Introduction
Atopic dermatitis (AD) is a common chronic
inflammatory skin disease (1). AD
generally presents in infancy and early childhood, and its
prevalence is increasing worldwide (2). Environmental, pharmacological and
genetic factors may play a role in AD by disturbing the balance of
the immune system (3). The main
symptoms of AD include erythema, itching, eczematous lesions,
excoriation, edema and thickening of the skin (4,5). In
severe cases, fluid extravasation and bacterial infections may
develop (5). Steroids,
antihistamines and immunosuppressive agents are currently used to
treat AD (6,7). However, these agents are associated
with a variety of adverse effects when used in the long term or at
high doses (6–8). Therefore, new, effective alternative
medicines are needed for AD (7,8).
AD symptoms, such as skin thickening and cracking,
are caused by T helper 1 (Th1) and T helper 2 (Th2) cells (9). The production of cytokines by Th2
cells increases the production of IgE, as well as the infiltration
of eosinophils and mast cells into inflamed skin tissue (10–12).
IgE is a major mediator of mast cell activation and contributes to
the release of inflammatory mediators, including histamine and
cytokines (13). The MAPK signaling
cascade plays a key role in the differentiation of inflammatory
cells (14). The transcription
factor NF-κB also plays a major role in regulating several
inflammatory mediators (15).
AD is induced by various chemokines and inflammatory
cytokines. Thymus and activation-regulated chemokine [TARC/C-X-C
motif chemokine ligand 17 (CXCL17)] is produced by dendritic cells,
endothelial cells, keratinocytes and fibroblasts, and induces Th2
cell migration to the inflammatory site, as these cells express the
receptor for TARC/CCL17. Therefore, TARC is an important chemokine
in inflammatory skin diseases (16)
and is used as a clinical biomarker to measure atopic diseases
(16). Granulocyte-macrophage
colony-stimulating factor (GM-CSF) is also involved in the
initiation and maintenance of chronic inflammation by activating
Langerhans cells (17), as well as
by causing hyperproliferation (18)
and apoptosis of keratinocytes (19). In addition, high levels of GM-CSF
were observed in the skin of patients with AD (20). Monocyte chemoattractant protein-1
(MCP-1) plays a key role in mast cell degranulation, histamine
release from basophils and transformation of undifferentiated T
cells to Th2 cells (21). In
addition, scratching of itchy skin causes secretion of various
pro-inflammatory cytokines, including IL-1β, TNF-α and IL-4, from
keratinocytes, thereby aggravating the inflammatory reaction
(11,22). According to previous reports, it is
known that the expression of TNF-α/IFN-γ-induced adhesion proteins
and pro-inflammatory cytokines/chemokines in keratinocytes is
suppressed by inhibiting the activity of MAPKs and NF-κB (23,24).
Lycopus lucidus Turcz (LLT) is a perennial
herb known as ‘Taekran’ in Korea (25). Traditionally, LLT has been used to
treat amenorrhea, dysmenorrhea, edema, carbuncles and sores
(26). Recently, LLT has been
reported to have various biological properties, including effects
on the blood circulation (25),
anti-inflammatory (27),
antioxidant (28), anti-vascular
inflammation and wound-healing effects (27,29),
suggesting that LLT may also affect the mechanism underlying
AD.
In the present study, the thickness of the epidermis
and dermis and the infiltration by mast cells and eosinophils were
evaluated in mice with 2,4-dinitrochlorobenzene (DNCB)-induced AD.
In addition, the expression levels of serum IgE and IL-6 and
members of the NF-κB and MAPK signaling pathways were investigated.
To examine the mechanism of AD inhibition, the expression of
chemokines and pro-inflammatory cytokines and NF-κB/MAPK signaling
pathway molecules was examined in TNF-α/IFN-γ-stimulated HaCaT
cells. Furthermore, the expression of Th1 cytokines (IFN-γ and
TNF-α) and NF-κB/MAPK signaling pathway-related proteins was
examined in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells.
The results of these experiments were integrated to determine the
potential applicability of LCL in the treatment of AD.
Materials and methods
Reagents
DNCB, toluidine blue, hematoxylin, eosin, human
TNF-α, human IFN-γ, LPS, caffeic acid, protease inhibitor cocktail
and phosphatase inhibitor cocktail were purchased from
Sigma-Aldrich (Merck KGaA). Mouse IgE (cat. no. 555248), mouse IL-6
(cat. no. 555240), human GM-CSF (cat. no. 555126), human TNF-α
(cat. no. 555212), human IL-1β (cat. no. 557953), human MCP-1 (cat.
no. 555179), human IL-6 (cat. no. 555220) and human IL-8 (cat. no.
555244) ELISA kits were purchased from BD Biosciences. Polink-2
Plus AP rabbit kit (cat. no. D70-18) was purchased from GBI Labs.
Antibodies against CD4 (cat. no. ab183685; type, monoclonal;
species, anti-mouse) and CD8 (cat. no. ab209775; type, monoclonal;
species, anti-mouse) were obtained from Abcam. Antibodies against
phosphorylated (p)-ERK (cat. no. cs-4370; type, monoclonal,
species, anti-rabbit), total (t)-ERK (cat. no. cs-4695; type,
monoclonal; species, anti-rabbit), p-JNK (cat. no. cs-4668; type,
monoclonal; species, anti-rabbit), t-JNK (cat. no. cs-9258; type,
monoclonal; species, anti-rabbit), NF-κB (cat. no. cs-8242; type,
monoclonal; species, anti-rabbit), p-NF-κB (cat. no. cs-3033; type,
monoclonal; species, anti-rabbit) and IkB (cat. no. cs-4814S; type,
monoclonal; species, anti-rabbit) were purchased from Cell
Signaling Technology, Inc. Anti-lamin B antibody (cat. no. sc-6216;
type, polyclonal; species, anti-mouse) and anti-actin antibody
(cat. no. sc-8432; type, polyclonal; species, anti-mouse) were
purchased from Santa Cruz Biotechnology, Inc. A SuperScript™ IV
reverse transcriptase was purchased from Invitrogen (Thermo Fisher
Scientific, Inc.). Taq polymerase was obtained from Kapa Biosystems
(Roche Diagnostics). PCR primers were purchased from GenoTech Corp.
DMEM was purchased from Welgene, Inc. Goat serum (16210064 for
IHC), FBS, penicillin/streptomycin (P/S) and Dulbecco's PBS (DPBS)
were purchased from Gibco (Thermo Fisher Scientific, Inc.). An
aqueous non-radioactive cell proliferation assay (MTS) was
purchased from Promega Corporation.
Preparation of LLT
LLT was purchased from Kyung Hee University
Healthcare System (Seoul, Korea). LLT extract was prepared by
decocting 500 g of the dried herb with 5 l boiling distilled water
for 2 h, then filtering the mixture using filter paper. The extract
was concentrated in a rotary evaporator and lyophilized. The powder
(57.1 g; yield ratio, 11.4%) was stored at −20°C until use.
Animals and induction of AD-like
lesions and drug treatment in mice
All animal experiments were performed with the
approval of the Institutional Animal Care and Use Committee of
Kyung Hee University Institutional Animal Care and Use Committee
[KHUASP (SE)-15-116]. A total of 32 male 6-week-old BALB/c mice
(weighing 18–20 g) were obtained from Nara Biotech. Animal
experiments were performed in an air-conditioned room, with a
temperature of 23±2°C, and food and water were provided ad
libitum. After an acclimatization period of 7 days, the mice
were divided into four groups (n=8 per group;) as follows: Normal
(vehicle-treated), Control (DNCB-sensitized), LLT low (LLT-L; DNCB
+ 1 mg/kg LLT) and LLT high (LLT-H; DNCB + 5 mg/kg LLT). The mice
were anesthetized with isoflurane diluted in 100% oxygen (4%
induction and 2% maintenance). The dorsal skin of the mice was
shaved with a clipper 1 day before the experiment. In the first
sensitization, the mice (control, LLT-L and LLT-H groups) were
treated with 200 µl 0.5% DNCB solution (dissolved in a 3:1 mixture
of acetone and olive oil) for 3 days. The normal group was treated
with PBS applied to the backs of the mice during the first
sensitization period. In the second sensitization, 200 µl 1% DNCB
solution was applied to the dorsal skin of the control, LLT-L and
LLT-H groups (once every 3 days, 6 times in total). The normal
group was treated with 200 µl 9:1 mixture of PBS and olive oil to
the dorsal skin. The LLT-treated groups were challenged with 200 µl
LLT in a 9:1 mixture of PBS and olive oil (LLT-L, DNCB + 1 mg/kg
LLT; and LLT-H, DNCB + 5 mg/kg LLT) 2 h after the application of
DNCB daily. The normal and control groups were treated with a 200
µl 9:1 mixture of PBS and olive oil (Fig. 1A). The health and behavior of the
mice were monitored daily and no animals died during the
experiment. The following humane endpoints were used: i) Walking
uncomfortably and difficulty consuming feed or water; ii)
difficulty maintaining a normal posture due to weakness; iii)
decrease in weight of >20% compared with the control group of
the same age; iv) coarse breathing, cyanosis, chronic discomfort or
constipation; v) hematological or blood biochemistry parameters
indicating organ function decline that compromises survival
ability; vi) falling into an unconscious state and not responding
to external stimuli. After the experiment was completed, the mice
were deeply anesthetized intraperitoneally with 80 mg/kg
pentobarbital sodium, and 800–1,000 µl blood was collected by
cardiac puncture. After death was confirmed by cessation of
breathing and blood circulation, a dorsal skin sample was
collected.
