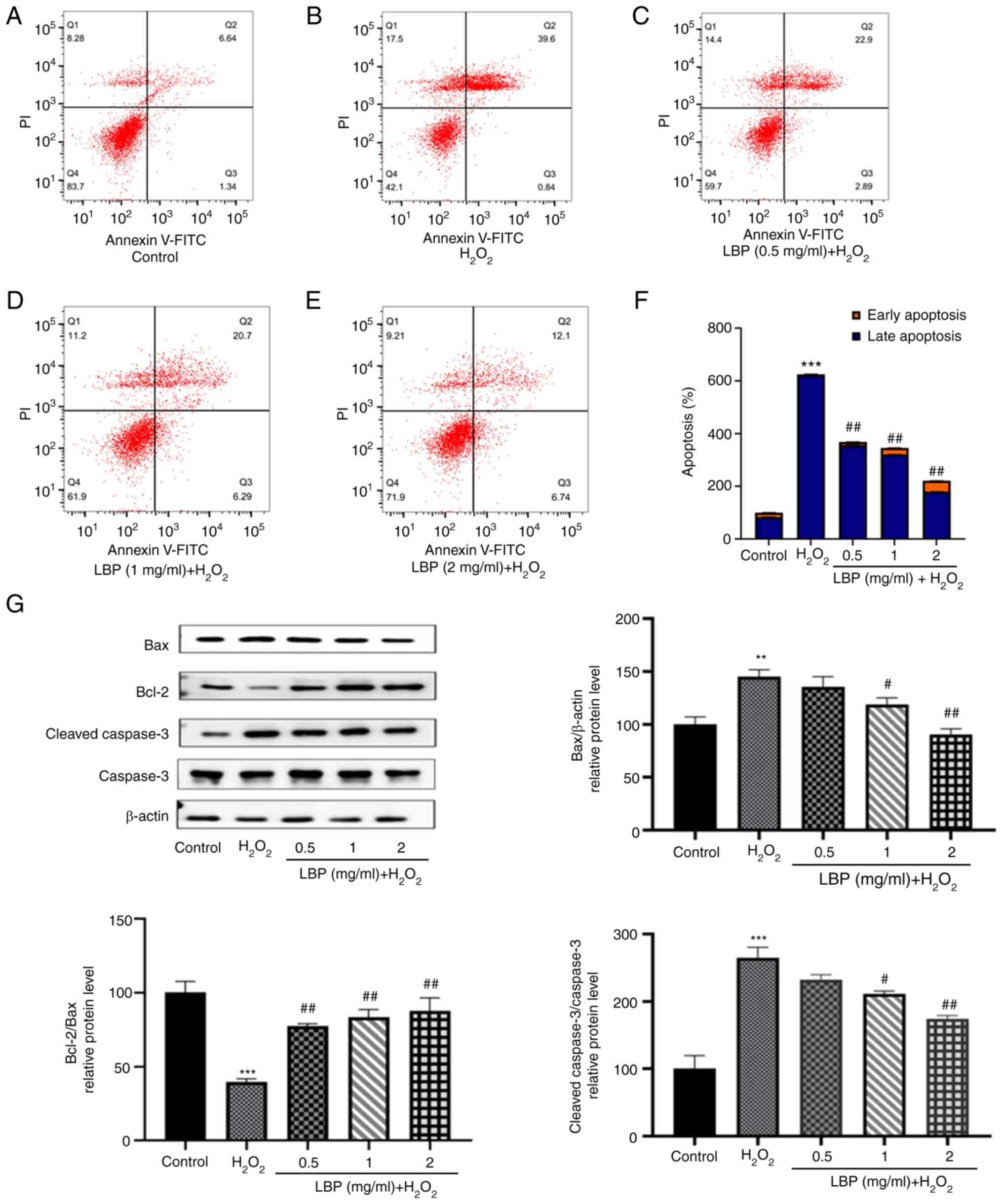
LBP prevents
H2O2-induced apoptosis
Flow cytometry was performed to determine the extent
of apoptosis occurring in ARPE-19 cells under different
experimental conditions. As presented in Fig. 3A and B, the extent of apoptosis in
the H2O2 group was markedly higher compared
with the control group. Notably, H2O2-induced
apoptosis in ARPE-19 cells was inhibited following treatment with
different concentrations of LBP (Fig.
