Mental depression is a common psychiatric disorder,
where ~264 million individuals of different ages suffered from
depressive behavior at January 2020 with ~800,000 annual suicides
(World Health Organization, WHO) (1). Although depression has been
extensively studied, its mechanisms remain to be elucidated.
Norepinephrine and serotonin are two neurotransmitters considered
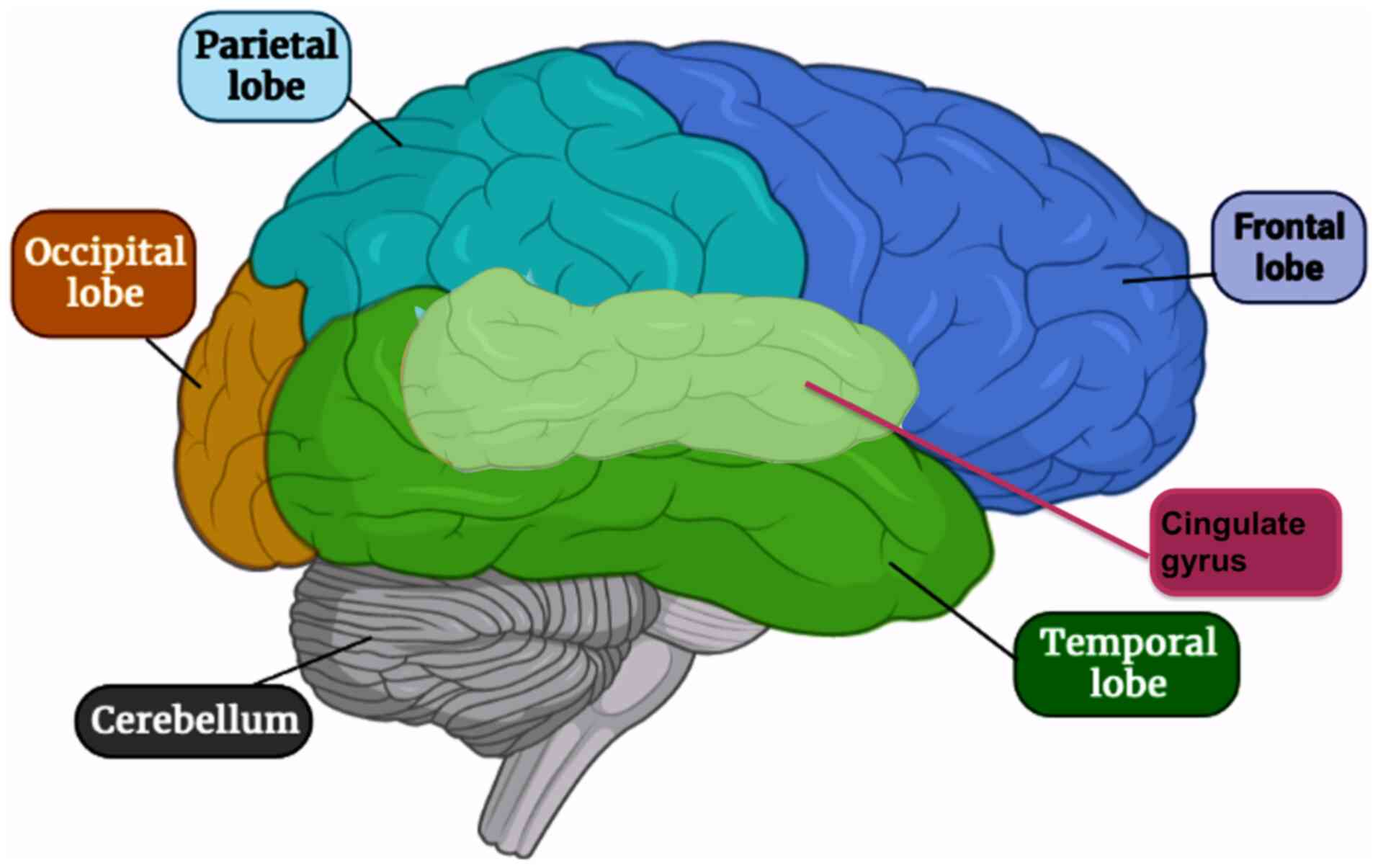
to be the most efficient in mental depression (2). The areas in the brain supposed to be
affected include the temporal and frontal lobes and the cingulate
gyrus of the limbic system (Fig.
1) (3). The symptoms of
depression are mostly mild and unpredicted either by patients or
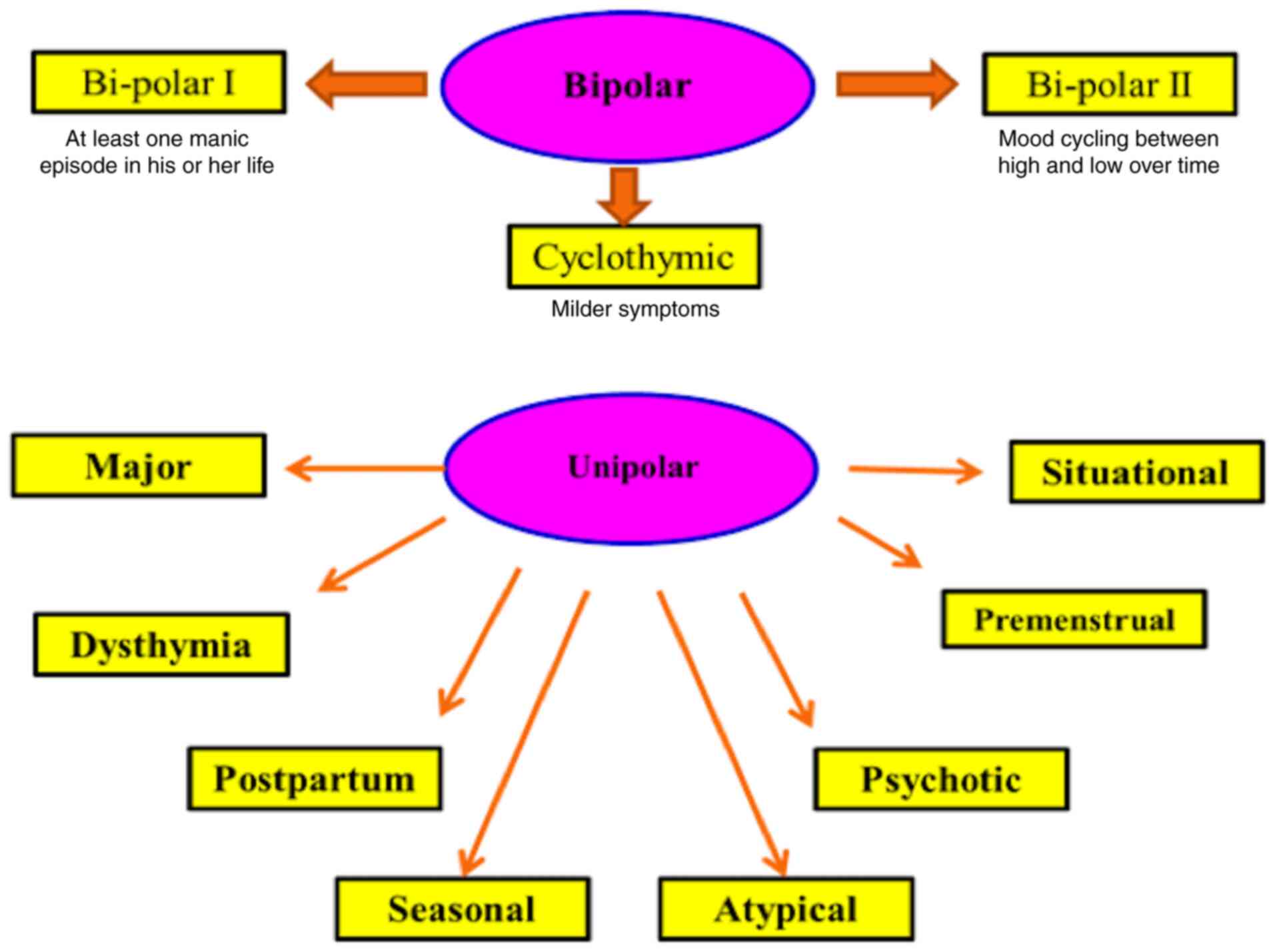
even physicians. The typical types of depression, whether major or
minor, have only symptoms of depression (4). However, bipolar and cyclothymic
types are accompanied by manic symptoms (5). According to the American Psychiatric
Associations of the Diagnostic and Statistical Manual of Mental
Disorders (DSM-5), depressive disorders are classified into three
common types based on a theoretical basis; the first type usually
occurs due to stressful stimuli. The second type is genetically
based and termed endogenous depression; it is demonstrated through
patient difficulties in dealing with normal life (6). The third type is bipolar depression
(or manic-depressive disorder).
By contrast, the International Alliance on Mental
Illness classifies depression into nine common types; Major
Depression: patients with this type feel excessive sadness, lack of
energy, irritability, hopelessness, physical pain, lower
concentration, alterations in eating or sleep habits, feelings of
guilt and attempted suicide. Only two weeks of suffering these
symptoms are needed to officially diagnose major depression
(7). Dysthymia is a type of
depression that causes persistent low mood with changes in sleep
habits, sadness, trouble in concentration, fatigue and appetite
loss. Talk therapy, not medication, is usually suitable for this
type of depression (8).
Postpartum depression (PPD) usually happens after birth where the
patient typically suffers from loneliness, hopelessness, deep
sadness, fatigue, fear about baby disconnection and fears about
harming her newborn (9). Seasonal
Affective Disorder generally occurs in winter, probably due to a
shortage of sunlight. Symptoms are generally mild to severe. This
type usually lasts in spring and natural or artificial light is
usually the suitable treatment (10).
Atypical Depression is commonly associated with
physical symptoms, including a sense of heaviness in the legs or
arms together with being overweight and problems with social
communication (11). Psychotic
Depression is characterized by psychological symptoms including
delusions, and auditory and visual hallucinations (12). In Bipolar Disorder, patients have
a cycle of depression and mania symptoms. It is classified into
bipolar I (where patients have at least one manic episode)
(13); bipolar II (where patients
have hypomania as well as depression) (14) and cyclothymia, a cycle of
depression followed by a cycle of mania (15). Premenstrual Dysphoric Disorder
occurs in females during the last 2 weeks of the menstrual cycle
(16). Situational Depression is
related to certain stressful situations and the symptoms are
unhappiness, anxiety and perhaps major depression (17) (Fig.
2).
Depression can be treated by non-drug or
drug-containing therapy. None-drug treatment includes encouraging
creativity, physical or alternative therapy including exposure to
sunlight, St. John's Wort and acupuncture (18). On the other hand, well-known
antidepressant drugs including selective serotonin reuptake
inhibitors (SSRIs), tricyclic antidepressants (TCAs),
norepinephrine reuptake inhibitors (NRIs), serotonin antagonist and
reuptake inhibitors (SARIs) and serotonin-norepinephrine reuptake
inhibitors (SNRIs) are used (19). However, efforts have been made to
find new antidepressants with high efficacy and faster response,
fewer side effects, able the preserve sexual functions in patients,
improved efficacy, rapid onset and improved safety profile. Mental
health problems resulting from the COVID-19 pandemic comprise
insomnia, anxiety, stress, fear and depressive behavior (20). So far, there have been no data
about the changes in cognitive functioning or emotions from the
direct effects of the virus on the brain. A proportion of the
world's population are in health quarantine and isolated at home
and have lost their jobs, family members, relatives, colleagues, or
friends. Consequently, depression associated with COVID-19 can be
included under a situational or emotional type of depression.