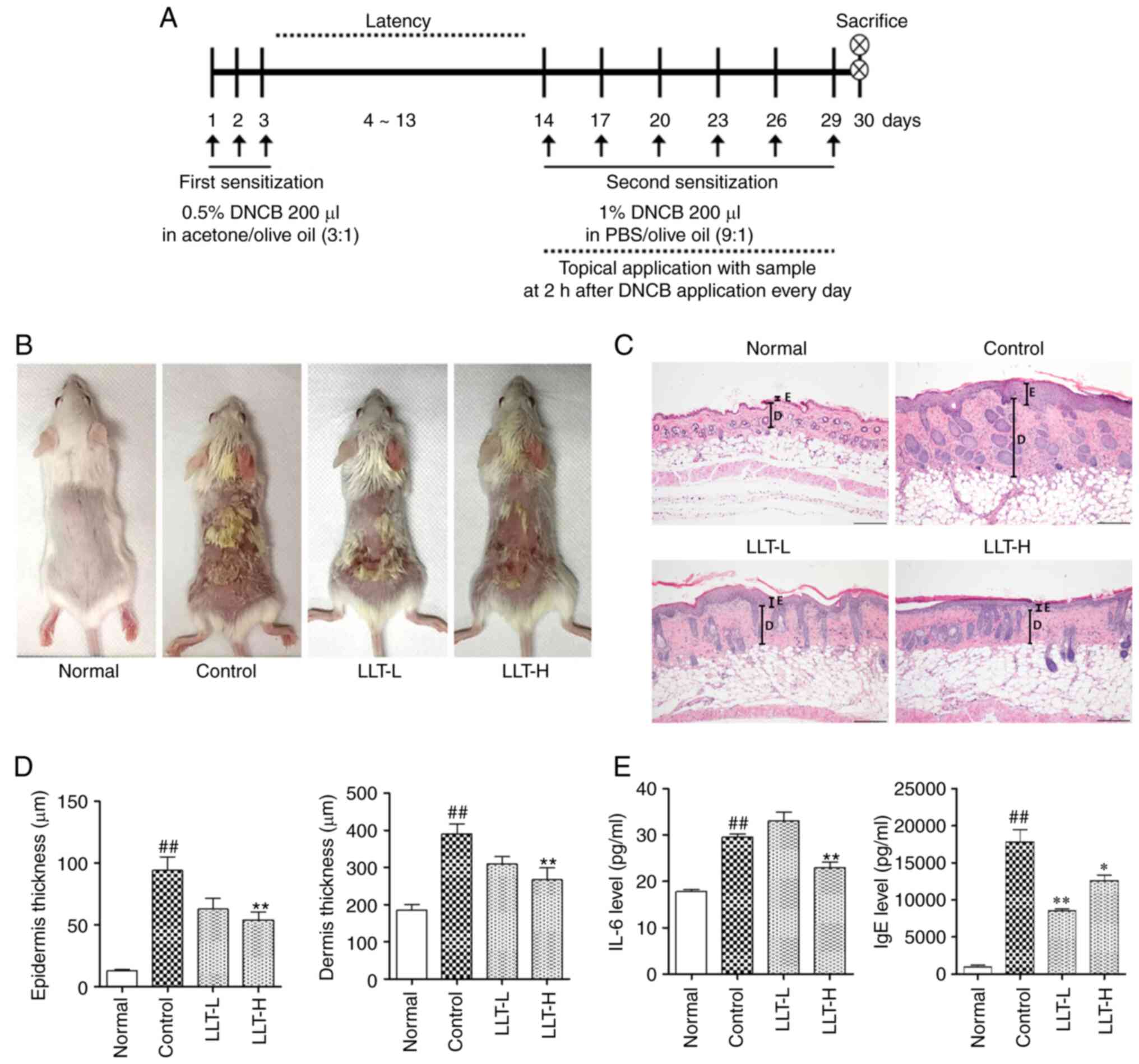 | Figure 1.Effects of LLT on the histological
characteristics of mice with DNCB-induced AD. (A) Schematic diagram
of the experimental schedule. (B) Clinical characteristics of each
treatment group on day 30. (C) Epidermal and dermal thickness was
examined by H&E staining of skin sections. Magnification, ×100.
Scale bar, 200 µm. (D) Measurement of epidermal and dermal
thickness. (E) Serum IgE and IL-6 levels were quantified by ELISA.
Data represent the mean ± SEM. n=8. ##P<0.01 vs.
normal group; *P<0.05 and **P<0.01 vs. control group. Normal
group, vehicle-treated; control group, DNCB-sensitized; LLT-L
group, DNCB + 1 mg/kg LLT; LLT-H group, DNCB + 5 mg/kg LLT. AD,
atopic dermatitis; LLT, Lycopus lucidus Turcz; DNCB,
2,4-dinitrochlorobenzene; E, epidermis; D, dermis. |
Histological analysis
The dorsal skin was fixed in 10% neutral buffered
formalin (NBF) at room temperature for 1 day, and the tissues were
washed with tap water for 1 day. The tissues were embedded in
paraffin and cut into 5-µm sections using a rotary microtome (Zeiss
AG). The tissues were deparaffinized for 3 min, rehydrated for 3
min, and stained with hematoxylin and eosin (H&E) for 3 min or
toluidine blue for 3 min. All staining reactions were performed at
room temperature. The infiltration by eosinophils was examined
under a light microscope (magnification, ×400; 10 fields per
section). The infiltration of mast cells was observed
(magnification, ×200; 3 fields per section). In addition, the
thickness of the dermis and epidermis were also analyzed
(magnification, ×100; 3 fields per section). The skin thickness and
inflammatory cell count were measured using ImageJ software
(version 1.46; National Institutes of Health).
Immunohistochemistry (IHC)
staining
The dorsal skin was fixed in 10% NBF at room
temperature for 1 day, and the tissues were washed with tap water
for 1 day. The tissues were embedded in paraffin and cut into 5-µm
sections using a rotary microtome (Zeiss AG). The tissues were
deparaffinized and rehydrated. For epitope retrieval, the tissue
was placed in sodium citrate buffer (0.1 M citric acid; 0.1 M
sodium citrate) and heated using an Electric Pressure Cooker
(CPC-600; Cuisinart). After the endogenous peroxidase activity was
blocked using 0.3% (v/v) H2O2 in methanol at
room temperature for 30 min, 10% goat serum (Gibco; Thermo Fisher
Scientific, Inc.) in PBS was used for blocking at room temperature
for 10 min. Subsequently, the tissues were incubated with primary
antibodies (dilution 1:100) at 4°C for overnight. The tissue was
incubated for 1 h at room temperature with a biotinylated secondary
antibody (dilution 1:100). Finally, the tissues were stained with
Polink-2 Plus AP rabbit kit for 30 min at room temperature, and the
nuclei were counterstained with Harris hematoxylin for 5 min at
room temperature. The stained tissue was observed under a light
microscope (BX51; Olympus Corporation) at a magnification of ×400
(10 fields per section). The CD4+ and CD8+
cells were counted using ImageJ software (version 1.51j8; National
Institutes of Health).
Cell culture
HaCaT cells were purchased from the CLS Cell Lines
Service GmbH (cat. no. CLS 300493). HaCaT cells were cultured in
DMEM with 10% FBS and 1% P/S. RAW 264.7 cells were obtained from
the Korean Cell Line Bank (Korean Cell Line Research Foundation;
cat. no. KCLB 40071). RAW 264.7 cells were cultured in DMEM with
10% FBS and 1% P/S. Both cell lines were cultured in a cell
incubator at 37°C in a humidified atmosphere of 5% CO2
in air.
Ex vivo ELISA
To prepare serum, blood collected from the mice was
separated by centrifugation at 14,310 × g and 4°C for 10 min. The
concentrations of IgE and IL-6 in the serum were detected using
ELISA kits in vitro. The HaCaT cells were seeded in a 6-well
plate at a density of 1×106 cells/well. After 24 h, the
cells were pretreated with LLT at concentrations of 1, 10 and 100
µg/ml for 1 h and stimulated with TNF-α/IFN-γ (10 ng/ml) for 24 h
in a CO2 incubator maintained at 37°C. The culture
medium was not changed for pretreatment and stimulant treatment.
The concentrations of various pro-inflammatory cytokines and
chemokines (GM-CSF, TNF-α, IL-1β, MCP-1, IL-6 and IL-8) in the
culture medium were also detected using ELISA kits. All experiments
were carried out according to the manufacturer's protocol.
Western blot analysis
Ex vivo
To extract nuclear fraction, the frozen skin tissue
was homogenized with NE-PER nuclear and cytoplasmic extraction
reagent (cat. no. 78835, Thermo Fisher Scientific Inc.). To extract
whole protein, the frozen skin tissue was homogenized with lysis
buffer (20 mM HEPES, pH 7.5; 1.5 mM MgCl2; 0.2 mM EDTA;
0.1 M NaCl; 0.2 mM DTT; 0.5 mM Na3VO4).
Proteins were obtained after centrifugation at 58,440 × g for 30
min at 4°C. In vitro. HaCaT cells were seeded in a 6-well
plate, with a density of 1×106 cells/well. After 24 h,
the cells were pretreated with LLT at concentrations of 1, 10 and
100 µg/ml for 1 h and stimulated with TNF-α/IFN-γ (10 ng/ml) for 5
min (nuclear and cytoplasmic protein) and 30 min (whole protein) in
a CO2 incubator maintained at 37°C. The culture medium
was not changed for pretreatment and stimulant treatment. RAW 264.7
cells were seeded in a 6-well plate, with a density of
1×106 cells/well. After 24 h, the cells were pretreated
with LLT at concentrations of 1, 10 and 100 µg/ml for 1 h and
stimulated with LPS (1 µg/ml) for 5 min (nuclear and cytoplasmic
protein) and 30 min (total protein) in a CO2 incubator
maintained at 37°C. The culture medium was not changed for
pretreatment and stimulant treatment. When extracting the nuclear
and cytoplasmic protein, the HaCaT and RAW 264.7 cells were washed
with PBS and lysed with nuclear and cytoplasmic extraction
reagents. To extract the whole protein, the cells were washed with
DPBS and proteins were extracted using RIPA buffer (50 mM Tris-Cl,
150 nM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, protease
inhibitor cocktail and phosphatase inhibitor cocktail) for total
protein lysis.
The same method was used for ex vivo and
in vitro experiments after protein extraction. The protein
concentration was determined using a BCA assay. The protein samples
(30 µg) were separated by SDS-PAGE on 10% gels, then transferred to
a nitrocellulose membrane. The membrane was blocked with 5% skimmed
milk for 1 h at room temperature. After blocking, the membrane was
incubated overnight with primary antibodies against t-ERK (dilution
1:1,000), p-ERK (dilution 1:1,000), t-JNK (dilution 1:1,000), p-JNK
(dilution 1:1,000), t-p38 (dilution 1:1,000), p-p38 (dilution
1:1,000), NF-κB (dilution 1:1,000), p-NF-κB (dilution 1:1,000),
lamin B (dilution 1:1,000), IκB (dilution 1:1,000) and actin
(dilution 1:1,000) at 4°C. The membrane was then incubated with
secondary antibodies (dilution 1:10,000) for 1 h at room
temperature. The protein bands were visualized using an enhanced
chemiluminescence (cat. no. RPN2106; Cytiva) detection reagent and
semi-quantified using ImageJ software (version 1.51j8, National
Institutes of Health).
Cell viability assay
The MTS assay was used to evaluate the cytotoxicity
of LLT in HaCaT and RAW 264.7 cells. The cells were seeded in a
96-well plate at a density of 5×104 cells/well. After 24
h, LLT (1, 10 or 100 µg/ml) was added to the medium for 24 h in a
CO2 incubator maintained at 37°C. In addition, in order
to confirm the toxicity of LLT in HaCaT cells stimulated with
TNF-α/IFN-γ, the cells were seeded in a 96-well plate at a density
of 5×104 cells/well. After 24 h, LLT (1, 10 or 100
µg/ml) and TNF-α/IFN-γ (10 ng/ml) was added to the medium for 24 h
in a CO2 incubator maintained at 37°C. After each
reaction was completed, a volume of 20 µl MTS solution was then
added to each well for 2 h, and then the optical density was
measured using a microplate reader at a wavelength of 562 nm.
