Introduction
Gastric cancer, one of the commonest malignancies,
is the third leading cause of cancer-related deaths worldwide
(1). The incidence and mortality
rates of gastric cancer are declining every year due to the
improved control of related risk factors and the development of
screening techniques (2). However,
the incidence rate of gastric cancer remains high due to the large
population base of the world and the trend of population aging
(3). The gastric cancer cases and
deaths in China account for 50% of the total gastric cancer cases
in the world. In China, the incidence rate of gastric cancer is
higher in northwestern and eastern coastal areas than in southern
regions (4,5). Gastric cancer not only damages the
digestive system but also affects the liver, kidney and respiratory
functions once metastasis occurs. In severe cases, gastric cancer
may lead to cachexia and ultimately become life-threatening
(6). Surgery is the main treatment
method for gastric cancer. Numerous patients with gastric cancer
have witnessed an improvement in their conditions following surgery
combined with chemo- or radiotherapies. However, a number of
patients still suffer from recurrences and metastases after initial
treatment. The effects of treatment and the 5-year survival rate
are unsatisfactory (7). Therefore,
new drugs and therapies for the clinical treatment of gastric
cancer need to be urgently developed.
A number of studies have demonstrated that Chinese
herbal medicines can effectively kill cancer cells and possess
fewer problems in drug-resistance and toxic side effects (8). Naringin
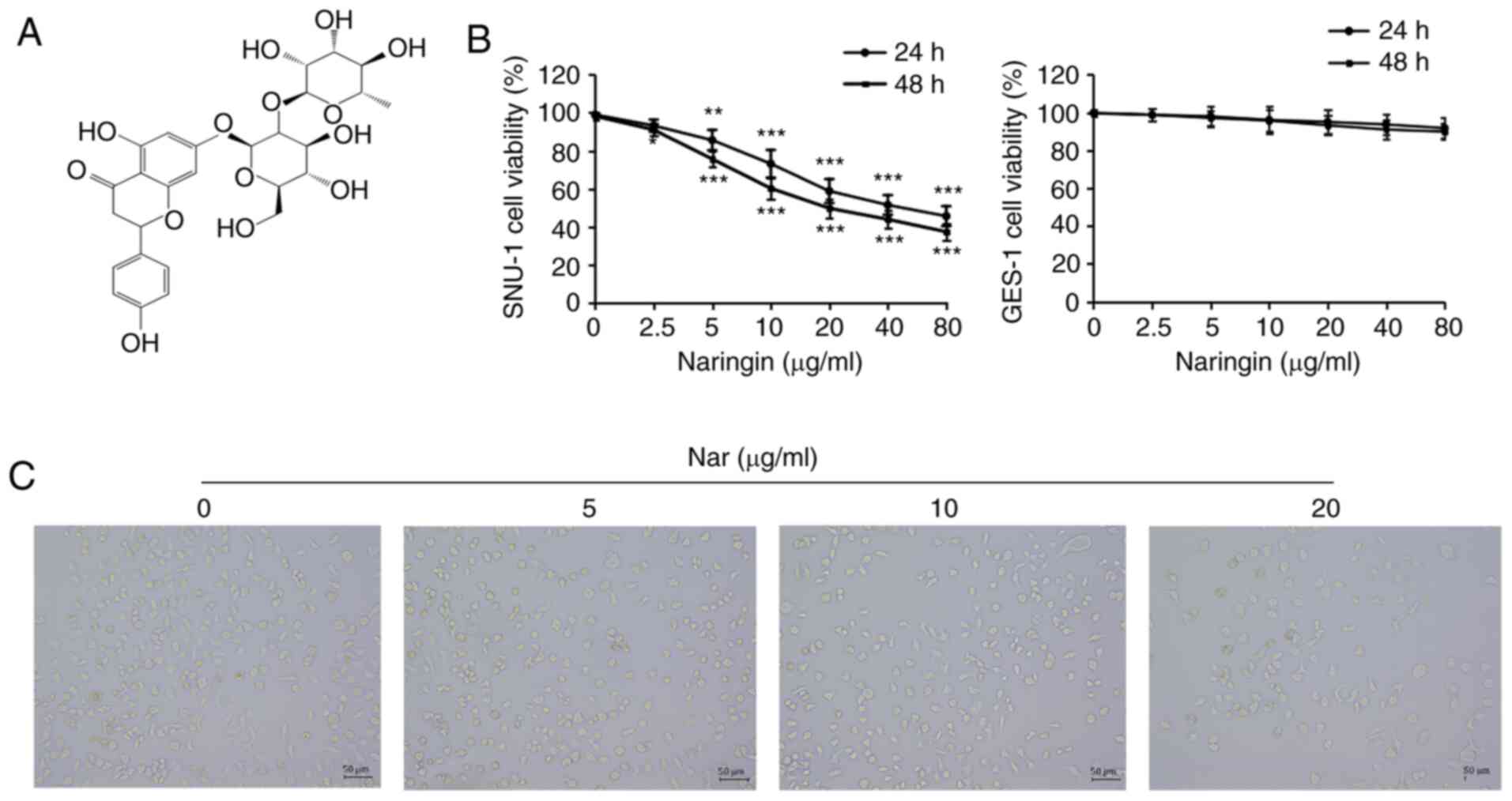
(4′,5,7-trihydroxyflavanone-7-rhamnoside, Nar; Fig. 1A) is a natural glycoside, also
called bioflavonoid, found in pomelo and other citrus fruits
(9). Previous studies have shown
that Nar has various pharmacological activities, including
anti-inflammatory, antioxidant and cardioprotective effects and can
regulate glucose and lipid metabolism (10,11).
Accumulating studies have also confirmed the anti-cancer activities
of Nar through eliminating free radicals via its antioxidant
effects and inhibiting oncogene expression and then inhibiting
cancer cell proliferation and inducing cancer cell apoptosis
(12–14). Nar can induce apoptosis and inhibit
cancer cell growth in colorectal cancer, cervical cancer, ovarian
cancer, liver cancer and other types of cancer (15–18).
However, the effect of Nar on gastric cancer SNU-1 cells and its
mechanism remain unclear.
The present study demonstrated the anti-cancer
effect of Nar and its potential molecular mechanism through
experimental research on gastric cancer cell line SNU-1, thus
providing a new theoretical basis for further research on the
anti-cancer effect of Nar and related molecular mechanisms.
Materials and methods
Experimental reagents
Gastric carcinoma cell lines (SNU-1; cat. no.
CL-0474), normal human gastric epithelial cells (GES-1; cat. no.
CL-0563) and fetal bovine serum (FBS) were purchased from Procell
Life Science & Technology Co., Ltd. RPMI-1640 medium was
purchased from Gibco (Thermo Fisher Scientific, Inc.). The Hoechst
33258 staining solution (C1017), TUNEL Apoptosis Assay kit (C1088)
and BCA Protein Assay Kit (P0012) were procured from Beyotime
Institute of Biotechnology. The rabbit anti-human cysteinyl
aspartate specific proteinase (caspase 3) antibody (1:1,000; cat.
no. ab179517), rabbit anti-human Bax (1:1,000; cat. no. ab32503),
rabbit anti-human Bcl-2 (1:2,000; cat. no. ab182858), rabbit
anti-human microtubule-associated protein 1 light chain 3β (LC3B;
1:3,000; cat. no. ab51520), rabbit anti-human Beclin (1:2,000; cat.
no. ab207612), rabbit anti-human p62 (1:500; cat. no. ab155686),
rabbit anti-human phosphorylated (p-)-PI3K (1:1,000; cat. no.
ab138364), rabbit anti-human PI3K (1:1,000; cat. no. ab32089),
rabbit anti-human AKT antibody (1:500; cat. no. ab8805), rabbit
anti-human p-AKT antibody (1:500; cat. no. ab38449) and rabbit
anti-human GAPDH antibody (1:2,500; cat. no. ab9485) were obtained
from Abcam. Horseradish peroxidase (HRP)-labeled goat anti-rabbit
immunoglobulin G (IgG) antibody (1:2,000; cat. no. CW0103) was
purchased from Cwbio. Naringin (cat. no. HY-N0153) and
3-methyladenine (3-MA; cat. no. HY-19312) were purchased from
MedChemExpress. Annexin V-fluorescein isothiocyanate (FITC)
apoptosis detection kits (cat. no. KGA106) and cell cycle detection
kits (cat. no. KGA511) were purchased from Keygentec.