According to the World Health Organization (WHO),
264 million individuals worldwide suffer from major depression.
Almost 800,000 suicides are counted each year and it is the second
leading cause of death among young individuals (21). Adolescents aged between 12 and 17
years exhibit the highest rate of major depressive episodes (11.3%)
followed by young adults between 12 and 20 years old at 9.6%
(22). It has been observed that
adults >50 years of age are less likely to have major depressive
episodes (4.5%) (23). The rate
of moderate to severe depression increased from 23.2 to 41.1% from
2007 to 2018 (24). It is noted
that the prevalence of depression among women is almost twice as
high as that of men (25).
Despite the availability of effective antidepressants with multiple
mechanisms of action, it has been observed that between 76 and 85%
of individuals who belong to developing countries do not receive
any treatment for depression (25,26).
There are no specific biological markers for
depression. Various medical and psychiatric disorders, as well as
certain drugs, have been noted in patients with depressive illness
(Table I) (27).
In all, 25% of patients with a chronic medical
history, including cancer, diabetes, or cardiovascular diseases,
develop depressive disorder without a clear and accurate diagnosis
for depression (28). According
to the DSM-5, the symptoms of major depression are sadness, anxiety
or irritability, feelings of guilt and suicidal thoughts (29). The clinical presentation could be
classified into emotional and physical symptoms. Emotional symptoms
include stress, sadness, anxiety, or feeling guilty, whereas
physical symptoms may include sleep disturbance, appetite changes,
headache, pain, or fatigue (Table
II).
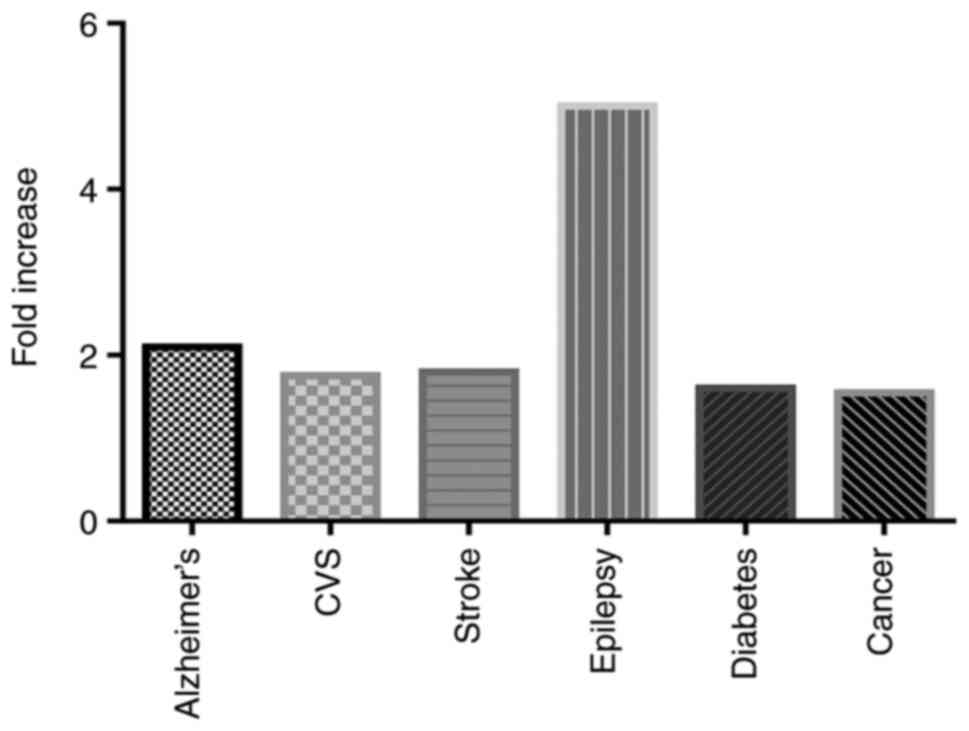
Depression can cause several medical diseases,
including Alzheimer's (by 2.0-fold) (30), cardiovascular diseases
(1.5–2.0-fold) (31), stroke
(1.8-fold) (31), epilepsy
(4.0–6.0-fold) (32), diabetes
(1.6-fold) (33) and cancer
(1.3–1.8-fold) (34) (Fig. 3). Drugs used in controlling
depressive illness have multiple unwanted effects, including
increased weight, sedation, sexual dysfunction, indigestion, or
decreased blood pressure. Incompliance or stopping medication may
cause a relapse of the symptoms of depression and pose a risk of
attempted suicide (35).
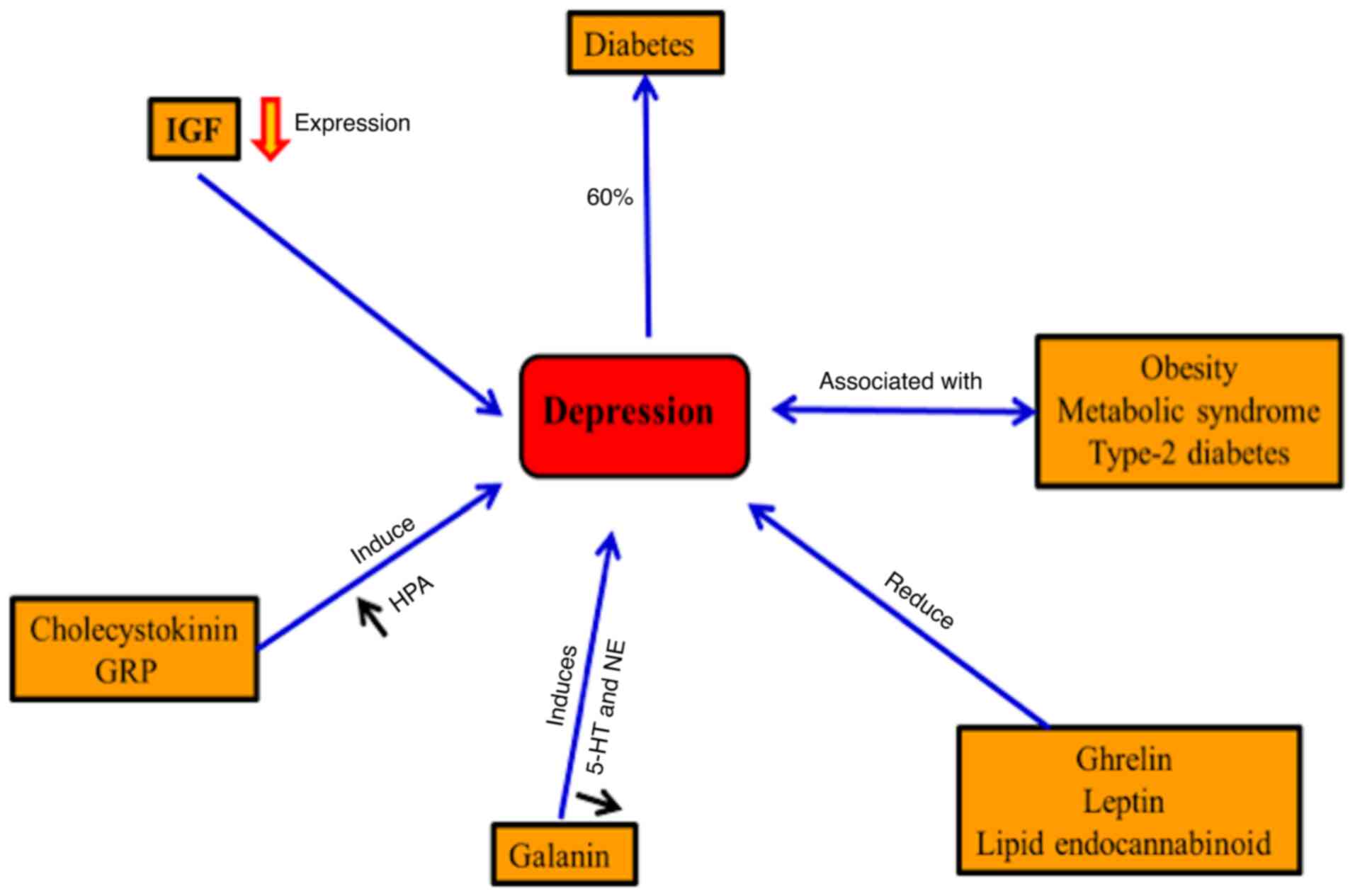
Depression is usually accompanied by metabolic
syndrome, obesity and type-2 diabetes (36,37). In diabetes, ~20.9% of males and
27% of females exhibit major depression in western Kenya (38). By contrast, depression may
increase the risk for diabetes (33). Gastrointestinal hormones,
including ghrelin, leptin and the lipid endocannabinoid, may
regulate mood in the case of depression (39). Improving the depression symptoms
has been accompanied by inhibition of cholecystokinin receptors in
mice and also inhibits the hyperactivity of the
hypothalamic-pituitary-adrenal axis (40). Neuroendocrine, noradrenergic and
serotonergic are controlled by the galanin neuropeptide systems in
depression and anxiety-related behaviors (41). Furthermore, hippocampal
neurogenesis is induced by an insulin-like growth factor (IGF) and
the disturbance of neuronal IGF results in depression (42). Regulation of synaptic plasticity
and improving depressive behavior results from releasing
gastrin-releasing peptide in the hippocampus (43) (Fig.
4).
