Introduction
Incident cases of prostate cancer (PCa) have
increased by 3.7-fold from 1990 to 2015, and the numbers continue
to increase with an increase in screening popularity and life
expectancy (1). PCa is the one of
leading cause of cancer-associated mortality in men (2). In 2017, the number of newly
diagnosed cases of PCa was 161,360, which was the first among the
newly diagnosed cancer cases in men, accounting for 19% cancer
cases, and the cases of death were 26,730, which was the
third-leading cause of cancer-related mortality in men, accounting
for 8% (3). By 2020, the new
diagnoses of PCa increase to 191,930 cases, and the number of
deaths was 33,330, becoming the second-leading cause of
cancer-related mortality in men and aggravating the global burden
of PCa (4). The development of
disease severity increases genome changes and molecular complexity,
thereby affecting PCa development and precision therapy (5). It is important to understand the
carcinogenic effects and underlying mechanisms associated with PCa
for accurate detection and to provide intervention approaches.
The role of non-coding RNA in the occurrence,
progression and treatment of PCa has previously been reported
(6). Long non-coding RNAs
(lncRNAs) and microRNAs (miRNAs/miRs) are essential participants in
the field of non-coding RNAs, and they have received great interest
due to their abundance, specific expression, functional role in
diseases and potential clinical applications (7). lncRNAs are >200 bases in length
and transcribed from the genomic intergenic regions, which regulate
the expression of genes at epigenetic, transcriptional and
post-transcriptional levels (8).
For example, LINC00844 inhibits the progression and metastasis of
PCa, whereas HOXD-AS1 and PCA3 promote tumor growth, castration
resistance and chemoresistance (9–11).
lncRNAs act as competitive endogenous RNA (ceRNA) to regulate miRNA
expression (12,13). miRNAs are a type of small
non-coding RNA, 17–22 nucleotides in length. Mature miRNAs suppress
gene expression by recognizing the complementary target site in the
3′-untranslated region (UTR) of the target mRNA (14). miRNAs can also act as activators
or inhibitors of cancer (15).
According to reports, 19,075 lncRNA-miRNA-mRNA regulatory networks
have been screened, and may be involved in the pathogenesis of PCa
(16).
lncRNA small nucleolar RNA host gene 16 (SNHG16) is
a novel oncogene identified in different types of cancer, such as
colorectal cancer, hepatocellular carcinoma and glioma (17–20). A previous study has reported that
SNHG16, which is upregulated in patients with PCa, can promote the
expression of glucose transporter 1 and induce glucose uptake and
cell proliferation (21). SNHG16
regulates glucose metabolism and participates in tumor lipid
metabolism via ceRNA (17).
Glucose and lipid metabolism are the major metabolic pathways of
tumor cells (22). SNHG16
carcinogenic signal may be one of the ways for tumor cells to
obtain energy (17,21,23). SNHG16 also acts as a ceRNA to
drive vascular endothelial cell proliferation, migration, invasion
and vasoformation by the modulating miR-520d-3p/STAT3 axis
(24). Angiogenesis plays a key
role in promoting tumor growth and metastasis (25). SNHG16 also promotes tumor
proliferation and metastasis by directly targeting the
miR-216A-5p/ZEB1 and miR-17-5p/p62 axes (18,19). SNHG16 is considered a central
regulator in diverse biological processes controlling
tumorigenesis, including promoting metabolic energy, growth,
metastasis and chemoresistance (23). A recent study confirmed that
SNHG16 is highly expressed in glioma, and regulates epidermal
growth factor receptor by sponging miR-373-3p via the PI3K/AKT
pathway to exert its carcinogenic function (20). In addition, miR-373-3p expression
is downregulated by testicular nuclear receptor 4, which increases
PCa cell invasion (26); thus,
miR-373 may be a therapeutic target for PCa (27). In choriocarcinoma, upregulated
miR-373-3p expression partly accounts for the downregulation of
transforming growth factor-β receptor type 2 (TGF-β-R2), which
suppresses epithelial-to-mesenchymal transition (EMT) and migration
(28). Dysregulation of miR-373
has been reported in different types of cancer, and it is involved
in nearly all cellular processes, acting as an oncogene or a tumor
suppressor (29). For example,
miR-373-3p is significantly upregulated in tongue squamous cell
carcinoma tissues, and by directly targeting DKK1, it
constitutively activates Wnt/β-catenin signaling, thereby promoting
EMT-induced tumor metastasis (30). However, miR-373 is downregulated
in lung cancer and acts as a tumor suppressor to attenuate cell
proliferation, migration, invasion and mesenchymal phenotype by
targeting interleukin 1 receptor associated kinase 2 and lysosomal
associated membrane protein 1 (31).
TGF-β-R2 is a potential PCa suppressor (32). Deletion of TGF-β-R2 may increase
the stemness capacity of PCa cells and cause rapid tumor
development (33). The aberrant
expression levels of lncRNAs and miRNAs, which contribute to cell
proliferation, metastasis and drug resistance in PCa, are potential
biomarkers and therapeutic targets (34). However, the role of the
SNHG16/miR-373-3p signal axis in regulating the proliferation and
metastasis of PCa cells remains unclear. Thus, the present study
aimed to investigate the function of the SNHG16/miR-373-3p axis in
the progression of PCa.
Materials and methods
Clinical tissue collection
A total of 80 patients with PCa at the Mindong
Hospital Affiliated to Fujian Medical University were enrolled in
the present study between February 2019 and January 2020. PCa tumor
tissues and paired adjacent normal tissues (2 cm away from the
lesion) were collected by resection. All patients, without history
of preoperative radiotherapy or chemotherapy, were confirmed to
have PCa via analysis from three pathologists. The inclusion
criteria were: i) Patients underwent surgical treatment; ii)
pathologically diagnosed as PCa; and iii) no radiotherapy,
chemotherapy and immunotherapy before surgery. Exclusion criteria
were: i) Combined with other malignant tumors; ii) received
radiotherapy and chemotherapy; iii) history of prostate surgery;
and iv) blood system diseases. All specimens were immediately
stored in liquid nitrogen at −80°C until subsequent
experimentation. The pathological type for all patients was
adenocarcinoma. The clinicopathological characteristics of all
patients, including age, tumor-node-metastasis (TNM) stage,
International Society of Urological Pathology (ISUP) grade and
serum prostate-specific antigen (sPSA) were recorded and analyzed
to assess the associations between the in vitro findings and
clinical presentations (35). The
present study was approved by the Ethics Committee of Mindong
Hospital Affiliated to Fujian Medical University [batch no. (2019)
NingMin Medical Ethics approval no. (0110-1)], and performed in
accordance with the Declaration of Helsinki. Written informed
consent was provided by all patients prior to the study start.
Cell culture
The human PCa cell lines, DU-145 (HTB-81), PC-3
(CRL-1435), 22Rv-1 (CRL-2505) and LNCaP (CRL-1740), and the normal
prostate epithelial cell line, RWPE1 (CRL-11609) were purchased
from the American Type Culture Collection. DU-145, 22Rv-1 and LNCaP
cells were maintained in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.). PC-3 cells were cultured in F-12K medium
(American Type Culture Collection). PCa cells were maintained in
RPMI-1640 or F-12K medium supplemented with 10% fetal bovine serum
(PAN Biotech Ltd.), 4 mM L-glutamine (MedChemExpress), 100 U/ml
penicillin (MedChemExpress) and 100 µg/ml streptomycin
(MedChemExpress). RWPE1 cells were cultured in keratinocyte
serum-free medium (Gibco; Thermo Fisher Scientific, Inc.), and the
medium included 0.05 mg/ml extractive from bovine pituitary and 5
ng/ml human recombinant epidermal growth factor. All cells were
incubated at 37°C in a humidified atmosphere of 5%
CO2.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from PCa tissues and cells
using NucleoZOL® (Gene Co., Ltd.; http://www.genetech.com.cn/). Total RNA (10 µg) was
reverse transcribed into cDNA using the RT System kit (Takara Bio,
Inc.) and PCR instrument (Heal Force; http://www.healforce.com/cn/). The reaction conditions
were as follows: 25°C for 5 min, 42°C for 60 min and 75°C for 15
min. The primer sequences used for qPCR were designed and
synthesized by Shanghai GenePharma Co., Ltd. The primer sequences
were resuspended in 250 µl RNase-free water to a final
concentration of 10 µM. qPCR reaction was subsequently performed
using 12.5 µl SYBR-Green qPCR Master Mix (Takara Bio, Inc.), 1 µl
upstream and downstream primers, 2 µl RT product and 8.5 µl RNase
free water. The following thermocycling conditions were used: 40
PCR amplification cycles were performed with initial incubation at
95°C for 10 min and final extension at 72°C for 5 min. Each cycle
comprised denaturation at 95°C for 10 sec, annealing at 60°C for 30
sec and extension at 72°C for 30 sec. The following primer
sequences were used for qPCR: SNHG16 forward,
5′-GTGCCTCAGGAAGTCTTGCC-3′ and reverse,
5′-ATCCAAACAAGTTATCAGCAGCAGCAC-3′; TGF-β-R2 forward,
5′-GTAGCTCTGATGAGTGCAATGAC-3′ and reverse,
5′-CAGATATGGCAACTCCCAGTG-3′; miR-373-3p forward,
5′-GCGGAAGTGCTTCGATTTTG-3′ and reverse, 5′-AGTGCAGGGTCCGAGGTATT-3′;
RT primer,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACACCC-3′; GAPDH
forward, 5′-CACCCACTCCTCCACCTTTGA-3′ and reverse,
5′-TCTCTCTTCCTCTTGTGCTCTTGC-3′; U6 forward,
5′-CTCGCTTCGGCAGCACATATACT-3′ and reverse,
5′-ACGCTTCACGAATTTGCGTGTC-3′; and RT primer,
5′-AAAATATGGAACGCTTCACGAATTTG-3′. Relative expression levels were
calculated using the 2−ΔΔCq method (36). SNHG16 and TGF-β-R2 were normalized
to the internal reference gene GAPDH, while miR-373-3p expression
was normalized to U6.