Radio-Immunoprecipitation Assay (RIPA) solution (cat. no. P0013)
was obtained from Beyotime Institute of Biotechnology.
Cell culture
All cells were maintained in the RPMI-1640 medium
supplemented with 10% FBS and 100 U/ml penicillin and 100 µg/ml
streptomycin in an incubator at 37°C with a 5% CO2
atmosphere. In the present study, SNU-1 cells without costunolide
(Cos) treatment (0 µg/ml) group served as the control group.
Cell Counting Kit-8 (CKK-8) assay
Cell proliferation was analyzed using CCK-8
solution. SNU-1 and GES-1 cells (100 µl/well, 1.5×105
cells/ml) were seeded in a 96-well plate and incubated in a
CO2 incubator for 24 h at 37°C. After aspiration, the
cells were incubated with different concentrations (0, 2.5, 5, 10,
20, 40 and 80 µg/ml) of Nar in FBS-free RPMI 1640 for 24 h and then
CCK-8 assay was used according to the manufacturer's protocol.
Optical density values were measured with a microplate reader at
450 nm.
Observation of cell morphology
The SNU-1 cells were treated with different
concentrations (0, 5, 10 and 20 µg/ml) of Nar in FBS-free RPMI 1640
for 24 h and observed and images captured using an inverted light
microscope (magnification, ×100; Olympus Corporation).
Hoechst 33258 staining
Cell apoptosis was analyzed using Hoechst 33258
staining. SNU-1 cells were seeded into 12-well plates and treated
with different concentrations (0, 5, 10 and 20 µg/ml) of Nar for 24
h. The adherent cells were washed twice with phosphate-buffered
saline. The cells were then stained with Hoechst 33258 for 5 min at
room temperature and washed twice. The blue-stained nuclei was
observed under the BX41 fluorescence microscope (magnification,
×100; Olympus Corporation). Images were captured and used to
quantitatively analyze the apoptosis of cells using Image-Pro Plus
analysis software 6.0 (Media Cybernetics, Inc.).
TUNEL staining
Cell apoptosis was analyzed using TUNEL staining.
SNU-1 cells were plated onto 12-well plates for 24 h and treated
with different concentrations (0, 5, 10 and 20 µg/ml) of Nar for 24
h. Then, apoptosis was evaluated using the TUNEL apoptosis assay
kit and observed under the BX41 fluorescence microscope
(magnification, ×100; Olympus Corporation). Images were captured
and used to quantitatively analyze the apoptosis of cells using
Image-Pro Plus analysis software 6.0 (Media Cybernetics, Inc.).
Flow cytometry
Cell cycle and apoptosis were measured by flow
cytometry. The cell cycle was analyzed using an Annexin
V-FITC/propidium iodide (PI) apoptosis kit. The six-well plate was
seeded with SNU-1 cells (2.0 ml/well, 1.0×106 cells/ml)
cultured for 24 h. After aspiration, the cells were incubated with
2.0 ml of different concentrations (0, 5, 10 and 20 µg/ml) of Nar
for 24 h, or treated with Nar before pretreatment with 3-MA,
following which the collected cells were fixed with 75% ethanol at
4°C overnight. The cell cycle detection kit was used following the
manufacturer's protocols. The cell samples were mixed with PI for
15 min at 37°C in the dark and apoptotic cells were examined with a
BD FACSaria Fusion flow cytometer (BD Biosciences) and ModFit
software version 3.2 (BD Biosciences). The apoptotic rate was
calculated as the percentage of early and late apoptotic cells.
Western blot analysis
The changes in apoptosis-related proteins (caspase
3, Bax and Bcl2), signaling pathway-related proteins (AKT,
phosphorylated (p-)AKT, PI3K and p-PI3K) and autophagy-related
proteins (LC3B, Beclin-1 and p62) in SNU-1 cells were analyzed
using western blot analysis. After incubation with Nar, the cells
were collected and proteins were extracted on ice with RIPA lysis
buffer containing protease inhibitors. Proteins were quantified
with the BCA Protein Assay kit. The collected lysate samples (20
µg/well) were separated by sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) on 12% gels and transferred to
nitrocellulose membranes blocked with 50 g/l skimmed milk for 2 h
at room temperature. Following overnight incubation with the
primary antibodies of caspase 3, Bax, Bcl-2, AKT, p-AKT, PI3K,
p-PI3K, LC3B, Beclin-1, p62 and GAPDH at 4°C, the membranes were
incubated with an HRP-labeled goat anti-rabbit IgG antibody for 2 h
at room temperature. In a darkroom, the SuperSignal ELISA Femto
Substrate was added onto the membranes which were subsequently
exposed to x-ray films. Protein bands were imaged using an Alpha
Innotech FluorChem FC2 Imaging System (ProteinSimple). The
densitometric analysis was performed using ImageJ software v1.46
(National Institutes of Health) and GAPDH expression was used to
normalize the data.
Wound-healing assay
SNU-1 cells (2.0 ml/well, 1×106 cells/ml)
were seeded to confluence in 6-well plates; when the confluence of
the cells reached ~90% wounds were made by a 200-µl pipette tip,
then the cells were incubated in a serum-free medium with different
concentrations (0, 10, 20, 40 µg/ml) of Cos for 24 h, observed and
images captured using an inverted light microscope (magnification,
×100; Olympus Corporation). Quantitation of wound healing assay
results were analyzed by Image-Pro Plus software v 6.0 (Media
Cybernetics, Inc.).
Statistical analysis
All data were shown as mean ± standard error of
mean. The intergroup deviations were evaluated using one-way
analysis of variance (ANOVA) implemented in the GraphPad Prism 6.0
software. Comparison between groups was performed using one-way
ANOVA followed by Tukey's test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of Nar on SNU-1 cell
proliferation
The changes in cell proliferation were determined
using CCK-8 assay to demonstrate the effects of Nar on SNU-1 cell
proliferation. As shown in Fig. 1B,
Nar could significantly inhibit the proliferation of SNU-1 cells in
a dose-dependent manner, but the effect of Nar on normal gastric
cells (GES-1 cells) was not as sensitive as that on SNU-1 cells
(Fig. 1B). The half-maximal
inhibitory concentration (IC50) for both 24 and 48 h was
~20 µg/ml. Therefore, the concentrations of 0, 5, 10 and 20 µg/ml
were used for subsequent in vitro assays. The results of
observing the cell morphology with a phase-contrast microscope
showed that Nar induced shrinkage, nuclear lysis and rupture of
SNU-1 cells (Fig. 1C).
Nar induces SNU-1 cell cycle
arrest
Based on the results of anti-proliferative assays,
SNU-1 cells were treated with 0, 5, 10 and 20 µg/ml Nar to reveal
the effect of Nar on the cell cycle. The flow cytometry results
showed that Nar could significantly induce cell cycle arrest in the
G0/G1 phase in SNU-1 cells dose-dependently
(Fig. 2).
