Introduction
Gentamicin (GM) is an aminoglycoside antibiotic that
is commonly used in the clinic (1). For more than half a century, despite
the advantages of aminoglycosides in terms of their stable
properties, broad antibacterial spectrum, strong bactericidal power
and low cost, a large number of clinical reports and animal
experiments have also proven that aminoglycosides induce
ototoxicity (2), which severely
limits their clinical application. Current studies have found that
the mechanism underlying aminoglycoside ototoxicity may be that
aminoglycoside antibiotics can accumulate in cochlear hair cells,
and mitochondria are the main site of accumulation (3,4).
Moreover, the hair cells located in the basal turn of the basilar
membrane have a stronger absorption capacity than those located in
the apical turn (5). The damage
to cochlear hair cells caused by aminoglycosides is associated with
the activity of oxygen radicals, and the expression of caspase-3 is
also subsequently increased (6).
Thus, GM-induced ototoxicity may be closely related to the
overproduction of reactive oxygen species (ROS) in cochlear hair
cells and the activation of endogenous mitochondrial apoptosis
pathways.
Puerarin (PU) is the main active ingredient among
the isoflavones of wild Pueraria and dried Pueraria
(7). Isoflavones are aromatic
oxyheterocyclic compounds and effective antioxidants that prevent
the formation of oxygen free radicals and exert biological
antioxidant effects (8). Based on
a number of animal experiments and clinical studies, PU has
antioxidant, anti-ageing, anti-inflammatory and anti-osteoporosis
activities and is also used to treat hangovers and hypoglycaemia
(9,10); in addition, PU is mainly used in
the treatment of neurological diseases and cardiovascular diseases
(11,12). The mechanism underlying the
various pharmacological effects of PU has been thoroughly studied
and has attracted increasing attention from researchers. PU can
improve the learning and memory abilities of ageing mice, and the
mechanism underlying its anti-ageing effects is related to the
inhibition of mitochondrial dysfunction and the enzyme activity of
caspase-3 (13). PU can also
significantly improve the activity of superoxide dismutase in
ageing mice and enhance its ROS scavenging function. PU may inhibit
the expression of caspase-3 and Bax, which are closely related to
cell apoptosis, and promote the protein expression of Bcl-2
(11,12). In addition, it has been reported
that PU is useful in treating alcoholic liver disease as an
antidote and anti-drinking agent (14). However, the effects of PU on
GM-induced ototoxicity have not yet been reported.
Apoptosis is a pathophysiological process in which
various proteins or factors are involved. p53 is considered to be
an important gene that mediates cell apoptosis and plays an
important regulatory role in various cellular stress responses
(15). The mechanism by which p53
functions is extremely complex, and p53 plays vital roles in the
signalling pathways mediated by endogenous regulatory factors. When
cells are stimulated by apoptotic signals, the expression of p53 is
increased; p53 regulates the expression of Bcl-2 family proteins
through transcription-dependent pathways, and p53 can also directly
act on mitochondria-mediated apoptosis pathways. In fact,
H2O2, the most important ROS in redox
signalling, has been shown to trigger a typical DNA damage response
pathway and the subsequent activation of p53 and apoptosis
(16,17). GM-induced ototoxicity may be
closely related to the overproduction of ROS in cochlear hair
cells, the upregulation of p53 protein expression and the
activation of endogenous mitochondrial apoptosis pathways. Thus, in
the present study it was hypothesized that PU may protect cochlear
hair cells from GM-mediated damage by reducing ROS production and
inhibiting the mitochondria-dependent apoptosis pathway.
Materials and methods
Animal experiments and drugs
The animal protocols followed the guidelines of the
Institutional Animal Care and Use Committee of University of
Science and Technology of China (Jinan, China), and the experiments
were conducted in accordance with the National Institutes of Health
(NIH) Guide for the Care and Use of Animals in Laboratory
Experiments (18). The study was
approved by the Institutional Animal Care and Use Committee of
University of Science and Technology of China. A total of 24 male
C57BL/6J mice (8 days old; 4.5±0.3 g) were purchased from Changzhou
Cavens Experimental Animal Co., Ltd. The mice were housed in an SPF
facility, where they were under a controlled temperature of 25±2°C,
a humidity of 55±5% and a 12-h day/night cycle, and where the mice
were allowed to eat and drink ad libitum. The health and
behaviour of the mice were checked every day. Animals were
maintained in accordance with the Guidelines for the Care and Use
of Laboratory Animals (18). When
the mice were 6 weeks old, the 24 mice were divided into four
groups (six mice in each group): The control group; PU group; GM
group; and GM + PU group. The mice in the control group were
intraperitoneally injected with 1 ml physiological saline once a
day for 10 days. The mice in the PU group were intraperitoneally
injected with 100 mg/kg PU (Sigma-Aldrich; Merck KGaA) once a day
for 10 days (19). The mice in
the GM group were first intraperitoneally injected with 1 ml
physiological saline once a day for 3 consecutive days and then
intraperitoneally injected with 200 mg/kg GM (Sigma-Aldrich; Merck
KGaA) once a day for 7 days (20). The mice in the GM + PU group were
first intraperitoneally injected with 100 mg/kg PU once a day for 3
consecutive days and then intraperitoneally injected with 100 mg/kg
PU and 200 mg/kg GM once a day for 7 consecutive days. In the
entire study, no mice died accidentally. All efforts were made to
minimize suffering. All the 24 mice were anaesthetized with an
intraperitoneal injection of a combination of ketamine (100 mg/kg)
and xylazine (15 mg/kg) (21) At
the end of the experiments, the mice were administrated with 30%
volume displacement rate of CO2 for euthanasia. Animal
death was confirmed by observing cardiac arrest and mydriasis.
Auditory brainstem response (ABR)
test
The ABR thresholds of the four groups were measured
in the 8th week. The mice were anaesthetized by intraperitoneal
injection with a combination of ketamine (100 mg/kg) and xylazine
(15 mg/kg) (21), and the mice
were kept warm during the ABR recording. ABR measurements were
performed by using Tucker-Davis Technology System hardware and
software (Tucker-Davis Technologies). The recording electrode was
subcutaneously inserted into the tissue at the vertex, and the
reference and ground electrodes were subcutaneously placed behind
the ears. The ABR was measured with broadband clicks and pure tones
at frequencies of 8, 12, 16, 24 and 32 kHz with 1,024 stimulus
repetitions per record, and the stimulus sound was decreased from
90 dB SPL to 10 dB SPL successively at 10-dB SPL intervals. When
the electrophysiological response to the stimulus sound
disappeared, the lowest stimulus sound that triggered a response
was defined as the auditory threshold of the mouse tested at this
frequency.
Treatment of cochlear basal membranes
and staining
After the ABR was detected, the mice were
anaesthetized, and perfusion was performed with normal saline and
4% paraformaldehyde. The head was removed, the temporal bone was
removed and the cochlea was separated. The cochlea was immersed in
a 10% EDTA solution and stored at 4°C for 7 days. The basilar
membrane was then isolated from the cochlea under an anatomical
microscope. The basilar membrane was fixed in 10% formaldehyde
solution at room temperature for 15 min and fluorescence staining
was performed with phalloidin (1:100, red) at room temperature in
the dark for 30 min. Fluorescence microscopy was used for
observation and imaging. The number of outer and inner hair cells
in each segment of the basilar membrane was quantified per 100 µm.