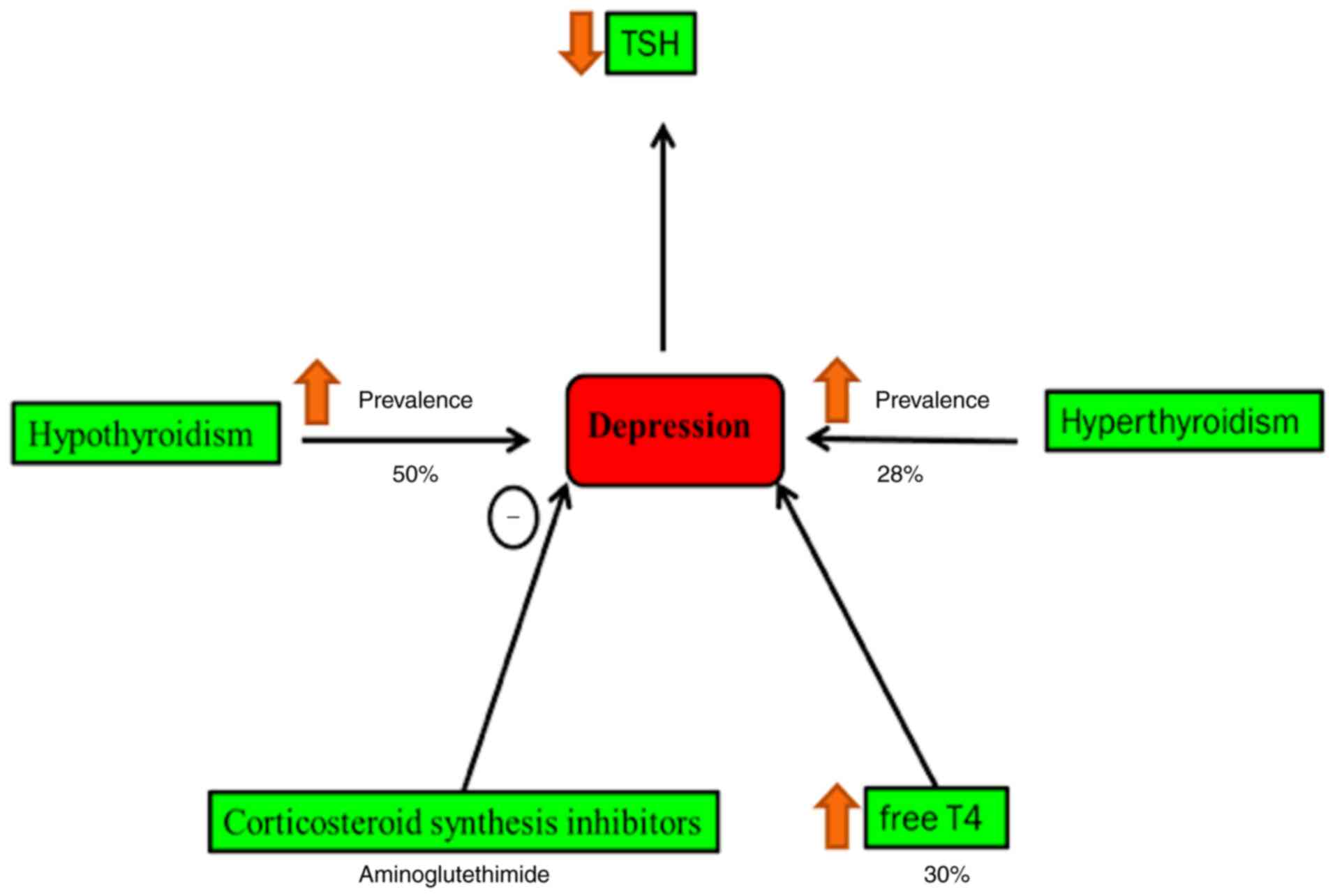
Cushing's syndrome and hypothyroidism are
accompanied by a high rate of psychiatric morbidity (44). Secondary hypothyroidism is
normally characterized by decreased blood level thyroid-stimulating
hormone, which is concomitant with an increased risk of depressive
episodes (45). It is found that
40% of patients with hypothyroidism suffer from clinical depression
(46,47). Hyperthyroidism patients have a
high risk of depression by 28% compared with normal individuals,
whereas it occurs in 50% of all cases of patients with
hypothyroidism (46,48). Blood and cerebrospinal levels of
T4 are relatively increased during the depression (46) (Fig.
5). The raised concentration of corticotropin-releasing factor
in cerebrospinal fluid in patients with depression is noted in a
previous study (49).
Decreased plasma volume and elevation in blood
viscosity lead to a higher risk of depression (50,51). Patients with depression have a
high risk for hypertension, congestive heart failure and myocardial
infarction (52).
Hypercoagulability, platelet activation and D-dimer proteins can be
detected in patients with depression (50,53,54). In clinical trials, the risk of
depression is significantly reduced in cardiovascular disease
patients treated with statins (50,55). It has been demonstrated that
vascular endothelial growth factor has neurogenic/neuroprotective
effects (50,56,57). Furthermore, its level is notably
increased in patients diagnosed with depression (58,59).
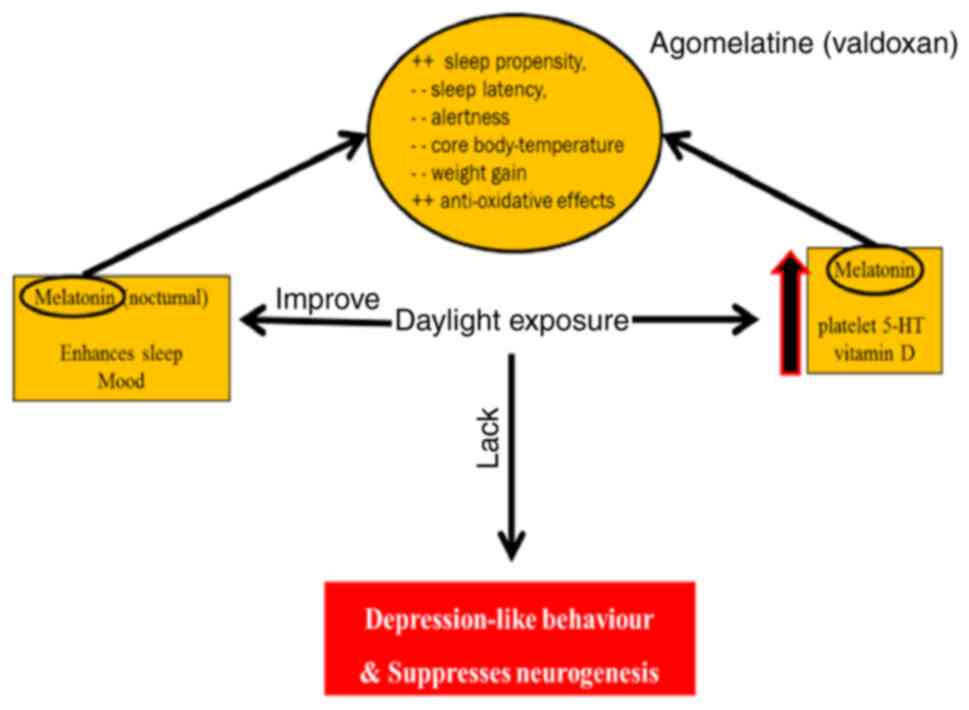
Deficiency of light has been shown to suppress
neurogenesis and increase symptoms of depression (65). Nocturnal melatonin, vitamin D and
platelet 5-HT values are induced by environmental light and improve
depression symptoms (66,67). Melatonin has anti-oxidative
effects, increases sleep propensity and has been known to decrease
alertness and attenuate weight gain (68) (Fig.
7).
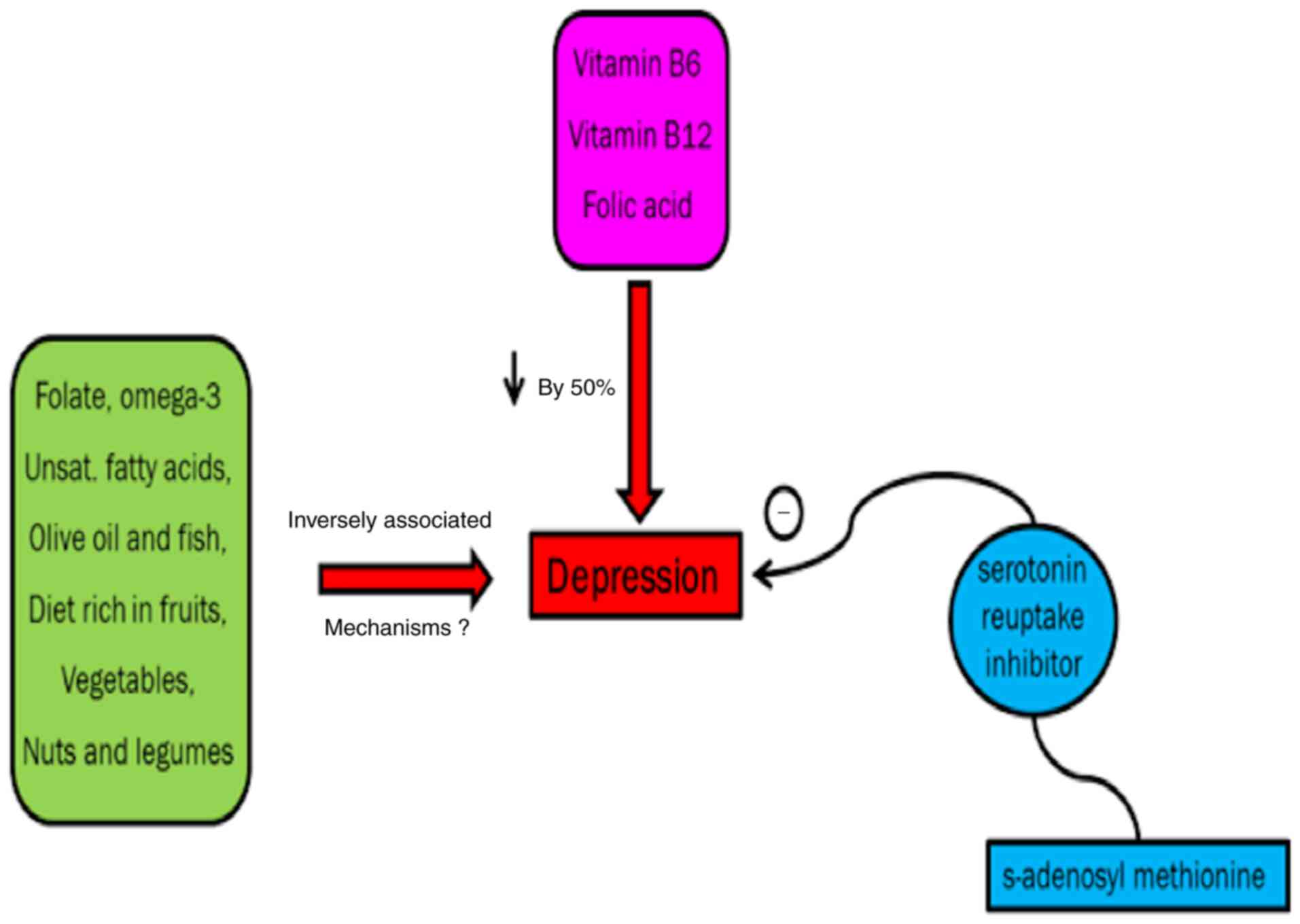
Intake of omega-3 fatty acids, fish, vitamin B6,
vegetables, fruits, folate, olive oil, vitamin B12, nuts and
legumes improves depressive symptoms although the exact mechanism
remains to be elucidated (69,70).
A previous study demonstrates that unipolar
depression can be effectively controlled by folic acid (71). A study conducted on >70
patients showed that major depressive disorder in non-responders to
SSRIs could be improved by s-adenosyl methionine if taken
concurrently with serotonin reuptake inhibitor (72) (Fig.
8).
The ionotropic glutamate receptors
N-methyl-D-aspartate (NMDA),
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid and
metabotropic glutamate receptors (mGluR1 to mGluR8) activation
regulate synaptic plasticity and transmission. Glutamate is cleaned
by high-affinity excitatory amino acid transporters (EAAT) 1 and 2
from extracellular space found in glial cells (73).
Following the conversion of the glial cells
glutamate to glutamine, by glutamine synthetase, glutamine is
hydrolyzed into glutamate via glutaminase (74). In experimental animal models of
depression, drugs that increase glutamate clearance can improve
stress and possess antidepressant effects (75,76).
Ketamine N-methyl-D-aspartate receptor antagonist in
subanesthetic dose (0.5 mg/kg) has a short onset antidepressant
effect (77). Another
rapid-acting antidepressant is dextromethorphan (NMDA receptor
antagonist) (78). The
antimuscarinic drug scopolamine has short onset with a potent
antidepressant effect for bipolar and major depression (79,80). As with NMDA receptor antagonists,
zinc and magnesium have an antidepressant effect in experimental
animal models (81). It has been
stated that magnesium has an antidepressant effect in type II
diabetic patients, compared with imipramine (82).
Long-time stress may negatively affect the process
of neurogenesis and cause neuronal loss in the hippocampus
(83,84). In depressive behaviors, synaptic
plasticity has been revealed to be controlled by brain-derived
neurotrophic factor (BDNF) (85,86). The blood level of BDNF is
decreased in patients with depression and its level is increased by
antidepressant drugs (87,88).