Reverse transcription PCR (RT-PCR)
analysis
HaCaT cells were seeded in a 6-well plate at a
density of 1×106 cells/well. After 24 h, the cells were
pretreated with LLT at concentrations of 1, 10 and 100 µg/ml for 1
h at 37°C CO2 incubator and stimulated with TNF-α/IFN-γ
(10 ng/ml) for 24 h in a CO2 incubator maintained at
37°C. The culture medium was not changed prior to pretreatment and
stimulation. RAW 264.7 cells were seeded in a 6-well plate at a
density of 2×106 cells/well. After 24 h, the cells were
co-treated with LLT at concentrations of 1, 10 and 100 µg/ml and
LPS (1 µg/ml) for 6 h in a CO2 incubator maintained at
37°C.
Total RNA was extracted using RNAiso Plus (cat. no.
9108; Takara Bio, Inc.), according to the manufacturer's protocol,
and the mass of RNA (2 µg) was equalized after measuring the
concentration of the samples with a NanoDrop (Thermo Fisher
Scientific, Inc.) at 250–260 nm. cDNA was prepared from total RNA
using a reverse transcription kit, then amplified using a Taq
polymerase and target primers. The PCR thermocycling conditions
were: 30–44 cycles of 1 min at 94°C (denaturation), 1 min at
50–58°C (annealing) and 1 min at 72°C (extension)]. The sequences
of the primers and specific annealing temperatures are listed in
Table I. The amplified samples were
separated on a 1.2% agarose gel, stained with SYBR green
(Invitrogen; Thermo Fisher Scientific, Inc.). The stained agarose
gel was captured using NαBI (NeoScience). Semi-quantification of
the bands was performed using ImageJ software (version 1.51j8;
National Institutes of Health). The expression of each target gene
was quantified using GAPDH.
 | Table I.Primer sets for reverse transcription
PCR. |
Table I.
Primer sets for reverse transcription
PCR.
| Primer name | Primer sequence,
5′→3′ | Gene name | Number of
cycles | Annealing
temperature,°C | Genbank accession
no. |
|---|
| h-TARC F |
ACTGCTCCAGGGATGCCATCGTTTTT | CCL17 | 44 | 57.5 | NM_002987.3 |
| h-TARC R |
ACAAGGGGATGGGATCTCCCTCACTG |
|
|
|
|
| h-GAPDH F |
CGTCTAGAAAAACCTGCCAA | GAPDH | 30 | 50 | NM_001256799.3 |
| h-GAPDH R |
TGAAGTCAAAGGAGACCACC |
|
|
|
|
| m-TNF-α F |
GCAGAAGAGGCACTCCCCCA | Tnf | 30 | 58 | NM_001278601.1 |
| m-TNF-α R |
GATCCATGCCGTTGGCCAGG |
|
|
|
|
| m-IFN-γ F |
CTCAAGTGGCATAGATGT | Ifng | 38 | 57 | NM_008337.4 |
| m-IFN-γ R |
GAGATAATCTGGCTCTGCAGGATT |
|
|
|
|
| m-GAPDH F |
AACTTTGGCATTGTGGAAGG | GAPDH | 30 | 58 | NM_008084.3 |
| m-GAPDH R |
ACACATTGGGGGTAGGAACA |
|
|
|
|
High performance liquid chromatography
(HPLC) analysis
HPLC analysis was carried out on the Waters 2695
system with a 2996 dual λ absorbance detector (Waters Corporation).
The system was equipped with the XBridge™ C18 column (250×4.6 mm; 5
mm; Waters Corporation). The mobile phase consisted of acetonitrile
(solvent A) and 1% acetic acid (solvent B) at a flow rate of 1.0
ml/min. The injection volume of the extract was 10 µl. The elution
phase consisted of 0–40 min of 10–40% solvent A and 90–60% of
solvent B. Caffeic acid (also known as 3,4-Dihydroxybenzeneacrylic
acid; cat. no. C0625; Sigma-Aldrich; Merck KGaA), an indicator
component of LLT, was used as a standard. The elution was monitored
at 368 nm.
Statistical analysis
Data are presented as the mean ± SEM. All
experiments were repeated at least three times. Statistical
analysis was performed using GraphPad Prism Software (version 5.01;
GraphPad Software, Inc.). One-way ANOVA was used to evaluate the
treatment effect, followed by Tukey's multiple-comparisons test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of LLT on the skin and serum
of DNCB-induced AD mice
To evaluate the therapeutic efficacy of LLT in the
DNCB-induced AD mouse model, DNCB was applied to the dorsal skin of
Balb/c mice. As shown Fig. 1B, it
was confirmed that the control group exhibited erythema, edema and
eczematous skin lesions on the dorsal skin, and LLT treatment
improved these atopic-like symptoms. To evaluate the effect of LLT
on histological characteristics, the thickness of the epidermis and
dermis of the dorsal skin was measured in H&E-stained sections
(Fig. 1C). The thickness of the
epidermis and dermis was increased in the DNCB-induced control
group compared with the normal group (P<0.01). The LLT-H group
exhibited significantly reduced epidermal and dermal thickness
compared with the control group (P<0.01) (Fig. 1D). To assess the clinical symptoms
of AD, the serum levels of IgE and IL-6 were measured using ELISA
(Fig. 1E). The serum IL-6 levels
were significantly increased in the control group compared with the
normal group (P<0.01). The serum IL-6 levels were significantly
reduced in the LLT-H groups compared with the control group
(P<0.01). The serum IgE levels were significantly increased in
the control group compared with the normal group (P<0.01). The
serum IgE levels were significantly reduced in the LLT-L and LLT-H
groups compared with the control group (P<0.01 and P<0.05,
respectively).
Effects of LLT on histological changes
in mice with DNCB-induced AD
To examine eosinophil and mast cell infiltration in
the dermis, sections of dorsal skin were stained with H&E and
toluidine blue (Fig. 2A and B).
Eosinophil infiltration significantly increased in the control
group compared with the normal group (P<0.01); however,
application of LLT significantly inhibited eosinophil infiltration
(P<0.01; Fig. 2E). In addition,
mast cell infiltration was significantly increased in the control
group compared with the normal group (P<0.01), and application
of LLT significantly inhibited mast cell infiltration (P<0.01;
Fig. 2F). The effects of LLT
application on CD4+ and CD8+ cell
infiltration induced by DNCB was demonstrated by IHC staining
(Fig. 2C and D). The infiltration
by CD4+ cells increased in the control group compared
with the normal group (P<0.01). However, application of LLT did
not significantly affect CD4+ cell infiltration
(Fig. 2G). In addition, the
infiltration by CD8+ cells were increased in the control
group compared with the normal group (P<0.01), and application
of LLT-H significantly inhibited CD8+ cell infiltration
(P<0.05; Fig. 2H).
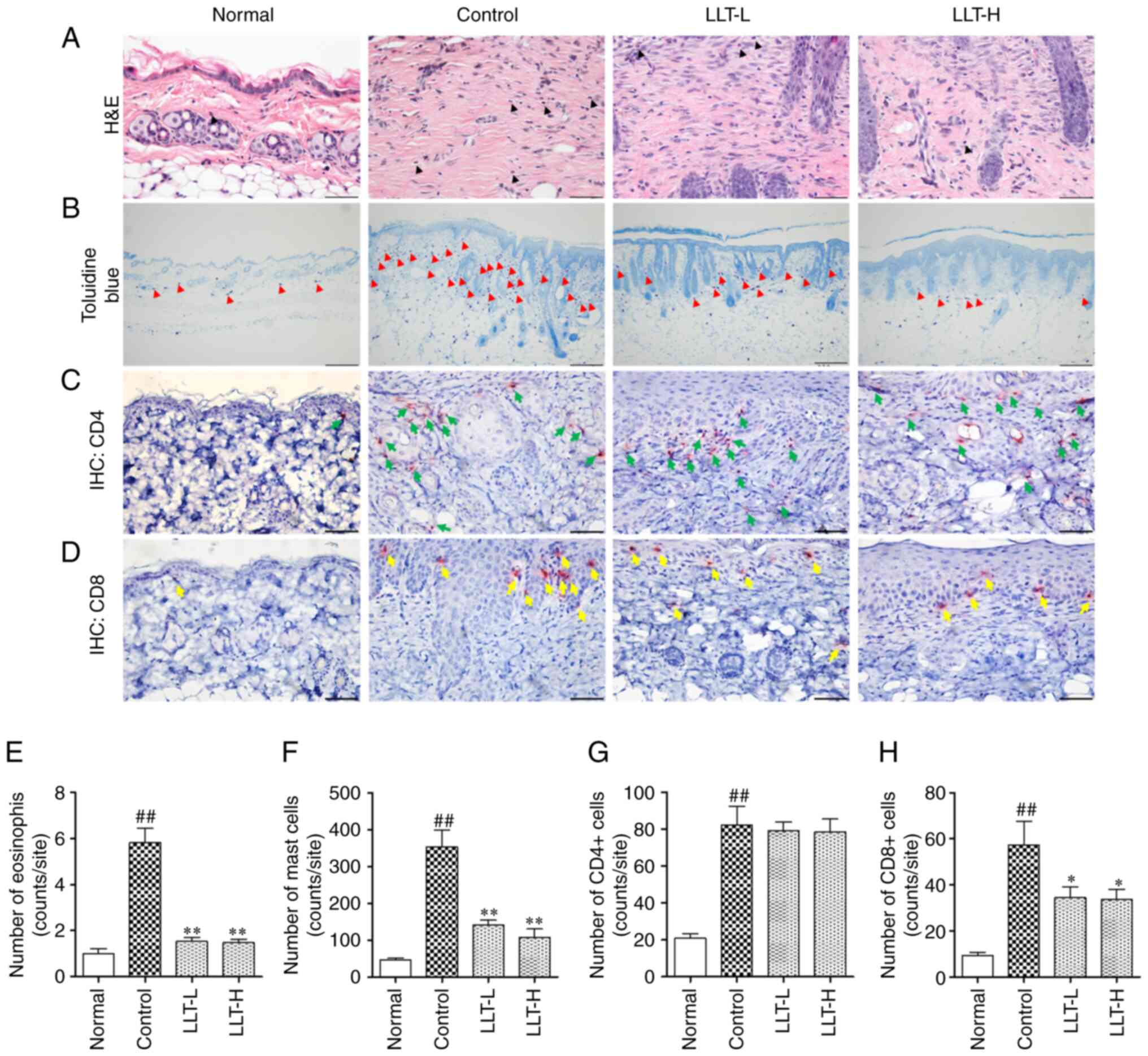 | Figure 2.Effect of LLT on immune cell
infiltration and the numbers of CD4+ and CD8+
cells in mice with DNCB-induced AD. Infiltration by (A) eosinophils
(black arrow; magnification, ×400; scale bar, 50 µm) and (B) mast
cells (red arrow; magnification, ×100; scale bar, 200 µm) in dermal
lesions was examined by H&E and toluidine blue staining of skin
sections. (C) CD4+ (green arrow) and (D) CD8+
(yellow arrow) cells were examined by IHC. Magnification, ×400.
Scale bar, 50 µm. The number of (E) eosinophil cells, (F) mast
cells, (G) CD4+ cells and (H) CD8+ cells was
counted using ImageJ software. Data represent the mean ± SEM. n=8.