3C-E). The percentage of apoptotic cells of each experimental
group are shown in Fig. 3F,
differences were significant (P<0.001). To identify the cause of
this anti-apoptotic effect at the protein level, the present study
detected the expression levels of apoptosis-related proteins,
including pro-apoptotic Bax and cleaved caspase-3 and the
anti-apoptotic protein Bcl-2 via western blotting (Fig. 3G). Compared with the controls, cells
treated with 500 µM H2O2 exhibited higher
levels of Bax and cleaved caspase-3 expression (P<0.01 or
P<0.001), which is consistent with the results obtained from
flow cytometry. Furthermore, an increased expression of Bcl-2 with
a concomitant decrease in Bax and cleaved caspase-3 (P<0.01 or
P<0.001) was observed 24 h after pretreatment with LBP,
suggesting that LBP exhibits a significant dose-dependent reversal
of H2O2-induced apoptosis (Fig. 3G). In addition, the Bcl-2/Bax ratio
markedly increased in the LBP pretreatment group (P<0.001), the
effects of which were reversed following treatment with
H2O2, suggesting its effective protection
against H2O2-induced apoptosis in ARPE-19
cells.
LBP alleviates
H2O2-induced cell damage via the Nrf2/HO-1
pathway
To determine the molecular mechanism involved in
this protection against H2O2-induced
oxidative damage and apoptosis, a potential signaling effect
induced by LBP upon Nrf2/HO-1 was investigated. Western blot
analysis demonstrated that treatment with
H2O2 increased the nuclear transcriptional
expression of Nrf2 protein (P<0.05; Fig. 4A), the main regulator of the
cellular antioxidant response (8).
Compared with the H2O2 group, pretreatment
with LBP increased this nuclear transcriptional expression of Nrf2
protein, in a dose-dependent manner (P<0.01 or P<0.001;
Fig. 4A). In addition, the
downstream gene, HO-1 also exhibited a similar trend in expression
to that of Nrf2 (P<0.01 or P<0.001; Fig. 4B). To further investigate the
molecular mechanism of LBP on H2O2-induced
ARPE-19 cell damage, treatment with LBP exhibited a negligible
influence on the expression of nuclear Nrf2 and HO-1 (Fig. 4C). However, a statistically
significant increase was observed in nuclear Nrf2 and HO-1 upon
induction of damage by H2O2 (P<0.01;
Fig. 4C), suggesting that the
combination of LBP and OxS contributed to increasing the nuclear
translocation of the Nrf2 protein in a synergistic manner.
 | Figure 4.LBP alleviates
H2O2-induced RPE cell damage via the
Nrf2/HO-1 pathway. ARPE-19 cells were incubated in the presence or
absence of LBP for 24 h, and subsequently treated with 500 µM
H2O2 for 2 h. (A) The relative protein
expression levels of nuclear Nrf2 were determined via western
blotting. (B) The relative protein expression levels of HO-1 were
determined via western blotting. (C) Western blot analysis was
performed to detect the protein expression levels of nuclear Nrf2
and HO-1. (D) ARPE-19 cells were transfected with siRNA (NC or
Nrf2) for 12 h. Protein expression levels of Nrf2 were analyzed via
western blotting. ARPE-19 cells were transfected with siRNA (NC or