Images were captured using a fluorescence microscope (Leica
Corporation).
Cell culture and treatment
House Ear Institute-Organ of Corti 1 (HEI-OC1) cells
were derived from a murine Corti organ; this cell line is a type of
auditory cell line and has properties similar to those of hair
cells. HEI-OC1 cells were donated by Professor Renjie Chai of
Southeast University. HEI-OC1 cells were cultured in high-glucose
medium (Gibco; Thermo Fisher Scientific, Inc.) containing 10%
foetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) in a
10% CO2 incubator at 33°C. The HEI-OC1 cells in the
control group were treated with no drugs. The cells in the GM, PU
and H2O2 groups were treated with 1.0 mM GM,
100 µg/ml PU and 0.1 mM H2O2 for 24 h,
respectively. The cells in the GM + PU group were pre-treated with
100 µg/ml PU for 2 h, followed by a combination treatment
with 1.0 mM GM for 24 h. The cells in the
H2O2 + PU group were pre-treated with 100
µg/ml PU for 2 h, followed by a combination treatment with
0.1 mM H2O2 for 24 h. All treatments were at
33°C.
Cell viability
HEI-OC1 cells were seeded in a 96-well plate (5,000
cells per well) and incubated overnight at 33°C in 10%
CO2. The cells were treated with different
concentrations of GM (0.1, 0.2, 0.5, 1.0 and 2.0 mM) and PU (5, 10,
20, 50, 100, 200 and 300 µg/ml) for 24 h. A total of 10
µl Cell Counting Kit-8 (CCK-8; Sangon Biotech Co., Ltd.) was
added to the treated cells in each well and incubated at 37°C for 1
h. The absorbance at 450 nm was determined with an ELISA reader
(Multiskan MK3). The cell viability was calculated as (%) (OD assay
- OD blank) / (OD control - OD blank) ×100%.
Flow cytometry analysis of cell
apoptosis
The treated cells were collected and transferred
into a centrifuge tube. After centrifugation (1,000 × g, 4°C, 5
min), the cells were resuspended in 100 µl binding buffer.
An Annexin V-APC/7-AAD apoptosis kit (BD Biosciences) was used for
double staining in the dark. The apoptosis rate of the HEI-OC1
cells was measured by flow cytometry (CytoFLEX S; Beckman Coulter,
Inc.). CytExpert (v2.0; Beckman Coulter, Inc.) was used to analyze
the data.
Detection of ROS
The intracellular ROS levels were detected by
DCFH-DA staining (cat. no. E004; Nanjing Jiancheng Bioengineering
Institute). DCFH-DA (10 µM) was added to the treated cells
in a 6-well plate, at 1×106 cells per well, and
incubated at 33°C in 10% CO2 for 2 h. The cells were
observed and photographed under a fluorescence microscope. Then,
ROS production of the HEI-OC1 cells was measured by flow cytometry
(Beckman Coulter, Inc.) (22).
CytExpert (v2.0; Beckman Coulter, Inc.) was used to analyze the
data.
TUNEL assay
A TUNEL assay was used to investigate the
morphological characteristics of apoptosis. The treated cells were
fixed with 4% paraformaldehyde for 30 min at 20°C. After 20 min of
treatment with 0.1% Triton X-100, TUNEL working solution (Beyotime
Institute of Biotechnology) was added to the cells for 1 h at 37°C.
DAPI staining was performed for 2 min at 20°C. The treated HEI-OC1
cells were then photographed and analyzed under a fluorescence
microscope. Images were captured using a fluorescence microscope
(Leica Corporation). For each sample, >5 areas were randomly
selected for analysis.
Mitochondrial fluorescent probe
staining analysis
A JC-1 probe was used to measure mitochondrial
depolarization in HEI-OC1 cells. Briefly, cells were treated in
6-well plates, at 1×106 cells per well, as indicated,
and then the cells were incubated with an equal volume of JC-1
staining solution (5 pg/ml; Beyotime Institute of Biotechnology) at
37°C for 20 min and washed twice with PBS. The mitochondrial
membrane potentials were monitored by determining the relative
amounts of the emissions of mitochondrial JC-1 monomers or
aggregates using fluorescence microscopy and flow cytometry. The
excitation and emission wavelengths of 514 and 529 nm,
respectively, were used to detect the monomeric form of JC-1. JC-1
aggregation was detected at 585 and 590 nm (23). CytExpert (v2.0; Beckman Coulter,
Inc.) was used to analyse the data.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
RT-qPCR was performed to detect the mRNA expression
levels of Bax, caspase-3 and Bcl-2 in the HEI-OC1 cells. Total RNA
was isolated from HEI-OC1 cells using RNAiso Plus®
reagent (Takara Biotechnology Co., Ltd.). Next, according to the
product specification, ReverTra-Plus™ (Toyobo Life
Science) was used to reverse transcribe the RNA into cDNA. qPCR was
performed using used TB Green® Premix Ex Taq™
II (Takara Biotechnology Co., Ltd.). The following thermocycling
conditions were used for the qPCR: Initial denaturation at 95°C for
30 sec; followed by 40 cycles at 95°C for 5 sec and 60°C for 34
sec. The primers used in this experiment (24) are shown in Table I. The mRNA expression levels were
normalized to GAPDH expression, and gene expression was calculated
by using the 2−ΔΔCq method in this study (25).
 | Table I.Primers used in this study. |
Table I.
Primers used in this study.
| Gene | Sequences
(5′-3′) |
|---|
| GAPDH | F:
GTATGACTCCACTCACGG |
|
| R:
GGTCTGGCTCCTGGAAGA |
| Bcl-2 | F:
ATCGCCCTGTGGATGACTGAGT |
|
| R:
GCCAGGAGAAATCAAACAGAGGC |
| Bax | F:
TCAGGATGCGTCCACCAAGAAG |
|
| R:
TGTGTCCACGGCGGCAATCATC |
| p53 | F:
TCCGAAGACTGGATGACTGC |
|
| R:
GATCGTCCATGCAGTGAGGT |
| Caspase-3 | F:
GGAAGCGAATCAATGGACTCTGG |
|
| R:
GCATCGACATCTGTACCAGACC |
Western blotting
HEI-OC1 cells were lysed in RIPA buffer (Wuhan
Servicebio Technology Co., Ltd.) or Cell Mitochondria Isolation kit
(Beyotime Institute of Biotechnology). The total protein content
was determined using a BCA Protein assay kit (Beyotime Institute of
Biotechnology). Protein samples (20 µg) were resolved by 10%
SDS-PAGE (Wuhan Servicebio Technology Co., Ltd.), and subsequently
transferred to polyvinylidene fluoride membranes (MilliporeSigma).
The membranes were blocked with 5% skimmed milk (P0216; Beyotime
Institute of Biotechnology) for 1 h at room temperature. Then, the
proteins were incubated with primary antibodies overnight in a 4°C
refrigerator, followed by incubation with secondary antibodies
[HRP-conjugated Affinipure Goat Anti-Rabbit IgG(H+L); cat. no.
