Introduction
Sepsis is a life-threatening organ dysfunction
caused by the host's dysfunctional response to infection (1). Myocardial injury is one of the most
common complications of sepsis, which mostly occurs in the middle
and late stages of the disease (2).
It was shown that ~50% of patients with sepsis also experience
complications with myocardial depression (2), which is manifested by left and right
ventricular cardiac dysfunction and reduced left ventricular
ejection fraction; the mortality rate is as high as 70% (3). Cardiac dysfunction may complicate the
course of sepsis and septic shock, and cardiac dysfunction caused
by myocardial injury is an important causes of death in patients
with sepsis (3,4). At present, the specific mechanism of
septic cardiomyopathy is not clear, but activation of the apoptosis
pathway has been reported to serve an important role in septic
myocardial injury (5). A previous
study confirmed that activation of caspases and release of
mitochondrial cytochrome c can be found in septic
cardiomyocytes (6). Inhibition of
apoptosis can reverse the occurrence of septic myocardial injury
(5). Maintaining myocardial
mitochondrial membrane potential and inhibiting the activation of
the apoptotic proteins caspase-3 and caspase-7 can reduce the
apop-totic rate of myocardial cells (7). Therefore, methods to improve
myocardial injury in sepsis and to reduce the apoptosis of
myocardial cells were explored in the present study.
Thrombospondin (THBS; also known as TSP) is a
secretory glycoprotein that serves a role in embryonic development,
wound healing, angiogenesis and the inflammatory response (8,9). THBS
has a multimodular structure, with each region performing different
functions through specific binding of different factors;
high-resolution determination revealed its unique and interesting
pro-tein structure (8).
Thrombospondins (THBSs) are divided into two groups, A and B. Group
A includes THBS1 and THBS2, which can form trimers; group B
includes THBS3, THBS4 and THBS5, which can form pentamers (10). Based on the THBS molecular
structure, THBSs can be divided into two groups, the THBS1 type and
the THBS2 I repetitive sequence TSRs (group A), whereas the other
members do not contain this sequence (group B) (10). TSRs, also known as lysine repeat
sequences, are involved in cell attachment, inhibition of
angiogenesis, protein-protein and protein-mucopolysaccharide
interactions (11); thus, THBS1 can
regulate the adhesion, migration, proliferation and
neovascularization of vascular endothelial cells. Group B members
lack the TSR sequence and therefore have no antiangiogenic effect
in the tumor microenvironment (12).
Among the THBS family, THBS1 can regulate tumor
growth, cell migration and vascular formation (13). Research on THBS1 in septic
cardiomyocytes has rarely been reported (11–13).
The mechanism of organ damage induced by sepsis, especially
myocardial injury, has been examined previously (14); however, the specific effects of
THBS1 on myocardial cell injury, oxidative stress and apoptosis in
sepsis are not entirely understood. In the present study, THBS1
small interfering (si)RNA was transfected into primary cardiac
cells, which are known to have relatively high THBS1 protein
expression, and the effects of THBS1 gene silencing on myocardial
cell injury, oxidative stress, the inflammatory response and
apoptosis were observed.
Materials and methods
Animals, cells and main reagents
Male C57dx newborn mice and male C57BL/6 mice were
pur-chased from the Experimental Animal Center of Shanghai Jiao
Tong University (Shanghai, China). All of the experimental
procedures were performed after obtaining the approval of the
Ethical Committee for Animal Experiments of Shanghai General
Hospital (Shanghai, China; 2020AWS006); all experimental procedures
were carried out according with the guidelines of the Institutional
Animal Care and Use Committee of Shanghai General Hospital.
RPMI-1640 medium and fetal bovine serum were purchased from Gibco
(Thermo Fisher Scientific, Inc.). Lipopolysaccharide (LPS). BCA
protein quantitative kit was purchased from Vazyme Biotech Co.,
Ltd. siTHBS1 and control siRNA were purchased from Santa Cruz
Biotechnology, Inc. Mouse anti-human THBS1 monoclonal antibody
(cat. no. ab1823) was purchased from Abcam. Anti-β-actin (cat. no.
ab8226) was purchased from Abcam and goat anti-rabbit polyclonal
antibodies (HRP-conjugated) (cat. no. 31460; Thermo Fisher
Scientific, Inc.).
Cell culture
A total of 12 male C57dx mice (age, 3 days; weight
1.5–2 g) were randomly selected. After cervical dislocation, the
hearts were extracted and the atria were cut off; the ventricles
were rinsed with 1 ml collagenase, and the heart tissue was cut
into ~1 mm3 pieces. The pieces of heart tissue were
mixed with collagenase and transferred into a 15-ml centrifuge tube
and were repeated-ly shaken and mixed at 37°C for 1 min. After
allowing the tubes to stand and for the solid tissue to settle, the
supernatant was removed, and this was repeated until the tissue
mass was dissolved completely. The obtained supernatant was
centrifuged at 2,000 × g for 10 min (37°C), the supernatant was
discarded and the precipitate was mixed with 2 ml of medium, which
was inoculated into a 75 ml culture bottle. After incubating at
37°C for 90 min, the non-myocardial cells had adhered to the
bottle, and the cell suspension (5×105−6×105
cells/ml), which contained the myocardial primary cells, was
subsequently inoculated into another culture bottle and incubated
at 37°C for 24 h.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the cardiomyocytes
(5×105−6×105 cells/ml) using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The concentration
of RNA was measured by a NanoDrop 8000 spectrophotometer (Thermo
Fisher Scientific, Inc.) and its purity was determined. Total RNA
was reverse transcribed into cDNA using a TransScript All-in-One
First-Strand cDNA Synthesis SuperMix for qPCR (One-Step gDNA
Removal) (Agrisera) for 30 min at 42°C, then 5 sec at 85°C. qPCR
was subsequently performed using Unique Aptamer qPCR SYBR Green
Master Mix (MedChemExpress), according to the manufacturer's
protocol. After the reaction, the amplification and dissolution
curves were verified, and the results were interpreted strictly in
accordance with the instructions of the kit. The 2−ΔΔCq
method (15) was used to calculate
the level of THBS1 mRNA expression. The primer sequences were as
follows: THBS1, forward 5′-GGAAAGAUUUCACUGCAUATT-3′, reverse
5′-UAUGCAGUGAAAUCUUUCCAG-3′; and GAPDH, forward
5′-GTCAAGGCTGAGAACGGGAA-3′, reverse, 5′-AAATGAGCCCCAGCCTTCTC-3′.