Nrf2) for 12 h, incubated with LBP for 24 h and subsequently
treated with H2O2 for 2 h. Protein expression
levels of Nrf2 and HO-1 were analyzed via western blotting. (E)
ARPE-19 cells were transfected with siRNA (NC or Nrf2) for 12 h,
incubated in the presence or absence of LBP for 24 h and
subsequently treated with H2O2 for 2 h. The
cytoprotective effect of LBP was analyzed via the Cell Counting
Kit-8 assay. +, presence of H2O2 or LBP; -,
absence of H2O2 or LBP. *P<0.05,
**P<0.01, ***P<0.001 vs. control; #P<0.01,
##P<0.001 vs. H2O2-treated
cells with no LBP pretreatment. LBP, Lycium barbarum
polysaccharide; RPE, retinal pigment epithelium; Nrf2, nuclear
factor erythroid 2-related factor 2; HO-1, heme oxygenase-1; si,
small interfering; NC, negative control. |
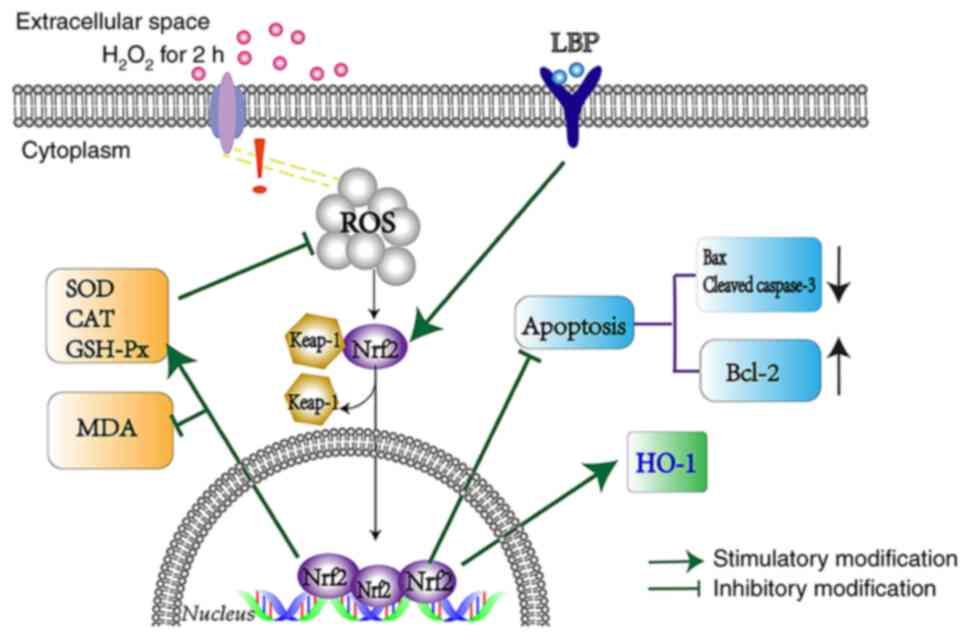
To determine the molecular mechanisms involved in
this process, siRNA transfection was performed to silence Nrf2
expression. The results demonstrated that Nrf2 protein expression
significantly decreased in ARPE-19 cells (P<0.01; Fig. 4D) and LBP-mediated expression of
HO-1 was almost eliminated (P<0.001; Fig. 4D). In addition, transfection with
Nrf2-siRNA enhanced H2O2-induced cell death,
thereby offsetting the protection by LBP (P<0.05; Fig. 4E). Taken together, these results
suggest that LBP activates the Nrf2/HO-1 pathway, and thus protects
ARPE-19 cells from H2O2-induced cell damage
(Fig. 5).
Discussion
RPE cells are critical for maintaining the
structural integrity of the retina (16,17)
and are particularly susceptible to the negative effects of OxS,
and are generally exposed to high levels of ROS (18) due to the high oxygen demands of the
retina. Previous studies (7,19,20)
have reported the association between ROS-induced damage in RPE
cells and AMD, suggesting that early intervention is important for
the prevention of OxS-induced damage. The anti-oxidative and
anti-apoptotic functions of LBP have been reported in several eye
diseases, including glaucoma (21),
retinal ischemia-reperfusion injury (22) and diabetic retinopathy (23). Studies on the chemical composition
of LBP have revealed that glycopeptides within its structure can
alleviate lipid peroxidation (24–26).
Thus, the present study aimed to investigate how LBP prevents OxS
and apoptosis in ARPE-19 cells and its potential mechanism of
action.
The present study used H2O2 to
mimic the pathogenesis of AMD to determine the influence of LBP on
OxS (27–29). The results of the CCK-8 assay
demonstrated that treatment with 500 µM H2O2
significantly reduced the viability of ARPE-19 cells, the effects
of which were reversed following pretreatment with LBP, in a
concentration-dependent manner.
It has been reported that
H2O2-induced OxS is associated with increased
ROS levels, which can be eliminated by enhancing the activity of
antioxidant enzymes, thereby reducing the apoptotic state of aging
RPE cells (30,31). The present study performed DCFH-DA
staining to detect ROS levels, and flow cytometric analysis
demonstrated that the fluorescence intensity of ROS in the
H2O2 group significantly increased.