In patients with depression, a decreased level of
vascular growth factor (VGF) has been shown (89) and an antidepressant effect is
produced by treatment with recombinant VGF in an experimental
animal model (90).
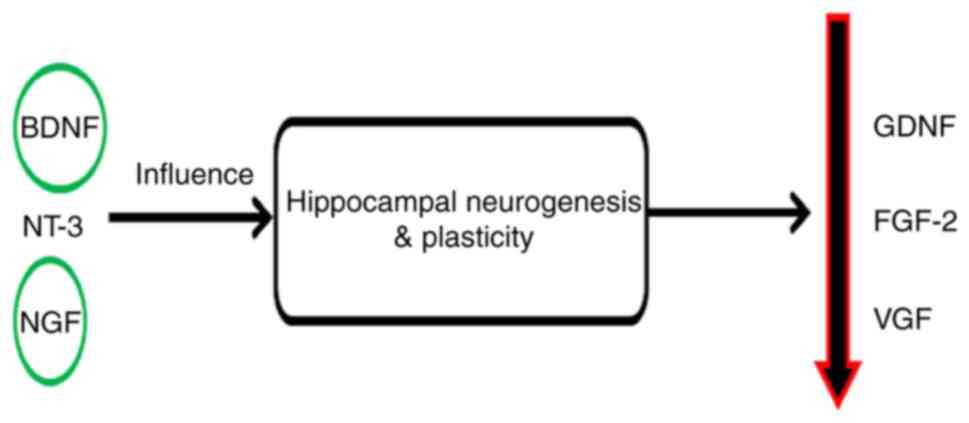
Neurotrophin-3 and nerve growth factor may contribute to the
antidepressant responses by improving plasticity and neurogenesis
in the hippocampus (91,92). Other factors such as glial cell
line-derived neurotrophic factor and fibroblast growth factor-2 are
modulated in depression (58,93) (Fig.
9).
In patients with depression, numerous
enzyme-mediated reactive oxygen species (ROS) production is
increased (94). Superoxide and
hydrogen peroxide are products of oxidation of xanthine mediated by
xanthine oxidase enzyme that has been found to increase in the
thalamus in postmortems of patients with depression (95). Metabolism and oxidative
deamination of serotonin are exaggerated by monoamine oxidase,
which can cause ROS production leading to mitochondrial dysfunction
and neuronal apoptosis (96). In
addition, COX-2 is involved in the inflammatory cascades and its
inhibitors can lead to neuronal inflammation that may worsen
depression (97). Decreased
superoxide dismutase activity is shown in patients diagnosed with
depression (98). Peroxides, such
as hydrogen peroxide catalyzed by glutathione peroxidase, have
lower activity in patients with depression compared to healthy
individuals (99). Microglia are
the main inflammatory neuronal cells that are activated by the
increased levels of superoxide and other ROS (100). This activated cell releases a
number of inflammatory cytokines, mostly TNF-α and IL-6 (101).
It has been demonstrated that an increased level of
nitric oxide (NO) mediates the 3′,5′-cyclic guanosine monophosphate
(cGMP) signaling pathway leading to depressive symptoms; however,
the reduction of NO and/or cGMP results in improvement in
depression-like symptoms in experimental animal models (102). The elevated level of NO may
react with superoxide anions and produce highly reactive
inflammogenic and neurotoxic peroxynitrite and consequently a local
surge in inflammatory cytokines (103). The previous NO effects are
involved in the pathogenesis of major depression (104), Parkinson's disease and
Alzheimer's (103).
There are differences in the cortical gene
expression in patients with schizophrenia, bipolar disorder and
autism including differentiation of the glial cell and metabolism
of fatty acid (105,106). Notably, the pathophysiology of
major depression (MD) involves inflammatory pathways and the
hypothalamic-pituitary axis (107). Another study found low
expression of two genes, ATP binding cassette subfamily G member 2
and prominin 1 in patients with MD (108). Similarly, a low level of mRNA
encoding the gene Fli-1 proto-oncogene, ETS transcription factor
has been found and it serves a role in the growth of the neuronal
crest in patients with depression (109). Reduction in the expression of
SLC1A2 and SLC1A3 genes has been found in the dorsolateral
prefrontal cortex and anterior cingulate of patients with MD and
bipolar disorder (110). A lower
expression of NMDA receptor subunits was found in patients with MD
including PSD-95, NR1, NR2A and NR2B in the anterior prefrontal
cortex (111). GABA
signaling-related genes such as ALDH9A1 and glutamate ammonia
ligase, are changed in patients with depression (112). ALDH9A1, which catalyzes the
conversion of γ-aminobutyraldehyde into GABA, was increased in
depressed subjects, whereas glutamate ammonia ligase, which
increases glutamate brain clearance by its conversion to glutamine,
was decreased in depressed brain. Furthermore, decreased activation
and functions in the gene expression of the extracellular
signal-regulated kinase (ERK) cascade is recorded in MD (113). Several deviations in the gene
expression in MD were found to be gender-specific, occurring mostly
in females. For instance, hub gene Dusp6 (female-specific gene) is
downregulated in female, but not male mice prefrontal cortex
simulated stress by increasing pyramidal neuron excitability and
ERK signaling. This gender-specific feature in MD patients could be
due to changes in the specificity of ventromedial cortex content of
phosphatase 6 in female patients and empty spiracles homeobox 1 in
males (113).
Reading books or journals, gardening, painting and
playing music can offer a passage to drive out the painful thoughts
and bad moods. It is known that creative individuals are at high
risk for depression than non-creative ones (114).
In this, patients with depression are directed to
perform physical exercise. The only challenge facing this strategy
is that patients with depression often suffer from a lack of
energy. However, starting a new physical activity can be
influential in managing such symptoms. Performing physical exercise
is known to produce euphoria by releasing endorphins (115).
Cognitive Behavioral Therapy is a common
psychotherapy strategy used in the treatment of depression. This
strategy directs patients to correct negative non-constructive
thinking (116).
A 40 min exposure to sunlight by patients suffering
from seasonal depression results in a good response (117). This type of therapy may be
helpful in patients suffering from seasonal depression. St. John's
Wort and acupuncture are used and can improve depressive behavioral
symptoms (118).
Most antidepressants employ important activities on
the metabolism of monoamine neurotransmitters and their receptors,
mainly norepinephrine and serotonin (119). The well-known antidepressant
classes include SSRIs, TCAs, NRIs, SARIs, SNRIs and noradrenaline
and dopamine reuptake inhibitors (Table III,Table IV,Table V,Table VI,Table VII,Table VIII,Table IX) (120).
Ansofaxine is developed by Luye Pharma Group for the
treatment of major depression. The American food and drug
administration (FDA) approved the serotonin-norepinephrine-dopamine
triple reuptake inhibitor LY03005 in December 2020 under the name
of Ansofaxine (121). It was
developed under Luye pharma's new chemical and therapeutic entities
research and development platform (122). Triple reuptake inhibitors
encourage a class of antidepressants and are considered to have
high efficacy and faster antidepressant response. They have
relative advantages in preserving patients' sexual functions,
improved efficacy, rapid onset and improved safety profile
(122).
Duloxetine hydrochloride (Cymbalta) is an oral
selective serotonin and norepinephrine reuptake inhibitor. It has
been accepted in the treatment of different mental disorders,
including depressive behavioral illness (123). One important benefit that leads
to the use of duloxetine in the treatment of depression is its
effect in the prevention of suicidal thoughts and attempts
(124). Wu et al
(125) demonstrate that
duloxetine may be effective in the treatment of epileptic patients
with depression. A recent study demonstrated that a spray-dried
complexation-based transdermal patch of duloxetine functions as a
possible new drug delivery system in the treatment of depression
(126).
Vilazodone was approved by the FDA in January 2011
and it is the first and only selective serotonin reuptake inhibitor
and 5-HT1A receptor partial agonist (127). A large dose of vilazodone is
accompanied by acute serotonin syndrome. Some serious uncommon
adverse effects include suicidal thoughts, sexual dysfunction,
manic behavior, serotonin syndrome and hyponatremia (128).
GLYX-13 finalized phase II in 2015 and on January
29, 2016 Allergan company reported that Rapastinel had received
approval from the FDA for treatment of major depression (129). GLYX-13 functions by controlling
the neuronal N-methyl-D-aspartate (NMDA) receptor. Although it is
similar to ketamine receptor antagonists of NMDA, GLYX-13 does not
cause severe adverse effects such as schizophrenic symptoms and
hallucinations effects (130).
Brintellix, used for the treatment of major
depression, is recently available in the United States markets
(131). The antidepressant
mechanism of Brintellix remains to be elucidated. Vortioxetine is a
5-HT1D, 5-HT3 and 5-HT7 receptors antagonist, 5-HT1B partial
receptors agonist and 5-HT1A receptor agonist (132). Plasma concentration of BDNF
shows a significant increase and the concentration of platelet 5-HT
displays significant reduction following Brintellix administration
in patients with major depression (129).
Valdoxan targets the melatonin system in the brain
and is considered a melatonergic drug. It was approved for the
treatment of major depression in Europe in 2009. It has been
recently reported that Agomelatine is effective in PPD treatment
and is safe during breastfeeding (133). Agomelatine works by targeting
the neuronal effects of melatonin, which has an essential role as
an antidepressant. Similar to serotonin, agomelatine appears to be
important in controlling sleep and circadian rhythms (134,135).
It is an anesthetic drug that has an antidepressant
effect but is not yet accepted as an antidepressant by the FDA.
Ketamine acts on a number of central nervous system receptors
responsible for learning and memory. These receptors work on the
excitatory neurotransmitter, glutamate (136), which activates mTORC1 kinase via
inactivation of eukaryotic initiation factor 4E-binding proteins
(137). Intranasal spray of
ketamine was FDA approved as an antidepressant. Finally, the safety
and efficacy of ketamine in depression control requires further
investigation (138).
Levomilnacipran is a rapid onset drug used for
treatment of depression that belongs to a class of SNRIs. There is
no evidence to indicate any relative advantages of levomilnacipran
compared with available SNRIs (139). A recent study demonstrated that
levomilnacipran in older patients with depression has lower
tolerability than in older adults with depression. Levomilnacipran
requires to be more deeply studied to analyze its efficacy and
tolerability in patients with depression (140).
Although it inhibits nerve communication between the
brain areas, like other atypical anti-psychotics, the mechanism of
quetiapine remains to be elucidated. A promising result of SNRI
(Venlafaxine) and Quetiapine co-treatment in patients with
depression has been reported (141). The benefits of Quetiapine may be
due to inhibition of the serotonin type 2 and dopamine type 2
receptors (142). Hazards of
mortality, suicidal thoughts and stroke in the elderly are common
among patients treated with Quetiapine (143).
The outbreak of COVID-19 seriously influences mental
health. The pandemic represents a stressful situation worldwide and
at the time of writing this review, the confirmed cases are
>177,108,695 including 3,840,223 mortalities (WHO; http://covid19.who.int). Mental health problems
resulting from this pandemic include insomnia, anxiety, depressive
behavior, stress and fear (144). In the aftermath of the COVID-19
pandemic, depression and anxiety are common psychological
complaints (145). Lai et
al (146) observed the
mental health status during the COVID-19 pandemic in 1,257 doctors
and other healthcare staff in China. It was found that 71.5% of the
study sample claimed distress, 50.4% depression, 44.6% anxiety and
34.0% insomnia. In addition, a possible correlation between the
symptoms in COVID-19 patients and potential neurological
consequences has been found (147). At present, nothing is known
about the changes in cognitive functioning or emotions from the
direct effects of the virus on the brain. A number of individuals
are in health quarantine and isolated at home and have lost their
jobs, family members, relatives, colleagues, or friends.