SA00001-2; 1:5,000, ProteinTech Group, Inc.] at room temperature
for 1 h. An ECL kit (Wuhan Servicebio Technology Co., Ltd.) was
used to detect the immunoreactive bands. Protein expression was
detected using a Clinx ChemiScope 3300 (Clinx, Inc.). ImageJ
software (v1.8; National Institutes of Health, Inc.) was used to
quantify protein expression levels. GAPDH (cat. no. 5174; 1:1,000;
Cell Signaling Technology, Inc.) and Cox IV (cat. no. 4850;
1:1,000; Cell Signaling Technology, Inc.) (26) were used as the loading controls.
The primary antibodies used were specific for p53 (cat. no. 2524;
1:1,000; Cell Signaling Technology, Inc.), Bcl-2 (cat. no. sc-7382;
1:1,000; Santa Cruz Biotechnology, Inc.), cleaved-caspase-3 (cat.
no. 9661; 1:1,000; Cell Signaling Technology, Inc.), caspase-3
(cat. no. 14220; 1:1,000; Cell Signaling Technology, Inc.), Bax
(cat. no. 2772; 1:1,000; Cell Signaling Technology, Inc.) and
cytochrome c (Cyto C; cat. no. 11940; 1:1,000; Cell
Signaling Technology, Inc.).
Statistical analysis
The statistical software GraphPad Prism 7 (GraphPad
Software, Inc.) was used to analyse the significance of the data.
All experimental results were independently repeated at least three
times. All the data were collected from six or more samples in each
of the experimental groups. The data are expressed as the mean ±
standard error of the mean, and one-way ANOVA followed by Tukey's
post hoc test was performed. P<0.05 was considered to indicate a
statistically significant difference.
Results
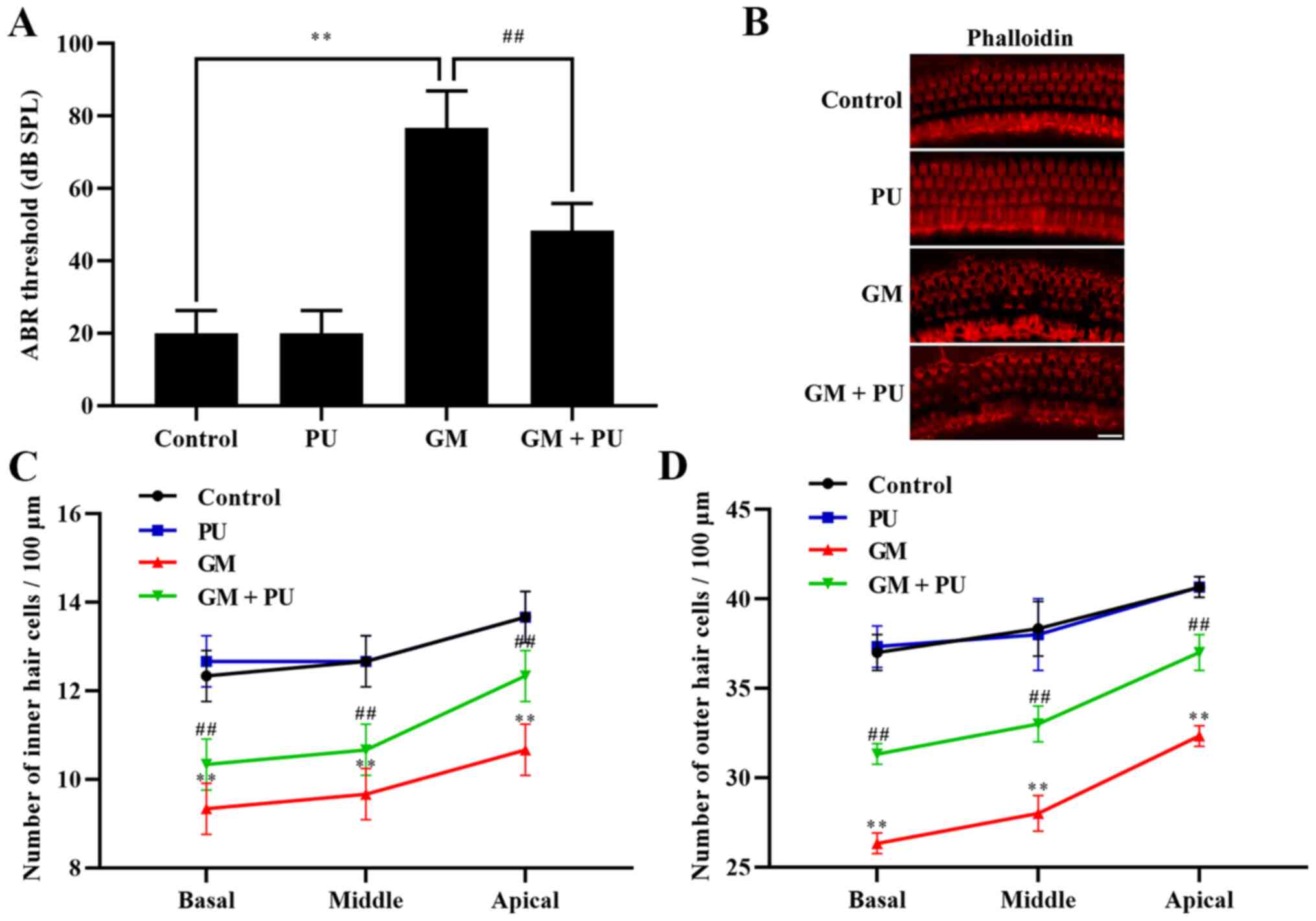
PU protects against GM-induced hearing
loss in C57BL/6J mice
The ABR threshold recording results showed that
there was no difference between the PU and control groups. The
threshold value of the GM group was significantly increased, with
an increasing trend within the frequency range of 4 to 32 kHz, and
the increase was more obvious at high frequencies (Fig. 1A). The threshold value of the GM +
PU group was lower than that of the GM group.
PU attenuates GM-induced cochlear hair
cell damage
The basal membrane of the cochlea and hair cells
were stained with phalloidin (Fig.
1B). The results showed that the hair cells in the Control and
PU groups were closely and neatly arranged. In the GM group, the
hair cells were disorganized and partially absent, and the cilia
were prostrate. Furthermore, the damage to hair cells in the basal
and middle turns was more serious than that in the apical turn
(Fig. 1C and D). The degree of
hair cell damage in the GM + PU group was lower than that in the GM
group.