Conversely, pretreatment with LBP reduced
H2O2-triggered ROS enhancement. The level of
MDA was also consistent with the level of ROS, and the antioxidant
levels in ARPE-19 cells, including SOD, CAT and GSH-Px, were
maintained at high levels in response to LBP pretreatment.
Previous studies have demonstrated that the
activation of apoptosis triggered by ROS represents a contributing
factor for AMD pathogenesis (32,33).
The effect of H2O2 exposure resulted in an
increase in pro-apoptotic proteins (Bax and cleaved caspase-3), and
a decrease in the anti-apoptotic protein, Bcl-2. However, 24 h of
LBP pretreatment before H2O2 addition
reversed the previously observed phenomenon, as shown by the
reduced expression of Bax and cleaved caspase-3 and increased
expression of Bcl-2.
Furthermore, Nrf2 is heavily involved in the process
of cell redox homeostasis, which serves to reduce OxS by promoting
the expression of antioxidant enzymes (34,35).
However, few studies have focused on the association between LBP
and the Nrf2 pathway during oxidative damage (36,37).
It has been reported that once stimulated by OxS, Nrf2 becomes
dissociated from Keap1 and translocates to the nucleus where it
activates the HO-1 gene (38,39).
The results of the present study demonstrated that LBP pretreatment
alone did not increase nuclear translocation of Nrf2, and as a
result, HO-1 expression was not affected. However, when OxS was
induced, LBP increased the nuclear translocation of Nrf2 and HO-1
expression. Thus, H2O2 is essential for Nrf2
translocation and the expression of antioxidant proteins; these
results are consistent with previous findings (40). The results of the present study
demonstrated that transfection with Nrf2 siRNA partially reversed
the protective effect of LBP on H2O2-induced
cell death. However, only one retinal cell line was assessed in the
present study. Thus, other retinal cell lines and in vivo
studies are required to verify the results presented here.
In conclusion, the results of the present study
suggest that LBP exerts a protective effect on ARPE-19 cells,
particularly by inhibiting H2O2-induced OxS
and apoptosis, enhancing antioxidant enzymes and activating the
Nrf2/HO-1 pathway. Thus, LBP, a nutritional supplement (41), has the ability to reduce the risk of
AMD and OxS-associated retinal disorders.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
QZhao and RL designed the present study. RL, MG and
QZhu performed the experiments. XH analyzed the data. YW helped
perform the analysis with constructive discussions. XH and YW
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AMD
|
age-related macular degeneration
|
|
LBP
|
Lycium barbarum polysaccharide
|
|
RPE
|
retinal pigment epithelium
|
|
OxS
|
oxidative stress
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
Mitchell P, Liew G, Gopinath B and Wong
TY: Age-related macular degeneration. Lancet. 392:1147–1159. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang M, So KF, Lo ACY and Lam WC: The
effect of Lycium barbarum polysaccharides on pyroptosis-associated
amyloid β1-40 oligomers-induced adult retinal pigment epithelium 19
cell damage. Int J Mol Sci. 21:46582020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bhutto IA, Baba T, Merges C, McLeod DS and
Lutty GA: Low nitric oxide synthases (NOSs) in eyes with
age-related macular degeneration (AMD). Exp Eye Res. 90:155–167.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Strauss O: The retinal pigment epithelium
in visual function. Physiol Rev. 85:845–881. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anderson RE, Rapp LM and Wiegand RD: Lipid
peroxidation and retinal degeneration. Curr Eye Res. 3:223–227.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Catalá A: An overview of lipid
peroxidation with emphasis in outer segments of photoreceptors and
the chemiluminescence assay. Int J Biochem Cell Biol. 38:1482–1495.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cai J, Nelson KC, Wu M, Sternberg P Jr and
Jones DP: Oxidative damage and protection of the RPE. Prog Retin
Eye Res. 19:205–221. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bellezza I, Giambanco I, Minelli A and
Donato R: Nrf2-Keap1 signaling in oxidative and reductive stress.