##P<0.01 vs. normal grou; *P<0.05 and **P<0.01
vs. control group. Normal group, vehicle-treated; control group,
DNCB-sensitized; LLT-L group, DNCB + 1 mg/kg LLT; LLT-H group, DNCB
+ 5 mg/kg LLT. AD, atopic dermatitis; DNCB,
2,4-dinitrochlorobenzene; LLT, Lycopus lucidus Turcz;
H&E, hematoxylin and eosin; IHC, immunohistochemistry. |
Effect of LLT on the translocation and
of NF-κB and phosphorylation of ERK and JNK in dorsal skin tissue
of AD mice
To investigate the anti-inflammatory role of LLT,
proteins from dorsal skin tissue were extracted and the
translocation of NF-κB and phosphorylation of ERK and JNK
were measured by western blotting (Fig.
3A). The expression of NF-κB was significantly increased
in the control group compared with the normal group. In addition,
application of LLT significantly inhibited the expression of
NF-κB in a concentration-dependent manner (P<0.01;
Fig. 3B). DNCB treatment in Balb/c
mice induced phosphorylation of ERK and JNK in the dorsal skin
tissue, and LLT application significantly inhibited this
phosphorylation (P<0.01; Fig. 3C and
D).
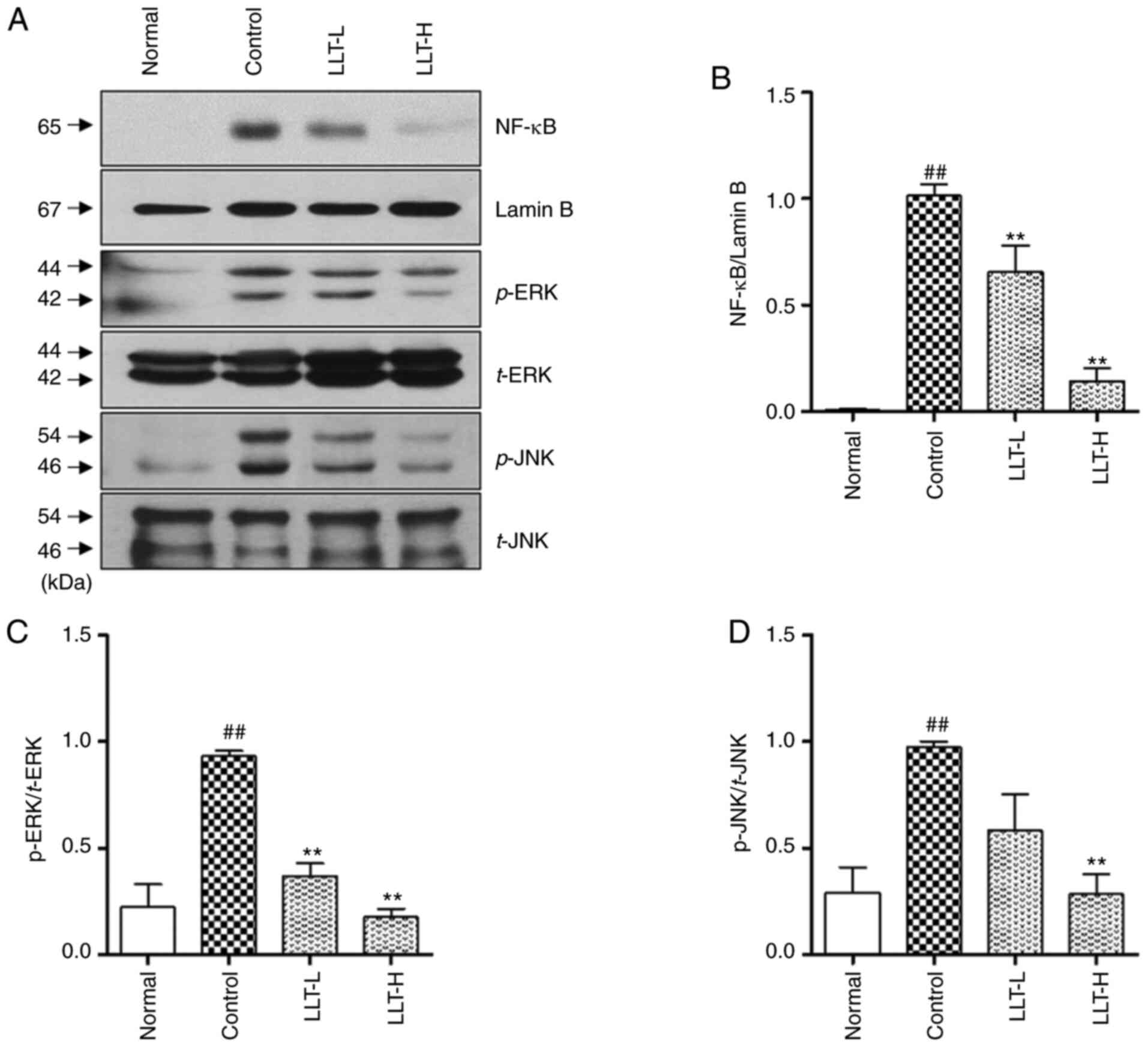 | Figure 3.Effects of LLT on NF-κB and
MAPK protein expression in BALB/c mice with DNCB-induced AD. (A)
NF-κB, p-ERK1/2 and p-JNK expression levels were examined by
western blotting. (B) NF-κB expression level was normalized
to lamin B, (C) p-ERK expression level was normalized to t-ERK and
(D) p-JNK expression level was normalized to t-JNK using ImageJ
software. Data represent the mean ± SEM (n=8).
##P<0.01 vs. normal group and **P<0.01 vs. control
group. Normal group, vehicle-treated; control group,
DNCB-sensitized; LLT-L group, DNCB + 1 mg/kg LLT; LLT-H group, DNCB
+ 5 mg/kg LLT. DNCB, 2,4-dinitrochlorobenzene; LLT, Lycopus
lucidus Turcz; AD, atopic dermatitis; p-, phosphorylated; t-,
total; LPS, lipopolysaccharide. |
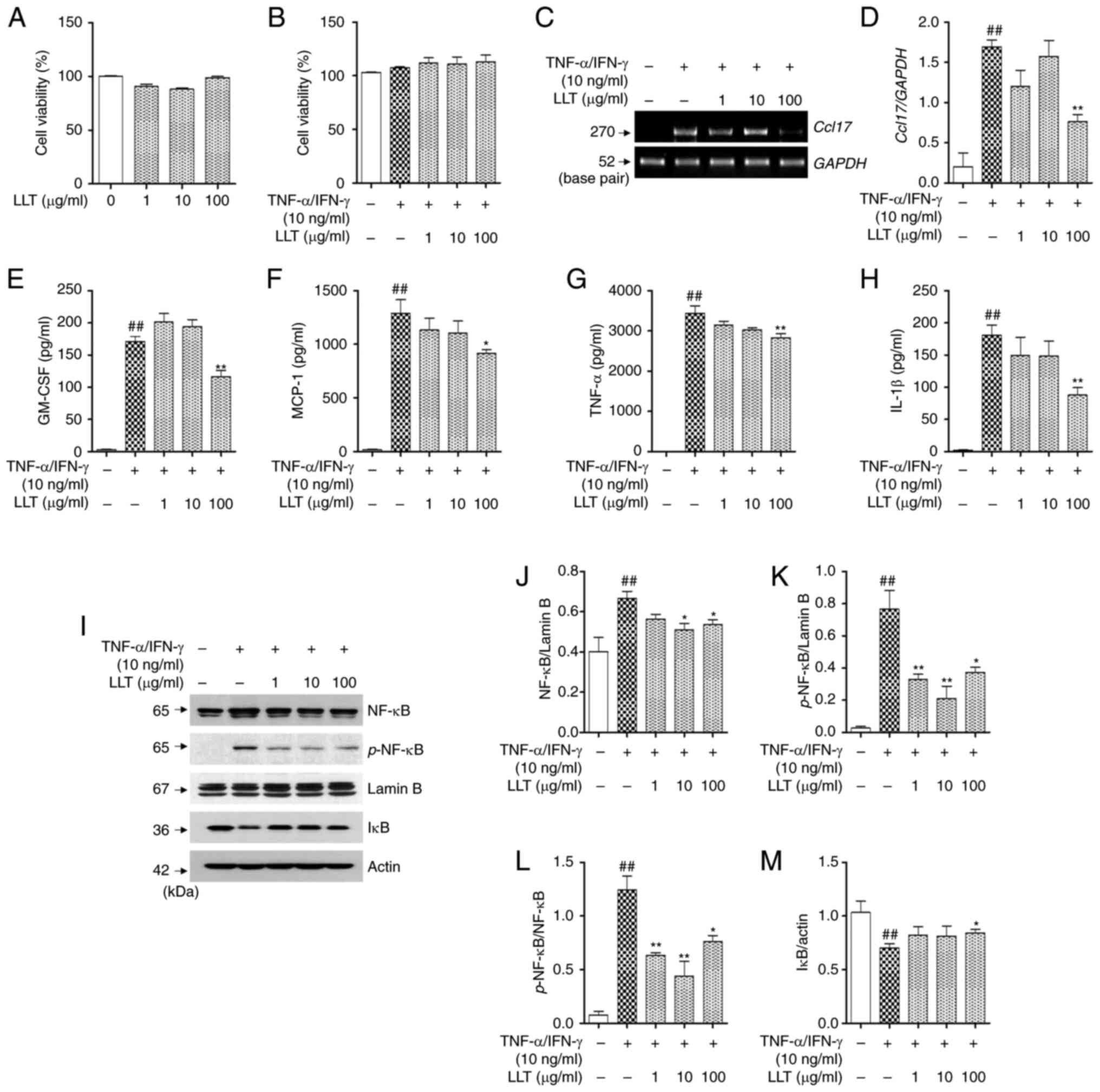
Effect of LLT on the expression of
pro-inflammatory cytokines and chemokines and the NF-κB signaling
following TNF-α/IFN-γ stimulation in HaCaT cells
Prior to the in vitro experiment, an MTS
assay was conducted to measure the toxicity of LLT in HaCaT cells.
After treatment for 24 h, it was observed that LLT did not
significantly affect the viability of HaCaT cells (Fig. 4A). LLT and TNF-α/IFN-γ stimulation
were then used to treat HaCaT cells, and cytotoxicity was also
measured. No significant change in cell viability was observed
(Fig. 4B). To investigate the
effect of LLT on the production of pro-inflammatory cytokines and
chemokines in AD, their mRNA expression levels were measured using
RT-PCR and their levels in the culture medium using ELISA. As shown
in Fig. 4C and CD, the mRNA
expression of TARC significantly increased following treatment with
TNF-α/IFN-γ in HaCaT cells (P<0.01). Moreover, the addition of
100 µg/ml LLT significantly reduced the expression of TARC
(P<0.01). As shown Fig. 4E-H,
the expression of GM-CSF, MCP-1, TNF-α and IL-1β in the cell medium
significantly increased following treatment with TNF-α/IFN-γ
(P<0.01), and 100 µg/ml LLT significantly inhibited this effect
(P<0.01, P<0.05, P<0.01 and P<0.01, respectively).