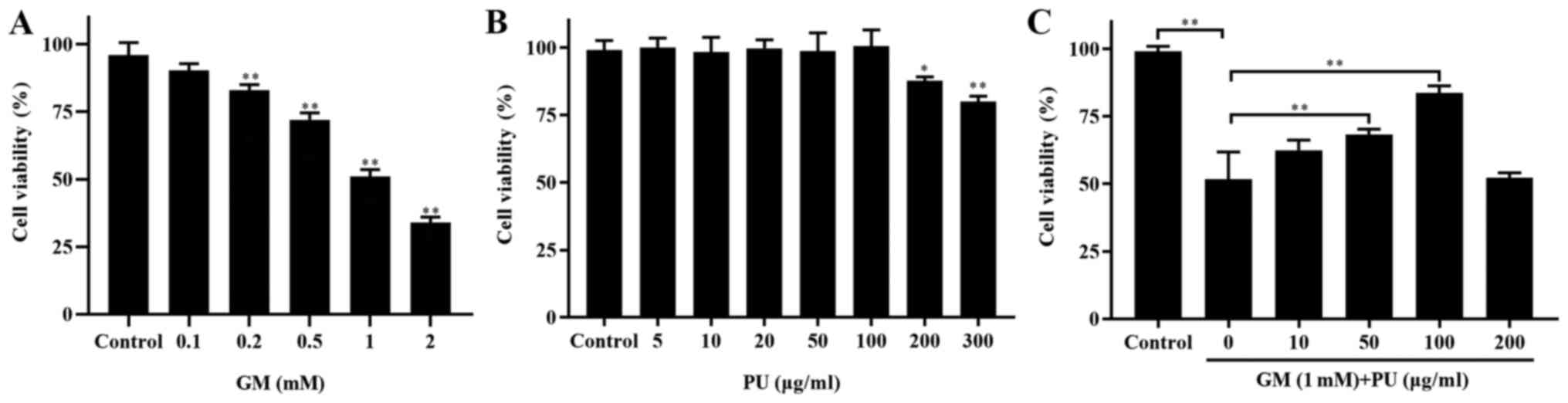
PU improves the survival rate of
HEI-OC1 cells treated with GM
CCK-8 assays were used to detect the viability of
HEI-OC1 cells (Fig. 2). The
results showed that the viability of the HEI-OC1 cells treated with
1 mM GM was ~50% (Fig. 2A). The
HEI-OC1 cell viability was not affected by treatment with 5, 10,
20, 50 or 100 µg/ml PU, while 200 and 300 µg/ml PU
decreased the viability of the HEI-OC1 cells (Fig. 2B). HEI-OC1 cells were pre-treated
with different concentrations of PU (10, 50, 100 and 200
µg/ml) for 2 h, followed by a combination treatment with 1.0
mm GM for 24 h. It was demonstrated that the viability of the cells
treated with 100 µg/ml PU was the highest (Fig. 2C). Based on these results,
treatment with 1 mM GM for 24 h was considered to be the
appropriate condition for the HEI-OC1 cell damage model, and 100
µg/ml PU was the optimal concentration for protecting
HEI-OC1 cells from GM-mediated damage. Therefore, the
aforementioned treatment conditions were selected for subsequent
cell experiments.
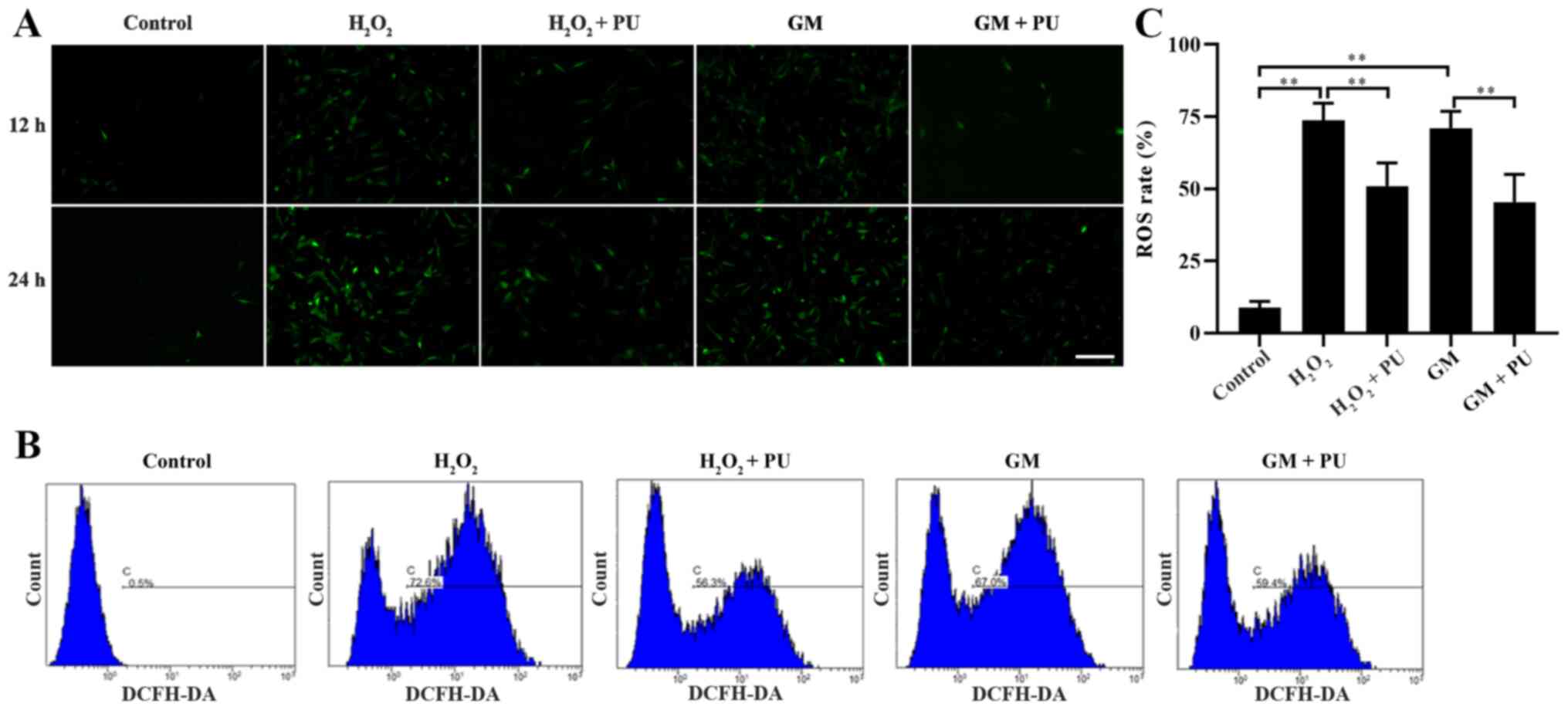
PU attenuates GM-induced oxidative
stress in HEI-OC1 cells
The cells in the Control,
H2O2, H2O2 + PU, GM and
GM + PU groups were stained with fluorescent DCFH-DA for 12 and 24
h, according to the manufacturer's instructions, and the cells were
photographed under a fluorescence microscope (Fig. 3A). ROS were detected by DCFH-DA,
and the intensity of green fluorescence was proportional to the
level of ROS in the cells. The results indicated that the amount of
intracellular ROS in the H2O2 and GM groups
were notably increased in the 24 h staining group compared with the
12 h staining group, and PU could reduce the amount of
intracellular ROS production induced by H2O2
and GM. Flow cytometry was used to detect the green fluorescence
intensity of DCFH-DA in the cells after 24 h of treatment in each
group (Fig. 3B). The statistical
results also showed that PU could significantly reduce the ROS
levels in the HEI-OC1 cells after treatment with
H2O2 and GM (Fig. 3C).