Biochim Biophys Acta Mol Cell Res. 1865:721–733. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kobayashi M and Yamamoto M: Molecular
mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene
regulation. Antioxid Redox Signal. 7:385–394. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shelton LM, Kevin Park B and Copple IM:
Role of Nrf2 in protection against acute kidney injury. Kidney Int.
84:1090–1095. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou J, Chen F, Yan A and Xia X:
Madecassoside protects retinal pigment epithelial cells against
hydrogen peroxide-induced oxidative stress and apoptosis through
the activation of Nrf2/HO-1 pathway. Biosci Rep.
40:BSR201943472020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cameron BD, Sekhar KR, Ofori M and Freeman
ML: The role of Nrf2 in the response to normal tissue radiation
injury. Radiat Res. 190:99–106. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu W, Liu Y, Zhu R, Yu J, Lu W, Pan C,
Yao W and Gao X: Structure characterization, chemical and enzymatic
degradation, and chain conformation of an acidic polysaccharide
from Lycium barbarum L. Carbohydr Polym. 147:114–124. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qi B, Ji Q, Wen Y, Liu L, Guo X, Hou G,
Wang G and Zhong J: Lycium barbarum polysaccharides protect human
lens epithelial cells against oxidative stress-induced apoptosis
and senescence. PLoS One. 9:e1102752014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pan H, Shi Z, Yang TG, Yu LM and Xu AL:
The protective effects of lycium barbarum polysaccharides on
retinal neurons in diabetic rats and its mechanism. Zhongguo Ying
Yong Sheng Li Xue Za Zhi. 35:55–59. 2019.(In Chinese). PubMed/NCBI
|
|
16
|
Curcio CA, Zanzottera EC, Ach T,
Balaratnasingam C and Freund KB: Activated retinal pigment
epithelium, an optical coherence tomography biomarker for
progression in age-related macular degeneration. Invest Ophthalmol
Vis Sci. 58:BIO211–BIO226. 2017.PubMed/NCBI
|
|
17
|
Kopitz J, Holz FG, Kaemmerer E and Schutt
F: Lipids and lipid peroxidation products in the pathogenesis of
age-related macular degeneration. Biochimie. 86:825–831. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li S, Gaur U, Chong CM, Lin S, Fang J,
Zeng Z, Wang H and Zheng W: Berberine protects human retinal
pigment epithelial cells from hydrogen peroxide-induced oxidative
damage through activation of AMPK. Int J Mol Sci. 19:17362018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Golestaneh N, Chu Y, Xiao YY, Stoleru GL
and Theos AC: Dysfunctional autophagy in RPE, a contributing factor
in age-related macular degeneration. Cell Death Dis. 8:e25372017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Golestaneh N, Chu Y, Cheng SK, Cao H,
Poliakov E and Berinstein DM: Repressed SIRT1/PGC-1α pathway and
mitochondrial disintegration in iPSC-derived RPE disease model of
age-related macular degeneration. J Transl Med. 14:3442016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mi XS, Chiu K, Van G, Leung JW, Lo AC,
Chung SK, Chang RC and So KF: Effect of Lycium barbarum
Polysaccharides on the expression of endothelin-1 and its receptors
in an ocular hypertension model of rat glaucoma. Neural Regen Res.
7:645–651. 2012.PubMed/NCBI
|
|
22
|
Li SY, Yang D, Yeung CM, Yu WY, Chang RC,
So KF, Wong D and Lo AC: Lycium barbarum polysaccharides reduce
neuronal damage, blood-retinal barrier disruption and oxidative
stress in retinal ischemia/reperfusion injury. PLoS One.
6:e163802011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yao Q, Yang Y, Lu X, Zhang Q, Luo M, Li PA
and Pan Y: Lycium barbarum polysaccharides improve retinopathy in
diabetic sprague-dawley rats. Evid Based Complement Alternat Med.