Cell transfection
Prior to transfection, DU-145 cells were seeded into
24-well plates at a density of 1×105 cells/well.
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for cell transfection. For SNHG16 gene
knockdown experiments, short hairpin (sh)RNA was amplified using
the following primer sequences: shRNA-SNHG16,
5′-GATCCGGATGAGACTTAACTTAAATTCAAGAGATTTAAGTTAAGTCTCATCCTTTTTG-3′,
and shRNA-negative control (NC),
5′-GATCCGTGTAGATGCGTTGTGATATTCAAGAGATATCACAACGCATCTACACTTTTTG-3′
(Thermo Fisher Scientific, Inc.). The product was subsequently
digested using BamhI and EcoRI restriction enzymes
(Fermentas; Thermo Fisher Scientific, Inc.), and the fragment was
inserted into the BamhI/EcoRI sites of the
pLVX-shRNA2-Puro vector (Zolgene Biotechnology Co., Ltd.,
http://www.zolgene.com/). For SNHG16
overexpression, cells were transfected with SNHG16 overexpression
construct (pcDNA3.1-SNHG16) (Zolgene Biotechnology Co., Ltd.), and
the NC was a pcDNA3.1 vector (Invitrogen; Thermo Fisher Scientific,
Inc.). Specifically, the full length of SNHG16 was obtained via PCR
amplification using the following primer sequences: SNHG16 forward,
5′-CGGGATCCCGGCGTTCTTTTCGAGGTCGGCCG-3′ and reverse,
5′-CCCTCGAGGGTGACGGTAGTTTCCCAAGTTTA-3′. The amplified product was
subsequently digested using BamhI and XhoI
restriction enzymes, and the fragment was inserted into the
BamhI/XhoI sites of the pcDNA3.1 vector to obtain the SNHG16
overexpression plasmid. When the DU-145 cells reached 30–50%
confluence, 10 nM pLVX-shRNA2-Puro-NC, pLVX-shRNA2-Puro-SNHG16,
pcDNA3.1 and pcDNA3.1-SNHG16 plasmids were used to transfect cell
at 37°C for 48 h.
Next, miR-373-3p mimic, inhibitor and the respective
NCs were purchased from Shanghai GenePharma, Co., Ltd. The
following primer sequences were used: miR-373-3p mimic,
5′-GAAGUGCUUCGAUUUUGGGGUGU-3′; mimic NC,
5′-UCACAACCUCCUAGAAAGAGUAGA-3′; miR-373-3p inhibitor,
5′-ACACCCCAAAAUCGAAGCACUUC-3′; and inhibitor NC,
5′-UCUACUCUUUCUAGGAGGUUGUGA-3′. When the DU-145 cells reached
30–50% confluence, 10 nM miR-373-3p mimic, inhibitor and the NC
were used to transfect cells, which were cultured in a 5%
CO2 incubator at 37°C for 48 h. After confirming the
transfection efficiency of miR-373-3p mimic and inhibitor,
pLVX-shRNA2-Puro-SNHG16-NC (10 nM) and miR-373-3p inhibitor NC (10
nM), pLVX-shRNA2-Puro-SNHG16 (10 nM), miR-373-3p inhibitor (10 nM),
pLVX-shRNA2-Puro-SNHG16 (10 nM) and miR-373-3p inhibitor (10 nM)
plasmids were used to transfect DU-145 cell for 48 h at 37°C in an
incubator (SANYO Trading Co., Ltd.; http://www.sanyo-si.com/).
Third, The shRNA and shRNA-NC sequences targeting
TGF-β-R2 were synthesized by Shanghai Sangon Biotech, Co., Ltd. The
shRNA targeting TGF-β-R2 was
5′-GGTGGGAACTGCAAGATACATCTGTGCTGTCCATGTATCTTGCAGTTCCCACCTTTTT-3′,
and the shRNA-NC was
5′-GATCCGATCAATACTATTCATCAATTCAAGAGATTGATGAATAGTATTGATCTTTTTG-3′.
The pLVX-shRNA2-Puro vector, EcoRI and BamhI were
used to construct the TGF-β-R2 knockdown and NC plasmids. Then,
miR-373-3p inhibitor NC (10 nM) and pLVX-shRNA2-Puro-TGF-β-R2-NC
(10 nM), pLVX-shRNA2-Puro-TGF-β-R2 (10 nM), miR-373-3p inhibitor
(10 nM), pLVX-shRNA2-Puro-TGF-β-R2 (10 nM) and miR-373-3p inhibitor
(10 nM) were used for transfection at 37°C when the DU-145 cells
reached 30–50% confluence. Subsequent experiments were performed 48
h post-transfection. The experiments were performed in
triplicate.
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation was assessed via the CCK-8 assay
(Dojindo Laboratories, Inc.). DU-145 cell suspensions were
transferred into 96-well plates at a density of 1×104
cells/ml and incubated for 24, 48, 72 and 96 h at 37°C.
Subsequently, 10 µl of CCK-8 reagent was added to each well and
further incubated for 4 h. Absorbance was measured at a wavelength
of 450 nm, using a microplate reader (Perlong; http://ziyeyl.chemdrug.com/sell/).
Apoptosis analysis
Cells were seeded into 24-well plates at a density
of 1×105 cells/well and transfected until they reached
60–70% confluence. After transfecting with plasmid for 48 h, cells
were washed twice with PBS and centrifuged at room temperature and
220 × g for 5 min. Cells were subsequently incubated with 5 µl of
Annexin V-FITC for 15 min and 10 µl of PI for 5 min at room
temperature, in the dark (BD Biosciences), according to the
manufacturer's instructions. Apoptotic cells were subsequently
analyzed via flow cytometry (Becton-Dickinson and Company).
Gap closure assay
Cell migration was assessed via the gap closure
assay. Following transfection with plasmid for 48 h, cells were
seeded into both sides of the Ibidi-cell plug-in (Corning, Inc.) in
24-well plates at a density of 3×105 cells/well. After 24 h of cell
culture, the Ibili-cell plug-in was removed and the culture in
serum-free medium was continued. Following incubation for 24 h at
37°C, cells were observed under a florescence microscope at ×100
magnification (Mshot; http://www.mshot.com.cn/). The gap closure migrated
area was measured using ImageJ software (version number, 1.42;
National Institutes of Health). The formula used was: Gap closure
area (%) = (0 h area-24 h area)/0 h area ×100%.
Transwell assay
Cell invasion was assessed using a Transwell chamber
(8 µm pore size, Corning, Inc.) deposited with Matrigel (BD
Biosciences). The melted Martrigel in a 4°C refrigerator was
diluted in serum-free medium at 1:8. Then, 50 µl diluted Martrigel
was used to coat the upper chamber of Transwell, which was air
dried at 4°C and solidify at 37°C for 30 min. Following
transfection with plasmid for 48 h, cells were resuspended in
serum-free medium and seeded in the upper chamber with
1×105 cells, while complete media (RPMI-1640 medium with
10% fetal bovine serum, 4 mM L-glutamine, 100 U/ml penicillin and
100 µg/ml streptomycin) was plated in the lower chamber. Following
incubation for 24 h at 37°C, cells in the upper membrane were
removed. At room temperature, the invasive cells were fixed with 4%
paraformaldehyde for 30 min, stained with 0.1% crystal violet for
15 min and observed under the florescence microscope at ×100
magnification (MSHOT; http://www.mshot.com.cn/mf52.htm).