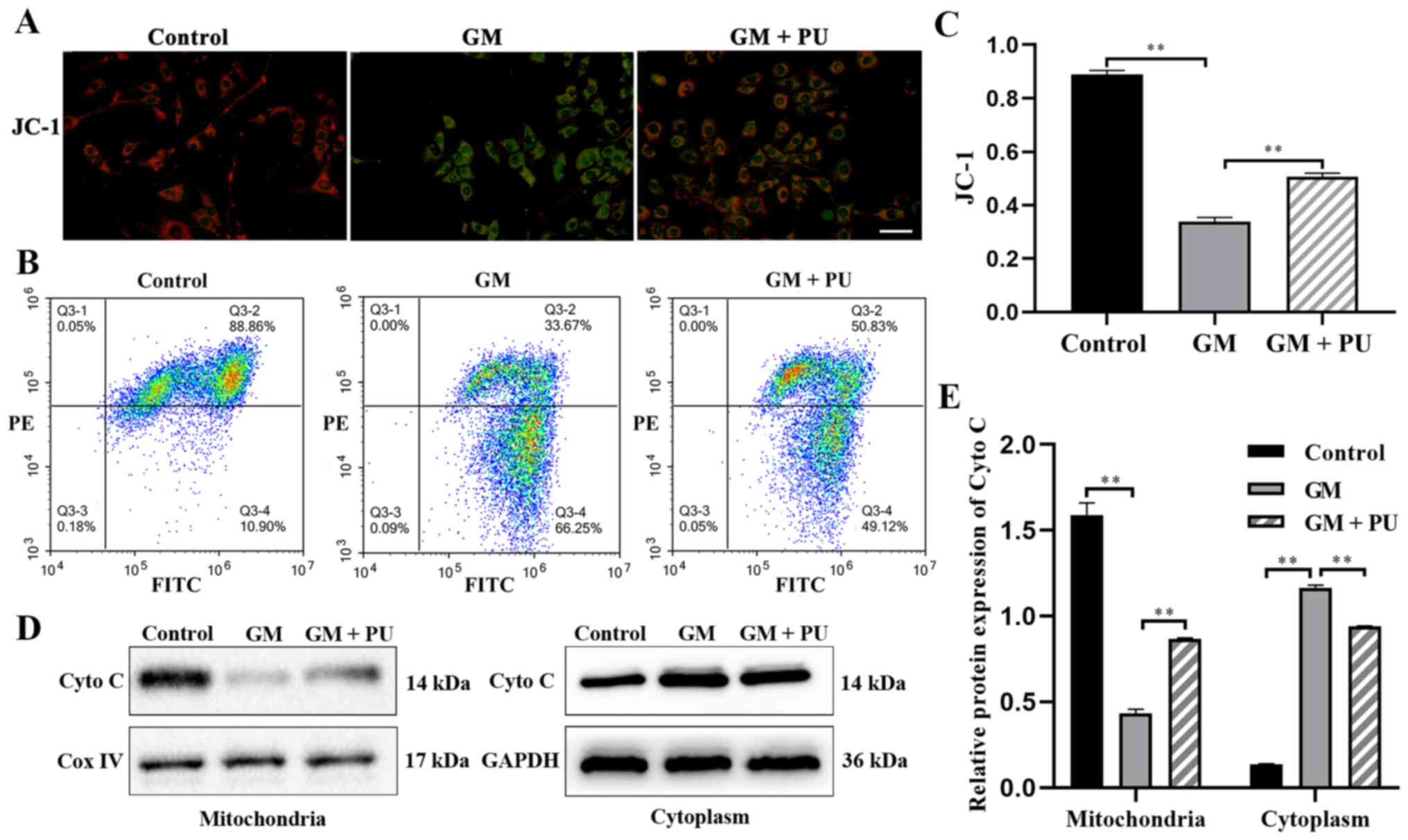
PU attenuates GM-induced damage to
mitochondrial membrane in HEI-OC1 cells
JC-1 could aggregate in normal mitochondria and emit
red fluorescence. The exposure of HEI-OC1 cells to GM (1 mM) for 24
h resulted in the dissipation of the ΔΨm, which was shown by
increased green fluorescence and decreased red fluorescence after
JC-1 staining. Pre-treatment with PU (100 µM) moderated this
dissipation, indicating the protective effect of PU (Fig. 4A). The mitochondrial membrane
potential of each group was evaluated with JC-1 stain by flow
cytometry. Cells in the GM group displayed a lower ratio of
mitochondria with normal potential. Pre-treatment of the cells with
PU displayed a higher ratio (Fig. 4B
and C). The western blotting results suggested that the Cyto C
expression levels were significantly reduced in the mitochondria in
the GM group compared with the Control group, but increased in the
cytoplasm in the GM group. These changes were reversed following
pre-treatment with PU (Fig. 4D and
E).
PU inhibits the GM-induced apoptosis
of HEI-OC1 cells
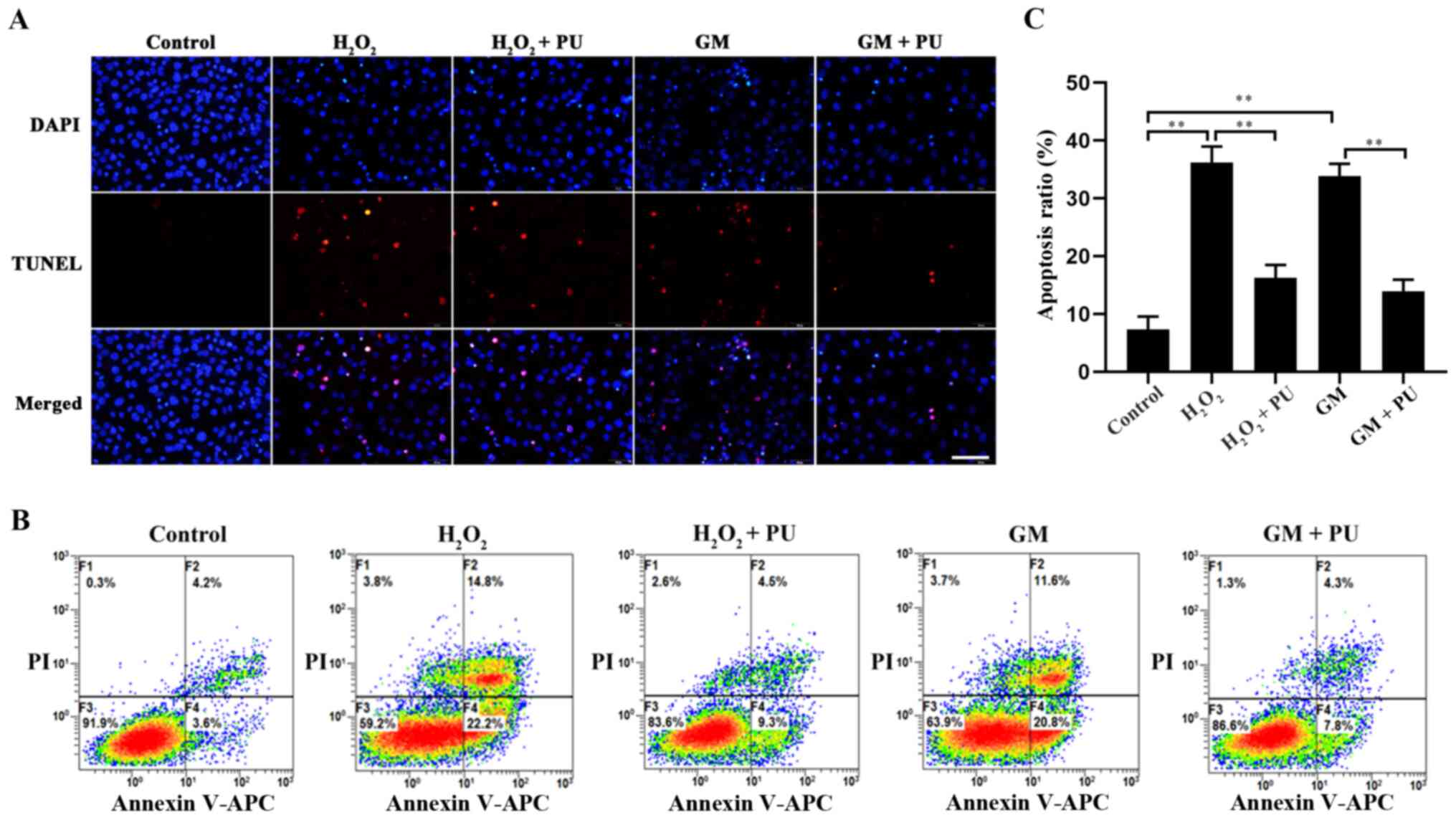
The apoptosis of HEI-OC1 cells in each group was
detected via the TUNEL method (Fig.