The thermocycling conditions used for qPCR were as follows: Initial
denaturation at 94°C for 5 min; followed by 30 cycles of 5 sec at
94°C and annealing and extension at 65°C for 30 sec.
Western blotting
The cells (5×105−6×105
cells/ml) were placed in RIPA lysis buffer (Beyotime Institute of
Biotechnology). The total protein concentration was detected using
a BCA kit. The protein samples (60 µg/well) were separated by 8%
SDS-PAGE and then transferred to a PVDF membrane. The membrane was
blocked with 5% skimmed milk powder (37°C for 1 h) and then
incubated with the indicated primary antibodies, anti-THBS1 (1:200;
cat. no. ab1823) or β-actin (1:200; cat. no. ab8226) at 4°C for 12
h. Following the primary antibody incubation, the cells were washed
with TBS-Tween 20 and incubated with the secondary antibody
(1:2,000) at 37°C for 1 h. Protein bands were visualized with an
ECL system (Abcam) using a western blot gel imager (ChemiDoc;
Bio-Rad Laboratories, Inc.). Protein expression levels were
calculated using ImageJ V1.8.0.112 software (National Institutes of
Health) using actin as the internal reference.
Effects of siTHBS1 on myocardial cell
injury, oxidative stress, inflammatory response and apoptosis
Cell grouping
Primary cells (5×105−6×105
cells/ml) were inoculated into a 25 cm2 culture flask
containing RPMI-1640 medium supplemented with 10% fetal bovine
serum by volume. Then, cells were digested with trypsin and
inoculated into 6-well plates. Four experimental groups were
prepared: i) Control group; ii) LPS group; iii) siTHBS1 group; and
iv) siTHBS1 + LPS group. There was no intervention in the Control
group. Cells were treated at 37°C for 24 h with 1 mg/l LPS to
stimulate the cardiomyocytes. siTHBS1 was transfected into the
cardiomyocytes using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) at 37°C at 24 h with or without
previously stimulating with LPS, aforementioned.
Detection of THBS1 mRNA in cells after
transfection for 48 h
The expression of THBS1 mRNA in the cells was
detected by RT-qPCR, aforementioned. Each group was tested with
three biological replicates twice.
Detection index
Cell injury, oxidative stress, inflammatory response
and apoptosis were detected 48 h after transfection. The cells were
inoculated into a 96-well plate. After routine culture for 48 h,
expression of cardiac troponin I (cTNI) (mouse TNNI3/cTn-I ELISA
kit; cat. no. D721149-0048; Sangon Biotech Co., Ltd.), pro-brain
natriuretic peptide (proBNP; mouse NT-proBNP ELISA kit; cat. no.
E-EL-M0834; Elabscience Biotechnology, Inc.), reactive oxygen
species (ROS; ROS ELISA kit; cat. no. ZK-M5156; ZIKER), IL-6 (IL-6
ELISA kit; cat. no. ab46100; Abcam), TNF-α (TNF-α ELISA kit; cat.
no. SEKM-0034; Beijing Solarbio Science & Technology Co., Ltd.)
and caspase-3 (caspase-3 ELISA kit; cat. no. BH-E0764; Thermo
Fisher Scientific, Inc.) were detected by ELISA. Supernatant was
used in the ELISA, which was obtained by centrifugation of the cell
culture at 4°C at 6,000 × g for 5–10 min.
Sepsis model
Healthy specific pathogen free male C57BL/6 mice
(n=18; age, 8 weeks; weight ~20±2 g) were used. Before the
experiment, the mice were provided free access to food and water,
and maintained at an ambient temperature of 20°C and 40–60%
humidity under a 14/10-h light/dark cycle. Intraperitoneal
injection of LPS 15 mg/kg was used to make the model, and
successful model establishment was determined based on a previous
study (16); sepsis modelling was
confirmed successful by histopathological changes in cardiac muscle
24 h after modelling, as well as determination of cTNI, proBNP,
Il-6, and TNF-α levels by ELISA using serum which was collected
following euthanasia. Three groups (n=6 mice/group) were used in
the in vivo experiments: i) Control group; ii) LPS group;
and iii) siTHBS1 + LPS group (15 nmol/20 g weight siTHBS1 was
injected through the caudal vein). Euthanasia was performed in a
CO2 chamber with a flow rate of 50% volume
displacement/min. The means used to verify mouse death were whether
the heart stopped completely and whether the pupils were
dilated.
H&E, TUNEL and caspase-3
staining
Upright fluorescence Metallurgical Microscope was
NIKON ECLIPSE C1 and inverted fluorescence microscope was NIKON
ECLIPSE TI-SR (magnifications, ×200 and 400). H&E staining and
TUNEL analysis were performed to assess the histopathological
changes in myocardial tissue. Mouse myocardial tissues were fixed
with 4% paraformaldehyde (Beijing Solarbio Science & Technology
Co., Ltd.) for 12 h at 4°C. The nuclei were counterstained in the
TUNEL and caspase-3 staining assay with DAPI for 10 min at 37°C.
Paraffin embedding, tissue sections and H&E staining were
performed (the width of the sections used was 0.5-mm and H&E
staining was performed at 37°C for 30 min). A TUNEL assay kit
(Roche Diagnostics; cat. no. 11684817910) was used to measure the
rate of apoptosis of the cardiomyocytes, according to the
manufacturer's specifications. A caspase-3 fluorometry kit (Wuhan
Servicebio Technology Co., Ltd.) was used for detection of
caspase-3 levels. LDH activity, which is a marker of cell injury,
was not examined because LDH is greatly affected by sepsis
(17).
Collection of clinical samples
A total of 2 ml serum was collected from patients
with sepsis-induced myocardial injury (n=84), those without
sepsis-induced myocardial injury (n=84) and healthy individuals
(n=10) and stored at −80°C. The inclusion criteria included
patients who were diagnosed with sepsis and were 18–80 years old.
The exclusion criteria included the presence of malignant tumors
and post-transplant patients. THBS1 was detected by ELISA,
aforementioned. The prognosis of the patients was recorded during
the 28-day follow-up. Ethics approval was obtained for the use of
humans/human tissues prior to the start of the study from the
ethics committee based at Shanghai General Hospital (approval no.
2020sq049). Informed consent was obtained from each patient prior
to participation. Basic clinicopatholgical features, including age
and sex, of the patients with sepsis-induced myocardial injury
(n=84) are shown in Table I.
 | Table I.Clinicopathological characteristics of
the patients with sepsis-induced myocardial injury. |
Table I.