The effect of LLT on the translocation and
phosphorylation of NF-κB, which regulates the expression of
pro-inflammatory cytokines and chemokines, was investigated.
Treatment with TNF-α/IFN-γ induced the expression and
phosphorylation of NF-κB in the nuclear protein fraction
(P<0.01) and degradation of IκB in the cytoplasmic protein
fraction (P<0.01). LLT inhibited the effects of TNF-α/IFN-γ
(P<0.01) (Fig. 4I). Following
standardization with lamin B, LLT inhibited the expression (10 and
100 µg/ml, both P<0.05; Fig. 4J)
and phosphorylation of NF-κB (1, 10 and 100 µg/ml P<0.01,
P<0.01 and P<0.05, respectively; Fig. 4K). In addition, the ratio of
p-NF-κB/NF-κB was significantly increased through TNF-α/IFN-γ
stimulation (P<0.01), LLT inhibited the expression (1, 10 and
100 µg/ml, P<0.01, P<0.01 and P<0.05, respectively;
Fig. 4L). It also inhibited the
degradation of IκB (100 µg/ml, P<0.05; Fig. 4M).
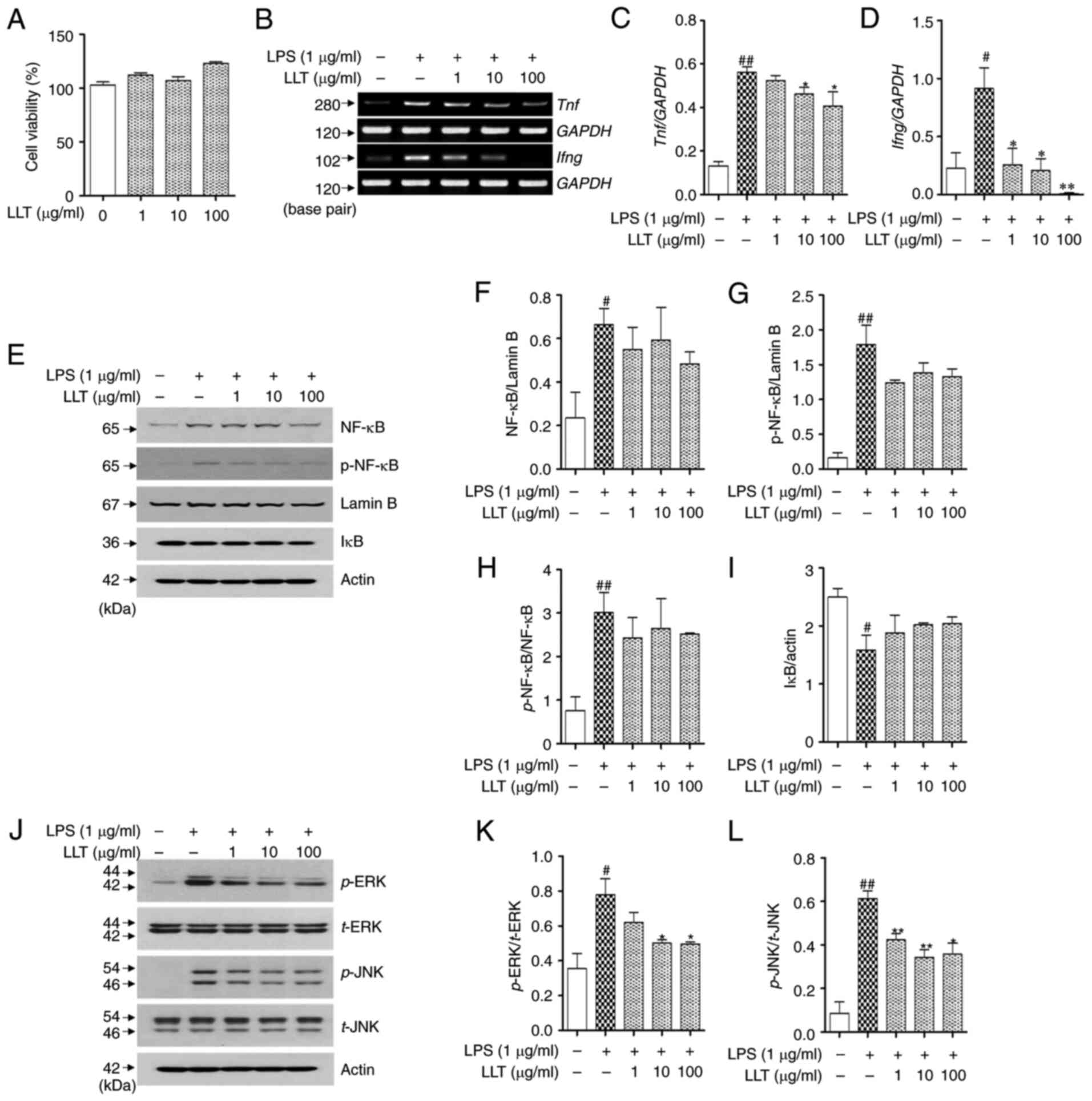
Effects of LLT on the expression of
inflammatory cytokines, MAPKs and NF-κB in LPS-stimulated RAW 264.7
cells
The potential cytotoxicity of LLT was measured in
RAW 264.7 cells using an MTS assay. As shown in Fig. 5A, none of the concentrations of LLT
(1, 10 and 100 µg/ml) affected the viability of the RAW 264.7
cells. The inhibitory effects of LLT on the mRNA expression of
inflammatory cytokines were confirmed in LPS-stimulated RAW 264.7
cells (Fig. 5B). The expression of
TNF-α and IFN-γ was increased in the LPS-stimulated control group
compared with that in the normal group (P<0.01 and P<0.05).
TNF-α expression was significantly downregulated in the LLT-treated
groups (10 and 100 µg/ml; P<0.01 and P<0.05, respectively;
Fig. 5C). LLT at concentrations of
1, 10 and 100 µg/ml significantly reduced the levels of IFN-γ in
RAW 264.7 cells (1, 10 and 100 µg/ml P<0.05, P<0.05 and
P<0.01, respectively; Fig.
5D).
To determine the effect of LLT on the NF-κB pathway
in RAW 264.7 cells, the effect of LLT on the expression and
phosphorylation of NF-κB and degradation of IκB was investigated
(Fig. 5E). Treatment with LPS
induced the expression (P<0.05) and phosphorylation of NF-κB
(P<0.01) in the nuclear protein and degradation of IκB in the
cytoplasmic protein (P<0.05). The expression and phosphorylation
of NF-κB in the LLT-treated groups was reduced compared with the
control, but the difference was not statistically significant
(Fig. 5F and G). In addition, the
ratio of p-NF-κB/NF-κB was significantly increased through LPS
stimulation (P<0.01), but no significant change was observed
with LLT treatment (Fig. 5H).
Although LLT appeared to inhibit the degradation of IκB following
LPS treatment, the difference was not significant (Fig. 5I).
To determine the effect of LLT on the MAPK pathways,
the protein levels of the MAPKs ERK and JNK were assessed using
western blot analysis (Fig. 5J).
The levels of p-ERK and p-JNK were significantly increased in the
control group compared with those in the normal group (P<0.01
and P<0.05, respectively). The phosphorylation of ERK and JNK
was significantly reduced in the LLT treatment groups compared with
the control group (P<0.05 and P<0.01, respectively). The
level of p-ERK was significantly decreased in the LLT-treated
groups compared with the control group (10 and 100 µg/ml; both
P<0.05; Fig. 5K). Similar to the
results of p-ERK expression, the level of p-JNK was decreased in
the LLT-treated groups compared with the control group (1, 10 and
100 µg/ml P<0.01, P<0.01 and P<0.05, respectively;
Fig. 5L).
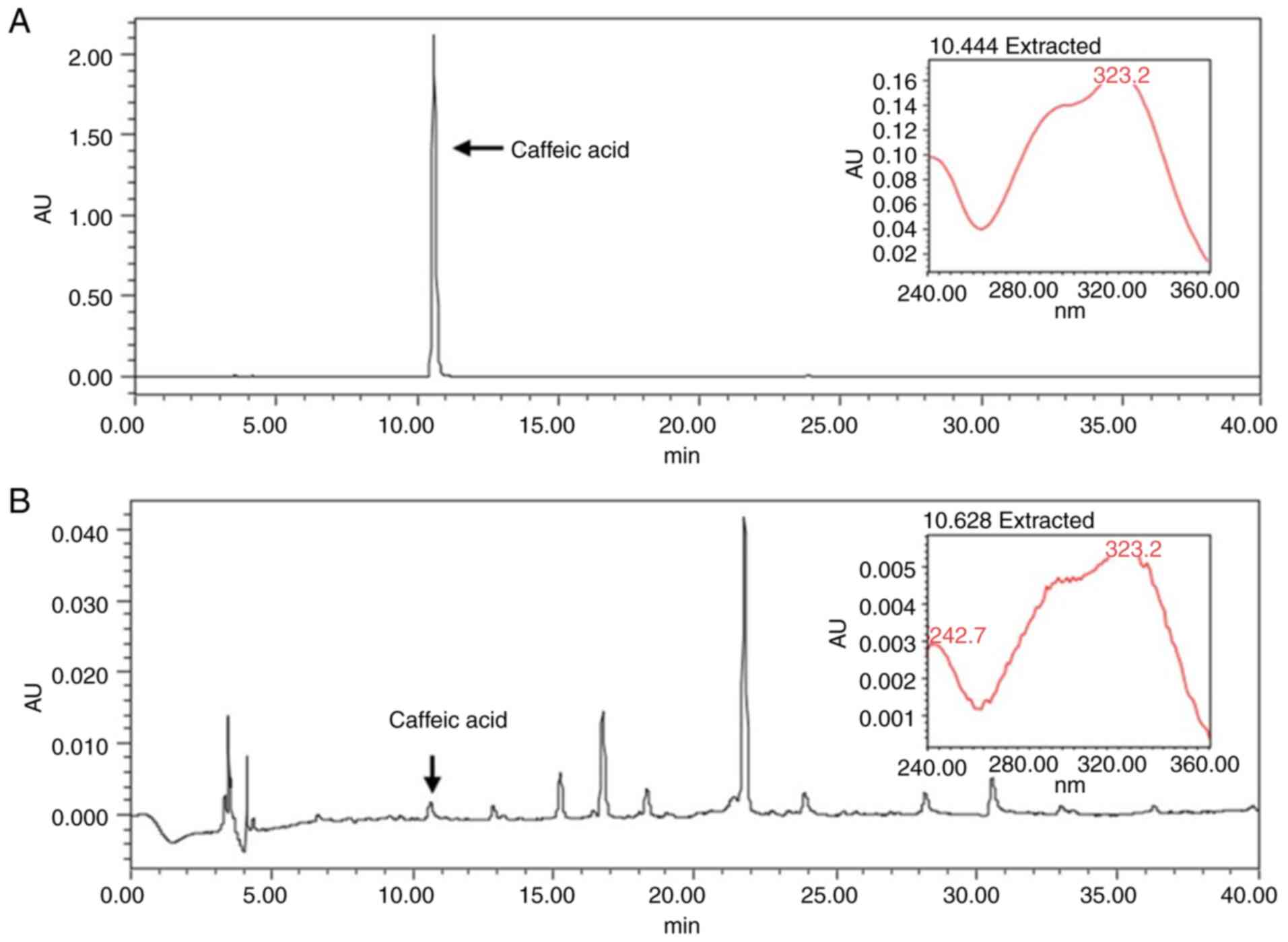
HPLC analysis
Caffeic acid has been used as a standard marker for
LLT in various studies (30–32).