Accordingly, depression due to COVID-19 may be included under the
situational or emotional type of depression.
There are a number of drugs under investigation in
different research institutes, universities, or under clinical
trials in hospitals. The investigational drugs include Baricitinib,
interferon-β 1b, Bemcentinib, Bevacizumab, chloroquine phosphate,
Colchicine, Dexamethasone, EIDD-2801, Favipiravir,
Hydroxychloroquine, Azithromycin, anticoagulants, Lopinavir,
Ritonavir and Remdesivir. The current use of some of these drugs in
certain areas is marked with hopes and promises without attention
being payed to their behavioral side effects and lethality. For
example, Baricitinib (Olumiant R which is a Janus kinase inhibitor
now in phase III clinical trials for COVID-19 treatment and has
been used in the treatment of rheumatoid arthritis. Olumiant may
occasionally lead to depression as a side effect (148).
Another drug that has been used recently in the
treatment of COVID-19 is interferon-β 1b. Clinical studies on the
efficacy of interferon I α and interferon β in SARS-CoV had
differing results. Interferon-β 1b may increase the speed of
COVID-19 viral clearance (149).
The most serious side effects of interferon-β-1b in clinical trials
are attempted suicide, depressive behavior and necrosis at the site
of injection (150).
Dexamethasone is a common and widely used synthetic steroid for a
number of diseases, which can reduce mortality of seriously ill
COVID-19 patients although with no improving effect in less serious
cases (151). Fortunately,
Dexamethasone level has previously been shown to possess a good
prognosis for endocrine dysfunction in depression (152).
The United States FDA has approved the use of the
antimalarial drugs chloroquine
(N4-(7-chloro-4-quinolinyl)-N1,N1-diethyl-1,4-pentanediamine) and
hydroxychloroquine sulfate (a derivative of chloroquine) in
COVID-19 treatment regardless of a questionable clinical proof of
their effectiveness (153).
Behavioral changes, delirium and depression have been associated
with chloroquine use (153). One
of the most important points that must be taken into account is the
older population, who are directed to self-isolate for a long time.
This is to protect over-loaded health systems and to diminish the
outbreak of COVID-19. This social isolation not only increases the
risk for neurocognitive, cardiovascular and autoimmune diseases but
also exposes elder populations to a greater risk of depression
(154). Decision makers and
leaders need to realize the resultant mental health changes. In
addition, they must take the situational depression existing at
present into their consideration and give it the care and attention
it deserves.
Alteration in consciousness is one of COVID-19
manifestations and accounts for 20% of patients (155). Furthermore, increased incidence
of psychiatric disorders has been identified during and after the
infection with COVID-19 (156).
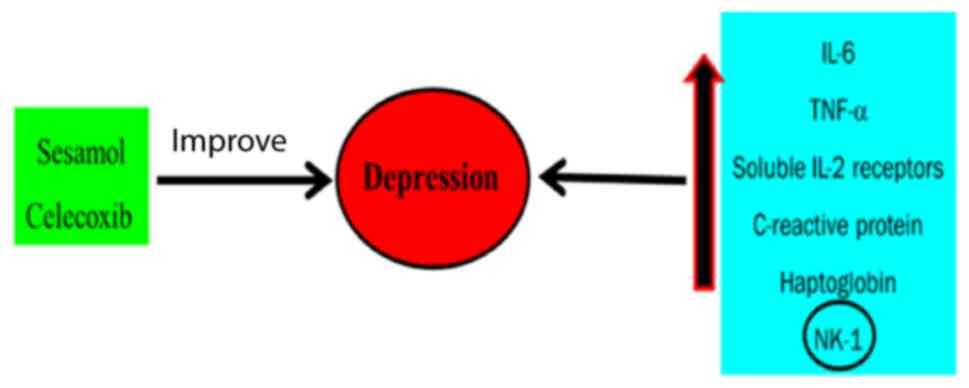
Most of these patients display cytokine storm or cytokine release
syndrome and showed raised plasma levels of pro-inflammatory
cytokines including IL-2R, IL-6, IL-8, IL-10 and TNF-α (157). Depression is known to be an
inflammatory state in which the patients manifested high levels of
inflammatory cytokines, normalized after anti-depressants treatment
(158). In the central nervous
system and the peripheral blood, elevated levels of IL-1β, IL-6,
IL-8, CRP and TNF-α cytokines are identified in patients with
depression (159). Blocking of
cytokines by IL-1β receptor antagonist, Anakinra or IL-6 receptor
antagonist Tocilizumab (160)
has benefits on the inflammation-mediated respiratory failure in
patients with depression. Based on the previous reports, the
present study suggested the protective effect of cytokine blockers
against depression symptoms in COVID-19 patients.
Treatment of depression remains a great public
mental health problem. Depression comprises different types, some
of which are related to events in a person's life while others are
related to the chemical changes in the brain. In some cases,
depression may be considered as a risk factor for certain diseases
(e.g. type 1 or 2 diabetes), and in other cases, certain diseases
(e.g. Alzheimer's and epilepsy) may lead to depressive episodes.
Thus, the relationship between such diseases and depression was
discussed in the present study. In addition, the newer treatments
of depression, such as modulation of glutamatergic, neuronal NMDA
and serotonergic systems and triple reuptake inhibitors, were also
presented. The newer treatments result in a lower risk of suicidal
attempts compared to older treatments. However, they still have
some risks including the possibility of drug abuse. Cooperation
between governmental organizations, researchers, clinicians,
patients and family is crucial in future progress.
Until now, nothing has been known about the direct
effect of COVID-19 on the brain. What health care staff can do is
to treat depression resulting from COVID-19 infection and its
complications, if there are any. The challenge facing health care
staff is to treat the so-called defeat stress resulting from the
consequences of COVID-19 infection. Defeat stress consequences may
not be able to respond to the classical antidepressants which
include maladaptive behaviors (161,162). COVID-19 patients need both
pharmacological treatment and mental care. Mental health personnel
have to be close to COVID-19 patients directly or indirectly
through the internet video media or telephone to soothe or prevent
waves depression or low moods. Decision-makers and officials all
over the world need to know that emotional or situational
depression has to be taken seriously.
Researchers would like to thank the Deanship of
Scientific Research, Qassim University for funding the publication
of this project.
Researchers would like to thank the Deanship of
Scientific Research, Qassim University for funding the publication
of this project.
Not applicable.
MSAB conceived, designed, analyzed and presented
diagrams, wrote and revised the review. AHA and EA constructed
diagrams, wrote and revised the review. TMF wrote and revised the
review. Data authentication is not applicable. All authors read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Averina OV, Zorkina YA, Yunes RA, Kovtun
AS, Ushakova VM, Morozova AY, Kostyuk GP, Danilenko VN and
Chekhonin VP: Bacterial metabolites of human gut microbiota
correlating with depression. Int J Mol Sci. 21:92342020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Delgado PL and Moreno FA: Role of
norepinephrine in depression. J Clin Psychiatry. 61 (Suppl
1):S5–S12. 2000.PubMed/NCBI
|
|
3
|
Zhang FF, Peng W, Sweeney JA, Jia ZY and
Gong QY: Brain structure alterations in depression:
Psychoradiological evidence. CNS Neurosci Ther. 24:994–1003. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fox ME and Lobo MK: The molecular and
cellular mechanisms of depression: A focus on reward circuitry. Mol
Psychiatry. 24:1798–1815. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Muller JK and Leweke FM: Bipolar disorder:
Clinical overview. Med Monatsschr Pharm. 39:363–369.
2016.PubMed/NCBI
|
|
6
|
American Psychiatric Association A and
Association AP, . Diagnostic and Statistical Manual of Mental
Dsorders (DSM-5). American Psychiatric Association; Washington, DC:
2013
|
|
7
|
Assari S: Chronic medical conditions and
major depressive disorder: Differential role of positive religious
coping among African Americans, Caribbean blacks and non-hispanic
whites. Int J Prev Med. 5:405–413. 2014.PubMed/NCBI
|
|
8
|
Devanand DP: Dysthymic disorder in the
elderly population. Int Psychogeriatr. 26:39–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Molyneaux E, Poston L, Ashurst-Williams S
and Howard LM: Obesity and mental disorders during pregnancy and
postpartum: A systematic review and meta-analysis. Obstet Gynecol.
123:857–867. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sanassi LA: Seasonal affective disorder:
Is there light at the end of the tunnel? JAAPA. 27:18–23. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gremaud-Heitz D, Riemenschneider A, Walter
M, Sollberger D, Kuchenhoff J and Dammann G: Comorbid atypical
depression in borderline personality disorder is common and
correlated with anxiety-related psychopathology. Compr Psychiatry.
55:650–656. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Østergaard SD, Meyers BS, Flint AJ,
Mulsant BH, Whyte EM, Ulbricht CM, Bech P and Rothschild AJ;
STOP-PD Study Group, : Measuring psychotic depression. Acta
Psychiatr Scand. 129:211–220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Muralidharan K, Torres IJ, Silveira LE,
Kozicky JM, Bücker J, Fernando N and Yatham LN: Impact of
depressive episodes on cognitive deficits in early bipolar
disorder: Data from the systematic treatment optimization programme
for early mania (STOP-EM). Br J Psychiatry. 205:36–43. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Benti L, Manicavasagar V, Proudfoot J and
Parker G: Identifying early indicators in bipolar disorder: A
qualitative study. Psychiatr Q. 85:143–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harnic D, Pompili M, Innamorati M, Erbuto
D, Lamis DA, Bria P, Girardi P and Janiri L: Affective temperament
and attachment in adulthood in patients with bipolar disorder and
cyclothymia. Compr Psychiatry. 55:999–1006. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Craner JR, Sigmon ST, Martinson AA and
McGillicuddy ML: Premenstrual disorders and rumination. J Clin
Psychol. 70:32–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Simpson T and Ivey J: Pediatric management
problems. Situational depression. Pediatr Nurs. 31:312–313.
2005.PubMed/NCBI
|
|
18
|
Jorm AF, Christensen H, Griffiths KM and
Rodgers B: Effectiveness of complementary and self-help treatments
for depression. Med J Aust. 176:S84–S96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baldwin DS: Unmet needs in the
pharmacological management of depression. Hum Psychopharmacol.
16:S93–S99. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hossain MM, Tasnim S, Sultana A, Faizah F,
Mazumder H, Zou L, McKyer ELJ, Ahmed HU and Ma P: Epidemiology of
mental health problems in COVID-19: A review. F1000Res. 9:6362020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pedersen CB, Mors O, Bertelsen A, Waltoft
BL, Agerbo E, McGrath JJ, Mortensen PB and Eaton WW: A
comprehensive nationwide study of the incidence rate and lifetime
risk for treated mental disorders. JAMA Psychiatry. 71:573–581.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mojtabai R, Olfson M and Han B: National
trends in the prevalence and treatment of depression in adolescents
and young adults. Pediatrics. 138:e201618782016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Substance Abuse and Mental Health Services
Association, . 2018.
|
|
24
|
Duffy ME, Twenge JM and Joiner TE: Trends
in mood and anxiety symptoms and suicide-related outcomes among
U.S. undergraduates, 2007–2018: Evidence from two national surveys.