Clinicopathological characteristics of
the patients with sepsis-induced myocardial injury.
| Clinicopathological
characteristic | Total (n=84) |
|---|
| Males (%) | 51 (60.7) |
| Age, years | 58.68±14.94 |
| Location: Shanghai,
China | 84 (100) |
| Dates of
admission | Jan 2018-Dec
2020 |
| Comorbidities, n
(%) |
|
|
Hypertension | 37 (44.0) |
|
Diabetes | 14 (16.7) |
| Immune
diseases | 8 (9.5) |
|
CKD | 10 (11.9) |
| Liver
disease | 7 (8.3) |
|
COPD | 11 (13.1) |
| Days (every 24 h)
of noradrenaline use | 2.226±3.507 |
| Days (every 24 h)
of mechanical ventilation | 6.333±7.630 |
| SOFA | 7.643±3.998 |
| APACHE II | 16.90±7.108 |
| Mortality (%) | 23 (27.4) |
| Need for dialysis,
day | 1.464±3.276 |
Statistical analysis
SPSS v17.0 (SPSS, Inc.) was used to process the
data. The expression levels of THBS1 mRNA and cTNI, proBNP, ROS,
IL-6, TNF- α and caspase-3 levels in the different groups were
compared by one-way ANOVA; Fisher's LSD test was used for pairwise
comparisons. Kaplan-Meier survival curves were analyzed by
log-rank; the cut-off value used to separate high vs. low THBS1 was
the mean value of expression 271.179 ng/ml. The optimal cutoff
values were determined by the highest values of sensitivity and
specificity indicated in the area under the receiver operatic
characteristic (ROC) curve (AUC) analysis. P≤0.05 was used to
indicate a statistically significant difference.
Results
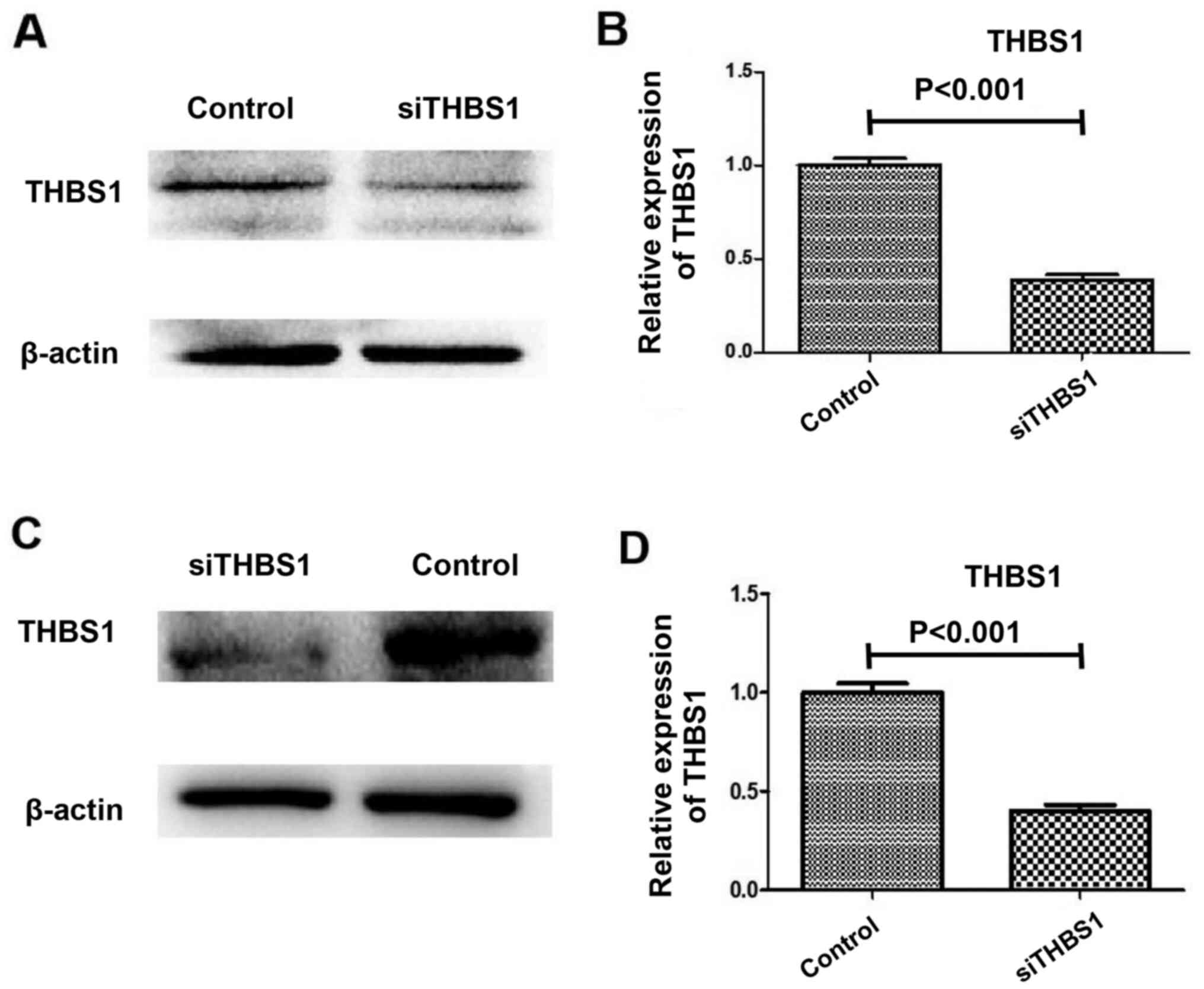
THBS1 knockdown
Successful knockdown of THBS1 by siRNA is shown in
Fig. 1. THBS1 expression was
reduced in the in vivo model mice injected with siTHBS1
compared with the control group, as determined using western
blotting and RT-qPCR (Fig. 1A and
B). Similarly, THBS1 expression was reduced in the in
vitro primary myocardial cell cultures following siTHBS1
transfection compared with the control group, as determined using
western blotting and RT-qPCR (Fig. 1C
and D).