As shown Fig. 6, the retention time
of the caffeic acid was 10.62 min (Fig.
6A). The chromatographic peak of the LLT was 10.62 min at a
wavelength of 254 nm (Fig. 6B).
Since the peaks of LLT and caffeic acid were detected at the same
time period, the LLT used in this experiment was proved to be the
same as that used in other studies.
Discussion
To the best of the authors' knowledge, the present
study is the first to confirm the beneficial effect of LLT on
DNCB-induced AD and its effect on the expression of cytokines and
chemokines in HaCaT and RAW 264.7 cells. It was demonstrated that
LLT inhibited DNCB-induced hyperkeratosis in the epidermis and
dermis, and infiltration by eosinophils, mast cells and
CD8+ cells. LLT also inhibited the expression of IgE and
IL-6 in the serum, and the phosphorylation of MAPK and NF-κB in the
skin tissues. In addition, LLT inhibited the expression of
inflammatory chemokines and cytokines in HaCaT and RAW 264.7 cells.
Taken together, these findings confirmed that LLT has the potential
of becoming a new alternative AD treatment. Balb/c mice, in which
AD was induced through DNCB stimulation, are characterized by a
Th2-dominated immune response and are widely used in the field of
Immunology (33). In addition, DNCB
was found to promote the expression of various cytokines (34) and chemokines (35) that induce AD. HaCaT cells are a
naturally immortalized line of human keratinocytes. These cells are
widely used in skin biology and differentiation studies. RAW 264.7
cells are macrophages derived from Abelson leukemia virus-infected
mice, and they are used in various studies to verify the effects on
the inflammatory response (36).
LPS is a gram-negative bacterium, which increases various
inflammatory cytokines and a large amount of nitric oxide (NO) to
cause tissue damage, edema and inflammatory response (37). Therefore, the LPS-induced RAW 264.7
cell model has been used in various studies to examine the
mechanism of AD (38,39).
Skin thickening is a notable clinical symptom of AD
(40,41). Continuous allergic and inflammatory
responses may result in hardened and thickened skin (42). In the present study, LLT inhibited
the thickening of the epidermis and dermis, indicating that LLT
exerts an inhibitory effect on hyperkeratosis and alleviates skin
thickening, which is one of the main symptoms of AD. The cytokines
secreted by Th1 and Th2 cells promote the development of AD
(43). Th2-cell allergic
inflammation predominates in the acute stage of AD, resulting in
increased IL-6 and IgE expression, whereas Th1-cell inflammation
predominates in the chronic stage of AD, resulting in increased
expression of IFN-γ and TNF-α (5,44,45).
Th2 cells activate B cells, which produce IgE (46). IgE binds to the IgE receptor, FcεRI,
and initiates the activation of mast cells, leading to the release
of histamine and other mediators of inflammation (47,48).
In the present study, the levels of IgE and IL-6 were significantly
increased in the serum of mice with DNCB-induced AD, whereas LLT
decreased the production of IgE and IL-6.
AD is associated with high IgE levels and
infiltration by inflammatory cells, such as mast cells and
eosinophils (49). The majority of
patients with AD are pathologically characterized by infiltration
of the skin lesions by eosinophils and mast cells (50,51).
The activation of mast cells has been associated with allergic
inflammation and the expression of various inflammatory mediators,
including histamine, which induces AD-like skin lesions (52,53).
Eosinophils are cells of the immune system, which, together with
mast cells, act as mediators of allergic reactions (13). Mast cells and eosinophils are
recruited and activated in the inflamed dermis, where they play a
major role in aggravating allergic skin conditions (54). Research has shown that these cells
modulate pruritus in AD and induce allergic inflammation (55,56).
Increased numbers of eosinophils and mast cells are a
characteristic feature of AD in both humans and DNCB-induced AD
mice (57–59). To examine the effects of LLT on the
infiltration of eosinophils and mast cells, the present study
performed H&E and toluidine blue staining of dorsal skin
tissues obtained from DNCB-induced AD mice. It was observed that
LLT decreased the number of eosinophils and mast cells in the skin
of AD mice, indicating that LLT may inhibit the infiltration of the
dermis by mast cells and eosinophils by suppressing IgE and the
release of pro-inflammatory cytokines.
CD4+ T cells (Th cells) induce humoral
immunity by acting on specific B lymphocytes to promote responses
to antigens (60,61). Humoral immunity is an
antigen-antibody reaction caused by the activation of B cells and T
cells by CD4+ T cells and the mechanism is as follows.
The antigen-memory CD4+ T cells induce the
differentiation of B cells. Differentiated B cells meet with T
cells to form antibodies in T cells and these antibodies circulate
in body fluids and cause antigen/antibody reactions (62). In a normal allergic environment, Th2
cells induce the production of Th2 cytokines responsible for the
expression of allergen-specific IgE (63). CD8+ cells
(cytotoxic/suppressor T cells) release toxic substances, such as
perforin, granzyme and granulicin, that can directly target
antigen-bearing cells, and when stimulated, Th1 cells are activated
to induce the expression of inflammatory cytokines such as TNF-α
(64); therefore, the numbers of
CD4+ and CD8+ cells are important indicators
for measuring the immune function (65). In the present study, LLT reduced the
number of CD8+ cells in the skin tissue with
DNCB-induced AD, but did not significantly affect the number of
CD4+ cells. Because CD4+ cells are not under
the control of LLT in the present study, the reason why the
expression of IgE mediated by Th2 cytokines is suppressed may be
related to the suppression of IL-6 expression. A previous study has
shown that the expression of IL-6 upregulates the expression of IgE
(66). From this study, the IgE
expression inhibitory effect of LLT is hypothesized to be
suppressed through IL-6, not through CD4+ cells. In
addition, the expression of IgE induces the degradation of the mast
cells to express various inflammatory cytokines. In this study, the
invasion of the mast cells in the tissue was inhibited, as
evidenced by toluidine blue staining. Therefore, following the
results of these studies, it was demonstrated that the infiltration
of mast cells was induced by the expression of IgE and that LLT
inhibited them. Combining the experimental results of serum and
tissue analysis, these results indicated that the inhibition of the
inflammatory response by LLT in AD is mediated through inhibition
of CD8+ cell, but not CD4+ cell
infiltration.
The activation of NF-κB is a key step in the
progression of inflammatory skin conditions (15,67,68).
Therefore, the inhibition of the NF-κB expression may represent a
target for the treatment of inflammatory diseases, including AD
(69). The activation of MAPK
signaling plays a major role in NF-κB activation (70,71).
The MAPK (ERK/1/2 and JNK) signaling pathway plays an important
role in the proliferation, degranulation and activation of diverse
immune cells (72). In the present
study, LLT inhibited the expression of NF-κB in the dorsal skin
tissues obtained from mice with DNCB-induced AD. Furthermore, LLT
inhibited the expression and phosphorylation of NF-κB in HaCaT
cells induced by TNF-α/IFN-γ in nuclear protein and it also
inhibited the degradation of IκB in the cytoplasmic protein. In
addition, LLT treatment appeared to show a positive effect on the
NF-κB pathway in RAW 264.7 cells stimulated with LPS, although the
difference was not significant. These results indicated that the
effect of LLT may be mediated through inhibition of expression and
phosphorylation of NF-κB, a target in AD treatment. In addition,
LLT significantly inhibited the phosphorylation of ERK and JNK in
tissues and phosphorylation of LPS-induced MAPK pathway-related
proteins in RAW 264.7 cells.
MAPK and NF-κB signaling induce AD through the
expression of various pro-inflammatory cytokines and chemokines
(73). TARC is a representative Th2
chemokine that acts on the C-C chemokine receptor 4 expressed on T
cells to induce the migration and invasion of Th2 cells in
inflammatory lesions (74). TARC is
produced by a variety of cells, including endothelial cells,
dendritic cells and keratinocytes. In AD, TARC induces
integrin-dependent adhesion and passage of T cells through the
blood vessel wall, acting in the first stage of T-cell recruitment
to the lesion (75). The expression
of TARC is high in the basal layer of the epidermis in skin lesions
(74), and serum TARC levels are
markedly elevated in patients with AD, which is directly
proportional to the severity of the symptoms (76) and may be used as an index of AD
severity. In the present study, LLT was shown to inhibit TARC
expression in HaCaT cells stimulated with TNF-α/IFN-γ. GM-CSF is a
cytokine secreted by lymphocytes, and its expression is increased
in chronic AD, which is known to inhibit apoptosis and promote
survival of lymphocytes, causing chronic inflammation of skin
lesions (77). MCP-1 is a member of
the CC family of chemokines and it serves as a chemotactic factor
for monocytes. MCP-1 has been demonstrated to affect the migration
and invasion of monocytes, macrophages, memory T cells and natural
killer cells, and an increase in MCP-1 has been observed in studies
on patients with chronic and idiopathic urticaria, a chronic
inflammatory skin disease (78). In
the present study, LLT inhibited GM-CSF and MCP-1 expression in
HaCaT cells stimulated by TNF-α/IFN-γ.
When HaCaT cells are exposed to stress, cytokines,
such as TNF-α and IL-1β, activate several inflammation-related cell
signaling pathways (79). TNF-α is
a pro-inflammatory cytokine that is involved in the initiation of
inflammatory reactions. TNF-α regulates the lipid barrier function
of the skin, either by acting alone or in combination with Th2
cytokines, and is also involved in the proliferation of thymic
stromal lymphopoietin (80). IL-1β
is mainly secreted by activated mononuclear phagocytic cells
(81). As a lymphocyte-activating
factor, IL-1β promotes the activation and proliferation of
CD4+ T lymphocytes and secretion of IFN-γ, and activates
vascular endothelial cells to cause leukocyte adhesion (82). Therefore, IL-1β plays an important
role in the inflammatory process and is involved in type I
hypersensitivity reactions in allergic diseases (11). In the present study, LLT inhibited
the expression of TNF-α and IL-1β in stimulated HaCaT cells, and
suppressed the expression of TNF-α and IFN-γ in stimulated RAW
264.7 cells. Collectively, these experimental results indicate that
LLT may suppress the expression of chemokines and inflammatory
cytokines by inhibiting the expression of MAPKs and NF-κB
signaling. Finally, LLT may improve AD by increasing the thickness
of the skin barrier and inhibiting infiltration by eosinophils and
mast cells.