5A) and flow cytometry (Fig.
5B). The results showed that apoptosis was significantly
increased after H2O2 and GM treatment and
that PU pre-treatment significantly reduced this apoptosis
(Fig. 5C). The RT-qPCR results
suggested that compared with the GM group, the GM + PU group
exhibited significantly reduced mRNA expression of Bax, caspase-3
and p53, and increased mRNA expression of Bcl-2 (Fig. 6A). The western blotting results
(Fig. 6B) showed that p53
expression was increased after the HEI-OC1 cells were treated with
GM; moreover, PU could decrease the GM-induced expression of p53.
Compared with that in the GM group, the Bax and cleaved-caspase-3
expression in the GM + PU group was significantly decreased, while
the Bcl-2 expression was increased (Fig. 6C). Moreover, the Bax/Bcl-2 ratio
and cleaved-caspase-3/caspase-3 ratio in the GM + PU group was
significantly lower than that in the GM group (Fig. 6D and E).
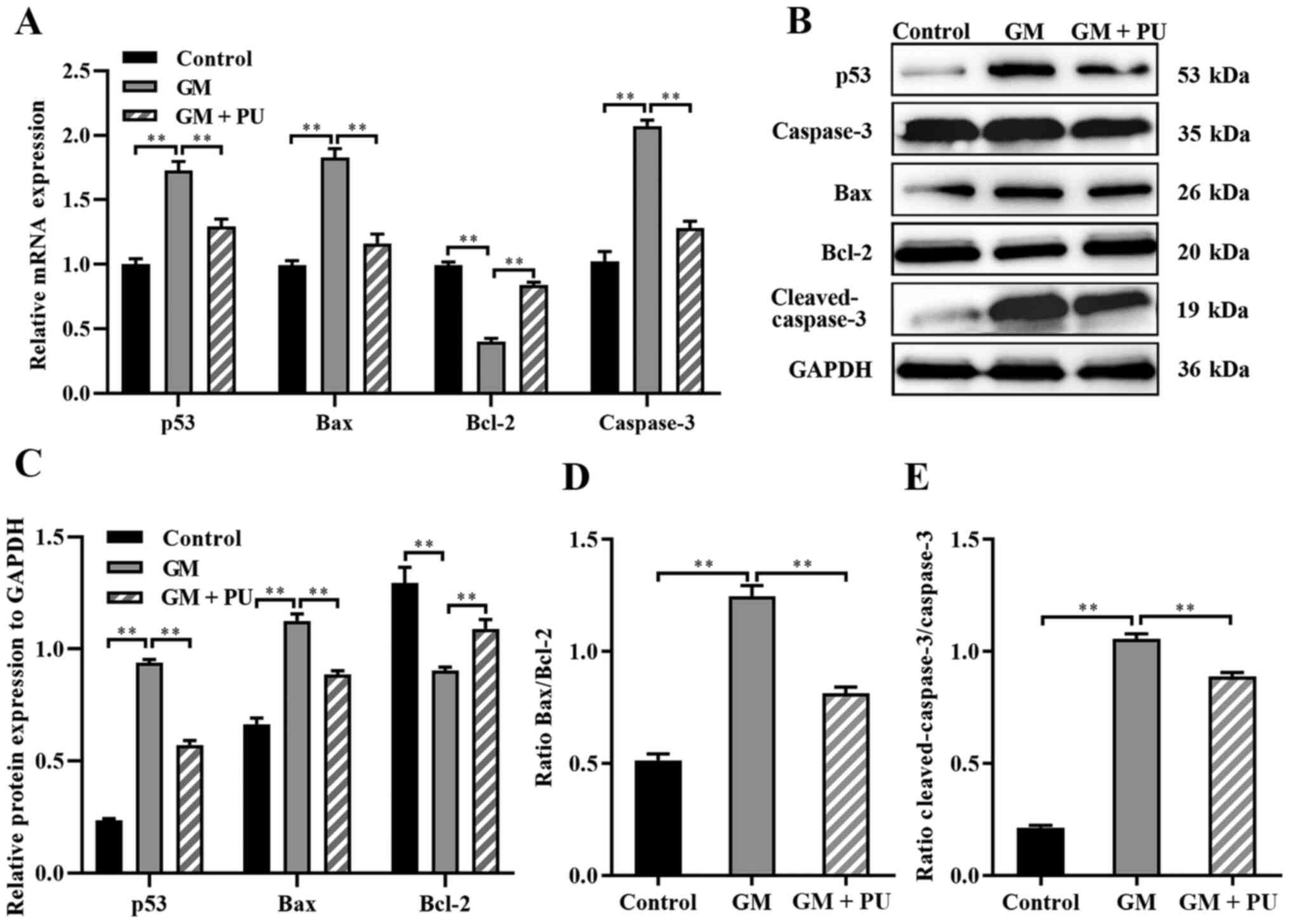 | Figure 6.Expression levels of caspase-3,
cleaved-caspase-3, Bcl-2, Bax and p53 were analysed via RT-qPCR and
western blotting. (A) The mRNA expression levels of caspase-3,
Bcl-2, Bax and p53 in each group were detected by RT-qPCR. n=6.