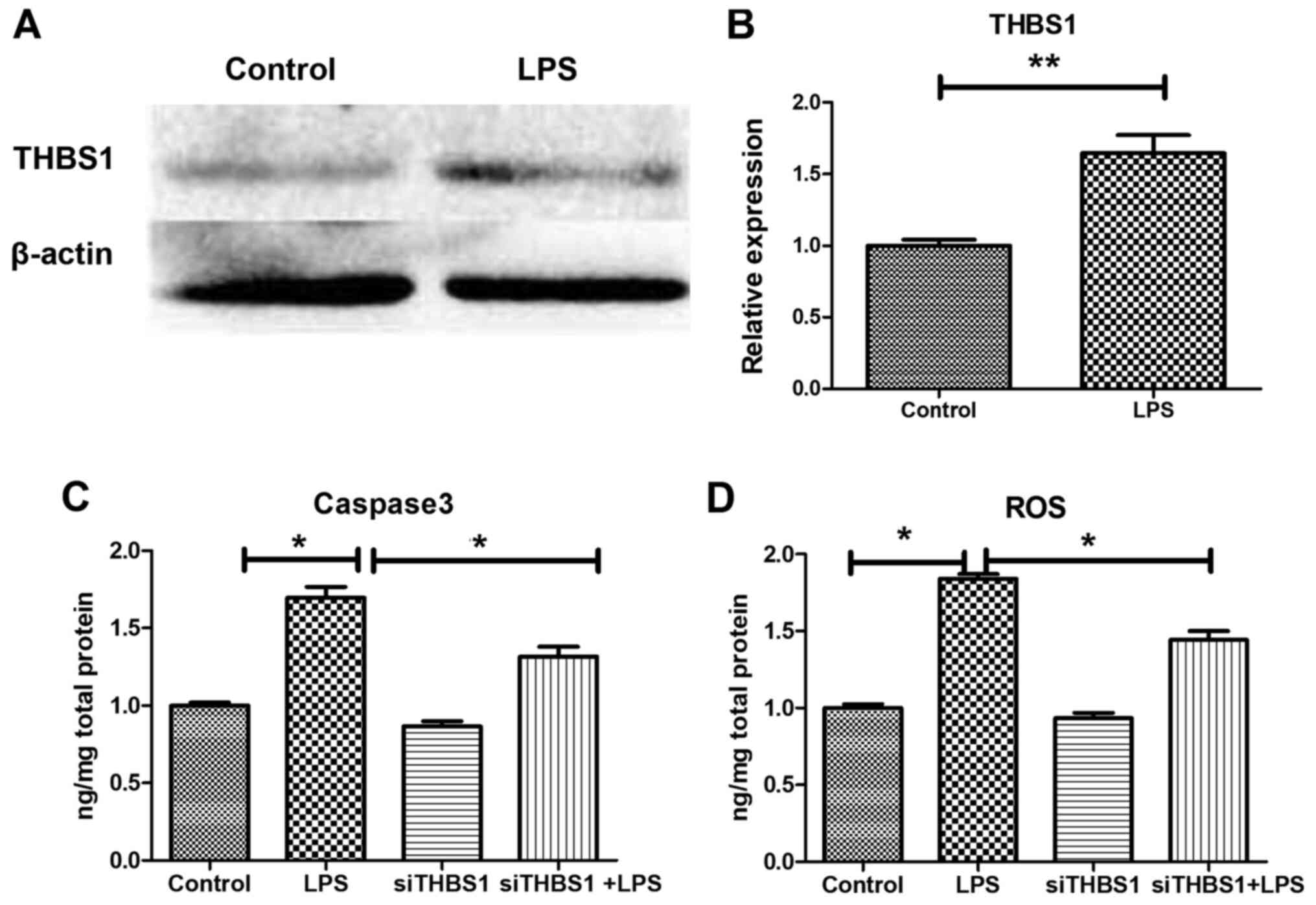
THBS1 expression in vitro
In the LPS-stimulated primary cardiomyocytes,
RT-qPCR analysis demonstrated a significant increase in THBS1 mRNA
expression compared with the Control group (Fig. 2B); a similar increase was observed
for THBS1 protein expression detected by western blotting (Fig. 2A).
Caspase-3 and ROS levels determined by
ELISA in vitro
In LPS-stimulated primary cardiomyocytes, ELISA
demonstrated a significant increase in caspase-3 and ROS levels in
the LPS group compared with the control group. Caspase-3 was
significantly decreased compared with the LPS group, indicating
that myocardial cell apoptosis was decreased (Fig. 2C), and ROS levels were significantly
decreased compared with the LPS group, indicating that the
oxidative stress response was decreased (Fig. 2D).
cTNI, proBNP, Il-6, and TNF-α levels
detected by ELISA in vitro
In the LPS-stimulated primary myocardial cells,
ELISA results demonstrated that the cTNI, proBNP, Il-6 and TNF-α
levels were significantly increased compared with the control group
(Fig. 3A-D, respectively). cTNI,
proBNP, IL-6 and TNF-α levels were significantly reduced in the
siTHBS1 + LPS group compared with the LPS group.
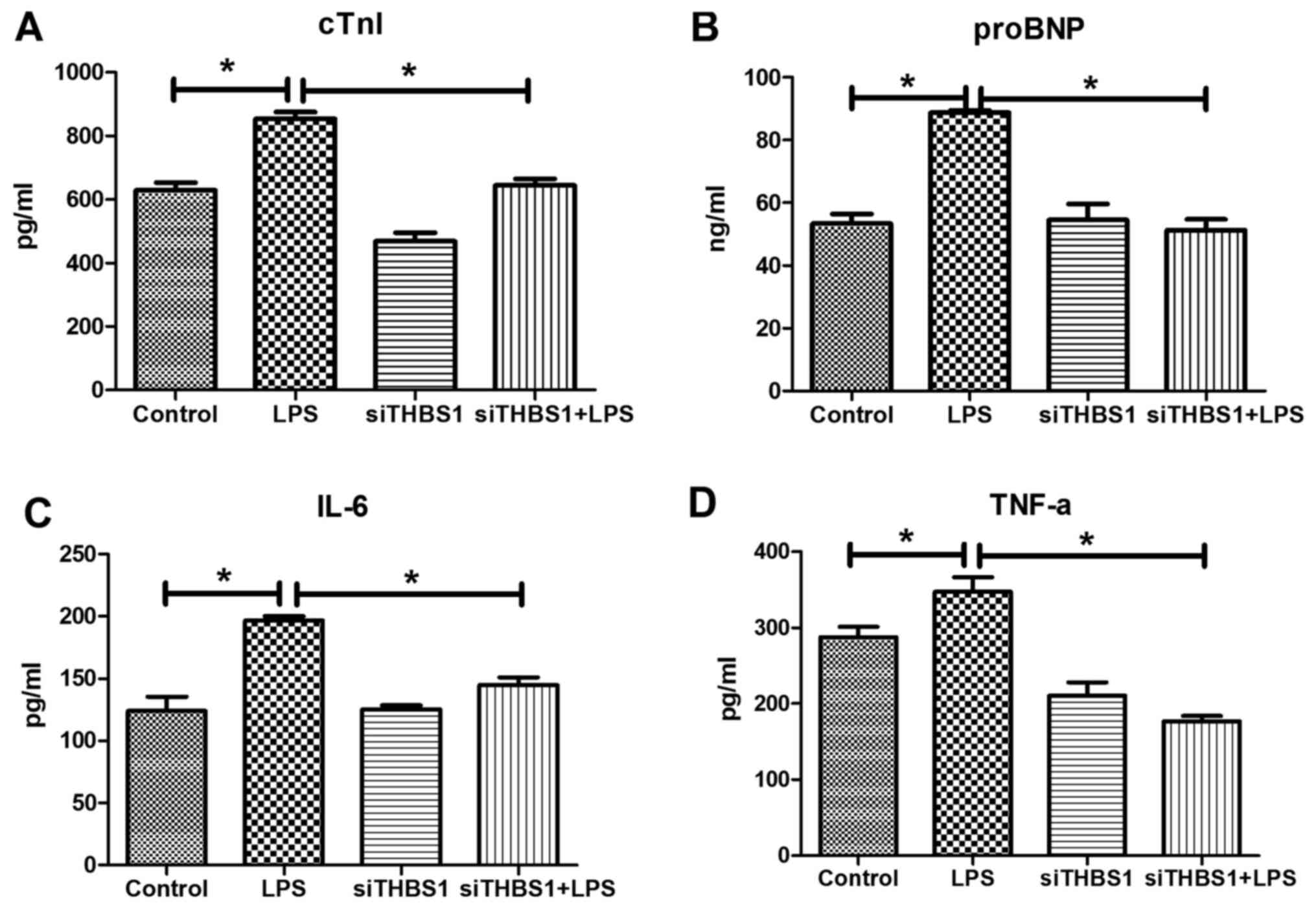 | Figure 3.Levels of cTNI, proBNP, IL-6, and
TNF-α in LPS-stimulated primary cardiomyocytes in vitro.