This study has the following limitations. First, in
an in vitro assay, it was verified the NF-κB pathway in
HaCaT cells and RAW 264.7 cells. LLT showed a significant
inhibitory effect on the expression and phosphorylation of NF-κB in
HaCaT cells, and showed a positive effect compared to the induced
group, although the difference was not significant in RAW 264.7
cells. However, in the study of NF-κB in animal tissues, the
expression of NF-κB was only measured in the nuclear protein
fraction. As a result, the role of the NF-κB pathway in AD remains
unconfirmed. In future studies, additional research on NF-κB
phosphorylation or IκB degradation may provide further insight into
the ability of LLT to inhibit AD. Moreover, this study verified the
anti-atopic effect of LLT, a herbal medicine, but it is unclear
which ingredients in LLT play an anti-atopic role. In previous
study, LLT was shown to possess various active ingredients such as
schizotenuin A, 3-O-(caffeoyl)-rosmarinic acid, rosmarinic acid
ethyl ester, apigenin, acacetin,
acacetin-7-O-b-D-glucuronopyranoside, luteolin, scutellarin and
hispidulinc (83). Among these
ingredients, rosmarinic acid improves the scoring atopic dermatitis
(SCORAD) index (a standardized method for the severity of atopic
dermatitis in the European atopic dermatitis task force consensus
report) (84) and skin moisture
index in clinical trials (85),
luteolin was effective in treating skin diseases by inhibiting the
expression of pro-inflammatory mediators in the NF-κB pathway
(86). In addition, hispidulin
inhibited AD by regulating the expression of IL-1β, IL-6, IL-8 and
chemokine C-C (87). However, the
pharmacological effects of the remaining ingredients on atopic
diseases have not yet been verified. In the future, directly
separating the components contained in LLT, may further confirm and
improve our understanding of the pharmacological effect of LLT in
each atopic disease.
Acknowledgements
Not applicable.
Funding
This work was supported by a grant from The National
Research Foundation of Korea (NRF) funded by the Korean Government
[Ministry of Science and ICT (MSIT); grant no.
2020R1A2C2005836].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HSJ designed the experiments. EYK, GYM and SH
performed the in vivo experiments. GYM, JHK, MK and EJK
performed the in vitro experiments. YS, HSJ and JHP
performed the data analysis. GYM wrote the manuscript. GYM and HSJ
confirm the authenticity of the raw data in this manuscript. All
the authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were performed with the
approval of The Institutional Animal Care and Use Committee of
Kyung Hee University [approval no. KHUASP (SE)-15-116].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Choi JH, Kim HG, Jin SW, Han EH, Khanal T,
Do MT, Hwang YP, Choi JM, Chun SS, Chung YC, et al: Topical
application of Pleurotus eryngii extracts inhibits
2,4-dinitrochlorobenzene-induced atopic dermatitis in NC/Nga mice
by the regulation of Th1/Th2 balance. Food Chem Toxicol. 53:38–45.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johansson SG, Hourihane JO, Bousquet J,
Bruijnzeel-Koomen C, Dreborg S, Haahtela T, Kowalski ML, Mygind N,
Ring J, van Cauwenberge P, et al: A revised nomenclature for
allergy. An EAACI position statement from the EAACI nomenclature
task force. Allergy. 56:813–824. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cookson W: The immunogenetics of asthma
and eczema: A new focus on the epithelium. Nat Rev Immunol.
4:978–988. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Denby KS and Beck LA: Update on systemic
therapies for atopic dermatitis. Curr Opin Allergy Clin Immunol.
12:421–426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bieber T: Atopic dermatitis. N Engl J Med.
358:1483–1494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim JH, Kim MH, Yang G, Huh Y, Kim SH and
Yang WM: Effects of topical application of Astragalus membranaceus
on allergic dermatitis. Immunopharmacol Immunotoxicol. 35:151–156.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuan XY, Ma HM, Li RZ, Wang RY, Liu W and
Guo JY: Topical application of aloperine improves
2,4-dinitrofluorobenzene-induced atopic dermatitis-like skin
lesions in NC/Nga mice. Eur J Pharmacol. 658:263–269. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim EC, Lee HS, Kim SK, Choi MS, Lee S,
Han JB, An HJ, Um JY, Kim HM, Lee NY, et al: The bark of Betula
platyphylla var. Japonica inhibits the development of atopic
dermatitis-like skin lesions in NC/Nga mice. J Ethnopharmacol.
116:270–278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watanabe H, Unger M, Tuvel B, Wang B and
Sauder DN: Contact hypersensitivity: The mechanism of immune
responses and T cell balance. J Interferon Cytokine Res.
22:407–412. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bieber T, Cork M and Reitamo S: Atopic
dermatitis: A candidate for disease-modifying strategy. Allergy.
67:969–975. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brandt EB and Sivaprasad U: Th2 cytokines
and atopic dermatitis. J Clin Cell Immunol. 2:1102011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tokura Y: Extrinsic and intrinsic types of
atopic dermatitis. J Dermatol Sci. 58:1–7. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu FT, Goodarzi H and Chen HY: IgE, mast
cells, and eosinophils in atopic dermatitis. Clin Rev Allergy
Immunol. 41:298–310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seitz CS, Lin Q, Deng H and Khavari PA:
Alterations in NF-kappaB function in transgenic epithelial tissue
demonstrate a growth inhibitory role for NF-kappaB. Proc Natl Acad
Sci USA. 95:2307–2312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Smahi A, Courtois G, Rabia SH, Doffinger
R, Bodemer C, Munnich A, Casanova JL and Israel A: The NF-kappaB
signalling pathway in human diseases: From incontinentia pigmenti
to ectodermal dysplasias and immune-deficiency syndromes. Hum Mol
Genet. 11:2371–2375. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kataoka Y: Thymus and activation-regulated
chemokine as a clinical biomarker in atopic dermatitis. J Dermatol.
41:221–229. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Emile JF, Fraitag S, Andry P, Leborgne M,
Lellouch-Tubiana A and Brousse N: Expression of GM-CSF receptor by
Langerhans' cell histiocytosis cells. Virchows Arch. 427:125–129.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rajagopalan LE, Burkholder JK, Turner J,
Culp J, Yang NS and Malter JS: Granulocyte-macrophage
colony-stimulating factor mRNA stabilization enhances transgenic
expression in normal cells and tissues. Blood. 86:2551–2558. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Breuhahn K, Mann A, Muller G, Wilhelmi A,
Schirmacher P, Enk A and Blessing M: Epidermal overexpression of
granulocyte-macrophage colony-stimulating factor induces both
keratinocyte proliferation and apoptosis. Cell Growth Differ.
11:111–121. 2000.PubMed/NCBI
|
|
20
|
Pastore S, Fanales-Belasio E, Albanesi C,
Chinni LM, Giannetti A and Girolomoni G: Granulocyte macrophage
colony-stimulating factor is overproduced by keratinocytes in
atopic dermatitis. Implications for sustained dendritic cell
activation in the skin. J Clin Invest. 99:3009–3017. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaburagi Y, Shimada Y, Nagaoka T, Hasegawa
M, Takehara K and Sato S: Enhanced production of CC-chemokines
(RANTES, MCP-1, MIP-1alpha, MIP-1beta, and eotaxin) in patients
with atopic dermatitis. Arch Dermatol Res. 293:350–355. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Klonowska J, Glen J, Nowicki RJ and
Trzeciak M: New cytokines in the pathogenesis of atopic
dermatitis-new therapeutic targets. Int J Mol Sci. 19:30862018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leung DY: Atopic dermatitis: New insights
and opportunities for therapeutic intervention. J Allergy Clin
Immunol. 105:860–876. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tan Q, Yang H, Liu E and Wang H: P38/ERK
MAPK signaling pathways are involved in the regulation of filaggrin
and involucrin by IL17. Mol Med Rep. 16:8863–8867. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cui HZ, Oh HC, Li X, Lee YJ, Cho KW, Kang
DG and Lee HS: Ethanol extract of Lycopus lucidus elicits
positive inotropic effect via activation of Ca2+ entry
and Ca2+ release in beating rabbit atria. J Med Food.
16:633–640. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jeong DW, Kim EY, Kim JH, Lee B, Hong S,
Park JH, Jung HS and Sohn Y: Lycopus lucidus Turcz Inhibits the
Osteoclastogenesis in RAW 264.7 Cells and Bone Loss in
Ovariectomized Rat Model. Evid Based Complement Alternat Med.
2019:32317842019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shin TY, Kim SH, Suk K, Ha JH, Kim I, Lee
MG, Jun CD, Kim SY, Lim JP, Eun JS, et al: Anti-allergic effects of
Lycopus lucidus on mast cell-mediated allergy model. Toxicol
Appl Pharmacol. 209:255–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Woo ER and Piao MS: Antioxidative
constituents from Lycopus lucidus. Arch Pharm Res.
27:173–176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee YJ, Kang DG, Kim JS and Lee HS:
Lycopus lucidus inhibits high glucose-induced vascular
inflammation in human umbilical vein endothelial cells. Vascul
Pharmacol. 48:38–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim KY, Oh TW, Ma JY and Park KI: Ethanol
extract of Lycopus lucidus Turcz. ex benth inhibits
metastasis by downregulation of Runx-2 in mouse colon cancer cells.
Evid Based Complement Alternat Med. 2018:95132902018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ren Q, Ding L, Sun SS, Wang HY and Qu L:
Chemical identification and quality evaluation of Lycopus
lucidus Turcz by UHPLC-Q-TOF-MS and HPLC-MS/MS and hierarchical
clustering analysis. Biomed Chromatogr. 31:2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Slusarczyk S, Hajnos M, Skalicka-Woźniak K
and Matkowski A: Antioxidant activity of polyphenols from
Lycopus lucidus Turcz. Food Chem. 113:134–138. 2009.