Western blotting
Following transfection for 48 h, DU-145 cells were
collected by centrifuging at 220 × g room temperature for 5 min.
RIPA buffer (Takara Bio, Inc,) and protease inhibitor [Roche
Diagnostics (Shanghai) Co., Ltd.] were used for cell lysis. The BCA
assay kit was used to detect protein concentration (Epizyme, Inc.).
Then, 10 µl protein samples were separated via 10% SDS-PAGE,
transferred onto PVDF membranes and blocked with 5% skim milk in 20
mM Tris-HCl, 150 mM NaCl, 0.1% Tween-20 for 1 h at room temperature
The membranes were incubated with primary antibodies against
TGF-β-R2 (cat. no. 41896, 85 kDa), c-Myc (cat. no. 18583, 65 kDa),
E2F4 (cat. no. 40291, 62 kDa), SMAD2 (cat. no. 5339, 60 kDa),
phosphorylated (p)-SMAD2 (cat. no. 3108, 60 kDa), SMAD3 (cat. no.
9523, 52 kDa), p-SMAD3 (cat. no. 9520, 52 kDa) and β-actin (cat.
no. 4970, 45 kDa) overnight at 4°C (all 1:1,000 and Cell Signaling
Technology, Inc.). The membranes were washed twice with PBS and
subsequently incubated with HRP-conjugated Affinipure Goat
Anti-Rabbit IgG(H+L) (ProteinTech Group, Inc., 1:5,000, cat. no.
SA00001-2) for 2 h at room temperature. Protein bands were
visualized using ECL Western blotting reagents (Cytiva), imaged
using Tanon 5200 Biotanon (Tanon Science & Technology Co.,
Ltd.) and analyzed using ImageJ software (version number, 1.42;
National Institutes of Health).
Dual-luciferase reporter assay
According to TargetScan (http://www.targetscan.org/vert_72/, version number,
7.2) and StarBase (http://starbase.sysu.edu.cn/, version no. 2.0)
databases, the fragments from SNHG16 and TGF-β-R2 containing the
predicted miR-373-3p binding sites and its mutant (MUT) sequence
were directly synthesized (Table
SI). The sequences of SNHG16-3′-UTR-wild-type (WT),
SNHG16-3′-UTR-MUT, TGF-β-R2 3′-UTR-WT and TGF-β-R2 3′-UTR-MUT were
synthesized by Shanghai, GenePharma, Co., Ltd., and digested using
XhoI and NotI restriction enzymes (Fermentas; Thermo
Fisher Scientific, Inc.). The 3′-UTR fragments were subsequently
inserted into the XhoI/NotI sites of the psiCHECK-2
vector (Promega Corporation) to obtain luciferase reporter
plasmids.
DU-145 cell suspensions were seeded into 24-well
plates at a density of 1×105 cells/well and incubated for 24 h at
37°C. The psiCHECK-2-SNHG16-WT or psiCHECK-2-SNHG16-MUT (Zolgene
Biotechnology Co., Ltd.; http://www.zolgene.com/) reporter plasmids (20 ng)
were co-transfected with miR-373-3p mimics or mimic NC (10 nM) into
cells, using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) to verify the targeting effect of SNHG16
and miR-373-3p. Similarly, the psiCHECK-2-TGF-β-R2-WT or
psiCHECK-2-TGF-β-R2-MUT reporter plasmids (Zolgene Biotechnology
Co., Ltd.; http://www.zolgene.com/) (20 ng) were
co-transfected with miR-373-3p mimics or mimic NC (10 nM) into
cells, using Lipofectamine® 2000 to verify the targeting
effect of TGF-β-R2 and miR-373-3p. Following transfection for 36 h
at 37°C, cell lysates were prepared using lysis buffer (Promega
Corporation). Firefly and Renilla luciferase activities were
detected via the dual-luciferase reporter assay (Promega
Corporation). Renilla luciferase values were divided by the
Firefly luciferase values to normalize the difference in
transfection efficiency.
Statistical analysis
Statistical analysis was performed using SPSS 20.0
software (IBM Corp.). The experiment was repeated three times, and
data were presented as the mean ± SD. If the data followed the
normal distribution, Student's t-test was used for pairwise
comparison, and one-way ANOVA followed by Tukey's post hoc test was
used to compare differences between multiple groups. If the data
did not follow the normal distribution, Wilcoxon signed-rank test
was used for paired data, while Mann-Whitney U test was used for
unpaired data. Spearman's correlation coefficient analysis was
performed to assess the correlation between SNHG16 and miR-373-3p,
and TGF-β-R2 and miR-373-3p. Patients with PCa were divided into
high expression group (n=40) and low expression group (n=40) based
on the median expression of SNHG16, miR-373-3p and TGF-β-R2. Then,
Pearson's χ2 test and Fisher's exact test were used for
descriptive analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Correlation between the expression
levels of SNHG16, miR-373-3p and TGF-β-R2 and the
clinicopathological characteristics of patients with PCa
Patient characteristics are presented in Table I. According to SNHG16 expression,
80 cases of PCa tissues were divided into: Low SNHG16 expression
(below the median SNHG16 expression, n=40), and high SNHG16
expression (above the median SNHG16 expression, n=40) groups.
According to miR-373-3p expression, PCa tissues were divided into:
Low miR-373-3p expression (below the median, n=40), and high
miR-373-3p expression (above the median, n=40) groups. Grouping
according to TGF-β-R2 gene expression: Low TGF-β-R2 expression
(below the median, n=40) and high TGF-β-R2 expression (above the
median, n=40) groups. The expression levels of SNHG16, miR-373-3p
and TGF-β-R2 had no significant correlations with age (<60 vs.
≥60 years), TNM stage (≤T2 vs. ≥T3) and sPSA (<20 vs. ≥20 ng/ml)
(all P>0.05). However, the expression levels of SNHG16,
miR-373-3p and TGF-β-R2 were significantly correlated with ISUP
grade (≤3 vs. ≥4; P=0.044, P=0.004 and P<0.001,
respectively).
 | Table I.Correlation between the expression
levels of SNHG16, hsa-miR-373-3p and TGF-β-R2 and the
clinicopathological characteristics of patients with prostate
cancer (n=80). |
Table I.
Correlation between the expression
levels of SNHG16, hsa-miR-373-3p and TGF-β-R2 and the
clinicopathological characteristics of patients with prostate
cancer (n=80).
|
|
|
| SNHG16
expression |
| miR-373-3p
expression |
| TGF-β-R2
expression |
|---|
|
|
|
|
|
|
|
|
|
|---|
|
Characteristics | Cases, n (%) | P-value | Low, n | High, n | P-value | Low, n | High, n | P-value | Low, n | High, n |
|---|
| Age, years |
|
|
|
|
|
|
|
|
|
|
|
<60 | 55 (68.75) | 28 | 27 | 0.809 | 26 | 29 | 0.469 | 29 | 26 | 0.469 |
|
≥60 | 25 (31.25) | 12 | 13 |
| 14 | 11 |
| 11 | 14 |
|
| ISUP grade |
|
|
|
|
|
|
|
|
|
|
| ≤3 | 41 (51.25) | 25 | 16 | 0.044a | 14 | 27 | 0.004b | 29 | 12 |
<0.001c |
| ≥4 | 39 (48.75) | 15 | 24 |
| 26 | 13 |
| 11 | 28 |
|
| TNM stage |
|
|
|
|
|
|
|
|
|
|
|
≤T2 | 53 (66.25) | 25 | 28 | 0.478 | 27 | 26 | 0.813 | 25 | 28 | 0.478 |
|
≥T3 | 27 (33.75) | 15 | 12 |
| 13 | 14 |
| 15 | 12 |
|
| sPSA, ng/ml |
|
|
|
|
|
|
|
|
|
|
|
<20 | 11 (13.75) | 7 | 4 | 0.518 | 3 | 8 | 0.193 | 8 | 3 | 0.193 |
|
≥20 | 69 (86.25) | 33 | 36 |
| 37 | 32 |
| 32 | 37 |
|
High SNHG16 expression promotes PCa
development by regulating the TGF-β-R2/SMAD signaling pathway
SNHG16 expression was significantly upregulated in
PCa tissues compared with adjacent normal tissues (P<0.001;
Fig. 1A). Similarly, SNHG16 and
TGF-β-R2 expression levels were upregulated in DU-145 and PC-3
cells compared with RWPE1 cells. However, no significant
differences in expression levels were observed in 22Rv-1 and LNCaP
cells (P>0.05; Fig. S1A).