ELISA was used to determine the levels of (A) cTNI, (B) proBNP, (C)
IL-6 and (D) TNF-α in the various groups. *P<0.05, **P<0.001.
cTnI, cardiac troponin I; LPS, lipopolysaccharide; pro-BNP,
pro-brain natri-uretic peptide; si, small interfering RNA; THBS1,
thrombospondin-1. |
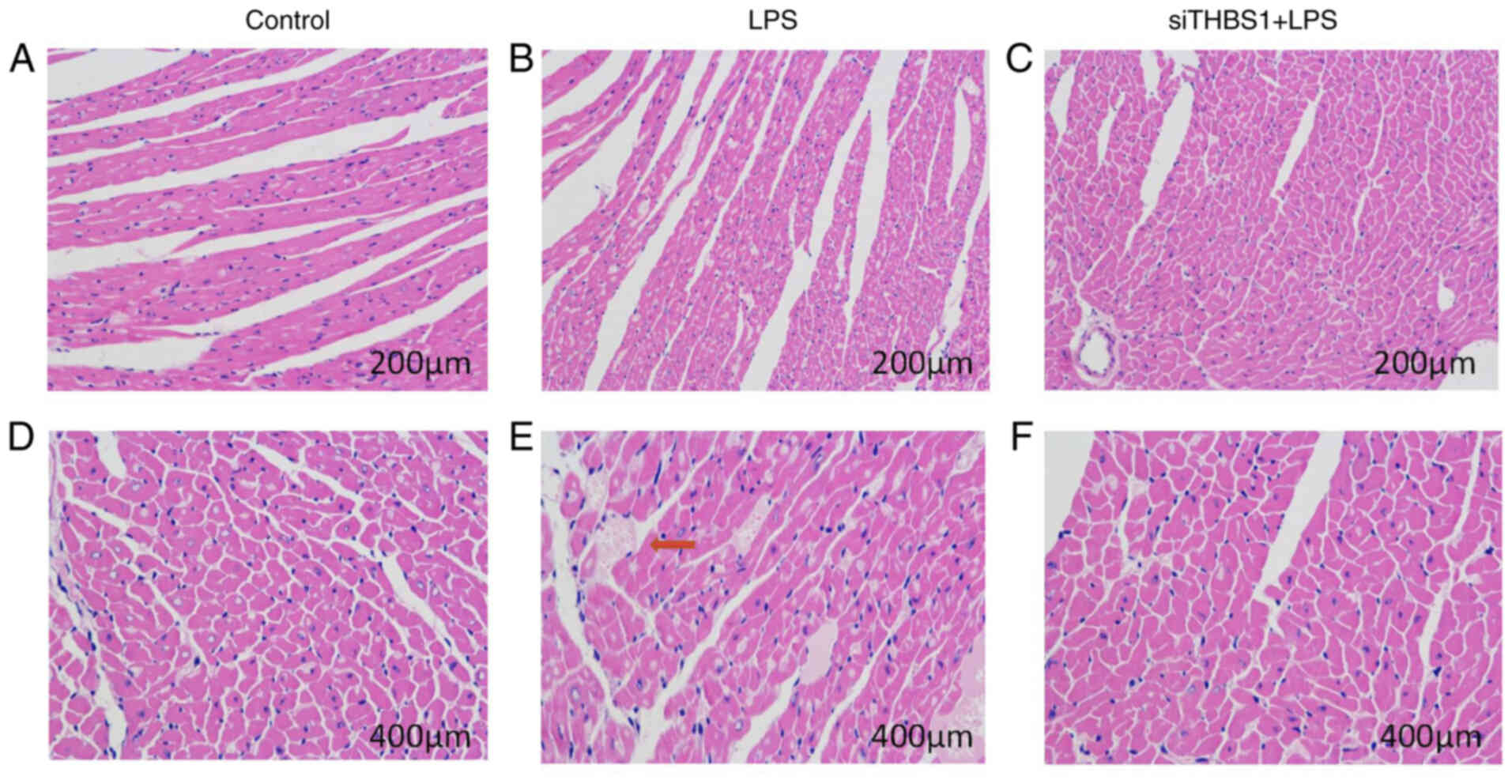
Histopathological changes in cardiac
muscle 24 h after sepsis modelling in vivo
In the mouse cardiac pathology sections from mice
intraperitoneally injected with LPS (15 mg/kg), myocardial
histopathological changes were observed 24 h after mouse modeling
under a light microscope. In the Control group (Fig. 4A and D), the myocardial fibers were
arranged neatly with clear horizontal stripes; the cardiomyocytes
displayed normal morphology, the cytoplasm was pink, the nuclear
membrane was intact, the chromatin was dense and deeply stained,
and there were no significant changes in the myocardial
interstitium. In the LPS group (Fig. 4B
and E) the myocardial fibers were irregular; there was
interstitial edema, hemorrhage, and inflammatory cell infiltration.