Nar induces the apoptosis of SNU-1
cells
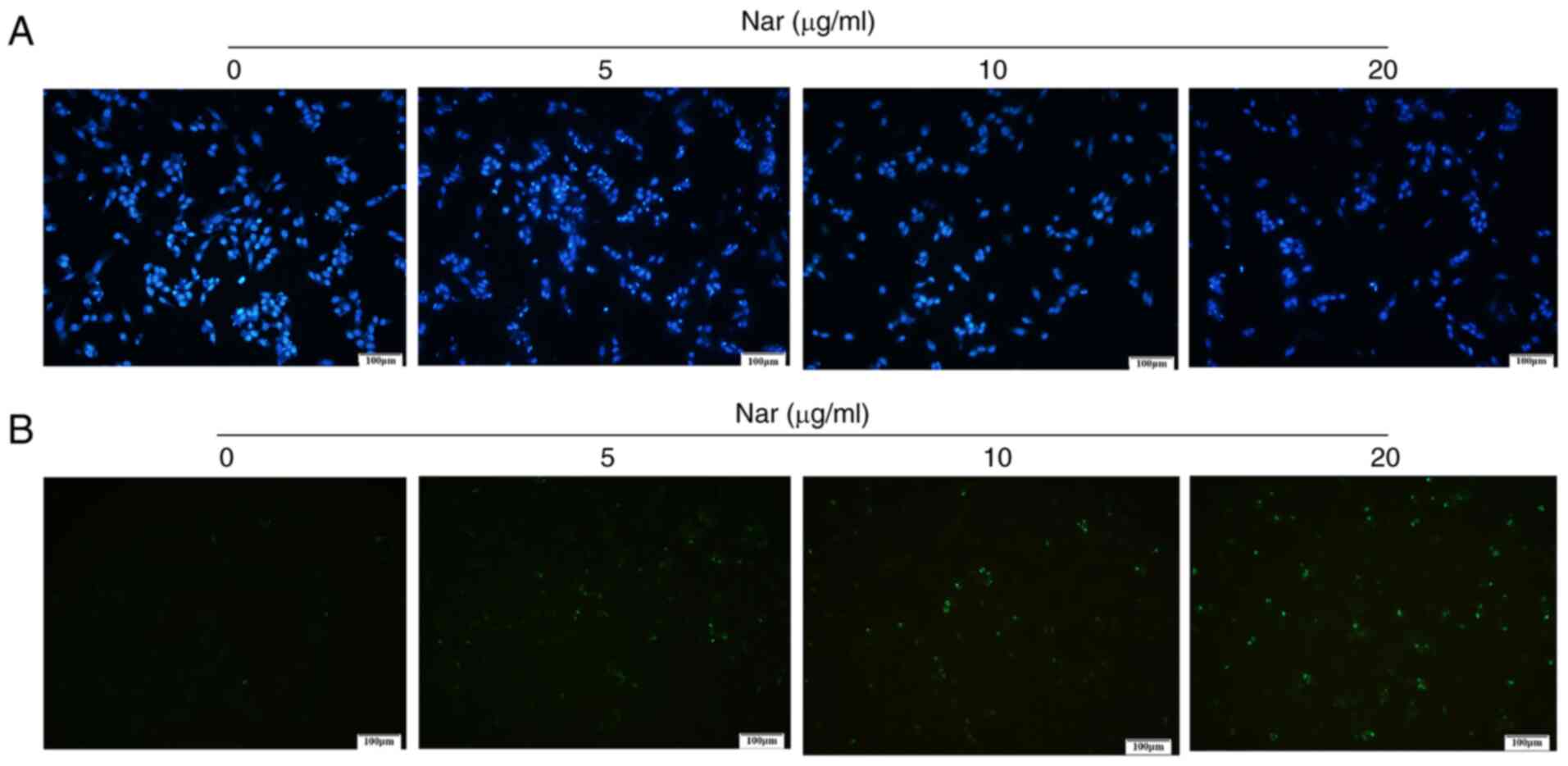
Hoechst 33258 and TUNEL staining demonstrated that
the rate of apoptosis increased with an increase in Nar
concentration (Fig. 3A and B). The
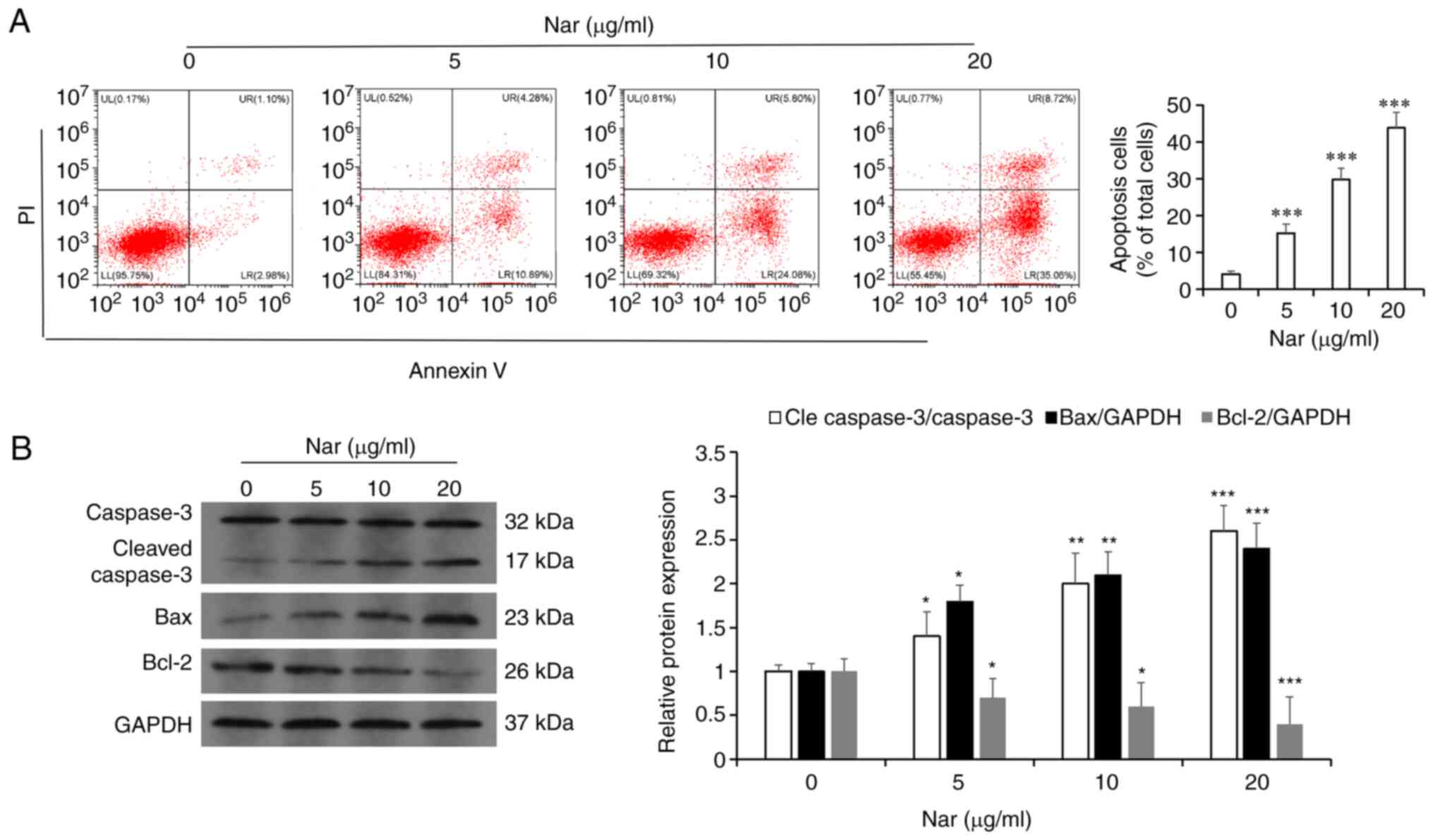
flow cytometry results showed that Nar could dose-dependently
induce the apoptosis of SNU-1 cells (Fig. 4A). Western blot analysis results
also demonstrated that Nar upregulated the expression of caspase 3
and Bax, but downregulated the expression of Bcl-2 in a
dose-dependent manner (Fig.