**P<0.01. (B) The relative expression levels of p53, caspase-3,
Bax, Bcl-2 and cleaved-caspase-3 were analysed via western
blotting. (C) Statistical analysis of protein expression in each
group. n=6. **P<0.01. (D) The Bax to Bcl-2 ratio. Statistical
analysis of protein expression n=6. **P<0.01. (E) The
cleaved-caspase-3 to caspase-3 ratio. Statistical analysis of
protein expression. n=6. **P<0.01. PU, puerarin; GM, gentamicin;
RT-qPCR, reverse transcription-quantitative PCR. |
Discussion
PU is mainly used in the treatment of neurological
diseases, cardiovascular diseases and hepatic impairment (7). In the present study, it was found
that PU could protect the hearing of GM-treated C57BL/6J mice. We
will build more samples and conduct further animal experiments
based on this mouse model in follow-up studies. The HEI-OC1 cell
line is extremely sensitive to ototoxic drugs. Moreover, the
HEI-OC1 cell line is a very useful cell model that has been widely
used in a number of studies investigating the molecular mechanism
underlying ototoxicity and in studies that screen novel drugs with
protective effects in vitro (27,28). After treatment with high
concentrations of PU, cell viability was decreased. This finding
indicated the potential toxicity of high concentrations of PU. We
will conduct further in-depth research on this toxicity in the
future.
The main sites of aminoglycoside aggregation in
cochlear hair cells are the mitochondria, which are the main
organelles that produce oxygen free radicals (29). Appropriate ROS levels can promote
immunity, repair and growth of the body, but excessive ROS levels
can cause damage to mitochondria and lead to programmed cell death
(30). ROS include
H2O2, OH-, NO and ONOO-. Among these ROS,
H2O2 easily penetrates the plasma membrane
and produces the highly reactive free radical OH-, which leads to
damage to cells and tissues and induces cell apoptosis.
H2O2 has been widely used to establish in
vitro models of oxidative stress damage (31). In the present study,
H2O2 was used to establish a control group
for oxidative stress injury.
Although the mechanism underlying GM-induced injury
has not been determined, ROS may be key players in this mechanism
(32), and ROS can also cause
oxidative stress and cell damage. The ototoxicity induced by GM is
related to the production of ROS, which leads to damage to the
auditory hair cells in Corti organs. For a long time, cochlear
sensory cells have been known to produce ROS after exposure to
aminoglycoside compounds (33).
p53 is an important protein that mediates cell apoptosis, and p53
is directly regulated by redox signals due to its readily oxidized
cysteine (16). Previous studies
have shown that p53 is activated when ROS levels increase (16,34). Moreover, p53 expression has been
demonstrated to be positively correlated with Bax expression
(35). Bax not only antagonizes
the inhibitory effect of Bcl-2 on apoptosis, but also promotes
apoptosis. Caspase-3 is a regulator of cell death and plays an
important role in the progression of apoptosis. Caspase-3 is a
protease that directly leads to the disintegration of apoptotic
cells and plays a central role in the network of apoptotic
mechanisms. As an upstream protein that regulates caspase-3, Bax
can activate caspase-3 and initiate the caspase cascade (36). The cytotoxicity of GM in hair
cells is mainly due to the initiation of the mitochondrial
apoptosis pathway (29). In the
present study, p53 expression was increased, and p53 regulated the
expression of Bcl-2 family proteins when ROS accumulated in HEI-OC1
cells. Bax and Bcl-2 form heteropolymers, enhance mitochondrial
membrane permeability, release apoptotic factors into the cytoplasm
and stimulate the caspase apoptotic pathway, leading to the
programmed death of HEI-OC1 cells (11). Investigation into the regulatory
roles of ROS and p53 was not performed in the present study, so
these results may have limited generalizability. In future studies,
p53 and ROS inhibitors will be used to clarify the protective
effect of ROS and p53 on PU in auditory hair cell damage induced by
GM.
Previous studies have shown that PU has a variety of
pharmacological activities and exerts antioxidant effects (11,37). The present study found that PU
protected against GM-induced hearing damage in C57BL/6J mice and
ameliorated the morphological changes in mouse cochlear hair cells
after GM-mediated damage. It was also observed that PU could reduce
the production of ROS, downregulate the expression of p53, Bax and
caspase-3, and upregulate the expression of Bcl-2 in GM- and
H2O2-treated HEI-OC1 cells. After treatment
of HEI-OC1 cells with GM and H2O2, the
expression of p53 was significantly increased. It was demonstrated
that PU may ameliorate GM-induced ototoxicity, which is at least
partially mediated by p53-regulated apoptotic signalling pathways,
by inhibiting the mitochondria-dependent apoptosis pathway. This
study provided novel insights into potential therapeutic targets
for protecting against GM-induced ototoxicity. PU could be used as
a protective agent against the ototoxicity caused by GM.
Acknowledgements
The authors would like to thank Dr Panpan Huang, Dr
Wenxiang Fang and Dr Guodong Shen from the University of Science
and Technology of China (Hefei, China), for their technical
assistance. This work was supported by Anhui Provincial Key
Laboratory of Tumor Immunotherapy and Nutrition Therapy (Hefei,
China), which provided experimental equipment.
Funding
This research was supported by the National Natural
Sciences Foundation of China (grant nos. 81800911 and 81470699),
the Anhui Natural Science Foundation (grant no. 1808085QH248) and
Fundamental Research Funds for the Central Universities (grant no.
WK9110000053).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PN and YS contributed equally to the work and should
be regarded as co-first authors. CP and Jing S conceived and
designed the study. PN, YS, SW, GL, XT and Jia S performed the cell
and animal experiments. PN and YS performed the statistical
analysis and drafted the manuscript. CP reviewed and edited the
manuscript. PN and CP confirm the authenticity of all the raw data.
All the authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal protocols followed the guidelines of the
Institutional Animal Care and Use Committee of University of
Science and Technology of China (Jinan, China), and the experiments
were conducted in accordance with the National Institutes of Health
(NIH) Guide for the Care and Use of Animals in Laboratory
Experiments. The study was approved by the Institutional Animal
Care and Use Committee of University of Science and Technology of
China [approval no. 2020-N(A)-032]. Anhui Provincial Hospital is
the other name for the First Affiliated Hospital of the University
of Science and Technology of China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu J, Kachelmeier A, Dai C, Li H and
Steyger PS: Uptake of fluorescent gentamicin by peripheral
vestibular cells after systemic administration. PLoS One.
10:e01206122015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ding D, Zhang J, Jiang H, Xuan W, Qi W and
Salvi R: Some Ototoxic Drugs Destroy Cochlear Support Cells Before
Damaging Sensory Hair Cells. Neurotox Res. 37:743–752. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yao L, Zhang JW, Chen B, Cai M, Feng D,
Wang Q, Wang X, Sun J, Zheng Y, Wang G and Zhou F: Mechanisms and
pharmacokinetic/pharmacodynamic profiles underlying the low
nephrotoxicity and ototoxicity of etimicin. Acta Pharmacol Sin.