Notably, miR-373-3p expression was significantly downregulated in
DU-145 and PC-3 cells compared with RWPE1 cells (P<0.05;
Fig. S1A). Thus, SNHG16
expression was knocked down and overexpressed in DU-145 cells. The
expression of SNHG16 in the knockdown group was significantly lower
than that in the sh-NC group, and in the overexpression group it
was significantly increased compared with oe-NC group (P<0.01;
Fig. 1B). The results
demonstrated that overexpression of SNHG16 significantly promoted
cell proliferation (P<0.001; Fig.
1C), invasion (P<0.05; Fig.
1E) and gap closure (P<0.001; Fig. 1F). However, overexpression of
SNHG16 significantly inhibited cell apoptosis (P<0.01; Figs. 1D and S1B). Western blot analysis demonstrated
that overexpression of SNHG16 significantly increased the protein
expression levels of c-Myc, TGFBR2, E2F4, p-SMAD2/SMAD2 and
p-SMAD3/SMAD3, the effects of which were reversed following SNHG16
knockdown (P<0.001; Fig.
1G).
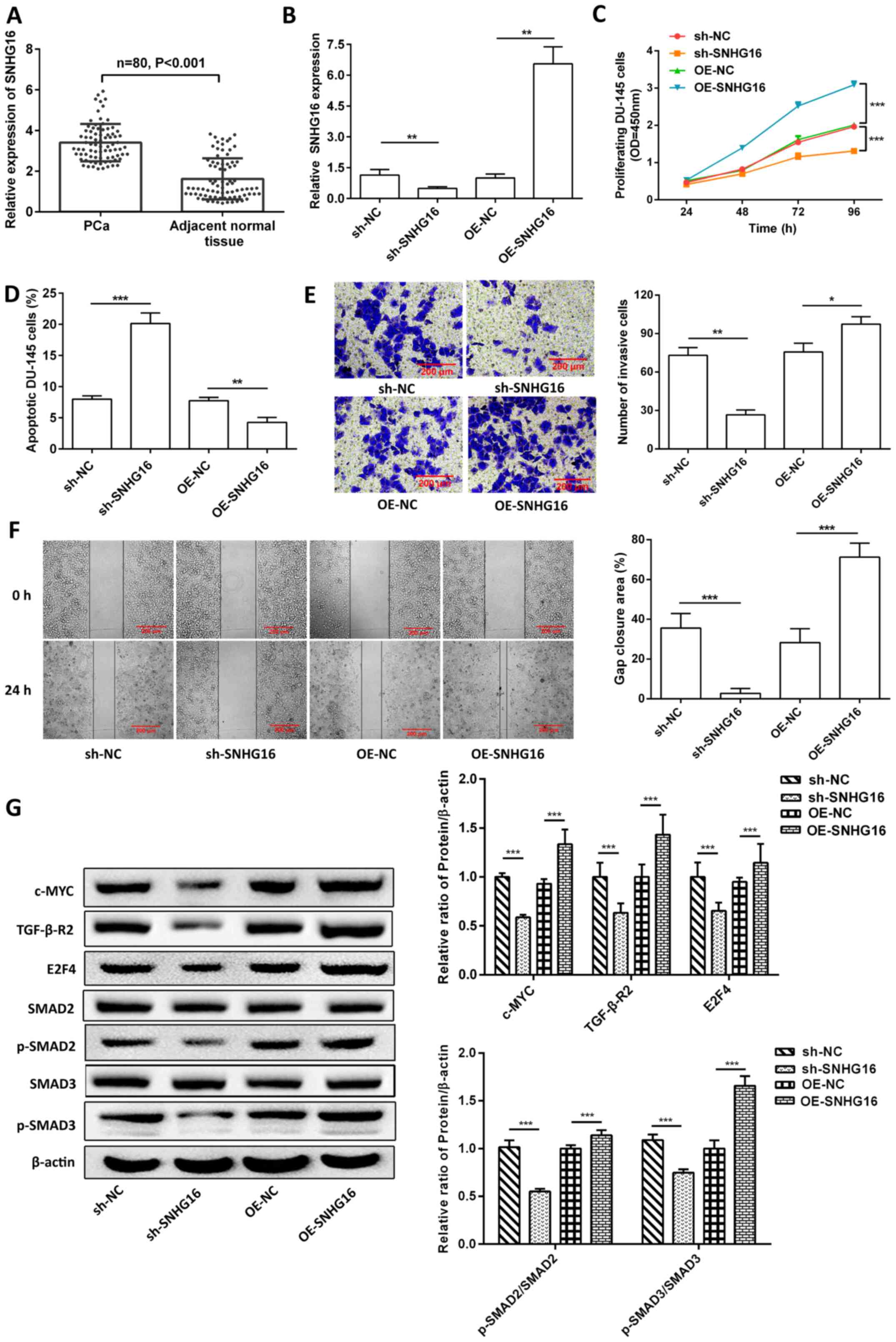 | Figure 1.SNHG16 promotes the tumor process by
regulating TGF-β-R2/SMAD signaling. (A) Reverse
transcription-quantitative PCR analysis was performed to detect
SNHG16 expression in PCa tissues and adjacent normal tissues. (B)
SNHG16 expression was detected following knockdown or
overexpression of SNHG16 in DU-145 cells. SNHG16 regulated (C) cell
proliferation, (D) apoptosis, (E) invasion and (F) migration. (G)
Western blot analysis was performed to detect the protein
expression levels of c-Myc, TGF-β-R2, E2F4, SMAD2, p-SMAD2, SMAD3
and p-SMAD3. Data are presented as the mean ± SD (n=3). *P<0.05;
**P<0.01; ***P<0.001. SNHG16, small nucleolar RNA host gene
16; TGF-β-R2, transforming growth factor-β receptor type 2; PCa,
prostate cancer; p, phosphorylated; sh, short hairpin; NC, negative
control; OE, overexpression; OD, optical density; lncRNA, long
non-coding RNA. |
SNHG16 acts as miR-373-3p sponge to
affect DU-145 cell biological processes by regulating TGF-β-R2/SMAD
signaling
TargetScan and StarBase analyses revealed that a
binding site exists between SNHG16 and miR-373-3p (Fig. 2A). miR-373-3p expression was
significantly downregulated in PCa tissues compared with adjacent
normal tissues (P<0.001; Fig.
2B), and was negatively correlated with SNHG16 expression (rho,
−0.631; P<0.001; Fig. 2C).
DU-145 cells were transfected with miR-373-3p mimics and inhibitor.
The expression of miR-373-3p in mimic group was significantly
increased than NC group, while it was significantly decreased in
inhibitor group. (P<0.001; Fig.
2D). The results of the dual-luciferase reporter assay revealed
that miR-373-3p mimic transfection caused a significant reduction
in the luciferase activity of SNHG16-WT (P<0.01), but not of
SNHG16-MUT (P>0.05; Fig. 2E).
Thus, SNHG16 showed biological binding to miR-373-3p. In addition,
SNHG16 knockdown significantly increased miR-373-3p expression, the
effects of which were reversed following transfection with
miR-373-3p inhibitor (P<0.05; Fig.
2F).