4B).
Nar induces apoptosis of SNU-1 cells
via inhibiting the PI3K/AKT pathway
The changes in PI3K/AKT pathway-related proteins
treated with different concentrations of Nar were detected using
western blot analysis. The results showed that the ratios
p-PI3K/PI3K and p-AKT/AKT were downregulated in a dose-dependent
manner (Fig. 5A). After
pretreatment with 3-MA, an inhibitor of PI3K, the ratios of
p-PI3K/PI3K and p-AKT/AKT in cells co-treated with 3-MA and Nar
were markedly downregulated compared with those in cells treated
with Nar or 3-MA alone (Fig.
5B).
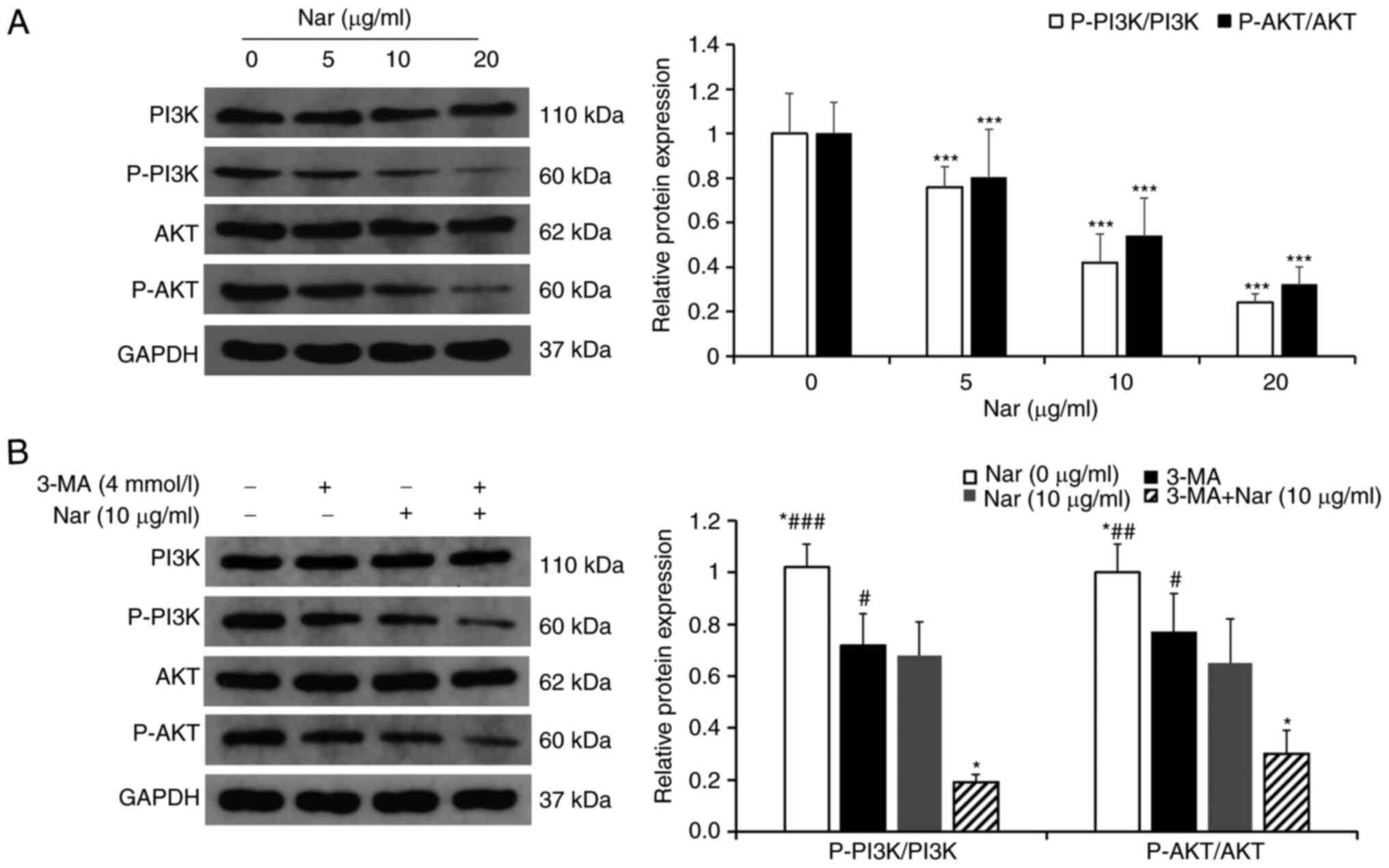 | Figure 5.Nar induces apoptosis and autophagy
of SNU-1 cells by inhibiting the PI3K/AKT signaling pathway. (A)
Changes in cellular PI3K, p-PI3K, AKT and p-AKT levels were
determined by western blot analysis. GAPDH expression was used to
normalize the data. (B) Changes in PI3K, p-PI3K, AKT and p-AKT
levels in SNU-1 cells following Nar (10 µg/ml), 3-MA and Nar (10
µg/ml) plus 3-MA treatment. GAPDH expression was used to normalize
the data. *P<0.05, ***P<0.001 vs. Nar (10 µg/ml) group;
#P<0.05, ##P<0.01,
###P<0.001 vs. Nar (10 µg/ml) plus 3-MA group. Nar,
naringin; p-, phosphorylated. |