J Adolesc Health. 65:590–598. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuehner C: Why is depression more common
among women than among men? Lancet Psychiatry. 4:146–158. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang PS, Aguilar-Gaxiola S, Alonso J,
Angermeyer MC, Borges G, Bromet EJ, Bruffaerts R, de Girolamo G, de
Graaf R, Gureje O, et al: Use of mental health services for
anxiety, mood, and substance disorders in 17 countries in the WHO
world mental health surveys. Lancet. 370:841–850. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Katon W and Sullivan MD: Depression and
chronic medical illness. J Clin Psychiatry. 51 (Suppl):S3–S14.
1990.PubMed/NCBI
|
|
28
|
Lebowitz BD, Pearson JL, Schneider LS,
Reynolds CF III, Alexopoulos GS, Bruce ML, Conwell Y, Katz IR,
Meyers BS, Morrison MF, et al: Diagnosis and treatment of
depression in late life. Consensus statement update. JAMA.
278:1186–1190. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Slavich GM and Irwin MR: From stress to
inflammation and major depressive disorder: A social signal
transduction theory of depression. Psychol Bull. 140:774–815. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Green RC, Cupples LA, Kurz A, Auerbach S,
Go R, Sadovnick D, Duara R, Kukull WA, Chui H, Edeki T, et al:
Depression as a risk factor for Alzheimer disease: The MIRAGE
study. Arch Neurol. 60:753–759. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ramasubbu R and Patten SB: Effect of
depression on stroke morbidity and mortality. Can J Psychiatry.
48:250–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hesdorffer DC, Hauser WA, Annegers JF and
Cascino G: Major depression is a risk factor for seizures in older
adults. Ann Neurol. 47:246–249. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nouwen A, Lloyd CE and Pouwer F:
Depression and type 2 diabetes over the lifespan: A meta-analysis.
Response to Mezuk et al. Diabetes Care. 32:e56–e57. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Penninx BW, Guralnik JM, Pahor M, Ferrucci
L, Cerhan JR, Wallace RB and Havlik RJ: Chronically depressed mood
and cancer risk in older persons. J Natl Cancer Inst. 90:1888–1893.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Keller MB, Hirschfeld RM, Demyttenaere K
and Baldwin DS: Optimizing outcomes in depression: Focus on
antidepressant compliance. Int Clin Psychopharmacol. 17:265–271.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bouwman V, Adriaanse MC, van't Riet E,
Snoek FJ, Dekker JM and Nijpels G: Depression, anxiety and glucose
metabolism in the general dutch population: The New Hoorn Study.
PLoS One. 5:e99712010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shelton RC and Miller AH: Eating ourselves
to death (and despair): The contribution of adiposity and
inflammation to depression. Prog Neurobiol. 91:275–299. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shirey K, Manyara SM, Atwoli L, Tomlin R,
Gakinya B, Cheng S, Kamano J, Laktabai J and Pastakia S: Symptoms
of depression among patients attending a diabetes care clinic in
rural western Kenya. J Clin Transl Endocrinol. 2:51–54.
2015.PubMed/NCBI
|
|
39
|
Häfner S, Baumert J, Emeny RT, Lacruz ME,
Thorand B, Herder C, Koenig W, Rupprecht R and Ladwig KH: Sleep
disturbances and depressed mood: A harmful combination associated
with increased leptin levels in women with normal weight. Biol
Psychol. 89:163–169. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Becker C, Zeau B, Rivat C, Blugeot A,
Hamon M and Benoliel JJ: Repeated social defeat-induced
depression-like behavioral and biological alterations in rats:
Involvement of cholecystokinin. Mol Psychiatry. 13:1079–1092. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kozlovsky N, Matar MA, Kaplan Z, Zohar J
and Cohen H: The role of the galaninergic system in modulating
stress-related responses in an animal model of posttraumatic stress
disorder. Biol Psychiatry. 65:383–391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cline BH, Steinbusch HW, Malin D,
Revishchin AV, Pavlova GV, Cespuglio R and Strekalova T: The
neuronal insulin sensitizer dicholine succinate reduces
stress-induced depressive traits and memory deficit: Possible role
of insulin-like growth factor 2. BMC Neurosci. 13:1102012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Roesler R, Henriques JA and Schwartsmann
G: Gastrin-releasing peptide receptor as a molecular target for
psychiatric and neurological disorders. CNS Neurol Disord Drug
Targets. 5:197–204. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Musselman DL and Nemeroff CB: Depression
and endocrine disorders: Focus on the thyroid and adrenal system.
Br J Psychiatry Suppl. 123–128. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Frey A, Lampert A, Dietz K, Striebich S,
Locher C, Fedorenko O, Möhle R, Gallinat J, Lang F and Lang UE:
Thyrotropin serum concentrations in healthy volunteers are
associated with depression-related personality traits.
Neuropsychobiology. 56:123–126. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bahls SC and de Carvalho GA: The relation
between thyroid function and depression: A review. Braz J
Psychiatry. 26:41–49. 2004.(In Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cleare AJ, McGregor A and O'Keane V:
Neuroendocrine evidence for an association between hypothyroidism,
reduced central 5-HT activity and depression. Clin Endocrinol
(Oxf). 43:713–719. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Holtzheimer PE III and Nemeroff CB: Future
prospects in depression research. Dialogues Clin Neurosci.
8:175–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Banki CM, Bissette G, Arato M, O'Connor L
and Nemeroff CB: CSF corticotropin-releasing factor-like
immunoreactivity in depression and schizophrenia. Am J Psychiatry.
144:873–877. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lippi G, Montagnana M, Favaloro EJ and
Franchini M: Mental depression and cardiovascular disease: A
multifaceted, bidirectional association. Semin Thromb Hemost.
35:325–336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tiemeier H, van Dijck W, Hofman A,
Witteman JC, Stijnen T and Breteler MM: Relationship between
atherosclerosis and late-life depression: The rotterdam study. Arch
Gen Psychiatry. 61:369–376. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Nemeroff CB and Goldschmidt-Clermont PJ:
Heartache and heartbreak-the link between depression and
cardiovascular disease. Nat Rev Cardiol. 9:526–539. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Geiser F, Meier C, Wegener I, Imbierowicz
K, Conrad R, Liedtke R, Oldenburg J and Harbrecht U: Association
between anxiety and factors of coagulation and fibrinolysis.
Psychother Psychosom. 77:377–383. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Nemeroff CB and Musselman DL: Are
platelets the link between depression and ischemic heart disease?
Am Heart J. 140 (Suppl 4):S57–S62. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Stafford L and Berk M: The use of statins
after a cardiac intervention is associated with reduced risk of
subsequent depression: Proof of concept for the inflammatory and
oxidative hypotheses of depression? J Clin Psychiatry.
72:1229–1235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jin K, Zhu Y, Sun Y, Mao XO, Xie L and
Greenberg DA: Vascular endothelial growth factor (VEGF) stimulates
neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA.
99:11946–11950. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Warner-Schmidt JL and Duman RS: VEGF is an
essential mediator of the neurogenic and behavioral actions of
antidepressants. Proc Natl Acad Sci USA. 104:4647–4652. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kahl KG, Bens S, Ziegler K, Rudolf S,
Kordon A, Dibbelt L and Schweiger U: Angiogenic factors in patients
with current major depressive disorder comorbid with borderline
personality disorder. Psychoneuroendocrinology. 34:353–357. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Nakataki M, Iga J, Numata S, Yoshimoto E,
Kodera K, Watanabe SY, Song H, Ueno S and Ohmori T: Gene expression
and association analysis of the epithelial membrane protein 1 gene
in major depressive disorder in the Japanese population. Neurosci
Lett. 489:126–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Dantzer R: Cytokine, sickness behavior,
and depression. Immunol Allergy Clin North Am. 29:247–264. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Akhondzadeh S, Jafari S, Raisi F, Nasehi
AA, Ghoreishi A, Salehi B, Mohebbi-Rasa S, Raznahan M and
Kamalipour A: Clinical trial of adjunctive celecoxib treatment in
patients with major depression: A double blind and placebo
controlled trial. Depress Anxiety. 26:607–611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Müller N, Schwarz MJ, Dehning S, Douhe A,
Cerovecki A, Goldstein-Müller B, Spellmann I, Hetzel G, Maino K,
Kleindienst N, et al: The cyclooxygenase-2 inhibitor celecoxib has
therapeutic effects in major depression: Results of a double-blind,
randomized, placebo controlled, add-on pilot study to reboxetine.
Mol Psychiatry. 11:680–684. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Nery FG, Monkul ES, Hatch JP, Fonseca M,
Zunta-Soares GB, Frey BN, Bowden CL and Soares JC: Celecoxib as an
adjunct in the treatment of depressive or mixed episodes of bipolar
disorder: A double-blind, randomized, placebo-controlled study. Hum
Psychopharmacol. 23:87–94. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kumar B, Kuhad A and Chopra K:
Neuropsychopharmacological effect of sesamol in unpredictable
chronic mild stress model of depression: Behavioral and biochemical
evidences. Psychopharmacology (Berl). 214:819–828. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lau BW, Ren C, Yang J, Yan SW, Chang RC,
Pu M and So KF: Light deprivation induces depression-like behavior
and suppresses neurogenesis in diurnal mongolian gerbil (Meriones
unguiculatus). Cell Transplant. 20:871–881. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ljubicić D, Stipcević T, Pivac N,
Jakovljević M and Mück-Seler D: The influence of daylight exposure
on platelet 5-HT levels in patients with major depression and
schizophrenia. J Photochem Photobiol B. 89:63–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ubbenhorst A, Striebich S, Lang F and Lang
UE: Exploring the relationship between vitamin D and basic
personality traits. Psychopharmacology (Berl). 215:733–737. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
LeBourgeois MK, Carskadon MA, Akacem LD,
Simpkin CT, Wright KP Jr, Achermann P and Jenni OG: Circadian phase
and its relationship to nighttime sleep in toddlers. J Biol
Rhythms. 28:322–331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Almeida OP, Marsh K, Alfonso H, Flicker L,
Davis TM and Hankey GJ: B-vitamins reduce the long-term risk of
depression after stroke: The VITATOPS-DEP trial. Ann Neurol.
68:503–510. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sanhueza C, Ryan L and Foxcroft DR: Diet
and the risk of unipolar depression in adults: Systematic review of
cohort studies. J Hum Nutr Diet. 26:56–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lazarou C and Kouta C: The role of nurses
in the prevention and management of obesity. Br J Nurs. 19:641–647.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Papakostas GI, Mischoulon D, Shyu I,
Alpert JE and Fava M: S-adenosyl methionine (SAMe) augmentation of
serotonin reuptake inhibitors for antidepressant nonresponders with
major depressive disorder: A double-blind, randomized clinical
trial. Am J Psychiatry. 167:942–948. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
O'Shea RD: Roles and regulation of
glutamate transporters in the central nervous system. Clin Exp
Pharmacol Physiol. 29:1018–1023. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Erecińska M and Silver IA: Metabolism and
role of glutamate in mammalian brain. Prog Neurobiol. 35:245–296.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Banasr M, Chowdhury GM, Terwilliger R,
Newton SS, Duman RS, Behar KL and Sanacora G: Glial pathology in an
animal model of depression: Reversal of stress-induced cellular,
metabolic and behavioral deficits by the glutamate-modulating drug
riluzole. Mol Psychiatry. 15:501–511. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Gourley SL, Espitia JW, Sanacora G and
Taylor JR: Antidepressant-like properties of oral riluzole and
utility of incentive disengagement models of depression in mice.