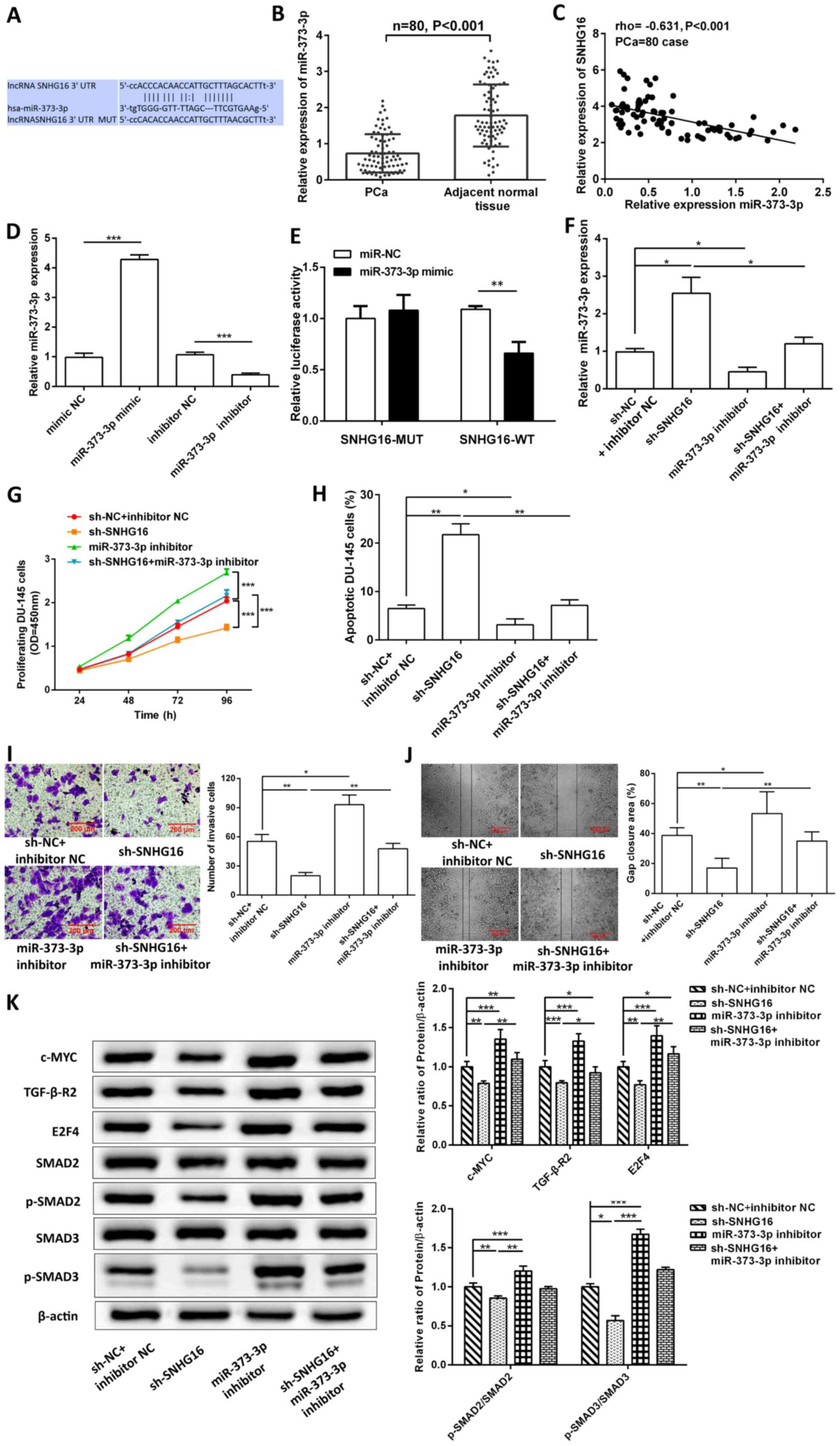 | Figure 2.SNHG16 acts as a miR-373-3p sponge to
affect DU-145 cell biological processes by regulating TGF-β-R2/SMAD
signaling. (A) Binding sites between SNHG16 and miR-373-3p. (B)
Reverse transcription-quantitative PCR analysis was performed to
detect miR-373-3p expression in PCa tissues and adjacent normal
tissues. (C) Spearman's correlation coefficient analysis was
performed to assess the correlation between SNHG16 and miR-373-3p.
(D) miR-373-3p expression was detected following overexpression or
knockdown of miR-373-3p. (E) The dual-luciferase reporter assay was
performed to verify the interaction between miR-373-3p and SNHG16.
Transfection with miR-373-3p inhibitor partially reversed
sh-SNHG16-regulated (F) miR-373-3p expression, (G) cell
proliferation, (H) apoptosis, (I) invasion and (J) migration. (K)
Western blot analysis was performed to detect the protein
expression levels of c-Myc, TGF-β-R2, E2F4, SMAD2, p-SMAD2, SMAD3
and p-SMAD3. Data are presented as the mean ± SD (n=3). *P<0.05;
**P<0.01; ***P<0.001. SNHG16, small nucleolar RNA host gene
16; miR, microRNA; PCa, prostate cancer; TGF-β-R2, transforming
growth factor-β receptor type 2; sh, short hairpin; p,
phosphorylated; N, negative control; MUT, mutant, WT, wild-type;
OD, optical density. |
The results demonstrated that transfection with
miR-373-3p inhibitor significantly improved cell proliferation
(P<0.001; Fig. 2G), and
rescued invasion (P<0.01; Fig.
2I) and migration (P<0.01; Fig. 2J) inhibited by sh-SNHG16, and
decreased sh-SNHG16-induced apoptosis (P<0.01; Figs. 2H and S1C). In addition, transfection with
miR-373-3p inhibitor significantly increased the protein expression
levels of c-Myc, TGFBR2, E2F4, p-SMAD2/SMAD2 and p-SMAD3/SMAD3
(P<0.001), and rescued the inhibition of sh-SNHG16 (Fig. 2K).
miR-373-3p targets TGF-β-R2 to mediate
DU-145 cell biological processes
The binding site between miR-373-3p and TGF-β-R2 is
presented in Fig. 3A. TGF-β-R2
mRNA expression was significantly upregulated in PCa tissues
(P<0.001; Fig. 3B), and was
negatively correlated with miR-373-3p expression (rho, −0.516;
P<0.001; Fig. 3C). Luciferase
activity was significantly reduced in the miR-373-3p mimic and
TGF-β-R2-WT co-transfection group (P<0.001), but not after
TGF-β-R2-MUT co-transfection (P>0.05; Fig. 3D). miR-373-3p showed biological
binding to TGF-β-R2. TGF-β-R2 expression was knocked down in DU-145
cells (Fig. 3E). Notably,
overexpression of miR-373-3p significantly decreased TGF-β-R2 mRNA
expression (P<0.001; Fig. 3F).
miR-373-3p inhibitor partially reversed the effects of TGF-β-R2
knockdown on cell proliferation (P<0.05; Fig. 3G), invasion (P<0.01; Fig. 3I) and migration (P<0.001;
Fig. 3J), and increased
miR-373-3p-inhibitor-suppressed apoptosis (P<0.001; Figs. 3H and S1D).
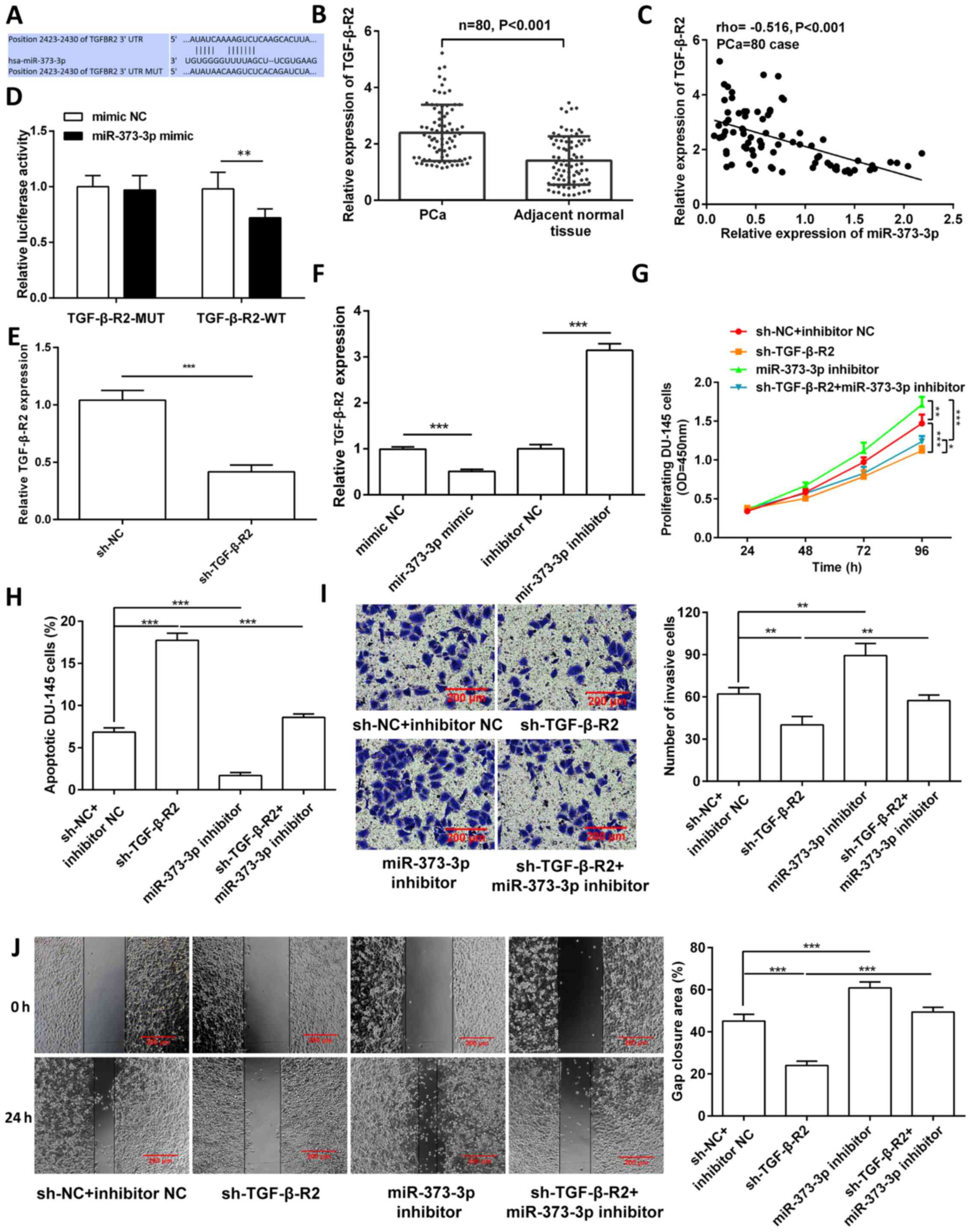 | Figure 3.miR-373-3p targets TGF-β-R2 to
mediate DU-145 cell biological processes. (A) Binding sites between
miR-373-3p and TGF-β-R2. (B) Reverse transcription-quantitative PCR
analysis was performed to detect TGF-β-R2 expression in PCa tissues
and adjacent normal tissues. (C) Spearman's correlation coefficient
analysis was performed to assess the correlation between TGF-β-R2
and miR-373-3p. (D) The dual-luciferase reporter assay was
performed to verify the interaction between TGF-β-R2 and
miR-373-3p. (E) TGF-β-R2 knockdown in DU-145 cells. (F) TGF-β-R2
expression was detected following overexpression or knockdown of
miR-373-3p. TGF-β-R2 knockdown partially reversed the effects of
miR-373-3p inhibitor-regulated (G) cell proliferation, (H)
apoptosis, (I) invasion and (J) migration. Data are presented as
the mean ± SD (n=3). **P<0.01; ***P<0.001. miR, microRNA;
TGF-β-R2, transforming growth factor-β receptor type 2; PCa,
prostate cancer; NC, negative control; MUT, mutant; WT, wild-type;
sh, short hairpin; OD, optical density. |
Discussion
lncRNAs can function as modulators of biological
processes, and act as oncogenes or tumor suppressor genes in PCa
(9–11,37). The results of the present study
confirmed that lncRNA SNHG16 is highly expressed in PCa. It also
acted as a ceRNA to target modulation of miR-373-3p/TGF-β-R2/SMAD
signaling, and exerted a carcinogenic effect. Previous studies have
also shown that SNHG16 was upregulated in PCa tissues and promoted
tumor proliferation (21), and
that it exerted a carcinogenic function by sponging miR-373-3p in
glioma (20). miR-373 was
decreased in PCa (27), and
targeting TGF-β-R2/p-SMAD3 signals inhibited PCa cell invasion and
migration (26). To summarize,
the current results were consistent with those of previous
research.