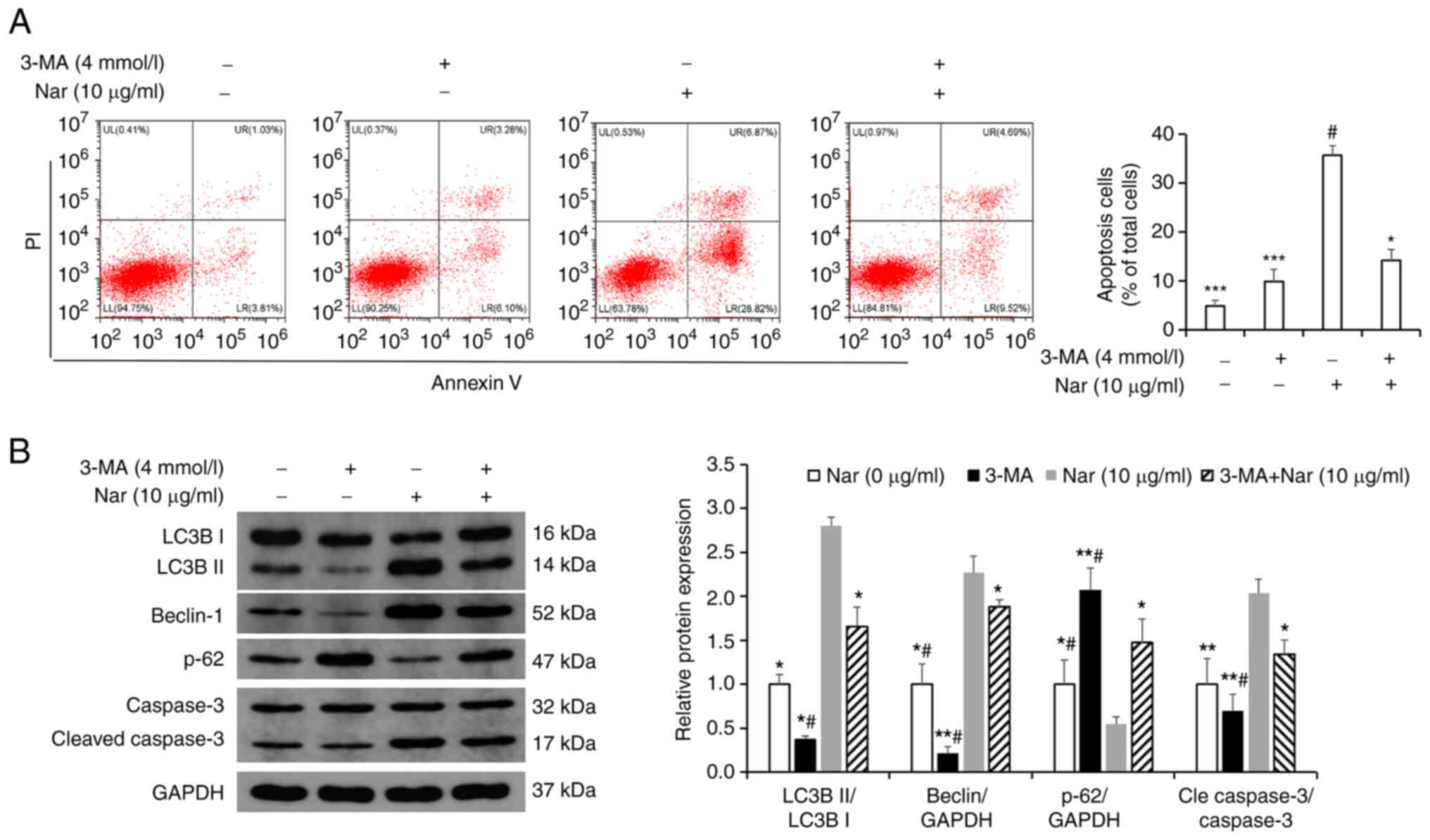
Nar induces apoptosis via activating
autophagy in SNU-1 cells
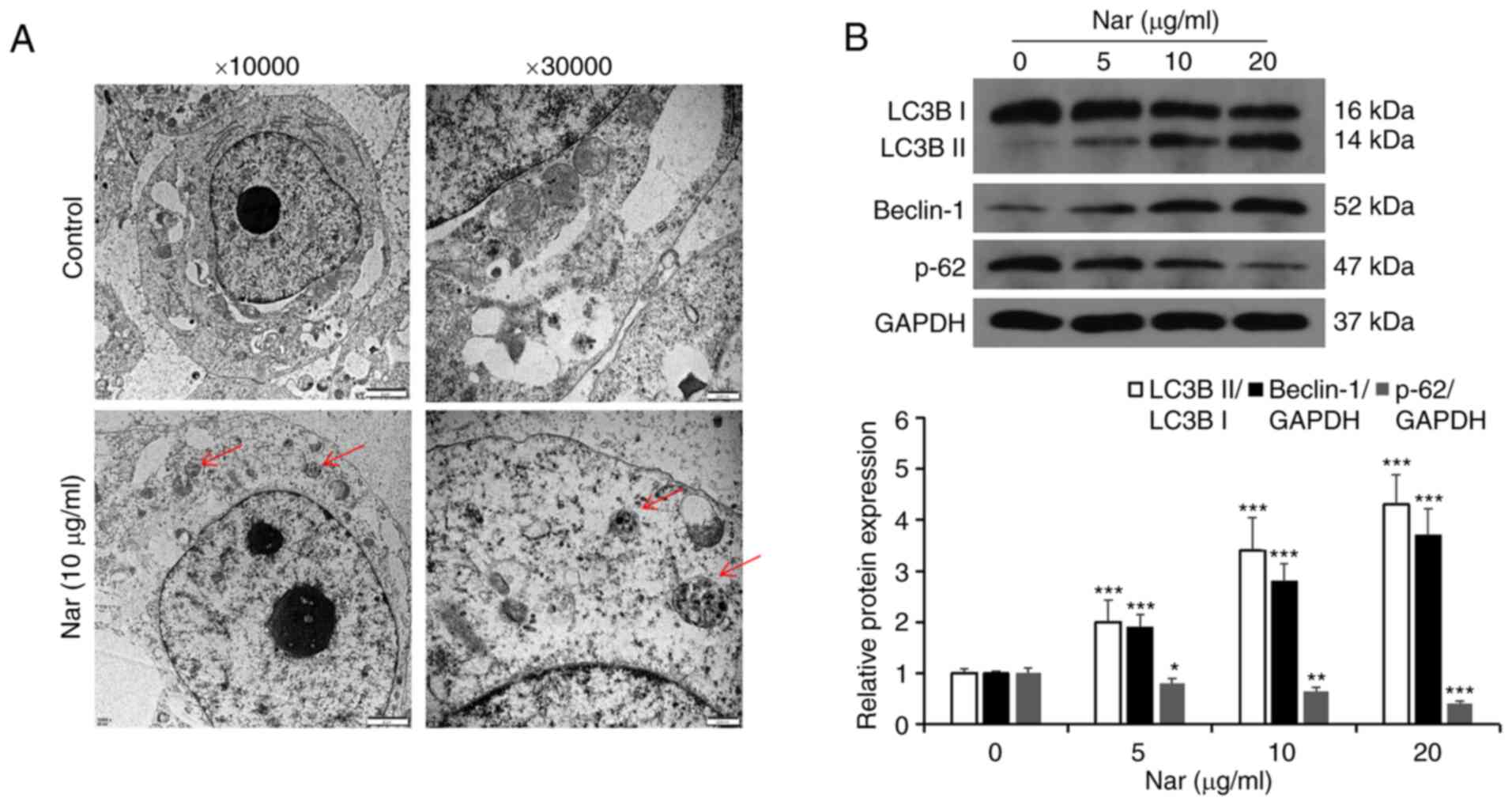
Transmission electron microscopy results showed the
formation of autophagic vacuoles in SNU-1 cells following treatment
with Nar (Fig. 6A). Western blot
analysis results showed that Nar could promote the expression of
Beclin-1, increase the LC3BII/LC3BI ratio and inhibit the
expression of p62 (Fig. 6B). SNU-1
cells were treated with 10 µg/ml Nar for 24 h before incubating
with 4 mmol/l 3-MA (an autophagy inhibitor) for 1 h and the
apoptosis were measured by flow cytometry. The results showed that
Nar-induced apoptosis was significantly attenuated in the 3-MA- and
Nar-co-treated group compared with the Nar-treated group (Fig. 7A). Western blot analysis results
also showed that the Beclin-1 expression level and the LC3BII/LC3BI
ratio were markedly downregulated and the expression of p62 was
upregulated in 3-MA-treated SNU-1 cells. However, when cells were
co-stimulated with 3-MA and Nar, Beclin-1 expression level and
LC3BII/LC3BI ratio significantly increased and the p62 degradation
was rescued compared with those in cells treated with 3-MA alone
(Fig. 7B).