In the siTHBS1 + LPS group (Fig. 4C and
F), there was interstitial edema, but inflammatory cell
infiltration was reduced compared with the control. Successful
knockdown of THBS1 by siRNA is shown in Fig. 1A and B.
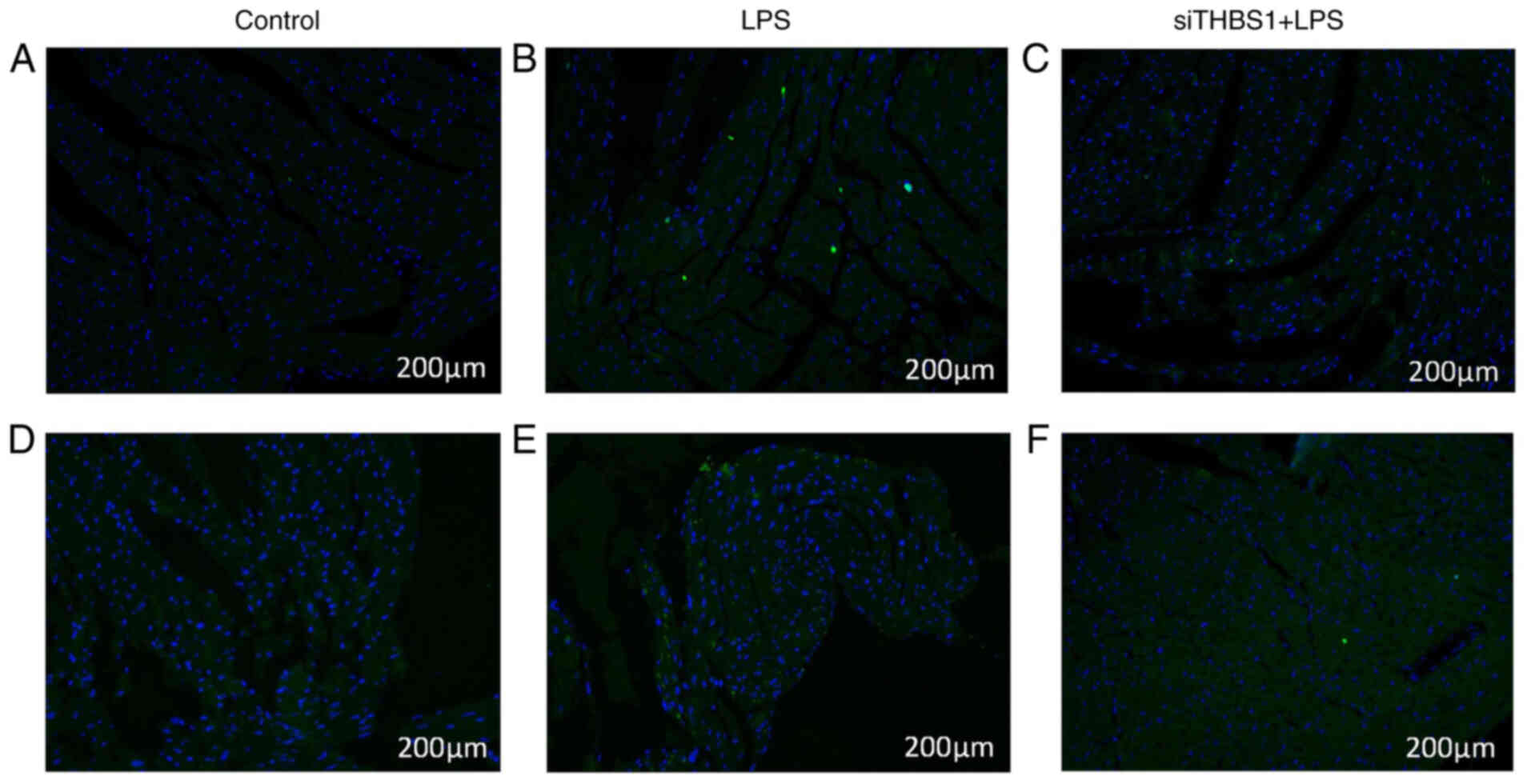
TUNEL analysis and caspase-3
immunofluorescence for myocardial injury in sepsis model mice
TUNEL staining was used to observe the changes of
the myocardial cells in mice 24 h after modeling. LPS (15 mg/kg)
treatment (Fig. 5B) led to
increased apoptosis compared with the Control group (Fig. 5A), whereas the siTHBS1 + LPS group
(Fig. 5C) showed decreased
apoptosis compared with the LPS group.
Caspase-3 immunostaining was used to observe the
changes of myocardial cells in mice 24 h after modeling. The LPS
group (Fig. 5E) displayed increased
caspase-3 expression compared with the Control group (Fig. 5D), and there was decreased caspase-3
expression in the siTHBS1 + LPS group compared with the LPS group
(Fig. 5F).