SNHG16 has been reported to act as a tumor activator
in neuroblastoma (38). The
results of the present study demonstrated that SNHG16 was
abnormally expressed at high levels in PCa. SNHG16 also promoted
tumor cell proliferation, migration and invasion. High SNHG16
expression induces PCa cell proliferation by promoting glucose
metabolism (21). SNHG16 is also
a prognostic indicator of gastric cancer, and its overexpression is
significantly associated with tumor invasion depth, lymph node
metastasis, TNM stage and histological differentiation (39). In ovarian cancer cell lines,
overexpression of SNHG16 is essential for tumor diagnosis and
development (40). SNHG16
expression is upregulated in several malignancies and associated
with tumor cell lines (41).
However, reports of SNHG16 expression in colorectal cancer and
hepatocellular carcinoma were conflicting (42,43). High-quality RNA and tumor cell
specific gravity may explain the inconsistencies of SNHG16
expression (17). The results of
the present study confirmed the oncogenic effect of SNHG16 in PCa
cells. Notably, SNHG16 promoted PCa cell proliferation by
regulating TGF-β-R2/SMAD signaling. Furthermore, SNHG16 upregulated
TGF-β-R2 expression and promoted PCa cell viability. TGF-β-R2/SMAD
signaling plays a crucial role in regulating the proliferation,
differentiation and metastasis of PCa cells (32). TGF-β-R2 promotes EMT, migration
and invasion of PCa cells (44,45).
SMAD2/3 interacts with PKCε, causing SMAD3 to bind
to the promoter of glycolysis genes, inducing glycolysis gene
expression and promoting aerobic glycolysis and PCa cell
proliferation (46). However,
SMAD3 or TGF-β-R2 significantly reduce the mass and microvascular
density of PCa xenograft tumors (47). In addition, SMAD2/3 activates
Rb/E2F4 and upregulates survivin to induce PCa progression and
chemotherapy resistance (48).
E2F4, which were upregulated in PCa cells, promotes cell cycle by
forming complexes with P130 (49,50). c-Myc, a proto-oncogene, plays a
key role in cell cycle progression, apoptosis and cellular
transformation (51). Cancer stem
cell-derived exosome-SNHG16 promotes cancer progression by
activating TLR7/MyD88/NF-κB/c-Myc signaling (52). High c-Myc expression can promote
PCa development by inducing the transcription of the androgen
receptor gene (53). Taken
together, these findings suggest that SNHG16 increases the
expression levels of c-Myc, TGF-β-R2, E2F4, p-SMAD2 and p-SMAD3,
and this development is an essential mechanism for its carcinogenic
effects.
Increasing evidence suggest that SNHG16 can function
as a ceRNA by sequestering miR-373-3p in glioma (20). The results of the present study
demonstrated that low miR-373-3p expression was negatively
correlated with SNHG16, and SNHG16 acted as a miR-373-3p sponge to
promote PCa progression by upregulating TGF-β-R2/SMAD signaling.
Pang et al (27) reported
that miR-373 expression is downregulated in PCa cell lines and
tissues, which suppresses the TGF-β-R2/p-SMAD3 pathway to inhibit
the invasion of PCa C4-2, PC3 and CWR22Rv1 cells (26). Overexpression of miR-373 decreases
the invasion, migration and EMT potential of PCa cells (27). Thus, miR-373-3p may be used as a
promising biomarker for PCa. In addition, the carcinogenic effect
of miR-373-3p has been confirmed in several tumors. For example,
miR-373 suppresses the progression, metastasis and inflammation of
breast cancer by inhibiting NF-κB and the TGF-β/TGF-β-R2/SMAD
pathway (54). Furthermore,
miR-373 inhibits the invasion and peritoneal dissemination of
pancreatic cancer cells by suppressing the EMT process (55). However, miR-373 acted as a
carcinogen in testicular germ cell carcinoma by regulating p53/CDK
(56). Thus, miR-373-3p has a
dual function as a promoter or suppressor in different types of
cancer. miRNAs are single-stranded non-coding RNAs that regulate
gene expression through a conservative mechanism across metazoans
(57). Furthermore, miR-373-3p
directly targets TGF-β-R2 3′-UTR to regulate PCa cell proliferation
and metastasis (26); thus,
TGF-β-R2 is an important cancer driver (58,59). Downregulation of TGF-β-R2 inhibits
the expression of SMAD-dependent metastasis-promoting genes and
invasion (54). High expression
of TGF-β-R2 enhances PCa cell proliferation by promoting
p-SMAD2/3/c-MYC signaling (60).
TGF-β-R2 is associated with NF-κB and TGF-β signals to drive the
progression, EMT and metastasis of castration-resistant PCa
(61).
In conclusion, the results of the present study
demonstrated that SNHG16, miR-373-3p and TGF-β-R2 are important
factors in PCa pathogenesis. SNHG16 promotes the proliferation,
migration and invasion of PCa cell by sponging miR-373-3p to
regulate the TGF-β-R2/SMAD pathway. Thus, targeting SNHG16 may be
an effective strategy for the treatment of PCa.
The present study is not without limitations. First,
although the expression levels of SNHG16, miR-373-3p and TGF-β-R2
were analyzed in RWPE1, DU-145, PC-3, 22Rv-1 and LNCaP cells, the
contribution of SNHG16/miR-373-3p/TGF-β-R2 signaling to
tumorigenesis was verified only in DU-145 cells. Thus, prospective
studies will aim to analyze the role of SNHG16/miR-373-3p/TGF-β-R2
signaling in other PCa cell lines. Secondly, the present study
failed to construct an animal model to verify the role of SNHG16 in
PCa. Thus, additional animal experiments are required to determine
whether SNHG16 acts as a ceRNA to regulate the miR-373-3p/TGF-β-R2
axis, and that it affects the biological functions of PCa
cells.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Fujian province (grant no. 2018J01220).