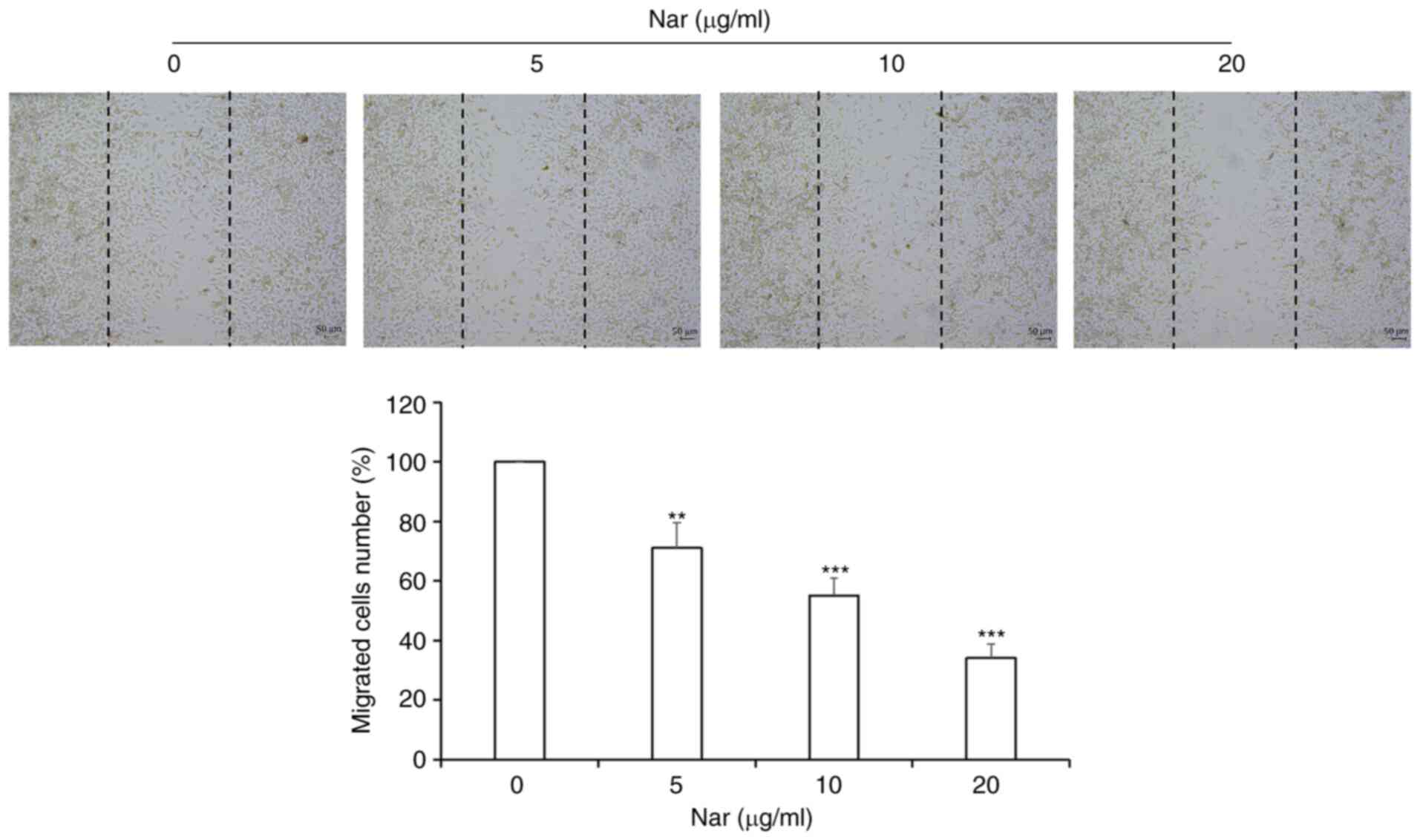
Nar inhibits migration of SNU-1
cells
Wound-healing assay results demonstrated that Nar
inhibited the migration of SNU-1 cells in a dose-dependent manner
(Fig. 8).
Discussion
Gastric cancer is one of the commonest and most
malignant tumors in the digestive system (19). Although the treatment of gastric
cancer has improved to a certain level with the advancement of
medical technology, the survival rate of patients with gastric
cancer remains poor. Therefore, seeking more effective adjuvant
treatment to improve the therapeutic effect on GC is urgently
required for patients undergoing surgery and chemotherapy. Some
studies have shown that ~60% of cancer patients in the United
States take high-dose complex antioxidant nutrients while
undergoing conventional treatment to improve the effect of
conventional anti-cancer treatment and reduce the side effects of
radiotherapy and chemotherapy (20,21).
Oxidative nutrients (antioxidants) refer to a class of nutrients
that have antioxidant capacity, inhibit the generation of free
radicals, accelerate the elimination of free radicals and inhibit
the oxidative damage of biological macromolecules caused by free
radicals (20). Green vegetables
such as cauliflower are rich sources of antioxidant nutrients, such
as vitamin A, vitamin C, vitamin E and their derivatives, while
citrus fruits are rich in flavonoids, isoflavones and saponins
(9). At present, a large number of
cell culture experiments, animal experiments and some clinical
trials have confirmed that compound antioxidant nutrients have
positive effects in anti-cancer treatment (20). In addition, studies have found that
a number of plant-derived molecules can exert their anti-cancer
effects by targeting specific signaling pathways (22,23).
Therefore, extracting pure natural drugs from plants to develop
more effective and nontoxic anti-cancer agents is an ideal and
promising avenue. The Nar used in the present study is a
dihydroflavonoid compound extracted from citrus fruits. Its
biologically active substances are not only are plentiful in
content but various, mainly including flavonoids, limonoids,
carotenoids, coumarins, essential oils, dietary fiber and pectin
(24). Nar has various biological
activities, such as anti-inflammatory, anti-viral, anti-cancer,
anti-mutation, anti-allergic, anti-ulcer, analgesic and
antihypertensive. Gastric cancer has a number of risk factors,
including gastric ulcer, atrophic gastritis and Helicobacter
pylori infection. Nar can suppress these risk factors, which is
particularly important in the prevention and adjuvant treatment of
gastric cancer. In recent years, some anti-cancer active
ingredients in Nar have become research targets in the food and
medical fields (10,25). They can inhibit the growth of cancer
cells via different molecular mechanisms, such as triggering cell
cycle arrest, apoptosis, necrosis and autophagy (26). Studies have found that Nar initiates
the release of TNF by inducing lipopolysaccharides, reduces the
incidence of liver cancer, induces cancer cell apoptosis and
inhibits oncogene expression through anti-oxidative and anti-free
radical effects (27,28). However, the effect of Nar on gastric
cancer SNU-1 cells and the related mechanism have been seldom
studied. The present study demonstrated that Nar inhibited SNU-1
cell proliferation in a dose- and time-dependent manner. The
inhibition of cell proliferation is considered to be specific to
gastric cancer because Nar has no obvious inhibitory effect on
normal gastric mucosal GES-1 cells, indicating that it has no
cytotoxicity on human normal gastric cells. In addition, the
present study found that Nar had an anti-proliferative effect on
SNU-1 cells at low concentrations, as observed under an inverted
microscope. This indicated that if Nar is supplemented continuously
for a period of time, it would be effective even at lower
concentrations. Nar has an anti-cancer effect on esophageal cancer
stem cell xenotransplanted tumor mouse models without altering the
body and liver weight and the combination of Nar and doxorubicin
can reduce the side effects of doxorubicin (29). Nar is both effective and safe as an
anti-cancer drug (30). The present
study found that Nar arrested SNU-1 cells in the
G0/G1 phase, promoting apoptosis in a
dose-dependent manner. Further mechanistic studies demonstrated
that Nar significantly blocked the PI3K/AKT signaling pathway and
activated autophagy. 3-MA pretreatment significantly attenuated
Nar-induced apoptosis. The results suggested the potential
relevance of consuming Nar-rich foods or nutrients in reducing the
development of gastric cancer.
Tumors are characterized by abnormal cell
proliferation. Various anti-tumor drugs induce tumor cell cycle
arrest and apoptosis, thereby inhibiting the abnormal proliferation
of tumor cells and exerting anti-cancer activities (31–33).
Nar can induce cell cycle arrest and apoptosis in human breast
cancer (34). In cervical cancer
cells, Nar induces G0/G1 phase arrest and
activated endoplasmic reticulum-mediated apoptosis (35). It also induces
G0/G1 phase arrest and apoptosis in human
osteosarcoma MG63 and U2OS cells (36). The results confirmed that Nar
significantly inhibited SNU-1 cells and induced
G0/G1 phase arrest and apoptosis in a
concentration-dependent manner. In addition, the results confirmed
that Nar promoted the generation of cleaved caspase 3 in SNU-1
cells. The aforementioned results indicated that Nar inhibited
SNU-1 cell proliferation by promoting the
G0/G1 phase arrest and apoptosis. This was
consistent with the results of previous studies showing that
flavonoids, such as apigenin, luteolin and myricetin, induced
exogenous apoptosis in different cancer cell lines (37–39).