41:866–878. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kros CJ and Steyger PS: Aminoglycoside-
and Cisplatin-Induced Ototoxicity: Mechanisms and Otoprotective
Strategies. Cold Spring Harb Perspect Med. 9:a0335482019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Y, Yu Y, Chu H, Bing D, Wang S, Zhou
L, Chen J, Chen Q, Pan C, Sun Y, et al: 17-DMAG induces Hsp70 and
protects the auditory hair cells from kanamycin ototoxicity in
vitro. Neurosci Lett. 588:72–77. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang Q, Zhou Y, Yin H, Li H, Zhou M, Sun
G, Cao Z, Man R, Wang H and Li J: PINK1 Protects Against
Gentamicin-Induced Sensory Hair Cell Damage: Possible Relation to
Induction of Autophagy and Inhibition of p53 Signal Pathway. Front
Mol Neurosci. 11:4032018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mahdy HM, Mohamed MR, Emam MA, Karim AM,
Abdel-Naim AB and Khalifa AE: The anti-apoptotic and
anti-inflammatory properties of puerarin attenuate
3-nitropropionic-acid induced neurotoxicity in rats. Can J Physiol
Pharmacol. 92:252–258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Umeno A, Horie M, Murotomi K, Nakajima Y
and Yoshida Y: Antioxidative and Antidiabetic Effects of Natural
Polyphenols and Isoflavones. Molecules. 21:7082016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiong FL, Sun XH, Gan L, Yang XL and Xu
HB: Puerarin protects rat pancreatic islets from damage by hydrogen
peroxide. Eur J Pharmacol. 529:1–7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim J, Kim KM, Kim CS, Sohn E, Lee YM, Jo
K and Kim JS: Puerarin inhibits the retinal pericyte apoptosis
induced by advanced glycation end products in vitro and in vivo by
inhibiting NADPH oxidase-related oxidative stress. Free Radic Biol
Med. 53:357–365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Yang X, Ge X and Zhang F:
Puerarin attenuates neurological deficits via Bcl-2/Bax/cleaved
caspase-3 and Sirt3/SOD2 apoptotic pathways in subarachnoid
hemorrhage mice. Biomed Pharmacother. 109:726–733. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu B, Zhao C, Li H, Chen X, Ding Y and Xu
S: Puerarin protects against heart failure induced by pressure
overload through mitigation of ferroptosis. Biochem Biophys Res
Commun. 497:233–240. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu S, Cao XL, Liu GQ, Zhou T, Yang XL and
Ma BX: The in silico and in vivo evaluation of puerarin against
Alzheimer's disease. Food Funct. 10:799–813. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu YS, Yuan MH, Zhang CY, Liu HM, Liu JR,
Wei AL, Ye Q, Zeng B, Li MF, Guo YP, et al: Puerariae Lobatae radix
flavonoids and puerarin alleviate alcoholic liver injury in
zebrafish by regulating alcohol and lipid metabolism. Biomed
Pharmacother. 134:1111212021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He L, Chen Y, Feng J, Sun W, Li S, Ou M
and Tang L: Cellular senescence regulated by SWI/SNF complex
subunits through p53/p21 and p16/pRB pathway. Int J Biochem Cell
Biol. 90:29–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi T and Dansen TB: Reactive Oxygen
Species Induced p53 Activation: DNA Damage, Redox Signaling, or
Both? Antioxid Redox Signal. 33:839–859. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Valverde M, Lozano-Salgado J, Fortini P,
Rodriguez-Sastre MA, Rojas E and Dogliotti E: Hydrogen
Peroxide-Induced DNA Damage and Repair through the Differentiation
of Human Adipose-Derived Mesenchymal Stem Cells. Stem Cells Int.
2018:16154972018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
National Research Council, . Guide for the
Care and Use of Laboratory Animals. National Academies Press;
Washington, DC: 2010, https://www.ncbi.nlm.nih.gov/books/NBK54050/
|
|
19
|
Chen X, Qian L, Wang B, Zhang Z, Liu H,
Zhang Y and Liu J: Synergistic Hypoglycemic Effects of Pumpkin
Polysaccharides and Puerarin on Type II Diabetes Mellitus Mice.
Molecules. 24:9552019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang X and Yu J: Baicalin attenuates
gentamicin-induced cochlear hair cell ototoxicity. J Appl Toxicol.
39:1208–1214. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Svorc P Jr, Bačová I, Svorc P and Bužga M:
Autonomic nervous system under ketamine/xylazine and pentobarbital
anaesthesia in a Wistar rat model: a chronobiological view. Prague
Med Rep. 114:72–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eruslanov E and Kusmartsev S:
Identification of ROS using oxidized DCFDA and flow-cytometry.
Methods Mol Biol. 594:57–72. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin H, Xiong H, Su Z, Pang J, Lai L, Zhang
H, Jian B, Zhang W and Zheng Y: Inhibition of DRP-1-Dependent
Mitophagy Promotes Cochlea Hair Cell Senescence and Exacerbates
Age-Related Hearing Loss. Front Cell Neurosci. 13:5502019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu X, Liu W, Fan Z, Qian F, Zhang D, Han
Y, Xu L, Sun G, Qi J, Zhang S, et al: c-Myb knockdown increases the
neomycin-induced damage to hair-cell-like HEI-OC1 cells in vitro.
Sci Rep. 7:410942017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stevens JM: Cytochrome c as an
experimental model protein. Metallomics. 3:319–322. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kalinec G, Thein P, Park C and Kalinec F:
HEI-OC1 cells as a model for investigating drug cytotoxicity. Hear
Res. 335:105–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park C, Thein P, Kalinec G and Kalinec F:
HEI-OC1 cells as a model for investigating prestin function. Hear
Res. 335:9–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kros CJ and Steyger PS: Aminoglycoside-
and Cisplatin-Induced Ototoxicity: Mechanisms and Otoprotective
Strategies. Cold Spring Harb Perspect Med. 9:a0335482019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Circu ML and Aw TY: Reactive oxygen
species, cellular redox systems, and apoptosis. Free Radic Biol
Med. 48:749–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Z, Wei D and Xiao H: Methods of
cellular senescence induction using oxidative stress. Methods Mol
Biol. 1048:135–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Choung YH, Taura A, Pak K, Choi SJ, Masuda
M and Ryan AF: Generation of highly-reactive oxygen species is
closely related to hair cell damage in rat organ of Corti treated
with gentamicin. Neuroscience. 161:214–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou M, Sun G, Zhang L, Zhang G, Yang Q,
Yin H, Li H, Liu W, Bai X, Li J, et al: STK33 alleviates
gentamicin-induced ototoxicity in cochlear hair cells and House Ear
Institute-Organ of Corti 1 cells. J Cell Mol Med. 22:5286–5299.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mishina NM, Bogdanova YA, Ermakova YG,
Panova AS, Kotova DA, Bilan DS, Steinhorn B, Arnér ESJ, Michel T
and Belousov VV: Which Antioxidant System Shapes Intracellular
H2O2 Gradients? Antioxid Redox Signal.
31:664–670. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qi X, Davis B, Chiang YH, Filichia E,
Barnett A, Greig NH, Hoffer B and Luo Y: Dopaminergic
neuron-specific deletion of p53 gene is neuroprotective in an
experimental Parkinson's disease model. J Neurochem. 138:746–757.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao H, Yenari MA, Cheng D, Sapolsky RM
and Steinberg GK: Bcl-2 overexpression protects against neuron loss
within the ischemic margin following experimental stroke and
inhibits cytochrome c translocation and caspase-3 activity.
J Neurochem. 85:1026–1036. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xing ZH, Ma YC, Li XP, Zhang B and Zhang
MD: Research progress of puerarin and its derivatives on
anti-inflammatory and anti-gout activities. Zhongguo Zhongyao
Zazhi. 42:3703–3708. 2017.(In Chinese). PubMed/NCBI
|