Psychopharmacology (Berl). 219:805–814. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Diazgranados N, Ibrahim L, Brutsche NE,
Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z,
Luckenbaugh DA, Salvadore G, et al: A randomized add-on trial of an
N-methyl-D-aspartate antagonist in treatment-resistant bipolar
depression. Arch Gen Psychiatry. 67:793–802. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lauterbach EC: Dextromethorphan as a
potential rapid-acting antidepressant. Med Hypotheses. 76:717–719.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Drevets WC and Furey ML: Replication of
scopolamine's antidepressant efficacy in major depressive disorder:
A randomized, placebo-controlled clinical trial. Biol Psychiatry.
67:432–438. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Furey ML and Drevets WC: Antidepressant
efficacy of the antimuscarinic drug scopolamine: A randomized,
placebo-controlled clinical trial. Arch Gen Psychiatry.
63:1121–1129. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Sawada T and Yokoi K: Effect of zinc
supplementation on mood states in young women: A pilot study. Eur J
Clin Nutr. 64:331–333. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Barragán-Rodríguez L, Rodríguez-Morán M
and Guerrero-Romero F: Efficacy and safety of oral magnesium
supplementation in the treatment of depression in the elderly with
type 2 diabetes: A randomized, equivalent trial. Magnes Res.
21:218–223. 2008.PubMed/NCBI
|
|
83
|
Schmidt HD and Duman RS: The role of
neurotrophic factors in adult hippocampal neurogenesis,
antidepressant treatments and animal models of depressive-like
behavior. Behav Pharmacol. 18:391–418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Schmidt HD, Shelton RC and Duman RS:
Functional biomarkers of depression: Diagnosis, treatment, and
pathophysiology. Neuropsychopharmacology. 36:2375–2394. 2011.
View Article : Google Scholar : PubMed/NCBIPubMed/NCBI
|
|
85
|
Pittenger C and Duman RS: Stress,
depression, and neuroplasticity: A convergence of mechanisms.
Neuropsychopharmacology. 33:88–109. 2008. View Article : Google Scholar : PubMed/NCBIPubMed/NCBI
|
|
86
|
Schinder AF and Poo M: The neurotrophin
hypothesis for synaptic plasticity. Trends Neurosci. 23:639–645.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Aydemir O, Deveci A and Taneli F: The
effect of chronic antidepressant treatment on serum brain-derived
neurotrophic factor levels in depressed patients: A preliminary
study. Prog Neuropsychopharmacol Biol Psychiatry. 29:261–265. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Sen S, Duman R and Sanacora G: Serum
brain-derived neurotrophic factor, depression, and antidepressant
medications: Meta-analyses and implications. Biol Psychiatry.
64:527–532. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Cattaneo A, Sesta A, Calabrese F, Nielsen
G, Riva MA and Gennarelli M: The expression of VGF is reduced in
leukocytes of depressed patients and it is restored by effective
antidepressant treatment. Neuropsychopharmacology. 35:1423–1428.
2010. View Article : Google Scholar : PubMed/NCBIPubMed/NCBI
|
|
90
|
Hunsberger JG, Newton SS, Bennett AH,
Duman CH, Russell DS, Salton SR and Duman RS: Antidepressant
actions of the exercise-regulated gene VGF. Nat Med. 13:1476–1482.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
91
|
Hellweg R, Lang UE, Nagel M and
Baumgartner A: Subchronic treatment with lithium increases nerve
growth factor content in distinct brain regions of adult rats. Mol
Psychiatry. 7:604–608. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
von Richthofen S, Lang UE and Hellweg R:
Effects of different kinds of acute stress on nerve growth factor
content in rat brain. Brain Res. 987:207–213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Rosa AR, Frey BN, Andreazza AC, Ceresér
KM, Cunha AB, Quevedo J, Santin A, Gottfried C, Gonçalves CA, Vieta
E and Kapczinski F: Increased serum glial cell line-derived
neurotrophic factor immunocontent during manic and depressive
episodes in individuals with bipolar disorder. Neurosci Lett.
407:146–150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Scapagnini G, Davinelli S, Drago F, De
Lorenzo A and Oriani G: Antioxidants as antidepressants: Fact or
fiction? CNS Drugs. 26:477–490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Leonard B and Maes M: Mechanistic
explanations how cell-mediated immune activation, inflammation and
oxidative and nitrosative stress pathways and their sequels and
concomitants play a role in the pathophysiology of unipolar
depression. Neurosci Biobehav Rev. 36:764–785. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Lopresti AL, Hood SD and Drummond PD: A
review of lifestyle factors that contribute to important pathways
associated with major depression: Diet, sleep and exercise. J
Affect Disord. 148:12–27. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Galecki P: Oxidative stress in depression.
Systems Biology of Free Radicals and Antioxidants. Laher I:
Springer; Berlin: pp. 2369–2395. 2014, View Article : Google Scholar
|
|
98
|
Lukic I, Mitic M, Djordjevic J, Tatalovic
N, Bozovic N, Soldatovic I, Mihaljevic M, Pavlovic Z, Radojcic MB,
Maric NP and Adzic M: Lymphocyte levels of redox-sensitive
transcription factors and antioxidative enzymes as indicators of
pro-oxidative state in depressive patients. Neuropsychobiology.
70:1–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Maes M, Galecki P, Chang YS and Berk M: A
review on the oxidative and nitrosative stress (O&NS) pathways
in major depression and their possible contribution to the
(neuro)degenerative processes in that illness. Prog
Neuropsychopharmacol Biol Psychiatry. 35:676–692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Halliwell B: Free radicals and
antioxidants-quo vadis? Trends Pharmacol Sci. 32:125–130. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Sperner-Unterweger B, Kohl C and Fuchs D:
Immune changes and neurotransmitters: Possible interactions in
depression? Prog Neuropsychopharmacol Biol Psychiatry. 48:268–276.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Kudlow P, Cha DS, Carvalho AF and McIntyre
RS: Nitric oxide and major depressive disorder: Pathophysiology and
treatment implications. Curr Mol Med. 16:206–215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Steinert JR, Chernova T and Forsythe ID:
Nitric oxide signaling in brain function, dysfunction, and
dementia. Neuroscientist. 16:435–452. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Tobe EH: Mitochondrial dysfunction,
oxidative stress, and major depressive disorder. Neuropsychiatr Dis
Treat. 9:567–573. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Gandal MJ, Haney JR, Parikshak NN, Leppa
V, Ramaswami G, Hartl C, Schork AJ, Appadurai V, Buil A, Werge TM,
et al: Shared molecular neuropathology across major psychiatric
disorders parallels polygenic overlap. Focus (Am Psychiatr Publ).
17:66–72. 2019.PubMed/NCBI
|
|
106
|
Gandal MJ, Haney JR, Parikshak NN, Leppa
V, Ramaswami G, Hartl C, Schork AJ, Appadurai V, Buil A, Werge TM,
et al: Shared molecular neuropathology across major psychiatric
disorders parallels polygenic overlap. Science. 359:693–697. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Dean B: Understanding the role of
inflammatory-related pathways in the pathophysiology and treatment
of psychiatric disorders: Evidence from human peripheral studies
and CNS studies. Int J Neuropsychopharmacol. 14:997–1012. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Akula N, Barb J, Jiang X, Wendland JR,
Choi KH, Sen SK, Hou L, Chen DT, Laje G, Johnson K, et al:
RNA-sequencing of the brain transcriptome implicates dysregulation
of neuroplasticity, circadian rhythms and GTPase binding in bipolar
disorder. Mol Psychiatry. 19:1179–1185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhao Z, Xu J, Chen J, Kim S, Reimers M,
Bacanu SA, Yu H, Liu C, Sun J, Wang Q, et al: Transcriptome
sequencing and genome-wide association analyses reveal lysosomal
function and actin cytoskeleton remodeling in schizophrenia and
bipolar disorder. Mol Psychiatry. 20:563–572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Bernard R, Kerman IA, Thompson RC, Jones
EG, Bunney WE, Barchas JD, Schatzberg AF, Myers RM, Akil H and
Watson SJ: Altered expression of glutamate signaling, growth
factor, and glia genes in the locus coeruleus of patients with
major depression. Mol Psychiatry. 16:634–646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Feyissa AM, Chandran A, Stockmeier CA and
Karolewicz B: Reduced levels of NR2A and NR2B subunits of NMDA
receptor and PSD-95 in the prefrontal cortex in major depression.
Prog Neuropsychopharmacol Biol Psychiatry. 33:70–75. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Kang HJ, Adams DH, Simen A, Simen BB,
Rajkowska G, Stockmeier CA, Overholser JC, Meltzer HY, Jurjus GJ,
Konick LC, et al: Gene expression profiling in postmortem
prefrontal cortex of major depressive disorder. J Neurosci.
27:13329–13340. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Labonté B, Engmann O, Purushothaman I,
Menard C, Wang J, Tan C, Scarpa JR, Moy G, Loh YE, Cahill M, et al:
Sex-specific transcriptional signatures in human depression. Nat
Med. 23:1102–1111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Ando V, Claridge G and Clark K: Psychotic
traits in comedians. Br J Psychiatry. 204:341–345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Heyman E, Gamelin FX, Goekint M,
Piscitelli F, Roelands B, Leclair E, Di Marzo V and Meeusen R:
Intense exercise increases circulating endocannabinoid and BDNF
levels in humans-possible implications for reward and depression.
Psychoneuroendocrinology. 37:844–851. 2012. View Article : Google Scholar : PubMed/NCBIPubMed/NCBI
|
|
116
|
Blanke U and te Wildt B: Depressive
disorders in the context of biographical and historical
changes-experiences within a psychiatric outpatient service.
Psychiatr Prax. 34 (Suppl 3):S269–S272. 2007.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wang SJ and Chen MY: The effects of
sunlight exposure therapy on the improvement of depression and
quality of life in post-stroke patients: A RCT study. Heliyon.
6:e043792020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Bouron A and Lorrain E: Cellular and
molecular effects of the antidepressant hyperforin on brain cells:
Review of the literature. Encephale. 40:108–113. 2014.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Owens MJ, Morgan WN, Plott SJ and Nemeroff
CB: Neurotransmitter receptor and transporter binding profile of
antidepressants and their metabolites. J Pharmacol Exp Ther.
283:1305–1322. 1997.PubMed/NCBI
|
|
120
|
Menkes D, Bosanac P and Castle D:
MAOIs-does the evidence warrant their resurrection? Australas
Psychiatry. 24:371–373. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Dale E, Bang-Andersen B and Sánchez C:
Emerging mechanisms and treatments for depression beyond SSRIs and
SNRIs. Biochem Pharmacol. 95:81–97. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Sharma H, Santra S and Dutta A: Triple
reuptake inhibitors as potential next-generation antidepressants: A
new hope? Future Med Chem. 7:2385–2406. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Cipriani A, Koesters M, Furukawa TA, Nosè
M, Purgato M, Omori IM, Trespidi C and Barbui C: Duloxetine versus
other anti-depressive agents for depression. Cochrane Database Syst
Rev. 10:CD0065332012.PubMed/NCBI
|
|
124
|
Parker L, Huelin R, Khankhel Z, Wasiak R
and Mould J: A systematic review of pharmacoeconomic studies for
pregabalin. Pain Pract. 15:82–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Wu Y, Huang Y, Song M, Zhang Z, Liang Z
and Deng X: Anticonvulsive activity of duloxetine: A new choice for
the epileptic patients with depression. Pak J Pharm Sci.