The PI3K/AKT pathway is a classic signaling pathway
involved in regulating a variety of cellular processes, including
proliferation, migration, differentiation and apoptosis (40). Previous studies have identified
anti-cancer drugs inducing apoptosis by blocking the PI3K/AKT
signaling pathway (41,42). In colorectal cancer, Nar inhibits
cell growth and induces apoptosis via blocking the PI3K/AKT
signaling pathway (15). By
targeting this pathway, Nar inhibits the proliferation of thyroid
cancer cells and induces apoptosis (43). The mechanism may be that activated
Akt can promote the Ser184 phosphorylation of Bax, which can
negatively regulate the pro-apoptotic function and could also
inactivate caspase-9 Ser196 phosphorylation and inhibit apoptosis
(32,33). The results of the present study
showed that Nar could significantly induce SNU-1 cell apoptosis by
inhibiting the PI3K/AKT signaling pathway in SNU-1 cells.
Autophagy is a lysosomal degradation pathway of the
cell. It is characterized by an increase in the number of acidic
vesicle organelles associated with autophagosomes, which is often
dysregulated in cancer as another important form of programmed cell
death (44). Autophagy can both
promote cell death and inhibit cell death. In tumor cells, the
effect of autophagy often depends on the cell type (45). Previous studies have confirmed
autophagy as an important signal downstream of the PI3K/AKT
pathway; it is involved in drug-induced cancer cell apoptosis
(46,47). Recent studies have confirmed that
Nar activates autophagy by inhibiting PI3K/AKT signal, thereby
inhibiting the growth of gastric cancer cells (48). The results of the present study
confirmed that Nar significantly activated autophagy, featured by
the expression of autophagy-related proteins LC3BII and Beclin 1
increased, while the expression of p62 decreased in a
dose-dependent manner. This was inconsistent with reports that
apigenin (which is a flavonoid) could induce autophagy and promote
the increase in p62 expression. The p62 protein is located on the
autophagosome by LC3 binding and it is degraded by autophagy
(49). The overexpression of p62
can activate caspase 8 and promote cell apoptosis, which is related
to the ubiquitin-associated domain at the C terminal (50). This indicates that besides being a
marker of autophagy activation, p62 protein also served as an
important regulator of apoptosis. Inhibiting autophagy
significantly attenuated the generation of caspase 3 spliceosome
induced by Nar. Based on these results, it was confirmed that
PI3K/AKT signaling and autophagy were involved in the process of
Nar-induced apoptosis in SNU-1 cells. Finally, the anti-gastric
cancer metastatic effect of Nar was investigated using a wound
healing test. The drug inhibited the migration of SNU-1 cells,
further confirming that Nar had an anti-gastric cancer effect.
However, its mechanism and whether Nar has the potential to
overcome drug resistance in gastric cancer in the same manner as
S-adenosyl-1-methionine needs to be studied in follow-up
experiments (50). In addition, the
experiments were performed only in a specific cancer cell line,
SNU-1. At least one key finding should be reproduced in a different
cancer cell line. Therefore, the use of one cell line as a
limitation of the present study will be improved in the future
studies. These future studies intend to use single-cell sequencing
to screen out the targets of Nar acting on gastric cancer cells and
further reveal its mechanism of action. In addition, the
combination of Nar and other Chinese medicine monomers is being
currently studied, to find the best combination for the treatment
of gastric cancer.
In summary, Nar significantly inhibited the growth
of SNU-1 cells, inducing G0/G1 phase arrest
and apoptosis. Moreover, it induced SNU-1 cell apoptosis by
inhibiting the PI3K/AKT signaling and activating autophagy. The
present study was based on the potential application of Nar as a
protective nutrient and chemopreventive and therapeutic molecule,
confirming that Nar was a potential drug for the treatment of
gastric cancer.
Our research group has conducted research on the
anti-tumor mechanisms of luteolin, costanolactone and oleandrin.
The combined effect and mechanism of several drugs will be further
explored in detail. The findings are expected to lay the foundation
for the development of anti-cancer plant nutrition sources in food
science.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Shaanxi
Science and Technology Innovation Team (grant no. 2017KCT-28), the
Shaanxi Province Key R&D Project (grant no. 2019ZDLSF02-09-01)
and the National Natural Science Foundation of China (grant no.
81900686).
Availability of data and materials
All data generated or analyzed in the present study
are included in this published article.
Authors' contributions
CX, XH, JW and XD conceived and designed the
experiments. CX, XH, YH, XL and MW performed the experiments. CX,
XH and JW analyzed the data. CX, JW and XD performed data
interpretation and made critical manuscript revisions. CX and XH
wrote the manuscript and confirmed the authenticity of all the raw
data. All authors reviewed and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Strand MS, Lockhart AC and Fields RC:
Genetics of gastric cancer. Surg Clin North Am. 97:345–370. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hamashima C: Current issues and future
perspectives of gastric cancer screening. World J Gastroenterol.
20:13767–13774. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zong L, Abe M, Seto Y and Ji J: The
challenge of screening for early gastric cancer in China. Lancet.
388:26062016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roder DM: The epidemiology of gastric
cancer. Gastric Cancer. 5 (Suppl 1):S5–S11. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ang TL and Fock KM: Clinical epidemiology
of gastric cancer. Singapore Med J. 55:621–628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuo YT, Chang TT, Muo CH, Wu MY, Sun MF,
Yeh CC and Yen HR: Use of complementary traditional Chinese
medicines by adult cancer patients in Taiwan: A nationwide
population-based study. Integr Cancer Ther. 17:531–541. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singh N, Bansal Y, Bhandari R, Marwaha L,
Singh R, Chopra K and Kuhad A: Naringin reverses neurobehavioral
and biochemical alterations in intracerebroventricular
collagenase-induced intracerebral hemorrhage in rats. Pharmacology.
100:172–187. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen R, Qi QL, Wang MT and Li QY:
Therapeutic potential of naringin: An overview. Pharm Biol.
54:3203–3210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bharti S, Rani N, Krishnamurthy B and Arya
DS: Preclinical evidence for the pharmacological actions of
naringin: A review. Planta Med. 80:437–451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jeon SM, Bok SH, Jang MK, Kim YH, Nam KT,
Jeong TS, Park YB and Choi MS: Comparison of antioxidant effects of
naringin and probucol in cholesterol-fed rabbits. Clin Chim Acta.
317:181–190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jagetia GC and Reddy TK: Modulation of
radiation-induced alteration in the antioxidant status of mice by
naringin. Life Sci. 77:780–794. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rajadurai M and Stanely Mainzen Prince P:
Preventive effect of naringin on lipid peroxides and antioxidants
in isoproterenol-induced cardiotoxicity in Wistar rats: Biochemical
and histopathological evidences. Toxicology. 228:259–268. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng H, Jiang X, Zhang Q, Ma J, Cheng R,
Yong H, Shi H, Zhou X, Ge L and Gao G: Naringin inhibits colorectal
cancer cell growth by repressing the PI3K/AKT/mTOR signaling
pathway. Exp Ther Med. 19:3798–3804. 2020.PubMed/NCBI
|
|
16
|
Ramesh E and Alshatwi AA: Naringin induces
death receptor and mitochondria-mediated apoptosis in human
cervical cancer (SiHa) cells. Food Chem Toxicol. 51:97–105. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai L, Wu H, Tu C, Wen X and Zhou B:
Naringin inhibits ovarian tumor growth by promoting apoptosis: An
in vivo study. Oncol Lett. 16:59–64. 2018.PubMed/NCBI
|
|
18
|
Banjerdpongchai R, Wudtiwai B and Khawon
P: Induction of human hepatocellular carcinoma HepG2 cell apoptosis
by naringin. Asian Pac J Cancer Prev. 17:3289–3294. 2016.PubMed/NCBI
|
|
19
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ilghami R, Barzegari A, Mashayekhi MR,
Letourneur D, Crepin M and Pavon-Djavid G: The conundrum of dietary
antioxidants in cancer chemotherapy. Nutr Rev. 78:65–76. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McDermott JH: Antioxidant nutrients:
Current dietary recommendations and research update. J Am Pharm
Assoc (Wash). 40:785–799. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nijveldt RJ, van Nood E, van Hoorn DE,
Boelens PG, van Norren K and van Leeuwen PA: Flavonoids: A review
of probable mechanisms of action and potential applications. Am J
Clin Nutr. 74:418–425. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Courdavault V, O'Connor SE, Oudin A,
Besseau S and Papon N: Towards the microbial production of
plant-derived anticancer drugs. Trends Cancer. 6:444–448. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang X, Ouyang Y, Liu J, Zhu M, Zhao G,
Bao W and Hu FB: Fruit and vegetable consumption and mortality from
all causes, cardiovascular disease, and cancer: Systematic review
and dose-response meta-analysis of prospective cohort studies. BMJ.