Availability of data and materials
All datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
WW and CL made substantial contributions to the
conception and design of the present study. WW, CL, GL, QR, HL, NL
and GC made substantial contributions to the acquisition, analysis
and interpretation of the data. WW and CL confirm the authenticity
of all the raw data. WW participated in drafting the initial
manuscript, and CL critically revised the manuscript for important
intellectual content. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Mindong Hospital Affiliated to Fujian Medical
University [batch no. (2019) NingMin Medical Ethics approval no
(0110-1)], and performed in accordance with the Declaration of
Helsinki. Written informed consent was provided by all patients
prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pishgar F, Ebrahimi H, Saeedi Moghaddam S,
Fitzmaurice C and Amini E: Global, regional and national burden of
prostate cancer, 1990 to 2015: Results from the global burden of
Disease Study 2015. J Urol. 199:1224–1232. 2018. View Article : Google Scholar
|
|
2
|
Litwin MS and Tan HJ: The diagnosis and
treatment of prostate cancer: A review. JAMA. 317:2532–2542. 2017.
View Article : Google Scholar
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar
|
|
5
|
Arora K and Barbieri CE: Molecular
subtypes of prostate cancer. Curr Oncol Rep. 20:582018. View Article : Google Scholar
|
|
6
|
Hua JT, Chen S and He HH: Landscape of
noncoding RNA in prostate cancer. Trends Genet. 35:840–851. 2019.
View Article : Google Scholar
|
|
7
|
Dragomir MP, Kopetz S, Ajani JA and Calin
GA: Non-coding RNAs in GI cancers: From cancer hallmarks to
clinical utility. Gut. 69:748–763. 2020. View Article : Google Scholar
|
|
8
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar
|
|
9
|
Lingadahalli S, Jadhao S, Sung YY, Chen M,
Hu L, Chen X and Cheung E: Novel lncRNA LINC00844 regulates
prostate cancer cell migration and invasion through AR signaling.
Mol Cancer Res. 16:1865–1878. 2018. View Article : Google Scholar
|
|
10
|
Gu P, Chen X, Xie R, Han J, Xie W, Wang B,
Dong W, Chen C, Yang M, Jiang J, et al: lncRNA HOXD-AS1 regulates
proliferation and chemo-resistance of castration-resistant prostate
cancer via recruiting WDR5. Mol Ther. 25:1959–1973. 2017.
View Article : Google Scholar
|
|
11
|
Salameh A, Lee AK, Cardó-Vila M, Nunes DN,
Efstathiou E, Staquicini FI, Dobroff AS, Marchiò S, Navone NM,
Hosoya H, et al: PRUNE2 is a human prostate cancer suppressor
regulated by the intronic long noncoding RNA PCA3. Proc Natl Acad
Sci USA. 112:8403–8408. 2015. View Article : Google Scholar
|
|
12
|
Liao W and Zhang Y: MicroRNA-381
facilitates autophagy and apoptosis in prostate cancer cells via
inhibiting the RELN-mediated PI3K/AKT/mTOR signaling pathway. Life
Sci. 254:1176722020. View Article : Google Scholar
|
|
13
|
Bhatia V, Yadav A, Tiwari R, Nigam S, Goel
S, Carskadon S, Gupta N, Goel A, Palanisamy N and Ateeq B:
Epigenetic silencing of miRNA-338-5p and miRNA-421 drives
SPINK1-positive prostate cancer. Clin Cancer Res. 25:2755–2768.
2019.
|
|
14
|
Lee YS and Dutta A: MicroRNAs in cancer.
Annu Rev Pathol. 4:199–227. 2009. View Article : Google Scholar
|
|
15
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar
|
|
16
|
He JH, Han ZP, Zou MX, Wang L, Lv YB, Zhou
JB, Cao MR and Li YG: Analyzing the LncRNA, miRNA, and mRNA
regulatory network in prostate cancer with bioinformatics software.
J Comput Biol. 25:146–157. 2018. View Article : Google Scholar
|
|
17
|
Christensen LL, True K, Hamilton MP,
Nielsen MM, Damas ND, Damgaard CK, Ongen H, Dermitzakis E, Bramsen
JB, Pedersen JS, et al: SNHG16 is regulated by the Wnt pathway in
colorectal cancer and affects genes involved in lipid metabolism.
Mol Oncol. 10:1266–1282. 2016. View Article : Google Scholar
|
|
18
|
Zhu H, Zeng Y, Zhou CC and Ye W:
SNHG16/miR-216-5p/ZEB1 signal pathway contributes to the
tumorigenesis of cervical cancer cells. Arch Biochem Biophys.
637:1–8. 2018. View Article : Google Scholar
|
|
19
|
Zhong JH, Xiang X, Wang YY, Liu X, Qi LN,
Luo CP, Wei WE, You XM, Ma L, Xiang BD, et al: The lncRNA SNHG16
affects prognosis in hepatocellular carcinoma by regulating p62
expression. J Cell Physiol. 235:1090–1102. 2020. View Article : Google Scholar
|
|
20
|
Zhou XY, Liu H, Ding ZB, Xi HP and Wang
GW: lncRNA SNHG16 promotes glioma tumorigenicity through
miR-373/EGFR axis by activating PI3K/AKT pathway. Genomics.
112:1021–1029. 2020. View Article : Google Scholar
|
|
21
|
Shao M, Yu Z and Zou J: LncRNA-SNHG16
silencing inhibits prostate carcinoma cell growth, downregulate
GLUT1 expression and reduce glucose uptake. Cancer Manag Res.
12:1751–1757. 2020. View Article : Google Scholar
|
|
22
|
Tang Z, Xu Z, Zhu X and Zhang J: New
insights into molecules and pathways of cancer metabolism and
therapeutic implications. Cancer Commun (Lond). 41:16–36. 2021.
View Article : Google Scholar
|
|
23
|
Yang M and Wei W: SNHG16: A novel long-non
coding RNA in human cancers. OncoTargets Ther. 12:11679–11690.
2019. View Article : Google Scholar
|
|
24
|
Zhao W, Fu H, Zhang S, Sun S and Liu Y:
LncRNA SNHG16 drives proliferation, migration, and invasion of
hemangioma endothelial cell through modulation of miR-520d-3p/STAT3
axis. Cancer Med. 7:3311–3320. 2018. View Article : Google Scholar
|
|
25
|
Albini A, Bruno A, Noonan DM and Mortara
L: Contribution to tumor angiogenesis from innate immune cells
within the tumor microenvironment: Implications for immunotherapy.
Front Immunol. 9:5272018. View Article : Google Scholar
|
|
26
|
Qiu X, Zhu J, Sun Y, Fan K, Yang DR, Li G,
Yang G and Chang C: TR4 nuclear receptor increases prostate cancer
invasion via decreasing the miR-373-3p expression to alter
TGFβR2/p-Smad3 signals. Oncotarget. 6:15397–15409. 2015. View Article : Google Scholar
|
|
27
|
Pang J, Dai L, Zhang C and Zhang Q:
MiR-373 inhibits the epithelial-mesenchymal transition of prostatic
cancer via targeting runt-related transcription factor 2. J Healthc
Eng. 2021:69742252021. View Article : Google Scholar
|
|
28
|
Lu Y, Li X, Zuo Y, Xu Q, Liu L, Wu H, Chen
L, Zhang Y, Liu Y and Li Y: miR-373-3p inhibits
epithelial-mesenchymal transition via regulation of TGFβR2 in
choriocarcinoma. J Obstet Gynaecol Res. 47:2417–2432. 2021.
View Article : Google Scholar
|
|
29
|
Wei F, Cao C, Xu X and Wang J: Diverse
functions of miR-373 in cancer. J Transl Med. 13:1622015.
View Article : Google Scholar
|
|
30
|
Weng J, Zhang H, Wang C, Liang J, Chen G,
Li W, Tang H and Hou J: miR-373-3p targets DKK1 to promote
EMT-induced metastasis via the Wnt/β-catenin pathway in tongue
squamous cell carcinoma. BioMed Res Int. 2017:60109262017.
View Article : Google Scholar
|
|
31
|
Seol HS, Akiyama Y, Shimada S, Lee HJ, Kim
TI, Chun SM, Singh SR and Jang SJ: Epigenetic silencing of
microRNA-373 to epithelial-mesenchymal transition in non-small cell
lung cancer through IRAK2 and LAMP1 axes. Cancer Lett. 353:232–241.
2014. View Article : Google Scholar
|
|
32
|
Zhu B and Kyprianou N: Transforming growth
factor beta and prostate cancer. Cancer Treat Res. 126:157–173.