32:997–1003. 2019.PubMed/NCBI
|
|
126
|
Kumar R, Sinha VR, Dahiya L and Sarwal A:
Transdermal delivery of duloxetine-sulfobutylether-β-cyclodextrin
complex for effective management of depression. Int J Pharm.
594:1201292021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ashby CR Jr, Kehne JH, Bartoszyk GD, Renda
MJ, Athanasiou M, Pierz KA and Seyfried CA: Electrophysiological
evidence for rapid 5-HT1A autoreceptor inhibition by vilazodone, a
5-HT1A receptor partial agonist and 5-HT reuptake inhibitor. Eur J
Pharmacol. 714:359–365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Bathla M and Anjum S: A 12-week
prospective randomized controlled comparative trial of vilazodone
and sertraline in Indian patients with depression. Indian J
Pharmacol. 52:10–15. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Hashimoto K, Malchow B, Falkai P and
Schmitt A: Glutamate modulators as potential therapeutic drugs in
schizophrenia and affective disorders. Eur Arch Psychiatry Clin
Neurosci. 263:367–377. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Burgdorf J, Zhang XL, Weiss C, Matthews E,
Disterhoft JF, Stanton PK and Moskal JR: The N-methyl-D-aspartate
receptor modulator GLYX-13 enhances learning and memory, in young
adult and learning impaired aging rats. Neurobiol Aging.
32:698–706. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Mørk A, Montezinho LP, Miller S,
Trippodi-Murphy C, Plath N, Li Y, Gulinello M and Sanchez C:
Vortioxetine (Lu AA21004), a novel multimodal antidepressant,
enhances memory in rats. Pharmacol Biochem Behav. 105:41–50. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Katona C, Hansen T and Olsen CK: A
randomized, double-blind, placebo-controlled,
duloxetine-referenced, fixed-dose study comparing the efficacy and
safety of Lu AA21004 in elderly patients with major depressive
disorder. Int Clin Psychopharmacol. 27:215–223. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Xiao L: Agomelatine for postpartum
depression and breastfeeding. Ther Adv Psychopharmacol.
11:204512532110221722021. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
de Bodinat C, Guardiola-Lemaitre B, Mocaër
E, Renard P, Muñoz C and Millan MJ: Agomelatine, the first
melatonergic antidepressant: Discovery, characterization and
development. Nat Rev Drug Discov. 9:628–642. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Gumuslu E, Mutlu O, Sunnetci D, Ulak G,
Celikyurt IK, Cine N, Akar F, Savlı H and Erden F: The
antidepressant agomelatine improves memory deterioration and
upregulates CREB and BDNF gene expression levels in unpredictable
chronic mild stress (UCMS)-exposed mice. Drug Target Insights.
8:11–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Diamond PR, Farmery AD, Atkinson S, Haldar
J, Williams N, Cowen PJ, Geddes JR and McShane R: Ketamine
infusions for treatment resistant depression: A series of 28
patients treated weekly or twice weekly in an ECT clinic. J
Psychopharmacol. 28:536–544. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Aguilar-Valles A, De Gregorio D,
Matta-Camacho E, Eslamizade MJ, Khlaifia A, Skaleka A, Lopez-Canul
M, Torres-Berrio A, Bermudez S, Rurak GM, et al: Antidepressant
actions of ketamine engage cell-specific translation via eIF4E.
Nature. 590:315–319. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Corriger A and Pickering G: Ketamine and
depression: A narrative review. Drug Des Devel Ther. 13:3051–3067.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Palmer EC, Binns LN and Carey H:
Levomilnacipran: A new serotonin-norepinephrine reuptake inhibitor
for the treatment of major depressive disorder. Ann Pharmacother.
48:1030–1039. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Krause-Sorio B, Kilpatrick L, Siddarth P,
Ercoli L, Laird KT, Aguilar-Faustino Y, Milillo MM, Narr KL and
Lavretsky H: Cortical thickness increases with levomilnacipran
treatment in a pilot randomised double-blind placebo-controlled
trial in late-life depression. Psychogeriatrics. 20:140–148. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Anthonis E and Sienaert P:
Farmacotherapeutic treatment of psychotic depression: A review.
Tijdschr Psychiatr. 63:358–365. 2021.(In Dutch). PubMed/NCBI
|
|
142
|
Greer TL, Grannemann BD, Chansard M, Karim
AI and Trivedi MH: Dose-dependent changes in cognitive function
with exercise augmentation for major depression: Results from the
TREAD study. Eur Neuropsychopharmacol. 25:248–256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Reutfors J, Brenner P, Brody B, Wray H,
Andersen M and Brandt L: A post-authorization safety study of
quetiapine as antidepressant treatment in Sweden: Nested
case-control analyses of select outcomes. Drug Saf. 43:135–145.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Torales J, O'Higgins M, Castaldelli-Maia
JM and Ventriglio A: The outbreak of COVID-19 coronavirus and its
impact on global mental health. Int J Soc Psychiatry. 66:317–320.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Sensoy B, Gunes A and Ari S: Anxiety and
depression levels in Covid-19 disease and their relation to
hypertension. Clin Exp Hypertens. 43:237–241. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Lai J, Ma S, Wang Y, Cai Z, Hu J, Wei N,
Wu J, Du H, Chen T, Li R, et al: Factors associated with mental
health outcomes among health care workers exposed to coronavirus
disease 2019. JAMA Netw Open. 3:e2039762020. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Mahalakshmi AM, Ray B, Tuladhar S, Bhat A,
Paneyala S, Patteswari D, Sakharkar MK, Hamdan H, Ojcius DM, Bolla
SR, et al: Does COVID-19 contribute to development of neurological
disease? Immun Inflamm Dis. 9:48–58. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Combe B, Balsa A, Sarzi-Puttini P, Tony
HP, de la Torre I, Rogai V, Durand F, Witt S, Zhong J and Dougados
M: Efficacy and safety data based on historical or pre-existing
conditions at baseline for patients with active rheumatoid
arthritis who were treated with baricitinib. Ann Rheum Dis.
78:1135–1138. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Lipworth B, Kuo CR and Chan R: Emerging
pharmacotherapy for COVID-19. J R Coll Physicians Edinb.
50:133–137. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Reder AT, Oger JF, Kappos L, O'Connor P
and Rametta M: Short-term and long-term safety and tolerability of
interferon β-1b in multiple sclerosis. Mult Scler Relat Disord.
3:294–302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Theoharides TC and Conti P: Dexamethasone
for COVID-19? Not so fast. J Biol Regul Homeost Agents.
34:1241–1243. 2020.PubMed/NCBI
|
|
152
|
Menke A, Arloth J, Best J, Namendorf C,
Gerlach T, Czamara D, Lucae S, Dunlop BW, Crowe TM, Garlow SJ, et
al: Time-dependent effects of dexamethasone plasma concentrations
on glucocorticoid receptor challenge tests.
Psychoneuroendocrinology. 69:161–171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Shader RI: COVID-19 and depression. Clin
Ther. 42:962–963. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Armitage R and Nellums LB: COVID-19 and
the consequences of isolating the elderly. Lancet Public Health.
5:e2562020. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Kumar S, Veldhuis A and Malhotra T:
Neuropsychiatric and cognitive sequelae of COVID-19. Front Psychol.
12:5775292021. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Rogers JP, Chesney E, Oliver D, Pollak TA,
McGuire P, Fusar-Poli P, Zandi MS, Lewis G and David AS:
Psychiatric and neuropsychiatric presentations associated with
severe coronavirus infections: A systematic review and
meta-analysis with comparison to the COVID-19 pandemic. Lancet
Psychiatry. 7:611–627. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Liu J, Li S, Liu J, Liang B, Wang X, Wang
H, Li W, Tong Q, Yi J, Zhao L, et al: Longitudinal characteristics
of lymphocyte responses and cytokine profiles in the peripheral
blood of SARS-CoV-2 infected patients. EBioMedicine. 55:1027632020.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Tsamakis K, Mueller C, Tsirigotis P,
Tsiptsios D, Tsamakis C, Charakopoulos E, Charalampous C, Spandidos
DA, Douzenis A, Papageorgiou C, et al: Depression following
graft-versus-host disease in a patient with acute lymphoblastic
leukaemia: A case report. Mol Clin Oncol. 12:208–211.
2020.PubMed/NCBI
|
|
159
|
Cavalli G, De Luca G, Campochiaro C,
Della-Torre E, Ripa M, Canetti D, Oltolini C, Castiglioni B, Tassan
Din C, Boffini N, et al: Interleukin-1 blockade with high-dose
anakinra in patients with COVID-19, acute respiratory distress
syndrome, and hyperinflammation: A retrospective cohort study.
Lancet Rheumatol. 2:e325–e331. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Campochiaro C, Della-Torre E, Cavalli G,
De Luca G, Ripa M, Boffini N, Tomelleri A, Baldissera E,
Rovere-Querini P, Ruggeri A, et al: Efficacy and safety of
tocilizumab in severe COVID-19 patients: A single-centre
retrospective cohort study. Eur J Intern Med. 76:43–49. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Gonda X, Pompili M, Serafini G, Carvalho
AF, Rihmer Z and Dome P: The role of cognitive dysfunction in the
symptoms and remission from depression. Ann Gen Psychiatry.
14:272015. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Martin V, Allaïli N, Euvrard M, Marday T,
Riffaud A, Franc B, Mocaër E, Gabriel C, Fossati P, Lehericy S and
Lanfumey L: Effect of agomelatine on memory deficits and
hippocampal gene expression induced by chronic social defeat stress
in mice. Sci Rep. 8:459072017. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Yamada M and Yasuhara H: Clinical
pharmacology of MAO inhibitors: Safety and future. Neurotoxicology.
25:215–221. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Andrade C: Selective serotonin reuptake
inhibitor drug interactions in patients receiving statins. J Clin
Psychiatry. 75:e95–e99. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Voican CS, Corruble E, Naveau S and
Perlemuter G: Antidepressant-induced liver injury: A review for
clinicians. Am J Psychiatry. 171:404–415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Nelson JC: A review of the efficacy of
serotonergic and noradrenergic reuptake inhibitors for treatment of
major depression. Biol Psychiatry. 46:1301–1308. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Fagiolini A, Comandini A, Catena Dell'Osso
M and Kasper S: Rediscovering trazodone for the treatment of major
depressive disorder. CNS Drugs. 26:1033–1049. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Jensen NH, Rodriguiz RM, Caron MG, Wetsel
WC, Rothman RB and Roth BL: N-desalkylquetiapine, a potent
norepinephrine reuptake inhibitor and partial 5-HT1A agonist, as a
putative mediator of quetiapine's antidepressant activity.
Neuropsychopharmacology. 33:2303–2312. 2008. View Article : Google Scholar : PubMed/NCBIPubMed/NCBI
|
|
169
|
Ketter TA, Jenkins JB, Schroeder DH,
Pazzaglia PJ, Marangell LB, George MS, Callahan AM, Hinton ML, Chao
J and Post RM: Carbamazepine but not valproate induces bupropion
metabolism. J Clin Psychopharmacol. 15:327–333. 1995. View Article : Google Scholar : PubMed/NCBI
|