349:g44902014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alam MA, Subhan N, Rahman MM, Uddin SJ,
Reza HM and Sarker SD: Effect of citrus flavonoids, naringin and
naringenin, on metabolic syndrome and their mechanisms of action.
Adv Nutr. 5:404–417. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mani JS, Johnson JB, Hosking H, Ashwath N,
Walsh KB, Neilsen PM, Broszczak DA and Naiker M: Antioxidative and
therapeutic potential of selected Australian plants: A review. J
Ethnopharmacol. 268:1135802021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blankson H, Grotterød EM and Seglen PO:
Prevention of toxin-induced cytoskeletal disruption and apoptotic
liver cell death by the grapefruit flavonoid, naringin. Cell Death
Differ. 7:739–746. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kawaguchi K, Kikuchi S, Hasegawa H,
Maruyama H, Morita H and Kumazawa Y: Suppression of
lipopolysaccharide-induced tumor necrosis factor-release and liver
injury in mice by naringin. Eur J Pharmacol. 368:245–250. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tajaldini M, Samadi F, Khosravi A,
Ghasemnejad A and Asadi J: Protective and anticancer effects of
orange peel extract and naringin in doxorubicin treated esophageal
cancer stem cell xenograft tumor mouse model. Biomed Pharmacother.
121:1095942020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tarun EI, Kurchenko VP and Metelitsa DI:
Flavonoids as effective protectors of urease from ultrasonic
inactivation in solutions. Bioorg Khim. 32:391–398. 2006.(In
Russian). PubMed/NCBI
|
|
31
|
Liu Y, Kang X, Niu G, He S, Zhang T, Bai
Y, Li Y, Hao H, Chen C, Shou Z and Li B: Shikonin induces apoptosis
and prosurvival autophagy in human melanoma A375 cells via
ROS-mediated ER stress and p38 pathways. Artif Cells Nanomed
Biotechnol. 47:626–635. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kang X, Wang H, Li Y, Xiao Y, Zhao L,
Zhang T, Zhou S, Zhou X, Li Y, Shou Z, et al: Alantolactone induces
apoptosis through ROS-mediated AKT pathway and inhibition of
PINK1-mediated mitophagy in human HepG2 cells. Artif Cells Nanomed
Biotechnol. 47:1961–1970. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qiu C, Zhang T, Zhang W, Zhou L, Yu B,
Wang W, Yang Z, Liu Z, Zou P and Liang G: Licochalcone a inhibits
the proliferation of human lung cancer cell lines A549 and H460 by
inducing G2/M cell cycle arrest and ER stress. Int J Mol Sci.
18:17612017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kabala-Dzik A, Rzepecka-Stojko A, Kubina
R, Iriti M, Wojtyczka RD, Buszman E and Stojko J: Flavonoids,
bioactive components of propolis, exhibit cytotoxic activity and
induce cell cycle arrest and apoptosis in human breast cancer cells
MDA-MB-231 and MCF-7-a comparative study. Cell Mol Biol
(Noisy-le-grand). 64:1–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin R, Hu X, Chen S, Shi Q and Chen H:
Naringin induces endoplasmic reticulum stress-mediated apoptosis,
inhibits β-catenin pathway and arrests cell cycle in cervical
cancer cells. Acta Biochim Pol. 67:181–188. 2020.PubMed/NCBI
|
|
36
|
Ming H, Chuang Q, Jiashi W, Bin L,
Guangbin W and Xianglu J: Naringin targets Zeb1 to suppress
osteosarcoma cell proliferation and metastasis. Aging (Albany NY).
10:4141–4151. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sang Eun H, Seong Min K, Ho Jeong L,
Vetrivel P, Venkatarame Gowda Saralamma V, Jeong Doo H, Eun Hee K,
Sang Joon L and Gon Sup K: Scutellarein induces Fas-mediated
extrinsic apoptosis and G2/M cell cycle arrest in Hep3B
hepatocellular carcinoma cells. Nutrients. 11:2632019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang Y, Xu X, Li W, Miao H, Huang S, Zhou
Y, Sun Y, Li Z, Guo Q and Zhao L: Activation of endoplasmic
reticulum stress and the extrinsic apoptotic pathway in human lung
cancer cells by the new synthetic flavonoid, LZ-205. Oncotarget.
7:87257–87270. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tavsan Z and Kayali HA: Flavonoids showed
anticancer effects on the ovarian cancer cells: Involvement of
reactive oxygen species, apoptosis, cell cycle and invasion. Biomed
Pharmacother. 116:1090042019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Porta C, Paglino C and Mosca A: Targeting
PI3K/Akt/mTOR signaling in cancer. Front Oncol. 4:642014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang J, Ren X, Zhang L, Li Y, Cheng B and
Xia J: Oridonin inhibits oral cancer growth and PI3K/Akt signaling
pathway. Biomed Pharmacother. 100:226–232. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu ML, Zhang PM, Jiang M, Yu SW and Wang
L: Myricetin induces apoptosis and autophagy by inhibiting
PI3K/Akt/mTOR signalling in human colon cancer cells. BMC
Complement Med Ther. 20:2092020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhou J, Xia L and Zhang Y: Naringin
inhibits thyroid cancer cell proliferation and induces cell
apoptosis through repressing PI3K/AKT pathway. Pathol Res Pract.
215:1527072019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yun CW and Lee SH: The roles of autophagy
in cancer. Int J Mol Sci. 19:34662018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Towers CG, Wodetzki D and Thorburn A:
Autophagy and cancer: Modulation of cell death pathways and cancer
cell adaptations. J Cell Biol. 219:e2019090332020.PubMed/NCBI
|
|
46
|
Wei M, Wu Y, Liu H and Xie C: Genipin
induces autophagy and suppresses cell growth of oral squamous cell
carcinoma via PI3K/AKT/MTOR pathway. Drug Des Devel Ther.
14:395–405. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhou J, Jiang YY, Chen H, Wu YC and Zhang
L: Tanshinone I attenuates the malignant biological properties of
ovarian cancer by inducing apoptosis and autophagy via the
inactivation of PI3K/AKT/mTOR pathway. Cell Prolif. 53:e127392020.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Raha S, Yumnam S, Hong GE, Lee HJ,
Saralamma VV, Park HS, Heo JD, Lee SJ, Kim EH, Kim JA and Kim GS:
Naringin induces autophagy-mediated growth inhibition by
downregulating the PI3K/Akt/mTOR cascade via activation of MAPK
pathways in AGS cancer cells. Int J Oncol. 47:1061–1069. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu WJ, Ye L, Huang WF, Guo LJ, Xu ZG, Wu
HL, Yang C and Liu HF: p62 links the autophagy pathway and the
ubiqutin-proteasome system upon ubiquitinated protein degradation.
Cell Mol Biol Lett. 21:292016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang YB, Gong JL, Xing TY, Zheng SP and
Ding W: Autophagy protein p62/SQSTM1 is involved in HAMLET-induced
cell death by modulating apotosis in U87MG cells. Cell Death Dis.
4:e5502013. View Article : Google Scholar : PubMed/NCBI
|