2005. View Article : Google Scholar
|
|
33
|
Zhao W, Zhu Q, Tan P, Ajibade A, Long T,
Long W, Li Q, Liu P, Ning B, Wang HY, et al: Tgfbr2 inactivation
facilitates cellular plasticity and development of Pten-null
prostate cancer. J Mol Cell Biol. 10:316–330. 2018. View Article : Google Scholar
|
|
34
|
Ma G, Tang M, Wu Y, Xu X, Pan F and Xu R:
LncRNAs and miRNAs: Potential biomarkers and therapeutic targets
for prostate cancer. Am J Transl Res. 8:5141–5150. 2016.
|
|
35
|
Moris L, Cumberbatch MG, Van den Broeck T,
Gandaglia G, Fossati N, Kelly B, Pal R, Briers E, Cornford P, De
Santis M, et al: Benefits and risks of primary treatments for
high-risk localized and locally advanced prostate cancer: An
international multidisciplinary systematic review. Eur Urol.
77:614–627. 2020. View Article : Google Scholar
|
|
36
|
Maruyama T, Nishihara K, Umikawa M,
Arasaki A, Nakasone T, Nimura F, Matayoshi A, Takei K, Nakachi S,
Kariya KI, et al: MicroRNA-196a-5p is a potential prognostic marker
of delayed lymph node metastasis in early-stage tongue squamous
cell carcinoma. Oncol Lett. 15:2349–2363. 2018.
|
|
37
|
Prensner JR, Iyer MK, Sahu A, Asangani IA,
Cao Q, Patel L, Vergara IA, Davicioni E, Erho N, Ghadessi M, et al:
The long noncoding RNA SChLAP1 promotes aggressive prostate cancer
and antagonizes the SWI/SNF complex. Nat Genet. 45:1392–1398. 2013.
View Article : Google Scholar
|
|
38
|
Yu M, Ohira M, Li Y, Niizuma H, Oo ML, Zhu
Y, Ozaki T, Isogai E, Nakamura Y, Koda T, et al: High expression of
ncRAN, a novel non-coding RNA mapped to chromosome 17q25.1, is
associated with poor prognosis in neuroblastoma. Int J Oncol.
34:931–938. 2009.
|
|
39
|
Lian D, Amin B, Du D and Yan W: Enhanced
expression of the long non-coding RNA SNHG16 contributes to gastric
cancer progression and metastasis. Cancer Biomark. 21:151–160.
2017. View Article : Google Scholar
|
|
40
|
Yang XS, Wang GX and Luo L: Long
non-coding RNA SNHG16 promotes cell growth and metastasis in
ovarian cancer. Eur Rev Med Pharmacol Sci. 22:616–622. 2018.
|
|
41
|
Xiao Y, Xiao T, Ou W, Wu Z, Wu J, Tang J,
Tian B, Zhou Y, Su M and Wang W: LncRNA SNHG16 as a potential
biomarker and therapeutic target in human cancers. Biomark Res.
8:412020. View Article : Google Scholar
|
|
42
|
Qi P, Xu MD, Ni SJ, Shen XH, Wei P, Huang
D, Tan C, Sheng WQ, Zhou XY and Du X: Down-regulation of ncRAN, a
long non-coding RNA, contributes to colorectal cancer cell
migration and invasion and predicts poor overall survival for
colorectal cancer patients. Mol Carcinog. 54:742–750. 2015.
View Article : Google Scholar
|
|
43
|
Xu F, Zha G, Wu Y, Cai W and Ao J:
Overexpressing lncRNA SNHG16 inhibited HCC proliferation and
chemoresistance by functionally sponging hsa-miR-93. OncoTargets
Ther. 11:8855–8863. 2018. View Article : Google Scholar
|
|
44
|
Qi JC, Yang Z, Zhang YP, Lu BS, Yin YW,
Liu KL, Xue WY, Qu CB and Li W: miR-20b-5p, TGFBR2, and E2F1 form a
regulatory loop to participate in epithelial to mesenchymal
transition in prostate cancer. Front Oncol. 9:15352020. View Article : Google Scholar
|
|
45
|
Liu JJ, Zhang X and Wu XH: miR-93 promotes
the growth and invasion of prostate cancer by upregulating its
target genes TGFBR2, ITGB8, and LATS2. Mol Ther Oncolytics.
11:14–19. 2018. View Article : Google Scholar
|
|
46
|
Xu W, Zeng F, Li S, Li G, Lai X, Wang QJ
and Deng F: Crosstalk of protein kinase C ε with Smad2/3 promotes
tumor cell proliferation in prostate cancer cells by enhancing
aerobic glycolysis. Cell Mol Life Sci. 75:4583–4598. 2018.
View Article : Google Scholar
|
|
47
|
Yang F, Strand DW and Rowley DR:
Fibroblast growth factor-2 mediates transforming growth factor-beta
action in prostate cancer reactive stroma. Oncogene. 27:450–459.
2008. View Article : Google Scholar
|
|
48
|
Yang J, Song K, Krebs TL, Jackson MW and
Danielpour D: Rb/E2F4 and Smad2/3 link survivin to TGF-beta-induced
apoptosis and tumor progression. Oncogene. 27:5326–5338. 2008.
View Article : Google Scholar
|
|
49
|
Waghray A, Schober M, Feroze F, Yao F,
Virgin J and Chen YQ: Identification of differentially expressed
genes by serial analysis of gene expression in human prostate
cancer. Cancer Res. 61:4283–4286. 2001.
|
|
50
|
DuPree EL, Mazumder S and Almasan A:
Genotoxic stress induces expression of E2F4, leading to its
association with p130 in prostate carcinoma cells. Cancer Res.
64:4390–4393. 2004. View Article : Google Scholar
|
|
51
|
Littler S, Sloss O, Geary B, Pierce A,
Whetton AD and Taylor SS: Oncogenic MYC amplifies mitotic
perturbations. Open Biol. 9:1901362019. View Article : Google Scholar
|
|
52
|
Zhang R, Li P, Lv H, Li N, Ren S and Xu W:
Exosomal SNHG16 secreted by CSCs promotes glioma development via
TLR7. Stem Cell Res Ther. 12:3492021. View Article : Google Scholar
|
|
53
|
Bai S, Cao S, Jin L, Kobelski M, Schouest
B, Wang X, Ungerleider N, Baddoo M, Zhang W, Corey E, et al: A
positive role of c-Myc in regulating androgen receptor and its
splice variants in prostate cancer. Oncogene. 38:4977–4989. 2019.
View Article : Google Scholar
|
|
54
|
Keklikoglou I, Koerner C, Schmidt C, Zhang
JD, Heckmann D, Shavinskaya A, Allgayer H, Gückel B, Fehm T,
Schneeweiss A, et al: MicroRNA-520/373 family functions as a tumor
suppressor in estrogen receptor negative breast cancer by targeting
NF-κB and TGF-β signaling pathways. Oncogene. 31:4150–4163. 2012.
View Article : Google Scholar
|
|
55
|
Nakata K, Ohuchida K, Mizumoto K, Aishima
S, Oda Y, Nagai E and Tanaka M: Micro RNA-373 is down-regulated in
pancreatic cancer and inhibits cancer cell invasion. Ann Surg
Oncol. 21 (Suppl 4):S564–S574. 2014. View Article : Google Scholar
|
|
56
|
Voorhoeve PM, le Sage C, Schrier M, Gillis
AJ, Stoop H, Nagel R, Liu YP, van Duijse J, Drost J, Griekspoor A,
et al: A genetic screen implicates miRNA-372 and miRNA-373 as
oncogenes in testicular germ cell tumors. Cell. 124:1169–1181.
2006. View Article : Google Scholar
|
|
57
|
Bartel DP: Metazoan MicroRNAs. Cell.
173:20–51. 2018. View Article : Google Scholar
|
|
58
|
Nadauld LD, Garcia S, Natsoulis G, Bell
JM, Miotke L, Hopmans ES, Xu H, Pai RK, Palm C, Regan JF, et al:
Metastatic tumor evolution and organoid modeling implicate TGFBR2
as a cancer driver in diffuse gastric cancer. Genome Biol.
15:4282014. View Article : Google Scholar
|
|
59
|
Moon H, Ju HL, Chung SI, Cho KJ, Eun JW,
Nam SW, Han KH, Calvisi DF and Ro SW: Transforming growth factor-β
promotes liver tumorigenesis in mice via up-regulation of snail.
Gastroenterology. 153:1378–1391.e6. 2017. View Article : Google Scholar
|
|
60
|
Ayub SG, Kaul D and Ayub T: An
androgen-regulated miR-2909 modulates TGFβ signalling through
AR/miR-2909 axis in prostate cancer. Gene. 631:1–9. 2017.
View Article : Google Scholar
|
|
61
|
Pollard BS, Suckow MA, Wolter WR, Starr
JM, Eidelman O, Dalgard CL, Kumar P, Battacharyya S, Srivastava M,
Biswas R, et al: Digitoxin inhibits
epithelial-to-mesenchymal-transition in hereditary castration
resistant prostate cancer. Front Oncol. 9:6302019. View Article : Google Scholar
|

