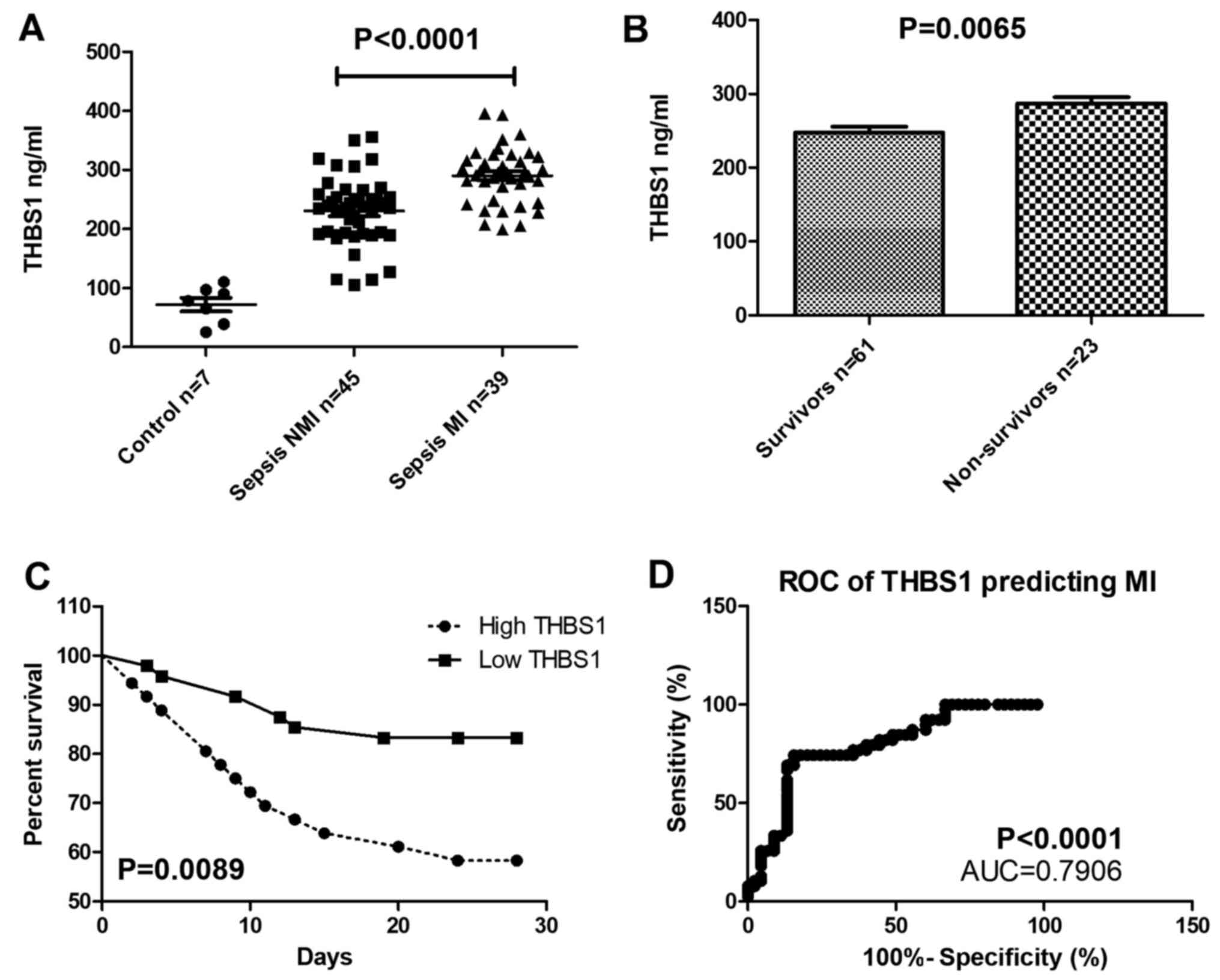
THBS1 expression in patients with
sepsis-induced myocardial injury
THBS1 serum levels were significantly increased in
patients with sepsis accompanied by myocardial injury compared with
the control group (Fig. 6A), and
THBS1 was also higher in deceased patients compared with those who
survived (Fig. 6B). The survival
rate of patients with high THBS1 was lower compared with that of
patients with low THBS1 over the 28-day follow-up (Fig. 6C). THBS1 expression can be used to
predict myocardial injury, the cutoff value of THBS1 is 271.2
ng/ml. When THBS1>271.2 ng/ml, myocardial injury may happen (AUC
=0.7906; P<0.001; Fig. 6D).
Discussion
Although THBS1 has been shown to have positive roles
on various biological functions (18), the effects of THBS1 for the
treatment of cardiac injury are unclear. THBS1 promotes the over
production of free radicals and ROS (18). Free radicals attack the cell
membrane, increase cell death and result in the production of
caspase-3 (18). Results from the
present study demonstrated that decreased THBS1 expression by siRNA
effectively reducing caspase-3 levels and decreasing ROS
concentration and cTnI activity following LPS treatment. These
results indicated that decreased THBS1 may decrease oxidative
damage in LPS-induced cardiac injury. Thus, whether THBS1 takes
part in the process of LPS-induced cardiac injury requires further
investigation.
Apoptosis is a type of programmed cell death that
can be triggered by environmental or chemical irritants (1,16,17).
In the early stage of apoptosis, caspase-3 is activated. The
activated caspase-3 consists of two large subunits (17 kDa) and two
small subunits (12 kDa), which eventually leads to apoptosis
(4). The present study demonstrated
that decreased THBS1 expression leads to decreased levels of
caspase-3. Therefore, the results suggested that THBS1 may promote
LPS-induced myocardial apoptosis.
As a potent inflammatory mediator, TNF-α can be
activated by oxidative stress (5).
Under the stimulation of pro-inflammatory cytokines, TNF-α is
activated, it accumulates and subsequently accelerates the
production of IL-6, which is a pro-inflammatory cytokine (5). Owing to the adverse impact of
oxidative stress, the cell upregulates genes involved in
inflammation to activate or amplify the inflammatory response
(17). The present study results
showed that the LPS-induced increases in TNF-α and IL-6 levels were
substantially suppressed by siTHBS1 co-treatment in vitro.
Therefore, decrease THBS1 expression may protect against
LPS-induced cardiac injury by reducing the expression of
inflammatory cytokines to weaken the inflammatory response.
Studies on THBS1 in human sepsis are extremely
limited, with a few studies showing no clear correlation between
THBS1 expression and the prognosis of sepsis (19,20),
and a single-center cohort study in intensive care showed no
correlation between baseline THBS1 concentration and mortality in
patients with sepsis (19).
However, in the present study, THBS1 was found to be related to
myocardial damage, and may be an important area for further
multicenter, large sample confirmation.
Individual studies have confirmed that THBS1 may
contribute to mortality in murine sepsis by affecting innate
immunity (20). Regarding
inflammation, Xing et al (21) found that the production of THBS1
regulates the secretion of inflammatory cytokines in the THP-1
human monocyte cell line through the NF-κB signaling pathway
(21). It has been shown that
gingival stem cells can improve LPS-induced inflammation by
secreting TGF-β3 and THBS1, which can induce M2-polarization of
macrophages (22). In the present
study, siTHBS1 led to reduced inflammation, reduced oxidative
stress, reduced myocardial injury and reduced apoptosis of
cardiomyocytes. The specific mechanism still needs further
investigation.
Results from the present study demonstrated that the
expression level of THBS1 mRNA in LPS-induced primary
cardiomyocytes was higher compared with the expression levels in
untreated cardiomyocytes. This study also confirmed in clinical
samples that the expression level of THBS1 in sepsis patients with
myocardial injury was also higher compared with that in the normal
control group. An siRNA was constructed and transfected it into
primary cardiomyocytes with the highest THBS1 expression. THBS1
mRNA expression in the cells was significantly downregulated
compared with the control, which resulted in lower oxidative stress
and apoptosis. To the best of our knowledge, this is the first
study to report the effects of THBS1 expression on myocardial cell
injury, oxidative stress and apoptosis in sepsis.