View Article : Google Scholar
|
|
33
|
Kwon B, Hong SY, Kim EY, Kim JH, Kim M,
Park JH, Sohn Y and Jung HS: Effect of cone of pinus densiflora on
DNCB-induced allergic contact dermatitis-like skin lesion in Balb/c
Mice. Nutrients. 13:8392021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang EY, Chen AY and Zhu BT: Mechanism of
dinitrochlorobenzene-induced dermatitis in mice: Role of specific
antibodies in pathogenesis. PLoS One. 4:e77032009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han MH, Yoon WK, Lee H, Han SB, Lee K,
Park SK, Yang KH, Kim HM and Kang JS: Topical application of
silymarin reduces chemical-induced irritant contact dermatitis in
BALB/c mice. Int Immunopharmacol. 7:1651–1658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Raschke WC, Baird S, Ralph P and Nakoinz
I: Functional macrophage cell lines transformed by Abelson leukemia
virus. Cell. 15:261–267. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bhardwaj M, Sali VK, Mani S and Vasanthi
HR: Neophytadiene from turbinaria ornata suppresses LPS-induced
inflammatory response in RAW 264.7 macrophages and sprague dawley
rats. Inflammation. 43:937–950. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee DH, Park JK, Choi J, Jang H and Seol
JW: Anti-inflammatory effects of natural flavonoid diosmetin in
IL-4 and LPS-induced macrophage activation and atopic dermatitis
model. Int Immunopharmacol. 89:1070462020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee HN, Shin SA, Choo GS, Kim HJ, Park YS,
Kim BS, Kim SK, Cho SD, Nam JS, Choi CS, et al: Antiinflammatory
effect of quercetin and galangin in LPS-stimulated RAW264.7
macrophages and DNCB-induced atopic dermatitis animal models. Int J
Mol Med. 41:888–898. 2018.PubMed/NCBI
|
|
40
|
Choi JK, Oh HM, Lee S, Kwon TK, Shin TY,
Rho MC and Kim SH: Salvia plebeia suppresses atopic dermatitis-like
skin lesions. Am J Chin Med. 42:967–985. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Proksch E, Folster-Holst R and Jensen JM:
Skin barrier function, epidermal proliferation and differentiation
in eczema. J Dermatol Sci. 43:159–169. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yarbrough KB, Neuhaus KJ and Simpson EL:
The effects of treatment on itch in atopic dermatitis. Dermatol
Ther. 26:110–119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang Q, Gao S, Wu GZ, Yang N, Zu XP, Li
WC, Xie N, Zhang RR, Li CW, Hu ZL and Zhang WD: Total sesquiterpene
lactones isolated from Inula helenium L. attenuates 2,
4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions
in mice. Phytomedicine. 46:78–84. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim JE, Kim JS, Cho DH and Park HJ:
Molecular mechanisms of cutaneous inflammatory disorder: Atopic
dermatitis. Int J Mol Sci. 17:12342016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cesare AD, Meglio PD and Nestle FO: A role
for Th17 cells in the immunopathogenesis of atopic dermatitis? J
Invest Dermatol. 128:2569–2571. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Deo SS, Mistry KJ, Kakade AM and Niphadkar
PV: Role played by Th2 type cytokines in IgE mediated allergy and
asthma. Lung India. 27:66–71. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Stone KD, Prussin C and Metcalfe DD: IgE,
mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 125
(Suppl 2):S73–S80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Metcalfe DD, Baram D and Mekori YA: Mast
cells. Physiol Rev. 77:1033–1079. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Inagaki N, Shiraishi N, Igeta K, Itoh T,
Chikumoto T, Nagao M, Kim JF and Nagai H: Inhibition of scratching
behavior associated with allergic dermatitis in mice by tacrolimus,
but not by dexamethasone. Eur J Pharmacol. 546:189–196. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Plager DA, Henke SA, Matsuwaki Y, Madaan
A, Squillace DL, Dierkhising RA and Kita H: Pimecrolimus reduces
eosinophil activation associated with calcium mobilization. Int
Arch Allergy Immunol. 149:119–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fujii Y, Takeuchi H, Sakuma S, Sengoku T
and Takakura S: Characterization of a
2,4-dinitrochlorobenzene-induced chronic dermatitis model in rats.
Skin Pharmacol Physiol. 22:240–247. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Galli SJ and Tsai M: IgE and mast cells in
allergic disease. Nat Med. 18:693–704. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sismanopoulos N, Delivanis DA,
Alysandratos KD, Angelidou A, Therianou A, Kalogeromitros D and
Theoharides TC: Mast cells in allergic and inflammatory diseases.
Curr Pharm Des. 18:2261–2277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Won TJ, Kim B, Lee Y, Bang JS, Oh ES, Yoo
JS, Hyung KE, Yoon J, Hwang S, Park ES, et al: Therapeutic
potential of Lactobacillus plantarum CJLP133 for house-dust
mite-induced dermatitis in NC/Nga mice. Cell Immunol. 277:49–57.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ikoma A, Steinhoff M, Stander S,
Yosipovitch G and Schmelz M: The neurobiology of itch. Nat Rev
Neurosci. 7:535–547. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Paus R, Schmelz M, Biro T and Steinhoff M:
Frontiers in pruritus research: Scratching the brain for more
effective itch therapy. J Clin Invest. 116:1174–1186. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
James EA and Kwok WW: Autoreactive CD4(+)
T cells in patients with atopic dermatitis. J Allergy Clin Immunol.
128:100–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Murota H, El-latif MA, Tamura T, Amano T
and Katayama I: Olopatadine hydrochloride improves dermatitis score
and inhibits scratch behavior in NC/Nga mice. Int Arch Allergy
Immunol. 153:121–132. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yamanaka K and Mizutani H: The role of
cytokines/chemokines in the pathogenesis of atopic dermatitis. Curr
Probl Dermatol. 41:80–92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Barcena J and Blanco E: Design of novel
vaccines based on virus-like particles or chimeric virions. Subcell
Biochem. 68:631–665. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Eisenbarth SC, Baumjohann D, Craft J,
Fazilleau N, Ma CS, Tangye SG, Vinuesa CG and Linterman MA:
CD4+ T cells that help B cells-a proposal for uniform
nomenclature. Trends Immunol. 42:658–669. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mitchison NA: T-cell-B-cell cooperation.
Nat Rev Immunol. 4:308–312. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Gri G, Piconese S, Frossi B, Manfroi V,
Merluzzi S, Tripodo C, Viola A, Odom S, Rivera J, Colombo MP and
Pucillo CE: CD4+CD25+ regulatory T cells
suppress mast cell degranulation and allergic responses through
OX40-OX40L interaction. Immunity. 29:771–781. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Fong TA and Mosmann TR: Alloreactive
murine CD8+ T cell clones secrete the Th1 pattern of
cytokines. J Immunol. 144:1744–1752. 1990.PubMed/NCBI
|
|
65
|
Yagi R, Nagai H, Iigo Y, Akimoto T, Arai T
and Kubo M: Development of atopic dermatitis-like skin lesions in
STAT6-deficient NC/Nga mice. J Immunol. 168:2020–2027. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Tan HP, Lebeck LK and Nehlsen-Cannarella
SL: Regulatory role of cytokines in IgE-mediated allergy. J Leukoc
Biol. 52:115–118. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Barnes PJ and Karin M: Nuclear
factor-kappaB: A pivotal transcription factor in chronic
inflammatory diseases. N Engl J Med. 336:1066–1071. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Finco TS and Baldwin AS: Mechanistic
aspects of NF-kappa B regulation: The emerging role of
phosphorylation and proteolysis. Immunity. 3:263–272. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Choi YY, Kim MH, Lee JY, Hong J, Kim SH
and Yang WM: Topical application of Kochia scoparia inhibits the
development of contact dermatitis in mice. J Ethnopharmacol.
154:380–385. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Arthur JS and Ley SC: Mitogen-activated
protein kinases in innate immunity. Nat Rev Immunol. 13:679–692.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hommes DW, Peppelenbosch MP and van
Deventer SJ: Mitogen activated protein (MAP) kinase signal
transduction pathways and novel anti-inflammatory targets. Gut.
52:144–151. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Jeon YD, Kee JY, Kim DS, Han YH, Kim SH,
Kim SJ, Um JY and Hong SH: Effects of Ixeris dentata water extract
and caffeic acid on allergic inflammation in vivo and in vitro. BMC
Complement Altern Med. 15:1962015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Venuprasad K, Elly C, Gao M,
Salek-Ardakani S, Harada Y, Luo JL, Yang C, Croft M, Inoue K, Karin
M and Liu YC: Convergence of Itch-induced ubiquitination with
MEKK1-JNK signaling in Th2 tolerance and airway inflammation. J
Clin Invest. 116:1117–1126. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Campbell JJ, Haraldsen G, Pan J, Rottman
J, Qin S, Ponath P, Andrew DP, Warnke R, Ruffing N, Kassam N, et
al: The chemokine receptor CCR4 in vascular recognition by
cutaneous but not intestinal memory T cells. Nature. 400:776–780.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hijnen D, De Bruin-Weller M, Oosting B,
Lebre C, De Jong E, Bruijnzeel-Koomen C and Knol E: Serum thymus
and activation-regulated chemokine (TARC) and cutaneous T
cell-attracting chemokine (CTACK) levels in allergic diseases: TARC
and CTACK are disease-specific markers for atopic dermatitis. J
Allergy Clin Immunol. 113:334–340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Shimada Y, Takehara K and Sato S: Both Th2
and Th1 chemokines (TARC/CCL17, MDC/CCL22, and Mig/CXCL9) are
elevated in sera from patients with atopic dermatitis. J Dermatol
Sci. 34:201–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cooper KD: Atopic dermatitis: Recent
trends in pathogenesis and therapy. J Invest Dermatol. 102:128–137.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Deshmane SL, Kremlev S, Amini S and Sawaya
BE: Monocyte chemoattractant protein-1 (MCP-1): An overview. J
Interferon Cytokine Res. 29:313–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Homey B, Steinhoff M, Ruzicka T and Leung
DY: Cytokines and chemokines orchestrate atopic skin inflammation.
J Allergy Clin Immunol. 118:178–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Danso MO, van Drongelen V, Mulder A, van
Esch J, Scott H, van Smeden J, El Ghalbzouri A and Bouwstra JA:
TNF-α and Th2 cytokines induce atopic dermatitis-like features on
epidermal differentiation proteins and stratum corneum lipids in
human skin equivalents. J Invest Dermatol. 134:1941–1950. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Pacheco KA, Tarkowski M, Sterritt C, Negri
J, Rosenwasser LJ and Borish L: The influence of diesel exhaust
particles on mononuclear phagocytic cell-derived cytokines: IL-10,
TGF-beta and IL-1 beta. Clin Exp Immunol. 126:374–383. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Luna JD, Chan CC, Derevjanik NL, Mahlow J,
Chiu C, Peng B, Tobe T, Campochiaro PA and Vinores SA:
Blood-retinal barrier (BRB) breakdown in experimental autoimmune
uveoretinitis: Comparison with vascular endothelial growth factor,
tumor necrosis factor alpha, and interleukin-1beta-mediated
breakdown. J Neurosci Res. 49:268–280. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Murata T, Watahiki M, Tanaka Y, Miyase T
and Yoshizaki F: Hyaluronidase inhibitors from Takuran, Lycopus
lucidus. Chem Pharm Bull (Tokyo). 58:394–397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kunz B, Oranje AP, Labreze L, Stalder JF,
Ring J and Taieb A: Clinical validation and guidelines for the
SCORAD index: Consensus report of the European task force on atopic
dermatitis. Dermatology. 195:10–19. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Lee J, Jung E, Koh J, Kim YS and Park D:
Effect of rosmarinic acid on atopic dermatitis. J Dermatol.
35:768–771. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Gendrisch F, Esser PR, Schempp CM and
Wolfle U: Luteolin as a modulator of skin aging and inflammation.
Biofactors. 47:170–180. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kang J, Lee S, Kim N, Dhakal H, Choi YA,
Kwon TK, Khang D and Kim SH: Hispidulin alleviates
2,4-dinitrochlorobenzene and house dust mite extract-induced atopic
dermatitis-like skin inflammation. Biomed Pharmacother.
137:1113592021. View Article : Google Scholar : PubMed/NCBI
|