2018:79432122018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Varoni MV, Pasciu V, Gadau SD, Baralla E,
Serra E, Palomba D and Demontis MP: Possible antioxidant effect of
Lycium barbarum polysaccharides on hepatic cadmium-induced
oxidative stress in rats. Environ Sci Pollut Res Int. 24:2946–2955.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen L, Li W, Qi D and Wang D: Lycium
barbarum polysaccharide protects against LPS-induced ARDS by
inhibiting apoptosis, oxidative stress, and inflammation in
pulmonary endothelial cells. Free Radic Res. 52:480–490. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schoppet M, Tailhades J, Kulkarni K and
Cryle MJ: Precursor manipulation in glycopeptide antibiotic
biosynthesis: Are β-amino acids compatible with the oxidative
cyclization cascade? J Org Chem. 83:7206–7214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaczara P, Sarna T and Burke JM: Dynamics
of H2O2 availability to ARPE-19 cultures in models of oxidative
stress. Free Radic Biol Med. 48:1064–1070. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Geiger RC, Waters CM, Kamp DW and
Glucksberg MR: KGF prevents oxygen-mediated damage in ARPE-19
cells. Invest Ophthalmol Vis Sci. 46:3435–3442. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zareba M, Raciti MW, Henry MM, Sarna T and
Burke JM: Oxidative stress in ARPE-19 cultures: Do melanosomes
confer cytoprotection? Free Radic Biol Med. 40:87–100. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao H, Wang R, Ye M and Zhang L: Genipin
protects against H2O2-induced oxidative damage in retinal pigment
epithelial cells by promoting Nrf2 signaling. Int J Mol Med.
43:936–944. 2019.PubMed/NCBI
|
|
31
|
Pintea A, Rugină DO, Pop R, Bunea A and
Socaciu C: Xanthophylls protect against induced oxidation in
cultured human retinal pigment epithelial cells. J Food Compos
Anal. 24:830–836. 2011. View Article : Google Scholar
|
|
32
|
Musat O, Ochinciuc U, Gutu T, Cristescu TR
and Coman C: Pathophysiology and treatment of ARMD. Oftalmologia.
56:45–50. 2012.(In Romanian). PubMed/NCBI
|
|
33
|
Qu S, Zhang C, Liu D, Wu J, Tian H, Lu L,
Xu GT, Liu F and Zhang J: Metformin protects ARPE-19 cells from
glyoxal-induced oxidative stress. Oxid Med Cell Longev.
2020:17409432020.PubMed/NCBI
|
|
34
|
Sakai E, Shimada-Sugawara M, Yamaguchi Y,
Sakamoto H, Fumimoto R, Fukuma Y, Nishishita K, Okamoto K and
Tsukuba T: Fisetin inhibits osteoclastogenesis through prevention
of RANKL-induced ROS production by Nrf2-mediated up-regulation of
phase II antioxidant enzymes. J Pharmacol Sci. 121:288–298. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiang P, Chen L, Sun J, Li J, Xu J, Liu W,
Feng F and Qu W: Chotosan ameliorates cognitive impairment and
hippocampus neuronal loss in experimental vascular dementia via
activating the Nrf2-mediated antioxidant pathway. J Pharmacol Sci.
139:105–111. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiong GF, Li DW, Zheng MB and Liu SC: The
effects of Lycium barbarum polysaccharide (LBP) in a mouse model of
cerulein-induced acute pancreatitis. Med Sci Monit. 25:3880–3886.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang Y, Zhou F, Shen C, Wang H and Xiao
Y: LBP reduces theinflammatory injuryof kidney in septic rat and
regulates the Keap1-Nrf2/ARE signaling pathway1. Acta Cir Bras.
34:e201900100000032019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen B, Lu Y, Chen Y and Cheng J: The role
of Nrf2 in oxidative stress-induced endothelial injuries. J
Endocrinol. 225:R83–R99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hiramatsu K, Tsuneyoshi T, Ogawa T and
Morihara N: Aged garlic extract enhances heme oxygenase-1 and
glutamate-cysteine ligase modifier subunit expression via the
nuclear factor erythroid 2-related factor 2-antioxidant response
element signaling pathway in human endothelial cells. Nutr Res.
36:143–149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hao Y, Liu J, Wang Z, Yu LL and Wang J:
Piceatannol protects human retinal pigment epithelial cells against
hydrogen peroxide induced oxidative stress and apoptosis through
modulating PI3K/Akt signaling pathway. Nutrients. 11:15152019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cao S, Du J and Hei Q: Lycium barbarum
polysaccharide protects against neurotoxicity via the Nrf2-HO-1
pathway. Exp Ther Med. 14:4919–4927. 2017.PubMed/NCBI
|