In conclusion, the results of the present study
suggested that THBS1 may be closely related to the occurrence and
development of sepsis-induced myocardial injury, which may lay a
foundation for research into THBS1 as a therapeutic target for
myocardial injury in sepsis.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Second
Round of Key Support Projects of The Three-Year Action Plan for
Promoting Clinical Skills and Clinical Innovation in Municipal
Hospitals (grant no. SHDC2020CR5010-003), The Emerging Advanced
Technology Joint Research Project of Shanghai Shenkang Hospital
Development Center (grant no. SHDC12019131), The Clinical Research
Innovation Plan of Shanghai General Hospital (grant no.
CTCCR-2016B01), The Wu Jieping Medical Foundation (grant no.
320.6750.18546), The Songjiang District Science and Technology
Project (grant no. 19SJKJGG92) and The Clinical Research Innovation
Plan of Shanghai Shenkang Center (grant no. SHDC2020CR2013A), 3rd
Sansheng TCP Young and Middleaged Scientific Research
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YX and RW confirm the authenticity of all the raw
data. YX, JZ, RT, WJ and RW contributed to the study conception and
design, analysis and interpretation of data, drafting of the
article and critical revision for important intellectual content.
All of the authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All of the experimental procedures were performed
after obtaining the approval of the Ethical Committee for Animal
Experiments of Shanghai General Hospital (Shanghai, China;
2020AWS006); all experimental procedures were carried out according
with the guidelines of the Institutional Animal Care and Use
Committee of Shanghai General Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rhodes A, Evans LE, Alhazzani W, Levy MM,
Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally
ME, et al: Surviving Sepsis Campaign: International Guidelines for
Management of Sepsis and Septic Shock: 2016. Crit Care Med.
45:486–552. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frencken JF, Donker DW, Spitoni C,
Koster-Brouwer ME, Soliman IW, Ong DSY, Horn J, van der Poll T, van
Klei WA, Bonten MJM, et al: Myocardial Injury in Patients With
Sepsis and Its Association With Long-Term Outcome. Circ Cardiovasc
Qual Outcomes. 11:e0040402018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aneman A and Vieillard-Baron A: Cardiac
dysfunction in sepsis. Intensive Care Med. 42:2073–2076. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie Y, Tian R, Jin W, Xie H, Du J, Zhou Z
and Wang R: Antithrombin III Predicts Acute Kidney Injury in Septic
Elderly Patients. Exp Ther Med. 19:1024–1032. 2020.PubMed/NCBI
|
|
5
|
Liu YC, Yu MM, Shou ST and Chai YF:
Sepsis-induced cardiomyopathy: Mechanisms and treatments. Front
Immunol. 8:10212017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Buerke U, Carter JM, Schlitt A, Russ M,
Schmidt H, Sibelius U, Grandel U, Grimminger F, Seeger W,
Mueller-Werdan U, et al: Apoptosis contributes to septic
cardiomyopathy and is improved by simvastatin therapy. Shock.
29:497–503. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peng S, Xu J, Ruan W, Li S and Xiao F:
PPAR- γ activation prevents septic cardiac dysfunction via
inhibition of apoptosis and necroptosis. Oxid Med Cell Longev.
2017:1–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lopez-Dee Z, Pidcock K and Gutierrez LS:
Thrombospondin-1: Multiple paths to inflammation. Mediators
Inflamm. 2011:2960692011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bornstein P: Thrombospondins function as
regulators of angiogenesis. J Cell Commun Signal. 3:189–200. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stenina-Adognravi O: Thrombospondins: Old
players, new games. Curr Opin Lipidol. 24:401–409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bigé N, Boffa JJ, Lepeytre F and Shweke N:
Rôle de la thrombospondine-1 dans le développement des maladies
rénales [Role of thrombospondin-1 in the development of kidney
diseases. Med Sci (Paris). 29:1131–1137. 2013.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuzmanov A, Wielockx B, Rezaei M,
Kettelhake A and Breier G: Overexpression of factor inhibiting
HIF-1 enhances vessel maturation and tumor growth via
platelet-derived growth factor-C. Int J Cancer. 131:E603–E613.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weng TY, Wang CY, Hung YH, Chen WC, Chen
YL and Lai MD: Differential Expression Pattern of THBS1 and THBS2
in Lung Cancer: Clinical Outcome and a Systematic-Analysis of
Microarray Databases. PLoS One. 11:e01610072016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Larche J, Lancel S, Hassoun SM, Favory R,
Decoster B, Marchetti P, Chopin C and Neviere R: Inhibition of
mitochondrial permeability transition prevents sepsis-induced
myocardial dysfunction and mortality. J Am Coll Cardiol.
48:377–385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takehara K, Murakami T, Kuwahara-Arai K,
Iba T, Nagaoka I and Sakamoto K: Evaluation of the effect of
recombinant thrombomodulin on a lipopolysaccharide-induced murine
sepsis model. Exp Ther Med. 13:2969–2974. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu J, Wei Z, Jiang H, Cheng L, Chen Q,
Chen M, Yan J and Sun Z: Lactate dehydrogenase is associated with
28-day mortality in patients with sepsis: A retrospective
observational study. J Surg Res. 228:314–321. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao JB, Tang WD, Wang HX and Xu Y:
Predictive value of thrombospondin-1 for outcomes in patients with
acute ischemic stroke. Clin Chim Acta. 450:176–180. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van der Wekken RJ, Kemperman H, Roest M
and de Lange DW: Baseline thrombospondin-1 concentrations are not
associated with mortality in septic patients: A single-center
cohort study on the intensive care unit. Intensive Care Med Exp.
5:72017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McMaken S, Exline MC, Mehta P, Piper MC,
Wang Y, Fischer SN, Newland CA, Schrader CA, Basler SR, Sarkar A,
et al: Thrombospondin-1 Contributes to Mortality in Murine Sepsis
through Effects on Innate Immunity. PLOS ONE.
6:e196542011.https://doi.org/10.1371/journal.pone.0019654
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xing T, Wang Y, Ding WJ, Li YL, Hu XD,
Wang C, Ding A and Shen JL: Thrombospondin-1 Production Regulates
the Inflammatory Cytokine Secretion in THP-1 Cells Through NF-κB
Signaling Pathway. Inflammation. 40:1606–1621. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen X, Yang B, Tian J, Hong H, Du Y, Li
K, Li X, Wang N, Yu X and Wei X: Dental Follicle Stem Cells
Ameliorate Lipopolysaccharide-Induced Inflammation by Secreting
TGF-β3 and TSP-1 to Elicit Macrophage M2 Polarization. Cell Physiol
Biochem. 51:2290–2308. 2018. View Article : Google Scholar : PubMed/NCBI
|